Introduction
Propofol is always utilized as a sedative and
anesthetic agent before medical procedures, which can modulate
different GABA receptors in the central nervous system (1). Various studies have demonstrated
that propofol can induce developmental neurotoxicity in mice
(2,3) and induces the apoptosis of neuronal
stem cells in the hippocampus of neonatal mice (4). Thus, the reproductive system in the
fetal period can also be highly possible affected by anesthetic
agents. It has been demonstrated that Leydig cells are derived from
the neural system (5) and the
essential role of Leydig cells is to produce testosterone (6). For normal reproductive functions in
males, Leydig stem/progenitor cells are essential for testis
development in the fetal period (7). It has been demonstrated that
different factors influence the development of Leydig
stem/progenitor cell in the fetal period, resulting in infertility
(7). However, it remains unknown
as to whether propofol induces the death of Leydig stem/progenitor
cells, which would suppress male reproductive function and
development.
Cell number regulation is essential in tissue
homeostasis controlled by cell death, proliferation and
differentiation (8). Cell death
can be categorized into 3 categories based on morphological
appearance, which includes necrosis, apoptosis and autophagy
(9). Cell apoptosis is uniformly
featured with nuclear condensation and fragmentation, cell
shrinking, membrane blebbing and separation of the cellular
components into apoptotic bodies (10). Apoptosis is regulated by 2
pathways, the extrinsic and intrinsic pathways, respectively
(11). The extrinsic pathway is
known as the death receptor pathway, stimulated by the activation
of death receptors with pro-apoptotic ligands, such as Fas ligand
(FasL), tumor necrosis factor (TNF)-α and TNF-related
apoptosis-inducing ligand (TRAIL). As ligands bind, the
intracellular death domains of the receptors associate with
Fas-associated death domain (FADD), resulting in the death-induced
signaling complex (DISC) recruitment, and hence leading to
caspase-8 activation and further triggering caspase-3 or -7
activation (12). The intrinsic
pathway is associated with the mitochondrial pathway, which is
triggered by a number of stimuli (chemotherapeutic agents,
radiation and growth factor withdrawal) leading to the release of
cytochrome c from the mitochondria into the cytosol
(13). Cytochrome c binds
with the adapter molecule, apoptotic protease activating factor 1
(Apaf-1), and recruits procaspase-9 to form the apoptosome to
further activate caspase-9 and caspase-3, orchestrating apoptosis
(14). Both the intrinsic and
extrinsic pathways can lead to the cleavage of poly(ADP-ribose)
polymerase (PARP), which impedes the effects of DNA repair
(11).
The mitogen-activated protein-serine/threonine
kinase (MAPK) pathway plays a key role in regulating cell
proliferation, growth, differentiation, invasion, migration and
apoptosis (15). MAPKs include
extracellular-regulated kinase (ERK)1/2, Janus kinase (JNK) and
p38, which can be stimulated by phosphorylation, commencement with
MAPK kinase kinase kinase (MAP3K) activation to control cell fate
(16). The Akt signaling pathway
is considered as a pro-survival pathway, which can inhibit
apoptotic cascades and activate pro-survival signals (17). The Akt pathway can inhibit a
number of pro-apoptotic Bcl-2 family members (Bad, Bax and Bim) and
positively regulate anti-apoptotic pathways [nuclear factor (NF)-κB
transcription factor], promoting the transcription of a number of
anti-apoptotic genes, such as Bcl-2 and Bcl-xL (18). It has been illustrated that the
reduction of Akt expression can induce the apoptosis of various
cells (19).
TM3 cells are derived from 11-13-day-old testicular
interstitial cells of neonatal BALB/c mouse, which can be
stimulated by luteinizing hormone (LH) to secret testosterone
(20-22), and TM3 cells exhibit progenitor
cell characteristics (7,20). Consequently, TM3 cells are
appropriate for use in the investigation of the apoptotic effects
of propofol with mechanistic analyses. In the present study,
propofol induced TM3 progenitor cell membrane blebbing with
detachment phenomena, a decrease in cell number, activated the
caspase cascade, activated MAPK pathways and inhibited Akt pathway,
which promoted cell apoptosis. The findings of the present study
demonstrated that propofol regulates TM3 mouse Leydig
stem/progenitor cell apoptosis, which deepens the knowledge of the
mechanisms through which anesthetic agent exposure can affect
testicular normal development.
Materials and methods
Chemicals and reagents
Propofol, penicillin-streptomycin, ethylene diamine
tetraacetic acid (EDTA), propidium iodide (P), MTT, RNase A, 30%
acrylamide/Bis-acrylamide solution, Waymouth MB 752/1 medium,
sodium orthovanadate, monoclonal antibody against β-actin (#A5441)
and Triton X-100 were purchased from Sigma-Aldrich; Merck KGaA.
EGTA, Tween 20, dimethyl sulfoxide (DMSO) and DS were purchased
from Merck KGaA. An Annexin V-fluorescein isothiocyanate (FITC)
apoptosis detection kit was purchased from Strong Biotech
Corporation. Dulbecco's modified Eagle medium/F12, fetal bovine
serum and trypsin-EDTA were purchased from Gibco; Thermo Fisher
Scientific Inc. Potassium chloride, sodium chloride, Tris base and
glycine 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid were
purchased from J.T. Baker (Avantor Performance Materials). Donkey
anti-rabbit (NEF81200-1EA) and anti-mouse (NEF82200-1EA) IgG
conjugated with horseradish peroxidase (HRP) were purchased from
PerkinElmer, Inc. A Micro BCA protein assay kit was purchased from
Thermo Fisher Scientific, Inc. An enhanced chemiluminescence
detection kit was purchased from EMD Millipore. Polyclonal
antibodies against cleaved caspase-8 (#9429; 1/1,000), cleaved
caspase-9 (#9509; 1/1,000), cleaved PARP (#9542; 1/1,000),
phospho-JNK (#9251; 1/4,000), JNK (#9252; 1/1,000), phospho-ERK1/2
(#9101; 1/4,000), ERK1/2 (#9102; 1/4,000), phospho-p38 (#9215;
1/1,000), p38 (#9212; 1/4,000), phospho-Akt (#9271; 1/4,000) and
Akt (#9272; 1/1,000) and phospho-mechanistic target of rapamycin
(mTOR) (#2971; 1/1,000) were purchased from Cell Signaling
Technology, Inc. ISOTON™ II Diluent was purchased from Beckman
Coulter, Inc. Monoclonal antibody against cleaved caspase-3 (#9661;
1/1,000) was purchased from Cell Signaling Technology, Inc.
Cells and cell culture
TM3 cells were obtained from ATCC and sustained in
DMEM/F12 medium with 10% FBS at 37˚C in a humidified incubator
containing 95% air and 5% CO2 for all experiments.
Morphological observation
TM3 cells were seed at 6×105/ml and
treated with various concentrations of propofol (0, 300, 350 and
400 µM) for 3 h, respectively. Propofol was diluted with
DMSO. The changes in cell morphology were then investigated with an
Olympus CK40 light microscopy at ×100 magnification and the images
were logged using an Olympus DP20 digital camera (Olympus
Corporation).
MTT cell viability assay
MTT assay is a colorimetric assay for measuring cell
viability (23). TM3 cells were
cultured at 8×103 cells. After reaching 70-80%
confluence, cells were treated with various concentrations of
propofol (0, 10, 50, 100, 300, 400, 500 and 600 µM) for 1,
3, 6, 12 and 24 h, respectively. Subsequently, each well was
supplemented with MTT (0.5 mg/ml) for different periods of time
followed by incubation at 37°C for 4 h. The media was discarded and
50 µl DMSO were added to dissolve the crystals using a
shaker to vibrate the plate at 37°C for 20 min in the dark. Cell
viability was examined at λ=570 nm using a VersaMax ELISA reader
(Molecular Devices, Inc.), as previously described (23).
Cell cycle analysis
TM3 cells were seeded at 6×105 with 2 ml
culture medium, and treated with various concentrations of propofol
(0, 100, 300 and 400 µM) for 3, 6, 12 and 24 h,
respectively. Cells were collected through trypsin digestion and
centrifugation (400 x g for 12 min at 4°C), and washed with isoton
II and fixed with 70% ethanol for at least 2 h at -20°C. Following
fixation, cells were washed with cold isoton II and collected by
centrifugation (400 × g for 12 min at 4°C). Cell suspensions were
then mixed with 100 µg/ml RNase and stained with 40
µg/ml propidium iodine (PI) solution for 30 min at 25°C.
Stained cells were determined at λ=488 nm for PI detection by
FACScan flow cytometer (BD Biosciences). SubG1 phase cells with
lower DNA contents are considered as apoptotic cells (24). The percentages of sub-G1, S and
G2/M phase cells were analyzed using FACStation v6.1x and Modfit LT
v3.3 software (BD Biosciences).
Annexin V/PI double staining assay
After the TM3 cells were collected by trypsin, cell
suspensions were centrifuged (400 × g for 12 min at 4°C). The
pellets were resuspended with cold isoton II and centrifuged again
(400 × g for 12 min at 4°C), and then mixed with 100 µl
staining solution for 15 min at 25°C following the user's manual of
Annexin V-FITC apoptosis detection kit from Srong Biotech
Corporation. Stained cells were determined at λ=488 nm excitation
using 515 nm band pass filter for FITC detection and >600 nm
band pass filter for PI detection using a FACScan flow cytometer
(BD Biosciences). The double-positive cells (late apoptotic),
Annexin V single-positive cells (early apoptotic), PI
single-positive cells (necrotic) and double-negative cells (viable)
are illustrated in 4 quadrants (25,26). The percentage of cells in the 4
quadrants were analyzed using FACStation v6.1x software.
Protein extraction and western blot
analysis
Following the treatments, cells in the medium were
removed and washed with cold PBS. Attached cells were lysed by 20
µl lysis buffer (150 mM NaCl, 20 mM Tris pH7.5, 1 mM EGTA, 1
mM EDTA, 1% Triton X-100, 1 mM sodium orthovanadate and 2.5 mM
sodium pyrophosphate) with proteinase inhibitor cocktail (cat. no.
P5655; Sigma-Aldrich; Merck KGaA). The pellets were then
resuspended with 10 µl lysis buffer and centrifuged at
12,000 × g for 12 min at 4°C. The supernatants were saved and
stored at −80°C. The protein concentrations of cell lysates were
analyzed by Lowry assay (27).
For western blot analysis, 30 µg protein were
separated by 12% SDS-PAGE gel with running buffer (0.1% SDS, 192 mM
glycine and 25 mM Tris; pH 8.3) at 25°C, and electrophoretically
transferred to polyvinylidene difluoride membranes at 4°C. After
blocking the membranes with 1% milk at 25°C for 3 h, the membranes
were incubated with primary antibodies for 24 h at 4°C. The
membranes were then washed 3 times and incubated with
HRP-conjugated secondary antibodies (1/2,000) at 25°C for 1 h.
Bands were detected using an enhanced chemiluminescence kit and the
UVP EC3 BioImaging system (UVP, LLC). Quantification of the western
blotting data was performed using ImageJ version 1.50 software
(National Institutes of Health) (28).
Statistical analysis
Data are presented as the means ± standard error of
the mean among 3 experiments. Significance of differences between
the control and treatment groups were determined by one-way
analysis of variance followed by all pairs Tukey's honestly
significant difference (HSD) post-hoc test comparisons. Statistical
analysis was accomplished using GraphPad Prism 6 software (GraphPad
Software, Inc.). P<0.05 was considered to indicate a
statistically significant difference.
Results
Cell morphology is altered by propofol
associated with the death of TM3 cells
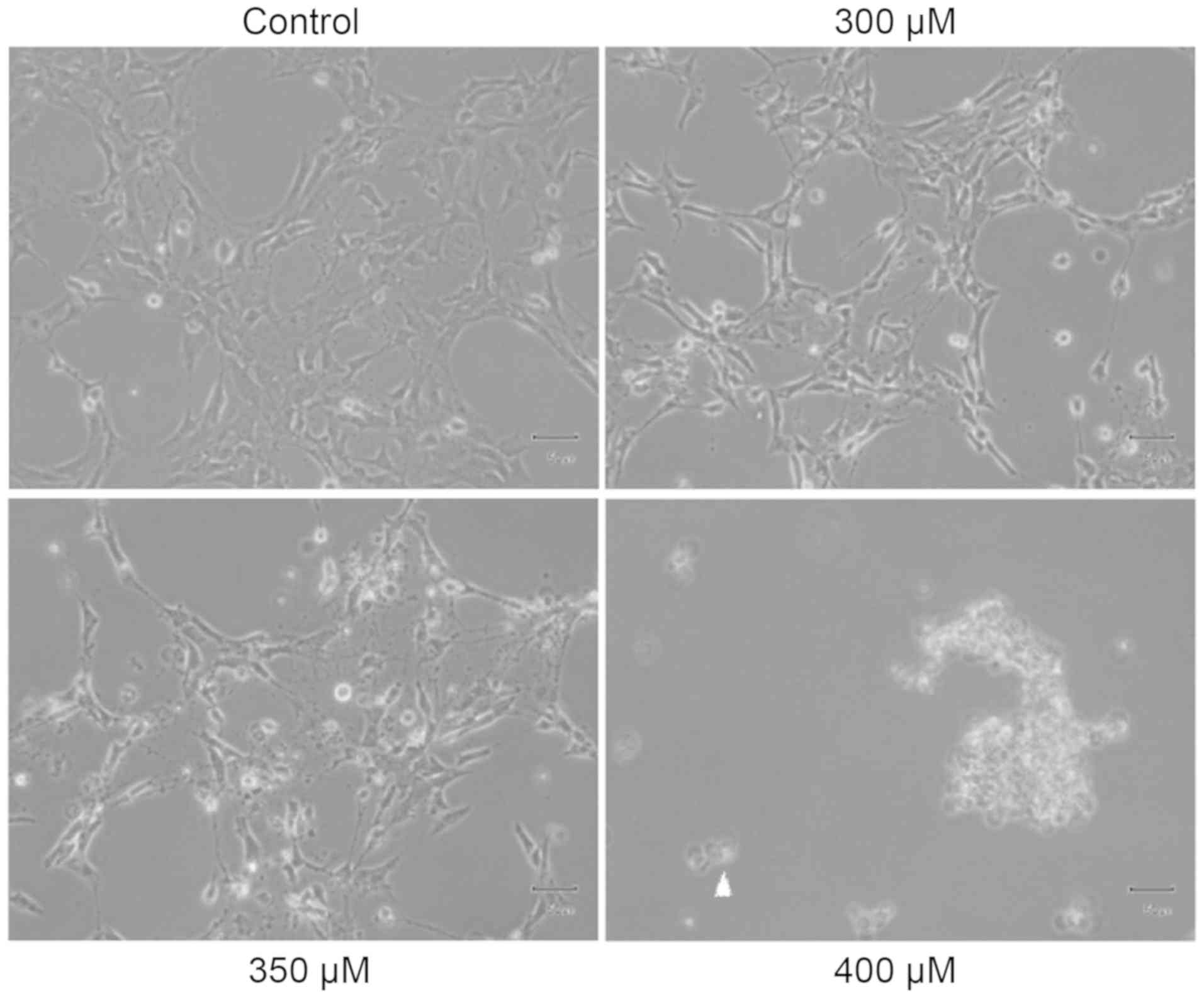
TM3 cells were treated with various concentrations
(0, 300, 350 and 400 µM) of propofol (Fig. 1) for 3 h, respectively.
Morphological changes of cells were examined by light microscopy.
The TM3 cells shrank in size following treatment with 300 µM
propofol for 24 h (Fig. 1) and
350 µM propofol for 3 h (Fig.
1); in addition, treatment with 400 µM propofol for 3 h
caused the detachment of most cells (Fig. 1). These results suggested that
propofol can cause membrane blebbing in TM3 cells, which indicates
that propofol can promote cell death possibly via apoptosis in TM3
mouse Leydig stem/progenitor cells.
Viability of TM3 cells is reduced by
propofol in a time- and dose-dependent manner
The viability of TM3 cells affected by propofol was
investigated by MTT assay. TM3 cells were treated with propofol (0
to 600 µM) for 1, 3, 6, 12 and 24 h, respectively. The
viability of the TM3 cells decreased significantly following
treatment with propofol from 100 to 600 µM for 6 to 24 h
(P<0.05) (Fig. 2). In fact,
TM3 cell viability decreased to 49±8.1% with 300 µM propofol
for 12 h treatment (Fig. 2).
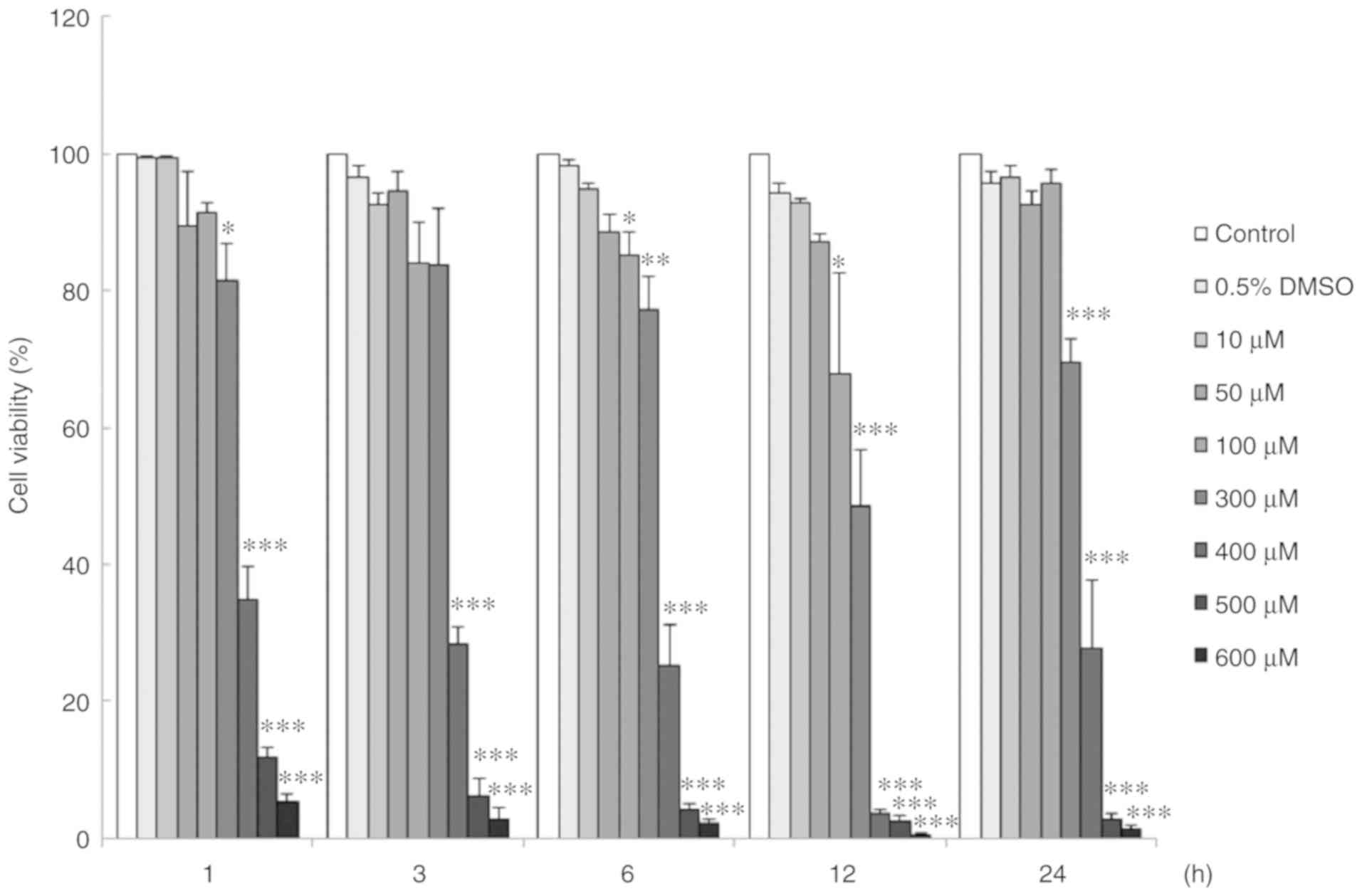 | Figure 2Viability of TM3 cells was reduced by
propofol in a time- and dose-dependent manner. Cells were treated
with 0 (Control), 0.5% DMSO, 10, 50, 100, 300, 400, 500 and 600
µM propofol for 1, 3, 6, 12 and 24 h, respectively. MTT
viability assay was used to determine cell viability. Data are
illustrated as a percentage of cell growth relative to the control
groups. Each data point represents the mean ± SEM of 3 separate
experiments. *P<0.05, **P<0.01 and
***P<0.001 indicate statistically significant
differences compared to the control group. DMSO, dimethyl
sulfoxide. |
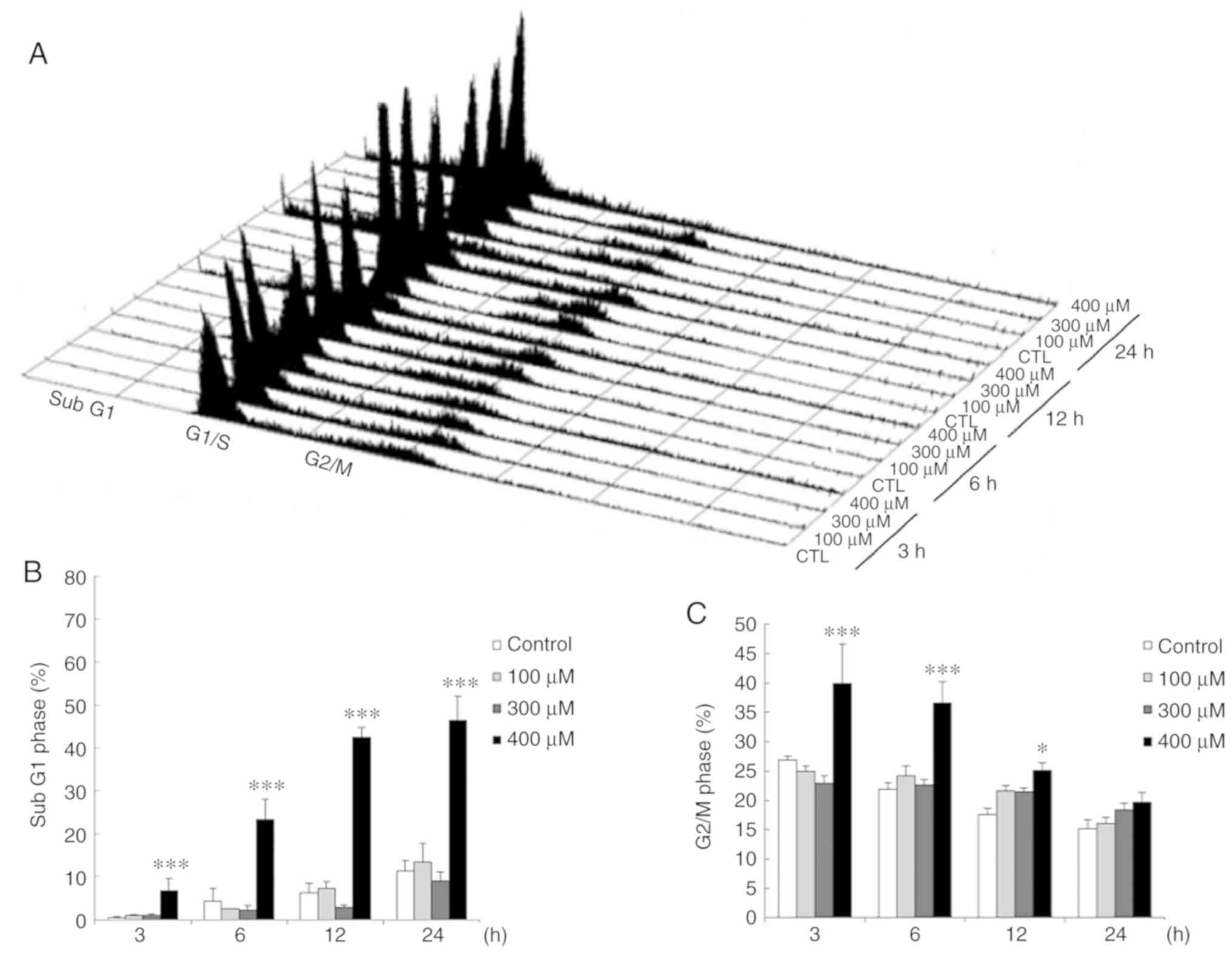
Propofol regulates the TM3 cell cell
cycle
TM3 cells were treated with various concentrations
of propofol (0, 100, 300 and 400 µM) for 3, 6, 12 and 24 h,
and the DNA contents in each treatment were investigated by flow
cytometry. The data illustrated that the percentage of TM3 cells in
the subG1 phase significantly increased by treatment with 400
µM propofol for 3 to 24 h (P<0.05) (Fig. 3A and B). Moreover, the percentage
of TM3 cells in the G2/M phase markedly increased following
treatment with 400 µM propofol for 3 and 12 h (P<0.05)
(Fig. 3A and C).
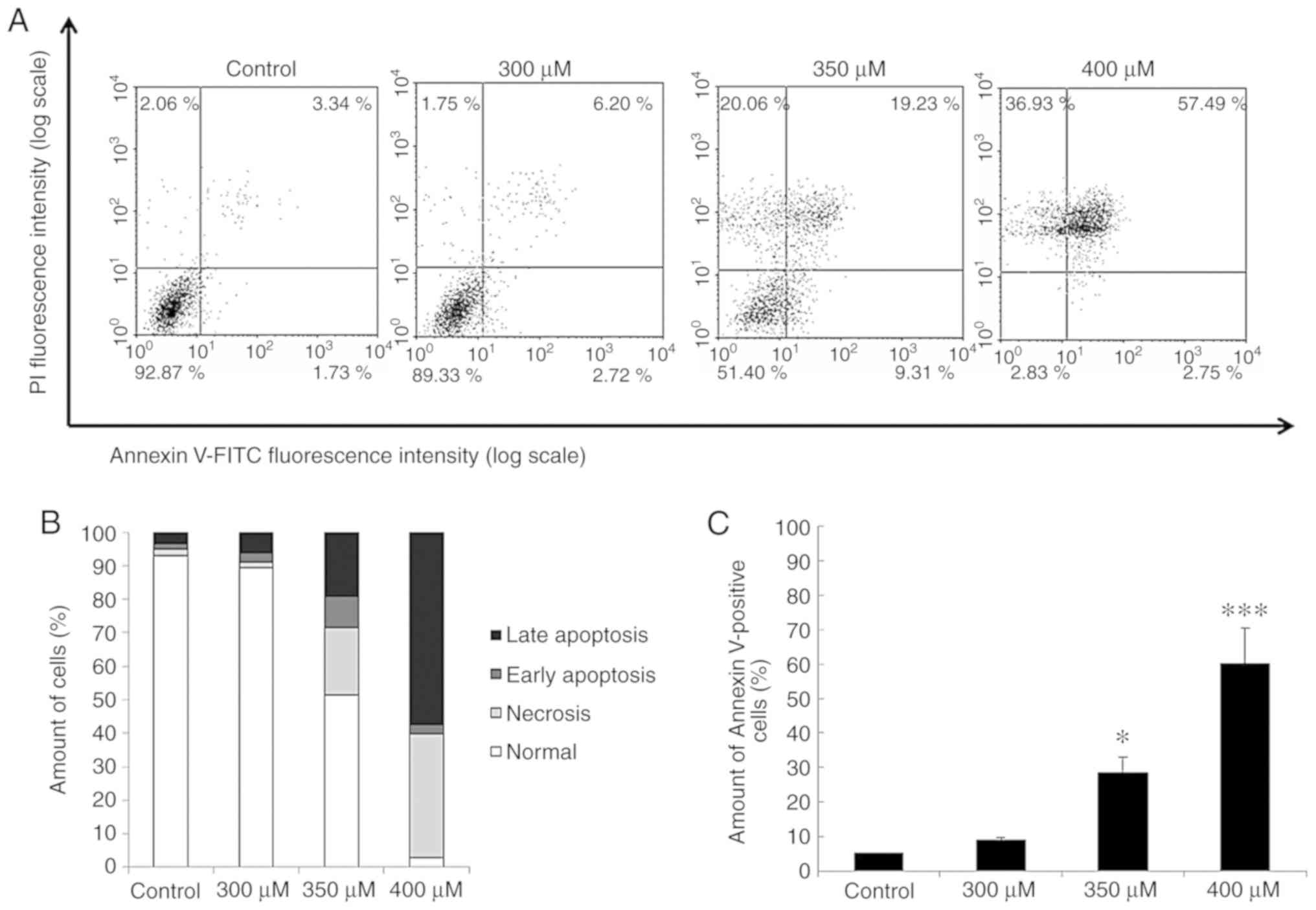
Propofol induces the apoptosis of TM3
cells
The results revealed that propofol induced cell
death with DNA fragmentation plus membrane blebbing and an increase
in the number of TM3 cells in the subG1 phase. To further examine
whether propofol induces the apoptosis of TM3 cells, Annexin V/PI
double staining assay was used. It is acknowledged that percentages
of double-positive cells (late apoptotic), Annexin V
single-positive cells (early apoptotic), PI single-positive cells
(necrotic) and double-negative cells (viable) are illustrated in 4
quadrants with the double staining illustrating different cell
apoptotic phenomena (29). The
cell distributions of viable, early apoptotic, necrotic and late
apoptotic TM3 cells are illustrated in Fig. 4A, and the percentages of 4
different statuses among the cells treated with 0, 300, 350 and 400
µM propofol, respectively, are demonstrated in Fig. 4B. In addition, the number of
Annexin V-positive TM3 cells (early and late apoptosis) was
significantly induced by treatment with propofol at 350 and 400
µM for 24 h (P<0.05) (Fig.
4C). These data indicate that propofol induces the apoptosis of
TM3 cells.
Propofol activates the caspase cascade
associated with the apoptosis of TM3 cells
The extrinsic and intrinsic caspase cascade is an
imperative inducer of apoptotic pathway (30). As demonstrated above, propofol
induced the apoptosis of TM3 cells. Thus, the present study then
determined whether propofol induces apoptosis by activating caspase
extrinsic and intrinsic pathways to induce the cleavage of
caspase-9, caspase-8, caspase-3 plus PARP. The results revealed
that treatment with 350 and 400 µM propofol for 3 h
significantly induced the expression of cleaved caspase-8 (Fig. 5A and B). Treatment with 350 and
400 µM propofol for 3 and 6 h also significantly induced the
expression of cleaved caspase-9 (Fig.
5A and C), and treatment with 350 µM propofol for 3 h
and 400 µM propofol for 3, 6 and 12 h significantly induced
the expression of cleaved caspase-3 (Fig. 5A and D). It was also found that
treatment with 350 µM propofol for 3 h significantly induced
the expression of cleaved PARP (P<0.05) (Fig. 5A and E). These data illustrate
that caspase pathways were activated by propofol to stimulate
apoptosis in TM3 cells.
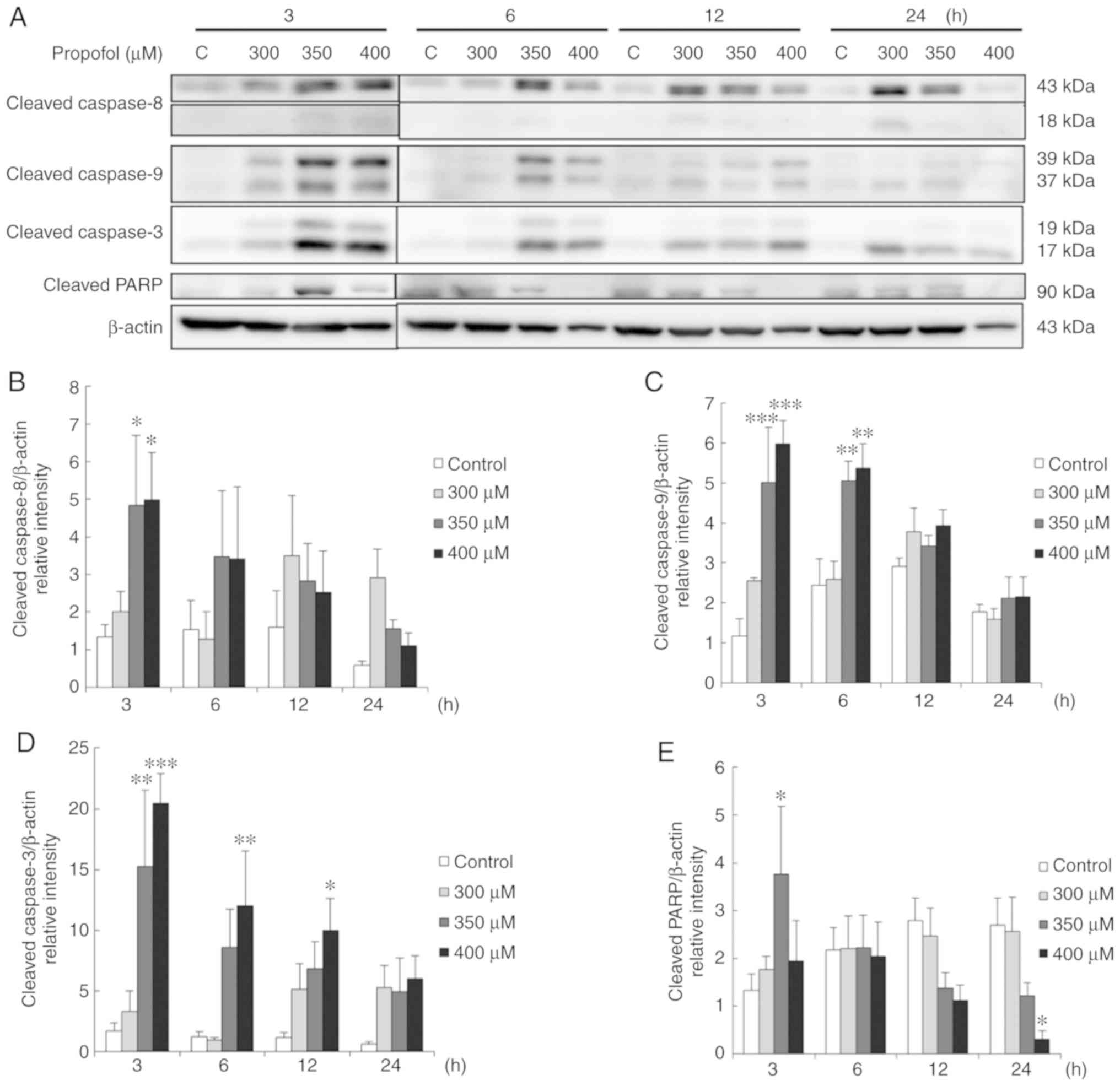 | Figure 5Propofol activates the caspase
cascade associated with the apoptosis of TM3 cells. Cells were
treated with various concentrations of propofol (control, 300, 350
and 400 µM) for 3, 6, 12 and 24 h, respectively. (A) Cleaved
caspase-8 (43/18 kDa), cleaved caspase-9 (39/37 kDa), cleaved
caspase-3 (17/19 kDa) and cleaved PARP (85-90 kDa) were examined by
western blot analyses. The integrated optical densities (IOD) of
(B) cleaved caspase-8, (C) cleaved caspase-9, (D) cleaved caspase-3
and (E) cleaved PARP proteins were normalized to β-actin (43 kDa)
in each lane, respectively. Each data point represents the mean ±
SEM of 3 separate experiments. *P<0.05,
**P<0.01 and ***P<0.001 indicate
statistically significant differences compared to the control
group. C, control. |
It should be noted that treatment with 400 µM
propofol for 24 h significantly reduced the expression of cleaved
PARP (P<0.05) (Fig. 5A and E),
suggesting that treatment of the TM3 cells for a long period of
time with a high concentration of propofol suppressed the apoptotic
phenomenon. In addition, the results revealed that treatment with
350 µM propofol for 3 h activated the caspase cascade in TM3
cells (Fig. 5), but not in the
MA-10 cells, as previously demonstrated (26), suggesting that the TM3 cells are
more sensitive to propofol compared to MA-10 cells.
Propofol activates MAPK pathways
associated with the apoptosis of TM3 cells
A previous study demonstrated that the MAPK pathways
control cell proliferation, growth and apoptosis (15). In the present study, to examine
whether propofol regulates MAPK pathways to induce TM3 cell
apoptosis, MAPK proteins were examined by western blot analysis.
The results demonstrated that treatment with 300 µM propofol
for 12 h and 350 µM propofol for 3, 6 and 12 h significantly
induced the expression of phospho-JNK (P<0.05) (Fig. 6A and B). In addition, treatment
with 300 µM propofol for 3 h and 350 µM propofol for
3 and 6 h significantly induced the expression of phospho-ERK
(P<0.05) (Fig. 6A and C). It
was also found that treatment with 350 µM propofol for 3, 6
and 12 h, and 400 µM propofol for 3 h significantly induced
the expression of phospho-p38 (P<0.05) (Fig. 6A and D). These data clearly
illustrate that propofol activates MAPK pathways to stimulate the
apoptosis of TM3 cells.
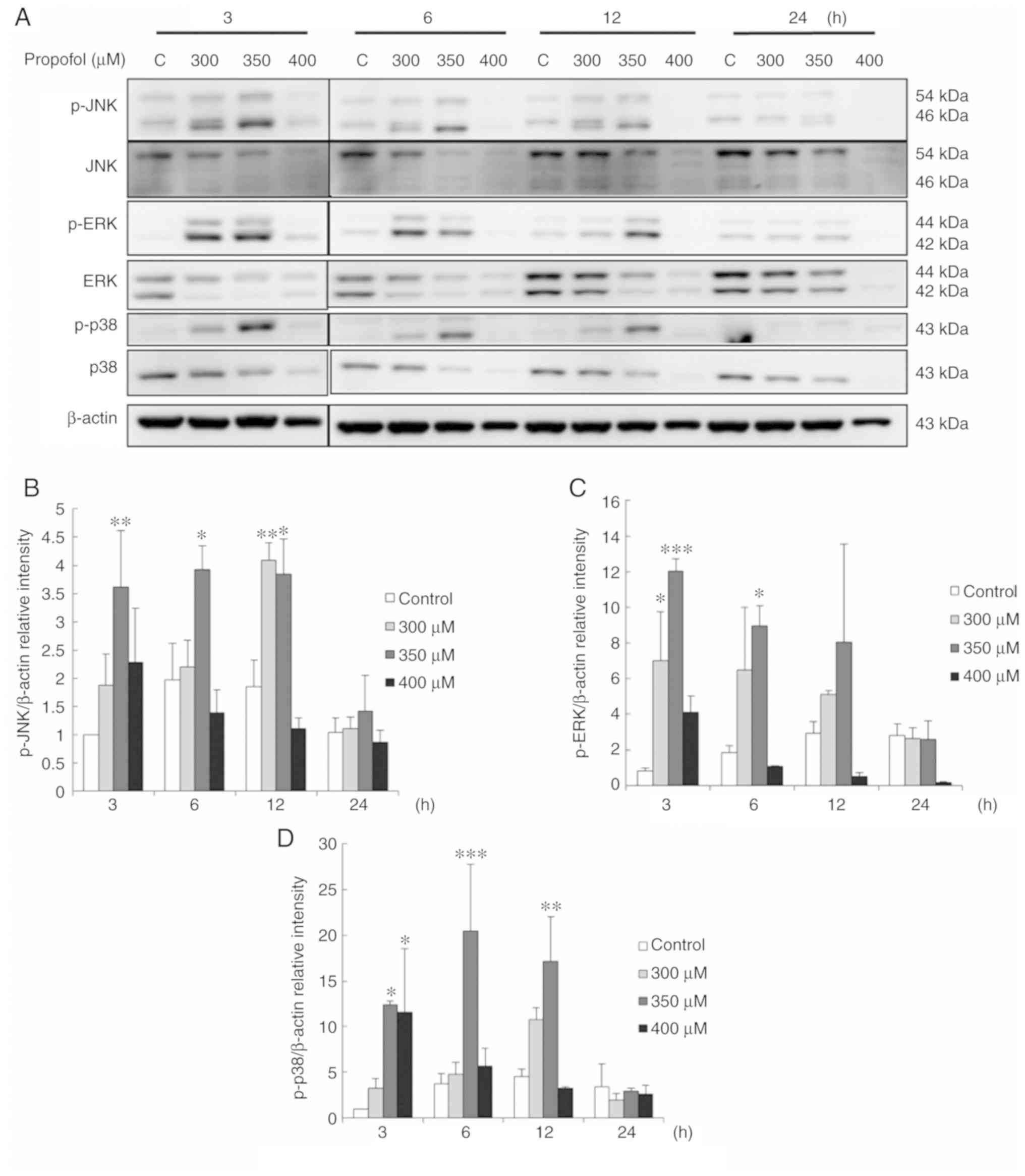 | Figure 6Propofol activates MAPK pathways
associated with the apoptosis of TM3 cells. Cells were treated with
various concentrations of propofol (control, 300, 350 and 400
µM) for 3, 6, 12 and 24 h, respectively. (A) Phospho-JNK
(p-JNK; 54/46 kDa), JNK (54/46 kDa), phospho-ERK (p-ERK; 44/42
kDa), ERK (44/42 kDa), phospho-p38 (p-P38; 43 kDa) and p38 (43 kDa)
were examined by western blot analysis. The integrated optical
densities (IOD) of (B) p-JNK, (C) p-ERK and (D) p-p38 proteins were
normalized to β-actin (43 kDa) in each lane, respectively. Each
data point represents the mean ± SEM of 3 separate experiments.
*P<0.05, **P<0.01 and
***P<0.001 indicate statistically significant
differences compared to the control group. C, control. |
Propofol suppresses the Akt pathway
associated with the apoptosis of TM3 cells
A previous study demonstrated that the Akt pathway
inhibit apoptotic signal cascades and activate pro-survival signal
cascades for cell survival (17).
Thus, in the present study, to further examine whether propofol
stimulates TM3 cell apoptosis through the inhibition of the Akt
pathway, phospho-Akt, Akt, phospho-mTOR and mTOR expression
patterns were examined by western blot analysis. The data
demonstrated that treatment with 350 µM propofol for 3 h,
and 400 µM propofol for 3 and 6 h significantly reduced
phospho-Akt expression (P<0.05) (Fig. 7A and B). In addition, treatment
with 400 µM propofol for 24 h reduced phospho-mTOR
expression (P<0.05) (Fig. 7A and
C). These observations suggested that propofol induced
apoptosis by inhibiting the Akt pathway in TM3 cells.
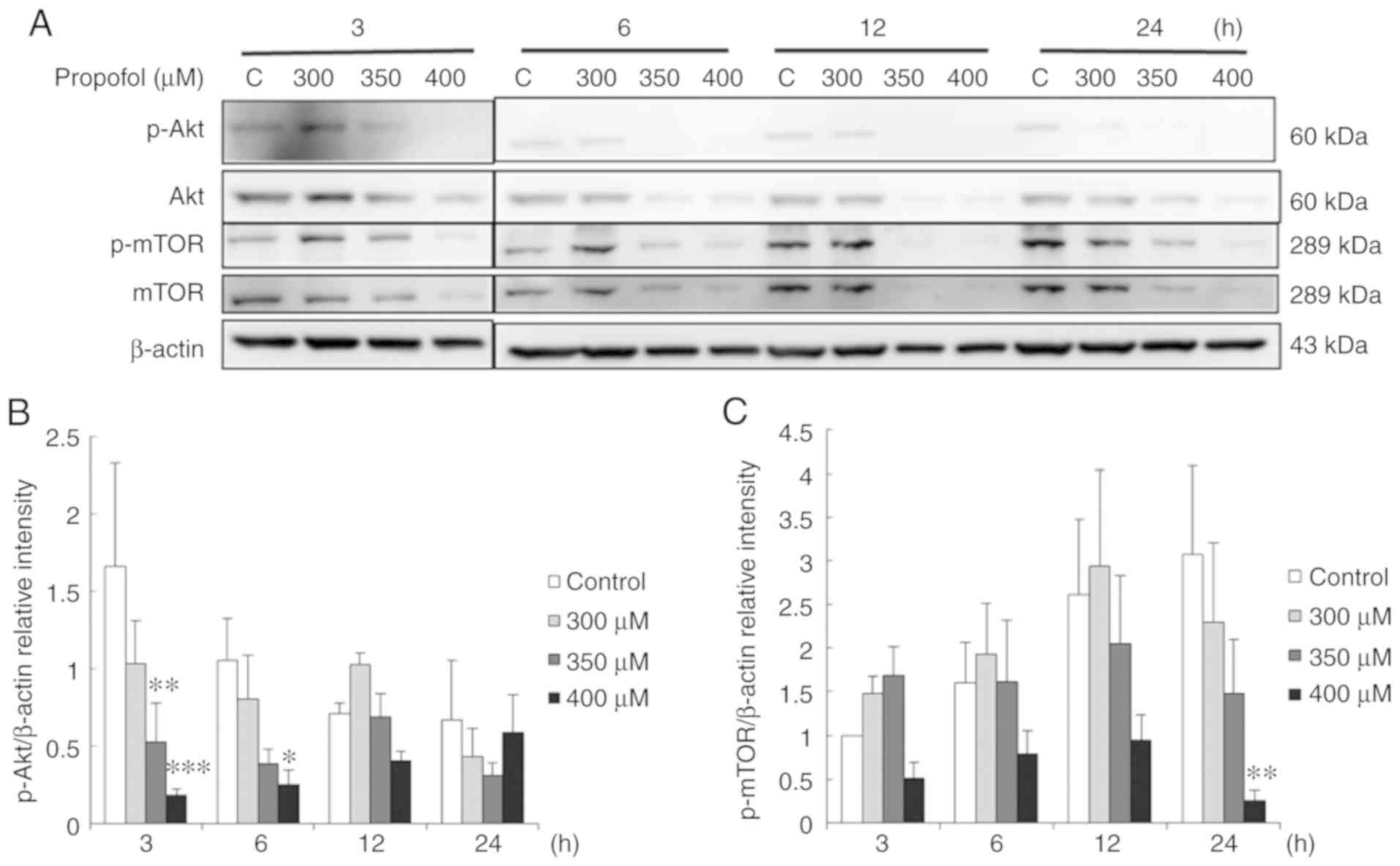 | Figure 7Propofol suppresses the Akt pathway
associated with the apoptosis of TM3 cells. TM3 cells were treated
with various concentrations of propofol (control, 300, 350 and 400
µM) for 3, 6, 12 and 24 h, respectively. (A) Phospho-Akt
(p-Akt; 60 kDa), Akt (60 kDa), phospho-mTOR (p-mTOR; 289 kDa) and
mTOR (289 kDa) were examined by western blot analysis. The
integrated optical densities (IOD) of (B) p-Akt and (C) p-mTOR
proteins were normalized to β-actin (43 kDa) in each lane,
respectively. Each data point represents the mean ± SEM of 3
separate experiments. *P<0.05, **P<0.01
and ***P<0.001 indicate statistically significant
differences compared to the control group. C, control. |
Discussion
A previous study indicated that propofol induced
neurotoxicity (31). Moreover,
studies have further demonstrated that propofol cause developmental
neurotoxicity in mice (2,3) and induces the apoptosis of neuronal
stem cells in the hippocampus in neonatal mice (4). It is highly possible that propofol
disrupts the development of Leydig stem/progenitor cells during the
fetal and neonatal periods. Thus, in the present study, TM3 mouse
Leydig stem/progenitor cells were used to examine the effects of
propofol with a brief mechanistic investigation; it was found that
propofol induced TM3 cell apoptosis.
Apoptosis is associated with intense transformations
in the cellular architecture, and the activation of caspase
cascades weakens the cell cytoskeleton, causing morphological
modifications, such as membrane blebbing and cell shrinkage
(32). The findings of the
present study demonstrated that propofol induced membrane blebbing
and cell shrinkage, illustrating that propofol affects the
cytoskeleton and induces morphological changes to stimulate cell
death associated with the apoptosis of TM3 cells. The authors have
previously demonstrated that membrane blebbing occurs in MA-10
cells treated with a higher concentration (400 µM) of
propofol (26). However, in the
present study, a lower concentration (350 µM) of propofol
was needed in the TM3 cells, indicating that the TM3 cells were
more sensitive to propofol than the MA-10 cells. In a cell
viability assay, it was observed TM3 cell viability decreased to
49% following treatment with 300 µM propofol for 12 h.
However, as previously demonstrated, MA-10 cell viability decreased
to 67% following treatment with 300 µM propofol for 12 h
(26). These phenomena indicate
that the TM3 cells are more sensitive to propofol than the MA-10
cells, which is similar to the morphological results.
The substantial increase in subG1 phase cell numbers
with propofol treatment illustrated that propofol regulates the
distribution of the cell cycle to induce DNA fragmentation and the
apoptosis of TM3 cells. Of note, propofol induced G2/M phase arrest
in the TM3 cells, but not in the MA-10 cells (26). Previous studies have demonstrated
that the increase in subG1 and G2/M phase arrest results in cell
death through apoptosis (33,34). Henceforth, the results of the
present study suggest that propofol-induced apoptosis may be
associated with cell cycle regulation.
In an Annexin V/PI double staining assay, the data
also revealed the stimulation of apoptosis by propofol in a
dose-dependent manner in TM3 cells, signifying that propofol
actually induced TM3 cell apoptosis. However, propofol induced
necrosis in TM3 cells in a dose-dependent manner, but not in the
MA-10 cells (26). It has been
shown that an overdose of propofol causes endothelial cytotoxicity
by apoptosis plus necrosis (35).
Thus, the findings of the present study are in accordance with
those of other studies. It would be of interest to further
investigate the mechanisms related to the stimulation of necrosis
by propofol in TM3 cells.
It has been demonstrated that apoptosis principally
commences by extrinsic and intrinsic signals, followed by the
activation of the caspase cascade (11). The findings of the present study
demonstrated that propofol activated caspase extrinsic and
intrinsic signals to promote the apoptosis of TM3 cells. However,
the same apoptotic effect with the activation of caspase pathways
occurred in TM3 cells at a lower concentration (350 µM) as
in the MA-10 cells with a higher concentration (400 µM)
(26). Therefore, it was again
observed that the TM3 cells were more sensitive to propofol than
the MA-10 cells.
Studies have demonstrated that the MAPK pathways
(ERK1/2, JNK and p38) regulated the apoptotic pathway to induce the
apoptosis of various cell types (15,36-38). In fact, JNK can cause a shift to
autophagy from apoptosis to regulate the survival of
choriocarcinoma cells (37) and
JNK can prevent the apoptosis of acute myeloid leukemia cells
(39). It has also been
demonstrated that ERK is able to promote apoptosis by facilitating
cell cycle arrest in response to DNA damage in different cell types
(38) and p38 can suppress
caspase-3 activity in neural cells to induce apoptosis (36,40). Studies have indicated that
propofol can inhibit the MAPK pathways in different cell types to
inhibit cell migration and inflammation (41,42). The findings of the present study
demonstrated that propofol significantly increased the
phosphorylation of ERK, JNK and p38 in TM3 cells, suggesting that
propofol can stimulate the MAPK pathways to induce the apoptosis of
TM3 cells. It should be noted that propofol did reduce the
expression of total MAPKs, as propofol induced p-JNK, p-ERK and
p-p38 expression levels. These phenomena indicate that porpofol may
cause the instability of MAPKs, which would result in MAPK
degradation in TM3 cells. Further studies are warranted to reveal
the underlying mechanisms.
Akt signaling can prevent the apoptotic signal and
stimulate pro-survival signals to maintain cell survival (17). It has been demonstrated that
PI3K/Akt pathway activation is highly associated with the formation
of certain types of cancer (43).
Actually, the function of propofol in the Akt pathway remains
undetermined. Studies have found that propofol suppresses Akt
signaling in macrophages to stimulate apoptosis (44), as propofol can activate the Akt
pathway to protect rat cardio-myocytes from doxorubicin-induced
toxicity (45). The present study
found that propofol reduced the phosphorylation of Akt in TM3
cells. Thus, it is suggested propofol attenuates the activity of
Akt and mTOR to stimulate the apoptosis of TM3 cells.
In conclusion, the present study demonstrates that
propofol induces cell apoptosis through the activation of caspases
and MAPKs pathways and the inhibition of the Akt pathway in TM3
Leydig stem/progenitor cells. This indicates that exposure to
anesthetic agents can affect testicular normal Leydig cell
development.
Funding
The present study was supported by grants from the
Ministry of Science and Technology MOST 105-2320-B-006- 028-MY3 (to
BMH) and the Chi Mei-NCKU hospital grant CMNCKU10811 (to FCK and
BMH), Taiwan, R.O.C.
Availability of data and materials
The data used and analyzed in this study are always
accessible from authors on reasonable request.
Authors' contributions
FCK, YCC and SCW contributed to conducting all the
experiments with statistical analysis. ECS and BMH contributed to
all the experimental designs plus rationale setup, data analysis,
manuscript writing, and ensuring the accuracy integrity of the
whole study. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Busettini C and Frolich MA: Effects of
mild to moderate sedation on saccadic eye movements. Behav Brain
Res. 272:286–302. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jiang C, Logan S, Yan Y, Inagaki Y, Arzua
T, Ma P, Lu S, Bosnjak ZJ and Bai X: Signaling network between the
dysregulated expression of microRNAs and mRNAs in propofol-induced
developmental neurotoxicity in mice. Sci Rep. 8:141722018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Logan S, Jiang C, Yan Y, Inagaki Y, Arzua
T and Bai X: Propofol alters long non-coding RNA profiles in the
neonatal mouse hippocampus: Implication of novel mechanisms in
anesthetic-induced developmental neurotoxicity. Cell Physiol
Biochem. 49:2496–2510. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yan Y, Qiao S, Kikuchi C, Zaja I, Logan S,
Jiang C, Arzua T and Bai X: Propofol induces apoptosis of neurons
but not astrocytes, oligodendrocytes, or neural stem cells in the
neonatal mouse hippocampus. Brain Sci. 7:E1302017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Davidoff MS, Schulze W, Middendorff R and
Holstein AF: The leydig cell of the human testis-a new member of
the diffuse neuroendocrine system. Cell Tissue Res. 271:429–439.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Habert R, Lejeune H and Saez JM: Origin,
differentiation and regulation of fetal and adult leydig cells. Mol
Cell Endocrinol. 179:47–74. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen H, Ge RS and Zirkin BR: Leydig cells:
From stem cells to aging. Mol Cell Endocrinol. 306:9–16. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yu FX and Guan K: The Hippo pathway:
Regulators and regulations. Genes Dev. 27:355–371. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kroemer G, Galluzzi L, Vandenabeele P,
Abrams J, Alnemri ES, Baehrecke EH, Blagosklonny MV, El-Deiry WS,
Golstein P and Green DR: et al Classification of cell death:
Recommendations of the nomenclature committee on cell death 2009.
Cell Death Differ. 16:3–11. 2009. View Article : Google Scholar :
|
|
10
|
Kerr JF, Wyllie AH and Currie AR:
Apoptosis: A basic biological phenomenon with wide-ranging
implications in tissue kinetics. Br J Cancer. 26:239–257. 1972.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Oliveira JB and Gupta S: Disorders of
apoptosis: Mechanisms for autoimmunity in primary immunodeficiency
diseases. J Clin Immunol. 1(Suppl 28): S20–S28. 2008. View Article : Google Scholar
|
|
12
|
Lewis-Wambi JS and Jordan VC: Estrogen
regulation of apoptosis: How can one hormone stimulate and inhibit?
Breast Cancer Res. 11:2062009. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cossarizza A, Baccarani-Contri M,
Kalashnikova G and Franceschi C: A new method for the
cytofluorimetric analysis of mitochondrial membrane potential using
the J-aggregate forming lipophilic cation
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-benzimidazolcarbocyanine
iodide (JC-1). Biochem Biophys Res Commun. 197:40–45. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Green DR and Reed JC: Mitochondria and
apoptosis. Science. 281:1309–1312. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Johnson GL and Lapadat R:
Mitogen-activated protein kinase pathways mediated by ERK, JNK, and
p38 protein kinases. Science. 298:1911–1912. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Son Y, Cheong YK, Kim NH, Chung HT, Kang
DG and Pae HO: Mitogen-activated protein kinases and reactive
oxygen species: How can ROS activate MAPK pathways? J Signal
Transduct. 2011:7926392011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Markman B, Dienstmann R and Tabernero J:
Targeting the PI3K/Akt/mTOR pathway-beyond rapalogs. Oncotarget.
1:530–543. 2010. View Article : Google Scholar
|
|
18
|
Hein AL, Ouellette MM and Yan Y:
Radiation-induced signaling pathways that promote cancer cell
survival (review). Int J Oncol. 45:1813–1819. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou C, Zhao XM, Li XF, Wang C, Zhang XT,
Liu XZ, Ding XF, Xiang SL and Zhang J: Curcumin inhibits
AP-2γ-induced apoptosis in the human malignant testicular germ
cells in vitro. Acta Pharmacol Sin. 34:1192–1200. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Geigerseder C, Doepner RF, Thalhammer A,
Krieger A and Mayerhofer A: Stimulation of TM3 Leydig cell
proliferation via GABA(A) receptors: A new role for testicular
GABA. Reprod Biol Endocrinol. 2:132004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Goldenberg RC, Fortes FS, Cristancho JM,
Morales MM, Franci CR, Varanda WA and Campos de Carvalho AC:
Modulation of gap junction mediated intercellular communication in
TM3 leydig cells. J Endocrinol. 177:327–335. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mather JP: Establishment and
characterization of two distinct mouse testicular epithelial cell
lines. Biol Reprod. 23:243–252. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Green LM, Reade JL and Ware CF: Rapid
colorimetric assay for cell viability: Application to the
quantitation of cytotoxic and growth inhibitory lymphokines. J
Immunol Methods. 70:257–268. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chang MM, Lai MS, Hong SY, Pan BS, Huang
H, Yang SH, Wu CC, Sun HS, Chuang JI, Wang CY and Huang BM:
FGF9/FGFR2 increase cell proliferation by activating ERK1/2,
Rb/E2F1 and cell cycle pathways in mouse leydig tumor cells. Cancer
Sci. 109:3503–3518. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kang FC, Wang SC, Chang MM, Pan BS, Wong
KL, Cheng KS, So EC and Huang BM: Midazolam activates caspase,
MAPKs and endoplasmic reticulum stress pathways, and inhibits cell
cycle and Akt pathway, to induce apoptosis in TM3 mouse leydig
progenitor cells. Onco Targets Ther. 11:1475–1490. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kang FC, Wang SC, So EC, Chang MM, Wong
KL, Cheng KS, Chen YC and Huang BM: Propofol could increase
caspases and MAPKs pathways and suppress Akt pathway to induce
apoptosis in MA-10 mouse leydig tumor cells. Oncol Rep.
41:3565–3574. 2019.PubMed/NCBI
|
|
27
|
Lowry OH, Rosebrough NJ, Farr AL and
Randall RJ: Protein measurement with the folin phenol reagent. J
Biol Chem. 193:265–275. 1951.PubMed/NCBI
|
|
28
|
Mu YF, Chen YH, Chang MM, Chen YC and
Huang BM: Arsenic compounds induce apoptosis through the caspase
pathway in MA-10 leydig tumor cells. Oncol Lett. 18:944–954.
2019.PubMed/NCBI
|
|
29
|
van Engeland M, Ramaekers FC, Schutte B
and Reutelingsperger CP: A novel assay to measure loss of plasma
membrane asymmetry during apoptosis of adherent cells in culture.
Cytometry. 24:131–139. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Creagh EM and Martin SJ: Caspases:
Cellular demolition experts. Biochem Soc Trans. 29:696–702. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yu D, Jiang Y, Gao J, Liu B and Chen P:
Repeated exposure to propofol potentiates neuroapoptosis and
long-term behavioral deficits in neonatal rats. Neurosci Lett.
534:41–46. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Taylor RC, Cullen SP and Martin SJ:
Apoptosis: Controlled demolition at the cellular level. Nat Rev Mol
Cell Biol. 9:231–241. 2008. View Article : Google Scholar
|
|
33
|
Paul-Samojedny M, Suchanek R, Borkowska P,
Pudelko A, Owczarek A, Kowalczyk M, Machnik G, Fila-Danilow A and
Kowalski J: Knockdown of AKT3 (PKBγ) and PI3KCA suppresses cell
viability and proliferation and induces the apoptosis of
glioblastoma multiforme T98G cells. Biomed Res Int.
2014:7681812014. View Article : Google Scholar
|
|
34
|
Zhang C, Chen Z, Zhou X, Xu W, Wang G,
Tang X, Luo L, Tu J, Zhu Y and Hu W: et al Cantharidin induces G2/M
phase arrest and apoptosis in human gastric cancer SGC-7901 and
BGC-823 cells. Oncol Lett. 8:2721–2726. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lin MC, Chen CL, Yang TT, Choi PC, Hsing
CH and Lin CF: Anesthetic propofol overdose causes endothelial
cytotoxicity in vitro and endothelial barrier dysfunction in vivo.
Toxicol Appl Pharmacol. 265:253–262. 2012. View Article : Google Scholar
|
|
36
|
Lee JM, Lee JM, Kim KR, Im H and Kim YH:
Zinc preconditioning protects against neuronal apoptosis through
the mitogen-activated protein kinase-mediated induction of heat
shock protein 70. Biochem Biophys Res Commun. 459:220–226. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shen Y, Yang J, Zhao J, Xiao C, Xu C and
Xiang Y: The switch from ER stress-induced apoptosis to autophagy
via ROS-mediated JNK/p62 signals: A survival mechanism in
methotrexate-resistant choriocarcinoma cells. Exp Cell Res.
334:207–218. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tang D, Wu D, Hirao A, Lahti JM, Liu L,
Mazza B, Kidd VJ, Mak TW and Ingram AJ: ERK activation mediates
cell cycle arrest and apoptosis after DNA damage independently of
p53. J Biol Chem. 277:12710–12717. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lin X, Fang Q, Chen S, Zhe N, Chai Q, Yu
M, Zhang Y, Wang Z and Wang J: Heme oxygenase-1 suppresses the
apoptosis of acute myeloid leukemia cells via the JNK/c-JUN
signaling pathway. Leuk Res. 39:544–552. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang B, Wu T, Wang Z, Zhang Y, Wang J,
Yang B, Zhao Y, Rao Z and Gao J: p38MAPK activation mediates tumor
necrosis factor-α-induced apoptosis in glioma cells. Mol Med Rep.
11:3101–3107. 2015. View Article : Google Scholar
|
|
41
|
Wu KC, Yang ST, Hsia TC, Yang JS, Chiou
SM, Lu CC, Wu RS and Chung JG: Suppression of cell invasion and
migration by propofol are involved in down-regulating matrix
metal-loproteinase-2 and p38 MAPK signaling in A549 human lung
adenocarcinoma epithelial cells. Anticancer Res. 32:4833–4842.
2012.PubMed/NCBI
|
|
42
|
Li D, Wang C, Li N and Zhang L: Propofol
selectively inhibits nuclear factor-κB activity by suppressing p38
mitogen-activated protein kinase signaling in human EA.hy926
endothelial cells during intermittent hypoxia/reoxygenation. Mol
Med Rep. 9:1460–1466. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Vivanco I and Sawyers CL: The
phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev
Cancer. 2:489–501. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hsing CH, Chen YH, Chen CL, Huang WC, Lin
MC, Tseng PC, Wang CY, Tsai CC, Choi PC and Lin CF: Anesthetic
propofol causes glycogen synthase kinase-3β-regulated
lysosomal/mitochondrial apoptosis in macrophages. Anesthesiology.
116:868–881. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sun X, Gu J, Chi M, Li M, Lei S and Wang
G: Activation of PI3K-Akt through taurine is critical for propofol
to protect rat cardiomyocytes from doxorubicin-induced toxicity.
Can J Physiol Pharmacol. 92:155–161. 2014. View Article : Google Scholar : PubMed/NCBI
|