Introduction
Oxidative stress produces excessive reactive oxygen
species (ROS), such as hydrogen peroxide
(H2O2), superoxide radical
(O2-) and hydroxyl radical (•OH).
The insufficient elimination of ROS by antioxidant mechanisms
perturbs cellular redox homeostasis (1-3).
Previous studies have demonstrated that excessive ROS generation
leads to the peroxidation of membrane lipids, disturbs cellular
antioxidant signaling and induces apoptosis (4). ROS also play critical roles as
pathogenic factors in the development of cardiovascular disease by
promoting the apoptosis of endothelial cells (5,6).
Therefore, protecting endothelial cells from apoptosis induced by
oxidative stress appears to be an effective strategy for the
prevention and treatment of cardiovascular disease.
Cinnamaldehyde (CA) is a major compound in cinnamon
obtained from the stem bark of Cinnamomum cassia (7). CA exhibits various biological
properties, such as antibacterial, anti-inflammatory and anticancer
activities (8-10). Additionally, Song et al
reported the antioxidative and anti-inflammatory properties of CA
in a model of acute myocardial ischemia (11). However, the precise molecular
mechanisms responsible for the protective effects of CA on cells
remain to be elucidated.
Atherosclerosis and its complications are the most
common causes of mortality in humans, and oxidative stress and
inflammation play critical roles in the development of
cardiovascular diseases. During the complex developmental process
of atherosclerosis, damage to vascular endothelial cells is
considered an initiator of vascular disease (12-14). The authors have recently reported
that CA actively protects human dental pulp cells (hDPCs) under
oxidative stress conditions by inducing nuclear factor erythroid
2-related factor 2 (Nrf2) activation and subsequent heme
oxygenase-1 (HO-1) expression (15). As a transcription factor, Nrf2
regulates the expression of antioxidant proteins that protect cells
against oxidative stress. Under conditions of stress, Nrf2 is
activated and translocated from the cytoplasm to the nucleus where
it induces the expression of antioxidant defense enzymes, such as
HO-1 (16,17). CA also exerts protective effects
against the oxidized low-density lipoprotein (ox-LDL)-induced
proliferation and migration of vascular smooth muscle cells (VSMCs)
(18). Additionally,
2-methoxycinnamaldehyde (MCA), a natural derivative of CA, has been
shown to exhibit anti-atherosclerotic activity by inhibiting the
proliferation and migration of human aortic smooth muscle cells
(HASMCs) (19). These findings
strongly suggest that CA is a candidate against for the treatment
of cardiovascular diseases. However, to the best of our knowledge,
no available studies to date have described the effects of CA on
vascular endothelial cells exposed to oxidative stress.
In the present study, the value of CA as an
anti-atherosclerotic agent was assessed. The cytoprotective effects
of CA and the underlying molecular mechanisms in
H2O2-exposed human umbilical vein endothelial
cells (HUVECs) were examined. In addition, the anti-inflammatory
effects of CA in HUVECs and Sprague-Dawley rats were
investigated.
Materials and methods
Materials
CA, zinc protoporphyrin (ZnPP) and SB202190 were
obtained from Sigma-Aldrich; Merck KgaA. MCA and
2′-hydroxycinnamaldehyde (HCA) were purchased from Santa Cruz
Biotechnology, Inc., and H2O2 was supplied by
CalBiochem. Cobalt protoporphyrin (CoPP) was purchased from MP
Biomedicals and calcein AM was supplied by Invitrogen; Thermo
Fisher Scientific, Inc. Recombinant human tumor necrosis factor
(TNF)-α was obtained from R&D Systems, Inc.
Cell culture and viability assay
HUVECs (ATCC, CRL-1730) were maintained at 37°C
under 5% CO2 in endothelial cell medium (ECM)
supplemented with reagents provided in a complete kit (ScienCell
Research Laboratories, Inc.). Human monocytic U937 cells (ATCC,
CRL-1593) were maintained in RPMI-1640 medium supplemented with 10%
FCS, 100 units/ml penicillin and 100 µg/ml streptomycin. For
viability assays, HUVECs were seeded in 12-well plates at a density
of 5×104 cells/ml, cultured overnight and treated with
various concentrations of CA, MCA or HCA for 24 h. Cell viability
was measured using a 3-(4,5-dimethylthiazol-
2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay according to a
previously described method (20).
Cytotoxicity assay
Cell cytotoxicity was determined by measuring
lactate dehydrogenase (LDH) activity following treatment with CA,
MCA or HCA using a CytoTox 96® nonradioactive assay kit
(Promega Corporation) according to the manufacturer's
instructions.
Western blot analysis
HUVECs were treated with various concentrations of
CA or MCA for various periods of time. The cells were treated with
SB202190 (20 µM) 2 h prior to CA treatment. The cells were
washed twice with cold PBS and lysed in RIPA buffer (PBS
supplemented with 1% NP-40, 0.5% sodium deoxycholate, 1 mM
phenylmethylsulfonyl fluoride, 1 µg/ml aprotinin and 1 mM
sodium orthovanadate). The cell lysates were incubated at 4°C for
30 min and then cleared by centrifugation at 10,000 x g for 10 min.
Alternatively, cytoplasmic and nuclear fractions were obtained
using a previously described subcellular fractionation method
(21). The protein concentration
was determined using Protein Assay Dye Reagent Concentrate (Bio-Rad
Laboratories, Inc.). A total of 10 µg of proteins were
resolved on 10 or 12% SDS-PAGE and then transferred to a
nitrocellulose membrane. The membrane was blocked in Tris-buffered
saline (TBS) containing 5% non-fat milk for 4 h at room
temperature. Immunoblotting was performed overnight at 4°C using
the following antibodies: Anti-HO-1 (1:1,000; cat. no. ADI-SPA-896;
Enzo Life Sciences, Inc.), anti-AKT (1:2,000; cat. no. 4691s; Cell
Signaling Technology, Inc.), anti-p-AKT (1:2,000; cat. no. 4060s;
Cell Signaling Technology, Inc.), anti-ERK (1:2,000; cat. no.
4695s; Cell Signaling Technology, Inc.), anti-p-ERK (1:2,000; cat.
no. 4370s; Cell Signaling Technology, Inc.), anti-p38 (1:2,000;
cat. no. 8690s; Cell Signaling Technology, Inc.), anti-p-p38
(1:2,000; cat. no. 4511s; Cell Signaling Technology, Inc.),
anti-p-nuclear factor (NF)-κB (1:2,000; cat. no. 3033s; Cell
Signaling Technology, Inc.), anti-poly(ADP-ribose) polymerase
(PARP) (1:2,000; cat. no. 9542s; Cell Signaling Technology, Inc.),
anti-cleaved-PARP (1:2,000; cat. no. 5625s; Cell Signaling
Technology, Inc.), anti-Nrf2 (1:1,000; cat. no. sc365949; Santa
Cruz Biotechnology, Inc.), anti-NF-κB (1:2,000; cat. no. sc8008;
Santa Cruz Biotechnology, Inc.), anti-intercellular adhesion
molecule 1 (ICAM-1) (1:2,000; cat. no. sc8439; Santa Cruz
Biotechnology, Inc.), anti-vascular cell adhesion protein 1
(VCAM-1) (1:2,000; cat. no. sc8304; Santa Cruz Biotechnology,
Inc.), anti-TATA-binding protein (TBP) (1:2,000; cat. no. ab818;
Abcam) and anti-β-actin (1:5,000; cat. no. A1978; Sigma-Aldrich;
Merck KgaA). The blots were then incubated with goat anti-rabbit
horse-radish peroxidase (HRP)-conjugated secondary antibodies
(1:5,000; cat. no. ADI-SAB-300-J; Enzo Life Sciences, Inc.) or goat
anti-mouse HRP-conjugated secondary antibodies (1:5,000; cat. no.
ADI-SAB-100-J; Enzo Life Sciences, Inc.) at room temperature for
1.5 h and then developed using Immobilon® Western
Chemiluminescent HRP substrate (Merck KgaA).
RNA isolation and RT-PCR
HUVECs were treated with 20 µM CA or MCA for
various periods of time. Total RNA was isolated using TRI-solution
(Bio Science Technology) according to the manufacturer's
instructions. Semi-quantitative RT-PCR was performed using an
ONE-STEP RT-PCR PreMix kit (iNtRON Biotechnology) according to the
manufacturer's instructions. The specific primers and cycling
conditions for RT-PCR have been previously described (15). The RT-PCR products were
electrophoresed on 1.7% agarose gels and visualized by ethidium
bromide staining.
Detection of ROS
HUVECs were treated with 10 µM ZnPP. After 2
h, the cells were treated with 20 µM CA for 24 h and then
further treated with 350 µM H2O2 for
an additional 6 h. The levels of generated ROS were measured by
staining the cells with 10 µM dihydroethidium for 30 min
according to a previously described method (15). Fluorescence was analyzed using a
conventional fluorescence microscope (Axio Observer D1, Carl Zeiss
AG).
Transfection with siRNA
HUVECs were seeded in a 12-well plate at a density
of 7×104 cells/ml, cultured overnight and transfected
with either 25 nM of HO-1 siRNA (5′-GGC AGA GAA UGC UGA GUU C-3′)
or 5 nM of Nrf2 siRNA (sc-37030, Santa Cruz Biotechnology, Inc.)
using DharmaFECT transfection reagent (GE Healthcare Dharmacon,
Inc.) according to the manufacturer's instructions. After 6 h, the
media were changed, and the cells were further incubated for 18 h
before being treated with 20 µM CA. siGENOME Non-Targeting
siRNA Pool (GE Healthcare Dharmacon, Inc.) was used as a
control.
Annexin V apoptosis assay
HUVECs were treated with 20 µM CA or 5
µM CoPP for 24 h and then with 350 µM
H2O2 for 24 h. An Annexin V apoptosis assay
was performed with a Muse™ Annexin V & Dead Cell kit according
to the manufacturer's protocol (Merck KGaA). Briefly, 100 µl
of a cell suspension was mixed with 100 µl of Muse™ Annexin
V and Dead Cell reagent and then incubated at room temperature for
20 min. Apoptotic cells were analyzed using a Muse Cell Analyzer
(Merck KGaA).
U937 cell adhesion assay
U937 cells were labeled with calcein AM fluorescent
dye (1 µg/ml) for 30 min at 37°C. The cells were then washed
with PBS and resuspended in ECM. HUVECs were cultured in a 35 mm
dish until confluent, after which the cells were treated with CA
(20 µM) and/or TNF-α (10 ng/ml) for 6 h. The HUVECs were
then co-cultured with calcein AM-labeled U937 cells for 3 h.
Non-adhering U937 cells were removed by washing twice with PBS.
Finally, the HUVECs were fixed with 4% paraformaldehyde in PBS for
10 min and then mounted using CRYSTAL/MOUNT™ mounting medium
(Biomeda Corp.). Fluorescence analysis was performed using a
conventional fluorescence microscope.
Animals, lipopolysaccharide (LPS)
administration and histological analysis
A total of 25 male Sprague-Dawley rats (4 weeks old,
weighing 80-100 g, Samtaco Bio) were used in the present study. The
animals were housed in a controlled environment with a temperature
of 24±1°C and a humidity of 50±5% with a 12 h light and dark cycle.
Food and water were provided ad libitum. The rats (n=5 in
each group) were intraperitoneally injected with CA (50 mg/kg).
After 3 h, LPS (0.5 mg/kg) was inoculated subcutaneously into the
right flanks of the rats. After 24 h, the rats were sacrificed,
subcutaneous tissues were excised, and then fixed in 10% buffered
formalin and embedded in paraffin. Tissue sections
(4-µm-thick) were stained with Hematoxylin (Sigma-Aldrich;
Merck KgaA) for 5 min and eosine (Sigma-Aldrich; Merck KgaA) for 10
sec at RT and then examination by light microscopy.
Ethics statement
Animal care procedures were approved by the Chosun
University Institutional Animal Care and Use Committee
(CIACUC2020-A0012). All research was conducted in accordance with
the provided protocol.
Statistical analysis
The data are expressed as the means ± SD of 3
independent experiments. Statistical analysis was performed using
SPSS 24 (IBM, Corp.). Differences between groups were analyzed by
ANOVA followed by Tukey's post hoc test. A P-value <0.05 was
considered to indicate a statistically significant difference.
Results
Effect of CA and its derivatives on HUVEC
viability and cytotoxicity
To evaluate CA and its natural derivatives, MCA and
HCA, as possible candidates for the treatment of atherosclerosis,
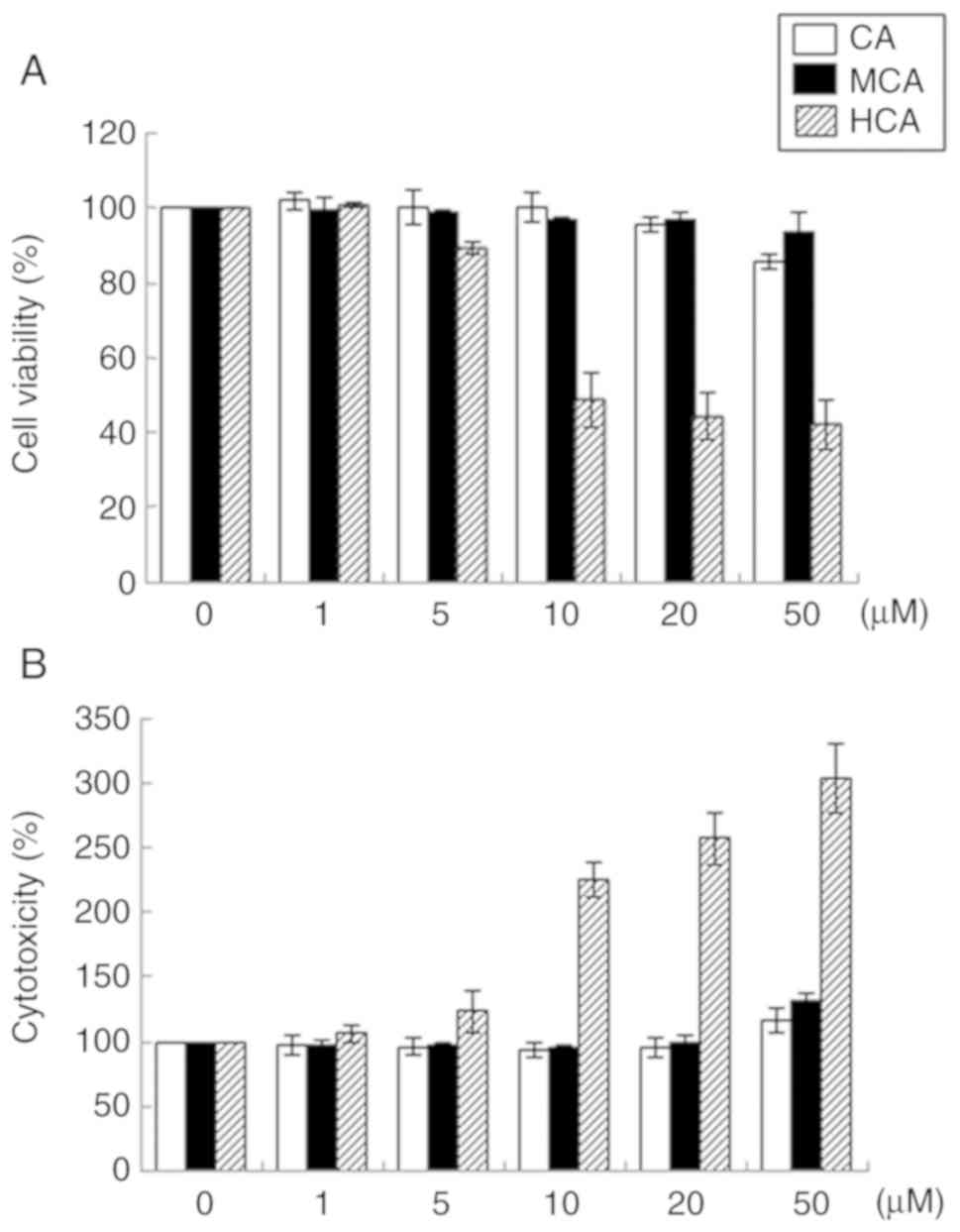
their effects on HUVEC viability were examined. HCA markedly
decreased cell viability in a dose-dependent manner (Fig. 1A). Cells treated with 50 µM
HCA exhibited 57.7% lower viability compared with the untreated
control HUVECs. HCA also markedly induced cytotoxicity in a
dose-dependent manner (Fig. 1B).
However, CA and MCA at concentrations of up to 50 µM did not
induce any notable changes in cell viability or cytotoxicity.
Therefore, CA and MCA were used in the subsequent experiments.
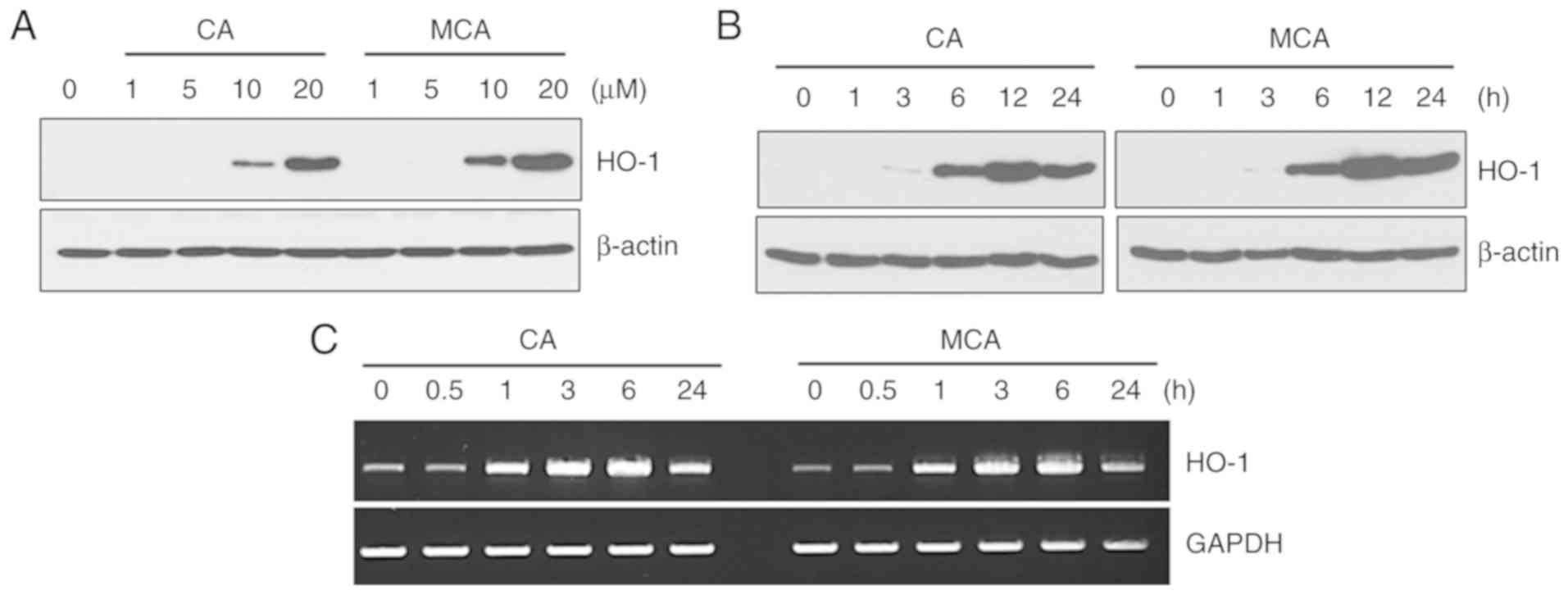
CA and MCA induce the expression of
HO-1
Recently, it was reported that CA induces HO-1
expression in hDPCs (15).
Therefore, the present study examined the effects of CA and MCA on
HO-1 expression in HUVECs. As shown in Fig. 2A and B, both CA and MCA induced
the expression of HO-1 in a dose- and time-dependent manner. By
showing that CA and MCA increased the mRNA level of HO-1 (Fig. 2C), it was confirmed that CA and
MCA regulate HO-1 at the transcriptional level.
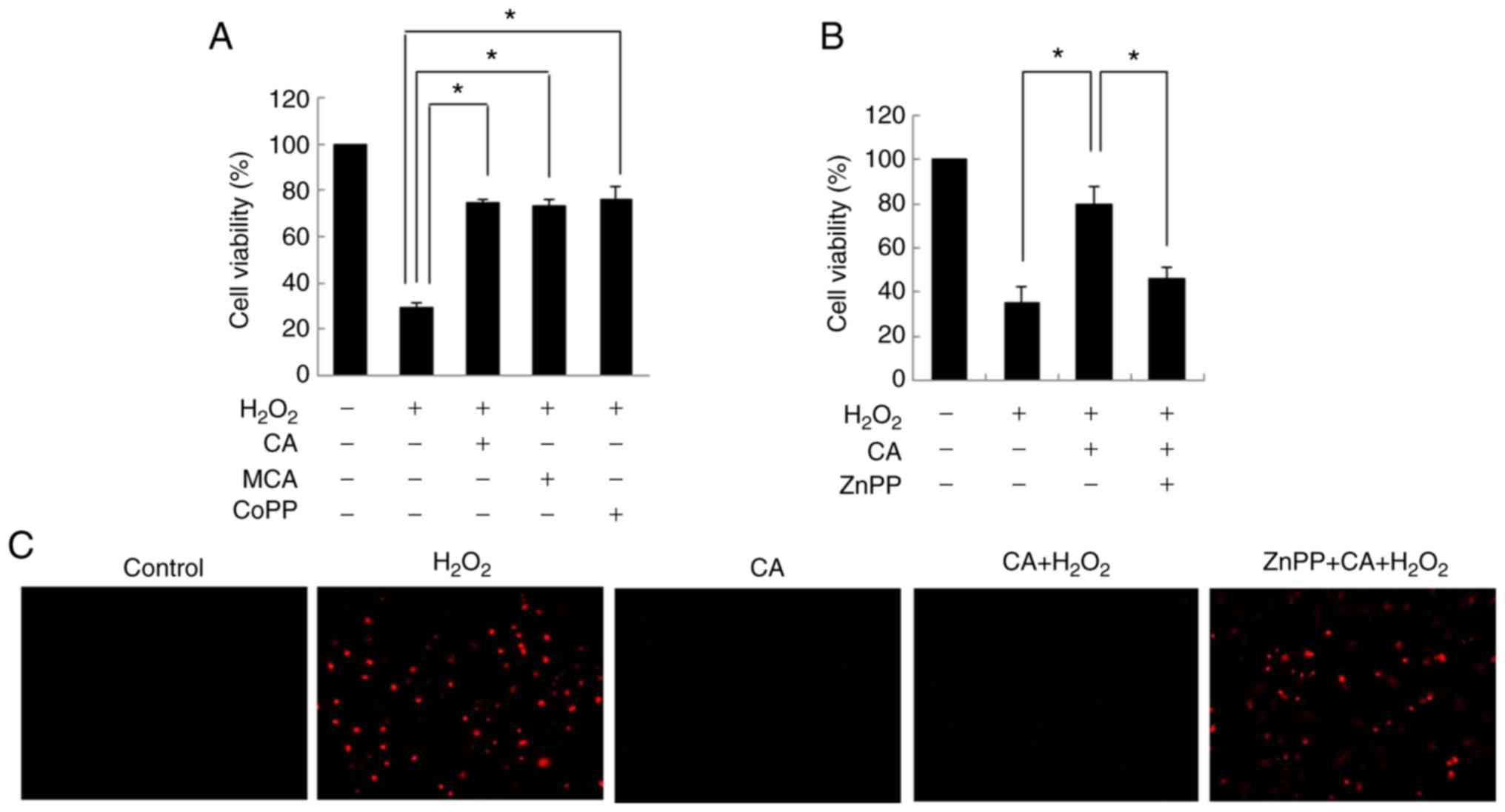
CA protects cells from oxidative stress
by inducing HO-1 expression
Previous studies have demonstrated that HO-1 exerts
cytoprotective effects against oxidative stress (22,23). Therefore, the cytoprotective
effects of CA and MCA on HUVECs were evaluated in the present
study. Exposure to H2O2 decreased cell
viability. However, pre-treatment with both CA and MCA inhibited
H2O2-induced cell cytotoxicity (Fig. 3A). CoPP, an HO-1 inducer, exerted
an inhibitory effect similar to that of CA and MCA, suggesting that
the cytoprotective effects of CA and MCA are mainly mediated via
the induction of HO-1.
To confirm the role of HO-1 in
H2O2-induced cytotoxicity, HUVECs were
treated with ZnPP, an inhibitor of HO-1. As shown in Fig. 3B, the cytoprotective effects of CA
were almost completely inhibited by ZnPP pre-treatment. ROS
staining also demonstrated that CA effectively reduced the
H2O2-induced intracellular ROS levels
(Fig. 3C), and ZnPP completely
reversed the inhibitory effects of CA on ROS generation induced by
H2O2, confirming that HO-1 plays a role in
the CA-mediated cytoprotective effect on HUVECs.
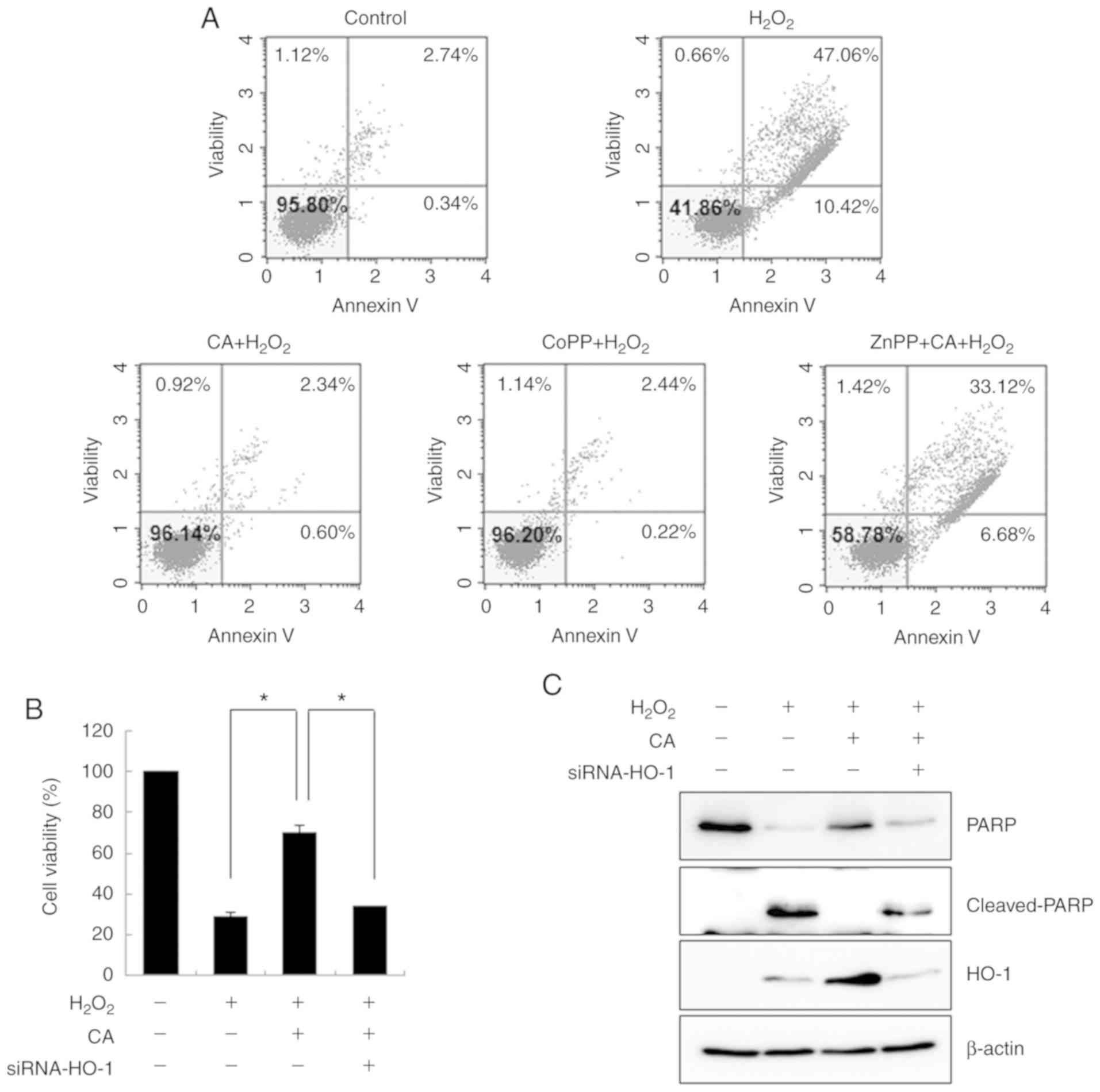
The cytoprotective effects of CA against
H2O2-induced apoptosis was also evaluated by
flow cytometry. H2O2 markedly increased
apoptotic cell death compared with the untreated HUVECs (Fig. 4A). However, CA effectively
inhibited H2O2-induced apoptosis. Although
pre-treatment with ZnPP failed to protect the cells from oxidative
stress, CoPP exerted a cytoprotective effect to an extent similar
to that of CA, revealing that HO-1 plays an important role in
oxidative stress-induced cell death.
To verify the role of HO-1 in
H2O2-induced cell death, HUVECs were
transfected with HO-1-specific siRNA. As depicted in Fig. 4B, transfection with HO-1-siRNA
abrogated the cytoprotective effects of CA on
H2O2-induced apoptosis. By demonstrating that
CA treatment decreased the level of cleaved PARP and that this
cytoprotective effect was abolished by HO-1-siRNA, the role of HO-1
in CA-mediated cytoprotection was confirmed (Fig. 4C). The transfection efficiency of
the siRNA was confirmed by demonstrating that the induced level of
HO-1 was effectively inhibited by HO-1-siRNA.
CA induces HO-1 expression through the
p38-mediated Nrf2 signaling pathway
To determine the signaling mechanisms responsible
for CA-induced HO-1 expression, western blot analysis was
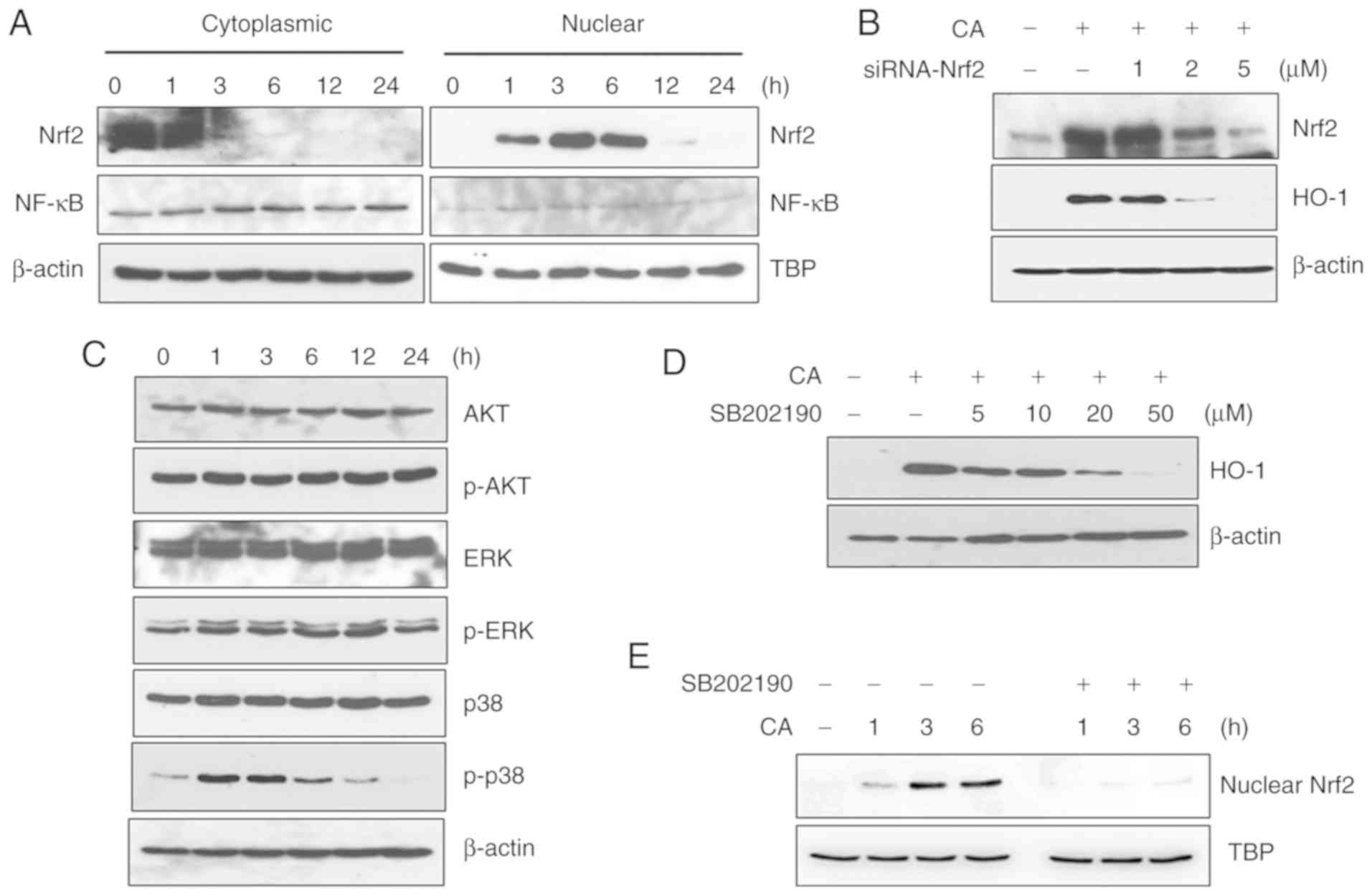
performed. CA markedly induced the nuclear translocation of Nrf2,
although it did not affect NF-κB nuclear translocation, as
illustrated in Fig. 5A. As
Nrf2-specific siRNA inhibited HO-1 expression in a dose-dependent
manner, CA was confirmed to induce HO-1 expression through Nrf2
activation (Fig. 5B).
The involvement of MAPKs in CA-induced Nrf2
activation was then examined. CA did not affect the phosphorylation
levels of AKT and ERK; however, the phosphorylated levels of p38
were markedly increased (Fig.
5C). JNK was not detected under the same experimental
conditions (data not shown). Treatment with SB202190, an inhibitor
of p38, decreased HO-1 expression in a dose-dependent manner,
suggesting the involvement of the p38 signaling pathway in
CA-induced HO-1 expression (Fig.
5D). Furthermore, treatment with SB202190 inhibited the nuclear
translocation of Nrf2 (Fig. 5E),
confirming the involvement of the p38 signaling pathway in
CA-induced Nrf2/HO-1 activation.
CA inhibits U937 cell adhesion to
HUVECs
To further evaluate CA as a candidate
anti-atherosclerotic agent, a U937 cell adhesion assay with HUVECs
was performed. As shown in Fig.
6A, TNF-α-induced U937 cell adhesion was markedly inhibited by
CA treatment.
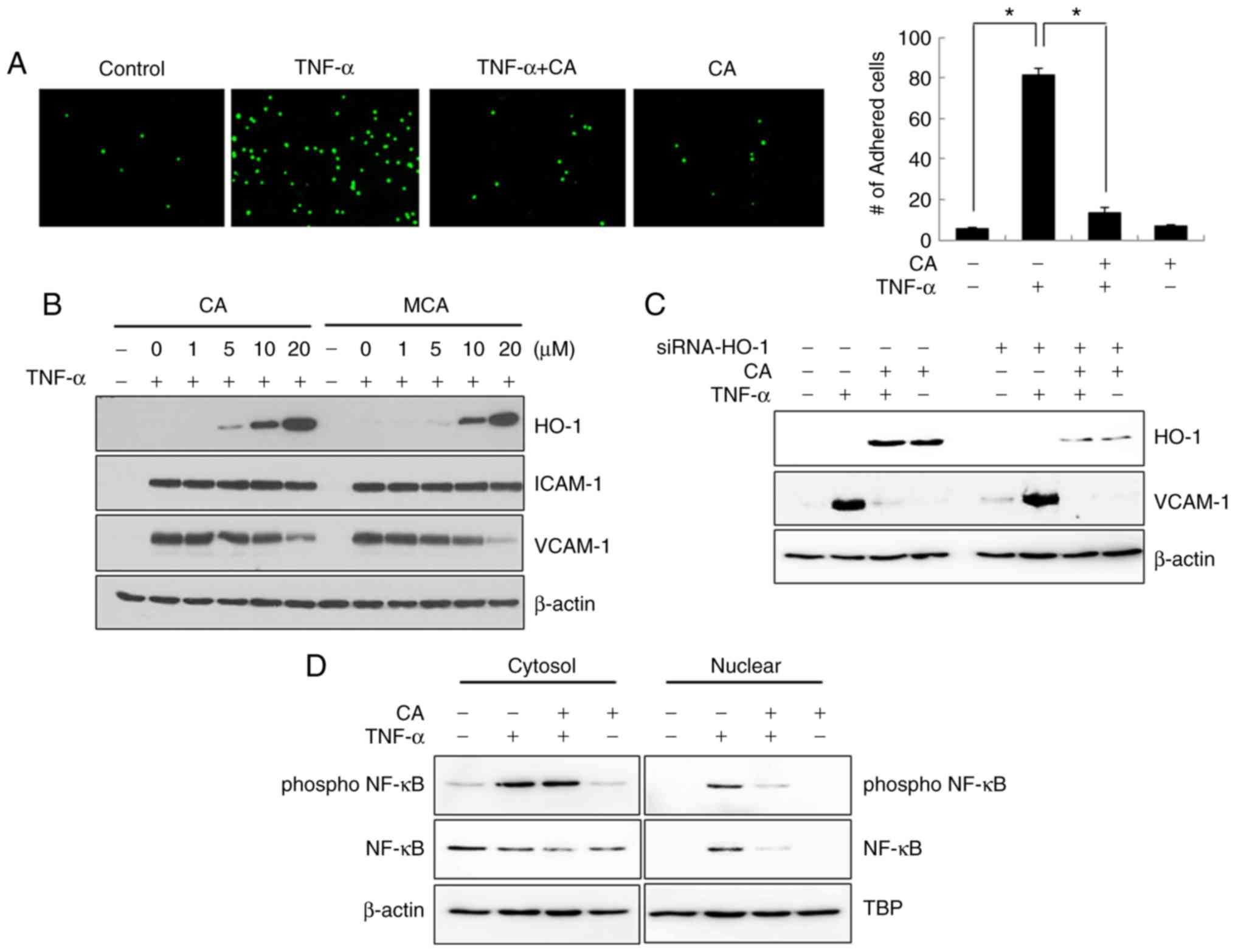 | Figure 6CA inhibits TNF-α-induced U937 cell
adhesion. (A) HUVECs were treated with TNF-α and/or CA for 3 h. The
cells were then co-cultured with calcein-AM-labeled U937 cells for
3 h, after which they were fixed with paraformaldehyde and analyzed
by fluorescence microscopy. The data are expressed as the means ±
SD of 3 individual experiments. *P<0.05. (B) HUVECs
were treated with TNF-α and/or various concentrations of CA or MCA
for 24 h. Total cell lysates were prepared, and the levels of HO-1,
ICAM-1 and VCAM-1 were detected by western blot analysis. (C) Cells
were transfected with siRNA against HO-1. After 6 h, the media were
changed, and the cells were incubated for an additional 6 h. The
cells were then treated with TNF-α and/or CA for 24 h. Total cell
lysates were prepared, and western blot analysis was performed. (D)
Cells were treated with TNF-α and/or CA for 24 h. Cytoplasmic and
nuclear fractions were prepared, and the levels of NF-κB and
p-NF-κB were detected by western blot analysis. CA, cinnamaldehyde;
HO-1, heme oxygenase-1; ICAM-1, intercellular adhesion molecule 1;
VCAM-1, vascular cell adhesion molecule 1; TNF-α, tumor necrosis
factor α. |
The adhesion of monocytes/macrophages to the
endothelium is induced by adhesion molecules, such as ICAM-1 and
VCAM-1. Therefore, the expression levels of these adhesion
molecules in CA-treated HUVECs were examined. CA treatment induced
a dose-dependent decrease in VCAM-1 expression, whereas it has no
effect on ICAM-1 expression (Fig.
6B). MCA exerts effects similar to those of CA. As the
expression of HO-1 and VCAM-1 was inversely regulated by CA, the
association between HO-1 and VCAM-1 expression was then assessed.
As shown in Fig. 6C, the level of
HO-1 did not affect the level of VCAM-1, suggesting that CA
independently regulates the expression levels of HO-1 and VCAM-1.
The transfection efficiency of HO-1-siRNA was confirmed by
examining the level of HO-1. NF-κB is a well-known transcription
factor that modulates inflammatory cytokines and adhesion
molecules. Although CA did not affect the phosphorylation of NF-κB
induced by TNF-α, it effectively inhibited the TNF-α-induced
nuclear translocation of NF-κB (Fig.
6D).
CA inhibits inflammatory cell
infiltration
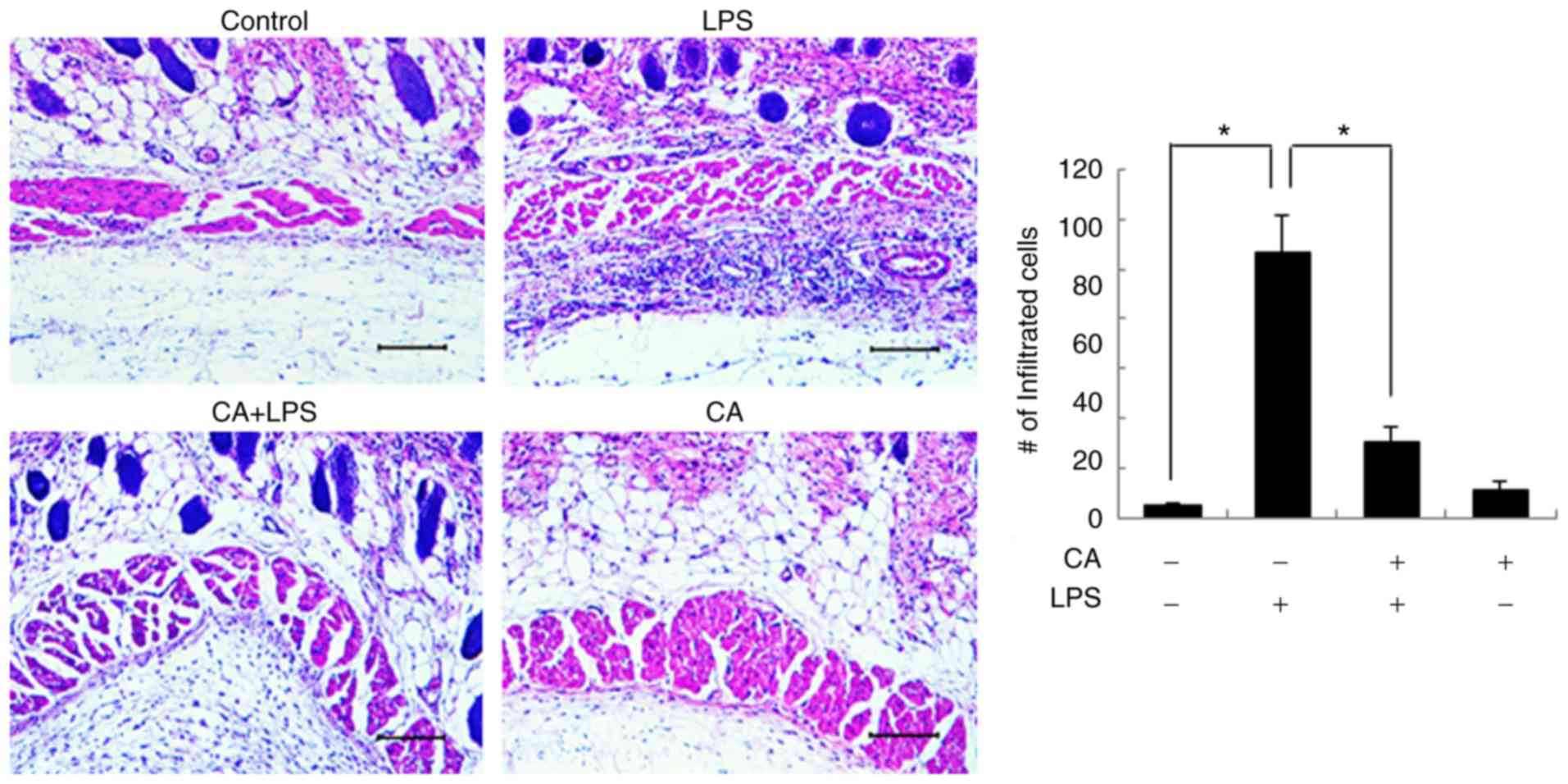
To examine the anti-inflammatory effects of CA in
vivo, Sprague-Dawley rats were pre-treated with CA and then
exposed to LPS, as described in the Materials and methods section.
Various concentrations (20 mg to 100 mg/kg) of CA have been applied
to tumor and inflammatory animal models (24,25). Based on previous studies, the
present study examined the anti-inflammatory effects of CA (20 and
50 mg/kg) in an animal model. In a preliminary experiment, the 50
mg/kg treated group exhibited a more effective anti-inflammatory
effect than the 20 mg/kg-treated group (data not shown). Therefore,
CA 50 mg/kg was used in the in vivo experiments. After 24 h,
subcutaneous tissues were subjected to histological examination.
LPS induced excessive inflammatory cell infiltration in
subcutaneous tissues (Fig. 7).
However, CA pre-treatment decreased the LPS-induced inflammatory
cell infiltration, verifying the anti-inflammatory effects of CA.
Treatment with CA alone did not affect subcutaneous tissues.
Discussion
ROS are oxygen-containing compounds that are highly
reactive with biomolecules, such as lipids, proteins and DNA.
H2O2 is a key ROS, along with
O2- and •OH. Endogenous
H2O2 is generated through the respiratory
chain and as a byproduct of cellular metabolism. Exogenous
H2O2 can also enter cells through simple
diffusion or facilitated diffusion by aqua-porins (26,27). As ROS are an important risk factor
for the development of cardiovascular disease, antioxidant
therapies that scavenge ROS are considered an effective strategy
for anti-atherosclerotic treatment (5,6).
Vascular endothelial cells play a critical role in
the maintenance of vascular homeostasis, and endothelial
dysfunction is involved in the pathogenesis of diverse
cardiovascular and inflammatory diseases (28). To the best of our knowledge, the
present study evaluated, for the first time, the antioxidant
activity of CA and its derivatives in HUVECs. Stem bark-derived
cinnamon is composed of 45-65% CA (7). HCA and MCA, natural derivatives of
CA, contain hydroxy and methoxy groups, respectively, at the CA 2′
site (19). The authors of the
present study, as well as others have demonstrated that HCA
exhibits potent anti-tumor activity in various types of cancer
cells by inducing cell cycle arrest and subsequent apoptosis
(29-31). Consistent with these studies, the
data of the present study clearly demonstrated that HCA inhibited
cell viability and induced cytotoxicity (Fig. 1). Therefore, HCA was ruled out as
a candidate anti-atherosclerotic agent.
Recently, it was demonstrated that CA exhibits
antioxidant activity by enhancing the expression of HO-1 through
the Nrf2 signaling pathway in hDPCs (15). The present study verified that
both CA and MCA induced the expression of HO-1 and protected HUVECs
against oxidative stress through an HO-1-mediated mechanism. It was
also demonstrated that CA activated Nrf2 nuclear translocation and
subsequent HO-1 expression. Nrf2 plays a critical role in
protecting cells from various toxic insults, such as oxidative
stress. Under normal conditions, Nrf2 is inactive and localized in
the cytosol. Upon cellular insult, activated Nrf2 is translocated
to the nucleus, where it binds antioxidant response elements (AREs)
to activate target gene expression (16,17). Several signaling pathways, such as
the AKT and ERK pathways, have been reported to activate the
Nrf2/HO-1 pathway (32). In the
present study, it was demonstrated that CA induced the
phosphorylation of p38 and that a specific inhibitor of p38,
SB202190, completely abolished not only Nrf2 nuclear translocation,
but also subsequent HO-1 expression (Fig. 5). These data suggest that the
induction of the p38 pathway by CA induces Nrf2 nuclear
translocation, leading to HO-1 expression. However, it was
previously reported by the authors that CA-induced activation of
Nrf2/HO-1 signaling in hDPCs does not involve MAPK signaling
pathways (15). Furthermore,
Huang et al demonstrated that CA activated the ERK, AKT and
JNK pathways, but not the p38 pathway in HepG2 hepatoma cells
(33). Therefore, the activation
of Nrf2 by CA appears to be controversial and cell-type
specific.
Inflammation is also a critical factor in the
development of atherogenic processes (12,34). TNF-α, a well-known major
inflammatory cytokine that induces inflammatory responses in the
vascular endothelium, has been identified in atherosclerotic plaque
specimens (35). TNF-α stimulates
endothelial cell adhesion to circulating monocytes by inducing the
expression of the cell adhesion molecules ICAM-1 and VCAM-1. The
present study demonstrated that both CA and MCA effectively reduced
the expression of VCAM-1 and led to the inhibition of U937
monocytic cell adhesion to endothelial cells (Fig. 6). Nonetheless, siRNA specific for
HO-1 did not affect the level of VCAM-1, suggesting that the
anti-inflammatory effect of CA was not related to HO-1 expression.
Consistent with these results, CA inhibited the TNF-α-induced
nuclear translocation of NF-κB.
In the present study, it was found that CA
effectively protected HUVECs from
H2O2-induced oxidative stress through the
activation of the Nrf2 signaling pathway and the subsequent
induction of HO-1. The anti-inflammatory effects of CA were also
verified by demonstrating that CA inhibits monocyte adhesion to
endothelial cells by decreasing the expression of VCAM-1. Taken
together, these findings strongly suggest that CA and MCA are
possible candidates for atherosclerosis treatment.
Funding
The present study was supported by the National
Research Foundation of Korea (NRF) grant funded by the Korea
government (MEST) (grant no. NRF-2017R1A2B4009239).
Availability of data and materials
All data generated or analyzed during this study are
available from the corresponding author on reasonable request.
Authors' contributions
NYK and NTT performed the experiments and analyzed
the data. SGA designed, analyzed and performed the animal
experiment. SAK designed the study, analyzed the data and wrote the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Animal care procedures were approved by the Chosun
University Institutional Animal Care and Use Committee
(CIACUC2020-A0012). All research was conducted in accordance with
the provided protocol.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Apel K and Hirt H: Reactive oxygen
species: Metabolism, oxidative stress, and signal transduction.
Annu Rev Plant Biol. 55:373–399. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
D'Autreaux B and Toledano MB: ROS as
signaling molecules: Mechanisms that generate specificity in ROS
homeostasis. Nat Rev Mol Cell Biol. 8:813–824. 2007. View Article : Google Scholar
|
|
3
|
Kim SM, Hwang KA and Choi KC: Potential
roles of reactive oxygen species derived from chemical substances
involved in cancer development in the female reproductive system.
BMB Rep. 51:557–562. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pisoschi AM and Pop A: The role of
antioxidants in the chemistry of oxidative stress: A review. Eur J
Med Chem. 97:55–74. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ochoa CD, Wu RF and Terada LS: ROS
signaling and ER stress in cardiovascular disease. Mol Aspects Med.
63:18–29. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pignatelli P, Menichelli D, Pastori D and
Violi F: Oxidative stress and cardiovascular disease: New insights.
Kardiol Pol. 76:713–722. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shen Y, Jia LN, Honma N, Hosono T, Ariga T
and Seki T: Beneficial effects of cinnamon on the metabolic
syndrome, inflammation, and pain, and mechanisms underlying these
effects-a review. J Tradit Complement Med. 2:27–32. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ahn SG, Jin YH, Yoon JH and Kim SA: The
anticancer mechanism of 2′-hydroxycinnamaldehyde in human head and
neck cancer cells. Int J Oncol. 47:1793–1800. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chao LK, Hua KF, Hsu HY, Cheng SS, Lin IF,
Chen CJ, Chen ST and Chang ST: Cinnamaldehyde inhibits
pro-inflammatory cytokines secretion from monocytes/macrophages
through suppression of intracellular signaling. Food Chem Toxicol.
46:220–231. 2008. View Article : Google Scholar
|
|
10
|
Kwon JA, Yu CB and Park HD: Bacteriocidal
effects and inhibition of cell separation of cinnamic aldehyde on
Bacillus cereus. Lett Appl Microbiol. 37:61–65. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Song F, Li H, Sun J and Wang S: Protective
effects of cinnamic acid and cinnamic aldehyde on
isoproterenol-induced acute myocardial ischemia in rats. J
Ethnopharmacol. 150:125–130. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fan J and Watanabe T: Inflammatory
reactions in the pathogenesis of atherosclerosis. J Atheroscler
Thromb. 10:63–71. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Incalza MA, D'Oria R, Natalicchio A,
Perrini S, Laviola L and Giorgino F: Oxidative stress and reactive
oxygen species in endothelial dysfunction associated with
cardiovascular and metabolic diseases. Vascul Pharmacol. 100:1–19.
2018. View Article : Google Scholar
|
|
14
|
Kim SM, Huh JW, Kim EY, Shin MK, Park JE,
Kim SW, Lee W, Choi B and Chang EJ: Endothelial dysfunction induces
atherosclerosis: Increased aggrecan expression promotes apoptosis
in vascular smooth muscle cells. BMB Rep. 52:145–150. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim NY, Ahn SG and Kim SA: Cinnamaldehyde
protects human dental pulp cells against oxidative stress through
the Nrf2/HO-1-dependent antioxidant response. Eur J Pharmacol.
815:73–79. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen XL and Kunsch C: Induction of
cytoprotective genes through Nrf2/antioxidant response element
pathway: A new therapeutic approach for the treatment of
inflammatory diseases. Curr Pharm Des. 10:879–891. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Niture SK, Khatri R and Jaiswal AK:
Regulation of Nrf2-an update. Free Radic Biol Med. 66:36–44. 2014.
View Article : Google Scholar
|
|
18
|
Li W, Zhi W, Zhao J, Yao Q, Liu F and Niu
X: Cinnamaldehyde protects VSMCs against ox-LDL-induced
proliferation and migration through S arrest and inhibition of p38,
JNK/MAPKs and NF-κB. Vascul Pharmacol. 108:57–66. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jin YH and Kim SA: 2-methoxycinnamaldehyde
inhibits the TNF-α-induced proliferation and migration of human
aortic smooth muscle cells. Int J Mol Med. 39:191–198. 2017.
View Article : Google Scholar
|
|
20
|
Kim SA, Kim YC, Kim SW, Lee SH, Min JJ,
Ahn SG and Yoon JH: Antitumor activity of novel indirubin
derivatives in rat tumor model. Clin Cancer Res. 13:253–259. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jin YH, Ahn SG and Kim SA: BAG3 affects
the nucleocytoplasmic shuttling of HSF1 upon heat stress. Biochem
Biophys Res Commun. 464:561–567. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mo L, Yang C, Gu M, Zheng D, Lin L, Wang
X, Lan A, Hu F and Feng J: PI3K/Akt signaling pathway-induced heme
oxygenase-1 upregulation mediates the adaptive cytoprotection of
hydrogen peroxide preconditioning against oxidative injury in PC12
cells. Int J Mol Med. 30:314–320. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Takahashi T, Morita K, Akagi R and Sassa
S: Heme oxygenase-1: A novel therapeutic target in oxidative tissue
injuries. Curr Med Chem. 11:1545–1561. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chiang YF, Chen HY, Huang KC, Lin PH and
Hsia SM: Dietary antioxidant trans-cinnamaldehyde reduced
visfatin-induced breast cancer progression: In vivo and in vitro
study. Antioxidants (Basel). 8:E6252019. View Article : Google Scholar
|
|
25
|
Patra K, Jana S, Sarkar A, Mandal DP and
Bhattacharjee S: The inhibition of hypoxia-induced angiogenesis and
metastasis by cinnamaldehyde is mediated by decreasing HIF-1α
protein synthesis via PI3K/Akt pathway. Biofactors. 45:401–415.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Holmstrom KM and Finkel T: Cellular
mechanisms and physiological consequences of redox-dependent
signalling. Nat Rev Mol Cell Biol. 15:411–421. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schieber M and Chandel NS: ROS function in
redox signaling and oxidative stress. Curr Biol. 24:R453–R462.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li B, Lee YJ, Kim YC, Yoon JJ, Lee SM, Lee
YP, Kang DG and Lee HS: Sauchinone from saururus chinensis protects
vascular inflammation by heme oxygenase-1 induction in human
umbilical vein endothelial cells. Phytomedicine. 21:101–108. 2014.
View Article : Google Scholar
|
|
29
|
Lee CW, Lee SH, Lee JW, Ban JO, Lee SY,
Yoo HS, Jung JK, Moon DC, Oh KW and Hong JT:
2-hydroxycinnamaldehyde inhibits SW620 colon cancer coll growth
through AP-1 inactivation. J Pharmacol Sci. 104:19–28. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim SA, Sung YK, Kwon BM, Yoon JH, Lee H,
Ahn SG and Hong SH: 2′-hydroxycinnamaldehyde shows antitumor
activity against oral cancer in vitro and in vivo in a rat tumor
model. Anticancer Res. 30:489–494. 2010.PubMed/NCBI
|
|
31
|
Nguyen HA and Kim SA:
2′-hydroxycinnamaldehyde induces apoptosis through HSF1-mediated
BAG3 expression. Int J Oncol. 50:283–289. 2017. View Article : Google Scholar
|
|
32
|
Luo Y, Lu S, Dong X, Xu L, Sun G and Sun
X: Dihydromyricetin protects human umbilical vein endothelial cells
from injury through ERK and Akt mediated Nrf2/HO-1 signaling
pathway. Apoptosis. 22:1013–1024. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Huang TC, Chung YL, Wu ML and Chuang SM:
Cinnamaldehyde enhances Nrf2 nuclear translocation to upregulate
phase II detoxifying enzyme expression in HepG2 cells. J Agric Food
Chem. 59:5164–5171. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim EJ, Park WH, Ahn SG, Yoon JH, Kim SW
and Kim SA: 5′-nitro-indirubinoxime inhibits inflammatory response
in TNF-alpha stimulated human umbilical vein endothelial cells.
Atherosclerosis. 211:77–83. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Barath P, Fishbein MC, Cao J, Berenson J,
Helfant RH and Forrester JS: Detection and localization of tumor
necrosis factor in human atheroma. Am J Cardiol. 65:297–302. 1990.
View Article : Google Scholar : PubMed/NCBI
|