Introduction
Renal cell carcinoma (RCC) is a common disease of
the urinary tract which accounts for 2–3% of all adult malignant
tumors (1). Clear cell RCC is the
most common type of RCC and is present in 30% of patients with RCC
with evidence of metastasis at the initial diagnosis (2). In China, the morbidity of patients
with kidney cancer has increased in recent years, which clearly
poses a serious public health problem. Surgery remains the most
effective option for localized treatment as RCC is often
insensitive to traditional chemotherapy and radiotherapy (3). Targeted therapy has been widely used
in patients with advanced metastatic RCC; however, a significant
number of patients exhibited limited benefits. Therefore, a novel
and more efficient therapeutic regimen is required.
Silibinin, a flavonoid extracted from milk thistle
seeds, is widely used as a hepatoprotective and antioxidant agent
in Asia and Europe (4).
Previously, accumulating evidence from the authors’ and other
research laboratories has demonstrated the anti-cancer effects of
silibinin in various models of cancer (4–16).
The authors’ laboratory has focused on the mechanisms underlying
silibinin mediated suppression of metastasis in different types of
urological cancer, including RCC, prostate cancer and bladder
cancer. In RCC, the authors’ previously demonstrated that silibinin
decreased invasion and migration of RCC 786-O cells in vitro
and this was associated with downregulation of matrix
metalloproteinase (MMP)-2 and 9, and urokinase plasminogen
activator, and inhibition of the mitogen-activated protein kinase
pathway. Additionally, silibinin decreased migration and invasion
of RCC cells by suppressing epidermal growth factor receptor/MMP-9
signaling (17) and decreased the
metastatic capacity of RCC by activating autophagy through
adenosine 5′-monophosphate-activated protein kinase
(AMPK)/mammalian target of rapamycin (mTOR) pathway (18). However, the molecular mechanisms
underlying regulation of autophagy are largely unknown.
In the present study, the anti-metastatic effects of
silibinin on RCC were determined, with a focus on
autophagy-dependent Wnt/β-catenin signaling. The results suggest
that silibinin exerted its anti-metastatic effects on RCC through
inhibition of the epithelial-mesenchymal transition (EMT).
Mechanistically, autophagy-dependent Wnt/β-catenin signaling is
involved in the inhibition of metastasis and EMT by silibinin in
RCC.
Materials and methods
Cell culture
Human RCC cell lines, 786-O and ACHN were obtained
from the American Type Culture Collection, and maintained in
RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.) with 10%
fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) at
37°C with 5% CO2 in a humidified atmosphere. The culture
medium was supplemented with 100 U/ml penicillin and 0.1 mg/ml
streptomycin (Gibco; Thermo Fisher Scientific, Inc.).
Reagents and antibodies
Silibinin was purchased from Sigma-Aldrich; Merck
KGaA and dissolved to a final concentration of 50 mM in DMSO.
Hydroxychloroquine sulfate (cat. no. H0915), 3-Methyladenine (3-MA;
cat. no. M9281) and lithium chloride (LiCl; cat. no. L9650) were
purchased from Sigma-Aldrich; Merck KGaA. Recombinant human
transforming growth factor-β1 (TGF-β1; cat. no. 240-B) was
purchased from R&D Systems, Inc., and used at a concentration
of 5 ng/ml. Rabbit primary antibodies against vimentin (cat. no.
5471; 1:2,000), Wnt3a (cat. no. 2721; 1:500), phosphorylated
β-catenin (Ser33/37; cat. no. 2009; 1:500), total β-catenin (cat.
no. 8480; 1:1,000), phosphorylated glycogen synthase kinase (GSK)3β
(Ser9; cat. no. 5558; 1:1,000), total GSK3β (cat. no. 12456;
1:1,000), autophagy-associated gene 5 (ATG5; cat. no. 9980;
1:1,000) and β-actin (cat. no. 4970; 1:5,000) were all purchased
from Cell Signaling Technology, Inc. Antibodies against LC3B (cat.
no. ab48394; 1:1,000), epithelial (E)-cadherin (cat. no. ab15148;
1:2,000), neural (N)-cadherin (cat. no. ab76057; 1:1,000), Histone
H3 (cat. no. ab176842; 1:1,000) and GAPDH (cat. no. ab181602;
1:5,000) were purchased from Abcam.
MTT assay
RCC cells were plated into 96-well culture plates
and treated with various concentrations of silibinin for 24 h. The
supernatant of each well was replaced with fresh medium containing
10% MTT (5 mg/ml) and incubated for a further 4 h. Subsequently,
the medium was removed and 150 μl DMSO was added to each well. A
96-well microplate reader (Bio-Rad Laboratories, Inc.) was used to
detect the absorbance at 570 nm.
Wound healing assay
RCC cells were seeded at a density of
20.0×104 cells/well into 6-well plates and cultured.
Wounds were created by scratching with a 200-μl pipette tip when
the cells had grown to 90–100% confluence. The fragments of RCC
cells were washed with PBS and incubated in serum-free media with
or without silibinin. Wound closure was observed at 0, 12, 24 and
36 h using an inverted microscope (magnification, ×40). The average
area of the wound was calculated using ImageJ v1.47 software
(National Institute of Health). The wound closure (% of control)
was calculated using the following formula: Wound closure (% of
control)=(gap closure of silibinin treatment group/gap closure of
control group) ×100.
Transwell migration and invasion
assays
For migration assays, 0.2 ml FBS-free RMPI-1640
medium suspension with 2×104 RCC cells were seeded into
the upper chamber in a 24-well plate and 0.8 ml supplemented
RMPI-1640 medium was added to the lower chamber. After incubating
for 24 h, the chamber was washed with PBS and fixed at room
temperature with 4% formalin for 15 min. Subsequently, the chamber
was stained at room temperature with crystal violet (0.1%,
dissolved in the ethanol) for 25 min. For invasion assays, a 50 μl
mixture of FBS-free RPMI-1640/Matrigel at 10:1 ratio (Matrigel was
obtained Sigma-Aldrich; Merck KGaA) was plated onto the upper
chamber. RCC cells were incubated for 36 h and the rest protocol
was performed as described for the migration assay. An inverted
microscope was used to count the number of cells which had migrated
or invaded in 5 randomly selected fields (magnification, ×100). The
migration or invasion index (%) was calculated using the following
formula: Migration/invasion index (%)=(average transmembrane number
of silibinin treatment group/average transmembrane number of
control group) ×100.
Western blot analysis
Cells were washed with ice-cold PBS and lysed with
radioimmunoprecipitation assay buffer (50 mM Tris, 150 mM NaCl,
0.1% SDS, 1% NP40 and 0.5% sodium deoxycholate; pH 7.4) containing
proteinase inhibitors (cat. no. 04693132001; Sigma-Aldrich; Merck
KGaA) and phosphatase inhibitors (cat. no. 04906837001;
Sigma-Aldrich; Merck KGaA). The lysates were centrifuged at 15,000
× g at 4°C for 15 min and 5 μg cell supernatant lysate was used to
detect the concentration of proteins using a Bradford assay. A
total of 30 μg proteins was loaded onto a 10 or 15% SDS gel and
resolved by SDS-PAGE. Proteins were transferred onto PVDF
membranes. The membranes were blocked with 5% BSA for 1 h at room
temperature and subsequently incubated with primary antibodies
[vimentin (cat. no. 5741; dilution, 1:2,000; Cell Signaling
Technology, Inc.), Wnt3a (cat. no. 2721; dilution, 1:500; Cell
Signaling Technology, Inc.), phosphorylated β-catenin (Ser33/37;
cat. no. 2009; dilution, 1:500; Cell Signaling Technology, Inc.),
total β-catenin (cat. no. 8480; dilution, 1:1,000; Cell Signaling
Technology, Inc.), phosphorylated glycogen synthase kinase (GSK)3β
(Ser9; cat. no. 5558; dilution, 1:1,000; Cell Signaling Technology,
Inc.), total GSK3β (cat. no. 12456; dilution, 1:1,000; Cell
Signaling Technology, Inc.), autophagy-associated gene 5 (ATG5;
cat. no. 9980; dilution, 1:1,000; Cell Signaling Technology, Inc.)
and β-actin (cat. no. 4970; dilution, 1:5,000; Cell Signaling
Technology, Inc.), LC3B (cat. no. ab48394; dilution, 1:1,000;
Abcam), epithelial (E)-cadherin (cat. no. ab15148; dilution,
1:2,000; Abcam), neural (N)-cadherin (cat. no. ab76057; dilution,
1:1,000; Abcam), Histone H3 (cat. no. ab176842; dilution, 1:1,000;
Abcam) and GAPDH (cat. no. ab181602; dilution, 1:5,000; Abcam)]
overnight at 4°C. After incubation with the primary antibodies the
membranes were washed with TBS with 0.1% Tween-20 and incubated
with the anti-rabbit IgG peroxidase antibody produced in goat (cat.
no. A9169; dilution, 1:5,000; Sigma-Aldrich; Merck KGaA) for 1 h at
room temperature. Signals were visualized using Clarity Max Western
ECL substrate (cat. no. 1705062; Bio-Rad Laboratories, Inc.),
followed by exposure to X-ray films. β-actin was used as the
loading control.
Co-immunoprecipitation
Cells were lysed with immunoprecipitation (IP)
buffer (50 mM Tris HCl, 150 mM NaCl, 1 mM
ethylenediaminetetraacetic acid, 1% Triton X-100) with protease
inhibitors and phosphatase inhibitors (5). After incubation with an LC3B
antibody (20 μg per 500 μg of protein; cat. no. ab48394; Abcam) for
12 h at 4°C, Protein G Dynabeads were applied to bind with the LC3B
antibody for 3 h at 4°C. Subsequently, cell lysates were washed
twice with IP buffer and boiled at 95°C for 5 min. Finally,
proteins were subjected to western blotting.
Small interfering (si)RNA and plasmid
transfections
siRNAs targeting specific genes and non-specific
control (NC) were purchased from Shanghai GenePharma Co., Ltd.
siRNA transfections were used to silence the expression of
β-catenin and ATG5 in RCC cells. The corresponding negative control
(si-NC) 5′-UUCUCCGAACGUGUCACGUTT-3′ was designed and synthesized by
Guangzhou RiboBio Co., Ltd. The sequences of the siRNAs against
ATG5 were: ATG5 siRNA (si-ATG5) sequence 1,
5′-GAAGTTTGTCCTTCTGCTA-3′ and sequence 2,
5′-CAAUCCCAUCCAGAGUUGCTT-3′. The sequences of the siRNAs against
β-catenin were: siRNA β-catenin (si-β-catenin) sequence 1,
5′-CCUUCACUCAAGAACAAGUTT-3′ and sequence 2,
5′-GCUCAUCAUACUGGCUAGUTT-3′. β-catenin cDNA was cloned into a
pcDNA3.1 vector. For transfection, a 1:1 mixture of the siRNAs or
plasmid with Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) was added to the serum free medium
according to the manufacturer’s protocol. At 48 h after
transfection, reverse transcription-quantitative PCR and western
blot analyses were used to determine transfection efficiency.
Immunofluorescence staining
786-O and ACHN cells were plated on glass coverslips
and treated with silibinin for 24 h. Cells were fixed at room
temperature with 4% formaldehyde for 20 min and washed with PBS
three times. A 0.5% Triton X-100 solution was used to permeate the
cells for 20 min, after which the cells were washed with PBS three
times. The cells were incubated with primary antibodies against
β-catenin (cat. no. 8480; dilution, 1:200; Cell Signaling
Technology, Inc.) overnight at 4°C and subsequently washed with PBS
three times. Cells were subsequently incubated with goat
anti-rabbit IgG H&L fluorescein isothiocyanate (FITC) (cat. no.
ab6717; cat. no. 1:200; Abcam) for 1 h at room temperature. RCC
cells were counterstained with DAPI (1 μg/ml) for 5 min. β-catenin
expression was detected on a confocal laser scanning
microscope.
Preparation of cytoplasmic and nuclear
extracts
786-O and ACHN cells were treated with the indicated
doses of silibinin for 24 h. Cytoplasmic and nuclear proteins were
extracted using Nuclei EZ Prep Nuclei Isolation kit (Sigma-Aldrich;
Merck KGaA) according to the manufacturer’s protocol. Western-blot
analysis was used to detect the cytoplasmic and nuclear expression
of various proteins.
Dual-luciferase reporter assay
786-O cells were seeded in 6-well plates and
transfected with Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.), along with TCF-responsive promoter
reporter (TOP-flash) or nonresponsive control reporter (FOP-flash)
β-catenin firefly luciferase reporter gene constructs (provided by
Professor Mien-Chie Hung, University of Texas M. D. Anderson Cancer
Center, Houston, USA), and a pRL-SV40 Renilla luciferase
construct was used as the internal control for the reporter gene
assay as previously described (12). Subsequently, cells were treated
with silibinin and the luciferase activity was determined using a
Dual-Luciferase Reporter Assay System (Promega Corporation). The
ratio of TOP and FOP luciferase activity represented the
transcriptional activity of β-catenin. Relative luciferase activity
was represented as the mean ± standard error of mean after
normalizing to the control.
Xenograft animal model
A total of 10, 4-week-old BALB/c male nude mice
(weight, 15–20 g) were purchased from the Laboratory Animal Center
of Xi’an Jiaotong University. The nude mice were maintained in
specific pathogen-free rooms that are carefully monitored for the
presence of mouse pathogens. The rooms were kept at a temperature
of 22–25°C, with a 12-h light/dark cycle and with free access to
water and food. We operated the mice during the light phase in the
daytime. The permission number for in vivo animal study is
no. XJTULAC2019-1151. All animal care and experiments were approved
by the Institutional Animal Care and Use Committee of Xi’an
Jiaotong University. Animal health and behavior were monitored
daily. Briefly, 786-O cells (2×106) were resuspended in
0.1 ml PBS and subcutaneously injected into the right flank of nude
mice. When the tumor volume reached ~100 mm3, the nude
mice were randomly divided into two groups (n=5 mice per group):
Control group and silibinin-treated group. The control group
received treatment with the vehicle (oral gavage with saline) and
the silibinin-treated group were fed by oral gavage with silibinin
(150 mg/kg) every other day. The tumor sizes were measured every
three days and the tumor volume was calculated as follows: Volume
(mm3)=0.5 × (length) × (width)2. After 30
days, the mice were sacrificed using carbon dioxide
(CO2) with a CO2 displacement rate of 17.5%
of chamber volume/min. the animals were exposed to CO2
until complete cessation of breathing was observed for 10 min.
Visually inspection of the animals for the absence of movement and
respiration was performed. Death was assured by subsequent use of
cervical dislocation. The tumors were weighed by electronic scales
and prepared for immunohistochemical staining and western blot
analysis.
For the metastatic model, 786-O cells were
transfected with luciferase lentivirus and injected into the mice
via the tail vein. The mice were randomly divided into the 2
aforementioned groups and treated as above. After 4 weeks, the mice
were intraperitoneally injected with D-luciferin (150 mg/kg) and
anesthetized with 10% chloral hydrate at a dose of 400 mg/kg by
intrapenitoneal injection. Then the mice were imaged using an IVIS
Lumina II (PerkinElmer, Inc.) with Living Image software v4.5.4
(PerkinElmer, Inc.). The lung metastatic tumors were stained with
hematoxylin for 10 min at room temperature and eosin for 1 min at
room temperature.
Statistical analysis
All data are presented as the mean ± standard
deviation of at least three independent experiments. All
statistical analyses were performed using GraphPad Prism 5.2
software (GraphPad Software, Inc.). The difference between various
groups were analyzed using a one-way analysis of variance (ANOVA).
Tukey’s honestly significant difference post hoc test was used
following one-way ANOVA. A Student’s t-test was used for the
comparisons between two groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
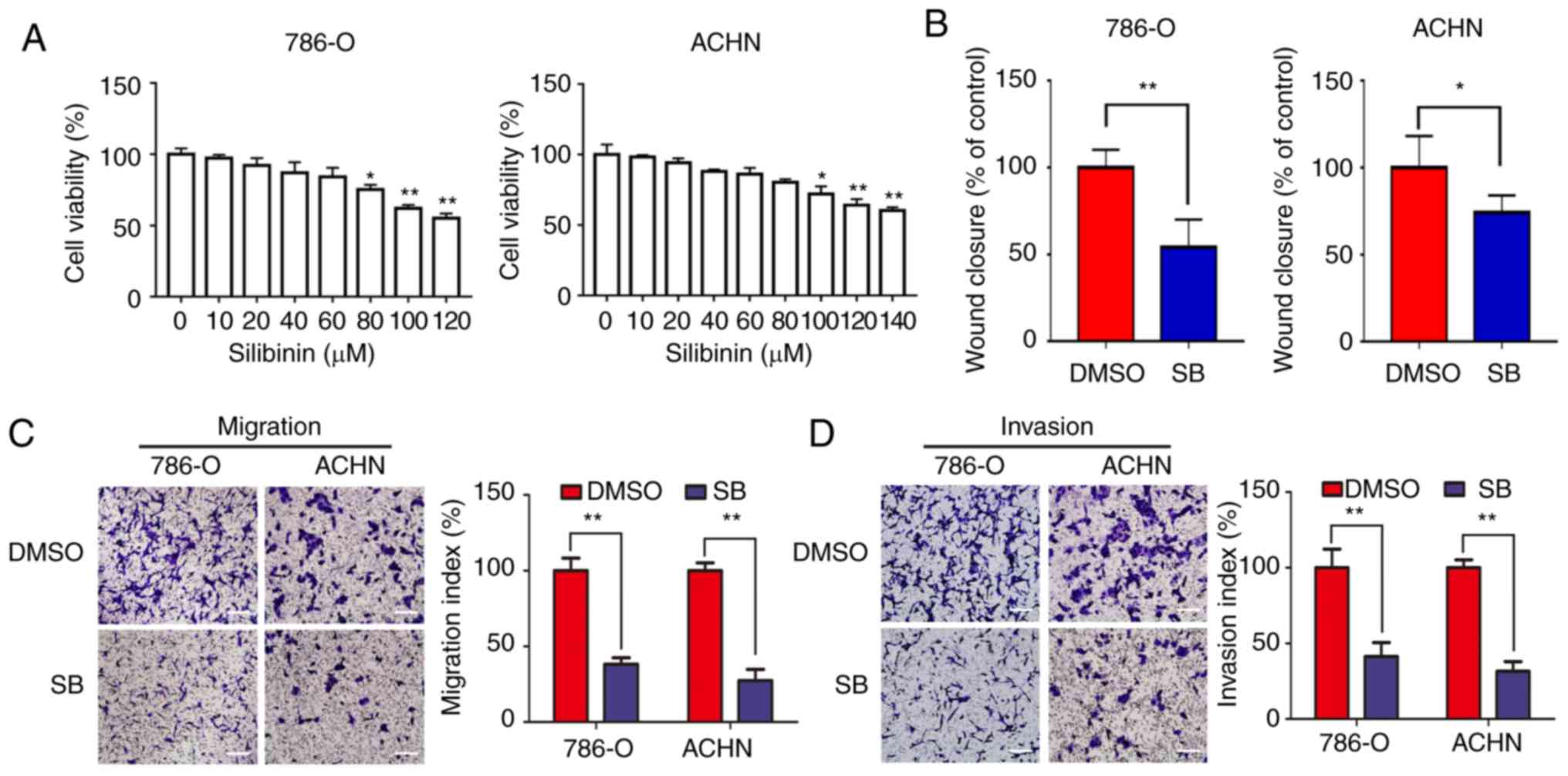
Silibinin inhibits migration and invasion
in vitro
In order to assess the inhibitory effects of
silibinin on RCC cells, 786-O and ACHN cells were treated with
different concentrations of silibinin for 24 h. The results
indicated that a concentration <60 μM silibinin (SB) doesn’t
have significant effect on the proliferation of 786-O and
concentration <80 μM SB doesn’t have significant effect on the
proliferation of ACHN. As the concentration >60 μM in 786-O and
>80 μM in ACHN can affect the cell viability, so 20/40/60 μM SB
was chosen in 786-O and 40/60/80 μM SB in ACHN (Fig. 1A).
Migration of 786-O and ACHN cells was significantly
inhibited by 60 μM SB as determined by a wound healing assay
(Fig. 1B). Similar results were
obtained from the Transwell migration and invasion assays (Fig. 1C and D). Consistent with the
authors’ previous study (18), a
low dose of SB (<80 μM) significantly inhibited migration and
invasion of RCC cells in vitro.
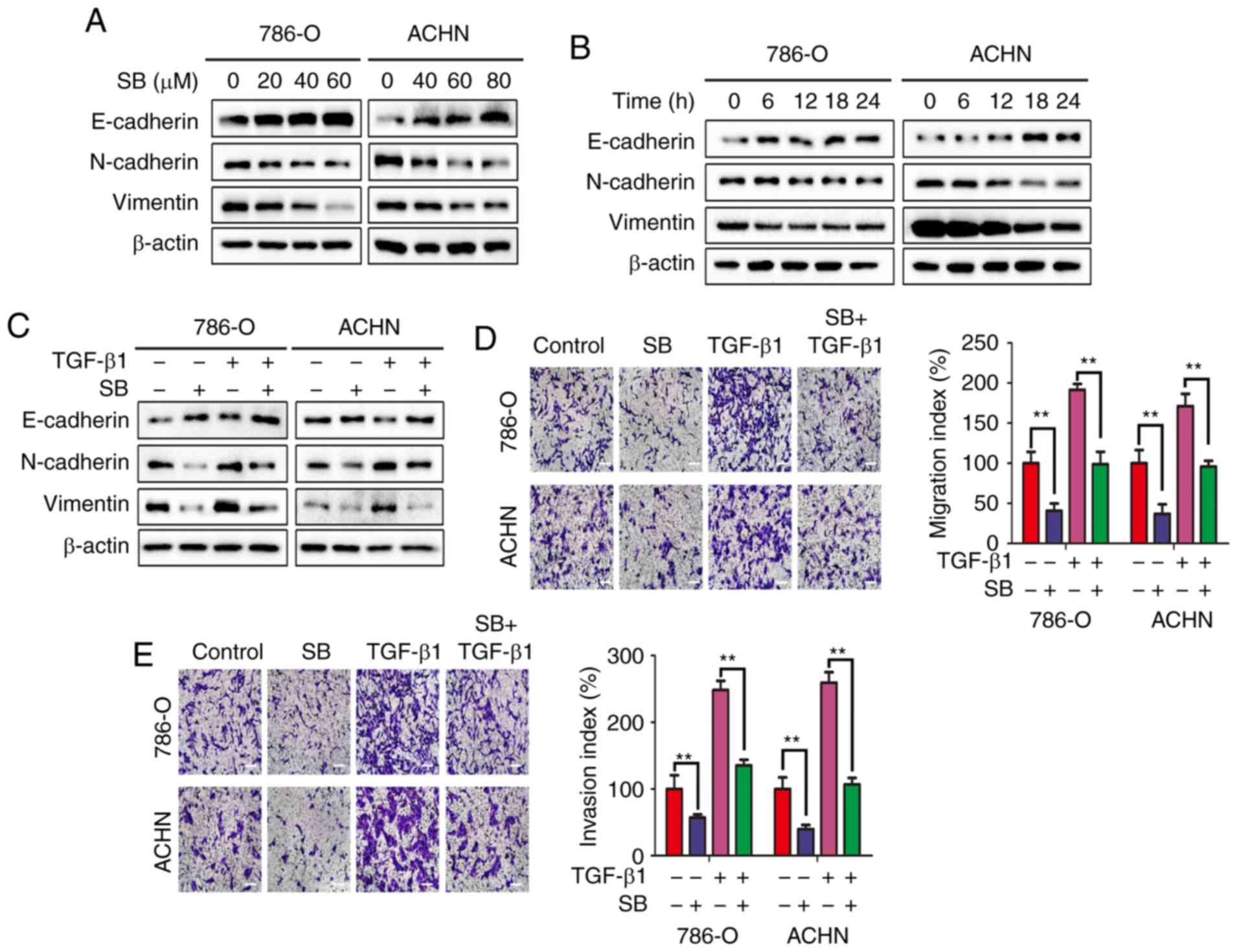
SB suppresses EMT in RCC cells
As described previously, EMT is one of the major
mechanisms that regulates the metastatic progression of cancer
(19). In RCC, EMT is known to be
associated with migration and invasion (20). To evaluate whether the inhibitory
effects of SB on migration and invasion was associated with EMT,
the expression of EMT markers was measured using western blotting.
As shown in Fig. 2A and B, SB
increased the expression of E-cadherin and decreased the expression
of the mesenchymal markers N-cadherin and vimentin in both a
concentration- and time-dependent manner. To further demonstrate
the effects of SB on EMT, cells were treated with TGF-β1, a
well-known EMT inducer (21).
TGF-β1-induced EMT, as determined by an increase in N-cadherin and
vimentin expression and decrease in E-cadherin expression, and this
was prevented by treatment with SB (Fig. 2C). The Transwell migration and
invasion assays also showed that SB prevented TGF-β1-induced cell
migration and invasion (Fig. 2D and
E). Together, these results confirm that SB may inhibit RCC
cell migration and invasion through preventing EMT.
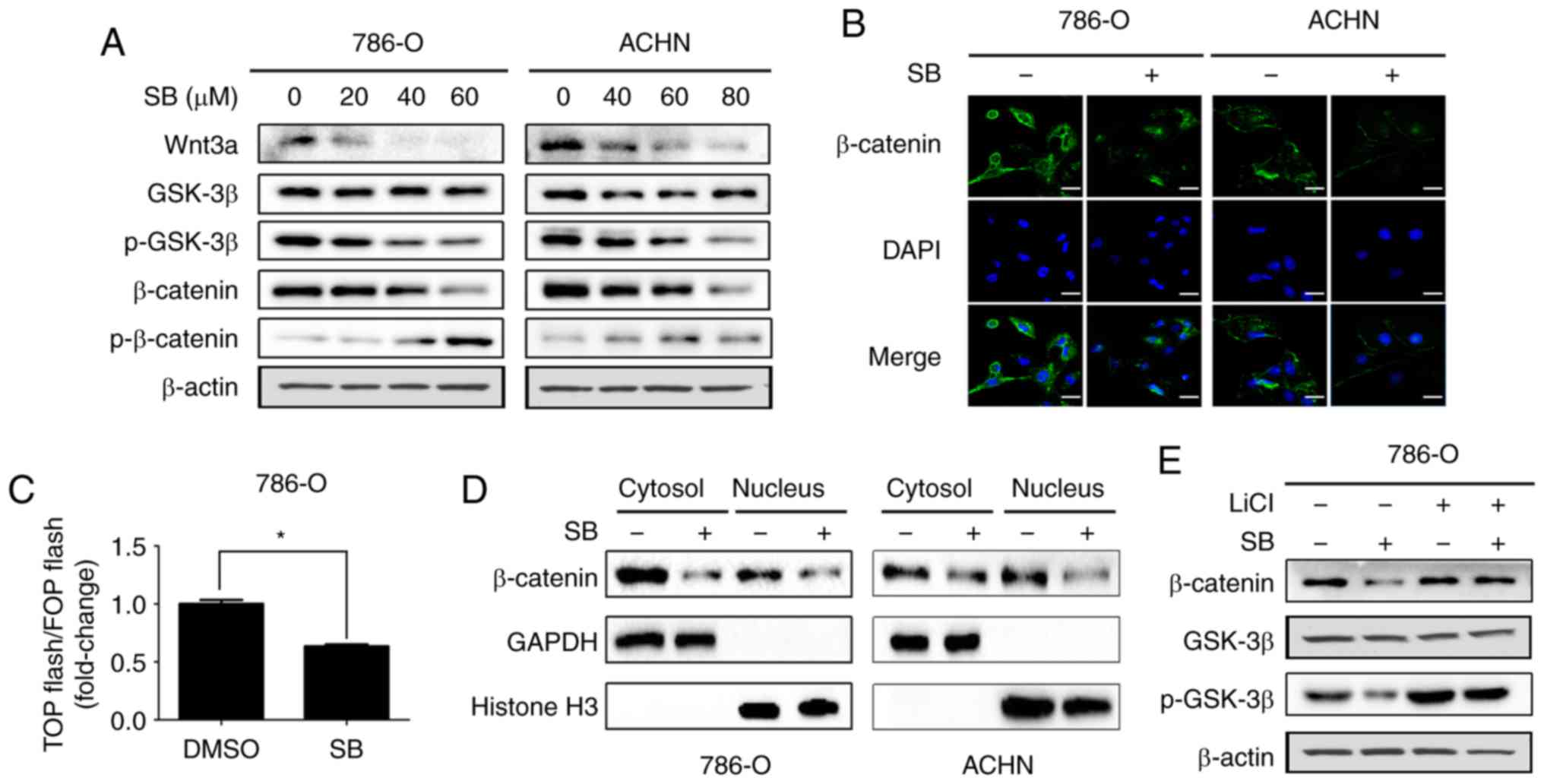
SB inhibits Wnt/β-catenin signaling in
RCC cells
Wnt/β-catenin signaling has been demonstrated to
contribute to EMT and metastasis of RCC (22). Therefore, it was next determined
if inhibition of EMT and metastasis by SB was associated with the
Wnt/β-catenin signaling pathway. In the present study, together
with upregulation of p-β-catenin, downregulation of Wnt3a, p-GSK3β
and β-catenin were also observed following treatment with SB
(Fig. 3A). Decreased expression
of total β-catenin was further confirmed by immunofluorescence
analysis (Fig. 3B). In addition,
a TOP-flash/FOP-flash luciferase reporter gene assay also showed
that SB decreased the transcriptional activity of β-catenin
(Fig. 3C). Consistent with this,
SB decreased both the cytosolic and nuclear expression levels of
β-catenin (Fig. 3D).
Additionally, pretreatment with LiCl (a GSK3β kinase inhibitor)
prevented the suppressive effects of SB on β-catenin (Fig. 3E).
SB inhibits metastasis of RCC through
downregulation of the Wnt/β-catenin signaling pathway
To confirm the role of SB in regulating the
Wnt/β-catenin signaling pathway, β-catenin was overexpressed in
786-O cells using plasmid transfections. As shown in Fig. 4A and B, overexpression of
β-catenin reversed the effects of SB on EMT markers and metastatic
activity. Although the inhibitory effects of SB on invasion showed
no difference following knockdown of β-catenin using siRNA,
β-catenin knockdown further enhanced the suppressive effects of SB
on EMT markers and migration (Fig. 4C
and D).
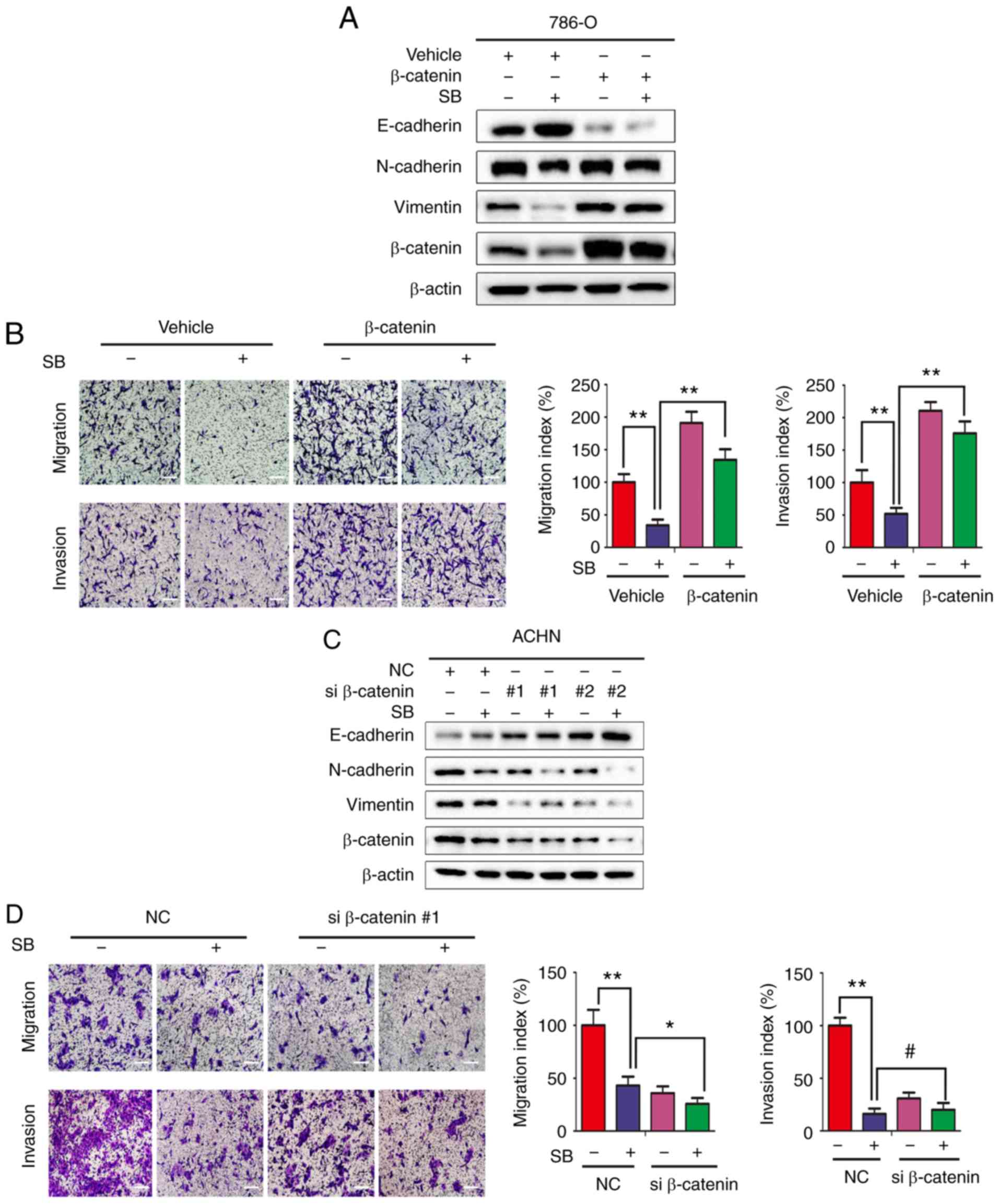 | Figure 4SB inhibits RCC metastasis through
downregulating the Wnt/β-catenin pathway. (A)
β-catenin-overexpressing 786-O cells were treated with 60 μM SB for
24 h. Expression of EMT associated markers were determined by
western blotting. (B) Transwell migration and invasion assays were
performed in β-catenin-overexpressing 786-O cells following
treatment with 60 μM SB. Magnification, ×100. Scale bar, 20 μm. The
experiment was repeated three times. **P<0.01. (C)
ACHN cells were transfected with two different siRNA targeting
β-catenin for 24 h and treated with DMSO or SB for another 24 h.
Expression of EMT associated markers were determined by western
blotting. (D) Transwell migration and invasion assays were
performed on ACHN cells transfected with β-catenin siRNA sequence 1
following treatment with 60 μM SB treatment. Magnification ×100.
Scale bar, 20 μm. The experiment was repeated three times.
#P>0.05, *P<0.05 and
**P<0.01. RCC, renal cell carcinoma; EMT,
epithelial-mesenchymal transition; si, small interfering; SB,
silibinin; N, neural; E, epithelial. |
SB inhibits EMT of RCC cells through
autophagy-dependent Wnt/β-catenin signaling
In the authors’ previous study, it was demonstrated
that autophagy induction by SB contributed to its reduction of
metastasis in RCC cells (18);
however, the underlying molecular mechanism was unknown. Consistent
with the authors’ previous findings (18), SB induced autophagy in RCC cells,
as determined by the upregulation of LC3-II and p62 protein
expression levels (Fig. 5A). As
the interplay between Wnt/β-catenin signaling and autophagy has
been identified previously (23,24), and β-catenin is known to be
degraded through autophagy-lysosome system, the relationship
between SB -induced autophagy and β-catenin downregulation was
assessed. Inhibition of autophagy by chloroquine (CQ), a lysosome
inhibitor, resulted in increased expression of β-catenin in the
presence of SB compared with SB treatment alone (Fig. 5B). Previous studies have shown
that LC3 forms a complex with β-catenin, which promotes the
lysosomal degradation of β-catenin (23,24). To further elucidate the underlying
molecular mechanism, the effects of SB on the interaction between
LC3 and β-catenin were determined using an immunoprecipitation
assay. There was an increased level of interaction between LC3 and
β-catenin following treatment with SB (Fig. 5C). Additionally, inhibition of
initiation of autophagy by either 3-MA (Fig. 5D and E) or ATG5 knockdown
(Fig. 5F and G) significantly
attenuated the SB-induced suppression of cell migration and
invasion, inhibition of EMT and downregulation of β-catenin,
suggesting a vital role of autophagy-regulated β-catenin signaling
in the anti-cancer effects of SB.
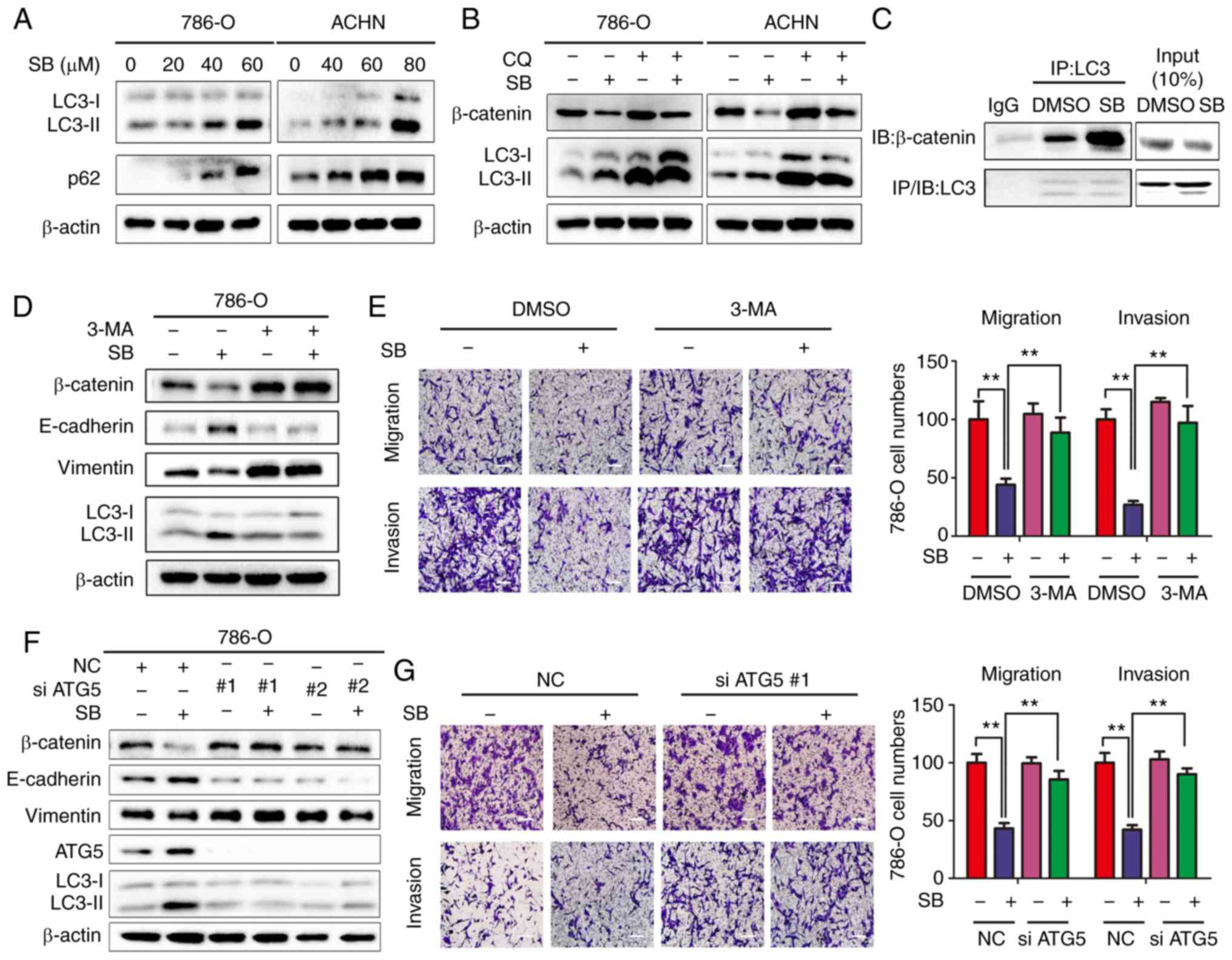 | Figure 5SB induces inhibition of EMT via
autophagy-dependent Wnt/β-catenin signaling in RCC cells. (A)
Western blot analysis of LC3-I/II protein levels in 786-O and ACHN
cells treated with different doses of SB. β-actin was used as the
loading control. (B) 786-O and ACHN cells were treated with 60 μM
of SB for 24 h in the presence of 50 μM CQ, and the protein
expression levels of β-catenin and LC3-I/II were measured. β-actin
was used as the loading control. (C) Co-immunoprecipitation of
endogenous β-catenin and LC3 was assayed in 786-O cells following
treatment with 60 μM SB. (D) 786-O cells were pretreated with 3 mM
3-MA for 1 h, followed by treatment with 60 μM of SB for 24 h.
Western blot analysis was used to detect the expression levels of
total β-catenin, E-cadherin, vimentin and LC3-I/II. (E) Transwell
migration and invasion assays were performed on treated cells.
Magnification, ×100. Scale bar, 20 μm. The experiment was repeated
three times. **P<0.01. (F) 786-O cells were
transfected with two siRNA sequences targeting ATG5 for 24 h and
treated with DMSO or 60 μM SB for another 24 h. Western blot
analysis was used to detect the expression levels of total
β-catenin, E-cadherin, vimentin, ATG5 and LC3-I/II. β-actin was
used as the loading control. (G) Transwell migration and invasion
assays were performed on treated cells under similar conditions.
Magnification, ×100. Scale bar, 20 μm. The experiment was repeated
three times. **P<0.01. EMT, epithelial-mesenchymal
transition; CQ, chloroquine; IB, immunoblotting; IP,
immunoprecipitation; ATG5, autophagy-associated gene 5; SB,
silibinin; RCC, renal cell carcinoma; 3-MA, 3-methyladenine; NC,
negative control; E, epithelial. |
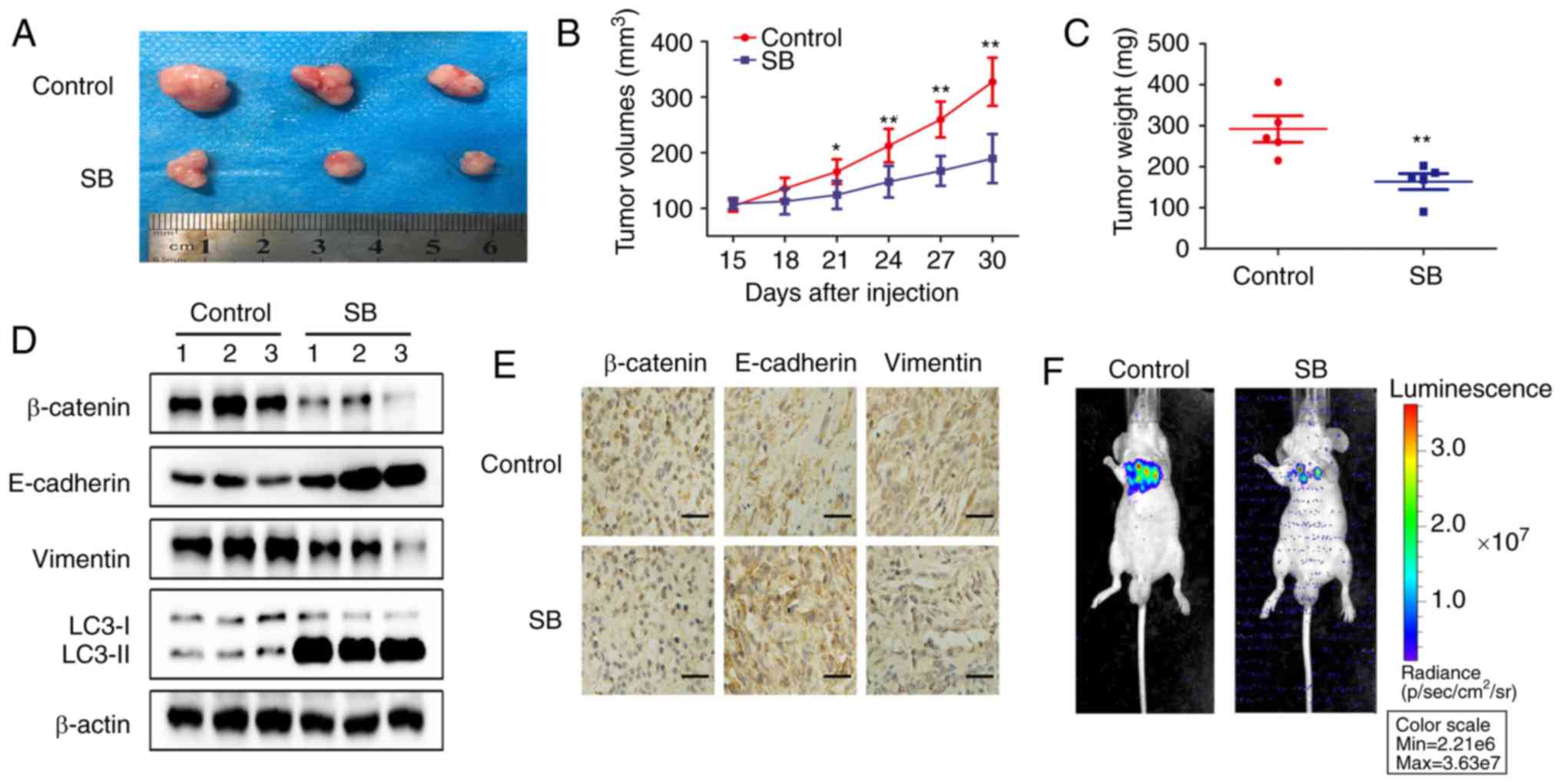
SB inhibits RCC EMT and metastasis in
vivo
To verify the in vitro results, RCC
subcutaneous and metastatic xenografts in nude mice were used as
the in vivo model system. SB significantly decreased tumor
growth and volume in RCC subcutaneous xenografts (P<0.01;
Fig. 6A and B) without affecting
the body weights of the nude mice (Fig. 6C). After 30 days, the average
tumor weight of the control group was 292.14±72.02 mg, whereas in
the SB-treated group it was 163.68±43.23 mg. Western blotting
indicated that SB inhibited the expression of β-catenin and
vimentin, whilst promoting the levels of E-cadherin and LC3-II
(Fig. 6D). Downregulation of
β-catenin and vimentin and upregulation of E-cadherin were further
confirmed by immunohistochemistry (Fig. 6E).
Additionally, treatment with SB for 4 weeks
inhibited lung metastasis induced by tail vein injection of 786-O
cells in vivo (Fig. 6F).
Collectively, these results were consistent with the in
vitro results and suggested that SB inhibited RCC EMT and
metastasis in vivo by regulating the autophagy-dependent
Wnt/β-catenin signaling pathway.
Discussion
SB is a traditional medicine extracted from milk
thistle seeds and has been widely used in clinical practice as a
hepatoprotective and antioxidative agent. Previous studies have
demonstrated the anti-cancer properties of SB in various types of
cancer, including breast cancer, gastric cancer, bladder cancer and
prostate cancer (4–16). A previous study from the authors’
laboratory has demonstrated the potential anti-cancer effects of SB
against RCC (18), although the
molecular mechanisms are yet to be identified. In the present
study, a focus was placed on the interplay between β-catenin and
autophagy. SB inhibited Wnt/β-catenin signaling in vitro and
in vivo in an autophagy-dependent manner, which contributed
to metastasis and EMT of 786-O and ACHN cells.
TGF-β1 treatment resulted in marginal change of both
E-Cadherin and N-cadherin expression in 786-O cells, although
enhanced invasion and migration ability of 786-O cells were
observed. Complete EMT involves almost complete loss of epithelial
markers and an increase in levels of several mesenchymal markers.
Basically, the magnitude of change in the levels of the epithelial
and mesenchymal markers can be used to distinguish between complete
EMT and partial EMT (25).
Therefore, a possible induction of partial EMT by TGF-β1 was
proposed in the present study based on the changes of EMT markers.
Partial EMT has received great attention by oncologists when
compared with complete EMT (25,26). For partial EMT, cells
simultaneously express epithelial and mesenchymal traits. Hence,
they can initiate metastasis with incomplete loss of epithelial
traits and/or incomplete gain of mesenchymal traits (27). Partial EMT in cancer cells is
thought to enhance their invasive properties, generate circulating
tumor cells and cancer stem cells, and promote resistance to
anti-cancer drugs (26).
Therefore, the ability of cancer cells to undergo partial EMT,
rather than complete EMT, poses a higher metastatic risk. In the
current study, enhanced invasive potential induced by TGF-β1 was
observed in both 786-O and ACHN cells. Interestingly, this effect
could be significantly attenuated by SB treatment. Further studies
focusing on the effects of SB on circulating tumor cells and cancer
stem cells are needed to identify the exact role of SB on partial
EMT in RCC induced by TGF-β1.
In the complex biological process of invasion and
metastasis, tumor cells must adapt to different survival pressures.
Autophagy, an intracellular physiological reaction, is regulated by
numerous genes and their expression products. Autophagy can be
activated to adapt to the metabolic stress and microenvironmental
changes, and it is associated with EMT, inflammation, apoptosis and
mechanisms of cancer metastasis (28,29). For cancer metastasis, autophagy
serves varying roles at different stages. During the early stages,
autophagy inhibits metastasis through maintaining genomic stability
and reducing tumor inflammation; whereas, in the later stages of
tumor progression, autophagy promotes metastasis by improving the
survival ability of tumor cells (28). In the authors’ previous study, SB
decreased the metastatic capacity of RCC by activating autophagy
through the AMPK/mTOR pathway (18). Autophagy induction by SB
positively contributed to the anti-metastatic effects of SB against
RCC. Although existing research has demonstrated the role of
autophagy in promoting cell survival and therapeutic resistance
(30–32), the relationship between autophagy
and metastasis is obscure. In the present study, it was
consistently demonstrated that SB decreased metastasis and EMT of
RCC cells by inducing autophagy.
The Wnt/β-catenin signaling pathway participates in
proliferation, invasion and metastasis of renal cancer cells, and
effectively induces resistance and regeneration of renal cancer
(33). Targeting the
Wnt/β-catenin pathway inhibits the growth and metastasis of renal
cancer and increases the sensitivity to chemotherapy. In bladder
cancer, the authors’ laboratory previously demonstrated that SB
inhibited β-catenin/ZEB1 signaling and decreased metastasis of
bladder cancer (12). However,
the effect of SB on β-catenin/ZEB1 signaling is still unclear. In
the present study, it was demonstrated that SB-induced inactivation
of Wnt/β-catenin signaling was associated with the inhibitory
effects of SB on metastasis and EMT. Interestingly, in the present
study, there was no statistical significance between the invasion
results of SB and SB+siRNA β-catenin groups. The reasons that
contributed to this phenomenon are still unclear. One possible
mechanism is that different signaling pathways may play different
roles in mediating cancer cell migration and invasion. Further
study is needed to explore the possible mechanisms. β-catenin
negatively regulates the formation of the autophagosome and has
direct inhibitory effects on the expression of p62 via TCF4
(23). Furthermore, LC3 or p62
directly interacts with β-catenin for lysosomal-autophagic
degradation (23,24). In the present study, increased
lysosomal degradation of β-catenin and enhanced interactions
between LC3 and β-catenin were observed following SB treatment in
RCC cells.
In summary, the present study identified a novel
mechanism by which SB regulated metastasis and EMT of RCC in
vitro and in vivo, in which activation of autophagy by
SB treatment resulted in degradation of β-catenin. The data
highlight the clinical potential of SB for treating patients with
RCC and further demonstrates that increasing β-catenin degradation
in autophagy-lysosome pathway may be a promising target for
treating RCC.
Acknowledgements
The authors would like to thank Professor Mien-Chie
Hung (China Medical University, Taichung, Taiwan, China) for
supplying the TOP-flash and FOP-flash β-catenin firefly luciferase
reporter gene constructs.
Funding
The present study was funded by National Natural
Science Foundation of China (grant nos. 81101936 and 81672538).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors’ contributions
YF, TH, LL and JZ conceived and designed
experiments. YF, TH, WD, TL, JL and BL performed all experiments.
YF, TH, LL and JZ analyzed the data. YF, LL and JZ wrote and
revised the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All animal care and experiments were approved by the
Institutional Animal Care and Use Committee of Xi’an Jiaotong
University. The permission number for in vivo animal study
is no. XJTULAC2019-1151.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ljungberg B, Bensalah K, Canfield S,
Dabestani S, Hofmann F, Hora M, Kuczyk MA, Lam T, Marconi L,
Merseburger AS, et al: EAU guidelines on renal cell carcinoma: 2014
update. Eur Urol. 67:913–924. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Capitanio U, Bensalah K, Bex A, Boorjian
SA, Bray F, Coleman J, Gore JL, Sun M, Wood C and Russo P:
Epidemiology of renal cell carcinoma. Eur Urol. 75:74–84. 2019.
View Article : Google Scholar
|
|
3
|
Singer EA, Gupta GN and Srinivasan R:
Update on targeted therapies for clear cell renal cell carcinoma.
Curr Opin Oncol. 23:283–289. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cheung CW, Gibbons N, Johnson DW and Nicol
DL: Silibinin-a promising new treatment for cancer. Anticancer
Agents Med Chem. 10:186–195. 2010. View Article : Google Scholar
|
|
5
|
Zeng J, Liu W, Fan YZ, He DL and Li L:
PrLZ increases prostate cancer docetaxel resistance by inhibiting
LKB1/AMPK-mediated autophagy. Theranostics. 8:109–123. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li F, Sun Y, Jia J, Yang C, Tang X, Jin B,
Wang K, Guo P, Ma Z, Chen Y, et al: Silibinin attenuates TGF β1
induced migration and invasion via EMT suppression and is
associated with COX2 downregulation in bladder transitional cell
carcinoma. Oncol Rep. 40:3543–3550. 2018.PubMed/NCBI
|
|
7
|
Dheeraj A, Rigby CM, O’Bryant CL, Agarwal
C, Singh RP, Deep G and Agarwal R: Silibinin treatment inhibits the
growth of Hedgehog inhibitor-resistant basal cell carcinoma cells
via targeting EGFR-MAPK-Akt and Hedgehog signaling. Photochem
Photobiol. 93:999–1007. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rigby CM, Roy S, Deep G, Guillermo-Lagae
R, Jain AK, Dhar D, Orlicky DJ, Agarwal C and Agarwal R: Role of
p53 in silibinin-mediated inhibition of ultraviolet B
radiation-induced DNA damage, inflammation and skin carcinogenesis.
Carcinogenesis. 38:40–50. 2017. View Article : Google Scholar :
|
|
9
|
Deep G, Kumar R, Nambiar DK, Jain AK,
Ramteke AM, Serkova NJ, Agarwal C and Agarwal R: Silibinin inhibits
hypoxia-induced HIF-1alpha-mediated signaling, angiogenesis and
lipogenesis in prostate cancer cells: In vitro evidence and in vivo
functional imaging and metabolomics. Mol Carcinog. 56:833–848.
2017. View
Article : Google Scholar
|
|
10
|
Ting H, Deep G, Kumar S, Jain AK, Agarwal
C and Agarwal R: Beneficial effects of the naturally occurring
flavonoid silibinin on the prostate cancer microenvironment: Role
of monocyte chemotactic protein-1 and immune cell recruitment.
Carcinogenesis. 37:589–599. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bosch-Barrera J and Menendez JA: Silibinin
and STAT3: A natural way of targeting transcription factors for
cancer therapy. Cancer Treat Rev. 41:540–546. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu K, Ning Z, Zeng J, Fan J, Zhou J, Zhang
T, Zhang L, Chen Y, Gao Y, Wang B, et al: Silibinin inhibits
beta-catenin/ZEB1 signaling and suppresses bladder cancer
metastasis via dual-blocking epithelial-mesenchymal transition and
stemness. Cell Signal. 25:2625–2633. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Raina K, Agarwal C, Wadhwa R, Serkova NJ
and Agarwal R: Energy deprivation by silibinin in colorectal cancer
cells: A double-edged sword targeting both apoptotic and autophagic
machineries. Autophagy. 9:697–713. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zeng J, Sun Y, Wu K, Li L, Zhang G, Yang
Z, Wang Z, Zhang D, Xue Y, Chen Y, et al: Chemopreventive and
chemotherapeutic effects of intravesical silibinin against bladder
cancer by acting on mitochondria. Mol Cancer Ther. 10:104–116.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li L, Zeng J, Gao Y and He D: Targeting
silibinin in the antiproliferative pathway. Expert Opin Investig
Drugs. 19:243–255. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cui W, Gu F and Hu KQ: Effects and
mechanisms of silibinin on human hepatocellular carcinoma
xenografts in nude mice. World J Gastroenterol. 15:1943–1950. 2019.
View Article : Google Scholar
|
|
17
|
Liang L, Li L, Zeng J, Gao Y, Chen YL,
Wang ZQ, Wang XY, Chang LS and He D: Inhibitory effect of silibinin
on EGFR signal-induced renal cell carcinoma progression via
suppression of the EGFR/MMP-9 signaling pathway. Oncol Rep.
28:999–1005. 2012.PubMed/NCBI
|
|
18
|
Li F, Ma Z, Guan Z, Chen Y, Wu K, Guo P,
Wang X, He D and Zeng J: Autophagy induction by silibinin
positively contributes to its anti-metastatic capacity via
AMPK/mTOR pathway in renal cell carcinoma. Int J Mol Sci.
16:8415–8429. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Smith BN and Bhowmick NA: Role of EMT in
metastasis and therapy resistance. J Clin Med. 5:E172016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Piva F, Giulietti M, Santoni M, Occhipinti
G, Scarpelli M, Lopez-Beltran A, Cheng L, Principato G and
Montironi R: Epithelial to mesenchymal transition in renal cell
carcinoma: Implications for cancer therapy. Mol Diagn Ther.
20:111–117. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tsuda T: Extracellular interactions
between fibulins and transforming growth factor (TGF)-β in
physiological and pathological conditions. Int J Mol Sci.
19:27872018. View Article : Google Scholar
|
|
22
|
Xu Q, Krause M, Samoylenko A and Vainio S:
Wnt signaling in renal cell carcinoma. Cancers (Basel). 8:572016.
View Article : Google Scholar
|
|
23
|
Petherick KJ, Williams AC, Lane JD,
Ordonez-Moran P, Huelsken J, Collard TJ, Smartt HJ, Batson J, Malik
K, Paraskeva C and Greenhough A: Autolysosomal β-catenin
degradation regulates Wnt-autophagy-p62 crosstalk. Embo J.
32:1903–1916. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jia Z, Wang J, Wang W, Tian Y, XiangWei W,
Chen P, Ma K and Zhou C: Autophagy eliminates cytoplasmic
beta-catenin and NICD to promote the cardiac differentiation of
P19CL6 cells. Cell Signal. 26:2299–2305. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Grigore AD, Jolly MK, Jia D, Farach-Carson
MC and Levine H: Tumor Budding: The Name is EMT. Partial EMT J Clin
Med. 5:512016. View Article : Google Scholar
|
|
26
|
Saitoh M: Involvement of partial EMT in
cancer progression. J Biochem. 164:257–264. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Christiansen JJ and Rajasekaran AK:
Reassessing epithelial to mesenchymal transition as a prerequisite
for carcinoma invasion and metastasis. Cancer Res. 66:8319–8326.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mowers EE, Sharifi MN and Macleod KF:
Autophagy in cancer metastasis. Oncogene. 36:1619–1630. 2017.
View Article : Google Scholar :
|
|
29
|
Levy J, Towers CG and Thorburn A:
Targeting autophagy in cancer. Nat Rev Cancer. 17:528–542. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li YJ, Lei YH, Yao N, Wang CR, Hu N, Ye
WC, Zhang DM and Chen ZS: Autophagy and multidrug resistance in
cancer. Chin J Cancer. 36:522017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Smith AG and Macleod KF: Autophagy, cancer
stem cells and drug resistance. J Pathol. 247:708–718. 2019.
View Article : Google Scholar :
|
|
32
|
Das CK, Mandal M and Kögel D: Pro-survival
autophagy and cancer cell resistance to therapy. Cancer Metastasis
Rev. 37:749–766. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Guillen-Ahlers H: Wnt signaling in renal
cancer. Curr Drug Targets. 9:591–600. 2008. View Article : Google Scholar : PubMed/NCBI
|