Since the discovery of the clustered regularly
interspaced short palindromic repeats (CRISPR) system in 1987
(1), there have been significant
advances in the field of gene therapy. The CRISPR system is an
adaptive prokaryotic immune system, which serves as a bacterial
defence mechanism against insertion of foreign genomic material and
prevents the destructive impacts of mobile genetic elements
delivered by phages and plasmids (2). CRISPR/Cas9 has been shown to boost
the host immune system using the invading organisms' genetic
material in order to protect the host from further invasion. The
protective mechanism is completed with the acquisition of spacer
sequences by CRISPR-associated spacer (Cas) proteins (3). Cas proteins are guided to the
exogenous spacer sequences of foreign nucleic acids by
CRISPR-associated RNA (crRNA) (4). The mechanism involves spacer
identification and anchoring by Cas proteins, providing protection
against further invasion. The existence of CRISPR was discovered in
1987 by Ishino et al (1),
who cloned a portion of the CRISPR sequence together with the
inhibitor of apoptosis gene (1).
This discovery resulted in a novel method for gene-based
therapeutics for the treatment of challenging disorders (5). Further analysis of prokaryotes, such
as Archaeoglobus fulgidus, revealed other constituents of
the CRISPR system, including non-messenger RNA sequencing,
transcription of DNA repeats loci (target DNA sequences that are
acquired and preserved in CRISPR loci) to small RNAs (Fig. 1) (6), and the Cas gene family (which is
associated with CRISPR loci during immune processes) (7). Furthermore, identification of
specific spacer sequences from the viral genome revealed how
bacterial systems exhibited phenotypic resistance against the phage
(7).
The aim of the present review was to describe the
challenges and achievements of CRISPR customisation, taking into
consideration various factors that may affect therapeutic outcomes
in vitro and in vivo.
As a therapeutic method, CRISPR is used for both
gene knockout and gene knock-in to correct a specific gene.
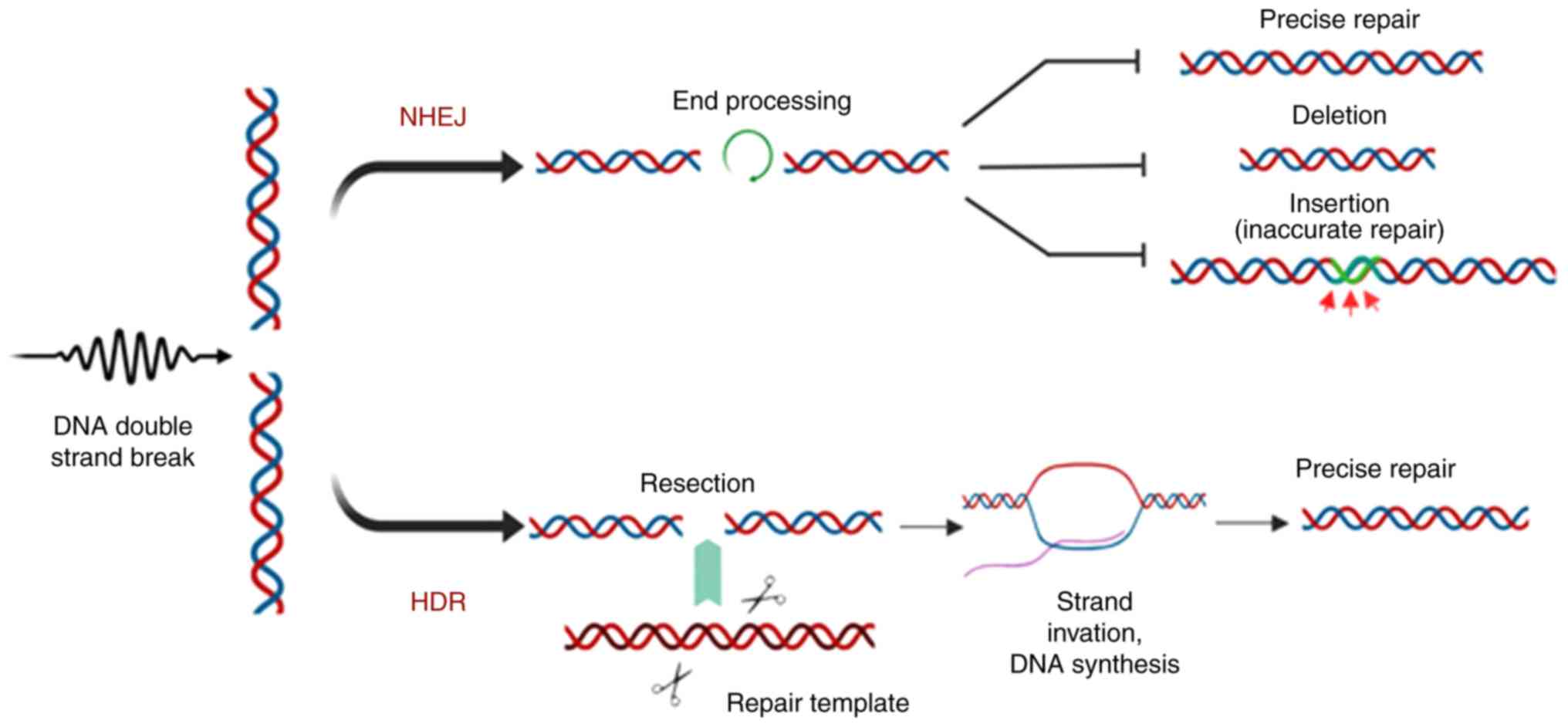
Generation of double-strand breaks (DSBs) in the target gene
results in the initiation of two endogenous DNA repair mechanisms:
Non-homologous end joining (NHEJ) and homology-directed repair
(HDR).
Various tools have been developed to perform
knock-in and knockout in target genes to improve gene editing, and
these have been used as therapeutic tools to treat certain
diseases.
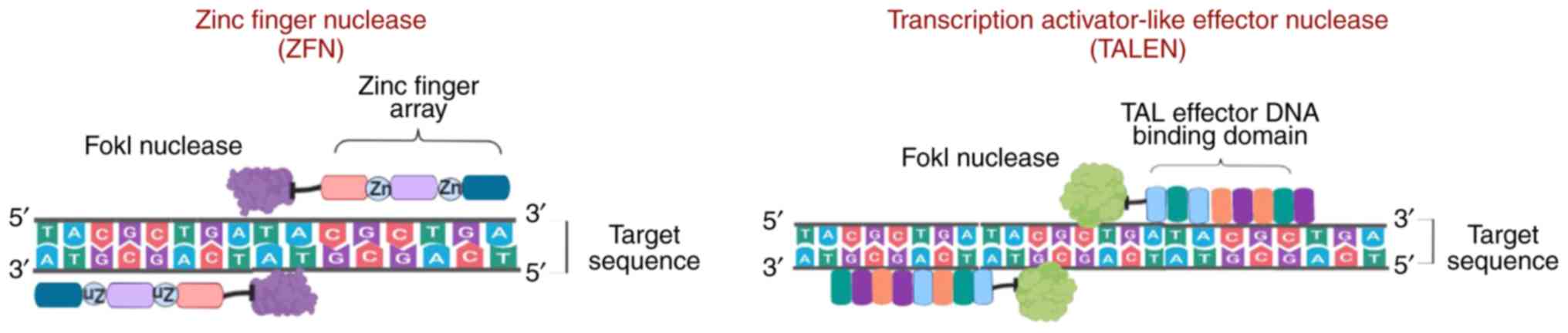
ZFNs are sequence-specific nucleases that are
frequently found in eukaryotes and were first discovered as
repetitive zinc-binding domains in Xenopus oocytes (17), which are currently known as zinc
finger motifs. Analysis of the ZFN crystal structure revealed the
presence of 30 cysteine and histidine residues (each in pair;
Cys2-His2) bound to zinc ions that provide stability to their ββα
structure (18). ZFNs also
contain a non-specific Fok-1 restriction enzyme, which is bound to
the DNA-binding domain of eukaryotic transcription factors known as
zinc finger proteins (ZFPs) containing Cys2-His2 fingers. Each
finger identifies almost three base pairs of DNA sequences of
target DNA and assists the binding of the ZFN to a particular
sequence (17,19). However, there are difficulties in
assembling ZFP fingers to bind to specific extended DNA sequences.
Maeder et al (20,21) designed and assembled ZPFs that
could bind to a specific 200-bp DNA sequence. However, binding may
occur at random sites of the genome and, thus, may complicate gene
correction. Furthermore, frequent off-target effects in the number
of loci is another concern (22,23). Researchers successfully addressed
this issue by designing ZFN pairs where one ZFN binds to the
forward strand and the other to the reverse strand. Each pair
contains distinct heterodimer Fok-1 domains with opposite charges,
assisting in the formation of DSBs (Fig. 3) (24-26).
Subsequently, another method for gene editing,
termed TALENs, was developed, which is considered more efficient,
and has more advanced potential gene therapy applications (27,28). TALENs are fusion proteins composed
of TALE and Fok-1 nucleases. The proteins, 33-35 amino acids in
length, contain repeat variable di-residues, which are central
binding protein regions that may be customized (29,30). Compared with ZFNs, TALENs are more
suitable for therapeutic use due to the 1:1 TALE-DNA binding
affinity (31) and lower rates of
cytotoxicity (32). However,
TALENs delivery is dependent on vectors, and developing suitable
vectors has proved challenging, as the size of the cDNA that
encodes TALENs is larger than the cDNA which encodes ZFNs (Fig. 3) (33).
The CRISPR/Cas system is classified into two types,
with each type comprising various subtypes (I-V) based on their
flanking Cas genes and location of the target on foreign DNA
(37), which have improved our
under-standing of and ability to develop phage-resistant strains
and the phylogenetic classification of bacteria. During
phage/plasmid invasion, CRISPR/Cas functions in three phases:
Adaptation, expression and interference. Each stage is associated
with specific characteristics that result in antiplasmid or
antiviral immunity (38).
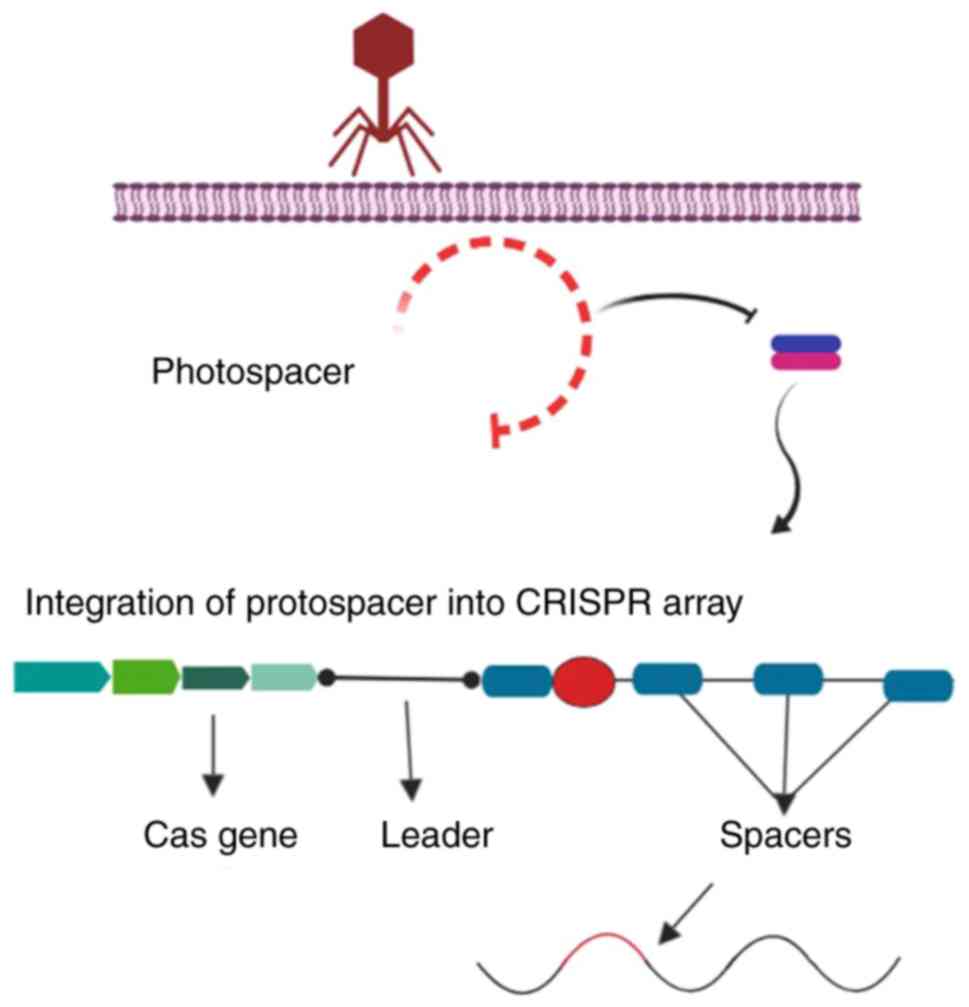
Adaptation consists of an integration process by which the
invader-derived spacers (known as the spacer sequence) merge with
the CRISPR array. In the next step, the CRISPR loci are transcribed
into CRISPR-associated RNA (crRNA), which contains the spacer
sequence. Subsequently, an endonuclease is produced and uses the
spacer sequences as a guide to cleave the invader genome (Fig. 4) (39).
The functional characteristics of the CRISPR/Cas
system are defined by the properties of the Cas1 and Cas2 genes.
Taxonomic studies initially classified the CRISPR/Cas system into
three types based on the particular marker proteins: Cas3 (type I),
Cas9 (type II) and Csm/Cmr (type III) (40). Subsequently, classes IV and V of
the CRISPR editing system were added (41). The general classification of the
CRISPR system is based on the genes that encode the functional
proteins and factors (Cascade, Csm, Cmr complex or Cas9). Class 1
CRISPR systems functions consist of multi-subunit crRNA-effector
complexes, and includes type I, III and putative type IV (41). Class 2 only consists of Cas9 as a
single protein, which performs all the functions of the CRISPR
system, a feature also observed in putative class V (40).
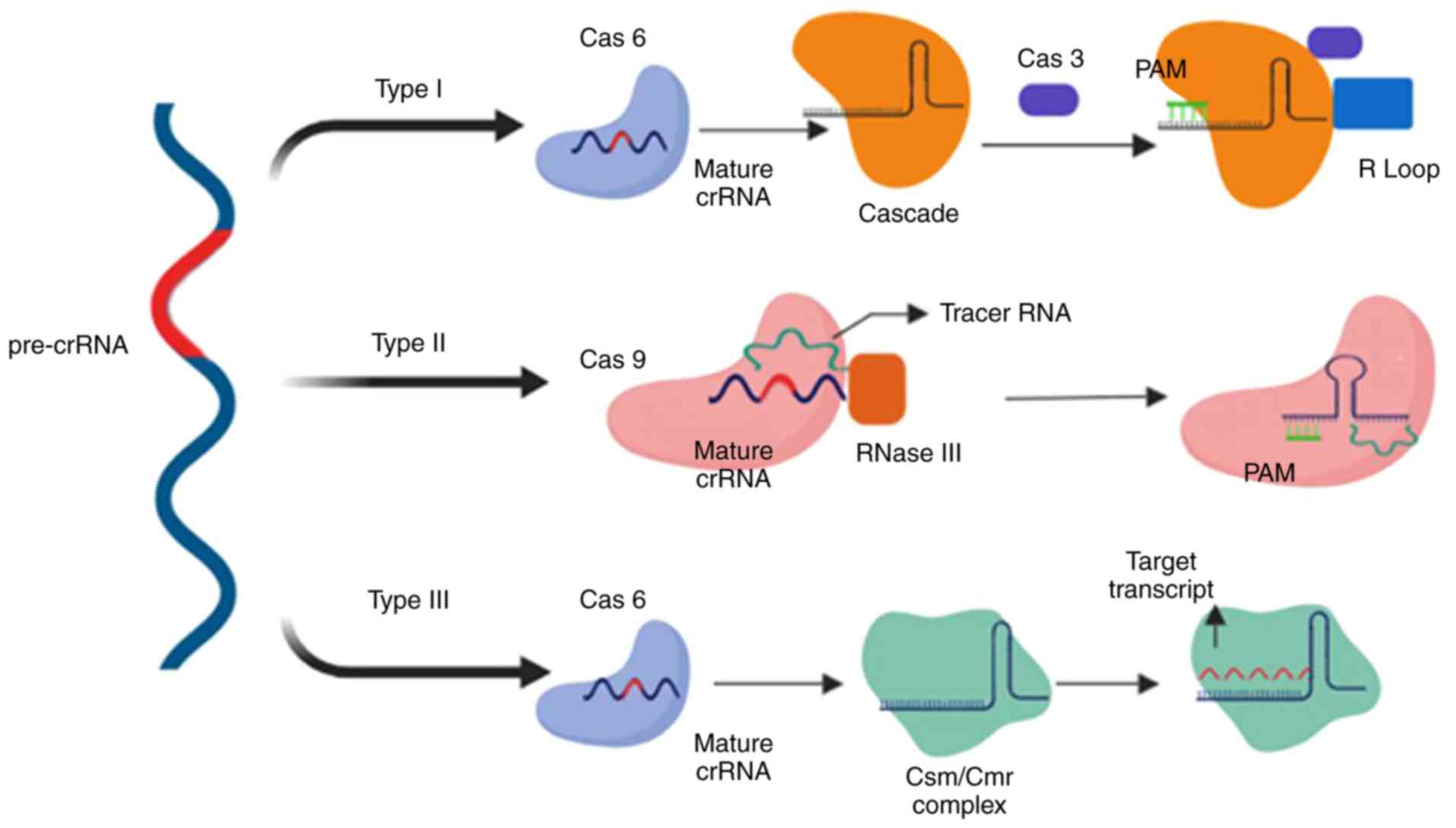
Type I CRISPR is defined by the significance of the
Cascade complex followed by the Cas3 nuclease (42). Pre-crRNA, which is the product of
a transcribed CRISPR array, is cleaved by Cas6e, resulting in the
production of crRNA (43). crRNA,
which is associated with Cascade, is responsible for locating the
protospacer in the target DNA. Furthermore, another subunit of
Cascade, Cas8, identifies and locates protospacer adjacent motif
(PAM), which is a short sequence located near the target sequence
(44). The PAM sequence is the
crucial factor for type I Cascade-Cas immune defence mechanisms;
its dysfunction results in an inability of crRNA to recognise the
spacers in target DNA by Cascade proteins (45,46), and inhibits R-loop formation
between crRNA and target DNA, a process which eventually results in
viral evasion from CRISPR screening (47,48). In the presence of a fully
functional CRISPR system, recognition leads to activation of the
Cas3 nuclease, which creates nicks on the single-stranded DNA of
the target (virus or plasmid), resulting in its degradation
(Fig. 4) (49).
Unlike type I, type II relies on Cas9 as the sole
Cas protein, two types of RNAs, RNase III and tracer RNA (13), and a PAM, which is located
downstream of the protospacer sequence in target DNA and recognised
by the Cas9 protein (50). A DSB
is introduced by HNH nuclease domain cleaving one strand, and RuvC
nuclease domain cleaving another (51). However, cleavage requires
identification of a PAM sequence by Cas9, which results in
dissociation of dsDNA and the formation of an R-loop between the
crRNA and DNA. This structural change results in the binding of the
tracer RNA to the cleavage target sequence (52,53).
Type III is unique to Cas6, whose endoribonuclease
mechanism produces crRNA by cleaving pre-crRNA (5). Unlike previous models of the CRISPR
system, this type introduces 8-nucleotide repeat sequences, known
as crRNA tags, as a result of crRNA cleavage by Cas6. The crRNA
tag, which is located downstream of the spacer sequence (54-56), undergoes maturation and is
modified into six nucleotides (53). The size of the crRNA complex
increases and forms Cas10-Csm in type III-A and Cas10-Cmr complex
III-B systems (54). In contrast
to type I and type II, type III targets both DNA and RNA, producing
co-transcriptional crRNA-guided cleavage of the target DNA
(57,58). The palm domain of Cas10 cleaves
DNA strands (58) and Csm3 (type
III-A), and Cmr4 (type III-B) cleave RNA transcripts (59,60). Another notable difference of type
III is that the PAM is not necessarily essential for the system to
initiate immune mechanisms (Fig.
4).
The identification of the CRISPR system allowed for
improvements to gene editing technologies, and may improve current
therapeutic techniques in the field of medicine, genetics,
embryology, bioinformatics and pathology. Compared with the other
genome editing techniques, CRISPR/Cas9 is more cost-effective,
easier to use and can be used to perform specific gene knockdown,
or base insertions and substitutions with lower rates of mutations
with the known mechanism of CRISPR system.
Neurodegenerative diseases are characterised by
disruption of neuronal function or loss of neurons that cause
progressive central nervous system dysfunction and, thus, are
frequently difficult to treat (61). There are numerous pathological
neurodegenerative disorders, including Alzheimer's disease (AD),
which is a fatal form of progressive dementia, and Parkinson's
disease, which is a progressive movement disorder. These
neurodegenerative diseases are relatively common (62). Familial AD, which may be caused by
point mutations or deletions in the genes encoding amyloid
precursor protein (APP), presenilin (PSEN)1 or PSEN2 (62), accounts for <5% of AD cases.
Sporadic AD, which develops due to environmental factors, such as
ageing or injury following brain ischemia, accounts for >90% of
AD cases (63). In vitro
analyses of induced pluripotent stem cells (iPSCs) that have been
treated with CRISPR/Cas9 provide future perspectives in the
treatment of neurodegenerative diseases, such as AD (64). Several studies have used the
CRISPR/Cas9 system to identify defective and upregulated genes in
early- and late-onset AD. Analysis of chromosome 1q31-42 in
early-onset familial AD revealed a point mutation in PSEN2
(65), which was found to be
correlated with a significant increase in the β-amyloid peptide
ratio (Aβ42-43/40) (66), a
change in voltage-gated potassium channel expression (67), and an increase in the
concentration of calcium in the endoplasmic reticulum of neurons
(68), which leads to
neurotoxicity, cognitive deterioration and short-term memory loss
in patients with AD, primarily due to basal forebrain cholinergic
neuron (BFCN) damage. When healthy BFCNs were transplanted into an
AD mouse model, a significant improvement in learning ability was
observed (69). These findings
led to further analyses of BFCNs, which were established from in
vitro development of iPSCs obtained from the fibroblasts of
humans with a PSEN2 mutation (70). The PSEN2N141 iPSC-derived BFCNs,
characterised by a lack of electrophysiological properties, were
observed to exhibit an improvement in neural activity and amyloid
ratio following correction of the PSEN2 gene using CRISPR/Cas9
(71). Other studies performing
similar analyses confirmed the effect of the CRISPR/Cas9 genome
editing system on cells, the damage of which was associated with
AD.
Scientists from the New York Stem Cell Foundation
Research Institute expanded their studies on PSEN1 and PSEN2 point
mutations in familial AD (72).
They demonstrated a correlation between the inflammasome (a
component of an innate immune system in a myeloid cell) and PSEN2
mutations using CRISPR/Cas9 (71). A correction of the point mutation
on the PSEN2 gene in iPSCs derived from AD patients was performed
using CRISPR/Cas9 and template sgRNA, as well as single-stranded
oligonucleotides (ssODNs) that could edit the sequence in exon 5 of
the PSEN2 gene located on chromosome 1 (1q42.13) (73). In vitro gene editing using
the CRISPR/Cas9 system reversed the effect of the mutated PSEN2
gene on the increase of amyloid plaques (Aβ42/40) in iPSC-derived
BFCNs in the cerebrospinal fluid, which was directly associated
with the onset of AD (71). The
in vivo effect of CRISPR/Cas9 on the mutated APP gene shows
the system's ability to reduce neurotoxicity in AD patients. The
APPsw allele in the fibroblasts of human patients' with an APPsw
mutation was transfected using CRISPR/Cas9 with a gRNA designed to
specifically mutate the allele. There was a considerable decrease
in heterozygous APP alleles (APPsw/WT) and in the β-amyloid ratio
(73). Additionally, the S.
pyogenes CRISPR system has been shown to target specific
desired genes or alleles (e.g., the APPsw allele) without
disruption or indel formation in the other alleles, such as APPWT
(73). However, in vivo
analysis in a Tg2576 mouse model (mice with an age-deficient
cognitive ability that over-express mutant APP) using
exosome-adeno-associated virus (AAV)1 with CRISPR/Cas9 and gRNA,
showed indel formation and DNA frameshifts in the APPsw alleles
(73). Although CRISPR/Cas9 may
successfully alter the APPsw allele and reduce the β-amyloid ratio
in brain cells, further investigations and clinical trials are
required to improve the system and to reduce or ideally nullify
indel formation.
Before the discovery of gene editing tools, several
complications in cancer therapy could not be efficiently addressed,
as drug resistance had not been effectively identified as a
possible cause (74-76). CRISPR/Cas9 has facilitated the
identification of genes associated with drug resistance by exposing
drug-resistant cells to CRISPR/Cas9 gRNAs, each of which
individually knock out a single gene at a time in each cell
(77), and the results may be
used to introduce alternative drugs with increased efficacy for
improved outcomes (77).
For drug design, animal models and human cell lines
are the optimal platforms for testing specific drug toxicity and
efficacy prior to its use in humans. However, animal models, as
well as in vitro analysis of human cell lines, may not
provide representative and conclusive results regarding the
effectiveness of a particular drug. CRISPR/Cas9 has provided a
means to modify cells to allow them to more accurately represent
human models for different types of cancer. ID8 (mouse ovarian
surface epithelium) cells, which were obtained from ID8 mice with
ovarian cancer with the TP53 and BRCA2 genes knocked out using
CRISPR/Cas9, displayed characteristics of a high-grade serous
carcinoma (78). High-grade
serous ovarian carcinomas (HGSCs) exhibit reduced activity of TP53
and BRCA genes, loss of ability to form Rad51 foci (associated with
DSB repair), and sensitivity to poly(ADP-ribose) polymerase
inhibition (79). CRISPR/Cas9 has
assisted in the development of more representative models of cancer
that are likely to be increasingly used for evaluating drug safety
and eliminating drug resistance in human diseases.
In addition to drug design, CRISPR/Cas9 has been
proposed as a promising gene editing tool in cancer therapy. The
dCas9 (mutated Cas9 without endonuclease activity, with added
transcriptional activators on dCas9 or gRNA) is used to target
specific genes by either activating or knocking them out, when
combined with transcriptional activation or inhibition (80). Epigenome editing is another
approach to cancer treatment, in which dCas9 is tethered to histone
modifiers involved in DNA methylation to disturb processes
associated with cancer progression, as DNA methylation is observed
in the majority of cancers (81).
To determine the effects of the CRISPR/Cas9 system
on different malignancies, such as leukaemia, K562 human myeloid
leukaemia cells, which do not normally harbour a mutation in the
isocitrate dehydrogenase (IDH2) gene, under-went a DSB and point
mutation on the IDH2 R140Q locus following transfection with a
plasmid which contained a sgRNA and CRISPR/Cas9. Following the
mutation, gene repair was performed using another transfection with
a pBS-SK+ vector with a CRISPR/Cas9 and
fluorescent-template DNA followed by sgRNA to check gene correction
rate on point-mutated cells (82). The results revealed high levels of
H3K9me2, H3K27me2 and H3K4me3 expression, which indicated
hyper-methylation of chromatin in mutated cells (83). In other studies, CRISPR/Cas9 was
used as a tracking device to determine the effect of IL
(interleukin)4-induced signal transducer and activator of
transcription (Stat)6 activation on the elimination of leukaemia
cells by using lentiviral vectors containing Cas9 and sgRNA for
Stat6 (84). The results
indicated that IL4 and its antileukemic effects were dependent on
the ability of acute myeloid leukaemia cells to activate Stat6
(84), highlighting the potential
of the CRISPR/Cas9 system as a therapeutic and diagnostic tool in
various diseases. Several studies have been performed to assess the
therapeutic potential of the CRISPR system for treating different
types of cancer. 2CT-CRISPR assay is used to identify the genes
causing resistance to immune cells (85). The test consists of two types of
target cells and effector cells, such as melanoma cells and
CD8+ T cells, respectively. The aim of the assay is to
identify factors mediating the growth of melanoma cells following
an immune system response, and to design efficient therapeutic
methods to augment immunotherapy against cancer cells. These assays
were performed in vivo on C57BL/6J mice (85). Other studies focused on modifying
chimeric antigen receptor (CAR) T cells in order to target cancer
cells, and showed that CRISPR/Cas9 was more effective compared with
RNA interference, which is only partially effective (86). Thus, scientists improved the
efficiency of the technique by using CRISPR/Cas9, TALENs and ZFN,
which reduced the off-target effects (87-93). However, the possibility of
recurrence following treatment is considered to be a notable
disadvantage that should be taken into account when designing the
most effective therapy to decrease the possibility of the need for
repeated treatments (88). Recent
studies have demonstrated that CAR T-cell therapy may a promising
treatment for various diseases, including cancer. CRISPR is a
system that may be used for improving CAR T cells. CAR permanent
tonic signalling has been shown to reduce antitumor activity
(89-93). Human CAR T cells modified with
CRISPR/Cas9 gene editing, which contained 4-1BB and CD3z
intracellular signalling domains, eliminated tumour cells by
targeting the CD19+ B cells that are associated with
increased tumorigenesis (89).
DMD is an X-linked recessive muscular disorder
caused by certain mutations of the DMD gene, which is located on
chromosome 21. The mutation leads to a decrease in the levels of
dystrophin, which is the protein responsible for normal muscular
integrity (94). Men are more
prone to this disease, as they carry one X chromo-some (95). Of all the mutations identified in
the DMD gene, ~86% are deletions and are present in an exon
(96). In an attempt to
regenerate muscle tissues to replace damaged tissue, haematopoietic
therapy using haematopoietic stem cells obtained using ex
vivo expansion of myoblasts from satellite cells has been
developed. However, this technique was not found to be beneficial
for patients with DMD (97), as
myoblasts lose their ability to engraft into muscle tissues
(98). iPSCS and embryonic stem
cells (ESCs) are capable of producing a vast quantity of skeletal
myogenic progenitors, exhibit in vivo regenerative capacity,
as well the ability to synthesize dystrophin (99,100); therefore, ESCs are the optimal
cells for performing genome editing to reduce DMD symptoms
(99). Different therapies have
been developed to replace dystrophin deficiency, such as
anti-inflammatory-based techniques, or to restore DMD gene
expression, such as cell-based therapies (101). Genome editing techniques,
including the ZFN, TALENs and CRISPR systems, are the most
efficient of these techniques. In vitro, TALENs and CRISPR
function by restoring dystrophin synthesis via gene knock-in
(insertion of exon 44 in the DMD gene), and has been demonstrated
to be effective, with minimal off-target effects. iPSCs that were
collected from DMD fibroblasts of a specific patient with DMD with
exon 44 missing were transfected with TALENs and CRISPR separately
to insert exon 44 by creating indels in adjacent exons (101). In vitro removal of exons
45-55, instead of a single exon in the DMD gene in the patient's
iPSC-derived cardiomyocytes and skeletal myotubes using sgRNA and
CRISPR/Cas9 system, resulted in restoration of dystrophin protein
synthesis and, consequently, creatine kinase levels, whose linkage
causes muscle instability and disintegration (102). Such a deletion was observed to
normalise miR32 (miRNA32) (102), which reduces dystrophin levels
in muscular dystrophies, including Becker muscular dystrophy
(103). These results indicate
the potential benefits of larger deletions, which rectify the
dysfunction of other factors affecting the function of dystrophin.
In vivo, CRISPR/Cas9 has been used to correct mutations of
the DMD gene, in mdx mice (mice with a point mutation in the DMD
gene and lacking dystrophin expression). sgRNA vector plasmids were
used to target exon 23 of the DMD gene and ssODN to activate HDR
repair mechanisms and repair the lesions of the DMD gene. However,
the results indicated a higher rate of NHEJ-based repair, which
resulted in the formation of indels (12).
The HIV-1 virus invades host immune cells through
the CD4 receptor and interaction with CC chemokine receptor 5
(CCR5) and CXC chemokine receptor 4. Although developmental
treatment for HIV-1 with combinational retroviral therapy has
improved the quality of life of the patients, it fails to eradicate
the HIV-1 virus from the body, resulting in high rates of morbidity
and mortality (104). A 32-bp
deletion in the CCR5 allele results in the deactivation of the CCR5
gene, which results in a high degree of resistance to HIV-1
infection (104,105). In 2000, a patient with acute
myeloid leukaemia and HIV-1 infection underwent bone marrow
transplantation (using allogeneic stem cells) from a donor carrying
CCR5Δ32/Δ32 cells, which resulted in abrogation of HIV replication,
and the HIV-1 virus was not detected in the body (106). Research has focused on the
development of homozygous cells using gene editing technologies,
such as zinc-finger nuclease (107-109). Human CD34+
hematopoietic stem/progenitor cells (HSPCs) from umbilical cord
blood were transfected with ZFNs to knock out the CCR5 locus on
chromosome 3 to establish a CCR5−/− clone (108-110). These cells were grafted into
non-obese diabetic/severe combined immunodeficient/interleukin
2Rγnull (NOD/SCID/IL2Rγnull; NSG) mice, an
ideal rodent model for examining HIV-1 infections (111) and haematopoiesis (112). The results of the experiment
revealed HIV-1 replication control (110). Similar studies on
CD34+ HSPCs using adenoviral vectors carrying CCR5-ZFN
resulted in a more effective knockdown of CCR5−/− and
fewer off-target effects compared with plasmid DNA ZFNs (109). These studies were extended to
human patients with HIV infection who received CD4 T cells with
dysfunctional CCR5 using ZFN60. The results indicated a high number
of CD4 T cells. However, the rate of viral replication in cells
with non-mutated CCR5 alleles (homozygous) was faster compared with
cells with mutated CCR5 allele (heterozygous), which indicates that
cells with homogenicity require knockout in both alleles to
participate in the disease prognosis (107). Furthermore, using modified cells
with ZFN does not result in permanent changes in vivo, as
modified cells fail to control HIV-1 replication due to the
presence of unmodified cells (113). Additionally, the adverse effects
of adenoviral vectors must be considered (94). Wild-type (WT) iPSCs have been used
to generate homozygous cells that harbour mutations in CCR5, termed
CCR5-Δ32 (114). Generation of
these cells was performed by transfecting WT iPSCs with a
CRISPR/Cas9 plasmid with a specific sgRNA (115). The matured monocytes or
macrophages from the modified iPSCs expressed resistance to HIV-1
infection (114-116). Another study on HIV-1-positive
patients demonstrated that the presence of Cas9 and gRNA together
in T-cells (specifically CD4+ T cells) that have been
manipulated genetically by Cas9/gRNA confer resistance to HIV-1
infection (117). The same study
experimented with excision of pro-viral HIV-1 DNA from T cells
using Cas9/gRNA on the RSBN1 gene, without disrupting the normal
function of the gene, which encodes histone demethylase, which is
responsible for chromatin structure (117). Astrocyte cell lines were
transfected with Cas9 protein with and without plasmids, and double
fluorescent protein HIV-1 reporter RGH was utilised to determine
the excision sites of pro-viral HIV-1 DNA using a gRNA; the results
demonstrated there was a reduction in fluorescent protein in
astrocytes with no alterations to their regular function and
morphology (118).
In SCD, a recessive genetic disorder with a
prevalence of 250,000 annually worldwide (119), a modified CRISPR/Cas9 system was
used with guide strands that specifically target the HBB and CCR5
genes using the pX330 plasmid in human kidney cells (120). Off-target effects were found to
be directly associated with the presence of adjacent acquisition
motifs (AAMs) in the PAM sequence, which reduces or nullifies the
cleavage of target genes via CRISPR/Cas9 (120). The rate of mutations due to
interception of the correction of the genes by Cas9 was directly
correlated with the distance between sgRNA and the PAM of the
specific protospacer recognised by the particular sgRNA (121). Furthermore, to correct the
mutations in patients with SCD, ribonucleoprotein (RNP) consisting
of Cas9 protein and sgRNA trG10 (truncated sgRNA G10 that targets
the first exon of the HBB gene) along with ssODNs was introduced
into human HSPCs collected from blood samples of patients with SCD
(121). The results demonstrated
that the use of CRISP/CAS9 resulted in efficient gene correction
with reduced off-target effects and with optimum activation of HDR,
compared with previous studies that used ZFN mRNA electroporation
with ssODNs (13). The various
diseases that have been treated using the CRISPR/Cas9 system are
listed in Table I.
CRISPR/Cas9 using AAV vectors has been assessed for
the development of novel therapeutic methods to treat X-linked
genetic diseases (122), such as
haemophilia, a challenging disease with a high mortality rate,
which is characterised by mutation on the coagulation factor IX
(FIX). To restore the function of the F9 gene in patients with
haemophilia, an AAV8 vector system carrying codon-optimized SaCas9
cDNA and sgRNA was transfected into hepatocytes in an in
vivo model of mice with haemophilia B to create DSBs in the
exon near the F9 gene (exon 2-8), and insert cDNA into an intron of
the gene (123). The results
revealed a genotypic and phenotypic correction of haemophilia B
mice by targeting hepatocytes without disrupting epithelial cells
of the liver morphologically or phenotypically (123). Additional studies extended the
therapeutic design using other vectors with capacity for larger
constructs. A low dose of adenovirus (Adv) containing Cas9 and
specific sgRNA containing a correct donor template (dsDNA) was
transfected into hepatocytes of haemophilia B mice, which resulted
in F9 gene correction with improved efficacy compared with other
vectors (14). However, lack of
restoration in coagulation factor was an unfortunate outcome, as
the Adv resulted in an adverse immune response due to the presence
of vector genome immunogenicity, and an insignificant rate of HDR.
Thus, it was hypothesized that recombinant Adv may be more suitable
(14). However, integration of
cDNA into the host genome can result in genotoxicity by either
activating potential oncogenes or damaging functional genes
(124). Therefore, using iPSCs
compared with vector may be more suitable. In vitro and
in vivo CRISPR studies using human iPSCs from patients with
haemophilia B reported interesting results. iPSC cell lines
prepared from peripheral blood mononuclear cells from patients with
haemophilia B were modified by inserting the complete F9 human cDNA
using CRISPR/Cas9; these cells differentiated into hepatocytes, and
were subsequently injected into NOD/SCID mice. Analysis of the mice
following transplantation revealed secretion of human FIX (125), a promising result that may serve
as the basis for future studies.
ASD is a primarily inherited neurodevelopmental
condition that is characterised by difficulty in social
interactions, with language and communication abnormalities, which
may be identified in children during early development. The
symptoms have been found to be genetically associated with fragile
X, maternal 15q11-13 duplication (83) and 2q37 and 22q13.3 deletion
(126). AAVs, specifically AAV9,
improves the treatment of Rett syndrome, as AAV9 can effectively
penetrate into brain cells (127). To enhance the ability of the
vectors for gene delivery, a self-complementary AAV9 vector, along
with a codon-optimized version of the major methyl CpG binding
protein 2 (Mecp2) gene (a mutation in the Mecp2 gene causing Rett
syndrome has been observed in almost 1% of ASD patients) (128). The function of this gene
involves transcription regulation by activating and repressing
neuron function, and its brain isoform, referred to as MCO, was
injected intravenously into Mecp2 knockout mice and was shown to
improve behavioural development (22). However, an increased level of
Mecp2 in liver cells, as a result of an off-target effect, was
observed when a high dose was used for treatment (129), which disrupted liver metabolism
and function (130). Different
types of AAV vectors, such as AAV8, were used to transfect enhanced
green fluorescent protein in astrocytes, which are cells abundantly
present in the mouse striatum. The results demonstrated a high rate
of protein transport due to the high penetrative ability of AAV8 in
brain cells (131), which may be
used to design methods to transfer target genes to astrocytes in
patients with ASD.
The CRISPR/Cas9 genome editing system has been used
in neural and brain cells to deliver specific genes to target
cells. RNP induced Cas9 with Simian vacuolating virus 40 nuclear
localisation sequence in Ai9 tdTomato mouse neural progenitor cells
in the hippocampus, striatum and cortex, and this demonstrated
marked gene editing ability in vitro and in vivo,
indicating successful neuron-specific targeting for future ASD
treatment (132). CRISPR/Cas9
has been used for Huntington's disease to suppress mutant HTT
(mHTT) gene, which is located on chromosome 4 and produces
Huntington's disease protein when mutated. In vivo HTT gene
targeting in HD140Q-KI mice with mHTT using an AVV vector
containing the CRISPR/Cas9 system with four gRNAs resulted in a
significant reduction of mHTT expression and, thus, a reduction in
the expression of Huntington's disease protein in HD140Q-KI mice
(133). Therefore, it may be
possible to design an efficient gene editing tool to treat ASD or
minimise the severity of the symptoms.
One of the most successful studies on Thy1-YFP and
Ai9 mice with X-fragile syndrome resulted in a significant
reduction in repetitive symptoms of X-fragile syndrome (XFS) by
using CRISPR-Gold (CRISPR designed with gold nanoparticles) to
deliver Cas9 or Cpf1 to the striatum via local intracranial
injection. CRISPR system side effects included minimal off-target
effects and no impact on the immune system, unlike AAVs.
Additionally, the metabotropic glutamate receptor subtype 5 gene
was selected as an editing target (134,135), since its signalling was found to
be overactivated in XFS and other ASD syndromes (136,137). The study reported minimal side
effects, indicating successful Cas9 or Cpf1 delivery and gene
editing. The same study used CRSPR-Gold effect for gene editing of
other cell types, such as glial cells, dysfunction of which is
observed in numerous neurological and brain disorders (137,138). Such accomplishments have
provided novel insights into CRISPR genome editing technology, and
may be used to treat other rare diseases.
Although the abilities of CRISPR/Cas9 system are
clearly established and have been used in various applications,
there are concerns regarding off-target mutations, which may limit
its future perspectives. Data from several studies indicate that
the off-target effects of the CRISPR/Cas9 system are among the most
important consequences of this method, regardless of the cell type
and target genes (5,14,16,33,36,43). Hybrid R-loop formation between
sgRNA and the target DNA may result in double-stranded cleavage of
DNA due to RNA-guided nucleases, the recognition of PAM sequences
and the presence of adjacent AAMs (139). Additionally, it was demonstrated
that such activity results in an increased degree and a high volume
of off-target effects by CRISPR/Cas9 during gene treatment,
specifically due to dsDNA break and NHEJ function (140). Various techniques and protocols
have been designed to optimise the low specificity of CRISPR/Cas9
and to promote HDR-based repair over NHEJ, in order to reduce the
mutation rate. Exposure of mini circle-iPSCs to cold shock or low
temperatures after treatment with CRISPR/Cas system resulted in
increased HDR function and, thus, reduced off-target effects.
However, the rate of indel formation was not significantly affected
(15). Another study designed to
reduce the off-target effects investigated changing the ratio of
sgRNA to Cas9 protein, and demonstrated that a higher ratio of
sgRNA to Cas9 resulted in reduced incidence of off-target effects
(139).
Selection of bacteria for harvesting Cas9 markedly
affects the performance of CRISPR/Cas9. For example, several
studies investigated the impact of the CRISPR/Cas9 system using
three different species of bacteria; Streptococcus pyogenes
Cas9 (SpCas9), S. thermophilus Cas9 (St1Cas9) and
SaCas9 (139,141). The analysis of human cells
transfected with Cas9 plasmids from bacteria exhibited increased
activity, as well as reduced mutation rates, compared with SpCas9
and SaCas9 (124). In addition
to the findings mentioned above, the base sequence of the AAM
upstream of PAM plays a key role in sgRNA binding with protospacers
on the target DNA (142). sgRNAs
with a higher ratio of guanine and a lower ratio of adenine are
more stable in binding with target DNA compared with sgRNAs with a
higher ratio of cytosine (143).
Other challenges include plasmids with low
specificity and random integration into the target DNA, which
creates tracking obstacles (139).
Limitations of previous genome editing tools led
scientists to develop the CRISPR system, which has reduced the
undesired effects whilst increasing efficiency compared with
previous methods. CRISPR was designed to reduce off-target effects
caused by mutations as a result of DNA breaks, thus resulting in a
reduction of unwanted errors. CpfI endonuclease was
introduced into the CRISPR system (Class II) to overcome the
challenges mentioned above. CfpI is a single RNA-guided
endonuclease that does not require tracer RNA, and studies using
Cpf1 of Francisella novicida bacterium showed inactivation
of RuvC-like domain avoiding dsDNA cleavage (16). In addition to the findings
mentioned above, Cpf1 creates 5' overhangs, which can efficiently
add a DNA sequence during genome editing via a non-HDR system, in
contrast to Cas9, which forms blunt end cuts on target DNA
(16).
CRPSR has helped overcome the challenges of disease
therapies by locating target genes that are the causes of drug
resistance (88). Techniques,
such as 2CT-CRISPR and dCas9, have increased the efficacy of drug
therapy. Several factors, such as vector/plasmid and CRISPR
selection based on size, have exerted a notable effect on the
efficacy and delivery of CRISPR endonucleases to target genes
(37,45). Therefore, selecting the most
suitable vector with good penetration and a low rate of host immune
activation for CRISPR delivery is required to carefully address the
treatment of various diseases, such as AIDS, haemophilia, ASD and
SCD.
Scientists at the University of Washington used
vectors with CRISPR/Cas9 components from either Streptococcus
pyogenes or Staphylococcus aureus to treat DMD (146). The results demonstrated that the
expression of dystrophin using dual vectors exhibited increased
efficiency compared with a single vector. Furthermore, they
achieved a higher yield with SpCas9 compared with SaCas9, as
demonstrated by the reduction in the off-target effects in both DMD
and SCD (146,147). In addition, certain factors,
such as size and PAM sequence recognition, further improve the
efficiency of SpCas9 compared with SaCas9 for CRISPR/Cas9 therapy
(147). The impact of the
CRISPR/Cas9 system on other diseases has been shown to involve
off-target effects and, eventually, the formation of indels due to
the involvement of NHEJ-based repair, which is associated with an
increased risk of mutations (148).
The effects of CRISPR/Cas9 on humans requires
further investigation, as human iPSCs and mouse iPSCs vary in
response based on the specific type of CRISPR system used.
Furthermore, the issue of indels as a result of NHEJ-based repair
must be reduced in order to reduce the off-target effects caused by
indels. Therefore, studies are focusing on designing a CRISPR/Cas
system for gene editing with a lower risk of mutations utilizing
HDR.
Numerous studies have demonstrated the use of
CRISPR, ZFNs and TALENS as powerful gene editing tools. Although
ZFNs and TALENS represent important advances in gene editing, their
capacity is currently limited for effective use. The discovery of
CRISPR, which exhibits higher efficiency and fewer off-target
effects, has provided opportunities for scientists to use this
technique widely and develop CRISPR-based gene therapy. However,
the issue of off-target effects must be addressed and, thus, should
be the focus of future studies, with the aim of further developing
this technology for use in human gene therapy.
No funding was received.
Not applicable.
MA drafted the paper and designed the manuscript
structure. NK drafted the manuscript and arranged the data of the
studies. Both authors have read and approved the final version of
the manuscript for publication.
Not applicable.
Not applicable.
All the authors declare that they have no competing
interests.
Not applicable.
|
1
|
Ishino Y, Shinagawa H, Makino K, Amemura M
and Nakata A: Nucleotide sequence of the iap gene, responsible for
alkaline phosphatase isozyme conversion in Escherichia coli, and
identification of the gene product. J Bacteriol. 169:5429–5433.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sorek R, Lawrence CM and Wiedenheft B:
CRISPR-mediated adaptive immune systems in bacteria and archaea.
Annu Rev Biochem. 82:237–266. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Karginov FV and Hannon GJ: The CRISPR
system: Small RNA-guided defense in bacteria and archaea. Mol Cell.
37:7–19. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Barrangou R and Marraffini LA: CRISPR-Cas
systems: Prokaryotes upgrade to adaptive immunity. Mol Cell.
54:234–244. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cong L, Ran FA, Cox D, Lin S, Barretto R,
Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA and Zhang F:
Multiplex genome engineering using CRISPR/Cas systems. Science.
339:819–823. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Barrangou R, Fremaux C, Deveau H, Richards
M, Boyaval P, Moineau S, Romero DA and Horvath P: CRISPR provides
acquired resistance against viruses in prokaryotes. Science.
315:1709–1712. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tang TH, Bachellerie JP, Rozhdestvensky T,
Bortolin ML, Huber H, Drungowski M, Elge T, Brosius J and
Hüttenhofer A: Identification of 86 candidates for small
non-messenger RNAs from the archaeon Archaeoglobus fulgidus. Proc
Natl Acad Sci USA. 99:7536–7541. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hefferin ML and Tomkinson AE: Mechanism of
DNA double-strand break repair by non-homologous end joining. DNA
Repair (Amst). 4:639–648. 2005. View Article : Google Scholar
|
|
9
|
Davis AJ and Chen DJ: DNA double strand
break repair via non-homologous end-joining. Transl Cancer Res.
2:130–143. 2013.PubMed/NCBI
|
|
10
|
Bibikova M, Golic M, Golic KG and Carroll
GD: Targeted chromosomal cleavage and mutagenesis in Drosophila
using zinc-finger nucleases. Genetics. 161:1169–1175.
2002.PubMed/NCBI
|
|
11
|
Takata M, Sasaki MS, Sonoda E, Morrison C,
Hashimoto M, Utsumi H, Yamaguchi-Iwai Y, Shinohara A and Takeda S:
Homologous recombination and non-homologous end-joining pathways of
DNA double-strand break repair have overlapping roles in the
maintenance of chromosomal integrity in vertebrate cells. EMBO J.
17:5497–5508. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Long C, McAnally JR, Shelton JM, Mireault
AA, Bassel-Duby R and Olson EN: Prevention of muscular dystrophy in
mice by CRISPR/Cas9-mediated editing of germline DNA. Science.
345:1184–1188. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hoban MD, Cost GJ, Mendel MC, Romero Z,
Kaufman ML, Joglekar AV, Ho M, Lumaquin D, Gray D, Lill GR, et al:
Correction of the sickle cell disease mutation in human
hematopoietic stem/progenitor cells. Blood. 125:2597–2604. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guan Y, Ma Y, Li Q, Sun Z, Ma L, Wu L,
Wang L, Zeng L, Shao Y, Chen Y, et al: CRISPR/Cas9-mediated somatic
correction of a novel coagulator factor IX gene mutation
ameliorates hemophilia in mouse. EMBO Mol Med. 8:477–488. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guo Q, Mintier G, Ma-Edmonds M, Storton D,
Wang X, Xiao X, Kienzle B, Zhao D and Feder JN: 'Cold shock'
increases the frequency of homology directed repair gene editing in
induced pluripotent stem cells. Sci Rep. 8:20802018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zetsche B, Gootenberg JS, Abudayyeh OO,
Slaymaker IM, Makarova KS, Essletzbichler P, Volz SE, Joung J, van
der Oost J, Regev A, et al: Cpf1 is a single RNA-guided
endonuclease of a class 2 CRISPR-Cas system. Cell. 163:759–771.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Miller J, McLachlan AD and Klug A:
Repetitive zinc-binding domains in the protein transcription factor
IIIA from Xenopus oocytes. EMBO J. 4:1609–1614. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pavletich NP and Pabo CO: Zinc finger-DNA
recognition: Crystal structure of a Zif268-DNA complex at 2.1 A.
Science. 252:809–817. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wolfe SA, Nekludova L and Pabo CO: DNA
recognition by Cys2His2 zinc finger proteins. Annu Rev Biophys
Biomol Struct. 29:183–212. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Maeder ML, Thibodeau-Beganny S, Osiak A,
Wright DA, Anthony RM, Eichtinger M, Jiang T, Foley JE, Winfrey RJ,
Townsend JA, et al: Rapid 'open-source' engineering of custom-ized
zinc-finger nucleases for highly efficient gene modification. Mol
Cell. 31:294–301. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Maeder ML, Thibodeau-Beganny S, Sander JD,
Voytas DF and Joung JK: Oligomerized pool engineering (OPEN): An
'open-source' protocol for making customized zinc-finger arrays.
Nat Protoc. 4:1471–1501. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pattanayak V, Ramirez CL, Joung JK and Liu
DR: Revealing off-target cleavage specificities of zinc-finger
nucleases by in vitro selection. Nat Methods. 8:765–770. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gabriel R, Lombardo A, Arens A, Miller JC,
Genovese P, Kaeppel C, Nowrouzi A, Bartholomae CC, Wang J, Friedman
G, et al: An unbiased genome-wide analysis of zinc-finger nuclease
specificity. Nat Biotechnol. 29:816–823. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Doyon Y, Vo TD, Mendel MC, Greenberg SG,
Wang J, Xia DF, Miller JC, Urnov FD, Gregory PD and Holmes MC:
Enhancing zinc-finger-nuclease activity with improved obligate
heterodimeric architectures. Nat Methods. 8:74–79. 2011. View Article : Google Scholar
|
|
25
|
Szczepek M, Brondani V, Buchel J, Serrano
L, Segal DJ and Cathomen T: Structure-based redesign of the
dimerization interface reduces the toxicity of zinc-finger
nucleases. Nat Biotechnol. 25:786–793. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Miller JC, Holmes MC, Wang J, Guschin DY,
Lee YL, Rupniewski I, Beausejour CM, Waite AJ, Wang NS, Kim KA, et
al: An improved zinc-finger nuclease architecture for highly
specific genome editing. Nat Biotechnol. 25:778–785. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bogdanove AJ, Schornack S and Lahaye T:
TAL effectors: Finding plant genes for disease and defense. Curr
Opin Plant Biol. 13:394–401. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Scholze H and Boch J: TAL effectors are
remote controls for gene activation. Curr Opin Microbiol. 14:47–53.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Christian M, Cermak T, Doyle EL, Schmidt
C, Zhang F, Hummel A, Bogdanove AJ and Voytas DF: Targeting DNA
double-strand breaks with TAL effector nucleases. Genetics.
186:757–761. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Miller JC, Tan S, Qiao G, Barlow KA, Wang
J, Xia DF, Meng X, Paschon DE, Leung E, Hinkley SJ, et al: A TALE
nuclease archi-tecture for efficient genome editing. Nat
Biotechnol. 29:143–148. 2011. View Article : Google Scholar
|
|
31
|
Zhu H, Lau CH, Goh SL, Liang Q, Chen C, Du
S, Phang RZ, Tay FC, Tan WK, Li Z, et al: Baculoviral transduction
facilitates TALEN-mediated targeted transgene integration and
Cre/LoxP cassette exchange in human-induced pluripotent stem cells.
Nucleic Acids Res. 41:e1802013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mussolino C, Alzubi J, Fine EJ, Morbitzer
R, Cradick TJ, Lahaye T, Bao G and Cathomen T: TALENs facilitate
targeted genome editing in human cells with high specificity and
low cytotoxicity. Nucleic Acids Res. 42:6762–6773. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gupta RM and Musunuru K: Expanding the
genetic editing tool kit: ZFNs, TALENs, and CRISPR-Cas9. J Clin
Invest. 124:4154–4161. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Deltcheva E, Chylinski K, Sharma CM,
Gonzales K, Chao Y, Pirzada ZA, Eckert MR, Vogel J and Charpentier
E: CRISPR RNA maturation by trans-encoded small RNA and host factor
RNase III. Nature. 471:602–607. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jinek M, Chylinski K, Fonfara I, Hauer M,
Doudna JA and Charpentier E: A programmable dual-RNA-guided DNA
endonu-clease in adaptive bacterial immunity. Science. 337:816–821.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liang P, Xu Y, Zhang X, Ding C, Huang R,
Zhang Z, Lv J, Xie X, Chen Y, Li Y, et al: CRISPR/Cas9-mediated
gene editing in human tripronuclear zygotes. Protein Cell.
6:363–372. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shmakov S, Smargon A, Scott D, Cox D,
Pyzocha N, Yan W, Abudayyeh OO, Gootenberg JS, Makarova KS, Wolf
YI, et al: Diversity and evolution of class 2 CRISPR-Cas systems.
Nat Rev Microbiol. 15:169–182. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shabbir MA, Hao H, Shabbir MZ, Hussain HI,
Iqbal Z, Ahmed S, Sattar A, Iqbal M, Li J and Yuan Z: Survival and
evolution of CRISPR-Cas system in prokaryotes and its applications.
Front Immunol. 7:3752016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sander JD and Joung JK: CRISPR-Cas systems
for editing, regulating and targeting genomes. Nat Biotechnol.
32:347–355. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Makarova KS, Haft DH, Barrangou R, Brouns
SJ, Charpentier E, Horvath P, Moineau S, Mojica FJ, Wolf YI,
Yakunin AF, et al: Evolution and classification of the CRISPR/Cas
systems. Nat Rev Microbiol. 9:467–477. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Makarova KS, Wolf YI, Alkhnbashi OS, Costa
F, Shah SA, Saunders SJ, Barrangou R, Brouns SJ, Charpentier E,
Haft DH, et al: An updated evolutionary classification of
CRISPR/Cas systems. Nat Rev Microbiol. 13:722–736. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Brouns SJ, Jore MM, Lundgren M, Westra ER,
Slijkhuis RJ, Snijders AP, Dickman MJ, Makarova KS, Koonin EV and
van der Oost J: Small CRISPR RNAs guide antiviral defense in
prokaryotes. Science. 321:960–964. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Haurwitz RE, Jinek M, Wiedenheft B, Zhou K
and Doudna JA: Sequence- and structure-specific RNA processing by a
CRISPR endonuclease. Science. 329:1355–1358. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sashital DG, Wiedenheft B and Doudna JA:
Mechanism of foreign DNA selection in a bacterial adaptive immune
system. Mol Cell. 46:606–615. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sinkunas T, Gasiunas G, Waghmare SP,
Dickman MJ, Barrangou R, Horvath P and Siksnys V: In vitro
reconstitution of Cascade-mediated CRISPR immunity in Streptococcus
thermophilus. EMBO J. 32:385–394. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Westra ER, van Erp PB, Künne T, Wong SP,
Staals RH, Seegers CL, Bollen S, Jore MM, Semenova E, Severinov K,
et al: CRISPR immunity relies on the consecutive binding and
degradation of negatively supercoiled invader DNA by Cascade and
Cas3. Mol Cell. 46:595–605. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Szczelkun MD, Tikhomirova MS, Sinkunas T,
Gasiunas G, Karvelis T, Pschera P, Siksnys V and Seidel R: Direct
observation of R-loop formation by single RNA-guided Cas9 and
Cascade effector complexes. Proc Natl Acad Sci USA. 111:9798–9803.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Semenova E, Jore MM, Datsenko KA, Semenova
A, Westra ER, Wanner B, van der Oost J, Brouns SJ and Severinov K:
Interference by clustered regularly interspaced short palindromic
repeat (CRISPR) RNA is governed by a seed sequence. Proc Natl Acad
Sci USA. 108:10098–11103. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hochstrasser ML, Taylor DW, Bhat P,
Guegler CK, Sternberg SH, Nogales E and Doudna JA: CasA mediates
Cas3-catalyzed target degradation during CRISPR RNA-guided
interference. Proc Natl Acad Sci USA. 111:6618–6623. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Nishimasu H, Ran FA, Hsu PD, Konermann S,
Shehata SI, Dohmae N, Ishitani R, Zhang F and Nureki O: Crystal
structure of Cas9 in complex with guide RNA and target DNA. Cell.
156:935–949. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Gasiunas G, Barrangou R, Horvath P and
Siksnys V: Cas9-crRNA ribonucleoprotein complex mediates specific
DNA cleavage for adaptive immunity in bacteria. Proc Natl Acad Sci
USA. 109:E2579–E2586. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Jiang W, Bikard D, Cox D, Zhang F and
Marraffini LA: RNA-guided editing of bacterial genomes using
CRISPR/Cas systems. Nat Biotechnol. 31:233–239. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Sternberg SH, Redding S, Jinek M, Greene
EC and Doudna JA: DNA interrogation by the CRISPR RNA-guided
endonuclease Cas9. Nature. 507:62–67. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Carte J, Wang R, Li H, Terns RM and Terns
MP: Cas6 is an endoribonuclease that generates guide RNAs for
invader defense in prokaryotes. Genes Dev. 22:3489–3496. 2008.
View Article : Google Scholar
|
|
55
|
Chen F, Ding X, Feng Y, Seebeck T, Jiang Y
and Davis GD: Targeted activation of diverse CRISPR-Cas systems for
mammalian genome editing via proximal CRISPR targeting. Nat Commun.
8:149582017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Hatoum-Aslan A, Maniv I and Marraffini LA:
Mature clustered, regularly interspaced, short palindromic repeats
RNA (crRNA) length is measured by a ruler mechanism anchored at the
precursor processing site. Proc Natl Acad Sci USA. 108:21218–21222.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Peng W, Feng M, Feng X, Liang YX and She
Q: An archaeal CRISPR type III-B system exhibiting distinctive RNA
targeting features and mediating dual RNA and DNA interference.
Nucleic Acids Res. 43:406–417. 2015. View Article : Google Scholar :
|
|
58
|
Samai P, Pyenson N, Jiang W, Goldberg GW,
Hatoum-Aslan A and Marraffini LA: Co-transcriptional DNA and RNA
Cleavage during Type III CRISPR/Cas Immunity. Cell. 161:1164–1174.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Staals RH, Zhu Y, Taylor DW, Kornfeld JE,
Sharma K, Barendregt A, Koehorst JJ, Vlot M, Neupane N, Varossieau
K, et al: RNA targeting by the type III-A CRISPR/Cas Csm complex of
thermus thermophilus. Mol Cell. 56:518–530. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Tamulaitis G, Kazlauskiene M, Manakova E,
Venclovas Č, Nwokeoji AO, Dickman MJ, Horvath P and Siksnys V:
Programmable RNA shredding by the type III-A CRISPR-Cas system of
streptococcus thermophilus. Mol Cell. 56:506–517. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Cicero CE, Mostile G, Vasta R, Rapisarda
V, Signorelli SS, Ferrante M, Zappia M and Nicoletti A: Metals and
neurodegenerative diseases. A systematic review. Environ Res.
159:82–94. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Bertram L, McQueen MB, Mullin K, Blacker D
and Tanzi RE: Systematic meta-analyses of Alzheimer disease genetic
association studies: The AlzGene database. Nat Genet. 39:17–23.
2007. View
Article : Google Scholar
|
|
63
|
Armstrong RA: What causes Alzheimer's
disease? Folia Neuropathol. 51:169–188. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Heidenreich M and Zhang F: Applications of
CRISPR/Cas systems in neuroscience. Nat Rev Neurosci. 17:36–44.
2016. View Article : Google Scholar
|
|
65
|
Levy-Lahad E, Wasco W, Poorkaj P, Romano
DM, Oshima J, Pettingell WH, Yu CE, Jondro PD, Schmidt SD, Wang K,
et al: Candidate gene for the chromosome 1 familial Alzheimer's
disease locus. Science. 269:973–977. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Tomita T, Maruyama K, Saido TC, Kume H,
Shinozaki K, Tokuhiro S, Capell A, Walter J, Grünberg J, Haass C,
et al: The presenilin 2 mutation (N141I) linked to familial
Alzheimer disease (Volga German families) increases the secretion
of amyloid beta protein ending at the 42nd (or 43rd) residue. Proc
Natl Acad Sci USA. 94:2025–2030. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Sachse CC, Kim YH, Agsten M, Huth T,
Alzheimer C, Kovacs DM and Kim DY: BACE1 and presenilin/γ-secretase
regulate proteolytic processing of KCNE1 and 2, auxiliary subunits
of voltage-gated potassium channels. FASEB J. 27:2458–2467. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Stutzmann GE, Caccamo A, LaFerla FM and
Parker I: Dysregulated IP3 signaling in cortical neurons of
knock-in mice expressing an Alzheimer's-linked mutation in
presenilin1 results in exaggerated Ca2+ signals and
altered membrane excitability. J Neurosci. 24:508–513. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Yue W, Li Y, Zhang T, Jiang M, Qian Y,
Zhang M, Sheng N, Feng S, Tang K, Yu X, et al: ESC-derived basal
forebrain cholinergic neurons ameliorate the cognitive symptoms
associated with Alzheimer's disease in mouse models. Stem Cell
Reports. 5:776–790. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Paull D, Sevilla A, Zhou H, Hahn AK, Kim
H, Napolitano C, Tsankov A, Shang L, Krumholz K, Jagadeesan P, et
al: Automated, high-throughput derivation, characterization and
differentiation of induced pluripotent stem cells. Nat Methods.
12:885–892. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Ortiz-Virumbrales M, Moreno CL, Kruglikov
I, Marazuela P, Sproul A, Jacob S, Zimmer M, Paull D, Zhang B,
Schadt EE, et al: CRISPR/Cas9-Correctable mutation-related
molecular and phys-iological phenotypes in iPSC-derived Alzheimer's
PSEN2N141I neurons. Acta Neuropathol Commun. 5:772017.
View Article : Google Scholar
|
|
72
|
Liu C, Zhang L, Liu H and Cheng K:
Delivery strategies of the CRISPR/Cas9 gene-editing system for
therapeutic applications. J Control Release. 266:17–26. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
György B, Lööv C, Zaborowski MP, Takeda S,
Kleinstiver BP, Commins C, Kastanenka K, Mu D, Volak A, Giedraitis
V, et al: CRISPR/Cas9 mediated disruption of the swedish app allele
as a therapeutic approach for early-onset Alzheimer's disease. Mol
Ther Nucleic Acids. 11:429–440. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Mirzaei HR, Sahebkar A and Salehi R,
Nahand JS, Karimi E, Jaafari MR, Mirzaei H, Sahebkar A and Salehi
R: Boron neutron capture therapy: Moving toward targeted cancer
therapy. J Cancer Res Ther. 12:520–525. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Pourhanifeh MH, Mohammadi R, Noruzi S,
Hosseini SA, Fanoudi S, Mohamadi Y, Hashemzehi M, Asemi Z, Mirzaei
HR, Salarinia R and Mirzaei H: The role of fibromodulin in cancer
pathogenesis: Implications for diagnosis and therapy. Cancer Cell
Int. 19:1572019. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Shafabakhsh R, Pourhanifeh MH, Mirzaei HR,
Sahebkar A, Asemi Z and Mirzaei H: Targeting regulatory T cells by
curcumin: A potential for cancer immunotherapy. Pharmacol Res.
147:1043532019. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Scott A: How CRISPR is transforming drug
discovery. Nature. 555:S10–S11. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Walton J, Blagih J, Ennis D, Leung E,
Dowson S, Farquharson M, Tookman LA, Orange C, Athineos D, Mason S,
et al: CRISPR/Cas9-mediated Trp53 and Brca2 knockout to generate
improved murine models of ovarian high-grade serous carcinoma.
Cancer Res. 76:6118–6129. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Cruz C, Castroviejo-Bermejo M,
Gutiérrez-Enríquez S, Llop-Guevara A, Ibrahim YH, Gris-Oliver A,
Bonache S, Morancho B, Bruna A, Rueda OM, et al: RAD51 foci as a
functional biomarker of homologous recombination repair and PARP
inhibitor resistance in germline BRCA-mutated breast cancer. Ann
Oncol. 29:1203–1210. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Chen B, Gilbert LA, Cimini BA,
Schnitzbauer J, Zhang W, Li GW, Park J, Blackburn EH, Weissman JS,
Qi LS and Huang B: Dynamic imaging of genomic loci in living human
cells by an optimized CRISPR/Cas system. Cell. 155:1479–1491. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Klann TS, Black JB, Chellappan M, Safi A,
Song L, Hilton IB, Crawford GE, Reddy TE and Gersbach CA:
CRISPR-Cas9 epigenome editing enables high-throughput screening for
functional regulatory elements in the human genome. Nat Biotechnol.
35:561–568. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Brabetz O, Alla V, Angenendt L, Schliemann
C, Berdel WE, Arteaga MF and Mikesch JH: RNA-guided CRISPR/Cas9
system-mediated engineering of acute myeloid leukemia mutations.
Mol Ther Nucleic Acids. 6:243–248. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Xu J, Zwaigenbaum L, Szatmari P and
Scherer SW: Molecular cytogenetics of autism. Curr Genomics.
5:347–364. 2004. View Article : Google Scholar
|
|
84
|
Peña-Martínez P, Eriksson M, Ramakrishnan
R, Chapellier M, Högberg C, Orsmark-Pietras C, Richter J, Andersson
A, Fioretos T and Järås M: Interleukin 4 induces apoptosis of acute
myeloid leukemia cells in a Stat6-dependent manner. Leukemia.
32:588–596. 2018. View Article : Google Scholar :
|
|
85
|
Patel SJ, Sanjana NE, Kishton RJ,
Eidizadeh A, Vodnala SK, Cam M, Gartner JJ, Jia L, Steinberg SM,
Yamamoto TN, et al: Identification of essential genes for cancer
immunotherapy. Nature. 548:537–542. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Mirzaei H, Yazdi F, Salehi R and Mirzaei
HR: SiRNA and epigenetic aberrations in ovarian cancer. J Canc Res
Ther. 12:498–508. 2016. View Article : Google Scholar
|
|
87
|
Mirzaei HR, Pourghadamyari H, Rahmati M,
Mohammadi A, Nahand JS, Rezaei A, Mirzaei H and Hadjati J:
Gene-knocked out chimeric antigen receptor (CAR) T cells: Tuning up
for the next generation cancer immunotherapy. Cancer Lett.
423:95–104. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Dai WJ, Zhu LY, Yan ZY, Xu Y, Wang QL and
Lu XJ: CRISPR-Cas9 for in vivo gene therapy: Promise and hurdles.
Mol Ther Nucleic Acids. 5:e3492016. View Article : Google Scholar :
|
|
89
|
Mirzaei HR, Jamali A, Jafarzadeh L,
Masoumi E, Alishah K, Fallah Mehrjardi K, Emami SAH, Noorbakhsh F,
Till BG and Hadjati J: Construction and functional characterization
of a fully human anti-CD19 chimeric antigen receptor
(huCAR)-expressing primary human T cells. J Cell Physiol.
234:9207–9215. 2019. View Article : Google Scholar
|
|
90
|
Gomes-Silva D, Mukherjee M, Srinivasan M,
Krenciute G, Dakhova O, Zheng Y, Cabral JMS, Rooney CM, Orange JS,
Brenner MK and Mamonkin M: Tonic 4-1BB costimulation in chimeric
antigen receptors impedes T Cell survival and is vector-dependent.
Cell Rep. 21:17–26. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Mirzaei HR, Mirzaei H, Lee SY, Hadjati J
and Till BG: Prospects for chimeric antigen receptor (CAR) γδ T
cells: A potential game changer for adoptive T cell cancer
immunotherapy. Cancer Lett. 380:413–423. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Mirzaei HR, Mirzaei H, Namdar A, Rahmati
M, Till BG and Hadjati J: Predictive and therapeutic biomarkers in
chimeric antigen receptor T-cell therapy: A clinical perspective. J
Cell Physiol. 234:5827–5841. 2019. View Article : Google Scholar
|
|
93
|
Mirzaei HR, Rodriguez A, Shepphird J,
Brown CE and Badie B: Corrigendum: Chimeric antigen receptors T
cell therapy in solid tumor: Challenges and clinical applications.
Front Immunol. 10:7802019. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Mah JK: Current and emerging treatment
strategies for Duchenne muscular dystrophy. Neuropsychiatr Dis
Treat. 12:1795–1807. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Lee SH, Lee JH, Lee KA and Choi YC:
Clinical and genetic characterization of female dystrophinopathy. J
Clin Neurol. 11:248–251. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Bladen CL, Salgado D, Monges S, Foncuberta
ME, Kekou K, Kosma K, Dawkins H, Lamont L, Roy AJ, Chamova T, et
al: The TREAT-NMD DMD Global Database: Analysis of more than 7,000
Duchenne muscular dystrophy mutations. Hum Mutat. 36:395–402. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Mendell JR, Kissel J, Amato AA, King W,
Signore L, Prior TW, Sahenk Z, Benson S, McAndrew PE, Rice R, et
al: Myoblast transfer in the treatment of Duchenne's muscular
dystrophy. N Engl J Med. 333:832–838. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Montarras D, Morgan J, Collins C, Relaix
F, Zaffran S, Cumano A, Partridge T and Buckingham M: Direct
isolation of satellite cells for skeletal muscle regeneration.
Science. 309:2064–2067. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Darabi R, Arpke RW, Irion S, Dimos JT,
Grskovic M, Kyba M and Perlingeiro RC: Human ES- and iPS-derived
myogenic progenitors restore DYSTROPHIN and improve contractility
upon transplantation in dystrophic mice. Cell Stem Cell.
10:610–619. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Shimizu-Motohashi Y, Miyatake S, Komaki H,
Takeda S and Aoki Y: Recent advances in innovative therapeutic
approaches for Duchenne muscular dystrophy: From discovery to
clinical trials. Am J Transl Res. 8:2471–2489. 2016.PubMed/NCBI
|
|
101
|
Li HL, Fujimoto N, Sasakawa N, Shirai S,
Ohkame T, Sakuma T, Tanaka M, Amano N, Watanabe A, Sakurai H, et
al: Precise correction of the dystrophin gene in duchenne muscular
dystrophy patient induced pluripotent stem cells by TALEN and
CRISPR-Cas9. Stem Cell Reports. 4:143–154. 2015. View Article : Google Scholar :
|
|
102
|
Young CS, Hicks MR, Ermolova NV, Nakano H,
Jan M, Younesi S, Karumbayaram S, Kumagai-Cresse C, Wang D, Zack
JA, et al: A single CRISPR/Cas9 deletion strategy that targets the
majority of DMD patients restores dystrophin function in
hiPSC-derived muscle cells. Cell Stem Cell. 18:533–540. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Cacchiarelli D, Incitti T, Martone J,
Cesana M, Cazzella V, Santini T, Sthandier O and Bozzoni I: miR-31
modulates dystro-phin expression: New implications for Duchenne
muscular dystrophy therapy. EMBO Rep. 12:136–114. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Marmor M, Sheppard HW, Donnell D, Bozeman
S, Celum C, Buchbinder S and Koblin B: Homozygous and heterozygous
CCR5-Delta32 genotypes are associated with resistance to HIV
infection. J Acquir Immune Defic Syndr. 27:472–481. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Liu R, Paxton WA, Choe S, Ceradini D,
Martin SR, Horuk R, MacDonald ME, Stuhlmann H, Koup RA and Landau
NR: Homozygous defect in HIV-1 coreceptor accounts for resistance
of some multiply-exposed individuals to HIV-1 infection. Cell.
86:367–377. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Hütter G, Nowak D, Mossner M, Ganepola S,
Müssig A, Allers K, Schneider T, Hofmann J, Kücherer C, Blau O, et
al: Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell
transplantation. N Engl J Med. 360:692–698. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Tebas P, Stein D, Tang WW, Frank I, Wang
SQ, Lee G, Spratt SK, Surosky RT, Giedlin MA, Nichol G, et al: Gene
editing of CCR5 in autologous CD4 T cells of persons infected with
HIV. N Engl J Med. 370:901–910. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
DiGiusto DL, Cannon PM, Holmes MC, Li L,
Rao A, Wang J, Lee G, Gregory PD, Kim KA, Hayward SB, et al:
Preclinical development and qualification of ZFN-mediated CCR5
disruption in human hematopoietic stem/progenitor cells. Mol Ther
Methods Clin Dev. 3:160672016. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Li L, Krymskaya L, Wang J, Henley J, Rao
A, Cao LF, Tran C A, Torres-Coronado M, Gardner A, Gonzalez N, et
al: Genomic editing of the HIV-1 coreceptor CCR5 in adult
hematopoietic stem and progenitor cells using zinc finger
nucleases. Mol Ther. 21:1259–1269. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Holt N, Wang J, Kim K, Friedman G, Wang X,
Taupin V, Crooks GM, Kohn DB, Gregory PD, Holmes MC and Cannon PM:
Human hematopoietic stem/progenitor cells modified by zinc-finger
nucleases targeted to CCR5 control HIV-1 in vivo. Nat Biotechnol.
28:839–847. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Kumar P, Ban HS, Kim SS, Wu H, Pearson T,
Greiner DL, Laouar A, Yao J, Haridas V, Habiro K, et al: T
cell-specific siRNA delivery suppresses HIV-1 infection in
humanized mice. Cell. 134:577–586. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Ishikawa F, Yasukawa M, Lyons B, Yoshida
S, Miyamoto T, Yoshimoto G, Watanabe T, Akashi K, Shultz LD and
Harada M: Development of functional human blood and immune systems
in NOD/SCID/IL2 receptor {gamma} chain(null) mice. Blood.
106:1565–1573. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Cavazzana-Calvo M, Payen E, Negre O, Wang
G, Hehir K, Fusil F, Down J, Denaro M, Brady T, Westerman K, et al:
Transfusion independence and HMGA2 activation after gene therapy of
human β-thalassaemia. Nature. 467:318–322. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Ye L, Wang J, Beyer AI, Teque F, Cradick
TJ, Qi Z, Chang JC, Bao G, Muench MO, Yu J, et al: Seamless
modification of wild-type induced pluripotent stem cells to the
natural CCR5Δ32 mutation confers resistance to HIV infection. Proc
Natl Acad Sci USA. 111:9591–9596. 2014. View Article : Google Scholar
|
|
115
|
Hsu PD, Lander ES and Zhang F: Development
and applications of CRISPR/Cas9 for genome engineering. Cell.
157:1262–1278. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Hütter G: Stem cell transplantation in
strategies for curing HIV/AIDS. AIDS Res Ther. 13:312016.
View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Kaminski R, Chen Y, Fischer T, Tedaldi E,
Napoli A, Zhang Y, Karn J, Hu W and Khalili K: Elimination of HIV-1
genomes from human T-lymphoid cells by CRISPR/Cas9 gene editing.
Sci Rep. 6:225552016. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Huang Z and Nair M: A CRISPR/Cas9 guidance
RNA screen platform for HIV provirus disruption and HIV/AIDS gene
therapy in astrocytes. Sci Rep. 7:59552017. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Lervolino LG, Baldin PE, Picado SM, Calil
KB, Viel AA and Campos LA: Prevalence of sickle cell disease and
sickle cell trait in national neonatal screening studies. Rev Bras
Hematol Hemoter. 33:49–54. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Cradick TJ, Fine EJ, Antico CJ and Bao G:
CRISPR/Cas9 systems targeting β-globin and CCR5 genes have
substantial off-target activity. Nucleic Acids Res. 41:9584–9592.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
DeWitt MA, Magis W, Bray NL, Wang T,
Berman JR, Urbinati F, Heo SJ, Mitros T, Muñoz DP, Boffelli D, et
al: Selection-free genome editing of the sickle mutation in human
adult hematopoietic stem/progenitor cells. Sci Transl Med.
8:360ra1342016. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Stephens CJ, Kashentseva E, Everett W,
Kaliberova L and Curiel DT: Targeted in vivo knock-in of human
alpha-1-anti-trypsin cDNA using adenoviral delivery of CRISPR/Cas9.
Gene Ther. 25:139–156. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Ohmori T, Nagao Y, Mizukami H, Sakata A,
Muramatsu SI, Ozawa K, Tominaga SI, Hanazono Y, Nishimura S, Nureki
O and Sakata Y: CRISPR/Cas9-mediated genome editing via post-natal
administration of AAV vector cures haemophilia B mice. Sci Rep.
7:41592017. View Article : Google Scholar
|
|
124
|
Bergmann T, Ehrke-Schulz E, Gao J, Schiwon
M, Schildgen V, David S, Schildgen O and Ehrhardt A: Designer
nuclease-medi-ated gene correction via homology-directed repair in
an in vitro model of canine hemophilia. B J Gene Med. 20:e30202018.
View Article : Google Scholar
|
|
125
|
Lyu C, Shen J, Wang R, Gu H, Zhang J, Xue
F, Liu X, Liu W, Fu R, Zhang L, et al: Targeted genome engineering
in human induced pluripotent stem cells from patients with
hemophilia B using the CRISPR-Cas9 system. Stem Cell Res Ther.
9:922018. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Jacquemont ML, Sanlaville D, Redon R,
Raoul O, Cormier-Daire V, Lyonnet S, Amiel J, Le Merrer M, Heron D,
de Blois MC, et al: Array-based comparative genomic hybridisation
identifies high frequency of cryptic chromosomal rearrangements in
patients with syndromic autism spectrum disorders. J Med Genet.
43:843–849. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Gray SJ, Matagne V, Bachaboina L, Yadav S,
Ojeda SR and Samulski RJ: Preclinical differences of intravascular
AAV9 delivery to neurons and glia: A comparative study of adult
mice and nonhuman primates. Mol Ther. 19:1058–1069. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Amir RE, Van den Veyver IB, Wan M, Tran
CQ, Francke U and Zoghbi HY: Rett syndrome is caused by mutations
in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat
Genet. 23:185–188. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
129
|
Matagne V, Ehinger Y, Saidi L,
Borges-Correia A, Barkats M, Bartoli M, Villard L and Roux JC: A
codon-optimized Mecp2 transgene corrects breathing deficits and
improves survival in a mouse model of Rett syndrome. Neurobiol Dis.
99:1–11. 2017. View Article : Google Scholar
|
|
130
|
Kyle SM, Saha PK, Brown HM, Chan LC and
Justice MJ: MeCP2 co-ordinates liver lipid metabolism with the
NCoR1/HDAC3 corepressor complex. Hum Mol Genet. 25:3029–3041.
2016.PubMed/NCBI
|
|
131
|
Pignataro D, Sucunza D, Vanrell L,
Lopez-Franco E, Dopeso-Reyes IG, Vales A, Hommel M, Rico AJ,
Lanciego JL and Gonzalez-Aseguinolaza G: Adeno-associated viral
vectors serotype 8 for cell-specific delivery of therapeutic genes
in the central nervous system. Front Neuroanat. 11:122017.
View Article : Google Scholar
|
|
132
|
Staahl BT, Benekareddy M, Coulon-Bainier
C, Banfal AA, Floor SN, Sabo JK, Urnes C, Munares GA, Ghosh A and
Doudna JA: Efficient genome editing in the mouse brain by local
delivery of engineered Cas9 ribonucleoprotein complexes. Nat
Biotechnol. 35:431–434. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Yang S, Chang R, Yang H, Zhao T, Hong Y,
Kong HE, Sun X, Qin Z, Jin P, Li S and Li XJ: CRISPR/Cas9-mediated
gene editing ameliorates neurotoxicity in mouse model of
Huntington's disease. J Clin Invest. 127:2719–2724. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Lee B, Lee K, Panda S, Gonzales-Rojas R,
Chong A, Bugay V, Park HM, Brenner R, Murthy N and Lee HY:
Nanoparticle delivery of CRISPR into the brain rescues a mouse
model of fragile X syndrome from exaggerated repetitive behaviours.
Nat Biomed Eng. 2:497–507. 2018. View Article : Google Scholar
|
|
135
|
Silverman JL, Smith DG, Rizzo SJ, Karras
MN, Turner SM, Tolu SS, Bryce DK, Smith DL, Fonseca K, Ring RH and
Crawley JN: Negative allosteric modulation of the mGluR5 receptor
reduces repetitive behaviors and rescues social deficits in mouse
models of autism. Sci Transl Med. 4:131ra512012. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Tao J, Wu H, Coronado AA, de Laittre E,
Osterweil EK, Zhang Y and Bear MF: Negative allosteric modulation
of mGluR5 partially corrects pathophysiology in a mouse model of
Rett syndrome. J Neurosci. 36:11946–11958. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Almad AA and Maragakis NJ: Glia: An
emerging target for neurological disease therapy. Stem Cell Res
Ter. 3:372012. View Article : Google Scholar
|
|
138
|
Alagoz M, Kherad N, Gavaz M and Yuksel A:
New genetic approaches for early diagnosis and treatment of autism
spectrum disorders. Rev J Autism Dev Disoed. 6:367–380. 2019.
View Article : Google Scholar
|
|
139
|
Kim S, Kim D, Cho SW, Kim J and Kim JS:
Highly efficient RNA-guided genome editing in human cells via
delivery of purified Cas9 ribonucleoproteins. Genome Res.
24:1012–1019. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Klein M, Eslami-Mossallam B, Arroyo DG and
Depken M: Hybridization kinetics explains CRISPR/Cas Off-targeting
rules. Cell Rep. 22:1413–1423. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Chari R, Mali P, Moosburner M and Church
GM: Unraveling CRISPR-Cas9 genome engineering parameters via a
library-on-library approach. Nat Methods. 12:823–826. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Matsoukas IG: Commentary: CRISPR-Cas
encoding of a digital movie into the genomes of a population of
living bacteria. Front Bioeng Biotechnol. 5:572017. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Moreno-Mateos MA, Vejnar CE, Beaudoin JD,
Fernandez JP, Mis EK, Khokha MK and Giraldez AJ: CRISPRscan:
Designing highly efficient sgRNAs for CRISPR-Cas9 targeting in
vivo. Nat Methods. 12:982–988. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Kleinstiver BP, Prew MS, Tsai SQ, Topkar
VV, Nguyen NT, Zheng Z, Gonzales AP, Li Z, Peterson RT, Yeh JR, et
al: Engineered CRISPR/Cas9 nucleases with altered PAM
specificities. Nature. 523:481–485. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Rutkauskas M, Sinkunas T, Songailiene I,
Tikhomirova MS, Siksnys V and Seidel R: Directional R-Loop
formation by the CRISPR-Cas surveillance complex cascade provides
efficient Off-target site rejection. Cell Rep. 10:1534–1543. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Bengtsson NE, Hall JK, Odom GL, Phelps MP,
Andrus CR, Hawkins RD, Hauschka SD, Chamberlain JR and Chamberlain
JS: Muscle-specific CRISPR/Cas9 dystrophin gene editing ameliorates
pathophysiology in a mouse model for Duchenne muscular dystrophy.
Nat Commun. 8:144542017. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Morsy SG, Tonne JM, Zhu Y, Lu B, Budzik K,
Krempski JW, Ali SA, El-Feky MA and Ikeda Y: Divergent
susceptibilities to AAV-SaCas9-gRNA vector-mediated genome-editing
in a single-cell-derived cell population. BMC Res Notes.
10:7202017. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Fu Y, Foden JA, Khayter C, Maeder ML,
Reyon D, Joung JK and Sander JD: High-frequency off-target
mutagenesis induced by CRISPR-Cas nucleases in human cells.
Divergent susceptibilities to AAV-SaCas9-gRNA vector-mediated
genome-editing in a single-cell-derived cell population. Nat
Biotechnol. 31:822–826. 2013. View Article : Google Scholar : PubMed/NCBI
|