Introduction
Alcoholic hepatitis (AH) is an acute inflammatory
syndrome that can result in high morbidity and mortality (1). AH, as a severe liver injury, is
associated with both acute hepatocellular dysfunction and chronic
liver failure that can finally lead to death (2). Present evidence demonstrated that AH
results from the complicated interaction between alcohol
metabolism, inflammation and innate immunity (3). People with chronic or active alcohol
abuse have the potential to suffer from AH and there will be 30-40%
mortality after a month in certain severe cases (4). However, corticosteroids or
pentoxifylline which are the current drug treatment options only
result in ~50% survival (5).
Alcoholic liver disease (ALD) is a common liver disease worldwide
and acute AH is characterized by the fact that macrophage
inflammatory cytokines disproportionately respond to bacterial
lipopolysaccharide (6). Alcohol
also has an influence on the intestine, contributing to the
increase of intestinal permeability and the change of bacterial
microflora (7). The human
intestine embodies a large number of microbes promoting metabolism
and digestion and the alterations of intestinal microbiota is
associated with liver diseases (8). In addition, previous evidence
suggests that endotoxemia has a close association with ALD in that
circulating endotoxins stem from intestinal microflora and gut
barrier dysfunction can cause increased intestinal permeability
(9).
At present, it is reported that microRNAs (miRs)
serve an important role in ALD (10). Certain miRs (like miR-223, miR-155
and miR-125) exhibited an abnormal miRNA profile that was
influenced by alcohol and is associated with the pathogenesis of
ALD (11). miR-182 serves a
crucial role in disease severity and liver injury of AH and is a
potential therapeutic target and biomarker of AH (12). A previous study revealed that
miR-122, rich in hepatocytes, is associated with acute
alcohol-induced liver injury and miR-155 regulated inflammation in
ALD and targeted Toll-like receptor (TLR) signaling concerning
pro-and anti-inflammatory cytokines (13). As previously reported, the TLR
signaling pathway serves a role in a number of diseases including
neuro-inflammatory disorders, alcohol abuse and chronic
inflammation (14). miR-141 was
identified to be involved in the process of epithelial-mesenchymal
transition (15), which was
decreased in hepatocellular carcinoma (HCC) and overexpression of
miR-141 could inhibit the growth and metastasis of HCC cells
(16). In addition, Huang et
al (17) have identified the
crucial antagonistic roles of miR-141-3p in the modulation of
invasive potential in HCC cells. Furthermore, miR-141 seems to
serve a role in the bowel inflammation of individuals with active
ulcerative colitis via downregulation of C-X-C motif chemokine 5
(CXCL5) expression (18).
However, there are few studies on the role of miR-141 in AH.
Therefore, the present study would like to investigate the role of
miR-141 in intestinal injury and intestinal endotoxemia (IETM) of
AH and its interaction with partially TLR4-mediated signaling
pathway. The present study aimed to test the hypothesis that
miR-141 could inhibit the activation of the TLR pathway by
targeting TLR4, therefore alleviating the intestinal injury of AH
and inhibit the occurrence of IETM.
Materials and methods
Ethics statement
All involved animal experiments were in line with
the principles of the approval of the clinical ethical committee of
the Affiliated Huai'an Hospital of Xuzhou Medical University
(Huai'an, China). Significant efforts were made in order to
minimize the number of animals used as well as their respective
suffering.
Study subjects
A total of 45 C57BL/6 male mice (aged 6 weeks;
weighing 18-22 g) were provided by the experimental animal center
of Peking University Health Science Center (Beijing, China) and
randomly assigned into normal group (10 mice) and AH group (35
mice). The mice in the normal group were fed with Lieber-Decarli
alcohol-free liquid while mice in the AH group were fed with
Lieber-Decarli alcohol. The two types of liquid contained an equal
calorie content. The mice were kept in a specific pathogen-free
animal laboratory with 40-70% humidity, 24±2°C and day and night
cycle of artificial lighting (day time: 08:00 a.m.-08:00 p.m.). The
criteria for validated AH model were as follows (19): Mice exhibited unkempt and
lackluster hair, decreased activity, slow reaction and slow weight
gain. After 6 weeks of alcohol feeding, five mice were euthanized
in the normal group and the AH group, respectively. The
pathological alterations of liver tissues were observed by
hematoxylin and eosin (H&E) staining to determine whether the
model was successfully constructed. The remaining mice were
randomly selected and fasted for 12 h. Mice were anaesthetized by
intraperitoneal injection with 2% sodium pentobarbital (50 mg/kg)
and then the 1 ml cardiac blood was collected, mixed in vacuum tube
(Becton, Dickinson and Company), centrifuged at 600 × g for 5 min
at 4°C and preserved at -20°C. Intestinal tissues were collected at
3 cm from the anus, a part of which were kept in liquid nitrogen
and the other part were fixed in 10% neutral formalin for 24 h at
room temperature, which were then dehydrated by gradient alcohol
(100, 95, 80 and 70%, each for 2 min), immersed in a wax box at
60°C for 12 h and embedded for 2 h for further use.
Animal grouping
According to the mmu-miR-141 sequence at www.miRbase.org website and the RNA sequence design
principle, the miR-141 irrelevant sequences
(5′-UAGAAAUGGAGUAGCAAUGAU-3′), miR-141 mimic sequence
(5′-UAGAAAUGGUCUAGUCACAAU-3′) and miR-141 inhibitor sequence
(5′-AUUGUGACUAGACCAUUUCUA-3′) were designed and synthesized by
Shanghai Genechem Co., Ltd. The amplified target fragments and the
linearized expression plasmid pcDNA3.1(−) (Invitrogen; Thermo
Fisher Scientific, Inc.) following enzyme digestion with
BamHI and HindIII (Invitrogen; Thermo Fisher
Scientific, Inc.) were reacted overnight at 16°C by T4 DNA ligase.
Next, the products were incubated with DH5α competent cells (CB101;
Tiangen Biochemical Technology Co., Ltd.) on ice for 30 min, at
42°C for 90 sec and again on ice for 5 min. Next, the mixture was
supplemented with 800 µl lysogeny broth (LB) liquid medium
without antibiotics and incubated at 37°C for 45 min, resuspended
in 200 µl LB liquid medium without antibiotics, and then
placed on a MAC culture plate (cat. no. HB8458; Hope Biotechnology
Co., Ltd.) containing antibiotics (ampicillin) for 12-16 h at 37°C.
The next day, the positive clones were selected and sequenced. The
cells that failed to be transformed could not grow on the MAC
culture plate; cells without recombinant plasmid transformation
appeared red on the MAC culture plate, and cells with recombinant
plasmid transformation appeared white on the MAC culture plate. The
white colonies were the positive clones that were selected.
C57BL/6 mice were further classified into 7 groups:
Normal group (normal mice without any treatment), blank group (AH
mice without treatment), negative control group (NC group, AH mice
injected with blank plasmid), miR-141 mimic group (AH mice injected
with miR-141 mimics), miR-141 inhibitor group (AH mice injected
with miR-141 inhibitors), TLR4mAb group (AH mice injected with TLR
pathway inhibitor TLR4mAb), miR-141 inhibitor + TLR4mAb group (AH
mice co-injected with miR-141 inhibitors and TLR4mAb), with 5 mice
in each group. Mice were anesthetized with 2% sodium pentobarbital,
fixed with their limbs supine on the operating table and injected
with plasmids (50 nM) via the tail vein. A total of 4 weeks
following treatment, all mice were fasted for 12 h. The cardiac
blood was collected, mixed in vacuum tube (Becton, Dickinson and
Company), centrifuged at 604 × g for 5 min at 4°C and preserved at
−20°C. Intestinal tissues were collected at 3 cm from the anus,
washed by phosphate buffer saline (PBS), fixed by 4% formalin at
room temperature for 24 h, embedded by paraffin and then sliced
(3-5 µm) for further use.
Dual-luciferase reporter gene assay
The possible target genes of miR-141 were predicted
at microRNA.org and the sequences of targeting sites
were obtained. The 3′untranslated region (UTR) of TLR4 was
amplified and cloned to pmirGLO (E1330; Promega Corp.) Luciferase
vector, named pTLR4-wild type (Wt). The binding sites of miR141 on
TLR4 were predicted using an online database (microRNA.org) and
mutated. The mutant form of 3′UTR of TLR4 was also cloned into
pmirGLO vector to construct pTLR4-mutant (Mut). The pRL-TK vector
expressing Renilla luciferase (cat. no. E2241; Promega
Corp.) was regarded as an internal reference. Using the
Lipofectamine® 2000 transfection reagent (Invitrogen;
Thermo Fisher Scientific, Inc.), the miR-141 mimic or miR-141 NC
were respectively co-transfected with pTLR4-Wt or pTLR4-Mut into
293T cells (CRL-1415; American Type Culture Collection). Following
36 h of transfection, the fluorescence detector (Glomax20/20;
Promega Corp.) was used to detect luciferase activity. The ratio of
firefly luciferase to Renilla luciferase was used to
calculate the relative luciferase activity.
H&E staining
A total of 5 paraffin-embedded intestinal tissues
were selected from each group and cut into 5-µm serial
sections. After incubating for 1 h at 60°C, the sections were
dewaxed with xylene I and II for 5 min. The sections were then
dehydrated by gradient alcohol (alcohol concentration of 70, 80, 95
and 100%, each for 2 min) and washed by distilled water 2 times for
5 min each time. Following hydration, the sections were stained
with routine H&E (Beijing Solarbio Technology Co., Ltd.) at
room temperature for 3 min, washed by running water for 5 min,
dehydrated by 70 and 80% ethanol (5 min each), cleared by xylene I
and II (5 min each) and sealed with neutral balsam. Lastly, a
transmitted polarizing microscope (XP-330; Shanghai Bing Yu Optical
Instrument Co., Ltd.) was used to observe the pathological
alterations of sections and then the sections were scored according
to the scoring standard by Geboes et al (20) (Table
I). A total of 10 sections were selected from each group and
the average value was taken.
 | Table IPathological judgement criteria for
intestinal injury. |
Table I
Pathological judgement criteria for
intestinal injury.
| Histological
appearance | No | Light | Moderate | Severe |
|---|
| Mucosal injury | 0 | 1 | 2 | 3 |
| Crypt
destruction | 0 | 1 | 2 | 3 |
| Mucosal
bleeding | 0 | 1 | 2 | 3 |
| Interstitial
injury | 0 | 1 | 2 | 3 |
| Inflammatory cell
infiltration | 0 | 1 | 2 | 3 |
Activities of serum endotoxin, D lactic
acid, diamineoxidase (DAO) and alanine aminotransferase (ALT)
The activities of serum endotoxin, D lactic acid and
DAO were measured from the serum of 5 mice in each group. The
content of endotoxin was determined by modified substrate azo dye
method using kit purchased from Shanghai Biochemical Institute.
Colorimetric detection was conducted using the 721
spectrophotometer (Shanghai Precision and Scientific Instrument
Corporation). D lactic acid was detected by modified enzyme
spectrophotometric method. D lactic acid standard solution (69806)
and lactate dehydrogenase (D-LDH; 61309) were purchased from
Sigma-Aldrich; Merck KGaA. DAO was determined by o-dianisidine
reagent method. Cadaverine dihydrochloride (cat. no. D9143),
o-dianisidine (cat. no. 540765), DAO standard solution (cat. no.
77332) and horseradish peroxidase (cat. no. MAK037) were purchased
from Sigma-Aldrich; Merck KGaA. ALT in serum was determined using
96-well spectrophotometry using the ALT kit (cat. no. SEA207Mu;
Shanghai Chao Yan Biotechnology Co., Ltd.). The operation was
performed according to the kit protocol and the experiment was
repeated 3 times.
Immunohistochemistry
A total of 5 paraffin-embedded mice intestinal
tissues of each group were randomly selected and the sections (3-4
µm) were prepared. The sections were put into 3%
H2O2, dewaxed by xylene I and II (10 min
each), dehydrated by gradient alcohol (alcohol concentration of 70,
80, 95 and 100% for 2 min each), and washed by distilled water 2
times, 5 min each (placed in a shaker). The sections were immersed
with 3% H2O2 for 10 min and washed by
distilled water. After the antigen was repaired at high pressure
for 90 sec, the sections were cooled to room temperature and washed
with PBS. The sections were blocked with 5% bovine serum albumin
(cat. no. A1933; Sigma-Aldrich; Merck KGaA) at 37°C for 30 min and
incubated at 4°C overnight with the monoclonal antibody rabbit
anti-mouse TLR4 (cat. no. ab13556; Abcam) or CD14 (cat.
no. ab78333; Abcam; 1:100). The sections were then washed and
incubated with biotinylated goat anti-rabbit immunoglobulin G,
horse radish peroxidase (HRP) secondary antibody (cat. no. SE134;
1:1,000, Beijing Solarbio Science & Technology Co., Ltd.) for
30 min at 37°C. After that, the sections were treated with
hematoxylin (cat. no. C0105; Beyotime Institute of Biotechnology)
at room temperature to counterstain the nucleus for 30 sec,
developed by diaminobenzidine (DAB; cat. no. P0202; Beyotime
Institute of Biotechnology) at room temperature for 3-10 min,
dehydrated by hydrochloric acid ethanol, sealed with neutral balsam
and observed and images were captured under the light microscope. A
total of 5 fields under high-power lens were randomly selected. The
judgment standards of immunohistochemistry were as follows
(21): The brownish yellow signal
mainly located in the cytoplasm or cell membrane was considered to
indicate positively stained cells. The percentage of cells with
positive expression of TLR4 and CD14 in the total number
of cells was calculated. The experiment was repeated 3 times.
ELISA
The serum was taken from 5 mice of each group. The
tumor necrosis factor-α (TNF-α; cat. no. 69-25328), interleukin-6
(IL-6; cat. no. 69-40133) and IL 1β (cat. no. 69-30375) ELISA kits
were purchased form Wuhan Moshake Biotechnology Co., Ltd. Serum
samples (100 µl) were added into the reaction wells and
incubated at 37°C for 90 min. The wells were then incubated with
100 µl freshly-prepared biotinylated antibody at 37°C for 60
min. After washing, the wells were incubated with 100 µl
freshly-prepared enzyme bound reactant at 37°C for 30 min avoiding
exposure to light. The substrate (100 µl) was then added to
the samples and incubated at 37°C for 15 min avoiding exposure to
light. After that, the stop solution was added immediately to
terminate the reaction. Within 3 min, the OD value was measured at
450 nm wavelength by Microplate Reader (Multiskan GO; Thermo Fisher
Scientific, Inc.). The experiment was repeated 3 times.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The intestinal tissues preserved in liquid nitrogen
from 5 mice in each group were randomly selected, ground and 1 ml
TRIzol reagent was added (Invitrogen; Thermo Fisher Scientific,
Inc.) to extract total RNA according to the instructions (total RNA
extraction from cells was also conducted according to this method).
The purity and concentration of RNA were detected by ultraviolet
spectrophotometry (cat. no. UV1901; Shanghai AuCybest Technology
Instrument Co., Ltd.). The RNAs were reverse transcribed into cDNAs
(50 ng/µl) by PrimeScript™ RT reagent kit (cat. no. RR047A;
Beijing Think-Far Technology Co., Ltd.). The reaction condition was
as follows: Reverse transcription reaction at 37°C 3 times, each
for 15 min, reverse transcriptase inactivation reaction at 85°C for
5 sec and cryopreservation at -80°C for later use. The primers for
RT-qPCR (LightCycler FastStart DNA Master PLUS SYBR Green I kit;
Roche Diagnostics) were designed using Premier 5.0 software
(Premier Biosoft International) and synthesized by Beijing Qingke
Xinye Biological Technology Co., Ltd. (22) (Table II). The reaction was conducted in
an ABI 7900HT RT-qPCR instrument (ABI 7900, Shanghai Pudi
Biological Technology Co. Ltd.) in the light of two-step method. U6
was used as the internal reference of miR-141 and GAPDH was
regarded as the internal reference for other genes. The reaction
conditions were as follows: Pre-denaturation at 95°C for 30 sec, 40
cycles of denaturation at 95°C for 5 sec, annealing at 58°C for 30
sec, followed by extension at 72°C for 15 sec. The
2-ΔΔCq method (23)
was applied to calculate the relative mRNA expression of miR-141,
TLR4, CD14, NF-κBp65, myeloid differentiation primary
response 88 (MyD88), TIR-domain-containing adapter-inducing
interferon-β (TRIF) and proliferating cell nuclear antigen (PCNA)
in intestinal tissues. A total of three duplicated wells were set
for each sample. The experiment was repeated 3 times.
 | Table IIPrimer sequence for reverse
transcription quantitative polymerase chain reaction. |
Table II
Primer sequence for reverse
transcription quantitative polymerase chain reaction.
| Gene | Sequence |
|---|
| miR-141 | Forward:
5′-CCGGGTAACACTGTCTGGTAAAG-3′ |
| Reverse:
5′-GTGCAGGGTCCGAGGT-3′ |
| U6 | Forward:
5′-GCTTCGGCAGCACATATACTAAAAT-3′ |
| Reverse:
5′-CGCTTCACGAATTTGCGTGTCAT-3′ |
| TLR4 | Forward:
5′-GCAATGTCTCTGGCAGGTGTA-3′ |
| Reverse:
5′-CAAGGGATAAGAACGCTGAGA-3′ |
|
CD14 | Forward:
5′-TTGCCGAGGTTCAAGATC-3′ |
| Reverse:
5′-TGTGGACACGGAAGCAGA-3′ |
| NF-κBp65 | Forward:
5′-TGCGAATGGAGCGACAGG-3′ |
| Reverse:
5′-AGGCCAAATGAAAGGAGTGG-3′ |
| MyD88 | Forward:
5′-GGCATTTCACTGCTTGATGT-3′ |
| Reverse:
5′-TGACATTCCCATGAAACCTC-3′ |
| TRIF | Forward:
5′-TCAGAGAGTCCATCATTCGG-3′ |
| Reverse:
5′-TACACGCCCACTCTTCTGAG-3′ |
| PCNA | Forward:
5′-AGGGCTGAAGATAATGCTGATA-3′ |
| Reverse:
5′-CTCATTCATCTCTATGGTCACAG-3′ |
| GAPDH | Forward:
5′-GCAAGTTCAACGGCACAG-3′ |
| Reverse:
5′-CGCCAGTAGACTCCACGAC-3′ |
Western blot analysis
The liquid nitrogen preserved intestinal tissues
were randomly selected from 5 mice in each group. The tissues were
ground, washed with PBS 3 times, lysed with 100 µl tissue
RIPA lysis buffer (2 µg/µl; cat. no. 20101ES60;
Yeasen Biotechnology Co. Ltd.) on ice for 5 min and centrifuged at
9,668 × g for 20 min at 4°C. The concentration of total protein was
detected using bicinchoninic acid kit (P0012-1; Beyotime Institute
of Biotechnology). Cells in logarithmic growth phase were
collected, centrifuged at 604 × g for 20 min at 4°C with
supernatant discarded and lysed with 100 µl lysate
(containing 20 mM Tris, 150 mM NaCl and 1% Triton) and 1 µl
phosphatase inhibitor (1111111; Roche Diagnostics) for 30 min on
ice, and then centrifuged at 9,668 × g for 10 min at 4°C. A total
of 50 µg proteins were dissolved in 2X SDS loading buffer,
boiled at 100°C for 5 min, separated by 10% SDS-PAGE gel and
transferred to a polyvinylidene fluoride (PVDF) membrane. The PVDF
membrane was blocked in 5% skimmed milk powder at room temperature
for 1 h and washed with PBS for 2 min. The membrane was incubated
with rabbit anti-mouse primary antibodies (TLR4; diluted at 1:500;
cat. no. ab13556; CD14, 1:100; cat. no. ab18322;
NF-κBp65; 1:2,000; cat. no. ab86299; MyD88; 1:1,000; cat. no.
ab2064; TRIF; 1:500; cat. no. ab13810; PCNA; 1:1,000; cat. no.
ab92552; GAPDH, 1:1,000; cat. no. ab8245) at 4°C overnight. After
washing 3 times with Tris-Buffered Saline and 0.05% Tween-20
(TBST), the membrane was incubated with HRP-labeled secondary
antibody goat anti-rabbit (1:5,000; cat. no. ab97051; Abcam) for 1
h. Then, the membrane was rinsed with TBST 3 times for 5 min each
and developed using an ECL fluorescent detection kit (cat. no.
C10001; Pierce; Thermo Fisher Scientific, Inc.), exposed by X-ray
and the image was captured. Gel imaging analysis system (GelDoc
XR+, Bio-Rad Laboratories Co., Ltd.) was applied to analyze the OD
of color band. The relative content of proteins was expressed as
the ratio of average OD of the sample to that of the corresponding
internal reference according to the statistical analysis chart that
was calculated. The experiment was repeated 3 times.
Cell treatment
The mice intestinal tissues in each group were
washed several times with PBS containing antibiotics (1% penicillin
and streptomycin) under aseptic conditions. The intestinal wall was
cut into pieces <1 mm3, transferred to 50 ml
centrifugal tubes, washed in DMEM (Invitrogen; Thermo Fisher
Scientific, Inc.) without serum, mixed repeatedly with a pipette
and centrifuged at 67 × g for 3 min at 4°C. The above steps were
repeated until the supernatant was clear. The tissue blocks were
suspended by DMEM containing 5% fetal bovine serum (FBS;
Invitrogen; Thermo Fisher Scientific, Inc.) and cultured in a
culture plate or a culture flask for several days. Next, cells were
washed using PBS, detached by 0.05%
trypsin-ethylenediaminetetraacetic acid (EDTA) for 1-2 min, treated
again with 0.05% trypsin-EDTA for 2-3 min, mixed with 0.05%
FBS-DMEM and cultured.
MTT Assay
The cell suspension was inoculated onto a 96-well
plate with a density of 1×104 cells per well (200
µl in each well). The culture plate was placed in an
incubator with 5% CO2 at 37°C with the culture medium
changed every other day. When the cells adhered to the wall, cells
in each well were incubated with 10 µl 5 mg/ml MTT solution
(Sigma-Aldrich; Merck KGaA) for 4 h. Then 150 µl
dimethylsulfoxide was added to the cells in each well were added
and agitated for 10 min to fully dissolve. The OD value of each
well was determined at 490 nm wavelength by an automatic enzyme
reading meter (Bio-Rad Laboratories, Inc.). The experiment was
repeated 3 times.
Permeability assay
Serum-free DMEM was used to resuspend and adjust the
cell density to 3×105 cells/ml. The apical chamber of
the Transwell was supplemented with 200 µl cell suspension.
DMEM medium (600 ml) containing 20% FBS was added into the
basolateral chamber. When the barrier model of intestinal
epithelial cells in vitro was formed, 100 mM luteolin was
added to the cells in each group and cultured at 37°C with 5%
CO2 for 1 h. The cells were washed by Hank's balanced
salt solution (HBSS) 3 times and incubated with HBSS for 30 min in
an incubator, the HBSS was then discarded. Then the cells in the
apical chamber were incubated with 40 mg/ml fluorescein sodium at
37°C for 1 h. The liquid on the basolateral chamber was collected
and the fluorescence intensity was detected by multi-functional
fluorescence analyzer. Fluorescein sodium transmissivity=the
fluorescence amount of fluorescein sodium on the basolateral
chamber/the fluorescence amount of fluorescein sodium in the apical
chamber.
Statistical analysis
All data were processed by SPSS 21.0 (IBM Corp.)
statistical software. All data were tested and conformed to the
normal distribution and homogeneity of variance. The measurement
data were expressed as the mean ± standard deviation. A t-test was
used for comparison between two groups and one-way analysis of
variance was used for comparisons among multiple groups, with
Tukey's post hoc test conducted. P<0.05 was considered to
indicate a statistically significant difference.
Results
Successful establishment of mouse model
of AH
Initially, H&E staining was performed to observe
the liver tissues in mice. The results demonstrated that compared
with the normal group, the liver tissues of mice in the AH group
exhibited immune cell infiltration and fatty accumulation, which
was typical of AH. The result demonstrated that the model of mice
with AH was successful established (Supplementary Fig. S1).
miR-141 ameliorates AH-induced
pathological alterations of the intestinal tissues
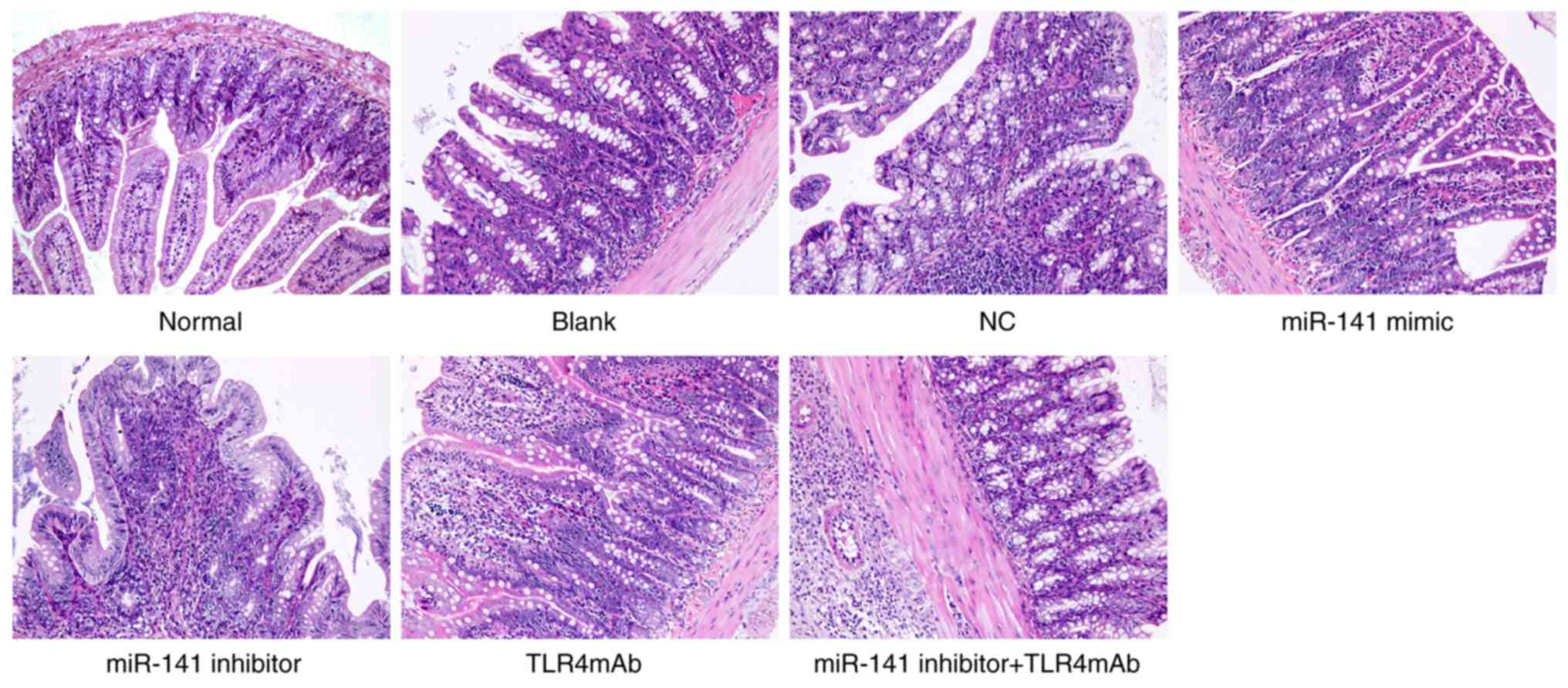
H&E staining was also performed to observe
whether miR-141 could affect AH-induced pathological changes in the
intestinal tissues (Fig. 1). The
structure of intestinal tissues of mice in the normal group was
clear, without any abnormality. In the blank, NC and miR-141
inhibitor + TLR4mAb groups, adhesions were identified between the
intestinal wall and surrounding tissues as well as hyperemia and
edema in surrounding mucosa, the intestinal canal was thickened,
ulcer foci was identified with thickening intestinal wall, severe
mucosal edema was observed in the colon, plasma cells, lymphocytes
and neutrophilic infiltration occurred in the mucosa and plasma
layer, and an obvious necrosis layer and granulation tissue were
identified on the ulcer surface. The intestinal tissue injury was
ameliorated in the miR-141 mimic and TLR4mAb groups whereas it was
aggravated in the miR-141 inhibitor group. As presented in Table III, in comparison with the
normal group, all the other groups exhibited a significantly
increased pathological score of intestinal tissue injury (all
P<0.05). In comparison with the blank and NC groups, the miR-141
mimic and TLR4mAb groups exhibited a decreased pathological score
while the miR-141 inhibitor group demonstrated an significantly
increased pathological score (all P<0.05). There was no
significant difference in terms of pathological score in the
miR-141 inhibitors + TLR4mAb group (P>0.05). Therefore, it was
suggested that miR-141 or inhibiting TLR pathway could ameliorate
AH-induced pathological changes in the intestinal tissues.
 | Table IIImiR-141 ameliorates alcoholic
hepatitis-induced pathological changes in the intestinal tissues,
reflected in a lower pathological score of intestinal injury. |
Table III
miR-141 ameliorates alcoholic
hepatitis-induced pathological changes in the intestinal tissues,
reflected in a lower pathological score of intestinal injury.
| Group | n | Pathological
score |
|---|
| Normal | 5 | 0 |
| Blank | 5 | 11.00±1.14a |
| NC | 5 | 11.80±1.10a |
| miR-141 mimic | 5 |
4.60±0.55a-c |
| miR-141
inhibitor | 5 |
14.20±0.84a-c |
| TLR4mAb | 5 |
4.80±0.45a-c |
| miR-141 inhibitor +
TLR4mAb | 5 | 11.00±0.71a |
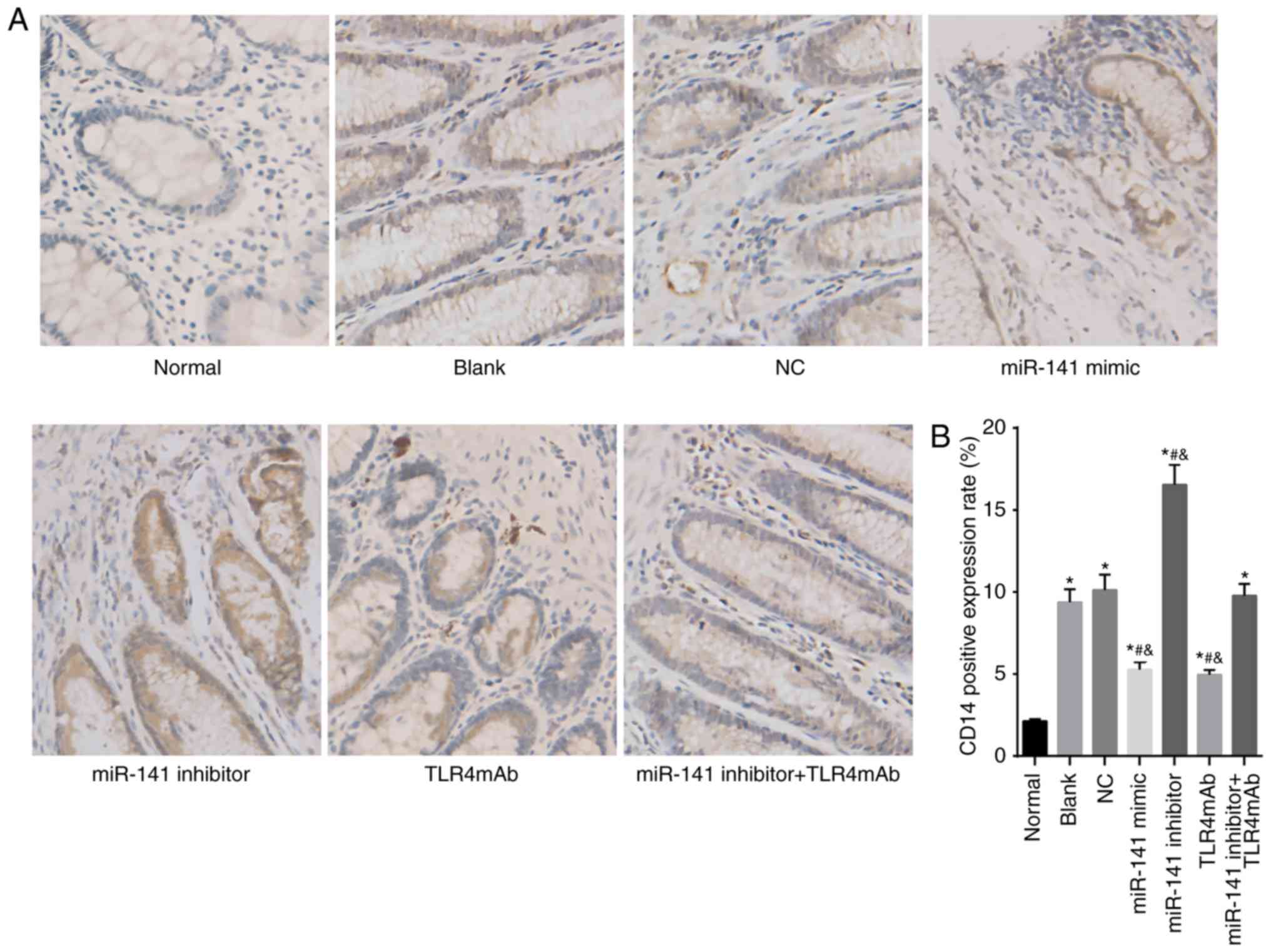
miR-141 decreases expression of TLR4 and
CD14 in the intestinal tissues
In order to evaluate the protein levels of TLR4 and
CD14, immunohistochemistry was performed. As displayed
in Fig. 2, TLR4 and
CD14 were mainly localized in the cytoplasm. Compared
with the normal group, the expression of TLR4 and CD14
was significantly increased in all the other groups (all
P<0.05). In comparison to the blank and NC groups, the miR-141
mimic and TLR4mAb groups demonstrated significantly downregulated
positive expression of TLR4 and CD14 while the miR-141
inhibitor group exhibited significantly upregulated positive
expression of TLR4 and CD14 (all P<0.05). There was
no significant difference in terms of positive expression of TLR4
and CD14 between the miR-141 inhibitor + TLR4mAb group
and the blank and NC groups (P>0.05). Taken together, miR-141 or
TLR pathway inhibitor could decrease the expression of TLR4 and
CD14 in the intestinal tissues.
miR-141 inhibits serum levels of
endotoxin, D lactic acid, DAO and ALT in AH mice
Subsequently, the contents of endotoxin, D lactic
acid, DAO and ALT in the serum of AH mice were measured. As
presented in Table IV, compared
with the normal group, all the other groups exhibited significantly
increased levels of endotoxin, D lactic acid, DAO and ALT in serum
(all P<0.05), suggesting a successful establishment of model of
AH mice. In comparison to the blank and NC groups, the miR-141
mimic and TLR4mAb groups presented significantly decreased contents
of serum endotoxin, D lactic acid, DAO, and ALT while the miR-141
inhibitor group exhibited significantly increased levels of those
substances (all P<0.05), which indicated that overexpressed
miR-141 or inhibited TLR pathway could ameliorate the intestinal
injury and IETM induced by AH. There was no significant difference
in the levels of all the above indicators in the miR-141 inhibitors
+ TLR4mAb group compared with the blank or NC groups (P>0.05),
which was caused by inhibiting the miR-141 and the TLR pathway. The
above results demonstrated that over-expression of miR-141
alleviated the AH-induced intestinal injury and IETM through
targeting the TLR4 pathway.
 | Table IVmiR-141 inhibits content of
endotoxin, D lactic acid, DAO and ALT in the serum of alcoholic
hepatitis mice. |
Table IV
miR-141 inhibits content of
endotoxin, D lactic acid, DAO and ALT in the serum of alcoholic
hepatitis mice.
| Group | n | Serum endotoxin
(kEU/l) | D lactic acid
(µg/ml) | DAO (kU/l) | ALT (U/l) |
|---|
| Normal | 5 | 0.30±0.02 | 0.76±0.08 | 1.90±0.20 | 42.40±4.20 |
| Blank | 5 | 0.71±0.06a | 2.07±0.17a | 4.50±0.40a |
354.40±32.40a |
| NC | 5 | 0.72±0.06a | 2.12±0.16a | 4.60±0.40a |
351.40±31.10a |
| miR-141 mimic | 5 | 0.47±0.04a,b | 1.41±0.10a-c | 3.20±0.30a-c |
213.70±21.30a-c |
| miR-141
inhibitor | 5 | 0.90±0.08a,b | 2.79±0.18a-c | 6.50±0.60a-c |
466.50±45.60a-c |
| TLR4mAb | 5 | 0.49±0.04a,b | 1.43±0.12a-c | 3.30±0.20a-c |
211.70±20.20a-c |
| miR-141 inhibitor +
TLR4mAb | 5 | 0.73±0.06a | 2.10±0.19a | 4.60±0.50a |
359.40±29.70a,c |
miR-141 reduces TNF-α, IL-6 and IL-1
contents in the intestinal tissues of AH mice
An ELISA was conducted to measure the levels of
inflammatory factors TNF-α, IL-6 and IL-1 in the intestinal tissues
of AH mice (Table V). Compared
with the normal group, all the other groups demonstrated
significantly upregulated contents of TNF-α, IL-6 and IL-1β in the
intestinal tissues (all P<0.05). In comparison to the blank and
NC groups, the contents of TNF-α, IL-6 and IL-1β were notably
decreased in the miR-141 mimic and TLR4mAb groups while
significantly increased in the miR-141 inhibitor group (all
P<0.05). No significant difference was identified in the
contents of TNF-α, IL-6 and IL-1β in the miR-141 inhibitor +
TLR4mAb group compared with the NC or the blank group (P>0.05).
The aforementioned results indicated that upregulation of miR-141
or inhibit TLR could reduce contents of TNF-α, IL-6 and IL-1 in the
intestinal tissues of AH mice.
 | Table VmiR-141 reduces content of TNF-α,
IL-6 and IL-1 in the intestinal tissues of alcoholic hepatitis
mice, determined by ELISA. |
Table V
miR-141 reduces content of TNF-α,
IL-6 and IL-1 in the intestinal tissues of alcoholic hepatitis
mice, determined by ELISA.
| Group | n | TNF-α (pg/ml) | IL-6 (pg/ml) | IL-1β (pg/kg) |
|---|
| Normal | 5 | 10.8±0.92 | 47.8±3.24 | 27.5±2.4 |
| Blank | 5 | 26.3±2.1a | 189.5±
11.26a | 128.6±13.3a |
| NC | 5 | 27.5±1.97a |
191.16±10.64a | 129.2±11.2a |
| miR-141 mimic | 5 | 19.4±1.42a,b | 135.28±9.23a-c | 84.5±8.4a-c |
| miR-141
inhibitor | 5 | 35.2±2.81a,b |
253.92±18.87a-c | 162.7±15.8a-c |
| TLR4mAb | 5 | 19.7±1.35a,b | 138.31±8.79a-c | 87.4±7.9a-c |
| miR-141 inhibitor +
TLR4mAb | 5 | 26.9±2.04a |
190.66±12.70a | 131.7±11.3a |
miR-141 downregulates the mRNA level of
TLR4, CD14, NF-κBp65, MyD88 and TRIF but upregulates the
mRNA level PCNA in the intestinal tissues
RT-qPCR was performed to measure the miR-141 level
and mRNA levels of TLR4, CD14, NF-κBp65, MyD88, TRIF and
PCNA in the intestinal tissues of mice in each group. As presented
in Fig. 3, in contrast to the
normal group, the levels of miR-141 and PCNA were significantly
downregulated and the levels of TLR4, CD14, NF-κBp65,
MyD88 and TRIF were significantly upregulated in the other groups
(all P<0.05). In comparison to the blank and NC groups, the
miR-141 mimic and TLR4mAb groups exhibited significantly
upregulated expression of miR-141 and PCNA but significantly
downregulated expression of TLR4, CD14, NF-κBp65, MyD88
and TRIF (all P<0.05), while the miR-141 inhibitor group
exhibited significantly increased expression of TLR4,
CD14, NF-κBp65, MyD88 and TRIF but decreased expression
of miR-141 and PCNA (all P<0.05). The miR-141 inhibitor +
TLR4mAb group revealed decreased expression of miR-141 but
exhibited no obvious differences in mRNA levels of other genes
compared with the blank or NC group (P>0.05). All the obtained
data indicated that miR-141 could downregulate the mRNA levels of
TLR4, CD14, NF-κBp65, MyD88 and TRIF and upregulate that
of PCNA in the intestinal tissues.
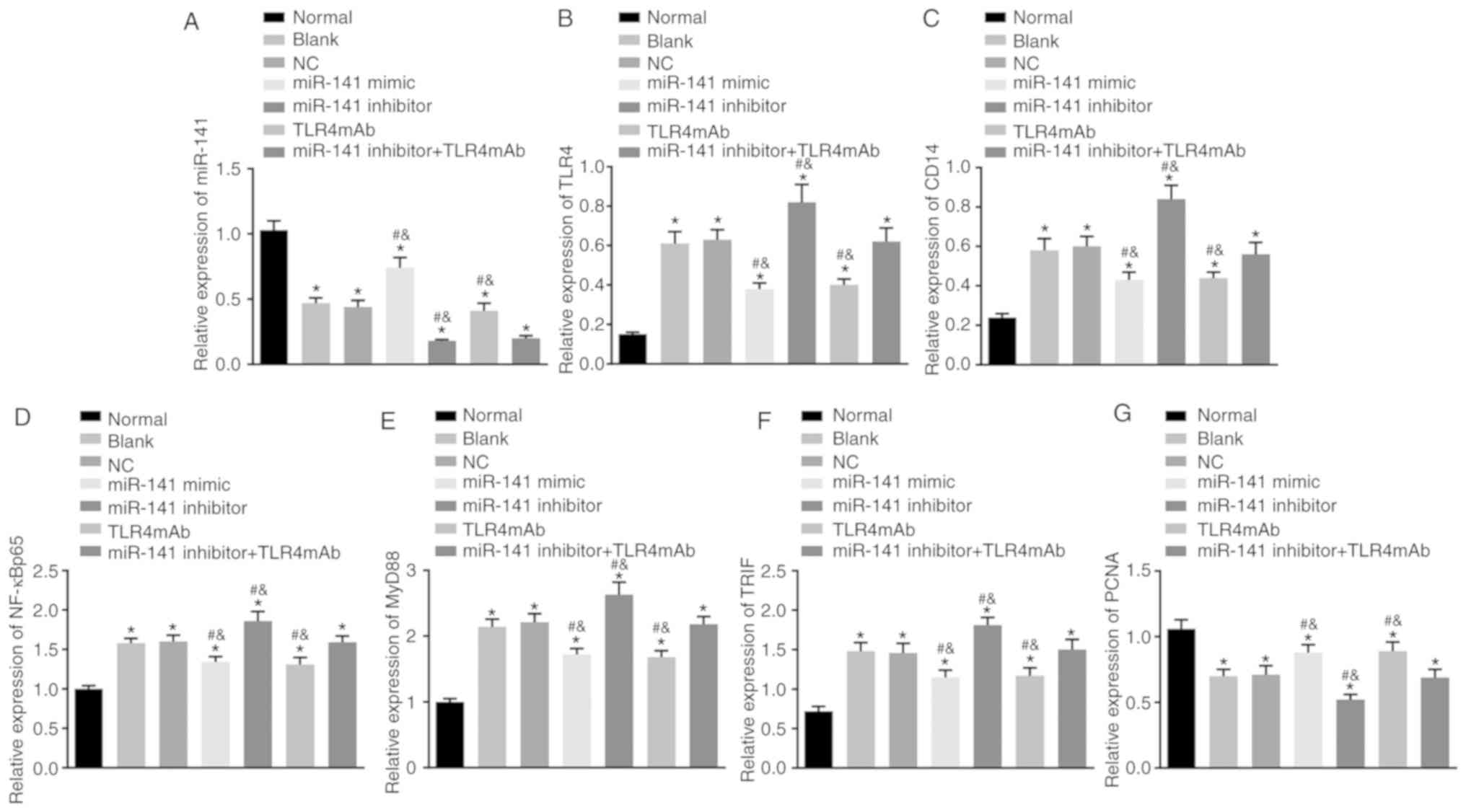 | Figure 3miR-141 downregulates the mRNA
expression of TLR4, CD14, NF-κBp65, MyD88 and TRIF but
upregulates that of PCNA in the intestinal tissues of alcoholic
hepatitis mice. (A) The miR-141 expression, the mRNA expression of
(B) TLR4, (C) CD14, (D) NF-κBp65, (E) MyD88, (F) TRIF
and (G) PCNA in intestinal tissues following different treatments
examined by reverse transcription quantitative polymerase chain
reaction. *P<0.05 vs. the normal group;
#P<0.05 vs. the blank group;
&P<0.05 vs. the NC group. The measurement data
were expressed as the mean ± standard deviation and analyzed by
one-way analysis of variance. n=5. miR-141, microRNA-141; NC,
negative control; TLR4mAb, Toll-like receptor 4 monoclonal
antibodies; TLR4, Toll-like receptor 4; CD14, cluster of
differentiation 14; NF-κBp65, nuclear factor-κBp65; MyD88, myeloid
differentiation factor 88; TRIF, TIR-domain-containing adaptor
inducing interferon-β; PCNA, proliferating cell nuclear
antigen. |
miR-141 inhibits the protein levels of
TLR4, CD14, NF-κBp65, MyD88 and TRIF but increases
protein level of PCNA in the intestinal tissues
Western blot analysis was conducted to determine
protein levels of TLR4, CD14, NF-κBp65, MyD88, TRIF and
PCNA in the intestinal tissues of mice in each group. As presented
in Fig. 4, compared with the
normal group, the protein level of PCNA was decreased while the
protein levels of TLR4, CD14, NF-κBp65, MyD88 and TRIF
were increased in all the other groups (all P<0.05). In
comparison to the blank and NC groups, the miR-141 mimic and
TLR4mAb groups presented downregulated protein levels of TLR4,
CD14, NF-κBp65, MyD88 and TRIF however significantly
upregulated levels of PCNA protein were identified (all P<0.05).
The miR-141 inhibitor group demonstrated increased expression of
TLR4, CD14, NF-κBp65, MyD88 and TRIF while decreased
expression of PCNA (all P<0.05). The miR-141 inhibitor + TLR4mAb
group exhibited no obvious differences in the protein levels of
TLR4, CD14, NF-κBp65, MyD88, TRIF and PCNA compared with
the blank or NC group (P>0.05). These results suggested that
miR-141 could inhibit expression of TLR4, CD14,
NF-κBp65, MyD88 and TRIF but promote expression of PCNA at protein
levels in the intestinal tissues.
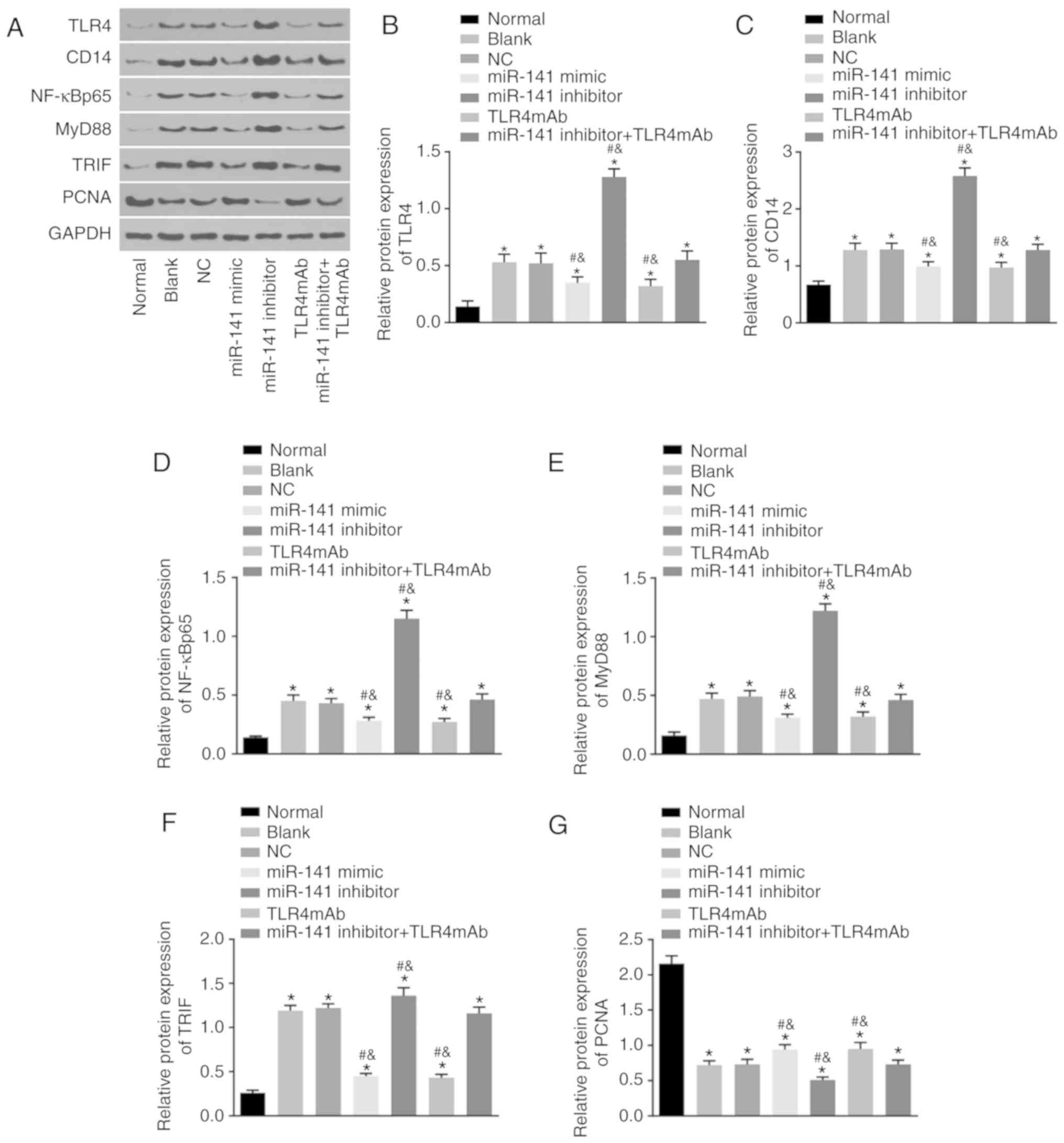 | Figure 4miR-141 inhibits the protein
expression of TLR4, CD14, NF-κBp65, MyD88 and TRIF but
promotes the protein expression of PCNA in the intestinal tissues
of AH mice. (A) The protein bands of TLR4, CD14,
NF-κBp65, MyD88 and TRIF and PCNA in intestinal tissues following
different treatments examined western blot analysis. The protein
expression of (B) TLR4, (C) CD14, (D) NF-κBp65, (E)
MyD88, (F) TRIF and (G) PCNA in intestinal tissues following
different treatments examined western blot analysis.
*P<0.05 vs. the normal group; #P<0.05
vs. the blank group; &P<0.05 vs. the NC group.
The measurement data were expressed as the mean ± standard
deviation and analyzed by one-way analysis of variance. n=5.
miR-141, microRNA-141; NC, negative control; TLR4mAb, Toll-like
receptor 4 monoclonal antibodies; TLR4, Toll-like receptor 4;
CD14, cluster of differentiation 14; NF-κBp65, nuclear
factor-κBp65; MyD88, myeloid differentiation factor 88; TRIF,
TIR-domain-containing adaptor inducing interferon-β; PCNA,
proliferating cell nuclear antigen; AH, alcoholic hepatitis. |
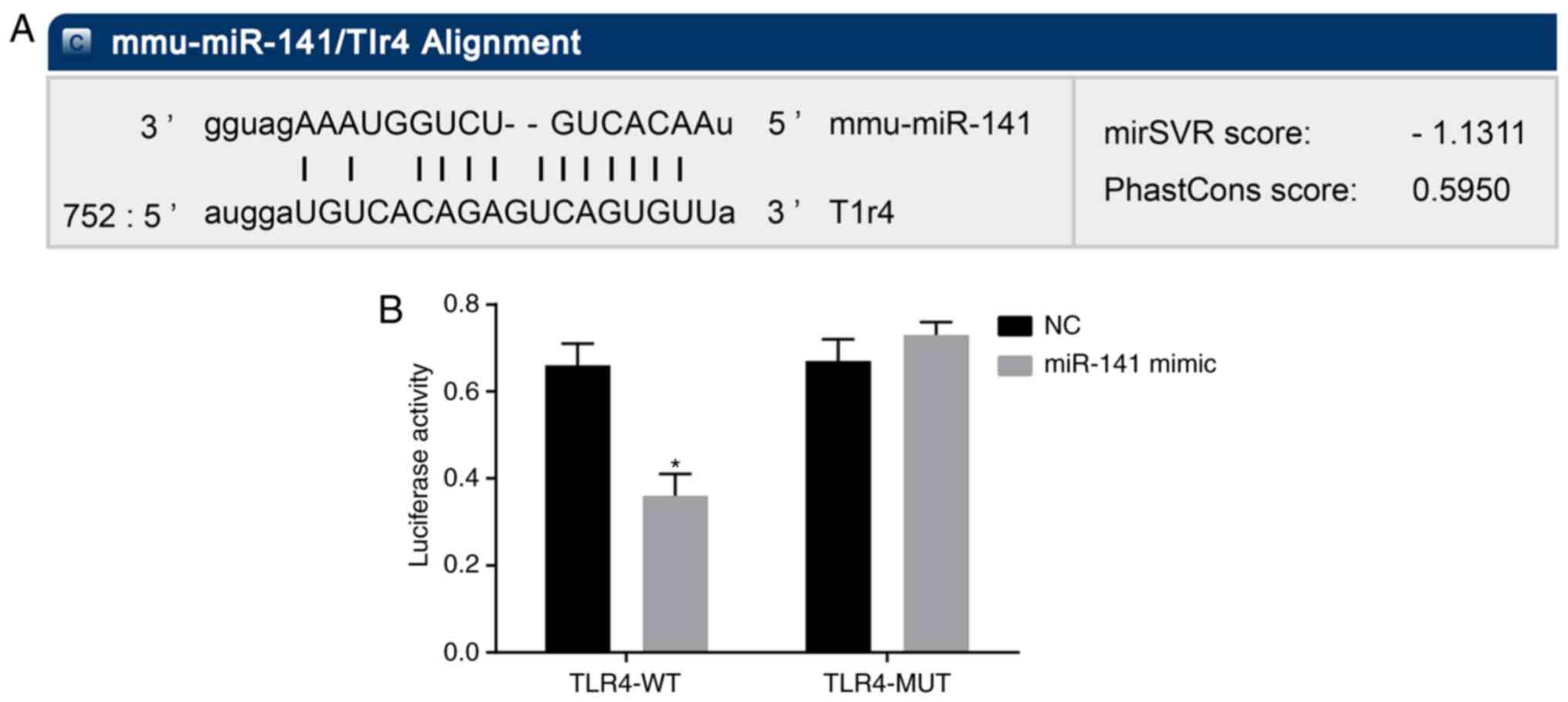
TLR4 is a target gene of miR-141
Furthermore, whether miR-141 could directly regulate
TLR4 was examined by target prediction program and luciferase
assay. miR-141 was predicted to target TLR4 based on the biological
prediction website MicroRNA.org. Moreover, the luciferase report
assay results suggested that compared with the NC group, the
luciferase activity of TLR4 wild-type 3′UTR was significantly
inhibited by miR-141 (P<0.05), whereas that of the mutant 3′UTR
was not affected (Fig. 5). These
results indicated that miR-141 could specifically bind to 3′UTR of
TLR4 and downregulate the expression of TLR4.
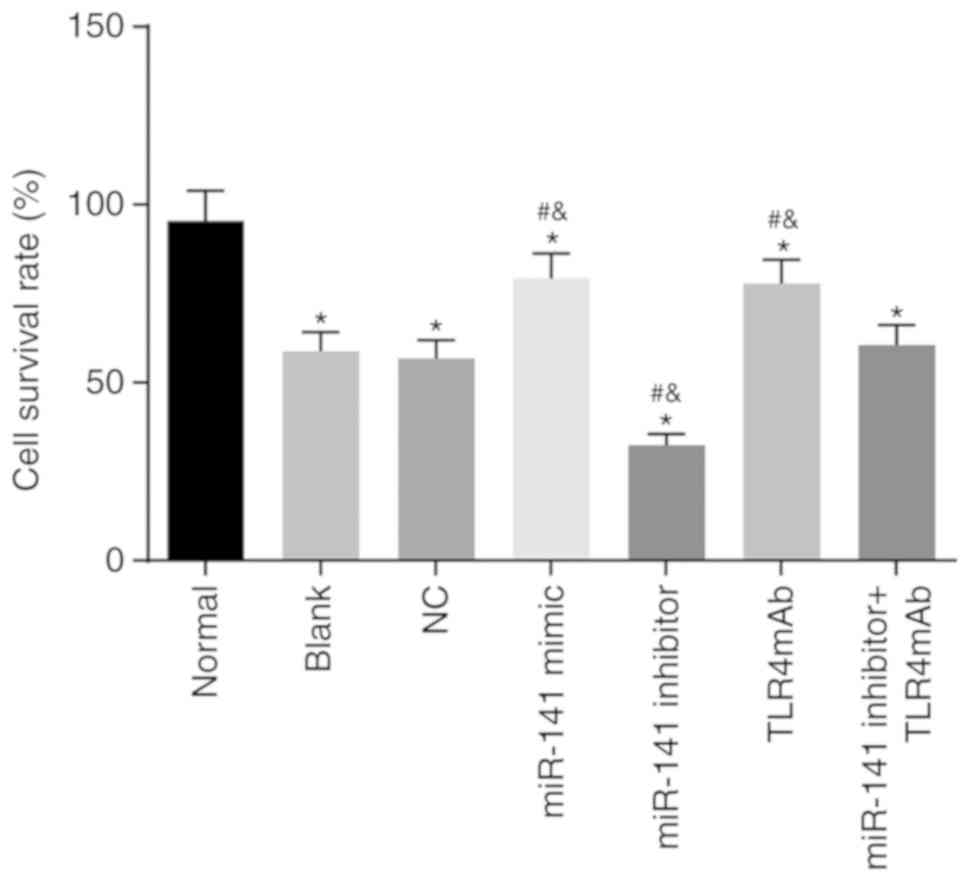
miR-141 promotes viability of intestinal
epithelial cells
An MTT assay was applied to investigate the effect
of miR-141 on viability of intestinal epithelial cells. As
presented in Fig. 6, in
comparison to the normal group, all the other groups exhibited
significantly decreased viability of intestinal epithelial cells
(all P<0.05). In contrast to the blank and NC groups, the
miR-141 mimic and TLR4mAb groups demonstrated significantly
enhanced viability of intestinal epithelial cells while the miR-141
inhibitor group exhibited significantly lower viability of
intestinal epithelial cells (all P<0.05). No significant
difference in the viability of intestinal epithelial cells was
identified in the miR-141 inhibitor + TLR4mAb group compared with
the blank or NC group (P<0.05). Therefore it was concluded that
miR-141 could promote viability of intestinal epithelial cells.
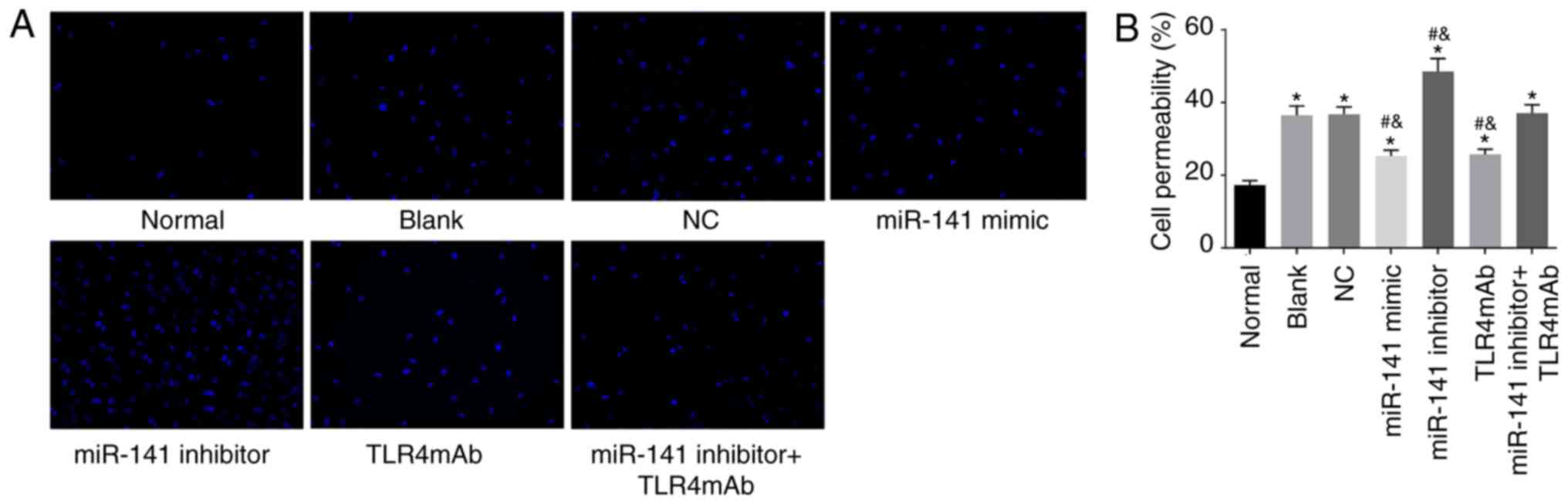
miR-141 suppresses the permeability of
intestinal epithelial cells
In order to examine whether miR-141 could influence
the permeability of intestinal epithelial cells, permeability assay
was performed. As presented in Fig.
7, the intestinal epithelial cell permeability was increased in
all the other groups compared with the normal group (all
P<0.05). In comparison to the blank and NC groups, the cell
permeability was significantly decreased in the miR-141 mimic and
TLR4mAb groups while significantly increased in the miR-141
inhibitor group (all P<0.05). No obvious change in the cell
permeability was identified in the miR-141 inhibitor + TLR4mAb
group compared with the blank or normal group (P>0.05). The
above findings revealed that miR-141 could suppress the
permeability of intestinal epithelial cells.
Discussion
AH manifests the features of hepatocellular damage,
fibrosis and inflammation, which has a high mortality rate without
effective treatment (24,25). Corticosteroids serve as a widely
used treatment for AH, but the effects differ considerably and
patients with AH mainly die of infection though the infiltration of
neutrophils which can cause injury to the liver (26). Previously, a study has
demonstrated that miRs contribute to the pathogenesis of liver
diseases and can function as diagnostic and prognostic markers as
well as therapeutic targets for liver diseases (27). Therefore, the present study
investigated the role of miR-141 in intestinal injury and IETM of
AH and identified that miR-141 reversed intestinal injury and IETM
of AH by targeting TLR4 and inhibiting the TLR signaling
pathway.
Alcohol metabolism activates the innate immune cells
including monocytes and macrophages in the liver, thereby promoting
the progression and pathogenesis of ALD. It is reported that
miR-27a is associated with the activation and polarization of
alcohol-induced monocytes via the extracellular signal regulated
kinase signaling pathway (28). A
previous study demonstrated that miR-146b improved intestinal
injury in colitis through the activation of the NF-κB signaling
pathway (29). miR-122, with
various functions in hepatocytes, is also reported to be associated
with acute alcohol-induced liver injury and to serve a role in
regulating cholesterol metabolism (13). A previous study indicates that
miR-141 exerts influence on inflammatory cell trafficking in
colonic inflammation by targeting CXCL12β (30). miR-141 is also reported to be
involved in the improvement of carrageenan-induced prostatitis
through the regulation of the Kelch-like ECH-associated
protein1/Nuclear factor erythroid 2-related factor 2 signaling
pathway (31).
The present study revealed that upregulation of
miR-141 ameliorated intestinal injury of AH by suppressing the TLR
signaling pathway. It has been found that the induction of miR-155
is involved in the regulation of tenascin-C in the inflammatory
axis via the TLR signaling pathway (32). TLR4, a pattern recognition
receptor, serves a key part in the pathogenesis of liver disease
and the CD14/TLR4 receptor complex is associated with
the release of inflammatory cytokines induced by lipopolysaccharide
(LPS) (33). It is also
demonstrated that interferon regulatory factor 3 results in liver
inflammation and hepatocellular damage by mediating
ischemia-reperfusion injury-induced TLR4 (34). Previous studies demonstrate that
C-C motif chemokine 20 (CCL20) is a crucial mediator associated
with hepatic inflammation and injury in AH and TLR1 in the
intestinal epithelium resulted in the upregulation of CCL20
(35,36). Additionally, TLR3-mediated
intestinal tissue injury can be decreased by immunobiotic
lactobacilli via the regulation of intraepithelial lymphocytes
response (37). Another study
demonstrated that that TLRs serve a protective role in necrotizing
enterocolitis by producing anti-inflammatory cytokines and also
have positive effects on intestinal mucosal damage by improving
intestinal homeostasis (38).
In addition, the present study demonstrated that
miR-141 inhibited TNF-α, IL-6 and IL-1β, as well as the expression
of TLR4, CD14, NF-κBp65, MyD88, and TRIF. TNF-α, IL-6
and IL-1β are pro-inflammatory cytokines that are secreted by glial
cells in the central nervous system (39). IL-1β is closely associated with
inflammation responses and cell death in a pro-inflammatory form
(40). Another study also
demonstrated that the miR-141-3p mimic group exhibited decreased
levels of IL-1β, TNF-α and IL-6, and miR-141-3p was capable of
mitigating chronic inflammatory pain by downregulating the
high-mobility group box1 gene, which is consistent with the present
results (41). MyD88 is rapidly
produced when M1 myeloleukemic cells differentiate into
macrophages, which is stimulated by IL-6 (42). TLR4 combined with LPS induces a
signaling cascade via the MyD88-dependent and/or MyD88-independent
pathway, which results in activating mitogen activated protein
kinase 1 and nuclear factor (NF)-kB, thereby contributing to
producing pro-inflammatory cytokines (43).
In addition, the present study demonstrated that
miR-141 inhibited the occurrence of IETM in AH by targeting the
TLR4 gene. It was identified that endotoxins may induce the release
of pro-inflammatory cytokines since they can activate the
TLR4/NF-κB signaling pathway (44). When epithelial mesenchymal
transition (ETM) occurs, pro-inflammatory cytokines are reported to
be secreted into the intestinal lumen, which promotes intestinal
dysmotility and intestinal failure (45). One study revealed that miR-146a
serves a significant role in endotoxin-induced cross-tolerance for
monocytic cells in vitro (46). Another study also
demonstrated that miR-98 remarkably reduces the triggering of
endotoxin tolerance and is closely associated with the regulation
of IL-10 production in endotoxin tolerance (47). Furthermore, it has been reported
that LRG47 in LPS-stimulated murine macrophages inhibits the
production of pro-inflammatory cytokines by downregulating TLR4 and
prevents ETM by inhibiting MyD88/NF-κB/p38-induced TNF/IL-6
synthesis (48).
In conclusion, overexpression of miR-141 contributed
to the inhibition of the TLR signaling pathway by targeting the
TLR4 gene, thereby rescuing intestinal injury and IETM of AH, which
will provide a novel basis for the treatment of intestinal injury
and IETM in AH.
Supplementary Data
Acknowledgments
Not applicable.
Funding
The present study was funded by the Huai'an Natural
Science Foundation (grant no. HAB201722) and Nanjing Medical
University Natural Science Foundation (grant no. 2016NJMU137).
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WQ and YL designed the study. WQ, XL, and YP
collated the data, designed and developed the database, carried out
data analyses and produced the initial draft of the manuscript. WQ
and YL contributed to drafting the manuscript. All authors
participated in the revised manuscript and have read and approved
the final submitted manuscript.
Ethics approval and consent to
participate
All animal experiments involved in the present study
were in line with the principles of the approval of the clinical
ethical committee of the Affiliated Huai'an Hospital of Xuzhou
Medical University. Significant efforts were made in order to
minimize the number of animals used as well as their respective
suffering.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Damgaard Sandahl T: Alcoholic hepatitis.
Dan Med J. 61:B47552014.PubMed/NCBI
|
|
2
|
Kendrick SF, O'Boyle G, Mann J, Zeybel M,
Palmer J, Jones DE and Day CP: Acetate, the key modulator of
inflammatory responses in acute alcoholic hepatitis. Hepatology.
51:1988–1997. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chayanupatkul M and Liangpunsakul S:
Alcoholic hepatitis: A comprehensive review of pathogenesis and
treatment. World J Gastroenterol. 20:6279–6286. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Singal AK, Kamath PS, Gores GJ and Shah
VH: Alcoholic hepatitis: Current challenges and future directions.
Clin Gastroenterol Hepatol. 12:555–564; quiz e31-e32. 2014.
View Article : Google Scholar :
|
|
5
|
Raff E and Singal AK: Optimal management
of alcoholic hepatitis. Minerva Gastroenterol Dietol. 60:25–38.
2014.PubMed/NCBI
|
|
6
|
Torok NJ: Update on alcoholic hepatitis.
Biomolecules. 5:2978–2986. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yan AW and Schnabl B: Bacterial
translocation and changes in the intestinal microbiome associated
with alcoholic liver disease. World J Hepatol. 4:110–118. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schnabl B and Brenner DA: Interactions
between the intestinal microbiome and liver diseases.
Gastroenterology. 146:1513–1524. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rao R: Endotoxemia and gut barrier
dysfunction in alcoholic liver disease. Hepatology. 50:638–644.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
McDaniel K, Herrera L, Zhou T, Francis H,
Han Y, Levine P, Lin E, Glaser S, Alpini G and Meng F: The
functional role of microRNAs in alcoholic liver injury. J Cell Mol
Med. 18:197–207. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Szabo G and Satishchandran A: MicroRNAs in
alcoholic liver disease. Semin Liver Dis. 35:36–42. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Blaya D, Coll M, Rodrigo-Torres D,
Vila-Casadesús M, Altamirano J, Llopis M, Graupera I, Perea L,
Aguilar-Bravo B, Diaz A, et al: Integrative microRNA profiling in
alcoholic hepatitis reveals a role for microRNA-182 in liver injury
and inflammation. Gut. 65:1535–1545. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bala S, Petrasek J, Mundkur S, Catalano D,
Levin I, Ward J, Alao H, Kodys K and Szabo G: Circulating microRNAs
in exosomes indicate hepatocyte injury and inflammation in
alcoholic, drug-induced, and inflammatory liver diseases.
Hepatology. 56:1946–1957. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lucas K and Maes M: Role of the Toll Like
receptor (TLR) radical cycle in chronic inflammation: Possible
treatments targeting the TLR4 pathway. Mol Neurobiol. 48:190–204.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou X, Ye F, Yin C, Zhuang Y, Yue G and
Zhang G: The interaction between MiR-141 and lncRNA-H19 in
regulating cell proliferation and migration in gastric cancer. Cell
Physiol Biochem. 36:1440–1452. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xue J, Niu YF, Huang J, Peng G, Wang LX,
Yang YH and Li YQ: miR-141 suppresses the growth and metastasis of
HCC cells by targeting E2F3. Tumour Biol. 35:12103–12107. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang XY, Huang ZL, Xu YH, Zheng Q, Chen
Z, Song W, Zhou J, Tang ZY and Huang XY: Comprehensive circular RNA
profiling reveals the regulatory role of the
circRNA-100338/miR-141-3p pathway in hepatitis B-related
hepatocellular carcinoma. Sci Rep. 7:54282017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cai M, Chen S and Hu W: MicroRNA-141 is
involved in ulcerative colitis pathogenesis via aiming at CXCL5. J
Interferon Cytokine Res. 37:415–420. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lowe PP, Gyongyosi B, Satishchandran A,
Iracheta-Vellve A, Ambade A, Kodys K, Catalano D, Ward DV and Szabo
G: Alcohol-related changes in the intestinal microbiome influence
neutrophil infiltration, inflammation and steatosis in early
alcoholic hepatitis in mice. PLoS One. 12:e01745442017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Geboes K, Riddell R, Ost A, Jensfelt B,
Persson T and Löfberg R: A reproducible grading scale for
histological assessment of inflammation in ulcerative colitis. Gut.
47:404–409. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang X, Sun L, Wang X, Kang H, Ma X, Wang
M, Lin S, Liu M, Dai C and Dai Z: Acidified bile acids enhance
tumor progression and telomerase activity of gastric cancer in mice
dependent on c-Myc expression. Cancer Med. 6:788–797. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li X, Li D, Zhuang Y, Shi Q, Wei W and Ju
X: Overexpression of miR-708 and its targets in the childhood
common precursor B-cell ALL. Pediatr Blood Cancer. 60:2060–2067.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ayuk SM, Abrahamse H and Houreld NN: The
role of photo-biomodulation on gene expression of cell adhesion
molecules in diabetic wounded fibroblasts in vitro. J Photochem
Photobiol B. 161:368–374. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dominguez M, Miquel R, Colmenero J, Moreno
M, García-Pagán JC, Bosch J, Arroyo V, Ginès P, Caballeria J and
Bataller R: Hepatic expression of CXC chemokines predicts portal
hypertension and survival in patients with alcoholic hepatitis.
Gastroenterology. 136:1639–1650. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Boetticher NC, Peine CJ, Kwo P, Abrams GA,
Patel T, Aqel B, Boardman L, Gores GJ, Harmsen WS, McClain CJ, et
al: A randomized, double-blinded, placebo-controlled multicenter
trial of etanercept in the treatment of alcoholic hepatitis.
Gastroenterology. 135:1953–1960. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mookerjee RP, Stadlbauer V, Lidder S,
Wright GA, Hodges SJ, Davies NA and Jalan R: Neutrophil dysfunction
in alcoholic hepatitis superimposed on cirrhosis is reversible and
predicts the outcome. Hepatology. 46:831–840. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang XW, Heegaard NH and Orum H: MicroRNAs
in liver disease. Gastroenterology. 142:1431–1443. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Saha B, Bruneau JC, Kodys K and Szabo G:
Alcohol-induced miR-27a regulates differentiation and M2 macrophage
polarization of normal human monocytes. J Immunol. 194:3079–3087.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nata T, Fujiya M, Ueno N, Moriichi K,
Konishi H, Tanabe H, Ohtake T, Ikuta K and Kohgo Y: MicroRNA-146b
improves intestinal injury in mouse colitis by activating nuclear
factor-κB and improving epithelial barrier function. J Gene Med.
15:249–260. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huang Z, Shi T, Zhou Q, Shi S, Zhao R, Shi
H, Dong L, Zhang C, Zeng K, Chen J and Zhang J: miR-141 Regulates
colonic leukocytic trafficking by targeting CXCL12β during murine
colitis and human Crohn's disease. Gut. 63:1247–1257. 2014.
View Article : Google Scholar
|
|
31
|
Wang LL, Huang YH, Yan CY, Wei XD, Hou JQ,
Pu JX and Lv JX: N-acetylcysteine ameliorates prostatitis via
miR-141 regulating keap1/Nrf2 signaling. Inflammation. 39:938–947.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Piccinini AM and Midwood KS: Endogenous
control of immunity against infection: Tenascin-C regulates
TLR4-mediated inflammation via microRNA-155. Cell Rep. 2:914–926.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Von Hahn T, Halangk J, Witt H, Neumann K,
Müller T, Puhl G, Neuhaus P, Nickel R, Beuers U, Wiedenmann B and
Berg T: Relevance of endotoxin receptor CD14 and TLR4 gene variants
in chronic liver disease. Scand J Gastroenterol. 43:584–592. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhai Y, Shen XD, O'Connell R, Gao F,
Lassman C, Busuttil RW, Cheng G and Kupiec-Weglinski JW: Cutting
edge: TLR4 activation mediates liver ischemia/reperfusion
inflammatory response via IFN regulatory factor 3-dependent
MyD88-independent pathway. J Immunol. 173:7115–7119. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Affò S, Morales-Ibanez O, Rodrigo-Torres
D, Altamirano J, Blaya D, Dapito DH, Millán C, Coll M, Caviglia JM,
Arroyo V, et al: CCL20 mediates lipopolysaccharide induced liver
injury and is a potential driver of inflammation and fibrosis in
alcoholic hepatitis. Gut. 63:1782–1792. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sugiura Y, Kamdar K, Khakpour S, Young G,
Karpus WJ and DePaolo RW: TLR1-induced chemokine production is
critical for mucosal immunity against Yersinia enterocolitica.
Mucosal Immunol. 6:1101–1109. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tada A, Zelaya H, Clua P, Salva S, Alvarez
S, Kitazawa H and Villena J: Immunobiotic Lactobacillus strains
reduce small intestinal injury induced by intraepithelial
lymphocytes after Toll-like receptor 3 activation. Inflamm Res.
65:771–783. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tatum PM Jr, Harmon CM, Lorenz RG and
Dimmitt RA: Toll-like receptor 4 is protective against neonatal
murine ischemia-reperfusion intestinal injury. J Pediatr Surg.
45:1246–1255. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Choi BM, Lee SH, An SM, Park DY, Lee GW
and Noh GJ: The time-course and RNA interference of TNF-α, IL-6,
and IL-1β expression on neuropathic pain induced by L5 spinal nerve
transection in rats. Korean J Anesthesiol. 68:159–169. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Peng Y, French BA, Tillman B, Morgan TR
and French SW: The inflammasome in alcoholic hepatitis: Its
relationship with Mallory-Denk body formation. Exp Mol Pathol.
97:305–313. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shen WS, Xu XQ, Zhai NN, Zhou ZS, Shao J
and Yu YH: Potential mechanisms of microRNA-141-3p to alleviate
chronic inflammatory pain by downregulation of downstream target
gene HMGB1: In vitro and in vivo studies. Gene Ther. 24:353–360.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Akira S and Takeda K: Toll-like receptor
signalling. Nat Rev Immunol. 4:499–511. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen Q, Wang H, Liu Y, Song Y, Lai L, Han
Q, Cao X and Wang Q: Inducible microRNA-223 down-regulation
promotes TLR-triggered IL-6 and IL-1β production in macrophages by
targeting STAT3. PLoS One. 7:e429712012. View Article : Google Scholar
|
|
44
|
Grimaldi D, Guivarch E, Neveux N, Fichet
J, Pène F, Marx JS, Chiche JD, Cynober L, Mira JP and Cariou A:
Markers of intestinal injury are associated with endotoxemia in
successfully resuscitated patients. Resuscitation. 84:60–65. 2013.
View Article : Google Scholar
|
|
45
|
Sonnier DI, Bailey SR, Schuster RM,
Gangidine MM, Lentsch AB and Pritts TA: Proinflammatory chemokines
in the intestinal lumen contribute to intestinal dysfunction during
endotoxemia. Shock. 37:63–69. 2012. View Article : Google Scholar
|
|
46
|
Nahid MA, Satoh M and Chan EK: Mechanistic
role of microRNA-146a in endotoxin-induced differential
cross-regulation of TLR signaling. J Immunol. 186:1723–1734. 2011.
View Article : Google Scholar
|
|
47
|
Liu Y, Chen Q, Song Y, Lai L, Wang J, Yu
H, Cao X and Wang Q: MicroRNA-98 negatively regulates IL-10
production and endotoxin tolerance in macrophages after LPS
stimulation. FEBS Lett. 585:1963–1968. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Bafica A, Feng CG, Santiago HC, Aliberti
J, Cheever A, Thomas KE, Taylor GA, Vogel SN and Sher A: The
IFN-inducible GTPase LRG47 (Irgm1) negatively regulates
TLR4-triggered proinflammatory cytokine production and prevents
endotoxemia. J Immunol. 179:5514–5522. 2007. View Article : Google Scholar : PubMed/NCBI
|