Introduction
Asthma is a chronic condition that causes
intermittent inflammation and narrowing of the airways in the
lungs. One of the major risk factor of asthma development is
exposure to environmental allergens (1). In addition, house dust mites (HDM)
can induce DNA damage and cause asthma (2). Oxidative stress can also cause DNA
damage, playing a pivotal role in the development of human
immunological diseases (3,4).
Asthma is a chronic inflammatory airway disease and oxidative
stress may also be involved in its pathogenesis (5). To date, several reports demonstrated
that repair of DNA damage is controlled by epigenetics and plays an
important role in the process of asthma (6,7).
It was previously demonstrated that DNA damage and apoptosis of
airway epithelial cells may be caused by cigarette smoke extract
(8,9).
Resveratrol (RES; trans-3,5,4′-trihydroxystilbene)
is produced by several plants and may be found in red grape skins,
red wine and peanuts (10). It
was previously reported that cardiovascular protection,
anti-inflammatory and anti-aging activities may be regulated by
antioxidant status, and anti-apoptotic activity may affect several
biological processes (11-15).
Additionally, RES can regulate lipid metabolism and affect cytokine
expression in the immune system (16-18). Studies indicated that a diet
supplemented with RES could improve health and survival in mice
with metabolic syndrome (19,20). Previous epidemiological studies
demonstrated that elderly individuals on a Mediterranean diet,
which is rich in RES, display a markedly reduced risk of
cardiovascular disease (11,12,21). Chen et al (22) reported that RES can inhibit DNA
damage in cultured human mammary epithelial cells. Previous
evidence indicated that RES exerted anti-inflammatory and
anti-asthmatic effects on a mouse model of allergic asthma
(23-26). Rhee and Lee demonstrated that RES
exerts inhibitory effects on airway remodeling through the
transforming growth factor-β/mothers against decapentaplegic
homolog signaling pathway in chronic asthma models (27). However, the underlying mechanism
and the protective role of RES against cell apoptosis in bronchial
epithelial cells remain elusive.
The aim of the present study was to investigate the
possible mechanism underlying HDM-induced airway epithelial injury
and the protective role of RES against cell apoptosis in the
bronchial epithelial cells, in order to determine whether RES can
prevent HDM-induced DNA damage and cell apoptosis and whether it
represents a novel approach to asthma treatment.
Materials and methods
Asthma mouse model
A total of 24 C57BL/6J female mice (Beijing Hfk
Bioscience Co., Ltd.), aged 6-8 weeks and weighing 22-26 g, were
used in this study. All mice were maintained in a specific
pathogen-free facility in the Animal Experimental Center of
Southwest Medical University. All animals had ad libitum
access to food and water and were maintained in a stable
environment at 25±1°C, 60±5% humidity and a 12-h light/dark cycle.
The mice were intraperitoneally sensitized on days 1 and 8 with 20
µg HDM (cat. no. 326779, Greer Laboratories, Inc.) and 1 mg
aluminum hydroxide. One week after the final injection, the mice
were treated with HDM intranasal (i.n.), alone or combined with RES
(100 mg/kg, intragastric; cat. no. R5010, Sigma-Aldrich; Merck
KGaA) daily for 7 days. After 7 days of treatment, the mice were
sacrificed by intraperitoneal injection of sodium pentobarbital
(100 mg/kg of body weight). The mice were confirmed dead by no
spontaneous breathing for 2-3 min and no blink reflex. The lung
tissues and bronchoalveolar lavage fluid (BALF) were collected for
further study. All animal experiments (including euthanasia) were
in compliance with the regulations and guidelines of the Southwest
Medical University Institutional Animal Care Committee (Approval
no. 20160041) and were conducted according to the AAALAC and IACUC
guidelines.
Measurement of airway
hyperresponsiveness
A total of 24 h after the final challenge, whole
body plethysmography (Buxco Europe Ltd.) was used to assess total
respiratory system resistance after administration of increasing
doses of methacholine (0, 6.25, 12.5, 25 and 50 mg/ml). Data are
reported as peak Penh values.
Histological analysis
Lung tissues were fixed in 10% neutral-buffered
formalin for 24 h at room temperature and then embedded in
paraffin. The tissues were then cut in 5-µm sections and
subjected to standard hematoxylin-eosin staining (28).
TUNEL assay
Lung tissues were harvested and fixed with 10%
formalin for 24 h at room temperature, and then embedded in
paraffin and cut into 5-µm sections. The tissue sections
were deparaffinized and rehydrated, and then treated with citric
acid for antigen retrieval for 10 min at room temperature. The
TUNEL Assay Apoptosis Detection kit (cat. no. C1088; Beyotime
Institute of Biotechnology) was used to detect DNA fragmentation.
In brief, the tissue sections were treated with formaldehyde on ice
for 15 min. Subsequently, the tissue sections were washed with
phosphate-buffered saline (PBS) and 70% ice-cold ethanol was added
followed by incubation for 30 min. The tissue sections were again
washed with PBS three times for 5 min each time, staining solution
was added and the sections were incubated at 37°C for 60 min. The
tissue sections were then washed and treated with ribonuclease A
(RNase A) for 30 min at room temperature, then visualized under a
fluorescence microscope (SP5 Leica confocal microscope; Leica
Microsystems GmbH). The number of TUNEL-positive cells were counted
in five different fields for each stained section.
Cell culture and treatment
The bronchial epithelial cells (16HBE) were obtained
from Cell Bank of the Chinese Academy of Sciences, and were
cultured in DMEM (cat. no. SH30022.01; GE Healthcare Life Sciences)
supplemented with 10% fetal bovine serum (cat. no. 35-076-CV;
Corning Inc.) in a humidified atmosphere containing 5%
CO2 at 37°C. Cells were seeded at 0.5 million cells per
well in 6-well plates. At 24 h after seeding, cells were treated
with 10 µM RES, 10 mM N-acetyl-L-cysteine (NAC; cat. no.
A9165, Sigma-Aldrich; Merck KGaA), or 2.5 µM NU7441 (cat.
no. S2638; Selleck Chemicals) and then (2 h later) with HDM (200
µg/ml) and incubated for an additional 12 h. Control cells
were incubated with an equal amount of DMSO.
Single-cell gel electrophoresis
assay
A single-cell gel electrophoresis assay or Comet
assay was used to detect DNA damage. Bronchial epithelial cells
(3×105 cells) were cultured in 6-well plates and exposed
to different treatment conditions. Cells were cultured with HDM,
with or without the presence of RES or NAC and DMSO was used for
the control group. After 12 h of treatment, the Comet assay was
performed using CometChip Reagent kit (Trevigen, Inc.) according to
the manufacturer's protocol.
Paraffin-embedded tissue
immunohistochemistry staining
For immunofluorescence (IF) analysis, the sections
of lung tissues were fixed with 4% formaldehyde and permeabilized
with 0.3% Triton X-100 in PBS for 10 min at room temperature.
Subsequently, the slides were incubated with 2% serum-blocking
buffer for 20 min at room temperature and then incubated with
specific antibodies. The primary antibodies against γH2AX (1:200;
cat. no. 05-636, EMD Millipore) and 8-OHdG (1:200; cat. no.
ab48508, Abcam) were added and incubated at room temperature for 2
h, followed by incubation with the Alexa Fluor 555 conjugated
secondary antibody (1:500; cat. no. A32727; Invitrogen; Thermo
Fisher Scientific, Inc.) for 1 h. The nuclei were stained with DAPI
(cat. no. C1005; Beyotime Institute of Biotechnology) at room
temperature for 5 min. Differences in immunostaining were detected
by using SP5 Leica confocal microscope with Leica Application Suite
Software (version 14.0.0.162, Leica Microsystems GmbH).
IF staining
The bronchial epithelial cells (1×105
cells) were cultured in the plate on glass slides and cells were
subjected to different treatments as follows: i) Control group; ii)
HDM group; and iii) HDM combined with RES group. Following
treatment, cells were fixed with ice-cold methanol for 10 min at
room temperature and incubated with primary antibody targeting
γH2AX (1:200; cat. no. 05-636; EMD Millipore) and 8-OHdG (1:200;
cat. no. ab48508; Abcam) at room temperature for 2 h, followed by
Alexa Fluor 555 conjugated secondary antibody (1:500; cat. no.
A32727; Invitrogen; Thermo Fisher Scientific, Inc.) at room
temperature for 1 h. The nuclei were stained with DAPI at room
temperature for 5 min. Following staining, the differences were
observed by using SP5 Leica confocal microscope with Leica
Application Suite Software (version no. 14.0.0.162; Leica
Microsystems GmbH).
Western blotting
Western blot analysis was performed as previously
described (28). The tissue
samples or the cells from various treatment groups were lysed with
ice-cold cell lysis buffer plus protease inhibitor, obtained from
Thermo Fisher Scientific, Inc. The samples were boiled 10 min
before loading on SDS-PAGE gel. The same amount (20 µg) of
protein from each group was loaded and separated by 12% SDS/PAGE
electrophoresis and then transferred to a polyvinylidene fluoride
membrane. The membranes were blocked by using 5% skim milk for 60
min and probed at 4°C with specific antibodies overnight, including
antibodies against γH2AX (1:1,000; cat. no. 05-636; EMD Millipore),
cleaved caspase-3 (1:1,000; cat. no. 9664; Cell Signaling
Technology, Inc.) and GAPDH (1:1,000; cat. no. AF0006; Beyotime
Institute of Biotechnology). Immunoreactive protein bands were
visualized using horseradish peroxidase-conjugated secondary
antibodies (anti-mouse IgG, HRP-linked antibody, 1:1,000; cat. no.
7076; anti-rabbit IgG, HRP-linked Antibody, 1:1,000; cat. no. 7074;
all from Cell Signaling Technology., Inc.) and a Clarity Western
ECL Substrate (cat. no. 170-5061; Bio-Rad Laboratories, Inc.). The
protein bands were analyzed using FluorChem 8900
(ProteinSimple).
Flow cytometry
As previously described, bronchial epithelial cells
were cultured in 6-well plates, HDM was used to induce cell
apoptosis and HDM combined with RES, NAC or NU7441 were used to
determine the effects of RES, NAC and NU7441 on cell apoptosis.
After 12 h of treatment, each group of cells was gently trypsinized
and collected (1×105 cells were collected in the tube).
Each group of cells was suspended in 100 µl binding buffer.
Subsequently, 5 µl Annexin V-FITC and 5 µl propidium
iodide (cat. no. FXP018; Beijing 4A Biotech Co., Ltd) were added
and the cells were incubated at room temperature for 15 min in the
dark. After incubation, 400 µl of binding buffer was added
to each tube and the percentage of apoptotic cells was analyzed by
afluorescence-activated cell sorting instrument (NovoCyte; ACEA
Biosciences, Inc.) with NovoExpressTM software (version
no. 1.0.0; ACEA Biosciences, Inc.).
Reactive oxygen species (ROS)
measurement
ROS generation in 16HBE cells was measured using the
oxidant-sensitive fluorometric probe DCFH-DA (cat. no. S0033;
Beyotime Institute of Biotechnology) according to the
manufacturer's protocol. In brief, the cells were cultured in the
plate on glass slides and subjected to different treatments as
follows: i) Control group; ii), HDM group; iii) RES group; iv) HDM
combined with RES group; v) NAC group; and vi) HDM combined with
NAC group. After treatment, cells were washed with PBS and then
incubated with 10 µM DCFH-DA in DMEM for 30 min at 37°C. The
cells were then washed with PBS and images were captured using a
fluorescence microscope (SP5 Leica confocal microscope; Leica
Microsystems GmbH).
Frozen lung tissues were incubated with 25 µM
dihydroethidium (DHE) (cat. no. BB-470515, BestBio) in PBS for 15
min at 37°C. The sections were washed with PBS for 3 min and then
imaged using a fluorescence microscope (SP5 Leica confocal
microscope; Leica Microsystems GmbH).
ELISA
ELISA assessed the BALF levels of 8-OHdG/8-oxoG in
the mice using Mouse 8-OHdG ELISA kit (cat. no. JL12294; Shanghai
Jianglai Industrial Limited by Share Ltd.) following the
manufacturer's protocol.
Bronchial epithelial cells were cultured in 6-well
plates and were subjected to different treatments. The cells were
cultured with HDM, with or without the presence of RES and DMSO was
used for the control group. After 12 h of treatment, the cell
culture supernatant was collected and the levels of 8-OHdG/8-oxoG
were determined using ELISA (cat. no. JL11850, Shanghai Jianglai
Industrial Limited by Share Ltd.) according to the manufacturer's
protocol.
Statistical analysis
All values are expressed as means ± standard
deviation. Data statistical analysis was performed by SPSS 16.0
(SPSS, Inc.). Student's t-test or one-way analysis of variance
(Tukey-Kramer test or Dunnett's T3 post hoc tests) was used to
compare data between two groups or multiple groups, respectively.
P<0.05 was considered to indicate a statistically significant
difference.
Results
RES attenuates cell apoptosis induced by
HDM
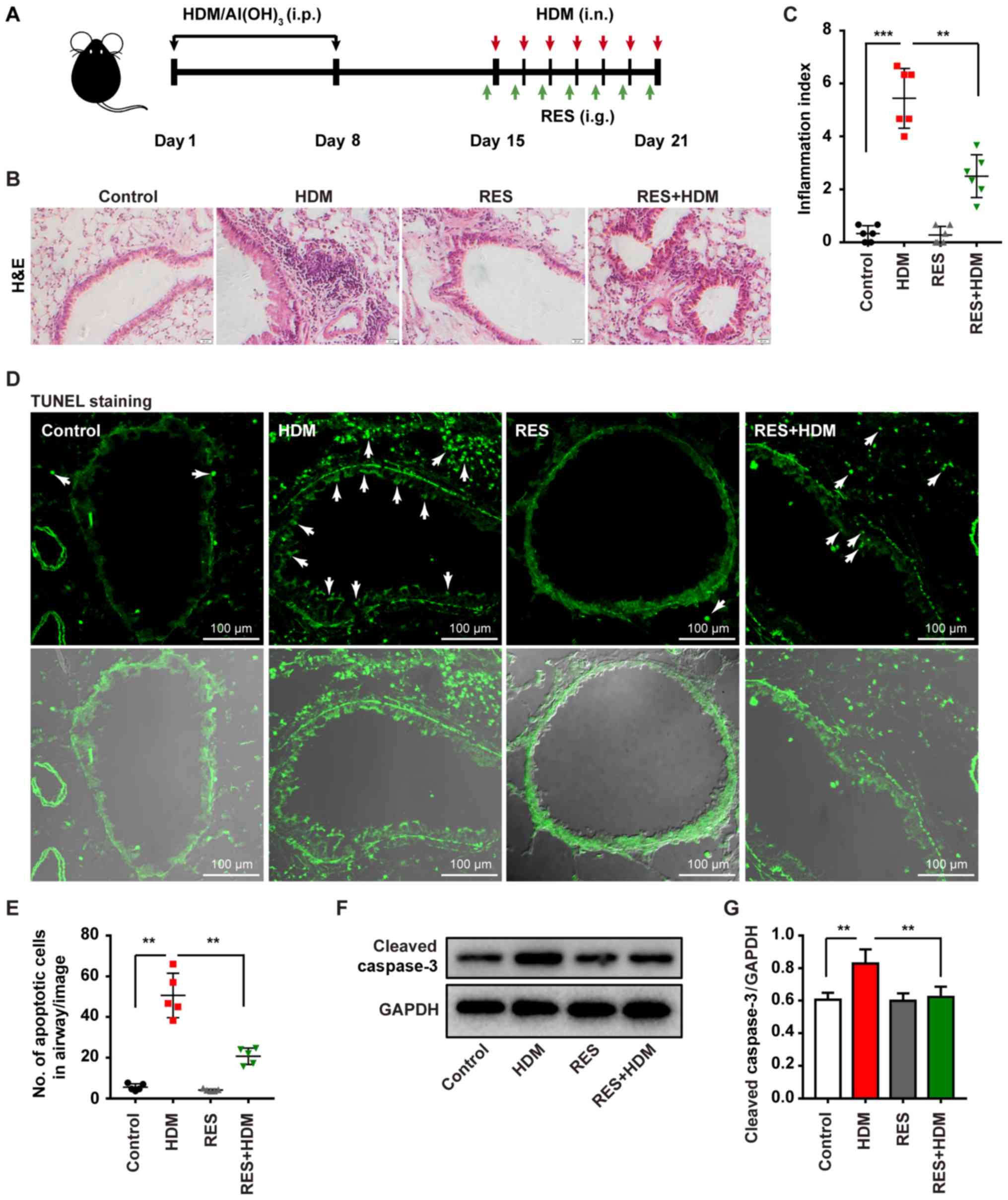
In the present study, an asthma mouse model was
established in the authors' laboratory and the mice were treated
with HDM/AI(OH)3, followed by HDM (i.n.) with or without
RES (i.p.) (Fig. 1A) to detect
the pathogenetic mechanism triggered by this exposure. Airway
hyperresponsiveness was measured using plethysmographs at 24 h
after the final challenge. The bronchial airway hyper-response was
markedly elevated in the mice that are repeatedly exposed to HDM.
However, the airway response declined in the mice exposed to HDM in
addition to RES compared with the mice exposed to HDM, when the
mice were treated with atomized methacholine (Fig. S1). Less extensive inflammatory
cell infiltration was observed around the airway in the mice
exposed to HDM combined with RES, compared with mice exposed to HDM
alone (Fig. 1B). Quantification
of airway inflammation index also proved that RES suppressed
HDM-induced inflammation (Fig.
1C). Furthermore, treatment with HDM resulted in an increase of
the apoptotic cells in airway epithelial cells of lung tissues
(Fig. 1D and E). However, mice
treated with HDM combined with RES exhibited a significantly
decreased percentage of apoptotic cells in the airway epithelial
cells of lung tissues. Western blotting revealed that HDM treatment
increased the expression level of cleaved caspase-3, whereas
treatment with RES resulted in a decrease in the level of cleaved
caspase-3 in mice with HDM-induced asthma (Fig. 1F and G). Taken together, these
findings indicate that RES attenuates cell apoptosis induced by
HDM.
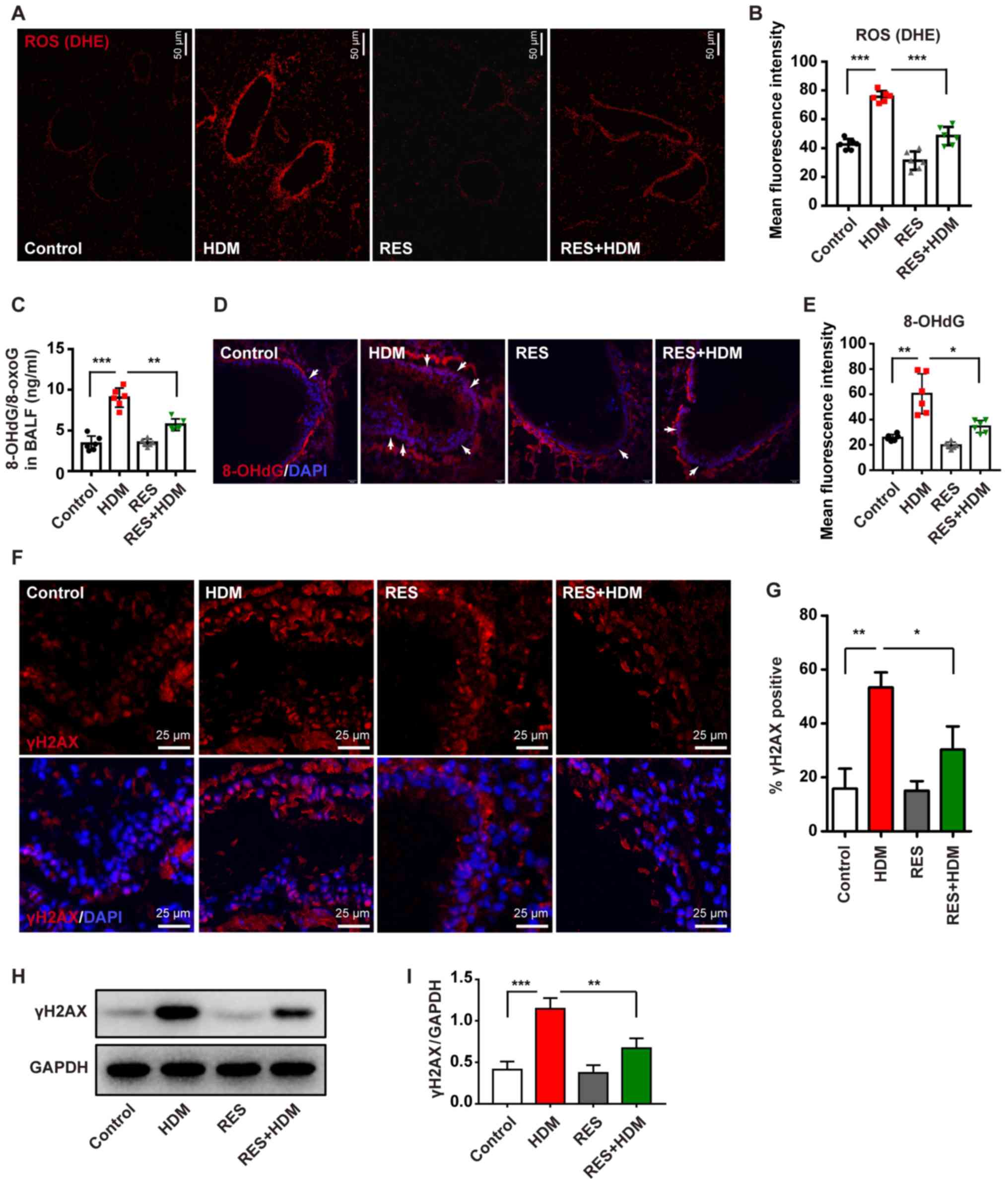
RES inhibits oxidative damage and DNA
double-strand breaks in the lungs of asthmatic mice
It was revealed that exposure to HDM caused a
significant increase in ROS levels (Fig. 2A and B) in a mouse model of asthma
and ROS is known to cause DNA damage (29,30). To quantify oxidative damage to
nucleic acids, 8-OHdG/8-oxoG levels were detected in BALF and lung
tissue. The levels of 8-OHdG were markedly increased in the BALF of
mice with HDM-induced asthma and 8-OHdG/8-oxoG could be
simultaneously inhibited via treatment with RES (Fig. 2C). IF staining also revealed that
RES attenuated 8-OHdG levels in airway epithelial cells of mice
with HDM-induced asthma (Fig. 2D and
E). To further confirm this finding, another DNA damage-related
gene, γH2AX, was analyzed. HDM increased the expression level of
γH2AX, which was detected by IF staining (Fig. 2F and G) and western blotting
(Fig. 2H and I); however, these
processes were blocked by treatment with RES, and the expression
level of γH2AX was decreased. Collectively, these findings
suggested that RES can inhibit DNA damage in a mouse model of
asthma.
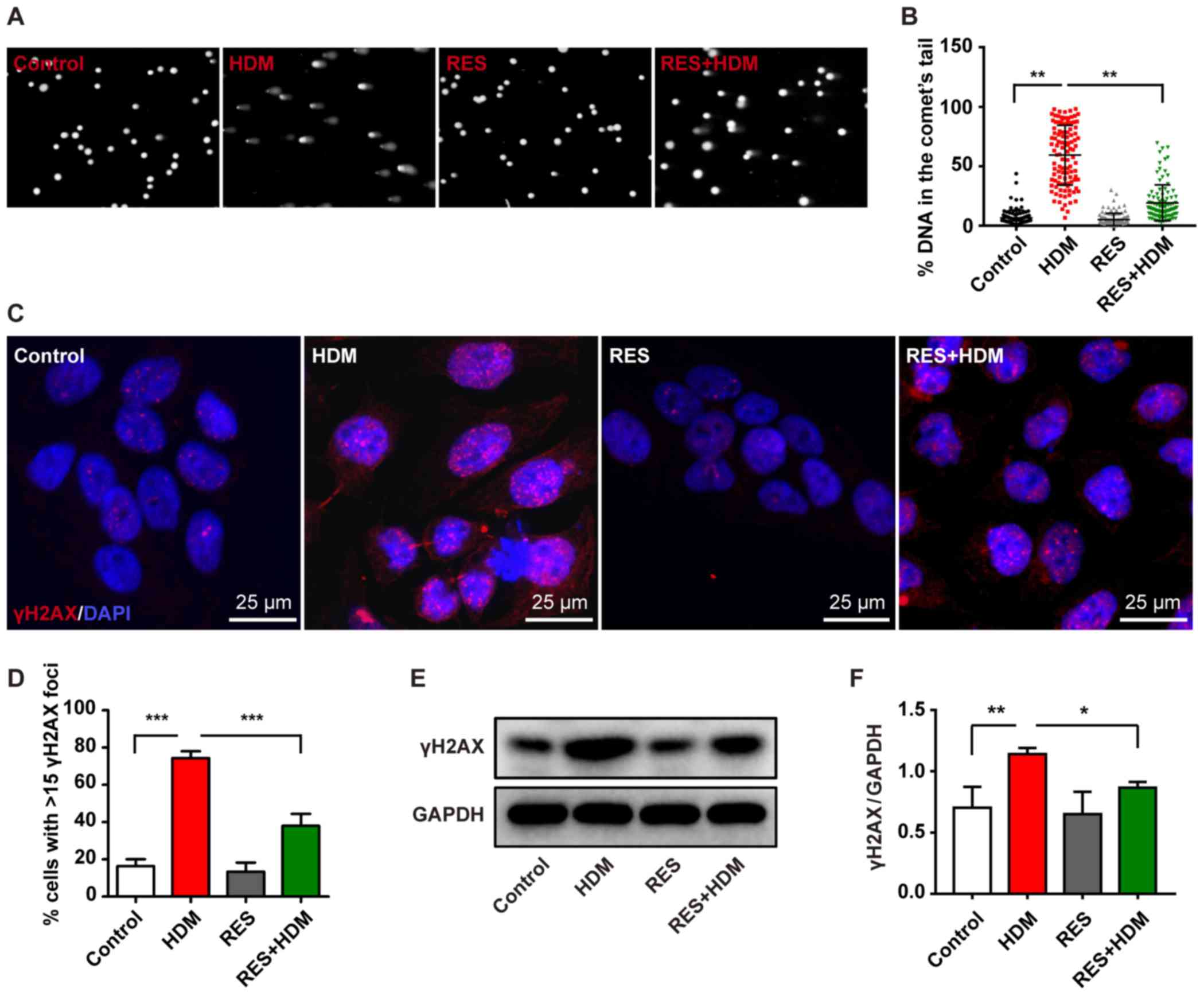
RES attenuates DNA damage in bronchial
epithelial cells
The results of the present study demonstrated that
HDM can promote cell apoptosis and the increased ROS levels can
cause DNA damage in a mouse asthma model in the airway of lung
tissues. However, treatment with RES may attenuate cell apoptosis
promoted by HDM and inhibit DNA damage in bronchial epithelial
cells therefore these cells were used to further investigate the
present hypothesis. It was revealed that DNA damage occurred in the
bronchial epithelial cells treated with HDM, as shown in Fig. 3A and B. Increased γH2AX expression
level was detected by immunofluorescence staining (Fig. 3C and D) and western blotting
(Fig. 3E and F) in bronchial
epithelial cells treated with HDM. However, the level of DNA damage
was decreased by combination treatment with HDM and RES (Fig. 3A and B), which was accompanied by
a reduction in the expression of γH2AX (Fig. 3C-F). Taken together, these results
indicate that RES attenuates DNA damage induced by HDM in bronchial
epithelial cells.
RES decreases ROS generation to inhibit
oxidative DNA damage in bronchial epithelial cells
One of characteristics of oxidative stress is
increased ROS levels, which may continuously affect the structure
of DNA and cause oxidative DNA damage (31). It was previously indicated that
treatment of aged rats with RES attenuated oxidative stress in
bronchial epithelial cells (32).
The present study investigated whether RES could attenuate DNA
damage caused by exposure to ROS.
Through detection by DCFH-DA assay, ROS in bronchial
epithelial cells were significantly increased after treatment with
HDM and the increased ROS levels in bronchial epithelial cells were
restored via combination treatment with HDM and RES (Fig. 4A and B). Further testing
demonstrated that 8-OHdG/8-oxoG in bronchial epithelial cells,
which serves as a marker of oxidative DNA damage, was increased by
treatment with HDM and the 8-OHdG/8-oxoG could be simultaneously
protected via treatment with RES (Fig. 4C). IF staining also proved that
RES attenuated the 8-OHdG level increased by HDM in bronchial
epithelial cells (Fig. 4D and E).
To further explore the damaging effects of excessive ROS generation
on DNA, NAC was used to inhibit the oxidative stress induced by HDM
(Fig. 4F). As expected, an
elevation in ROS levels was accompanied by increased DNA damage
(Fig. 4F-I) following treatment
with HDM, while inhibition of ROS generation by NAC was associated
with a decrease in DNA damage (Fig.
4F-I). These results suggested that RES inhibits oxidative DNA
damage by reducing ROS levels.
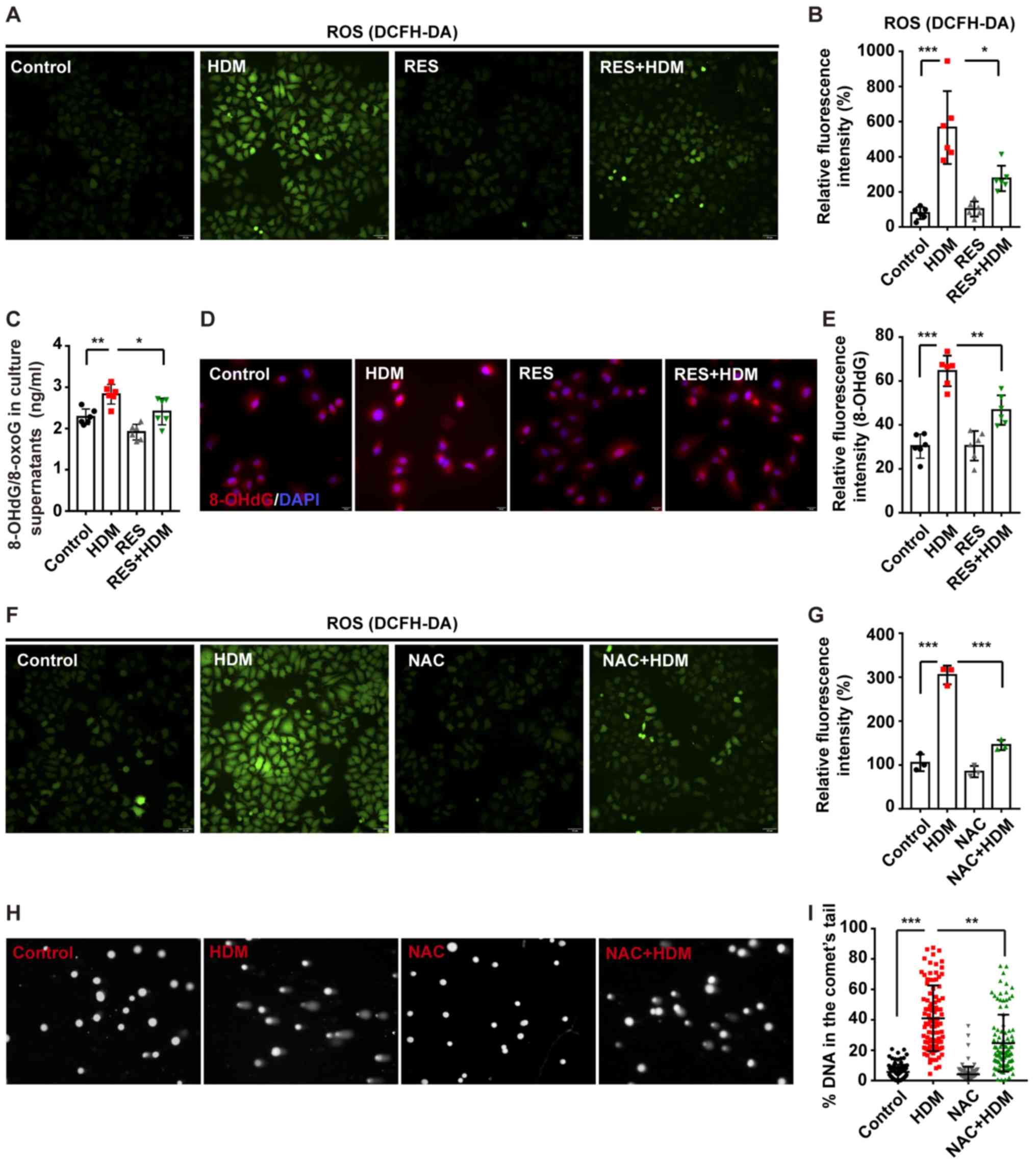 | Figure 4RES decreases ROS to inhibit
oxidative DNA damage in bronchial epithelial cells. 16HBE cells
were incubated with RES for 2 h and then treated with HDM for
another 12 h. (A) Cells were stained with DCFH-DA assay to measure
the oxidative stress level and (B) this was quantified.
Representative images of DCFH-DA and the fold-change of ROS signal
intensity are shown (magnification, ×200). (C) The expression level
of 8-OHdG/8-oxoG in the culture supernatant was measured by ELISA.
(D) Staining with 8-OHdG (red) and DAPI (blue). Representative
immunofluorescence images of 8-OHdG (magnification, ×400). (E)
Quantitation of the fluorescence intensity of 8-OHdG. (F) 16HBE
cells were incubated with NAC for 2 h and then treated with HDM for
another 12 h and (G) the results were quantified. Cells were
stained with DCFH-DA to measure the oxidative stress level.
Representative images of DCFH-DA and the fold-change of ROS signal
intensity are displayed (magnification, ×200). (H) Representative
images of the Comet assay (magnification, ×400). (I) The percentage
of DNA intensity in the Comet tail, reflecting the severity of DNA
damage, was quantified. At least 30 individual Comets were analyzed
in each treatment group (n=3). Data are presented as mean ±
standard deviation. One-way analysis of variance with Tukey-Kramer
test or Dunnett's T3 test was used. *P<0.05,
**P<0.01 and ***P<0.001. RES,
resveratrol; HDM, house dust mites; ROS, reactive oxygen species;
NAC, N-acetyl-L-cysteine. |
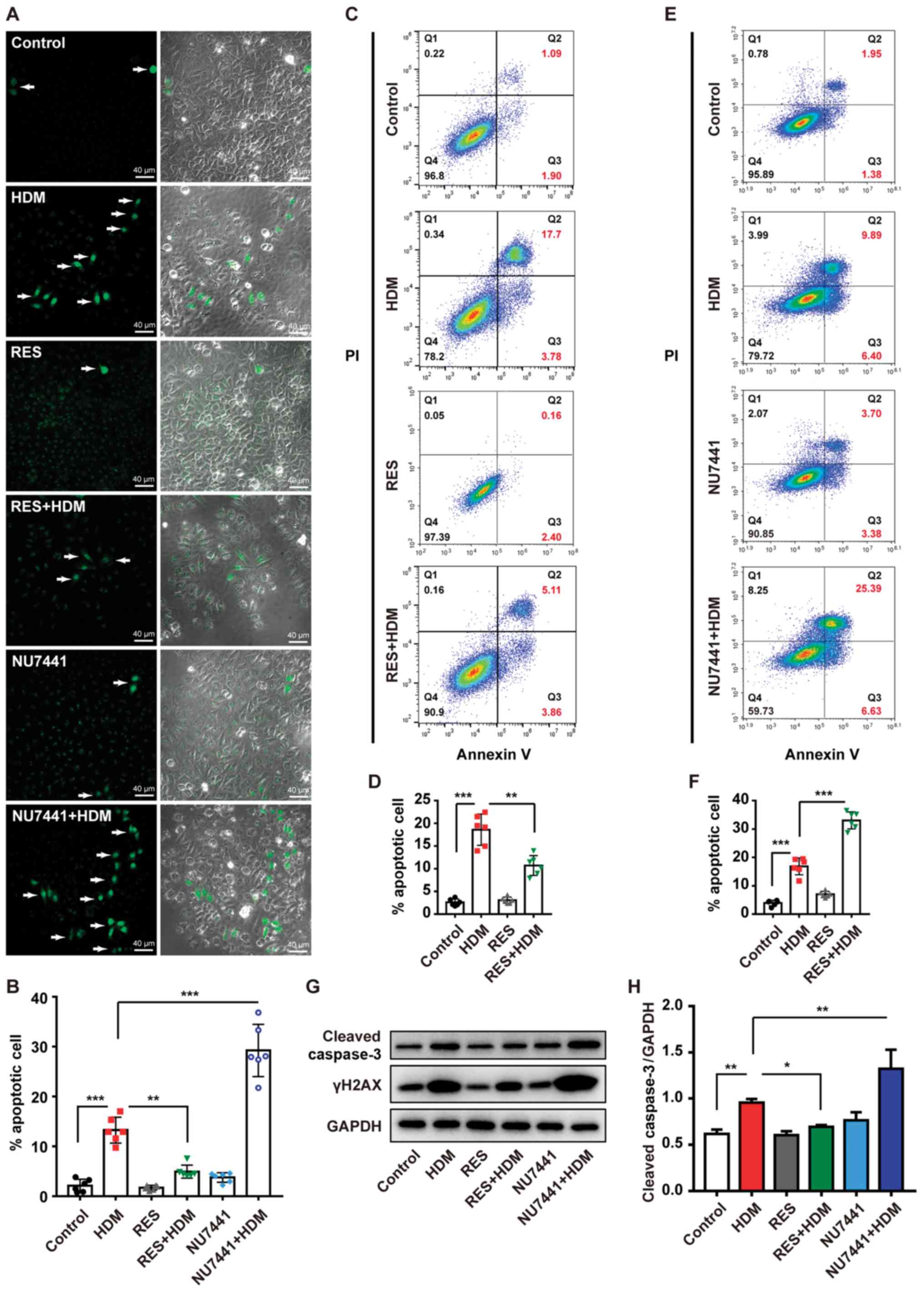
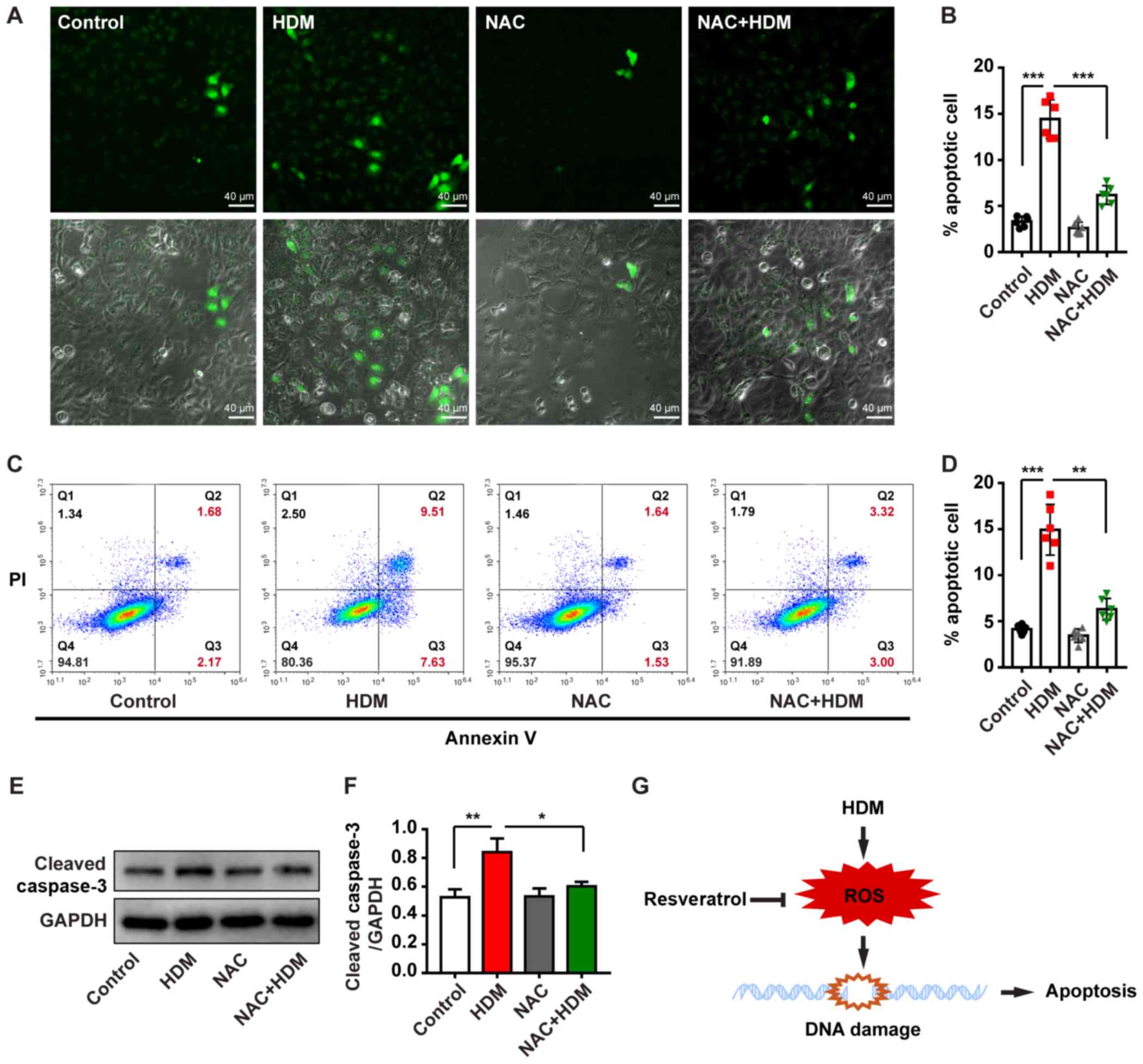
RES protects bronchial epithelial cells
from HDM-induced apoptosis
Of note, 8-OHdG/8-oxoG is a marker of DNA damage
(33,34) and γH2AX also plays a key role in
chromatin remodeling and DNA repair, which has already been
demonstrated in the current mouse model and bronchial epithelial
cell model (30,35). In the present study, it was
hypothesized that RES can protect bronchial epithelial cells from
apoptosis caused by exposure to HDM. IF staining revealed that the
percentage of apoptotic cells was significantly increased following
treatment with HDM compared with the PBS control group and this
process was inhibited by treatment with RES (Fig. 5A and B). Flow cytometry analysis
also confirmed this result (Fig. 5C
and D), as the percentage of apoptotic cells among bronchial
epithelial cells was increased from 1.09 to 17.7% following
treatment with HDM, whereas it decreased to 5.11% by the
combination of HDM with RES, indicating that RES can attenuate
apoptosis of bronchial epithelial cells exposed to HDM. Western
blotting also revealed that RES protected cells from apoptosis,
resulting in lower expression levels of cleaved caspase-3 with
combination treatment with HDM and RES compared with treatment with
HDM alone (Fig. 5G and H).
To investigate the effects of DNA damage on
apoptosis induced by HDM, DNA double-strand breaks repair inhibitor
NU7441 was used to inhibit DNA repair. IF staining revealed that
HDM-induced apoptosis was further enhanced by NU7441 (Fig. 5A and B). Flow cytometry analysis
also demonstrated that NU7441 led to a higher level of HDM-induced
apoptosis (Fig. 5E and F).
Furthermore, western blotting revealed that NU7441 treatment
resulted in an increase in the levels of cleaved caspase-3 and
γH2AX following combination treatment with HDM and NU7441 compared
with treatment with HDM alone (Fig.
5G and H). In addition, RES inhibited γH2AX and cleaved
caspase-3 in bronchial epithelial cells (Fig. 5G and H). Taken together, these
results indicate that RES protects bronchial epithelial cells from
apoptosis through preventing DNA damage.
In order to further examine the role of oxidative
stress in apoptosis induced by HDM, NAC was used to inhibit the
oxidative stress induced by HDM. IF staining revealed that
apoptosis was significantly reduced in bronchial epithelial cells
treated with HDM and NAC compared with bronchial epithelial cells
treated with HDM alone (Fig. 6A and
B). Flow cytometry analysis also demonstrated that NAC could
attenuate apoptosis of bronchial epithelial cells exposed to HDM
(Fig. 6C and D). Furthermore, the
western blotting results revealed that NAC reduced cleaved
caspase-3 level in the epithelial cells subjected to HDM treatment
(Fig. 6E and F). Collectively,
these data indicate that RES protects bronchial epithelial cells
from apoptosis through inhibiting oxidative DNA damage (Fig. 6G).
Discussion
RES is a known antioxidant, which is produced by
plants and it can affect multiple human chronic diseases, in
addition to interacting with the immune system (3,4).
In addition, HDM is a major perennial allergen source and a
significant cause of allergic rhinitis and allergic asthma. In the
present study, it was hypothesized that HDM can directly induce a
high level of ROS generation and cause DNA damage to bronchial
epithelial cells, while the antioxidant properties of RES
attenuated this process. However, the detailed mechanisms of HDM
triggering asthma remain elusive and further investigation may
assist physicians to effectively treat patients with asthma through
controlling ROS-induced damage to bronchial epithelial cells.
The anti-apoptotic and antioxidant stress-protective
effects of RES have been widely investigated. In 2018, Hsu et
al (36) revealed that RES
could protect A549 human lung epithelial cells against carbon black
nanoparticle (CBNP)-induced inflammation and oxidative stress, as
CBNPs are known to promote pulmonary toxicity through inflammation
and oxidative stress. A previous study used cigarette smoke extract
(CSE), which induced apoptosis in a human bronchial epithelial cell
model and studied the effects of treatment with or without RES
(37). Their results demonstrated
that RES exerted a protective effect against CSE-induced apoptosis
and a molecular pathway involving Sirtuin 1 (SIRT1) and
oxygen-regulated protein 150, may be associated with the
anti-apoptotic function of RES. HBE1 human bronchial epithelial
cells were exposed to combined treatment with RES and
4-hydroxynonenal, which acted protectively against cell death
caused by oxidative stress, and the Nrf2-EpRE signaling pathway was
also involved in this combined therapeutic effect (38). Furthermore, RES also decreased
high glucose-induced endothelial cell apoptosis by inhibition of
Nox/ROS (39). The results of the
present study indicated the anti-apoptotic function of RES in
bronchial epithelial cells. Therefore, it may be concluded that RES
helps protect cells from apoptosis caused by HDM.
ROS are highly reactive molecules and can damage
cell structures such as carbohydrates, nucleic acids, lipids and
proteins and alter their functions. The shift in the balance
between oxidants and antioxidants in favor of oxidants is termed
'oxidative stress'. Oxidative stress is characterized by the
presence of increased ROS levels, either as a result of increased
production of ROS or decreased amounts of anti-oxidants. ROS create
a variety of pathological changes in the airways, including
increased airway reactivity and increased mucous production,
factors that have important implications in asthma (40). The present study demonstrated that
exposure to HDM induced high levels of ROS in bronchial epithelial
cells in both the mouse model and the cellular model. ROS have been
shown to inactivate histone deacetylase-2, which is an essential
factor for the inflammatory response (41). RES can improve the expression
level of SIRT1 and increase antioxidant production to reduce
mitochondrial-associated apoptotic signaling pathways and cell
apoptosis and prevent ROS-induced cell damage in myoblasts
(42). In the present study, high
expression levels of 8-OHdG/8-oxoG were detected, which indicated
that the bronchial epithelial cells were damaged. In vitro
studies have shown that RES induces the production of antioxidants
to reduce the impact of ROS (43-45). A study on RES indicated that
treatment of aged rats with RES can activate Nrf2 and attenuate
oxidative stress in endothelial cells. It was observed that
combined treatment with HDM and RES resulted in lower expression
level of ROS and 8-OHdG/8-oxoG. In addition, the Comet assay for
DNA damage confirmed that RES can attenuate DNA damage in bronchial
epithelial cells caused by HDM. This evidence demonstrated that RES
can protect bronchial epithelial cells from oxidative DNA damage
due to HDM exposure. DNA repair protects bronchial epithelial cells
from HDM-induced DNA damage and apoptosis (2). In the present study, the results
also proved that DNA double-strand breaks repair inhibitor NU7441
led to a higher level of HDM-induced apoptosis. Furthermore, the
inhibition of ROS production by NAC in HDM-activated bronchial
epithelial cells decreased apoptosis.
In conclusion, as one of the most common and
important allergens in the environment, HDM can affect the immune
system and cause airway allergic diseases, such as asthma. During
this process, HDM triggers ROS production and increases DNA damage,
which may cause apoptosis of bronchial epithelial cells. RES
exerted protective antioxidant effects that prevented HDM-induced
DNA damage and apoptosis in bronchial epithelial cells.
Supplementary Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Health and
Family Planning Commission of Sichuan Province (grant nos. 18PJ402
and 16PJ543) and the Doctoral Research Initiation Fund of
Affiliated Hospital of Southwest Medical University (grant no.
19045). The funders had no say in the study design, data
collection, data analysis, interpretation, or writing of the
manuscript.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
YZ, LG, VKWW and XingW conceived and designed the
research. LG and XingW drafted the manuscript. YZ, LG, BYKL, XL,
NM, GX, XiaoyunW and XY performed the experiments. YZ, VKWW, HT, QC
and XingW analyzed the data. YZ, LG, VKWW and XingW edited the
article. All authors read and approved the final version of the
article.
Ethics approval and consent to
participate
All animal experiments (including euthanasia) were
in compliance with the regulations and guidelines of the Southwest
Medical University Institutional Animal Care Committee and were
approved by this committee (approval no. 20160041). In addition,
the assays were conducted according to the AAALAC and IACUC
guidelines.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sleiman PM, Flory J, Imielinski M,
Bradfield JP, Annaiah K, Willis-Owen SA, Wang K, Rafaels NM, Michel
S, Bonnelykke K, et al: Variants of DENND1B associated with asthma
in children. N Engl J Med. 362:36–44. 2010. View Article : Google Scholar
|
|
2
|
Chan TK, Loh XY, Peh HY, Tan WNF, Tan WSD,
Li N, Tay IJJ, Wong WSF and Engelward BP: House dust mite-induced
asthma causes oxidative damage and DNA double-strand breaks in the
lungs. J Allergy Clin Immunol. 138:84–96. e812016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mukhopadhyay P, Mukherjee S, Ahsan K,
Bagchi A, Pacher P and Das DK: Restoration of altered microRNA
expression in the ischemic heart with resveratrol. PLoS One.
5:e157052010. View Article : Google Scholar
|
|
4
|
Vang O, Ahmad N, Baile CA, Baur JA, Brown
K, Csiszar A, Das DK, Delmas D, Gottfried C, Lin HY, et al: What is
new for an old molecule? Systematic review and recommendations on
the use of resveratrol. PLoS One. 6:e198812011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sahiner UM, Birben E, Erzurum S, Sackesen
C and Kalayci O: Oxidative stress in asthma. World Allergy Organ J.
4:151–158. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ba X, Aguilera-Aguirre L, Sur S and
Boldogh I: 8-Oxoguanine DNA glycosylase-1-driven DNA base excision
repair: Role in asthma pathogenesis. Curr Opin Allergy Clin
Immunol. 15:89–97. 2015. View Article : Google Scholar :
|
|
7
|
Toussaint M, Jackson DJ, Swieboda D,
Guedán A, Tsourouktsoglou TD, Ching YM, Radermecker C, Makrinioti
H, Aniscenko J, Bartlett NW, et al: Host DNA released by NETosis
promotes rhinovirus-induced type-2 allergic asthma exacerbation.
Nat Med. 23:681–691. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Haswell LE, Hewitt K, Thorne D, Richter A
and Gaca MD: Cigarette smoke total particulate matter increases
mucous secreting cell numbers in vitro: A potential model of goblet
cell hyperplasia. Toxicol In Vitro. 24:981–987. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
White SR: Apoptosis and the airway
epithelium. J Allergy (Cairo). 2011:9484062011.
|
|
10
|
Jang M, Cai L, Udeani GO, Slowing KV,
Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta
RG, et al: Cancer chemopreventive activity of resveratrol, a
natural product derived from grapes. Science. 275:218–220. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Keys A, Menotti A, Karvonen MJ, Aravanis
C, Blackburn H, Buzina R, Djordjevic BS, Dontas AS, Fidanza F and
Keys MH: The diet and 15-year death rate in the seven countries
study. Am J Epidemiol. 124:903–915. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
de Lorgeril M, Salen P, Martin JL, Monjaud
I, Delaye J and Mamelle N: Mediterranean diet, traditional risk
factors, and the rate of cardiovascular complications after
myocardial infarction: Final report of the Lyon Diet Heart Study.
Circulation. 99:779–785. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tyagi A, Singh RP, Agarwal C, Siriwardana
S, Sclafani RA and Agarwal R: Resveratrol causes Cdc2-tyr15
phosphorylation via ATM/ATR-Chk1/2-Cdc25C pathway as a central
mechanism for S phase arrest in human ovarian carcinoma Ovcar-3
cells. Carcinogenesis. 26:1978–1987. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vergara D, Simeone P, Toraldo D, Del
Boccio P, Vergaro V, Leporatti S, Pieragostino D, Tinelli A, De
Domenico S, Alberti S, et al: Resveratrol downregulates Akt/GSK and
ERK signalling pathways in OVCAR-3 ovarian cancer cells. Mol
Biosyst. 8:1078–1087. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang H, Peng Y, Wang J, Gu A, Li Q, Mao D
and Guo L: Effect of autophagy on the resveratrol-induced apoptosis
of ovarian cancer SKOV3 cells. J Cell Biochem. Nov 18–2018.Epub
ahead of print.
|
|
16
|
Falchetti R, Fuggetta MP, Lanzilli G,
Tricarico M and Ravagnan G: Effects of resveratrol on human immune
cell function. Life Sci. 70:81–96. 2001. View Article : Google Scholar
|
|
17
|
Ahn J, Cho I, Kim S, Kwon D and Ha T:
Dietary resveratrol alters lipid metabolism-related gene expression
of mice on an athero-genic diet. J Hepatol. 49:1019–1028. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hou X, Xu S, Maitland-Toolan KA, Sato K,
Jiang B, Ido Y, Lan F, Walsh K, Wierzbicki M, Verbeuren TJ, et al:
SIRT1 regulates hepatocyte lipid metabolism through activating
AMP-activated protein kinase. J Biol Chem. 283:20015–20026. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Baur JA, Pearson KJ, Price NL, Jamieson
HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K,
et al: Resveratrol improves health and survival of mice on a
high-calorie diet. Nature. 444:337–342. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lagouge M, Argmann C, Gerhart-Hines Z,
Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P,
Elliott P, et al: Resveratrol improves mitochondrial function and
protects against metabolic disease by activating SIRT1 and
PGC-1alpha. Cell. 127:1109–1122. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Carter LG, D'Orazio JA and Pearson KJ:
Resveratrol and cancer: Focus on in vivo evidence. Endocr Relat
Cancer. 21:R209–R225. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen ZH, Hurh YJ, Na HK, Kim JH, Chun YJ,
Kim DH, Kang KS, Cho MH and Surh YJ: Resveratrol inhibits
TCDD-induced expres-sion of CYP1A1 and CYP1B1 and catechol
estrogen-mediated oxidative DNA damage in cultured human mammary
epithelial cells. Carcinogenesis. 25:2005–2013. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee M, Kim S, Kwon OK, Oh SR, Lee HK and
Ahn K: Anti-inflammatory and anti-asthmatic effects of resveratrol,
a polyphenolic stilbene, in a mouse model of allergic asthma. Int
Immunopharmacol. 9:418–424. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Royce SG, Dang W, Yuan G, Tran J, El Osta
A, Karagiannis TC and Tang ML: Resveratrol has protective effects
against airway remodeling and airway hyperreactivity in a murine
model of allergic airways disease. Pathobiol Aging Age Relat Dis.
1:2011.PubMed/NCBI
|
|
25
|
Aich J, Mabalirajan U, Ahmad T, Khanna K,
Rehman R, Agrawal A and Ghosh B: Resveratrol attenuates
experimental allergic asthma in mice by restoring inositol
polyphosphate 4 phosphatase (INPP4A). Int Immunopharmacol.
14:438–443. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
André DM, Calixto MC, Sollon C, Alexandre
EC, Leiria LO, Tobar N, Anhê GF and Antunes E: Therapy with
resveratrol attenuates obesity-associated allergic airway
inflammation in mice. Int Immunopharmacol. 38:298–305. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee HY, Kim IK, Yoon HK, Kwon SS, Rhee CK
and Lee SY: Inhibitory effects of resveratrol on airway remodeling
by transforming growth factor-β/smad signaling pathway in chronic
asthma model. Allergy Asthma Immunol Res. 9:25–34. 2017. View Article : Google Scholar
|
|
28
|
Zhang Y, Tang H, Yuan X, Ran Q and Wang X,
Song Q, Zhang L, Qiu Y and Wang X: TGF-β 3 promotes MUC5AC
hyper-expression by modulating autophagy pathway in airway
epithelium. Ebiomedicine. 33:242–252. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pan MR, Li K, Lin SY and Hung WC:
Connecting the dots: From DNA damage and repair to aging. Int J Mol
Sci. 17:E6852016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Leysen H, van Gastel J, Hendrickx JO,
Santos-Otte P, Martin B and Maudsley S: G protein-coupled receptor
systems as crucial regulators of DNA damage response processes. Int
J Mol Sci. 19:E29192018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Knaapen AM, Schins RP, Polat D, Becker A
and Borm PJ: Mechanisms of neutrophil-induced DNA damage in
respiratory tract epithelial cells. Mol Cell Biochem.
234-235:143–151. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Csiszar A, Csiszar A, Pinto JT, Gautam T,
Kleusch C, Hoffmann B, Tucsek Z, Toth P, Sonntag WE and Ungvari Z:
Resveratrol encapsulated in novel fusogenic liposomes activates
Nrf2 and attenuates oxidative stress in cerebromicrovascular
endothelial cells from aged rats. J Gerontol A Biol Sci Med Sci.
70:303–313. 2015. View Article : Google Scholar :
|
|
33
|
Whitaker AM, Schaich MA, Smith MR, Flynn
TS and Freudenthal BD: Base excision repair of oxidative DNA
damage: From mechanism to disease. Front Biosci (Landmrk Ed).
22:1493–1522. 2017. View
Article : Google Scholar
|
|
34
|
Zeng H, Nanayakkara GK, Shao Y, Fu H, Sun
Y, Cueto R, Yang WY, Yang Q, Sheng H, Wu N, et al: DNA checkpoint
and repair factors are nuclear sensors for intracellular organelle
stresses - inflammations and cancers can have high genomic risks.
Front Physiol. 9:5162018. View Article : Google Scholar
|
|
35
|
Rajendran P, Ho E, Williams DE and
Dashwood RH: Dietary phytochemicals, HDAC inhibition, and DNA
damage/repair defects in cancer cells. Clin Epigenetics. 3:42011.
View Article : Google Scholar
|
|
36
|
Hsu HT, Tseng YT, Wong WJ, Liu CM and Lo
YC: Resveratrol prevents nanoparticles-induced inflammation and
oxidative stress via downregulation of PKC-α and NADPH oxidase in
lung epithelial A549 cells. BMC Complement Altern Med. 18:2112018.
View Article : Google Scholar
|
|
37
|
Zhang L, Guo X, Xie W, Li Y, Ma M, Yuan T
and Luo B: Resveratrol exerts an anti-apoptotic effect on human
bronchial epithelial cells undergoing cigarette smoke exposure. Mol
Med Rep. 11:1752–1758. 2015. View Article : Google Scholar
|
|
38
|
Zhang H, Shih A, Rinna A and Forman HJ:
Resveratrol and 4-hydroxynonenal act in concert to increase
glutamate cysteine ligase expression and glutathione in human
bronchial epithelial cells. Arch Biochem Biophys. 481:110–115.
2009. View Article : Google Scholar :
|
|
39
|
Chen F, Qian LH, Deng B, Liu ZM, Zhao Y
and Le YY: Resveratrol protects vascular endothelial cells from
high glucose-induced apoptosis through inhibition of NADPH oxidase
activation-driven oxidative stress. Cns Neurosci Ther. 19:675–681.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lugogo NL, Bappanad D and Kraft M:
Obesity, metabolic dysregulation and oxidative stress in asthma.
Biochim Biophys Acta. 1810:1120–1126. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ito K, Hanazawa T, Tomita K, Barnes PJ and
Adcock IM: Oxidative stress reduces histone deacetylase 2 activity
and enhances IL-8 gene expression: Role of tyrosine nitration.
Biochem Biophys Res Commun. 315:240–245. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Haramizu S, Asano S, Butler DC, Stanton
DA, Hajira A, Mohamed JS and Always SE: Dietary resveratrol confers
apoptotic resistance to oxidative stress in myoblasts. J Nutr
Biochem. 50:103–115. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Montesano A, Luzi L, Senesi P, Mazzocchi N
and Terruzzi I: Resveratrol promotes myogenesis and hypertrophy in
murine myoblasts. J Transl Med. 11:3102013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cosín-Tomàs M, Senserrich J, Arumí-Planas
M, Alquézar C, Pallàs M, Martín-Requero Á, Suñol C, Kaliman P and
Sanfeliu C: Role of resveratrol and selenium on oxidative stress
and expression of antioxidant and anti-aging genes in immortalized
lymphocytes from Alzheimer's disease patients. Nutrients.
11:E17642019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhuang Y, Wu H, Wang X, He J, He S and Yin
Y: Resveratrol attenuates oxidative stress-induced intestinal
barrier injury through PI3K/Akt-mediated Nrf2 signaling pathway.
Oxid Med Cell Longev. 2019:75918402019. View Article : Google Scholar : PubMed/NCBI
|