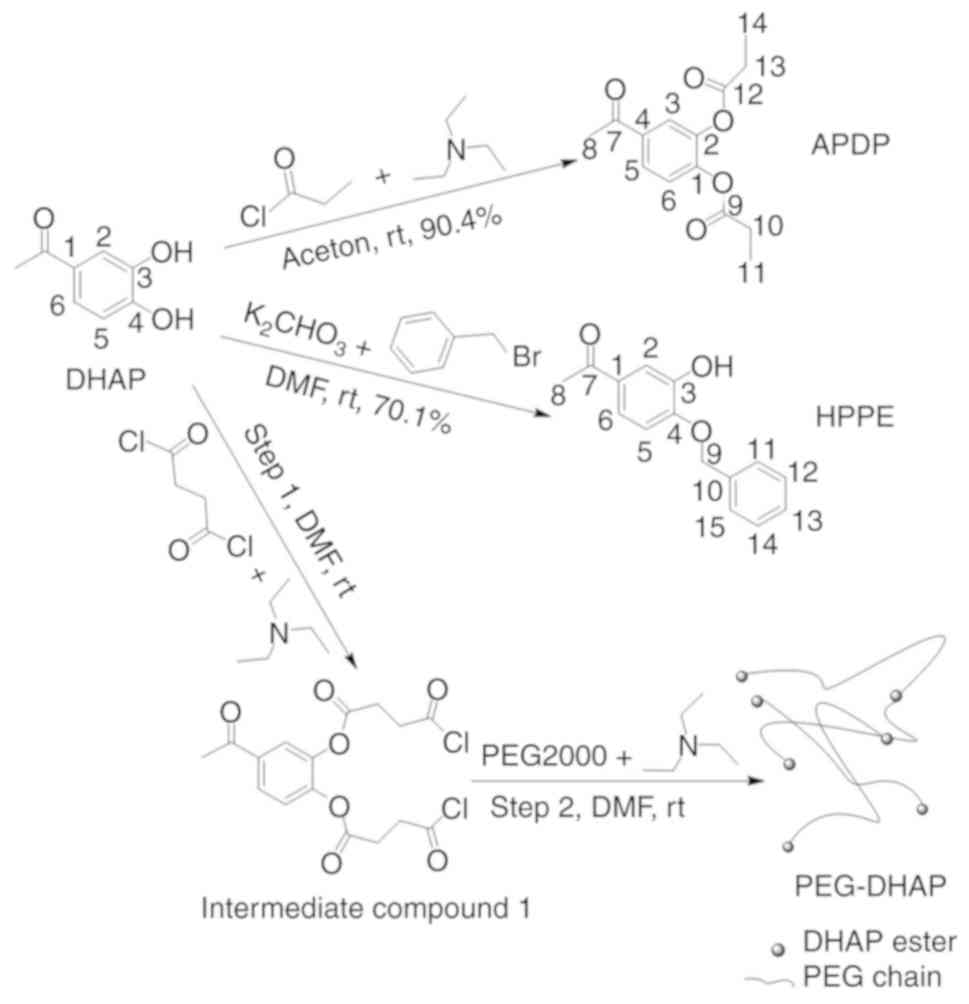
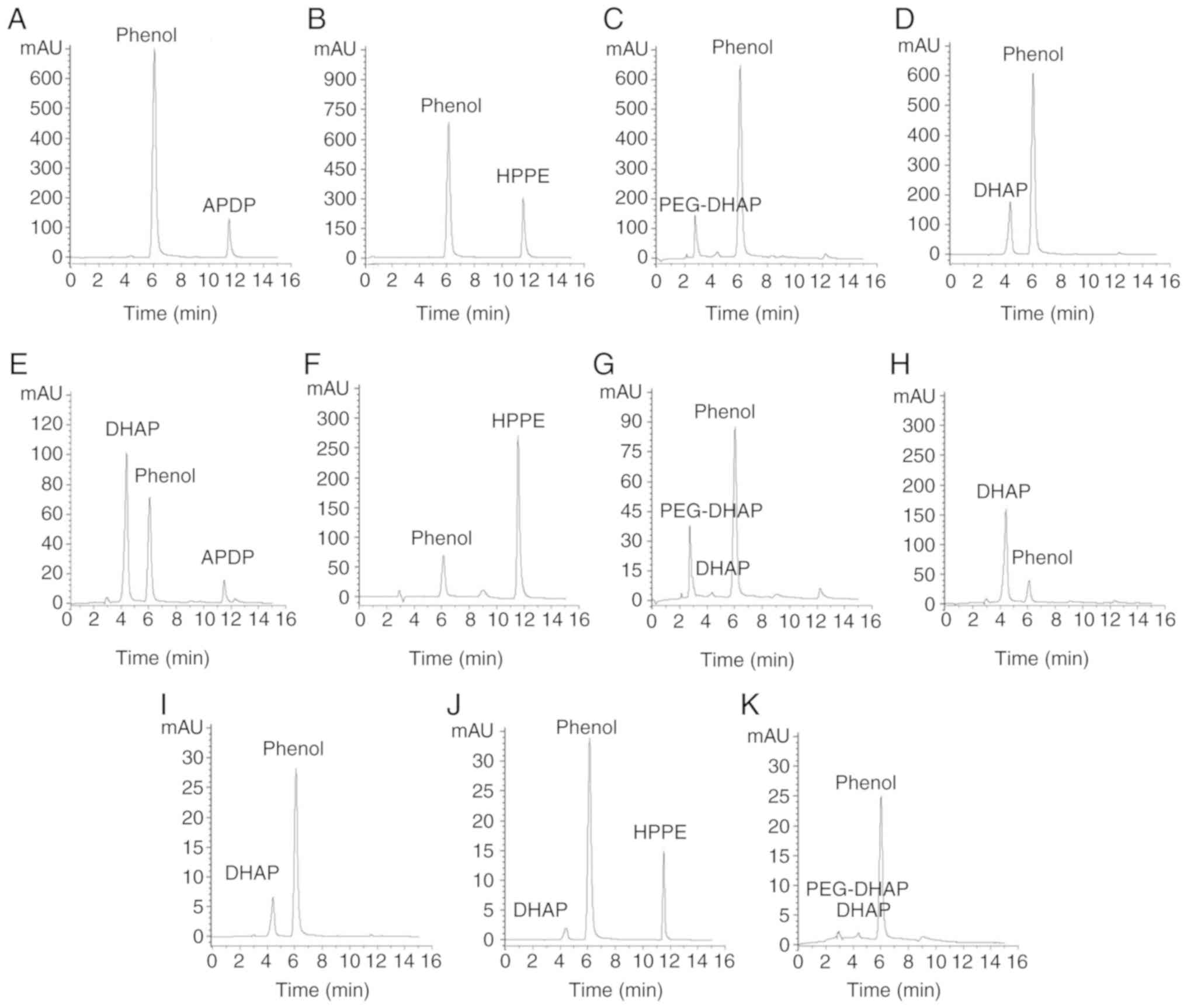
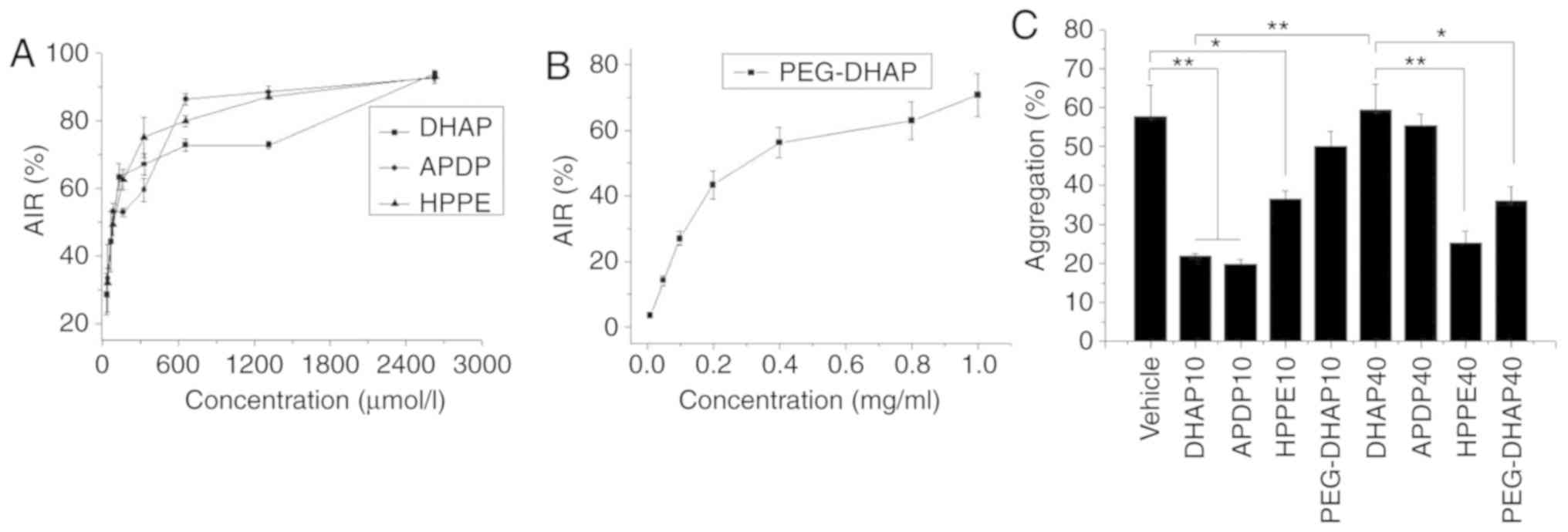
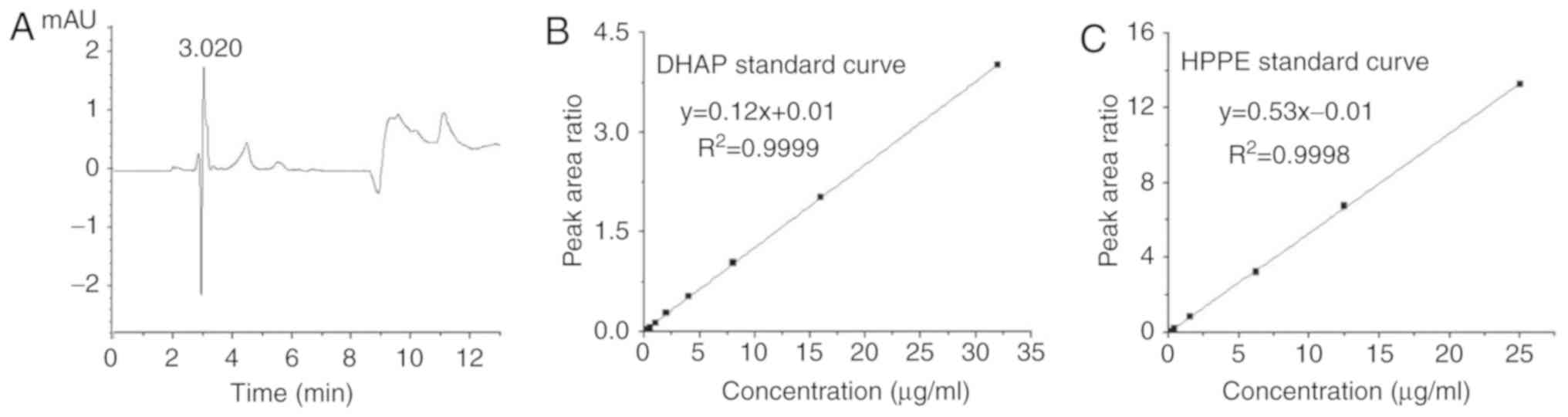
|
1
|
Beijing Pharmaceutical Industry Research
Institute, Chinese People's Liberation Army 157 Hospital: Study on
the active constituents of leaves of Ilex pubescens Hook et Arn var
glaber Chang. Chin Trad and Herb Drugs Commun. 8:7–10. 1977.In
Chinese.
|
|
2
|
Research Group of the Therapeutic Effects
of Qingxintong: Summary of the treatment effects of Qingxintong on
angina pectoris of coronary heart disease. Beijing Pharm Industry.
1:23–27. 1983.In Chinese.
|
|
3
|
Chinese People's Liberation Army 157
Hospital: Clinical observation of 50 cases of coronary heart
disease treated with Qingxintong injection. Beijing Pharm Industry.
1:27–29. 1983.In Chinese.
|
|
4
|
Beijing pharmaceutical industry research
institute: Effect of Qingxintong on thrombosis in patients with
coronary heart disease. Beijing Pharm Industry. 1:16–17. 1983.In
Chinese.
|
|
5
|
Beijing Pharmaceutical Research Institute:
Research on the pharmacologic effect of leaves of Qingxintong on
coronary heart disease. Chin Trad and Herb Drugs. 11:358–366.
1980.In Chinese.
|
|
6
|
Lin CL, Zhang ZX and Xu YJ: Therapeutic
mechanism of qingxintong on chronic obstructive pulmonary disease.
Chin J Tuberculosis and Respirat. 18:97–98. 1995.In Chinese.
|
|
7
|
Lin CL, Zhang ZX, Xu YJ and Ni W: Effects
of qingxintong on hemodynamics and atrial natriuretic peptide and
cyclic glucosides in patients with chronic obstructive pulmonary
disease. Chin J Integrated Trad Chin Western Med. 15:131–133.
1995.In Chinese.
|
|
8
|
Lin CL, Zhang ZX and Xu YJ: Effects of
3.4-dihydroxyaceto-phenine on hemorheology and plasma levels of
TXA2, CD62P in patients with chronic pulmonary heart disease. J
Clin Internal Med. 19:196–198. 2002.In Chinese.
|
|
9
|
Sun YP, Ma TY and Wu XR: Clinical analysis
and mechanism of treatment of pregnancy-induced hypertension with
Qingxintong. Practical J Obstet Gynecol. 7:141–143. 1991.In
Chinese.
|
|
10
|
Huang YP and Ma TY: Treatment of
intrauterine growth retardation with qingxintong. Chin J Obstet
Gynecol. 28:333–336. 1993.In Chinese.
|
|
11
|
Wu XR, Li Y, Yang DS, Han BJ, Si YZ, Shan
ZZ, Ma TY, Wen LZ, Sun YP and Huang YP: The utero-placental
circulation, eugenics and the prevention and treatment of high risk
pregnancies. J Tongji Med Univ. 14:1–7. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang YP, Ye DY, Wu P, Huang YF, Ying HG
and Ma TY: The therapeutic effectiveness of inhaled DHAP in the
treatment of intrauterine growth retardation. Acta Med Univ Sci Et
Technol Huazhong. 31:192–194. 2002.In Chinese.
|
|
13
|
Wang Z, Gao HQ, An Y, Zhu GQ and Yang ZM:
Effect of 3,4-dihydroxyacetophenone on rabbit platelet function.
Zhongguo Yao Li Xue Bao. 5:187–192. 1984.In Chinese. PubMed/NCBI
|
|
14
|
Wang Z, Gao HQ, Zhu GQ, An Y and Huang RS:
Effect of 3,4-dihydroxyacetophenone on the generation of PGI2-like
substances in the rat aorta. Zhongguo Yao Li Xue Bao. 7:37–40.
1986.In Chinese. PubMed/NCBI
|
|
15
|
Xu H, Wang Z and An Y: Effect of
3,4-dihydroxyacetophenone on platelet phosphodiesterase in rabbits.
Acta Pharmacol Sin. 8:159–162. 1986.In Chinese.
|
|
16
|
Wang Z, An Y, Liu Z, Zhu GQ and Huang RS:
Effect of 3,4-dihy-droxyacetophenone on TXA2 release from rabbit
platelets. Yao Xue Xue Bao. 22:330–334. 1987.In Chinese. PubMed/NCBI
|
|
17
|
Li S, Li Y, Xiong Z and Wu X: Influence of
Qingxingtong on platelet function of PIH-patients. J Tongji Med
Univ. 23:305–307. 1994.In Chinese.
|
|
18
|
Wang Z, Huang RS, Gao YH, An Y and Qiang
ZG: 3,4-Dihy-droxyacetophenone-a cyclooxygenase inhibitor (brief).
Acta Pharmacol Sin. 1:351988.In Chinese.
|
|
19
|
Shi L, Qin ZH and Gao SX: Effect of
3,4-dihydroxyacetophe-none on the membrane fluidity of platelets by
fluorescence polarization analysis. Zhongguo Yao Li Xue Bao.
7:149–151. 1986.In Chinese. PubMed/NCBI
|
|
20
|
Shan Z, Chang C, Feng L and Wu X: The
regulatory effect of 3,4-dihydroxyacetophenone on tPA and PAI
activity of the vascular endothelial calls. Pharmacol Clin Chin
Materia Med. 6:33–35. 1995.In Chinese.
|
|
21
|
Beijing Institute of Pharmaceutical
Industry, The People's Liberation Army 157 Hospital:
Pharmacological study of bald holly leaves on coronary heart
disease. Chin Pharm J. 15:421980.In Chinese.
|
|
22
|
Wu P, Huang YP and Ye DJ: Advances in
research on the mechanism of qingxintong promoting blood
circulation and removing blood stasis. Chin Trad Herbal Drugs.
23:277–279. 2001.In Chinese.
|
|
23
|
Ullah F, Wang DX, Ming Z and Yu SB:
Effects of 3,4-dihydroxy-acetophenone (3,4-DHAP) on hypoxic
pulmonary and systemic vascular response in dogs. J Tongji Med
Univ. 15:26–30. 1995. View Article : Google Scholar
|
|
24
|
Hong ZG, Wang DX, Jin S, Zhang J, Wang XL
and Ye DY: Effect of 3,4-dihydroxyacetophenone on delayed rectifier
potassium current of the intrapulmonary artery smooth muscle cells
in rats. Chin Pharmacol Bull. 18:386–389. 2002.In Chinese.
|
|
25
|
Yang DS, Xi-rui W and Ting-yuan M: Effects
of 3,4-dihy-droxyacetophenone on the biosynthesis of TXA2 and PGI2
in human placental villus and umbilical artery segments in vitro.
Prostaglandins. 38:497–504. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hang YP, Ye DJ and Ma TJ: Effect of
Qingxintong on nitric oxide synthase and plasma endothelin in
placental vascular wall of patients with pregnancy induced
hypertension. Chin J Obstet Gynecol. 31:667–669. 1996.In
Chinese.
|
|
27
|
Li G, Wu P, Zhang DJ, Ye DY and Li F:
Mechanism of inhibition of 3, 4-dihydroxyacetophenone on
LPS-induced apoptosis of RAW264.7 macrophages in mice. Chin Trad
Herbal Drugs. 36:1835–1838. 2005.In Chinese.
|
|
28
|
Wu P, Zhang L, Zhou X, Li Y, Zhang D, Wan
J and Ye D: Inflammation pro-resolving potential of
3,4-dihydroxyaceto-phenone through 15-deoxy-Δ12,14-prostaglandin J2
in murine macrophages. Int Immunopharmacol. 7:1450–1459. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang D, Liu T, Liu J, Cui X, Guo J, Wang
J and Ye D: Relationship between therapeutic and preventive effect
of dihydroxyaceto-phenone in treating atherosclerosis and the
Toll-like-receptor 4 pathway. Trad Chin Drug Res Clin Pharmacol.
20:404–407. 2009.In Chinese.
|
|
30
|
Zhang D, Liu T, Cui X, Liu J, Guo J, Wang
J and Ye D: Preventive effect of 3,4-dihydroxyacetophenone on
atherosclerosis and role of visfatin expression. Chin J
Pathophysiol. 26:1700–1703. 2010.In Chinese.
|
|
31
|
Zhang D, Gong Y, Liu J, Wang J, Wang L and
Liu T: Effects of 3,4-dihydroxyacetophenone on 5-lipoxygenase in
macrophages of atherosclerosis plaque. Trad Chin Drug Res Clin
Pharmacol. 23:243–246. 2012.In Chinese.
|
|
32
|
Zhang DJ, Liu JY, Wang L, Wang J, Li W,
Zhuang B, Hou J and Liu T: Effects of 3,4-dihydroxyacetophenone on
the hypercho-lesterolemia-induced atherosclerotic rabbits. Biol
Pharm Bull. 36:733–740. 2013. View Article : Google Scholar
|
|
33
|
Li SX, Li Y, Shan ZZ, Xiong ZM, Wu XR and
Yin XG: Pharmacokinetics of Qingxintong in rabbits. Acta
Universitatis Medictnae Tangji. 22:91–93. 1993.In Chinese.
|
|
34
|
Pang X, Fu G and Zuo M: Comparison of
bioavailability of Qingxintong by three administration routes. Trad
Chin Drug Res Clin Pharmacol. 13:31–32. 2001.In Chinese.
|
|
35
|
Eun JK, Jong WA, Hye SL and Wolfram C:
Determination of peripheral catecholO-methyltransferase (COMT)
activity in vivo using
(2-14C)-3′,4′-dihyroxyacetophenone. Arch Pharm Res.
14:290–294. 1991. View Article : Google Scholar
|
|
36
|
Montenegro L, Carbone C, Maniscalco C,
Lambusta D, Nicolosi G, Ventura CA and Puglisi G: In vitro
evaluation of quercetin-3-O acyl esters as topical prodrugs. Int J
Pharm. 336:257–262. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jin YY, Zhang JS, Zhang Y and Zhang YH:
Studies on the intestinal adsorption of crocin in rats and
determination of the partition coefficient. J Chin Pharm Univ.
35:247–257. 2004.In Chinese.
|
|
38
|
Fang L: Pharmaceutics. 8th edition.
People's Medical Publishing House; Beijing: pp. 137–139. 2016, In
Chinese.
|
|
39
|
Zhou X, Zhang X, Han S, Dou Y, Liu M,
Zhang L, Guo J, Shi Q, Gong G, Wang R, et al: Yeast
microcapsule-mediated targeted delivery of diverse nanoparticles
for imaging and therapy via the oral route. Nano Lett.
17:1056–1064. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
You QD: Medicinal Chemistry. 8th edition.
People's Medical Publishing House; Beijing: pp. 462016, In
Chinese.
|
|
41
|
Liu CX: Introduction to Pharmacokinetics.
Liu D: China Academic Press Publication; Beijing: pp. 215–217.
1984, In Chinese.
|