Introduction
Kidney stone formation is a common disease with a
high morbidity (1). Kidney stones
can lead to the development of severe diseases such as
hydronephrosis, renal function impairment or insufficiency if
active treatment is not undertaken. In addition, kidney stones have
a high occurrence rate (2).
Calcium oxalate (CaOx)-induced kidney stones, which are the most
common types of kidney stones, account for approximately 60% of all
cases of kidney stones in China (3). CaOx-induced kidney stones can be
formed by crystal deposits in tubular epithelial cells, a high
level of reactive oxygen species (ROS) and the subsequent
inflammatory response. Oxidative stress has been recognized as a
major factor contributing to the pathogenesis of kidney injury and
the formation of kidney stones (4). A previous study also demonstrated
that oxidative stress produced by a high concentration of oxalate
can lead to tubular injury and stone formation (5).
There is evidence to indicate that renal tubular
cell injury induced by CaOx plays a key role in the formation of
kidney stones (6); specifically,
the increased expression of cellular apoptosis- and
apoptosis-associated genes induced by CaOx contribute to kidney
stone formation (7). CaOx
crystals also induce autophagy through the activation of the ROS
pathway in HK-2 cells (8).
Randall plaques (RPs) are considered a factor contributing to the
formation of idiopathic CaOx-induced kidney stones. In a previous
study, in a model of RP-CaOx-induced kidney stones, differentially
expressed genes were shown to be associated with the activation of
mitogen-activated protein kinase, the Akt/phosphatidylinositol
3-kinase pathway and pro-inflammatory cytokines that can cause
renal injury and oxidative stress, which points to their critical
roles in the development of CaOx-induced kidney stones (9). Another study also demonstrated that
the ROS/Akt/p38 MAPK signaling pathway is activated in the calcium
oxalate monohydrate (COM)-induced disruption of tight junction and
causes renal cell injury and kidney stone formation (10). Epithelial-to-mesenchymal
transition (EMT) induced by CaOx crystals and oxalate in proximal
tubular cells has also been shown to lead to kidney injury
(11). Thus, it was speculated
that multiple pathways are involved in cell damage induced by
CaOx.
MicroRNAs (miRNAs or miRs) are critical regulators
in various diseases, including kidney stones (12). Claudin-14 gene expression is a key
regulator of renal Ca(2+) homeostasis. It has previously been
reported that miRNAs can directly regulate the expression of
Claudin-14 in human kidney diseases, including nephrolithiasis
(13,14). Recently, an integrative analysis
of miRNA-mRNA expression profiles in CaOx-induced kidney stones
suggested that miRNAs play a critical role in the development of
kidney stones (15). Serum and
urinary levels of miR-155 are closely related to inflammatory
cytokine levels in patients with nephrolithiasis (16). However, the role of miRNAs in the
pathophysiology of nephrolithiasis has been less extensively
reported. Recent studies have demonstrated that miR-30c-5p
(miR-30c) is related to kidney injury induced by
ischemia-reperfusion (I/R) (17,18). miR-30c has been shown to regulate
the apoptosis of renal tubular epithelial cells by targeting Bnip3L
and Hspa5 (19). miR-30c can
affect the pathogenesis of diabetic nephropathy by targeting CTGF.
Three miR-30 family members, including miR-30c are biomarkers and
therapeutic candidates for acute kidney injury (20). Therefore, it was speculated that
miR-30c may be involved in the development of renal injury and in
the formation of kidney stones.
In this study, the authors aimed to investigate the
role of miR-30c-5p in sodium oxalate-induced renal tubular
epithelial injury, in order to provide a better understanding of
the role of miRNAs in the pathogenesis of kidney stone
formation.
Materials and methods
Cells, cell culture and treatment
Human renal tubular epithelial cells (HK-2 cells)
were obtained from the American Type Culture Collection (ATCC) and
cultured in DMEM-F12 (11320082, Gibco; Thermo Fisher Scientific)
containing 10% FBS (16140071, Invitrogen; Thermo Fisher Scientific)
and 1% penicillin/streptomycin (15070063, Gibco; Thermo Fisher
Scientific) at 37°C with 5% CO2. Sodium oxalate (O0136,
Sigma) was diluted to 100, 250, 500, 750 and 1,000 µM by
double distilled water. The cells were seeded in a 6-well plate
(1×106 cells/well) or 96-well plate (2×104
cells/well) as needed. Subsequently, the cells were incubated with
the oxalate solution at various concentrations as described above,
and those in the control group were treated with an equal volume of
double distilled water. Following incubation for 3 days at 37°C
with 5% CO2, the medium was refreshed in each well.
CCK-8 assay
The cells were stimulated by various concentrations
(100, 250, 500, 750 and 1,000 µM) of oxalate for 3 days in a
96-well plate (2×104 cells/well) and washed with PBS
twice. Subsequently, 10 µl CCK-8 solution (70-CCK801,
MultiSciences) mixed with 100 µl fresh medium were added to
each well. Following incubation of the cells at 37°C in the dark
for 4 days, the absorbance value at a wavelength of 450 nm was
detected using the SpectraMax Plus 384 Microplate Reader (PLUS 384,
Molecular Devices). A 10 µl CCK-8 solution mixed with 100
µl of the medium served as a negative control.
RNA isolation and RT-qPCR
The cells were washed with PBS and treated with
TRIzol regent (15596018, Invitrogen; Thermo Fisher Scientific) at
4°C for 2 min. The RNA was then isolated by chloroform and
islpropanol at 4°C. A NanoDrop 8000 spectrophotometer (ND-8000-GL,
Thermo Fisher Scientific) was used to determine the concentration
of the RNA. The RNA was then reverse transcribed into cDNA using
the PrimeScript™ II 1st Strand cDNA Synthesis kit (6210B, Takara).
SYBR®-Green PCR Master Mix (4312704, ABI) and the
Bio-Rad CFX 96 Touch Real-Time PCR Detection System (1855196,
Bio-Rad) were used for RT-qPCR. The sequences of the primers were
as follows: ATG5 forward, 5′-AAG CAA CTC TGG ATG GGA TT-3′ and
reverse, 5′-GCA GCC ACA GGA CGA-3′ (21); and GAPDH forward, 5′-AGG TCG GTG
TGA ACG GAT TTG-3 and reverse, 5′-GGG GTC GTT GAT GGC AAC A-3′. The
parameters of RT-qPCR were set as follows: Pre-denaturation at 95°C
for 5 min, followed by denaturation at 95°C for 30 sec, annealing
at 60°C for 30 sec and extension at 72°C for 30 sec. The
2−∆∆Cq method (22)
was used to calculate the relative expression. For the
quantification of miR-30c-5p, the One-Step miRNA RT kit (D1801,
HaiGene) was used to prepare the cDNA. SYBR-Green qPCR kits
(AP01370 and AP02055, HaiGene, China) were used for the RT-qPCR of
miR-30c-5p and U6 snRNA. The parameters in RT-qPCR were set as
follows: At 95°C for 5 min, 40 cycles at 95°C for 15 sec, at 60°C
for 30 sec, and 70°C for 10 sec. The relative expression were
calculated by 2−∆∆Cq method (22).
Cell transfection
miR-30c-5p mimic (miR1160713102113-1-5), miR-30c-5p
inhibitor (miR20000244-1-5), negative control oligos for mimics
(miR0190513015853) and inhibitors (miR2N0000003-1-5) were
synthesized by Ribobio Co., Ltd. ATG5 was synthesized by Tsingke
Co., Ltd. and cloned into the pcDNA 3.1 vector (V79020, Invitrogen;
Thermo Fisher Scientific). The cells were cultured at
4×105 cells/well in 6-well plates and respectively
transfected with 100 pmoles of miRNA mimic or inhibitor using
Lipofectamine® 2000 (11668019, Invitrogen; Thermo Fisher
Scientific) at room temperature after the cells reached 50-60%
confluence. Following incubation for 24 h at 37°C, the cells were
restored by the addition of fresh culture medium and collected for
subsequent functional detections.
Cell apoptosis
The cell apoptotic rate was determined using the
Annexin V-FITC/PI kit (70-AP101-100, MultiSciences). Briefly, the
cells were cultured in a 6-well plate (2×104 cells/well)
for 24 h. Following trypsinization, the cells were collected by
centrifugation at 450 × g at 4°C for 5 min and then resuspended in
300 µl of binding buffer and added with 5 µl Annexin
V-FITC solution. Following 15 min of incubation at room temperature
in the dark, 5 µl propidium iodide (PI) were added to the
cells and incubated together for 5 min. Finally, 200 µl
binding buffer were added to the cells. The cell apoptotic rate was
determined using a FACSCalibur flow cytometer (342973, BD
Biosciences) and analyzed using BD FACSCanto™ system software v2.4
(646602, BD Biosciences).
ROS assay
ROS was measured using the Total Reactive Oxygen
Species (ROS) assay kit (S0033, Beyotime). Briefly, the cells were
seeded in 6-well plates at a density of 8×104 cells/well
and collected by trypsinization at 37°C for 2 min and
centrifugation at 500 × g for 4 min at 4°C. The cells were then
incubated with DCFH-DA (at a final concentration of 10 µM)
for 30 min at 37°C. After washing the cells 3 times with PBS
solution, the fluorescence intensity of the cells was measured by
flow cytometry (342973, BD Biosciences).
Mitochondrial membrane potential
(MMP)
MMP (also known as ∆Ψm) was assessed using JC-1
fluorescent dye (C2006, Beyotime). The cells were then cultured at
8×104 cells/well in 6-well plates and incubated with 10
µl of 200 µM JC-1 (final concentration of 2
µM) at 37°C in 5% CO2 for 30 min. Subsequently,
the cells were washed twice with 1 ml cooled 1X JC-1 buffer. The
cells were then centrifuged at 600 × g for 5 min at 4°C, and
analyzed on a flow cytometer (342973, BD Biosciences) at a 525 nm
wavelength and 490 nm wavelength for the detection of JC-1 polymers
and monomers, respectively.
Determination of lactate dehydrogenase
(LDH)
The cells were cultured in 96-well plates, and the
medium in each well was then collected and centrifuged at 1,000 ×
g, at 4°C for 10 min. The LDH in the supernatant was measured using
a Cytotoxicity Detection kit (LDH, 11644793001). Subsequently, 100
µl reaction reagent were mixed with 100 µl
supernatant and incubated for 30 min at room temperature. The
absorbance was then read at 490 nm using a multimode reader (PLUS
384, Molecular Devices). The data were normalized to those of the
cells in control group.
Measurement of oxidative stress
The level of malondialdehyde (MDA) was determined
using the Lipid Peroxidation (MDA) assay kit (MAK085, Sigma).
Briefly, 1×106 cells were homogenized by 300 µl
MDA lysis buffer containing 3 µl BHT. The samples were
centrifuged at 13,000 × g, 4°C for 10 min. A total of 200 µl
of the supernatant was mixed with 600 µl of the TBA
solution, and following incubation at 95°C for 60 min, the mixture
was cooled down on ice for 10 min. Subsequently, 200 µl of
each reaction mixture was pipetted into a 96-well plate and
analyzed at a 532 nm wavelength using a multimode reader (PLUS 384,
Molecular Devices).
In addition, the activities of the antioxidant
enzymes, super oxide dismutase (SOD) and catalase (CAT), were
measured using the Total Superoxide Dismutase assay kit (S0109,
Beyotime) and Catalase assay kit (S0051, Beyotime). The cells were
cultured at 8×104 cells/well in 6-well plates and then
homogenized in 0 5 ml buffer solution. The cells were centrifuged
at 600 × g, 4°C for 10 min to measure the activities of SOD and CAT
in the supernatants. For SOD activity, 20 µl homogenates
were mixed with 160 µl NBT reagent and 20 µl reaction
reagent at 37°C and held for 30 min. An equal volume of detection
buffer mixed with 160 µl NBT reagent and 20 µl
reaction reagent served as the blank control. The level of SOD was
determined at a 560 nm wavelength using a multimode reader (PLUS
384, Molecular Devices). For CAT activity, 10 µl homogenates
were mixed with 10 µl hydrogen peroxide reagent and 30
µl hydrogen peroxide detection buffer. Subsequently, 40
µl hydrogen peroxide detection buffer mixed with 10
µl of 250 mM hydrogen peroxide reagent served as the blank
control. Following incubation at 25°C for 5 min, 450 µl of
stopping solution were added to the mixture to terminate the
reaction, and the mixture was then detected at a 240 nm wavelength
using a multimode reader (PLUS 384, Molecular Devices).
Crystal-cell adhesion assay
The treated cells were cultured in a 6-well plate.
As previously described, the cells with full confluence were
incubated in DMEM containing 100 µg/ml calcium oxalate
monohydrate (COM, C0350000, Sigma) for 10 min at 37°C in a
humidified atmosphere with 5% CO2 (23). The cells were then washed by PBS
twice to remove residual COM crystals. The images of the crystals
were captured under a microscope (ECLIPSE TS100, Nikon). A total of
10 randomized high-power fields per well were selected and the
numbers of adherent crystals in each field were counted. The
representative images were selected, at the same scale, and the
area of the field selected by the square was equal. The crystal
numbers in different groups were standardized with the control
group.
Bioinformatics and dual-luciferase
reporter assay
TargetScan7.2 (http://www.targetscan.org/vert_72/) was applied to
predict the target gene of miR-30c-5p. DNA sequences of ATG5-WT and
ATG5-MUT were obtained from Tsingke Co., Ltd. and separately
constructed into the luciferase reporter gene vector (pmirGLO,
E1330, Promega) to construct luciferase reporter plasmids.
Renilla luciferase vector (E6911, Promega, USA) was
co-transfected into the cells as a reporter control. Briefly, the
cells cultured in 96-well plates were transfected with luciferase
reporter plasmid (0.05 µg/well) or co-transfected with
luciferase reporter plasmid and miR-30c-5p mimic (15 nM). Following
transfection for 24 h, the cells were analyzed for luciferase
activity using the Dual-Glo® Luciferase assay system
(E2920, Promega) and Microplate Luminometer (11300010, Berthold).
The luciferase activity was normalized by firefly luciferase
activity in comparison with Renilla luciferase activity. For
each transfection, the luciferase activity was averaged from 6
replicates.
Statistical analysis
The data are presented as the means ± SD.
Differences between 2 groups were assessed by one-way ANOVA,
followed by Dunnett's post hoc test (version 19.0 software, SPSS,
Inc.). A P<0.05 was considered to indicate a statistically
significant difference.
Results
Effect of miR-30c-5p on oxalate-induced
cytotoxicity in HK-2 cells
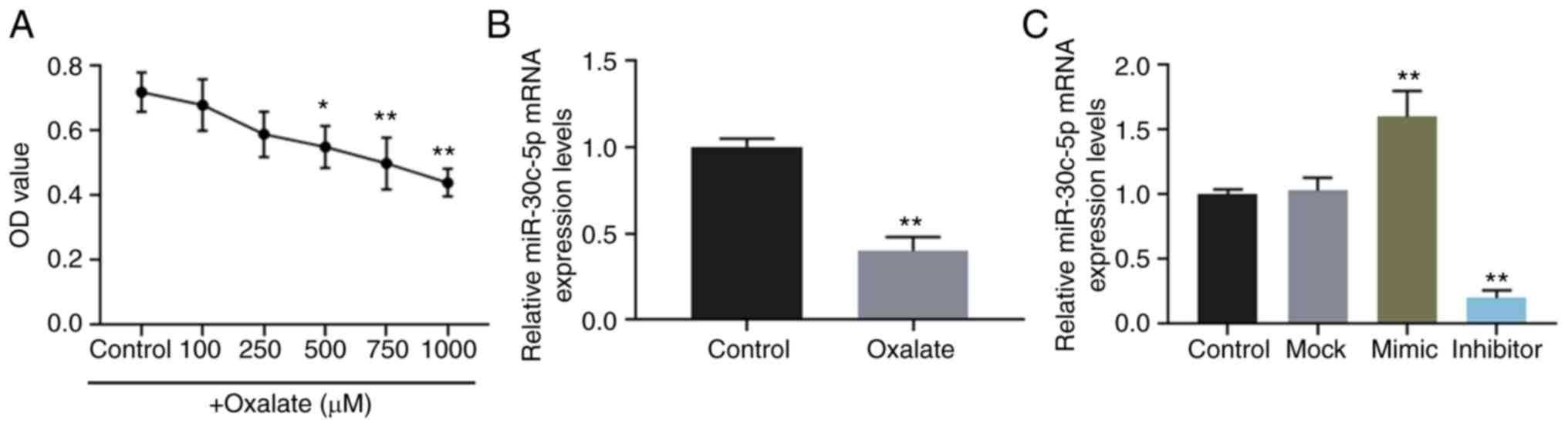
The viability of HK-2 cells stimulated by oxalate at
concentrations of 0, 100, 250, 500, 750 and 1,000 µM was
determined, and the results revealed that oxalate reduced cell
viability in a concentration-dependent manner (Fig. 1A). The results of RT-qPCR also
revealed that the expression level of miR-30c-5p was downregulated
in HK-2 cells exposed to 750 µM oxalate (Fig. 1B).
Furthermore, miR-30c-5p mimic and inhibitor were
successfully transfected into the HK-2 cells to upregulate and
downregulate the level of miR-30c-5p, respectively (Fig. 1C). The results of flow cytometry
indicated that oxalate increased the apoptotic rate of the HK-2
cells, which however, was reversed by transfection with miR-30c-5p
mimic and was enhanced by transfection with miR-30c-5p inhibitor,
respectively (Fig. 2A and B).
Oxalated stimulation also increased the activities of LDH and MDA,
and these effects were reverse by transfection with miR-30c-5p
mimic and aggravated by transfection with miR-30c-5p inhibitor (all
P<0.001, Fig. 2C and D).
However, the changes in SOD and CAT activities exhibited opposite
results (all P<0.001, Fig.
2D). JC-1 is used as an indicator of MMP (∆Ψm) in a variety of
cell types, and when the ∆Ψm is high, JC-1 accumulates in the
mitochondrial matrix and forms polymers, while at a low ∆Ψm, JC-1
is unable to accumulate in the mitochondrial matrix and thus forms
monomers. JC-1 polymers and monomers can be respectively detected
at a wavelength of 585/590 and 514/529 nm. In this study, the
levels of ROS and MMP were determined by flow cytometry, and the
results revealed that the increase in ROS production and the
decrease in MMP induced by oxalate were reversed by the
overexpression of miR-30c-5p, but were enhanced by the inhibition
of miR-30c-5p (Fig. 2E and F).
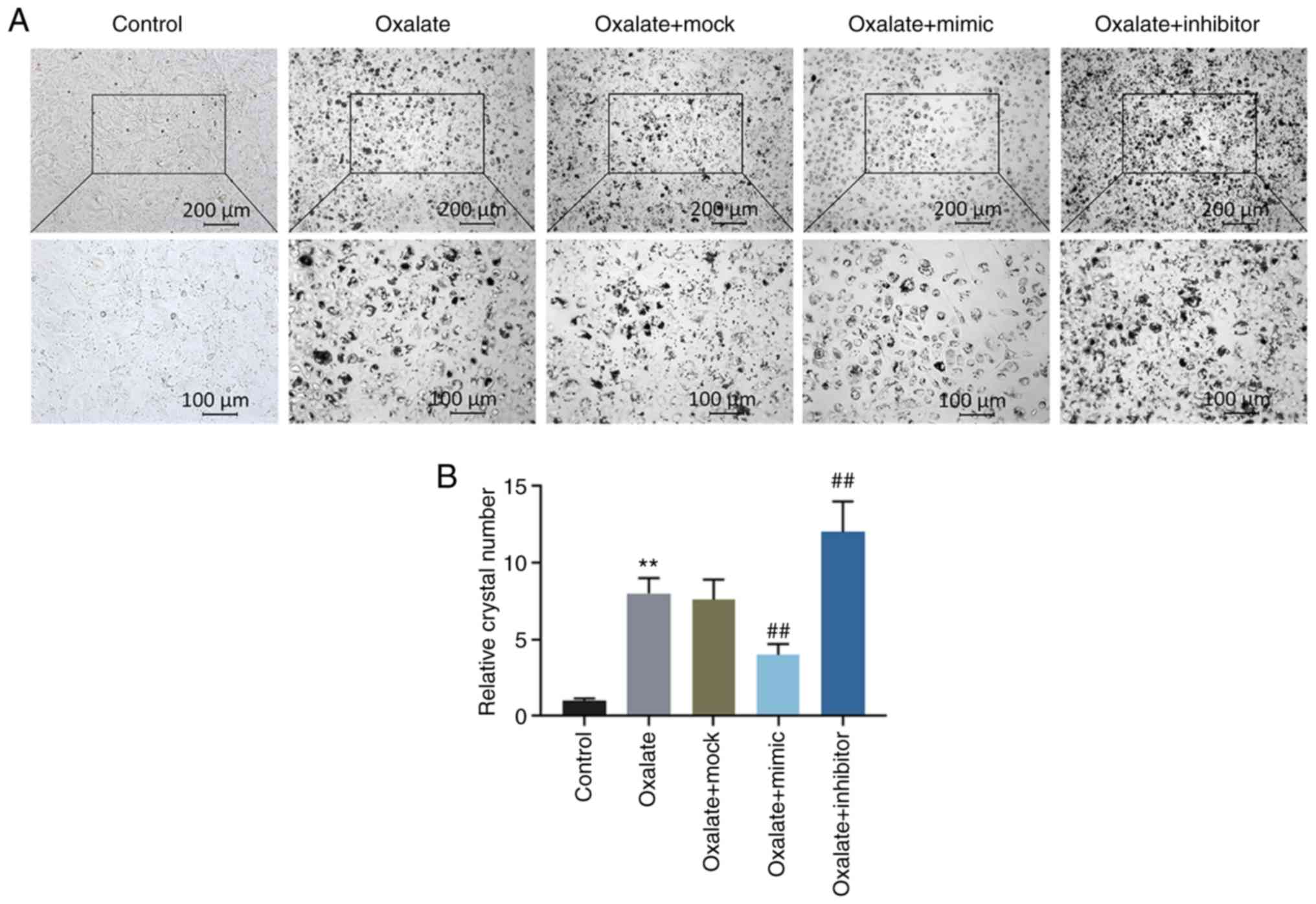
Crystal-cell adhesion assay also demonstrated that the crystal
number increased significantly in the HK-2 cells exposed to
oxalated, and this increase was abolished by transfection with
miR-30c-5p mimic, whereas it was promoted by transfection with
miR-30c-5p inhibitor (all P<0.001, Fig. 3).
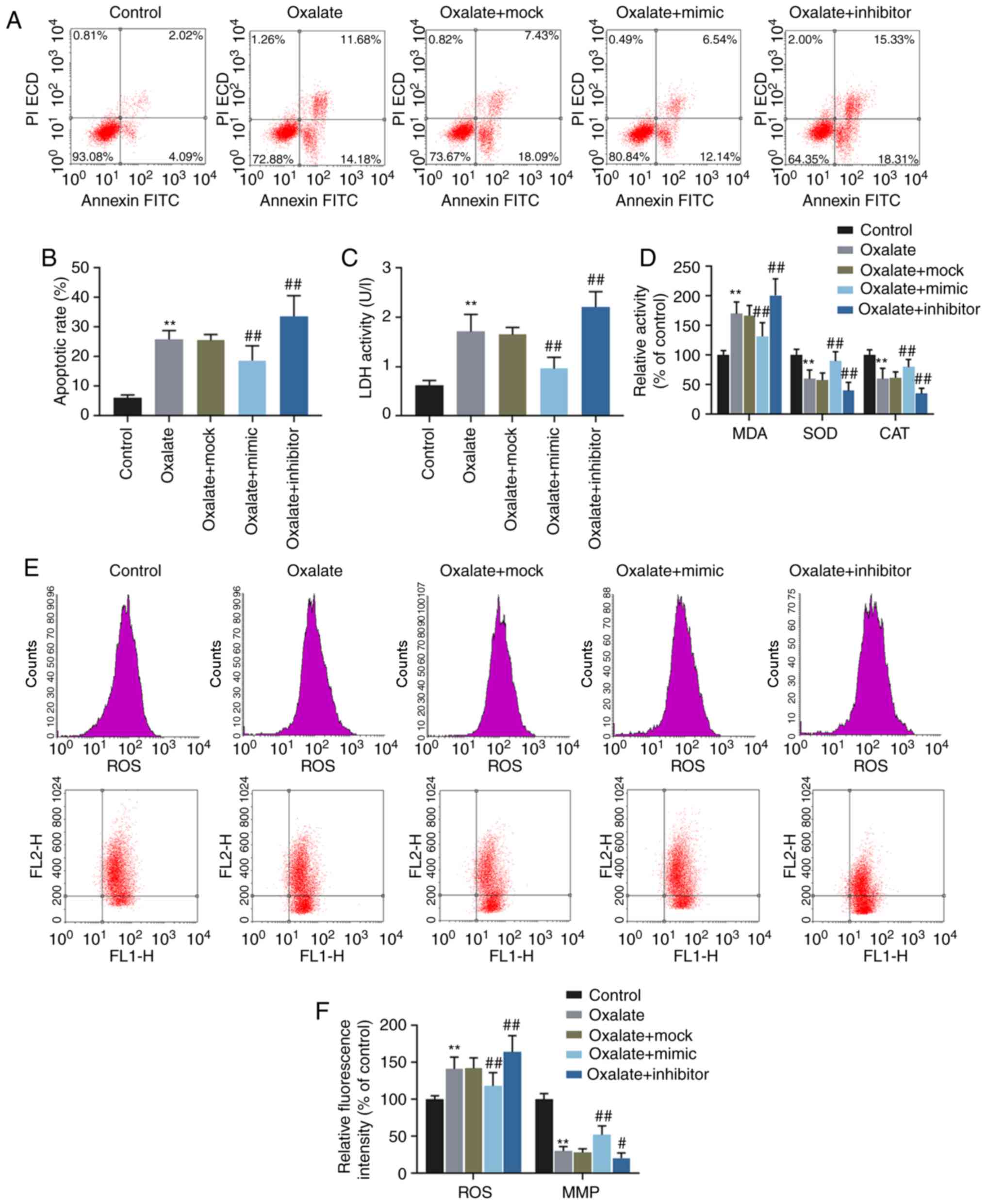 | Figure 2Effects of miR-30c-5p on cytotoxicity
in HK-2 cells induced by oxalate. (A and B) The apoptotic rates of
HK-2 cells determined by flow cytometry. (C) The LDH activity of
HK-2 cells was determined by LDH assay. (D) The relative activities
of MDA, SOD and CAT in HK-2 cells were determined by chemical
colorimetry. (E and F) The levels of ROS (upper panel in row in
ʻEʼ) and MMP (lower panel in ʻEʼ) in HK-2 cells were measured by
flow cytometry. FL1-H, fluorescence intensity of JC-1 monomers.
FL2-H, fluorescence intensity of JC-1 polymers.
**P<0.001 vs. control; #P<0.05 and
##P<0.001 vs. oxalate + mock. Control, transfection
with control mimic and control inhibitor; oxalate, incubation with
oxalate; oxalate + mock, incubation with oxalate and transfection
reagent; oxalate + mimic, co-incubation with oxalate and miR-30c-5p
mimic; oxalate + inhibitor, co-incubation with oxalate and
miR-30c-5p inhibitor; MMP, mitochondrial membrane potential; LDH,
lactate dehydrogenase; MDA, malondialdehyde; SOD, superoxide
dismutase; CAT, catalase. |
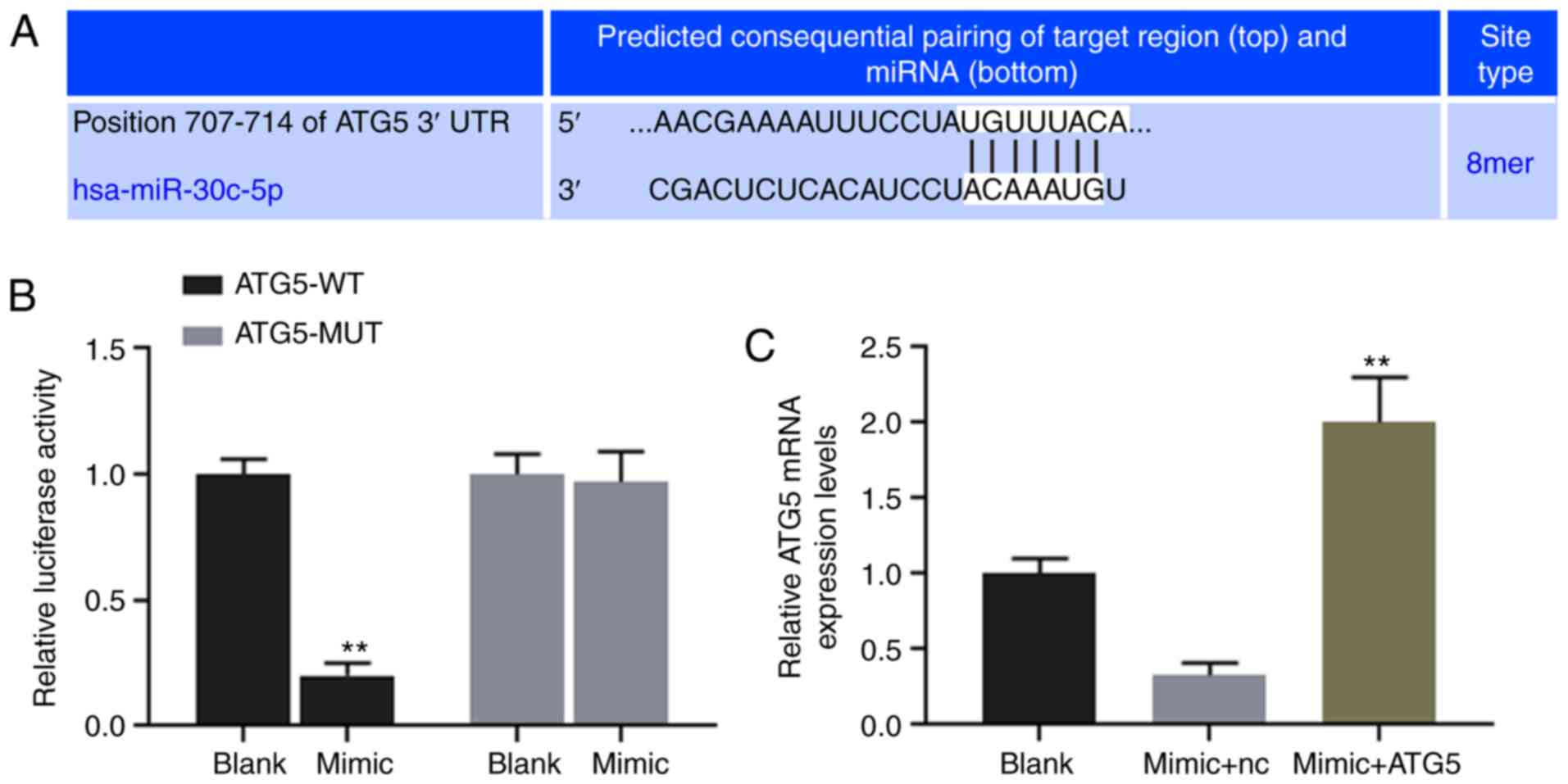
ATG5 is directly targeted and regulated
by miR-30c-5p
TargetScan7.2 was used to predict the binding site
for miR-30c-5p at position 707-714 of the ATG5 3′UTR (Fig. 4A), and dual-luciferase reporter
assay was performed to verify the association between miR-30c-5p
and ATG5. The results revealed that the luciferase activity of the
HK-2 cells transfected with ATG5-WT reporter plasmid was
significantly decreased by miR-30c-5p (P<0.001), while no
obvious change was observed in the cells transfected with the
ATG5-MUT reporter plasmid (Fig.
4B). The results of RT-qPCR demonstrated that the
overexpression of miR-30c-5p decreased the expression level of
ATG5, while ATG5 expression in the mimic + ATG5 group was
significantly higher than that in the mimic + NC group (Fig. 4C).
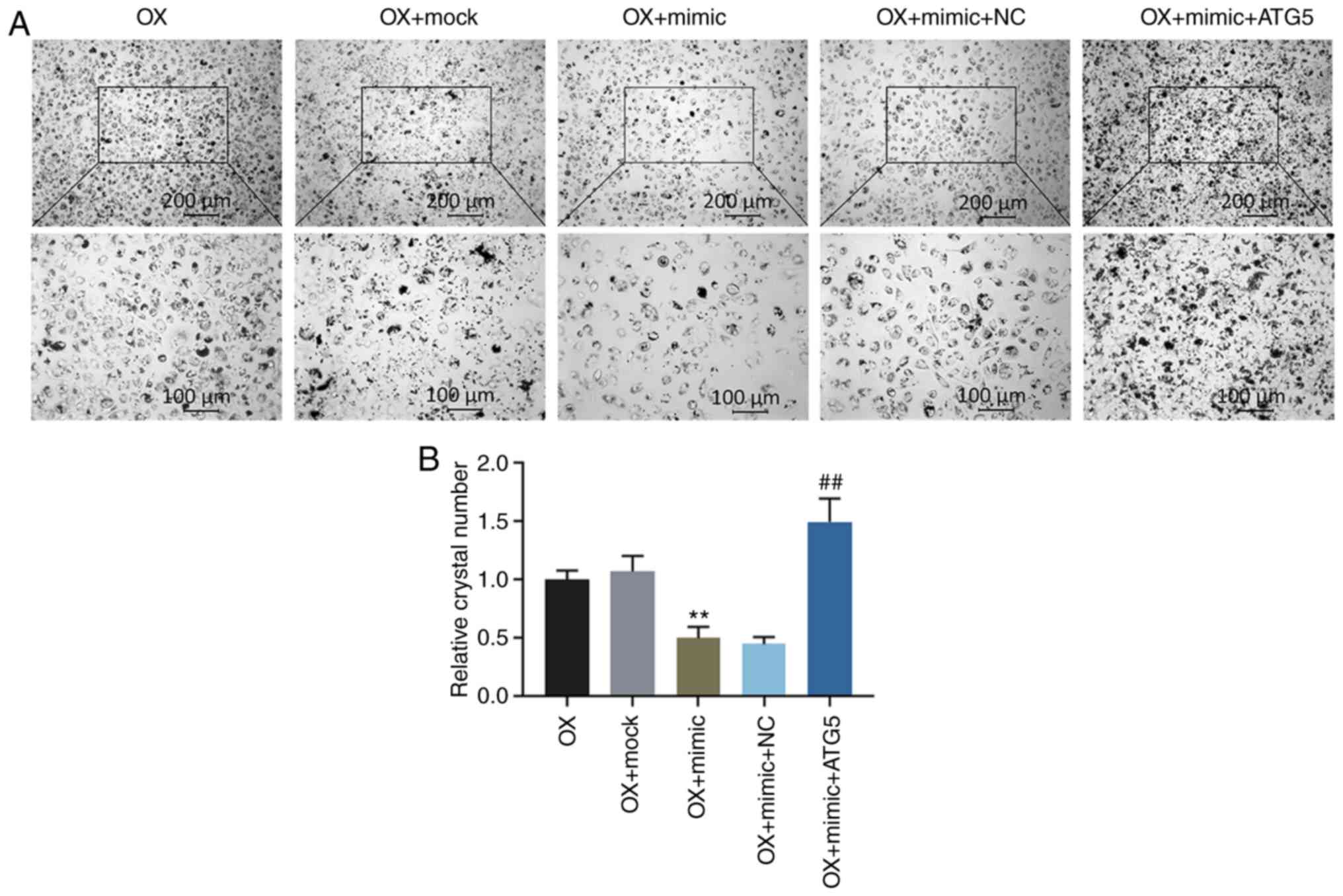
miR-30c-5p regulates the oxalate-induced
cytotoxicity in HK-2 cells by targeting ATG5
Functional rescue experiments were performed to
confirm the function of miR-30c-5p in targeting ATG5. Flow
cytometry was carried out to determine the cell apoptotic rate and
the levels of ROS and MMP in the HK-2 cells exposed to oxalate.
Additionally, the relative activities of LDH, MDA, SOD and CAT were
determined. The results demonstrated that the overexpression of
ATG5 ʻrescuedʼ the decrease in the cell apoptotic rate, the ROS
level, the activities of LDH and MDA and crystal number, the
increase in SOD and CAT activities and the upregulated level of MMP
induced by transfection with miR-30c-5p mimic (all P<0.001,
Fig. 5). Crystal-cell adhesion
assay was performed to determine the crystal-forming ability, and
the data indicated that transfection with miR-30c-5p mimic reduced
the crystal number in the HK-2 cells exposed to oxalate, whereas
the overexpression of ATG5 reversed the effects induced by
transfection with miR-30c-5p mimic (all P<0.001, Fig. 6).
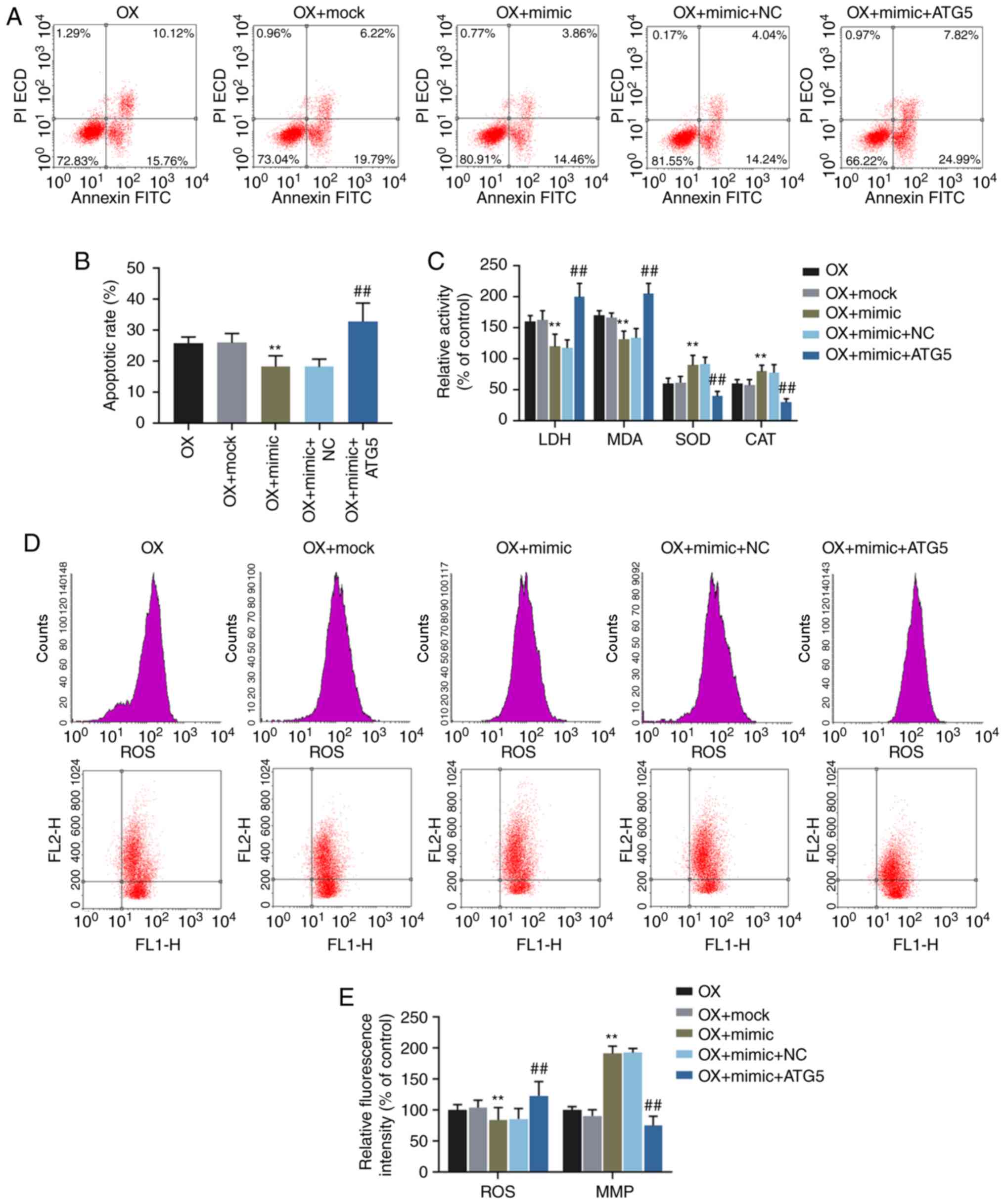 | Figure 5Effects of miR-30c-5p on
oxalate-induced cytotoxicity via the regulation of ATG5. (A and B)
Apoptotic rates of HK-2 cells were determined by flow cytometry.
(C) The relative activities of LDH, MDA, SOD and CAT in HK-2 cells
were determined by chemical colorimetry. (D and E) The levels of
ROS (upper panel in ʻDʼ) and MMP (lower panel in ʻDʼ) in HK-2 cells
were determined by flow cytometry. **P<0.001 vs. OX +
mock; ##P<0.001 vs. OX + mimic + NC. OX, incubation
with oxalate; OX + mock, incubation with oxalate and transfection
reagent; OX + mimic, co-incubation with oxalate and miR-30c-5p
mimic; oxalate + mimic + NC, co-incubation with oxalate, miR-30c-5p
mimic and empty plasmid; OX + mimic + ATG5, co-incubation with
oxalate, miR-30c-5p mimic and ATG5 overexpression plasmid. MMP,
mitochondrial membrane potential. |
Discussion
Oxalate is an end product of metabolism, and a high
level of oxalate can lead to hyperoxaluria and even in the
formation of CaOx-induced kidney stones (24). In this study, it was found that
oxalate at various concentrations led to a decrease in the
viability of human renal tubular epithelial cells, and such a
result was consistent with the findings of previous studies
(25,26). A previous study reported that
miR-30c alleviated diabetic nephropathy via the Snail1/TGF-β1
pathway (27). Of note, it was
found that miR-30c-5p was downregulated by oxalate stimulation,
suggesting that miR-30c-5p may be involved in oxalate-induced cell
injury.
Apoptosis is a type of programmed cell death, and a
previous study suggested that the reduction in cell viability
induced by CaOx was associated with cell apoptosis (28). Moreover, cell injury induced by
oxalate occurs due to apoptosis (29). In the early stages of apoptosis,
phosphatidylserine (PS) is exposed outwards to the cell surface.
FITC-labeled Annexin V selectively binds to PS and can be detected
by flow cytometry. MMP is another indicator of apoptosis, and a low
MMP (∆Ψm) causes JC-1 polymers to turn into monomers when apoptosis
occurs. JC-1 monomers can be detected at a wavelength of 514/529
nm, while JC-1 polymers can be detected at a wavelength of 585/590
nm. Thus, this study also detected the apoptotic rate and MMP by
flow cytometry. It has previously been reported that miR-30c is
closely related to cell apoptosis. For example, miR-30c regulates
the apoptosis and proliferation of renal tubular epithelial cells
(19), and a myocardial cell
model (30). This study indicated
that miR-30c-5p participates in cell apoptosis induced by
oxalate.
It has been demonstrated that ROS are produced
during CaOx-induced nephrolithiasis (31). The production of ROS is controlled
under normal conditions; however, it is increased when the
conditions change, such as for example, a high concentration of
oxalate and crystals of CaOx/calcium phosphate (CaP) can induce
excessive ROS production in renal epithelial cells (32). ROS are an important parameter
involved in oxidative and endoplasmic reticulum dysfunction of
cells (33). Therefore, in this
study, the effect of the miR-30c-5p on the ROS level was
investigated in HK-2 cells exposed to oxalate. The level of LDH
released by cells can indicate cell oxidative damage (34). As a product of lipid peroxidation,
MDA can be induced by oxidative stress and reflects the degree of
oxidative injury. SOD is an antioxidant enzyme, and is involved in
the antioxidant system. CAT can neutralize excessive ROS production
and catalyzes H2O2 into H2O and
O2 (35). Thus, the
levels of LDH, MDA, SOD and CAT were determined, and the results
revealed that oxalate causes severe oxidative dysfunction, while
miR-30c-5p mimic can relieve such an injury induced by oxalate.
Taken together, the findings of this study demonstrated that
miR-30c-5p is potentially a critical regulator of sodium
oxalate-induced kidney stones, and such a finding was further
confirmed by crystal-cell adhesion assay. These results suggested
that the overexpression of miR-30c-5p relieves cell cytotoxicity
and inhibits the formation of sodium oxalate-induced kidney
stones.
miRNAs play important roles in various diseases by
targeting and regulating mRNAs. In this study, ATG5 was targeted by
miR-30c-5p. There is evidence to indicate that ATG5 is involved in
the occurrence and development of cell apoptosis; for example,
knocking down ATG5 can alleviate the increase in cell apoptosis and
apoptosis-associated protein expression induced by hypoxia and
reoxygenation (36). Previous
studies have also demonstrated that ATG5 promoted cell apoptosis by
activating caspase-8 (37), and
that ATG5 is involved in the apoptosis of human cardiomyocytes
(38) and colorectal cancer cells
(39). The knockdown of ATG5
upregulates the apoptotic cell death of human vaginal epithelial
cells (40); moreover, ATG5
suppresses cellular proliferation and induces the apoptosis of DF-1
cells (41). In addition, the
inhibition of ATG5 can decrease oxidative stress-based cytotoxicity
in osteosarcoma cells (42). The
damage to renal tubular cells is a major factor contributing to COM
crystal adhesion (43). A
previous study demonstrated that the inhibition of the autophagy
pathway alleviated the oxidative injury to renal tubular cells and
reduced CaOx-induced crystal deposition via the p38 signaling
pathway (44). Therefore, as an
autophagy-related gene, ATG5 is directly regulated by miR-30c-5p
and is involved in renal cell oxidative stress and crystal
depositions induced by oxalate. To conclude, in this study, the
molecular mechanisms underlying the function of miR-30c-5p in
oxalate-induced HK-2 cell injury was examined in in vitro
experiments; however, these mechanisms need to be confirmed by
future in vivo studies.
In conclusion, this study demonstrates that
miR-30c-5p is downregulated in HK-2 cells exposed to oxalate, and
that miR-30c-5p mimic can alleviate the oxidative stress, cell
injury and crystal-cell adhesion caused by a high concentration of
oxalate through the regulation of ATG5.
Acknowledgments
Not applicable.
Funding
This study was supported by the 2018 Medical
Scientific Research Plan Project of Dalian City (1811117).
Availability of data and materials
The analyzed datasets generated during this study
are available from the corresponding author on reasonable
request.
Authors' contributions
XW and BG made substantial contributions to the
conception and design of the study. YZ, SH, HC, CC and LJ were
involved in data acquisition, data analysis and interpretation. XW
and BG were involved in the drafting of the article or critically
revising it for important intellectual content. All authors have
read and approved the final version of the manuscript to be
published. All authors agree to be accountable for all aspects of
the work in ensuring that questions related to the accuracy or
integrity of the work are appropriately investigated and
resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Stern JM, Moazami S, Qiu Y, Kurland I,
Chen Z, Agalliu I, Burk R and Davies KP: Evidence for a distinct
gut microbiome in kidney stone formers compared to non-stone
formers. Urolithiasis. 44:399–407. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Worcester EM and Coe FL: Clinical
practice. Calcium kidney stones. N Engl J Med. 363:954–963. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ye Z, Zeng G, Huan Y, Li J, Tang K, Wang
G, Wang S, Yu Y, Wang Y, Zhang T, et al: The status and
characteristics of urinary stone composition in China. BJU Int. Apr
8–2019.Epub ahead of print. View Article : Google Scholar
|
|
4
|
Khan SR: Reactive oxygen species as the
molecular modulators of calcium oxalate kidney stone formation:
Evidence from clinical and experimental investigations. J Urol.
189:803–811. 2013. View Article : Google Scholar
|
|
5
|
Ma MC, Chen YS and Huang HS: Erythrocyte
oxidative stress in patients with calcium oxalate stones correlates
with stone size and renal tubular damage. Urology. 83:510.e9–e17.
2014. View Article : Google Scholar
|
|
6
|
Asselman M, Verhulst A, De Broe ME and
Verkoelen CF: Calcium oxalate crystal adherence to hyaluronan-,
osteopontin-, and CD44-expressing injured/regenerating tubular
epithelial cells in rat kidneys. J Am Soc Nephrol. 14:3155–3166.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Miyazawa K, Suzuki K, Ikeda R, Moriyama
MT, Ueda Y and Katsuda S: Apoptosis and its related genes in renal
epithelial cells of the stone-forming rat. Urol Res. 33:31–38.
2005. View Article : Google Scholar
|
|
8
|
Liu Y, Li D, He Z, Liu Q, Wu J, Guan X,
Tao Z and Deng Y: Inhibition of autophagy-attenuated calcium
oxalate crystal-induced renal tubular epithelial cell injury in
vivo and in vitro. Oncotarget. 9:4571–4582. 2017.
|
|
9
|
Taguchi K, Hamamoto S, Okada A, Unno R,
Kamisawa H, Naiki T, Ando R, Mizuno K, Kawai N, Tozawa K, et al:
Genome-wide gene expression profiling of randall's plaques in
calcium oxalate stone formers. J Am Soc Nephrol. 28:333–347. 2017.
View Article : Google Scholar
|
|
10
|
Yu L, Gan X, Liu X and An R: Calcium
oxalate crystals induces tight junction disruption in distal renal
tubular epithelial cells by activating ROS/Akt/p38 MAPK signaling
pathway. Ren Fail. 39:440–451. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Convento MB, Pessoa EA, Cruz E, da Glória
MA, Schor N and Borges FT: Calcium oxalate crystals and oxalate
induce an epithelial-to-mesenchymal transition in the proximal
tubular epithelial cells: Contribution to oxalate kidney injury.
Sci Rep. 7:457402017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang B, Wu B, Liu J, Yao W, Xia D, Li L,
Chen Z, Ye Z and Yu X: Analysis of altered microRNA expression
profiles in proximal renal tubular cells in response to calcium
oxalate monohydrate crystal adhesion: implications for kidney stone
disease. PLoS One. 9:e1013062014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hou J: Lecture: New light on the role of
claudins in the kidney. Organogenesis. 8:1–9. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gong Y, Renigunta V, Himmerkus N, Zhang J,
Renigunta A, Bleich M and Hou J: Claudin-14 regulates renal
Ca++ transport in response to CaSR signalling via a
novel microRNA pathway. EMBO J. 31:1999–2012. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lan C, Chen D, Liang X, Huang J, Zeng T,
Duan X, Chen K, Lai Y, Yang D, Li S, et al: Integrative analysis of
miRNA and mRNA expression profiles in calcium oxalate
nephrolithiasis rat model. Biomed Res Int. 2017:83067362017.
View Article : Google Scholar
|
|
16
|
Hu YY, Dong WD, Xu YF, Yao XD, Peng B, Liu
M and Zheng JH: Elevated levels of miR-155 in blood and urine from
patients with nephrolithiasis. Biomed Res Int. 2014:2956512014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang C, Yu S, Zheng B, Liu D, Wan F, Ma
Y, Wang J, Gao Z and Shan Z: miR-30c-5p reduces renal
ischemia-reperfusion involving macrophage. Med Sci Monit.
25:4362–4369. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zou YF, Wen D, Zhao Q, Shen PY, Shi H,
Zhao Q, Chen YX and Zhang W: Urinary MicroRNA-30c-5p and
MicroRNA-192-5p as potential biomarkers of
ischemia-reperfusion-induced kidney injury. Exp Biol Med (Maywood).
242:657–667. 2017. View Article : Google Scholar
|
|
19
|
Du B, Dai XM, Li S, Qi GL, Cao GX, Zhong
Y, Yin PD and Yang XS: MiR-30c regulates cisplatin-induced
apoptosis of renal tubular epithelial cells by targeting Bnip3L and
Hspa5. Cell Death Dis. 8:e29872017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gutiérrez-Escolano A, Santacruz-Vázquez E
and Gómez-Pérez F: Dysregulated microRNAs involved in
contrast-induced acute kidney injury in rat and human. Ren Fail.
37:1498–1506. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zheng W, Xie W, Yin D, Luo R, Liu M and
Guo F: ATG5 and ATG7 induced autophagy interplays with UPR via PERK
signaling. Cell Commun Signal. 17:422019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
23
|
Manissorn J, Fong-Ngern K, Peerapen P and
Thongboonkerd V: Systematic evaluation for effects of urine pH on
calcium oxalate crystallization, crystal-cell adhesion and
internalization into renal tubular cells. Sci Rep. 7:17982017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Abhishek A, Benita S, Kumari M, Ganesan D,
Paul E, Sasikumar P, Mahesh A, Yuvaraj S, Ramprasath T and Selvam
GS: Molecular analysis of oxalate-induced endoplasmic reticulum
stress mediated apoptosis in the pathogenesis of kidney stone
disease. J Physiol Biochem. 73:561–573. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fishman AI, Green D, Lynch A, Choudhury M,
Eshghi M and Konno S: Preventive effect of specific antioxidant on
oxidative renal cell injury associated with renal crystal
formation. Urology. 82:489.e1–e7. 2013. View Article : Google Scholar
|
|
26
|
Cheraft-Bahloul N, Husson C, Ourtioualous
M, Sinaeve S, Atmani D, Stévigny C, Nortier JL and Antoine MH:
Protective effects of pistacia lentiscus L. fruit extract against
calcium oxalate monohydrate induced proximal tubular injury. J
Ethnopharmacol. 209:248–254. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao Y, Yin Z, Li H, Fan J, Yang S, Chen C
and Wang DW: MiR-30c protects diabetic nephropathy by suppressing
epithelial-to-mesenchymal transition in db/db mice. Aging Cell.
16:387–400. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Davalos M, Konno S, Eshghi M and Choudhury
M: Oxidative renal cell injury induced by calcium oxalate crystal
and renoprotection with antioxidants: A possible role of oxidative
stress in nephrolithiasis. J Endourol. 24:339–345. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jeong BC, Kwak C, Cho KS, Kim BS, Hong SK,
Kim JI, Lee C and Kim HH: Apoptosis induced by oxalate in human
renal tubular epithelial HK-2 cells. Urol Res. 33:87–92. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu X, Li M, Peng Y, Hu X, Xu J, Zhu S, Yu
Z and Han S: miR-30c regulates proliferation, apoptosis and
differentiation via the Shh signaling pathway in P19 cells. Exp Mol
Med. 48:e2482016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Khan SR: Hyperoxaluria-induced oxidative
stress and antioxidants for renal protection. Urol Res. 33:349–357.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Khan SR: Reactive oxygen species,
inflammation and calcium oxalate nephrolithiasis. Transl Androl
Urol. 3:256–276. 2014.PubMed/NCBI
|
|
33
|
Liang Y, Dong B, Pang N and Hu J: ROS
generation and DNA damage contribute to abamectin-induced
cytotoxicity in mouse macrophage cells. Chemosphere. 234:328–337.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ren J, Yuan L, Wang W, Zhang M, Wang Q, Li
S, Zhang L and Hu K: Tricetin protects against 6-OHDA-induced
neurotoxicity in Parkinson's disease model by activating Nrf2/HO-1
signaling pathway and preventing mitochondria-dependent apoptosis
pathway. Toxicol Appl Pharmacol. 378:1146172019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhao J, Zhang M, Zhang W, Liu F, Huang K
and Lin K: Insight into the tolerance, biochemical and
antioxidative response in three moss species on exposure to BDE-47
and BDE-209. Ecotoxicol Environ Saf. 181:445–454. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang C, Zhang C, Wang H, Qi Y, Kan Y and
Ge Z: Effects of miR-103a-3p on the autophagy and apoptosis of
cardiomyocytes by regulating Atg5. Int J Mol Med. 43:1951–1960.
2019.PubMed/NCBI
|
|
37
|
Young MM, Takahashi Y, Khan O, Park S,
Hori T, Yun J, Sharma AK, Amin S, Hu CD, Zhang J, et al:
Autophagosomal membrane serves as platform for intracellular
death-inducing signaling complex (iDISC)-mediated caspase-8
activation and apoptosis. J Biol Chem. 287:12455–12468. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhao P, Zhang BL, Liu K, Qin B and Li ZH:
Overexpression of miR-638 attenuated the effects of
hypoxia/reoxygenation treatment on cell viability, cell apoptosis
and autophagy by targeting ATG5 in the human cardiomyocytes. Eur
Rev Med Pharmacol Sci. 22:8462–8471. 2018.PubMed/NCBI
|
|
39
|
Zheng Y, Tan K and Huang H: Long noncoding
RNA HAGLROS regulates apoptosis and autophagy in colorectal cancer
cells via sponging miR-100 to target ATG5 expression. J Cell
Biochem. 120:3922–3933. 2019. View Article : Google Scholar
|
|
40
|
Shroff A and Reddy KVR: Autophagy gene
ATG5 knockdown upregulates apoptotic cell death during Candida
albicans infection in human vaginal epithelial cells. Am J Reprod
Immunol. 80:e130562018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liao Z, Dai Z, Cai C, Zhang X, Li A, Zhang
H, Yan Y, Lin W, Wu Y, Li H, et al: Knockout of Atg5 inhibits
proliferation and promotes apoptosis of DF-1 cells. In Vitro Cell
Dev Biol Anim. 55:341–348. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hollomon MG, Gordon N, Santiago-O'Farrill
JM and Kleinerman ES: Knockdown of autophagy-related protein 5,
ATG5, decreases oxidative stress and has an opposing effect on
camptothecin-induced cytotoxicity in osteosarcoma cells. BMC
Cancer. 13:5002013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Khan SR: Renal tubular damage/dysfunction:
Key to the formation of kidney stones. Urol Res. 34:86–91. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Duan X, Kong Z, Mai X, Lan Y, Liu Y, Yang
Z, Zhao Z, Deng T, Zeng T, Cai C, et al: Autophagy inhibition
attenuates hyperoxaluria-induced renal tubular oxidative injury and
calcium oxalate crystal depositions in the rat kidney. Redox Biol.
16:414–425. 2018. View Article : Google Scholar : PubMed/NCBI
|