Introduction
Pancreatic cancer (PCa) has a high degree of
malignancy (1). Early diagnosis
of the disease is often difficult due to the physiological location
and biological characteristics of the pancreas; furthermore, PCa
can rapidly progress into later stages and has poor prognosis and
high mortality rate in advanced stages (2,3).
At present, surgery is the main therapeutic option for treating
PCa. However, as PCa does not show specific symptoms in the early
stages, patients are often diagnosed with the disease at later
stages and therefore lose the optimal chance for surgery (4). Furthermore, patients with PCa
develop multi-drug resistance to chemotherapy drugs, which results
in poor outcomes for radiotherapy and chemotherapy (5,6).
In recent years, previously unrecognized miRNAs and mRNAs have been
detected and investigated as effective methods for the clinical
diagnosis and treatment of PCa (7-9).
As a class of single-stranded nucleotides, miRNAs
participate in the coding and synthesis of proteins and serve
critical roles in the physiological and pathological processes of
cancer (10). miRNAs can inhibit
the expression of target proteins by binding to the 3′-untranslated
region (3′-UTR) of the targeted genes, thereby restraining or
degrading the translation and expression of mRNAs (11). Previous studies have demonstrated
that miRNAs are associated with the migration and deterioration of
tumors (12-14). Previous studies also demonstrated
that miR-615-5p (15), miR-153
(16) and miR-206 (17) inhibited the metastasis of PCa,
while miR-10b (18), miR-212
(19) and miR-367 (20) promoted the migration of PCa.
miR-539 was revealed to act as a tumor suppressor
gene in different cancer types, including gastric cancer, prostate
cancer and lung cancer (21-24). Low expression of miR-539 has been
reported in gastric cancer tissues and cell lines, and this was
correlated with the differentiation, clinical stage, poor survival
outcome and lymph node metastasis of the disease (25). Although previous studies have
reported that the role of miR-539 in cancer is mainly acting as a
tumor suppressor, only few studies have investigated the specific
mechanisms of miR-539 in PCa. Specificity protein 1 (Sp1) was one
of the earliest transcription factors identified, and it belongs to
the Sp1/Krüppel-like factor transcription factor family of
sequence-specific DNA binding proteins (26,27). High expression of Sp1 has been
detected in gastric cancer and is associated with a poor prognosis
of the disease (28). In
addition, abnormal Sp1 activation may improve the growth,
metastasis and dedifferentiation of PCa and breast cancer (29,30). The results of these studies
indicated that Sp1 may serve an important role in cancer
development.
In order to further investigate the role of miRNAs
in PCa, the present study focused on the effects of miR-539 on PCa.
The roles of miR-539 in the proliferation, apoptosis, migration and
invasion of PCa cells were analyzed by upregulating and inhibiting
the expression of miR-539 in several PCa cell lines. Furthermore,
the target gene of miR-539 and the mechanism were studied. The
results of the present study provide a molecular mechanism and
alternative treating strategy to PCa.
Materials and methods
Tissue samples
PCa tissues and paired normal adjacent tissues were
collected from patients with PCa (36 males and 20 females; age
range, 29-70 years; median age, 61 years) who received surgical
resection in The First Affiliated Hospital, Zhejiang University
from January to December 2018. Those who had received radiotherapy,
chemotherapy, traditional Chinese medicine or immunotherapy prior
to surgery were excluded, and patients enrolled in the study
received routine preoperative auxiliary tests. Carcinoma tissues
and their paired normal adjacent tissues (2-3 cm from the margin of
the carcinoma tissue) were separated from surgical specimens within
30 min, temporarily frozen in liquid nitrogen and stored in a
refrigerator at −80°C for expression detection. The present study
was approved by the Ethics Committee of the First Affiliated
Hospital (approval no. ZJ2018121017), Zhejiang University and
written informed consent was obtained from all participants.
Cell culture
Non-cancerous pancreatic cells (hTRET-HPNE) and PCa
cell lines (CAPAN-2, BxPC3, CFPAC1, SW1990 and PANC1) were
purchased from American Type Culture Collection. All cells were
cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA), containing 10% fetal
bovine serum (FBS; HyClone; GE Healthcare Life Sciences), 100
µg/ml streptomycin, 100 units/ml penicillin and 2 mmol/l
glutamine (Gibco; Thermo Fisher Scientific, Inc.) in a humid
environment at 37°C with 5% CO2.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from tissues and cells using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.). The
purity and concentration of the extracted total RNAs were
determined by NanoDrop-2000c ultramicro spectrophotometer (Thermo
Fisher Scientific, Inc.) and 1% agarose modified gel
electrophoresis. Taqman microRNA RT kit (Applied Biosystems; Thermo
Fisher Scientific, Inc.) and corresponding primers were used to
reverse transcribe the total RNAs into cDNAs, the reverse
transcription condition as follows: At 42°C for 40 min, at 85°C for
5 min. SYBR-Green I mix reagent (Toyobo Life Science) and ABI Ⅶ A7
fluorescence quantitative PCR reactor were used for amplifying the
reaction under the following conditions: Pre-degeneration at 95°C
for 30 sec, denaturation at 95°C for 5 sec, annealing at 60°C for
34 sec for a total of 40 cycles. Primer sequences were synthesized
by Shanghai Gene Pharma Co., Ltd. (Shanghai, China) and are
presented in Table I.
Quantitative analysis was conducted using the 2-ΔΔCq
method (31), and the expression
of U6 served as the internal reference.
 | Table IPrimer base sequence. |
Table I
Primer base sequence.
| Gene | Forward
(5′-3′) | Reverse
(5′-3′) |
|---|
| miR-539 |
GAAGAGGCTAACGTGAGGTTG |
CACCATGACCAAGCCACGTAG |
| U6 |
GCTTCGGCAGCACATATACTAAAAT |
CGCTTCACGAATTTGCGTGTCAT |
Cell transfection
SW1990 and BxPC3 cells in the logarithmic growth
stage were inoculated into 6-well plates at a concentration of
1×105 cells/well. SW1990 cells were respectively
transfected with blank, miR-539 mimic (5′-GGA GAA AUU AUC CUU GGU
GUG U-3′; cat. no. 4464066; Ambion; Thermo Fisher Scientific, Inc.)
and mimic control (MC; 5′-UUU GUA CUA CAC AAA AGU ACU G-3′), while
BxPC3 cells were respectively transfected with blank, miR-539
inhibitor (5′-ACA CAC CAA GGA UAA UUU CUC C-3′; cat. no. 4464084;
Ambion; Thermo Fisher Scientific, Inc.) and inhibitor control (IC;
5′-CAG UAC UUU UGU GUA GUA CAA-3′). In addition, the SW1990 cells
were also co-transfected with MC and negative control (NC), MC and
SP1, mimic and NC (pcDNA3.1 empty plasmid), and mimic and SP1
(pcDNA3.1-SP1 plasmid; Ambion; Thermo Fisher Scientific, Inc.).
Similarly, the BxPC3 cells were co-transfected with IC and siNC
(5′-UUC UCC GAA CGU GUC ACG U-3′), IC and siSP1 (5′-CAG AUA CCA GAC
CUC UUC U-3′; cat. no. 4392420; Ambion; Thermo Fisher Scientific,
Inc.), inhibitor and siNC, and inhibitor and siSP1. A total of 5
µg/well Lipofectamine 2000 reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) was used for cell transfection. All the
cells were cultured in the medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc.) containing 10% FBS, 50 U/ml penicillin and 50
µg/ml streptomycin at 37°C with 5% CO2.
Cell activity
Cell Counting kit-8 (CCK-8; Dojindo Molecular
Technologies, Inc.) was mixed with the culture medium at a ratio of
1:10 to detect the proliferation abilities of SW1990 and BxPC3
cells following the manufacturer's protocol. The cells were
transferred into the 96-well plates at a density of
5×104/well, and 110 µl mixed medium was added.
The plates were maintained in the dark at 37°C with 5%
CO2, and optical density (OD) was recorded using a
microplate reader (BioTek Instruments, Inc.) at 450 nm after 24, 48
and 72 h of cultivation.
Cell apoptosis
Annexin V-FITC Apoptosis Detection kit (BD
Biosciences) was used to determine the apoptotic rates of SW1990
and BxPC3 cells. The culture medium was removed 48 h after
transfection. The cells were washed with pre-cooled
phosphate-buffered saline (PBS) at 4°C and digested by 0.25%
trypsin. Next, the supernatant was discarded (800 × g) by
centrifugation, and sediments were washed twice with PBS. According
to the manufacturer's protocol, 5 µl fluorescein
isothiocyanate (FITC) and 5 µl propidium iodide (PI) were
added into the cells and incubated together at room temperature in
the dark for 15 min. Cell apoptosis was analyzed by Beckman
CoulterFC500 (Beckman Coulter, Inc.).
Cell cycle
The transfected cells (SW1990 and BxPC3; at
1×105) were collected, washed with PBS and fixed using
70% ice ethanol overnight at 4°C. Next, cell DNA was stained with 1
mg/ml RNase A and 50 mg/ml PI at room temperature for 30 min. Cell
Lab Quanta SC flow cytometry (Beckman Coulter, Inc.) was used to
analyze the cell cycles in G1, S and G2 phases.
Cell migration
The cells (SW1990 and BxPC3) were inoculated at
1×105/ml into 6-well plates and cultured at 37°C with 5%
CO2 for 24 h. After the cells have been covered with
monolayer, scratches were created in the center of the 6-well
plates using a 1,000 µl spear head. Images were captured
using an inverted light microscope (Eclipse TS-100; Nikon
Corporation) at ×100 magnification at 0 and 48 h.
Cell invasion
Transwell invasion assays (Costar; Corning, Inc.)
were performed to detect cell invasion abilities. A total of 50
µl Matrigel (Costar; Corning, Inc.) at a dilution ratio of
1:8 was inserted into the upper chamber of the Transwell and
solidified at 37°C for 30 min. Cell suspension (serum-free medium)
at a density of 1×105/ml was added to the upper chamber,
while the lower chamber was covered with DMEM containing 20% FBS.
The chambers were cultured at 37°C with 5% CO2 for 48 h.
After the cells had been fixed with 70% ethanol for 30 min at room
temperature and stained with 0.1% crystal violet for 20 min at room
temperature, the number of cells was counted under a light
microscope (magnification, ×200; Zeiss AG).
Western blot analysis
Western blotting (WB) was performed to determine the
expression levels of proteins associated with
epithelial-mesenchymal transition (EMT). The total intracellular
proteins were extracted using radioimmunoprecipitation assay lysis
buffer (Beyotime Institute of Biotechnology, Haimen, China), and
the protein concentration was determined using bicinchoninic acid
(Pierce; Thermo Fisher Scientific, Inc.). A total of 50 µg
protein was separated on 10-12% SDS-PAGE (Beyotime Institute of
Biotechnology) and transferred to polyvinylidene difluoride
membranes (GE Healthcare) and then blocked with 5% non-fat milk at
room temperature for 2 h. Primary antibodies: E-cadherin (E-cad;
cat. no. 14472; Cell Signaling Technology, Inc.), N-cadherin
(N-cad; cat. no. 14215; Cell Signaling Technology, Inc.), Snail
(cat. no. ab53519; Abcam), SP1 (cat. no. ab13370; Abcam) and GAPDH
(cat. no. ab8245; Abcam) at a dilution of 1:1,000 at 4°C were used
to incubate the membranes overnight. GAPDH served as internal
reference. Next, a horseradish peroxidase-labeled secondary
antibody (dilution, 1:1,000; cat. no. ab6728; Abcam) was added to
incubate the membranes for another hour at room temperature. An
enhanced chemiluminescence kit (Thermo Fisher Scientific, Inc.) was
used for color development.
Dual-luciferase reporter
Targetscan 7.2 (http://www.targetscan.org/) predicted that SP1 was
possibly the target gene for miR-539, and the prediction was
further verified by double-luciferase reporter gene analysis. The
SP1 3′-UTR region containing the sequence of miR-539 binding site
was inserted into the pMIR-reporter vector (Guangzhou RiboBio Co.,
Ltd.) to construct mutant carrier (SP1-MUT) and wild-type vector
(SP1-WT). miR-539 mimic was transfected into SW1990 cells with
SP1-MUT or SP1-WT using Lipofectamine 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.), while BxPC3 cells were respectively
co-transfected with miR-539 inhibitor and the two plasmids. The
cells were collected 48 h after the transfection, and
double-luciferase reporter gene analysis system (Promega
Corporation) was performed to determine the activities of firefly
and renilla luciferase.
Statistical analysis
Statistical Package of the Social Sciences 20.0
software (IBM Corp.) was used for data analysis. The data are
presented as the mean ± standard deviation, and Student's t-test
was performed for comparison in two groups, while one-way analysis
of variance, followed by the Tukey's test, was conducted for
comparing differences among multiple groups. The association
between miR-539 expression and clinicopathological factors of PCa
was analyzed using the Pearson's χ2 test. All
independent experiments in vitro were performed in
triplicate. P<0.05 was considered to indicate a statistically
significant difference.
Results
miR-539 had a low expression in PCa
tissues and cell lines
The expression of miR-539 was significantly reduced
in cancer tissues compared with that in their paired normal
adjacent tissues of PCa (P<0.001; Fig. 1A). According to the median as the
segmentation point, the expression of miR-539 was divided into high
expression and low expression. As shown in Table II, miR-539 expression is
associated with tumor size, Tumor-Node-Metastasis (TNM) stage and
lymph node metastasis (LNM) of patients. In brief, patients with
tumor size ≥2, higher TNM stage and presenting LNM had lower
miR-539 expression. In PC cell lines, the expression level of
miR-539 was the lowest in SW1990 cells and the highest in BxPC3
cells (P<0.001; Fig. 1B). In
addition to the expression of miR-539 in different PCa cell lines,
cells with fast growth rates and good growth were selected.
Therefore, in subsequent experiments, miR-539 was overexpressed in
SW1990 cells, while BxPC3 cells were treated with an miR-539
inhibitor. The transfection results revealed that the expression of
miR-539 was increased markedly in the mimic group but was decreased
significantly in the inhibitor group, suggesting that the
transfection was successful (P<0.001; Fig. 1C and D).
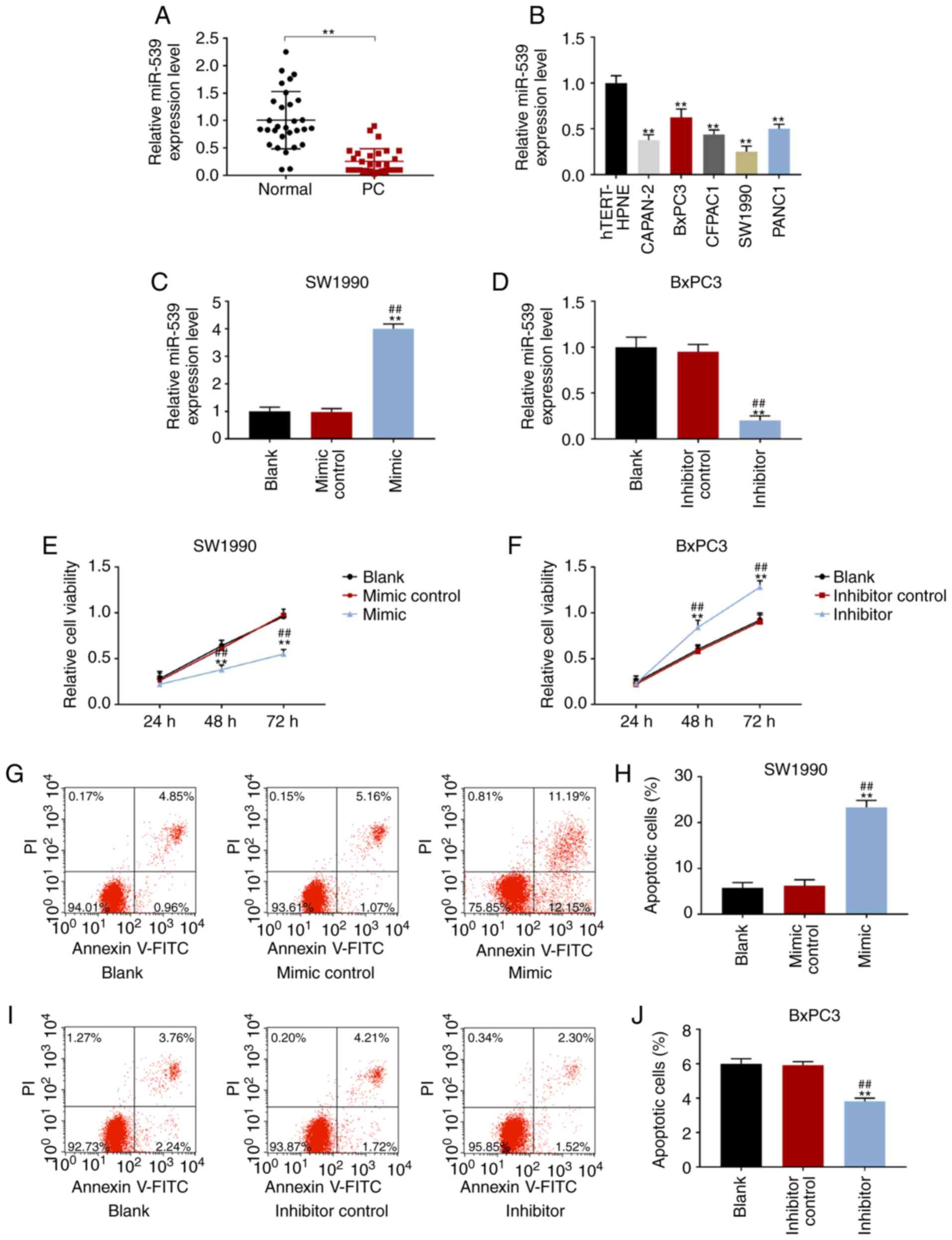 | Figure 1Overexpression of miR-539 inhibited
the activities of PCa cells and promoted apoptosis. According to
the results from RT-qPCR, the expression levels of miR-539 in (A)
cancerous tissues and their paired normal adjacent tissues from
patients with PCa (n=56) and in (B) non-cancerous pancreatic cells
(hTRET-HPNE) and PCa cell lines (CAPAN-2, BxPC3, CFPAC1, SW1990 and
PANC1). RT-qPCR assays showed the expression levels of miR-539 in
(C) SW1990 and (D) BxPC3 cells after transfection. The activities
of (E) SW1990 and (F) BxPC3 cells after transfection for 24, 48 and
72 h were detected by Cell Counting kit-8. Apoptosis figures and
corresponding quantitative analyses of (G and H) SW1990 and (I and
J) BxPC3 cells after transfection were tested by flow cytometry.
SW1990 cells were transfected with blank, mimic control and miR-539
mimic, while BxPC3 cells were transfected with blank, inhibitor
control and miR-539 inhibitor. **P<0.001, vs. normal,
hTRET-HPNE, or blank; ##P<0.001, vs. mimic control or
inhibitor control (n=3). PCa, pancreatic cancer; RT-qPCR, real
time-quantitative polymerase chain reaction. |
 | Table IIAssociations between miR-539
expression and clinicopathological characteristics of patients with
pancreatic cancer. |
Table II
Associations between miR-539
expression and clinicopathological characteristics of patients with
pancreatic cancer.
| Clinical
factor | miR-539
expression | P-value |
|---|
| Low expression
(n=28) | High expression
(n=28) |
|---|
| Age, years | | | 0.567 |
| <60 | 18 | 20 | |
| ≥60 | 10 | 8 | |
| Sex | | | 0.577 |
| Male | 19 | 17 | |
| Female | 9 | 11 | |
| Tumor size, cm | | | 0.016a |
| <2 | 9 | 18 | |
| ≥2 | 19 | 10 | |
| Tumor
differentiation | | | 0.179 |
| Well | 10 | 15 | |
| Poor | 18 | 13 | |
| TNM stage | | | 0.014a |
| I+II | 7 | 16 | |
| III+IV | 21 | 12 | |
| Lymph node
metastasis | | | 0.031a |
| Negative | 8 | 16 | |
| Positive | 20 | 12 | |
| Distant metastasis
(M) status | | | 0.105 |
| M0 | 13 | 19 | |
| M1 | 15 | 9 | |
Over-expression of miR-539 inhibited the
activities of PCa cells and promoted apoptosis
After transfection for 48 and 72 h, the activity of
SW1990 cells was significantly reduced in the mimic group, while
that of BxPC3 was notably increased in the inhibitor group
(P<0.001; Fig. 1E and F).
Furthermore, the apoptotic rate of SW1990 cells transfected with
miR-539 increased greatly, while that of BxPC3 cells transfected
with an miR-539 inhibitor markedly decreased (P<0.001; Fig. 1G-J). In addition, the cell
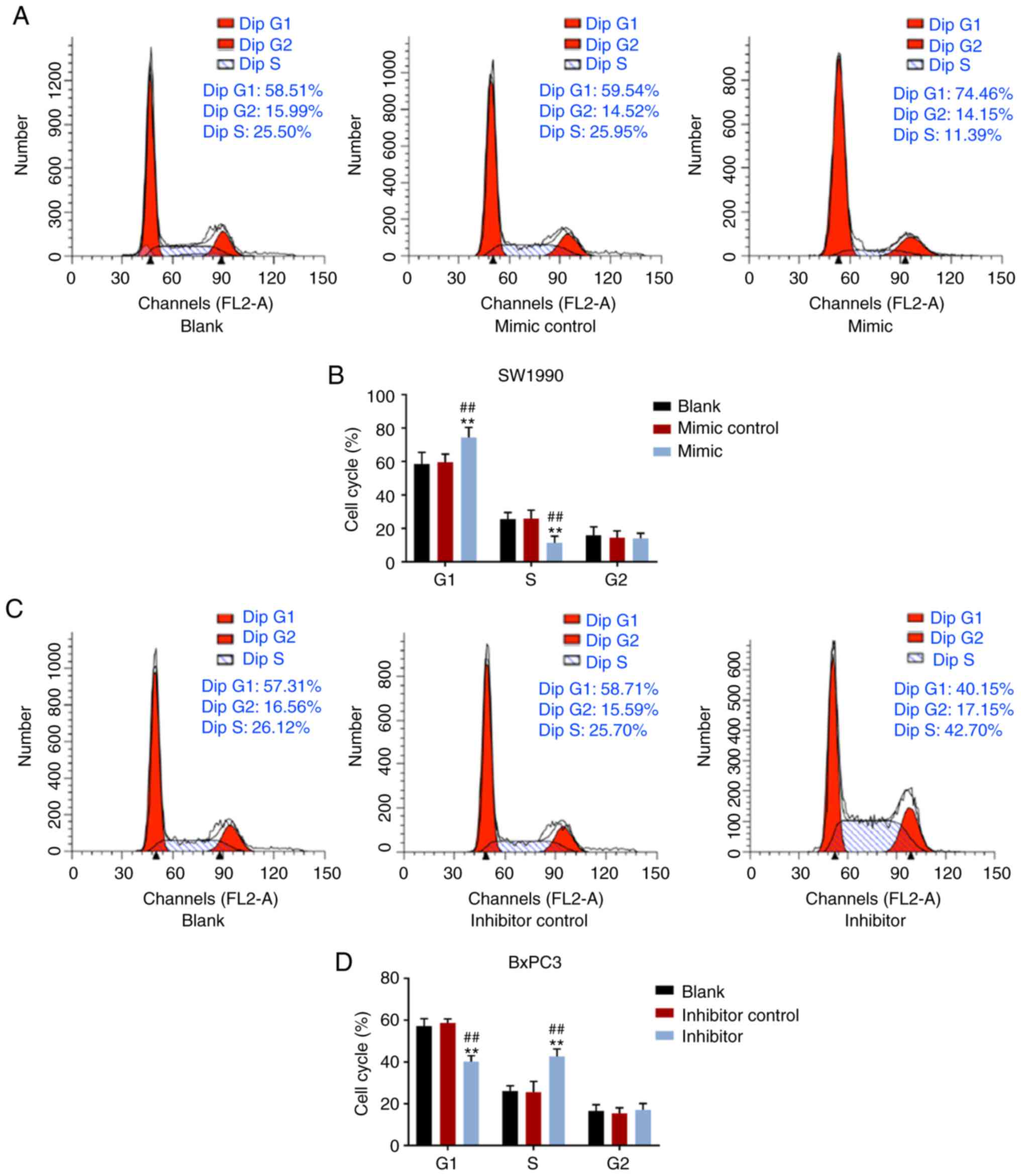
proportion in G1 phase was notably increased by elevated miR-539
expression, but decreased in S phase of SW1990 cells (P<0.001);
however, no significant effect of miR-539 on G2 phase was observed
(Fig. 2A and B). Additionally, in
BxPC3 cells, suppression of miR-539 significantly reduced cell
proportion in G1 phase, but increased cell proportion in the S
phase (P<0.001), and there was no notable difference in G2 phase
(Fig. 2C and D).
Over-expression of miR-539 suppressed the
migration and invasion abilities of PCa cells
The migration distance of SW1990 cells in the mimic
group was shorter than that in the blank and mimic control groups
48 h after the transfection (P<0.001; Fig. 2E and F), while the distance of
BxPC3 cells transfected with miR-539 inhibitor was significantly
longer than that of the blank and inhibitor control groups
(P<0.001; Fig. 2G and H). The
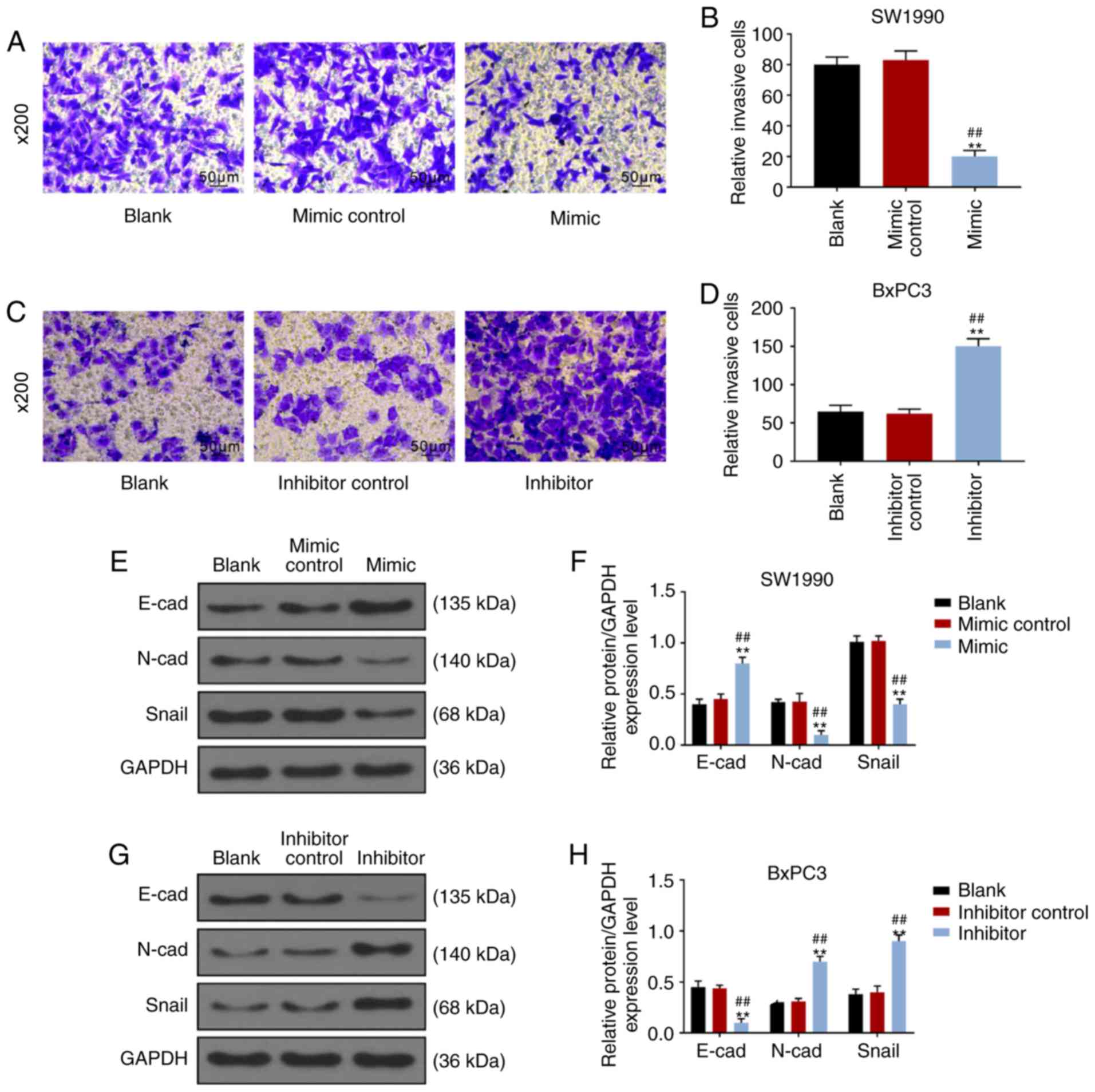
results of Transwell assays revealed that the number of SW1990
cells in the mimic group that penetrated into the lower chamber was
significantly fewer than that in the blank and control groups
(P<0.001; Fig. 3A and B),
while number of BxPC3 cells in the inhibitor group that penetrated
into the lower chamber was markedly more than the blank and control
groups (P<0.001; Fig. 3C and
D). Furthermore, WB of EMT-related proteins showed that
over-expression of miR-539 increased the expression of E-cad in
SW1990 cells, while the expressions of N-cad and Snail were
inhibited (P<0.001; Fig. 3E and
F). Correspondingly, in BxPC3 cells, low expression of miR-539
suppressed the expression of E-cad, but increased the expressions
of N-cad and Snail (P<0.001; Fig.
3G and H).
SP1 was the target gene of miR-539
Targetscan7.2 revealed that the position 3197-3204
of SP1 3′-UTR could be coupled with hsa-miR-539-5p, suggesting that
SP1 may be the target gene for miR-539 (Fig. 4A). Dual-luciferase reporter gene
analysis demonstrated that the luciferase activity of SW1990 cells
co-transfected with SP1-WT and miR-539 mimic decreased
significantly (P<0.001; Fig.
4B), while that of BxPC3 cells co-transfected with SP1-WT and
miR-539 inhibitor increased significantly (P<0.001; Fig. 4C), suggesting that SP1 was the
target gene for miR-539. Therefore, in the subsequent experiments,
miR-539 mimic, inhibitor and SP1, and siSP1 were co-transfected to
SW1990 and BxPC3 cells, respectively. The results of WB showed that
the expression of SP1 in MC+SP1 group was significantly higher than
that in MC+NC and mimic+SP1 groups, and that the expression of SP1
in mimic+SP1 group was significantly higher than that in mimic+NC
group (P<0.001; Fig. 4D and
E). In BxPC3 cells, SP1 expression was successfully inhibited
in IC+siSP1 and inhibitor+siSP1 groups (P<0.001; Fig. 4F and G).
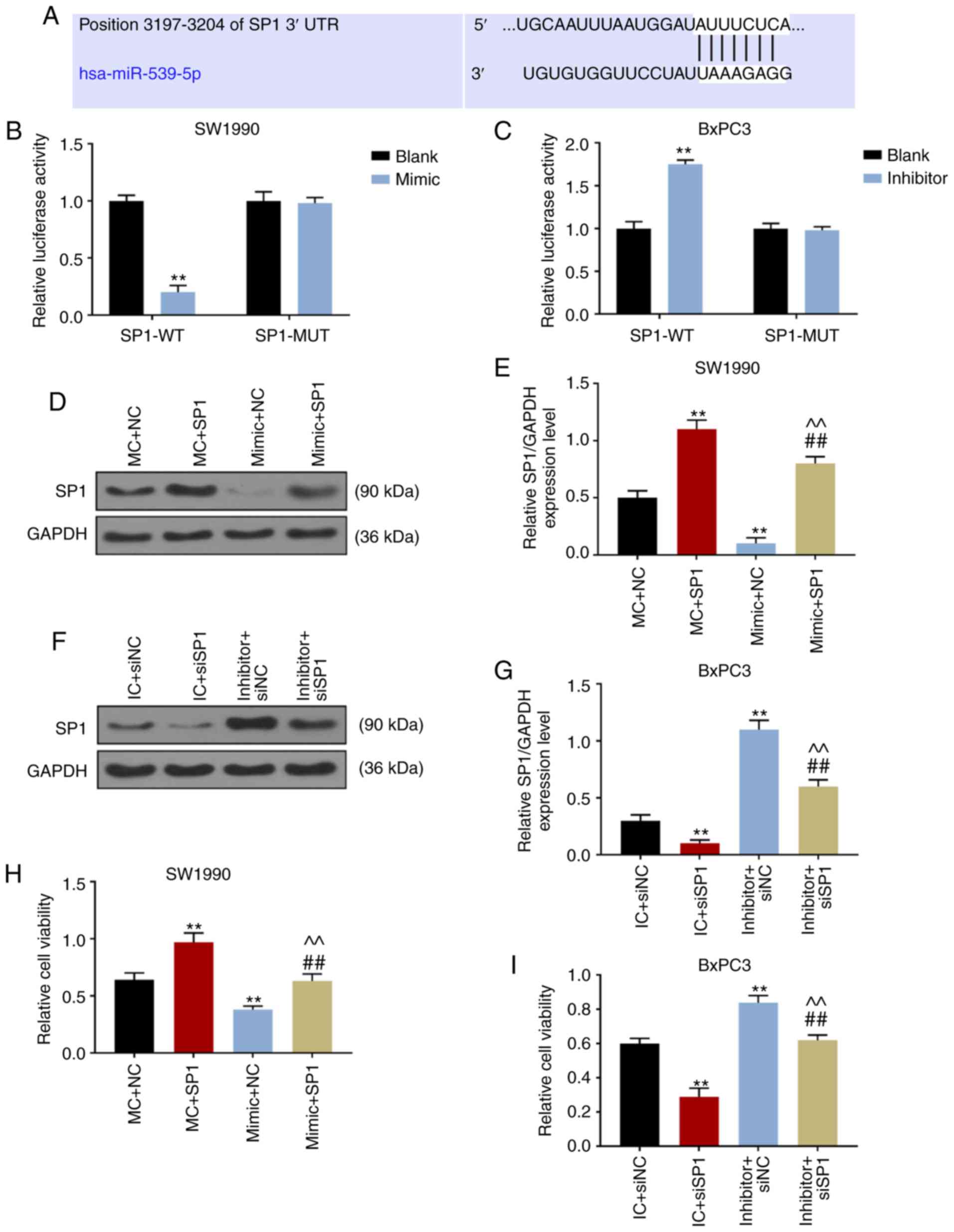 | Figure 4SP1 was the target gene of miR-539.
(A) Targetscan7.2 predicted that the position 3197-3204 of SP1
3′URT could be coupled with hsa-miR-539-5p. Results of
dual-luciferase reporter gene analysis in (B) SW1990 cells
co-transfected miR-539 mimic with wild-type SP1 (SP1-WT) and mutant
SP1 (SP1-MUT) and that in (C) BxPC3 cells co-transfected miR-539
inhibitor with SP1-WT or SP1-MUT. The expression levels of miR-539
in (D and E) SW1990 and (F and G) BxPC3 cells after transfection
were determined by the western blot analysis. The activities of (H)
SW1990 and (I) BxPC3 cells after 48-h transfection were detected
using Cell Counting kit-8. (D-I) SW1990 cells were co-transfected
with MC and negative control (NC, pcDNA3.1 empty plasmid), mimic
control (MC) and SP1, mimic and NC, and mimic and SP1. Similarly,
BxPC3 cells were co-transfected with inhibitor control (IC) and
siNC, IC and siSP1, inhibitor and siNC, and inhibitor and siSP1.
**P<0.001, vs. blank, MC + NC or IC + siNC;
##P<0.001, vs. MC + SP1 or IC + siSP1;
^^P<0.001, vs. mimic + NC or inhibitor + siNC
(n=3). |
Over-expression of miR-539 inhibited the
activity, migration and invasion of PCa cells and promoted cell
apoptosis by targeting SP1
CCK-8 results revealed that SP1 increased the
activity of SW1990 cells and reversed the inhibitory effect
produced by overexpressed miR-539 (P<0.001; Fig. 4H), while downregulation of SP1
reduced the activity of BxPC3 cells and weakened the promoted
effect produced by low expression of miR-539 (P<0.001; Fig. 4I). In cell apoptosis experiments,
the apoptosis rate of SW1990 cells transfected with SP1 decreased
significantly (P<0.05), which could be reversed by
over-expression of miR-539 (P<0.001; Fig. 5A and B). Additionally, the
apoptotic rate of BxPC3 cells transfected with siSP1 increased
significantly (P<0.001), and miR-539 inhibitor could reverse the
pro-apoptosis effect produced by low expression of SP1 (P<0.001;
Fig. 5C and D). Furthermore, it
was revealed that the migratory distance and number of invasive
SW1990 cells were increased by SP1. By contrast, the migratory
distance became shorter and the number of invasive BxPC3 cells was
reduced by inhibiting SP1. However, this phenomenon could be
reversed by overexpression of miR-539 or its inhibitor (P<0.001;
Figs. 5E-H and 6A-D). Furthermore, SP1 inhibited the
expression of E-cad and promoted the expression of N-cad and Snail,
while silencing P1 produced the opposite results (P<0.001).
Notably, overexpression of miR-539 attenuated the effects of SP1 on
EMT-related proteins, while miR-539 inhibitor reversed the effects
of siSP1 (P<0.001; Fig.
6E-H).
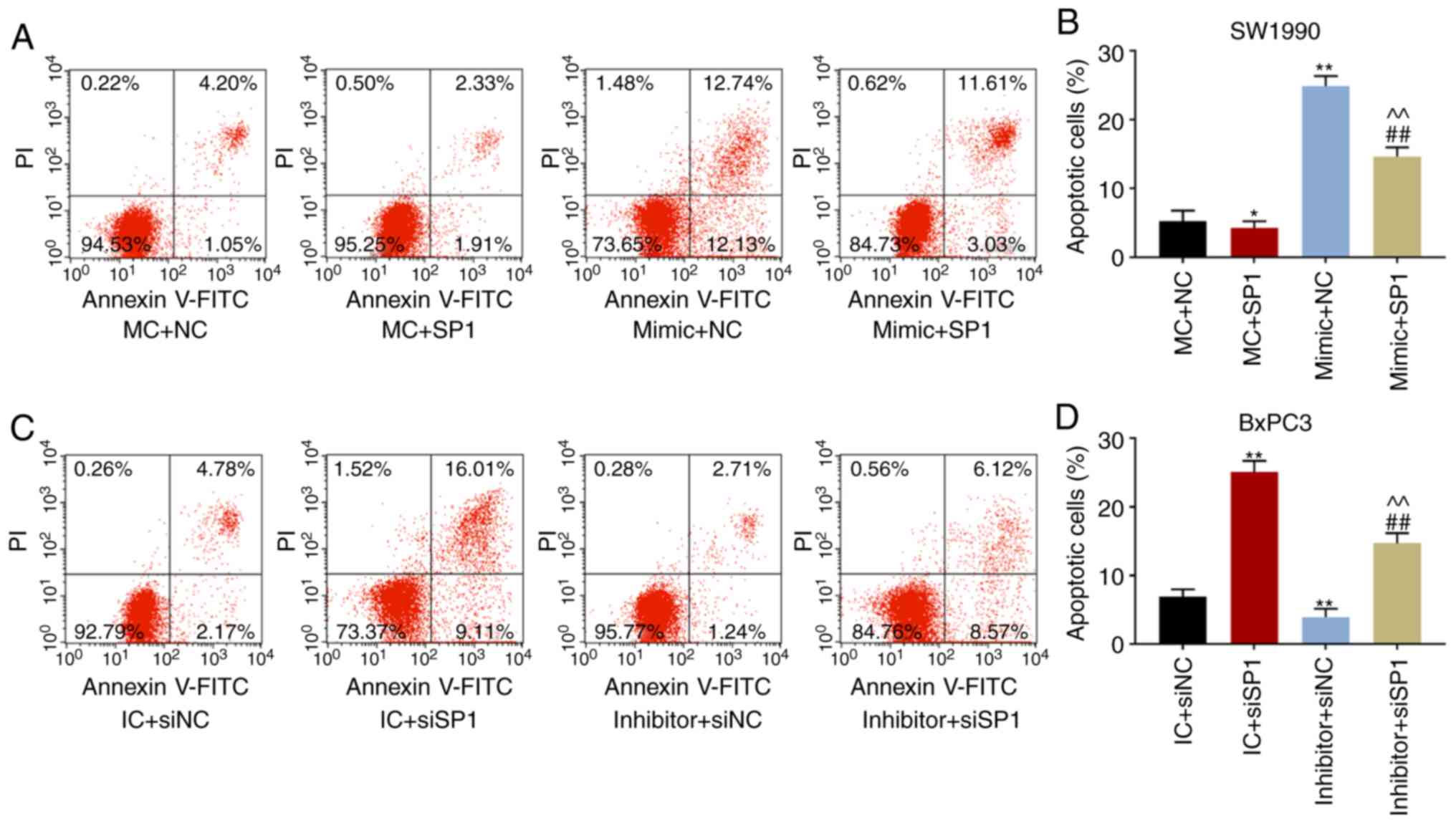 | Figure 5Over-expression of miR-539 suppressed
migration of pancreatic cancer cells and promoted apoptosis.
Apoptosis figures and corresponding quantitative analyses of (A and
B) SW1990 and (C and D) BxPC3 cells after transfection were
measured by flow cytometry. Migration distances under the
microscope and corresponding quantitative analyses of (E and F)
SW1990 and (G and H) BxPC3 cells at 0 or 48 h after transfection
were determined by scratch tests. SW1990 cells were co-transfected
with MC and negative control (NC, pcDNA3.1 empty plasmid), mimic
control (MC) and SP1, mimic and NC, and mimic and SP1. Similarly,
BxPC3 cells were co-transfected with inhibitor control (IC) and
siNC, IC and siSP1, inhibitor and siNC, and inhibitor and siSP1.
*P<0.05, **P<0.001, vs. MC + NC or IC +
siNC; ##P<0.001, vs. MC + SP1 or IC + siSP1;
^^P<0.001, vs. mimic + NC or inhibitor + siNC
(n=3). |
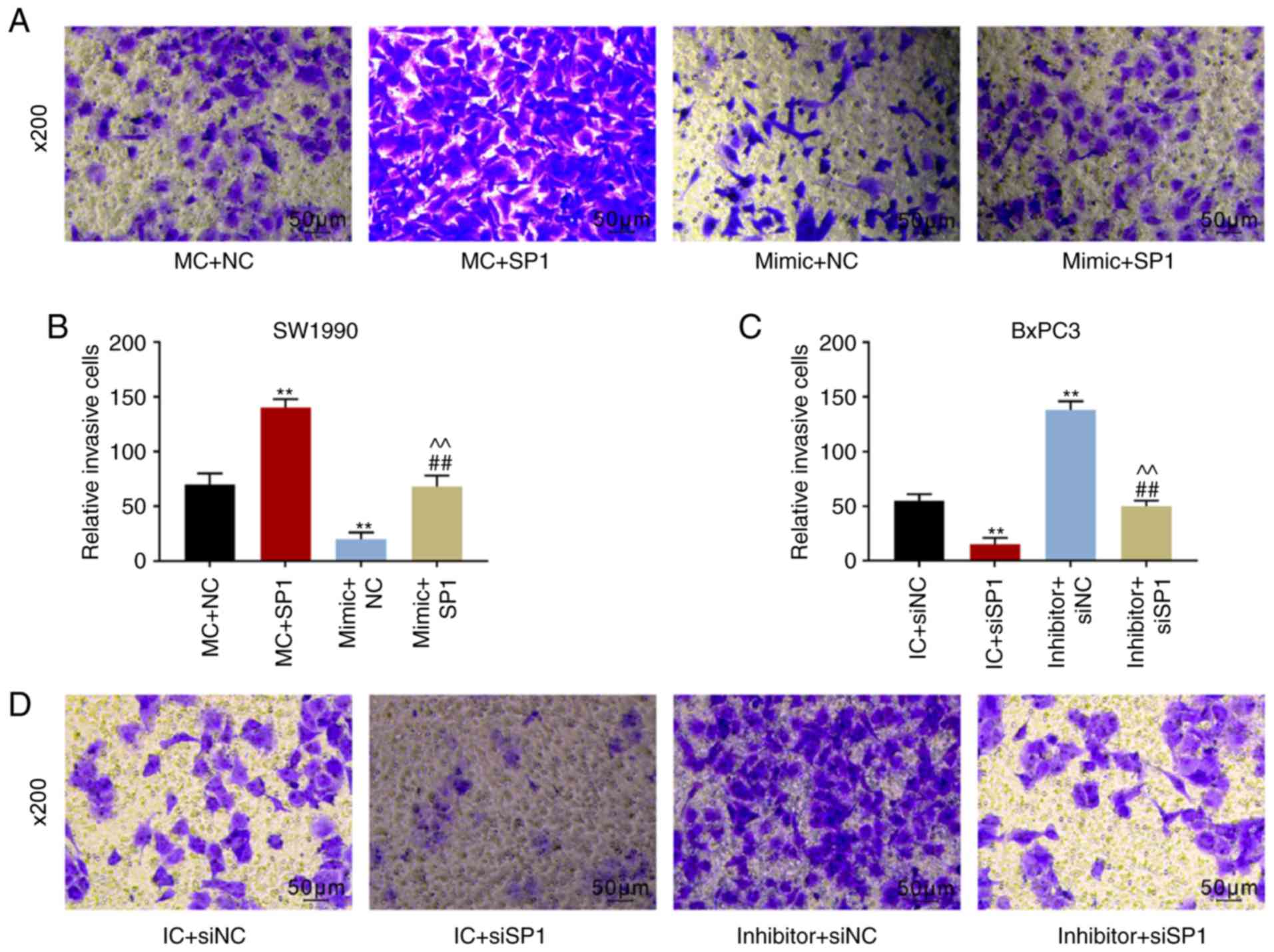 | Figure 6The numbers of invaded cells under
the microscope and corresponding quantitative analyses of (A and B)
SW1990 cells and (C and D) BxPC3 cells based on the Transwell
assays. Experimental results of western blotting showed the
expression levels of EMT-related proteins, including E-cadherin
(E-cad), N-cadherin (N-cad) and Snail, in (E and F) SW1990 and (G
and H) BxPC3 cells. GAPDH served as the internal reference. SW1990
cells were co-transfected with MC and negative control (NC,
pcDNA3.1 empty plasmid), mimic control (MC) and SP1, mimic and NC,
and mimic and SP1. Similarly, BxPC3 cells were co-transfected with
inhibitor control (IC) and siNC, IC and siSP1, inhibitor and siNC,
and inhibitor and siSP1. **P<0.001, vs. MC + NC or IC
+ siNC; ##P<0.001, vs. MC + SP1 or IC + siSP1;
^^P<0.001, vs. mimic + NC or inhibitor + siNC
(n=3). |
Discussion
PCa is a highly invasive cancer and its 5-year
survival rate is less than 5% (32). Surgical resection is an effective
method in treating PCa, but ~60% of PCa patients have lost the
optimal opportunities for undergoing surgery at the time of
diagnosis, and only 15% of patients are suitable for taking radical
surgery. Furthermore, median postoperative survival time for PCa
patients is only 20-23 months even they underwent surgery and
chemotherapy (33-35). Therefore, finding effective
methods for treating PCa is important. Previous studies have found
abnormal expression of miRNAs in PCa, and that this correlates with
the development, differentiation, invasion and metastasis of PCa
(36,37). The results of the present study
revealed that the expression level of miR-539 in cancerous tissues
from PCa patients was markedly lower than that in adjacent tissues
and PCa cell lines, suggesting that miR-539 may be associated with
the occurrence and progression of PCa.
A previous study reported that the expression of
miR-539 suppressed the proliferation, invasion and migration of PCa
cell lines (38). Jin and Wang
(39) also found that miR-539
could act as an inhibitor to control osteosarcoma progression. A
recent study reported that miR-539 overexpression suppressed the
invasion, migration and EMT of BXPC-3 and PANC-1 cells through
targeting TWIST1 (40).
Similarly, in this study, the overexpression of miR-539 reduced the
proliferation, migration and invasion of PCa cells, whereas the
inhibition of miR-539 produced the opposite effect. Furthermore,
the present study revealed that miR-539 induced the apoptosis of
PCa cell lines mainly through blocking the cell cycle in G1 phase,
which was consistent with the results obtained by Deng et al
(41) that miR-539 could cause
cell cycle arrest in non-small cell lung cancer cell lines. A
previous study also confirmed that miR-539 regulates the growth of
nasopharyngeal carcinoma cells through cell cycle arrest (42). Therefore, it was confirmed that
the upregulation of miR-539 played an active role in PCa.
EMT is the initial event indicating tumor metastasis
(43). E-cad, N-cad and Snail are
EMT-related proteins. E-cad is an adhesion protein, and
downregulating the expression of E-cad can decrease or even
eliminate adhesion between cells, thereby leading to tumor
detachment, local migration or distant spread via blood vessels and
lymphatic ducts from primary site (44). Notably, N-cad and Snail have
completely opposite effects to that of E-cad, and the upregulation
of N-cad and Snail accelerates tumor metastasis (45). A previous study revealed that
certain miRNAs could inhibit the occurrence of EMT by reducing the
expression of N-cad and Snail, and increasing the synthesis of
E-cad, thereby preventing tumor metastasis (46). The results of the present study
revealed that overexpression of miR-539 may promote the expression
of E-cad, while inhibiting the expression of N-cad and Snail.
Additionally, the suppression of miR-539 resulted in opposite
outcomes, suggesting that miR-539 could block the metastasis and
invasion of PCa cells through blocking EMT.
Furthermore, while investigating the mechanism of
miR-539 in PCa, the results of the present study suggested that SP1
was the target gene for miR-539. SP1, which is an important
transcription factor, is involved in cell proliferation, apoptosis
and differentiation, and associated with the expression of genes
related to tumor growth and metastasis (47,48). The results of the present study
demonstrated that increased SP1 promoted the proliferation,
migration, invasion and EMT of PCa cells and inhibited cell
apoptosis, while silencing of SP1 produced the opposite effects,
indicating that SP1 may facilitate the development and progression
of PCa. Xia et al (49)
reported that the downregulation of SP1 could reduce the
proliferation rate of ovarian cancer cells and restrain tumor
metastasis. Mei et al (50) revealed that certain miRNAs may
suppress the multiplication, migration and EMT of esophageal cancer
cells by targeting SP1. Notably, it was discovered that the
overexpression of miR-539 inhibited the effects of SP1 on PCa cell
lines, and that SP1-silencing reversed the effect of low expression
of miR-539, showing that miR-539 was involved in the occurrence and
development of PCa through targeting SP1. However, there are
questions that remain to be answered from basic research to
clinical application. The biological effects of miRNAs are
microenvironment-dependent or cell-type-dependent, and the
expression of miR-539 varies in different PCa cell lines, which may
also have different effects on the proliferation, invasion,
migration and EMT of different PCa cells. The expression of miR-539
differs in different PCa cells, and the effect of miR-539 on the
viability of different PCa cells may also vary, which requires
further investigation. However, miR-539 may still serve an
anti-cancer role in different PCa cells. Although there are
limitations to the present study, and in vivo experiments
should be performed to further support the present data, the
findings in the present study further elucidate the underlying
mechanism of miR-539 in PCa.
In conclusion, miR-539 is involved in the
development and progression of PCa through targeting SP1 to
suppress the proliferation, metastasis, invasion, apoptosis and EMT
of PCa cells, at least in SW1990 and BxPC3 cells. Therefore, we
hypothesize that miR-539 may be investigated as a novel therapeutic
strategy for PCa treatment in the future.
Abbreviations:
|
miR
|
microRNA
|
|
PCa
|
pancreatic cancer
|
|
EMT
|
epithelial-mesenchymal transition
|
|
miRNAs
|
microRNAs
|
|
MC
|
mimic control
|
|
IC
|
inhibitor control
|
|
NC
|
negative control
|
|
CCK-8
|
Cell Counting Kit-8
|
|
OD
|
optical density
|
|
FITC
|
fluorescein isothiocyanate
|
|
WB
|
western blotting
|
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LX and YS made substantial contributions to the
conception and design. ZZ was involved in data acquisition,
analysis and interpretation. LX was responsible for drafting the
article or critically revising it for important intellectual
content. SZ agreed to be accountable for all aspects of the work,
in ensuring that questions related to the accuracy or integrity of
the work are appropriately investigated and resolved. All authors
gave final approval of the version to be published.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the First Affiliated Hospital (approval no.
ZJ2018121017), Zhejiang University and written informed consent was
obtained from all participants. All procedures involving human
participants were performed in accordance with the 1964 Declaration
of Helsinki and its later amendments or comparable ethical
standards.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lin QJ, Yang F, Jin C and Fu DL: Current
status and progress of pancreatic cancer in China. World J
Gastroenterol. 21:7988–8003. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee ES and Lee JM: Imaging diagnosis of
pancreatic cancer: A state-of-the-art review. World J
Gastroenterol. 20:7864–7877. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ilic M and Ilic I: Epidemiology of
pancreatic cancer. World J Gastroenterol. 22:9694–9705. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Puleo F, Maréchal R, Demetter P, Bali MA,
Calomme A, Closset J, Bachet JB, Deviere J and Van Laethem JL: New
challenges in perioperative management of pancreatic cancer. World
J Gastroenterol. 21:2281–2293. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu Q, Yang Z, Nie Y, Shi Y and Fan D:
Multi-drug resistance in cancer chemotherapeutics: Mechanisms and
lab approaches. Cancer Lett. 347:159–166. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mansoori B, Mohammadi A, Davudian S,
Shirjang S and Baradaran B: The different mechanisms of cancer drug
resistance: A brief review. Adv Pharm Bull. 7:339–348. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tang Y, Tang Y and Cheng YS: miR-34a
inhibits pancreatic cancer progression through Snail1-mediated
epithelial-mesenchymal transition and the Notch signaling pathway.
Sci Rep. 7:382322017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wei W, Liu Y, Lu Y, Yang B and Tang L:
LncRNA XIST promotes pancreatic cancer proliferation through
miR-133a/EGFR. J Cell Biochem. 118:3349–3358. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huang B, Liu C, Wu Q, Zhang J, Min Q,
Sheng T, Wang X and Zou Y: Long non-coding RNA NEAT1 facilitates
pancreatic cancer progression through negative modulation of
miR-506-3p. Biochem Biophys Res Commun. 482:828–834. 2017.
View Article : Google Scholar
|
|
10
|
Feng YH and Tsao CJ: Emerging role of
microRNA-21 in cancer. Biomed Rep. 5:395–402. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao G, Wang B, Liu Y, Zhang JG, Deng SC,
Qin Q, Tian K, Li X, Zhu S, Niu Y, et al: miRNA-141, downregulated
in pancreatic cancer, inhibits cell proliferation and invasion by
directly targeting MAP4K4. Mol Cancer Ther. 12:2569–2580. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gambari R, Brognara E, Spandidos DA and
Fabbri E: Targeting oncomiRNAs and mimicking tumor suppressor
miRNAs: New trends in the development of miRNA therapeutic
strategies in oncology (Review). Int J Oncol. 49:5–32. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rupaimoole R and Slack FJ: MicroRNA
therapeutics: Towards a new era for the management of cancer and
other diseases. Nat Rev Drug Discov. 16:203–222. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tutar L, Özgür A and Tutar Y: Involvement
of miRNAs and pseudogenes in cancer. Methods Mol Biol. 1699:45–66.
2018. View Article : Google Scholar
|
|
15
|
Sun Y, Zhang T, Wang C, Jin X, Jia C, Yu S
and Chen J: Correction: miRNA-615-5p functions as a tumor
suppressor in pancreatic ductal adenocarcinoma by targeting AKT2.
PLoS One. 10:e01282572015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bai Z, Sun J, Wang X, Wang H, Pei H and
Zhang Z: MicroRNA-153 is a prognostic marker and inhibits cell
migration and invasion by targeting SNAI1 in human pancreatic
ductal adenocarcinoma. Oncol Rep. 34:595–602. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Keklikoglou I, Hosaka K, Bender C, Bott A,
Koerner C, Mitra D, Will R, Woerner A, Muenstermann E, Wilhelm H,
et al: MicroRNA-206 functions as a pleiotropic modulator of cell
proliferation, invasion and lymphangiogenesis in pancreatic
adenocarcinoma by targeting ANXA2 and KRAS genes. Oncogene.
34:4867–4878. 2015. View Article : Google Scholar :
|
|
18
|
Ouyang H, Gore J, Deitz S and Korc M:
microRNA-10b enhances pancreatic cancer cell invasion by
suppressing TIP30 expression and promoting EGF and TGF-beta
actions. Oncogene. 36:49522017. View Article : Google Scholar
|
|
19
|
Ma C, Nong K, Wu B, Dong B, Bai Y, Zhu H,
Wang W, Huang X, Yuan Z and Ai K: miR-212 promotes pancreatic
cancer cell growth and invasion by targeting the hedgehog signaling
pathway receptor patched-1. J Exp Clin Cancer Res. 33:542014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhu Z, Xu Y, Zhao J, Liu Q, Feng W, Fan J
and Wang P: miR-367 promotes epithelial-to-mesenchymal transition
and invasion of pancreatic ductal adenocarcinoma cells by targeting
the Smad7-TGF-β signalling pathway. Br J Cancer. 112:1367–1375.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ding S and Zhang Y: MicroRNA539 inhibits
the proliferation and migration of gastric cancer cells by
targeting SRYbox 5 gene. Mol Med Rep. 20:2533–2540. 2019.PubMed/NCBI
|
|
22
|
Sun B, Fan Y, Yang A, Liang L and Cao J:
MicroRNA-539 functions as a tumour suppressor in prostate cancer
via the TGF-β/Smad4 signalling pathway by down-regulating DLX1. J
Cell Mol Med. 23:5934–5948. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sun H and Sun Y: Lidocaine inhibits
proliferation and metastasis of lung cancer cell via regulation of
miR-539/EGFR axis. Artif Cells Nanomed Biotechnol. 47:2866–2874.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gong YB and Fan XH: miR-539-3p promotes
the progression of epithelial ovarian cancer by targeting SPARCL1.
Eur Rev Med Pharmacol Sci. 23:2366–2373. 2019.PubMed/NCBI
|
|
25
|
Jin W, Han H and Liu D: Downregulation
miR-539 is associated with poor prognosis of gastric cancer
patients and aggressive progression of gastric cancer cells. Cancer
Biomark. 26:183–191. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kadonaga JT, Carner KR, Masiarz FR and
Tjian R: Isolation of cDNA encoding transcription factor Sp1 and
functional analysis of the DNA binding domain. Cell. 51:1079–1090.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Roder K, Kim KH and Sul HS: Induction of
murine H-rev107 gene expression by growth arrest and histone
acetylation: Involvement of an Sp1/Sp3-binding GC-box. Biochem
Biophys Res Commun. 294:63–70. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen F, Zhang F, Rao J and Studzinski GP:
Ectopic expression of truncated Sp1 transcription factor prolongs
the S phase and reduces the growth rate. Anticancer Res.
20:661–667. 2000.PubMed/NCBI
|
|
29
|
Wei D, Wang L, He Y, Xiong HQ, Abbruzzese
JL and Xie K: Celecoxib inhibits vascular endothelial growth factor
expression in and reduces angiogenesis and metastasis of human
pancreatic cancer via suppression of Sp1 transcription factor
activity. Cancer Res. 64:2030–2038. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wright C, Angus B, Napier J, Wetherall M,
Udagawa Y, Sainsbury JR, Johnston S, Carpenter F and Horne CH:
Prognostic factors in breast cancer: Immunohistochemical staining
for SP1 and NCRC 11 related to survival, tumour epidermal growth
factor receptor and oestrogen receptor status. J Pathol.
153:325–331. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rao X, Huang X, Zhou Z and Lin X: An
improvement of the 2ˆ(-delta delta CT) method for quantitative
real-time polymerase chain reaction data analysis. Biostat
Bioinforma Biomath. 3:71–85. 2013.PubMed/NCBI
|
|
32
|
Vincent A, Herman J, Schulick R, Hruban RH
and Goggins M: Pancreatic cancer. Lancet. 378:607–620. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Witkowski ER, Smith JK and Tseng JF:
Outcomes following resection of pancreatic cancer. J Surg Oncol.
107:97–103. 2013. View Article : Google Scholar
|
|
34
|
Mohammed S, Van Buren G II and Fisher WE:
Pancreatic cancer: Advances in treatment. World J Gastroenterol.
20:9354–9360. 2014.PubMed/NCBI
|
|
35
|
Ansari D, Gustafsson A and Andersson R:
Update on the management of pancreatic cancer: Surgery is not
enough. World J Gastroenterol. 21:3157–3165. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Abreu FB, Liu X and Tsongalis GJ: miRNA
analysis in pancreatic cancer: The Dartmouth experience. Clin Chem
Lab Med. 55:755–762. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Vorvis C, Koutsioumpa M and Iliopoulos D:
Developments in miRNA gene signaling pathways in pancreatic cancer.
Future Oncol. 12:1135–1150. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang H, Li S, Yang X, Qiao B, Zhang Z and
Xu Y: miR-539 inhibits prostate cancer progression by directly
targeting SPAG5. J Exp Clin Cancer Res. 35:602016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jin H and Wang W: MicroRNA-539 suppresses
osteosarcoma cell invasion and migration in vitro and targeting
Matrix metal-lopeptidase-8. Int J Clin Exp Pathol. 8:8075–8082.
2015.
|
|
40
|
Yu H, Gao G, Cai J, Song H, Ma Z, Jin X,
Ji W and Pan B: miR-539 functions as a tumor suppressor in
pancreatic cancer by targeting TWIST1. Exp Mol Pathol. 108:143–149.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Deng H, Qianqian G, Ting J and Aimin Y:
miR-539 enhances chemosensitivity to cisplatin in non-small cell
lung cancer by targeting DCLK1. Biomed Pharmacother. 106:1072–1081.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lv LY, Wang YZ, Zhang Q, Zang HR and Wang
XJ: miR-539 induces cell cycle arrest in nasopharyngeal carcinoma
by targeting cyclin-dependent kinase 4. Cell Biochem Funct.
33:534–540. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kahlert UD, Joseph JV and Kruyt FAE: EMT-
and MET-related processes in nonepithelial tumors: Importance for
disease progression, prognosis, and therapeutic opportunities. Mol
Oncol. 11:860–877. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Liu JB, Feng CY, Deng M, Ge DF, Liu DC, Mi
JQ and Feng XS: E-cadherin expression phenotypes associated with
molecular subtypes in invasive non-lobular breast cancer: Evidence
from a retrospective study and meta-analysis. World J Surg Oncol.
15:1392017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Song PP, Qian XY, Zhou H, Shen XH, Liu DD,
Feng AN and Gao X: Expression of E-cadherin, N-cadherin,
beta-catenin and their clinical significance in laryngeal
carcinoma. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi.
51:440–445. 2016.In Chinese. PubMed/NCBI
|
|
46
|
Zhang L, Sun J, Wang B, Ren JC, Su W and
Zhang T: MicroRNA-10b triggers the epithelial-mesenchymal
transition (EMT) of laryngeal carcinoma Hep-2 cells by directly
targeting the E-cadherin. Appl Biochem Biotechnol. 176:33–44. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Vizcaíno C, Mansilla S and Portugal J: Sp1
transcription factor: A long-standing target in cancer
chemotherapy. Pharmacol Ther. 152:111–124. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Beishline K and Azizkhan-Clifford J: Sp1
and the 'hallmarks of cancer'. FEBS J. 282:224–258. 2015.
View Article : Google Scholar
|
|
49
|
Xia B, Hou Y, Chen H, Yang S, Liu T, Lin M
and Lou G: Long non-coding RNA ZFAS1 interacts with miR-150-5p to
regulate Sp1 expression and ovarian cancer cell malignancy.
Oncotarget. 8:19534–19546. 2017.PubMed/NCBI
|
|
50
|
Mei LL, Wang WJ, Qiu YT, Xie XF, Bai J and
Shi ZZ: miR-145-5p suppresses tumor cell migration, invasion and
epithelial to mesenchymal transition by Regulating the Sp1/NF-κB
signaling pathway in esophageal squamous cell carcinoma. Int J Mol
Sci. 18:E18332017. View Article : Google Scholar
|