Introduction
Bone is a metabolically dynamic tissue that
undergoes continuous renewal. The skeletal reconstruction process
consists of bone resorption by osteoclasts, and bone formation by
osteoblasts. The occurrence of resorption and formation ensures the
basal bone metabolism, thereby maintaining bone homeostasis.
Several factors, including systemic hormones, growth factors,
minerals and trace elements, have been shown to routinely regulate
the balance between these two processes. One of the trace elements
involved in these processes is strontium (Sr), which has long been
of particular interest due to its dual skeletal effects (1,2).
Strontium ranelate [RanSr; a strontium(II) salt with
ranelic acid] has been demonstrated to function as a medication for
postmenopausal osteoporosis (3-5).
This drug has been extensively used to inhibit massive bone loss
(6-8). Strontium (II) exhibits a dual
mechanism of action, inhibiting bone resorption and stimulating
bone formation. Although the beneficial effects of Sr on
osteogenesis in different models have been corroborated by numerous
previously published studies (1,9,10),
the mechanisms underpinning Sr action on bone reconstruction have
yet to be fully elucidated; indeed, an incomplete understanding of
the mechanism presents one of the major obstacles for the
successful application of Sr in clinical practice.
Macro-autophagy (henceforth, referred to as
autophagy) is known to be an ubiquitous intracellular degradation
process through which cells protect themselves. During autophagy,
the autophagosome, which contains dysfunctional proteins and futile
macromolecules, fuses with a lysosome to form the autolysosome,
where degradation occurs. In response to multiple stresses, such as
nutrition deficiency, tumor formation or aging, autophagy tends to
exert its function as a cell survival mechanism (11). In addition, accumulating evidence
has demonstrated that autophagy is also involved in osteogenesis
and bone development (12,13).
Furthermore, emerging evidence has suggested that AMP-activated
protein kinase (AMPK) and mammalian target of rapamycin (mTOR) are
crucial for autophagy (14-16).
The present study aimed to investigate the
interaction between Sr-inducing osteogenic differentiation and
autophagy. The underlying mechanisms, and the connection of the
AMPK/mTOR signaling pathway with this process, were also explored
in this study.
Materials and methods
Cell culture
MC3T3-E1 osteoblastic cells (subclone 14) were
purchased from the National Infrastructure of Cell Line Resource
(no. 3131C0001000300015) and routinely cultured at a density of
105 cells/well in HyClone™ α-modified Eagle's medium
(α-MEM) (Thermo Fisher Scentific, Inc.) supplemented with 10% (v/v)
Gibco™ fetal bovine serum (FBS) (Thermo Fisher Scientific, Inc.),
100 U/ml penicillin and 100 g/ml streptomycin (Sigma-Aldrich; now a
brand of Merck KGaA) at 37°C. To induce differentiation, the cells
were cultured in osteoinductive medium comprising α-MEM, 10% FBS,
1% penicillin-streptomycin, 10 mM β-glycerophosphate and 100
µg/ml ascorbic acid. For the experimental group, 3 mM
SrCl2 (Merck KGaA) was dissolved in normal saline before
being added to medium, and the cells were incubated for a different
number of days (3, 7 or 21 days). Cells grown in osteoinductive
medium containing the identical components, but without Sr, were
used the negative control.
MTT assay
Cell proliferation was assessed by MTT assay,
according to the manufacturer's protocol (Nanjing KeyGen Biotech
Co., Ltd.). The cells (5×103/ml) were seeded into
96-well plates. Following 24 h of incubation at 37°C, various
concentrations of Sr (0, 3, 6, 12, 24, 48, or 96 mM) were added to
the cells. After a further 72 h, the absorbance of the cells was
measured at 490 nm on a microplate reader (Synergy™ 2; BioTek
Instruments, Inc.).
Alkaline phosphatase (ALP) staining and
Alizarin red staining
For the analysis of mineralization, the MC3T3-E1
cells (5×104 cells/well) were seeded into 6-well plates.
Following 24 h of culture at 37°C, various concentrations of Sr
(i.e., 3, 6 or 12 mM) were added to the wells. ALP activity was
determined after a further 7 days by staining with
5-bromo-4-chloro-3-indolyl phosphate (BCIP)/nitro blue tetrazolium
(NBT/ALP) dyeing fluid for 30 min at room temperature (Beyotime
Institute of Biotechnology, Haimen, China). Mineralized nodules
were stained with Alizarin Red solution (Merck KGaA) for 30 min at
room temperature after 21 days.
RT-qPCR
MC3T3-E1 cells (5×104 cells/well) were
seeded in 12-well plates. Following 24 h of culture at 37°C,
various concentrations of Sr (3, 6 or 12 mM) were added to the
wells, and the cells were cultured for 72 h. Total RNA was
extracted using Invitrogen® TRIzol™ reagent (Thermo
Fisher Scientific, Inc.). A Nanodrop™ 2000c spectrophotometer
(Thermo Fisher Scientific, Inc.) was used to quantity the
concentration of total RNA. Aliquots (2 µg) of total RNA
were employed in RT reactions using a FastQuant RT Super Mix kit
(TianGen Biotech Co., Ltd., Beijing, China). RT-qPCR using the
Stratagene® MX3005P system (Agilent Technologies, Inc.)
was performed using SuperReal PreMix Plus (SYBR-Green; TianGen
Biotech Co., Ltd.) according to the manufacturers' protocol. The
thermal conditions were as follows: 95°C for 15 min, followed by 40
cycles at 95°C for 15 sec, 60°C for 20 sec and 72°C 20 sec. The
expression levels were normalized to GAPDH. The data obtained were
analyzed using the 2−ΔΔCq method, where ΔCq is the value
from the threshold cycle (Cq) of the treated sample subtracted from
the Cq value of control samples (17). The primers used in the present
study were obtained from General Biosystems and were as follows:
Runt-related transcription factor 2 (RUNX2) forward, 5′-GCT ATT AAA
GTG ACA GTG GAC GG-3′ and reverse, 5'-GGC GAT CAG AGA ACA AAC TAG
G-3'; osteocalcin (OCN) forward, 5'-AAG CAG GAG GGC AAT AAG GT-3'
and reverse, 5'-CAA GCA GGG TTA AGC TCA CA-3'; and GAPDH forward,
5'-CGT CCC GTA GAC AAA ATG GT-3' and reverse, 5'-AAT GGC AGC CCT
GGT GAC-3'.
Western blot analysis
The MC3T3-E1 cells were plated in 12-well plates at
a density of 5×l04 cells/well and treated with Sr (3, 6
or 12 mM) for 3 days. The cells were then lysed with SDS lysis
buffer (Nanjing KeyGen Biotech Co., Ltd., Nanjing, China). Equal
amounts of protein were resolved by SDS-PAGE, and the 10% gels were
transferred to PVDF membranes (Merck KGaA) using a semi-dry
transfer method. The membranes were blocked in 5% non-fat milk in
TBS/0.05% Tween-20 at room temperature for 2 h. Subsequently, the
blocked membranes were incubated with the appropriate specific
primary antibody overnight at 4°C. Primary antibodies against the
following targets were used: OCN antibody (polyclonal, rabbit
anti-mouse, 1:1,000 dilution, cat. no. DF12303, Affinity
Biosciences); sequestosome-1 (SQSTM1/p62) antibody (polyclonal,
rabbit anti-mouse, 1:1,000, cat. no. AF5384, Affinity Biosciences);
microtubule-associated protein 1 light chain 3 (LC3) A/B (D3U4C)
rabbit mAb (polyclonal, rabbit anti-mouse, 1:1,000, cat. no. 12741,
Cell Signaling Technology, Inc.); mTOR antibody (polyclonal, rabbit
anti-mouse, 1:1,000, cat. no. AF6308, Affinity Biosciences);
phosphor-mTOR (Ser-2448) antibody (polyclonal, rabbit anti-mouse,
1:1,000, cat. no. AF3308, Affinity Biosciences); AMPK1 antibody
(polyclonal, rabbit anti-mouse, 1:1,000, cat. no. AF6422, Affinity
Biosciences); phospho-AMPK-α (Thr-172) antibody (polyclonal, rabbit
anti-mouse, 1:1,000, cat. no. AF3423, Affinity Biosciences);
tubulin antibody (polyclonal, rabbit anti-mouse, 1:2,000, cat. no.
AF7011, Affinity Biosciences). The membranes were then incubated
with goat anti-rabbit horseradish peroxidase-conjugated secondary
antibody (cat. no. S0001; Affinity Biosciences) at a concentration
of 1:10,000 for 1 h at room temperature. Signals were detected with
Immobilon™ Western Chemiluminescent HRP substrate (Merck KGaA)
using the ChemiDoc XRS+ imaging system (Bio-Rad
Laboratories, Inc.). ImageJ software (National Institutes of
Health, v 1.8.0) was used to analyze protein band intensity. All
the western blot analysis experiments were repeated 3 times
independently.
Monodansylcadaverine (MDC) staining
MC3T3-E1 cells (105 cells/well) were
plated in 6-well plates and treated with 3 mM Sr. The cells were
cultured with osteogenic medium for 3 days at 37°C, and were then
stained with MDC for 45 min at room temperature (Nanjing KeyGen
Biotech Co., Ltd.) according to the manufacturer's protocol. The
fluorescence of the wells containing the attached cells was
measured using a fluorescence microscope (512 nm emission
wavelength; Nikon Corp.). The presence of acidic vesicles,
indicating the activated autophagosome, was determined by measuring
the level of green fluorescence.
Treatment with 3-methyladenine
(3-MA)
3-MA (100 mM; Merck KGaA) was dissolved in DMSO and
stored at −20°C prior to use. The stock was heated to 65°C in order
to obtain a clear solution, and subsequently diluted with α-MEM.
Prior to 3-MA treatment, the MC3T3-E1 cells were cultured in
12-well plates at 37°C until they reached ~80% confluence, and 10
mM 3-MA were added to the cells and continued to culture for 6 h.
Following pre-incubation of 3-MA, osteoinductive medium with or
without 3 mM Sr was added. The cells were subsequently incubated
for an additional 3 days.
Treatment with dorsomorphin (compound
C)
AMPK inhibitor compound C was purchased from Selleck
Chemicals, dissolved in DMSO (1 mM), and stored at −20°C prior to
use. The stock was diluted to 5 µM with α-MEM. The MC3T3-E1
cells were cultured in 12-well plates at 37°C until they reached
~80% confluence, and were then pre-incubated with 5 µM
compound C for 12 h. Following pre-incubation, osteoinductive
medium with or without 3 mM Sr was added. The cells were
subsequently incubated for an additional 3 days.
Statistical analysis
All the results are expressed as the means ± SD for
a minimum of 3 independently performed experiments. All data were
analyzed using GraphPad Prism 7.0 software (GraphPad Software,
Inc.). Statistical analysis was performed using either a two-tailed
Student's t-test or one-way ANOVA followed by post-hoc Tukey's test
for multiple comparisons. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effect of Sr on the viability of MC3T3-E1
cells
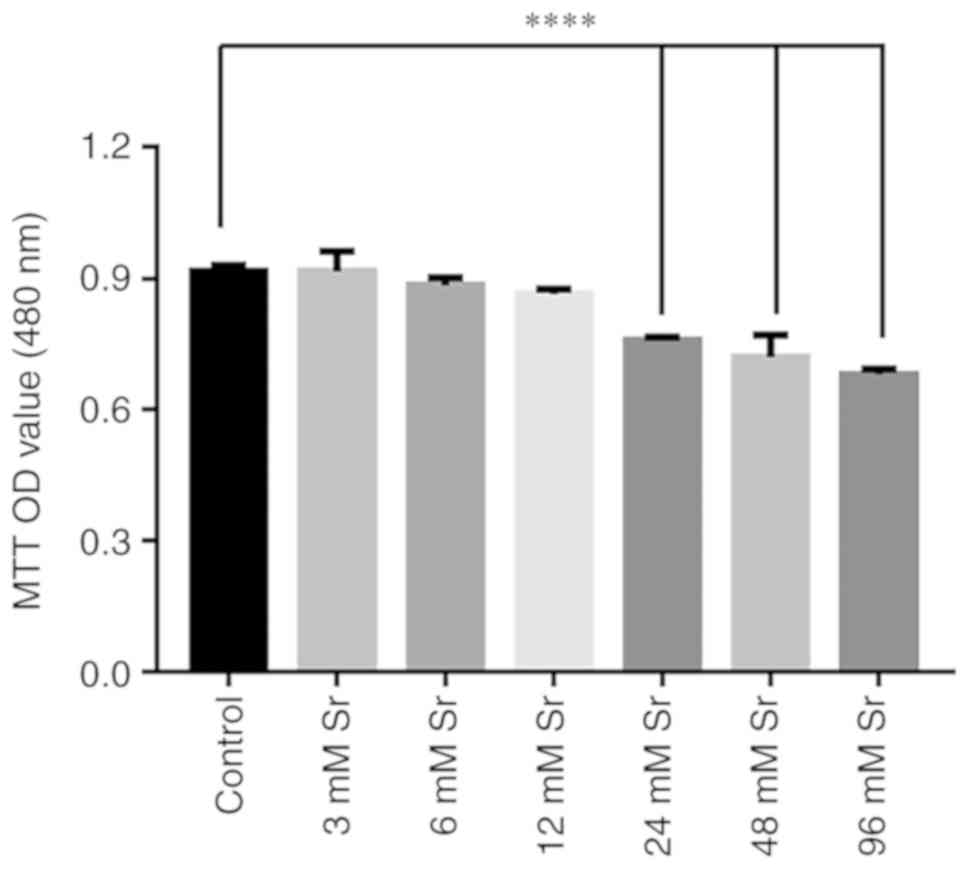
The effects of Sr at a wide concentration range on
the viability of the MC3T3-E1 cells was investigated by MTT assay.
As shown in Fig. 1, there was no
significant effect on cell viability observed when the cells were
treated with 3-12 mM Sr for 3 days. However, the exposure of
MC3T3-E1 to >24 mM Sr markedly decreased the viability of the
cells. As the viability of the MC3T3-E1 cells was markedly
decreased upon exposure to high levels of Sr, the safe range of
concentrations of Sr to be administered were selected to be 3-12 mM
for use in subsequent experiments to observe the osteogenic
differentiation of MC3T3-E1 cells.
Effect of Sr on the osteogenic
differentiation of MC3T3-E1 cells
Several assays were applied to examine the effects
of treatment with a low concentration of Sr on osteogenic
differentiation. First, the expression of genes associated with
osteogenic differentiation, namely RUNX2 and OCN, was assessed by
RT-qPCR. As shown in Fig. 2A and
B, treatment with 3 mM Sr significantly increased the
expression of both genes. The protein level of OCN was
correspondingly increased under 3 mM Sr treatment (Fig. 2C and D). Although Sr at the
concentrations of 6 and 12 mM exerted no toxic effects on cell
growth in the previous experiments shown above, these
concentrations had no marked effect on RUNX2 expression compared to
the control and suppressed the expression of OCN. In addition,
similar trends were observed based on the experiments involving ALP
staining and Alizarin Red staining. Following 7 days of Sr
treatment, the MC3T3-E1 cells exhibited the highest ALP activity at
the concentration of 3 mM Sr compared with the other groups
(Fig. 2E). The Alizarin Red
staining results revealed that the cells treated with 3 mM Sr
exhibited the optimal quantity and intensity of color (Fig. 2F). Taken together, these
experiments revealed that 3 mM Sr elicited the most pronounce
effects on the osteogenic differentiation of MC3T3-E1 cells;
therefore, 3 mM Sr was selected for use in subsequent
experiments.
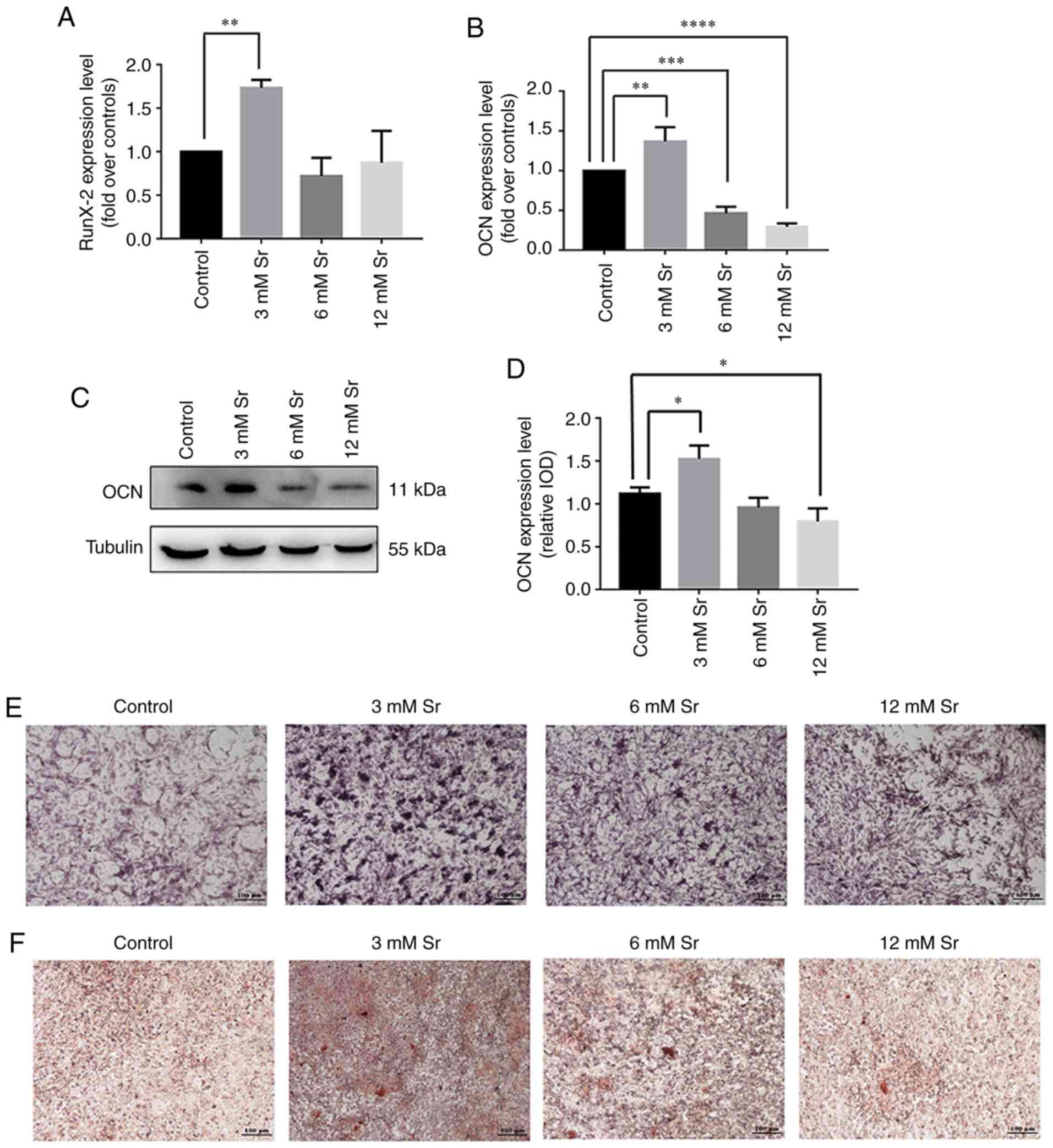 | Figure 2Effect of Sr on osteogenic
differentiation of MC3T3-E1 cells. The MC3T3-E1 cells were treated
with various concentrations of Sr (3, 6, or 12 mM). (A and B) Gene
expression of RUNX2 and OCN following an incubation period for the
cells of 3 days, detected by RT-qPCR, and normalized against GAPDH.
The results are expressed as the means ± SD (n=3 for each group).
**P<0.005; ***P<0.0005; and
****P<0.0001 compared with the control group. (C)
Western blot analysis of OCN following incubation of the cells for
3 days. (D) Grayscale analysis was used to compare the results of
western blot analysis among all concentrations of Sr and the
control groups. The results are expressed as the means ± SD (n=3
for each group). *P<0.05. (E) ALP staining on day 7
(original magnification, x4). (F) Alizarin Red staining on day 21
(original magnification, x4). Sr, strontium chloride; ALP, alkaline
phosphatase; OCN, osteocalcin; RUNX2, Runt-related transcription
factor 2. |
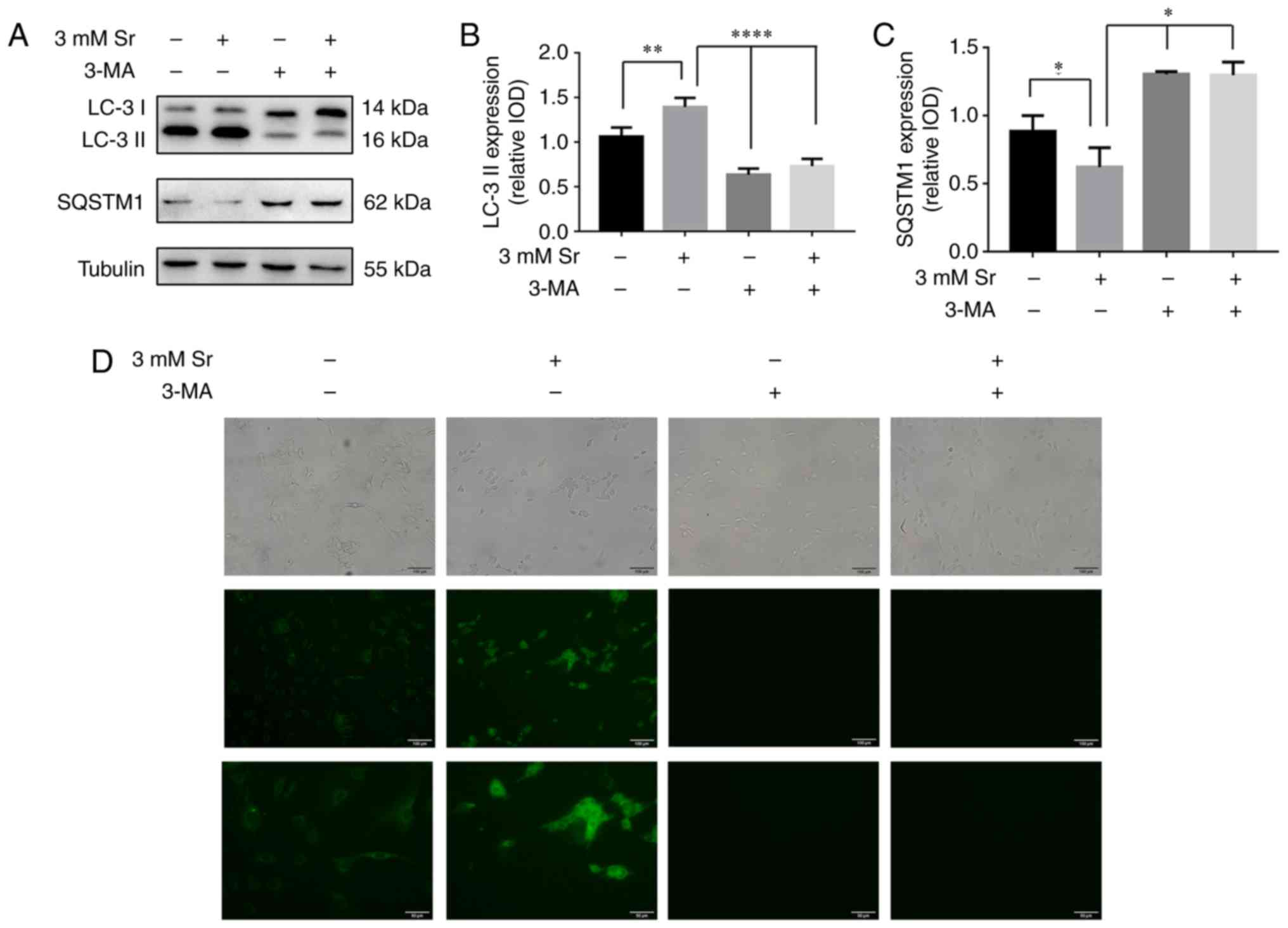
Autophagy participates in the process of
Sr-induced osteo- genic differentiation
Two markers for cellular autophagic activity, the
two isoforms of LC3, LC3-I/II, and SQSTM1/p62, were used to examine
the effects of autophagy on Sr-mediated osteogenic differentiation.
The essential autophagy-associated protein, SQSTM1/p62, functions
as an ubiquitin-binding protein, and is degraded during selective
autophagy progression. Upon the induction of autophagy, LC3-I
becomes acylated (i.e., it is converted into LC3-II), and inserts
itself into the autophagosomal membrane (18). In the present study, based on the
western blot analysis experiments, the conversion rate of LC3-I
into LC3-II was significantly increased, and this represents the
most critical event in autophagosome formation (Fig. 3A and B). Simultaneously, the
expression level of SQSTM1/p62 was significantly decreased
(Fig. 3A and C), and this protein
is involved in autolysosome degradation (18). To corroborate the current results,
the cells were also stained with MDC. These results confirmed that
the fluorescent Sr-treated cells exhibited a more obvious punctate
shape, which corroborated the protein expression results (Fig. 3D).
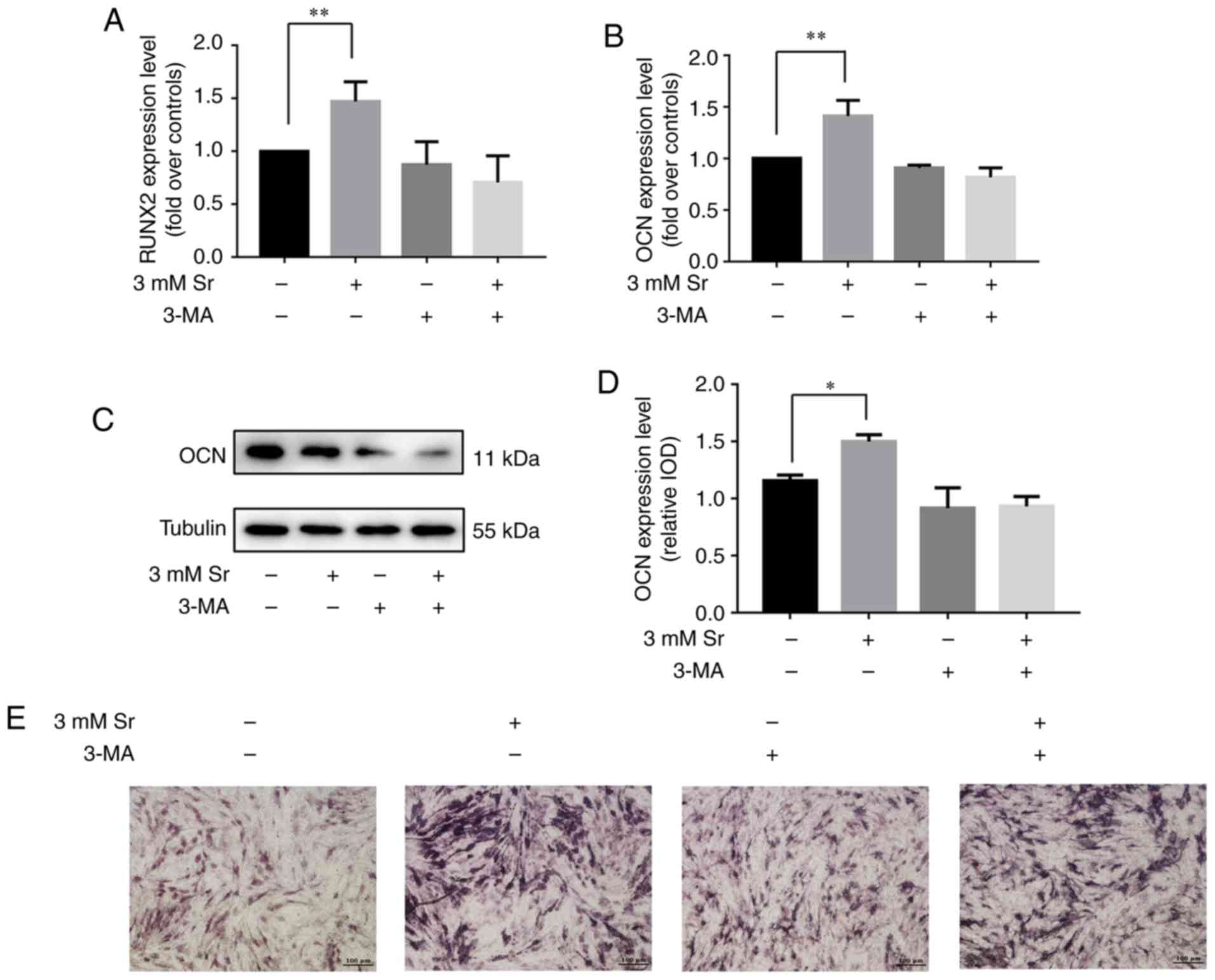
Inhibition of autophagy suppresses
Sr-induced osteogenic differentiation
3-MA has been shown to inhibit the progression of
autophagy by blocking autophagosome formation via the inhibition of
the type III phosphoinositide 3-kinase (PI3K) (19). Thus, in this study, to further
confirm whether autophagy is involved in the Sr-induced osteogenic
differentiation, the MC3T3-E1 cells were incubated with 10 mM 3-MA
for 6 h prior to the addition of 3 mM Sr. As shown in Fig. 3A-C, 3-MA successfully suppressed
the autophagy of the MC3T3-E1 cells with/without Sr. Subsequently,
osteogenic parameters were measured in order to demonstrate the
osteogenic conditions upon treatment with 3-MA. These results
indicated that treatment with 3-MA alone exerted no significant
effect on osteogenic differentiation. However, the osteogenic
differentiation induced by Sr was inhibited in the presence of
3-MA. Additionally, no significant differences were noted with
regard to the expression levels of RUNX2 and OCN, the activity of
ALP, and the formation of mineralized nodules in the 3-MA + Sr
experimental group compared with the control group (i.e., the cells
cultured only with osteogenic media) (Fig. 4).
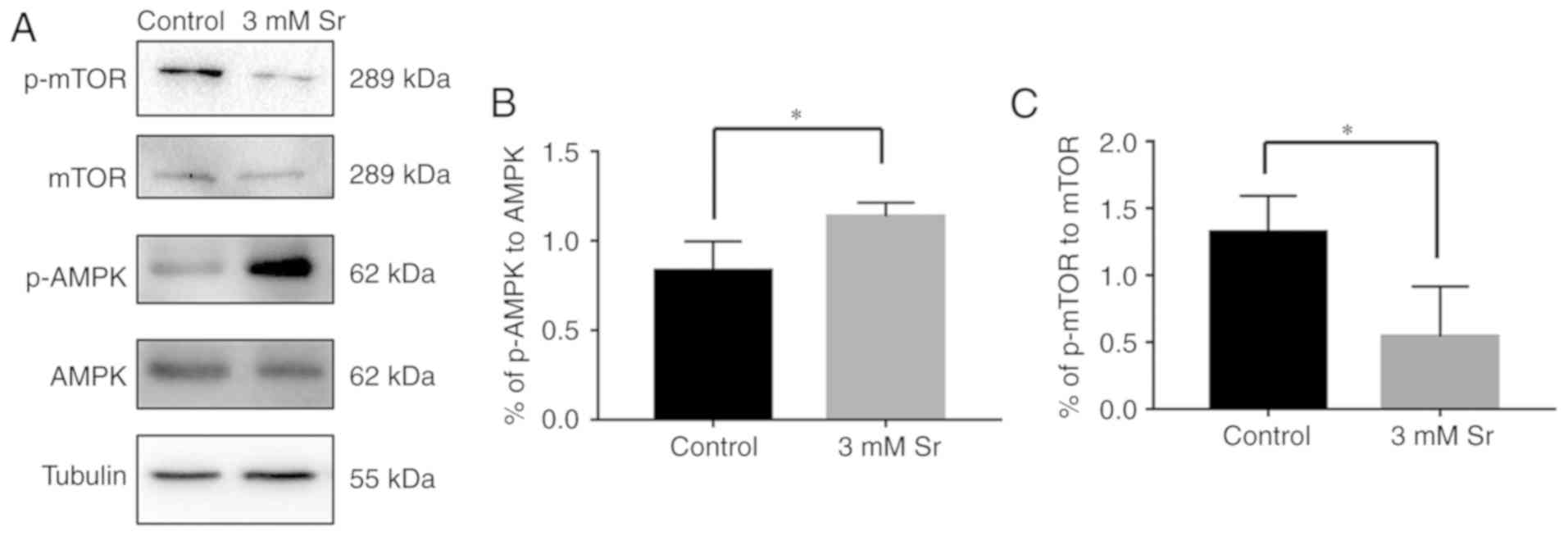
Sr-induced autophagy is activated by the
AMPK/mTOR signaling pathway
Finally, the molecular mechanisms involved in the
association between Sr-induced osteogenic differentiation and
autophagy was explored. To meet this aim, the AMPK/mTOR pathway
following Sr treatment was investigated. As shown in Fig. 5, compared with the control, the
phosphorylation level of AMPK was significantly increased in the
cells exposed to 3 mM Sr for 3 days. The higher ratio of
phosphorylated AMPK to AMPK suggested the activation of autophagy.
In addition, a low phosphorylation level of molecular mTOR
downstream was observed in the 3 mM Sr-treatment group, which was
consistent with the AMPK results. To further investigate the role
of the the AMPK/mTOR signaling pathway in Sr-induced autophagy, the
AMPK inhibitor, compound C, was used in the subsequent experiments.
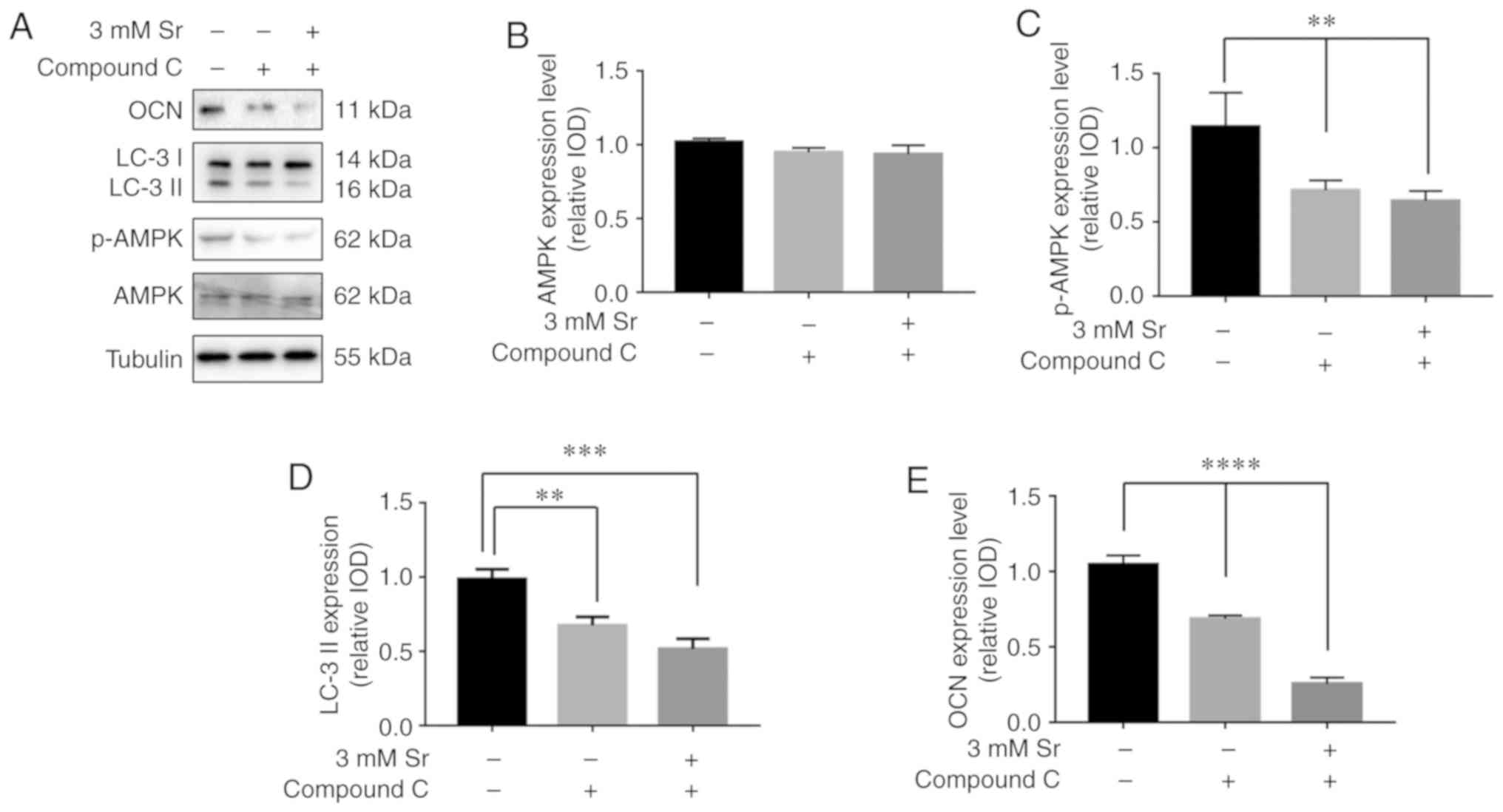
The results of western blot analysis revealed that pre-incubation
with compound C markedly inhibited the phosphorylation level of
AMPK and blocked the formation of LC-3 II (Fig. 6A-D). The conversion of LC3-I into
LC3-II, which represents inhibition of Sr-induced autophagy, was
not observed. Moreover, the expression level of OCN was
significantly decreased in the MC3T3-E1 cells upon treatment with
compound C (Fig. 6E). Taken
together, these data indicate that the AMPK/mTOR pathway is
involved in the mechanisms through which Sr induces autophagy and
the osteogenic differentiation of MC3T3-E1 cells.
Discussion
In the present study, the toxic effects of various
concentrations of Sr (3-96 mM) on MC3T3-E1 cells were investigated,
and the experiments confirmed that Sr at the concentration range of
3-12 mM exerted no marked effect on cell viability, whereas as the
concentration increased, a toxic effect on the cells was noted. The
results of the RT-qPCR, western blot analysis, ALP and Alizarin Red
staining also confirmed the effects of Sr on osteogenic
differentiation and mineralization, and these were consistent with
recently published studies (20,21). Previous studies have reported that
Sr is able to positively modulate osteogenic differentiation at
concentrations ranging from 1-10 mM (22,23). In the present study, the RT-qPCR
results of cells treated with 1 mM Sr exhibited no significant
changes in osteogenic differentiation compared with the control
(Fig. S1). Combining the PCR,
western blot analysis and Alizarin Red staining results, it was
possible to confirm that 3 mM Sr plays a positive role in the
osteogenic induction of MC3T3-E1 cells. In addition, the activity
and differentiation of cells at concentrations of Sr of 6 and 12 mM
were not as effective as that in the 3 mM Sr-treatment group. On
the basis of these data, 3 mM Sr was therefore selected as the
appropriate concentration of Sr, whereas concentrations of Sr
>12 mM could be toxic to cells.
During the course of the past decade, an increasing
number of studies have reported on the development of Sr
utilization, including pharmacological induction and biomaterial
substitution studies (3,24,25). Evidence from in vitro and
in vivo studies have shown that Sr may promote osteogenic
differentiation and mineralization in the dental pulp via PI3K/Akt
signaling (26,27). In addition, Wnt/β-catenin
signaling has been shown to mediate the protective effects of Sr in
mice (28-30). Although the beneficial effects of
Sr have been demonstrated in numerous studies (1,9,10),
no drug comprising Sr has yet been approved by the Food and Drug
Administration for osteoporosis treatment in the USA (4,20,31). During the year 2014, Sr also lost
its pre-eminent status in the European Medicines Agency owing to
the mounting concerns regarding the occurrence of cardiovascular
events associated with its long-term use (32). As previously reported by
Atteritano et al a 12-month treatment with Strontium
Ranelate did not alter hemostasis factors or markers of
cardiovascular risk (33). The
precise mechanisms of the drug-induced effect on the cardiovascular
risk is complex and requires further investigation in the future.
Currently, Sr is only cautiously allowed to be administered in the
treatment of severe osteoporosis. However, the balance between
treatment benefits and side-effect risk should always be considered
in drug application. Therefore, research into the molecular
mechanisms of Sr is urgently required in terms of its clinical
application.
Autophagy is an evolutionarily conserved cellular
pathway mediating cell metabolism under different conditions
(34,35). In addition to the function of
autophagy in cellular metabolism, survival and death, emerging
evidence has suggested an association between autophagy and cell
development (35-37). Liu et al (36) demonstrated that the suppression of
autophagy did lead to osteopenia in mice via the inhibition of
osteoblast differentiation. The activation of autophagy by Forkhead
box O3 (FOXO3) has been reported to regulate redox homeostasis
during osteogenic differentiation (37). Kang et al (38) reported that a deficiency in
autophagy may impair chondrogenesis via the PERK-ATF4-CHOP axis.
Furthermore, lipopolysaccharide-induced autophagy plays an
important role in osteoclastogenesis (39). An exploration of the observed
effects of autophagy and Sr in the literature stimulated our
investigation of the autophagy levels in Sr-treated MC3T3-E1 cells
in the present study. The results of LC-3 I/II conversion,
SQSTM1/p62 expression, and MDC staining in our study suggested that
the autophagic process may be activated during Sr-induced
osteogenic differentiation. In addition, the expression levels of
LC-3 II were significantly decreased in the 6-12 mM Sr-treated
cells, indicating that autophagy was not activated (Fig. S2). These results may help to
account for the osteogenic differentiation results determined
previously with the high-dose Sr group. The osteogenic
differentiation induced by Sr was attenuated when the cell
autophagy was inhibited by 3-MA. Taken together, these data suggest
that autophagic events in MC3T3-E1 cells are essential in terms of
the Sr-induced osteogenic differentiation process.
It has been well established that AMPK plays a
critical role in the regulation of osteogenic differentiation
(40-42). Several studies have demonstrated
that pharmacological AMPK activators induce the osteogenic
differentiation and mineralization of osteoblastic cell lines and
bone marrow progenitor cells, whereas AMPK gene knockdown can
reduce bone mass in mice (43-45). It is noteworthy that AMPK has also
been shown to be a well-established regulator in autophagy via the
inhibition of mTOR (14,45-47). In vitro studies have
reported crosstalk between the processes of osteogenic
differentiation and autophagy in human mesenchymal stem cells
mediated via the AMPK/mTOR signaling pathway (14,46). In the present study, the
phosphorylation level of AMPK in the Sr-treated cells was observed
to increase, suggesting that the activation of the autophagy
process had occurred. In addition, low phosphorylation levels of
molecular mTOR downstream were observed in the induction group,
which remained consistent with the preliminary results. As it had
already been observed that compound C could inhibit AMPK
phosphorylation, the western blot analysis results revealed the
occurrence of reduced autophagy and decreased osteogenic
differentiation in cells upon treatment with both compound C and
Sr. Therefore, it is evident that the AMPK/mTOR pathway is a
pivotal regulator involved in Sr-induced autophagy and osteogenic
differentiation. However, opposite results have also been reported,
i.e. that mTOR1 signaling promotes the maturation and
differentiation of pre-osteoblasts (47). The reason(s) for such a
discrepancy remains unclear, although there are two types of mTOR
complexes (mTORC1 and mTORC2) that possess different
characteristics, and the mTOR signal pathway may play distinctly
different roles during different stages of osteoblast
differentiation.
In conclusion, the findings of the present study
demonstrate that the AMPK/mTOR signaling pathway is involved in the
mechanisms of the autophagy process associated with the Sr-induced
osteogenic differentiation of MC3T3-E1 cells. Further clarification
of the Sr mechanism associated with autophagy may provide novel
opportunities for both drug development and a proper clinical
application for bone regeneration.
Supplementary Data
Acknowledgments
Not applicable.
Funding
This study was supported by the Natural Science
Foundation of Tianjin (grant no. 15JCYBJC27400) and Natural Science
Foundation of Tianjin (grant no. 14JCZDJC38500).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article or are available from the
corresponding author on reasonable request.
Authors' contributions
XL, YL and YC made substantial contributions to the
conception and design of the study; HB, ZL, XL and YC were involved
in the drafting of the manuscript; HB, YC, LH, QH and YS performed
the experiments for data acquisition; CY and LH performed the
statistical analysis; ZL, CY, YW and QH interpreted the
experimental results; YL, XL and YC wrote the manuscript. The final
version of the manuscript was read and approved by all the
authors.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare they have no competing
interests.
References
|
1
|
Chandran S, Babu SS, Vs HK, Varma HK and
John A: Osteogenic efficacy of strontium hydroxyapatite
micro-granules in osteopo-rotic rat model. J Biomater Appl.
31:499–509. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Querido W, Rossi AL and Farina M: The
effects of strontium on bone mineral: A review on current knowledge
and microanalytical approaches. Micron. 80:122–134. 2016.
View Article : Google Scholar
|
|
3
|
Scardueli CR, Bizelli-Silveira C,
Marcantonio RAC, Marcantonio E Jr, Stavropoulos A and Spin-Neto R:
Systemic administration of strontium ranelate to enhance the
osseointegration of implants: Systematic review of animal studies.
Int J Implant Dent. 4:212018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Reginster JY: Efficacy and safety of
strontium ranelate in the treatment of knee osteoarthritis: Results
of a double-blind randomised, placebo-controlled trial. Ann Rheum
Dis. 73:e82014. View Article : Google Scholar
|
|
5
|
Reginster JY, Beaudart C, Neuprez A and
Bruyère O: Strontium ranelate in the treatment of knee
osteoarthritis: New insights and emerging clinical evidence. Ther
Adv Musculoskelet Dis. 5:268–276. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chou J, Valenzuela SM, Santos J, Bishop D,
Milthorpe B, Green DW, Otsuka M and Ben-Nissan B: Strontium- and
magnesium-enriched biomimetic β-TCP macrospheres with potential for
bone tissue morphogenesis. J Tissue Eng Regen Med. 8:771–778. 2014.
View Article : Google Scholar
|
|
7
|
Pasqualetti S, Banfi G and Mariotti M: The
effects of strontium on skeletal development in zebrafish embryo. J
Trace Elem Med Biol. 27:375–379. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao S, Wang X, Li N, Chen Y, Su Y and
Zhang J: Effects of strontium ranelate on bone formation in the
mid-palatal suture after rapid maxillary expansion. Drug Des Devel
Ther. 9:2725–2734. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Henriques Lourenço A, Neves N,
Ribeiro-Machado C, Sousa SR, Lamghari M, Barrias CC, Trigo Cabral
A, Barbosa MA and Ribeiro CC: Injectable hybrid system for
strontium local delivery promotes bone regeneration in a rat
critical-sized defect model. Sci Rep. 7:50982017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Khan PK, Mahato A, Kundu B, Nandi SK,
Mukherjee P, Datta S, Sarkar S, Mukherjee J, Nath S, Balla VK and
Mandal C: Influence of single and binary doping of strontium and
lithium on in vivo biological properties of bioactive glass
scaffolds. Sci Rep. 6:329642016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mizushima N and Levine B: Autophagy in
mammalian development and differentiation. Nat Cell Biol.
12:823–830. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wan Y, Zhuo N, Li Y, Zhao W and Jiang D:
Autophagy promotes osteogenic differentiation of human bone marrow
mesenchymal stem cell derived from osteoporotic vertebrae. Biochem
Biophys Res Commun. 488:46–52. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Piemontese M, Onal M, Xiong J, Han L,
Thostenson JD, Almeida M and O'Brien CA: Low bone mass and changes
in the osteocyte network in mice lacking autophagy in the
osteoblast lineage. Sci Rep. 6:242622016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pantovic A, Krstic A, Janjetovic K, Kocic
J, Harhaji-Trajkovic L, Bugarski D and Trajkovic V: Coordinated
time-dependent modulation of AMPK/Akt/mTOR signaling and autophagy
controls osteogenic differentiation of human mesenchymal stem
cells. Bone. 52:524–531. 2013. View Article : Google Scholar
|
|
15
|
Wu SB and Wei YH: AMPK-mediated increase
of glycolysis as an adaptive response to oxidative stress in human
cells: Implication of the cell survival in mitochondrial diseases.
Biochim Biophys Acta. 1822:233–247. 2012. View Article : Google Scholar
|
|
16
|
Kim J, Kundu M, Viollet B and Guan KL:
AMPK and mTOR regulate autophagy through direct phosphorylation of
Ulk1. Nat Cell Biol. 13:132–141. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
18
|
Klionsky DJ, Abdalla FC, Abeliovich H,
Abraham RT, Acevedo-Arozena A, Adeli K, Agholme L, Agnello M,
Agostinis P, Aguirre-Ghiso JA, et al: Guidelines for the use and
interpretation of assays for monitoring autophagy. Autophagy.
8:445–544. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vinod V, Padmakrishnan CJ, Vijayan B and
Gopala S: 'How can I halt thee?' The puzzles involved in autophagic
inhibition. Pharmacol Res. 82:1–8. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Scaglione M, Fabbri L, Casella F and Guido
G: Strontium ranelate as an adjuvant for fracture healing:
Clinical, radiological, and ultrasound findings in a randomized
controlled study on wrist fractures. Osteoporos Int. 27:211–218.
2016. View Article : Google Scholar
|
|
21
|
Chao K, Xuxia W, Qianqian W, Yuanyuan H,
Shuya Z and Jun Z: Effects of strontium ranelate on the rats'
palatal suture after rapid maxillary expansion. Hua Xi Kou Qiang Yi
Xue Za Zhi. 34:336–340. 2016.In Chinese.
|
|
22
|
Querido W, Farina M and Anselme K:
Strontium ranelate improves the interaction of osteoblastic cells
with titanium substrates: Increase in cell proliferation,
differentiation and matrix mineralization. Biomatter.
5:e10278472015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Caverzasio J and Thouverey C: Activation
of FGF receptors is a new mechanism by which strontium ranelate
induces osteoblastic cell growth. Cell Physiol Biochem. 27:243–250.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Geng Z, Wang X, Zhao J, Li Z, Ma L, Zhu S,
Liang Y, Cui Z, He H and Yang X: The synergistic effect of
strontium-substituted hydroxyapatite and microRNA-21 on improving
bone remodeling and osseointegration. Biomater Sci. 6:2694–2703.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen YP, Tan A, Ho WP, Chuang TY, Chen WC
and Chen CH: Effectiveness of strontium ranelate in the treatment
of rat model of legg-calve-perthes disease. Indian J Orthop.
52:380–386. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bakhit A, Kawashima N, Hashimoto K, Noda
S, Nara K, Kuramoto M, Tazawa K and Okiji T: Strontium ranelate
promotes odonto-/osteogenic differentiation/mineralization of
dental papillae cells in vitro and mineralized tissue formation of
the dental pulp in vivo. Sci Rep. 8:92242018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guo X, Wei S, Lu M, Shao Z, Lu J, Xia L,
Lin K and Zou D: Dose-dependent effects of strontium ranelate on
ovariectomy rat bone marrow mesenchymal stem cells and human
umbilical vein endothelial cells. Int J Biol Sci. 12:1511–1522.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Qi HH, Bao J, Zhang Q, Ma B, Gu GY, Zhang
PL, Ou-Yang G, Wu ZM, Ying HJ and Ou-Yang PK: Wnt/β-catenin
signaling plays an important role in the protective effects of
FDP-Sr against oxidative stress induced apoptosis in MC3T3-E1 cell.
Bioorg Med Chem Lett. 26:4720–4723. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sun T, Li Z, Zhong X, Cai Z, Ning Z, Hou
T, Xiong L, Feng Y, Leung F, Lu WW and Peng S: Strontium inhibits
osteoclastogenesis by enhancing LRP6 and β-catenin-mediated OPG
targeted by miR-181d-5p. J Cell Commun Signal. 13:85–97. 2019.
View Article : Google Scholar
|
|
30
|
Geng T, Sun S, Yu H, Guo H, Zheng M, Zhang
S, Chen X and Jin Q: Strontium ranelate inhibits wear
particle-induced aseptic loosening in mice. Braz J Med Biol Res.
51:e74142018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Reginster JY, Brandi ML, Cannata-Andia J,
Cooper C, Cortet B, Feron JM, Genant H, Palacios S, Ringe JD and
Rizzoli R: The position of strontium ranelate in today's management
of osteoporosis. Osteoporos Int. 26:1667–1671. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cianferotti L, D'Asta F and Brandi ML: A
review on strontium ranelate long-term antifracture efficacy in the
treatment of postmenopausal osteoporosis. Ther Adv Musculoskelet
Dis. 5:127–139. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Atteritano M, Catalano A, Santoro D, Lasco
A and Benvenga S: Effects of strontium ranelate on markers of
cardiovascular risk in postmenopausal osteoporotic women.
Endocrine. 53:305–312. 2016. View Article : Google Scholar
|
|
34
|
Kim KH and Lee MS: Autophagy-a key player
in cellular and body metabolism. Nat Rev Endocrinol. 10:322–337.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Parzych KR and Klionsky DJ: An overview of
autophagy: Morphology, mechanism, and regulation. Antioxid Redox
Signal. 20:460–473. 2014. View Article : Google Scholar :
|
|
36
|
Liu F, Fang F, Yuan H, Yang D, Chen Y,
Williams L, Goldstein SA, Krebsbach PH and Guan JL: Suppression of
autophagy by FIP200 deletion leads to osteopenia in mice through
the inhibition of osteoblast terminal differentiation. J Bone Miner
Res. 28:2414–2430. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gómez-Puerto MC, Verhagen LP, Braat AK,
Lam EW, Coffer PJ and Lorenowicz MJ: Activation of autophagy by
FOXO3 regulates redox homeostasis during osteogenic
differentiation. Autophagy. 12:1804–1816. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kang X, Yang W, Feng D, Jin X, Ma Z, Qian
Z, Xie T, Li H, Liu J, Wang R, et al: Cartilage- specific autophagy
deficiency promotes ER stress and impairs chondrogenesis in
PERK-ATF4-CHOP-dependent manner. J Bone Miner Res. 32:2128–2141.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sul OJ, Park HJ, Son HJ and Choi HS:
Lipopolysaccharide (LPS)-induced autophagy is responsible for
enhanced osteoclastogenesis. Mol Cells. 40:880–887. 2017.PubMed/NCBI
|
|
40
|
Hu XK, Yin XH, Zhang HQ, Guo CF and Tang
MX: Liraglutide attenuates the osteoblastic differentiation of
MC3T3-E1 cells by modulating AMPK/mTOR signaling. Mol Med Rep.
14:3662–3668. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chen H, Liu X, Chen H, Cao J, Zhang L, Hu
X and Wang J: Role of SIRT1 and AMPK in mesenchymal stem cells
differentiation. Ageing Res Rev. 13:55–64. 2014. View Article : Google Scholar
|
|
42
|
Wang YG, Qu XH, Yang Y, Han XG, Wang L,
Qiao H, Fan QM, Tang TT and Dai KR: AMPK promotes osteogenesis and
inhibits adipogenesis through AMPK- Gfi1- OPN axis. Cell Signal.
28:1270–1282. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kim EK, Lim S, Park JM, Seo JK, Kim JH,
Kim KT, Ryu SH and Suh PG: Human mesenchymal stem cell
differentiation to the osteogenic or adipogenic lineage is
regulated by AMP-activated protein kinase. J Cell Physiol.
227:1680–1687. 2012. View Article : Google Scholar
|
|
44
|
Jang WG, Kim EJ, Bae IH, Lee KN, Kim YD,
Kim DK, Kim SH, Lee CH, Franceschi RT, Choi HS and Koh JT:
Metformin induces osteoblast differentiation via orphan nuclear
receptor SHP-mediated transactivation of Runx2. Bone. 48:885–893.
2011. View Article : Google Scholar
|
|
45
|
Jang WG, Kim EJ, Lee KN, Son HJ and Koh
JT: AMP-activated protein kinase (AMPK) positively regulates
osteoblast differentiation via induction of Dlx5-dependent Runx2
expression in MC3T3E1 cells. Biochem Biophys Res Commun.
404:1004–1009. 2011. View Article : Google Scholar
|
|
46
|
Heras-Sandoval D, Pérez-Rojas JM,
Hernández-Damián J and Pedraza-Chaverri J: The role of
PI3K/AKT/mTOR pathway in the modulation of autophagy and the
clearance of protein aggregates in neurodegeneration. Cell Signal.
26:2694–2701. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chen J and Long F: mTORC1 signaling
promotes osteoblast differentiation from preosteoblasts. PLoS One.
10:e1306272015.
|