Introduction
Acute kidney injury (AKI) is a disease with a high
mortality, not only due to renal dysfunction, but also due to
remote organ dysfunction, including the brain (1-3).
There is an urgent requirement to identify more effective therapies
to prevent or treat AKI. The induction of AKI by glycerol has been
widely used as a model in experimental studies (4,5).
The major pathophysiological process of AKI induced by glycerol is
rhabdomyolysis, which induces severe renal toxicity due to
oxidative damage, inflammation, endothelial dysfunction, ischemia,
cellular and tissue edema, vasoconstriction and apoptosis (3,5,6).
It has been demonstrated previously that renal disease may cause
oxidative stress, and biochemical and structural changes of the
brain through humoral and non-humoral crosstalk (1,7).
However, the molecular mechanisms underlying the crosstalk between
the kidney and the brain in AKI remain poorly understood.
Ginsenoside (GS) has also been demonstrated to
exhibit beneficial effects on the human body, including in brain
and renal tissues. GS has been previously investigated as a
protective agent against ischemia/reperfusion injury, metabolic
disorders, endothelial dysfunction, cardiotoxicity (8-11).
Recently, GS has also been demonstrated to be useful to neuronal
functions, and to protect the CNS against a variety of types of
brain injury (12-14). However, the mechanisms for the
protective effects of GS against AKI in the kidney-brain axis
remain unclear.
Hypoxia-inducible factor 1α (HIF-1α) is an important
modulator of cellular transcriptional response to low oxygen
conditions and is actively involved in the hypoxia induced by
kidney injury (15) and ischemic
brain damages (16).
Specifically, the predominant distribution of HIF-1α protein in
both the kidneys and brain highlights its essential role in
protecting against dysfunction of the kidney-brain axis (17,18). The production of HIF-1α markedly
increases in response to stimulation such as renal
ischemia/reperfusion injury (19), while knockdown of HIF-1α
aggravates ischemic damage (15,20). Collectively, these studies imply
that HIF-1α may be involved in the development of renal and brain
dysfunction induced by AKI.
Vascular endothelial growth factor A (VEGF-A) is an
angiogenesis and vascular permeability factor induced by hypoxia.
The increase in VEGF-A protein levels under hypoxic conditions is
partially due to the regulation of HIF-1α (21,22). Several studies have indicated the
beneficial effects of VEGF in animal models of brain injury such as
ischemic stroke (23) and
Alzheimer's disease (24),
including antiapoptotic, anti-inflammatory, antioxidant and
angiogenic effects. Furthermore, the expression of VEGF and VEGF
receptor (VEGFR), which has been observed in the hypothalamus, was
observed to be increased following hypoxia (24,25). However, it remains unclear whether
HIF-1α and VEGF-A are more extensively involved in the protective
effect of GS against AKI in rats.
Accordingly, in the present study, the effect of GS
on kidney function and oxidative stress in AKI rats was
investigated in the kidney-brain axis. It was hypothesized that GS
may rescue the impairment of oxidative stress induced by glycerol
injection in the kidney and hypothalamus of rats. Investigation of
the protective effect of GS in the kidney-brain axis and the
molecular mechanism of its action may suggest a potential
therapeutic intervention, and assist in designing novel agents that
may attenuate or prevent renal injury.
Materials and methods
Animals and groups
Adult male Sprague Dawley (SD) rats (age, 6-8 weeks)
were obtained from the Experimental Animal Center of Dalian Medical
University [permit no. SYXK (Liao) 2013-0006] and were housed under
controlled temperature (20-25°C), humidity (40-70%), specific
pathogen-free and 12 h light/dark cycle conditions with free access
to food and water. All treatment protocols were approved by the
Animal Care and Ethics Committee of Dalian Medical University. GS
was obtained from Hongjiu Biotech Co., Ltd., and is referred to as
the total saponins of Panax ginseng in the present study. As
described in previous studies (11,26), concentration gradient optimization
experiments were performed. By measuring renal function and
morphology, the dose of 250 mg/kg/day was selected, based on 4
ml/day, and GS was dissolved in normal saline (NS).
All the rats (n=138) were randomly allocated into 3
groups: NS + NS; AKI + NS; and AKI + GS. The AKI groups received
intramuscular injection of 50% glycerol [10 ml/kg body weight (BW)]
and the NS + NS group received an injection of NS. GS or NS (2 ml)
was administrated intragastrically for 2 consecutive days
(twice/day) following glycerol injection, as described in our
previous study (26). Rats were
sacrificed under general anesthesia using intra-peritoneal
injection of 4% chloral hydrate (400 mg/kg BW). A total of 30 rats
among all 138 rats were involved in the renal function, renal
histology and malondialdehyde (MDA) and superoxide dismutase (SOD)
analyses at 48 h following GS or NS treatment (3 groups; n=10 per
group). Blood samples (1 ml) were collected from the posterior
orbital venous plexus. The right kidneys and brains were collected
for MDA and SOD analyses. The left kidneys were removed for
histopathological analysis, and 18 rats among all 138 rats included
in immunohistochemistry analyses (3 groups; n=6 per group). For the
western blot analysis, each group was subdivided into 5 subgroups
at 0, 6, 12, 24 and 48 h following GS or NS treatment (n=6 per
subgroup).
Renal function assays
Blood urea nitrogen (BUN), creatinine (Cre) levels
were measured by the Fearon and the Jaffe methods (26), using a urea assay kit (cat. no.
C013-1-1) and creatinine assay kit (cat. no. C011-1-1),
respectively (both from Nanjing Jiancheng Bioengineering
Institute), according to the manufacturer's protocol.
Renal histology
The kidneys were fixed in 10% formalin at room
temperature (RT) for 24 h and embedded in paraffin. Paraffinized
kidney sections (4 µm) were stained with Hansen's
hematoxylin and eosin at RT. After deparaffinization and
rehydration, nuclei were stained with hematoxylin at RT for 10 min
and the cytoplasm were counterstained with eosin at RT for 5 min.
The sections were washed with distilled water in both steps. Then,
the sections were dehydrated in graded alcohol, cleared in xylene
and mounted. The extent of renal tissue damages was evaluated by
the extent of tubular injury, dilatation, vacuolation and necrosis.
A total of 5 fields of each slice (3 slides/animal) were randomly
selected for a blinded assessment of expression (n=10 per group)
using a light microscope (×20 magnification).
MDA and SOD analysis
Renal and hypothalamus tissues were collected at 48
h following GS or NS treatment. Tissue MDA levels were measured
using a commercial assay kit (cat. no. A003-1-1; Nanjing Jiancheng
Bioengineering Institute). Tissue superoxide dismutase (SOD)
activity was determined using the Xanthine oxidase method with a
commercial assay kit (cat. no. A001-1-1; Nanjing Jiancheng
Bioengineering Institute).
Western blot analysis
Total protein was isolated from brain or kidney
tissues at 5 time points following GS or NS treatment using a Total
Protein Extraction kit (Nanjing Keygen Biotech Co., Ltd.). Total
protein concentrations were quantified by BCA Protein Assay kit
(Nanjing Keygen Biotech Co., Ltd.). An equal amount of protein (40
µg) was separated by 10% SDS-PAGE, and transferred to a
polyvinylidene difluoride membrane following the manufacturer's
protocol. Membranes were incubated with 5% nonfat milk in TBS with
1% Tween-20 (TBST) for 60 min at RT. The primary antibodies were as
follows: Rabbit-HIF-1α antibody (1:1,000; cat. no. PB0245; Wuhan
Boster Biological Technology, Ltd.); rabbit VEGF-A antibody
(1:1,000; cat. no. PB9071; Wuhan Boster Biological Technology,
Ltd.); and rabbit β-actin antibody (1:1,000; cat. no. 20536-1-AP;
Wuhan Sanying Biotechnology). The membranes were incubated with the
primary antibodies at 4°C overnight, subsequently washed with TBST
3 times (10 min each), and then incubated with goat anti-rabbit
horseradish peroxidase-conjugated secondary antibody (1:5,000; cat.
no. 31460; Thermo Fisher Scientific, Inc.) for 2 h at RT. The
membranes were incubated with ECL reagent (Thermo Fisher
Scientific, Inc.) in the dark for 10 min at RT, and the bands were
visualized using a Universal Hood II Gel Doc system (Bio-Rad
Laboratories, Inc.). The mean grey values of the bands were
quantitatively analyzed using Image Lab software (v4.0; Bio-Rad
Laboratories, Inc.), and the band values were expressed as target
protein/β-actin ratio for each sample.
Immunohistochemical staining
Frozen kidney slices (7 µm) were
permeabilizated with 3% H2O2/methanol for 10
min at RT, and blocked with 2% BSA (Sigma-Aldrich; Merck KGaA) for
30 min at RT. Next, sections were incubated at 4°C overnight with
rabbit anti-HIF-1α (1:100) or rabbit anti-VEGF-A (1:100) primary
antibodies and then rinsed twice in PBS, and incubated with a
HRP-conjugated secondary antibody (ready to use; cat. no. SA1022;
Wuhan Boster Biological Technology, Ltd.) for 2 h at RT. The
sections were then counterstained with Mayer's hematoxylin for 1
min at RT. The number of positive granules in tissue sections were
imaged with a light microscope (×20 magnification; Leica DM 4000 B;
Leica Microsystems GmbH) and were semi-quantified by Image-Pro Plus
software (v6.0; Media Cybernetics, Inc.). The evaluation method was
the same as the aforementioned renal histology analysis (n=6 per
group).
Statistical analysis
Each experiment was replicated 3 times. All data are
presented as mean ± standard error of the mean and were performed
using the SPSS v17 software (SPSS, Inc.). Statistical significance
was determined by one-way analysis of variance followed by a
Student-Newman-Keuls post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Effect of GS on renal function and
structure in glycerol-induced AKI rats
The results presented in Table I indicated that glycerol injection
in rats induced a significant increase in the levels of renal
function, BUN and Cre compared with the control group (P<0.05).
In addition, GS attenuated the changes in BUN and Cre levels
induced by glycerol, but the levels of BUN and Cre in the AKI + GS
group were significantly increased compared with those in the
control group (Table I;
P<0.05). Overall, these data suggested that GS may alleviate
glycerol-induced renal impairment.
 | Table IEffect of GS treatment on renal
function in rats. |
Table I
Effect of GS treatment on renal
function in rats.
| Biomarkers | NS + NS | AKI + NS | AKI + GS |
|---|
| BUN (mmol/l) | 8.94±1.00 | 23.51±1.47a | 10.04±0.95b |
| Cre (mmol/l) | 95.68±4.32 |
157.34±11.22a | 80.00±6.33b |
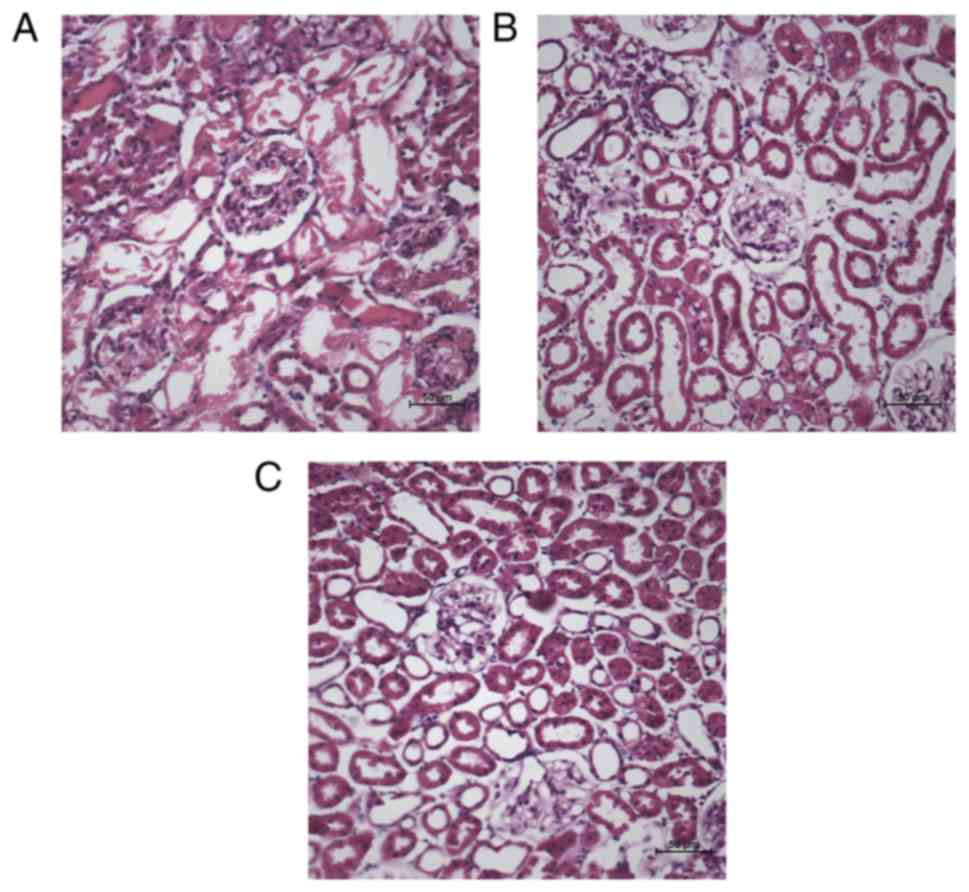
Histologic examination in AKI rats indicated that
glycerol treatment induced widespread degeneration and severe
necrosis in the majority of renal tubules, disintegration of the
tubular epithelial cells, tubular vacuolation and dilatation
(Fig. 1A). Concomitantly, GS
treatment resulted in decreased pathological changes, renal tubular
repair and protected renal tubules from morphological alterations
(Fig. 1B). Histological
evaluation suggested that glycerol treatment produced signifi-cant
renal structural abnormalities and functional impairment, and GS
may prevent the damage induced by AKI.
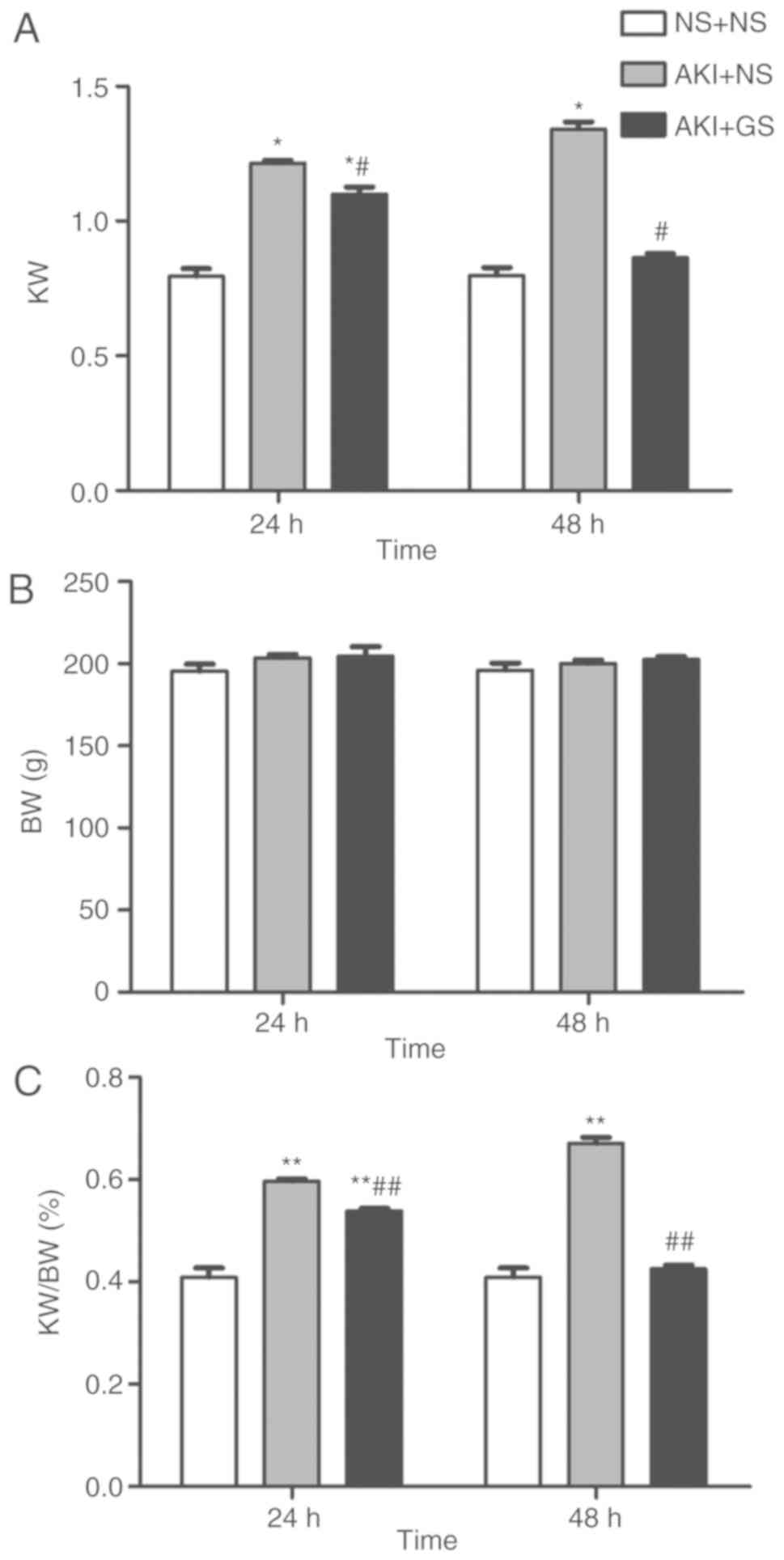
Effect of GS on kidney weight (KW), BW
and KW/BW ratio
In addition to the morphological changes observed,
KW and the ratio of KW to BW (KW/BW) were also altered. The results
presented in Fig. 2 indicated
that BW was unchanged in all groups at 24 and 48 h. At 24 h, there
were significant increases in KW and KW/BW in AKI + NS and AKI + GS
groups compared with the control group (P<0.05). GS treatment
also decreased KW and KW/BW in the AKI + GS 24 h group compared
with the AKI + NS 24 h group (P<0.05), but the KW and KW/BW in
AKI + GS 24 h group remained increased compared with those in the
control group 24 h (Fig. 2A and
C). Similarly, GS treatment inhibited the increase of KW and
KW/BW at 48 h (P<0.05), and there was no difference compared
with the control group 48 h (Fig.
2B).
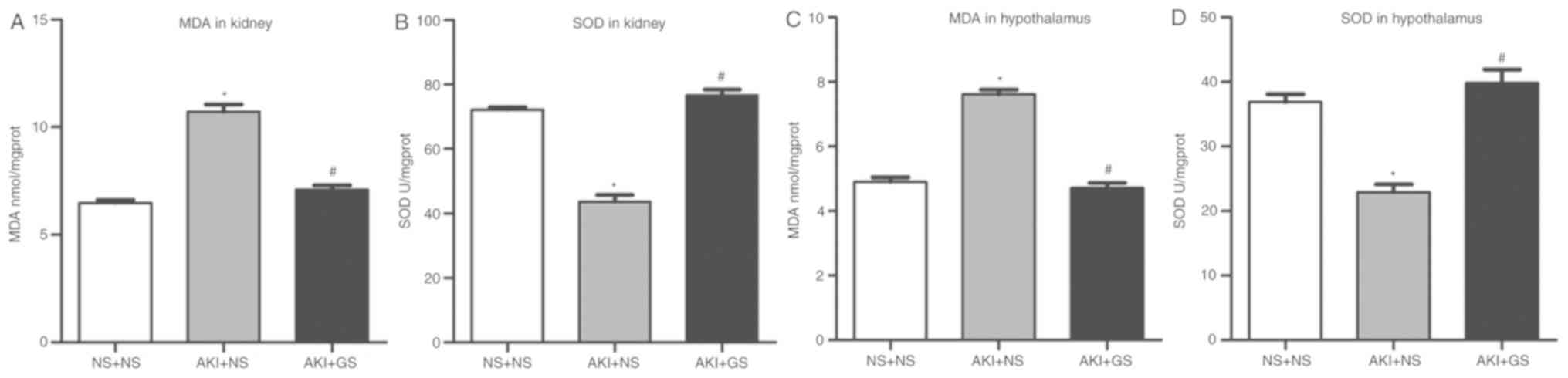
Effect of GS on the antioxidative
capacity in AKI rats
Kidney MDA level, an index of oxidant capacity, was
significantly increased in the AKI + NS group. Rats treated with GS
exhibited a significant decrease in kidney MDA level (Fig. 3A).
AKI rats exhibited a significant attenuation of
kidney SOD level compared with the AKI + NS and NS + NS groups. GS
treatment resulted in a significant increase of SOD levels in the
kidney tissues (Fig. 3B). There
were no significant differences in kidney MDA and SOD levels
between the NS + NS group and AKI + GS group (P>0.05). These
data demonstrated that oxidative stress is involved in the
impairment of kidney tissues following glycerol injection, and that
GS treatment attenuated the impairment.
In the hypothalamus, the level of MDA and SOD
exhibited a similar pattern. MDA levels were increased, whereas SOD
levels were decreased in the AKI + NS group. GS reversed the
impairments observed in the AKI + GS group (Fig. 3C and D; P<0.05). The results
suggested that GS serves a neuroprotective role in AKI rats,
presumably by attenuating damage caused by oxidative stress.
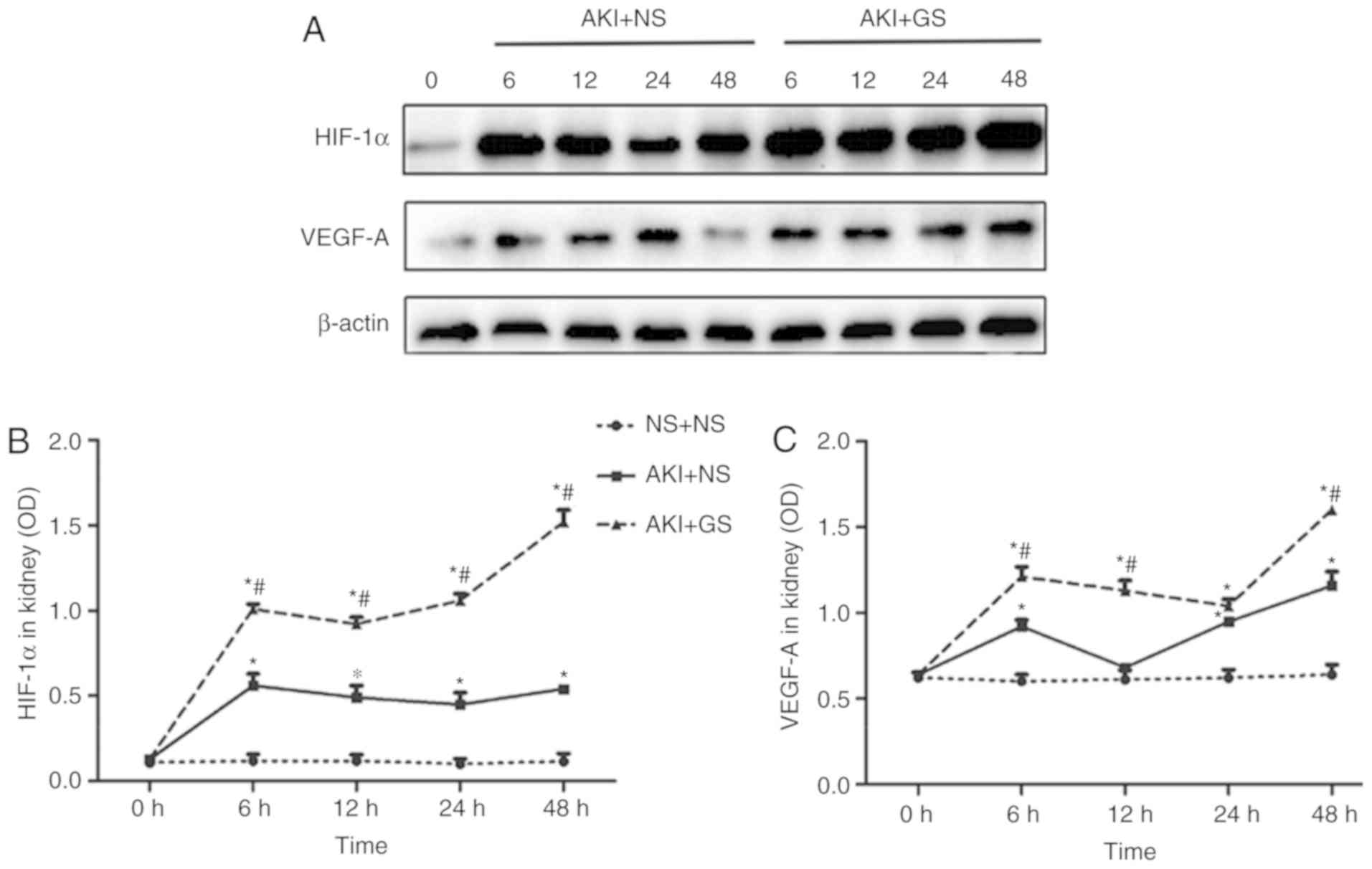
Effect of GS on the level of HIF-1α and
VEGF-A in kidney tissues of AKI rats
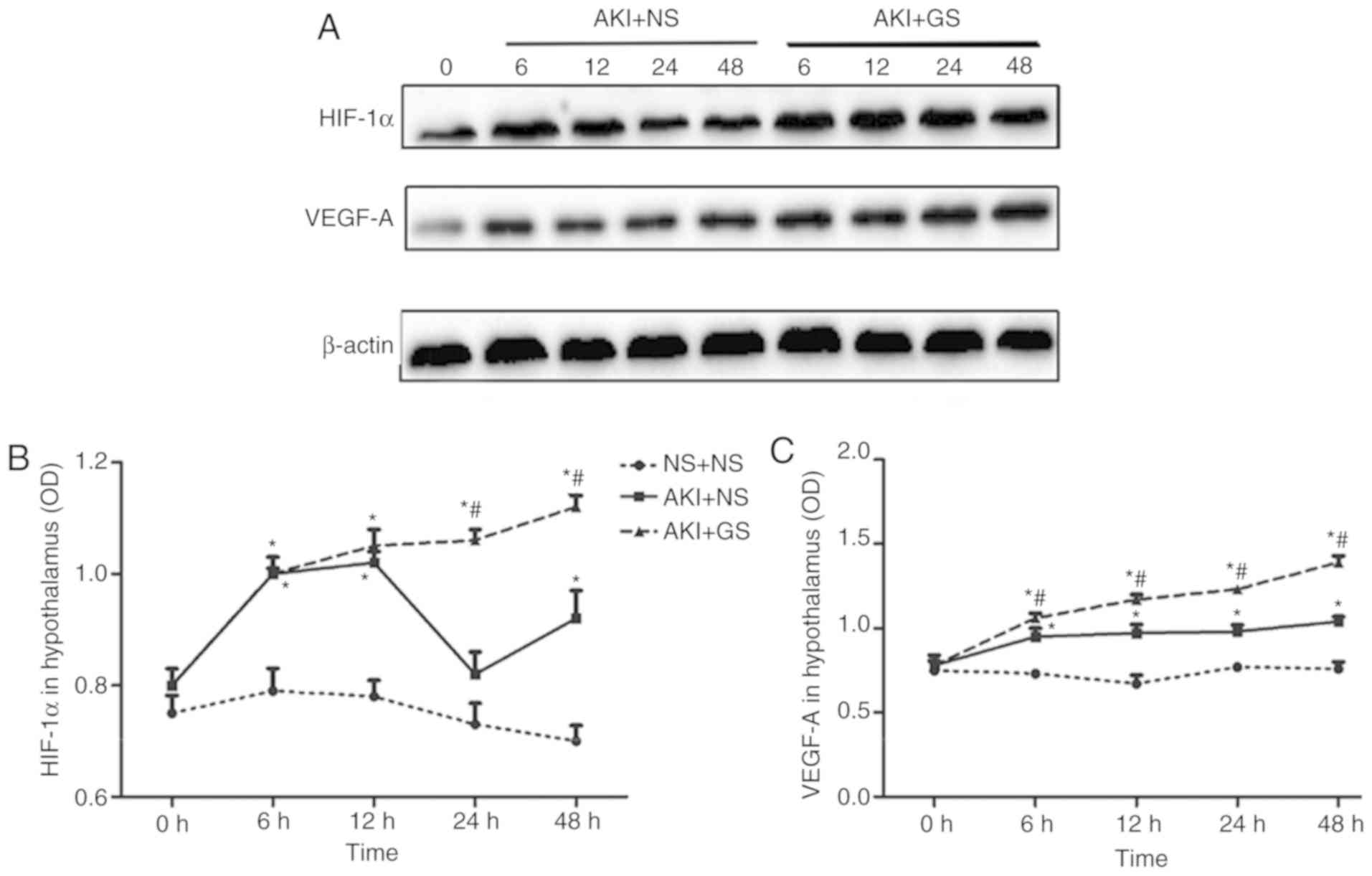
To fully understand the roles of HIF-1α and VEGF-A
during AKI, the changes in HIF-1α and VEGF-A expression levels in
kidney were investigated by western blot analysis at various time
points. A low expression of HIF-1α protein was observed in the
kidney at 0 h in all groups, whereas the expression of HIF-1α was
markedly increased in the AKI + GS group between 6 and 48 h,
compared with the AKI + NS group (Fig. 4A and B). HIF-1α expression in the
AKI + GS group was increased compared with that of the AKI + NS
group at 6 h, and this expression level was sustained between 12
and 24 h, peaking at 48 h (Fig.
4B; P<0.05).
Consistent with the changes in HIF-1α protein
levels, the expression levels of VEGF-A protein in the kidney also
exhibited similar changes (Fig. 4A
and C). The expression of VEGF-A protein in the AKI + GS group
was increased compared with the AKI + NS group at 6 h. VEGF-A
expression began to decrease slightly at 12 h, increasing again at
24 h, and peaking at 48 h (Fig.
4C). The results demonstrated that the HIF-1α and VEGF-A
expression levels were significantly increased in the AKI + NS
group, but that they were further increased following GS treatment
at 48 h.
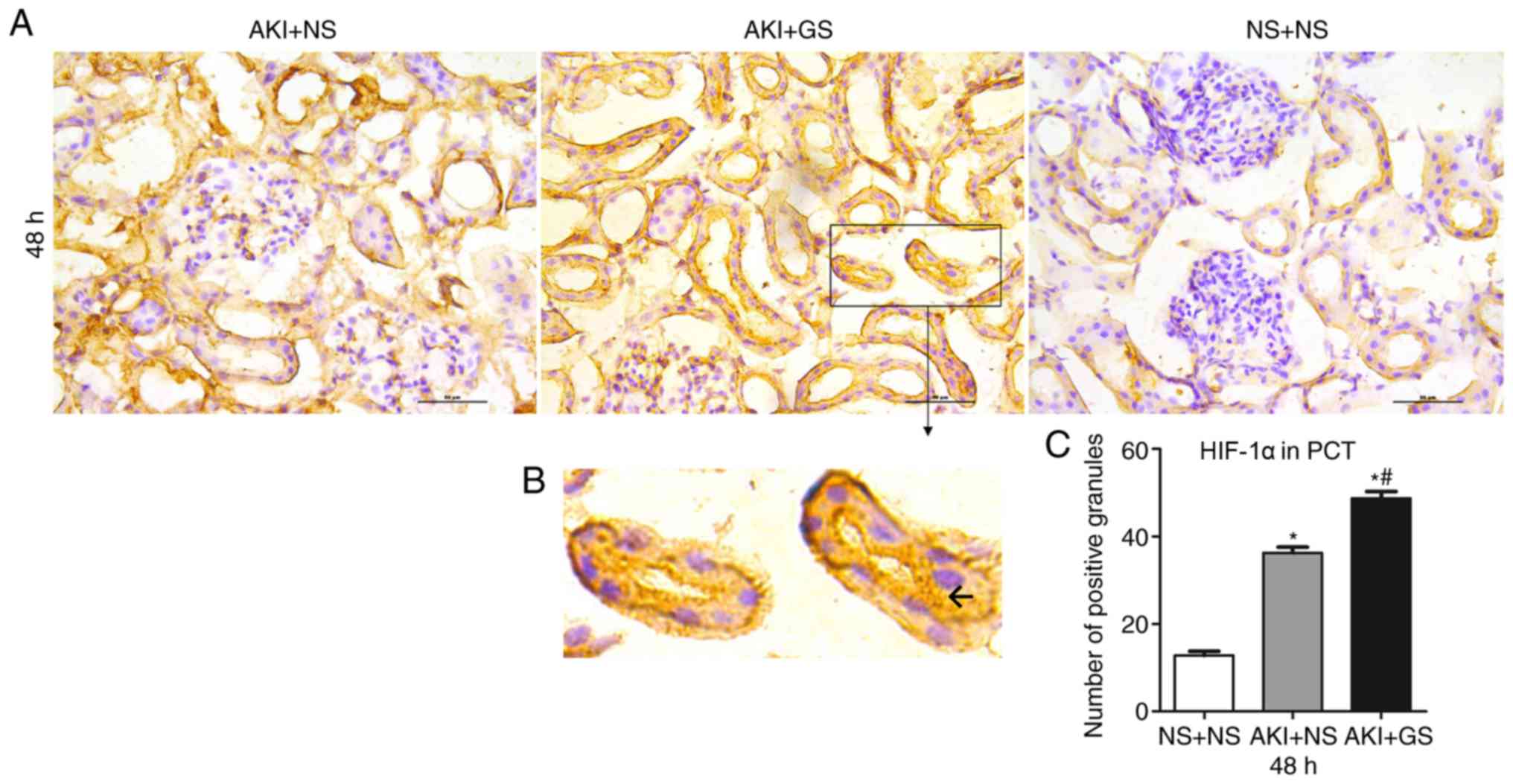
In addition, the expression of HIF-1α and VEGF-A in
kidney tissues was assessed using immunohistochemistry at 48 h.
HIF-1α-positive granules were stained brown and observed primarily
in the renal epithelial cells of proximal convoluted tubule (PCT)
(Fig. 5B). There were more
positive granules in the AKI + GS group compared with the AKI + NS
group, and the number of positive granules in the AKI + NS group
was markedly increased compared with the control group at 48 h
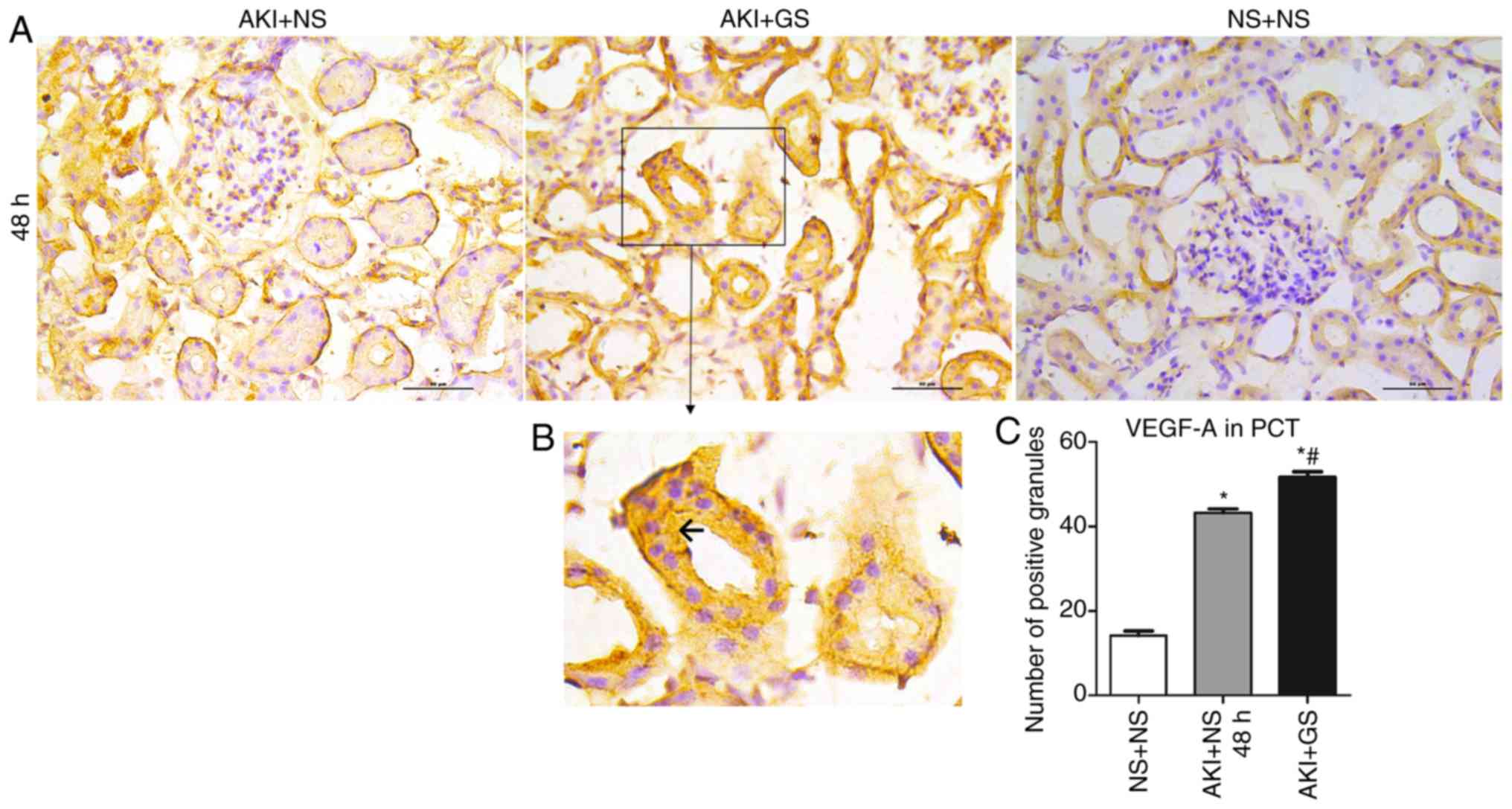
(Fig. 5A and C). Furthermore,
VEGF-A expression was also observed in the renal tubular epithelial
cells (Fig. 6B). These changes
were similar to those observed in HIF-1α expression (Fig. 6A and C).
Effect of GS on the levels of HIF-1α and
VEGF-A in the hypothalamus of AKI rats
In the present study, the expression levels of
HIF-1α and VEGF-A in the hypothalamus were examined following GS
exposure at 6, 12, 24 and 48 h after AKI. The expression levels of
the two proteins were increased in the AKI + NS group, and further
upregulated in the AKI + GS group at every time point (Fig. 7A-C), similar to the expression of
these proteins in the kidney tissues (Fig. 4A-C). These data suggested that
upregulation of HIF-1α and VEGF-A in the hypothalamus may
contribute to the protective effect of GS against kidney
dysfunctions following at 48 h after AKI.
Discussion
The results of the present study confirmed that
glycerol impaired renal function and induced AKI, as evidenced by
increased BUN and Cre levels and exacerbated renal structural
damage. In addition, rats with AKI exhibited notable kidney weight
abnormalities. Consistent with previous studies, the results of the
present study demonstrated that glycerol injection induced
oxidative stress damages, indicated by increased kidney MDA levels
and attenuated kidney SOD levels. Oxidative damages induced by
glycerol was also observed in the hypothalamus in the present
study. These results suggested that AKI not only induced renal
dysfunction, but also caused oxidative damages to the brain. The
present study provided novel evidence suggesting that AKI may
progress from single-organ failure to a multi-organ dysfunction
syndrome.
Previous studies have explored the protective role
of GS in AKI rats. The neuroprotective and renoprotective effects
of GS have been demonstrated in various studies (8,10).
GS protected neuron function against oxidative damage,
inflammation, ischemia and apoptosis (12,13) and improved cognitive function in
memory-impaired mice (12),
diabetic mice (27) and ageing
mice (14). In the present study,
GS was identified to attenuate AKI-induced oxidative neurotoxicity
in the kidney and in the hypothalamus. These data suggested that GS
may serve a protective role against AKI in the kidney-brain axis,
primarily in an antioxidative capacity.
To further investigate the protective effect of GS
in the kidney-brain axis, the potential molecular mechanisms were
investigated in the present study.
The response to ischemic or hypoxic conditions may
have a causal association with HIF-1α in the development and
progression of AKI. The results of the present study indicated that
HIF-1α and VEGF-A expression levels were increased in the kidney
and hypothalamus tissues during the processes of AKI. HIF-1α
regulates the adaptive response to hypoxia and other stresses
including glycolysis and angiogenesis (15,17,18,28). VEGF-A is a target gene of HIF-1α,
is a survival factor for renal tubule cells and has been implicated
in mediating protection against hypoxia and hypoglycemia (29). Recently, HIF-1α/VEGF-A activation
has also been demonstrated to have protective effects in multiple
animal models of renal injury, and in animal models of cerebral
ischemia (28,30,31). VEGF confers neuroprotection by
decreasing infarct size, delaying neuronal injury and stimulating
angiogenesis in ischemia brain injury (32), diabetic (33). The present study demonstrated that
the interaction between HIF-1α and VEGF-A may be involved in the
response of the kidney-brain axis to hypoxia following AKI. In
addition, HIF-1α and VEGF-A induction in the kidney-brain axis may
promote tissue adaptation and survival during renal injury, and
this effect may be a self-protective mechanism. However, it is
important to note that there were differences between the changes
in HIF-1α levels in kidney and hypothalamus tissues in the AKI rats
during the 48 h time period. The HIF-1α protein levels in the AKI +
NS group in kidney peaked at 6 h, and the hypothalamus HIF-1α
protein levels in the AKI + NS group peaked at 12 h. As the
interaction between kidney and brain has not been fully clarified,
additional studies are required.
In the present study, GS was identified to enhance
glycerol-induced upregulation of HIF-1α and VEGF-A in the
hypothalamus and kidney. The results indicated that, apart from
inhibiting the oxidative stress, the protective effect of GS may
partially be attributed to the involvement of HIF-1α and its
downstream gene VEGF-A in kidney-brain axis.
The molecular mechanisms underlying these results
are not completely understood. Previous studies have demonstrated
that the PI3K/Akt pathway was activated in response to hypoxia,
resulting in anti-apoptosis and renal cell survival (28,30,31). Whether GS promotes HIF-1α/VEGF-A
activation via the PI3K/Akt pathway, anti-apoptosis or
mitochondrial involvement requires further investigation.
In summary, the results of the present study
demonstrated that GS is a natural inducer of HIF-1α expression, and
that it protected the kidney and brain against AKI by decreasing
oxidative stress and upregulating VEGF-A. These data provide an
improved understanding of the neuroprotective and renal protective
role of GS in AKI, and indicate that HIF-1α may be a promising
therapeutic target for treating patients with AKI.
Funding
The present study was supported by Scientific
Research Cultivation Project, School of Medicine, Jiaxing
University and Scientific Research Project of Education Department
of Zhejiang Province (grant no. Y201942486).
Availability of data and materials
The data and materials generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
HM performed the majority of the experiments. CJ
designed the experiments, interpreted the data and reviewed the
manuscript. LX performed the experiments and analyzed the data. DC,
HL and YX provided the reagents/materials and technical assistance.
KM provided technical assistance. MW designed the experiments,
interpreted the data and wrote the manuscript. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the Animal
Care and Ethics Committee of Dalian Medical University and
performed according to the National Institute of Health Guide for
the care and Use of Laboratory Animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Malek M: Brain consequence of acute kidney
injury: Focusing on the hippocampus. Kidney Res Clin Pract.
37:315–322. 2018. View Article : Google Scholar
|
|
2
|
Makris K and Spanou L: Acute kidney
injury: Definition, pathophysiology and clinical phenotypes. Clin
Biochem Rev. 37:85–98. 2016.
|
|
3
|
Lu R, Kiernan MC, Murray A, Rosner MH and
Ronco C: Kidney-brain crosstalk in the acute and chronic setting.
Nat Rev Nephrol. 11:707–719. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu J, Pan X, Fu H, Zheng Y, Dai Y, Yin Y,
Chen Q, Hao Q, Bao D and Hou D: Effect of curcumin on
glycerol-induced acute kidney injury in rats. Sci Rep. 7:101142017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Al Asmari AK, Al Sadoon KT, Obaid AA,
Yesunayagam D and Tariq M: Protective effect of quinacrine against
glycerol-induced acute kidney injury in rats. BMC Nephrol.
18:412017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Panizo N, Rubio-Navarro A,
Amaro-Villalobos JM, Egido J and Moreno JA: Molecular mechanisms
and novel therapeutic approaches to rhabdomyolysis-induced acute
kidney injury. Kidney Blood Press Res. 40:520–532. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bugnicourt JM, Godefroy O, Chillon JM,
Choukroun G and Massy ZA: Cognitive disorders and dementia in CKD:
The neglected kidney-brain axis. J Am Soc Nephrol. 24:353–363.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen S, Li X, Wang Y, Mu P, Chen C, Huang
P and Liu D: Ginsenoside Rb1 attenuates intestinal
ischemia/reperfu-sion-induced inflammation and oxidative stress via
activation of the PI3K/Akt/Nrf2 signaling pathway. Mol Med Rep.
19:3633–3641. 2019.PubMed/NCBI
|
|
9
|
Xu ZM, Li CB, Liu QL, Li P and Yang H:
Ginsenoside Rg1 prevents doxorubicin-induced cardiotoxicity through
the inhibition of autophagy and endoplasmic reticulum stress in
mice. Int J Mol Sci. 19:pii: E3658. 2018. View Article : Google Scholar
|
|
10
|
Lü JM, Jiang J, Jamaluddin MS, Liang Z,
Yao Q and Chen C: Ginsenoside Rb1 blocks ritonavir-induced
oxidative stress and eNOS downregulation through activation of
estrogen receptor-beta and upregulation of SOD in human endothelial
cells. Int J Mol Sci. 20:pii: E294. 2019. View Article : Google Scholar
|
|
11
|
Van Kampen J, Robertson H, Hagg T and
Drobitch R: Neuroprotective actions of the ginseng extract G115 in
two rodent models of Parkinson's disease. Exp Neurol. 184:521–529.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang Q, Lin J, Zhang H, Liu Y, Kan M, Xiu
Z, Chen X, Lan X, Li X, Shi X, et al: Ginsenoside compound K
regulates amyloid β via the Nrf2/Keap1 signaling pathway in mice
with scopolamine hydrobromide-induced memory impairments. J Mol
Neurosci. 67:62–71. 2019. View Article : Google Scholar
|
|
13
|
Xu TZ, Shen XY, Sun LL, Chen YL, Zhang BQ,
Huang DK and Li WZ: Ginsenoside Rg1 protects against H2O2-induced
neuronal damage due to inhibition of the NLRP1 inflammasome some
signalling pathway in hippocampal neurons in vitro. Int J Mol Med.
43:717–726. 2019.
|
|
14
|
Chen L, Yao H, Chen X, Wang Z, Xiang Y,
Xia J, Liu Y and Wang Y: Ginsenoside Rg1 decreases oxidative stress
and down-regulates Akt/mTOR signalling to attenuate cognitive
impairment in mice and senescence of neural stem cells induced by
D-galactose. Neurochem Res. 43:430–440. 2018. View Article : Google Scholar
|
|
15
|
Qiu S, Chen X, Pang Y and Zhang Z:
Lipocalin-2 protects against renal ischemia/reperfusion injury in
mice through autophagy activation mediated by HIF1α and NF-κB
crosstalk. Biomed Pharmacother. 108:244–253. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang J, Liu C, Du X, Liu M, Ji X, Du H and
Zhao H: Hypoxia inducible factor 1α plays a key role in remote
ischemic preconditioning against stroke by modulating inflammatory
responses in rats. J Am Heart Assoc. 7:pii: e007589. 2018.
View Article : Google Scholar
|
|
17
|
Bergeron M, Gidday JM, Yu AY, Semenza GL,
Ferriero DM and Sharp FR: Role of hypoxia-inducible factor-1 in
hypoxia-induced ischemic tolerance in neonatal rat brain. Ann
Neurol. 48:285–296. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fan X, Heijnen CJ, van der Kooij MA,
Groenendaal F and van Bel F: The role and regulation of
hypoxia-inducible factor-1alpha expression in brain development and
neonatal hypoxic-ischemic brain injury. Brain Res Rev. 62:99–108.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Conde E, Alegre L, Blanco-Sánchez I,
Sáenz-Morales D, Aguado-Fraile E, Ponte B, Ramos E, Sáiz A, Jiménez
C, Ordoñez A, et al: Hypoxia inducible factor 1-alpha (HIF-1 alpha)
is induced during reperfusion after renal ischemia and is critical
for proximal tubule cell survival. PLoS One. 7:e332582012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hill P, Shukla D, Tran MG, Aragones J,
Cook HT, Carmeliet P and Maxwell PH: Inhibition of hypoxia
inducible factor hydroxy-lases protects against renal
ischemia-reperfusion injury. J Am Soc Nephrol. 19:39–46. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Palazon A, Tyrakis PA, Macias D, Veliça P,
Rundqvist H, Fitzpatrick S, Vojnovic N, Phan AT, Loman N, Hedenfalk
I, et al: An HIF-1α/VEGF-A axis in cytotoxic T cells regulates
tumor progression. Cancer cell. 32:669–683. 2017. View Article : Google Scholar
|
|
22
|
Ho QT and Kuo CJ: Vascular endothelial
growth factor: Biology and therapeutic applications. Int J Biochem
Cell Biol. 39:1349–1357. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang Q, Zhong W, Hu Z and Tang X: A
review of the role of cav-1 in neuropathology and neural recovery
after ischemic stroke. J Neuroinflammation. 15:3482018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Singh Angom R, Wang Y, Wang E, Pal K,
Bhattacharya S, Watzlawik JO, Rosenberry TL, Das P and Mukhopadhyay
D: VEGF receptor-1 modulates amyloid β 1-42 oligomer-induced
senescence in brain endothelial cells. FASEB J. 33:4626–4637. 2019.
View Article : Google Scholar
|
|
25
|
Greenberg DA and Jin K: From angiogenesis
to neuropathology. Nature. 438:954–959. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang HA, Wang M, Zhou J, Yao QY, Ma JM
and Jiang CL: Protective effect of ginsenoside against acute renal
failure and expression of tyrosine hydroxylase in the locus
coeruleus. Physiol Res. 59:61–70. 2010.
|
|
27
|
Tian Z, Ren N, Wang J, Zhang D and Zhou Y:
Ginsenoside ameliorates cognitive dysfunction in type 2 diabetic
gotokakizaki rats. Med Sci Monit. 24:3922–3928. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Song N, Zhang T, Xu X, Lu Z, Yu X, Fang Y,
Hu J, Jia P, Teng J and Ding X: miR-21 protects against
ischemia/reperfusion-induced acute kidney injury by preventing
epithelial cell apoptosis and inhibiting dendritic cell maturation.
Front Physiol. 9:7902018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang Y, Nakano D, Guan Y, Hitomi H,
Uemura A, Masaki T, Kobara H, Sugaya T and Nishiyama A: A
sodium-glucose cotransporter 2 inhibitor attenuates renal capillary
injury and fibrosis by a vascular endothelial growth
factor-dependent pathway after renal injury in mice. Kidney Int.
94:524–535. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tanaka T, Kojima I, Ohse T, Inagi R,
Miyata T, Ingelfinger JR, Fujita T and Nangaku M: Hypoxia-inducible
factor modulates tubular cell survival in cisplatin nephrotoxicity.
Am J Physiol Renal Physiol. 289:F1123–F1133. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang H, Misaki T, Taupin V, Eguchi A,
Ghosh P and Farquhar MG: GIV/girdin links vascular endothelial
growth factor signaling to Akt survival signaling in podocytes
independent of nephrin. J Am Soc Nephrol. 26:314–327. 2015.
View Article : Google Scholar :
|
|
32
|
Sun Y, Jin K, Xie L, Childs J, Mao XO,
Logvinova A and Greenberg DA: VEGF-induced neuroprotection,
neurogenesis, and angiogenesis after focal cerebral ischemia. J
Clin Invest. 111:1843–1851. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Storkebaum E, Lambrechts D and Carmeliet
P: VEGF: Once regarded as a specific angiogenic factor, now
implicated in neuroprotection. BioEssays. 26:943–954. 2004.
View Article : Google Scholar : PubMed/NCBI
|