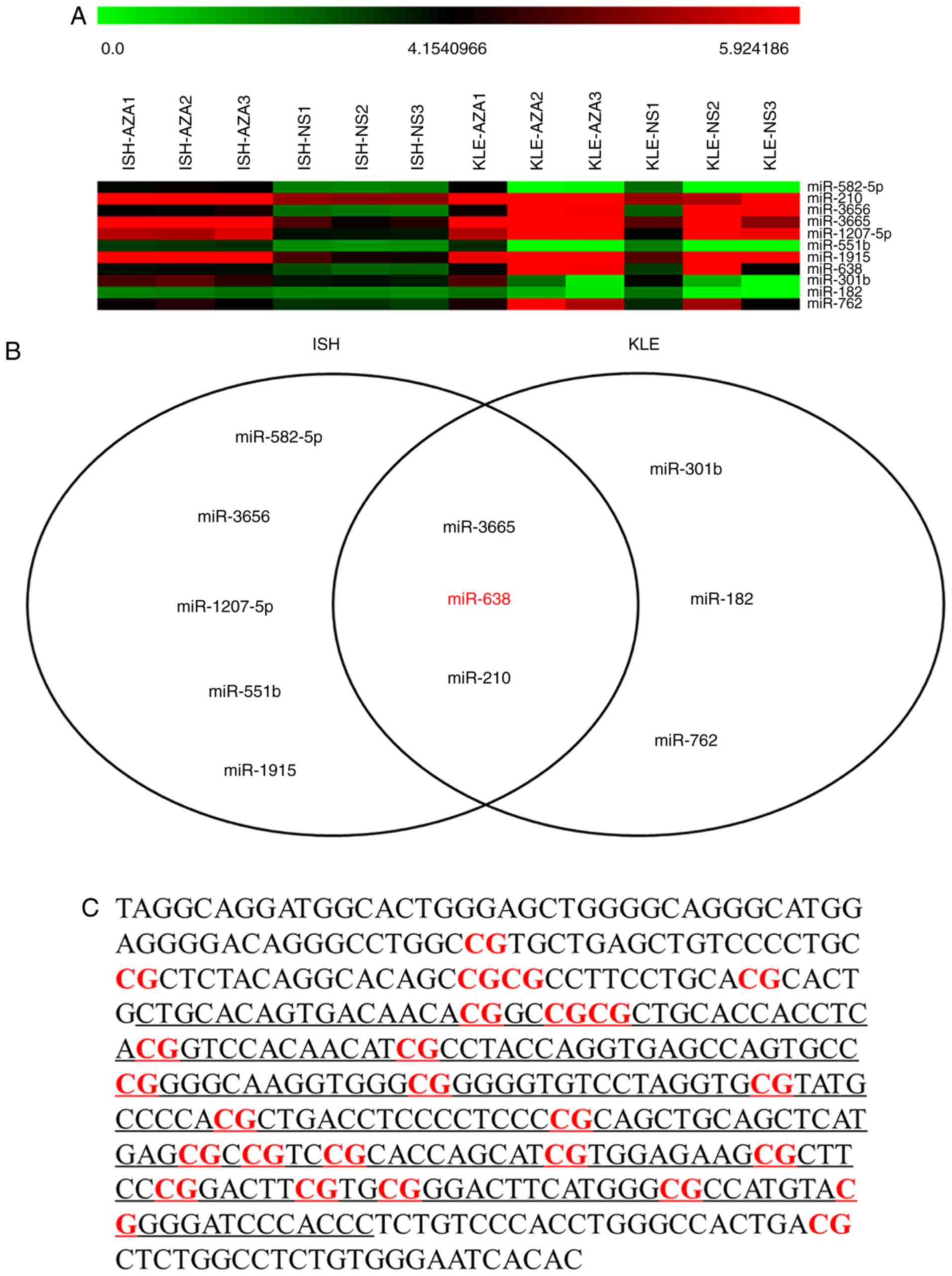
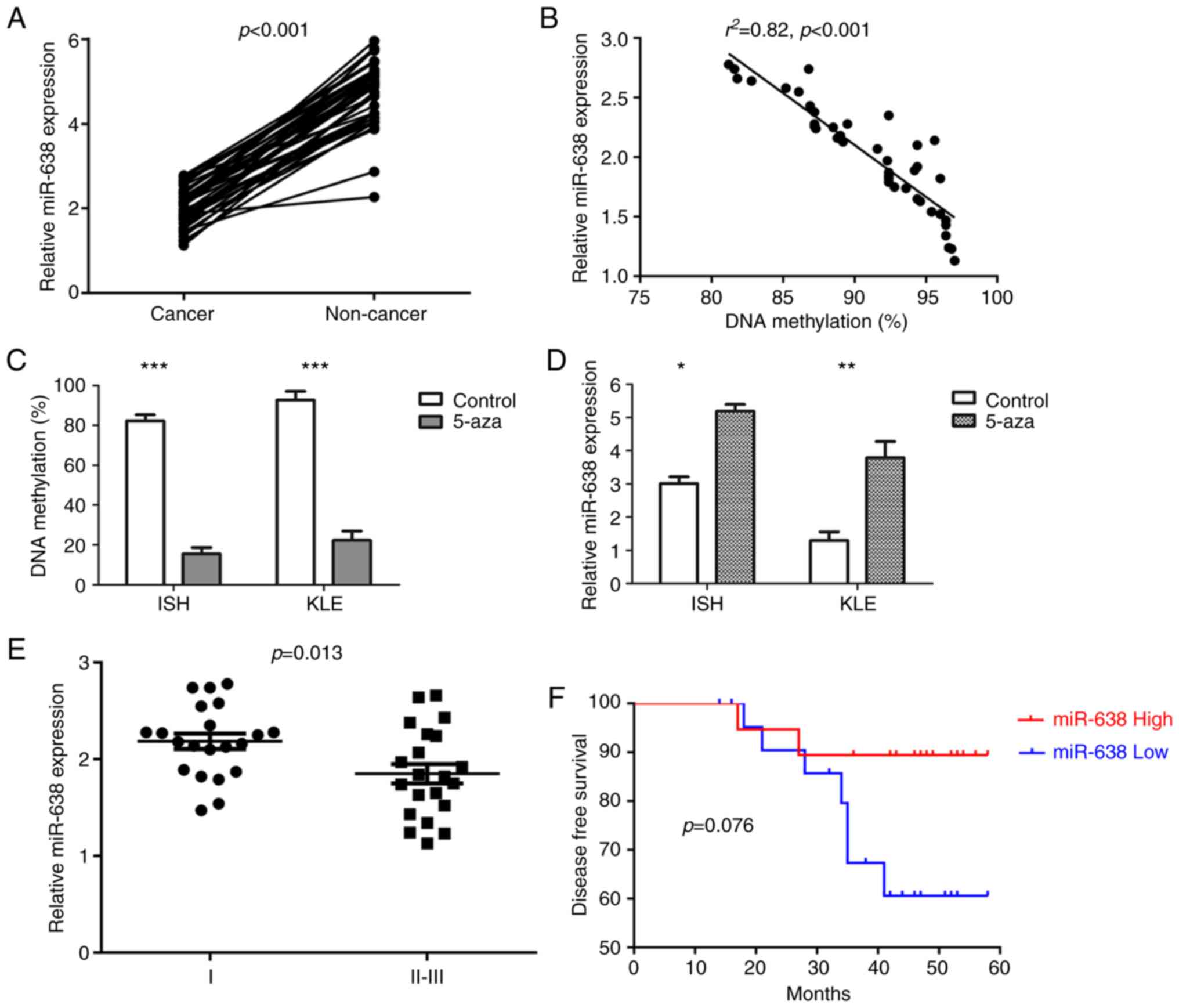
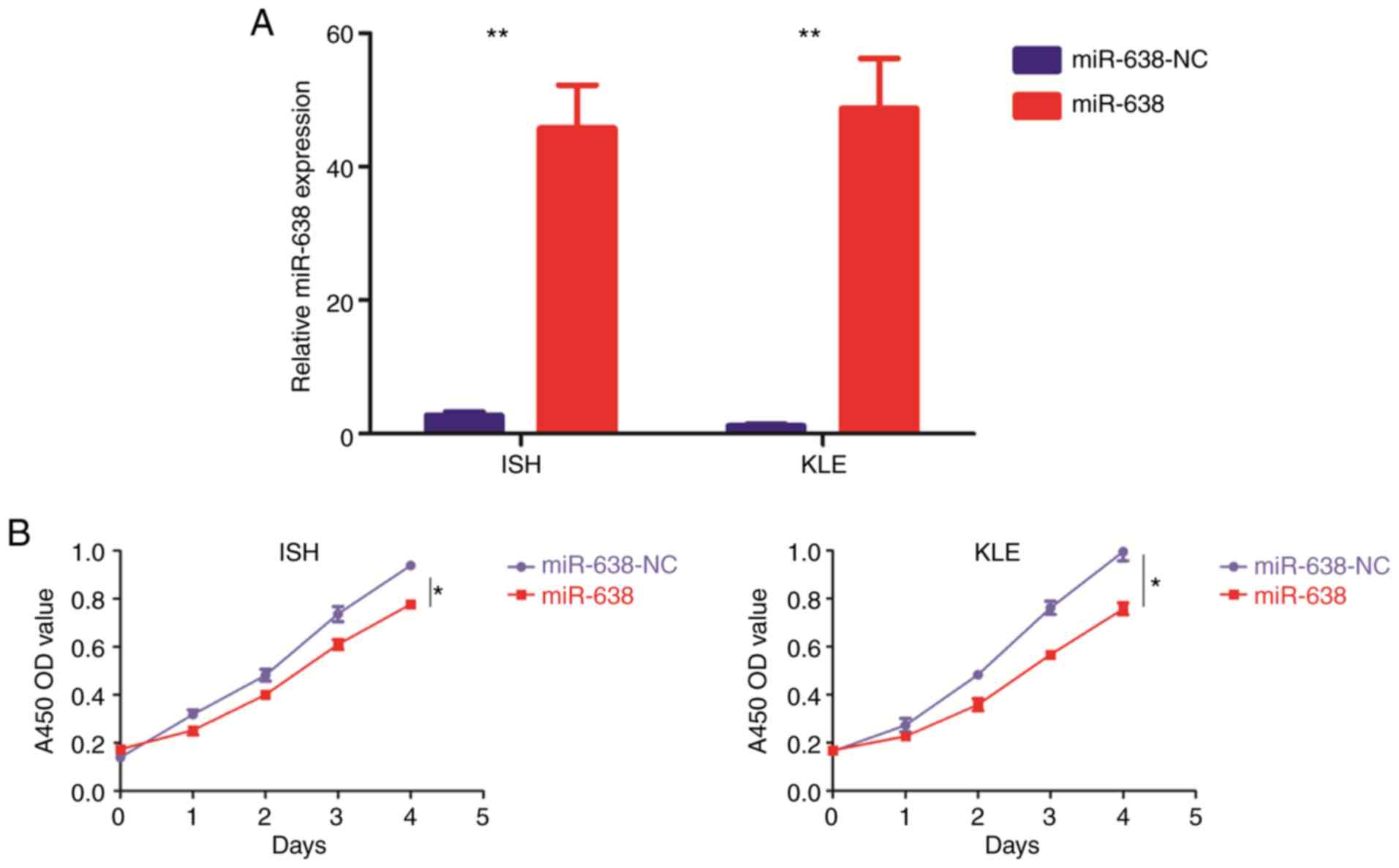
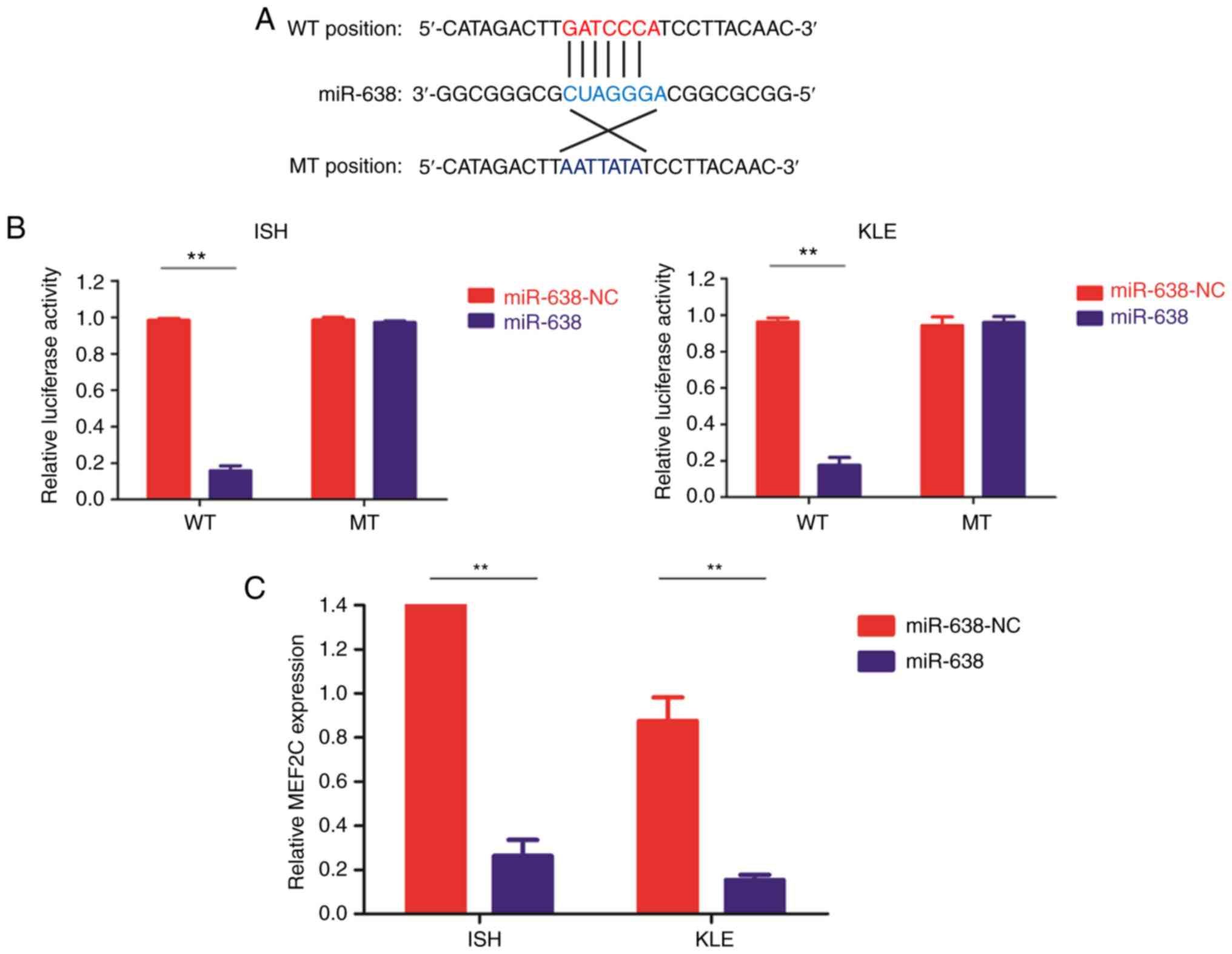
|
1
|
Holubekova V, Mendelova A, Jasek K,
Mersakova S, Zubor P and Lasabova Z: Epigenetic regulation by DNA
methylation and miRNA molecules in cancer. Future Oncol.
13:2217–2222. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ma J, Hong L, Chen Z, Nie Y and Fan D:
Epigenetic regulation of microRNAs in gastric cancer. Dig Dis Sci.
59:716–723. 2014. View Article : Google Scholar
|
|
3
|
Wang P, Chen L, Zhang J, Chen H, Fan J,
Wang K, Luo J, Chen Z, Meng Z and Liu L: Methylation-mediated
silencing of the miR-124 genes facilitates pancreatic cancer
progression and metastasis by targeting Rac1. Oncogene. 33:514–524.
2014. View Article : Google Scholar
|
|
4
|
Loginov VI, Rykov SV, Fridman MV and Braga
EA: Methylation of miRNA genes and oncogenesis. Biochemistry
(Mosc). 80:145–162. 2015. View Article : Google Scholar
|
|
5
|
Mei Q, Li X, Zhang K, Wu Z, Li X, Meng Y,
Guo M, Luo G, Fu X and Han W: Genetic and methylation-induced loss
of miR-181a2/181b2 within chr9q33.3 facilitates tumor growth of
cervical cancer through the PIK3R3/Akt/FoxO signaling pathway. Clin
Cancer Res. 23:575–586. 2017. View Article : Google Scholar
|
|
6
|
Ma K, Pan X, Fan P, He Y, Gu J, Wang W,
Zhang T, Li Z and Luo X: Loss of miR-638 in vitro promotes cell
invasion and a mesenchymal-like transition by influencing SOX2
expression in colorectal carcinoma cells. Mol Cancer. 13:1182014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tay Y, Tan SM, Karreth FA, Lieberman J and
Pandolfi PP: Characterization of dual PTEN and p53-targeting
microRNAs identifies microRNA-638/Dnm2 as a two-hit oncogenic
locus. Cell Rep. 8:714–722. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang J, Bian Z, Zhou J, Song M, Liu Z,
Feng Y, Zhe L, Zhang B, Yin Y and Huang Z: MicroRNA-638 inhibits
cell proliferation by targeting phospholipase D1 in human gastric
carcinoma. Protein Cell. 6:680–688. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ren Y, Chen Y, Liang X, Lu Y, Pan W and
Yang M: MiRNA-638 promotes autophagy and malignant phenotypes of
cancer cells via directly suppressing DACT3. Cancer Lett.
390:126–136. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bhattacharya A, Schmitz U, Raatz Y,
Schönherr M, Kottek T, Schauer M, Franz S, Saalbach A, Anderegg U,
Wolkenhauer O, et al: miR-638 promotes melanoma metastasis and
protects melanoma cells from apoptosis and autophagy. Oncotarget.
6:2966–2980. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao LY, Tong DD, Xue M, Ma HL, Liu SY,
Yang J, Liu YX, Guo B, Ni L, Liu LY, et al: MeCP2, a target of
miR-638, facilitates gastric cancer cell proliferation through
activation of the MEK1/2-ERK1/2 signaling pathway by upregulating
GIT1. Oncogenesis. 6:e3682017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wei H, Zhang JJ and Tang QL: MiR-638
inhibits cervical cancer metastasis through Wnt/β-catenin signaling
pathway and correlates with prognosis of cervical cancer patients.
Eur Rev Med Pharmacol Sci. 21:5587–5593. 2017.PubMed/NCBI
|
|
13
|
Li M, Wang J and Liu H: Downregulation of
miR-638 promotes progression of breast cancer and is associated
with prognosis of breast cancer patients. OncoTargets Ther.
11:6871–6877. 2018. View Article : Google Scholar
|
|
14
|
Zhang Y, Zhang D, Jiang J and Dong L: Loss
of miR-638 promotes invasion and epithelial-mesenchymal transition
by targeting SOX2 in hepatocellular carcinoma. Oncol Rep.
37:323–332. 2017. View Article : Google Scholar
|
|
15
|
Agarwal V, Bell GW, Nam JW and Bartel DP:
Predicting effective microRNA target sites in mammalian mRNAs.
Elife. 4:2015. View Article : Google Scholar
|
|
16
|
FIGO Committee on Gynecologic Oncology:
FIGO staging for carcinoma of the vulva, cervix, and corpus uteri.
Int J Gynaecol Obstet. 125:97–98. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang J, Fei B, Wang Q, Song M, Yin Y,
Zhang B, Ni S, Guo W, Bian Z, Quan C, et al: MicroRNA-638 inhibits
cell proliferation, invasion and regulates cell cycle by targeting
tetraspanin 1 in human colorectal carcinoma. Oncotarget.
5:12083–12096. 2014.PubMed/NCBI
|
|
18
|
Pecorelli S: Revised FIGO staging for
carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol
Obstet. 105:103–104. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ouldamer L, Bendifallah S, Body G, Touboul
C, Graesslin O, Raimond E, Collinet P, Coutant C, Lavoué V, Lévêque
J, et al: Predicting poor prognosis recurrence in women with
endometrial cancer: A nomogram developed by the FRANCOGYN study
group. Br J Cancer. 115:1296–1303. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cancer Genome Atlas Research Network;
Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, Shen H,
Robertson AG, Pashtan I, Shen R, et al: Integrated genomic
characterization of endometrial carcinoma. Nature. 497:67–73. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hecht JL and Mutter GL: Molecular and
pathologic aspects of endometrial carcinogenesis. J Clin Oncol.
24:4783–4791. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
McConechy MK, Ding J, Cheang MC, Wiegand
K, Senz J, Tone A, Yang W, Prentice L, Tse K, Zeng T, et al: Use of
mutation profiles to refine the classification of endometrial
carcinomas. J Pathol. 228:20–30. 2012.PubMed/NCBI
|
|
23
|
Murali R, Soslow RA and Weigelt B:
Classification of endometrial carcinoma: More than two types.
Lancet Oncol. 15:e268–278. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Setiawan VW, Yang HP, Pike MC, McCann SE,
Yu H, Xiang YB, Wolk A, Wentzensen N, Weiss NS, Webb PM, et al:
Type I and II endometrial cancers: Have they different risk
factors? J Clin Oncol. 31:2607–2618. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Marshall AD, van Geldermalsen M, Otte NJ,
Lum T, Vellozzi M, Thoeng A, Pang A, Nagarajah R, Zhang B, Wang Q,
et al: ASCT2 regulates glutamine uptake and cell growth in
endometrial carci-noma. Oncogenesis. 6:e3672017. View Article : Google Scholar
|
|
26
|
Gao J, Gao L, Li R, Lai Z, Zhang Z and Fan
X: Integrated analysis of microRNA-mRNA expression in A549 cells
infected with influenza A viruses (IAVs) from different host
species. Virus Res. 263:34–46. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Das K, Saikolappan S and Dhandayuthapani
S: Differential expression of miRNAs by macrophages infected with
virulent and avirulent mycobacterium tuberculosis. Tuberculosis
(Edinb). 93(Suppl): S47–S50. 2013. View Article : Google Scholar
|
|
28
|
Kiga K, Mimuro H, Suzuki M,
Shinozaki-Ushiku A, Kobayashi T, Sanada T, Kim M, Ogawa M, Iwasaki
YW, Kayo H, et al: Epigenetic silencing of miR-210 increases the
proliferation of gastric epithelium during chronic Helicobacter
pylori infection. Nat Commun. 5:44972014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xiong L, Wang F, Huang X, Liu ZH, Zhao T,
Wu LY, Wu K, Ding X, Liu S, Wu Y, et al: DNA demethylation
regulates the expression of miR-210 in neural progenitor cells
subjected to hypoxia. FEBS J. 279:4318–4326. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu N, Cui RX, Sun Y, Guo R, Mao YP, Tang
LL, Jiang W, Liu X, Cheng YK, He QM, et al: A four-miRNA signature
identified from genome-wide serum miRNA profiling predicts survival
in patients with nasopharyngeal carcinoma. Int J Cancer.
134:1359–1368. 2014. View Article : Google Scholar
|
|
31
|
Cante-Barrett K, Pieters R and Meijerink
JP: Myocyte enhancer factor 2C in hematopoiesis and leukemia.
Oncogene. 33:403–410. 2014. View Article : Google Scholar
|
|
32
|
Homminga I, Pieters R, Langerak AW, de
Rooi JJ, Stubbs A, Verstegen M, Vuerhard M, Buijs-Gladdines J, Kooi
C, Klous P, et al: Integrated transcript and genome analyses reveal
NKX2-1 and MEF2C as potential oncogenes in T cell acute
lymphoblastic leukemia. Cancer Cell. 19:484–497. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nagel S, Meyer C, Quentmeier H, Kaufmann
M, Drexler HG and MacLeod RA: MEF2C is activated by multiple
mechanisms in a subset of T-acute lymphoblastic leukemia cell
lines. Leukemia. 22:600–607. 2008. View Article : Google Scholar
|
|
34
|
Agatheeswaran S, Singh S, Biswas S, Biswas
G, Chandra Pattnayak N and Chakraborty S: BCR-ABL mediated
repression of miR-223 results in the activation of MEF2C and PTBP2
in chronic myeloid leukemia. Leukemia. 27:1578–1580. 2013.
View Article : Google Scholar
|
|
35
|
Schwieger M, Schuler A, Forster M,
Engelmann A, Arnold MA, Delwel R, Valk PJ, Löhler J, Slany RK,
Olson EN and Stocking C: Homing and invasiveness of MLL/ENL
leukemic cells is regulated by MEF2C. Blood. 114:2476–2488. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Brown FC, Still E, Koche RP, Yim CY, Takao
S, Cifani P, Reed C, Gunasekera S, Ficarro SB, Romanienko P, et al:
MEF2C phosphorylation is required for chemotherapy resistance in
acute myeloid leukemia. Cancer Discov. 8:478–497. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Laszlo GS, Alonzo TA, Gudgeon CJ,
Harrington KH, Kentsis A, Gerbing RB, Wang YC, Ries RE, Raimondi
SC, Hirsch BA, et al: High expression of myocyte enhancer factor 2C
(MEF2C) is associated with adverse-risk features and poor outcome
in pediatric acute myeloid leukemia: A report from the Children's
Oncology Group. J Hematol Oncol. 8:1152015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang JJ, Zhu Y, Xie KL, Peng YP, Tao JQ,
Tang J, Li Z, Xu ZK, Dai CC, Qian ZY, et al: Yin Yang-1 suppresses
invasion and metastasis of pancreatic ductal adenocarcinoma by
down-regulating MMP10 in a MUC4/ErbB2/p38/MEF2C-dependent
mechanism. Mol Cancer. 13:1302014. View Article : Google Scholar
|
|
39
|
Bai XL, Zhang Q, Ye LY, Liang F, Sun X,
Chen Y, Hu QD, Fu QH, Su W, Chen Z, et al: Myocyte enhancer factor
2C regulation of hepatocellular carcinoma via vascular endothelial
growth factor and Wnt/β-catenin signaling. Oncogene. 34:4089–4097.
2015. View Article : Google Scholar
|
|
40
|
Ignatius MS, Hayes MN, Lobbardi R, Chen
EY, McCarthy KM, Sreenivas P, Motala Z, Durbin AD, Molodtsov A,
Reeder S, et al: The NOTCH1/SNAIL1/MEF2C pathway regulates growth
and self-renewal in embryonal rhabdomyosarcoma. Cell Rep.
19:2304–2318. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mahecha AM and Wang H: The influence of
vascular endothelial growth factor-A and matrix metalloproteinase-2
and -9 in angiogenesis, metastasis, and prognosis of endometrial
cancer. OncoTargets Ther. 10:4617–4624. 2017. View Article : Google Scholar
|
|
42
|
Golsteyn RM: Cdk1 and Cdk2 complexes
(cyclin dependent kinases) in apoptosis: A role beyond the cell
cycle. Cancer Lett. 217:129–138. 2005. View Article : Google Scholar
|
|
43
|
Dosil MA, Mirantes C, Eritja N, Felip I,
Navaridas R, Gatius S, Santacana M, Colàs E, Moiola C,
Schoenenberger JA, et al: Palbociclib has antitumour effects on
Pten-deficient endometrial neoplasias. J Pathol. 242:152–164. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gong X, Litchfield LM, Webster Y, Chio LC,
Wong SS, Stewart TR, Dowless M, Dempsey J, Zeng Y, Torres R, et al:
Genomic aberrations that activate D-type cyclins are associated
with enhanced sensitivity to the CDK4 and CDK6 inhibitor
abemaciclib. Cancer Cell. 32:761–776. e7662017. View Article : Google Scholar : PubMed/NCBI
|