Introduction
Skin serves as the protective barrier of the body
and protects it from harmful agents, such as ultraviolet (UV)
radiation, heat and microorganisms (1,2).
Damage to the skin tissue not only causes damage to the
subcutaneous tissue, but also affects the internal balance of the
body (3,4). Thus, skin wound repair is crucial
for the restoration of the protective functions of the skin. Wound
repair requires a cascade of phases, including inflammation,
proliferation and remodeling (5,6).
Various growth factors and cytokines are released during these
processes. Transforming growth factor β1 (TGF-β1), a highly
multifunctional cytokine, affects all 3 phases of wound repair
(7-9). The extracellular matrix (ECM) of the
dermis is produced by fibroblasts and consists of collagen, elastin
and proteoglycans (10,11). Skin fibrosis is caused by an
imbalance between the generation and degradation of ECM proteins,
which results in the severe alteration of the skin connective
tissue and delays wound repair (12-14). However, the deposition of ECM
components is regulated by TGF-β1 in the wound repair process
(15). Its downstream signaling
Smad2 is phosphorylated by activated TGF-β1. The target genes are
then induced to promote wound repair (16,17). During wound repair, fibroblasts
can be activated and become myofibroblasts. These myofibroblast
cells can synthesize ECM and play a positive role in the
contraction of granulation tissue. The expression of α-smooth
muscle actin (α-SMA) is a specific marker of myofibroblasts.
Moreover, the differentiation of fibroblasts to α-SMA is promoted
by TGF-β1 (18).
Reactive oxygen species (ROS) function as secondary
messengers in a number of cells, including immunocytes and
non-lymphoid cells (19). These
cells play a positive role in tissue repair, and then promote wound
repair (19,20). ROS play a beneficial role in
protecting against invading bacteria in wound repair. Appropriate
levels of ROS are beneficial for wound repair. However, excessive
ROS production leads to oxidative stress, and inhibits the
proliferation and migration of cells in wound repair (21,22). In addition, high levels of
oxidative stress prolong the inflammatory process, induce tissue
damage and results in the delay of wound repair. Thus, it is
necessary to control the levels of ROS during wound repair
(23,24).
Spirulina, a blue-green algae, has been used as a
food source since ancient times. It is commercialized for abundant
proteins, vitamins and minerals. Moreover, spirulina has been
reported to exhibit pharmaceutical potential due to its
anti-inflammatory, antioxidant and anticancer properties (25,26). Recently, the promoting effects of
spirulina protein (SPCP) on the activity of CCD-986sk human
fibroblasts were demonstrated (27). Furthermore, SPCP was shown to
improve collagen formation in CCD-986sk cells and to decrease the
activity of elastase, which plays an important positive role in
wound repair (27). In addition,
the migration and proliferation of CCD-986sk cells have been shown
to be promoted by SPCP via phos-phoinositide 3-kinase
(PI3K)/protein kinase B (Akt) signaling pathway (28). These effects of SPCP on human
fibroblasts provide a possible application for promoting skin wound
repair.
Herein, the aim of the present study was to examine
the promoting effects of SPCP on skin wound repair. A mouse model
of full-thickness dermal excisional wounds using C57BL/6 mouse was
established. In addition, the underlying molecular mechanisms of
this process were investigated. The main findings suggested that
SPCP can promote the skin wound repair in C57BL/6 mice, and that
the extracellular signal-regulated kinase (ERK), Akt and TGF-β1
signaling pathways were activated by SPCP during this process.
Materials and methods
Experimental animals
All experiments procedures were approved by the
University Animal Care and Use Committee guidelines at Pukyong
National University (Busan, Korea; approval no. 2018-15) and
conducted according to the international regulations of the usage
and welfare of laboratory animals. A total of 20 C57BL/6 male mice
(6 weeks old; weighing 20-23 g) were obtained from IDEXX
Bioresearch, and maintained under controlled conditions with proper
temperature (22°C) and humidity (40-45%) under a light/dark cycle
of 12 h/12 h. They were kept in single-house and provided with
standard rodent food and water ad libitum.
Establishment of full-thickness
excisional wounds
All 20 mice were allowed to adapt to their new
environment for 1 week. Mice were anesthetized with ether (2-4% in
an inhaled mixture). The lack of a toe pinch reflex ensured that
the mouse was fully anesthetized. The hair of the dorsal surface
was removed using an electric clipper. The dorsal skin of each
mouse was rinsed using alcohol, and a 8-mm-diameter biopsy punch
was then used to create a full-thickness wound on the backs of the
mice. All the mice were randomly divided into 4 groups with 5 mice
in each group. The first group was the control group in which mice
were treated with vaseline (British-Dutch company, Unilever) only.
The second group was the positive control group in which mice were
treated with vaseline containing 10 µg/g epidermal growth
factor (EGF) (Sigma-Aldrich; Merck KGaA). The third group was the
sample group in which mice were treated with vaseline containing 2%
SPCP. The fourth group was the sample group in which mice were
treated with vaseline containing 4% SPCP. Vaseline, EGF or SPCP was
applied directly to the wound site once a day. Wound repair was
macroscopically monitored by obtaining images with a digital camera
(Sony HDR-XR260; Sony Corporation) at 1 p.m. each day. After 9
days, all 20 mice were euthanized by cervical dislocation.
Respiratory arrest and the absence of blinking confirmed the mouse
death. The skin around the wound was collected and treated with
liquid nitrogen. The collected skin tissues were stored at −70°C
for use in the subsequent experiments. The wound areas were
calculated using ImageJ software (version 1.40; National Institutes
of Health). The results were expressed as the percentage of the
original size.
Measurement of superoxide dismutase (SOD)
activity
The SOD Activity Assay kit (BioVision, Inc.) was
used to determine the activity of SOD in the mouse skin tissue by
ELISA. Firstly, PBS was used to remove any red blood cells. The
skin tissue was then homogenized with ice-cold (0-4°C) 0.1 mol/l of
Tris/HCl [pH 7.4, containing 5 mmol/l of β-mercaptoethanol (β-ME),
0.5% Triton X-100 and 0.1 mg/ml phenylmethylsulfonyl fluoride
(PMSF)]. It was subsequently centrifuged at 14,000 × g for 5 min at
4°C. The supernatant was used to determine the activity of SOD. The
supernatant from 4 groups was plated in 96-well plates; i.e.,
sample, blank 1, 2 and 3. A total of 20 µl of sample
solution were added to each well of the sample group and blank 2
group, respectively. This was followed by the addition of 20
µl of ddH2O to each well of the blank 1 group and
blank 3 group, respectively. Each of the above wells was then
supplemented with 200 µl of dilution buffer. A total of 20
µl of enzyme working solution (BioVision, Inc.) was then
added to each well of the sample group and blank 1 group. The plate
was then incubated at 37°C for 20 min. The Synergy HTX microplate
reader (BioTek Instruments, Inc.) was used to measure the
absorbance at 450 nm. The activity of SOD was calculated according
to the manufacturer's instructions.
Measurement of catalase (CAT)
activity
The activity of CAT was determined using the
Catalase Activity Colorimetric/Fluorometric Assay kit (BioVision,
Inc.) by ELISA. The skin tissue was homogenized with ice-cold assay
buffer. It was subsequently centrifuged at 10,000 x g for 5 min at
4°C. The supernatant was used to determine the activity of CAT.
Each of the sample wells was supplemented with 50 µl of
Sample Solution and the positive control well was supplemented with
3 µl of positive control solution (BioVision, Inc.). Each of
the wells was supplemented with assay buffer to the final volume of
78 µl. The sample high control well was supplemented with 50
µl of sample solution and then supplemented with assay
buffer to the final volume of 78 µl. The sample high control
well was then supplemented with 10 µl of stop solution.
After mixing, the plate was incubated at 25°C for 5 min to inhibit
the activity of CAT adequately. This was followed by the addition
of 12 µl of 1 mmol/l H2O2 to each well
(sample well, positive control well and sample high control well).
The plate was then incubated at 25°C for 30 min. Subsequently, 10
µl of stop solution were added to sample well and positive
control well. A total of 50 µl of Develop Mix (46 µl
of Assay Buffer, 2 µl of OxiRedTM Probe and 2 µl of
HRP solution) were then added to each well and incubated at 25°C
for 10 min. The Synergy HTX microplate reader (BioTek Instruments,
Inc.) was used to measure the absorbance at 570 nm. The activity of
CAT was calculated according to the manufacturer's
instructions.
Measurement of the malondialdehyde (MDA)
level
The level of MDA was determined using the Lipid
Peroxidation (MDA) Colorimetric/Fluorometric Assay kit (BioVision,
Inc.) by ELISA. The skin tissue was homogenized with MDA Lysis
Buffer. It was subsequently centrifuged at 13,000 x g for 10 min. A
total of 200 µl of supernatant was then place in a 1.5 ml
microcentrifuge tube. Thiobarbituric acid (TBA; 600 µl) was
then added to each well and incubated at 95°C for 60 min. The
sample was placed in the ice for 10 min and thawed to the room
temperature (20-25°C). A total of 200 µl was taken from 800
µl reaction mixture to a 96-well plate for analysis. The
Synergy HTX microplate reader (BioTek Instruments, Inc.) was used
to measure the absorbance at 532 nm. The level of MDA was
calculated according to the manufacturer's instructions.
Preparation of whole cell lysates
Skin tissue was minced and homogenized using RIPA
buffer (iNtRON Biotechnology) with 1% protease inhibitor in an
ice-bath. Subsequently, the extract was incubated in ice and then
centrifuged at 16,000 × g for 10 min at 4°C. The supernatant was
collected as the protein. The BCA protein assay kit (Pierce; Thermo
Fisher Scientific, Inc.) was used to analyze the concentration of
the protein. The protein concentration of different treatment
groups was adjusted to the same level. The protein solution was
then mixed with SDS sample buffer containing DTT and denatured at
100°C for 5 min.
Western blot analysis
SDS-PAGE gel (7.5-12.5%) was used to separate
proteins (30 µg per lane). The proteins were then
transferred to PVDF membranes (EMD Millipore). Membranes were
washed with methanol, and then blocked for 2 h with TBS-T [10 mm
Tris-HCl, 150 mm NaCl (pH 7.5) and 0.1% Tween-20] containing 1%
BSA. The membrane were then incubated at 4°C overnight (≥12 h) with
primary antibodies. After washing 2 times with TBS-T for 15 min
each time, the membranes were incubated at room temperature for a
further 2 h with the secondary antibodies (all 1:10,000). The
secondary antibodies used were HRP-conjugated anti-rabbit IgG (cat.
no. 7074S, Cell Signaling Technology, Inc.), donkey anti-goat IgG
(cat. no. A50-101p, Bethyl Laboratories, Inc.) and anti-mouse IgG
(cat. no. 7076S, Cell Signaling Technology, Inc.). The primary
antibodies used are listed in Table
I. Color development was performed using an enhanced
chemiluminescence western blot kit (Thermo Fisher Scientific,
Inc.). The bioanalytical imaging system (Azure Biosystems) was used
to detect the protein bands. Multi-Gauge software, version 3.0
(Fujifilm Life Science) was used to analyze the density of these
protein bands. Each density of these protein bands was normalized
to GAPDH.
 | Table IPrimary antibodies used in western
blot analysis. |
Table I
Primary antibodies used in western
blot analysis.
| Name of primary
antibody | Manufacturer and
cat. no. | Dilution rate |
|---|
| GAPDH | Santa Cruz
Biotechnology: sc-25778 | 1:1,000 |
| p-ERK | Santa Cruz
Biotechnology: sc-7383 | 1:1,000 |
| ERK1 | Santa Cruz
Biotechnology: sc-271269 | 1:1,000 |
| ERK2 | Santa Cruz
Biotechnology: sc-154 | 1:1,000 |
| p-Akt | Santa Cruz
Biotechnology: sc-514032 | 1:500 |
| Akt | Santa Cruz
Biotechnology: sc-8312 | 1:500 |
| α-actin | Santa Cruz
Biotechnology: sc-32251 | 1:1,000 |
| TGF-β1 | Santa Cruz
Biotechnology: sc-146 | 1:1,000 |
| p-Smad2 | Santa Cruz
Biotechnology: sc-135644 | 1:1,000 |
| Smad2 | Santa Cruz
Biotechnology: sc-6200 | 1:1,000 |
| COL1A1 | Santa Cruz
Biotechnology: sc-293182 | 1:500 |
| COL1A2 | Santa Cruz
Biotechnology: sc-376350 | 1:500 |
Statistical analysis
For all assays, at least 3 independent experiments
were performed. The mean ± standard deviations of the expression
values were calculated using Microsoft Excel. The differences
between 2 groups were evaluated with one-way analysis of variance
followed by the Bonferroni post hoc test using SPSS statistical
software for Windows, v.20.0 (IBM Corp.).
Results
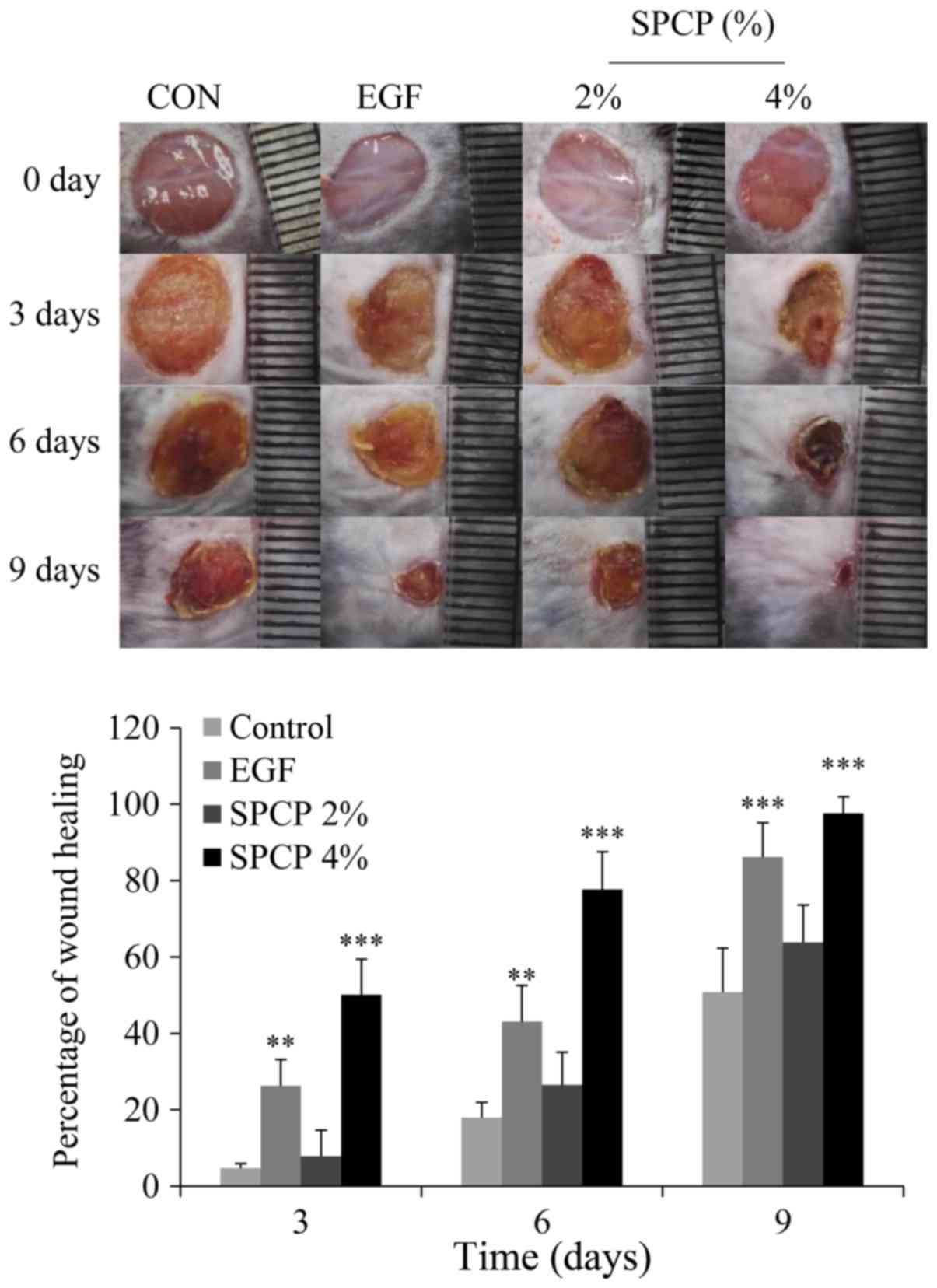
Treatment with SPCP accelerates wound
repair
In order to determine the effects of SPCP on skin
wound repair, C57BL/6 mice were used. To prove the hypothesis that
SPCP promotes wound healing, full-thickness excisional wounds were
created using mice. From the images obtained on days 0, 3, 6 and 9,
it can be seen that the percentage wound closure in the mice which
were treated with EGF or SPCP was higher than that of the mice
which were treated only with Vaseline as the control (Fig. 1).
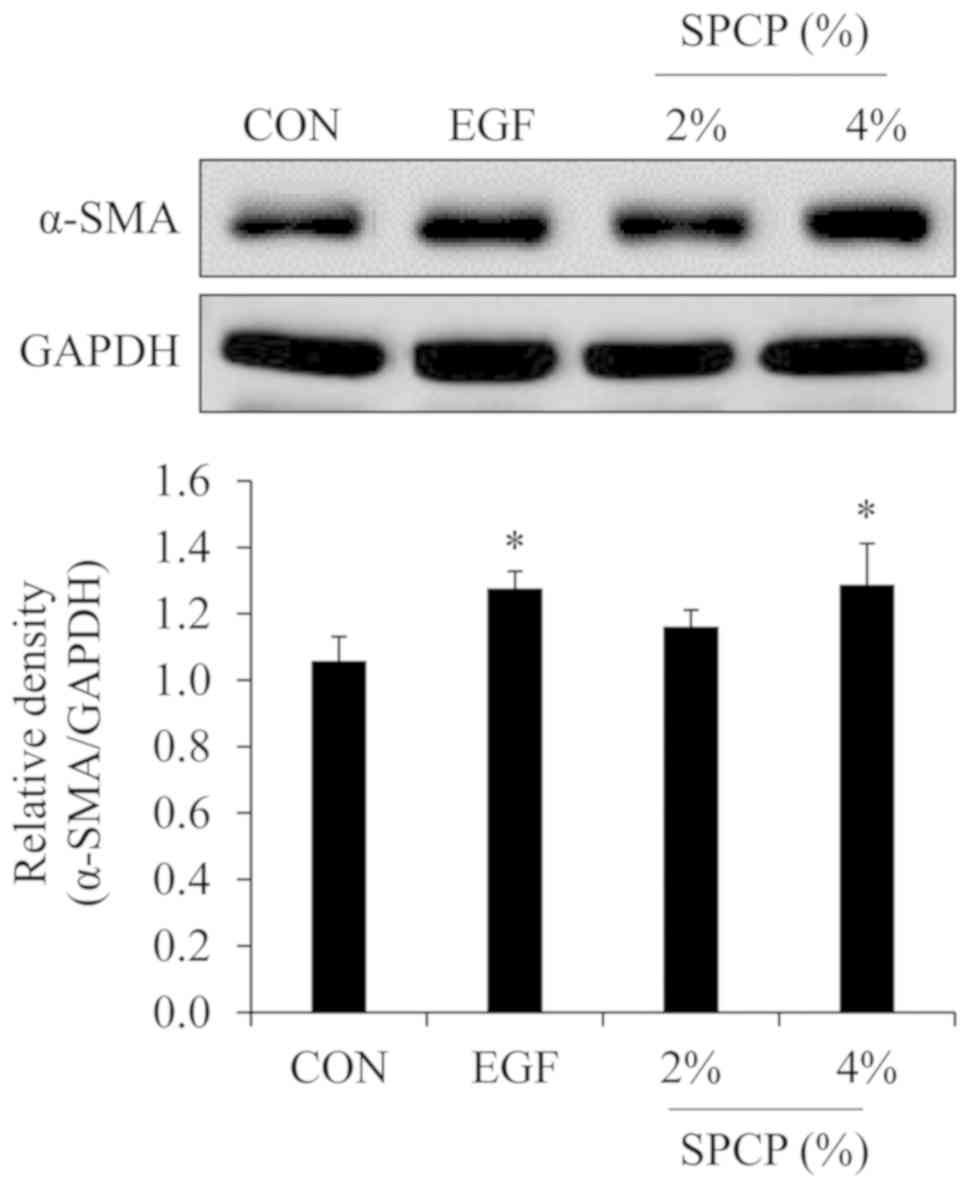
Myofibroblasts play an important role in skin wound
repair. One of the myofibroblast-specific markers is α-SMA
(18,29). Thus, the expression level of α-SMA
was determined by western blot analysis in the present study. As
shown in Fig. 2, the expression
level of α-SMA was higher in the EGF- or SPCP-treated groups than
in the control group. These results indicated that SPCP enhanced
wound repair by increasing the level of myofibroblasts.
Effect of SPCP on the body weight of
C57BL/6 mice
To determine the effects of SPCP on the body weight
of C57BL/6 mice, the body weights of the mice were recorded. From
the results (Table II) it can be
seen that the body weights of the mice in the SPCP-treated group
exhibited no marked differences with the mice in the control group.
These results indicated that SPCP exerted no effect on the body
weights of C57BL/6 mice.
 | Table IIEffect of SCPC on the body weights of
C57BL/6 mice. |
Table II
Effect of SCPC on the body weights of
C57BL/6 mice.
| Weight | Control | EGF | SPCP (%)
|
|---|
| 2% | 4% |
|---|
| Basal BW (g) | 22.86±0.40 | 21.66±0.74 | 23.00±0.41 | 22.40±0.35 |
| Final BW (g) | 22.92±0.68 | 22.18±0.66 | 23.08±0.77 | 22.76±0.58 |
Effect of 9 days of treatment with SPCP
on lipid peroxide and antioxidant enzyme levels in granulation
tissue homogenate
In order to determine the effects of SPCP on the
activity of SOD, enzyme-linked immunosorbent assay (ELISA) was
performed using an ELISA kit. As in shown in Table III, the mice which were treated
with SPCP exhibited a higher activity of SOD compared with those in
the control group. Furthermore, the activity of SOD was induced by
SPCP in a dose-dependent manner. These results indicated that SPCP
may exert a positive effect on antioxidants by enhancing the
activity of SOD during skin wound repair in mice.
 | Table IIIEffect of 9 days treatment with SPCP
on lipid peroxide and antioxidant enzyme levels in granulation
tissue homogenate. |
Table III
Effect of 9 days treatment with SPCP
on lipid peroxide and antioxidant enzyme levels in granulation
tissue homogenate.
| SOD activity (U/mg
protein) | CAT activity (mU/mg
protein) | MDA (nmol/mg
protein) |
|---|
| Control | 12.55±0.08 | 3.55±0.22 | 0.98±0.04 |
| EGF | 13.59±0.43a | 4.65±1.19 | 0.64±0.08b |
| 2% SPCP | 13.87±0.53a | 4.52±0.19 | 0.60±0.06c |
| 4% SPCP | 15.61±0.36c | 6.02±0.54b | 0.36±0.05c |
In order to determine the effects of SPCP on the
activity of CAT, ELISA was performed using an ELISA kit. As shown
in Table III, the mice which
were treated with SPCP exhibited a higher activity of CAT compared
with those in the control group. Furthermore, the activity of CAT
was induced by SPCP in a dose-dependent manner. These results
indicated that SPCP may exert a positive effect on antioxidants by
enhancing the activity of CAT during skin wound repair in mice.
In order to determine the effects of SPCP on the
level of MDA, ELISA was performed using an ELISA kit. As in shown
in Table III, the mice which
were treated with SPCP exhibited a lower level of MDA compared with
those in the control group. These results indicated that SPCP may
exert a positive effect on antioxidant by inhibiting the level of
MDA during skin wound repair in mice.
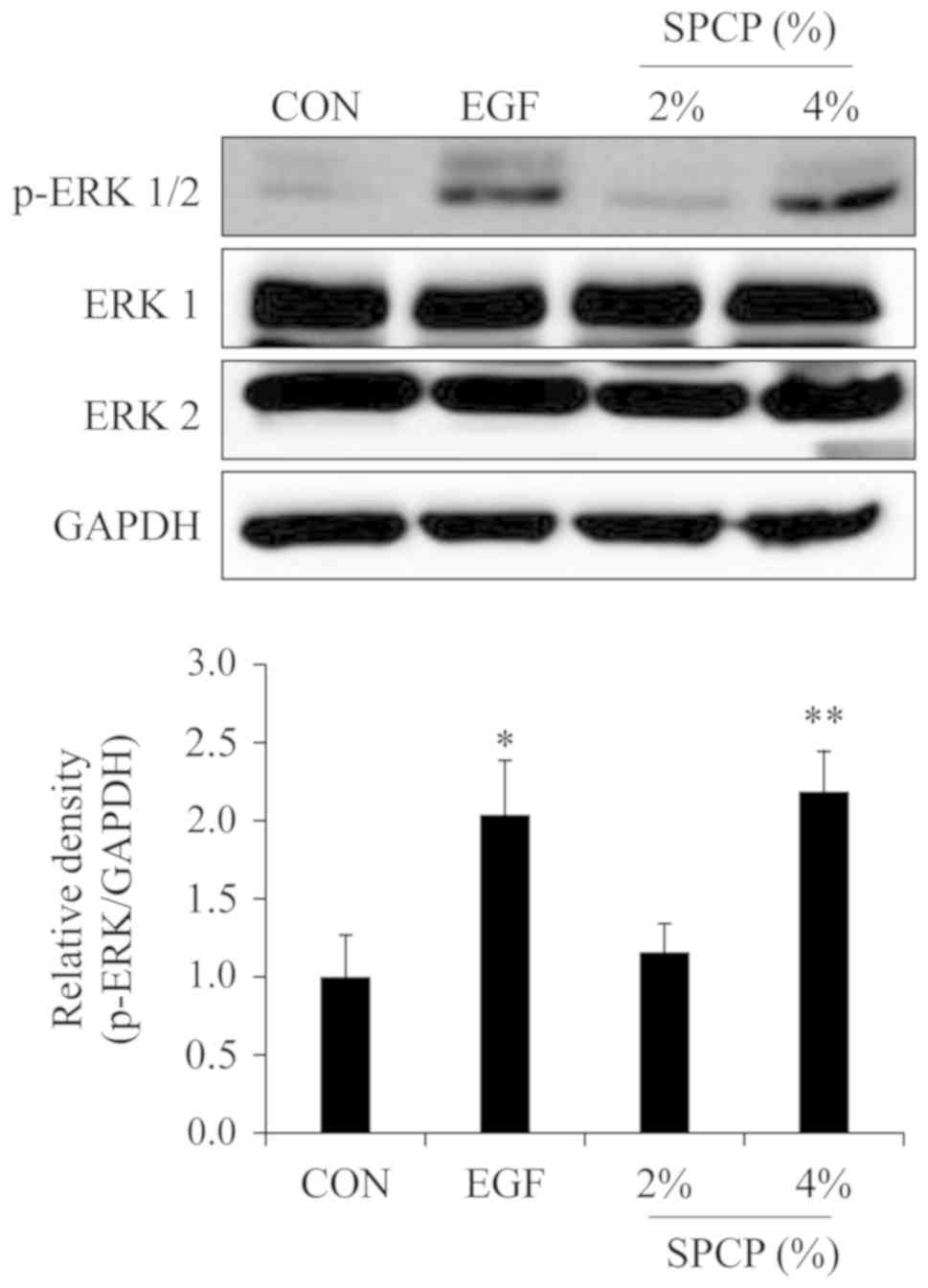
SPCP enhances wound repair via the ERK
signaling pathway in C57BL/6 mice
According to previous results in CCD-986sk cells
(27), it is known that the
EGFR/ERK signaling pathway is involved in the SPCP-induced
proliferation and migration of CCD-986sk cells. Thus, the effect of
SPCP on the phosphorylation level of ERK was determined by western
blot analysis. The results revealed that the phosphorylation level
of ERK was increased by treatment with SPCP in the skin granulation
tissue of C57BL/6 mice (Fig. 3).
This indicated that SPCP promoted skin wound repair in C57BL/6 mice
via the ERK signaling pathway.
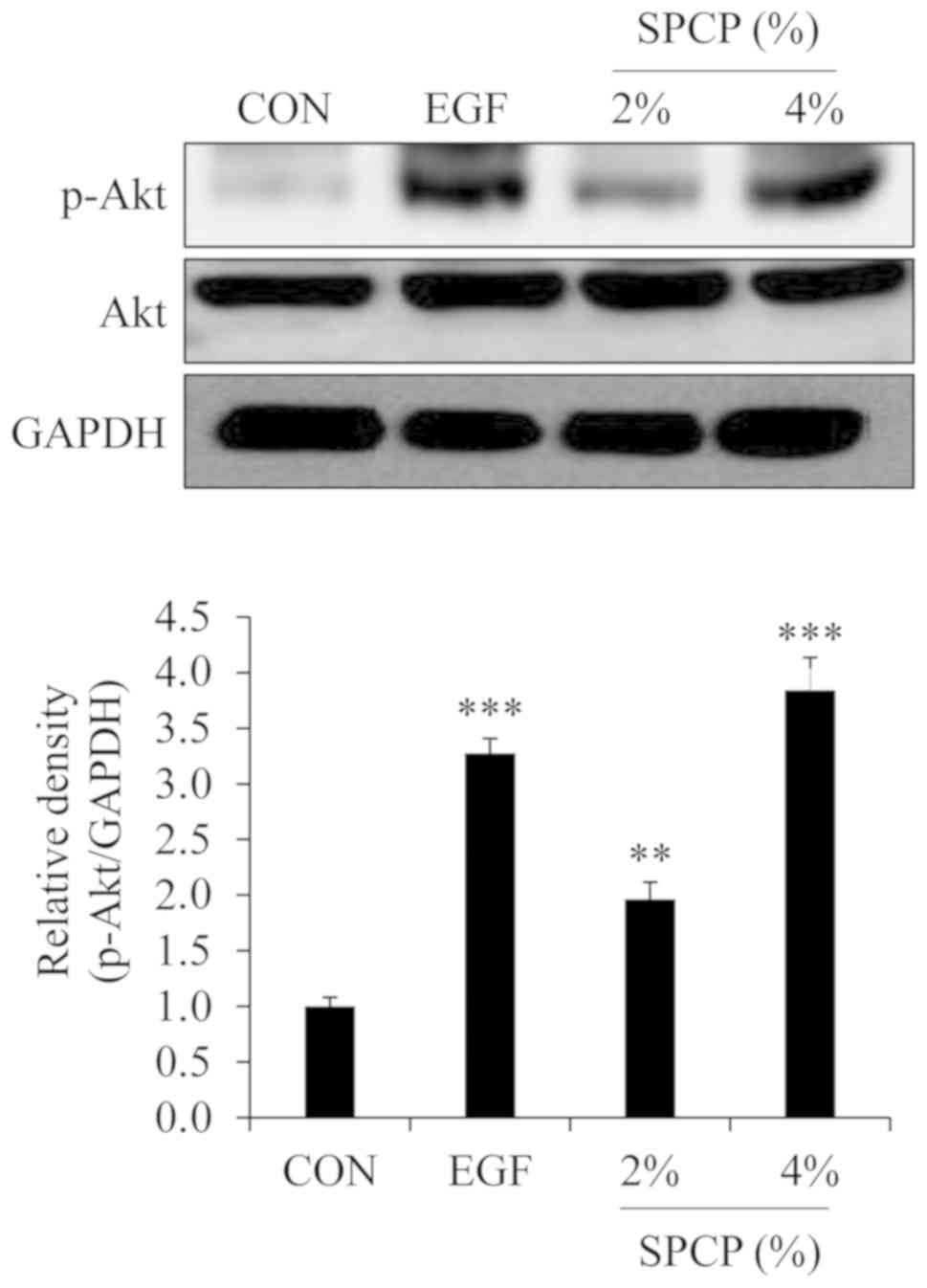
SPCP enhances wound repair via the Akt
signaling pathway in C57BL/6 mice
According to previous results in CCD-986sk cells
(28), it is known that the
PI3K/Akt signaling pathway is involved in the SPCP-induced
proliferation and migration of CCD-986sk cells. Thus, the effect of
SPCP on the phosphorylation level of Akt were determined by western
blot analysis in the present study. The results revealed that the
phosphorylation level of Akt was increased by treatment with SPCP
in the skin granulation tissue of C57BL/6 mice (Fig. 4). This indicated that SPCP
promoted skin wound repair in C57BL/6 mice via the Akt signaling
pathway.
SPCP enhances wound repair via the
TGF-β1/Smad signaling pathway
The TGF-β1/Smad signal transduction pathway is a
signal transduction pathway which plays an important role in tissue
repair (30). TGF-β1 is involved
in the whole process of inflammation, proliferative phase and
plasticization during wound repair (31). Thus, the effect of SPCP on the
TGF-β1/Smad signaling pathway was determined by western blot
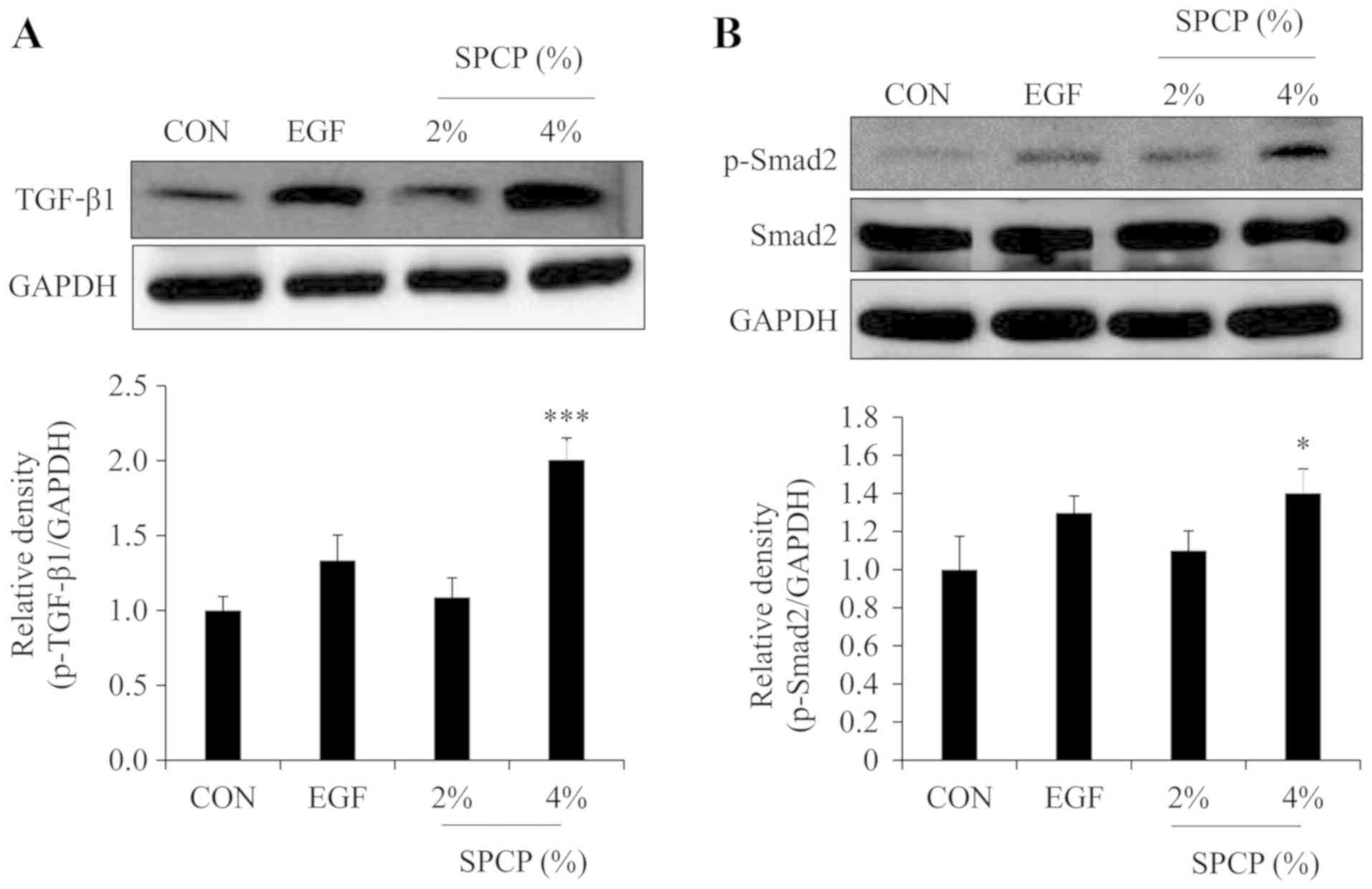
analysis in the present study. The results revealed that the level
of TGF-β1 was increased by treatment with SPCP in the skin
granulation tissue of C57BL/6 mice (Fig. 5A). Moreover, the level of p-Smad2
was increased by treatment with SPCP in the skin granulation tissue
of C57BL/6 mice (Fig. 5B). These
results indicated that SPCP promoted skin wound repair in C57BL/6
mice via the TGF-β1/Smad signaling pathway.
SPCP regulates the expression of
collagen
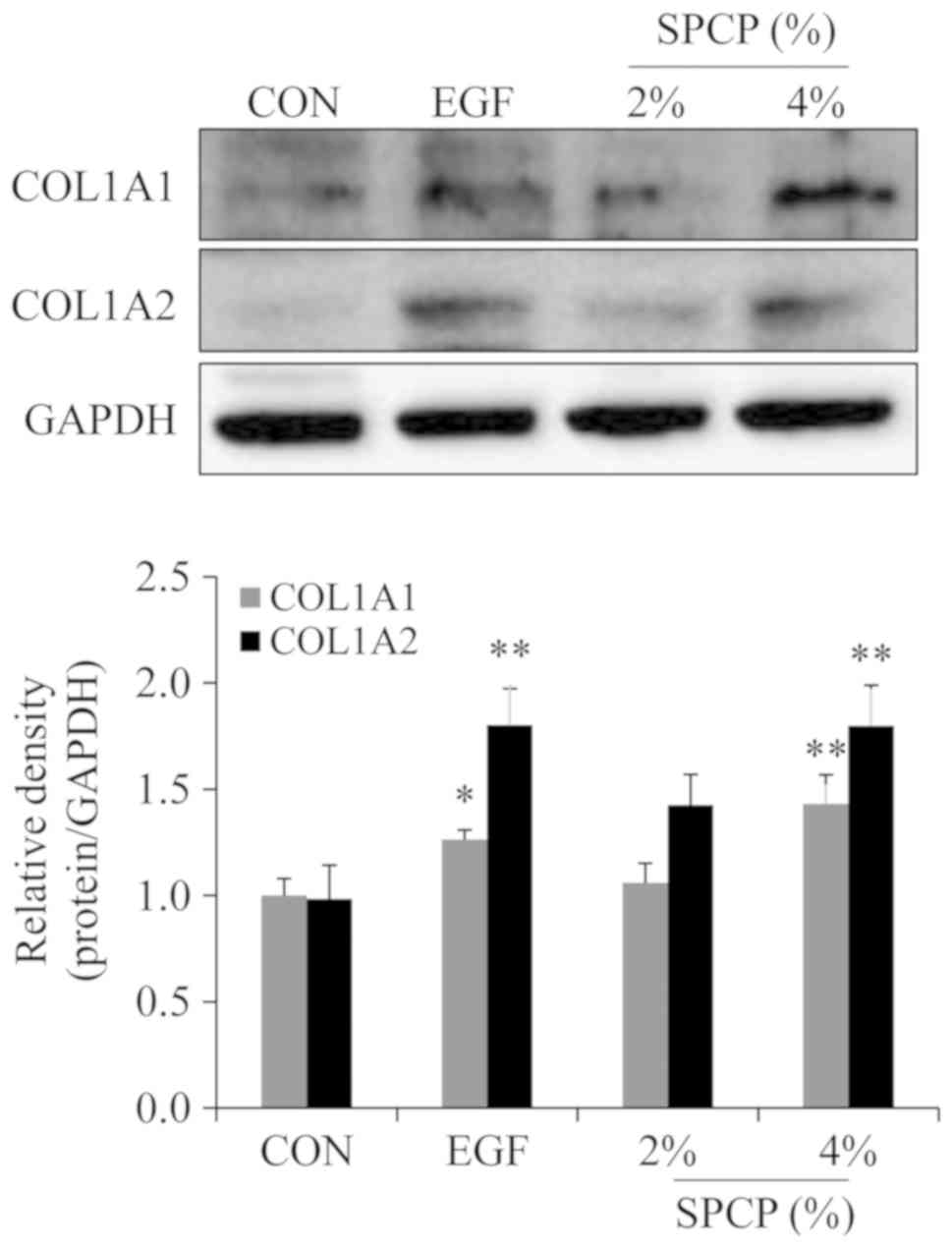
In order to determine the effects of SPCP on the
expression level of collagen in the granulation tissue of C57BL/6
mice, western blot analysis was performed. The results revealed
that the expression level of type I collagen was higher in
SPCP-treated group than that in the control group (Fig. 6). This result indicated that SPCP
enhanced the deposition of type I collagen during skin wound
repair.
Discussion
Normally, for the damage of the external
environment, skin can protect the integrity and function of
internal organs very effectively (22,32). Therefore, in the process of
resisting environmental stimuli, the skin will be damaged to
varying degrees. If the damage is severe, the function of internal
organs will change, and may even result in death (33,34). Therefore, it is crucial to
identify methods with which to promote the efficiency of skin wound
repair. As is known, the process of wound repair is very complex.
The key factor in this process is the process of forming and
reconstructing new tissue cells (1,35).
According to previous findings, SPCP enhances the proliferation and
migration of human fibroblasts (28), which play a crucial role in the
formation and remodeling of new tissues. In the present study, SPCP
was found to promote skin wound repair in C57BL/6 mice. It has been
reported that a low level of ROS is essential for wound repair.
However, excessive ROS production can inhibit wound repair
(36,37). Superoxide anion free radicals
(O2- are natural intermediates in various
physiological reactions of organisms. These are a type of ROS with
poten oxidation ability and are one of the important factors of
biological oxygen toxicity. SOD is a free radical scavenger, which
exists widely in various tissues of organisms and can scavenge free
radical O2- (38). CAT is an enzyme scavenger that can
decompose hydrogen peroxide into water and oxygen. Hydrogen
peroxide is scavenged by CAT to protect the body from oxidative
damage (39). Antioxidants play
an important role in wound repair due to its protection on the
wound from oxidative damage (32). Thus, the present study evaluated
the antioxidant effects of SPCP in wound repair by measuring the
SOD, CAT activity and MDA content. The results revealed that SPCP
reduced the MDA content. At the same time, the activities of SOD
and CAT in the granulation tissue of mice in the SPCP treatment
group were higher than those of the mice in the control group.
These results suggest that SPCP promotes the repair of skin wounds
in mice through antioxidation.
ERK1/2 can be phosphorylated by certain growth
factors and hydrogen peroxide, and then enters the nucleus to act
on transcription factors in the nucleus, such as c-Myc, c-Jun and
nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB)
(40). Therefore, ERK1/2 can
promote the activity of downstream genes, affect the transcription
and expression of downstream genes, regulate various functions of
cells, such as metabolism and survival, and ultimately affect the
corresponding biology of cells (41). It has been reported that only
phosphorylated ERK1/2 is active (42). In a previous study, it was
demonstrated that SPCP increased the phosphorylation level of
ERK1/2 (27). Thus, the
phosphorylation level of ERK1/2 in the granulation tissue of
C57BL/6 mice was determined in the present study. The results
revealed that SPCP activated ERK1/2 signaling in the skin
granulation tissue of C57BL/6 mice. Moreover, the PI3K/Akt
signaling pathway, as one of the more common signaling pathways
in vivo, is involved in regulating various cell activities,
such as cell inflammation, proliferation and differentiation
(43). PI3K/Akt pathway
integrates signals from growth factors and cytokines, and transmits
these signals through multiple downstream effectors (44). In turn, these effectors regulate
basic cellular functions, including growth, metabolism, survival
and proliferation (45). Previous
studies have found that SPCP activates the PI3K/Akt signaling
pathway in CCD-986sk cells. Therefore, the activation of Akt
signaling was examined in the present study. The results
demonstrated that SPCP activated Akt signaling in the skin
granulation tissue of C57BL/6 mice.
In the process of wound repair, wound contraction
and ECM recombination are crucial (46). In the process of wound
contraction, one of the most important factors is the expression
and differentiation of myofibroblasts. The expression of α-SMA is
an important marker of myofibroblasts (47). In the present study, on the 9th
day of wound repair, the expression level of α-SMA in granulation
tissue of SPCP treated mice was significantly higher than that of
the control group. Previous studies have indicated that in the
process of fibroblast differentiation into myofibroblasts, the
stimulation of TGF-β on wounds is crucial (7-9).
In the present study, on the 9th day following injury, SPCP
treatment significantly increased the expression of TGF-β1 in the
granulation tissue.
Moreover, the phosphorylation level of Smad2, which
was the downstream signal of TGF-β1, was enhanced by treatment with
SPCP in the granulation tissue of C57BL/6 mice. These results
suggested that SPCP promoted skin wound repair in mice by
increasing the expression of α-SMA and activating the TGF-β1/Smad
signaling pathway. For further research, the authors aim to
determine the later events in wound healing, such as tissue
normalization, reduction of α-SMA (positive cells), TGF-β1, and
associated components or reconstitution of ROS levels, to assess
whether SPCP may be used as an appropriate therapeutic. In
addition, the cutaneous ECM comprises a complex assortment of
proteins. The most abundant proteins in the ECM are collagens. In
particular, type I collagen is the most prevalent of the
fibril-forming collagens (48).
According to previous studies, it has been found that SPCP promotes
the secretion of collagen in CCD-986sk cells (27). In the present study, the
expression level of type I collagen was determined in granulation
tissue of C57BL/6 mice. The results revealed that the expression
level of type I collagen was induced by SPCP. In addition, other
components of the ECM also play an essential role in wound repair,
such as fibronectin which is an adhesive molecule that plays a
crucial role in ECM formation and skin wound repair (49). Thus, further studies are required
to determine the expression level of other components of the ECM in
granulation tissue following treatment with SPCP. Even though the
ECM plays an important role in skin wound repair, excessive ECM
deposition may result in fibrosis, scarring and the loss of tissue
function. Accordingly, it is important to maintain ECM production
in balance for a complete closure of a wound.
In conclusion, in the present tudy, following SPCP
treatment, the wound repair was enhanced in C57BL/6 mice. This
indicated that SPCP can effectively promote wound repair. In this
process, the ERK, Akt and TGF-β1 signaling pathways played an
important role. The results obtained herein provide evidence of the
promoting effects of SPCP on wound repair in C57BL/6 mice and the
underlying mechanisms were revealed. Furthermore, the results
obtained in the present study support the positive role of SPCP in
wound repair.
For further study, the authors aim to perform the
specific staining of tissue sections to address appearance and
location of critical components, cell and tissue fate of both skin
compartments. In addition, despite the fact that C57/BL6 mice are a
widely accepted wound repair model for experiments, the skin
structure and wound repair mechanisms of the mice differ from those
of humans. Further studies are thus required to evaluate the
therapeutic effets of SPCP on other wound repair models that are
more akin to the human skin.
Funding
The present study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF), funded by the Ministry of Education (grant no.
2012R1A6A1028677).
Availability of data and materials
The analyzed datasets of this study are available
from the corresponding author on reasonable request.
Authors' contributions
TJN was involved in the conceptualization of the
study. PL and YHC were involved in data analysis. PL, MKL and JWC
were involved in data analysis and investigation. PL was involved
in the writing of the original draft. YHC was involved in the
writing, reviewing and editing of the manuscript. TJN supervised
the study and was responsible for funding acquisition. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
All experiments procedures were approved by the
University Animal Care and Use Committee guidelines at Pukyong
National University (Busan, Korea; approval no. 2018-15) and
conducted according to the international regulations of the usage
and welfare of laboratory animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
Acknowledgments
Not applicable.
References
|
1
|
Kageyama H and Waditee-Sirisattha R:
Antioxidative, anti-inflammatory, and anti-aging properties of
mycosporine-like amino acids: Molecular and cellular mechanisms in
the protection of skin-aging. Mar Drugs. 17:pii: E222. 2019.
View Article : Google Scholar
|
|
2
|
Hyun YJ, Piao MJ, Kang KA, Zhen AX,
Madushan Fernando PDS, Kang HK, Ahn YS and Hyun JW: Effect of
fermented fish oil on fine particulate matter-induced skin aging.
Mar Drugs. 17:pii: E61. 2019. View Article : Google Scholar
|
|
3
|
Takeo M, Lee W and Ito M: Wound healing
and skin regeneration. Cold Spring Harb Perspect Med.
5:a0232672015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Martin P: Wound healing-aiming for perfect
skin regeneration. Science. 276:75–81. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hu L, Wang J, Zhou X, Xiong Z, Zhao J, Yu
R, Huang F, Zhang H and Chen L: Exosomes derived from human adipose
mensen-chymal stem cells accelerates cutaneous wound healing via
optimizing the characteristics of fibroblasts. Sci Rep.
6:329932016. View Article : Google Scholar
|
|
6
|
Chiquet M, Katsaros C and Kletsas D:
Multiple functions of gingival and mucoperiosteal fibroblasts in
oral wound healing and repair. Periodontol 2000. 68:21–40. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Barrientos S, Stojadinovic O, Golinko MS,
Brem H and Tomic-Canic M: Growth factors and cytokines in wound
healing. Wound Repair Regen. 16:585–601. 2008. View Article : Google Scholar
|
|
8
|
Crowe MJ, Doetschman T and Greenhalgh DG:
Delayed wound healing in immunodeficient TGF-beta 1 knockout mice.
J Invest Dermatol. 115:3–11. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Philipp K, Riedel F, Sauerbier M, Hörmann
K and Germann G: Targeting TGF-beta in human keratinocytes and its
potential role in wound healing. Int J Mol Med. 14:589–593.
2004.PubMed/NCBI
|
|
10
|
Tracy LE, Minasian RA and Caterson EJ:
Extracellular matrix and dermal fibroblast function in the healing
wound. Adv Wound Care (New Rochelle). 5:119–136. 2014. View Article : Google Scholar
|
|
11
|
Zhou ZQ, Chen Y, Chai M, Tao R, Lei YH,
Jia YQ, Shu J, Ren J, Li G, Wei WX, et al: Adipose extracellular
matrix promotes skin wound healing by inducing the differentiation
of adipose-derived stem cells into fibroblasts. Int J Mol Med.
43:890–900. 2019.
|
|
12
|
Xue M and Jackson CJ: Extracellular matrix
reorganization during wound healing and its impact on abnormal
scarring. Adv Wound Care (New Rochelle). 4:119–136. 2015.
View Article : Google Scholar
|
|
13
|
Guo S and Dipietro LA: Factors affecting
wound healing. J Dent Res. 89:219–229. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang Y, Xia T, Zhi W, Wei L, Weng J, Zhang
C and Li X: Promotion of skin regeneration in diabetic rats by
electrospun core-sheath fibers loaded with basic fibroblast growth
factor. Biomaterials. 32:4243–4254. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Moulin V: Growth factors in skin wound
healing. Eur J Cell Biol. 68:1–7. 1995.PubMed/NCBI
|
|
16
|
Derynck R, Zhang Y and Feng XH: Smads:
Transcriptional activators of TGF-beta responses. Cell. 95:737–740.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rolfe KJ, Richardson J, Vigor C, Irvine
LM, Grobbelaar AO and Linge C: A role for TGF-beta 1-induced
cellular responses during wound healing of the non-scarring early
human fetus? J Invest Dermatol. 127:2656–2667. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wynn TA: Cellular and molecular mechanisms
of fibrosis. J Pathol. 214:199–210. 2008. View Article : Google Scholar
|
|
19
|
Sauer H, Wartenberg M and Hescheler J:
Reactive oxygen species as intracellular messengers during cell
growth and differentiation. Cell Physiol Biochem. 11:173–186. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dunnill C, Patton T, Brennan J, Barrett J,
Dryden M, Cooke J, Leaper D and Georgopoulos NT: Reactive oxygen
species (ROS) and wound healing: The functional role of ROS and
emerging ROS-modulating technologies for augmentation of the
healing process. Int Wound J. 14:89–96. 2017. View Article : Google Scholar
|
|
21
|
Sen CK, Khanna S, Babior BM, Hunt TK,
Ellison EC and Roy S: Oxidant-induced vascular endothelial growth
factor expression in human keratinocytes and cutaneous wound
healing. J Biol Chem. 277:33284–33290. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Park HH, Park NY, Kim SG, Jeong KT, Lee EJ
and Lee E: Potential wound healing activities of galla rhois in
human fibroblasts and keratinocytes. Am J Chin Med. 43:1625–1636.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pozzolini M, Millo E, Oliveri C, Mirata S,
Salis A, Damonte G, Arkel M and Scarfì S: Elicited ROS scavenging
activity, photo-protective, and wound-healing properties of
collagen-derived peptides from the marine sponge chondrosia
reniformis. Mar Drugs. 16:pii: E465. 2018. View Article : Google Scholar
|
|
24
|
Son DH, Yang DJ, Sun JS, Kim SK, Kang N,
Kang JY, Choi YH, Lee JH, Moh SH, Shin DM and Kim KW: A novel
peptide, nicotinyl-isoleucine-valine-histidine (NA-IVH), promotes
antioxidant gene expression and wound healing in HaCaT cells. Mar
Drugs. 16:pii: E262. 2018. View Article : Google Scholar
|
|
25
|
Kubatka P, Kapinová A, Kružliak P, Kello
M, Výbohová D, Kajo K, Novák M, Chripková M, Adamkov M, Péč M, et
al: Antineoplastic effects of Chlorella pyrenoidosa in the breast
cancer model. Nutrition. 31:560–569. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sheih IC, Fang TJ, Wu TK and Lin PH:
Anticancer and antioxidant activities of the peptide fraction from
algae protein waste. J Agric Food Chem. 58:1202–1207. 2010.
View Article : Google Scholar
|
|
27
|
Liu P, Lee MK, Choi JW, Choi Y and Nam TJ:
Crude protein from spirulina increases the viability of CCD-986sk
cells via the EGFR/MAPK signaling pathway. Int J Mol Med.
43:771–778. 2019.
|
|
28
|
Liu P, Choi JW, Lee MK, Choi YH and Nam
TJ: Wound healing potential of spirulina protein on CCD-986sk
cells. Mar Drugs. 17:pii: E130. 2019.
|
|
29
|
Plikus MV, Guerrero-Juarez CF, Ito M, Li
YR, Dedhia PH, Zheng Y, Shao M, Gay DL, Ramos R, Hsi TC, et al:
Regeneration of fat cells from myofibroblasts during wound healing.
Science. 355:748–752. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Verrecchia F and Mauviel A: Transforming
growth factor-beta signaling through the Smad pathway: Role in
extracellular matrix gene expression and regulation. J Invest
Dermatol. 118:211–215. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu HY, Wu JL and Ni ZL: Overexpression of
microRNA-202-3p protects against myocardial ischemia-reperfusion
injury through activation of TGF-β1/Smads signaling pathway by
targeting TRPM6. Cell Cycle. 18:621–637. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Joshi A, Joshi VK, Pandey D and Hemalatha
S: Systematic investigation of ethanolic extract from Leea
macrophylla: Implications in wound healing. J Ethnopharmacol.
191:95–106. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhao X, Wu H, Guo B, Dong R, Qiu Y and Ma
PX: Antibacterial anti-oxidant electroactive injectable hydrogel as
self-healing wound dressing with hemostasis and adhesiveness for
cutaneous wound healing. Biomaterials. 122:34–47. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim HS, Park SY, Moon SH, Lee JD and Kim
S: Autophagy in human skin fibroblasts: Impact of age. Int J Mol
Sci. 19:pii: E2254. 2018.
|
|
35
|
Pereira RF and Bártolo PJ: Traditional
therapies for skin wound healing. Adv Wound Care (New Rochelle).
5:208–229. 2016. View Article : Google Scholar
|
|
36
|
Schäfer M and Werner S: Oxidative stress
in normal and impaired wound repair. Pharmacol Res. 58:165–171.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Landén NX, Li D and Ståhle M: Transition
from inflammation to proliferation: A critical step during wound
healing. Cell Mol Life Sci. 73:3861–3885. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nimse SB and Pal D: Free radicals, natural
antioxidants, and their reaction mechanisms. RSC Adv. 5:27986–8006.
2015. View Article : Google Scholar
|
|
39
|
Sies H: Hydrogen peroxide as a central
redox signaling molecule in physiological oxidative stress:
Oxidative eustress. Redox Biol. 11:613–619. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Meloche S and Pouysségur J: The ERK1/2
mitogen-activated protein kinase pathway as a master regulator of
the G1-to S-phase transition. Oncogene. 26:3227–3239. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
He Z, Jiang J, Kokkinaki M, Golestaneh N,
Hofmann MC and Dym M: Gdnf upregulates c-Fos transcription via the
Ras/Erk1/2 pathway to promote mouse spermatogonial stem cell
proliferation. Stem Cells. 26:266–278. 2008. View Article : Google Scholar
|
|
42
|
Mebratu Y and Tesfaigzi Y: How ERK1/2
activation controls cell proliferation and cell death: Is
subcellular localization the answer? Cell Cycle. 8:1168–1175. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Coutant A, Rescan C, Gilot D, Loyer P,
Guguen-Guillouzo C and Baffet G: PI3K-FRAP/mTOR pathway is critical
for hepatocyte proliferation whereas MEK/ERK supports both
proliferation and survival. Hepatology. 36:1079–1088. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Engelman JA, Luo J and Cantley LC: The
evolution of phospha-tidylinositol 3-kinases as regulators of
growth and metabolism. Nat Rev Genet. 7:606–619. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kornasio R, Riederer I, Butler-Browne G,
Mouly V, Uni Z and Halevy O: Beta-hydroxy-beta-methylbutyrate (HMB)
stimulates myogenic cell proliferation, differentiation and
survival via the MAPK/ERK and PI3K/Akt pathways. Biochim Biophys
Acta. 1793:755–763. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Schultz GS and Wysocki A: Interactions
between extracellular matrix and growth factors in wound healing.
Wound Repair Regen. 17:153–162. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hinz B: Formation and function of the
myofibroblast during tissue repair. J Invest Dermatol. 127:526–537.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kadler KE, Baldock C, Bella J and
Boot-Handford RP: Collagens at a glance. J Cell Sci. 120:1955–1958.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lenselink EA: Role of fibronectin in
normal wound healing. Int Wound J. 12:313–316. 2015. View Article : Google Scholar
|