Introduction
Osteoporosis (OP) is a chronic bone metabolism
disease that is clinically characterized by both bone mass
reduction and bone architecture alteration. OP increases bone
fragility and fracture risk worldwide (1,2).
With the ageing of the population, particularly in Asia, the number
of individuals who are aged ≥65 is projected to be 9.3% in 2025
(3). Patients with OP and
osteoporotic fracture (OPF) experience pain, inconvenience, low
quality of life, high economic burdens and mortality (4–6).
For OP prevention, controlling the risk factors is
necessary. Exercising, body mass index (BMI) monitoring, and taking
vitamin D and calcium are effective methods. In addition,
decreasing hyperkyphosis, and avoiding smoking, alcohol and
OP-inducing medications are preventive measures for OP (7,8).
The primary purpose of osteoporotic therapy is to decrease the risk
of fracture. The relevant medications are classified into two
groups, antiresorptive agents and anabolic agents, and include
bisphosphonates (BPs), estrogen replacement therapy (ERT) agents,
selective estrogen receptor modulators, and calcitonin (CT)
(9). However, the drugs available
at present have a number of adverse effects. ERT, for example, is
associated with coronary heart disease, breast cancer, stroke and
dementia (10,11), and CT leads to nausea and local
inflammation (12).
Osteoking is a Traditional Chinese Medicine (TCM)
compound originating from the Yi ethnic group in Yunnan that has
been used to treat bone diseases for decades (13). Initially, osteoking was approved
by the Chinese State Food and Drug Administration in 2002 to treat
femoral head necrosis, lumbar disc herniation and osteoarthritis in
the clinic (14). In our previous
study, osteoking improved these bone diseases by upregulating the
gene expression of Runt-related transcription factor 2 and vascular
endothelial growth factor (VEGF) in osteoporotic rabbit models
(15–18). In clinical settings, osteoking
prevents fracture in humans (19). Osteoking improves osteoporotic
fracture in rats (20).
Prevention of OP/OPF may be a new application for osteoking.
However, its exact mechanisms in vivo and in vitro
remains unclear.
With the development of bioinformatics and
network-pharmacology analyses, in particular component
characterization of TCM compounds, researchers can easily obtain
data, including chemical components, biological targets, and even
metabolic processes in vivo, including absorption,
distribution, metabolism and excretion. Researchers can analyze the
relevant gene data from the Gene Expression Omnibus (GEO) database
to obtain disease-associated targets (21). Investigating the components of
osteoking and its OP-associated targets by network pharmacology may
help elucidate the mechanisms of action of osteoking (22). The present study aimed to reveal
the mechanism of osteoking and provide a new strategy for OP or OPF
therapy.
Materials and methods
Drugs and reagents
Osteoking was prepared according to the Chinese
Pharmacopeia (China Pharmacopeia Committee, 2002) and was supplied
by Crystal Pharma Co., Ltd. (lot. no. 20160506) (23). Its formula was as follows:
Citrus reticulata Blanco, Carthamus tinctorius L.,
Panax notoginseng (Burk) F. H. Chen Ex C., Eucommia
ulmoides Oliv., Panax ginseng C. A. Mey., Astragalus
membranaceus (Fisch.) Bunge, and Trionycis Carapace
(Fig. S1). The above materials
were ground into a coarse powder, immersed in 10-fold distilled
water for 12 h at room temperature and then boiled using a
distillation apparatus for 1 h. This process was repeated twice,
and for the second and third extraction, the residue from the
previous extraction was filtered, and the same extraction
procedures were applied. Thereafter, the combined extracts were
filtrated and evaporated using a rotary evaporator at 50°C to a
relative density of 1.03 g/cm3 and centrifuged (1,450 ×
g; 30 min; room temperature) and the obtained supernatant was
centrifuged (1,450 × g; 30 min; room temperature) once again
following precipitation for 12 h. Subsequently, 0.36 g/ml was the
clinical concentration of crude osteoking used.
Recombinant human parathyroid hormone 1–34 (rhPTH
1–34) was obtained from Dailan Meilun Biotech Co., Ltd. (cat. no.
MB1241), Chemical Abstracts Service (CAS ID: 52232-67-4).
The antibodies used were anti-β-actin clone AC-15
(1:5,000; cat. no. A1978; Sigma-Aldrich; Merck KGaA), anti-BMP-2
(1:2,000; cat. no. 18933-1-AP; ProteinTech Group, Inc.), anti-heat
shock protein HSP 90-β (HSP90-β; 1:3,000; cat. no. 11405-1-AP;
ProteinTech Group, Inc.), HRP-conjugated Affinipure Goat Anti-Mouse
IgG (H+L; 1:5,000; cat. no. SA00001-1; ProteinTech Group, Inc.),
HRP-conjugated Affinipure Goat Anti-Rabbit IgG (H+L; 1:5,000; cat.
no. SA00001-2; ProteinTech Group, Inc.), and HRP-linked anti-Rabbit
IgG (1:5,000; cat. no. G1215; 1:5,000; Wuhan Servicebio Technology
Co., Ltd.).
OP model
All animal experiments were approved by the Animal
Study Committee of Kunming Medical University (approval no. KMMU
2015007) and were conducted according to the requirements of the
National Institutes of Health Guidelines for care and use of
laboratory animals (24). A total
of 62 female 3-month-old Sprague-Dawley rats (270±15 g; Dossy Co.)
were maintained in standard conditions with a controlled
temperature (21–23°C) and a strict 12:12 h light: Dark cycle. All
rats were fed standard rat chow and allowed ad libitum
access to distilled water at all times during acclimation and
experimental treatment periods. The health and behavior of rats was
monitored every day. After 7 days of adaptation, animals were
randomly divided into the bilateral ovariectomy (OVX) group (54
rats) and the sham-surgery group (8 rats). A total of 8 rats
underwent bilateral adipose tissue resection, in which adipose
tissue of a similar weight to the ovaries was removed (sham group),
and the remaining 54 rats were subjected to bilateral ovariectomy
(OVX rats). The animals were anesthetized with intraperitoneal
injection of 30 mg/kg sodium pentobarbital (Servio Co.).
Preoperatively, all animals were fasted for 12 h. Benzylpenicillin
sodium (60,000 IU/kg; Harbin Pharmaceutical Co.) was administered
for 3 consecutive days following the surgeries.
Experimental protocol
A total of 54 OP rats underwent OVX surgery,
randomly selected from the OP animals (450±20 g), were randomly
divided into three groups: A positive control group treated with
0.33 μg/kg/2 days subcutaneous (s.c.) rhPTH; a
negative control group treated with 0.59 ml/kg intragastric
(i.g.) 0.9% NaCl (Baxter Medicine Co., Ltd.); and osteoking
group treated with 0.59 ml/kg i.g. osteoking. The dosage used for
each animal was dependent on the weight and body surface area of
the rat, as well as the conversion coefficient of the human
clinical dosage for rats (25).
After 12 weeks of treatment, the rats were sacrificed via cervical
dislocation under deep anesthesia (intraperitoneal injection of 30
mg/kg sodium pentobarbital), as described previously. When animals
were sacrificed, serum and bone were collected and preserved at
−20°C.
BMD analysis
After 4, 8 and 12 weeks of treatment with osteoking,
the whole-body BMD was measured by dual-energy X-ray absorptiometry
using the Lunar Prodigy Advance (GE Healthcare). Specific software
for small animals (GE Medical Systems, enCORE2004 software;
v.8.80.001) was used (26), as
described previously.
Identification of active ingredients and
prediction of OP-associated targets by bioinformatics
The Traditional Chinese Medicine Systems
Pharmacology (TCMSP) Database (http://lsp.nwu.edu.cn/tcmsp.php) was used to obtain
the main active ingredients of osteoking, which were identified
using the cut-off values of oral bioavailability ≥30% and
drug-likeness ≥0.18. The target-prediction model of TCMSP was used
to predict the associated targets. Significantly different genes
were obtained through the secondary mining of the GEO database
(Gene accession numbers: GSE35955; GSE35958 and GSE35959) (27).
Cell culture
Rat bone mesenchymal stem cells (rBMSCs) were
obtained from the Chinese Academy of Science Kunming Cell Bank of
Type Culture Collection (Kunming Institute of Zoology, Chinese
Academy of Sciences). The cells were maintained in Dulbecco’s
modified Eagle’s medium (DMEM; Biological Industries) supplemented
with 10% FBS (Biological Industries). The cells (1×104
in 200 ml/well) in 96-well plates and cultured in different
concentration of osteoking. After 24 h of administration, the
proliferations in each well were cultured with MTS colorimetric
assay kit (Abcam Co., Ltd.) at 37°C for 1 h and measured using a
microplate reader (Tecan Group, Ltd.) on OD=490 nm (Fig. S2). Osteoking was filtered through
a 0.22 mm filter and diluted 16 times for culture. The final
concentration of osteoking was 22 g/l crude osteoking. There were
two groups: The control group (DMEM+10% FBS) and osteoking group
(22 g/l crude osteoking) for reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) and western blot analysis. The
cells in 25 cm2 culture flask were induced for 4 weeks.
Concomitantly, the cells (1×105 in 1 ml/well) were
divided into four groups: Control; 20 mmol/l HSP90-b inhibitor
NVP-AUY922 (Selleck Chemicals); osteoking (22 g/l crude osteoking);
and osteoking + HSP90-b inhibitor, and cultured in 6-well plates
for 4 weeks. After 4 weeks of administration, the plates were
stained by alizarin red at room temperature for 25 min and measured
using a microplate reader.
Histopathology and
immunohistochemistry
After 12 weeks of treatment, the 3rd lumbar
vertebrae were harvested and fixed in 10% paraformaldehyde at room
temperature for 14 days, dehydrated (60, 75, 95 and 100% ethyl
alcohol) and decalcified (300 ml 30% HCL; 700 ml 4%
paraformaldehyde; 140 g NaCl and 20 ml glacial acetic acid)
gradually. Then, 5-μm-thick sections were prepared using a Leica
RM2245 microtome (Leica Microsystems, Inc.). The anti-HSP90-β
antibody (ProteinTech Group, Inc) was diluted 1:1,000 in blocking
solution. After samples were washed 3 times in PBS, they were
incubated with HRP-linked anti-Rabbit IgG (1:5,000; cat. no. G1215;
Wuhan Servicebio Technology Co., Ltd.) at room temperature for 1 h.
The results were analyzed using ImageJ software (version 1.46R;
National Institutes of Health). The cells in six-well plates were
washed and fixed using 10% paraformaldehyde at room temperature for
15 min. Alizarin red S (100 ml/bottle; Wuhan Servicebio Technology
Co., Ltd.) was used at room temperature for 25 min and staining
intensities were measured by microplate reader at an absorbance
wavelength of 560 nm (Tecan Group, Ltd.).
Micro computed tomography (micro-CT)
analysis
Following removal of adherent soft tissues, the
bilateral femurs were preserved in 4% paraformaldehyde at room
temperature as described previously (28). Micro-CT analysis was performed
according to recent guidelines56 using a micro-CT
imaging system (Bruker microCT N.V.) with a spatial resolution of
17.75 μm (X-ray source 70 KV/357 μA; exposure time 250 msec;
magnification, ×15; 1.0 mm aluminum filter applied). Volumetric
reconstructions and analyses were performed using built-in software
NRecon 1.6 and CTAn 1.8 (Bruker microCT N.V.). The parameters
measured were: Percent bone volume; bone surface/volume ratio
(BS/BV); trabecular number (Tb.N); trabecular separation;
trabecular thickness (Tb.Th); and structure model index (29).
Proteome analysis
After 12 weeks of treatment with osteoking, the
protein expression levels of the osteoking group and the control
group were measured by label-free quantification (Genecreate). The
basic principle was based on the extraction peak area of the
peptide segment parent ion. Then, the peptide segments and proteins
in the sample were identified, and the identified peptide
fragments/proteins were quantitatively analyzed. The two groups
were designated as the osteoking group and the control group.
Following removal of redundant sequences, the data contained 36,728
protein sequences from the UniProt database (30). The proteins were quantitatively
analyzed by Skyline software (version 3.5; SkylineGlobal Co.), and
the differences were identified using the cut-off |fold change|
≥2.
RT-qPCR
Total RNA from cells and tissue samples was
separately isolated using TRIzol® reagent (Thermo
Fischer Scientific, Inc.) according to the manufacturer’s protocol.
The primer sequences refer to the National Center for Biotechnology
Information database (31) and
were assessed by Oligo Calc. (http://biotools.nubic.northwestern.edu/OligoCalc.html)
(32). The primers were designed
as following: β-actin, forward 5′-TCTGAACCCTAAGGCCAACC-3′, and
reverse 5′-TACGTACATGGCTGGGGTGT-3′; Bmp-2, forward
5′-GTGCCCCCTAGTGCTTCTTAG-3′, and reverse
5′-CACCATGGTCGACCTTTAGGA-3′; Hsp 90-β, forward
5′-GCCCTGGACAAGATTCGGTA-3′, and reverse
5′-ATCTTCAGCTCTTTCCCGCTG-3′. The thermocycling conditions: Initial
denaturation is 95°C for 30 sec; 30 of cycles of denaturation at
94°C for 15 sec, annealing at 60°C for 30 sec and elongation at
72°C for 20 sec; and a final extension is 72°C for 1 min). For
RT-qPCR, cDNA was prepared from 2 μg RNA using a Prime Script RT
Reagent kit (Takara Biotechnology Co., Ltd.) and analyzed with SYBR
Green Master Mix (Takara Bio, Inc.) in an LC480 real-time PCR
system (Roche Diagnostics). The data were quantified using the
relative quantitative 2−ΔΔCq method and were normalized
to β-actin expression data (33).
Western blot analysis
Protein lysate (1 ml) with 10% PMSF (Dalian Meilun
Biology Technology Co., Ltd.) was added into 100 mg bone or
1×106 cells on the ice for 30 min. The samples were
tested using BCA protein assay kit and adjusted as a same
concentration 1 μg/ul. The loading quality of β-actin was 5 μl, and
the loading quality of targets is 20 μl. Precast gel (4–20%) and
PVDF were used. BSA (10%) blocked the PVDF at room temperature for
1 h. Anti-β-actin (1:5,000; cat. no. A1978; Sigma-Aldrich; Merck
KGaA), anti-HSP 90-β (1:3,000; cat. no. 11405-1-AP; ProteinTech
Group, Inc.) and anti-BMP-2 (1:2,000; cat. no. 18933-1-AP;
ProteinTech Group, Inc.) were incubated at 4°C overnight, as
described previously. Then, 1:5,000 HRP-anti-Mouse (H+L; 1:5,000;
cat. no. SA00001-1; ProteinTech Group, Inc.) or HRP-anti-Rabbit IgG
(H+L; 1:5,000; cat. no. SA00001-2; ProteinTech Group, Inc.) was
incubated at room temperature for 2 h. One PVDF was added with 1ml
super HRP-reagent (Dalian Meilun Biology Technology Co., Ltd.) and
displayed using an automatic chemiluminiscence system (Tanon
Science and Technology Co., Ltd.). The degree of gray density was
analyzed using Image J software (version 1.46R, National Institute
of Health.).
Bio-layer interferometry (BLI)
Biomolecular interactions were measured by the BLI
method in vitro (34). All
experiments were performed in 37°C PBS and AR2G biosensors were
used in ForteBio Octet Red 96 (ForteBio Inc.). All samples were
added into the black 96-well plate (Greiner Bio-One Co., Ltd.).
Before protein immobilization, the baseline was established with
PBS prewetted AR2G biosensor. Then, HSP90-β and MGP (Matrix gla
protein) were fixed to the AR2G biosensors at 0.1 mg/ml. The
binding time was 600s and the dissociating time was 1,200 sec. The
curve was fitted globally with a 1:1 model (Octet Red system;
version 7.0).
Statistical analysis
All the experimental data were assessed using SPSS
v21.0 statistical software (SPSS, Inc.), and the values are
expressed as the mean ± standard deviation. Data distribution was
determined by measuring kurtosis and skewness. Statistical
significance was determined by a Student’s t-test or ANOVA with
Tukey’s post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
HSP90-β is the key target of osteoking in
OP
In our previous studies, osteoking was identified to
have positive effects on OP/OPF rats (19,23). The components of osteoking and its
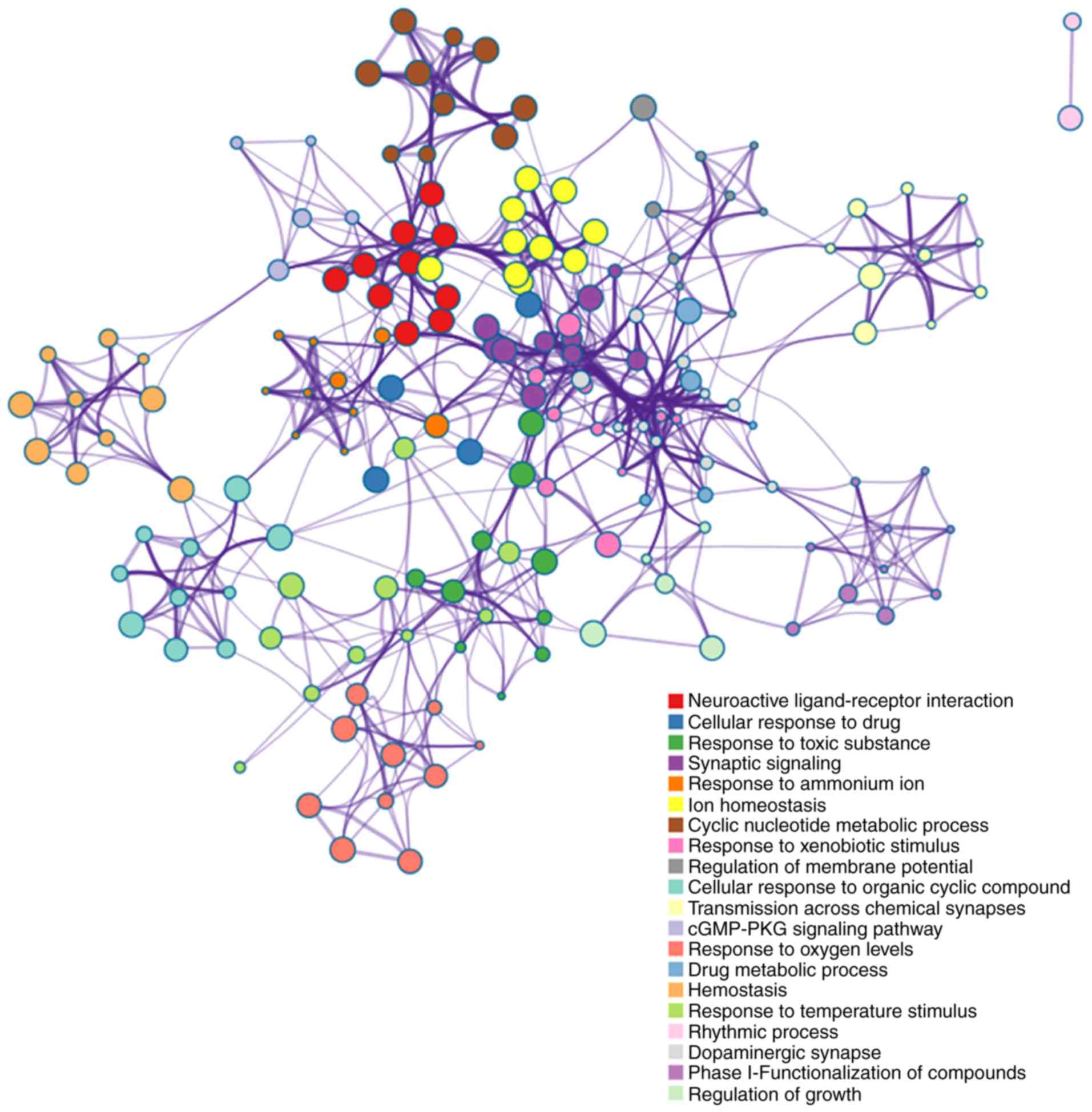
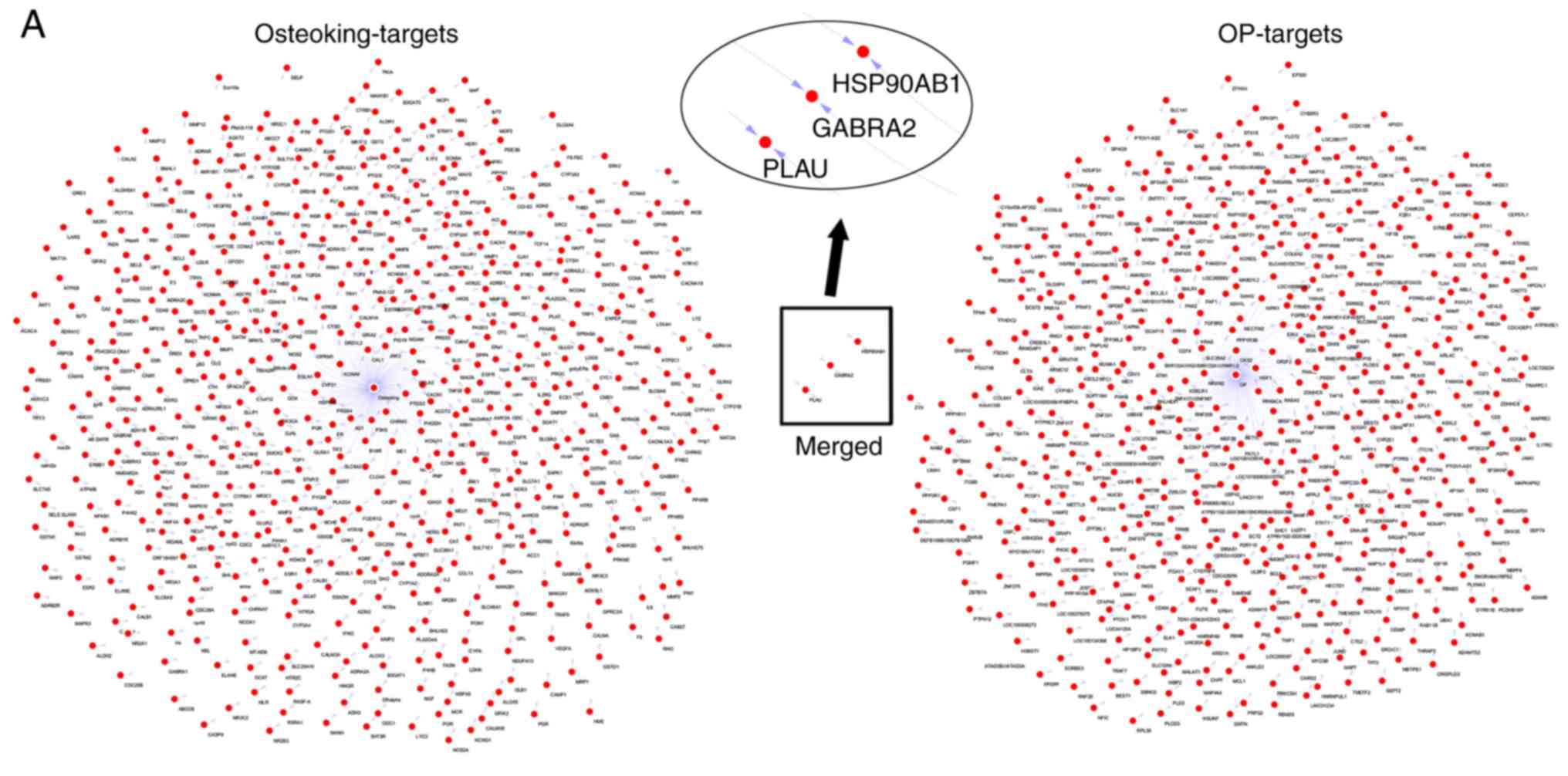
possible targets were obtained from the TCMSP database (Fig. 1). Notably, based on these targets,
functional enrichments suggested that osteoking was associated with
drug metabolic processes, blood circulation, hemostasis,
circulatory system processes and VEGF (Fig. 2). These targets are indirectly
associated with osteogenesis, but the related functions, including
growth, repair and angiogenesis, may benefit OP/OPF. Based on gene
data from the GEO database, the association between OP and
osteoking focused on HSP90-β (Fig.
3A). Importantly, HSP90-β was also confirmed to be highly
expressed, as determined by proteomic results in OP rat models
(Fig. 3B). Thus, HSP90-β may be a
key effector molecule of osteoking and serve an important role in
the pathophysiological process of OP, as indicated by
network-pharmacology analysis of the components of osteoking and
the in vivo proteomic results.
Osteoking leads to calcification
Postoperatively, the total BMD values of rats in the
OVX and sham groups were assayed using dual-energy X-ray. At the
23rd week, there was a significant difference (P<0.05) in BMD
between the OVX group (0.177±0.006 g/cm3) and the sham
group (0.188±0.008 g/cm3), suggesting that the OP rat
model was successfully established. In vivo, the rat
osteoporotic model was divided into three groups as aforementioned.
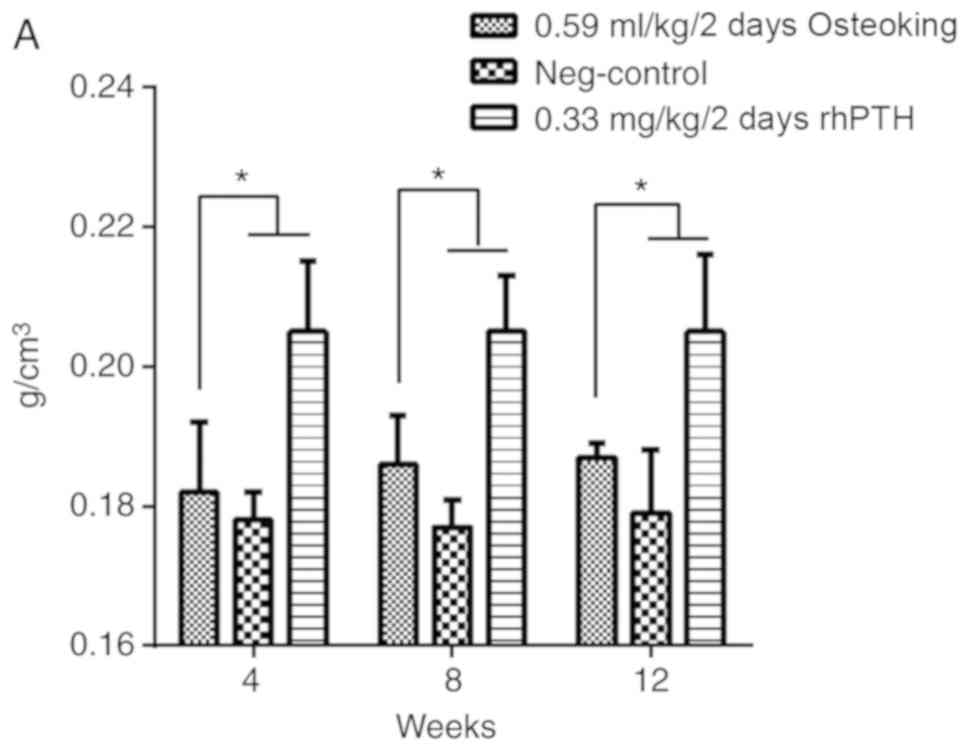
Following treatment with osteoking, changes in BMD, which is the
gold standard for evaluation of OP, were observed in each group
(Fig. 4A). The BMD in the
osteoking group significantly increased at the 8 and 12th weeks
compared with that of the negative control group (P<0.05);
however, the BMD in the osteoking group was decreased compared with
that in the positive control group (P<0.05). After 12 weeks of
administration, the micro-CT results indicated that the number of
bone trabeculae increased in the osteoking group (Fig. 4B). The BS/BV ratio, Tb.Th and Tb.N
in the osteoking group increased significantly compared with those
of the negative control group (P<0.05; Fig. 4C). However, these parameters were
all decreased compared with those in the positive control group
(P<0.05). At the initial time, all osteoporotic rats in the
three groups exhibited the same baseline bone alkaline phosphatase
(BALP; 21.55±1.54 U/l), PINP (280.63±75.27 pg/ml) and
tartrate-resistant acid phosphatase-5β (TRACP-5β; 0.71±0.01 mIU/ml)
values. BALP and PINP were used as bone formation markers. The
osteoking group demonstrated significantly increased BALP and PINP
after 12 weeks of treatment with osteoking compared with those of
the negative control group (P<0.05; Fig. 4D). In addition, in the osteoking
group, TRACP-5β, a bone resorption marker, was decreased compared
with in that of the other two groups.
HSP90-β improves OP by enhancing BMP-2
expression in vivo
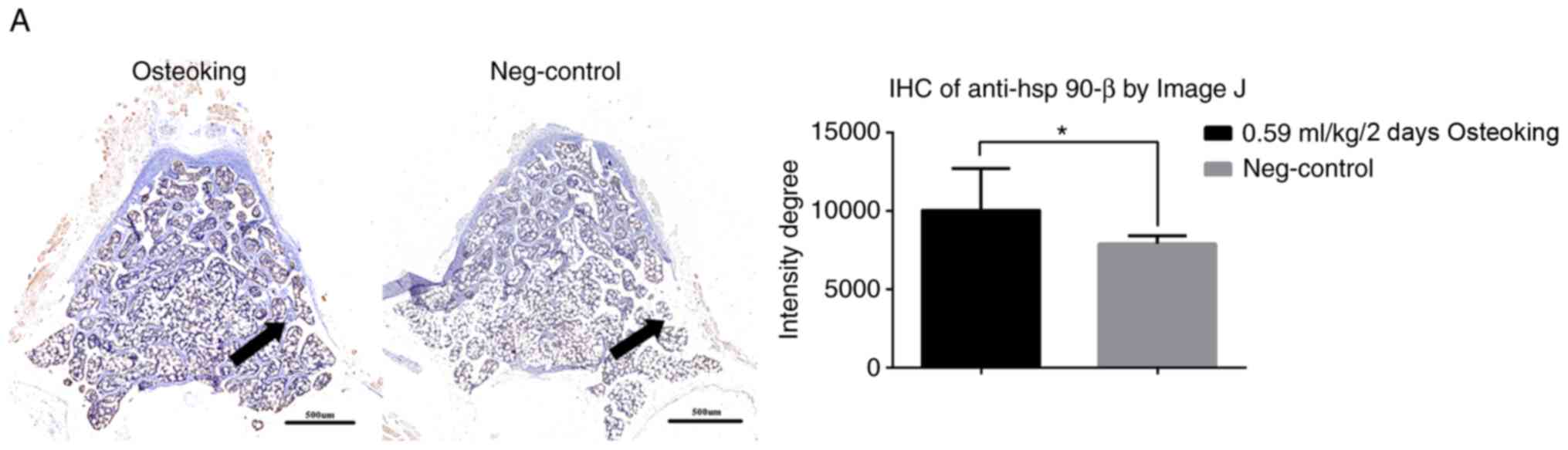
After 12 weeks of treatment, osteoking led to
calcification compared with that of the negative control group, and
its mechanism was associated with HSP90-β (Fig. 5A). Osteoporotic rats in the
osteoking group exhibited significantly increased HSP90-β
expression levels (Fig. 5B).
Moreover, increased BMP-2 expression levels were observed in the
osteoking group compared with those of the negative control
group.
Osteoking leads to high expression of
HSP90-β and BMP-2 after 4 weeks of culture in vitro
After 4 weeks of culture with osteoking, rBMSCs
demonstrated similar in vitro results (Fig. 5C). HSP90-β expression was
significantly increased in the osteoking group compared with that
of the control group, and BMP-2 expression was also increased.
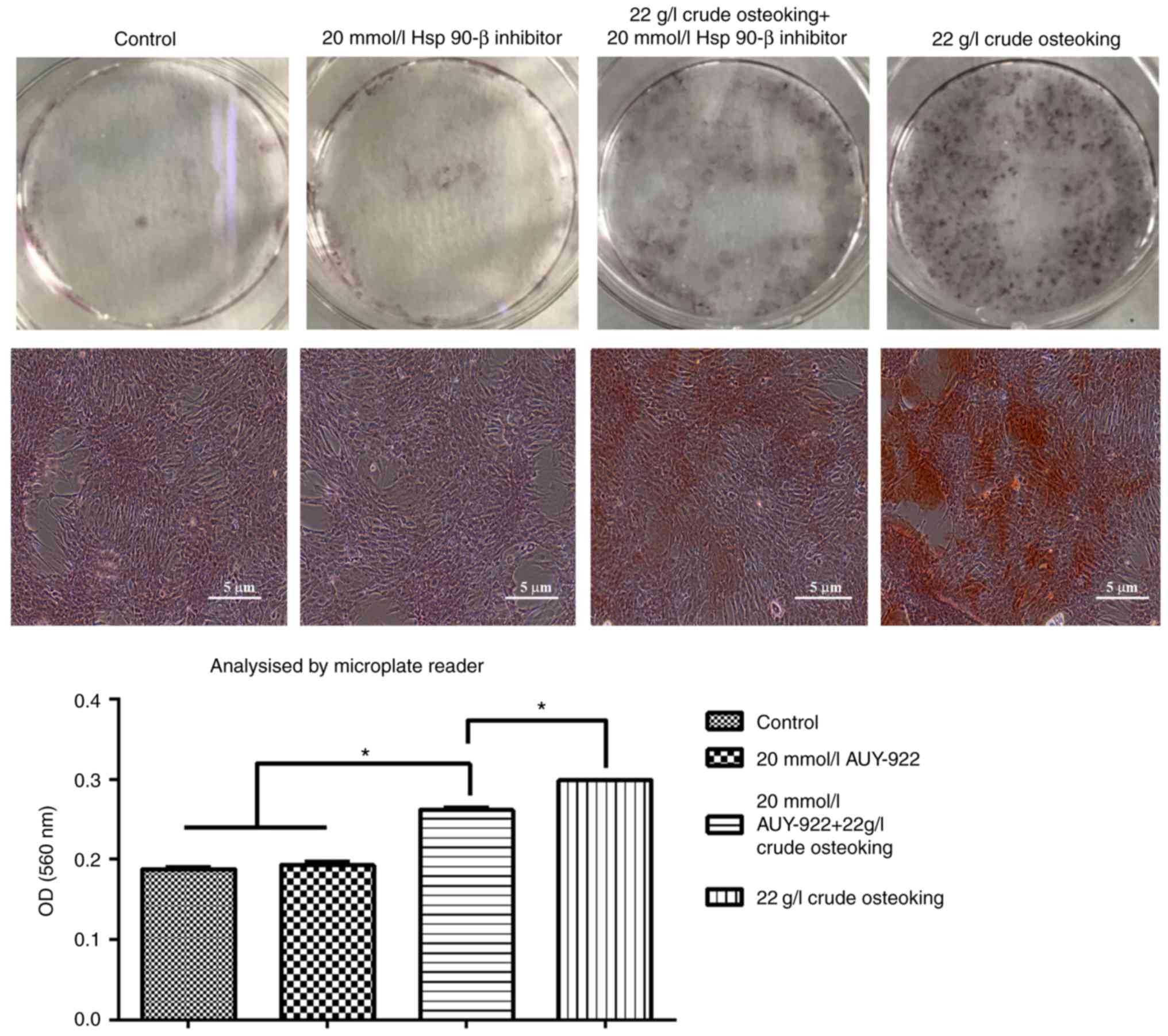
Blocking HSP90-β decreases calcium
deposition
After 4 weeks of culture, inhibiting HSP90-β in
vitro decreased calcium deposition in the osteoking + inhibitor
group compared with the osteoking group (P<0.05; Fig. 6). The number of calcified nodules
increased in the osteoking group compared with those of the control
group (P<0.05). In addition, the osteoking + inhibitor group
exhibited more calcified nodules compared with those of the control
group (P<0.05).
Discussion
At present, bioinformatics has contributed to the
development of TCM. Proteomics has validated the predicted results
of network pharmacology. Moreover, this method accurately
demonstrated marked changes between the osteoking and control
groups.
In the present study, osteoking improved OP in rats,
through increasing the BMD and enhancing the bone intensity. The
micro-CT results demonstrated improved micro-structure in the
osteoking group, which suggests an improved resistance to
biomechanical force in OP rats, which would help to avoid fracture.
Measurement of resistance to biomechanical force should have been
used in this study instead of the micro-CT. Previous studies have
indicated similar improvements of the OP/OPF model (13,15,18,19,20,23) and this is a limitation of the
present study. Measurement of biomechanical force could be better
to explain the effects of osteoking.
The results of the present study suggest that the
key protein involved in osteoking-mediated effects on and the
physiological process of OP is HSP90-β both in vivo and
in vitro. After 12 weeks of administration, osteoporotic
rats in the osteoking group had increased expression of HSP90-β
compared with that of the negative control rats. The results
demonstrated increased calcification and transiently increased
osteogenic activity in the osteoking group compared with that of
the negative control group. RBMSCs expressed increased HSP90-β
levels in vitro, and inhibition of HSP90-β decreased calcium
deposition. These data suggest that high HSP90-β is associated with
increased calcification in the osteoking group, inferring that
osteoking likely improves OP by regulating HSP90-β.
HSP90-β has been reported to contribute to the cell
cycle, proliferation, migration and apoptosis (35). HSP90-β affects endothelial cells
and their isoforms to promote angiogenesis (36,37). By contrast, an HSP90-β inhibitor
inhibited tumor growth through apoptosis, inducing cell cycle
arrest and downregulating target proteins (38,39). In addition, HSP90-β has been
demonstrated to regulate bone metabolism (40).
Firstly, the present study revealed that osteoking
directly stimulated rBMSCs, and that the increased HSP90-β
expression was a reaction to the drug (41). These results suggest that high
HSP90-β expression was not just a response to the drug, as
osteoking promoted rBMSC proliferation in vitro (Fig. S3).
Hsp 70 promotes calcium deposition by binding to
matrix Gla protein (Mgp), and Pro64 and Gla residues are required
for this binding (42). HSP90-β
has similar domains to those of Hsp 70, and HSP90-β binding to Mgp
was investigated. Notably, the molecular interaction results did
not suggest that HSP90-β binds to Mgp (Fig. S4).
Bioinformatics analysis indicated that the BMP
signaling pathway was associated with the mechanism of action of
osteoking. In vivo and in vitro data demonstrated
that high HSP90-β was accompanied by high BMP-2 levels. High BMP-2
levels led to calcium deposition and osteogenesis. Low-intensity
pulsed ultrasound, heating and cyclic tension have been reported to
promote osteogenesis through upregulation of the HSP90 and BMP
signaling pathways. High expressions of HSP90-β and BMP-2 was a
common phenomenon of physical treatments for OP. HSP90-β promotes
osteogenesis by enhancing BMP-2 (43,44).
In clinical settings, HSP90-β inhibitors have
demonstrated potential effects on disease, in particular HSP90-β
inhibitor-based suppression of tumor angiogenesis (45). HSP90-β inhibitors have been
studied for the treatment of atherosclerosis, due to their effects
on decreasing calcification (46). Following the inhibition of
HSP90-β, decreased calcium deposition was observed in the osteoking
+ inhibitor group compared with that of the osteoking group.
HSP90-β is an effective target of the osteoking-mediated effects on
OP, and high HSP90-β expression promoted calcium deposition.
However, calcium nodes were still observed following the inhibition
of HSP90-β. These data suggest that other components of osteoking
also promote calcium deposition.
In the in vitro experiments, a limitation of
the present study was the lack of a positive drug group. It has
been previously reported that osteoking promoted the
differentiation of BMSCs into osteoblasts, lipoblasts and
chondroblasts (47). In the
present study, high expression levels of HSP90-β promoted the
differentiation of rBMSCs into osteoblasts. High BMP-2 expression
levels leads to an improvement of the symptoms of OP (48). However, the mechanism between high
expressions of HSP90-β and BMP-2 is unknown. A positive drug group
could be helpful to explain the mechanism of action and further
study is required. HSP90-β was an effective osteoking target that
improved OP by enhancing BMP-2.
In addition, HSP90-β expression is also associated
with blood circulation. Osteoblasts are more effective compared
with osteoclasts in accelerating the exchange of oxygen-carbon
dioxide, nutrient-toxic substances and serum, and increasing BMD
(49–51). HSP90-β improves the bone
microenvironment by activating angiogenesis, which should be
investigated in future studies.
In conclusion, osteoking is a potential treatment
for OP. Osteoking contains 7 components, and its mechanism is
associated with the regulation of HSP90-β. Osteoking enhances
HSP90-β expression levels and improves OP by upregulating BMP-2.
Further examination of the association of HSP90-β and BMP-2 in OP
would be valuable.
Supplementary Information
Acknowledgements
Not applicable.
Funding
The present study was supported by National Science
Foundation of China-Yunnan Province Joint Fund (grant no.
U1502227).
Availability of data and materials
The data used to support the findings of this study
are available from the corresponding author upon request.
Competing interests
The authors declare that they have no competing
interests.
Abbreviations:
|
BMD
|
bone mineral density
|
|
BMC
|
bone mineral content
|
|
BALP
|
bone alkaline phosphatase
|
|
BPs
|
bisphosphonates
|
|
ERT
|
estrogen replacement
|
|
OVX
|
ovariectomized
|
|
PINP
|
procollagen I N-terminal peptide
|
|
rBMSC
|
rat bone mesenchymal stem cell
|
|
SERM
|
selective estrogen receptor
modulator
|
|
TCM
|
Traditional Chinese Medicine
|
Authors’ contributions
HBZ, WHL and ZQS designed this study. YS, DZ and RC
performed all the experiments. YS and WHL drafted the manuscript.
HBZ and ZQS gave suggestions and corrected the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study is approved by Animal Experimental
Ethical Committee of Kunming Medical University (approval no. KMMU
2015007).
Patient consent for publication
Not applicable.
References
|
1
|
Dunnewind T, Dvortsin EP, Smeets HM,
Konijn RM, Bos JHJ, de Boer PT, van den Bergh JP and Postma MJ:
Economic consequences and potentially preventable costs related to
osteoporosis in the Netherlands. Value Health. 20:762–768. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ensrud KE and Crandall CJ: Osteoporosis.
Ann Intern Med. 168:306–307. 2018. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
McClung MR: Denosumab for the treatment of
osteoporosis. Osteoporos Sarcopenia. 3:8–17. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fukumoto S and Matsumoto T: Recent
advances in the management of osteoporosis. F1000Res. 6:6252017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Qaseem A, Forciea MA, McLean RM and
Denberg TD; Clinical Guidelines Committee of the American College
of Physicians. Treatment of low bone density or osteoporosis to
prevent fractures in men and women: A clinical practice guideline
update from the American college of physicians. Ann Intern Med.
166:818–839. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hernlund E, Svedbom A, Ivergård M,
Compston J, Cooper C, Stenmark J, McCloskey EV, Jönsson B and Kanis
JA: Osteoporosis in the European Union: Medical management,
epidemiology and economic burden. A report prepared in
collaboration with the International Osteoporosis Foundation (IOF)
and the European Federation of Pharmaceutical Industry Associations
(EFPIA). Arch Osteoporos. 8:1362013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ramírez J, Nieto-González JC, Curbelo
Rodríguez R, Castañeda S and Carmona L: Prevalence and risk factors
for osteoporosis and fractures in axial spondyloarthritis: A
systematic review and meta-analysis. Semin Arthritis Rheum.
48:44–52. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tawaratsumida H, Setoguchi T, Arishima Y,
Ohtsubo H, Akimoto M, Ishidou Y, Nagano S, Taketomi E, Sunahara N
and Komiya S: Risk factors for bone loss in patients with
rheumatoid arthritis treated with biologic disease-modifying
anti-rheumatic drugs. BMC Res Notes. 10:7652017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Minisola S, Cipriani C, Occhiuto M and
Pepe J: New anabolic therapies for osteoporosis. Intern Emerg Med.
12:915–921. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rossini M, Adami S, Bertoldo F, Diacinti
D, Gatti D, Giannini S, Giusti A, Malavolta N, Minisola S, Osella
G, et al: Guidelines for the diagnosis, prevention and management
of osteoporosis. Reumatismo. 68:1–39. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kanis JA, Cooper C, Rizzoli R and
Reginster JY; Scientific Advisory Board of the European Society for
Clinical and Economic Aspects of Osteoporosis (ESCEO) and the
Committees of Scientific Advisors and National Societies of the
International Osteoporosis Foundation (IOF). European guidance for
the diagnosis and management of osteoporosis in postmenopausal
women. Osteoporos Int. 30:3–44. 2019. View Article : Google Scholar
|
|
12
|
Management of osteoporosis in
postmenopausal women: 2010 position statement of the North American
Menopause Society. Menopause. 17:25–54; quiz 55–26. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dai L, Wu H, Yu S, Zhao H, Xue L, Xu M,
Shen Z and Hu M: Effects of OsteoKing on osteoporotic rabbits. Mol
Med Rep. 12:1066–1074. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fan L, Ma J, Chen Y and Chen X:
Antioxidant and antimicrobial phenolic compounds from Setaria
viridis. Chem Nat Compounds. 50:433–437. 2014. View Article : Google Scholar
|
|
15
|
Hu M, Zhao HB, Qian CY, et al:
Ultrastructural evaluation of the SANFH rabbit animal models
intervened by Osteoking. Chin J Tradit Chin Med Pharm. 26:486–489.
2011.(In Chinese).
|
|
16
|
Zhao HB, Hu M and Liang HS: Experimental
study on osteoking in promoting gene expression of core binding
factor alpha 1 in necrotic femoral head of rabbits. Zhongguo Zhong
Xi Yi Jie He Za Zhi. 26:1003–1006. 2006.(In Chinese). PubMed/NCBI
|
|
17
|
Zhao H, Hu M, Wang W and Li L: Effects of
the VEGF gene expressions of Osteoking in the treatment of femoral
head necrosis. China J Orthop Traumatol. 20:757–759. 2007.
|
|
18
|
Zhao HB, Hu M, Zheng HY, Liang HS and Zhu
XS: Clinical study on effect of Osteoking in preventing
postoperational deep venous thrombosis in patients with
intertrochanteric fracture. Chin J Integr Med. 22:297–299.
2005.
|
|
19
|
Yan S: The effect of Osteoking on
ovariectomized female rat model of Osteoporosis in Chinese. Chin J
Osteoporosis. 12:21–24. 2016.
|
|
20
|
Hu M, Zhao HB, Wang B, Liang HS, Zhang CQ,
Zheng HY and Zhao XL: Clinical observation on promoting effect of
henggu gushang union agent on post-operational healing of
Gosselin’s fracture. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2:160–161.
2005.(In Chinese).
|
|
21
|
Hasan S, Bonde BK, Buchan NS and Hall MD:
Network analysis has diverse roles in drug discovery. Drug Discov
Today. 17:869–874. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Keiser MJ, Setola V, Irwin JJ, Laggner C,
Abbas AI, Hufeisen SJ, Jensen NH, Kuijer MB, Matos RC, Tran TB, et
al: Predicting new molecular targets for known drugs. Nature.
462:175–181. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Qin D, Zhang H, Zhang H, Sun T, Zhao H and
Lee WH: Anti-osteoporosis effects of osteoking via reducing
reactive oxygen species. J Ethnopharmacol. 244:1120452019.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jones-Bolin S: Guidelines for the care and
use of laboratory animals in biomedical research. Curr Protoc
Pharmacol. Appendix 4: Appendix 4B. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ahlquist RP: Experimental methods in
pharmacology. J Pharm Sci. 59:7282010. View Article : Google Scholar
|
|
26
|
Morgan SL and Prater GL: Quality in
dual-energy X-ray absorptiometry scans. Bone. 104:13–28. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Clough E and Barrett T: The gene
expression omnibus database. Methods Mol Biol. 1418:93–110. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gage GJ, Kipke DR and Shain W: Whole
animal perfusion fixation for rodents. J Vis Exp.
35642012.PubMed/NCBI
|
|
29
|
Bouxsein ML, Boyd SK, Christiansen BA,
Guldberg RE, Jepsen KJ and Müller R: Guidelines for assessment of
bone microstructure in rodents using micro-computed tomography. J
Bone Miner Res. 25:1468–1486. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
The UniProt Consortium. The universal
protein knowledgebase. Nucleic Acids Res. 45:D158–D169. 2017.
View Article : Google Scholar
|
|
31
|
NCBI Resource Coordinators. Database
resources of the National Center for Biotechnology Information.
Nucleic Acids Res. 46:D8–D13. 2018. View Article : Google Scholar :
|
|
32
|
Kibbe WA: OligoCalc: An online
oligonucleotide properties calculator. Nucleic Acids Res. 35(Web
Server issue): W43–W46. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
34
|
Guo XL, Liu LZ, Wang QQ, Liang JY, Lee WH,
Xiang Y, Li SA and Zhang Y: Endogenous pore-forming protein complex
targets acidic glycosphingolipids in lipid rafts to initiate
endolysosome regulation. Commun Biol. 2:592019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zuehlke AD, Moses MA and Neckers L: Heat
shock protein 90: Its inhibition and function. Philos Trans R Soc
Lond B Biol Sci. 373:201605272018. View Article : Google Scholar
|
|
36
|
Yan X, Hui Y, Hua Y, Huang L, Wang L, Peng
F, Tang C, Liu D, Song J and Wang F: EG-VEGF silencing inhibits
cell proliferation and promotes cell apoptosis in pancreatic
carcinoma via PI3K/AKT/mTOR signaling pathway. Biomed Pharmacother.
109:762–769. 2019. View Article : Google Scholar
|
|
37
|
Saryeddine L, Zibara K, Kassem N, Badran B
and El-Zein N: EGF-induced VEGF exerts a PI3K-dependent positive
feedback on ERK and AKT through VEGFR2 in hematological in vitro
models. PLoS One. 11:e01658762016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yasui H, Hideshima T, Ikeda H, Jin J, Ocio
EM, Kiziltepe T, Okawa Y, Vallet S, Podar K, Ishitsuka K, et al:
BIRB 796 enhances cytotoxicity triggered by bortezomib, heat shock
protein (Hsp) 90 inhibitor, and dexamethasone via inhibition of p38
mitogen-activated protein kinase/Hsp27 pathway in multiple myeloma
cell lines and inhibits paracrine tumour growth. Br J Haematol.
136:414–423. 2007. View Article : Google Scholar
|
|
39
|
Khong T and Spencer A: Targeting HSP90
induces apoptosis and inhibits critical survival and proliferation
pathways in multiple myeloma. Mol Cancer Ther. 10:1909–1917. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Miyasaka M, Nakata H, Hao J, Kim YK,
Kasugai S and Kuroda S: Low-intensity pulsed ultrasound stimulation
enhances heat-shock protein 90 and mineralized nodule formation in
mouse calvaria-derived osteoblasts. Tissue Eng Part A.
21:2829–2839. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tash JS, Chakrasali R, Jakkaraj SR, Hughes
J, Smith SK, Hornbaker K, Heckert LL, Ozturk SB, Hadden MK, Kinzy
TG, et al: Gamendazole, an orally active indazole carboxylic acid
male contraceptive agent, targets HSP90AB1 (HSP90BETA) and EEF1A1
(eEF1A), and stimulates Il1a transcription in rat Sertoli cells.
Biol Reprod. 78:1139–1152. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yao Y, Watson AD, Ji S and Bostrom KI:
Heat shock protein 70 enhances vascular bone morphogenetic
protein-4 signaling by binding matrix Gla protein. Circ Res.
105:575–584. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang Z, Ma Y, Guo S, He Y, Bai G and
Zhang W: Low-intensity pulsed ultrasound stimulation facilitates in
vitro osteogenic differentiation of human adipose-derived stem
cells via up-regulation of heat shock protein (HSP)70, HSP90, and
bone morphogenetic protein (BMP) signaling pathway. Biosci Rep.
38:BSR201800872018. View Article : Google Scholar :
|
|
44
|
Chung E, Sampson AC and Rylander MN:
Influence of heating and cyclic tension on the induction of heat
shock proteins and bone-related proteins by MC3T3-E1 cells. Biomed
Res Int. 2014:354260. 2014. View Article : Google Scholar
|
|
45
|
Graner MW: HSP90 and immune modulation in
cancer. Adv Cancer Res. 129:191–224. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Vázquez-Carrera M: HSP90 inhibitors as a
future therapeutic strategy in diabetes-driven atherosclerosis.
Clin Investig Arterioscler. 29:67–68. 2017.(In English, Spanish).
PubMed/NCBI
|
|
47
|
Yu C, Dai L, Ma Z, Zhao H, Yuan Y, Zhang
Y, Bao P, Su Y, Ma D, Liu C, et al: Effect of Osteoking on the
osteogenic and adipogenic differentiation potential of rat bone
marrow mesenchymal stem cells in vitro. BMC Complement Altern Med.
19:362019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Huang K, Wu G, Zou J and Peng S:
Combination therapy with BMP-2 and psoralen enhances fracture
healing in ovariectomized mice. Exp Ther Med. 16:1655–1662.
2018.PubMed/NCBI
|
|
49
|
Griffith JF, Wang YX, Zhou H, Kwong WH,
Wong WT, Sun YL, Huang Y, Yeung DK, Qin L and Ahuja AT: Reduced
bone perfusion in osteoporosis: Likely causes in an ovariectomy rat
model. Radiology. 254:739–746. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ding WG, Wei ZX and Liu JB: Reduced local
blood supply to the tibial metaphysis is associated with
ovariectomy-induced osteoporosis in mice. Connect Tissue Res.
52:25–29. 2011. View Article : Google Scholar
|
|
51
|
Peng J, Lai ZG, Fang ZL, Xing S, Hui K,
Hao C, Jin Q, Qi Z, Shen WJ, Dong QN, et al: Dimethyloxalylglycine
prevents bone loss in ovariectomized C57BL/6J mice through enhanced
angiogenesis and osteogenesis. PLoS One. 9:e1127442014. View Article : Google Scholar : PubMed/NCBI
|