Introduction
Preeclampsia (PE) is a hypertensive condition that
occurs during pregnancy. It is the major cause of maternal and
perinatal death, premature birth and fetal growth restriction; the
pathogenesis is complex and effective treatments are currently
lacking (1,2). PE is a chronic disease, which
frequently leads to multiple organ failure in the body of the
pregnant woman, increases the production of reactive oxygen
species, causes maternal inflammation and accelerates apoptosis,
leading to an imbalance between the formation of normal
placentation and pro-angiogenic factors (3). Previous studies have demonstrated
that compared with non-pregnant females, those with normal
pregnancy and patients with PE exhibit an inflammatory response,
and that patients with PE exhibit excessive activation of the
inflammatory response compared with normal pregnant subjects,
including increased levels of inflammatory cytokines and products
of oxidative stress (4,5). These results suggest that the
inflammatory response has a crucial role in the pathogenesis of
PE.
PE may lead to the dysfunction of vascular
endothelial cells and increase endothelin secretion (6). Under normal conditions, the
expression levels of intercellular adhesion molecule-1 (ICAM-1),
vascular cell adhesion molecule-1 (VCAM-1) and leukocyte
function-associated antigen (LFA-1) are low, allowing neutrophils
to roll along the endothelium without adhesion (7). However, the content of the
pro-inflammatory cytokines tumor necrosis factor-α (TNF-α),
interferon-γ (IFN-γ), interleukin (IL)-1 and monocyte
chemoattractant protein (MCP)-1 is increased in the blood of
patients with PE, which activates the molecular adhesion ligands on
the vascular endothelium and leukocytes, thus significantly
upregulating the levels of ICAM-1 and LFA-1, which interact with
each other. Endothelial cells firmly bind to neutrophils to induce
endothelial damage (7,8). In addition, vascular endothelial
growth factor, placental growth factor and soluble Fms-like
tyrosine kinase-1 (sFLT-1) lead to the general activation of the
maternal inflammatory system, dysfunction of widespread endothelial
cells and limitation of placental vascularization (9,10).
Several studies have indicated that the secretion levels of
multiple IL-1 family members in the maternal circulatory system and
placental chorionic trophoblast cells are significantly increased
(11,12), which serves an important role in
promoting the development of PE.
Previous studies have described the effects of two
steroid hormones, estrogen and progesterone, on vascular
endothelial and smooth muscle cells (13). As a vascular endothelial
protective factor maintains vascular tension and blood pressure
stability, the decrease in estradiol (E2) levels may be induce
cardiovascular disease (14). It
has been indicated that the levels of E2, propylene glycol and
progesterone are abnormal in PE (15), but the details of these changes
and specific effects remain elusive.
Although it has been reported that the expression of
E2 is low in patients with PE, the exact mechanism remains unclear
(16). The aim of the present
study was to investigate whether treatment with exogenous E2 may
improve inflammatory and endothelial dysfunction in a rat model of
PE.
Materials and methods
Animals
A total of 48 adult Sprague-Dawley rats weighing
210-250 g (age, 7 weeks) were purchased from the Department of
Experimental Zoology of Kunming Medical University (Kunming,
China). All rats had ad libitum access to food and water and
were maintained in a stable environment at 21±3°C, 62±3% humidity
and a 12-h light/dark cycle. The female rats were caged for 24 h
with a male rat, and mating was confirmed by the presence of a
vaginal plug and spermatozoa in the vaginal smear. The day on which
insemination was detected was designated as day 1 of pregnancy. The
protocols of the animal experiments followed the NIH Guide for the
Care and Use of Laboratory Animals and the study was approved by
the Experimental Animal Ethics Committee of Kunming Medical
University and the Commission for Animal Experimentation of The
People's Hospital of the Xishuangbanna Dai Nationality Autonomous
Prefecture.
Treatment and experimental grouping
The rats were randomly divided into four groups on
day 7 as follows: i) Normal pregnant rats receiving daily
intraperitoneal (i.p.) injections of equal volume of 0.9% normal
saline (NS) (Control group, n=12); ii) pregnant rats receiving
daily i.p. injections of 50 mg/kg L-NAME (L-NAME group, n=12); iii)
pregnant rats receiving daily i.p. injections of 50 mg/kg L-NAME
and NS from day 11 (L-NAME + NS group, n=12); iv) pregnant rats
receiving daily i.p. injections of 50 mg/kg L-NAME plus 100
µg/kg/day E2 from day 11 (L-NAME + E2 group, n=12).
On days 13 and 21 of gestation, 24-h urine of rats
was collected using metabolic cages (Yuyan Instruments) for the
determination of urinary protein. The level of urine protein was
measured using a Rat Urinary Protein kit (Sigma-Aldrich; Merck
KGaA).
Blood pressure (BP) measurement
On days 13 and 21, rat BP was measured using the
non-invasive tail cuff method after rats had been pre-warmed in a
heating chamber at 37°C for 15 min. The BP measurement was repeated
five times, and the mean value for each rat was obtained.
Determination of fetal weight and rate of
stillbirths
On day 21 of pregnancy, rats were anesthetized with
2% sodium pentobarbital at the dose of 50 mg/kg and placed in the
supine position for delivery by cesarean section. The following
parameters were determined: Litter size, number of stillbirths and
surviving neonates. Subsequently, fetal weight was determined by
dividing the total weight of all pups and placentas in each litter
by the number of pups in that litter. Under terminal anesthesia,
blood samples were collected from the maternal rat by intracardiac
puncture for ELISA and placenta tissue was harvested for
histopathologic analysis; placental homogenate was prepared for
mRNA and protein detection. At the end of the experiment, the
surviving pups were euthanized with 2% sodium pentobarbital.
H&E staining
For the histopathologic analysis of the placenta,
tissue samples were fixed in 10% phosphate-buffered formalin for 48
h at room temperature, embedded in paraffin, cut into 5-mm sections
and placed on slides. Subsequently, the sections were stained with
0.5% H&E solution and examined under a light microscope
(Olympus Corporation) under ×200 magnification. Five visual fields
of the placenta tissue on each section were selected randomly.
Cytokine determination using ELISA
Cytokine levels of IL-1β, IL-6, IFN-γ, MCP-1 and
sFlt-1 in the serum of each maternal rat and the activity levels of
nitric oxide (NO) and inducible NO synthase (iNOS) in the
homogenate of placenta tissue were determined by ELISA with rat
ELISA kits IL-1β (ml003057), IL-6 (ml102828), IFN-γ (ml064291),
MCP-1 (ml002960), sFlt-1 (ml059017), NO (ml059000) and iNOS
(ml003127) purchased from Shanghai Enzyme-linked Biotechnology Co.,
Ltd. according to the manufacturer's protocols. The absorbance in
each group was detected using a microplate reader at 450 nm.
Western blot analysis
Total proteins were extracted from the placenta
tissue homogenates using RIPA lysis buffer (Sigma-Aldrich; Merck
KGaA), and a bicinchoninic acid protein assay kit (Pierce; Thermo
Fisher Scientific, Inc.) was used to determine the protein
concentration. Proteins (45 µg/lane) were separated by 8-10%
SDS-PAGE and transferred onto polyvinylidene fluoride membranes
(Beyotime Institute of Biotechnology). The membranes were blocked
in 5% skimmed milk at room temperature for 1 h prior to incubation
with the primary antibodies against IL-1β (cat. no. ab2105;
1:1,000; Abcam), IL-6 (cat. no. 12153; 1:1,000; Cell Signaling
Technology, Inc.), IFN-γ (cat. no. 3159; 1:1,000; Cell Signaling
Technology, Inc.), MCP-1 (cat. no. ab25124; 1:2,000; Abcam), CD49D
(cat. no. ab75760; 1:1,000; Abcam), ICAM1 (cat. no. ab171123;
1:1,000; Abcam), LFA-1 (cat. no. ab52895; 1:2,000; Abcam), sFlt-1
(cat. no. ab9540; 1:1,000; Abcam), VCAM1 (cat. no. ab174279;
1:1,000; Abcam), MMP2 (cat. no. ab92536; 1:2,000; Abcam), MMP9
(cat. no. ab137867; 1:1,000; Abcam), ERα (cat. no. ab16460;
1:1,000; Abcam), ERβ (cat. no. ab3576; 1:1,000; Abcam), GPER (cat.
no. ab39742; 1:1,000; Abcam), TLR4 (cat. no. ab217274; 1:500;
Abcam), myeloid differentiation primary response 88 (MyD88; cat.
no. ab2064; 1:500; Abcam), phospho (p)-IL-1 receptor-associated
kinase 4 (IRAK4; cat. no; 11927, 1:1,000; Cell Signaling
Technology, Inc.), IRAK4 (cat. no. 4363; 1:1,000; Cell Signaling
Technology, Inc.), TNF receptor-associated factor 6 (TRAF6; cat.
no. ab33915; 1:2,000; Abcam) and ERα36 (cat. no. CY1109; 1:1,000;
Cell Applications, Inc.) at 4°C overnight. Subsequently, the
membranes were incubated with horseradish peroxidase-conjugated
goat anti-mouse (cat. no. ab205719; 1:2,000; Abcam) or anti-rabbit
(cat. no. ab205718; 1:2,000; Abcam) IgG secondary antibodies for 2
h at room temperature. The membranes were visualized using an
enhanced chemiluminescence system (Beyotime Institute of
Biotechnology) and densitometry analysis was performed using Image
J software (version 1.47; National Institutes of Health). GAPDH
(cat. no. ab181602; 1:10,000; Abcam) expression was used as an
internal control.
Total RNA isolation and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from the placenta tissue
homogenate of each rat using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). Total RNA was
reverse-transcribed into cDNA using SuperScript™ III Reverse
Transcriptase (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. The reverse transcription
conditions were 10 min at 25°C, 30 min at 48°C and 5 min a 95°C.
RT-qPCR was performed on a 7500 Fast Real-Time PCR System Light
Cycler (Applied Biosystems; Thermo Fisher Scientific, Inc.) using a
20-µl reaction system that was incubated as follows: 95°C
for 2 min, followed by 40 cycles of 95°C for 20 sec and 72°C for 30
sec. The PCR was repeated three times and the primer sequences were
as follows: ERα36 forward, 5′-CCAAGAATGTTCAACCACAACCT-3′ and
reverse, 5′-GCACGGTTCATTAACATCTTTCTG-3′; GADPH forward,
5′-ACAGTCAGCCGCATCTTCTT-3′ and reverse, 5′-GACAAGCTTCCCGTTCTCAG-3′.
The relative expression of mRNAs was determined using the
2−ΔΔCq method (17)
with normalization to GAPDH expression.
Immunofluorescence (IF) staining of rat
placenta tissue
IF staining was performed on placenta tissue by
cutting 5 µm-thick paraffin sections and incubating them
with an antibody against ERα36 (cat. no. CY1109; 1:100; Cell
Applications Inc.) at 4°C overnight. The sections were washed and
incubated at room temperature for 1 h with an Alexa Fluor
488-conjugated anti-rabbit IgG secondary antibody (cat. no.
ab150077; 1:500; Abcam). The nuclei were stained with DAPI
(Invitrogen; Thermo Fisher Scientific, Inc.). The images were
captured under a TCS SP5 confocal microscope (Leica Microsystems;
magnification, ×200). The intensity of green fluorescence in the
images represents the protein expression of ERα36.
Statistical analysis
Data are expressed as the mean ± standard
derivation. Statistical analyses were performed using SPSS software
(version 17.0; SPSS, Inc.). The statistical significance of
differences among multiple groups was determined by one-way
analysis of variance followed by Dunnett's post-hoc test. The
correlation between sFlt-1 expression and the level of other
cytokine levels, including IL-1β, IL-6, IFN-γ and MCP-1, was
examined using Pearson's correlation analysis. Each experiment was
performed at least three times. P<0.05 was considered to
indicate statistically significant difference.
Results
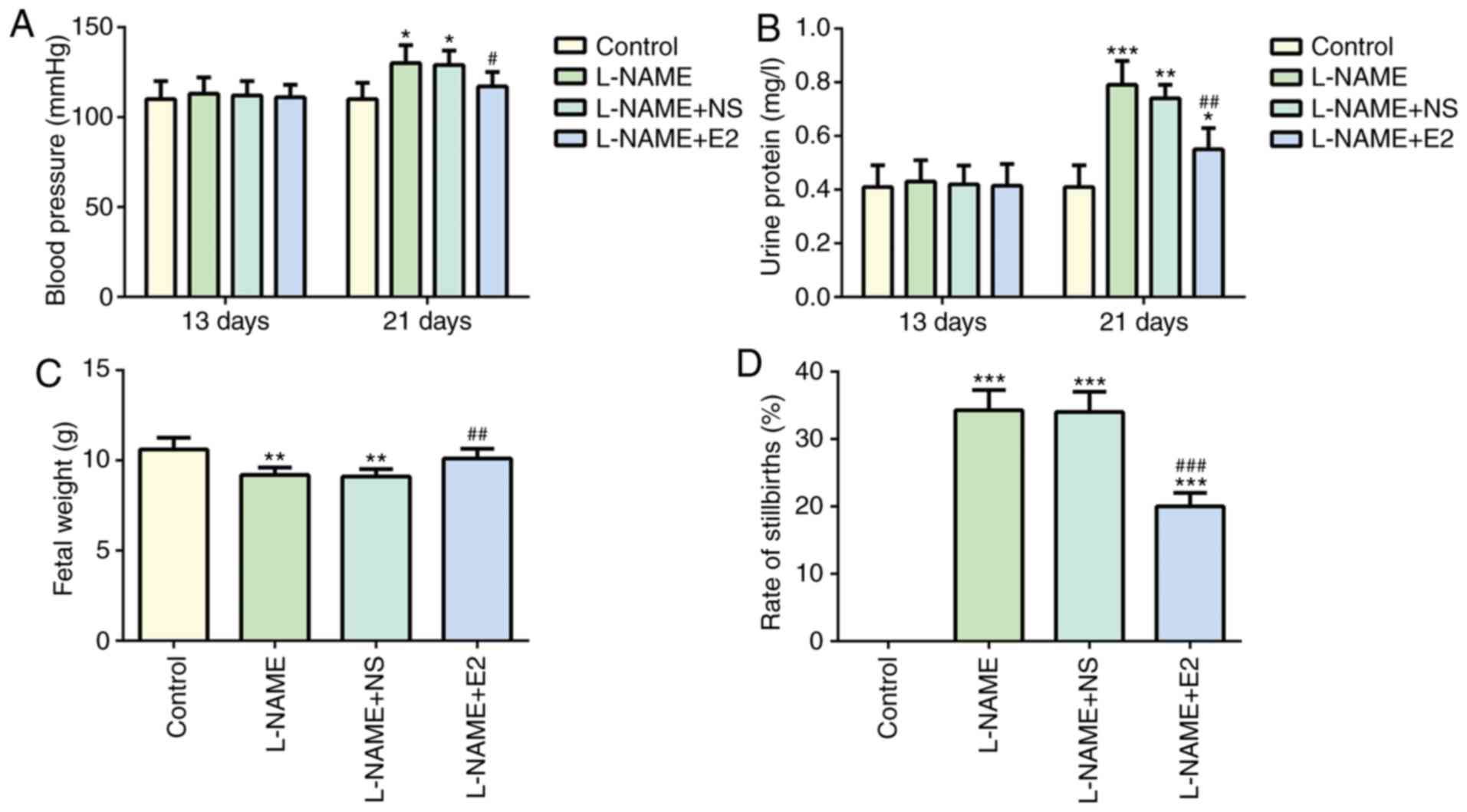
Effects of E2 on blood pressure and urine
protein content
No significant difference in systolic BP was
observed among the four groups on day 13 (P>0.05; Fig. 1A). On day 21, BP in the L-NAME
group was higher compared with that in the control group
(P<0.05; Fig. 1A), but it was
decreased by E2 treatment (P<0.05; Fig. 1A). These results suggested that E2
treatment prevented the elevation of BP in rats with PE (18).
Similarly, no significant difference in 24-h urine
protein was observed among the four groups on day 13 (P>0.05;
Fig. 1B). However, on day 21, the
24-h urine protein content was significantly higher in the L-NAME
group compared with that in the control group (P<0.001; Fig. 1B), whereas the L-NAME + E2 group
exhibited a significant decrease in urine protein content compared
with that in the L-NAME group (P<0.01; Fig. 1B). These results suggested that E2
was effective in preventing the elevation of urine protein in rats
with PE.
Effects of E2 on fetal weight and the
rate of stillbirths
Compared with the control group, a significant
decrease fetal weight was observed in the L-NAME group (P<0.01;
Fig. 1C). However, E2 treatment
restored the weight of neonates in the L-NAME + E2 group to the
level in the control group (P<0.01; Fig. 1C), whereas no significant
differences were identified between the L-NAME and L-NAME + NS
groups. In addition, the rate of stillbirths in the L-NAME group
was increased compared with that in the control group (P<0.001;
Fig. 1D), whereas the L-NAME + E2
group exhibited a decrease compared with the L-NAME group
(P<0.001; Fig. 1D); however,
E2 treatment did not restore the number of stillbirths to the
control level. Therefore, compared with that in the normal pregnant
rats in the control group, the rate of stillbirths in the L-NAME +
E2 group was still high. The results demonstrated that treatment
with E2 partially protected the fetuses of rats with PE against the
effects of L-NAME, increasing the fetal weight and reducing the
rate of stillbirths.
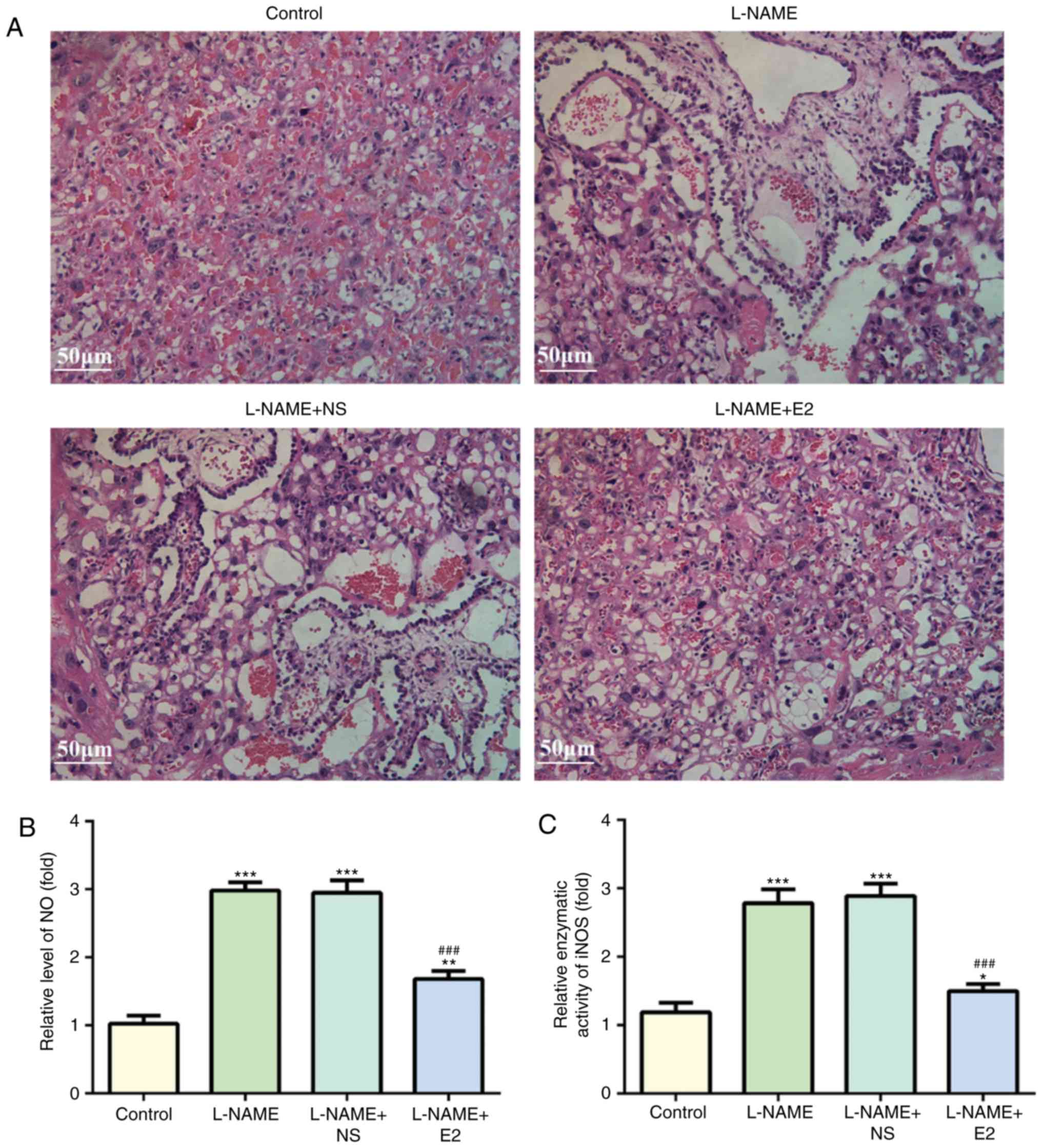
Effects of E2 on placental
histopathology
Histopathological examination with H&E staining
revealed degeneration in the placenta from the rats in the L-NAME
and L-NAME + NS groups, including necrotic changes and inflammatory
cell infiltration (Fig. 2A).
However, these were absent in the L-NAME + E2 group. Therefore, E2
treatment reduced inflammatory infiltration in placenta tissue of
rats with PE.
Effects of E2 on NO levels and iNOS
activity in placental homogenate
ELISAs were performed to detect NO levels and iNOS
activity in the placental homogenate using NO and iNOS kits for
rats. NO and iNOS levels were increased in the L-NAME group
compared with those in the control group (P<0.001; Fig. 2B and C). However, the NO and iNOS
activity did not significantly differ between the L-NAME and
L-NAME+NS groups. E2 administration partially inhibited the
L-NAME-induced elevation of placental NO and iNOS activity
(P<0.001; Fig. 2B and C).
Effects of E2 on inflammatory cytokines
in serum and placental homogenate
The levels of serum IL-1β (Fig. 3A), IL-6 (Fig. 3B), IFN-γ (Fig. 3C), MCP-1 (Fig. 3D) and sFlt-1 (Fig. 3E) in the L-NAME group were
significantly higher compared with those in the control group.
Following treatment with E2, the L-NAME + E2 group exhibited a
decrease in these inflammatory cytokines in the serum compared with
those in the L-NAME and L-NAME + NS groups. In addition, the
protein expression of inflammatory cytokines in the placenta
homogenate was detected using western blot analysis (Fig. 3F). The trends of the placental
protein expression of these inflammatory cytokines in the different
groups were consistent with the serum levels. The correlation
between sFlt-1 and IL-1β, IL-6, IFN-γ and MCP-1 expression in serum
was assessed using Pearson's correlation analysis and the results
revealed that sFlt-1 had a tendency towards a weak positive
correlation with IL-1β (Fig. 3G,
R2=0.3647, P=0.0376), IL-6 (Fig. 3H, R2=0.3372, P=0.0477),
IFN-γ (Fig. 3I,
R2=0.3863, P=0.0310) and MCP-1 (Fig. 3J, R2=0.5132, P=0.0088).
These results indicated that in rats with PE, E2 administration
effectively suppressed the inflammatory response.
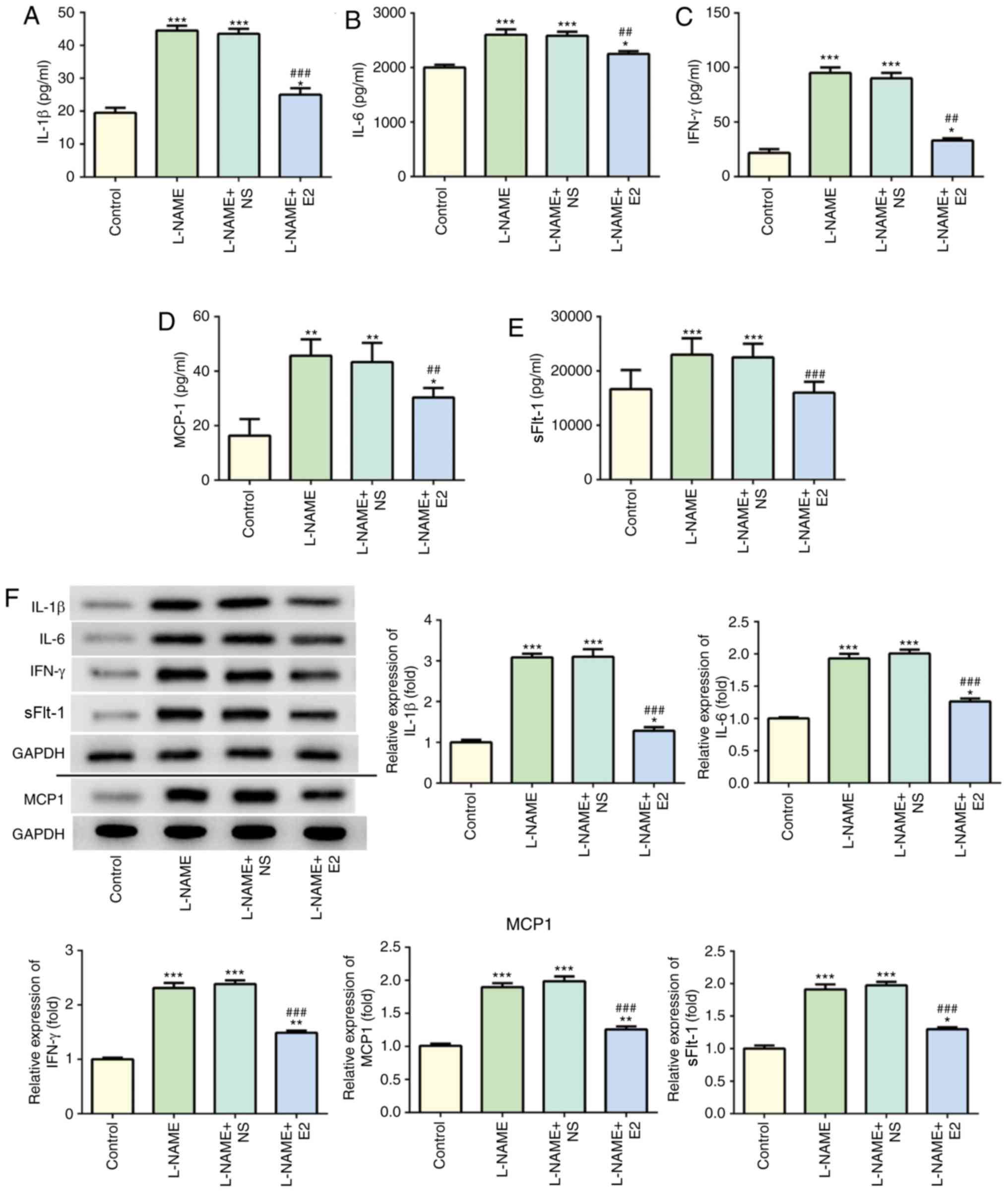 | Figure 3Effects of E2 on the levels of
inflammatory cytokines in the serum and placenta of rats with
preeclampsia. (A-E) Determination of serum (A) IL-1β, (B) IL-6, (C)
IFN-γ, (D) MCP-1 and (E) SFlt-1 levels. (F) Western blot analysis
of IL-1β, IL-6, IFN-γ, MCP-1 and SFlt-1 protein expressions among
the four groups. Data was presented as mean ± SD (n=12 per group).
*P<0.05, **P<0.01 and
***P<0.001 vs. control; ##P<0.01 and
###P<0.001 vs. L-NAME and L-NAME + NS. Effects of E2
on the levels of inflammatory cytokines in the serum and placenta
of rats with preeclampsia. (G-J) Pearson's correlation analysis of
sFlt-1 expression with (G) IL-1, (H) IL-6, (I) IFN-γ and (J) MCP-1
expression in the serum. n=12 rats/group. IL, interleukin; IFN-γ,
interferon-γ; MCP-1, monocyte chemoattractant protein 1; sFlt-1,
soluble Fms-like tyrosine kinase-1; L-NAME, N
(omega)-nitro-L-arginine methyl ester; NS, normal saline; E2,
estradiol. |
Effects of E2 on adhesion factors and
angiogenic factors in the placenta
Western blot analysis indicated that treatment with
L-NAME increased the expression levels of CD49d, ICAM1 and LFA-1
compared with those in the control group (P<0.001; Fig. 4A and B), whereas E2 treatment
decreased their expression levels compared with those in the L-NAME
and L-NAME + NS groups (P<0.001; Fig. 4A and B). In addition, the protein
levels of MMP2, MMP9 and VCAM1 were elevated in the L-NAME group
compared with those in the control group (P<0.001; Fig. 4C and D); however, the expression
of these proteins was downregulated in the L-NAME + E2 group
compared with that in the L-NAME and L-NAME + NS groups
(P<0.001; Fig. 4C and D).
Therefore, E2 treatment reduced endothelial dysfunction in rats
with PE.
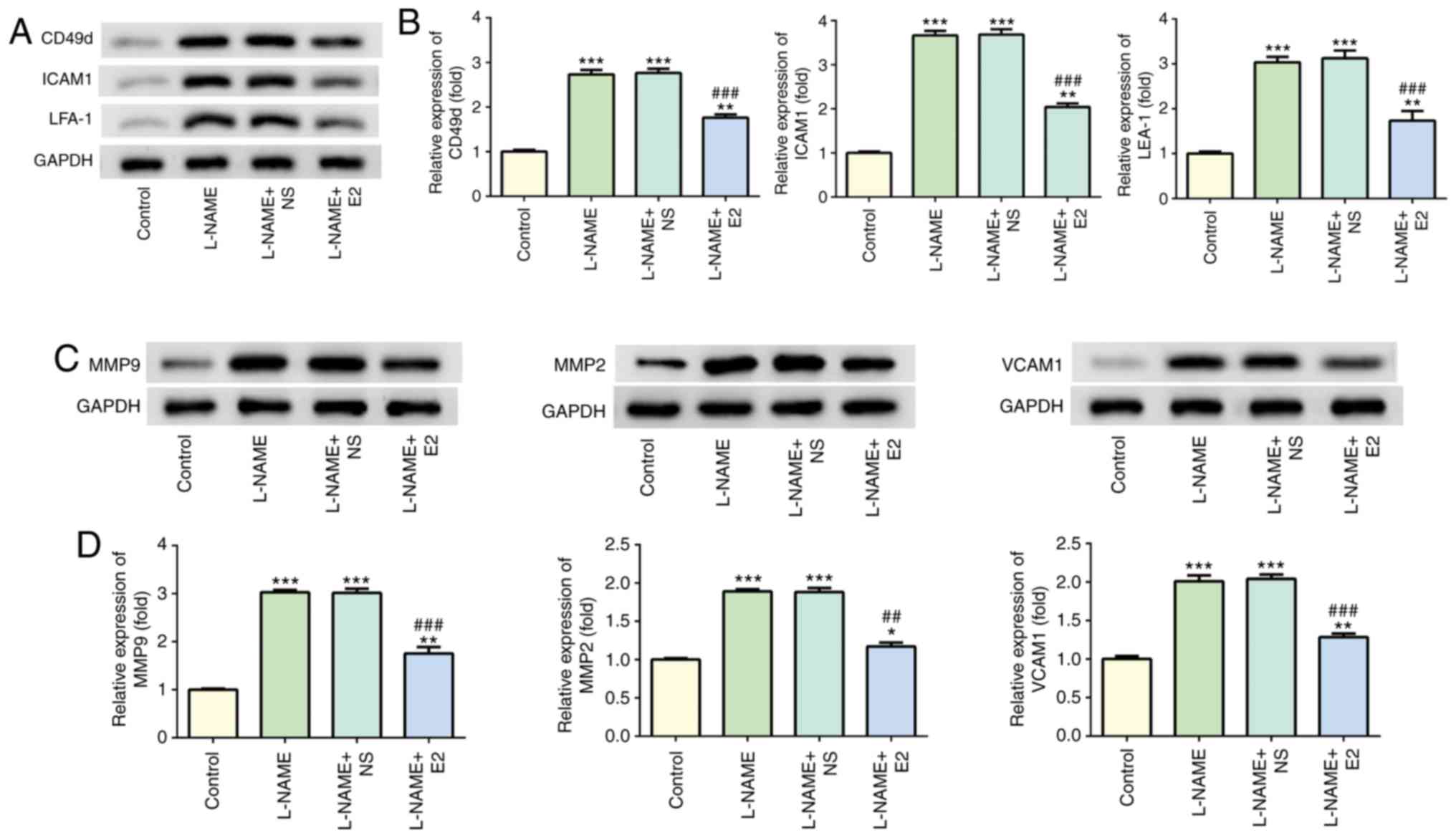 | Figure 4Effects of E2 on the levels of
adherence and angiogenic factors. (A) Representative western blot
images demonstrating the expression of CD49d, ICAM1 and LFA-1 in
the four groups. (B) Relative protein expression levels of CD49d,
ICAM1 and LFA-1. (C) Representative western blot images
demonstrating the expression of MMP2, MMP9 and VCAM1 in the
placenta in the four groups. (D) Relative protein expression of
MMP2, MMP9 and VCAM1. n=12 rats/group. *P<0.05,
**P<0.01 and ***P<0.001 vs. control;
##P<0.01 and ###P<0.001 vs. L-NAME and
L-NAME + NS. E2, estradiol; ICAM1, intercellular adhesion
molecule-1; LFA-1, leukocyte function-associated antigen; MMP,
matrix metallopeptidase; VCAM1, vascular cell adhesion molecule-1;
L-NAME, N (omega)-nitro-L-arginine methyl ester; NS, normal
saline. |
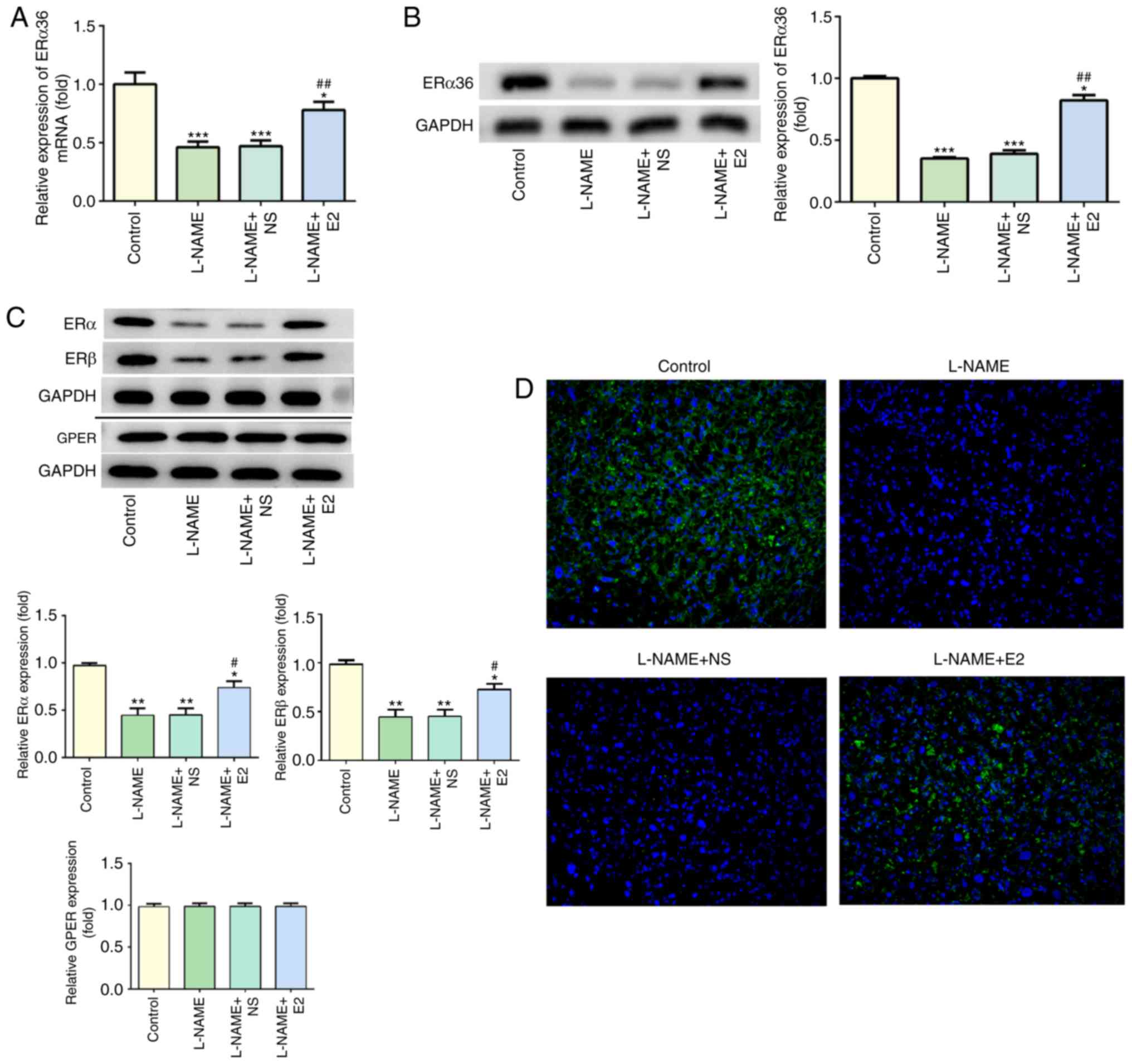
Effects of E2 on the expression of ERα36
in the placenta
As presented in Fig.
5A, compared with that in the control group, L-NAME treatment
significantly decreased the mRNA levels of ERα36 (P<0.001),
which were partially restored by E2 treatment (P<0.01). The
results of the western blot analysis of the protein levels of ERα36
were consistent with the RT-qPCR results (Fig. 5B). The protein levels of ERα, ERβ
and GPER were also assessed by western blot analysis; treatment
with L-NAME induced a decrease in the protein expression of ERα and
ERβ, whereas E2 partially reversed these effects (Fig. 5C). However, GPER expression was
not affected by L-NAME or E2, and no significant differences were
observed among the four groups. In addition, the IF assay indicated
high expression of ERα36 (green) in the placenta of the control
group, whereas L-NAME treatment caused a significant downregulation
of ERα36 expression (Fig. 5D). By
contrast, ERα36 expression (green) was upregulated in the L-NAME +
E2 group compared with that in the L-NAME and L-NAME + NS groups
(Fig. 5D). These results
indicated that the combination of E2 with ERs (especially ERα36)
was involved in the regulation of inflammation and endothelial
function in rats with PE.
Effects of E2 on the expression of key
molecules involved in the TLR4 signaling pathway in the
placenta
Western blot analysis indicated that following
treatment with L-NAME, the expression levels of TLR4, MyD88,
p-IRAK4 and TRAF6 were increased compared with those in the control
group (P<0.001; Fig. 6);
however, E2 administration significantly reduced the expression
levels of these proteins compared with those in the L-NAME and
L-NAME + NS groups (P<0.001; Fig.
6). Of note, total (t)-IRAK4 expression was not significantly
altered among the four groups. Overall, these results suggested
that the TLR4 signaling pathway may serve a functional role in rats
with PE.
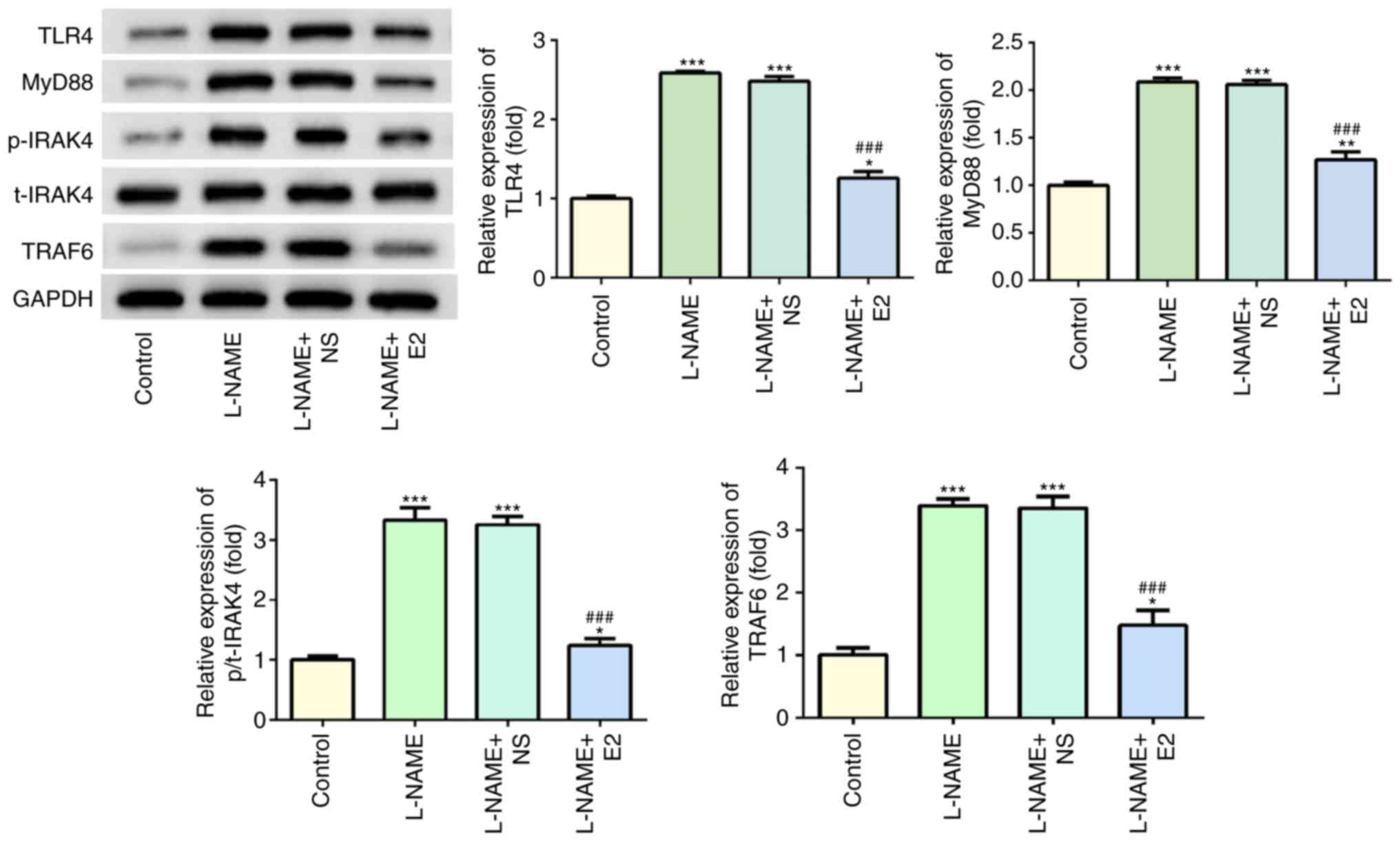 | Figure 6Effects of E2 on the activation of
the TLR4 signaling pathway. The expression and statistical analysis
of TLR4, MyD88, p-IRAK4, t-IRAK4 and TRAF6 by western blotting.
n=12 rats/group. *P<0.05, **P<0.01 and
***P<0.001 vs. control; ###P<0.001 vs.
L-NAME and L-NAME + NS. E2, estradiol; L-NAME, N
(omega)-nitro-L-arginine methyl ester; NS, normal saline; TLR4,
toll-like receptor 4; MyD88, myeloid differentiation primary
response 88; IRAK4, interleukin-1 receptor-associated kinase 4; p,
phospho; t, total; TRAF6, TNF receptor-associated factor 6. |
Discussion
The novel finding of the present study was that
treatment with E2 attenuated the adverse pregnancy outcomes in a
rat model of L-NAME-induced PE, and these effects were associated
with a decrease in inflammation and endothelial dysfunction, as
well as histological improvement in the placenta. In addition,
these results were supported by a significant decrease in the rate
of stillbirths and the levels of NO and iNOS, accompanied by a
reduction of the inflammatory cytokines IL-1β, IL-6, IFN-γ, MCP-1
and sFlt-1. The L-NAME-induced rat model of PE is accepted as a
method of inducing experimental hypertension, resulting in a
condition similar to clinical PE (19). To the best of our knowledge, the
present study was the first to explore the effect of exogenous E2
on rats with PE and the underlying molecular mechanisms of the
pathological process.
Previous studies have indicated abnormal changes in
serum biochemical indexes and pregnancy outcomes in rats with PE
(20,21), which were consistent with the
results of the present study. The levels of BP, urine protein and
fetal death were increased, whereas fetal body weight was decreased
in the L-NAME group in the present study. Hypertension is one of
the pathophysiological factors resulting in PE and injuries of the
systematic inflammatory response (22). E2 improved the biochemical indexes
and neonatal outcome by reducing BP, urine protein and the rate of
stillbirths, increasing fetal weight and attenuating pathological
injury, which indicated that the improvement of PE was associated
with E2 treatment. Previous studies have demonstrated that L-NAME
induced an increase in BP in rats and that the lower production of
iNOS and NO participated in the adaptive reconstruction of
vasculature occurring during normal pregnancy and served an
important role in maintaining constant vascular tension and the
stability of blood pressure (23,24). Akram et al (25) have reported that estrogen serves a
protective role in PE by affecting the
renin-angiotensin-aldosterone system to increase the blood volume
and regulating the activity of endothelial NO synthase to induce
the release of vasoconstrictors. In the present study, treatment of
pregnant rats with L-NAME elevated the production of NO and iNOS to
cause oxidative stress and impair endothelial function in early
hypertension. The results of the present study also suggested that
E2 may be a beneficial treatment for the symptoms of PE in
agreement with a previous study (25).
The high levels of IL-1β, IL-6, IFN-γ and MCP-1 in
the serum and placenta tissue of rats with PE may be linked to the
inflammatory response, suggesting that inflammatory cytokines may
participate in the adverse events in PE (26). In the present study, treatment
with E2 resulted in a reduction in the levels of IL-1β, IL-6, IFN-γ
and MCP-1 in the serum and placenta of rats with PE, indicating the
anti-inflammatory effect of exogenous estrogen. The major
alterations associated with PE are likely to trigger local
oxidative stress, and re-oxygenation may expand the local effects,
including the formation of reactive oxygen species, activation of
the maternal inflammatory system and acceleration of apoptosis that
may limit the establishment of normal placentation and cause
imbalance between pro-angiogenic factors, including sFLT-1 and
VCAM-1 (3). In addition, PE may
be associated with increased production of MCP-1 and IL-8, which
are regulated by signaling mechanisms sensitive to oxidative stress
(27). Inflammation is closely
associated with angiogenesis. Therefore, the correlation among
these factors was analyzed in the present study, and a weak
positive correlation between sFLT-1, inflammatory cytokines and
MCP-1 was revealed, indicating that sFlt-1 and MCP-1 may lead to
the general activation of the maternal inflammatory system,
endothelial dysfunction and the limitation of placental
vascularization. In addition, E2 may decrease the levels of sFlt1
and MCP-1 to reverse endothelial dysfunction and restricted
placental angiogenesis, which may achieve effective treatment of
PE.
High expression of ICAM-1 has been previously
detected in epithelial cells and the stroma of abortion-prone
fetuses in maternal rats, which causes increased recruitment of
lymphocytes expressing LFA-1 from the blood into the uterus
(28). The levels of IL-1β, TNF-α
and IFN-γ are increased in lymphocytes, and stress further
increases the expression of ICAM-1 in the endothelium (29). Inhibition of ICAM-1/LFA-1-mediated
intercellular adhesion events may restore the fetal immune
acceptance in challenged pregnancies (28). Studies have reported that ICAM-1
and VCAM-1 are increased in the serum or plasma of patients with PE
(30). CD49d, an
inflammation-associated adhesion molecule expressed on neutrophils
and monocytes, binds to VCAM-1 (31). The present result exhibited that
L-NAME induced an increase of CD49d, ICAM-1, LFA-1 and VCAM-1
expression levels, which were significantly reversed by E2
administration. This indicated that E2 may alleviate endothelial
dysfunction in PE rats by reducing the adhesion of placental tissue
and the process of angiogenesis.
ERα36, which is a membrane variant of ERα, is also
affected by the dysregulation of estrogen signaling (32). ERα has been demonstrated to
suppress the activity of ERα36 promoter in an estrogen-independent
manner (33). ERα36 has been
identified as a mediator of E2 action in the malignant liver and
may be involved in the occurrence and development of various tumors
(34,35). Consistent with the previous
results, the results of the present study indicated that the
induction of E2 after L-NAME treatment suppressed the expression of
ERα36, ERα and ERβ in the placenta of rats with PE compared with
that in normal pregnant rats. E2 administration induced
upregulation of ERα36, ERα and ERβ expression compared with that in
the L-NAME group. Among the ERs, the changes in ERα36 levels were
the most significant. The expression changes of other estrogen
receptors may eventually lead to changes in ERα36 expression, which
was why ERα36 was selected as the main research object of the
present study. A previous study reported that E2 suppressed the
binding activity of NF-κB mediated by ERα66 and reduced the
expression of MMP2, MMP9, cyclin A and cyclin D1, resulting in cell
cycle arrest and promoting cell apoptosis (36). Apoptotic processes and endothelial
dysfunction are common in PE (3).
The results of the present study indicated that that E2 suppressed
the expression of MMP9, MMP2 and VCAM-1 in placental tissue induced
by L-NAME treatment. ERα36 has been demonstrated to be located in
the cytoplasm as well as on the cell surface, where it mediates
non-genomic estrogen signaling through cross-talk with growth
factor receptors and other signaling molecules (including
mitogen-activated protein kinase/ERK and PI3K/AKT) to promote cell
proliferation, invasion and migration, as well as resistance to
endocrine therapy (37).
Therefore, it was hypothesized in the present study that E2 may
bind to ERα36 to regulate the expression of the above factors
through certain signaling pathways, such as the TRL4 signaling
pathway.
In the present study, the anti-inflammatory,
antioxidant and anti-vascular dysfunction properties of E2 were
indicated to be, at least in part, mediated by the suppression of
the TLR4 pathway through the inhibition of MyD88 and TRAF6
expression, as well as phosphorylation of IRAK4. A previous study
has reported that the TLR4 signaling pathway serves an important
role in autoimmune disease (38)
and the inflammatory response and pathological damage associated
with various diseases in humans (39,40). MyD88 and TRAF-6 are important
intracellular adaptor proteins that function as effectors of the
TLR4 signaling cascade (41).
MyD88 activates and phosphorylates IRAK4, leading to the
recruitment of TRAF6 and promoting inflammation (42). The intracellular signaling cascade
is activated by TLR4 by recruiting IRAK4 to the membrane (43). The TLR4-dependent signaling
pathway has been reported to be detrimental to trophoblast function
(44). These previous studies are
in accordance with the results of the present study, as the
TLR4/MyD88/IRAK4/TRAF6 signaling pathway was activated in rats with
PE. The results of the present study suggested that the regulation
of the TLR4/MyD88/IRAK4/TRAF6 pathway may be involved in the
effects of E2 against L-NAME-induced pathological processes of PE.
The binding of E2 to ERα36 may participate in the regulation of
angiogenesis and inflammation by mechanisms dependent on TLR4
activation in PE.
In summary, the results of the present study
demonstrated that E2 may improve adverse pregnancy outcomes induced
by L-NAME in rats. The protective effects of E2 may be partially
exerted by binding to ERα36, regulation of inflammatory, adherence
and placental angiogenic factors and deactivation of
TLR4/MyD88/IRAK4/TRAF6 signaling. Therefore, E2 may be a potential
therapeutic for treating PE.
Funding
No funding was received.
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZHL designed the experiments and wrote the
manuscript. ZHL, JJ and XYS performed the experiments and analyzed
the data. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The protocols of the animal experiments followed the
NIH Guide for the Care and Use of Laboratory Animals and were
approved by the Experimental Animal Ethics Committee of Kunming
Medical University (Kunming, China) and the Commission for Animal
Experimentation of The People's Hospital of the Xishuangbanna Dai
Nationality Autonomous Prefecture.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Walker CK, Krakowiak P, Baker A, Hansen
RL, Ozonoff S and Hertz-Picciotto I: Preeclampsia, placental
insufficiency, and autism spectrum disorder or developmental delay.
JAMA Pediatr. 169:154–162. 2015. View Article : Google Scholar :
|
|
2
|
Hod T, Cerdeira AS and Karumanchi SA:
Molecular mechanisms of preeclampsia. Cold Spring Harb Perspect
Med. 5:2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ramos JGL, Sass N and Costa SHM:
Preeclampsia. Rev Bras Ginecol Obstet. 39:496–512. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Borzychowski AM, Croy BA, Chan WL, Redman
CW and Sargent IL: Changes in systemic type 1 and type 2 immunity
in normal pregnancy and pre-eclampsia may be mediated by natural
killer cells. Eur J Immunol. 35:3054–3063. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Biondi C, Pavan B, Lunghi L, Fiorini S and
Vesce F: The role and modulation of the oxidative balance in
pregnancy. Curr Pharm Des. 11:2075–2089. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kiprono LV, Wallace K, Moseley J, Martin J
Jr and Lamarca B: Progesterone blunts vascular endothelial cell
secretion of endothelin-1 in response to placental ischemia. Am J
Obstet Gynecol. 209:44.e1–e6. 2013. View Article : Google Scholar
|
|
7
|
Wei J, Satomi M, Negishi Y, Matsumura Y,
Miura A, Nishi Y, Asakura H and Takeshita T: Effect of sera on the
adhesion of natural killer cells to the endothelium in severe
pre-eclampsia. J Obstet Gynaecol Res. 32:443–448. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mihu D, Razvan C, Malutan A and Mihaela C:
Evaluation of maternal systemic inflammatory response in
preeclampsia. Taiwan J Obstet Gynecol. 54:160–166. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
de Oliveira LG, Karumanchi A and Sass N:
Preeclampsia: Oxidative stress, inflammation and endothelial
dysfunction. Rev Bras Ginecol Obstet. 32:609–616. 2010.In
Portuguese.
|
|
10
|
Tranquilli AL, Dekker G, Magee L, Roberts
J, Sibai BM, Steyn W, Zeeman GG and Brown MA: The classification,
diagnosis and management of the hypertensive disorders of
pregnancy: A revised statement from the ISSHP. Pregnancy Hypertens.
4:97–104. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wei J, Chen Q, James JL, Stone PR and
Chamley LW: IL-1 beta but not the NALP3 inflammasome is an
important determinant of endothelial cell responses to
necrotic/dangerous trophoblastic debris. Placenta. 36:1385–1392.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Southcombe JH, Redman CW, Sargent IL and
Granne I: Interleukin-1 family cytokines and their regulatory
proteins in normal pregnancy and pre-eclampsia. Clin Exp Immunol.
181:480–490. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Khalil RA: Sex hormones as potential
modulators of vascular function in hypertension. Hypertension.
46:249–254. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Duckles SP and Miller VM: Hormonal
modulation of endothelial NO production. Pflugers Arch.
459:841–851. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Salas SP, Marshall G, Gutiérrez BL and
Rosso P: Time course of maternal plasma volume and hormonal changes
in women with preeclampsia or fetal growth restriction.
Hypertension. 47:203–208. 2006. View Article : Google Scholar
|
|
16
|
Zheng JJ, Wang HO, Huang M and Zheng FY:
Assessment of ADMA, estradiol, and progesterone in severe
preeclampsia. Clin Exp Hypertens. 38:347–351. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cikos S, Bukovská A and Koppel J: Relative
quantification of mRNA: Comparison of methods currently used for
real-time PCR data analysis. BMC Mol Biol. 8:1132007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Feng J, Wang X, Li H, Wang L and Tang Z:
Silencing of Annexin A1 suppressed the apoptosis and inflammatory
response of preeclampsia rat trophoblasts. Int J Mol Med.
42:3125–3134. 2018.PubMed/NCBI
|
|
19
|
Kanashiro CA, Cockrell KL, Alexander BT,
Granger JP and Khalil RA: Pregnancy-associated reduction in
vascular protein kinase C activity rebounds during inhibition of NO
synthesis. Am J Physiol Regul Integr Comp Physiol. 278:R295–R303.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Souza CO, Peracoli MT, Weel IC, Bannwart
CF, Romão M, Nakaira-Takahagi E, Medeiros LT, Silva MG and Peraçoli
JC: Hepatoprotective and anti-inflammatory effects of silibinin on
experimental preeclampsia induced by L-NAME in rats. Life Sci.
91:159–165. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Doering TP, Haller NA, Montgomery MA,
Freeman EJ and Hopkins MP: The role of AT1 angiotensin receptor
activation in the pathogenesis of preeclampsia. Am J Obstet
Gynecol. 178:1307–1312. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Beckers KF and Sones JL: Maternal
microbiome and the hypertensive disorder of pregnancy,
preeclampsia. Am J Physiol Heart Circ Physiol. 2019.PubMed/NCBI
|
|
23
|
de Moura RS, Resende AC, Moura AS and
Maradei MF: Protective action of a hydroalcoholic extract of a
vinifera grape skin on experimental preeclampsia in rats. Hypertens
Pregnancy. 26:89–100. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Conrad KP, Joffe GM, Kruszyna H, Kruszyna
R, Rochelle LG, Smith RP, Chavez JE and Mosher MD: Identification
of increased nitric oxide biosynthesis during pregnancy in rats.
FASEB J. 7:566–571. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Akram SK, Sahlin L, Ostlund E, Hagenäs L,
Fried G and Söder O: Placental IGF-I, estrogen receptor, and
progesterone receptor expression, and maternal anthropometry in
growth-restricted pregnancies in the Swedish population. Horm Res
Paediatr. 75:131–137. 2011. View Article : Google Scholar
|
|
26
|
Fodor P, White B and Khan R:
Inflammation-The role of ATP in pre-eclampsia. Microcirculation.
2019.PubMed/NCBI
|
|
27
|
Kauma S, Takacs P, Scordalakes C, Walsh S,
Green K and Peng T: Increased endothelial monocyte chemoattractant
protein-1 and interleukin-8 in preeclampsia. Obstet Gynecol.
100:706–714. 2002.PubMed/NCBI
|
|
28
|
Blois S, Tometten M, Kandil J, Hagen E,
Klapp BF, Margni RA and Arck PC: Intercellular adhesion
molecule-1/LFA-1 cross talk is a proximate mediator capable of
disrupting immune integration and tolerance mechanism at the
feto-maternal interface in murine pregnancies. J Immunol.
174:1820–1829. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Springer TA: Traffic signals for
lymphocyte recirculation and leukocyte emigration: The multistep
paradigm. Cell. 76:301–314. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chaiworapongsa T, Romero R, Yoshimatsu J,
Espinoza J, Kim YM, Park K, Kalache K, Edwin S, Bujold E and Gomez
R: Soluble adhesion molecule profile in normal pregnancy and
pre-eclampsia. J Matern Fetal Neonatal Med. 12:19–27. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Oggé G, Romero R, Chaiworapongsa T,
Gervasi MT, Pacora P, Erez O, Kusanovic JP, Vaisbuch E, Mazaki-Tovi
S, Gotsch F, et al: Leukocytes of pregnant women with
small-for-gestational age neonates have a different phenotypic and
metabolic activity from those of women with preeclampsia. J Matern
Fetal Neonatal Med. 23:476–487. 2010. View Article : Google Scholar
|
|
32
|
Xu Z, Liu J, Jianxin C, Yongliang Z and
Pan X: 17β-Estradiol inhibits testosterone-induced cell
proliferation in HepG2 by modulating the relative ratios of 3
estrogen receptor isoforms to the androgen receptor. J Cell
Biochem. 119:8659–8671. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zou Y, Ding L, Coleman M and Wang Z:
Estrogen receptor-alpha (ER-alpha) suppresses expression of its
variant ER-alpha 36. FEBS Lett. 583:1368–1374. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cocciadiferro L, Miceli V, Granata OM and
Carruba G: Abstract 1852: Merlin/NF2 is associated with elevated
aromatase expression and estrogen formation in human liver tissues
and liver cancer cells: An unifying model for hepatocellular
carcinoma development and progression. Cancer Res. 1852:2015.
|
|
35
|
Liu J, Xu Z, Ma X, Huang B and Pan X: Role
of ER-α36 in breast cancer by typical xenoestrogens. Tumour Biol.
36:7355–7364. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xu H, Wei Y, Zhang Y, Xu Y, Li F, Liu J,
Zhang W, Han X, Tan R and Shen P: Oestrogen attenuates tumour
progression in hepato-cellular carcinoma. J Pathol. 228:216–229.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang Z, Zhang X, Shen P, Loggie BW, Chang
Y and Deuel TF: Identification, cloning, and expression of human
estrogen receptor-alpha36, a novel variant of human estrogen
receptor-alpha66. Biochem Biophys Res Commun. 336:1023–1027. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rao Y and Su J: Insights into the
antiviral immunity against grass carp (Ctenopharyngodon idella)
reovirus (GCRV) in grass carp. J Immunol Res. 2015:6704372015.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Meng M, Wang H, Li Z, Guo M and Hou L:
Protective effects of polysaccharides from Cordyceps gunnii mycelia
against cyclophosphamide-induced immunosuppression to
TLR4/TRAF6/NF-κB signalling in BALB/c mice. Food Funct.
10:3262–3271. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang X, Han C, Qin J, Wei Y, Qian X, Bao Y
and Shi W: Pretreatment with salvia miltiorrhiza polysaccharides
protects from lipopolysaccharides/d-galactosamine-induced liver
injury in mice through inhibiting TLR4/MyD88 signaling pathway. J
Interferon Cytokine Res. 39:495–505. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Verstak B, Nagpal K, Bottomley SP,
Golenbock DT, Hertzog PJ and Mansell A: MyD88 adapter-like
(Mal)/TIRAP interaction with TRAF6 is critical for TLR2- and
TLR4-mediated NF-kappaB proinflammatory responses. J Biol Chem.
284:24192–24203. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shi S, Liang D, Chen Y, Xie Y, Wang Y,
Wang L, Wang Z and Qiao Z: Gx-50 reduces β-amyloid-induced TNF-α,
IL-1β, NO, and PGE2 expression and inhibits NF-κB signaling in a
mouse model of Alzheimer's disease. Eur J Immunol. 46:665–676.
2016. View Article : Google Scholar
|
|
43
|
Song J, Han X, Yao YL, Li YM, Zhang J,
Shao DY, Hou LS, Fan Y, Song SZ, Lian LH, et al: Acanthoic acid
suppresses lipin1/2 via TLR4 and IRAK4 signalling pathways in EtOH-
and lipopolysaccharide-induced hepatic lipogenesis. J Pharm
Pharmacol. 70:393–403. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Leon-Martinez D, Mulla MJ, Han CS, Chamley
LW and Abrahams VM: Modulation of trophoblast function by
concurrent hyperglycemia and antiphospholipid antibodies is in part
TLR4-dependent. Am J Reprod Immunol. 80:e130452018. View Article : Google Scholar : PubMed/NCBI
|