Introduction
Liver cancer is the fourth most common malignant
tumor in the world and the third leading cause of cancer-associated
death (1). China has a high
incidence of chronic hepatitis B, and persistent infection and
replication of hepatitis B virus causes liver fibrosis and
cirrhosis or possibly liver cancer as the diseases progresses
(2). A recent analysis reported
that 45.69% of liver cancer deaths were due to HBV infection, so
the incidence and mortality of hepatocellular carcinoma (HCC) is
high as a result (3). Studies
have found that due to an increase in the incidence of
non-alcoholic fatty liver disease and chronic hepatitis C, the
incidence of HCC in western countries is also increasing annually
(4,5). According to the statistics, ~600,000
individuals are diagnosed with HCC every year worldwide, and
~500,000 individuals die of HCC-associated diseases (1,6).
Therefore, it is of great significance to explore the pathogenesis
of liver cancer and develop new therapeutic targets for the
treatment of liver cancer.
Targeting protein for Xenopus kinesin-like protein 2
(TPX2) is a nuclear proliferation microtubule-associated protein
that can regulate spindle formation and stabilize spindle
microtubules by promoting chromatin microtubule nucleation
(7). TPX2 is the activating
protein of Aurora A and can localize with Aurora A in mitotic
spindle microtubules (8).
However, upregulation of TPX2 can cause centrosome amplification
and lead to DNA polyploidy (9).
Previous studies have found that TPX2 is upregulated in a wide
range of malignant tumors, including esophageal cancer (10), colon cancer (11,12), breast cancer (13), cervical cancer (14,15), ovarian cancer (16), bladder carcinoma (17) and medullary thyroid carcinoma
(18). To the best of our
knowledge, there are no studies on the association between TPX2 and
the occurrence and development of HCC. The role of TPX2 in liver
cancer progression and its potential molecular mechanism are
unclear.
In the present study, the mechanism by which TPX2
participated in the development of HCC was investigated. RNA
interference was used to silence TPX2 expression in HCC cells and
changes in the molecular biological behavior of HCC cells were
observed. Additionally, the molecular mechanism underlying
TPX2-mediated regulation of growth of HCC cells was elucidated.
Materials and methods
Reagents
DMEM, FBS and trypsin were purchased from HyClone
(GE Healthcare Life Sciences). The empty vector, pMagic4.1 with a
construct for green fluorescent protein was purchased from Clontech
Laboratories, Inc., and the recombinant the plasmids
pMagic4.1-shRNA-TPX2 and pMagic4.1-shRNA-NC were constructed in our
laboratory and verified by PCR, endonuclease cleavage and
sequencing in our previous study (19). Lipofectamine™ 2000 and
TRIzol® were purchased from Invitrogen (Thermo Fisher
Scientific, Inc). Cell Counting Kit-8 (CCK-8) solution was
purchased from Dojindo Molecular Technologies, Inc. The Annexin
V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) kit was
purchased from Beijing Solarbio Science & Technology Co., Ltd.
Transwell chambers were purchased from BD Biosciences. DMSO was
obtained from Sigma-Aldrich (Merck KGaA). The reverse transcription
kit was purchased from Fermentas (Thermo Fisher Scientific, Inc.).
PCR primers were synthesized by Generay Biotech Co. Ltd. Total
protein extraction kit was purchased from Sangon Biotech Co. Ltd.
Primary antibodies against TPX2 (D2R5C) (cat. no. 12245), PI3K
(cat. no. 4249), phospho (p)-AKT (Ser 473) (cat. no. 4060), AKT
(cat. no. 4685), P21 (cat. no. 2947), Bcl-2 (cat. no. 15071), p38
MAPK (cat. no. 8690), p-p38 MAPK (Thr180/Tyr182) (cat. no. 4511),
STAT3 (cat. no. 12640), p-STAT3 (Tyr705) (cat. no. 9145) and
β-actin (cat. no. 4970) were purchased from Cell Signaling
Technology. Anti-TPX2 (cat. no. ab32795) primary antibody for
immunohistochemical staining was purchased from Abcam. Primary
antibodies against Smad2/3 (cat. no. sc-398844) and p-Smad2/3 (Ser
423/425) (cat. no. sc-11769) were purchased from Santa Cruz
Biotechnology, Inc. Horseradish peroxidase-conjugated secondary
antibodies were purchased from OriGene Technologies, Inc.
Clinical specimen
A total of 14 HCC tissue samples and adjacent normal
liver tissue samples (distance from tumor >5 cm) from patients
who underwent surgical resection were collected at Jiading District
Central Hospital of Shanghai University of Medicine & Health
Sciences (Shanghai, China) between May 2016 and March 2018. The 14
HCC cases included 10 males and 4 females aged 42-79 (55.86±2.81)
years. None of the patients had received chemotherapy or
radiotherapy prior to radical resection of liver cancer. The
present study was approved by the Human Ethics Committee at Jiading
District Central Hospital of Shanghai University of Medicine &
Health Sciences and prior written consent was obtained from all
patients.
Cell culture
The human liver cancer cell lines Huh7, Hep3B,
PLC/PRF/5 and MHCC97-H were obtained from The Cell Bank of Type
Culture Collection of the Chinese Academy of Sciences. All cells
were maintained in DMEM containing 10% FBS, 100 U/ml penicillin and
100 µg/ml streptomycin. The cells were routinely plated at a
density of 1x105 cells/ml in 6-well plates and incubated
in a humidified incubator at 37°C with 95% air and 5%
CO2.
Reverse transcription-quantitative
(RT-q)PCR
RT-qPCR was performed on all tissue samples and
cells. Total RNA was extracted from frozen tissue samples and cells
using TRIzol®, and 1 µg total RNA was used as a
template for the synthesis of the first-strand cDNA synthesis using
a reverse transcription kit (Fermentas; Thermo Fisher Scientific,
Inc.). The reverse transcription temperature protocol used was 37°C
for 15 min and 85°C for 30 sec. TPX2, PI3K, AKT, P21, Bcl-2, c-Myc,
P27, BCL2L1 and Cyclin D1 primers were designed using Primer
Premier version 5.0 (Premier Biosoft International) and synthesized
by Generay Biotech Co. Ltd. The sequences of the primers are
presented in Table I. A Takara
quantitative kit with TB Green Premix Ex Taq (Takara Bio, Inc.) was
used for quantitative PCR. The thermocycling conditions were:
Initial denaturation at 95°C for 5 min; followed by 40 cycles of
95°C for 30 sec and 60°C for 30 sec. Relative expression of TPX2,
PI3K, AKT, P21, Bcl-2, c-Myc, P27, BCL2L1 and Cyclin D1 mRNA was
calculated using the 2–ΔΔCq (20) method and β-actin was used as the
internal reference.
 | Table IPrimer sequences used for PCR. |
Table I
Primer sequences used for PCR.
| Gene | Forward sequence,
5'-3' | Reverse sequence,
5'-3' |
|---|
| TPX2 |
ACCTTGCCCTACTAAGATT |
AATGTGGCACAGGTTGAGC |
| PI3K |
TGGCCTTAGCTCTTAGCCAAACAC |
ATTGGAACACGGCCTTTGACA |
| AKT C |
TGTGCCTATGCTGCCCAT |
CAGTGCGATGTCGTGGAGG |
| P21 |
GACCTGTCACTGTCTTGTAC |
CTCTCATTCAACCGCCTAG |
| Bcl-2 |
GGATAACGGAGGCTGGGATGC |
GACTTCACTTGTGGCCCAGAT |
| C-Myc |
TGTGTTACGGTCGCGTCTTT |
AACAGCTCGGTCACCATCTC |
| Cyclin D1 |
CCAGACCCACGTTTCTTTGC |
ATCCCTAGAAACACCACGGC |
| P27 |
TGGAAAGCGGTCTGCAAGTG |
TCACTGTCACATTCAGGGGC |
| BCL2L1 |
TCCCCATGGCAGCAGTAAAG |
TCCACAAAAGTATCCTGTTCAAAGC |
| β-actin |
AAGGTGACAGCAGTCGGTT |
TGTGTGGACTTGGGAGAGG |
Immunohistochemistry
Immunohistochemical staining was performed on
paraffin-embedded HCC tissue samples to examine the level of TPX2
protein as described previously (13). Briefly, the HCC tissue samples
were fixed in 4% paraformaldehyde for 48 h at room temperature,
dehydrated in a graded series of ethanol (50, 75, 85, 95 and 100%),
embedded in paraffin and sectioned into 4 µm thick slices.
Xylene and a graded series of ethanol (100, 95, 85 and 75%) were
used to dewax and hydrate the samples, respectively, followed by 30
min of antigen retrieval in Tris-EDTA (pH 9.0) in a 720 W
microwave. Subsequently, the sections were blocked in 3%
H2O2 for 10 min and incubated with anti-TPX2
(1:400) primary antibody at room temperature for 2 h, washed with
TBS-Tween, and incubated with the horseradish peroxidase-linked
anti-goat immunoglobulin G secondary antibody (1:1,500;
Sigma-Aldrich; Merck KGaA) for 1 h at room temperature. Following
incubation with the antibodies, a diaminobenzidine substrate kit
(Vector Laboratories, Inc.) was used to visualize bound antibodies.
Hematoxylin was used to stain the cell nuclei at room temperature
for 4 min. The tissues were observed and imaged under an inverted
light microscope at a x200 and x400 magnification (Olympus
Corporation) and evaluated by a pathologist blinded to the
patient's information. The staining score was assessed as described
previously (13). Expression
grading was stratified according to the final score as follows:
0-3, low TPX2 expression and 4-7, high TPX2 expression (13).
Western blotting
Western blot analysis of all tissue samples and
cells were performed according to the manufacturers' protocols.
Briefly, total proteins were extracted from tissue samples or cells
using RIPA lysis buffer (Cell Signaling Technology, Inc.) and
protein concentration was determined using a bicinchoninic acid
protein assay kit (Pierce; Thermo Fisher Scientific, Inc.). A total
of 40 mg/lane of samples were loaded on a 10% SDS gel, resolved
using SDS-PAGE and transferred to nitrocellulose membranes.
Subsequently, membranes were blocked in 5% non-fat milk for 1 h at
room temperature and incubated with anti-TPX2 (1:1,000), anti-PI3K
(1:1,000), anti-p-AKT (1:2,000), anti-AKT (1:1,000), anti-P21
(1:2,000), anti-Bcl-2 (1:1,000), anti-Smad2/3 (1:1,000),
anti-p-Smad2/3 (1:1,000), anti-p38 MAPK (1:1,000), anti-p-p38 MAPK
(1:1,000), anti-STAT3 (1:1,000), anti-p-STAT3 (1:2,000) or
anti-β-actin (1:3,000) primary antibody overnight at 4°C, followed
by incubation with horseradish peroxidase-conjugated anti-rabbit
and anti-mouse immunoglobulin G secondary antibodies at a dilution
of 1:5,000 (cat. no. ab6721 and ab205719, respectively; Abcam) at
room temperature for 2 h. The signal was visualized using enhanced
chemiluminescence reagent (Beyotime Institute of Biotechnology)
according to manufacturer's protocol. The densities of the protein
bands were quantified using ImageJ 1.8.0 software (National
Institutes of Health). Expression of β-actin antibody was used as
the internal control.
TPX2 short hairpin (sh)RNA and cell
transfection
Huh7 or Hep3B cells were divided into three groups:
i) untrans-fected control group (Ctrl); ii)
pMagic4.1-shRNA-negative control plasmid transfected group
(shRNA-NC); and iii) pMagic4.1-shRNA-TPX2 plasmid transfected group
(shRNA-TPX2). The TPX2 shRNA sequence and the detailed procedures
of the transfection are described in our previous publication
(19). Green fluorescence was
observed under a fluorescence microscope (magnification, x100)
after plasmid transfection for 48 h. The TPX2 mRNA and protein
levels in Huh7 and Hep3B cells were detected using RT-qPCR and
western blot analysis.
Cell proliferation assay
Huh7 or Hep3B cells were plated at a density of
5x103 cells/well into 96-well plates following
transfection. After 0, 24, 48 or 72 h of culture, 10 ml CCK-8
solution was added to each well and further incubated at 37°C for 1
h. Absorbance was measured at 450 nm using a micro-plate
reader.
Cell apoptosis assay
Huh7 or Hep3B cells in each group were trypsinized
and collected by centrifugation at 37°C for 5 min at a speed of
1,000 x g. Cells were washed twice with PBS, and 1x105
cells were re-suspended in 500 ml binding buffer and incubated with
Annexin V-FITC/PI dual stain for 15 min at room temperature. A
FACSCalibur flow cytometer (BD Biosciences) was used to detect the
apoptotic rate of cells. BD CellQuest™ Pro software version 5.1 (BD
Biosciences) was used to analyze the data.
Transwell migration and invasion
assays
Cell migration and invasion assays were performed
using Transwell chambers with (invasion) or without (migration)
Matrigel according to the manufacturer's protocol. A total of
2x105 Huh7 or Hep3B cells were plated into the upper
chamber of the insert in serum-free DMEM. DMEM containing 10% FBS
was added to the lower chamber. The Huh7 or Hep3B cells remaining
on the insert's top layer were removed with a cotton swab after 48
h of incubation. The cells which had migrated or invaded to the
lower surface of the membrane were stained with crystal violet for
30 min at room temperature. The cells on the lower surface of the
membrane were imaged under an inverted light microscope at x50
magnification (Olympus Corporation), and cells in 5 fields of view
were counted to estimate cell migration/invasion. Each experiment
was performed three times independently.
Statistical analysis
Data are presented as the mean ± standard deviation
of at least three repeats. Differences in the expression levels of
TPX2 in HCC tissue samples were evaluated using a Pearson
χ2 test or Fisher's exact test. Other experimental data
were evaluated using a one-way ANOVA with a post-hoc least
significant difference test, using SPSS version 19.0 (IBM, Corp.).
P<0.05 was considered to indicate a statistically significant
difference.
Results
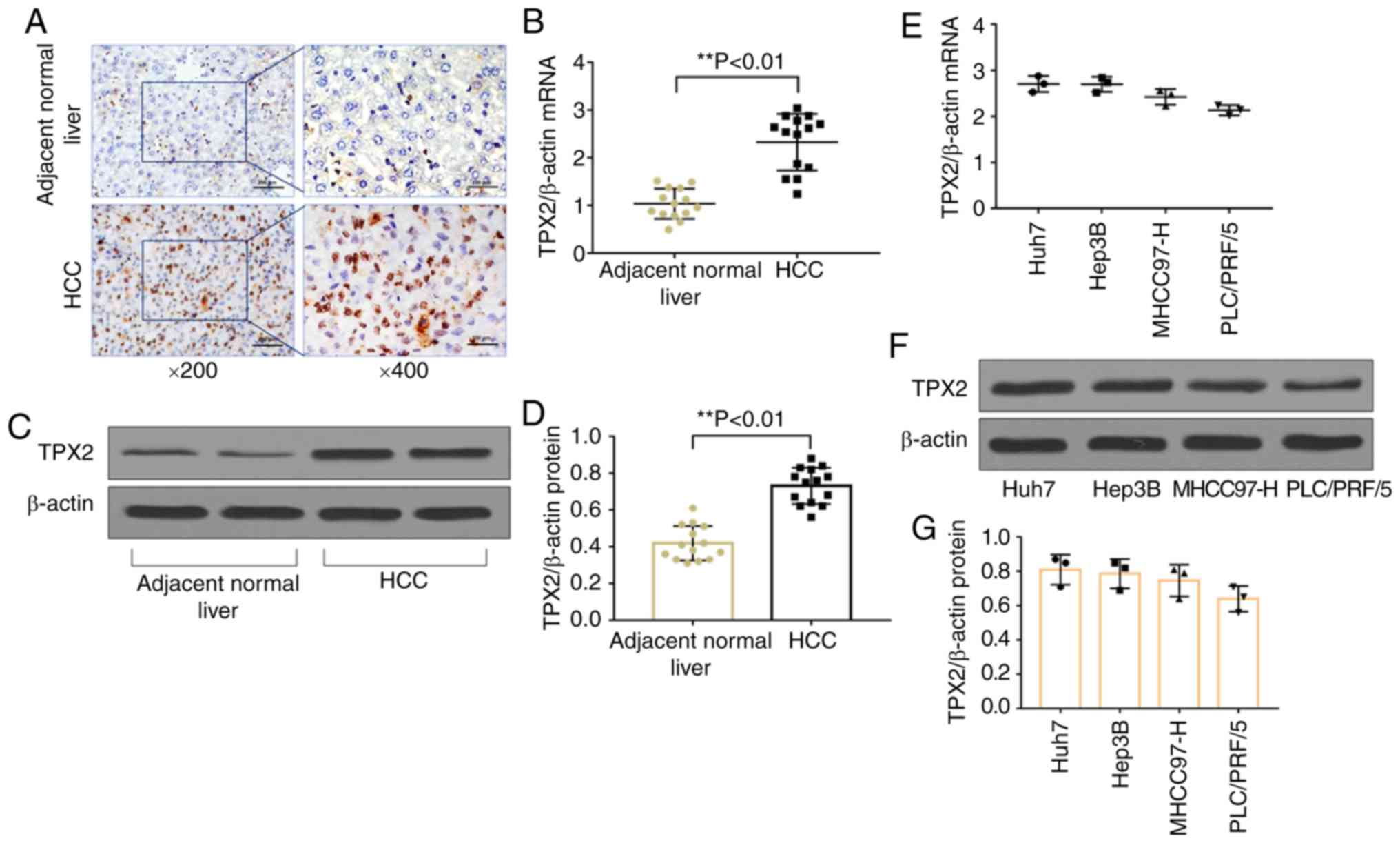
TPX2 expression is upregulated in HCC
tissues and human hepatoma cell lines
Immunohistochemistry staining, RT-qPCR and western
blot analysis demonstrated that the protein (P<0.01) and mRNA
(P<0.01) expression levels of TPX2 were significantly increased
in HCC tissues compared with adjacent normal liver tissues
(Fig. 1A-D). The correlation
between TPX2 expression and various clinicopathological
characteristics are shown in Table
II. TPX2 expression was correlated with tumor differentiation
(P=0.017) and clinical Tumor-Node-Metastasis stage (21) (P=0.016), suggesting that TPX2 may
be associated with carcinogenesis and progression of HCC.
Therefore, TPX2 mRNA and protein expression levels were assessed in
various human hepatoma cell lines, including Huh7, Hep3B, PLC/PRF/5
and MHCC97-H. TPX2 mRNA and protein expression levels were detected
in all human liver cancer cell lines (Fig. 1E-G). Huh7 and Hep3B cell lines had
the highest levels of expression of TPX2 and were thus used for
TPX2 knockdown and subsequent functional experiments.
 | Table IIAssociation between TPX2 expression
and clinicopathological features in patients with HCC. |
Table II
Association between TPX2 expression
and clinicopathological features in patients with HCC.
|
Characteristics | No. of
patients | Low TPX2
expression | High TPX2
expression | P-value |
|---|
| Sex | | | | 0.852 |
| Male | 10 | 3 | 7 | |
| Female | 4 | 1 | 3 | |
| Age, years | | | | 0.597 |
| ≤50 | 5 | 1 | 4 | |
| >50 | 9 | 3 | 6 | |
| Tumor size, cm | | | | 0.481 |
| ≤2 | 5 | 2 | 3 | |
| >2 | 9 | 2 | 7 | |
|
Differentiation | | | | 0.017a |
| Well | 1 | 1 | 0 | |
| Moderate | 5 | 3 | 2 | |
| Poor | 8 | 0 | 8 | |
| TNM stage | | | | 0.016a |
| I | 1 | 1 | 0 | |
| II | 4 | 3 | 1 | |
| III | 8 | 0 | 8 | |
| IV | 1 | 0 | 1 | |
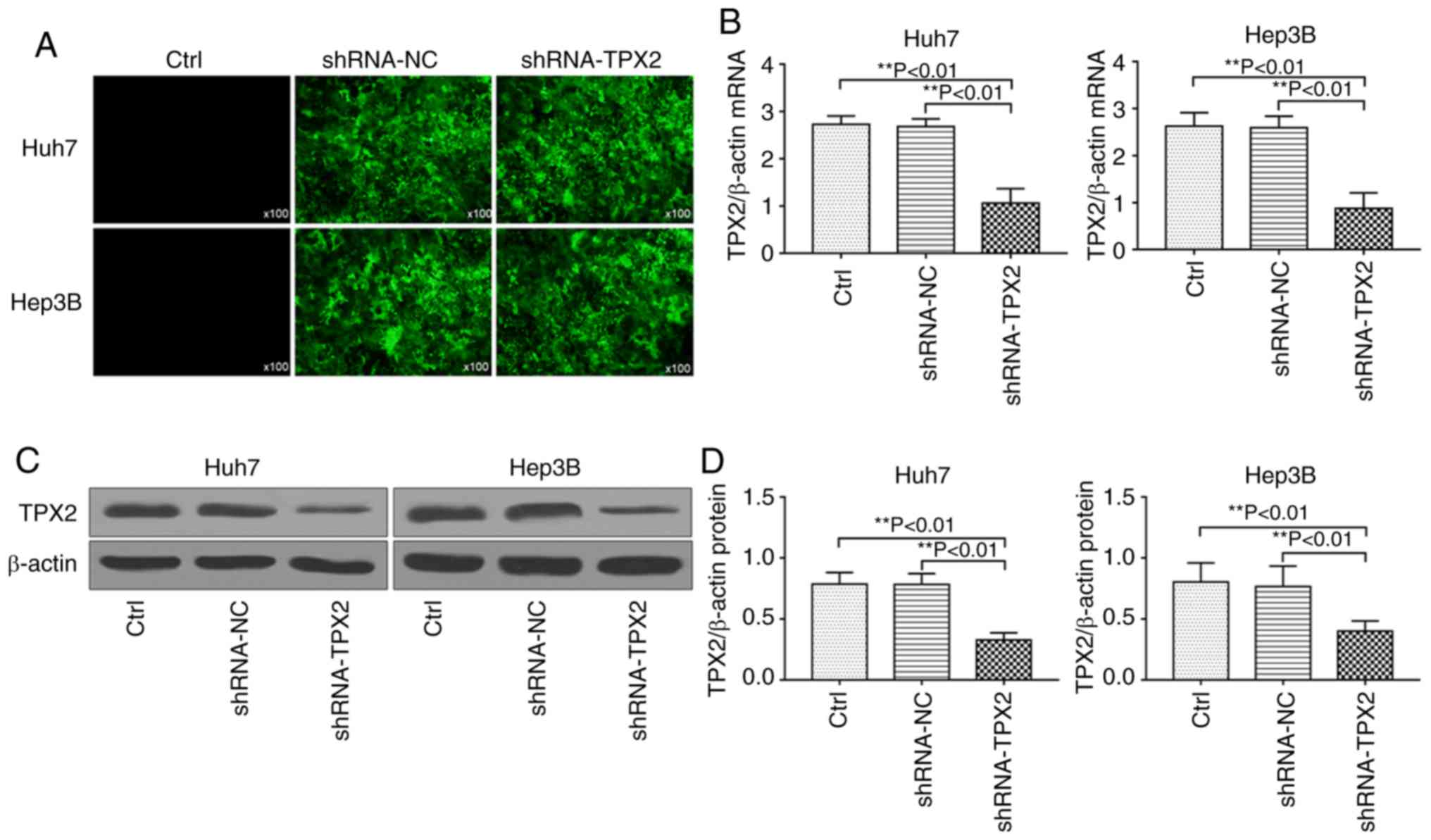
TPX2 shRNA plasmid transfection and
downregulation of TPX2
The pMagic4.1-shRNA-TPX2 plasmid or
pMagic4.1-shRNA-NC plasmid were transiently transfected into Huh7
and Hep3B cells. After 48 h, cells were observed under a
fluorescence microscope. As shown in Fig. 2A, the cells which were
successfully transfected with pMagic4.1-shRNA-TPX2 or
pMagic4.1-shRNA-NC plasmid showed green fluorescence. RT-qPCR and
western blot analysis demonstrated that the mRNA and protein
expression levels of TPX2 in Huh7 or Hep3B cells were significantly
downregulated (P<0.01; Fig.
2B-D).
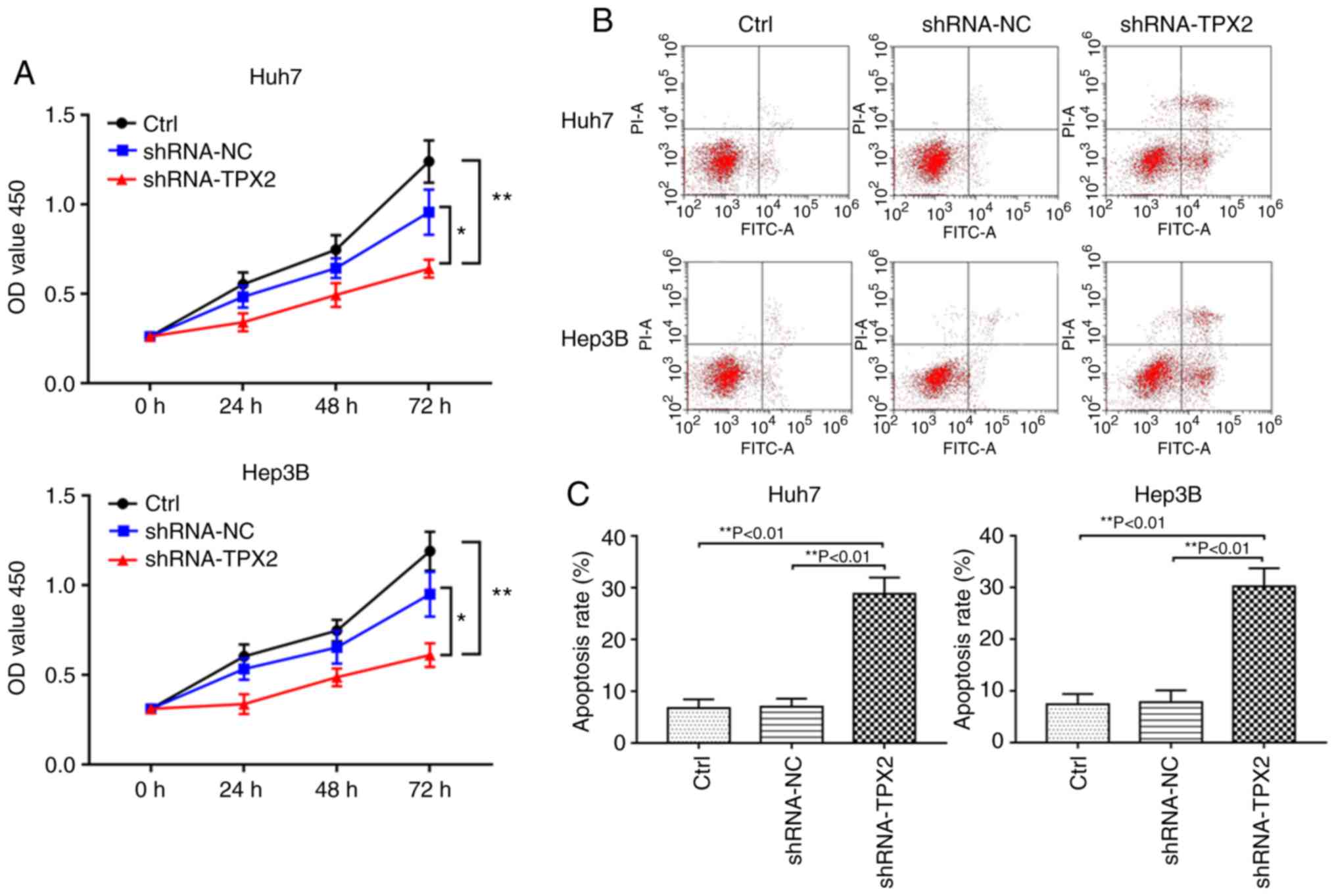
TPX2 silencing inhibits the proliferation
of Huh7 or Hep3B cells
A cell viability assay was used to investigate
whether TPX2 silencing affected proliferation of Huh7 and Hep3B
cells. As shown in Fig. 3A, the
optical density (OD) values of the shRNA-TPX2 group was
significantly lower compared with the shRNA-NC group and Ctrl group
(P<0.05 and P<0.01, respectively). There was no significant
difference in the OD value between the Ctrl group and shRNA-NC
group (P>0.05).
Downregulation of TPX2 increases
apoptosis of Huh7 or Hep3B cells
Flow cytometry showed that Huh7 or Hep3B cell
apoptosis were significantly increased when TPX2 was knocked down.
The apoptotic rate of the shRNA-TPX2 group was significantly higher
compared with the Ctrl group and the shRNA-NC group (P<0.01).
There was no significant difference in the apoptotic rate between
the Ctrl group and the shRNA-NC group (P>0.05; Fig. 3B and C).
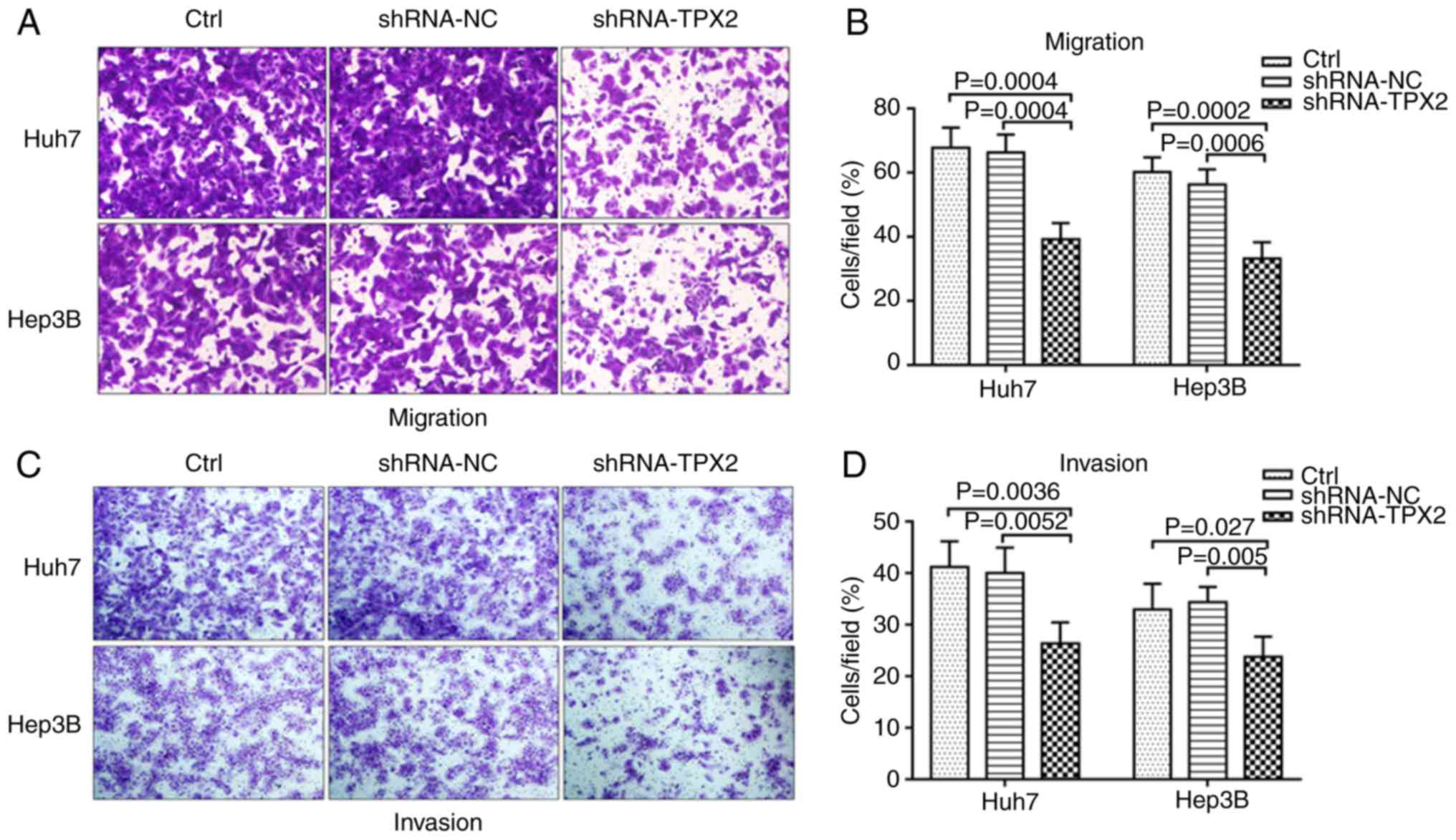
TPX2 knockdown suppresses the migration
and invasion of Huh7 and Hep3B cells
The effect of silencing TPX2 on the migration and
invasion of Huh7 and Hep3B cells was assessed using Transwell
migration and invasion assays. As shown in Fig. 4A and B, the migratory capacity of
Huh7 and Hep3B cells in the shRNA-TPX2 group was significantly
reduced compared with the Ctrl group and shRNA-NC group (both
P<0.01). The knockdown of TPX2 in Huh7 and Hep3B cells resulted
in decreased invasion compared with the shRNA-NC group and Ctrl
group (P<0.05 or P<0.01; Fig.
4C and D). There was no significant difference in the migratory
and invasive capacities between the Ctrl group and the shRNA-NC
group (P>0.05).
TPX2 silencing suppresses the PI3K/AKT
signaling pathway
The development and progression of HCC are
associated with multiple signaling pathways, such as the PI3K/AKT,
MAPK/P38, JAK2/STAT3, TGF-β/Smad and NF-κB signaling pathways
(22-26). Previous studies have found that
TPX2 can regulate the proliferation and apoptosis of a number of
different types of malignant tumors via the PI3K/AKT signaling
pathway (13,16). In the present study, the potential
effects of TPX2 silencing on the expression levels of PI3K/AKT
signaling pathway-associated factors in Huh7 or Hep3B cells were
assessed. As shown in Fig. 5A and
B, PI3K, Bcl-2, c-Myc and Cyclin D1 mRNA expression levels in
Huh7 or Hep3B cells were significantly decreased in the shRNA-TPX2
group compared with the Ctrl group and shRNA-NC group (P<0.05,
P<0.01), whereas P21 and P27 mRNA expression levels in the
shRNA-TPX2 group was significantly upregulated (P<0.05,
P<0.01). There were no significant differences in the BCL2L1
mRNA expression levels among the shRNA-TPX2, Ctrl and shRNA-NC
groups (P>0.05). Compared with the Ctrl group and shRNA-NC
group, PI3K, p-AKT and Bcl-2 protein expression levels were
significantly downregulated in the shRNA-TPX2 group (P<0.05 or
P<0.01), whereas P21 protein expression levels in the shRNA-TPX2
group were significantly increased (P<0.01; Fig. 5C-F). There was no significant
difference in the AKT mRNA and protein expression levels among the
shRNA-TPX2, Ctrl or shRNA-NC groups (P>0.05). Additionally,
there was no significant difference in the expression levels of
PI3K/AKT signaling pathway-associated factors between the Ctrl
group and the shRNA-NC group (P>0.05). To determine the
association between TPX2 and other signaling pathways in HCC,
Smad2/3, p-Smad2/3, p38 MAPK, p-p38 MAPK, STAT3 and p-STAT3 protein
expression levels were examined in the Huh7 cells following
silencing of TPX2. There were no significant differences in the
expression levels of TGFβ/Smad, MAPK/p38 and JAK2/STAT3 signaling
pathway-associated factors among the Ctrl group, shRNA-NC group and
shRNA-TPX2 group (P>0.05; Fig. 5G
and H). These results confirmed that TPX2 activated the
PI3K/AKT signaling pathway in liver cancer.
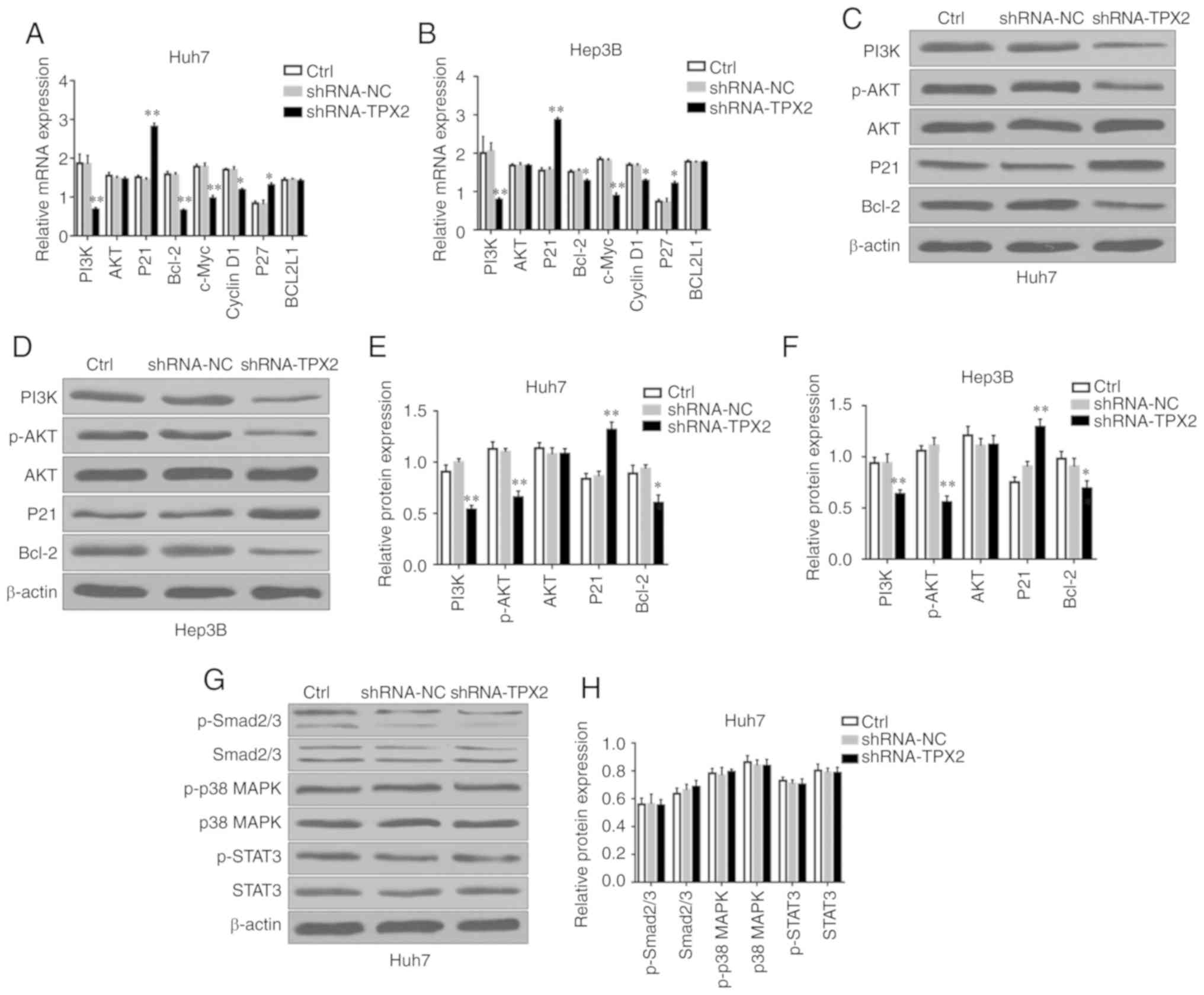 | Figure 5Effect of TPX2 knockdown on multiple
signaling pathways in Huh7 and Hep3B cells. mRNA expression levels
of PI3K, AKT, P21, Bcl-2, c-Myc, Cyclin D1, P27 and BCL2L1 in (A)
Huh7 and (B) Hep3B cells. *P<0.05,
**P<0.01. Western blots of PI3K, p-AKT, AKT, P21 and
Bcl-2 expression in (C) Huh7 and (D) Hep3B cells. Quantitative
analysis of protein expression levels of PI3K, p-AKT, AKT, P21 and
Bcl-2 in (E) Huh7 and (F) Hep3B cells. *P<0.05,
**P<0.01. (G) Western blots of p-Smad2/3, Smad2/3,
p-p38 MAPK, p38 MAPK, p-STAT3 and STAT3 expression in Huh7 cells.
β-actin was used as the loading control. (H) Quantitative analysis
of p-Smad2/3, Smad2/3, p-p38 MAPK, p38 MAPK, p-STAT3 and STAT3
expression in Huh7 cells. Data are presented as the mean ± standard
deviation. TPX2, targeting protein for Xenopus kinesin-like protein
2; sh, short hairpin; Ctrl, untransfected control; NC, negative
control plasmid. |
Discussion
According to the latest statistics from Global
Cancer 2018, liver cancer is one of the most common malignant
tumors and ranks 4th in incidence and 3rd in mortality rates of all
types of cancer (1). Over the
past few decades, with the development of radical hepatectomy and
liver transplantation, ~40% of patients with liver cancer have been
cured (27,28), but ~60% of patients with liver
cancer are still unable to receive effective treatment due to
advanced stages of liver cancer at initial diagnosis, poor economic
status or a lack of livers for transplants (29). In the majority of the patients who
undergo radical hepatectomy or liver transplantation, the tumor may
exhibit recurrence or may have metastasized (30,31). Although several biomarkers have
been thought to be associated with the occurrence and development
of liver cancer (32,33), the majority of these markers have
not proven beneficial for diagnosing or treating patients with
liver cancer. Recently, several novel molecular inhibitors were
approved for the treatment of advanced liver cancer, but the
overall survival rate of patients has not improved significantly,
and the efficacy of these drugs are not promising (34,35). Therefore, understanding the
molecular mechanisms underlying the occurrence of liver cancer may
assist in the development of new therapeutic strategies and targets
for treating liver cancer.
TPX2, a microtubule-associated protein located on
human chromosome 20q 11.2, serves an important role in regulating
mitotic spindles and chromosome segregation (36,37). TPX2 is a downstream effector of
the small GTPase Ran, which is involved in spindle formation
(38). Overexpression of TPX2 can
induce centrosome amplification, lead to DNA polyploidy and induce
tumor formation (36). A number
of studies have confirmed that TPX2 is closely associated with the
occurrence of tumors. Overexpression of TPX2 promotes the
occurrence and development of esophageal cancer, colon cancer,
breast cancer, cervical cancer, ovarian cancer, bladder carcinoma
and medullary thyroid carcinoma (10-18). In the present study it was also
demonstrated that the expression of TPX2 in human HCC tissues was
significantly upregulated compared with the adjacent normal
tissues. The expression of TPX2 in the normal liver cell line, LO2,
and compared with the different HCC cell lines Huh7, Hep3B,
PLC/PRF/5 and MHCC97-H. The expression levels of TPX2 in LO2 cells
were significantly lower compared with the different HCC cell
lines; however, the data from LO2 cells were removed as their
identity could not be verified using STR profiling. As such, the
fact that the data in the HCC cells were not compared to a normal
liver cell line is a limitation of the present study. Additionally,
silencing TPX2 gene expression using RNA interference,
significantly reduced proliferation, migration and invasion of Huh7
and Hep3B cells, whilst increasing apoptosis. These results suggest
that TPX2 may improve the viability of HCC cells and inhibit cell
apoptosis.
Previous studies have demonstrated that the
PI3K/AKT/P21 signaling pathway serves an important role in the
occurrence and development of malignant tumors (39-42). The activation of AKT is closely
associated with cell proliferation, survival, migration and
invasion of tumors (43).
Inhibiting the activation of AKT and promoting the expression of
P21 inhibits the proliferation of tumor cells, promotes cell
apoptosis and decreases tumor progression (44,45). In the present study, expression of
TPX2 was demonstrated to be associated with phosphorylation of AKT.
Following silencing of TPX2, the expression levels of p-AKT were
significantly decreased, suggesting that TPX2 may promote the
activation of AKT and PI3K/AKT signal transduction. P21 is an
inhibitor of cyclin-dependent kinase and an important cell cycle
regulator in the PI3K/AKT signaling pathway. Previous studies have
shown that P21 and P27 can inhibit cell cycle progression and
promote apoptosis, behaviors crucial for tumorigenesis (46,47). The present study also found that
the expression levels of P21 and P27 were significantly increased
when TPX2 expression was knocked down. Bcl-2, c-Myc, BCL2L1 and
Cyclin D1 are tumor-associated regulatory factors that are
significantly upregulated during tumorigenesis (48,49). TPX2 knockdown significantly
decreased the expression levels of Bcl-2, c-Myc and Cyclin D1.
Together, the present study demonstrated that downregulation of
TPX2 inhibited PI3K/AKT signal transduction, suppressed cell
proliferation and promoted cell apoptosis, and thus may prevent the
occurrence and development of HCC.
In summary, expression of TPX2 in human HCC was
significantly upregulated. Targeted silencing of TPX2 reduced cell
viability, abrogated cell cycle progress and promoted apoptosis of
HCC cells by inhibiting the PI3K/AKT signal transduction pathway.
Based on the results of the present study, TPX2 and its downstream
effectors may be potential targets for the diagnosis and treatment
of liver cancer and provide novel avenues for successful treatment
of malignant tumors.
Acknowledgements
The authors would like to thank Dr Xia Gan, Dr
Li-Hong Gan, Dr Fei Chen, Dr Li Zheng, Dr Ya-Qing Huang, and Dr
Ling Yao (Department of Gastroenterology, Third Affiliated Hospital
of Nanchang University) for their help.
Funding
The present study was supported by a grant from the
Scientific Research Project of Health system in Jiading District of
Shanghai (Shanghai, China; grant no. 2015-KY-04).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
DH, JJ and LL designed the experiments. DH, YZ and
SL performed the experiments. JJ contributed to the analysis of the
data and wrote the manuscript. LL corrected the manuscript. All
authors approved the final of the version manuscript.
Ethics approval and consent to
participate
The present study was approved by the Human Ethics
Committee at Jiading District Central Hospital of Shanghai
University of Medicine & Health Sciences (Shanghai, China) and
prior written consent was obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dandri M and Petersen J: Mechanism of
hepatitis B virus persistence in hepatocytes and its carcinogenic
potential. Clin Infect Dis. 62(Suppl 4): S281–S288. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang M, Wang Y, Feng X, Wang R, Wang Y,
Zeng H, Qi J, Zhao H, Li N, Cai J and Qu C: Contribution of
hepatitis B virus and hepatitis C virus to liver cancer in China
north areas: Experience of the Chinese National Cancer Center. Int
J Infect Dis. 65:15–21. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
de Martel C, Maucort-Boulch D, Plummer M
and Franceschi S: World-wide relative contribution of hepatitis B
and C viruses in hepatocellular carcinoma. Hepatology.
62:1190–1200. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Younossi ZM, Otgonsuren M, Henry L,
Venkatesan C, Mishra A, Erario M and Hunt S: Association of
nonalcoholic fatty liver disease (NAFLD) with hepatocellular
carcinoma (HCC) in the United States from 2004 to 2009. Hepatology.
62:1723–1730. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu Z, Jiang Y, Yuan H, Fang Q, Cai N, Suo
C, Jin L, Zhang T and Chen X: The trends in incidence of primary
liver cancer caused by specific etiologies: Results from the Global
Burden of Disease Study 2016 and implications for liver cancer
prevention. J Hepatol. 70:674–683. 2019. View Article : Google Scholar
|
|
7
|
Gruss OJ and Vernos I: The mechanism of
spindle assembly: Functions of Ran and its target TPX2. J Cell
Biol. 166:949–955. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rennie YK, McIntyre PJ, Akindele T,
Bayliss R and Jamieson AG: A TPX2 proteomimetic has enhanced
affinity for Aurora-A due to hydrocarbon stapling of a Helix. ACS
Chem Biol. 11:3383–3390. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pascreau G, Eckerdt F, Lewellyn AL,
Prigent C and Maller JL: Phosphorylation of p53 is regulated by
TPX2-Aurora A in xenopus oocytes. J Biol Chem. 284:5497–5505. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu HC, Zhang Y, Wang XL, Qin WS, Liu YH,
Zhang L and Zhu CL: Upregulation of the TPX2 gene is associated
with enhanced tumor malignance of esophageal squamous cell
carcinoma. Biomed Pharmacother. 67:751–755. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Takahashi Y, Sheridan P, Niida A, Sawada
G, Uchi R, Mizuno H, Kurashige J, Sugimachi K, Sasaki S, Shimada Y,
et al: The AURKA/TPX2 axis drives colon tumorigenesis cooperatively
with MYC. Ann Oncol. 26:935–942. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wei P, Zhang N, Xu Y, Li X, Shi D, Wang Y,
Li D and Cai S: TPX2 is a novel prognostic marker for the growth
and metastasis of colon cancer. J Transl Med. 11:3132013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen M, Zhang H, Zhang G, Zhong A, Ma Q,
Kai J, Tong Y, Xie S, Wang Y, Zheng H, et al: Targeting TPX2
suppresses proliferation and promotes apoptosis via repression of
the PI3k/AKT/P21 signaling pathway and activation of p53 pathway in
breast cancer. Biochem Biophys Res Commun. 507:74–82. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jiang P, Shen K, Wang X, Song H, Yue Y and
Liu T: TPX2 regulates tumor growth in human cervical carcinoma
cells. Mol Med Rep. 9:2347–2351. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chang H, Wang J, Tian Y, Xu J, Gou X and
Cheng J: The TPX2 gene is a promising diagnostic and therapeutic
target for cervical cancer. Oncol Rep. 27:1353–1359.
2012.PubMed/NCBI
|
|
16
|
Tian Y, Liu LL, Guo DM, Wang Y, Zha WH, Li
Y and Wu FJ: TPX2 gene silencing inhibits cell proliferation and
promotes apoptosis through negative regulation of AKT signaling
pathway in ovarian cancer. J Cell Biochem. 119:7540–7555. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yan L, Li Q, Yang J and Qiao B:
TPX2-p53-GLIPR1 regulatory circuitry in cell proliferation,
invasion, and tumor growth of bladder cancer. J Cell Biochem.
119:1791–1803. 2018. View Article : Google Scholar
|
|
18
|
Yang X, Liu G, Xiao H, Yu F, Xiang X, Lu
Y, Li W, Liu X, Li S and Shi Y: TPX2 overexpression in medullary
thyroid carcinoma mediates TT cell proliferation. Pathol Oncol Res.
20:641–648. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jian J, Huang Y, Liu LZ, Li SX and Deng F:
TPX2 gene-silencing inhibits the proliferation and invasion of
human colon cancer SW480 cells. TUMOR. 36:628–634. 2016.
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
21
|
Llovet JM, Bruix J, Fuster J, Castells A,
Garcia-Valdecasas JC, Grande L, Franca A, Brú C, Navasa M, Ayuso
MC, et al: Liver transplantation for small hepatocellular
carcinoma: The tumor-node-metastasis classification does not have
prognostic power. Hepatology. 27:1572–1577. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xue S, Zhou Y, Zhang J, Xiang Z, Liu Y,
Miao T, Liu G, Liu B, Liu X, Shen L, et al: Anemoside B4 exerts
anti-cancer effect by inducing apoptosis and autophagy through
inhibiton of PI3K/Akt/mTOR pathway in hepatocellular carcinoma. Am
J Transl Res. 11:2580–2589. 2019.PubMed/NCBI
|
|
23
|
Feng PC, Ke XF, Kuang HL, Pan LL, Ye Q and
Wu JB: BMP2 secretion from hepatocellular carcinoma cell HepG2
enhances angiogenesis and tumor growth in endothelial cells via
activation of the MAPK/p38 signaling pathway. Stem Cell Res Ther.
10:2372019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li SJ, Sui MH, Sun ZX and Zhang WW: LncRNA
00152 promotes the development of hepatocellular carcinoma by
activating JAK2/STAT3 pathway. Eur Rev Med Pharmacol Sci.
23:1038–1046. 2019.PubMed/NCBI
|
|
25
|
Zuo J, Ma H, Cai H, Wu Y, Jiang W and Yu
L: An inhibitory role of NEK6 in TGFβ/Smad signaling pathway. BMB
Rep. 48:473–478. 2015. View Article : Google Scholar :
|
|
26
|
Yang Y, Yang X, Li L, Yang G, Ouyang X,
Xiang J, Zhang T and Min X: LASS2 inhibits proliferation and
induces apoptosis in HepG2 cells by affecting mitochondrial
dynamics, the cell cycle and the nuclear factor-κB pathways. Oncol
Rep. 41:3005–3014. 2019.PubMed/NCBI
|
|
27
|
Thelen A, Benckert C, Tautenhahn HM, Hau
HM, Bartels M, Linnemann J, Bertolini J, Moche M, Wittekind C and
Jonas S: Liver resection for hepatocellular carcinoma in patients
without cirrhosis. Br J Surg. 100:130–137. 2013. View Article : Google Scholar
|
|
28
|
Rhu J, Kim JM, Choi GS, Kwon CHD and Joh
JW: Continuing five or more locoregional therapies before living
donor salvage liver transplantation for hepatocellular carcinoma is
related to poor recurrence-free survival. Ann Surg Treat Res.
95:152–160. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Anderson TN and Zarrinpar A: Hepatocyte
transplantation: Past efforts, current technology, and future
expansion of therapeutic potential. J Surg Res. 226:48–55. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Famularo S, Di Sandro S, Giani A, Lauterio
A, Sandini M, De Carlis R, Buscemi V, Uggeri F, Romano F, Gianotti
L and De Carlis L: Recurrence patterns after anatomic or
paren-chyma-sparing liver resection for hepatocarcinoma in a
western population of cirrhotic patients. Ann Surg Oncol.
25:3974–3981. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Meischl T, Rasoul-Rockenschaub S, Györi G,
Sieghart W, Reiberger T, Trauner M, Soliman T, Berlakovich G and
Pinter M: C-reactive protein is an independent predictor for
hepatocellular carcinoma recurrence after liver transplantation.
PLoS One. 14:e02166772019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Scaggiante B, Kazemi M, Pozzato G, Dapas
B, Farra R, Grassi M, Zanconati F and Grassi G: Novel
hepatocellular carcinoma molecules with prognostic and therapeutic
potentials. World J Gastroenterol. 20:1268–1288. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gao B, Li S, Tan Z, Ma L and Liu J: ACTG1
and TLR3 are biomarkers for alcohol-associated hepatocellular
carcinoma. Oncol Lett. 17:1714–1722. 2019.PubMed/NCBI
|
|
34
|
Augello G, Emma MR, Cusimano A, Azzolina
A, Mongiovì S, Puleio R, Cassata G, Gulino A, Belmonte B,
Gramignoli R, et al: Targeting HSP90 with the small molecule
inhibitor AUY922 (luminespib) as a treatment strategy against
hepatocellular carcinoma. Int J Cancer. 144:2613–2624. 2019.
View Article : Google Scholar
|
|
35
|
Pan W, Luo Q, Yan X, Yuan L, Yi H, Zhang
L, Li B, Zhang Y, Sun J, Qiu MZ and Yang DJ: A novel SMAC mimetic
APG-1387 exhibits dual antitumor effect on HBV-positive
hepatocellular carcinoma with high expression of cIAP2 by inducing
apoptosis and enhancing innate anti-tumor immunity. Biochem
Pharmacol. 154:127–135. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Neumayer G, Belzil C, Gruss OJ and Nguyen
MD: TPX2: Of spindle assembly, DNA damage response, and cancer.
Cell Mol Life Sci. 71:3027–3047. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wittmann T, Wilm M, Karsenti E and Vernos
I: TPX2, A novel xenopus MAP involved in spindle pole organization.
J Cell Biol. 149:1405–1418. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Moss DK, Wilde A and Lane JD: Dynamic
release of nuclear RanGTP triggers TPX2-dependent microtubule
assembly during the apoptotic execution phase. J Cell Sci.
122:644–655. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang H, Pan YZ, Cheung M, Cao M, Yu C,
Chen L, Zhan L, He ZW and Sun CY: LAMB3 mediates apoptotic,
proliferative, invasive, and metastatic behaviors in pancreatic
cancer by regulating the PI3K/Akt signaling pathway. Cell Death
Dis. 10:2302019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sun Y, Cao FL, Qu LL, Wang ZM and Liu XY:
MEG3 promotes liver cancer by activating PI3K/AKT pathway through
regulating AP1G1. Eur Rev Med Pharmacol Sci. 23:1459–1467.
2019.PubMed/NCBI
|
|
41
|
Cen D, Huang H, Yang L, Guo K and Zhang J:
Long noncoding RNA STXBP5-AS1 inhibits cell proliferation,
migration, and invasion through inhibiting the PI3K/AKT signaling
pathway in gastric cancer cells. Onco Targets Ther. 12:1929–1936.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yun WK, Hu YM, Zhao CB, Yu DY and Tang JB:
HCP5 promotes colon cancer development by activating AP1G1 via
PI3K/AKT pathway. Eur Rev Med Pharmacol Sci. 23:2786–2793.
2019.PubMed/NCBI
|
|
43
|
Li H, Zhang Q, Wu Q, Cui Y, Zhu H, Fang M,
Zhou X, Sun Z and Yu J: Interleukin-22 secreted by
cancer-associated fibroblasts regulates the proliferation and
metastasis of lung cancer cells via the PI3K-Akt-mTOR signaling
pathway. Am J Transl Res. 11:4077–4088. 2019.PubMed/NCBI
|
|
44
|
Chen T, Gu C, Xue C, Yang T, Zhong Y, Liu
S, Nie Y and Yang H: LncRNA-uc002mbe.2 interacting with hnRNPA2B1
mediates AKT deactivation and p21 up-regulation induced by
trichostatin in liver cancer cells. Front Pharmacol. 8:6692017.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chen T, Huang H, Zhou Y, Geng L, Shen T,
Yin S, Zhou L and Zheng S: HJURP promotes hepatocellular carcinoma
proliferation by destabilizing p21 via the MAPK/ERK1/2 and
AKT/GSK3β signaling pathways. J Exp Clin Cancer Res. 37:1932018.
View Article : Google Scholar
|
|
46
|
Zhang Y, Liu Y, Duan J, Yan H, Zhang J,
Zhang H, Fan Q, Luo F, Yan G, Qiao K and Liu J: Hippocalcin-like 1
suppresses hepatocellular carcinoma progression by promoting
p21(Waf/Cip1) stabilization by activating the ERK1/2-MAPK pathway.
Hepatology. 63:880–897. 2016. View Article : Google Scholar
|
|
47
|
Ohkoshi S, Yano M and Matsuda Y: Oncogenic
role of p21 in hepatocarcinogenesis suggests a new treatment
strategy. World J Gastroenterol. 21:12150–12156. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ma J, Ren Y, Zhang L, Kong X, Wang T, Shi
Y and Bu R: Knocking-down of CREPT prohibits the progression of
oral squamous cell carcinoma and suppresses cyclin D1 and c-Myc
expression. PLoS One. 12:e01743092017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chen Y, Fang L, Zhang J, Li G, Ma M, Li C,
Lyu J and Meng QH: Blockage of Glyoxalase I inhibits colorectal
tumorigenesis and tumor growth via upregulation of STAT1, p53, and
Bax and downregulation of c-Myc and Bcl-2. Int J Mol Sci.
18:2017.
|