Introduction
Due to their self-renewal capabilities,
multi-directional differentiation potential and the fact that they
are easily accessible, bone marrow-derived mesenchymal stem cells
(BMSCs) have been applied in tissue engineering, especially in the
construction of bone defects models (1). As bone defects such as osteoporosis
or osteonecrosis of femoral head are primarily due to the decreased
osteogenic differentiation of BMSCs (2), it is crucial to develop strategies
to promote the osteogenic differentiation of BMSCs in a clinical
setting (3).
Sclerostin (SOST), the protein product of the
SOST gene, serves a key role in inhibiting osteoblast
activity (4). It is also
well-known that SOST negatively regulates bone formation
through antagonizing the Wnt/β-catenin pathway (5). In addition, knockdown of the
SOST gene in mice results in a high bone mass phenotype and
resistance to bone loss. In addition, antibodies directed against
SOST stimulate bone formation and represent a novel therapeutic
option in the anabolic treatment of osteoporotic conditions
(6,7). Mirza et al (8) demonstrated that serum SOST levels
were inversely associated with estrogen levels (8). Kim et al (9) demonstrated that estrogen signaling
functions as an negative regulator of SOST expression
involving the Wnt/β-catenin/estrogen receptor α (ERα) pathway in
human osteoblasts.
Icariin, extracted from Herba epimedii, has
been suggested to significantly improve osteogenesis to decrease
bone loss in ovariectomized rats and steroid-induced osteonecrosis
of the femoral head (10).
However, recent studies have demonstrated that icariin is
enzymatically hydrolyzed, and then subsequently metabolized to
desmethyl icaritin and icaritin; desmethyl icaritin is suggested to
have more potent pharmacological effects compared with icariin
(11,12). Icaritin is able to promote the
proliferation and differentiation of osteoblasts and enhance matrix
calcification due to its estrogen-like activity (13). In addition, icaritin may decrease
the activity of osteoclasts in vitro and increase the
expression of osteogenic-associated mRNA levels in human BMSCs
(hBMSCs) (14). The osteogenic
effects of icaritin have been clearly described, but the underlying
mechanisms remain unclear. As icaritin has been suggested to
regulate the Wnt/β-catenin pathway (10) and ERα (9), which are closely associated with
SOST, we hypothesized that icaritin may promote osteogenesis
through inhibiting SOST.
The present study assessed the effects of icaritin
on osteogenesis of hBMSCs and explored the role of SOST in
icaritin-induced osteogenesis of hBMSCs.
Materials and methods
Reagents
Icaritin (>98% purity) was purchased from
Shanghai U-sea Biotech Co. Ltd. modified Eagle's medium of alpha
(α-MEM) and fetal bovine serum (FBS) were both obtained from
Hyclone (Hyclone; GE Healthcare Life Sciences); The Cell Counting
Kit-8 (CCK-8) was purchased from Dojindo Molecular Technologies,
Inc. Dimethyl sulfoxide (DMSO), trypsin and TRIzol®
reagent were purchased from Gibco; Thermo Fisher Scientific, Inc.;
the alkaline phosphatase (ALP) activity kit, enhanced
chemiluminescent detection reagent and micro-BCA assay kit were
obtained from Beyotime Institute of Biotechnology. DKK-1 and
ICI182780 were purchased from R&D Systems, Inc. The RNA
extraction kit was purchased from Takara Bio, Inc. Reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
primers were obtained from Invitrogen; Thermo Fisher Scientific,
Inc. The antibodies used were obtained from Cell Signaling
Technology, Inc., unless otherwise indicated. Other reagents used
in the experiment were purchased from Sigma-Aldrich; Merck KGaA.
Icaritin was dissolved in DMSO and the final concentration of DMSO
was 0.05% (v/v). ICI 182780 was dissolved in DMSO and stored at
4°C. Cells were pretreated with ICI 182780 (1 µM) for 30 min
at 37°C, followed by icaritin treatment.
Cell culture of hBMSCs and SOST
transfection
hBMSCs were purchased from Cyagen Biosciences, Inc.
(cat. no., HUXMA-01001). The hBMSCs were cultured in α-MEM
containing 10% FBS and 1% penicillin-streptomycin in a humidified
incubator with 5% CO2 at 37̊C. After reaching 80-90%
confluence, the adherent cells were harvested with trypsin for 1
min and sub-cultured at a ratio of 1:2. Cells from passages 3-6
were used in the following experiments.
The SOST gene overexpression lentivirus was
constructed and purchased from Shanghai GeneChem Co., Ltd. Briefly,
the SOST gene was amplified using PCR with the following
primers: SOST forward, CAC CGC TGC ACT TCA CCC GCT ACG TTT
CAA GAG AAC GTA GCG GGT GAA GTG CAG CTT TTT TG; and SOST
reverse, GAT CCA AAA AAG CTG CAC TTC ACC CGC TAC GTT CTC TTG AAA
CGT AGC GGG TGA AGT GCA GC. The gene was then cloned into plenti-U
bcP-IKZF2-V2-3xHA-pGK-Pur plasmid (Addgene; plasmid no. 107393) by
in vitro recombination. Lentiviruses were generated by
transient transfection of 293FT packaging cells (Invitrogen; Thermo
Fisher Scientific, Inc.) using the calcium phosphate method. After
72 h of transfection, the supernatant was collected. The
supernatant was filtered with a 0.45 µm syringe filter
(Sigma-Aldrich; cat. no. CLS431220), centrifuged for 90 min in at
4°C at 42,000 × g, and resuspended in 200 µl α-MEM. For
lentivirus transduction, hBMSCs from the third passage were seeded
in a 6-well plate (5×106 cells/well). After 24 h, the
lentivirus solution was added to each well in serum-free
Opti-MEM® (Invitrogen; Thermo Fisher Scientific, Inc.).
The medium was replaced with fresh complete medium after 8 h. The
cells were selected with puromycin (10 µg/ml) following
transduction for 48 h. Transduction efficiency was examined by PCR
and western blot analysis. The transduced cells were termed
BMSCs-vector (lentiviral vector only) and BMSCs-SOST (lentiviral
vector containing the SOST gene).
Identification of hBMSCs
An hBMSC suspension of 1×106 cells/ml was
prepared. The cells were washed twice with cold PBS, centrifuged at
1,000 × g for 5 min at 4°C and resuspended in 100 ml stain buffer
(BD Biosciences). The resuspended cells were incubated with
phycoerythrin (PE)-labeled primary antibodies against surface
markers integrin-β1 (CD29; cat. no. 34971T; 1:400) and
hematopoietic progenitor cell antigen CD34 (CD34; cat. no. 3569S;
1:400), as well as a corresponding isotype control antibody, at
room temperature according to the manufacturers' protocol. The
positively stained cells were immediately analyzed by flow
cytometry using FlowJo software 8.7.1 (FlowJo, LLC). hBMSCs from
passages 3-6 were used in the experiments.
Cell proliferation assay
To examine the effects of icaritin on hBMSC
proliferation, the cells were seeded into a 96-well plate (5000
cells/well). The medium was removed after 24 h, then the cells were
treated with complete medium with or without different
concentrations of icaritin (0.01, 0.1, 1 and 10 µM)
accordingly for 4 days. The cells were treated with 10% CCK-8 in
100 µl complete medium for 40 min at 37̊C each day. The
absorbance was determined at 450 nm using a microplate reader
(ELX808; BioTek Instruments, Inc.). Cell proliferative rate (%) was
calculated as: Optical density (OD) treatment/OD control x 100.
Osteogenic differentiation and
ALP/Alizarin Red S staining
hBMSCs were seeded onto a 24-well plate
(105 cells/well) and cultured in osteogenic induction
medium (OIM; Cyagen Biosciences, Inc.), consisting of 1 nM
dexamethasone, 50 mM L-ascorbic acid-2-phosphate and 20 mM
β-glycerolphosphate in complete medium, with various doses of
icaritin (0.01, 0.1 and 1 µM) for 21 days. The OIM was
changed every 3 days. ALP staining and Alizarin Red S staining were
conducted 7 and 14 days following the induction, respectively, and
were performed separately to evaluate the positive rates of ALP and
calcium deposit formation. For ALP staining, cells were fixed with
10% formaldehyde for 15 min, rinsed three times with deionized
water, and treated with the BCIP®/NBT solution
(Sigma-Aldrich; Merck KGaA) for 20 min. After an additional
washing, the stained cultures were photographed (magnification,
×10). To measure ALP activity, cell lysates were tested using a
commercial ALP assay kit. For Alizarin red S staining, hBMSCs were
stained with pH 4.2, 0.1% Alizarin red S (Aladdin Industries
Corporation) for 5 min and the images were captured using a
scanner. The calcium deposition was dissolved in 10 cetylpyridinium
chloride (Sigma-Aldrich; Merck KGaA), and the absorbance of the
extracts was determined at 570 nm.
RT-qPCR
Cellular RNA was extracted from the hBMSCs cultured
in OIM with or without icaritin using the RNA extraction kit, and
cDNA was synthesized with the M-MLV reverse transcriptase according
to the manufacturer's protocols. qPCR was conducted using Power
SYBR® Green PCR Master Mix on the ABI StepOnePlus System
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The
thermocycler conditions were as follows: Initial denaturation at
95°C for 5 min; 40 cycles at 95°C for 15 sec and 60°C for 1 min;
and a final extension at 72°C for 5 min. The primers are listed in
Table I. The target gene
expression was calculated using the 2-ΔΔCq method
(15), and were normalized using
the expression level of β-actin.
 | Table IPrimer sequences used in PCR
analysis. |
Table I
Primer sequences used in PCR
analysis.
| Genes | Forward primer
sequence (5′-3′) | Reverse primer
sequence (5′-3′) |
|---|
| β-actin |
GTCATCCATGGCGAACTGGT |
CGTCATCCATGGCGAACTGG |
| ALP |
CAAGGATGCTGGGAAGTCCG |
CTCTGGGCGCATCTCATTGT |
| Runx2 |
GCTTCATTCGCCTCACAAAC |
GTAGTGACCTGCGGAGATTAAC |
| OCN |
AAATAGCCCTGGCAGATTCC |
CAGCCTCCAGCACTGTTTAT |
|
β-catenin |
CTTCACCTGACAGATCCAAGTC |
CCTTCCATCCCTTCCTGTTTAG |
| SOST |
GGGCAACTGTAGATGTGGTT |
GTCCCGAAGGAGAATTGTGTAG |
Protein extraction and western blot
analysis
Cell samples were rinsed twice with cold PBS and
harvested in the extraction buffer (cat. no. P0013; Beyotime
Institute of Biotechnology). The lysate was centrifuged at 4°C with
16,000 × g for 30 min and the suspension was collected. Then, the
protein content was examined using a BCA kit. Total proteins (20
µg) were separated by 12% (w/v) SDS-PAGE. The proteins were
then transferred onto a polyvinylidene fluoride (PVDF) membrane.
The membrane was washed and blocked with freshly prepared TBST
containing 5% (w/v) non-fat dry milk for 90 min at room
temperature. The membrane was incubated with antibodies targeting
β-actin (1:1,000; cat. no. 4970S; Cell Signaling Technology, Inc.),
osteocalcin (OCN; 1:1,000; cat. no. MAB1419; R&D Systems,
Inc.), RUNX family transcription factor 2 (Runx2; 1:1,000; cat. no.
12556; Cell Signaling Technology, Inc.), ALP (1:1,000; cat. no.
AF2910; R&D Systems, Inc.), β-catenin (1:1,000; cat. no. 8480S;
Cell Signaling Technology, Inc.) and SOST (1:1,000; cat. no.
MAB1406; R&D Systems, Inc.) overnight at 4°C. Following three
washes, the membrane was incubated with horseradish
peroxidase-conjugated goat anti-rabbit or anti-mouse secondary
antibody (1:1,000; cat. nos. 93702 and 7076S, respectively; Cell
Signaling Technology, Inc.) for 1 h at room temperature. The
membrane was washed three times and then the protein-antibody
complexes were examined using an enhanced chemiluminescent
detection reagent. Antibody signals were developed using a Bio-Rad
XRS chemiluminescence detection system (Bio-Rad Laboratories,
Inc.). Band densities were analyzed using Quantity One Software
(Bio-Rad Laboratories, Inc.; v4.52). The mean expression levels of
the proteins relative to β-actin were presented.
Immunofluorescence staining
Following fixing with 4% paraformaldehyde at 4°C for
15 min, the hBMSCs were treated with 0.25% Triton X-100 and 2%
bovine serum albumin at 4°C for 15 and 30 min, respectively. hBMSCs
were washed and incubated overnight with primary anti-SOST antibody
(1:100; cat. no. MAB1406; R&D Systems, Inc.) at 4°C. After
washing three times with PBS, the samples were incubated with a
green fluorescence-labeled rabbit anti-mouse secondary antibody
(1:100; cat. no. ab150113; Abcam) for 2 h in the dark at 4°C. After
the nuclei were stained with DAPI (5 µg/ml) for 5 min, the
cells were observed using a fluorescence microscope (magnification,
×100; Leica Microsystems GbmH).
Statistical analysis
All data acquisitions were repeated 3 times and were
analyzed using SPSS v.18.0 (SPSS, Inc.). A one-way analysis of
variance followed by Bonferroni's post-hoc test was used for
multi-group comparison, and differences between two groups were
determined by Student's t-test. The data are presented as the mean
± standard deviation. P<0.05 was considered to indicate a
statistically significant difference.
Results
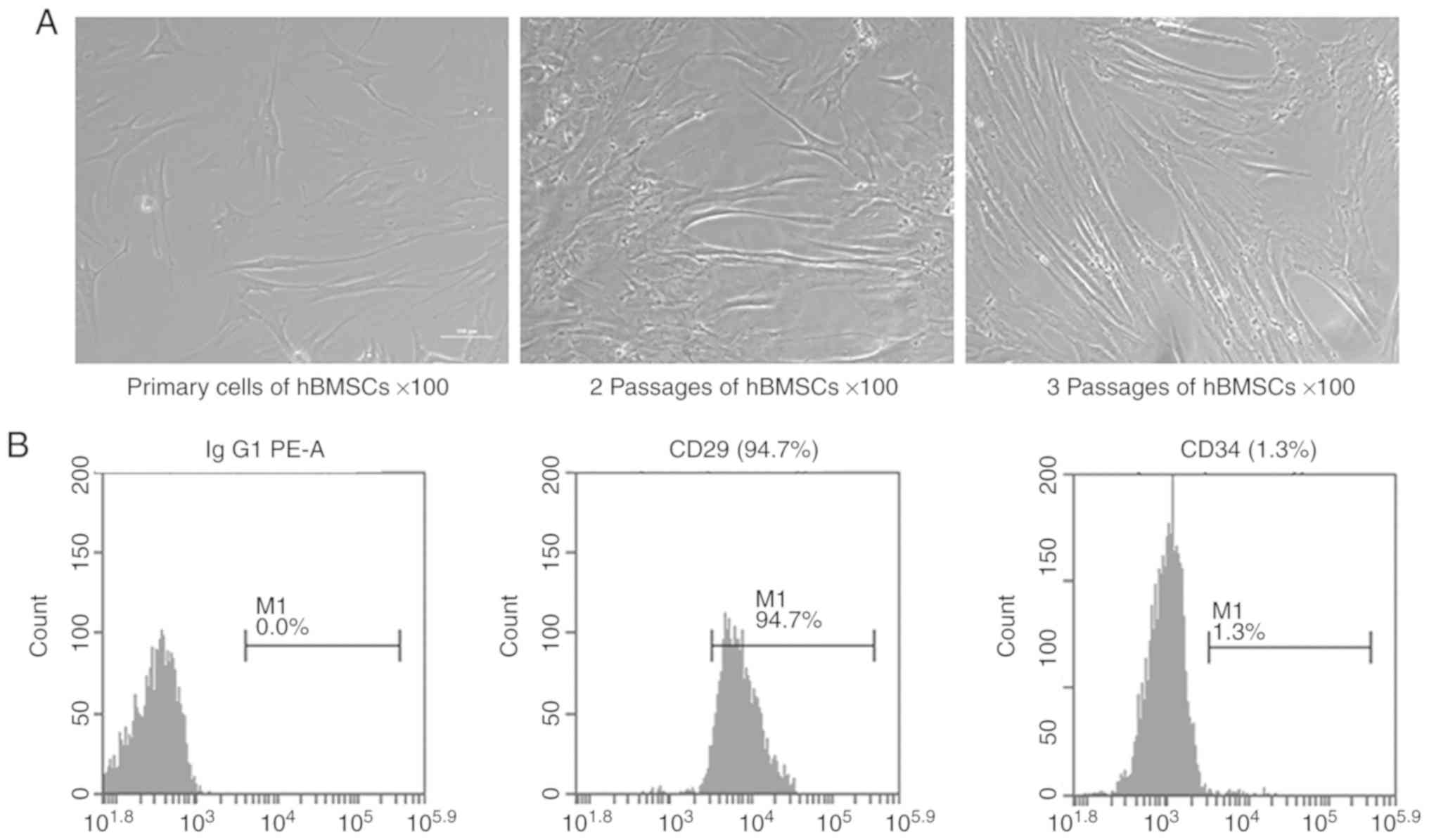
Identification of human MSCs
hBMSCs at passage 3 were harvested and the surface
phenotypes were assessed by flow cytometry (Fig. 1). The results showed that 94.7% of
the cells expressed CD29 and only 1.3% of the cells expressed CD34.
These results suggested that the isolated hBMSCs expressed standard
markers and were suitable for use in the following experiments.
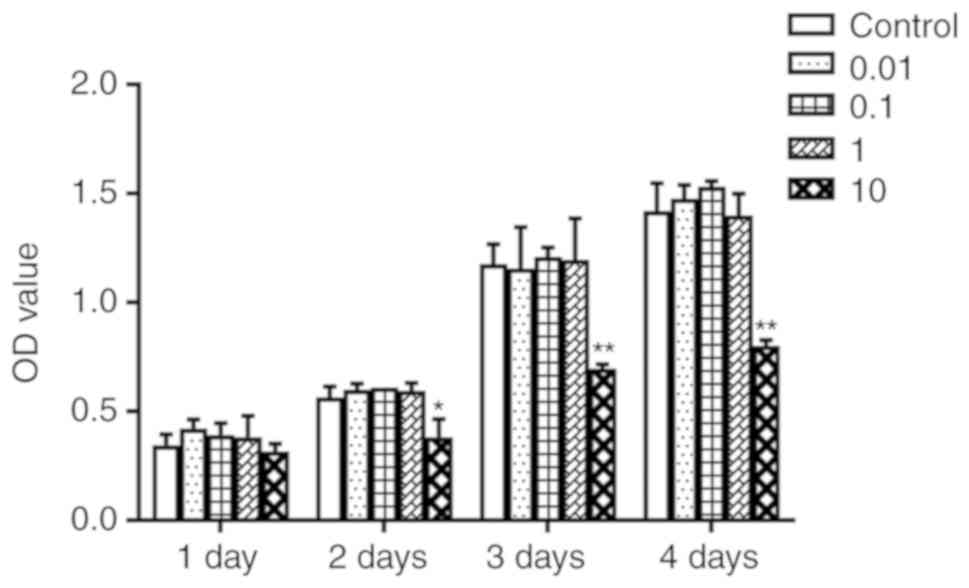
Icaritin did not affect proliferation of
hBMSCs
To determine whether icaritin would promote cell
proliferation of hBMSCs, a CCK-8 assay was used. hBMSCs were
treated with various concentrations of icaritin (0, 0.01, 0.1, 1
and 10 µM) for 1-4 days. The results indicated that icaritin
at 0.01-1 µM did not cause any significant toxicity to the
cells; however, icaritin started to exert toxic effects when the
dose reached 10 µM (Fig.
2). Therefore, concentrations of icaritin from 0.01 to 1
µM were chosen for further experimentation.
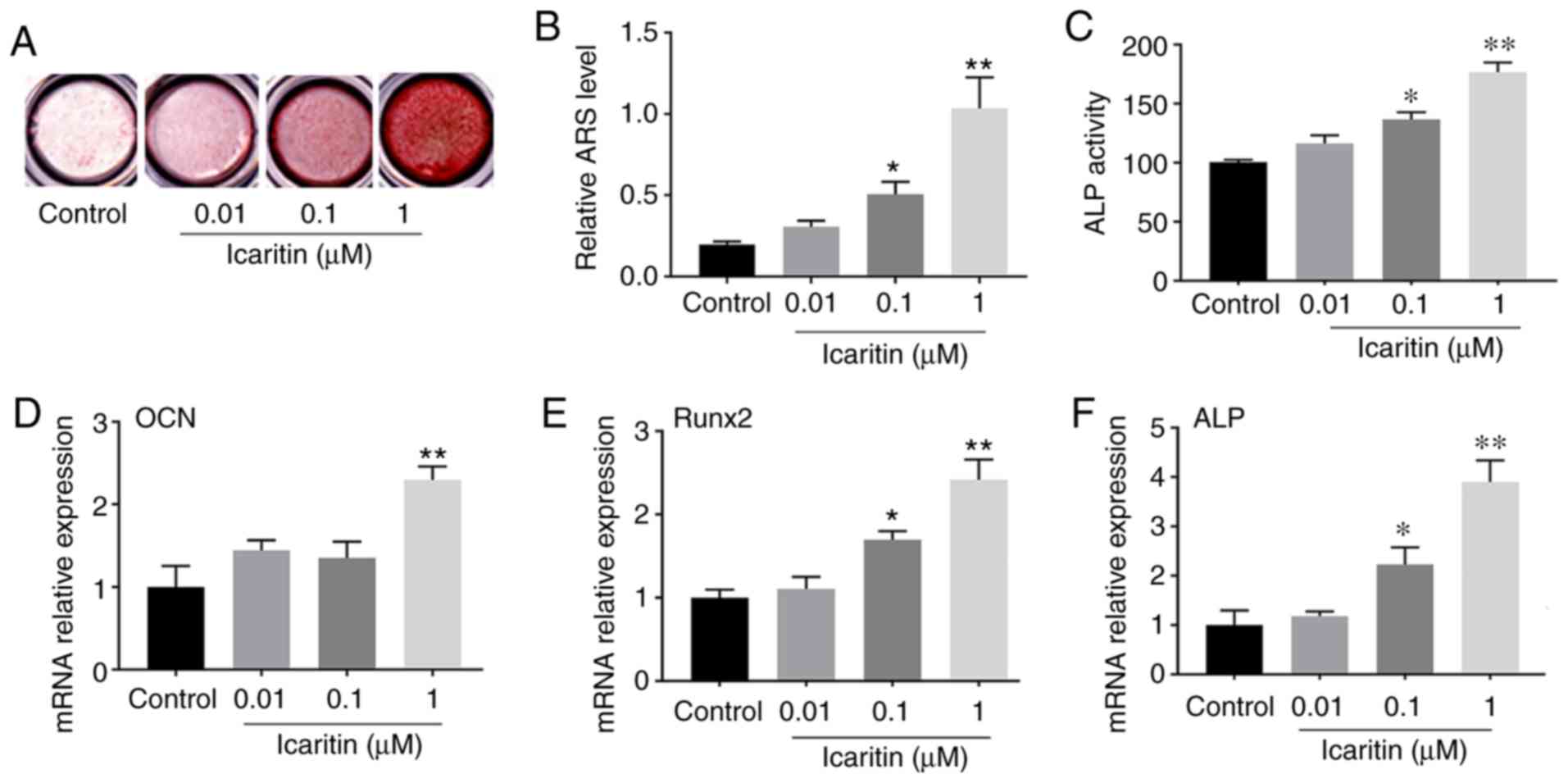
Icaritin enhanced osteogenesis of
hBMSCs
The data indicated that 0.01-1 µM icaritin
could induce the formation of calcified nodules of hBMSCs,
particularly at the concentration of 1 µM (Fig. 3A), which was confirmed by the
results from Alizarin red S quantitative assay (Fig. 3B). ALP activity can be used to
detect the early stage of osteogenesis. Therefore, the ALP activity
of BMSCs treated with or without icaritin was examined at 7 days
after osteogenic induction. The results demonstrated that ALP
activity in the icaritin group was increased almost 1.8-fold
compared with that in the control group (Fig. 3C). RT-qPCR was also used to detect
the osteogenic gene expression at 7 days. The results revealed that
the levels of osteogenic genes (OCN, Runx2 and
ALP) in the icaritin group were significantly increased
compared with those in the control group (Fig. 3D-F). The results suggested that
icaritin significantly enhanced osteogenesis of hBMSCs.
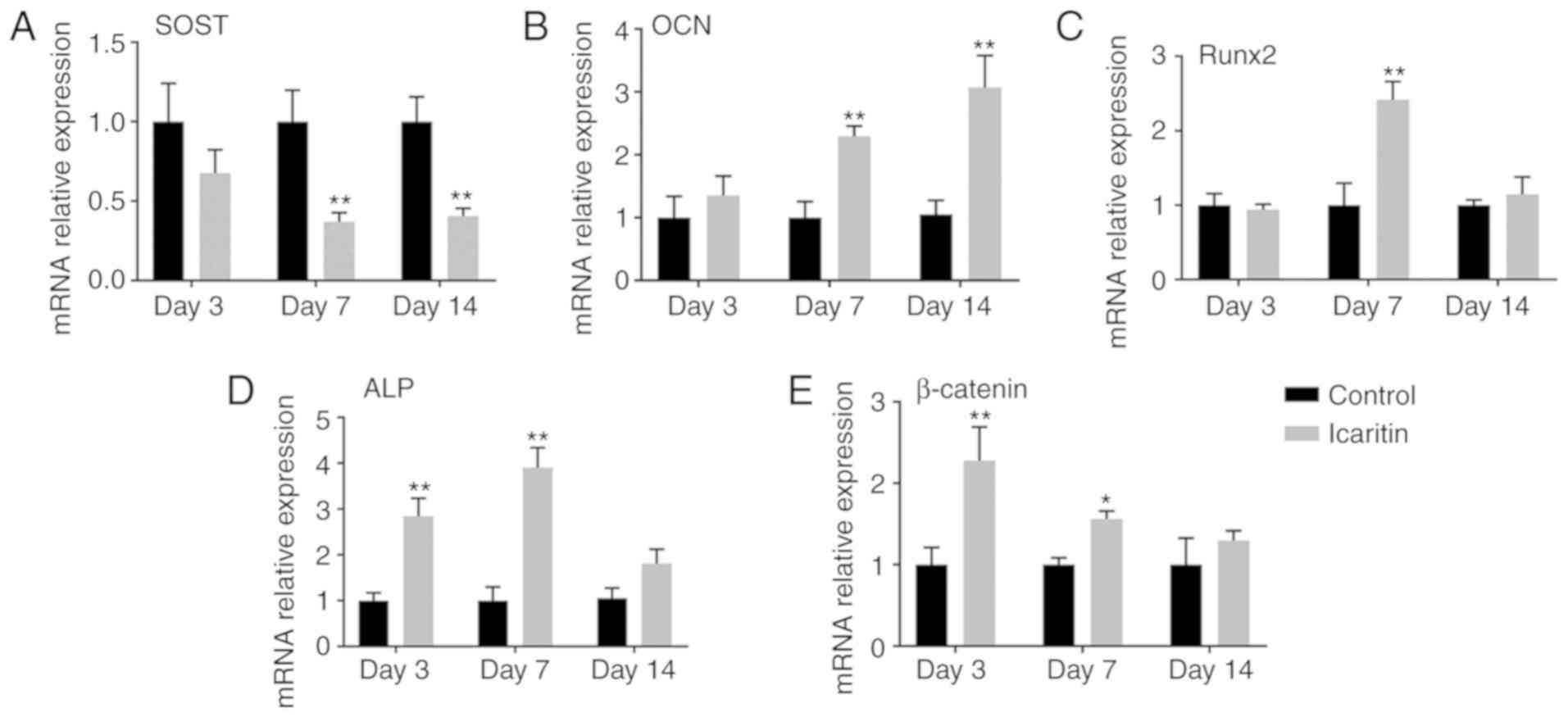
Effects of icaritin on mRNA and protein
levels of osteogenic genes
The effects of icaritin on the mRNA levels of
OCN, Runx2, ALP and β-catenin were
further determined at days 3, 7 and 14. The RT-qPCR results
suggested that icaritin upregulated OCN at days 7 and 14,
and Runx2 at day 7, and increased ALP and β-catenin
transcript levels at days 3 and 7 (Fig. 4A-D). However, icaritin decreased
the expression level of SOST at days 7 and 14 (Fig. 4E). In addition, the data from the
western blot analysis indicated that icaritin exhibit similar
effects on the expression levels of osteogenic proteins as with the
mRNA levels (Fig. 5).
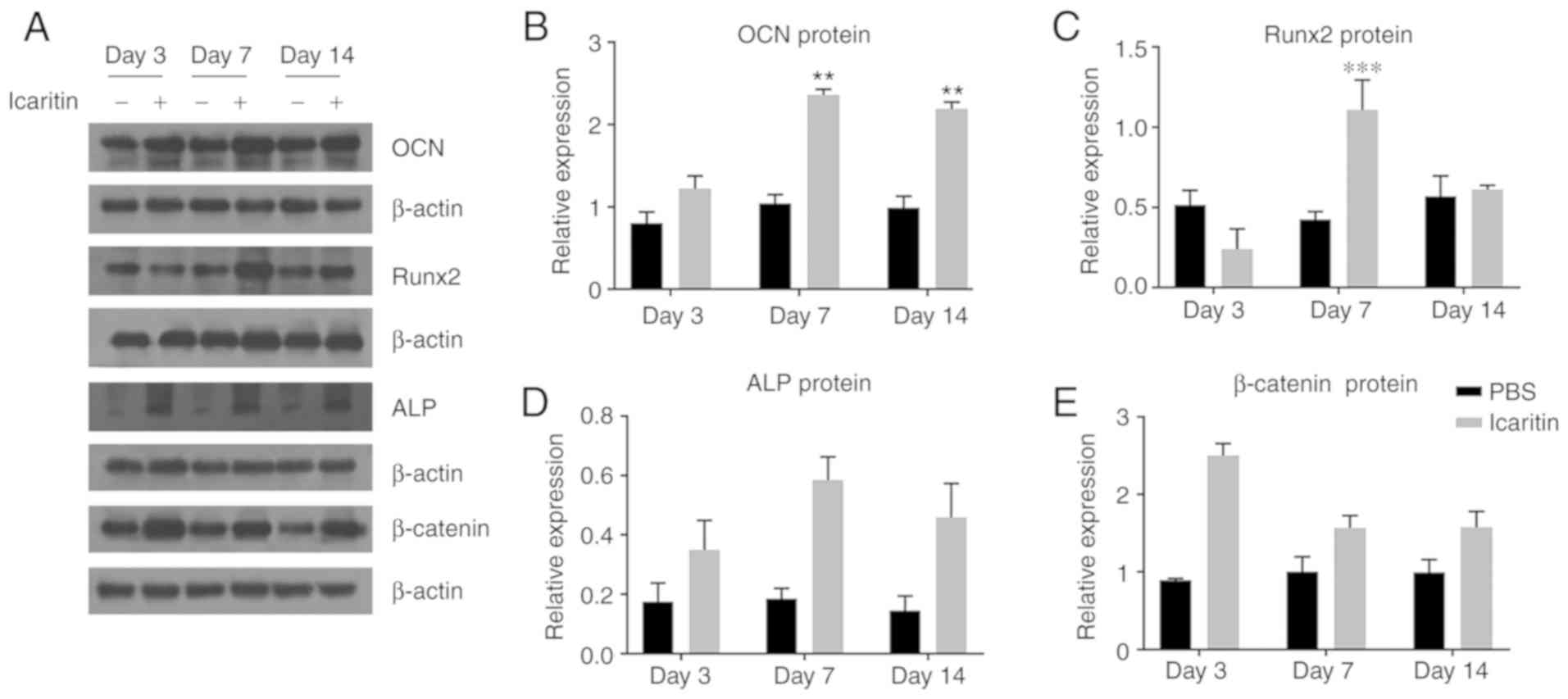 | Figure 5Icaritin (1 µM) increases
protein levels of osteogenic genes. (A) Human bone marrow-derived
mesenchymal stem cells were cultured in OIM for 3, 7, 14 days, and
OCN, Runx2, ALP, β-catenin protein levels were measured. DMSO was
used as control group. (B-E) Band density of (B) OCN, (C) Runx2,
(D) ALP and (E) β-catenin were quantified by densitometry. Data are
presented as mean ± standard deviation (n=3).
**P<0.01, ***P<0.001 vs. control group
at the same day. OCN, osteocalcin; Runx2, RUNX family transcription
factor 2; Alp, alkaline phosphatase. |
Lentivirus-mediated SOST overexpression
reverses the effects of icaritin on osteogenic differentiation
To investigate SOST function, hBMSCs were
transfected with lentiviruses encoded with the SOST gene to
overexpress SOST. The mRNA and protein expression levels of
SOST were significantly increased in the SOST overexpression
group compared with in the vector control group, as determined by
RT-qPCR and western blot analysis. Furthermore, overexpression of
SOST partly inhibited the icaritin-induced increase of ARS
level, ALP activity and osteogenic gene expression. As demonstrated
in Fig. 6, the ARS level and ALP
activity in the BMSCs-vector + icaritin group were significantly
increased compared with that in the BMSCs-SOST + icaritin group. In
addition, the BMSCs-SOST + icaritin group also exhibited decreased
levels of β-catenin and osteogenic genes including
OCN, Runx2 and ALP.
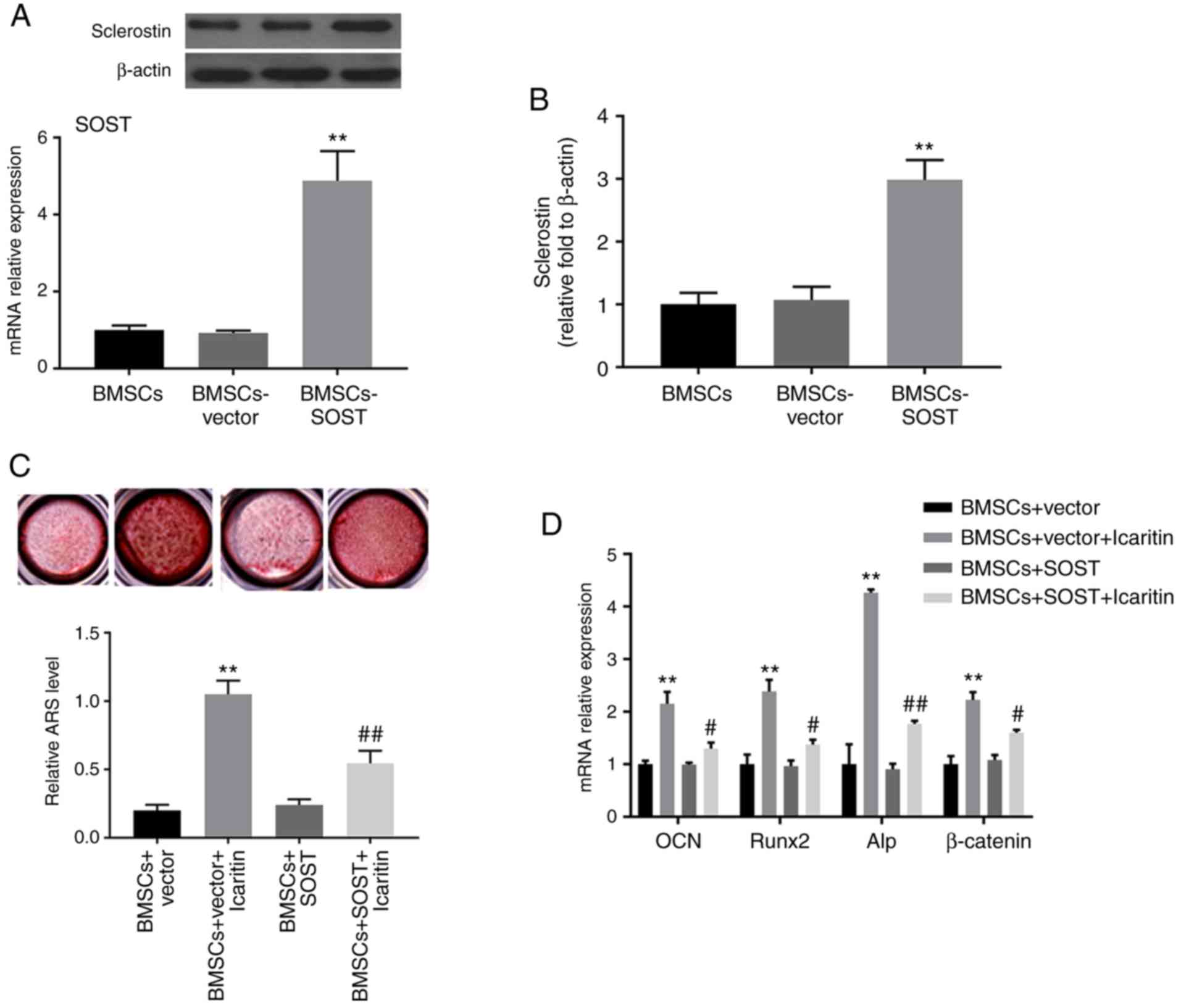 | Figure 6SOST overexpression reverses
icaritin-induced osteogenesis of hBMSCs. (A) Western blot analysis
for the protein level of SOST. (B) The mRNA expression of
SOST in BMSCs, BMSCs-vector, and BMSCs-SOST. (C)
Mineralization in cultured hBMSCs in BMSCs-vector and BMSCs-SOST
groups with or without icaritin were detected at day 14.
Magnification, ×10. (D) The mRNA levels of OCN, Runx2, Alp and
β-actin were determined by reverse transcription quantitative
polymerase chain reaction. Data are presented as mean ± standard
deviation (n=3). **P<0.01 vs. BMSCs group;
#P<0.05 and ##P<0.01 vs. BMSCs-vector
group. SOST, sclerostin; BMSCs, bone marrow-derived mesenchymal
stem cells; OCN, osteocalcin; Runx2, RUNX family transcription
factor 2; Alp, alkaline phosphatase. |
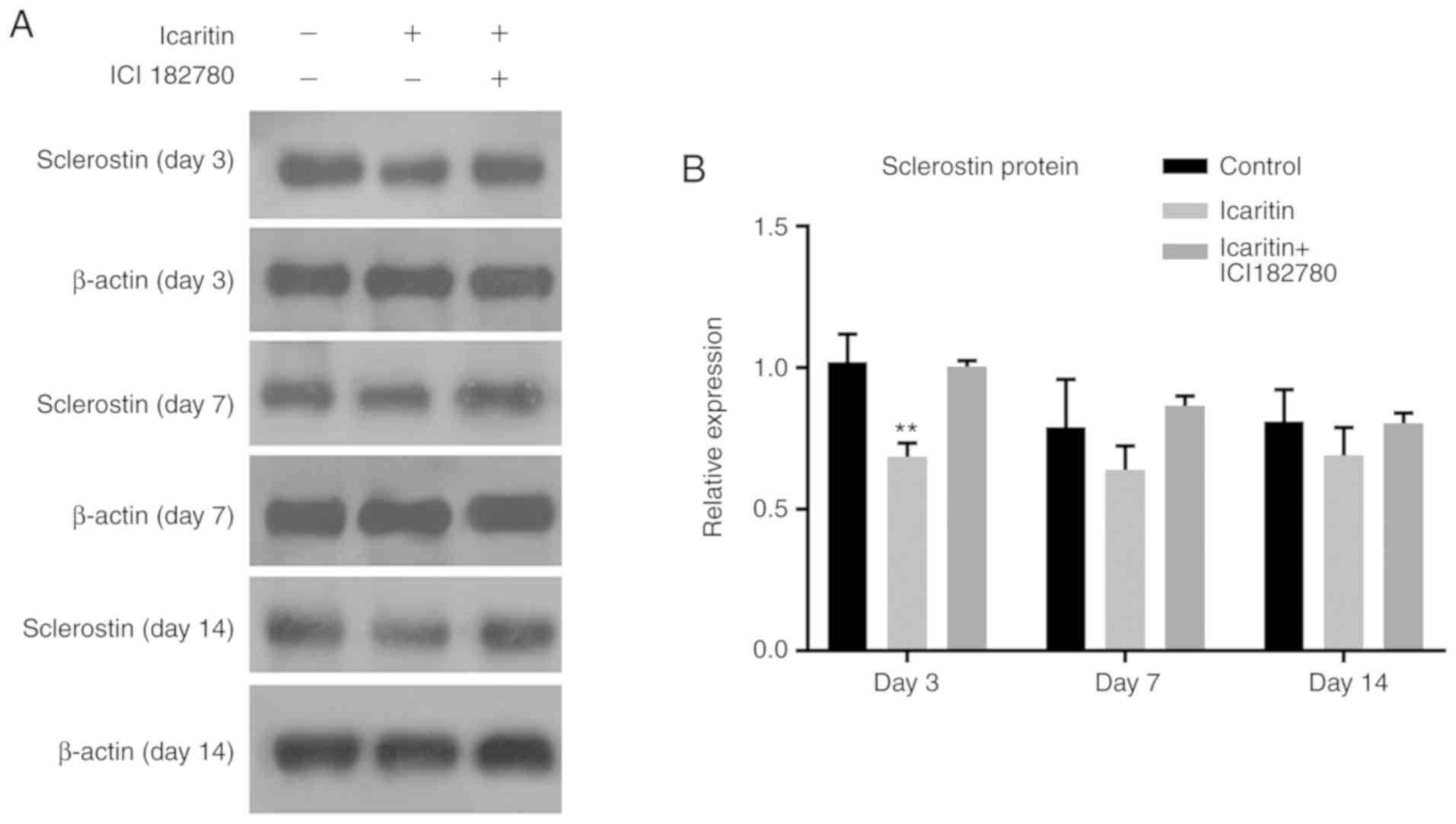
Icaritin suppresses SOST protein
expression mediated by the Wnt/ERα signaling pathway in hBMSCs
To examine whether the Wnt/ERα signaling pathway was
involved in icaritin-induced suppression of SOST, the protein level
of SOST in hBMSCs treated with icaritin and the Wnt inhibitor, ICI
182,780, which causes ERα degradation, were detected by western
blot analysis at days 3, 7 and 14. Icaritin decreased the protein
expression of SOST significantly at day 3, while the decrease was
not that marked at days 7 and 14. ICI 182,780 treatment partially
reversed the suppression of SOST caused by icaritin, to levels
close to those of the control group. The results of the present
study indicated that icaritin decreased SOST expression and was
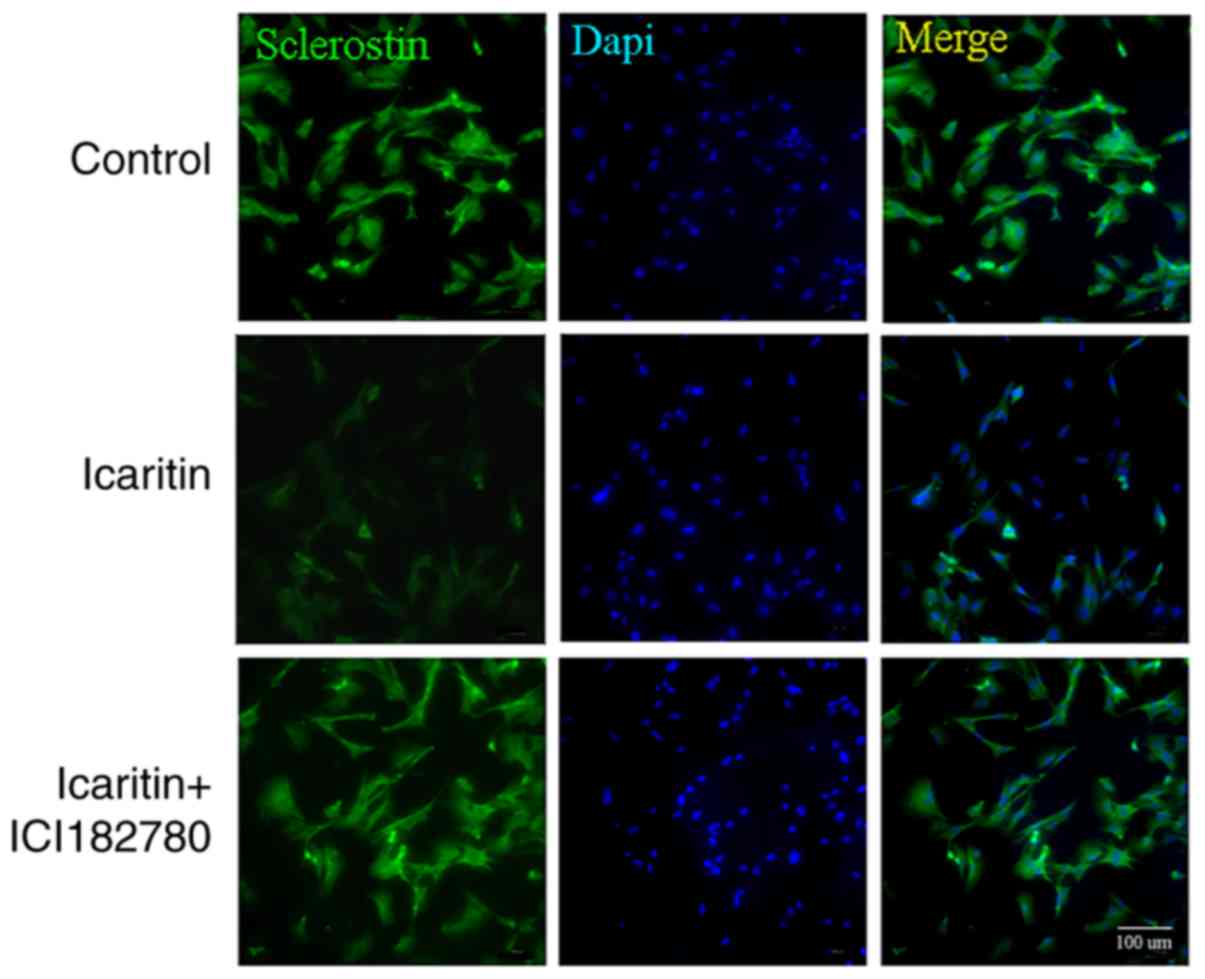
regulated via the Wnt/ERα signaling pathway (Fig. 7A and B). To confirm these data, a
immunofluorescence assay was conducted; a decreased SOST (green)
fluorescence signal in the cells treated with icaritin was
observed, and pretreatment with ICI 182780 was demonstrated to
reverse the process (Fig. 8).
Discussion
The results of the present study demonstrated that
the small phytomolecule icaritin enhanced osteogenic
differentiation of hBMSCs in vitro, with the greatest effect
demonstrated at 1 µM concentration. The results also
demonstrated that SOST served a crucial role in icaritin-induced
osteogenesis of hBMSCs. Icaritin downregulated SOST expression at
mRNA and protein level, as demonstrated by the RT-qPCR and western
blot analysis. Overexpression of SOST inhibited icaritin-induced
osteogenic differentiation. It was also identified that SOST
expression was mediated through icaritin-induced activation of the
Wnt/β-catenin and ERα signaling pathways. These results suggest
that SOST is a key factor in the icaritin-mediated osteogenesis of
hBMSCs.
Herba epimedii is used for curing
bone-associated disorders in China (10). Icariin is considered to be the
major pharmacologically active component and it promotes
osteogenesis of hBMSCs (10).
Recent studies have revealed that the pharmacological effects of
icaritin, which is an intestinal metabolism of icariin, were more
potent than icariin in vitro (11,12). In the present study, it was first
demonstrated icaritin had no effects on the proliferative ability
or viability of hBMSCs at a wide range of concentrations (0.01-1
µM). The dose would be much lower if used in vivo,
suggesting that icaritin may achieve a bio-safety level for
clinical use. Whether icaritin enhanced osteogenesis of BMSCs was
further explored through the detection of ALP activity and calcium
nodule formation. The results demonstrated that icaritin promoted
both of these osteoblast markers. Next, the effects of icaritin on
osteogenic marker genes of hBMSCs were examined. It was identified
that the levels of ALP, Runx2 and OCN were elevated following
icaritin treatment. ALP, an early marker of osteogenesis, is a key
regulator in bone formation (16), and is able to produce phosphate,
which reacts with calcium to form hydroxyapatite, further promoting
mineralization. Runx2, a pivotal transcription regulator, is the
runt family transcription factor, which serves an important role in
osteogenesis (17,18). OCN is a marker gene of late
stage osteoblast differentiation (17). Icaritin significantly increased
the mRNA and protein levels of these osteogenic marker genes,
suggesting that icaritin promoted osteogenesis of hBMSCs.
The Wnt/β-catenin pathway is well-known as a key
axis for modulating bone mass (13). SOST, the Wnt/β-catenin
regulator, inhibits bone formation by exerting antagonistic effects
on Wnt pathway. Antagonism or loss of the SOST will result in
elevated bone formation and a high bone mass (19,20). The SOST antibody (SclAb) has
attracted attention as an anabolic strategy for treating
post-menopausal osteoporosis (21). Furthermore, in phase II
(ClinicalTrials.gov identifier:
NCT01059435) (22) and III
clinical trials (ClinicalTrials. gov identifier: NCT01575834)
(23), SclAb increased bone mass
and strength in osteoporotic women. The results of the present
study indicated that icaritin downregulated the mRNA and protein
levels of SOST and upregulated the mRNA level of
β-catenin. When SOST was overexpressed in hBMSCs, the
mRNA level of β-catenin decreased, which is consistent with
previous data indicating that SOST antagonizes the Wnt signaling
pathway (9). To the best of our
knowledge, the present study demonstrated for the first time that
overexpression of SOST diminishes the icaritin-induced increases in
ALP activity, ARS level and osteogenic genes.
To determine whether the Wnt/ERα signaling pathway
was involved in icaritin-induced suppression of SOST, hBMSCs were
treated with icaritin and the Wnt inhibitor, ICI 182,780, which
causes ERα degradation (24). The
results demonstrated that icaritin treatment decreased the levels
of SOST protein expression, while treatment with ICI 182,780 partly
reversed the icaritin-mediated suppression of SOST, indicating that
the Wnt/β-catenin and ERα signaling pathways were involved in
icaritin-induced SOST suppression. These results were
consistent with previous data suggesting that estrogen-mediated
suppression of SOST expression was associated with the
Wnt/β-catenin/ERα axis in human osteoblasts (24).
Stem cell transplantation has been applied in
clinical settings for the treatment of multiple conditions, such as
diabetic neuropathy (25) and
leukemia (26). However, stem
cell transplantation alone has exhibited limited satisfactory
results Previously, the combination of stem cells with other
compounds with osteogenic effects may provide improved therapeutic
effects. Icariin, which has been suggested to exhibit weaker
pharmacological effects (12) was
demonstrated to result in significantly decreased levels of bone
loss in postmenopausal women (27). Based on these data, we
hypothesized that icaritin, which had significant osteogenic
effects, may also be used for decreasing the bone loss in
postmenopausal women.
In conclusion, icaritin induced osteogenesis in
hBMSCs by suppressing SOST expression, and icaritin-induced
suppression of SOST was regulated in part via the
Wnt/β-catenin and ERα pathways. The results of the present study
suggested that icaritin may be a promising agent for diseases
caused by decreased levels of osteogenic differentiation of BMSCs,
such as osteoporosis or osteonecrosis of the femoral head.
Funding
The present study was supported by grants from the
project of the National Natural Science Foundation of China (grant
nos. 8187031444 and 81574002).
Availability of data and materials
All the materials included in the manuscript,
including all relevant raw data, may be made freely available to
any researchers who wish to use them for non-commercial purposes,
while preserving any necessary confidentiality and anonymity.
Authors' contributions
YC conceived and supervised the project. BW and HH
were in charge of the PCR, western blotting, flow cytometry and
immunofluorescence staining. QW was responsible for the
experimental design, data analysis, drafting and revision of the
article. CX analyzed data. LL and JG were responsible for the
culture of the hBMSCs, ALP/Alizarin Red S staining, CCK-8 assay and
lentiviral transduction. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
We are thankful to Dr Liangliang Xu for providing
technical suggestions for the experiments.
References
|
1
|
Liu C, Zhang H, Tang X, Feng R, Yao G,
Chen W, Li W, Liang J, Feng X and Sun L: Mesenchymal stem cells
promote the osteo-genesis in collagen-induced arthritic mice
through the inhibition of TNF-α. Stem Cells Int. 2018:40690322018.
View Article : Google Scholar
|
|
2
|
Huang Z, Cheng C, Cao B, Wang J, Wei H,
Liu X, Han Y, Yang S and Wang X: Icariin protects against
glucocorticoid-induced osteonecrosis of the femoral head in rats.
Cell Physiol Biochem. 47:694–706. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wei Q, He M, Chen M, Chen Z, Yang F, Wang
H, Zhang J and He W: Icariin stimulates osteogenic differentiation
of rat bone marrow stromal stem cells by increasing TAZ expression.
Biomed Pharmacother. 91:581–589. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang D, Park BM, Kang M, Nam H, Kim EJ,
Bae C and Lim SK: The systemic effects of sclerostin overexpression
using ΦC31 integrase in mice. Biochem Biophys Res Commun.
472:471–476. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pietrzyk B, Smertka M and Chudek J:
Sclerostin: Intracellular mechanisms of action and its role in the
pathogenesis of skeletal and vascular disorders. Adv Clin Exp Med.
26:1283–1291. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
McDonald MM, Morse A, Birke O, Yu NYC,
Mikulec K, Peacock L, Schindeler A, Liu M, Ke HZ and Little DG:
Sclerostin antibody enhances bone formation in a rat model of
distraction osteogenesis. J Orthop Res. 36:1106–1113. 2018.
|
|
7
|
McClung MR: Sclerostin antibodies in
osteoporosis: Latest evidence and therapeutic potential. Ther Adv
Musculoskelet Dis. 9:263–270. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mirza FS, Padhi ID, Raisz LG and Lorenzo
JA: Serum sclerostin levels negatively correlate with parathyroid
hormone levels and free estrogen index in postmenopausal women. J
Clin Endocrinol Metab. 95:1991–1997. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim RY, Yang HJ, Song YM, Kim IS and Hwang
SJ: Estrogen modulates bone morphogenetic protein-induced
sclerostin expression through the Wnt signaling pathway. Tissue Eng
Part A. 21:2076–2088. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang JM, Bao Y, Xiang W, Jing XZ, Guo JC,
Yao XD, Wang R and Guo FJ: Icariin regulates the bidirectional
differentiation of bone marrow mesenchymal stem cells through
canonical Wnt signaling pathway. Evid Based Complement Alternat
Med. 2017:80853252017. View Article : Google Scholar
|
|
11
|
Wu T, Shu T, Kang L, Wu J, Xing J, Lu Z,
Chen S and Lv J: Icaritin, a novel plant-derived osteoinductive
agent, enhances the osteogenic differentiation of human bone
marrow- and human adipose tissue-derived mesenchymal stem cells.
Int J Mol Med. 39:984–992. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang ZQ and Lou YJ:
Proliferation-stimulating effects of icaritin and desmethylicaritin
in MCF-7 cells. Eur J Pharmacol. 504:147–153. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Baron R and Gori F: Targeting WNT
signaling in the treatment of osteoporosis. Curr Opin Pharmacol.
40:134–141. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sheng H, Rui XF, Sheng CJ, Li WJ, Cheng
XY, Jhummon NP, Yu YC, Qu S, Zhang G and Qin L: A novel
semisynthetic molecule icaritin stimulates osteogenic
differentiation and inhibits adipogenesis of mesenchymal stem
cells. Int J Med Sci. 10:782–789. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
16
|
Harrison G, Shapiro IM and Golub EE: The
phosphatidylinositol-glycolipid anchor on alkaline phosphatase
facilitates mineralization initiation in vitro. J Bone Miner Res.
10:568–573. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Orimo H: The mechanism of mineralization
and the role of alkaline phosphatase in health and disease. J
Nippon Med Sch. 77:4–12. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao Z, Zhao M, Xiao G and Franceschi RT:
Gene transfer of the Runx2 transcription factor enhances osteogenic
activity of bone marrow stromal cells in vitro and in vivo. Mol
Ther. 12:247–253. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Taylor S, Hu R, Pacheco E, Locher K, Pyrah
I, Ominsky MS and Boyce RW: Differential time-dependent
transcriptional changes in the osteoblast lineage in cortical bone
associated with sclerostin antibody treatment in ovariectomized
rats. Bone Rep. 8:95–103. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao W, Li X, Peng Y, Qin Y, Pan J, Li J,
Xu A, Ominsky MS, Cardozo C, Feng JQ, et al: Sclerostin antibody
reverses the severe sublesional bone loss in rats after chronic
spinal cord injury. Calcif Tissue Int. 103:443–454. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bhattacharyya S, Pal S and Chattopadhyay
N: Targeted inhibition of sclerostin for post-menopausal
osteoporosis therapy: A critical assessment of the mechanism of
action. Eur J Pharmacol. 826:39–47. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
McClung MR, Grauer A, Boonen S, Bolognese
MA, Brown JP, Diez-Perez A, Langdahl BL, Reginster JY, Zanchetta
JR, Wasserman SM, et al: Romosozumab in postmenopausal women with
low bone mineral density. N Engl J Med. 370:412–420. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lewiecki EM: Role of sclerostin in bone
and cartilage and its potential as a therapeutic target in bone
diseases. Ther Adv Musculoskelet Dis. 6:48–57. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mbalaviele G, Sheikh S, Stains JP, Salazar
VS, Cheng SL, Chen D and Civitelli R: Beta-catenin and BMP-2
synergize to promote osteoblast differentiation and new bone
formation. J Cell Biochem. 94:403–418. 2005. View Article : Google Scholar
|
|
25
|
Datta I, Bhadri N, Shahani P, Majumdar D,
Sowmithra S, Razdan R and Bhonde R: Functional recovery upon human
dental pulp stem cell transplantation in a diabetic neuropathy rat
model. Cytotherapy. 19:1208–1224. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Heidrich K, Thiede C, Schäfer-Eckart K,
Schmitz N, Aulitzky WE, Krämer A, Rösler W, Hänel M, Einsele H,
Baldus CD, et al: Allogeneic hematopoietic cell transplantation in
intermediate risk acute myeloid leukemia negative for FLT3-ITD,
NPM1- or biallelic CEBPA mutations. Ann Oncol. 28:2793–2798. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang Z, Wang D, Yang D, Zhen W, Zhang J
and Peng S: The effect of icariin on bone metabolism and its
potential clinical application. Osteoporos Int. 29:535–544. 2018.
View Article : Google Scholar
|