Introduction
Sepsis is a condition that is often caused by a
dysregulated host response to infection and can lead to
life-threatening organ dysfunction (1). Despite abundant investigative
attention towards advances in intensive care and supportive
technology, sepsis remains in the top 10 leading causes of death
and is a substantial cause of death among critically ill patients
in non-coronary intensive care units (2,3).
Sepsis is estimated to affect at least 19 million patients
worldwide, reminding scientists and clinicians that it remains a
serious global health burden and a major clinical challenge
(4–6). Sepsis-induced cardiomyopathy (SIC)
is a common complication of sepsis involving a combination of
dysregulated inflammatory response, oxidative stress, calcium
regulation disorder, dysregulated autonomic nervous system,
impaired autophagy, apoptotic damage, mitochondrial dysfunction and
endothelial dysfunction (7–9).
Despite more aggressive approaches to prevent the progression of
SIC, these strategies often turn out to be disappointing and the
mechanisms of SIC are currently poorly elucidated.
Luteolin, a common bioactive flavonoid polyphenolic
compound, can be isolated from numerous vegetables, fruits and
herbs (10). Luteolin has been
shown to have diverse pharmacological effects, including
antioxidant, anti-inflammatory, anticancer and other biological
properties (11,12). Luteolin plays a role in preventing
ischemia/reperfusion injury in adult rat cardiomyocytes by
improving contractile function and attenuating apoptosis (13,14), and can protect against
high-carbohydrate/high-fat diet-induced myocardial inflammation
through anti-inflammatory mechanisms (15). These cardiovascular protective
effects of luteolin may be due to its antioxidant activities. For
example, luteolin can improve angiotensin II-induced cardiac
remodeling by decreasing oxidative stress (16). While increasing evidence shows
that luteolin exerts cardiovascular protective effects, the
possible beneficial effects of luteolin on SIC have not yet been
fully clarified.
Autophagy is the main cellular pathway to maintain
protein quality and organelle function, through degradation of
damaged or dysfunctional cellular components (17,18). Autophagy has been shown to be
involved in numerous physiological processes, such as the
starvation response, cell growth control, anti-aging mechanisms and
innate immunity (19). Studies
have shown that autophagy is mobilized early in sepsis and is seen
in various organs by an increased accumulation of autophagic
vacuoles and enhanced expression of autophagy-associated proteins
(9,20,21). Various kinds of drugs administered
to reverse sepsis-induced immunoparalysis have been shown to be
autophagy-dependent (21).
AMP-activated protein kinase (AMPK) not only plays a key role in
sensing the energy status and regulating cellular energy
homeostasis, but is also an important positive regulator of
autophagy (22). Moreover,
activation of AMPK can ameliorate the organ injury induced by
sepsis through decreasing inflammatory cytokine levels and
endothelial activation (23).
The present study sought to demonstrate the
potential effects of luteolin on lipopolysaccharide (LPS)-induced
myocardial injury via increasing autophagy. Whether the protective
effect of luteolin on autophagy was mediated through AMPK signaling
was also explored.
Materials and methods
Experimental animals
Eight-week-old male C57BL/6 mice (n=75; weight,
25–30 g) were purchased from the Laboratory Animal Center of the
Fourth Military Medical University. All experimental procedures
were in accordance with the National Institutes of Health
Guidelines on the Care and Use of Laboratory Animal and were
approved by the Fourth Military Medical University Ethics Committee
on Animal Care. All mice were maintained in a
temperature-controlled room (22±2°C) under a 12-h light/dark cycle,
with relative humidity of 40–60%, and free access to food and
water.
Experimental protocol
The experimental mice were randomly selected and
allocated into the following 3 groups (25 mice in each group): The
control group (NC), the LPS group (LPS) and the LPS + luteolin
group (LPS + Lut). Before the model was induced, luteolin (10
μg/kg) (99%, Sigma-Aldrich; Merck KGaA) was dissolved in DMSO and
injected intraperitoneally for 10 days into LPS + Lut group
animals. NC group and the LPS group mice received an equal volume
of DMSO for 10 days. The animal model of sepsis-induced cardiac
dysfunction was induced on day 11 by intraperitoneal injection of
LPS (10 mg/kg) (Sigma-Aldrich; Merck KGaA) dissolved in normal
saline. NC group mice received an equivalent volume of normal
saline on day 11. Rational doses of luteolin (10 μg/kg) and LPS (10
mg/kg) were chosen according to the literature (24,25). A total of 12 h after LPS
injection, M-mode echocardiography was performed and the mice were
sacrificed.
Echocardiography
Echocardiography was performed using an
echocardiogram (15.0 MHz, VisualSonics Vero 2100) to measure the
changes in cardiac function. Two-dimensional guided M-mode
measurements of the left ventricular (LV) internal diameter were
obtained from the short-axis view at the level of the papillary
muscles over at least three beats and were averaged. Computer
algorithms were employed to measure the left ventricular
end-diastolic dimension (LVEDD), left ventricular end-systolic
dimension (LVESD), left ventricular ejection fraction (LVEF) and
left ventricular fraction shortening (LVFS). Millar Mikro-tip
catheter transducer was used and inserted into the left ventricular
cavity through the left carotid artery to measure the LV pressure.
Computer algorithms and an interactive videographics program
(Po-Ne-Mah Physiology Platform P3 Plus, Gould Instrument Systems)
were employed to obtain the first derivative of the left
ventricular pressure (±LV dp/dt max).
Determination of tissue interleukin
(IL)-1β, IL-6 and tumor necrosis factor (TNF)-α activity
Heart tissue samples were rinsed and perfused with
normal saline and samples were homogenized. The concentrations of
IL-1β (cat. no. PI301; Beyotime Institute of Biotechnology), IL-6
(cat. no. 88-7064-77; Thermo Fisher Scientific, Inc.) and TNF-α
(cat. no. BMS607-3; Thermo Fisher Scientific, Inc.) were assessed
using ELISA kits, following the manufacturer’s protocol. Values are
expressed as pg/mg of total protein.
Transmission electron microscopy
Heart tissues were removed from the animals and
quickly rinsed in PBS on wet ice. Samples removed from the left
ventricular myocardium were cut into 1 mm cubes and fixed with 2%
glutaraldehyde for 5 h at 4°C. Subsequently, samples were incubated
with 1% osmium tetroxide for 2 h in the dark. The samples were
embedded with fresh resin for 2 h at room temperature and then cut
into ultrathin sections (thickness, 50–100 nm). Uranyl acetate and
lead citrate were used to stain the ultrathin sections at 37°C, for
30 and 15 min respectively, and samples were then observed using a
JEOL JEM-2000EX transmission electron microscope (JEOL, Ltd.). For
an in vitro study, primary cardiomyocytes were collected by
centrifugation at 168 × g for 10 min at room temperature and fixed
with 2% glutaraldehyde for 5 h at 4°C. The fixed cells were then
stained with uranyl acetate and lead citrate at 37°C, for 30 and 15
min respectively, and images were captured using a JEOL JEM-2000EX
transmission electron microscope (JEOL, Ltd.) as described above.
Random sections were imaged and analyzed by two technicians blinded
to the experiment.
Determination of mitochondrial
transmembrane potential (ΔΨm)
Tetrachloro-tetraethyl benzimidazol carbocyanine
iodide (JC-1) was employed to evaluate the changes in ΔΨm. Primary
cardiomyocytes were cultured in disposable confocal dishes at a
density of 5xl04 cells/dish. The cells were incubated
with 5 μM JC-1 (Beyotime Institute of Biotechnology) for 30 min at
37°C in the dark and washed twice with PBS. JC-1-labelled cells
were visualized using an Olympus FV1000 laser confocal microscope.
Cellular mitochondria with normal ΔΨm emitted red fluorescence
(J-aggregate), while those with abnormal ΔΨm showed green
fluorescence (J-monomer).
Isolation of mitochondria
Mitochondria were isolated from hearts using the
Cell Mitochondria Isolation kit (Beyotime Institute of
Biotechnology) as previously described (26). Isolated mitochondria were
maintained on wet ice at 0°C before downstream processing.
Measurement of citrate synthase and
electron transport chain complex activities and ATP content
Citrate synthase (CS) and electron transport chain
complex activities were measured using the CS Assay kit
(Sigma-Aldrich; Merck KGaA) following the manufacturer’s protocol.
The ATP content of heart tissues was detected using an adenosine
triphosphate bioluminescence assay kit (Beyotime Institute of
Biotechnology).
Determination of mitochondrial calcium
retention capacity (mCRC)
The mCRC represents the capacity of mitochondria to
take in calcium before permeability transition. The mCRC level was
assessed as previously described (27).
Estimation of malondialdehyde (MDA) and
reactive oxygen species (ROS) production in heart tissue
Heart tissues were weighed and homogenized (1:10,
w/v) in phosphate buffer (50 mM, pH 7.4). The levels of ROS and MDA
were measured using the ROS Assay kit and Lipid Peroxidation MDA
assay kit according to the manufacturer’s protocol (Nanjing
Jiancheng Bioengineering Institute).
Western blot analysis
Cardiac tissues or cardiomyocytes were harvested and
homogenized with RIPA buffer containing protease inhibitor cocktail
(Roche Diagnostics) on ice. After centrifugation at 12,000 × g for
15 min at 4°C, the total protein content of the supernatant was
quantified using a bicinchoninic acid protein assay (Applygen
Technologies, Inc.) and the supernatant was stored at −80°C until
use. Protein samples (40 μg protein/lane) were separated using 10%
SDS-PAGE gels, transferred to polyvinylidene difluoride membrane
(EMD Millipore) and incubated overnight at 4°C with the primary
antibodies. The blots were then incubated with horseradish
peroxidase-conjugated secondary antibody for 1 h at 37°C.
Immunoreactive bands were detected and scanned with ECL™ Advance
Western Blotting Detection kit (Amersham Bioscience). ImageJ
Version 1.8.0 software (National Institutes of Health) was used to
analyze protein band intensity.
Reagents and antibodies
Antibodies against the following proteins were used:
Sequestosome 1 (p62; 1:1,000; cat. no. ab109012; Abcam), Beclin1
(1:1,000; cat. no. ab210498; Abcam), cleaved caspase-3 (1:1,000;
cat. no. AB3623; Sigma-Aldrich; Merck KGaA), cleaved caspase-9
(1:1,000; cat. no. AB3629; Sigma-Aldrich; Merck KGaA),
microtubule-associated protein light chain 3 A/B (LC3A/B; 1:1,000;
cat. no. 4108; Cell Signaling Technology, Inc.), AMPK (1:1,000;
cat. no. 5831; Cell Signaling Technology, Inc.), phosphorylated
(p)-AMPK (Thr172) (1:1,000; cat. no. 2535; Cell Signaling
Technology, Inc.), Unc-51 like autophagy activating kinase 1 (ULK1;
1:1,000; cat. no. 6439; Cell Signaling Technology, Inc.), p-ULK1
(Ser757) (1:1,000; cat. no. 14202; Cell Signaling Technology, Inc.)
and β-actin (1:5000; cat. no. sc-47778; Santa Cruz, Biotechnology.
Inc.). Horseradish peroxidase-conjugated secondary antibodies
(anti-mouse/rabbit IgG) (1:5,000; cat. nos. 7076 and 7074; Cell
Signaling Technology, Inc.) were used.
Culture of neonatal mouse ventricular
cardiomyocytes
Isolation of primary neonatal mouse cardiomyocytes
was described previously (26).
Male C57BL/6 mice (n=60; age, 1-day-old; weight, 1.5–3 g) were
purchased from the Laboratory Animal Center of the Fourth Military
Medical University. Neonatal mice were sacrificed and hearts were
quickly excised and minced into fragments prior to enzymatic
digestion with collagenase type 2 (Sigma-Aldrich; Merck KGaA).
After digestion for several minutes, the digested fragments were
placed on a sterilized platform to sediment for several minutes and
digested cells in supernatants were pre-plated for 1 h to remove
fibroblasts and endothelial cells. The residual supernatant with
abundant cardiomyocytes (10,000–12,000 cells/cm2) was
re-plated in DMEM (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 20% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.) and 1% penicillin-streptomycin at 37°C in 95%
O2 and 5% CO2. On the fourth day after
isolation, spontaneous beating of the primary neonatal mouse
cardiomyocytes was observed. Troponin T (cTnT) is a marker specific
to cardiomyocytes. A monoclonal antibody against cTnT (1:200; cat.
no. sc-20025; Santa Cruz Biotechnology, Inc.) was used to identify
the primary neonatal mouse cardiomyocytes (data not shown). To
determine whether luteolin could protect cardiomyocytes against
LPS-induced injury, cardiomyocytes were pretreated with luteolin in
the absence or presence of LPS. Luteolin (20 μM) was added to the
medium for 6 h and cells were then exposed to LPS (10 μg/ml) for 24
h. Cells were also pretreated with the AMPK activator
5-aminoimidazole-4-carboxamide ribonucleotide (AICAR; 1 mM), the
autophagy inhibitor 3-methyladenine (3-MA; 10 mM), or the AMPK
inhibitor dorsomorphin (compound C, CC, 10 mM) to evaluate the
changes in protein expression and autophagic activity.
Determination of cardiomyocyte
apoptosis
A TUNEL assay kit (In Situ Cell Death
Detection kit; Roche Diagnostics) was used to measure the apoptosis
ratio of the cardiomyocytes as described previously (26). TUNEL was performed with
fluorescein-dUTP for 1 h at 37°C to identify apoptotic cell nuclei
and 4′,6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich; Merck KGaA)
was used to stain all cell nuclei for 5 min at room temperature. A
monoclonal antibody against cTnI (Santa Cruz Biotechnology, Inc.)
was used to stain and identify the myocardium. The apoptotic index
was calculated as the ratio of TUNEL-positive cells to the total
number of DAPI-positive cells within the same area from five
randomly selected fields in each group.
Statistical analysis
All tests were repeated three times. Continuous
variables are presented as means ± standard error of the mean.
Differences between specific groups were determined by one-way
analysis of variance followed by the Student-Newman-Keuls test.
P<0.05 was considered to indicate a statistically significant
difference. SPSS software package version 14.0 (SPSS, Inc.) was
used for data analysis.
Results
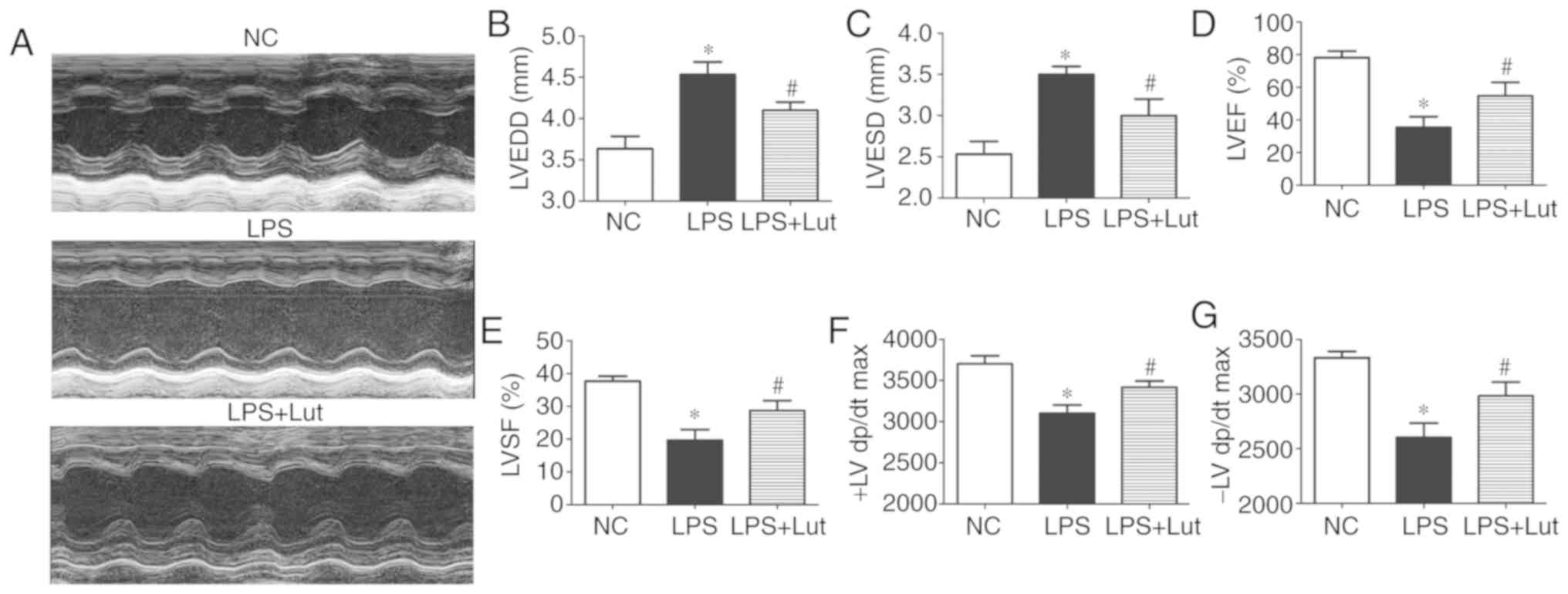
Luteolin improves cardiac function in
mice with LPS-induced sepsis
To investigate the effect of luteolin treatment on
cardiac function in mice with sepsis, echocardiography was used to
assess cardiac function parameters (Fig. 1A). LVEDD and LVESD were
significantly increased in the LPS group compared with the control
group. Luteolin treatment significantly suppressed the increases in
LVEDD and LVESD in mice with sepsis compared with the LVEDD and
LVESD in untreated mice with sepsis (Fig. 1B and C). LVEF, LVFS, and ±LV dp/dt
were decreased in the LPS group compared with in the control group.
Treatment with luteolin increased the LVEF, LVFS and ± LV dp/dt
values in mice with sepsis compared with those in untreated mice
with sepsis (Fig. 1D–G).
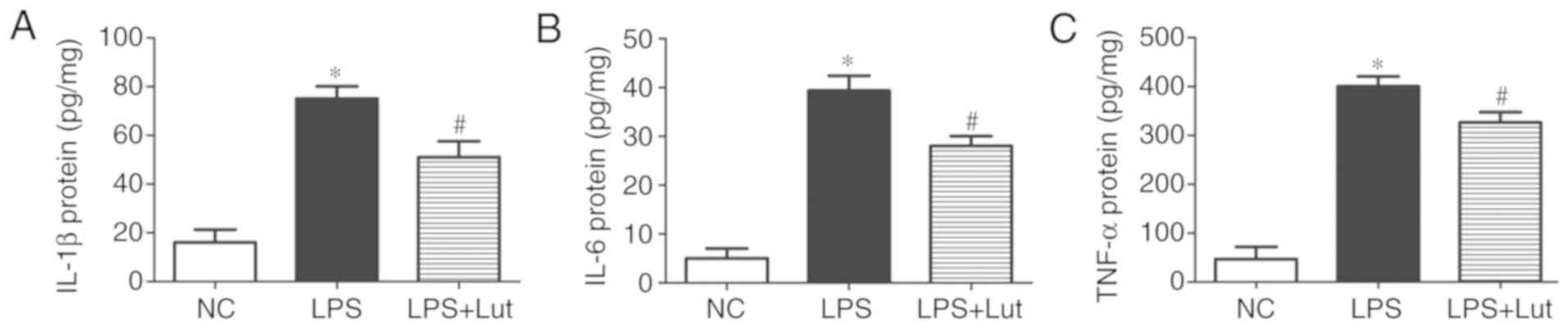
Luteolin attenuates inflammation in the
hearts of septic mice
Further experiments were done to examine the effects
of luteolin treatment on the inflammatory response in septic
hearts. The levels of the inflammatory factors IL-1β, IL-6 and
TNF-α were increased in the septic myocardium compared with in the
control myocardium. In contrast, lower levels of IL-1β, IL-6 and
TNF-α were detected in the LPS + luteolin group compared with in
the LPS group (Fig. 2–C).
Luteolin decreases cardiac apoptosis in
mice with LPS-induced sepsis
A TUNEL assay was performed to evaluate the effects
of luteolin on cardiac apoptosis (Fig. 3A). Significantly more
TUNEL-positive cardiomyocytes were detected in the LPS group than
in the control group, but this was attenuated by luteolin treatment
(Fig. 3B). Western blotting was
used to assess caspase enzyme activity (Fig. 3C). The levels of cleaved caspase-3
and cleaved caspase-9 were significantly increased in the LPS group
compared with in the control group, but luteolin treatment
decreased these levels in the myocardium of septic mice (Fig. 3D and E).
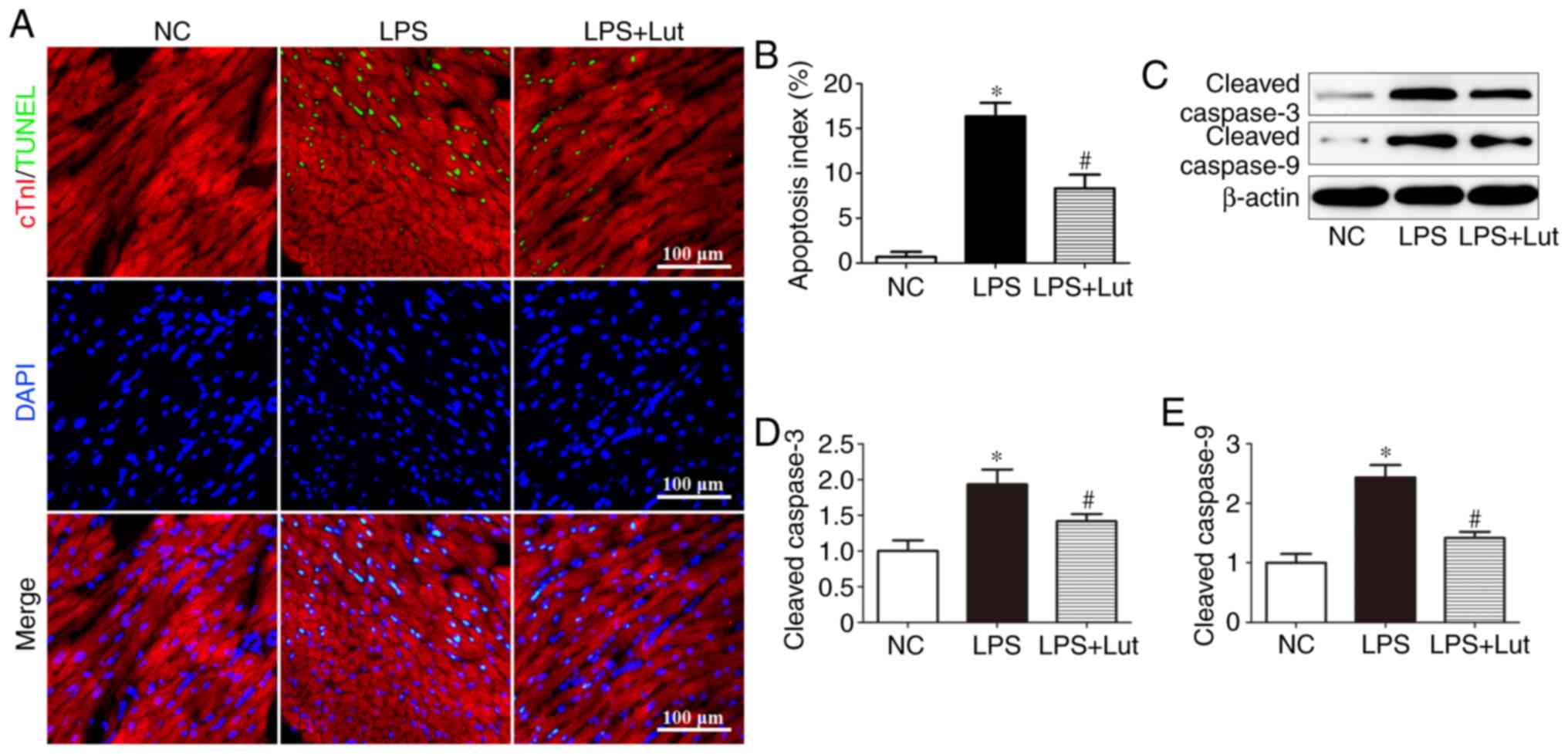 | Figure 3Effects of Lut on cardiac apoptosis
in LPS-induced septic mice. (A) Representative immunofluorescence
images of TUNEL (green), DAPI staining (blue) and cTnI antibody
staining (red) and (B) quantification of TUNEL-positive cells. The
columns and error bars represent the means and SEMs (n=5).
*P<0.05 vs. NC; #P<0.05 vs. LPS.
Representative (C) immunoblots of protein expression levels in the
myocardium, with quantitative analysis for cleaved (D) caspase-3,
(E) cleaved caspase-9 and β-actin. The columns and error bars
represent the means and SEMs (n=5). *P<0.05 vs. NC;
#P<0.05 vs. LPS. cTnI, Troponin I; LPS,
lipopolysaccharides; SEM, standard error of the mean; DAPI,
4′,6-diamidino-2-phenylindole; TUNEL, terminal
deoxynucleotidyl-transferase-mediated dUTP nick end labelling; NC,
negative control; Lut, luteolin. |
Luteolin attenuates mitochondrial injury
and oxidative stress in the hearts of septic mice
Mitochondrial dysfunction is correlated with the
deterioration of myocardial function during sepsis. Transmission
electron microscopy (TEM) was used to evaluate the effect of
luteolin treatment on mitochondrial ultrastructural changes in mice
with sepsis (Fig. 4A). Normal
mitochondria with organized cristae packed beside symmetric
myofibrils were seen in the hearts of control mice. In the
myocardium of septic mice, the mitochondria were swollen and
disarranged. In addition, rarefaction and vacuolization of the
mitochondrial structure, as well as rupture and disappearance of
the mitochondrial cristae, were seen. In the LPS + Lut group, most
mitochondria displayed sharply defined cristae. The ATP content, CS
activity and complex I/II/III/IV/V activities in the isolated
mitochondria were significantly decreased in the LPS group compared
with those in the control group and these activities were enhanced
in the LPS + Lut group (Fig. 4B, C
and D). The mCRC, determined as the capacity of mitochondria to
uptake calcium before permeability transition, was used to assess
the sensitivity of mitochondrial permeability transition pore
(mPTP) opening to calcium (Fig.
4E). The mCRC was significantly decreased in the LPS group
compared with in the control group. However, treatment with
luteolin significantly increased the mCRC, indicating that the
sensitivity to calcium-induced mPTP opening was decreased.
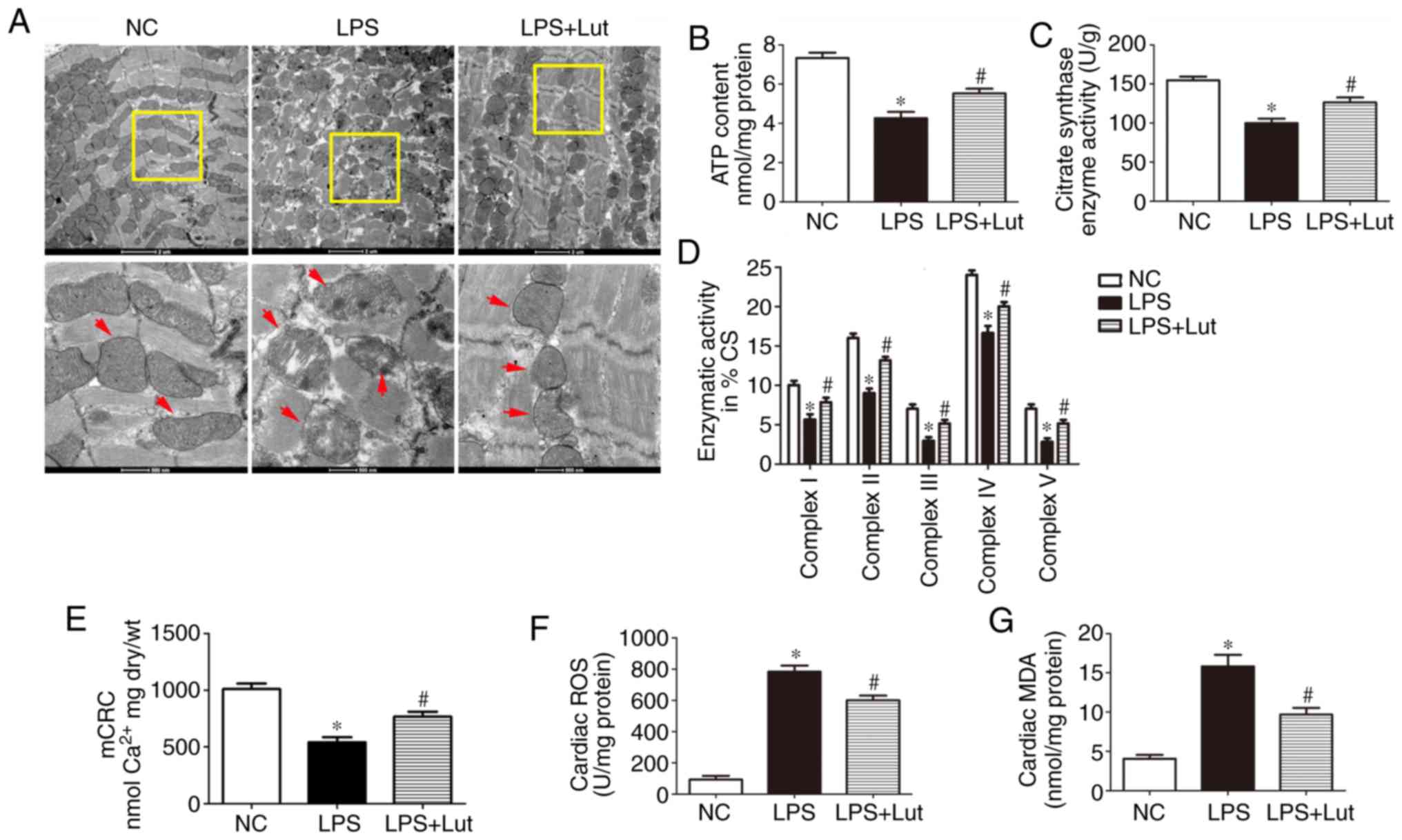 | Figure 4Effects of Lut on mitochondrial
function in LPS-induced septic mice. (A) Representative
transmission electron micrographs of left ventricular specimens
(magnification, ×8,200 and ×26,500, the red arrows indicate
mitochondria). (B) ATP content and (C) CS and (D) complex
I/II/III/IV/V activities in mitochondria isolated from mice. (E)
mCRC. (F) ROS and (G) MDA levels. The columns and error bars
represent the means and standard error of the mean (n=5).
*P<0.05 vs. NC; #P<0.05 vs. LPS. LPS,
lipopolysaccharides; MDA, malondialdehyde; NC, negative control;
mCRC, mitochondrial calcium retention capacity; CS, citrate
synthase; ROS, reactive oxygen synthase; Lut, luteolin. |
Oxidative stress contributes to myocardial injury
during sepsis. The ROS and MDA levels were measured to explore the
effect of luteolin treatment on oxidative stress in septic heart
tissue. The levels of ROS and MDA were significantly enhanced in
the LPS group compared with in the control group and luteolin
administration significantly inhibited these increases (Fig. 4F and G).
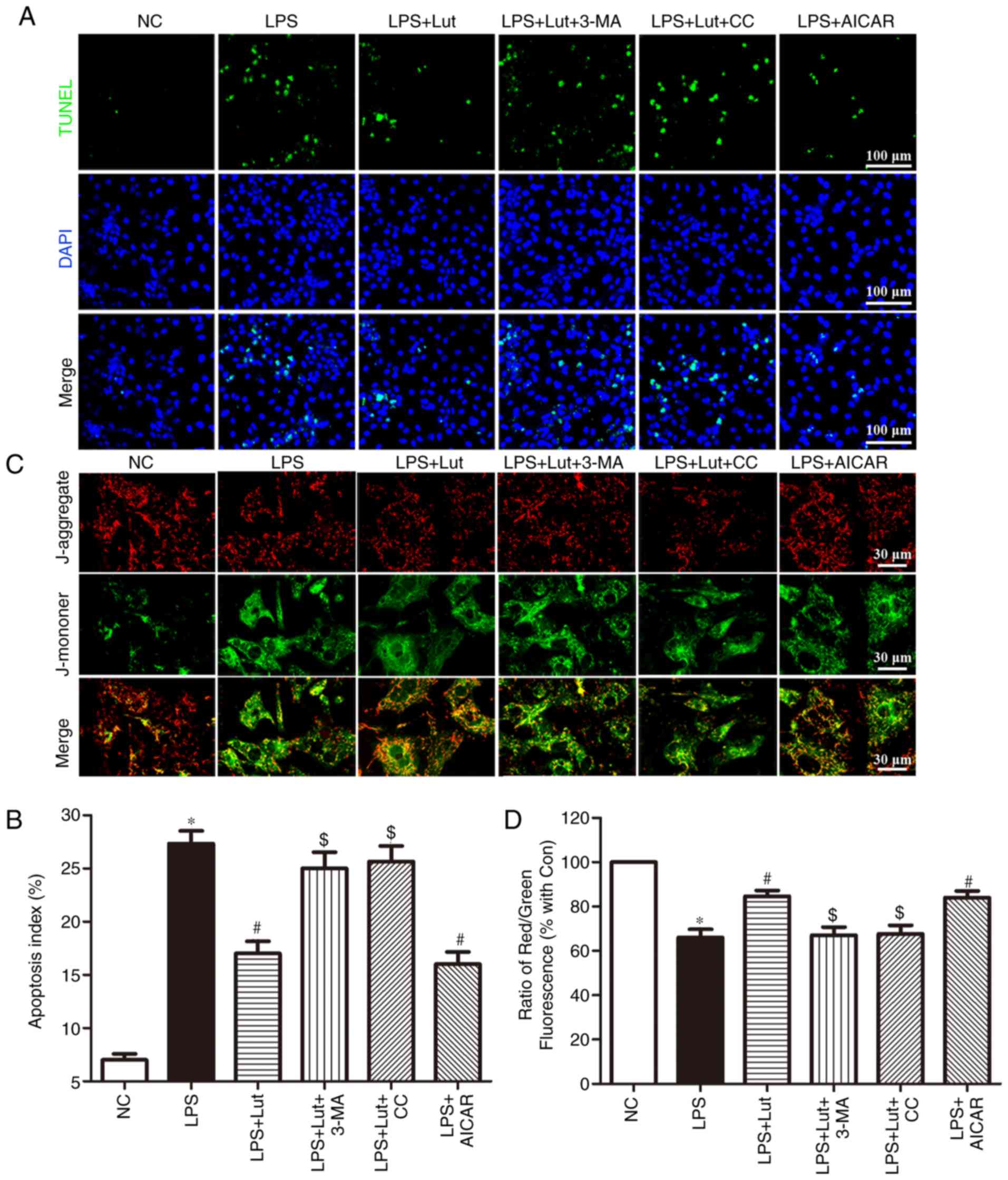
Luteolin enhances autophagy through AMPK
activation in the hearts of septic mice
Impairment of autophagy may contribute to
contractile dysfunction and apoptotic cardiomyocyte death in
sepsis. As shown by TEM, more autophagosomes were observed in the
LPS group compared with in the control group. Luteolin further
increased the number of autophagosomes in septic mice (Fig. 5A). Western blotting was used to
assess autophagic activity in the hearts of septic mice and in
LPS-treated cardiomyocytes (Fig. 5B
and H). The expression of LC3 II and Beclin1 was increased and
that of p62 was lower in the hearts of septic mice than in the
hearts of control mice. However, luteolin treatment further
increased LC3-II and Beclin1 expression and decreased p62
expression in septic mice (Fig.
5B–E). The effect of luteolin on LC3-II, p62 and Beclin1
expression in LPS-treated cardiomyocytes was consistent with the
in vivo effect (Fig.
5H–K). In addition, the autophagy inhibitor 3-MA abolished the
effect of luteolin on autophagic activity in cardiomyocytes
(Fig. 5H–K).
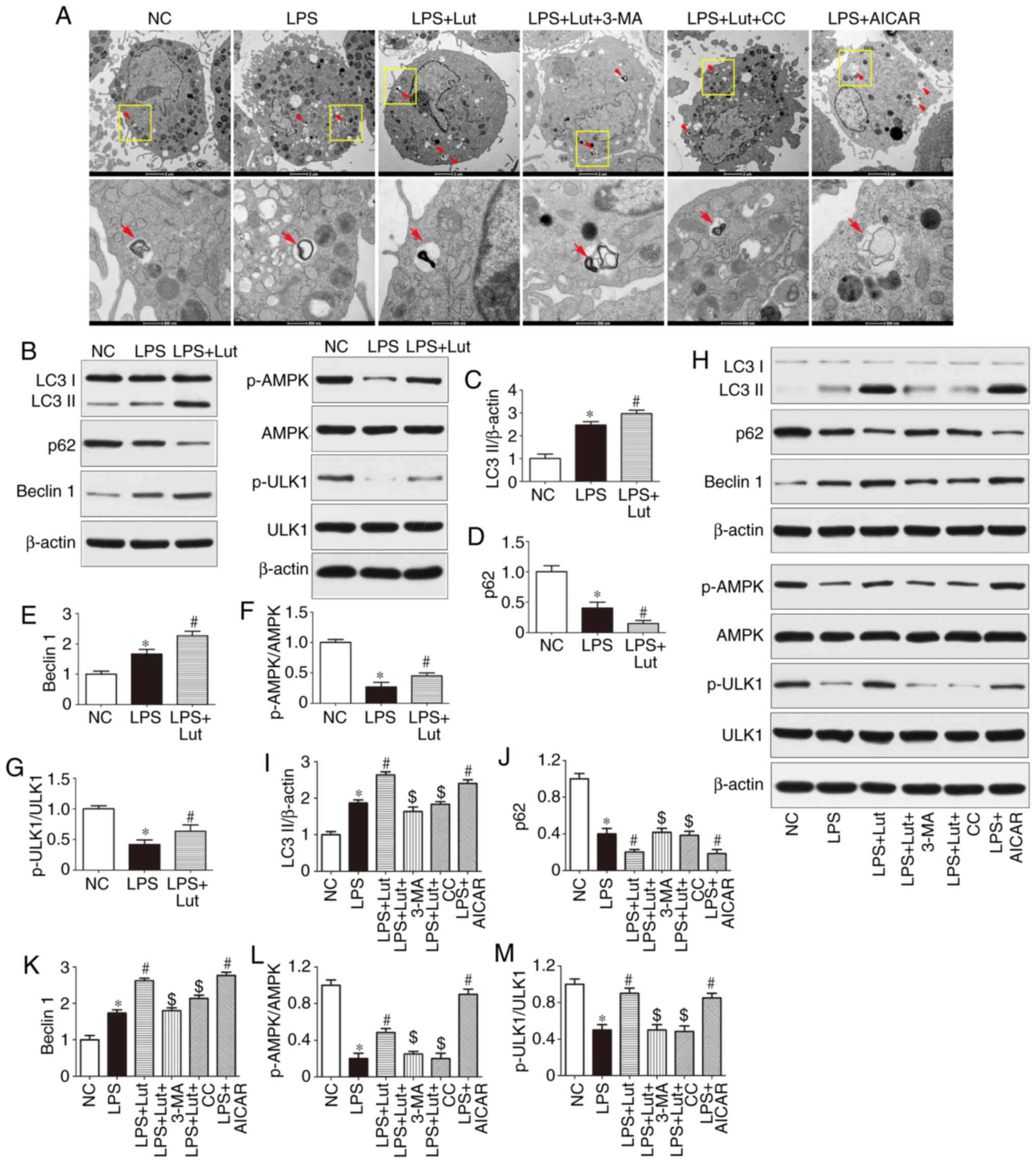 | Figure 5Effects of Lut on autophagy in the
hearts of septic mice and in LPS-treated cardiomyocytes. (A)
Representative images of ultrastructural morphology and typical
autophagosomes in cardiomyocytes subjected to different treatments
(magnification, ×6,000 and ×43,000, the red arrows indicate
autophagosomes). (B) Representative immunoblots and densitometric
quantification for (C) LC3II, (D) P62, (E) Beclin1, (F) p-AMPK,
AMPK, (G) p-ULK1, ULK1, and β-actin in myocardial tissues from the
indicated groups. The columns and error bars represent the means
and SEMs (n=5). *P<0.05 vs. NC; #P<0.05
vs. LPS. (H) Representative immunoblots and densitometric
quantification for (I) LC3II, (J) P62, (K) Beclin1, (L) p-AMPK,
AMPK, (M) p-ULK1, ULK1, and β-actin in cardiomyocytes from the
indicated groups. The columns and error bars represent the means
and SEMs. *P<0.05 vs. NC; #P<0.05 vs.
LPS; $P<0.05 vs. LPS + Lut. p62, sequestosome 1;
LC3II, microtubule-associated protein light chain 3II; AMPK,
AMP-activated protein kinase; ULK1, Unc-51 like autophagy
activating kinase 1; 3-MA, 3-methyladenine; AICAR,
5-aminoimidazole-4-carboxamide ribonucleotide; CC, dorsomorphin,
compound C; p-, phosphorylated; SEM, standard error of the mean;
NC, negative control; Lut, luteolin. |
Western blotting was used to measure the expression
of AMPK signaling proteins (Fig. 5B
and H). The phosphorylation of AMPK and ULK1 was reduced in the
hearts of septic mice compared with control mice and luteolin
enhanced the phosphorylation of AMPK and ULK1 in the hearts of
septic mice (Fig. 5B, F, and G).
Similar effects were found in vitro (Fig. 5H, L and M). More notably, the AMPK
inhibitor compound C abolished the effect of luteolin on autophagic
activity in LPS-treated cardiomyocytes, as evidenced by the
decrease in LC3 II and Beclin1 expression and the increase in p62
expression, while the AMPK activator AICAR mimicked the effect of
luteolin in LPS-treated cardiomyocytes (Fig. 5H–K).
Luteolin inhibits apoptosis in
LPS-treated cardiomyocytes
A TUNEL assay was employed to assess the effects of
luteolin on apoptosis in LPS-treated cardiomyocytes (Fig. 6A). Consistent with the in
vivo effect, luteolin treatment inhibited LPS-induced
cardiomyocyte apoptosis. Interestingly, 3-MA and compound C
reversed the anti-apoptotic effect of luteolin in LPS-treated
cardiomyocytes, while AICAR appreciably emulated the effect of
luteolin (Fig. 6A and B).
Luteolin stabilizes the mitochondrial
transmembrane potential in LPS-treated cardiomyocytes
Stability of the mitochondrial membrane potential is
helpful for maintaining the normal physiological function of
cardiomyocytes. LPS harms mitochondria and results in the loss of
mitochondrial membrane potential. JC-1 was employed to measure the
changes in the mitochondrial transmembrane potential (Fig. 6C). A decrease in the mitochondrial
membrane potential was noted in LPS-treated cardiomyocytes compared
with that in control cardiomyocytes. Luteolin treatment enhanced
the mitochondrial membrane potential in LPS-induced cardiomyocytes.
Notably, this effect of luteolin was abolished by 3-MA and compound
C but was mimicked by AICAR (Fig. 6C
and D).
Discussion
The major finding in the present study is that
luteolin treatment ameliorates LPS-induced myocardial injury by
increasing autophagy via AMPK signaling. The protective effects of
luteolin against SIC were associated with restored cardiac
function, reduced inflammation, ameliorated mitochondrial injury,
inhibited cardiac apoptosis and enhanced cardiac autophagy in
septic mice, and AMPK signaling was strongly involved in these
protective effects. In the present study, the cardioprotective
effect of luteolin against SIC was underscored by reduced levels of
the inflammatory cytokines IL-1β, IL-6 and TNF-α, as well as the
decrease in cardiac apoptosis in both in vitro and in
vivo models. Moreover, luteolin increased autophagic activity
in the hearts of septic mice. However, this action of luteolin was
abolished by 3-MA in cardiomyocytes treated with LPS, suggesting
that autophagy may be essential for luteolin to maintain cardiac
structure and function in response to sepsis. In addition, luteolin
treatment enhanced the phosphorylation of AMPK and ULK1 in
LPS-treated cardiomyocytes, while inhibition of AMPK by compound C
abolished the protective effect of luteolin and significantly
decreased autophagic activity in cardiomyocytes treated with LPS,
indicating the association between the protective effects of
luteolin, autophagic activity, and AMPK signaling activation.
Increasing evidence has demonstrated that luteolin
has a certain therapeutic effect on cardiovascular disease,
including preventing ischemia/reperfusion injury in adult rats
(14) and sodium fluoride-induced
hypertension in rats (28),
ameliorating inorganic mercury-induced cardiac injury in rats
(29), decreasing
high-carbohydrate/high-fat diet-induced myocardial inflammation
(15), and improving angiotensin
II-induced cardiac remodeling by decreasing oxidative stress
(16). SIC, first described by
Parker et al (30) in
1984, is a complication of severe sepsis and septic shock
characterized by an invertible myocardial depression. LV dilatation
and decreased ejection fraction as measured by echocardiography are
the major hemodynamic characteristics of SIC (31). The possible beneficial effects of
luteolin on SIC have not yet been fully demonstrated. In the
present study, treatment with luteolin increased the LVEF, LVFS and
±LV dp/dt values and decreased the LVEDD and LVESD values in mice
with sepsis. The present study to the best of our knowledge, is the
first to report that cardiac function was improved by luteolin
administration in septic mice.
The inflammatory response is the initial process in
and the hallmark of SIC development (7). In response to infection,
pro-inflammatory and anti-inflammatory mediators are released
(32). Excessive levels of
pro-inflammatory mediators result in the inflammatory response that
characterizes sepsis, while the compensatory anti-inflammatory
reaction fails to suppress the immune response (32). TNF-α, IL-1 and IL-6 are the
main inflammatory mediators that lead to myocardial depression in
sepsis (33). Antonucci et
al (34) argued that the
interaction between TNF-α/IL-1 and inducible nitric oxide synthase
exerts a negative inotropic effect on the septic myocardium. Pathan
et al (35) showed that
IL-6 contributed to myocardial depression manifested as induced
cardiac contractile dysfunction and inotrope insensitivity due to
the dysregulation of p38 mitogen-activated protein kinase
signaling. Vincent et al (36) reported that treatment with
anti-TNF antibodies improved LV function in patients with septic
shock. In the present study, the levels of the inflammatory factors
IL-1β, IL-6 and TNF-α were increased in the septic myocardium,
consistent with previous findings (33–36). Moreover, the current study found
that luteolin treatment attenuated inflammation in the hearts of
septic mice, suggesting that the cardioprotective effects of
luteolin are related to its anti-inflammatory activity in septic
mice.
Structural injury and functional impairment of
mitochondria play an important role in the pathogenesis of sepsis,
and the degree of mitochondrial injury is related to prognosis
(8). The decrease in the
mitochondrial membrane potential concurrent with the release of
hydrogen peroxide has been documented in experimental sepsis
(37–39). In addition, mitochondria-generated
ROS may serve as signaling molecules to communicate with other
cellular compartments (40).
Mitochondrial impairment could contribute to oxidative stress in
the septic myocardium, leading to myocardial injury (40). The present study revealed
mitochondrial ultrastructural damage in the hearts of septic mice.
Luteolin treatment ameliorated the pathological abnormalities of
mitochondria in septic mice, as evidenced by the sharply defined
cristae in the hearts of septic mice. Moreover, luteolin enhanced
the ATP content, CS activity, complex I/II/III/IV/V activities and
mCRC in LPS-treated mice and increased the mitochondrial membrane
potential in LPS-treated cardiomyocytes. Simultaneously, luteolin
administration inhibited the increases in ROS and MDA levels in
septic heart tissue. These results indicate that the effects of
luteolin against SIC may be associated with antioxidant
activity.
Apoptosis has been extensively implicated as a
determining process in sepsis-induced myocardial depression
(41). Cellular damage, such as
cardiomyocyte apoptosis and upregulated caspase activity, was
observed in the septic myocardium, while caspase inhibition
ameliorated cardiac function and apoptosis in the hearts of septic
rats (41). In the present study,
luteolin treatment decreased the percentage of TUNEL-positive
cardiomyocytes both in the hearts of septic mice and in LPS-treated
cells. In addition, the expression of caspase enzymes was measured
to demonstrate the molecular basis of the effects of luteolin.
Luteolin treatment attenuated the expression of cleaved caspase-3
and cleaved caspase-9 in the myocardium of septic mice. These data
indicate that caspase inhibition may be involved in the
anti-apoptotic effect of luteolin in the hearts of septic mice.
Autophagy is an important self-protective mechanism,
promoting cellular survival by controlling the degradation of
proteins and organelles, including the formation of
double-membraned autophagosomes and proteolytic degradation after
delivery to lysosomes (21,42). This process is the primary
mechanism for mitochondrial quality control, preventing apoptosis
and promoting antigen presentation (9), and dysregulation has been shown to
have significant harmful effects on the heart (42). Activation of autophagy has been
observed in a variety of heart diseases, including cardiac
hypertrophy and ischemia/reperfusion injury, suggesting that
autophagy may play an important role in myocardial dysfunction
(43). Previous in vitro
and in vivo models of sepsis showed that autophagy was
initially activated in sepsis, followed by a subsequent phase of
impairment (9,44). Incomplete activation of autophagy
may contribute to cardiac dysfunction during sepsis (44). In the present study, autophagic
activity was upregulated in the hearts of septic mice and in
LPS-treated cardiomyocytes, as revealed by the increased expression
of LC3II and Beclin1, decreased expression of p62, and accumulation
of autophagosomes, consistent with previous studies (9,44).
Moreover, autophagy modulation appears to be protective against
myocardial injury in murine sepsis models (9,44).
Multiple drugs and bioactive molecules exerting cardioprotective
effects in sepsis are correlated with the regulation of autophagy
(44). Rapamycin, an autophagy
inducer, exerts a cardioprotective effect in sepsis through the
complete induction of autophagy (44). P27, a canonical tumor suppressor,
protects cardiomyocytes from sepsis-induced cardiac depression via
the activation of autophagy and inhibition of apoptosis (45). In addition, luteolin regulates
autophagy in numerous other circumstances, including attenuation of
post-infarction cardiac dysfunction (25) and decreasing foam cell formation
and apoptosis in ox-LDL-stimulated macrophages by enhancing
autophagy (46), and promoting
apoptosis by inducing autophagy in hepatocellular carcinoma
(47). In the current study,
luteolin administration enhanced autophagy, as demonstrated by the
further increase in the expression of LC3II and Beclin1 and the
further decrease in the expression of p62 in vivo and in
vitro, as well as by the increased number of autophagosomes in
LPS-treated cardiomyocytes. It is worth noting that treatment with
the autophagy inhibitor 3-MA reversed the luteolin-induced
upregulation of autophagy, accompanied by decreased mitochondrial
membrane potential and increased cardiomyocyte apoptosis,
indicating that luteolin treatment may exert protective effects in
sepsis by increasing autophagy.
AMPK is a central metabolic sensor in all eukaryotes
that monitors glucose and lipid metabolism in response to changes
in nutrient and intracellular energy levels (48,49). AMPK, together with ULK1, plays a
vital role in promoting autophagy under nutrient or energy stress
conditions, including sepsis (50,51). AMPK directly activates ULK1
through the phosphorylation of Ser 317 and Ser 777 to facilitate
autophagy (22). In addition,
luteolin regulates AMPK signaling in numerous other circumstances,
including enhancing the survival of human umbilical vein
endothelial cells against oxidative stress by modulating
AMPK/protein kinase C pathway (52), improving atherosclerosis in mice
via regulating AMPK/sirtuin1 signaling in macrophages (53) and reducing obesity-associated
insulin resistance in mice by activating AMPK signaling in adipose
tissue macrophages (54). In the
present study, notably, the protective effect of luteolin was also
abolished by the AMPK inhibitor compound C, as demonstrated by the
decreased LC3 II and Beclin1 expression, enhanced p62 expression,
destabilized mitochondrial membrane potential, and increased
cardiomyocyte apoptosis. However, this effect was correspondingly
mimicked by the AMPK activator AICAR in LPS-treated cardiomyocytes,
suggesting that luteolin attenuates LPS-induced myocardial injury
by increasing autophagy through AMPK/ULK1 signaling. The present
study shows that AMPK/ULK1 signaling is involved in the role of
controlling autophagy blunted in LPS-treated cardiomyocytes.
Pharmacological activation of AMPK signaling improves cardiac
function in mice with LPS-induced sepsis. To modulate autophagy,
AMPK signaling might be a potential novel therapeutic target for
septic myocardial dysfunction. In the present study, AICAR
appreciably emulated the effect of luteolin in LPS-treated
cardiomyocytes, also suggesting that luteolin can be used as an
AMPK inducer to attenuate myocardial injury in mice with
LPS-induced sepsis.
However, there are still some limitations in this
study. Firstly, no control + luteolin group was set in this study.
Although it has been reported that luteolin has no myocardial
toxicity in normal mice (14,25), the lack of control + luteolin
group makes the study design incomplete. Secondly, primary neonatal
mouse cardiomyocytes were used in the in vitro experiment.
Some primary neonatal mouse cardiomyocytes have differentiation and
proliferation function, while the adult mouse cardiomyocytes belong
to terminal differentiation cells and have no differentiation and
proliferation function. The primary neonatal mouse cardiomyocytes
can’t fully mimic the adult cardiomyocytes. These problems should
be considered and solved in future experiments.
In conclusion, the present study confirms that
luteolin is a potential therapeutic agent for the treatment of SIC.
The protective effects of luteolin against SIC may be associated
with its pharmacological activities, including its antioxidant and
anti-inflammatory activities. The cardioprotective effects of
luteolin against SIC may also be attributed to decreasing apoptosis
and enhancing autophagy through the activation of AMPK signaling.
These findings may provide a promising and innovative therapeutic
strategy for SIC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81200189), the
Shaanxi Social Development and Scientific Program Tackling Program
(grant no. 2016SF021) and the New Technology Foundation of Xijing
Hospital (grant no. 2016-15).
Availability of data and materials
The data generated or analyzed during this study are
included in this published article.
Authors’ contributions
BW and MY made substantial contributions to the
conception and design of the experiments. BW, HS, MF, FY, LZ, JL,
JL, LW and CL conducted the experiments. BW, HS, and MF analyzed
the experimental data and wrote the manuscript. MY edited and
revised the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All experimental procedures were in accordance with
the National Institutes of Health Guidelines on the Care and Use of
Laboratory Animal and were approved by the Fourth Military Medical
University Ethics Committee on Animal Care.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Singer M, Deutschman CS, Seymour CW,
Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche
JD, Coopersmith CM, et al: The third international consensus
definitions for sepsis and septic shock (Sepsis-3). JAMA.
315:801–810. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mayr FB, Yende S and Angus DC:
Epidemiology of severe sepsis. Virulence. 5:4–11. 2014. View Article : Google Scholar :
|
|
3
|
Plevin R and Callcut R: Update in sepsis
guidelines: What is really new? Trauma Surg Acute Care Open.
2:e0000882017. View Article : Google Scholar
|
|
4
|
Cheng B, Hoeft AH, Book M, Shu Q and
Pastores SM: Sepsis: Pathogenesis, biomarkers, and treatment.
Biomed Res Int. 2015:846935. 2015. View Article : Google Scholar
|
|
5
|
Maloney PJ: Sepsis and septic shock. Emerg
Med Clin North Am. 31:583–600. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Adhikari NK, Fowler RA, Bhagwanjee S and
Rubenfeld GD: Critical care and the global burden of critical
illness in adults. Lancet. 376:1339–1346. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kakihana Y, Ito T, Nakahara M, Yamaguchi K
and Yasuda T: Sepsis-induced myocardial dysfunction:
Pathophysiology and management. J Intensive Care. 4:222016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu YC, Yu MM, Shou ST and Chai YF:
Sepsis-induced cardiomyopathy: mechanisms and treatments. Front
Immunol. 8:10212017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ho J, Yu J, Wong SH, Zhang L, Liu X, Wong
WT, Leung CC, Choi G, Wang MH, Gin T, et al: Autophagy in sepsis:
Degradation into exhaustion. Autophagy. 12:1073–1082. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Luo Y, Shang P and Li D: Luteolin: A
flavonoid that has multiple cardio-protective effects and its
molecular mechanisms. Front Pharmacol. 8:6922017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lopez-Lazaro M: Distribution and
biological activities of the flavonoid luteolin. Mini Rev Med Chem.
9:31–59. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Seelinger G, Merfort I and Schempp CM:
Anti-oxidant, anti-inflammatory and anti-allergic activities of
luteolin. Planta Med. 74:1667–1677. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu T, Li D and Jiang D: Targeting cell
signaling and apoptotic pathways by luteolin: Cardioprotective role
in rat cardiomyocytes following ischemia/reperfusion. Nutrients.
4:2008–2019. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qi L, Pan H, Li D, Fang F, Chen D and Sun
H: Luteolin improves contractile function and attenuates apoptosis
following ischemia-reperfusion in adult rat cardiomyocytes. Eur J
Pharmacol. 668:201–207. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Abu-Elsaad N and El-Karef A: The falconoid
luteolin mitigates the myocardial inflammatory response induced by
high-carbohydrate/ high-fat diet in wistar rats. Inflammation.
41:221–231. 2018. View Article : Google Scholar
|
|
16
|
Nakayama A, Morita H, Nakao T, Yamaguchi
T, Sumida T, Ikeda Y, Kumagai H, Motozawa Y, Takahashi T, Imaizumi
A, et al: A food-derived flavonoid luteolin protects against
angiotensin II-induced cardiac remodeling. PLoS One.
10:e01371062015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Parzych KR and Klionsky DJ: An overview of
autophagy: Morphology, mechanism, and regulation. Antioxid Redox
Signal. 20:460–473. 2014. View Article : Google Scholar :
|
|
18
|
Mialet-Perez J and Vindis C: Autophagy in
health and disease: Focus on the cardiovascular system. Essays
Biochem. 61:721–732. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Morel E, Mehrpour M, Botti J, Dupont N,
Hamai A, Nascimbeni AC and Codogno P: Autophagy: A druggable
process. Annu Rev Pharmacol Toxicol. 57:375–398. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Takahashi W, Watanabe E, Fujimura L,
Watanabe-Takano H, Yoshidome H, Swanson PE, Tokuhisa T, Oda S and
Hatano M: Kinetics and protective role of autophagy in a mouse
cecal ligation and puncture-induced sepsis. Crit Care. 17:R1602013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ren C, Zhang H, Wu TT and Yao YM:
Autophagy: A potential therapeutic target for reversing
sepsis-induced immunosuppression. Front Immunol. 8:18322017.
View Article : Google Scholar
|
|
22
|
Kim J, Kundu M, Viollet B and Guan KL:
AMPK and mTOR regulate autophagy through direct phosphorylation of
Ulk1. Nat Cell Biol. 13:132–141. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Escobar DA, Botero-Quintero AM, Kautza BC,
Luciano J, Loughran P, Darwiche S, Rosengart MR, Zuckerbraun BS and
Gomez H: Adenosine monophosphate-activated protein kinase
activation protects against sepsis-induced organ injury and
inflammation. J Surg Res. 194:262–272. 2015. View Article : Google Scholar
|
|
24
|
Li P, Chen XR, Xu F, Liu C, Li C, Liu H,
Wang H, Sun W, Sheng YH and Kong XQ: Alamandine attenuates
sepsis-associated cardiac dysfunction via inhibiting MAPKs
signaling pathways. Life Sci. 206:106–116. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hu J, Man W, Shen M, Zhang M, Lin J, Wang
T, Duan Y, Li C, Zhang R, Gao E, et al: Luteolin alleviates
post-infarction cardiac dysfunction by up-regulating autophagy
through Mst1 inhibition. J Cell Mol Med. 20:147–156. 2016.
View Article : Google Scholar
|
|
26
|
Wu B, Lin J, Luo J, Han D, Fan M, Guo T,
Tao L, Yuan M and Yi F: Dihydromyricetin protects against diabetic
cardiomyopathy in streptozotocin-induced diabetic mice. Biomed Res
Int. 2017:3764370. 2017.
|
|
27
|
Zhang M, Wang C, Hu J, Lin J, Zhao Z, Shen
M, Gao H, Li N, Liu M, Zheng P, et al: Notch3/Akt signaling
contributes to OSM-induced protection against cardiac
ischemia/reperfusion injury. Apoptosis. 20:1150–1163. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Oyagbemi AA, Omobowale TO, Ola-Davies OE,
Asenuga ER, Ajibade TO, Adejumobi OA, Afolabi JM, Ogunpolu BS,
Falayi OO, Saba AB, et al: Luteolin-mediated Kim-1/NF-kB/Nrf2
signaling pathways protects sodium fluoride-induced hypertension
and cardiovascular complications. Biofactors. 44:518–531. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Baiyun R, Li S, Liu B, Lu J, Lv Y, Xu J,
Wu J, Li J, Lv Z and Zhang Z: Luteolin-mediated PI3K/AKT/Nrf2
signaling pathway ameliorates inorganic mercury-induced cardiac
injury. Ecotoxicol Environ Saf. 161:655–661. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Parker MM, Shelhamer JH, Bacharach SL,
Green MV, Natanson C, Frederick TM, Damske BA and Parrillo JE:
Profound but reversible myocardial depression in patients with
septic shock. Ann Intern Med. 100:483–490. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sato R and Nasu M: A review of
sepsis-induced cardiomyopathy. J Intensive Care. 3:482015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sagy M, Al-Qaqaa Y and Kim P: Definitions
and pathophysiology of sepsis. Curr Probl Pediatr Adolesc Health
Care. 43:260–263. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chousterman BG, Swirski FK and Weber GF:
Cytokine storm and sepsis disease pathogenesis. Semin Immunopathol.
39:517–528. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Antonucci E, Fiaccadori E, Donadello K,
Taccone FS, Franchi F and Scolletta S: Myocardial depression in
sepsis: From pathogenesis to clinical manifestations and treatment.
J Crit Care. 29:500–511. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pathan N, Franklin JL, Eleftherohorinou H,
Wright VJ, Hemingway CA, Waddell SJ, Griffiths M, Dennis JL, Relman
DA, Harding SE and Levin M: Myocardial depressant effects of
interleukin 6 in meningococcal sepsis are regulated by p38
mitogen-activated protein kinase. Crit Care Med. 39:1692–1711.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vincent JL, Bakker J, Marecaux G,
Schandene L, Kahn RJ and Dupont E: Administration of anti-TNF
antibody improves left ventricular function in septic shock
patients. Results of a pilot study. Chest. 101:810–815. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Brealey D and Singer M: Mitochondrial
dysfunction in sepsis. Curr Infect Dis Rep. 5:365–371. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Unuma K, Aki T, Funakoshi T, Hashimoto K
and Uemura K: Extrusion of mitochondrial contents from
lipopolysaccharide-stimulated cells: Involvement of autophagy.
Autophagy. 11:1520–1536. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Duran-Bedolla J, Montes de Oca-Sandoval
MA, Saldana-Navor V, Villalobos-Silva JA, Rodriguez MC and
Rivas-Arancibia S: Sepsis, mitochondrial failure and multiple organ
dysfunction. Clin Invest Med Med. 37:E58–E69. 2014. View Article : Google Scholar
|
|
40
|
Garrabou G, Moren C, Lopez S, Tobias E,
Cardellach F, Miro O and Casademont J: The effects of sepsis on
mitochondria. J Infect Dis. 205:392–400. 2012. View Article : Google Scholar
|
|
41
|
Neviere R, Fauvel H, Chopin C, Formstecher
P and Marchetti P: Caspase inhibition prevents cardiac dysfunction
and heart apoptosis in a rat model of sepsis. Am J Respir Crit Care
Med. 163:218–225. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Terman A and Brunk UT: Autophagy in
cardiac myocyte homeostasis, aging, and pathology. Cardiovasc Res.
68:355–365. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gustafsson AB and Gottlieb RA: Eat your
heart out: Role of autophagy in myocardial ischemia/reperfusion.
Autophagy. 4:416–421. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hsieh CH, Pai PY, Hsueh HW, Yuan SS and
Hsieh YC: Complete induction of autophagy is essential for
cardioprotection in sepsis. Ann Surg. 253:1190–1200. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhao X, Qi H, Zhou J, Xu S and Gao Y: P27
protects cardiomyocytes from sepsis via activation of autophagy and
inhibition of apoptosis. Med Sci Monit. 24:8565–8576. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang BC, Zhang CW, Wang C, Pan DF, Xu TD
and Li DY: Luteolin attenuates foam cell formation and apoptosis in
Ox-LDL-stimulated macrophages by enhancing autophagy. Cell Physiol
Biochem. 39:2065–2076. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Cao Z, Zhang H, Cai X, Fang W, Chai D, Wen
Y, Chen H, Chu F and Zhang Y: Luteolin promotes cell apoptosis by
inducing autophagy in hepatocellular carcinoma. Cell Physiol
Biochem. 43:1803–1812. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Carling D: AMPK signalling in health and
disease. Curr Opin Cell Biol. 45:31–37. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Garcia D and Shaw RJ: AMPK: Mechanisms of
cellular energy sensing and restoration of metabolic balance. Mol
Cell. 66:789–800. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Khan SH and Kumar R: Role of an
intrinsically disordered conformation in AMPK-mediated
phosphorylation of ULK1 and regulation of autophagy. Mol Biosyst.
8:91–96. 2012. View Article : Google Scholar
|
|
51
|
Kurumbail RG and Calabrese MF: Structure
and regulation of AMPK. Exp Suppl. 107:3–22. 2016.PubMed/NCBI
|
|
52
|
Ou HC, Pandey S, Hung MY, Huang SH, Hsu
PT, Day CH, Pai P, Viswanadha VP, Kuo WW and Huang CY: Luteolin: A
natural flavonoid enhances the survival of HUVECs against oxidative
stress by modulating AMPK/PKC pathway. Am J Chin Med. 47:541–557.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Li J, Dong JZ, Ren YL, Zhu JJ, Cao JN,
Zhang J and Pan LL: Luteolin decreases atherosclerosis in LDL
receptor-deficient mice via a mechanism including decreasing
AMPK-SIRT1 signaling in macrophages. Exp Ther Med. 16:2593–2599.
2018.PubMed/NCBI
|
|
54
|
Zhang L, Han YJ, Zhang X, Wang X, Bao B,
Qu W and Liu J: Luteolin reduces obesity-associated insulin
resistance in mice by activating AMPKα1 signalling in adipose
tissue macrophages. Diabetologia. 59:2219–2228. 2016. View Article : Google Scholar : PubMed/NCBI
|