Introduction
Stroke is one of the main causes of death and
permanent disability worldwide (1). Cerebral artery occlusion can cause
acute ischemic stroke. Acute ischemic stroke accounts for >80%
of all strokes (2). Although some
progress has been made in post-stroke treatment, stroke
intervention is still insufficient (1). To date, no successful long-term
neuroprotective therapy has been identified in clinical trials
(3–5). The ischemic core is considered to be
an irreparably damaged area. Due to a serious lack of blood flow,
numerous nerve cells die within a few minutes after occlusion
(6). The ischemic penumbra was
first proposed by Astrup et al (7). The penumbra is an area of the brain
tissue that is damaged but not yet dead after local ischemia
(8). Clinically, the ischemic
penumbra is called the low perfusion area around the ischemic core.
If the cerebral blood flow is restored in a timely manner, the
damaged nerve cells can be saved (9). Neurogenesis (the birth of new
neurons) is a process involving the production of functional
neurons from precursor cells and occurs throughout the life cycle
of the mammalian brain, indicating it is an attractive target for
potential intervention (10,11). Most studies have focused on
newborn, perinatal and adult rodents, and few have evaluated
neurogenesis and myelin repair in adolescents after stroke. Adult
neurogenesis is different from developmental neurogenesis (12–14). In the developing brain, immature
neurons are extremely sensitive and vulnerable to widespread
insults and toxic exposures (15). A recent study reported that 3 to
4-week-old mice have fully developed brains and juvenile mice show
mature brain neurons like adult mice, and are not vulnerable to the
factors found in neonatal and perinatal brain development (10). Therefore, the juvenile brain is an
ideal choice for the study of neurogenesis (10). Elucidation of the signaling
molecules and related signaling pathways involved in the protection
of nerve cells in the ischemic penumbra after juvenile ischemic
stroke is needed.
However, whether in the development of the central
nervous system (CNS) or after CNS damage, the Wnt/β-catenin signal
transduction pathway plays a key role remains to be elucidated
(16). It has been found that
Wnt3a is an important protein in the Wnt family. It is involved in
neurogenesis in the hippocampus and cortex (17,18). Research showed that intranasal
administration of Wnt3a can enhance the neuroprotection and
regeneration of the Wnt signaling pathway after focal ischemic
stroke in mice (19). Previous
studies have shown that Wnt signal transduction is the main
regulator of hippocampal neurogenesis in adults (20–23). Activating the Wnt pathway in
vivo and in vitro was shown to increase neurogenesis,
and blocking the Wnt pathway inhibited the proliferation and
differentiation of rat neural progenitor cells (NPCs) (20). Moreover, Wnt signaling promotes
functional recovery by increasing neurogenesis (24).
Physical exercise can promote neurogenesis,
angiogenesis and enhance dendritic modification and synaptic
plasticity (25,26). Promoting brain-derived
neurotrophic factor (BDNF) expression during development can
regulate the cell signal transduction pathway, promote neuronal
regeneration and contribute to synaptic plasticity, learning,
memory and sensorimotor recovery (27). Treadmill exercise promotes
oligodendrocytes and, thus, myelination. In addition, the
improvement of ischemia-induced myelin injury by long-term exercise
is related to increased BDNF expression (28). Although the authors’ previous
studies (25,29,30) have focused on the neuroprotective
effect of treadmill exercise in adult rats its neuroprotective
effect on juvenile rats has not been explored.
In this study, it was shown that treadmill exercise
promoted neurogenesis and myelin repair in juvenile rats. The
inhibitor of the Wnt signaling pathway was used to elucidate the
role of the Wnt/β-catenin signaling pathway in treadmill
exercise-promoted neurogenesis and myelin repair. It was further
clarified that neurogenesis and myelin repair after ischemic stroke
are closely related to treadmill exercise and the Wnt/β-catenin
signaling pathway.
Materials and methods
Reagents
Anti-Wnt3a (1:1,000; EMD Millipore), anti-β-catenin
(1:5,000; Abcam), anti-BDNF (1:1,000; Abcam), anti-myelin basic
protein (MBP; 1:1,000; Abcam), anti-Dcx (1:200; Abcam), anti-NESTIN
(1:200; Abcam), anti-GAPDH (1:2,000; Abcam), anti-Lamin B1
(1;1,000; Abcam), XAV939 (Selleck Chemicals), 2,3,5-triphenyl
tetrazolium chloride (TTC; Sigma-Aldrich; Merck KGaA),
phenylmethylsulfonyl-fluoride (Beijing Solarbio Science &
Technology Co., Ltd.), Clarity™ Western ECL Substrate kit (Bio-Rad
Laboratories, Inc.), Alexa Fluor 488 AffiniPure Donkey Anti-Mouse
IgG and Alexa Fluor 594 AffiniPure Goat Anti-Rabbit IgG (1:200;
Yesen Bio), 4,6-diamidino-2-phenylindole (DAPI; Beyotime Institute
of Biotechnology).
Animals
The Shanghai Experimental Animal Center provided 171
juvenile Sprague-Dawley male rats (weight, 80–90 g; Sprague Dawley
rats were weaned for 21 days and matured for 6 to 7 weeks). The
Animal Research Committee of Wenzhou Medical University approved
the present experimental project and all the experimental followed
the guidelines of the National Institutes of Health on the Care and
Use of Animals (ethical no. wydw2019–0766). The animals were placed
in a suitable environment (4 rats per cage, relative humidity
55±5%, 22°C, 12-h light/dark cycle) and had free access to food and
water. The rats were randomly divided into 9 groups: Sham operation
group (n=19; S group); model group 7 and 14 days, respectively (M7:
n=19 and M14: n=19); treadmill training models (EM7: n=19 and EM14:
n=19); inhibitor treatment models (IM7: n=19 and IM14: n=19); and
inhibitor treatment and treadmill training models (IEM7: n=19 and
IEM14: n=19). The present research was conducted following these
criteria (31): i) Randomization,
ii) allocation concealment and iii) blinded assessment of
outcome.
Middle cerebral artery occlusion (MCAO)
model of focal brain ischemia
Briefly, rats were anesthetized with 3% isoflurane
vaporized in 30% O2/70% N2 until they were
unresponsive to the tail pinch test and then fitted with a nose
cone blowing 1.5% isoflurane for anesthesia maintenance (Shenzhen
RWD Life Science Co., Ltd.) during surgery. After the rats were
anesthetized, they were placed in the supine position on the
sterilized operating table and an incision was made in the right
side of the neck. The common carotid artery and external carotid
artery (ECA) was separated from the peripheral connective tissue
without injury to peripheral muscles and nerves. A small gap was
opened in the stump of the ECA and then a small silicon-coated
surgical nylon monofilament (0.24±0.02 mm/l, 2,400; Guangzhou
Jialing Biotechnology Co., Ltd.) was inserted into the lumen of the
internal carotid artery (ICA), and the operation was stopped ~17 mm
from the far end of the ICA bifurcation to block the middle
cerebral artery (MCA). The rats during and after the operation were
preserved under a 37°C heating pad. After 1.5 h of occlusion, the
filament in the rat ICA was removed under a light microscope
(Olympus Corporation) and the blood flow was restored, and the
reperfusion was allowed for 7 or 14 days. The rats in the sham
operation group were treated with the same surgical method as the
experimental group except for the insertion of the surgical nylon
monofilament. A total of 59 rats were excluded from the present
study, including 14 rats with low Zea Longa score (0 or 4)
(32) and 45 rats that died.
Among the 45 rats that died, 14 rats died of brain edema, 9 died of
subarachnoid hemorrhage, 13 died of massive haemorrhage and 9 died
of long operation time. In addition, all juvenile rats were
anesthetized by intraperitoneal injection of pentobarbital sodium
(65 mg/kg) before sacrifice.
Treadmill exercise and inhibitor
In this study, a small animal electric treadmill was
used (XR-PT-10A; Shanghai XinRuan Information Technology Co.,
Ltd.). Before MCAO, the animals received three days of adaptive
treadmill training. The EM group and IEM group underwent treadmill
training at 0 slope, 8 m/min, 30 min/d and 5 d/for 7 days or 14
days, respectively.
XAV939, a small molecule inhibitor of Wnt/β-catenin
signaling (40 mg/kg; intraperitoneal injection) was used. In short,
immediately after MCAO, XAV-939 was injected into the IM7 group and
the IEM7 group, and then injected every 24 h for 7 days. Also, in
IM14 group and IEM14 group, immediately after MCAO, XAV-939 was
injected into IM14 group and IEM14 group, and then injected every
24 h for 14 days. the dose of XAV939 was determined based on
dose-response studies and published reports (33,34). In addition, rats of the other
groups (S, M and EM) were simultaneously injected with the same
amount of physiological saline.
Evaluation of neurologic deficit
scores
A total of two independent examiners performed the
modified nerve severity score (mNSS) test (35) at 1, 7 and 14 days after MCAO. The
researchers were blinded to the treatment group (Table I).
 | Table IModified neurological severity score
points. |
Table I
Modified neurological severity score
points.
| Motor tests | |
| Raising rat by
tail | 3 |
| Flexion of
forelimb | 1 |
| Flexion of
hindlimb | 1 |
| Head moved 10 to
vertical axis within 30 sec | 1 |
| Placing rat on
floor (normal=0; maximum=3) | 3 |
| Normal walk | 0 |
| Inability to walk
straight | 1 |
| Circling toward
paretic side | 2 |
| Falls down to
paretic side | 3 |
| Sensory tests | 2 |
| Placing test
(visual and tactile test) | 1 |
| Proprioceptive
test (deep sensation, pushing paw against table edge to stimulate
limb muscles) | 1 |
| Beam balance tests
(normal=0; maximum=6) | 6 |
| Balances with
steady posture | 0 |
| Grasps side of
beam | 1 |
| Hugs beam and 1
limb falls down from beam | 2 |
| Hugs beam and 2
limbs fall down from beam, or spins on beam (>60 sec) | 3 |
| Attempts to
balance on beam but falls off (>40 sec) | 4 |
| Attempts to
balance on beam but falls off (>20 sec) | 5 |
| Falls off; no
attempt to balance or hang on to beam (<20 sec) | 6 |
| Reflex absence and
abnormal movements | 4 |
| Pinna reflex (head
shake when auditory meatus is touched) | 1 |
| Corneal reflex
(eye blink when cornea is lightly touched with cotton) | 1 |
| Startle reflex
(motor response to a brief noise from snapping a clipboard
paper) | 1 |
| Seizures,
myoclonus, myodystony | 1 |
| Maximum
points | 18 |
Infarct volume assessment
For determination of the volume of cerebral
infarction, TTC staining was performed on consecutive sections of
bregma +4.0–6.0 mm. the brain was cut with a blade into five
consecutive coronal sections separated by 2.0 mm. The obtained
brain tissue was frozen at −20°C for 10 min. Then, all brain slices
were immediately immersed in 2% TTC solutions, at 37°C for 30 min
and fixed at 4°C for 24 h in 4% paraformaldehyde buffer. In 5
sections, the total cerebral infarction volume was equal to the sum
of the infarction area. The correction formula for calculating the
infarction volume with minimization of the error caused by brain
edema is as follows (36):
Infarct percentage = [(contralateral hemisphere
region-non-infarcted region in the ipsilateral hemisphere)/volume
of the contralateral hemisphere] * 100%. Then, the analysis was
carried out using ImageJ software (ImageJ bundled with 64-bit Java
1.8.0_112; National Institutes of Health).
Bromodeoxyuridine injection and tissue
preparation
Bromodeoxyuridine (BrdU; 50 mg/kg/day) was injected
intraperitoneally into rats every day after MCAO for 7 or 14 days
to label newly formed cells. The proliferation of neural stem cells
(NSCs) and the differentiation of NSCs were observed by double
immunofluorescence.
At 7 and 14 days after MCAO, 3 rats in each group
were sacrificed. Under deep anesthesia with pentobarbital sodium
(65 mg/kg; intraperitoneally), the rats were perfused with 0.9%
sodium chloride (4°C) and then perfused with 4% paraformaldehyde
(Sigma-Aldrich; Merck KGaA) in 0.1 mol/l phosphate buffer (pH 7.4).
The whole brain was collected, fixed at 4°C for 24 h, dehydrated
and finally embedded in paraffin wax. Then, coronal sections of 5
μm were sliced by a microtome (Kedee Instrumental Equipment Co.
Ltd.) for hematoxylin-eosin (H&E) or Nissl staining. The rat
samples used for immunofluorescence labeling were removed and
stored in the same fixation solution at 4°C for 24 h and soaked
overnight in 0.1 M phosphate buffer (20 and 30% sucrose) at 4°C.
The brain tissue was embedded and frozen at −80°C in the optimal
cutting temperature complex. Finally, the coronal section of the
ischemic penumbra was cut with a cryostat (CM1900; Leica
Microsystems GmbH) (5 μm thick).
H&E staining
At least 3 slices were taken from each rat, dewaxed
in xylene and then dehydrated in alcohol. Finally, H&E staining
was performed at room temperature for 2 h, and observed under a
light microscope (Olympus Corporation). Pathological changes in
brain tissue were observed, images were captured and recorded.
Toluidine blue (Nissl) staining
The slices were washed 3 times. The slices were dyed
at 37°C with 1% toluene blue solution for 30 min. The tissue was
decolorized and dehydrated with ethanol and sealed with neutral
resin. Under light microscopy (Olympus Corporation), the Nissl
bodies of neurons were blue and purple.
Luxol fast blue (LFB) staining
The brain tissue was embedded in paraffin and then,
the 5 μm thick coronal slices were stained with 0.1% LFB (cat. no.
S3382; Sigma-Aldrich; Merck KGaA) at 60°C for 2 h. The slices were
soaked in 0.05% lithium carbonate solution to distinguish white
matter from gray matter. Finally, the slices were placed in
distilled water, re-dyed at room temperature in cresol purple
solution for 30–40 sec and then washed with distilled water.
Immunofluorescence
The slices were dried, then restored to room
temperature and soaked in 0.01 M phosphate buffer (PBS, pH 7.6) 3
times for 5 min. The sections were blocked for 1 h (20–22°C) with
10% goat serum (cat. no. C0265; Beyotime Institute of
Biotechnology) containing 0.3% Triton X-100. Dcx/BrdU and
Nestin/BrdU double immunofluorescence staining was performed.
Samples were incubated at 37°C with 1N HCL for 30 min before
blocking and then denatured twice for 10 min with borate buffer (pH
8.4). Then mixtures of mouse anti-BrdU antibody (dilution, 1:1,000;
cat. no. MAB4072; EMD Millipore) and rabbit anti-Dcx antibody
(dilution, 1:200; cat. no. ab207175; Abcam), mixtures of mouse
anti-BrdU antibody and rabbit anti-Nestin antibody (dilution,
1:200; cat. no. ab105389; Abcam), and rabbit anti-BDNF antibody
(dilution, 1:100; cat. no. ab108319; Abcam), used as antibodies for
Dcx/BrdU, Nestin/BrdU and BDNF, respectively, were separately added
for incubation overnight at 4°C. Next, the sections were restored
to room temperature. Then, the slices were soaked in 0.01 M PBS 3
times for 5 min and performed with the corresponding Alexa Fluor
488 AffiniPure Donkey Anti-Mouse IgG (dilution, 1:200; cat. no.
34106ES60; Shanghai Yeasen Biotechnology Co., Ltd.) and Alexa Fluor
594 AffiniPure Goat Anti-Rabbit IgG (dilution, 1:200; cat. no.
33112ES60; Shanghai Yeasen Biotechnology Co., Ltd.) secondary
antibodies for 1 h at room temperature. Nuclei were counterstained
at room temperature for 10 min with DAPI. The negative control
sections were incubated with 0.01 M PBS instead of primary antibody
and no positive fluorescence signal was found. According to the
present team’s previous explorations and classical anatomical
methods, the area of observation of the ischemic penumbra is as
shown in Fig. 1C and D (37). Brain sections were covered and the
ischemic penumbra was observed and analyzed by fluorescence
microscopy (BX51; Olympus Corporation) in all sections. Counting
the positive cells of 5 non-overlapping fields (x400-fold) on one
slice using Image-Pro Plus 5.0 analysis software (Media
Cybernetics, Inc.).
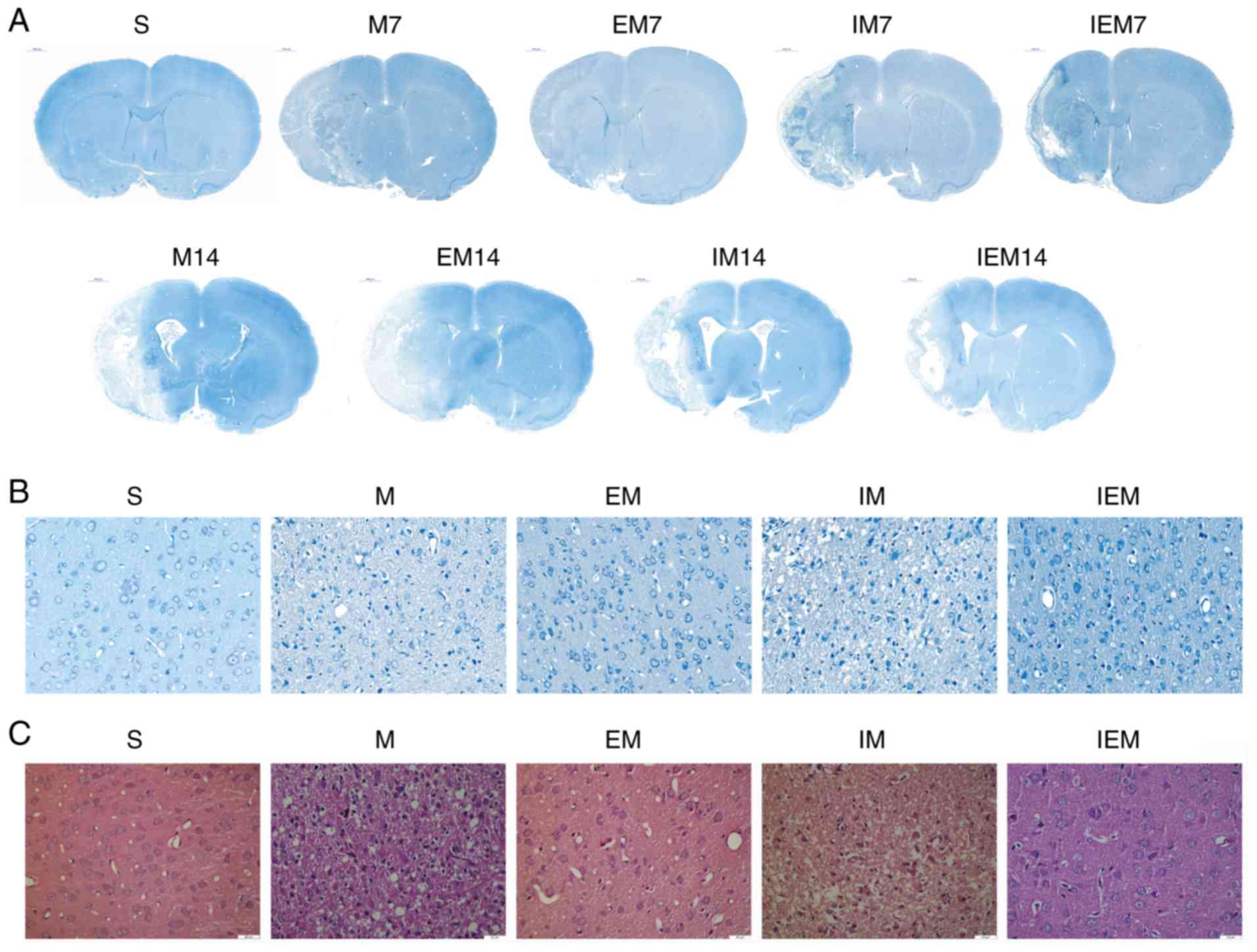
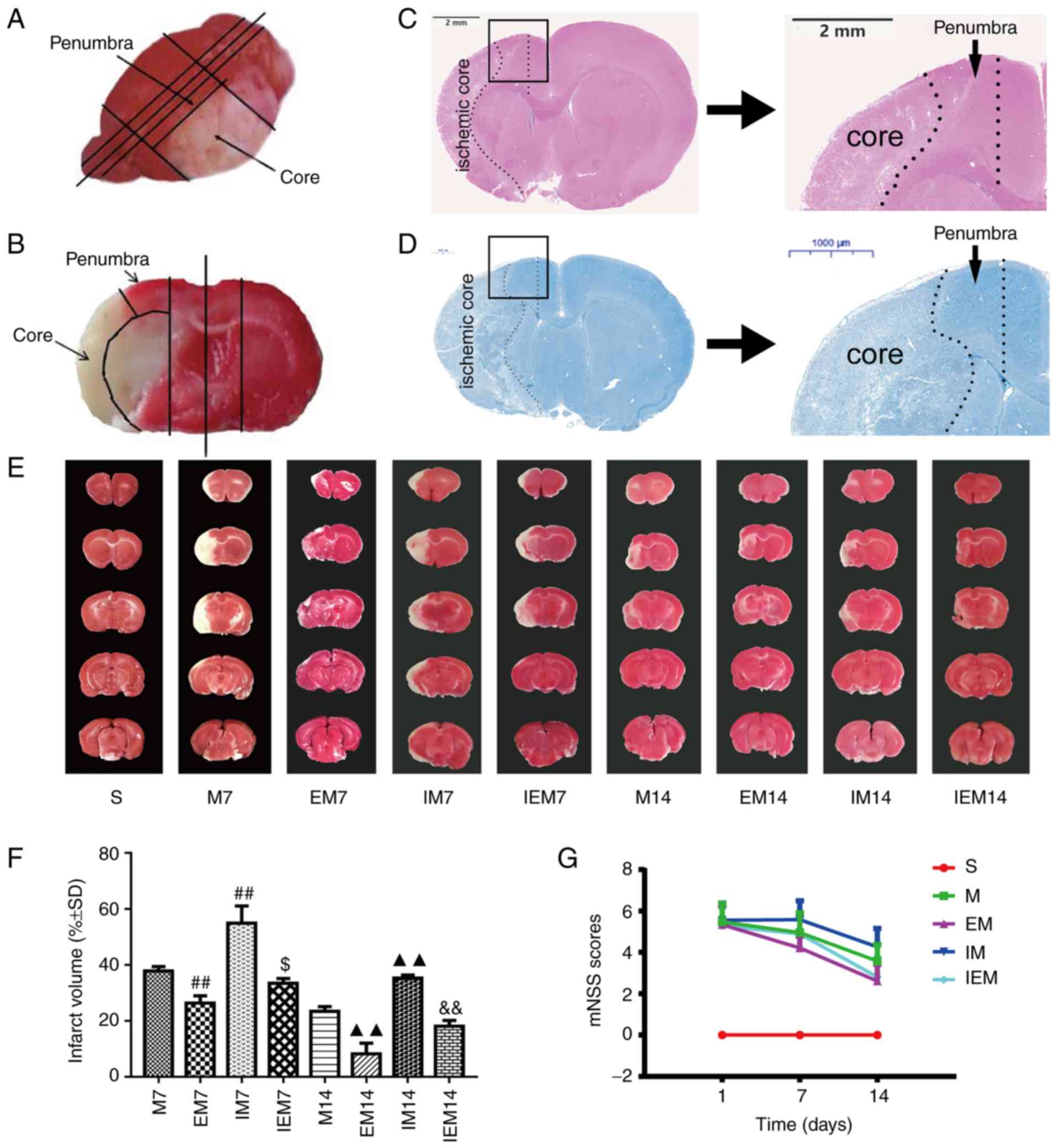 | Figure 1Treadmill training attenuates
neurological deficits and decreases infarct size, while the
inhibitor increased infarct injury. (A and B) Site of ischemic core
and ischemic penumbra area for the same rat. (A) Overall look for
the site of ischemic core and ischemic penumbra area. (B) Local
image on site of ischemic core and ischemic penumbra area; picture
from the previous paper of the current team (24). (C) Hematoxylin & eosin
staining. Scale bars=2 mm. (D) Nissl staining. Scale bars=1,000 μm.
(C) and (D) are shown the location of ischemic penumbra. (E) Showed
infarct volumes were assessed by 2,3,5-triphenyl tetrazolium
chloride. (F) Percentage of infarct volume in each group. Data are
presented as the mean ± standard deviation (n=5).
##P<0.001 vs. the M7 group; $P<0.05 vs.
the EM7 group; ▲▲P<0.01 vs. the M14 group;
&&P<0.01 vs. the EM14 group. (G) Neurological
scores in different groups, in which EM7 vs. M7, P<0.01; EM14
vs. M14, P<0.01; IM7 vs. M7, P<0.05; IM14 vs. M14, P<0.01;
IEM7 vs. EM7, P<0.01; IEM14 vs. EM14, P>0.05. M group, model
group; EM group, treadmill training model group; mNSS, modified
nerve severity score. |
Western blot analysis
As in the authors’ previous publication (26), coronal sections were performed at
4 and 9 mm from the prefrontal lobe, and then the left and right
brains were separated. Finally, sagittal sections were performed on
the 1.0–1.5 mm from the midline of the brain. a transverse diagonal
cut was then performed at ~’10 o’clock’ to separate the core from
the penumbra (38) (Fig. 1A and B). According to the protocol
of rat brain tissue extraction kit (Beyotime Institute of
Biotechnology), total protein and nuclear protein were extracted
respectively. The protein concentration was detected by a BCA
protein detection kit (Beyotime Institute of Biotechnology). The
same amount of protein (50 μg) was transferred to a polyvinylidene
difluoride (EMD Millipore) membrane after 10% SDS-PAGE analysis.
The membranes were blocked with 5% skim milk at room temperature
for 2 h. Wnt3a, β-catenin, BDNF, MBP, GAPDH and Lamin B1 were
incubated on a 4°C shaking table overnight. TBS with 0.05% Tween-20
was washed three times and then, the membranes were soaked in
horseradish peroxidase-conjugated secondary antibody (HRP
AffiniPure Goat Anti-Rabbit IgG (H+L); dilution, 1:10,000; cat. no.
E030120; EarthOx, LLC) at room temperature for 1.5 h. The protein
imaging was detected by a Clarity™ Western ECL Substrate kit (cat.
no. 1705060; Bio-Rad Laboratories, Inc.). ImageJ software (National
Institute of Health) was used for quantitative analysis.
Transmission electron microscopy
(TEM)
The rats were sacrificed at 7 and 14 days after
MCAO, and ultrastructural changes of neurons in the ischemic
penumbra cortex was observed by TEM. The specimens were fixed for
at least 2 h with 2.5% (w/v) glutaraldehyde overnight, washed three
times for 10 min in PBS and then soaked with 2% (v/v) osmium
tetroxide at 37°C for 1 h. The specimens were taken out and rinsed
twice, then stained at room temperature for 2 h with 1% uranyl
acetate and dehydrated with acetone. After dehydration, the tissue
was embedded in the mixture of acetone and entrapped liquid (1:1)
for incubation at 37°C for 2 h, followed by maintenance in mixture
of acetone and entrapped liquid (1:4) for incubation at 37°C
overnight. After that, the tissue was soaked in entrapped liquid
for 2 h at 45°C, followed by incubation at 45°C for 3 h and 65°C
for 48 h to obtain the coronal sections. Semi-thin sections and
toluidine blue staining were used for localization observation.
Finally, at least three ultra-thin sections in each sample were
observed by TEM (JEM-1200EX; JEOL, Ltd.).
Statistical analysis
All analyses were performed using GraphPad Prism 4.0
(GraphPad Software, Inc.) or IBM SPSS 25.0 statistical software
(IBM Corp.). Multiple comparisons were carried out by one-way
analysis of variance (ANOVA) with Tukey (when equal variances
assumed) or Dunnett’s T3 (when equal variances not assumed)
post-hoc analysis. P<0.05 was considered to indicate a
statistically significant difference. The data are reported as the
mean ± standard deviation.
Results
Treadmill training attenuates
neurological deficits, reduces infarct size and decreases the
damage of brain tissue and deformation of neurons, while the
inhibitor increases infarct injury
Treadmill training attenuates
neurologic deficits
As shown in the Table
II and Fig. 1G, the S group
was normal (the scores=0). One day after MCAO, there were no
significant differences in the scores of neurological impairments
among the four groups (P>0.05) and the scores decreased
gradually with the number of days. The score of the EM group was
decreased compared with the M group in the same period (EM7 vs. M7:
P<0.01; EM14 vs. M14: P<0.01). The scores of the IM group
were increased compared with the M group (IM7 vs. M7: P<0.05;
IM14 vs. M14: P<0.01). The score of the EM group was decreased
compared with the IEM group (IEM7 vs. EM7: P<0.01; IEM14 vs.
EM14: P>0.05).
 | Table IIModified neurological severity scores
post middle cerebral artery occlusion. |
Table II
Modified neurological severity scores
post middle cerebral artery occlusion.
| Group | N | 1 day | 7 days | 14 days |
|---|
| S | 19 | 0 | 0 | 0 |
| M | 38 | 5.47±0.89a | 4.95±0.96a | 3.58±0.79a |
| EM | 38 | 5.34±0.94 | 4.21±0.58b | 2.61±0.82b |
| IM | 38 | 5.55±0.83 | 5.58±0.92b | 4.26±0.89b |
| IEM | 38 | 5.29±0.90 | 4.89±0.83c | 2.81±0.90 |
Treadmill training reduces the infarct
volume
As shown in Fig. 1E
and F, the white area represents damaged brain cells, and the
red area represents functional cells. As expected, no infarction
was observed in group S. TTC staining showed that the infarct
volume in the EM group was smaller than that in the M group during
the same period (P<0.01), while the infarct volume in the IM
group was larger than that in the M group during the same period
(P<0.01). Compared with the EM group, the IEM group had larger
infarct volumes (IEM7 vs. EM7: P<0.05; IEM14 vs. EM14:
P<0.01) (Table III).
 | Table IIIResults of infarct volume in each
group after middle cerebral artery occlusion (%). |
Table III
Results of infarct volume in each
group after middle cerebral artery occlusion (%).
| Group | N | 7 days | 14 days |
|---|
| S | 3 | 0 | 0 |
| M | 5 | 37.92±1.50a | 23.37±1.75a |
| EM | 5 | 26.28±2.68b | 8.19±3.86b |
| IM | 5 | 54.89±6.21b | 35.25±1.20b |
| IEM | 5 | 33.41±1.77c | 18.05±2.12c |
Treadmill training decreases the
damage of brain tissue and deformation of neurons
As shown in Fig.
2, no infarction in the S group was found, but infarction was
discovered in the ischemic areas of the other groups. In group S,
as shown in Fig. 2, the neurons
in the cerebral cortex were regular in shape and normal in
structure. The nucleolus was clear. In the M and IM groups,
numerous neurons in the ischemic penumbra were deformed and
necrotic. Increased nuclear pyknosis and nuclear fragmentation
occurred compared with in the S group. The Nissl bodies were
narrowed, deeply stained and in some instances, disappeared. The
cells were swollen, the surrounding space was enlarged and the
shape was irregular. In the EM and IEM groups, the cells had a
normalized arrangement, and a few cells were deformed and
necrotic.
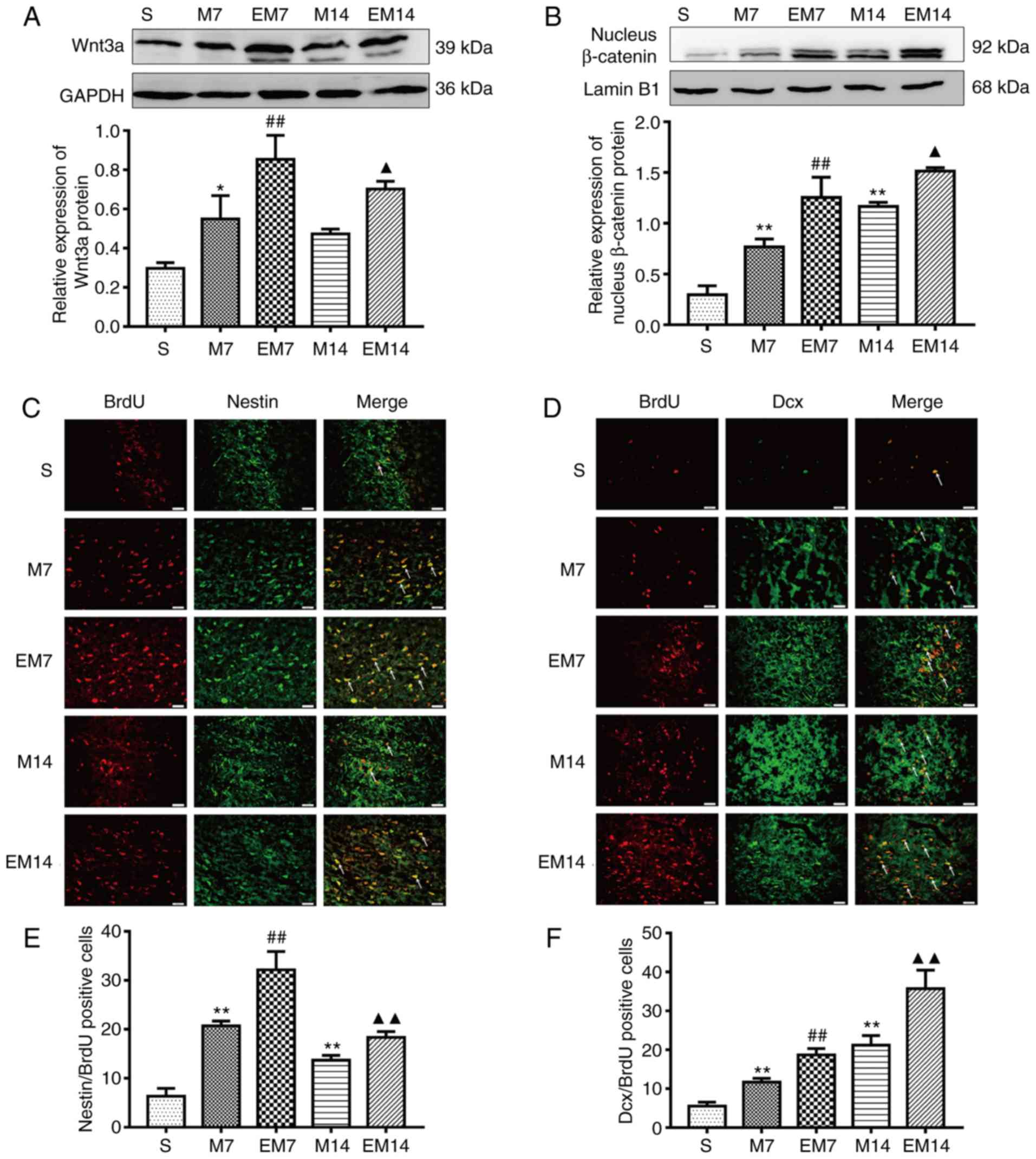
Treadmill training enhances the
activation of Wnt/β-catenin signaling pathway in the ischemic
penumbra
Compared with group M, the expression of Wnt3a and
nuclear β-catenin protein in the EM group was increased (Wnt3a, EM7
vs. M7: P<0.01; EM14 vs. M14: P<0.05; nuclear β-catenin, EM7
vs. M7: P<0.01; EM14 vs. M14: P<0.05; Fig. 3A and B). Compared with group S,
the expression of Wnt3a and nuclear β-catenin protein in the M
group was increased (Wnt3a, S vs. M7: P<0.05; S vs. M14:
P>0.05; nuclear β-catenin, S vs. M7: P<0.01; S vs. M14:
P<0.01; Fig. 3A and B)
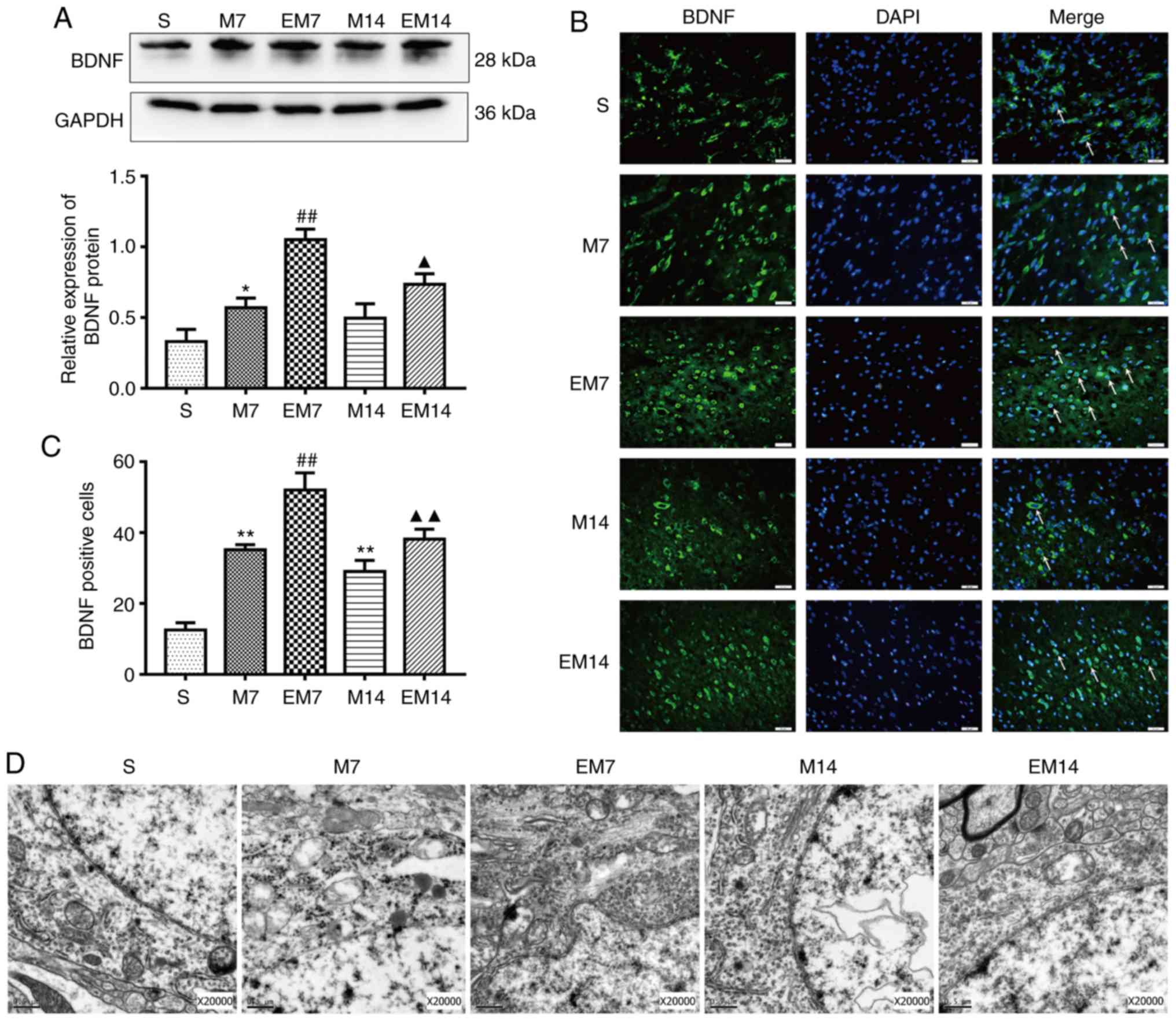
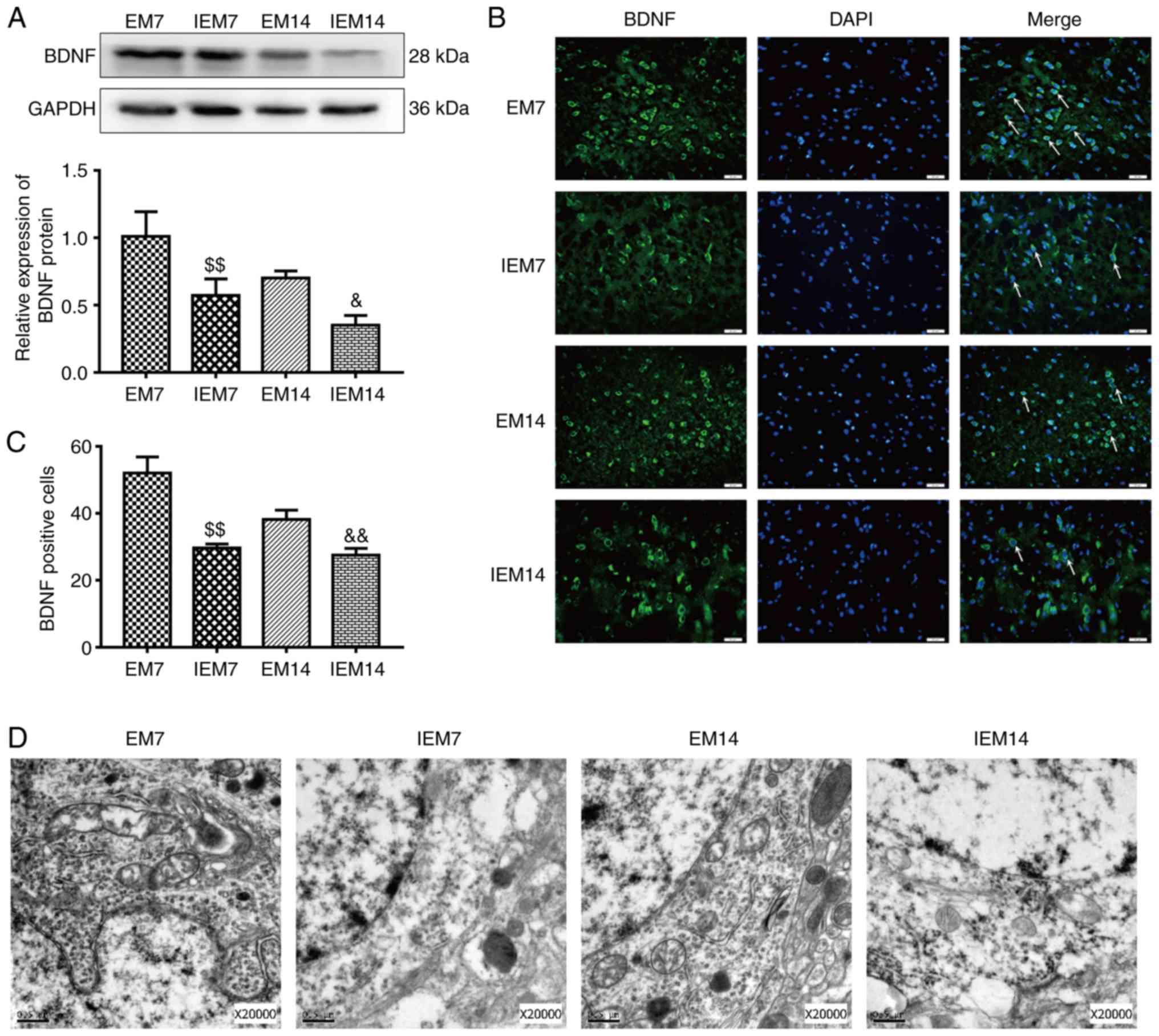
Treadmill training increases the
expression level of BDNF in the ischemic penumbra
The expression of BDNF protein and the number of
BDNF-positive cells in the M groups was increased compared with in
the S groups (western blotting: BDNF, S vs. M7: P<0.05; S vs.
M14: P>0.05; immunofluorescence: BDNF, S vs. M7: P<0.01; S
vs. M14: P<0.01). Similarly, the expression of the BDNF protein
and the number of BDNF-positive cells in EM group were increased
compared with in the M group (western blotting: BDNF, EM7 vs. M7:
P<0.01; EM14 vs. M14: P<0.05; immunofluorescence: BDNF, EM7
vs. M7: P<0.01; EM14 vs. M14: P<0.01; Fig. 4A–C).
Treadmill training improves
regenerative neurogenesis in the ischemic penumbra
Nestin/BrdU is a specific marker of proliferating
NSCs. Dcx/BrdU is a specific marker of proliferating neuroblasts
and differentiating neurons (14). There were almost no
Dcx/BrdU-positive cells and Nestin/BrdU-positive cells in the
ischemic penumbra of the S group in this study. Compared with that
of the sham group, the number of Nestin/BrdU-positive cells in the
MCAO group increased, peaked at 7 days and then decreased at 14
days (P<0.01 vs. sham). Similarly, compared with that of the M
group, the number of Nestin/BrdU-positive cells in the EM group
further increased and the number of cells in the EM group peaked at
7 days and showed a downward trend at 14 days (EM7 vs. M7:
P<0.01; EM14 vs. M14: P<0.01; Fig. 3C and E). The number of the
Dcx/BrdU-positive cells reached a peak at 14 days in the M group
and EM group. The Dcx/BrdU-positive cells in group M were
significantly increased compared with that of the sham operation
group (P<0.01 vs. sham). Similarly, on the 7 and 14th days, the
Dcx/BrdU-positive cells in the EM group were significantly
increased compared with in the M group (EM7 vs. M7: P<0.01; EM14
vs. M14: P<0.01; Fig. 3D and
F).
The present study used TEM to observe the
ultrastructure of the neuron nucleus (Fig. 4D). In the specimens of group S, it
was observed that the distribution of nuclear chromatin was
uniform, the double nuclear membrane of the neuron was clear and
complete. TEM analysis showed that compared with those of group S,
the chromatin distribution of group M was irregular, the nuclear
membrane structure was blurred and the mitochondrial crest was
deformed. In the EM group, the damage of nucleus was alleviated and
the contraction of nuclear membrane was not obvious. The edge of
the nucleus was smooth and a few of the inner cristae of
mitochondria were broken.
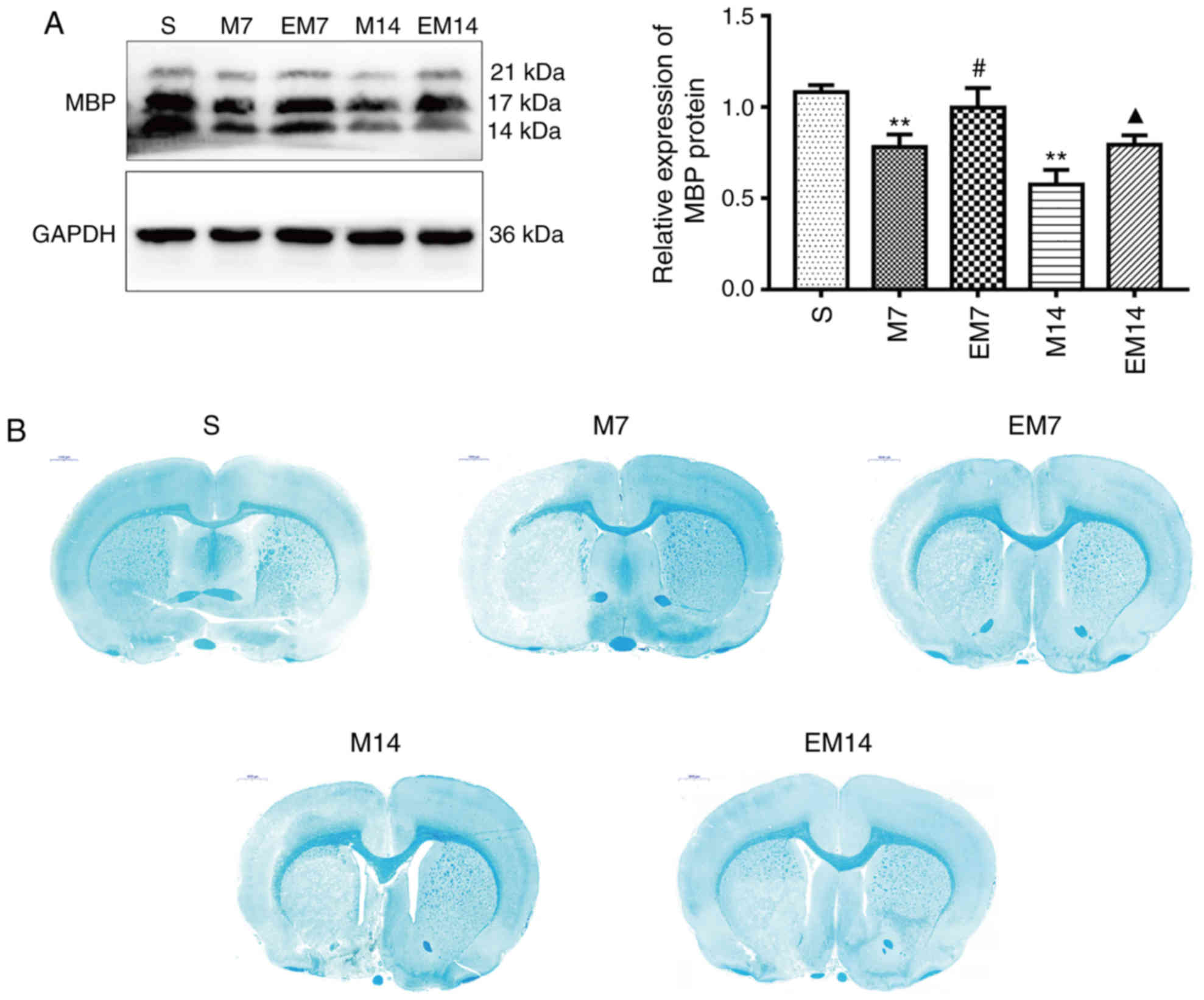
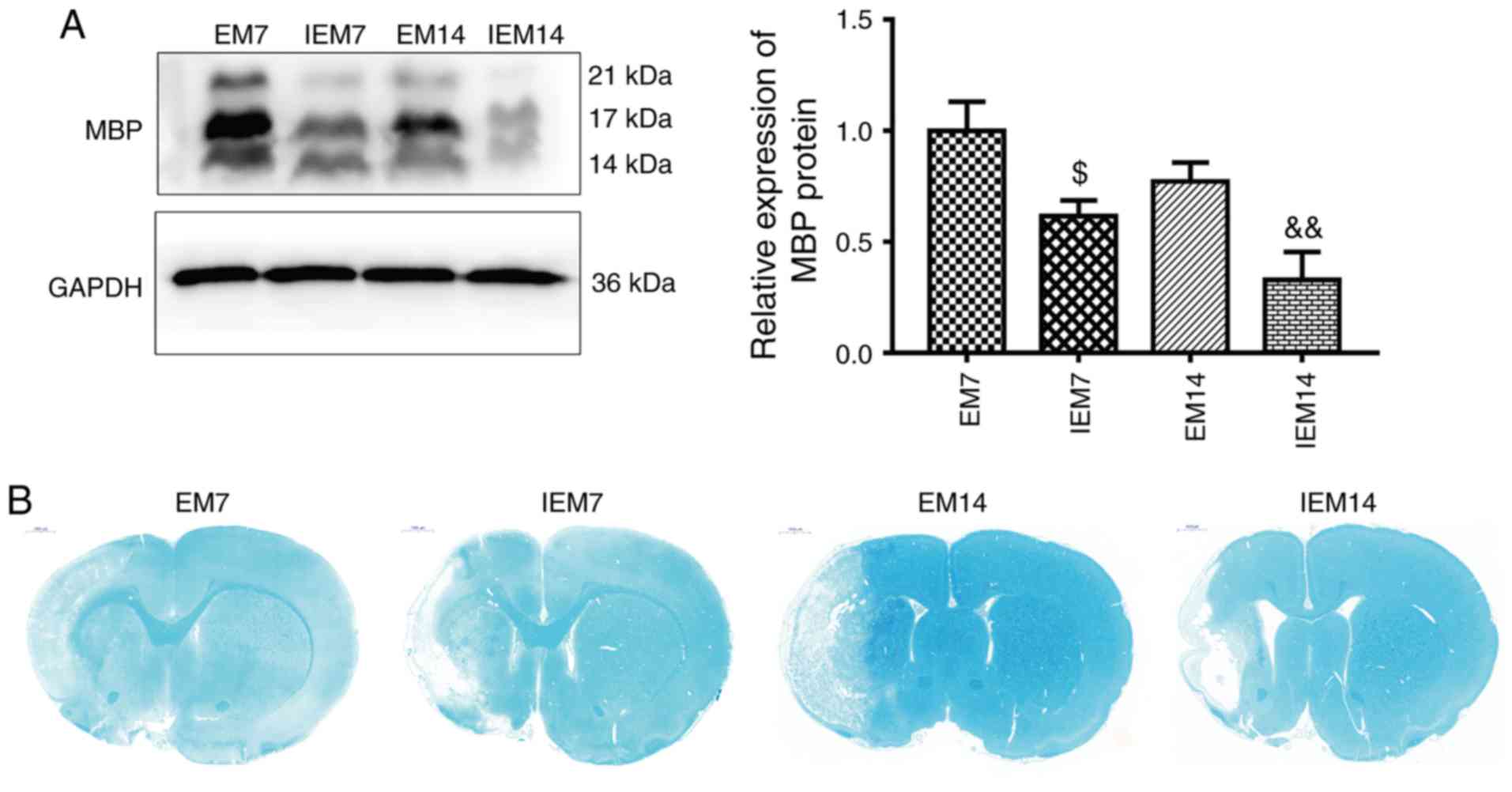
Treadmill training increases the
repair of myelin in the ischemic penumbra
To investigate the effect of treadmill exercise on
myelin repair, the expression level of the myelin marker MBP was
analyzed in the ischemic penumbra. As shown in Fig. 5A, western blot analysis showed
that the expression of MBP in the M group was significantly
decreased compared with the S group (P<0.01). At 7 or 14 days
after MCAO, compared with M group, the expression of MBP protein in
EM group was increased (EM7 vs. M7: P<0.05; EM14 vs. M14:
P<0.05). In sham brain sections, the myelinated fibers were
strongly stained by LFB, the myelinated fibers were dense and
uniform, and the morphology of the myelinated fibers was complete.
After 7 and 14 days of MCAO, all groups, except for the S group,
showed lesions in the cortex and subcortex. In the M group, LFB
staining analysis showed significant demyelination in the ischemic
penumbra. Demyelination was significantly decreased in the EM group
compared with the group M during the same period (Fig. 5B).
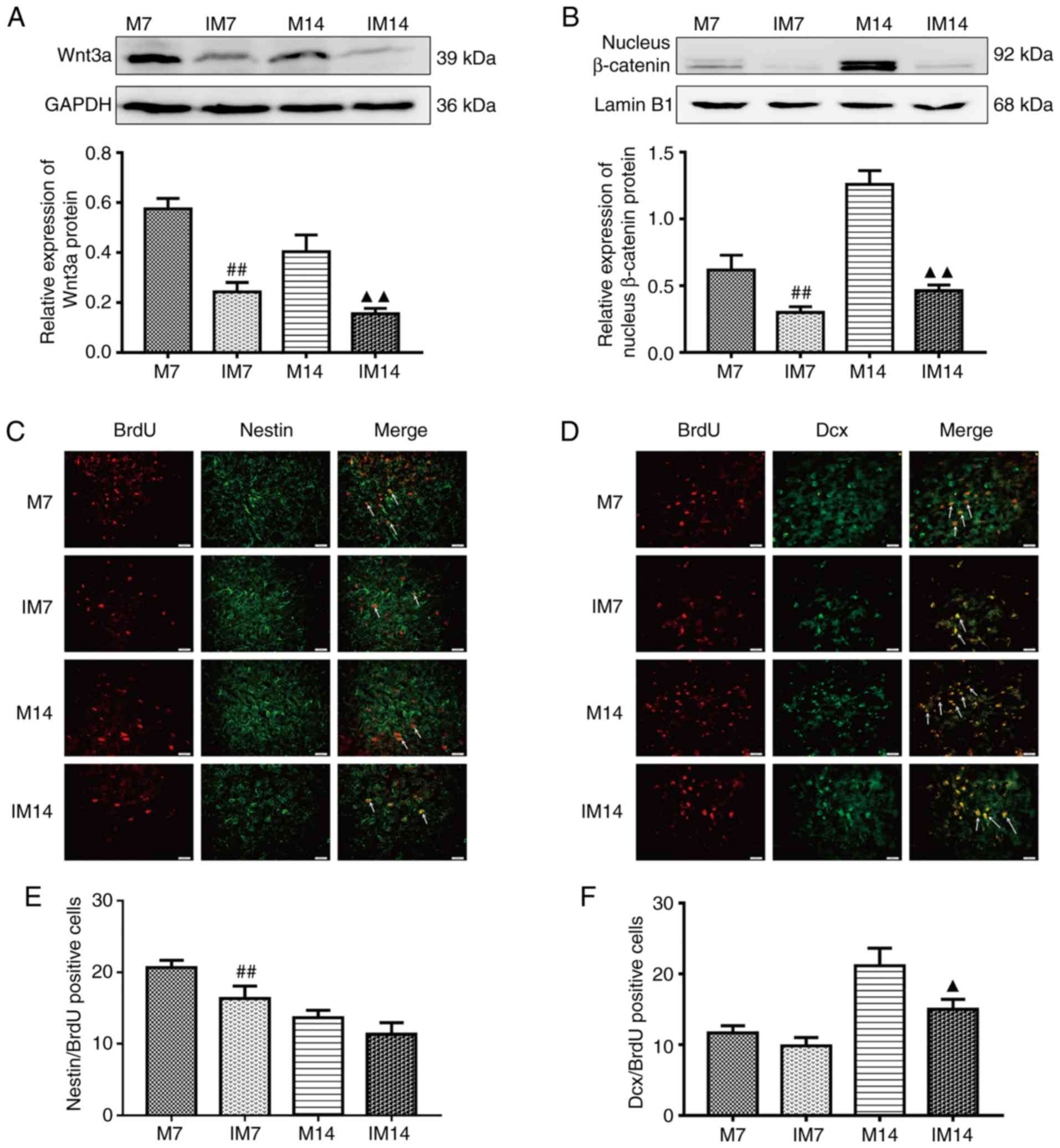
Inhibitor abolishes neurogenesis in
the ischemic penumbra
After the addition of the Wnt/β-catenin pathway
inhibitor XAV939, compared with the M group, the protein expression
of Wnt3a and nuclear β-catenin in the IM group was decreased.
(Wnt3a, IM7 vs. M7: P<0.01; IM14 vs. M14: P<0.01; nuclear
β-catenin, IM7 vs. M7: P<0.01; IM14 vs. M14: P<0.01; Fig. 6A and B). Compared with the M
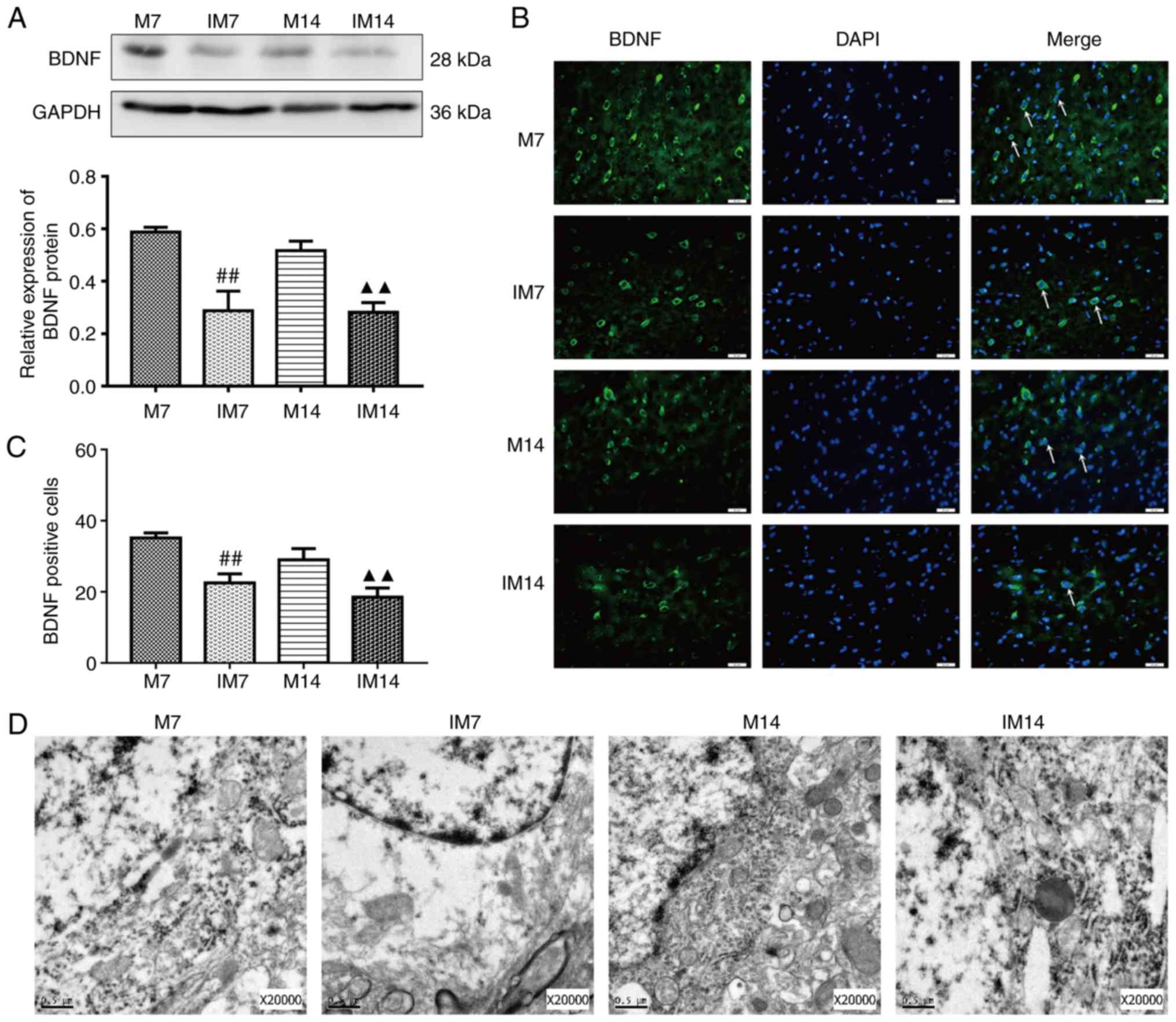
group, the expression of the BDNF protein in the IM group was
decreased during the same period (IM7 vs. M7: P<0.01; IM14 vs.
M14: P<0.01; Fig. 7A). The
same trend was observed in the BDNF-positive cells (IM7 vs. M7:
P<0.01; IM14 vs. M14: P<0.01; Fig. 7B and C). Compared with the M7
group, the number of Dcx/BrdU positive cells in the IM7 group had
no significant change, while there was a decrease in the number of
Dcx/BrdU-positive cells between group M14 and group IM14 (Dcx/BrdU,
IM7 vs. M7: P>0.05; IM14 vs. M14: P<0.05; Fig. 6D and F). There was a difference in
the number of Nestin/BrdU-positive cells between group M7 and group
IM7. Compared with the M14 group, the number of
Nestin/BrdU-positive cells in the IM14 group had no significant
change (IM7 vs. M7: P<0.01; IM14 vs. M14: P>0.05; Fig. 6C and E).
In terms of neuronal structure, TEM showed that
compared with group M, the IM group showed dissolved cytoplasm,
coacervation of nuclear chromatin and a shrunken or dissolved
nuclear membrane. Most of the organelles disappeared and only a
small number of denatured mitochondria (with focal cavitation) were
present (Fig. 7D).
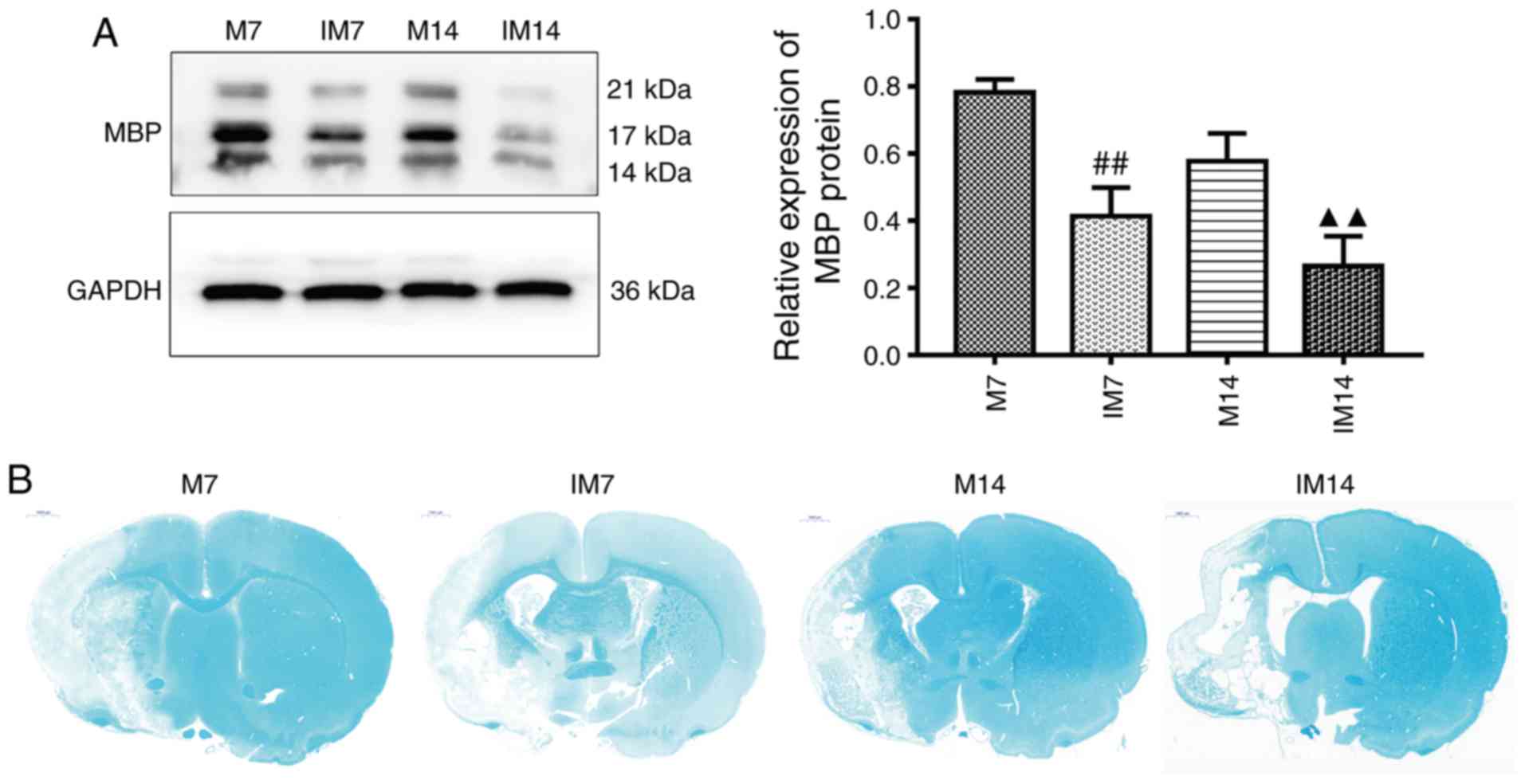
Inhibitor abolishes myelin repair in
the ischemic penumbra
The expression of MBP and the degree of
demyelination at 7 and 14 days after injection of the inhibitor was
further examined. Compared with group M, the expression of MBP
protein in the IM group was decreased (IM7 vs. M7: P<0.01; IM14
vs. M14: P<0.01; Fig. 8A). LFB
staining showed that compared with group M, the integrity of the
myelin sheath was decreased and demyelination was increased in
group IM (Fig. 8B).
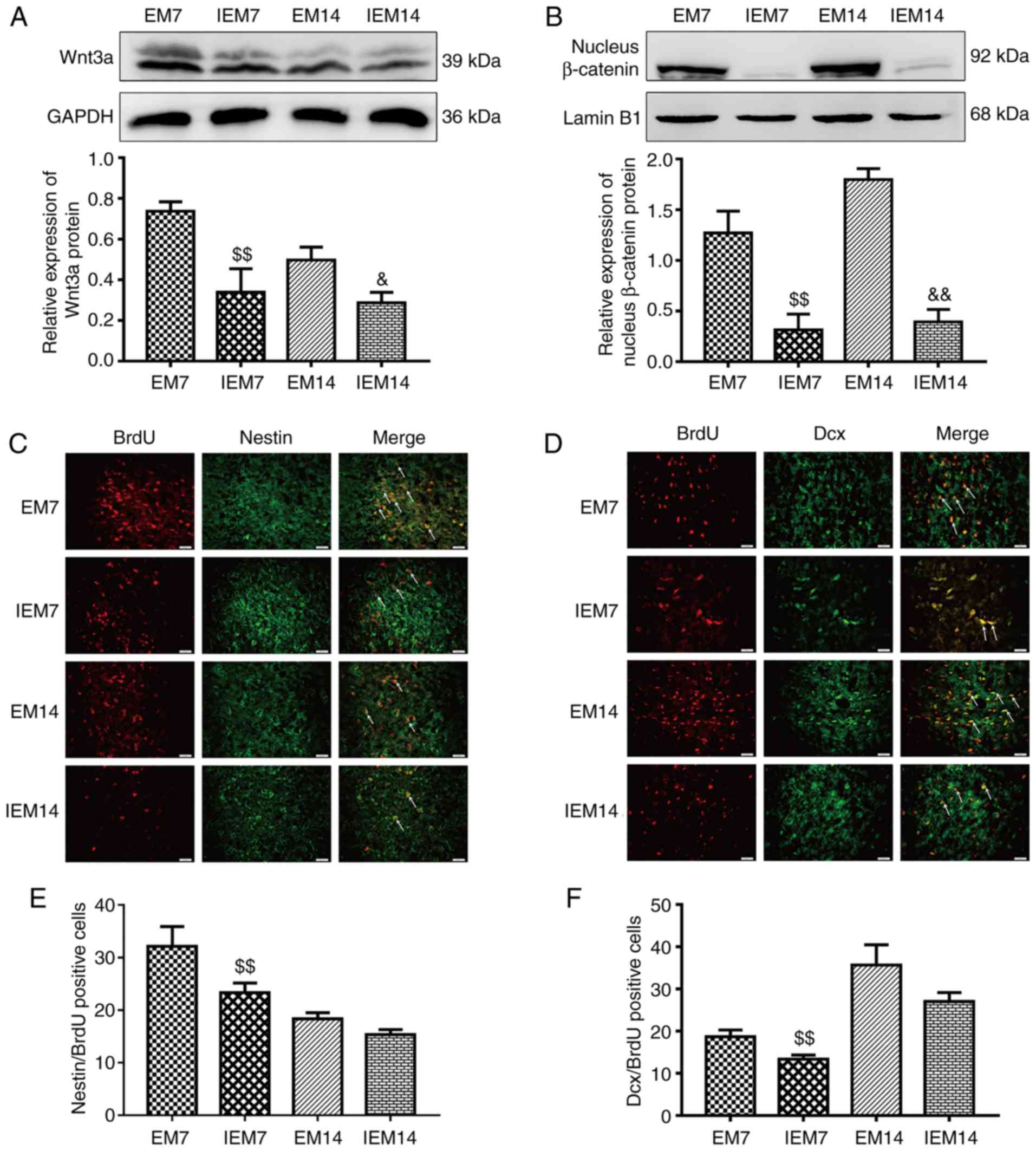
Inhibitor suppresses exercise-promoted
neurogenesis in the ischemic penumbra
After 7 and 14 days of MCAO, four groups of EM7,
IEM7, EM14 and IEM14 were designed to prove whether the
Wnt/β-catenin signaling pathway was involved in exercise-promoted
neurogenesis. The results showed that compared with group EM, the
expression of Wnt3a and nucleus β-catenin protein in group IEM were
decreased (Wnt3a, IEM7 vs. EM7: P<0.01; IEM14 vs. EM14:
P<0.05; nucleus β-catenin, IEM7 vs. EM7: P<0.01; IEM14 vs.
EM14: P<0.01; Fig. 9A and B).
During the same period, compared with group EM, the expression of
BDNF protein and the number of BDNF positive cells in group IEM
were decreased (western blotting: IEM7 vs. EM7: P<0.01; IEM14
vs. EM14: P<0.05; immunofluorescence: IEM7 vs. EM7: P<0.01;
IEM14 vs. EM14: P<0.01; Fig.
10A–C). At 7 and 14 days after MCAO, the expression levels of
Dcx/BrdU-and Nestin/BrdU-positive cells in the EM group were
different from those in the IEM group (Fig. 9C–F). At 7 days, there was a
significant difference in the number of Dcx/BrdU-positive cells
between IEM7 and EM7. However, at 14 days, there was no difference
in the IEM14 group compared with the EM14 group. Similarly, there
was no significant difference in the number of Nestin/BrdU-positive
cells between IEM14 and EM14. However, at 7 days, the number of
positive cells in the IEM7 group was reduced compared with the EM7
group (Dcx/BrdU, IEM7 vs. EM7: P<0.01; IEM14 vs. EM14:
P>0.05; Nestin/BrdU, IEM7 vs. EM7: P<0.01; IEM14 vs. EM14:
P>0.05).
By observing the ultrastructure of neuronal nuclei
under the TEM, the present study found that the IEM group showed
edema in the cytoplasm of the neurons, mitochondrial swelling,
mitochondrial crest deformation and organelle reduction compared
with the EM group (Fig.
10D).
Inhibitor abolishes exercise-promoted
myelin repair in the ischemic penumbra
The current study further detected the expression of
MBP and the changes in LFB staining at 7 or 14 days in group EM7,
IEM7, EM14 and IEM14. Compared with group EM, the expression of MBP
protein in group IEM was decreased (IEM7 vs. EM7: P<0.05; IEM14
vs. EM14: P<0.01; Fig. 11A).
The results of LFB staining showed that compared with those of the
EM group, the myelin staining in the IEM group became lighter, the
integrity became worse and the degree of demyelination increased
(Fig. 11B).
Discussion
The authors’ previous studies have shown that
treadmill exercise can promote angiogenesis and neurogenesis in the
ischemic penumbra of adult rats. In addition, treadmill exercise
can promote dendritic modification and synaptic plasticity
(25,29). In this study, the mechanism of
neurogenesis and myelin repair after ischemic stroke in juvenile
rats was focused on. The results showed that treadmill exercise can
promote neurogenesis and myelin repair after ischemic stroke in
juvenile rats. In addition, the Wnt/β-catenin signaling pathway was
involved in neurogenesis and myelin repair stimulated by treadmill
training.
For a long time, the focus of research has been on
the use of neurogenesis to promote neuronal replacement and enhance
endogenous brain repair. However, studies have found that only a
very small number of neurons survive in neurogenesis after adult
ischemic stroke (39). The latest
research shows that after cerebral ischemia, significant
reaggregation of neurons in the juvenile striatum occurs,
indicating that the juvenile brain has the ability to repair itself
(10). In the present study,
Nestin/BrdU- and Dcx/BrdU-positive cells increased in group M at 7
and 14 days after MCAO. Nestin/BrdU-positive cells reached the peak
on the 7th day and then decreased gradually. The number of
Dcx/BrdU-positive cells increased throughout and reached its peak
on the 14th day. In addition, the expression levels of Wnt3a,
nuclear β-catenin and BDNF increased on the 7 and 14th days after
MCAO. Physical exercise has a neuroprotective effect on both human
and animals. A previous study has shown that pre-ischemic treadmill
training reduces cerebral infarction volume, brain edema and
neurological deficit and improves brain injury after ischemic
stroke (40). The authors’
previous studies have shown that after ischemic stroke in adult
rats or mice, treadmill exercise enhanced neurogenesis by promoting
proliferation, migration and differentiation into mature neurons of
NPCs from the subventricular area to the ischemic penumbra
(29,30). In the present study, it was found
that treadmill exercise promoted the expression of Nestin/BrdU- and
Dcx/BrdU-positive cells in the ischemic penumbra of juvenile rats.
It is suggested that treadmill exercise enhances neurogenesis by
promoting the increment and migration of neuroblasts. More
importantly, how to stimulate the neurogenesis of the penumbra and
explore its mechanism is the focus of the current research.
After neonatal hypoxia-ischemia injury, treadmill
exercise helps improve the recovery of behavior after
hypoxia-ischemia via the upregulation of myelin components and
neurogenesis (41). In addition,
treadmill training combined therapy may improve motor and memory
function by increasing the oligodendroglia involved in the cyclic
AMP-responsive element-binding protein/BDNF signaling pathway, to
restore the myelin components of neonates after hypoxia-ischemia
(13). A study has identified
that long-term exercise can promote the expression of BDNF and
improve the myelin damage caused by ischemia (28). It was found that NPCs could
proliferate and differentiate into oligodendrocyte progenitor cells
(OPCs) after cerebral ischemia. OPCs can differentiate into mature
oligodendrocytes to repair damaged myelin sheaths (42). Oligodendrocyte injury and
demyelination can lead to neurological functional deficits
(43–45). A previous study has shown that
exercise can restore ischemia-induced myelinated hippocampal nerve
fiber injury (28). In the
present study, treadmill exercise promoted the expression of MBP in
the penumbra and reduced demyelination. In this study, treadmill
exercise may alleviate brain injury after stroke by promoting
neurogenesis and myelin repair. However, the mechanism of brain
protection by treadmill training remains to be studied.
A previous study has shown that there is a
pathological decrease in Wnt activity in subventricular zone (SVZ)
after ischemic stroke (19). More
importantly, administration of exogenous Wnt3a can promote the
migration and differentiation of newborn neuroblasts to the
ischemic cortex (19). It was
found that the increased expression of β-catenin could regulate
Wnt3a (46). Moreover, it was
found that the Wnt pathway is directly involved in the upregulation
of BDNF, indicating that the Wnt/β-catenin signaling pathway is one
of the upstream pathways of BDNF (19). BDNF plays an important role in
brain development and brain injury. BDNF can promote neurogenesis,
improve synaptic plasticity, and nourish neurons (47,48). Moreover, BDNF is released by
neurons and secreted mainly through dendrites (49–51). It is reported that exogenous BDNF
injection can promote neuronal regeneration, while the loss of BDNF
inhibited the differentiation of intermediate neurons in mice
(52–54). It has been found that activation
of the Wnt signaling pathway in SVZ can promote endogenous
neurogenesis, increase the number of new neurons and improve nerve
function after injury (19). A
previous study has shown that neurogenesis in the adult brain may
contribute not only to brain repair but also to overall plasticity
(55). Interestingly, in this
research, it was found that neurogenesis after ischemic stroke in
juvenile rats also promotes potential brain repair. Consistent with
a previous study (29), at 7 and
14 days after treadmill exercise in juvenile MCAO rats, numerous
newborn neuroblasts in the ischemic penumbra were observed.
Moreover, an increase in Wnt3a, nucleus β-catenin and BDNF protein
levels and BDNF-positive cells were found in the EM7 and EM14
groups.
Rehabilitation training after stroke is a long
process, which is consistent with previous studies. (25,29,56). The present study found that
compared with 7 days, the volume of cerebral infarction and mNSS
score decreased in 14 days. This change may be achieved through
some protective responses, strong collateral circulation and
favorable external intervention factors (26). These results suggest that
treadmill exercise promotes the repair of brain injury by
upregulating important molecules in the Wnt signaling pathway and
increasing neurogenesis in the ischemic penumbra.
The current study used XAV-939, an inhibitor of the
Wnt pathway, to determine the role of the Wnt pathway in the
ischemic penumbra. When ischemic stroke occurs, the Wnt signaling
pathway is activated. Inhibitors can aggravate neurological
deficits and increase the volume of cerebral infarction. The
present study found that on the 7 and 14th day after MCAO, the
number of Nestin/BrdU- and Dcx/BrdU-positive cells in the IM group
was decreased compared with the M group. This finding is consistent
with the results of a previous study (19). Nissl staining and TEM results
showed that neuronal damage was aggravated in the IM group compared
with the M group. LFB staining showed a decrease in myelinated
fibers in the IM group compared with the M group. The present
results showed that inhibition of Wnt/β-catenin pathway can reduce
neurogenesis and myelin repair in the ischemic penumbra.
In addition, inhibitors were used in the treadmill
exercise group to explore the relationship between neuroprotection
induced by treadmill exercise and Wnt/β-catenin signaling pathway.
At 7 and 14 days after MCAO, the number of Nestin/BrdU- and
Dcx/BrdU-positive cells in the IEM group was decreased compared
with the EM group, which was consistent with the low expression
levels of Wnt3a, nuclear β-catenin and BDNF. LFB staining showed
that compared with those in the EM group, the myelinated fibers in
the IEM group decreased and the staining became lighter, which was
consistent with the change in MBP expression. These results suggest
that the inhibitor reverses the upregulation of Wnt3a, nuclear
β-catenin and BDNF expression after treadmill training, reduces the
expression of MBP, and weakens treadmill exercise-induced
neurogenesis and myelin repair. It has been found that the
neurovascular unit (NVU) is involved in brain injury and brain
repair (26). Newly formed
neuroblasts after stroke are located in the peri-infarction cortex
and are closely related to the vascular endothelium (56). It has been found that the
Wnt/β-catenin signaling pathway can regulate vascular development
and angiogenesis (57).
Activation of the Wnt/β-catenin signaling pathway after MCAO was
reported to enhance the angiogenesis of the ischemic core (58). Wnt proteins are important
mediators of intercellular communication. Wnt3a is involved in
axonal regeneration after CNS injury. The latest studies on the CNS
after injury in adults have shown that the classical Wnt signal
transduction can induce axonal regeneration and neuronal growth,
which indicates that the developmental effect of Wnt can be reused
in adults through exogenous stimulation (59,60). It has been found that promoting
the expression of Wnt3a can increase vascular regeneration and
vascular repair in the peri-infarct region (19). In the model of crush injury of the
optic nerve in mice, intravitreal injection of classical
Wnt/β-catenin signal activator Wnt3a caused obvious axonal growth
beyond the site of axonal lesion (61). Wnt3a signal transduction promotes
neurite growth, increases neuronal function and induces repair
after spinal cord contusion in adult rats (62). Previous studies have shown that
exogenous Wnt3a mediated by lentivirus vectors enhances
neurogenesis in vivo (20). In addition, exogenous Wnt3a can
also promote the regeneration and differentiation of neural stem
cells in vitro (63). In
the present study, Wnt3a was focused on because of its established
roles in neural development during embryogenesis, as well as in
adult neurogenesis (64–66). Moreover, activation of Wnt3a/
β-catenin signaling directly regulates neurogenesis events, which
includes a set of transcriptional changes leading to enhanced
division and differentiation of Dcx+ neuroblasts out of
the SVZ (12,67,68). Furthermore, Wnt3a is
neuroprotective and may protect neurons during neurodegenerative
processes, such as Alzheimer’s disease and Huntington’s disease
(69–71). However, the relationship between
neurogenesis and angiogenesis was not investigated in this study.
Therefore, the current experiment can only prove that treadmill
training promotes neurogenesis and myelin repair through the
activation of the Wnt/β-catenin signaling pathway. In contrast,
inhibiting the conduction of the Wnt signaling pathway will weaken
the neuroprotective effect induced by treadmill training. However,
it is unclear whether angiogenesis or pairing of NVUs is directly
or indirectly involved in neurogenesis and myelin repair produced
by treadmill training through the activation of the Wnt/β-catenin
signaling pathway.
The present study has some limitations. First, the
conditions to use real-time monitoring of cerebral blood flow
unified modeling were not present, which may lead to modeling
instability. Second, only a few signal pathway proteins were
examined and no in vitro experiments were performed. Third,
only neurogenesis in two periods after stroke (7 and 14 days) was
studied; neurogenesis over a longer period of time, especially
newborn mature neurons should be further studied. Fourth, except
for LFB staining to observe the changes of myelin fibers and detect
the expression level of the myelin marker protein MBP, no other
analyses of proteins and cells related to myelin repair were
conducted. In future experiments, the authors will work hard to
improve the above deficiencies.
In conclusion, a new role for treadmill training in
promoting neurogenesis and myelin sheath repair in juvenile rats
was demonstrated. In addition, Wnt/β-catenin signaling pathway was
shown to not only mediate neurogenesis and myelin repair, but also
participate in exercise-mediated neurogenesis and myelin repair.
However, after ischemic stroke, treadmill-induced neurogenesis and
myelin repair is a complex process, involving numerous key factors,
which need to be fully clarified.
Acknowledgements
Not applicable.
Funding
The present study was funded by a project funded by
the Wenzhou Municipal Bureau of Science and Technology (grant nos.
Y20170070 and Y20180096).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors’ contributions
XC, HZ and JC conceived and designed the experiment,
and JC, QX and QH carried out the experiment. SW, JP, GP and YZ
helped in the execution of the experiment; WS and LJ analyzed the
data. JC and QX wrote the manuscript. All the authors discussed and
suggested the experiment. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All animal procedures in this study were approved by
the Experimental Animal Ethics Committee of Wenzhou Medical
University (ethical no. Wydw2019-0766) and carried out in
accordance with the guidelines for the National Institutes of
Health Guide for the Care and Use of Laboratory Animals.
Patient consent for publication
Not applicable.
Competing interest
The authors declare they have no competing
interests.
References
|
1
|
Feigin VL, Norrving B, George MG, Foltz
JL, Roth GA and Mensah GA: Prevention of stroke: A strategic global
imperative. Nat Rev Neurol. 12:501–512. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Moskowitz MA, Lo EH and Iadecola C: The
science of stroke: Mechanisms in search of treatments. Neuron.
67:181–198. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ginsberg MD: Neuroprotection for ischemic
stroke: Past, present and future. Neuropharmacology. 55:363–389.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Minnerup J, Sutherland BA, Buchan AM and
Kleinschnitz C: Neuroprotection for stroke: Current status and
future perspectives. Int J Mol Sci. 13:11753–11772. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Magnusson JP, Goritz C, Tatarishvili J,
Dias DO, Smith EM, Lindvall O, Kokaia Z and Frisén J: A latent
neurogenic program in astrocytes regulated by Notch signaling in
the mouse. Science. 346:237–241. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ji Y, Teng L, Zhang R, Sun J and Guo Y:
NRG-1β exerts neuroprotective effects against ischemia
reperfusion-induced injury in rats through the JNK signaling
pathway. Neuroscience. 362:13–24. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Astrup J, Siesjo BK and Symon L:
Thresholds in cerebral ischemia-the ischemic penumbra. Stroke.
12:723–725. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lo EH: A new penumbra: Transitioning from
injury into repair after stroke. Nat Med. 14:497–500. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jackman K and Iadecola C: Neurovascular
regulation in the ischemic brain. Antioxid Redox Signal.
22:149–160. 2015. View Article : Google Scholar :
|
|
10
|
Rodgers KM, Ahrendsen JT, Patsos OP,
Strnad FA, Yonchek JC, Traystman RJ, Macklin WB and Herson PS:
Endogenous neuronal replacement in the juvenile brain following
cerebral ischemia. Neuroscience. 380:1–13. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang Y, Zhao Z, Rege SV, Wang M, Si G,
Zhou Y, Wang S, Griffin JH, Goldman SA and Zlokovic BV:
3K3A-activated protein C stimulates postischemic neuronal repair by
human neural stem cells in mice. Nat Med. 22:1050–1055. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sun FL, Wang W, Zuo W, Xue JL, Xu JD, Ai
HX, Zhang L, Wang XM and Ji XM: Promoting neurogenesis via
Wnt/β-catenin signaling pathway accounts for the neurorestorative
effects of morroniside against cerebral ischemia injury. Eur J
Pharmacol. 738:214–221. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pak ME, Jung DH, Lee HJ, Shin MJ, Kim SY,
Shin YB, Yun YJ, Shin HK and Choi BT: Combined therapy involving
electroacupuncture and treadmill exercise attenuates demyelination
in the corpus callosum by stimulating oligodendrogenesis in a rat
model of neonatal hypoxia-ischemia. Exp Neurol. 300:222–231. 2018.
View Article : Google Scholar
|
|
14
|
Hao XZ, Yin LK, Tian JQ, Li CC, Feng XY,
Yao ZW, Jiang M and Yang YM: Inhibition of Notch1 signaling at the
subacute stage of stroke promotes endogenous neurogenesis and motor
recovery after stroke. Front Cell Neurosci. 12:2452018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Danzer SC: Postnatal and adult
neurogenesis in the development of human disease. Neuroscientist.
14:446–458. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Piccin D and Morshead CM: Wnt signaling
regulates symmetry of division of neural stem cells in the adult
brain and in response to injury. Stem Cells. 29:528–538. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yoshinaga Y, Kagawa T, Shimizu T, Inoue T,
Takada S, Kuratsu J and Taga T: Wnt3a promotes hippocampal
neurogenesis by shortening cell cycle duration of neural progenitor
cells. Cell Mol Neurobiol. 30:1049–1058. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Munji RN, Choe Y, Li G, Siegenthaler JA
and Pleasure SJ: Wnt signaling regulates neuronal differentiation
of cortical intermediate progenitors. J Neurosci. 31:1676–1687.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wei ZZ, Zhang JY, Taylor TM, Gu X, Zhao Y
and Wei L: Neuroprotective and regenerative roles of intranasal
Wnt-3a administration after focal ischemic stroke in mice. J Cereb
Blood Flow Metab. 38:404–421. 2018. View Article : Google Scholar :
|
|
20
|
Lie DC, Colamarino SA, Song HJ, Désiré L,
Mira H, Consiglio A, Lein ES, Jessberger S, Lansford H, Dearie AR
and Gage FH: Wnt signalling regulates adult hippocampal
neurogenesis. Nature. 437:1370–1375. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qu Q, Sun G, Murai K, Ye P, Li W, Asuelime
G, Cheung YT and Shi Y: Wnt7a regulates multiple steps of
neurogenesis. Mol Cell Biol. 33:2551–2559. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang J, Chen T and Shan G: MiR-148b
regulates proliferation and differentiation of neural stem cells
via Wnt/β-catenin signaling in rat ischemic stroke model. Front
Cell Neurosci. 11:3292017. View Article : Google Scholar
|
|
23
|
Xu D, Hou K, Li F, Chen S, Fang W and Li
Y: XQ-1H alleviates cerebral ischemia in mice through inhibition of
apoptosis and promotion of neurogenesis in a Wnt/β-catenin
signaling dependent way. Life Sci. 235:1168442019. View Article : Google Scholar
|
|
24
|
Adachi K, Mirzadeh Z, Sakaguchi M,
Yamashita T, Nikolcheva T, Gotoh Y, Peltz G, Gong L, Kawase T,
Alvarez-Buylla A, et al: Beta-catenin signaling promotes
proliferation of progenitor cells in the adult mouse subventricular
zone. Stem Cells. 25:2827–2836. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xie Q, Cheng J, Pan G, Wu S, Hu Q, Jiang
H, Wang Y, Xiong J, Pang Q and Chen X: Treadmill exercise
ameliorates focal cerebral ischemia/reperfusion-induced
neurological deficit by promoting dendritic modification and
synaptic plasticity via upregulating caveolin-1/VEGF signaling
pathways. Exp Neurol. 313:60–78. 2019. View Article : Google Scholar
|
|
26
|
Chen Z, Hu Q, Xie Q, Wu S, Pang Q, Liu M,
Zhao Y, Tu F, Liu C and Chen X: Effects of treadmill exercise on
motor and cognitive function recovery of MCAO mice through the
caveolin-1/VEGF signaling pathway in ischemic penumbra. Neurochem
Res. 44:930–946. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schabitz WR, Berger C, Kollmar R, Seitz M,
Tanay E, Kiessling M, Schwab S and Sommer C: Effect of
brain-derived neurotrophic factor treatment and forced arm use on
functional motor recovery after small cortical ischemia. Stroke.
35:992–997. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ahn JH, Choi JH, Park JH, Kim IH, Cho JH,
Lee JC, Koo HM, Hwangbo G, Yoo KY, Lee CH, et al: Long-term
exercise improves memory deficits via restoration of myelin and
microvessel damage, and enhancement of neurogenesis in the aged
gerbil hippocampus after ischemic stroke. Neurorehabil Neural
Repair. 30:894–905. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pang Q, Zhang H, Chen Z, Wu Y, Bai M, Liu
Y, Zhao Y, Tu F, Liu C and Chen X: Role of caveolin-1/vascular
endothelial growth factor pathway in basic fibroblast growth
factor-induced angiogenesis and neurogenesis after treadmill
training following focal cerebral ischemia in rats. Brain Res.
1663:9–19. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao Y, Pang Q, Liu M, Pan J, Xiang B,
Huang T, Tu F, Liu C and Chen X: Treadmill exercise promotes
neurogenesis in ischemic rat brains via caveolin-1/VEGF signaling
pathways. Neurochem Res. 42:389–397. 2017. View Article : Google Scholar
|
|
31
|
Saver JL, Albers GW, Dunn B, Johnston KC
and Fisher M; STAIR VI Consortium. Stroke Therapy Academic Industry
Roundtable (STAIR) recommendations for extended window acute stroke
therapy trials. Stroke. 40:2594–2600. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Longa EZ, Weinstein PR, Carlson S and
Cummins R: Reversible middle cerebral artery occlusion without
craniectomy in rats. Stroke. 20:84–91. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jean LeBlanc N, Menet R, Picard K, Parent
G, Tremblay ME and ElAli A: Canonical Wnt pathway maintains
blood-brain barrier integrity upon ischemic stroke and its
activation ameliorates tissue plasminogen activator therapy. Mol
Neurobiol. 56:6521–6538. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang YZ, Yamagami T, Gan Q, Wang Y, Zhao
T, Hamad S, Lott P, Schnittke N, Schwob JE and Zhou CJ: Canonical
Wnt signaling promotes the proliferation and neurogenesis of
peripheral olfactory stem cells during postnatal development and
adult regeneration. J Cell Sci. 124:1553–1563. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M
and Chopp M: Therapeutic benefit of intravenous administration of
bone marrow stromal cells after cerebral ischemia in rats. Stroke.
32:1005–1011. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ding YH, Ding Y, Li J, Bessert DA and
Rafols JA: Exercise pre-conditioning strengthens brain
microvascular integrity in a rat stroke model. Neurol Res.
28:184–189. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gao Y, Zhao Y, Pan J, Yang L, Huang T,
Feng X, Li C, Liang S, Zhou D, Liu C, et al: Treadmill exercise
promotes angiogenesis in the ischemic penumbra of rat brains
through caveolin-1/VEGF signaling pathways. Brain Res. 1585:83–90.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ashwal S, Tone B, Tian HR, Cole DJ,
Liwnicz BH and Pearce WJ: Core and penumbral nitric oxide synthase
activity during cerebral ischemia and reperfusion in the rat pup.
Pediatr Res. 46:390–400. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhao C, Deng W and Gage FH: Mechanisms and
functional implications of adult neurogenesis. Cell. 132:645–660.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang X, Zhang M, Yang SD, Li WB, Ren SQ,
Zhang J and Zhang F: Pre-ischemic treadmill training alleviates
brain damage via GLT-1-mediated signal pathway after ischemic
stroke in rats. Neuroscience. 274:393–402. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kim HN, Pak ME, Shin MJ, Kim SY, Shin YB,
Yun YJ, Shin HK and Choi BT: Comparative analysis of the beneficial
effects of treadmill training and electroacupuncture in a rat model
of neonatal hypoxia-ischemia. Int J Mol Med. 39:1393–1402. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gallo V and Armstrong RC: Myelin repair
strategies: A cellular view. Curr Opin Neurol. 21:278–283. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Dewar D, Underhill SM and Goldberg MP:
Oligodendrocytes and ischemic brain injury. J Cereb Blood Flow
Metab. 23:263–274. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Micu I, Jiang Q, Coderre E, Ridsdale A,
Zhang L, Woulfe J, Yin X, Trapp BD, McRory JE, Rehak R, et al: NMDA
receptors mediate calcium accumulation in myelin during chemical
ischaemia. Nature. 439:988–992. 2006. View Article : Google Scholar
|
|
45
|
McTigue DM and Tripathi RB: The life,
death, and replacement of oligodendrocytes in the adult CNS. J
Neurochem. 107:1–19. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yi H, Hu J, Qian J and Hackam AS:
Expression of brain-derived neurotrophic factor is regulated by the
Wnt signaling pathway. Neuroreport. 23:189–194. 2012. View Article : Google Scholar :
|
|
47
|
Lu B, Nagappan G, Guan X, Nathan PJ and
Wren P: BDNF-based synaptic repair as a disease-modifying strategy
for neurodegenerative diseases. Nat Rev Neurosci. 14:401–416. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Bekinschtein P, Oomen CA, Saksida LM and
Bussey TJ: Effects of environmental enrichment and voluntary
exercise on neurogenesis, learning and memory, and pattern
separation: BDNF as a critical variable? Semin Cell Dev Biol.
22:536–542. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Greenberg ME, Xu B, Lu B and Hempstead BL:
New insights in the biology of BDNF synthesis and release:
Implications in CNS function. J Neurosci. 29:12764–12767. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Brigadski T, Hartmann M and Lessmann V:
Differential vesicular targeting and time course of synaptic
secretion of the mammalian neurotrophins. J Neurosci. 25:7601–7614.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Matsuda N, Lu H, Fukata Y, Noritake J, Gao
H, Mukherjee S, Nemoto T, Fukata M and Poo MM: Differential
activity-dependent secretion of brain-derived neurotrophic factor
from axon and dendrite. J Neurosci. 29:14185–14198. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Scharfman H, Goodman J, Macleod A, Phani
S, Antonelli C and Croll S: Increased neurogenesis and the ectopic
granule cells after intrahippocampal BDNF infusion in adult rats.
Exp Neurol. 192:348–356. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Benraiss A, Chmielnicki E, Lerner K, Roh D
and Goldman SA: Adenoviral brain-derived neurotrophic factor
induces both neostriatal and olfactory neuronal recruitment from
endogenous progenitor cells in the adult forebrain. J Neurosci.
21:6718–6731. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Jones KR, Farinas I, Backus C and
Reichardt LF: Targeted disruption of the BDNF gene perturbs brain
and sensory neuron development but not motor neuron development.
Cell. 76:989–999. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Obernier K, Tong CK and Alvarez-Buylla A:
Restricted nature of adult neural stem cells: Re-evaluation of
their potential for brain repair. Front Neurosci. 8:1622014.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Carmichael ST, Ohab J and Nguyen J:
Post-stroke neurogenesis and the neurovascular niche: Newly born
neuroblasts localize to peri-infarct cortex in close association
with the vascular endothelium. J Cereb Blood Flow Metab. 25:S214.
2005. View Article : Google Scholar
|
|
57
|
Parmalee NL and Kitajewski J: Wnt
signaling in angiogenesis. Curr Drug Targets. 9:558–564. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Xu Y, Zhang G, Kang Z, Xu Y, Jiang W and
Zhang S: Cornin increases angiogenesis and improves functional
recovery after stroke via the Ang1/Tie2 axis and the Wnt/β-catenin
pathway. Arch Pharm Res. 39:133–142. 2016. View Article : Google Scholar
|
|
59
|
Ciani L and Salinas PC: WNTs in the
vertebrate nervous system: From patterning to neuronal
connectivity. Nat Rev Neurosci. 6:351–362. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Salinas PC: Wnt signaling in the
vertebrate central nervous system: From axon guidance to synaptic
function. Cold Spring Harb Perspect Biol. 4:pii: a008003. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Patel AK, Park KK and Hackam AS: Wnt
signaling promotes axonal regeneration following optic nerve injury
in the mouse. Neuroscience. 343:372–383. 2017. View Article : Google Scholar :
|
|
62
|
Yin ZS, Zu B, Chang J and Zhang H: Repair
effect of Wnt3a protein on the contused adult rat spinal cord.
Neurol Res. 30:480–486. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Kalani MY, Cheshier SH, Cord BJ, Bababeygy
SR, Vogel H, Weissman IL, Palmer TD and Nusse R: Wnt-mediated
self-renewal of neural stem/progenitor cells. Proc Natl Acad Sci
USA. 105:16970–16975. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Ikeya M and Takada S: Wnt-3a is required
for somite specification along the anteroposterior axis of the
mouse embryo and for regulation of cdx-1 expression. Mech Dev.
103:27–33. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Mastroiacovo F, Busceti CL, Biagioni F,
Moyanova SG, Meisler MH, Battaglia G, Caricasole A, Bruno V and
Nicoletti F: Induction of the Wnt antagonist, Dickkopf-1,
contributes to the development of neuronal death in models of brain
focal ischemia. J Cereb Blood Flow Metab. 29:264–276. 2009.
View Article : Google Scholar
|
|
66
|
Mussmann C, Hubner R, Trilck M, Rolfs A
and Frech MJ: HES5 is a key mediator of Wnt-3a-induced neuronal
differentiation. Stem Cells Dev. 23:1328–1339. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Giuliani D, Zaffe D, Ottani A, Spaccapelo
L, Galantucci M, Minutoli L, Bitto A, Irrera N, Contri M, Altavilla
D, et al: Treatment of cerebral ischemia with melanocortins acting
at MC4 receptors induces marked neurogenesis and long-lasting
functional recovery. Acta Neuropathol. 122:443–453. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Spaccapelo L, Galantucci M, Neri L, Contri
M, Pizzala R, D’Amico R, Ottani A, Sandrini M, Zaffe D, Giuliani D
and Guarini S: Up-regulation of the canonical Wnt-3A and Sonic
hedgehog signaling underlies melanocortin-induced neurogenesis
after cerebral ischemia. Eur J Pharmacol. 707:78–86. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Inestrosa NC, Urra S and Colombres M:
Acetylcholinesterase (AChE)-amyloid-beta-peptide complexes in
Alzheimer’s disease. The Wnt signaling pathway. Curr Alzheimer Res.
1:249–254. 2004. View Article : Google Scholar
|
|
70
|
Toledo EM, Colombres M and Inestrosa NC:
Wnt signaling in neuroprotection and stem cell differentiation.
Prog Neurobiol. 86:281–296. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Tourette C, Farina F, Vazquez-Manrique RP,
Orfila AM, Voisin J, Hernandez S, Offner N, Parker JA, Menet S, Kim
J, et al: The Wnt receptor Ryk reduces neuronal and cell survival
capacity by repressing FOXO activity during the early phases of
mutant huntingtin pathogenicity. PLoS Biol. 12:e10018952014.
View Article : Google Scholar : PubMed/NCBI
|