Introduction
Atherosclerosis is the basic pathological process of
athero-sclerotic cardiovascular disease (ASCVD), which remains a
common cause of morbidity and mortality globally (1). The vascular endothelium, which is
located between the blood and tissues, plays a major role in
maintaining body homeostasis. Upon exposure to proatherogenic
factors, such as oxidized low-density lipoprotein (ox-LDL),
endothelial cells (ECs) undergo a series of changes, including
increased permeability, induced inflammation and apoptosis,
ultimately disrupting the balance of EC homeostasis and initiating
of atherosclerosis (2,3). Apoptosis, a mode of programmed cell
death, is a direct consequence of impaired endothelial function.
Ox-LDL is associated with an increased risk of endothelial
apoptosis and contributes to the pathogenesis of atherosclerosis
(4).
Sonic hedgehog (Shh), initially identified in
Drosophila melanogaster, together with Indian hedgehog and
Desert hedgehog, acts as a growth factor, survival agent and
inductive signal in a gradient-dependent fashion to determine cell
fate (5). This process is
pivotally important to blood vessel development and adult
homeostasis. The Shh protein undergoes autocatalytic cleavage to
produce a mature Shh-N fragment with cholesterol and fatty
modifications (6). The canonical
Shh signaling pathway begins with the binding of Shh-N to the
transmembrane protein Patched (Ptch), relieving the inhibition of
Smoothened (Smo), which results in activation of glioma-associated
oncogene homolog transcription factors (Gli1, 2 and 3) (7,8).
Hedgehog signaling is critical for maintaining the
adult coronary vasculature and an impaired Shh pathway contributes
to cardiac dysfunction (9,10).
There is growing evidence that hedgehog components, such as Shh and
Gli1, are markedly downregulated in the atherosclerotic vasculature
(11-13). Exogenous Shh protein has
therapeutic potential for repairing oxidative stress-induced
endothelial injury by increasing endothelial nitric-oxide synthase
(eNOS) expression, inducing nitric oxide release and improving
endothelial function (14). Shh
signaling pathway activation could reduce astrocyte apoptosis
through increasing the expression of the anti-apoptotic protein
Bcl-2 (15).
The transcription factor NF-κB is an important
regulator of inflammatory responses and apoptosis in
atherosclerosis (16). Aberrant
NF-κB activation has been regarded as a proatherogenic factor and
inhibiting NF-κB signaling has been shown to protect against
atherosclerosis (16). Canonical
NF-κB activation is controlled by the inhibitor of κB kinase (IKK)
complex through phosphorylation. IKK activation phosphorylates
inhibitor of NF-κB (IκB), resulting in its degradation and allowing
NF-κB to be translocated to the nucleus (17). Upon extracellular stimulation,
such as hyperlipidemia or ox-LDL, the activated NF-κB triggers the
transcription of target genes, including adhesion molecules,
cytokines and apoptosis-associated proteins (18).
Hedgehog pathway components are downregulated in
atherosclerosis plaques (12),
and aberrant NF-κB signaling pathway activation plays a pivotal
role in the development of atherosclerosis (16). A previous study in multiple
myeloma demonstrated that hedgehog signaling can regulate the NF-κB
signaling pathways via classical and non-classical pathways
(19). However, to the best of
our knowledge, this relationship is still not clear in
atherosclerosis.
The present study investigated the role of Shh and
its molecular mechanisms in ox-LDL-induced endothelial apoptosis.
Additionally, the present study focused on the effects of Shh on
NF-κB pathway phosphorylation. It was found that Shh was
downregulated in ox-LDL-induced ECs. Overexpression of Shh
demonstrated its protective effect on ox-LDL-mediated cell
apoptosis via the NF-κB pathway and Bcl-2 mediated mitochondrial
signaling.
Materials and methods
Reagents
Ox-LDL was purchased from Yiyuan Biotechnologies
(cat. no. YB-002-1). Recombinant human Shh N-terminus protein
(rShh-N) was obtained from Sangon Biotech Co., Ltd. (cat. no.
C600310) and dissolved in ddH2O. Cyclopamine, a hedgehog
signaling inhibitor, was purchased from Cayman Chemical Company
(cat. no. 11321). Pyrrolidine dithiocarbamate (PDTC) was purchased
from Sigma-Aldrich; Merck KGaA (cat. no. P8765). Cell Counting
Kit-8 (CCK-8) was purchased from Dojindo Molecular Technologies,
Inc. (cat. no. CK04). Antibodies specific for Shh (cat. no. 2207;
1:1,000), cleaved caspase 3 (cat. no. 9662S; 1:500), Bcl-2 (cat.
no. 4223; 1:1,000), Bax (cat. no. 2773; 1:1,000), tublin (cat. no.
2148; 1:1,000) and β-actin (cat. no. 4970; 1:1,000), a NF-κB
pathway sampler kit including antibodies for IκBα, phosphorylated
(p)-IκBα, p-IKKα/β, NF-κB p65 and p-NF-κB p65 (cat. no. 9936; all
used at 1:1,000) and horseradish peroxidase-conjugated goat
anti-rabbit IgG (cat. no. 7074; 1:1,000) and horseradish
peroxidase-conjugated goat anti-mouse IgG (cat. no. 7076; 1:1,000)
were purchased from Cell Signaling Technology, Inc. Antibodies
specific for Smo (cat. no. ab113438; 1:1,000), Ptch (cat. no.
ab53715; 1;1,000), Gli1 (cat. no. ab49314; 1:500) and IKKα/β (cat.
no. ab178870; 1:1,000) were purchased from Abcam.
Cell culture
HUVECs were obtained from human umbilical cord veins
treated with a 0.25% trypsin solution (20). A total of 20 human umbilical cord
veins were collected from healthy newborns including 11 males and 9
females, at Fujian Provincial Hospital (Fuzhou, China) between
September 2018 and September 2019. The age of the mothers ranged
between 25 and 30 years. The research protocol was approved by the
Ethics Committee of Fujian Provincial Hospital (approval no.
K2018-09-008), informed consent forms were signed by the parents of
the newborns, and the procedures were all conducted in compliance
with the Declaration of Helsinki. HUVECs were cultured in
Endothelial Cell Medium (ScienCell Research Laboratories, Inc.)
consisting of basal medium, 5% FBS, 1% EC growth supplement and 1%
penicillin/streptomycin solution in a humidified incubator at 37°C
in a 5% CO2 atmosphere. Cells were used between passages
3 and 5.
Cell viability assay
HUVEC viability was analyzed by CCK-8 assay. A total
of 5×103 cells/well were seeded into 96-well plates and
incubated for 24 h. To assess the appropriate dose of ox-LDL for
further experiments, 10, 20, 50 or 100 µg/ml ox-LDL was
added to the cells and incubated at 37°C for 24 h. In addition, to
evaluate the appropriate stimulation time of ox-LDL, 50
µg/ml ox-LDL was added to the cells and incubated at 37°Cfor
4, 8, 12, 24 and 48 h. The absorbance at a wavelength of 450 nm was
measured after CCK-8 solution was added to the wells for 2 h.
Western blotting
HUVECs were incubated with 10, 20, 50 or 100
µg/ml ox-LDL for 24 h at 37°C or 1, 10 and 100 ng/ml rShh-N
for 24 h at 37°C. Alternatively HUVECs were treated with 20
µM cyclopamine for 2 h at 37°C or 20 µM PDTC for 1 h
at 37°C. Subsequently, total protein was extracted with RIPA lysis
buffer (cat. no. P0013C; Beyotime Institute of Biotechnology) added
protease/phosphatase inhibitor cocktail (cat. no. 5872; Cell
Signaling Technology, Inc.) on ice for 30 min. The samples were
then centrifuged at 12,000 × g for 15 min at 4°C, and protein
quantification was performed using BCA protein assay kit (cat. no.
23227; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. Following heat treatment at 99°C, 50
µg protein samples from the different groups were separated
by 8–12% SDS-PAGE and transferred to PVDF membranes. Blocking was
performed with 5% bovine serum albumin (cat. no. 9998; Cell
Signaling Technology, Inc.) for 2 h at room temperature. Primary
antibodies specific for Shh, Smo, Ptch, Gli1, cleaved caspase 3,
Bcl-2, Bax, IκBα, p-IκBα, IKKα/β, p-IKKα/β, NF-κB p65, p-NF-κB p65,
Tublin and β-actin were incubated with the membranes overnight at
4°C. The next day, secondary antibodies specific for horseradish
peroxidase-conjugated goat anti-rabbit IgG and horseradish
peroxidase-conjugated goat anti-mouse IgG were incubated with the
membranes at room temperature for 1 h and the proteins were
visualized using the WesternBright enhanced chemiluminescence (cat.
no. K-12045-D50; Advansta, Inc.). Image J (version 1.4; National
Institutes of Health) was used to quantify the western blots.
Plasmid transfection
Commercialized plasmid encoding the human Shh gene
(phShh) and pFlag were synthesized by General Biosystems Co., Ltd.
The control plasmid, pFlag, constructed by inserting Flag tag
sequence 5′-GATTACAAGGATGACGACGATAAG-3′ into the plasmid
pcDNA3.1/Hygro(+). phShh was constructed by inserting a 1,425 bp
Shh gene fused with Flag tag sequence into the plasmid
pcDNA3.1/Hygro(+). HUVECs were transfected with 2 µg phShh
or pFlag using Lipofectamine® 3000 transfection reagent
(cat. no. L3000015; Invitrogen; Thermo Fisher Scientific, Inc.),
and then cultured for 48 h before subsequent experiments.
Co-immunoprecipitation (Co-IP) assay
HUVECs were lysed with cell lysis buffer for western
and IP (cat. no. P0013; Beyotime Institute of Biotechnology) and
centrifuged at 12,000 × g for 15 min at 4°C. The protein lysates
were incubated with protein A/G plus agarose beads (cat. no.
sc-2003; Santa Cruz Biotechnology, Inc.) and antibodies specific
for Shh (1:100) or NF-κB p65 (1:100) overnight on a rotating device
at 4°C. The normal IgG (cat. no. 2729; Cell Signaling Technology,
Inc.; 1:100) was used for the control group. The following day, the
immunoprecipitated complexes consisting of antibody, targeted
protein and protein A/G plus agarose beads were centrifuged at
1,000 × g for 5 min at 4°C. Cell lysis buffer for western and IP
was used as the washing reagent and the cells were centrifuged at
1,000 × g for 5 min at 4°C in triplicate. Finally, the complexes
were analyzed by western blotting.
Apoptosis analysis by flow cytometry
FITC Annexin V Apoptosis Detection kit was purchased
from BD Biosciences (cat. no. 556547) and used for apoptosis
detection according to the manufacturer's protocol. Confluent cells
were treated under different conditions and then harvested,
centrifuged at 1,000 × g for 5 min at room temperature, washed
twice with PBS and then resuspended cell in Annexin V Binding
Buffer. The cells were stained with 5 µl propidium iodide
(PI) and 5 µl Alexa Fluor 488 Annexin V for 15 min at room
temperature in the dark. Subsequently, 400 µl Annexin V
Binding Buffer was added. Fluorescence was detected using a flow
cytometer (FACSVerse; BD Biosciences) and analyzed by BD FACSuite
software (Version 1.0; Becton, Dickinson and Company). The
unstained cells, cells stained with FITC Annexin V only and cells
stained with PI only were used to set up compensation and
quadrants.
Caspase 3 activity assay
Caspase 3 activity assay kit (cat. no. C1116;
Beyotime Institute of Biotechnology) was used to measure caspase 3
activity according to the manufacturer's protocol. Confluent cells
were harvested and lysed using 100 µl lysis buffer on ice
for 15 min, then centrifuged at 16,000 × g for 15 min at 4°C.
Subsequently, 50 µl supernatant or lysis buffer (as a
control) was mixed with 10 µl Ac-DEVD-pNA and 40 µl
detection buffer, followed by incubation at 37°C for 1 h. The
absorbance was detected at 405 nm.
Statistical analysis
All statistical analysis was performed using
GraphPad Prism 8.0 (GraphPad Software, Inc.). The data are
presented as the mean ± standard deviation (SD), and one-way ANOVA
was used to perform statistical comparisons, followed by Tukey's
post hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Shh expression is downregulated in
ox-LDL-induced HUVECs
To investigate the cytotoxicity of ox-LDL in HUVECs,
CCK-8 assays were used to examine cell viability. HUVEC viability
was decreased by ox-LDL in a dose- and time-dependent manner
(Fig. 1A and B). Cell apoptosis
was evaluated through the caspase 3 activity, the Bcl-2 and Bax
expression, and Annexin V/PI staining. A caspase 3 activity assay
kit was used to detect the caspase 3 activity and the results
demonstrated that caspase 3 activity was increased by ox-LDL in a
dose-dependent manner (Fig. 1C).
Consistent with this finding, ox-LDL increased cleaved caspase 3
protein expression in a dose-dependent manner, as indicated by
western blotting (Fig. 1D).
Additionally, ox-LDL reduced Bcl-2 expression and increased Bax
expression, as a consequence, the Bcl-2/Bax ratio was decreased
following treatment with ox-LDL. Ox-LDL reduced the Bcl-2/Bax ratio
significantly at a dose of 20, 50 or 100 µg/ml (Fig. 1E). Annexin V/PI staining indicated
that HUVEC apoptosis increased with increasing ox-LDL
concentrations. Following exposure to 25, 50 or 100 µg/ml
ox-LDL, the percentage of cells undergoing apoptosis was
significantly increased compared with the control group, indicating
that apoptosis was the main cause of HUVEC loss (Fig. 1G). Accordingly, 50 µg/ml
ox-LDL for 24 h was selected as the appropriate dose and duration
for observing ox-LDL-mediated HUVEC apoptosis. Ox-LDL significantly
induced the phosphorylation of NF-κB p65 in HUVECs (Fig. 1F). Additionally, the
ox-LDL-induced increase in cleaved caspase 3 protein expression was
significantly decreased by PDTC, a NF-κB inhibitor (Fig. 1H). Annexin V/PI staining indicated
that ox-LDL-induced HUVEC apoptosis was decreased by PDTC (Fig. 1I).
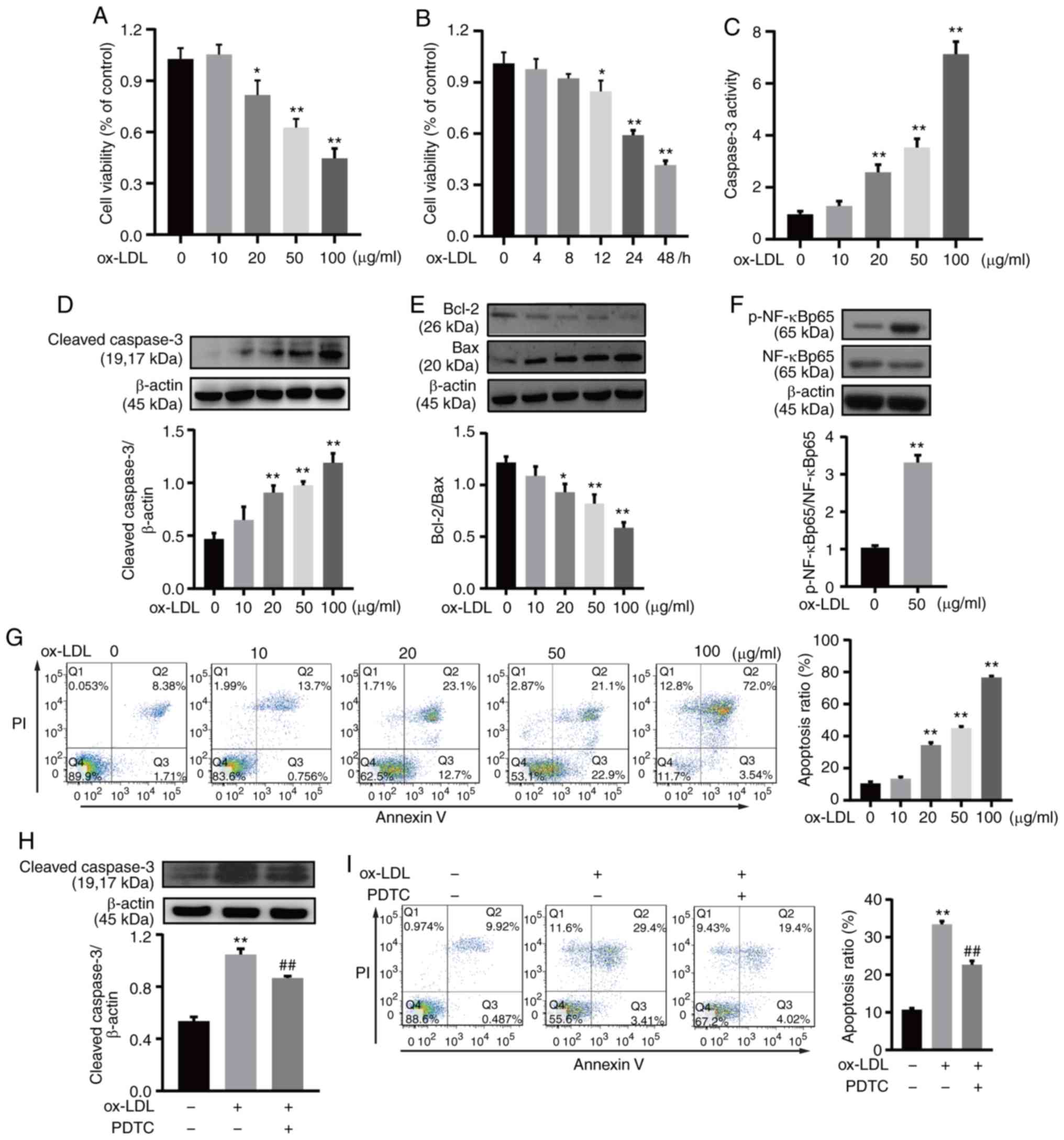 | Figure 1Ox-LDL induced HUVEC apoptosis and
the phosphorylation of NF-κB. (A) HUVECs were exposed to ox-LDL for
24 h. Cell viability was gradually downregulated in a
dose-dependent manner. (B) HUVECs exposed to 50 µg/ml ox-LDL
for 4-48 h, exhibited a progressive decrease in cell viability in a
time-dependent manner. (C) HUVECs were treated with ox-LDL for 24
h, and caspase 3 activity was determined at A405 nm using a caspase
3 activity assay kit. (D) HUVECs were treated with 50 µg/ml
ox-LDL for 24 h, then cell lysates were prepared and subjected to
western blot analysis for the detection of cleaved caspase 3
expression. β-actin was used as an internal loading control. (E)
HUVECs were treated with 50 µg/ml ox-LDL for 24 h, then
western blot analysis was performed to detect Bcl-2 and Bax
expression, and the Bcl-2/Bax ratio was calculated. (F) HUVECs were
treated with 50 µg/ml ox-LDL for 60 min, then western blot
analysis was used to detect p-NF-κB p65 and NF-κB p65 expression.
β-actin was used as an internal loading control. (G) HUVECs were
treated with ox-LDL for 24 h, and apoptosis was analyzed by Annexin
V/PI staining. Q1, dead cells; Q2, late apoptotic cells; Q3, early
apoptotic cells; and Q4, viable cells. Apoptotic cells were
considered as Q2 and Q3. (H) HUVECs were treated with 20 µM
PDTC and 50 µg/ml ox-LDL, followed by western blot analysis
for the detection of cleaved caspase 3 expression. β-actin was used
as an internal loading control. (I) HUVECs were treated with 20
µM PDTC and 50 µg/ml ox-LDL, and apoptosis was
analyzed by Annexin V/PI staining. Q1, dead cells; Q2, late
apoptotic cells; Q3, early apoptotic cells; and Q4, viable cells.
Apoptotic cells were considered as Q2 and Q3. All experiments were
repeated three times and the data are presented as the mean ±
standard deviation. *P<0.05, **P<0.01 vs.
untreated control. ##P<0.01 vs. ox-LDL only-treated
group. PDTC, pyrrolidine dithiocarbamate; ox-LDL, oxidized
low-density lipopro-tein; HUVEC, human umbilical vein endothelial
cell; p-, phosphorylated; PI, propidium iodide. |
The protein expression of hedgehog signaling family
members was evaluated by western blotting in HUVECs treated with
0–100 µg/ml ox-LDL for 24 h (Fig. 2A). HUVECs exposed to 20
µg/ml ox-LDL demonstrated significantly reduced protein
expression of both full-length Shh and the Shh-N fragment, which
was more notable with 50 or 100 µg/ml ox-LDL stimulation
(Fig. 2B and C). These
ox-LDL-induced reductions were not limited to Shh; the protein
expression levels of Ptch, Smo and Gli1 also decreased in a
concentration-dependent manner (Fig.
2D-F). Therefore, ox-LDL treatment reduced the protein
expression of Shh signaling components in HUVECs.
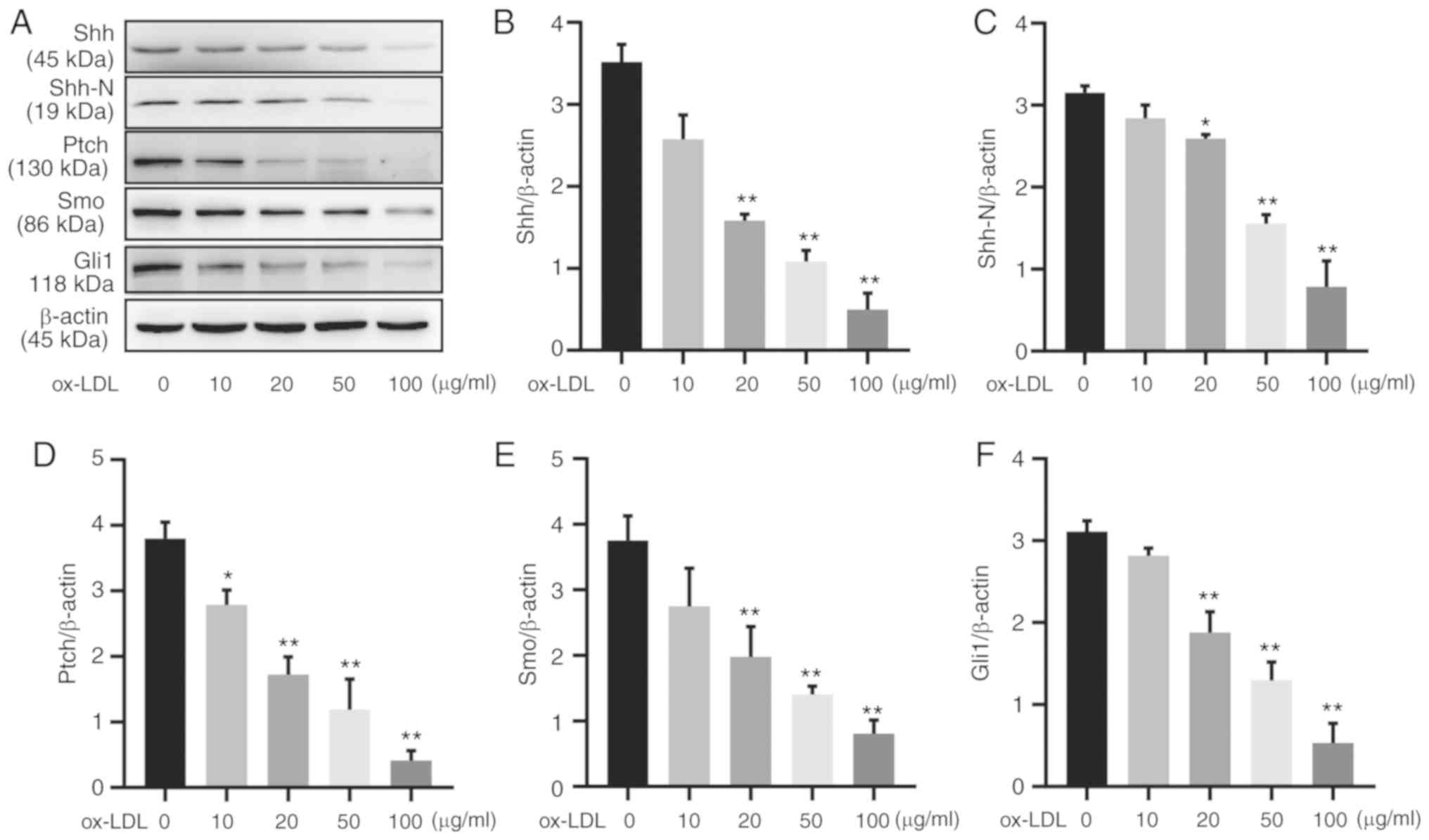 | Figure 2Hedgehog signaling is downregulated
in ox-LDL induced HUVECs. (A) HUVECs were treated with ox-LDL for
24 h, and western blot analysis of hedgehog signaling components
was performed. Quantification of (B) Shh, (C) Shh-N, (D) Ptch, (E)
Smo and (F) Gli1 protein expression. All experiments were repeated
three times and the data are presented as the mean ± standard
deviation. *P<0.05, **P<0.01 vs.
untreated control group. ox-LDL, oxidized low-density lipoprotein;
HUVEC, human umbilical vein endothelial cell; Shh, Sonic hedgehog;
Shh-N, Sonic hedgehog N-terminus; Ptch, patched; Smo, smoothened;
Gli1, glioma-associated oncogene homolog 1. |
Shh attenuates HUVEC apoptosis
To investigate whether Shh plays a role in
ox-LDL-induced endothelial apoptosis, rShh-N and phShh were used.
rShh-N protein (1, 10 and 100 ng/ml) was added 24 h prior to 50
µg/ml ox-LDL exposure, and Shh-N expression was
significantly enhanced by pretreatment with rShh-N, as determined
by western blotting (Fig. 3A and
B). Pretreatment with rShh-N significantly decreased cleaved
caspase 3 protein expression compared with the group only treated
with ox-LDL (Fig. 3C). In
addition, rShh-N significantly increased the Bcl-2/Bax ratio
compared with cells treated with ox-LDL alone (Fig. 3D). To corroborate this finding,
HUVECs were transfected with plasmid encoding either Shh or a
control sequence. HUVECs successfully overexpressed Shh and Shh-N
following phShh transfection for 48 h, as confirmed by western
blotting (Fig. 3E). Consistent
with the rShh-N data, following exposure to ox-LDL (50
µg/ml) for 24 h, phShh significantly decreased cleaved
caspase 3 protein expression and significantly enhanced the
Bcl-2/Bax ratio compared with the control group (Fig. 3F-H). The cells were incubated with
rShh-N protein for 24 h or transfected with phShh for 48 h, and
then exposed to 50 µg/ml ox-LDL, which demonstrated
significant downregulatory effects of rShh-N and phShh on caspase 3
activity compared with the group treated with ox-LDL alone
(Fig. 3I and J). The reduction in
cell apoptosis was also supported by the Annexin V/PI assay.
Following 50-µg/ml ox-LDL stimulation, HUVEC apoptosis was
significantly lower in the phShh group compared with the control
group (Fig. 3K). The apoptosis
rate was significantly increased in the ox-LDL-treated cells
pretreated with rShh compared with the cells only treated with
ox-LDL (Fig. 3L).
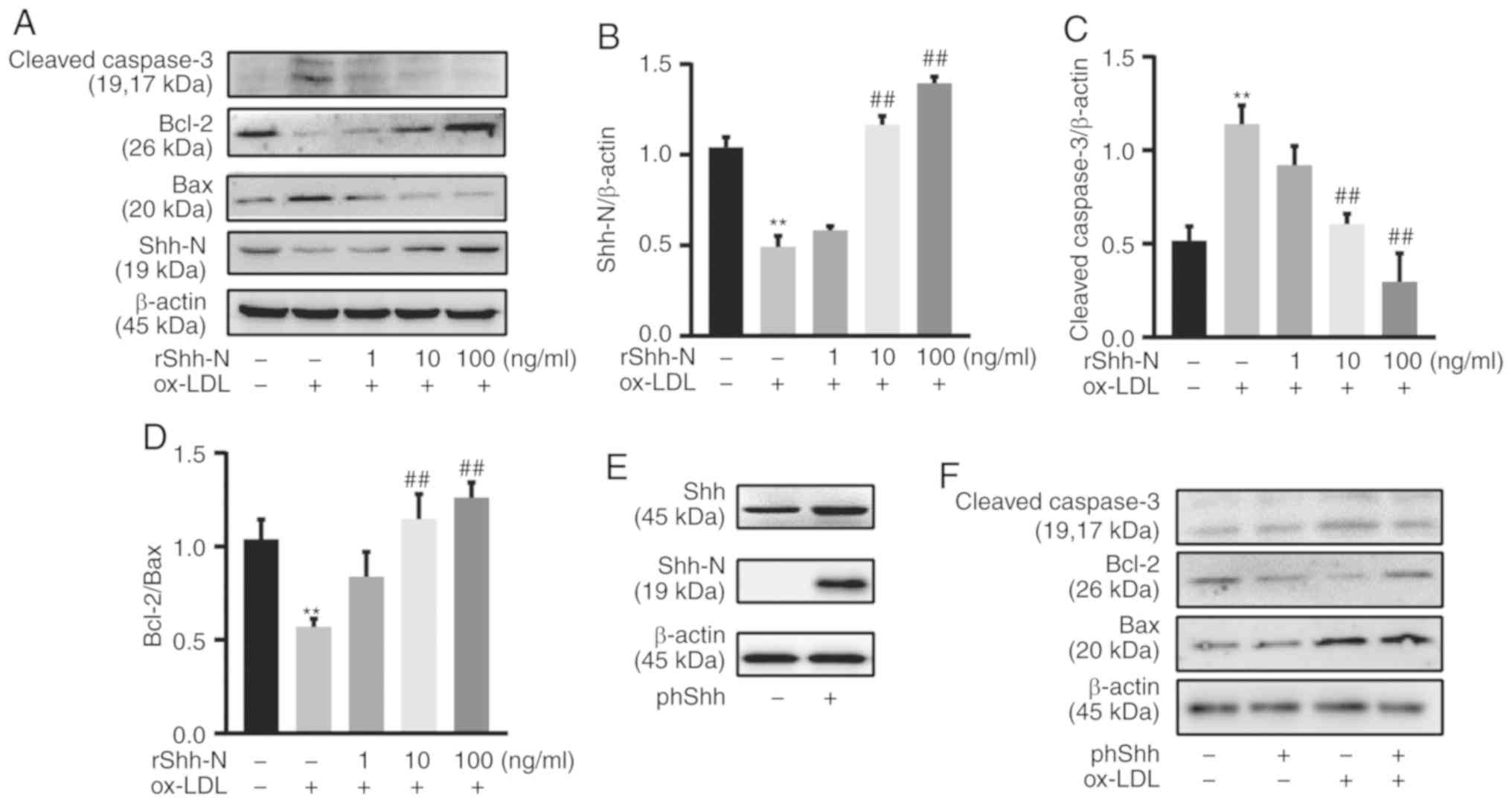 | Figure 3Sonic hedgehog attenuates HUVEC
apoptosis. (A) HUVECs were incubated with rShh-N for 24 h and then
stimulated with 50 µg/ml ox-LDL for 24 h. Representative
western blotting bands of cleaved caspase 3, Bcl-2, Bax and Shh-N
are presented. β-actin was used as an internal loading control.
Western blot analysis of (B) Shh-N and (C) cleaved caspase 3
relative protein expression and (D) Bcl-2/Bax ratio. (E) HUVECs
were transfected with phShh for 48 h, and western blotting
demonstrated that the transfection was successful. (F) HUVECs were
transfected with phShh for 48 h and then stimulated with 50
µg/ml ox-LDL for 24 h. Representative western blotting bands
of cleaved caspase 3, Bcl-2 and Bax are presented. β-actin was used
as an internal loading control. Western blot analysis of (G)
cleaved caspase 3 relative protein expression and (H) Bcl-2/Bax
ratio. (I) HUVECs were incubated with rShh-N for 24 h and then
stimulated with 50 µg/ml ox-LDL for 24 h, and caspase 3
activity was determined at A405 nm using a caspase 3 activity assay
kit. (J) HUVECs were transfected with phShh for 48 h, and then
stimulated with 50 µg/ml ox-LDL for 24 h. Caspase 3 activity
was determined at A405 nm using a caspase 3 activity assay kit. (K)
HUVECs were transfected with phShh for 48 h, and then stimulated
with 50 µg/ml ox-LDL for 24 h, and apoptosis was analyzed by
Annexin V/PI staining. Q1, dead cells; Q2, late apoptotic cells;
Q3, early apoptotic cells; and Q4m viable cells. Apoptotic cells
were considered as Q2 and Q3. (L) HUVECs were incubated with rShh-N
for 24 h and then stimulated with 50 µg/ml ox-LDL for 24 h,
and apoptosis was analyzed by Annexin V/PI staining. All
experiments were repeated three times and the data are presented as
the mean ± standard deviation. **P<0.01 vs. untreated
control. ##P<0.01 vs. ox-LDL only-treated group.
ox-LDL, oxidized low-density lipoprotein; HUVEC, human umbilical
vein endothelial cell; Shh, Sonic hedgehog; Shh-N, Sonic hedgehog
N-terminus; rShh-N, recombinant Shh-N protein; phShh, plasmid
encoding the human Shh gene; PI, propidium iodide. |
Cyclopamine abolishes the effects of
Shh-suppressed endothelial apoptosis
Cyclopamine, a hedgehog signaling inhibitor
(21), was used to investigate
the effects of Shh-suppressed endothelial apoptosis. The HUVECs
were pretreated with 20 µM cyclopamine for 2 h, then treated
with 10 ng/ml rShh-N for 24 h and ox-LDL for a further 24 h.
Following this treatment, cleaved caspase 3 expression increased
and the Bcl-2/Bax ratio decreased significantly compared with the
rShh-N and ox-LDL treatment group, suggesting that cyclopamine
inhibited the anti-apoptotic effect of rShh-N (Fig. 4A-C). Notably, cyclopamine
significantly reversed the Shh-mediated downregulation of caspase 3
activity, and even increased this activity to some extent (Fig. 4D). Finally, Annexin V/PI staining
demonstrated that compared with the rShh-N and ox-LDL co-culture
group, the percentage of apoptotic cells was increased in the group
also pretreated with cyclopamine, indicating that in the presence
of cyclopamine, the rShh-N treatment no longer decreases cell
apoptosis (Fig. 4E). Thus,
cyclopamine abolished the protective effect of the recombinant
Shh-N protein.
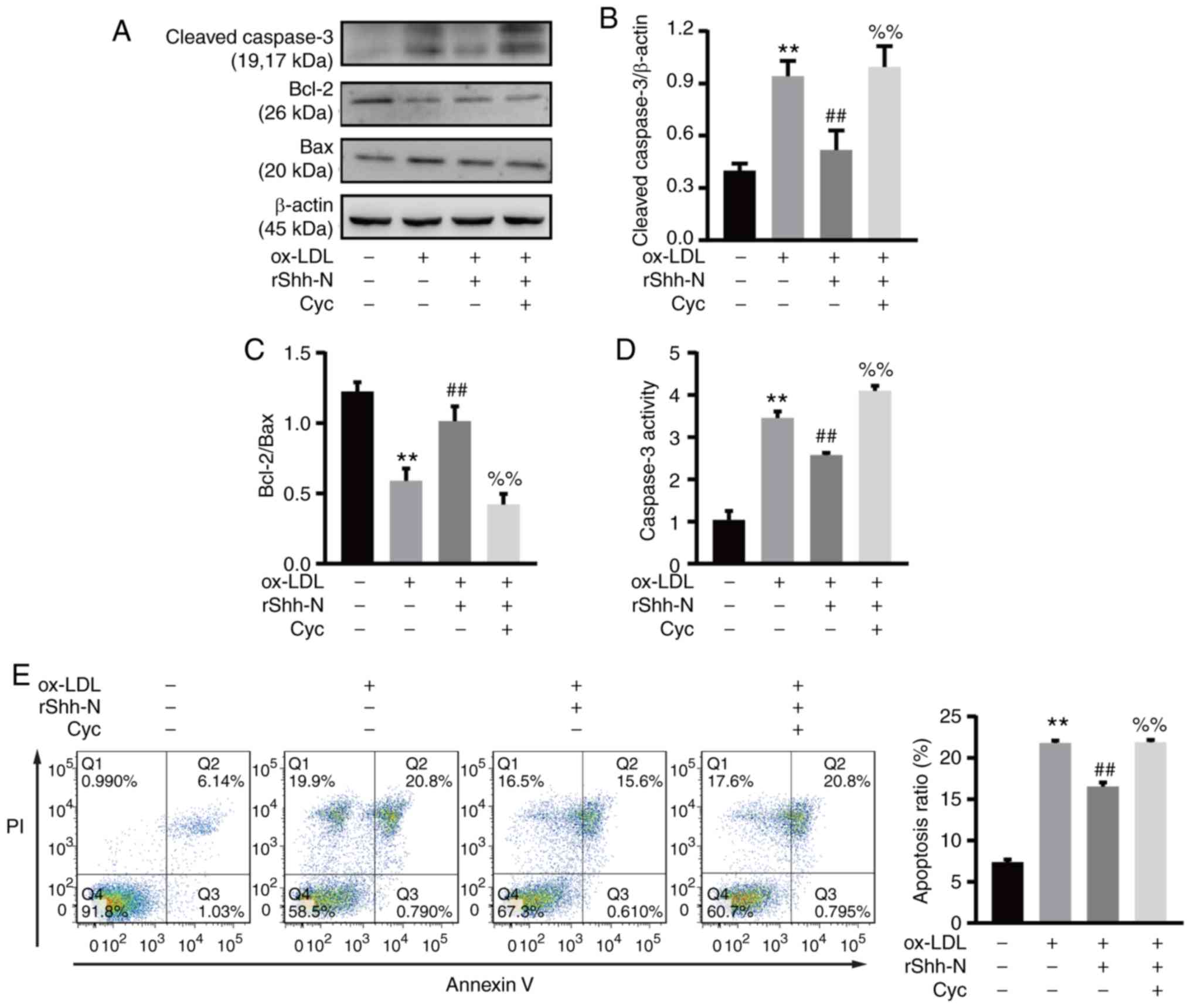 | Figure 4Cyc reverses the effects of
Shh-suppressed apoptosis. Human umbilical vein endothelial cells
were incubated with 20 µM Cyc for 2 h and then co-cultured
with 10 ng/ml rShh-N for 24 h and stimulated with 50 µg/ml
ox-LDL for 24 h. (A) Representative western blot bands of cleaved
caspase 3, Bcl-2 and Bax. β-actin was used as an internal loading
control. Analysis of (B) cleaved caspase 3 relative protein
expression and (C) Bcl-2/Bax ratio. (D) Caspase 3 activity was
determined at A405 nm by using a caspase 3 activity assay kit. (E)
The treated HUVECs were analyzed by Annexin V/PI staining. Q1, dead
cells; Q2, late apoptotic cells; Q3, early apoptotic cells; and Q4,
viable cells. Apoptotic cells were considered as Q2 and Q3. All
experiments were repeated three times and the data are presented as
the mean ± standard deviation. **P<0.01 vs. untreated
control group. ##P<0.01 vs. ox-LDL only-treated
group. %%P<0.01 vs. rShh-N and ox-LDL-treated group.
Cyc, cyclopamine; Shh, Sonic hedgehog; Shh-N, Sonic hedgehog
N-terminus; rShh-N, recombinant Shh-N protein; ox-LDL, oxidized
low-density lipoprotein; PI, propidium iodide. |
Shh suppresses NF-κB signaling pathway
phosphorylation in ox-LDL-induced HUVECs
To evaluate whether Shh could interact physically
with NF-κB p65, a co-IP experiment was performed.
Immunoprecipitated Shh from HUVEC lysates produced clear bands for
NF-κB p65 and, as expected, immunoprecipitated NF-κB p65 could bind
to Shh, revealing that Shh and NF-κB p65 formed a complex (Fig. 5A). Ox-LDL can induce NF-κB
activation within 30–60 min (22). To further investigate whether
NF-κB signaling was regulated by Shh, the canonical phosphorylation
targets in NF-κB signaling were analyzed. Treatment with rShh-N
significantly decreased p-IKKα/β and p-IκBα levels during a limited
period between 30 and 120 min after ox-LDL treatment (Fig. 5B-D). phShh transfection also
significantly decreased p-IKKα/β and p-IκBα levels at 60 and 120
min following ox-LDL treatment (Fig.
5E-G). Specific NF-κB p65 phosphorylation at its ser 536
residue increased progressively and reached a maximum at 60 min.
After only 30 min of ox-LDL treatment, rShh-N and phShh
significantly decreased NF-κB p65 phosphorylation compared with the
ox-LDL control group (Fig. 5H and
I). Cyclopamine significantly reversed the rShh-N-mediated
downregulation of NF-κB p65 phosphorylation (Fig. 5J). Together, these findings
revealed that Shh exerts its effects on NF-κB signaling by reducing
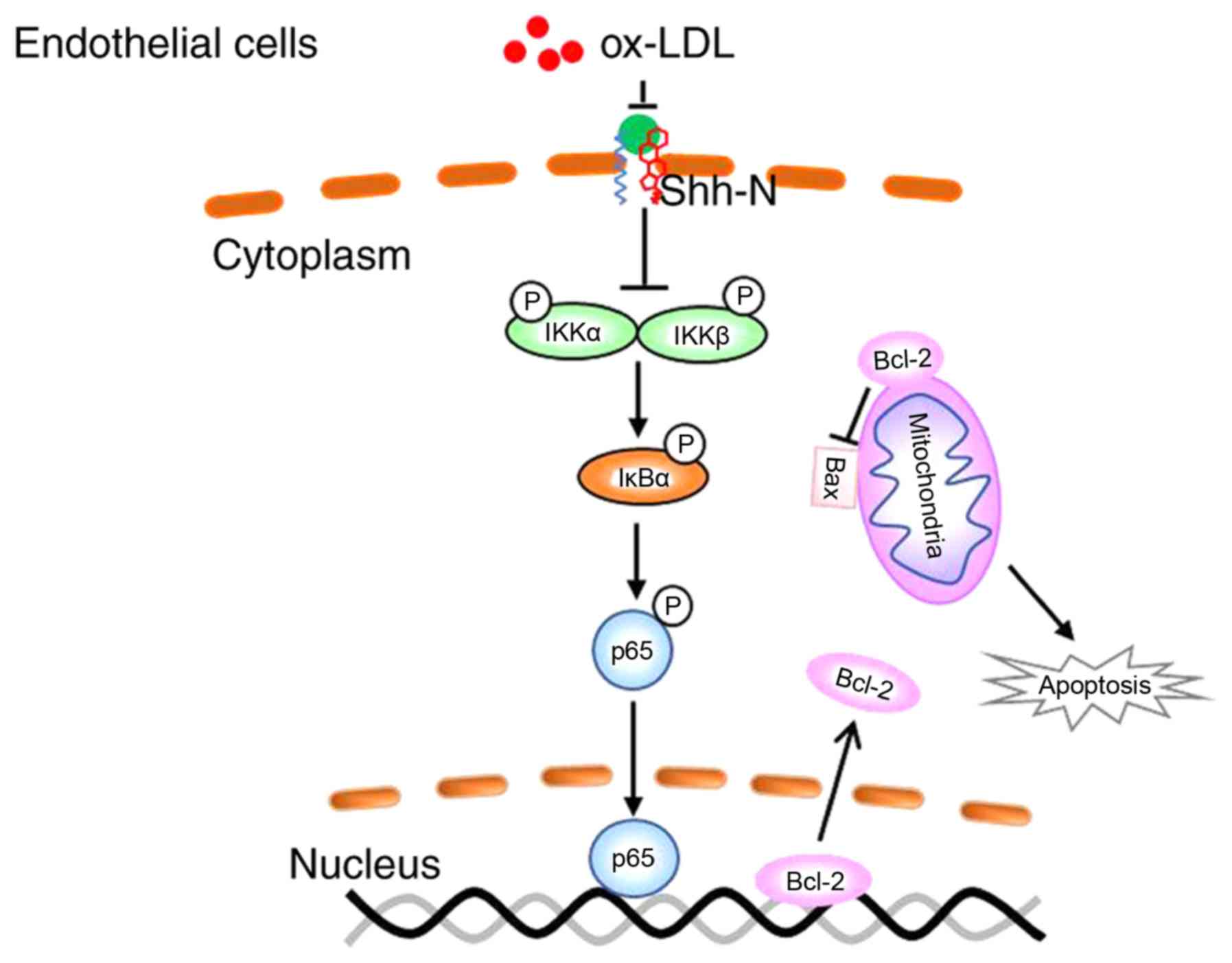
the phosphorylation of p65, IKKα/β and IκBα (Fig. 6).
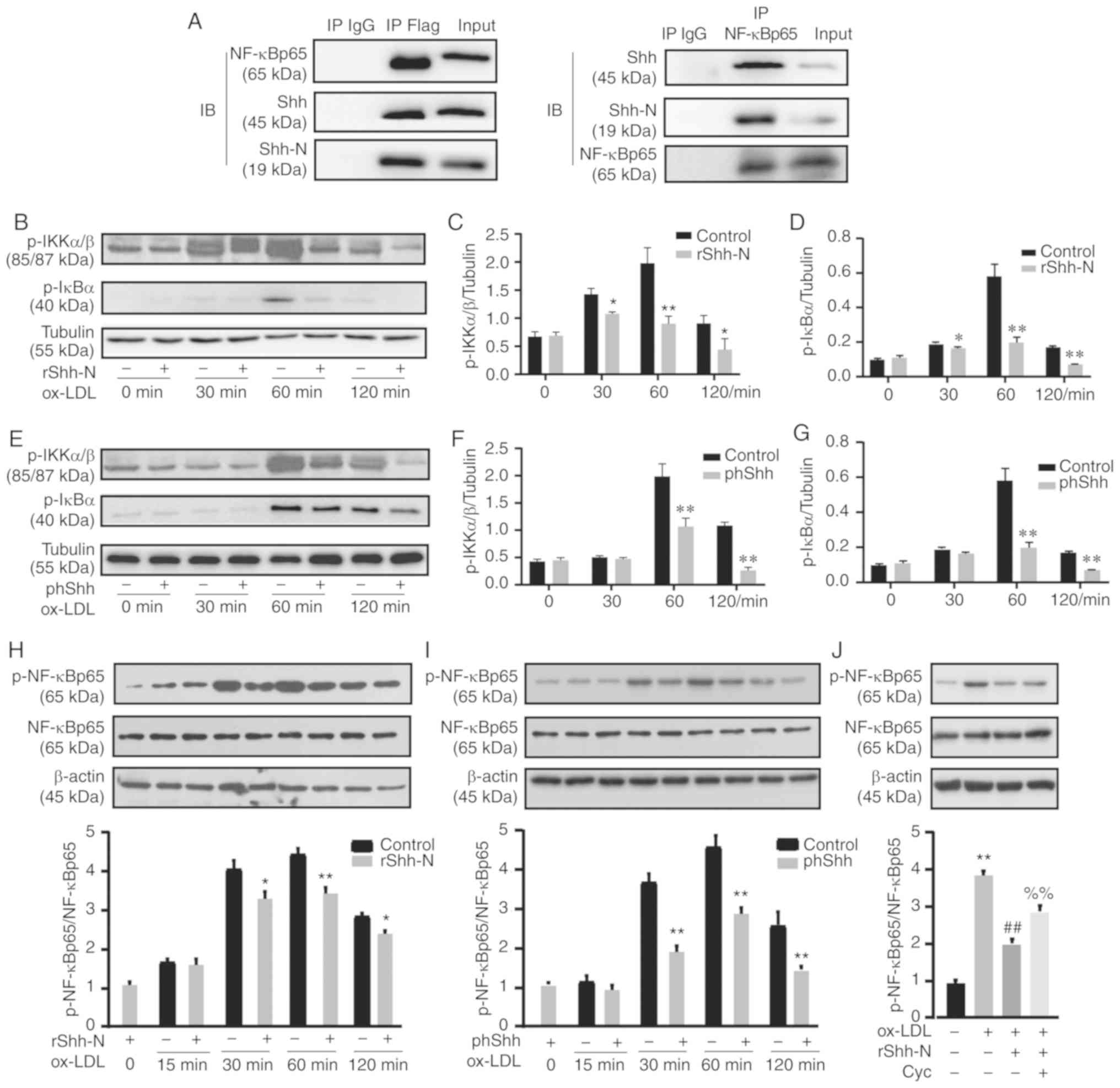 | Figure 5Shh suppresses the phosphorylation of
the NF-κB signaling pathway. (A) Co-immunoprecipitation assay was
used to reveal the association of Shh and NF-κB p65. (B) HUVECs
were co-cultured with 10 ng/ml rShh-N for 24 h and then stimulated
with 50 µg/ml ox-LDL for 30-120 min. Representative western
blotting bands of p-IKKα/β, total IKKα/β, p-IκBα and total IκBα.
Tublin was used as an internal loading control. Relative changes in
protein intensity were quantified for (C) p-IKKα/β and (D) p-IκBα.
(E) HUVECs were transfected with phShh for 48 h and then stimulated
with 50 µg/ml ox-LDL for 30-120 min. Representative western
blotting bands of p-IKKα/β, total IKKα/β, p-IκBα and total IκBα.
Tublin was used as an internal loading control. Relative changes in
protein intensity were quantified for (F) p-IKKα/β and (G) p-IκBα.
(H) HUVECs were co-cultured with 10 ng/ml rShh-N for 24 h and then
stimulated with 50 µg/ml ox-LDL for 15-120 min. Western blot
analysis of p-NF-κB p65 and NF-κB p65 relative protein expression.
β-actin was used as an internal loading control. (I) Following
transfection with phShh, HUVECs were stimulated with 50
µg/ml ox-LDL for 15–120 min, and western blot analysis of
p-NF-κB p65 and NF-κB p65 relative protein expression was
performed. β-actin was used as an internal loading control. (J)
Cells were incubated with Cyc 20 µM for 2 h and then
co-cultured with 10 ng/ml rShh-N for 24 h and stimulated with 50
µg/ml ox-LDL for 60 min. Western blot analysis of p-NF-κB
p65 and NF-κB p65 relative protein expression. β-actin was used as
an internal loading control All experiments were repeated three
times and the data are presented as the mean ± standard deviation.
*P<0.05, **P<0.01 vs. control.
##P<0.01 vs. ox-LDL only-treated group.
%%P<0.01 vs. rShh-N and ox-LDL-treated group. Cyc,
cyclo-pamine; IP, immunoprecipitate; Shh, Sonic hedgehog; Shh-N,
Sonic hedgehog N-terminus; rShh-N, recombinant Shh-N protein;
ox-LDL, oxidized low-density lipoprotein; p-, phosphorylated;
phShh, plasmid encoding the human Shh gene. |
Discussion
ASCVD is a global health problem that causes death
and a substantial economic burden. Perturbation of endothelial
integrity initiates atherosclerosis (20). EC apoptosis may result in the loss
and turnover of ECs, which contributes to increased vascular
permeability, and facilitates the migration and deposition of
lipids, further damaging the vasculature and propagating plaque
development (23). Additionally,
large-scale EC apoptosis results in the denudation of monolayer
cells, and exposure of the underlying extracellular matrix can
promote thrombosis and increase the risk of plaque disruption
(24). The inhibition of EC
apoptosis has become a vital target for the prevention and
treatment of atherosclerosis (25). The present study demonstrated that
activation of the Shh pathway is weakened in ox-LDL-induced HUVECs,
that administration of Shh has a protective effect against ox-LDL
induced HUVEC apoptosis, and that the protective effect of Shh is
mediated by NF-κB p65 pathway in HUVECs.
Previous studies revealed that classical Shh pathway
components are obviously decreased in the atherosclerotic
vasculature (12) and anti-CD31
antibody labeling of ECs produces Shh immunoreactivity that is not
detected in carotid and femoral artery plaques (13). Recently, structural biology
studies have focused on the modulated or interaction sites of
cholesterol in hedgehog signaling, revealing a direct interaction
between cholesterol and Smo, a critical component of the hedgehog
signaling cascade, which suggests potential drug-gable sites
(7,26-29). LDL is the main carrier of
cholesterol, and ox-LDL plays an important role in atherogenesis
(30). The present study used
ox-LDL simulation in a hyperlipid-emia-induced atherosclerosis in
EC, and the results suggested that the expression of Shh, Shh-N,
Ptch, Smo and Gli1 were downregulated in a dose-dependent manner,
which is consistent with the atherosclerotic vasculature studies
in vivo.
It has been reported that Shh is pivotally important
to vascular remodeling, and induces vascular endothelial growth
factor and angiopoietin levels (31). Intramyocardial gene transfer of
phShh could preserve left ventricular function by activating
hedgehog signaling, and enhanced neovascularization also reduces
fibrosis and cardiac apoptosis in acute and chronic myocardial
ischemia in adult animals (32).
A prior study revealed the protective effect of Shh in astrocytes
under oxidative stress and the protective effect of Shh in rat
astrocytes under H2O2 treatment, which is
considered a neuroprotective role against brain injury that is
mediated by the PI3K/AKT pathway (33). Compared with previous studies, the
present study suggested that NF-κB signaling is critical for the
regulation of HUVEC apoptosis by Shh. The effects of the pathway
under oxidative stress depends on the different factors, such as
cell types, cellular stimuli and environments condition. In
addition, Shh could increase eNOS expression and correct
angiotensin II-induced hypertension and endothelial dysfunction
(34). The present data clearly
demonstrate that overexpression of Shh, either by recombinant Shh-N
protein or the plasmid encoding the human Shh gene, reduces
ox-LDL-induced HUVEC apoptosis by enhancing Bcl-2 expression to
regulate mitochondrial apoptosis signaling, and a subsequent
downregulation of caspase 3 activity in ox-LDL-treated HUVECs.
Furthermore, the anti-apoptotic effect of Shh could be reversed by
blocking the Shh signaling pathway with cyclopamin.
Shh undergoes autoproteolysis and yields a mature
Shh-N fragment that is modified with N-terminal palmitoyl and
C-terminal cholesteryl moieties. Ptch is a 12-transmembrane domain
membrane protein and TM2-6 is annotated as its sterol-sensing
domain, which is involved in the interaction with Shh-N (35). The classical Shh signaling pathway
is initiated by the interaction between Shh-N and Ptch, which
relieves the suppression of Smo, subsequently leading to the
activation of Gli transcription factor (36). Shh induces angiogenesis in ECs,
which does not activate Gli expression or Gli-dependent
transcription, but is mediated by the Rho/ROCK pathway (37). Furthermore, several transduction
pathways have been reported to be stimulated by Shh. Shh induces
angiogenesis in fibroblasts and cardiomyocytes by targeting Notch
or Kruppel like factor 2, subsequently activating vascular
endothelial growth factor-A expression (38-40). Shh also exhibits an anti-apoptotic
effect mediated by the PI3K/AKT/Bcl-2 pathway in astrocytes
(33). In addition, Cai et
al (19) revealed that Shh
cross-talks with the NF-κB signaling pathway in multiple myeloma
cell lines, and Shh regulates the NF-κB signaling pathway by its
classical Smo/Ptch/Gli1 pathway and also by the non-classical
Gli1-independent pathway. In the present study, immunoprecipitated
Shh from HUVEC lysates revealed the interaction between Shh and
NF-κB p65. Shh was able to reduce the phosphorylation of IKKα/β,
IκBα and NF-κB p65 stimulated by ox-LDL. Furthermore, the
phosphorylation of NF-κB p65 could be reversed by blocking the Shh
signaling pathway with cyclopamin. These results confirmed that
hedgehog signaling is responsible for decreasing the
phosphorylation and activity of NF-κB signaling in ox-LDL-induced
HUVECs, which reveals a potential mechanism for improving
endothelial apoptosis caused by oxidative stress. The absence of
data on whether the enriched proteins could interact with other
known binding proteins under ox-LDL treatment is a limitation of
the present study.
The morphogen Shh promotes neovascularization in
adults (37). In arterial
occlusion conditions, blood vessels respond by forming a new
capillary network that benefits collateral circulation; however,
angiogenesis in atherosclerotic plaques may cause plaque
instability, which plays a critical role in the pathogenesis of
strokes. Intraplaque neovessels originating from the adventitial
vasa vasorum, monocytes or vascular smooth muscle cells may be
immature and hence susceptible to rupture (41–43). The mechanisms of angiogenesis in
atherosclerosis remain unknown, and its effects on ASCVD remain
controversial. However, these effects may be associated with when
and where angiogenesis occurs. Agonists and physical or chemical
stress can stimulate various cells to generate small
membrane-derived vesicles (0.05–1 µm diameter) expressing
Shh and the vesicles subsequently shed to neighboring cells,
therefore it is reasonable to consider that Shh has a feedback
effect on smooth muscle cells or monocytes; however, the detailed
mechanism still requires further investigation (44).
Collectively, the present results are relevant to
understanding the association between Shh and pathological
atherosclerosis. It was first identified that Shh is a protective
protein in ox-LDL-mediated endothelial apoptosis, and that Shh
markedly alleviates ox-LDL-induced endothelial apoptosis by
blocking the phosphorylation of NF-κB signaling pathway proteins
and Bcl-2-controlled mitochondrial signaling.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural
Science Foundation of China (grant nos. 81873515, 81670258 and
81373785), the Natural Science Foundation of Fujian (grant no.
2017J01247) and the Startup Fund for Scientific Research, Fujian
Medical University (grant no. 2017XQ2045).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HH designed the study. HH, HY and LL performed the
experiments and collected the data. HH, PZ and JC analyzed the data
and wrote the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The research protocol was approved by the Ethics
Committee of Fujian Provincial Hospital (Fuzhou, China; approval
no. K2018-09-008), and written informed consent was provided by the
parents of the newborns. All procedures were conducted in
compliance with the Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Arnett DK, Blumenthal RS, Albert MA,
Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A,
Lloyd-Jones D, McEvoy JW, et al: 2019 ACC/AHA Guideline on the
primary prevention of cardiovascular disease A Report of the
American College of Cardiology/American Heart Association Task
Force on Clinical Practice Guidelines. Circulation. 140. pp.
e596–e646. 2019
|
|
2
|
Gimbrone MA Jr and García-Cardeña G:
Endothelial cell dysfunction and the pathobiology of
atherosclerosis. Circ Res. 118:620–636. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bar A, Targosz-Korecka M, Suraj J,
Proniewski B, Jasztal A, Marczyk B, Sternak M, Przybyło M,
Kurpińska A, Walczak M, et al: Degradation of glycocalyx and
multiple manifestations of endothelial dysfunction coincide in the
early phase of endothelial dysfunction before atherosclerotic
plaque development in apolipoprotein E/low-density lipoprotein
receptor-deficient mice. J Am Heart Assoc. 8:e0111712019.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ference BA, Ginsberg HN, Graham I, Ray KK,
Packard CJ, Bruckert E, Hegele RA, Krauss RM, Raal FJ, Schunkert H,
et al: Low-density lipoproteins cause atherosclerotic
cardiovascular disease. 1. Evidence from genetic, epidemiologic,
and clinical studies. A consensus statement from the European
Atherosclerosis Society Consensus Panel. Eur Heart J. 38:2459–2472.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Falkenstein KN and Vokes SA:
Transcriptional regulation of graded Hedgehog signaling. Semin Cell
Dev Biol. 33:73–80. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Qi X, Schmiege P, Coutavas E, Wang J and
Li X: Structures of human Patched and its complex with native
palmitoylated sonic hedgehog. Nature. 560:128–132. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang P, Zheng S, Wierbowski BM, Kim Y,
Nedelcu D, Aravena L, Liu J, Kruse AC and Salic A: Structural basis
of smoothened activation in hedgehog signaling. Cell. 175:295–297.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Briscoe J and Therond PP: The mechanisms
of Hedgehog signalling and its roles in development and disease.
Nat Rev Mol Cell Biol. 14:416–429. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lavine KJ, Kovacs A and Ornitz DM:
Hedgehog signaling is critical for maintenance of the adult
coronary vasculature in mice. J Clin Invest. 118:2404–2414.
2008.PubMed/NCBI
|
|
10
|
Xiao Q, Hou N, Wang YP, He LS, He YH,
Zhang GP, Yi Q, Liu SM, Chen MS and Luo JD: Impaired sonic hedgehog
pathway contributes to cardiac dysfunction in type 1 diabetic mice
with myocardial infarction. Cardiovasc Res. 95:507–516. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Beckers L, Heeneman S, Wang L, Burkly LC,
Rousch MM, Davidson NO, Gijbels MJ, de Winther MP, Daemen MJ and
Lutgens E: Disruption of hedgehog signalling in ApoE −/− mice
reduces plasma lipid levels, but increases atherosclerosis due to
enhanced lipid uptake by macrophages. J Pathol. 212:420–428. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Queiroz KC, Bijlsma MF, Tio RA, Zeebregts
CJ, Dunaeva M, Ferreira CV, Fuhler GM, Kuipers EJ, Alves MM, Rezaee
F, et al: Dichotomy in Hedgehog signaling between human healthy
vessel and atherosclerotic plaques. Mol Med. 18:1122–1127. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dunaeva M, van Oosterhoud C and
Waltenberger J: Expression of Hedgehog signaling molecules in human
atherosclerotic lesions: An autopsy study. Int J Cardiol.
201:462–464. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Agouni A, Mostefai HA, Porro C, Carusio N,
Favre J, Richard V, Henrion D, Martínez MC and Andriantsitohaina R:
Sonic hedgehog carried by microparticles corrects endothelial
injury through nitric oxide release. FASEB J. 21:2735–2741. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen KY, Cheng CJ and Wang LC: Activation
of sonic Hedgehog leads to survival enhancement of astrocytes via
the GRP78-dependent pathway in mice infected with angio-strongylus
cantonensis. Biomed Res Int. 2015:6743712015.
|
|
16
|
Yu XH, Zheng XL and Tang CK: Nuclear
factor-κB activation as a pathological mechanism of lipid
metabolism and atherosclerosis. Adv Clin Chem. 70:1–30. 2015.
View Article : Google Scholar
|
|
17
|
Maziere C, Auclair M, Djavaheri-Mergny M,
Packer L and Maziere JC: Oxidized low density lipoprotein induces
activation of the transcription factor NF kappa B in fibroblasts,
endothelial and smooth muscle cells. Biochem Mol Biol Int.
39:1201–1207. 1996.PubMed/NCBI
|
|
18
|
Yurdagul A Jr, Sulzmaier FJ, Chen XL,
Pattillo CB, Schlaepfer DD and Orr AW: Oxidized LDL induces
FAK-dependent RSK signaling to drive NF-κB activation and VCAM-1
expression. J Cell Sci. 129:1580–1591. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cai K, Na W, Guo M, Xu R, Wang X, Qin Y,
Wu Y, Jiang J and Huang H: Targeting the cross-talk between the
hedgehog and NF-κB signaling pathways in multiple myeloma. Leuk
Lymphoma. 60:772–781. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lewis LJ, Hoak JC, Maca RD and Fry GL:
Replication of human endothelial cells in culture. Science.
181:453–454. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen JK: I only have eye for ewe: The
discovery of cyclopamine and development of Hedgehog
pathway-targeting drugs. Nat Prod Rep. 33:595–601. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dąbek J, Kułach A and Gąsior Z: Nuclear
factor kappa-light-chain-enhancer of activated B cells (NF-κB): A
new potential therapeutic target in atherosclerosis? Pharmacol Rep.
62:778–783. 2010. View Article : Google Scholar
|
|
23
|
Paone S, Baxter AA, Hulett MD and Poon
IKH: Endothelial cell apoptosis and the role of endothelial
cell-derived extracellular vesicles in the progression of
atherosclerosis. Cell Mol Life Sci. 76:1093–1106. 2019. View Article : Google Scholar
|
|
24
|
Luchetti F, Crinelli R, Cesarini E,
Canonico B, Guidi L, Zerbinati C, Di Sario G, Zamai L, Magnani M,
Papa S and Iuliano L: Endothelial cells, endoplasmic reticulum
stress and oxysterols. Redox Biol. 13:581–587. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Choy JC, Granville DJ, Hunt DW and McManus
BM: Endothelial cell apoptosis: Biochemical characteristics and
potential implications for atherosclerosis. J Mol Cell Cardiol.
33:1673–1690. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hedger G, Koldsø H, Chavent M, Siebold C,
Rohatgi R and Sansom MSP: Cholesterol interaction sites on the
transmembrane domain of the hedgehog signal transducer and Class F
G protein-coupled receptor smoothened. Structure. 27:549–559.e2.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Weiss LE, Milenkovic L, Yoon J, Stearns T
and Moerner WE: Motional dynamics of single Patched1 molecules in
cilia are controlled by Hedgehog and cholesterol. Proc Natl Acad
Sci USA. 116:5550–5557. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang P, Nedelcu D, Watanabe M, Jao C, Kim
Y, Liu J and Salic A: Cellular cholesterol directly activates
smoothened in hedgehog signaling. Cell. 166:1176–1187.e14. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang Y, Bulkley DP, Xin Y, Roberts KJ,
Asarnow DE, Sharma A, Myers BR, Cho W, Cheng Y and Beachy PA:
Structural basis for cholesterol transport-like activity of the
hedgehog receptor patched. Cell. 175:1352–1364.e1314. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rajman I, Eacho PI, Chowienczyk PJ and
Ritter JM: LDL particle size: An important drug target? Br J Clin
Pharmacol. 48:125–133. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bijlsma MF, Peppelenbosch MP and Spek CA:
Hedgehog morphogen in cardiovascular disease. Circulation.
114:1985–1991. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kusano KF, Pola R, Murayama T, Curry C,
Kawamoto A, Iwakura A, Shintani S, Ii M, Asai J, Tkebuchava T, et
al: Sonic hedgehog myocardial gene therapy: Tissue repair through
transient reconstitution of embryonic signaling. Nat Med.
11:1197–1204. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xia YP, Dai RL, Li YN, Mao L, Xue YM, He
QW, Huang M, Huang Y, Mei YW and Hu B: The protective effect of
sonic hedgehog is mediated by the phosphoinositide [corrected]
3-kinase/AKT/Bcl-2 pathway in cultured rat astrocytes under
oxidative stress. Neuroscience. 209:1–11. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Marrachelli VG, Mastronardi ML, Sarr M,
Soleti R, Leonetti D, Martínez MC and Andriantsitohaina R: Sonic
hedgehog carried by microparticles corrects angiotensin II-induced
hypertension and endothelial dysfunction in mice. PLoS One.
8:e728612013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Qi C, Di Minin G, Vercellino I, Wutz A and
Korkhov VM: Structural basis of sterol recognition by human
hedgehog receptor PTCH1. Sci Adv. 5:eaaw64902019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gong X, Qian H, Cao P, Zhao X, Zhou Q, Lei
J and Yan N: Structural basis for the recognition of Sonic Hedgehog
by human Patched1. Science. 361:eaas89352018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Renault MA, Roncalli J, Tongers J, Thorne
T, Klyachko Misener S, Volpert OV, Mehta S, Burg A, Luedemann C, et
al: Sonic hedgehog induces angiogenesis via Rho kinase-signaling in
endothelial cells. J Mol Cell Cardiol. 49:4902010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chinchilla P, Xiao L, Kazanietz MG and
Riobo NA: Hedgehog proteins activate pro-angiogenic responses in
endothelial cells through non-canonical signaling pathways. Cell
Cycle. 570–579. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Caradu C, Couffinhal T, Chapouly C,
Guimbal S, Hollier Ducasse E, Bura-Rivière A, Dubois M and Gadeau
Renault MA: Restoring endothelial function by targeting desert
Hedgehog downstream of Klf2 improves critical limb ischemia in
adults. Circ Res. 123:1053–1065. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Morrow D, Cullen JP, Liu W, Guha S,
Sweeney C, Birney Collins N, Walls D, Redmond EM and Cahill PA:
Sonic Hedgehog induces Notch target gene expression in vascular
smooth muscle cells via VEGF-A. Arterioscler Thromb Vasc Biol.
29:1112–1118. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jaipersad AS, Lip GY, Silverman S and
Shantsila E: The role of monocytes in angiogenesis and
atherosclerosis. J Am C Cardiol. 63:1–11. 2014. View Article : Google Scholar
|
|
42
|
Xu J, Lu X and Shi GP: Vasa vasorum in
atherosclerosis and clinical significance. Int J Mol Sci.
16:11574–11608. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zmysłowski A and Szterk A: Current
knowledge on mechanism of atherosclerosis and pro-atherosclerotic
properties of oxysterols. Lipids Health Dis. 16:1882017. View Article : Google Scholar
|
|
44
|
Soleti R and Martínez MC: Microparticles
harbouring Sonic Hedgehog: Role in angiogenesis regulation. Cell
Adh Migr. 293–295. 2009. View Article : Google Scholar : PubMed/NCBI
|