Introduction
Obesity is increasing worldwide. More than 1.9
billion adults are overweight and 13% of adults are obese (1). Obesity increases the risk of various
chronic diseases, such as cardiovascular diseases, arteriosclerosis
and type 2 diabetes, which are severe public health problems
(2). Obesity is characterized by
an accumulation of lipids via increasing adipogenesis, the
inhibition of lipolysis and adenosine monophosphate-activated
protein kinase (AMPK) activation (3-5).
Adipogenesis is the process of differentiation from preadipocytes
to mature adipocytes, which is mediated by several adipogenic
transcription factors, such as
γ-cytidine-cytidine-adenosine-adenosine-thy midine (CCAAT)/enhancer
binding protein (C/EBP)-α, -β and peroxisome proliferator-activated
receptor (PPAR)-γ (6,7). The expression of key transcription
regulators, including C/EBP-α, C/EBP-β and PPAR-γ, activates
various adipogenic genes, including fatty acid synthase (FAS),
fatty acid binding protein (FABP4), and glucose transporter 4
(GLUT4) (8,9). Furthermore, lipolysis is a catabolic
process that hydrolyzes triglycerides (TG) into glycerol and free
fatty acids, and it plays a crucial role in balancing the lipid
metabolism of adipose cells (4,10).
In particular, hormone-sensitive lipase (HSL) is a major lipolysis
gene that controls the hydrolysis of TG by its rate-limiting role
(10). Moreover, 5′-AMPK is a
regulator of energy homeostasis and plays an important role in
regulating adipocyte differentiation (5). Activation of AMPK induces fatty acid
oxidation, inhibition of fatty acids synthesis and a reduction in
the transcription of adipogenic genes such as C/EBP-α, C/EBP-β, and
PPAR-γ (5,11).
Numerous studies have demonstrated that extracts
from plants such as Aster glehni, Eclipta alba and
yellow capsicum cause a downregulation of adipogenesis and
lipogenesis as well as the induction of lipolysis (12-14). Acer okamotoanum is a plant
found on Ulleungdo Island (Korea) and contains a number of
antioxidant compounds including cleomiscosins A and C (15). Bioactive flavonoids from A.
okamotoanum, including quercitrin, isoquercitrin and afzelin
have been previously isolated (16). In addition, the sap of A.
okamotoanum is reported to have various biological activities
such as antioxidant, immune improvement and anti-hypertension
effects (17-19). In addition, the authors previously
demonstrated that the ethyl acetate (EtOAc) fraction from A.
okamotoanum exhibited antioxidant, neuroprotective and
cognitive improvement activities (20,21). According to Kim et al
(22), the A. okamotoanum
Nakai leaf extract suppressed the expression of PPAR-γ and C/EBP-α
via the inactivation of phosphatidylinositol 3 kinase
(PI3K)/protein kinase B (Akt) signaling and the activation of
β-catenin signaling. Therefore, the A. okamotoanum Nakai
leaf extract inhibited adipocyte differentiation via adipogenesis
in 3T3-L1 cells. Various mechanisms are involved in adipocyte
differentiation, such as adipogenesis, lipogenesis, lipolysis and
AMPK activation. This study is the first to the best of our
knowledge to report on the anti-adipocyte differentiation effects
of A. okamotoanum by the regulation of adipogenesis as well
as lipolysis and AMPK activation in 3T3-L1 cells.
In the present study, the anti-adipocyte
differentiation effect of the EtOAc fraction from A.
okamotoanum on the differentiation of 3T3-L1 cells was
investigated. Furthermore, the molecular mechanisms in the
protective role of adipocyte differentiation of the A.
okamotoanum EtOAc fraction were associated with the regulatory
pathways of adipogenesis, lipolysis and the activation of AMPK.
Materials and methods
Reagents
Dulbecco's modified eagle medium (DMEM), bovine calf
serum (BCS), fetal bovine serum (FBS), penicillin-streptomycin and
trypsin-EDTA solution were purchased from Welgene, Inc.
3-Isobutyl-1-methylxanthine (IBMX), dexamethasone and insulin were
purchased from Sigma-Aldrich; Merck KGaA.
3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide
(MTT) was obtained from Bio Basic, Inc., and dimethyl sulfoxide
(DMSO) was purchased from Bio Pure.eu GmbH. All primary and
secondary antibodies were purchased from Cell Signaling Technology,
Inc.
Sample preparation
A. okamotoanum was collected from Ulleung-do,
Korea. A voucher specimen was deposited at the Department of Plant
Science and Technology, Chung-ang University, Anseong, Korea
(Voucher no. LEE 2014-04). The dried aerial portion of A.
okamotoanum (995.4 g) was extracted eight times in methanol
(MeOH) using a rotary evaporator. The MeOH extract was suspended in
distilled water and then fractionated and dried successively with
n-hexane, CH2Cl2, EtOAc, and n-BuOH.
The EtOAc fraction (35.0 g) was used in the present study (16).
Cell culture and differentiation
The 3T3-L1 pre-adipocyte cells were obtained from
the American Type Culture Collection. The cells were cultured in
DMEM supplemented with 10% BCS and 1% penicillin-streptomycin at
37°C in humidified air with 5% CO2 in an incubator. To
induce differentiation, 100% confluent 3T3-L1 pre-adipocytes were
stimulated with 0.5 mM IBMX, 1 µM dexamethasone and 5
µg/ml insulin in DMEM containing 10% FBS
[methylisobu-tylxanthine, dexamethasone, insulin (MDI) media] for 2
days. The MDI media was replaced with differentiation media (5
µg/ml insulin in DMEM containing 10% FBS). The cell culture
media was changed 4 times every 2 days.
Cell viability
The 3T3-L1 cells were seeded at a density of
1×105 cells/ml in a 24 well plate and then incubated for
24 h. Afterward, the EtOAc fraction of A. okamotoanum was
added to the test wells at various concentrations (1-500
µg/ml) and then incubated at 37°C for 72 h. The cell
viability was determined using an MTT assay (23). The MTT solution was replaced with
DMEM media (5 mg/ml) in the wells followed by incubation at 37°C
for 4 h. The formazan crystals were dissolved in DMSO and the
absorbance was read at 540 nm using a microplate reader (Thermo
Fisher Scientific, Inc.).
Oil Red O staining
The cells were washed with PBS, fixed with 10%
formalin at 25°C for 10 min and washed with PBS and 60%
isopropanol. Cells were then stained with 0.6% Oil Red O solution
at 25°C for 20 min, washed 4 times with PBS and 60% isopropanol,
and images were captured. For quantitative analysis, Oil Red O
stain was eluted with 100% isopropanol and quantified by measuring
the absor-bance at 500 nm (24).
Western blot analysis
The cells were harvested using a cell scraper and
lysed with radioimmunoprecipitation assay buffer (Elpis Biotech,
Inc.) containing protease inhibitor cocktail at 4°C for 1 h. The
protein concentration was determined using a Bio-Rad protein assay
(Bio-Rad Laboratories, Inc.). Equal amounts of protein (15
µg) were separated with 8-13% SDS-PAGE and transferred onto
a polyvinylidene fluoride membrane. The membrane was blocked with
5% skim milk at room temperature for 1 h followed by incubation
with the following primary antibodies: β-actin (cat. no. 8457;
1:1,000), C/EBP-α (cat. no. 2295; 1:200), C/EBPβ (cat. no. 3087;
1:200), PPAR-γ (cat. no. 2430; 1:200), FAS (cat. no. 3189; 1:200),
FABP4 (cat. no. 2120; 1:200), GLUT4 (cat. no. 2213; 1:200),
phospho-HSL (cat. no. 4126; 1:200), HSL (cat. no. 4107; 1:200),
phospho-AMPK (cat. no. 2535; 1:200), or AMPK (cat. no. 2532; 1:200)
overnight at 4°C. Next, the membranes were incubated with the
secondary antibodies anti-rabbit IgG conjugated to horseradish
peroxidase (HRP) (cat. no. 7074; 1:500) and anti-mouse IgG
conjugated to HRP (cat. no. 7076; 1:500) at room temperature for 1
h, activated with ECL substrate solution (Clarity Western ECL
Substrate kit; Bio-Rad Laboratories, Inc.) and visualized with the
Davinchchemi™ Chemiluminescence Imaging system (Davinch Mini Chemi
Q6; Davinch-K Co. Ltd.). Quantification of western blot band
intensity was performed using ImageJ software (version 1.51p;
National Institutes of Health).
Statistical analyses
Each experiment was performed in triplicate (n=3).
All data are expressed as the mean ± standard deviation. The
results were assessed by one-way analysis of variance followed by
Duncan's multiple range test using IBM SPSS statistics software
(version 20.0, IBM Corporation). P<0.05 was considered to
indicate a statistically significant difference.
Results
Effect of A. okamotoanum on 3T3-L1 cell
viability
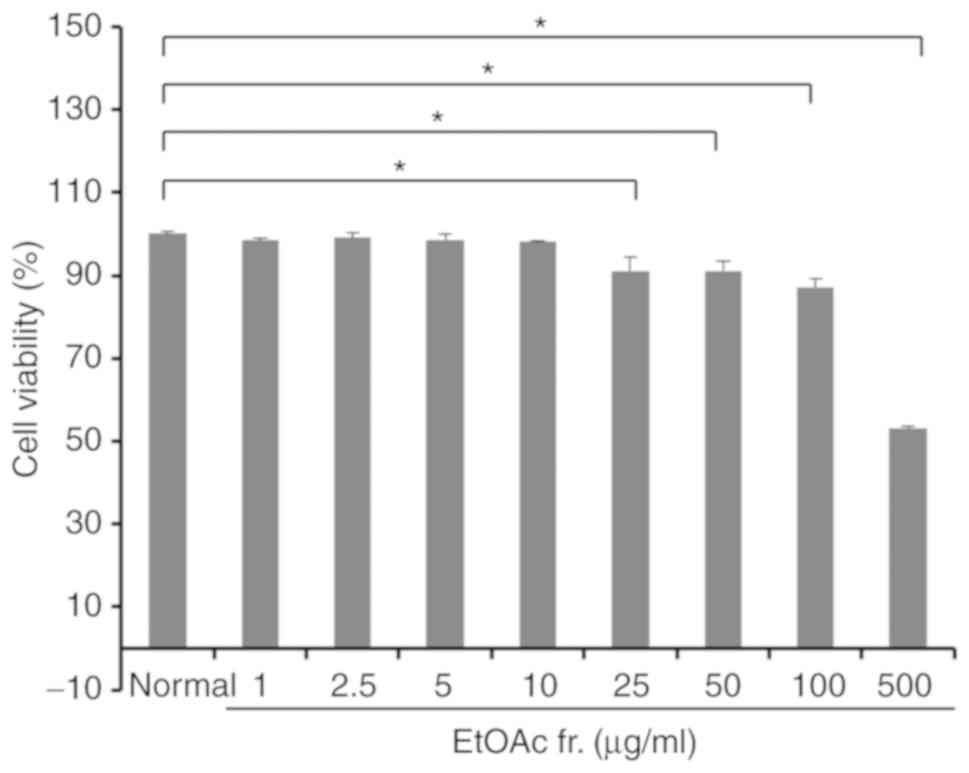
To evaluate the cytotoxicity of the EtOAc fraction
of A. okamotoanum on 3T3-L1 cells, cell viability was
investigated using an MTT assay. The 3T3-L1 adipocytes were treated
with various concentrations (1-500 µg/ml) of the EtOAc
fraction of A. okamotoanum for 72 h. As shown in Fig. 1, the EtOAc fraction of A.
okamotoanum at concentrations up to 10 µg/ml did not
exhibit significant cytotoxicity. However, cell viability was
significantly reduced with 25-500 µg/ml of the EtOAc
fraction of A. okamotoanum compared with the untreated
controls (P<0.05). Therefore, the EtOAc fractions of A.
okamotoanum at concentrations of 1, 2.5, 5 and 10 µg/ml
were used for further experiments.
Effect of A. okamotoanum on lipid
accumulation in 3T3-L1 cells
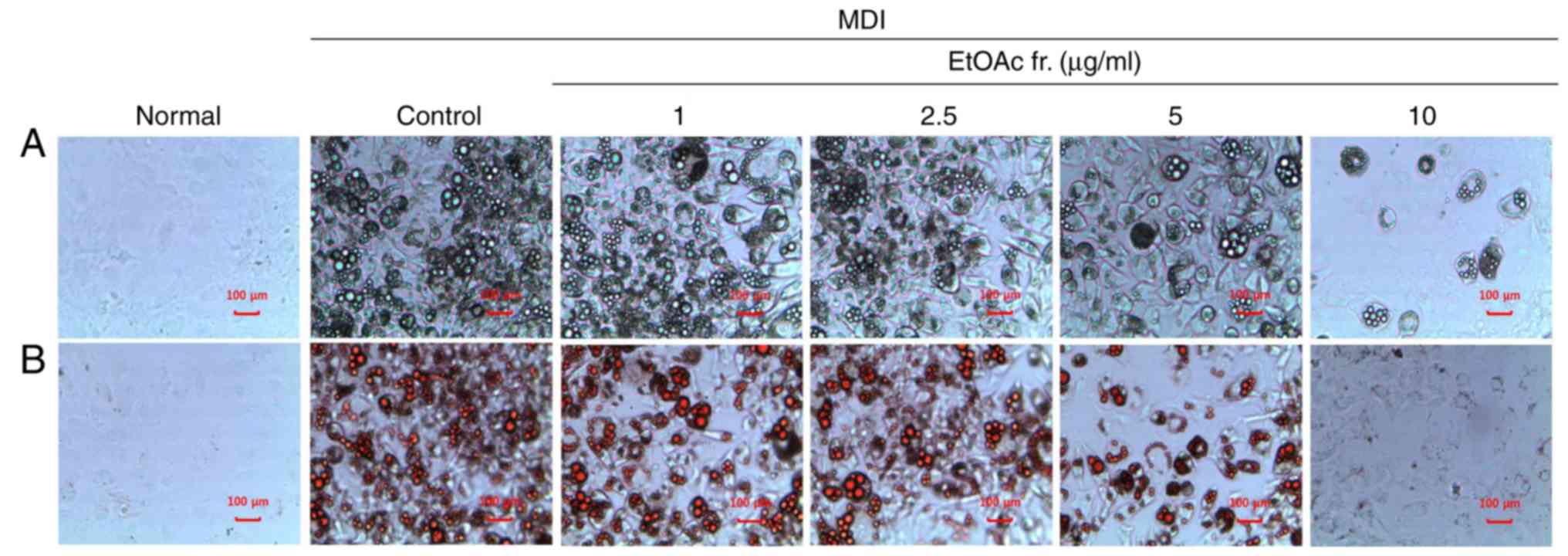
To confirm whether the EtOAc fraction of A.
okamotoanum inhibited adipocyte differentiation, differentiated
3T3-L1 cells were treated with various concentrations (1, 2.5, 5 or
10 µg/ml) of the EtOAc fraction of A. okamotoanum. As
shown in Fig. 2, the number of
lipid droplets increased in MDI-treated differentiated 3T3-L1 cells
when compared with the undifferentiated cells. However, treatment
with the EtOAc fraction of A. okamotoanum decreased lipid
accumulation; this was observed using Oil Red O staining. In
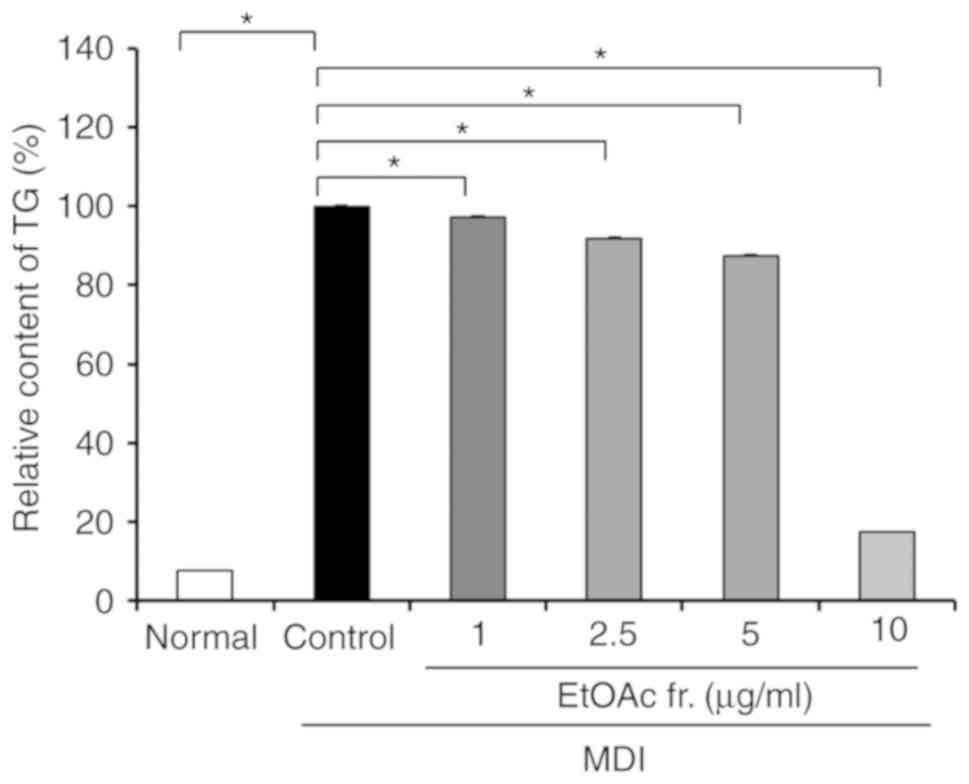
addition, the MDI-treated control had significantly increased
intracellular TG levels of 7.76-100.00% compared with the normal
group (P<0.05; Fig. 3). In
contrast, the treatment with the EtOAc fraction of A.
okamotoanum significantly decreased intracellular TG levels
(P<0.05). The treatment with the EtOAc fraction of A.
okamotoanum at doses of 1, 2.5 and 5 µg/ml slightly
decreased TG levels to 97.16, 91.75, and 87.29%, respectively.
However, treatment with 10 µg/ml of the EtOAc fraction of
A. okamotoanum markedly inhibited TG levels by 17.60% as
compared with the controls.
Effects of A. okamotoanum on the
expression of adipogenic transcription factors in 3T3-L1 cells
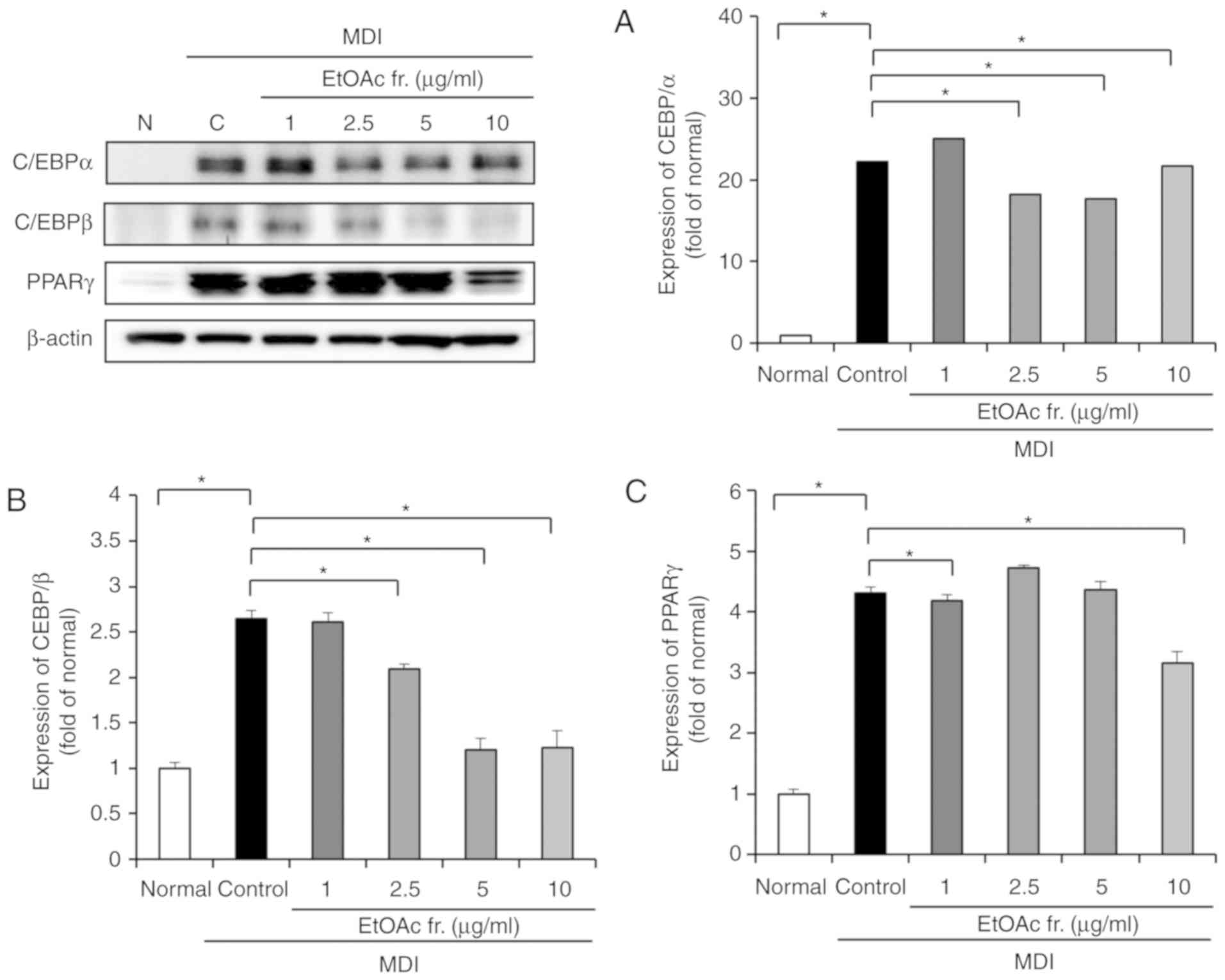
To investigate the anti-adipogenesis effects of the
EtOAc fraction of A. okamotoanum, the protein expression of
adipogenic transcription factors, including C/EBP-α, C/EB-Pβ and
PPAR-γ was measured. As shown in Fig.
4, the protein levels of C/EBP-α, C/EBP-β and PPAR-γ were
significantly upregulated in differentiated 3T3-L1 cells compared
with undifferentiated cells (P<0.05). However, the treatment
with the EtOAc fraction of A. okamotoanum inhibited the
protein expression of C/EBP-α, C/EBP-β and PPAR-γ (P<0.05). Of
the adipogenic transcription factors, the EtOAc fraction of A.
okamotoanum most effectively suppressed C/EBPβ in a
dose-dependent manner.
Effects of A. okamotoanum on
adipogenesis-related factors in 3T3-L1 cells
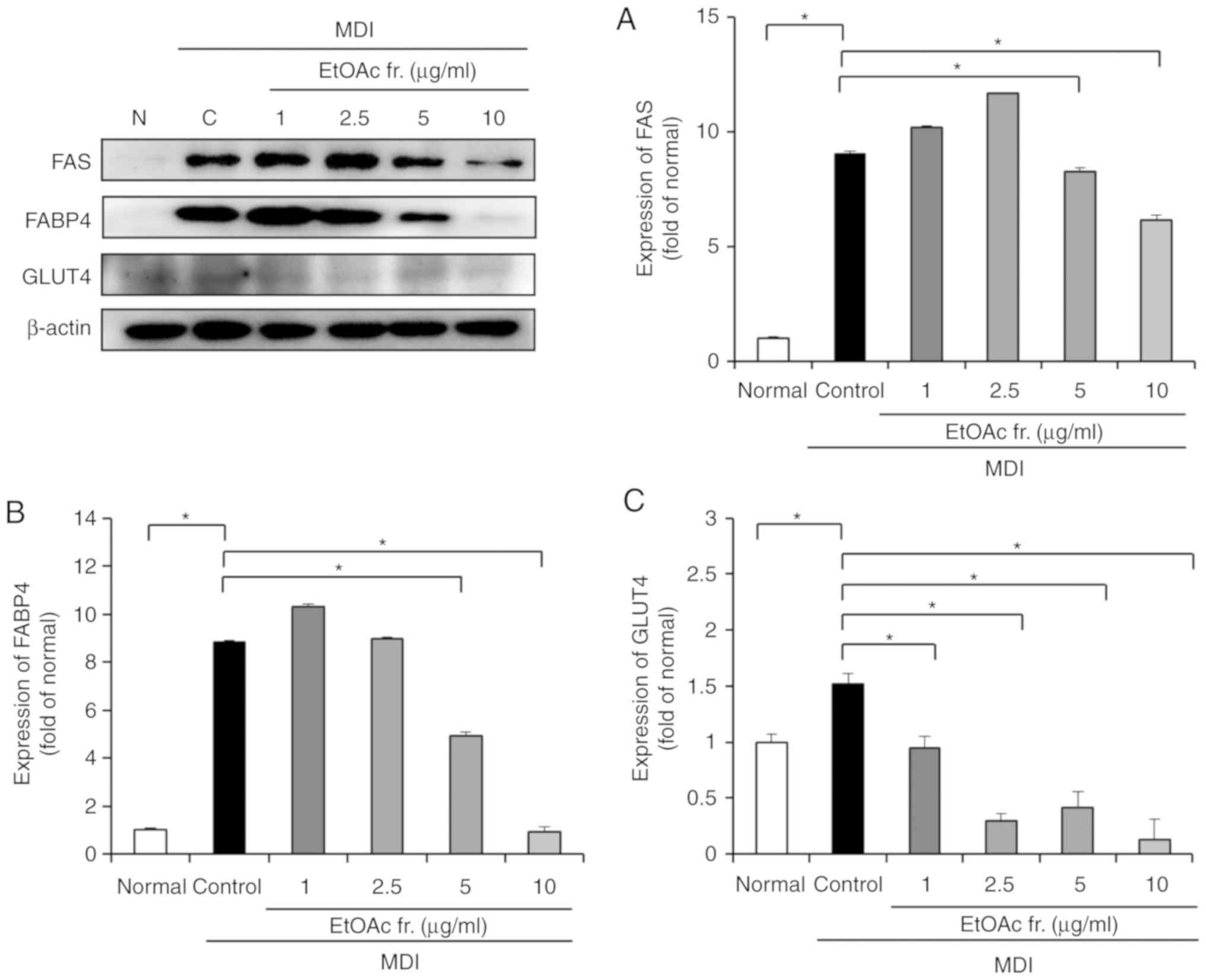
To investigate the effect of the EtOAc fraction of
A. okamotoanum on adipogenesis-related factors, the protein
expression of FAS, FABP4 and GLUT4 was confirmed (Fig. 5). The protein expression of FAS,
FABP4 and GLUT4 were increased in the differentiated 3T3-L1 cells
compared with the undifferentiated control cells (P<0.05).
However, the present results revealed that the treatment with the
EtOAc fraction of A. okamotoanum at concentrations of 5 and
10 µg/ml significantly downregulated FAS and FABP4
(P<0.05). GLUT4 protein expression was also significantly
decreased by the EtOAc fraction of A. okamotoanum compared
with the control (P<0.05).
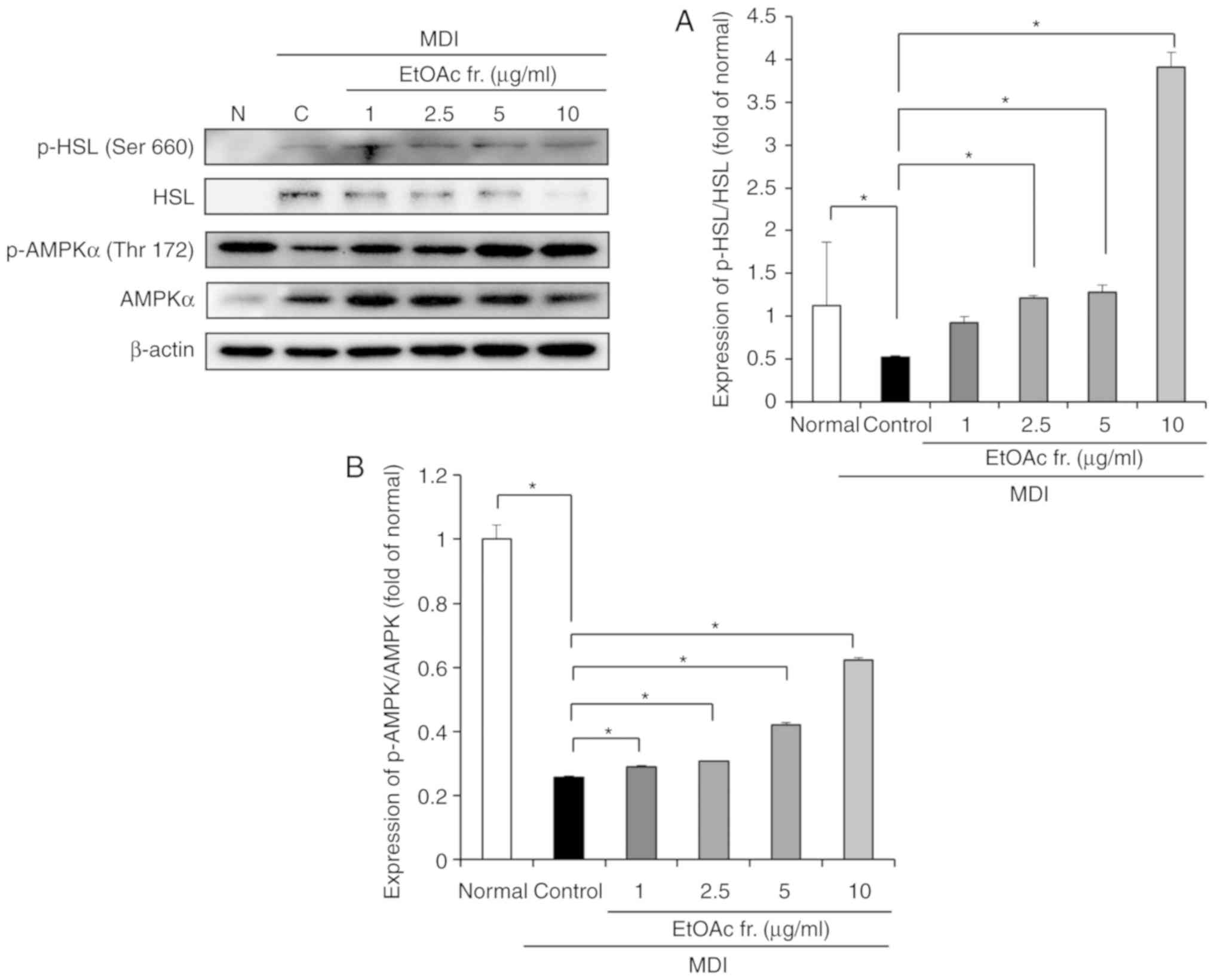
Effects of A. okamotoanum on lipolysis
related factors in 3T3-L1 cells
The effect of the EtOAc fraction of A.
okamotoanum on lipolysis was determined by measuring the
protein expression of HSL and phosphorylated HSL (Fig. 6A). The phosphorylation of HSL was
significantly reduced in the differentiated 3T3-L1 cells compared
with the undifferentiated cells (P<0.05). Treatment with the
EtOAc fraction of A. okamotoanum significantly upregulated
the phosphorylation of HSL (P<0.05).
Effects of A. okamotoanum on activation
of AMPK in 3T3-L1 cells
To examine whether the EtOAc fraction of A.
okamotoanum affected the activity of AMPK, the levels of AMPK
and phosphorylated AMPK were measured (Fig. 6B). The MDI-treated differentiated
3T3-L1 cells suppressed phosphorylation of AMPK compared with
undifferentiated 3T3-L1 cells (P<0.05). However, the EtOAc
fraction of A. okamotoanum significantly increased the
phosphorylation of AMPK compared with the control (P<0.05).
Discussion
In obesity, the accumulation of lipids and the
differentiation of adipocytes in adipose tissue can lead to
abnormal lipid metabolism, which can increase the risk of chronic
diseases (2). Adipose tissue is
dependent on the differentiation of preadipocytes to adipocytes;
therefore, 3T3-L1 preadipocytes have been widely used to study the
differentiation of adipocytes in vitro (25). During adipocyte differentiation,
key adipogenic transcription factors, including C/EBP-β, C/EBP-α
and PPAR-γ, are activated resulting in intracellular fat
accumulation (26). Activation of
adipogenic transcription factors induces target genes that
determine the phenotypes of mature adipocytes (26,27). These target genes are primarily
associated with lipogenesis, TG hydrolysis and glucose and fatty
acid metabolism (26,27). Differentiated 3T3-L1 cells treated
with MDI mimics the development of obesity in humans and also
possesses adipocyte structures similar to live adipose tissue
(25,28). In addition, several natural
extracts and bioactive compounds have been commonly used to produce
anti-obesity effects in a differentiated 3T3-L1 cell model
(12,13). The EtOAc fraction of A.
okamotoanum showed the highest protective effect from free
radicals and oxidative stress among other fractions and extracts
(20). In addition, the EtOAc
fraction from A. okamotoanum exhibited anti-oxidative
stress, neuroprotective effects and cognitive improvement activity
(20,21). Oxidative stress is closely
associated with obesity and antioxidant supplements are beneficial
in the management of obesity (29). In addition, the authors previously
reported determination of active compounds such as quercitrin,
isoquercitrin and afzelin from EtOAc fraction of A.
okamotoanum, which had antioxidant activity (16). Therefore, the anti-differentiation
effect of the EtOAc fraction of A. okamotoanum and its
molecular mechanisms were studied by measuring the expression of
adipogenesis and lipolysis-related factors in 3T3-L1 cells.
The present results showed that lipid droplets were
markedly increased in differentiated 3T3-L1 cells after stimulation
with MDI. However, the EtOAc fraction of A. okamotoanum
decreased the lipid droplets in differentiated 3T3-L1 cells.
Cytotoxicity of the EtOAc fraction of A. okamotoanum was
observed. Treatment with the EtOAc fraction of A. okamo-
toanum at concentrations up to 10 µg/ml did not show
significant cytotoxicity compared with untreated control cells;
therefore, concentrations of 1, 2.5, 5 and 10 µg/ml of the
EtOAc fraction of A. okamotoanum were used. TG levels were
measured quantitatively. Treatment with the EtOAc fraction of A.
okamotoanum at concentrations of 1, 2.5, 5 and 10 µg/ml
decreased TGs compared with the control. The inhibitory effect of
TG accumulation was dramatically elevated at the concentration of
10 µg/ml and it was relatively weak at the concentrations of
1-5 µg/ml. Therefore, it is suggested that A.
okamotoanum effectively inhibits lipid formation.
Adipogenesis is the differentiation process of
adipocytes from preadipocytes and it is characterized by
intracellular lipid accumulation. During adipocyte differentiation,
key adipogenic transcription factors, such as C/EBP-α, C/EBP-β and
PPAR-γ, are expressed. The expression of C/EBP-β activates C/EBP-α,
PPAR-γ and other adipogenic genes. The over-expression of C/EBP-α
and PPAR-γ is known to directly affect the development of fat cells
(8). In the present study,
differentiated 3T3-L1 cells treated with MDI showed an upregulation
of key transcription genes related to adipo-genesis, including
C/EBP-α, C/EBP-β and PPAR-γ. However, differentiated 3T3-L1 cells
treated with the EtOAc fraction of A. okamotoanum showed
downregulation of key transcription genes. The regulatory effect on
adipogenesis factors of the EtOAc fraction of A. okamotoanum
was not dose-dependent except with C/EBP-β. The activation of
C/EBP-α and PPAR-γ are associated with lipid metabolism factors
such as FAS, FABP4, and GLUT4. FAS is highly expressed in adipose
tissue; therefore, it plays an important role in lipogenesis
(30). FAS catalyzes the
synthesis of fatty acids and the cytoplasmic storage of TG
(30). FABP4, a terminal
adipocyte differentiation marker gene, is directly associated with
lipogenesis; it induces the accumulation of lipid droplets in the
cytoplasm of differentiated 3T3-L1 cells (31). GLUT4 plays a role in lipogenesis
through the insulin signaling pathway in differentiated 3T3-L1
adipocytes (32). GLUT4 is
involved in the insulin-stimulated glucose uptake by adipose tissue
and skeletal muscle, increasing the risk of developing obesity and
diabetes (33). The results of
the present study indicate that the EtOAc fraction of A.
okamotoanum inhibited adipogenesis in adipocytes through the
downregulation of FABP4, FAS and GLUT4 in differentiated 3T3-L1
cells.
Lipolysis is the catabolic process of releasing fat
from adipose tissue. Lipolysis is the chemical decomposition of TG
into glycerol and fatty acids, which blocks lipid accumulation
(34). In addition, lipolysis
controls lipid homeostasis in adipocytes (34). Therefore, the reduction of lipid
accumulation and the increase of lipid catabolism in adipocytes are
crucial to the development of anti-obesity agents. During the
lipolysis of adipocytes, HSL is an important lipolytic factor. HSL
hydrolyzes diglycerides and free fatty acids in TG hydrolysis. HSL
is activated via phosphorylation by cAMP-dependent protein kinase A
at Ser 660, which stimulates HSL to hydrolyze TGs (35). HSL is a rate-limiting enzyme for
lipid mobilization reactions and hydrolyzes diglycerides in the
mobilization of TG stored in adipocytes (36). In addition, elevated HSL
expression can catalyze adipose lipolysis in response to
β-adrenergic stimulation (37).
In this study, treatment with the EtOAc fraction of A.
okamotoanum increased lipolysis by upregulation of HSL
activation in differentiated 3T3-L1 cells. In particular, the
lipolysis factor ratio of phosphorylated-HSL/HSL was upregu-lated
dose-dependently compared with the control. Therefore, it is
suggested that the EtOAc fraction of A. okamotoanum could
play a potential role in the lipid catabolic process.
The present study also investigated AMPK protein
expression in the presence/absence of the EtOAc fraction of A.
okamotoanum in differentiated 3T3-L1 cells. AMPK is a metabolic
gene that is involved in the regulation of lipid metabolism
(38). The activation of AMPK in
adipose tissue suppresses lipid synthesis and lipogenesis,
regulates fatty acid synthesis, and enhances fatty acid oxidation
and glucose transport (38,39). Furthermore, AMPK activity enhances
lipolysis by phosphorylation of HSL (39,40). To develop anti-obesity agents,
numerous researchers have investigated the AMPK activity of natural
extracts and their bioactive compounds in adipocytes (41,42). Therefore, AMPK activity is
important to the development of an obesity treatment strategy. In
the present study, it was demonstrated that the EtOAc fraction of
A. okamotoanum induced the activation of AMPK signaling in
differentiated 3T3-L1 cells. This suggests that the EtOAc fraction
of A. okamotoanum inhibited adipogenesis and upregulated
lipolysis by the regulation of the AMPK signaling pathway. In the
present study, the EtOAc fraction of A. okamotoanum
inhibited TG accumulation during the differentiation of the 3T3-L1
cells. In addition, A. okamotoanum suppressed adipogenic
transcription factors and adipogenesis-related protein expression.
Furthermore, A. okamotoanum enhanced the expression of
lipolytic proteins, such as HSL and also activated AMPK signaling.
Conjugated linoleic acid (CLA) is widely used to treat obesity and
it has been reported that CLA is very effective at decreasing body
fat accumulation (43). Previous
studies demonstrated that CLA inhibited adipocyte differentiation
in 3T3-L1 cells by regulation of adipogenesis, lipolysis and AMPK
signaling (43-45). Treatment with 10 µg/ml
EtOAc fraction of A. okamotoanum inhibited intracellular TGs
more effectively than treatment with 3T3-L1 cells with 100
µM CLA (43). In addition,
A. okamotoanum at concentrations of 10 µg/ml
decreased the protein expression of PPAR-γ, which was similar to
that in 100 µM CLA-treated 3T3-L1 cells (43). Based on these findings, the
present study suggested that A. okamotoanum has regulatory
activity on adipocyte differentiation and its effect is similar to
that of CLA.
Acer (maple) is commonly used in commercial
products such as maple syrup and seed oil (46). In addition, several Acer
species have been used in traditional medicine for detoxification
and to treat rheumatism, eye disease, hepatic diseases, and
hemostasis (46). Recently,
numerous studies have reported the pharmacological activities of
Acer species, including antioxidant, anti-tumor,
anti-inflammation, antibacterial, antihyperglycemic,
hepatoprotective and anti-obesity activities (46). The EtOAc fraction of A.
truncatum Bunge significantly reduced body weight by the
inhibition of lipogenesis-related factors such as FAS in
vivo (47,48). In addition, Kim et al
(22) demonstrated that A.
okamotoanum regulated adipocyte differentiation by several
molecular mechanisms related to adipogenesis, including PI3K/Akt
and β-catenin/glycogen synthase kinase (GSK)3β. A previous study
demonstrated that the MeOH extract of leaves from A.
okamotoanum inhibited the phosphorylation of mammalian target
of rapamycin and P70S6K by attenuating the PI3K/Akt pathway,
thereby suppressing key adipogenic transcription genes (22). In addition, A. okamotoanum
induced the activation of β-catenin/GSK3β, promoting the
downregulation of PPAR-γ (22).
Therefore, A. okamotoanum inhibits adipogenesis via
regulation of PI3K/Akt and β-catenin/GSK3β signaling. The authors
previously isolated and identified flavonoid glyco-sylates such as
isoquercitrin (quercetin-3-β-D-glucoside; IQ), quercitrin
(quercetin-3-β-D-rhamnoside; QU), and afzelin
(keampferol-3-rhamnoside; AF) from the EtOAc fraction of A.
okamotoanum (16). IQ
inhibited adipocyte differentiation and lipogenesis by the
downregulation of adipogenic transcription factors in 3T3-L1
adipocyte cells (49). In
addition, IQ exhibited anti-obesity effects by reducing body weight
and regulating lipid metabolism in high fat diet-fed mice (49). Furthermore, aglycones of IQ, QU
and AF, such as quercetin and kaempferol, showed anti-obesity
activity through the regulation of adipogenesis, inflammation, and
oxidative stress (50-52). Therefore, flavonoid glycosylates
are primarily responsible for the anti-obesity effects of the EtOAc
fraction of A. okamotoanum.
In conclusion, the present study suggests that A.
okamotoanum inhibits adipocyte differentiation via
adipo-genesis and lipolysis in cells. A. okamotoanum could
be a promising therapeutic agent against obesity, although further
in vivo studies must be done.
Funding
The present study was supported by Basic Science
Research Program through the National Research Foundation of Korea
(NRF) funded by the Ministry of Education (grant no.
2015R1D1A1A01058868), Republic of Korea. This study was
additionally supported by the Global PH.D Fellowship Program
through the NRF funded by the Ministry of Education (grant no.
2016_H1A2A1906940).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
EJC planned and conceptualized the study. SL and HYK
designed the study. SL was involved in the preparation of samples.
JHK performed experiments and wrote the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
World Health Organization: Media Centre
(2017) Obesity and overweight: Key facts. http://www.who.int/mediacentre/fact-sheets/fs311/en/.
Accessed February 16, 2018.
|
|
2
|
Kopelman PG: Obesity as a medical problem.
Nature. 404:635–643. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jo J, Gavrilova O, Pack S, Jou W, Mullen
S, Sumner AE, Cushman SW and Periwal V: Hypertrophy and/or
hyperplasia: Dynamics of adipose tissue growth. PLoS Comput Biol.
5:e10003242009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Langin D, Dicker A, Tavernier G, Hoffstedt
J, Mairal A, Rydén M, Arner E, Sicard A, Jenkins CM, Viguerie N, et
al: Adipocyte lipases and defect of lipolysis in human obesity.
Diabetes. 54:3190–3197. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Muoio DM, Seefeld K, Witters LA and
Coleman RA: AMP-activated kinase reciprocally regulates
triacylglycerol synthesis and fatty acid oxidation in liver and
muscle: Evidence that sn-glycerol-3-phosphate acyltransferase is a
novel target. Biochem J. 338:783–791. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cowherd RM, Lyle RE and McGehee RE Jr:
Molecular regulation of adipocyte differentiation. Semin Cell Dev
Biol. 10:3–10. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lane MD, Lin FT, MacDougald OA and
Vasseur-Cognet M: Control of adipocyte differentiation by
CCAAT/enhancer binding protein alpha (C/EBP alpha). Int J Obes
Relat Metab Disord. 20(Suppl 3): S91–S96. 1996.PubMed/NCBI
|
|
8
|
Farmer SR: Regulation of PPARgamma
activity during adipo-genesis. Int J Obes (Lond). 29(Suppl 1):
S13–S16. 2005. View Article : Google Scholar
|
|
9
|
Shi Y and Burn P: Lipid metabolic enzymes:
Emerging drug targets for the treatment of obesity. Nat Rev Drug
Discov. 3:695–710. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zechner R, Kienesberger PC, Haemmerle G,
Zimmermann R and Lass A: Adipose triglyceride lipase and the
lipolytic catabolism of cellular fat stores. J Lipid Res. 50:3–21.
2009. View Article : Google Scholar
|
|
11
|
Gao Y, Zhou Y, Xu A and Wu D: Effects of
an AMP-activated protein kinase inhibitor, compound C, on
adipogenic differentiation of 3T3-L1 cells. Biol Pharm Bull.
31:1716–1722. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee HM, Yang G, Ahn TG, Kim MD, Nugroho A,
Park HJ, Lee KT, Park W and An HJ: Antiadipogenic effects of aster
glehni extract: In vivo and in vitro effects. Evid Based Complement
Alternat Med. 2013:8596242013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gupta A, Kumar A, Kumar D, Nandan S,
Shankar K, Varshney S, Rajan S, Srivastava A, Gupta S, Kanojiya S,
et al: Ethyl acetate fraction of eclipta alba: A potential
phytopharmaceutical targeting adipocyte differentiation. Biomed
Pharmacother. 96:572–583. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Feng Z, Hai-ning Y, Xiao-man C, Zun-chen
W, Sheng-rong S and Das UN: Effect of yellow capsicum extract on
proliferation and differentiation of 3T3-L1 preadipocytes.
Nutrition. 30:319–325. 2014. View Article : Google Scholar
|
|
15
|
Jin W, Thuong PT, Su ND, Min BS, Son KH,
Chang HW, Kim HP, Kang SS, Sok DE and Bae K: Antioxidant activity
of cleomiscosins A and C isolated from Acer okamotoanum. Arch Pharm
Res. 30:275–281. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee J, Lee DG, Rodriguez JP, Park JY, Cho
EJ, Jacinto SD and Lee S: Determination of flavonoids in Acer
okamotoanum and their aldose reductase inhibitory activities.
Hortic Environ Biotechnol. 59:131–137. 2018. View Article : Google Scholar
|
|
17
|
Yoo YM, Jung EM, Kang HY, Choi IG, Choi KC
and Jeung EB: The sap of Acer okamotoanum decreases serum alcohol
levels after acute ethanol ingestion in rats. Int J Mol Med.
28:489–495. 2011.PubMed/NCBI
|
|
18
|
An BS, Kang JH, Yang H, Yang MP and Jeung
EB: Effects of Acer okamotoanum sap on the function of
polymorphonuclear neutrophilic leukocytes in vitro and in vivo. Mol
Med Rep. 7:654–658. 2013. View Article : Google Scholar
|
|
19
|
Yang H, Hwang I, Koo TH, Ahn HJ, Kim S,
Park MJ, Choi WS, Kang HY, Choi IG, Choi KC and Jeung EB:
Beneficial effects of Acer okamotoanum sap on L-NAME-induced
hypertension-like symptoms in a rat model. Mol Med Rep. 5:427–431.
2012.
|
|
20
|
Choi SY, Kim JH, Lee J, Lee S and Cho EJ:
Protective effect of Acer okamotoanum from oxidative stress in C6
glial cells. J Appl Biol Chem. 60:141–147. 2017. View Article : Google Scholar
|
|
21
|
Choi SY, Lee J, Lee DG, Lee S and Cho EJ:
Acer okamotoanum improves cognition and memory function in
Aβ25-35-induced Alzheimer's mice model. Appl Biol Chem. 60:1–9.
2017. View Article : Google Scholar
|
|
22
|
Kim EJ, Kang MJ, Seo YB, Nam SW and Kim
GD: Acer okamotoanum nakai leaf extract inhibits adipogenesis via
suppressing expression of PPARγ and C/EBPα in 3T3-L1 cells. J
Microbiol Biotechnol. 28:1645–1653. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mosmann T: Rapid colorimetric assay for
cellular growth and survival: Application to proliferation and
cytotoxicity assays. J Immunol Methods. 65:55–63. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ramírez-Zacarías JL, Castro-Muñozledo F
and Kuri-Harcuch W: Quantitation of adipose conversion and
triglycerides by staining intracytoplasmic lipids with oil red O.
Histochemistry. 97:493–497. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zebisch K, Voigt V, Wabitsch M and
Brandsch M: Protocol for effective differentiation of 3T3-L1 cells
to adipocytes. Anal Biochem. 425:88–90. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tang QQ, Otto TC and Lane MD:
CCAAT/enhancer-binding protein beta is required for mitotic clonal
expansion during adipogenesis. Proc Natl Acad Sci USA. 100:850–855.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Moseti D, Regassa A and Kim WK: Molecular
regulation of adipogenesis and potential anti-adipogenic bioactive
molecules. Int J Mol Sci. 17:E1242016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ntambi JM and Young-Cheul K: Adipocyte
differentiation and gene expression. J Nutr. 130:3122S–3126S. 2000.
View Article : Google Scholar
|
|
29
|
Abdali D, Samson SE and Grover AK: How
effective are antioxidant supplements in obesity and diabetes? Med
Princ Pract. 24:201–215. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schmid B, Rippmann JF, Tadayyon M and
Hamilton BS: Inhibition of fatty acid synthase prevents
preadipocyte differentiation. Biochem Biophys Res Commun.
328:1073–1082. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gregoire FM, Smas CM and Sul HS:
Understanding adipocyte differentiation. Physiol Rev. 78:783–809.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ma X, Zhang H, Yuan L, Jing H, Thacker P
and Li D: CREBL2, interacting with CREB, induces adipogenesis in
3T3-L1 adipo-cytes. Biochem J. 439:27–38. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hernandez R, Teruel T and Lorenzo M:
Insulin and dexamethasone induce GLUT4 gene expression in foetal
brown adipocytes: Synergistic effect through CCAAT/enhancer-binding
protein alpha. Biochem J. 372:617–624. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shen WJ, Patel S, Miyoshi H, Greenberg AS
and Kraemer FB: Functional interaction of hormone sensitive lipase
and perilipin in lipolysis. J Lipid Res. 50:2306–2313. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sztalryd C and Kraemer FB: Regulation of
hormone-sensitive lipase in streptozotocin-induced diabetic rats.
Metabolism. 44:1391–1396. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zimmermann R, Strauss JG, Haemmerle G,
Schoiswohl G, Birner-Gruenberger R, Riederer M, Lass A, Neuberger
G, Eisenhaber F, Hermetter A and Zechner R: Fat mobilization in
adipose tissue is promoted by adipose triglyceride lipase. Science.
306:1383–1386. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yang X, Zhang X, Heckmann BL, Lu X and Liu
J: Relative contribution of adipose triglyceride lipase and
hormone-sensitive lipase to tumor necrosis factor-α (TNF-α)-induced
lipolysis in adipocytes. J Biol Chem. 286:40477–40485. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lim CT, Kola B and Korbonits M: AMPK as a
mediator of hormonal signalling. J Mol Endocrinol. 44:87–97. 2010.
View Article : Google Scholar
|
|
39
|
Daval M, Foufelle F and Ferré P: Functions
of AMP-activated protein kinase in adipose tissue. J Physiol.
574:55–62. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gaidhu MP, Fediuc S, Anthony NM, So M,
Mirpourian M, Perry RL and Ceddia RB: Prolonged AICAR-induced
AMP-kinase activation promotes energy dissipation in white
adipocytes: Novel mechanisms integrating HSL and ATGL. J Lipid Res.
50:704–715. 2009. View Article : Google Scholar :
|
|
41
|
Chen S, Li Z, Li W, Shan Z and Zhu W:
Resveratrol inhibits cell differentiation in 3T3-L1 adipocytes via
activation of AMPK. Can J Physiol Pharmacol. 89:793–799.
2011.PubMed/NCBI
|
|
42
|
Kang SW, Kang SI, Shin HS, Yoon SA, Kim
JH, Ko HC and Kim SJ: Sasa quelpaertensis nakai extract and its
constituent p-coumaric acid inhibit adipogenesis in 3T3-L1 cells
through activation of the AMPK pathway. Food Chem Toxicol.
59:380–385. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kang K, Liu W, Albright KJ, Park Y and
Pariza MW: Trans-10, cis-12 CLA inhibits differentiation of 3T3-L1
adipocytes and decreases PPAR gamma expression. Biochem Biophys Res
Commun. 303:795–779. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Moon HS, Lee HG, Seo JH, Chung CS, Kim TG,
Kim IY, Lim KW, Seo SJ, Choi YJ and Cho CS: Downregulation of
PPARgamma2-induced adipogenesis by PEGylated conjugated linoleic
acid as the pro-drug: Attenuation of lipid accumulation and
reduction of apoptosis. Arch Biochem Biophys. 456:19–29. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Jiang S, Chen H, Wang Z, Riethoven JJ, Xia
Y, Miner J and Fromm M: Activated AMPK and prostaglandins are
involved in the response to conjugated linoleic acid and are
sufficient to cause lipid reductions in adipocytes. J Nutr Biochem.
22:656–664. 2011. View Article : Google Scholar
|
|
46
|
Bi W, Gao Y, Shen J, He C, Liu H, Peng Y,
Zhang C and Xiao P: Traditional uses, phytochemistry, and
pharmacology of the genus Acer (maple): A review. J Ethnopharmacol.
189:31–60. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhao WH, Gao LF, Gao W, Yuan YS, Gao CC,
Cao LG, Hu ZZ, Guo JQ and Zhang YX: Weight-reducing effect of Acer
truncatum bunge may be related to the inhibition of fatty acid
synthase. Nat Prod Res. 25:422–431. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Gao L, Cao L, Tian M and Chen Z: Study on
the weight-reducing effect of Acer truncatum leave extract in
alimentary obesity rat. Wei Sheng Yan Jiu. 41:609–611. 2012.In
Chinese.
|
|
49
|
Lee CW, Seo JY, Lee J, Choi JW, Cho S, Bae
JY, Sohng JK, Kim SO, Kim J and Park YI: 3-O-Glucosylation of
quercetin enhances inhibitory effects on the adipocyte
differentiation and lipogenesis. Biomed Pharmacother. 95:589–598.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Nabavi SF, Russo GL, Daglia M and Nabavi
SM: Role of quer-cetin as an alternative for obesity treatment: You
are what you eat! Food Chem. 179:305–310. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chen S, Jiang H, Wu X and Fang J:
Therapeutic effects of quer-cetin on inflammation, obesity, and
type 2 diabetes. Mediators Inflamm. 2016:93406372016. View Article : Google Scholar
|
|
52
|
Lee YJ, Choi HS, Seo MJ, Jeon HJ, Kim KJ
and Lee BY: Kaempferol suppresses lipid accumulation by inhibiting
early adipogenesis in 3T3-L1 cells and zebrafish. Food Funct.
6:2824–2833. 2015. View Article : Google Scholar : PubMed/NCBI
|