Introduction
Patients with psoriasis exhibit chronic skin
inflammation, which is characterized by thick and scaly plaques
(1,2). Psoriasis is an autoimmune disease
mediated by T cells; studies using a range of immune antagonists
and human skin xenografts have identified that CD8+ and
CD4+ T cells infiltrate into psoriatic skin lesions
(1,3-6).
Programmed cell death 1 (PD-1), a member of the
superfamily of cytotoxic T-lymphocyte-associated protein 4 (CTLA-4)
immunoglobulins involved in immune regulation, has attracted
increasing attention in immune disease (7). PD-1 activation functions as a
rheostat to regulate immune activities, inhibiting T-cell
activation (8). PD-1 is activated
by binding its ligands, programmed cell death ligand 1 (PD-L1) and
PD-L2 (9-11), which signal T cells to inhibit
cell proliferation, cytotoxicity and cytokine production (12). PD-1 cannot normally be detected on
the surface of resting T cells; however, following activation by
ligand binding to T-cell receptors, activated T cells express high
levels of PD-1 (13,14). PD-1 signaling also affects
cytokine production, including interferon γ (IFN-γ), interleukin 2
(IL-2) and tumor necrosis factor α (TNF-α) (12). Mice lacking PD-1 are resistant to
viral infections, tumor growth and metastasis (15-17). Inhibiting PD-1 or its ligand PD-L1
has demonstrated promising benefits in metastatic melanoma
treatment by overcoming the immunosuppressive tumor
microenvironment and, consequently, has been approved by FDA for
use in patients (18-20).
Traditional therapeutics for patients with
psoriasis, including topical agents, systemic therapies and
phototherapy, are not always sufficiently satisfactory; topical
agents frequently result in short-term outcomes, and effective
phototherapy treatment demands high requirements for clinician
expertise and techniques to prevent skin erythema, photoaging or
burning by inadequate choice of phototherapy types, parameters or
unnecessary exposure (2,21). Despite the demonstrated efficacy
of biologic immune therapeutics in psoriasis treatment, which
consist of two main classes that target T cells and cytokines,
inconsistent efficacy is still an issue in clinical trials
(22). To maximize the
immunotherapeutic efficacy of psoriasis treatments, there is an
urgent need to understand the predominant mechanisms of
pathogenesis to provide experimental support for innovative
treatments and rational combinations.
The activation of PD-1 signaling has previously been
used to block T-cell activation, proliferation and cytotoxicity
(8,23). In addition, a previous study has
demonstrated that PD-1 upregulation occurs in mouse psoriatic
inflammatory skin induced by imiquimod (IMQ) (24). Treatment with recombinant PD-L1
protein significantly suppresses IMQ-induced psoriasis (25). Additionally, PD-1-null mice
exhibit severe psoriatic inflammation (24). Thus, the hypothesis of the present
study was that PD-1-targeted therapies may represent a promising
new therapeutic strategy in psoriasis treatment.
Materials and methods
Characteristics of patients with
psoriasis
Patients with psoriasis vulgaris were diagnosed
according to characteristic skin changes and histopathological
features. Prior to skin biopsy collection, systemic anti-psoriasis
medications or ultraviolet phototherapy were discontinued for at
least 8 weeks, and topical anti-psoriasis medications were
discontinued for at least 4 weeks. No evidence of autoimmune
disease other than psoriasis, malignant tumor and active viral or
bacterial infection was present at the time of patient recruitment.
Skin biopsies were collected from 10 patients [lesion skin; 6 (60%)
males, 4 (40%) females; age, 30-72 years; mean age, 49 years] for
IHC staining. The inflammatory level of psoriatic lesions was
evaluated using The Psoriasis Area and Severity Index (PASI; range,
0-72) (26), which included four
body regions: The head (h), trunk (t), upper extremities (u) and
lower extremities (l); and the levels of erythema (E), infiltration
(I), desquamation (D) and body surface area (A). The degree of
severity was defined as 0 (no symptoms), 1 (slight), 2 (moderate),
3 (marked), or 4 (very marked), with the surface area defined as 1
(<10%), 2 (10-29%), 3 (30-49%), 4 (50-69%), 5 (70-89%) or 6
(90-100%). The PASI was calculated using the following formula:
PASI = 0.1 x (Eh + Ih + Dh) x
Ah + 0.2 x (Eu + Iu +
Du) x Au + 0.3 x (Et +
It + Dt) x At + 0.4 x
(El + Il + Dl) x Al
(26). All participants signed
informed consent prior to enrollment. The study was approved by the
Beijing Chaoyang Hospital Scientific and ethics Committee (approval
no. 2016-11-4-5) and was conducted according to the Declaration of
Helsinki.
Animal experiments
For all the mouse experiments, C57BL/6 mice (8-12
weeks old; Jackson Laboratory) were treated every day with a
topical IMQ cream (62.5 mg in 5%; 3M Company) on the backs and
ears, whereas the control mice were treated with Vaseline Lanette
cream (Fagron, Inc.), and all mice were observed for the following
8 consecutive days as previously described (27). A total of 180 mice were used in
this experiment. PASI scoring was used to quantify the erythema,
thickness and scaling independently (score range, 0-4). The total
score was calculated as previously described (27). The experimental mice were
maintained under pathogen-free conditions, with ad libitum
food and water. Mice were euthanized by inhalation of
CO2 in a controllable manner at a flow rate of 15%
volume displacement per minute, and the skin tissues were obtained
and analyzed on the indicated days as described below.
Mouse in vivo experimental procedures
In the neutralizing anti-PD-1 mAb experiment, mice
(8-12 weeks old) were intraperitoneally injected with 200 µg
anti-PD-1 (RMP1-14) (n=20) or Igg (n=20) daily for 7 days starting
when IMQ treatment was initiated. A total of 40 mice were used for
this experiment. In the PD-1-Fc and anti-TNF-α intervention
experiment, mice of the same age were intraperitoneally injected
with 50 µg PD-1-Fc and/or anti-TNF-α on day 0 and day 3,
starting at IMQ treatment initiation. each individual group had 20
mice (5 mice per time point), with 80 mice in total used for this
experiment. All mice were monitored daily, and ear thickness was
measured with a dial thickness gauge caliper daily. Mice were
euthanized by inhalation of CO2, and the tissues were
obtained and analyzed on the indicated day as described. All mouse
experiments were approved by the Institutional Animal Care and Use
Committee at Beijing Chaoyang Hospital in Capital Medical
University in Beijing China (approval no. 2016-A-177).
Single-cell suspension preparation and
flow cytometry
The isolated skin-draining inguinal lymph nodes from
the mice were mashed with frosted glass slides and filtered through
a 70-µm cell strainer to produce a single-cell suspension
(1×107 cells/ml). The single-cell
suspension was fixed with 2% paraformaldehyde for 15 min at room
temperature prior to blocking with mouse Igg for 30 min on ice.
Subsequently, the cells (1×106 in
100 µl) were incubated with anti-CD45 PerCP-Cy5.5 (cat. no.
45-0451-82; Invitrogen; Thermo Fisher Scientific, Inc.), anti-CD4
FITC (cat. no. RM4-5; BD Biosciences), anti-CD8 PE (cat. no.
553032; BD Biosciences) and anti-PD-1 Pe-Cy7 (cat. no. 25-9985-82;
Invitrogen; Thermo Fisher Scientific, Inc.) antibodies on ice for 1
h. The cells were analyzed with an LSRII flow cytometer (BD
Biosciences), and the data were analyzed with FlowJo software v10
(FlowJo, LLC).
Reverse transcription-quantitative
(RT-q)PCR
Mouse skin was isolated for the extraction of total
RNA using the RNeasy Mini kit (cat. no. 74104; Qiagen GmbH). The
cDNA was synthesized with SuperScript II Reverse Transcriptase
(Invitrogen; Thermo Fisher Scientific, Inc.) with dNTPs and poly(A)
oligo(dT)25 as a template primer at 42°C for 50 min. A
total of 40 ng cDNA was used as the template for the RT-qPCR assay
with SyBR® green PCR master mix (Applied Biosystems;
Thermo Fisher Scientific, Inc.) in a 25-µl reaction system
using a Quantstudio 12K Flex qPCR System (Thermo Fisher Scientific,
Inc.) under the following thermocycling conditions: Denaturation at
95°C for 15 sec and annealing/extension at 60°C for 1 min for total
40 cycles. Control RT-qPCR was performed using the master mix
without reverse transcriptase. The relative gene expression levels
were normalized to the expression of Gapdh. The comparative Ct
method used was previously described (28). qPCR was performed three times.
The relative transcript levels of IL17, IFN-γ,
IL-22, and TNFα were determined by RT-qPCR as previously described
(29,30). The primers used were as follows:
Mouse IL17 forward, 5′-TTT AACTCCCTTGGCGCAAAA-3′ and reverse,
5′-CTTTCCCTCCGCATTGACAC-3′; mouse IFN-γ forward,
5′-ATGAACGCTACACACTGC ATC-3′ and reverse, 5′-CCATCCTTTTGC CAGTTC
CTC-3′; mouse TNFα forward, 5′-CCCTCACACTCAGATCATCTTCT-3′ and
reverse, 5′-GTCACGACGTGG GCTACAG-3′; mouse IL22 forward,
5′-ATGAGTTTTTCCCTTATGGGGAC-3′ and reverse,
5′-GCTGGAAGTTGGACACCTCAA-3′; and mouse Gapdh forward,
5′-TGTGTCCGTCGTGGATCTGA-3′ and reverse,
5′-TTGCTGTTGAAGTCGCAGGAG-3′.
Antibodies, cytokines and in vivo
treatment fusion proteins
The neutralizing monoclonal antibody (mAb) against
PD-1 (clone RMP1-14; cat. no. BE0146) and Rat IgG2a (clone 2A3;
cat. no. BE0089) in vivo were purchased from Bio X Cell. The
PD-1-Fc protein (cat. no. 50124-M03H) was obtained from Sino
Biological, Inc. Anti-mouse TNF-α (clone XT3.11; cat. no. BE0058)
mAb was purchased from Bio X Cell. Anti-CD3 (cat. no. sc-20047),
anti-CD8 (cat. no. sc-7188) and anti-CD4 (cat. no. sc-7219)
antibodies were purchased from Santa Cruz Biotechnology, Inc.
Anti-PD-1 and CD279 (cat. nos. PA5-2035 and PA5-32543) antibodies
were purchased from Thermo Fisher Scientific, Inc., and the
anti-PD-1 (clone J43; cat. no. 11-9985-81) antibody was purchased
from eBioscience.
Histology and immunohistochemical (IHC)
staining
Patient biopsies and mouse skin samples were fixed
in formalin at room temperature for 24 h and embedded in paraffin.
Hematoxylin and eosin (H&E) staining was performed as
previously described (29). IHC
staining was performed as previously described (29) with anti-CD4, anti-CD8 and
anti-PD-1 antibodies. An olympus light microscope was used to
examine the slides. Two independent researchers analyzed the
H&E and IHC staining slides. epidermal thickness was assessed
by quantification of keratinocyte numbers in the epidermis. Cells
positive for CD4, CD8 and PD-1 were quantified as the mean number
of positive cells in five high power fields (original
magnification, ×400).
Western blotting and ELISA
Mouse back skin with psoriatic lesions after IMQ
treatment for 6 days was isolated and lysed with RIPA buffer and
protease inhibitor cocktail (Thermo Fisher Scientific, Inc.),
followed by homogenization with a D1000 Handheld Homogenizer
(Benchmark Scientific, Inc.) for ELISA with the IL-17 Mouse ELISA
kit (cat. no. BMS6001; Thermo Fisher Scientific, Inc.) and IL-23
Mouse ELISA kit (cat. no. BMS6017; Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions.
The skin lysis protein concentration was measured
with the Braford Protein Assay kit I (Bio-Rad Laboratories, Inc.),
and 50 µg total protein of each sample was loaded into each
lane and separated with 12% SDS-PAGE. After transfer onto a PVDF
membrane, 5% (weight/volume) 5% BSA (Sigma-Aldrich; Merck KGaA) was
used for blocking at room temperature for 1 h, and then anti-IL17
(1:1,000, cat. no. ab218013; Abcam), anti-IL23 (1:400, cat. no.
ab45420; Abcam), and the control horseradish peroxidase
(HRP)-conjugated anti-β-actin (1:4,000, cat. no. ab49900, Abcam)
antibodies were used to blot the membranes at 4°C overnight. After
the 3 washes with 1X TBS buffer with 1% Tween-20 (TBST), a goat
anti-rabbit Igg secondary antibody (cat. no. 1706515; Bio-Rad
Laboratories, Inc.) was used to blot the membrane at room
temperature for 2 h. Subsequently, 1X TBST was used to wash the
membranes again prior to incubation with Pierce SuperSignal
chemiluminescent substrate (Thermo Fisher Scientific, Inc.). The
membranes were visualized by autoradiography into clear-blue X-ray
film (cat. no. 34089; Thermo Fisher Scientific, Inc.), and the
densitometry analysis was performed with Imagej 1.8.0 (national
Institutes of Health).
Statistical analysis
The experiments were repeated three times. Data are
presented as the mean ± SD. Statistical analysis was performed
using GraphPad Prism 7 (graphPad Software, Inc.). Statistical
significance was analyzed by a two-tailed unpaired Student's
t-test. Correlation was determined with Pearson's correlation
coefficient. For comparing multiple groups, one-way ANOVA and
Tukey's post hoc test was performed. P<0.05 was considered to
indicate a statistically significant difference.
Results
PD-1 is upregulated and correlates with
markers of psoriatic inflammation
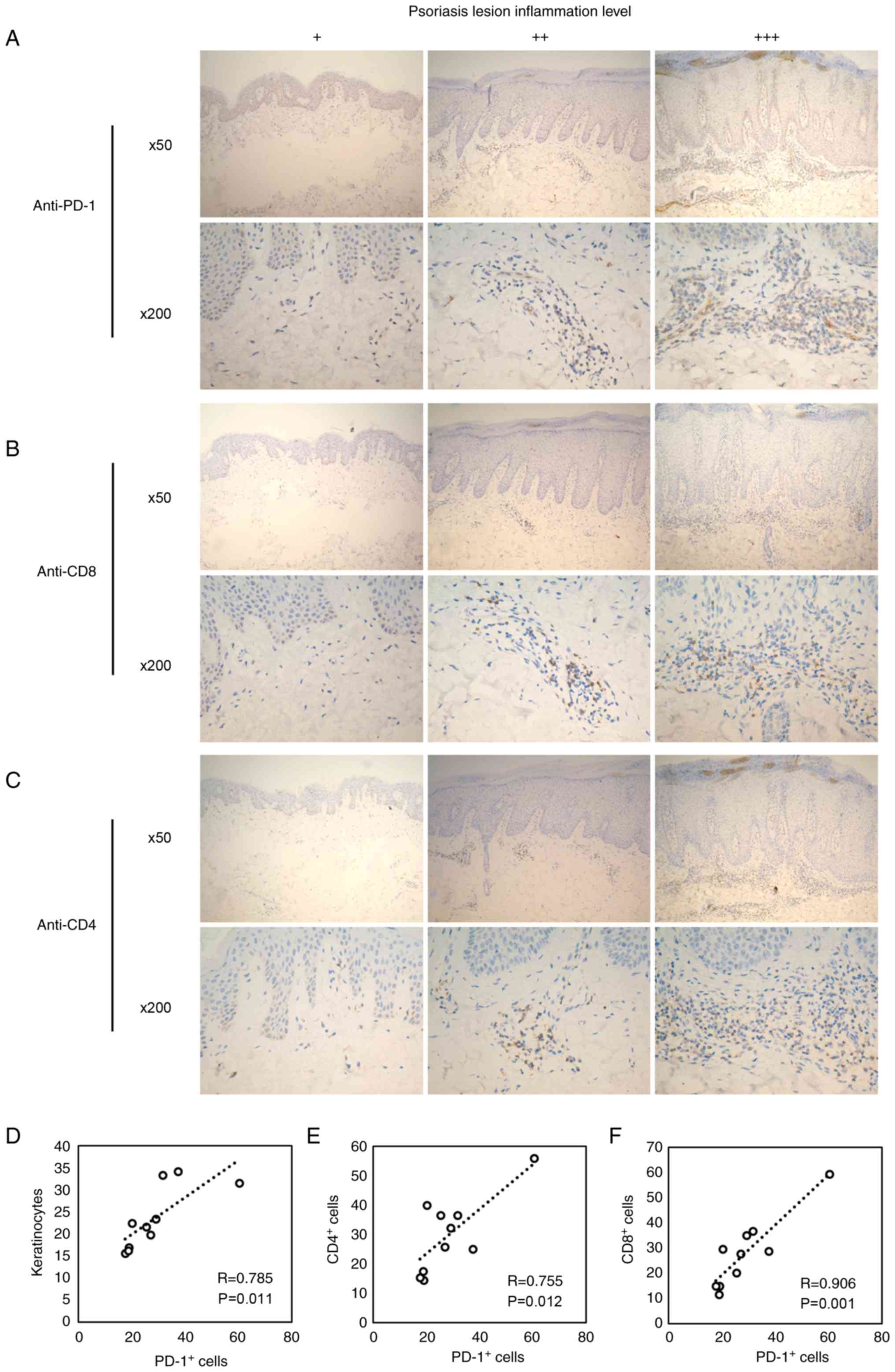
Immunohistochemical staining was performed on
patient psoriatic tissue samples using specific anti-PD-1
antibodies. The expression of PD-1 was detectable in all psoriatic
samples and was enhanced with increasing psoriatic inflammation
(Fig. 1A). In addition, the
numbers of CD4+ (Fig.
1B) and CD8+ (Fig.
1C) T cells increased in patients with high levels of psoriatic
lesions. These results indicated an inflammation-associated role of
PD-1 in psoriasis.
To identify the associations among epidermal
hyperplasia, inflammation and PD-1 expression, epidermal thickness
was measured by quantifying the keratinocyte number in the
epidermis in five different regions to indicate psoriatic
inflammation (31). The number of
Pd-1+ cells was significantly correlated with the number
of keratinocytes (Fig. 1D), which
suggested that Pd-1+ cells were associated with the
progression of skin inflammation. Furthermore, the number of
PD-1+ cells was significantly correlated with the number
of CD4+ and CD8+ T cells (Fig. 1E and F). Collectively, these
results indicated that PD-1 expression levels were associated with
skin inflammation in psoriasis.
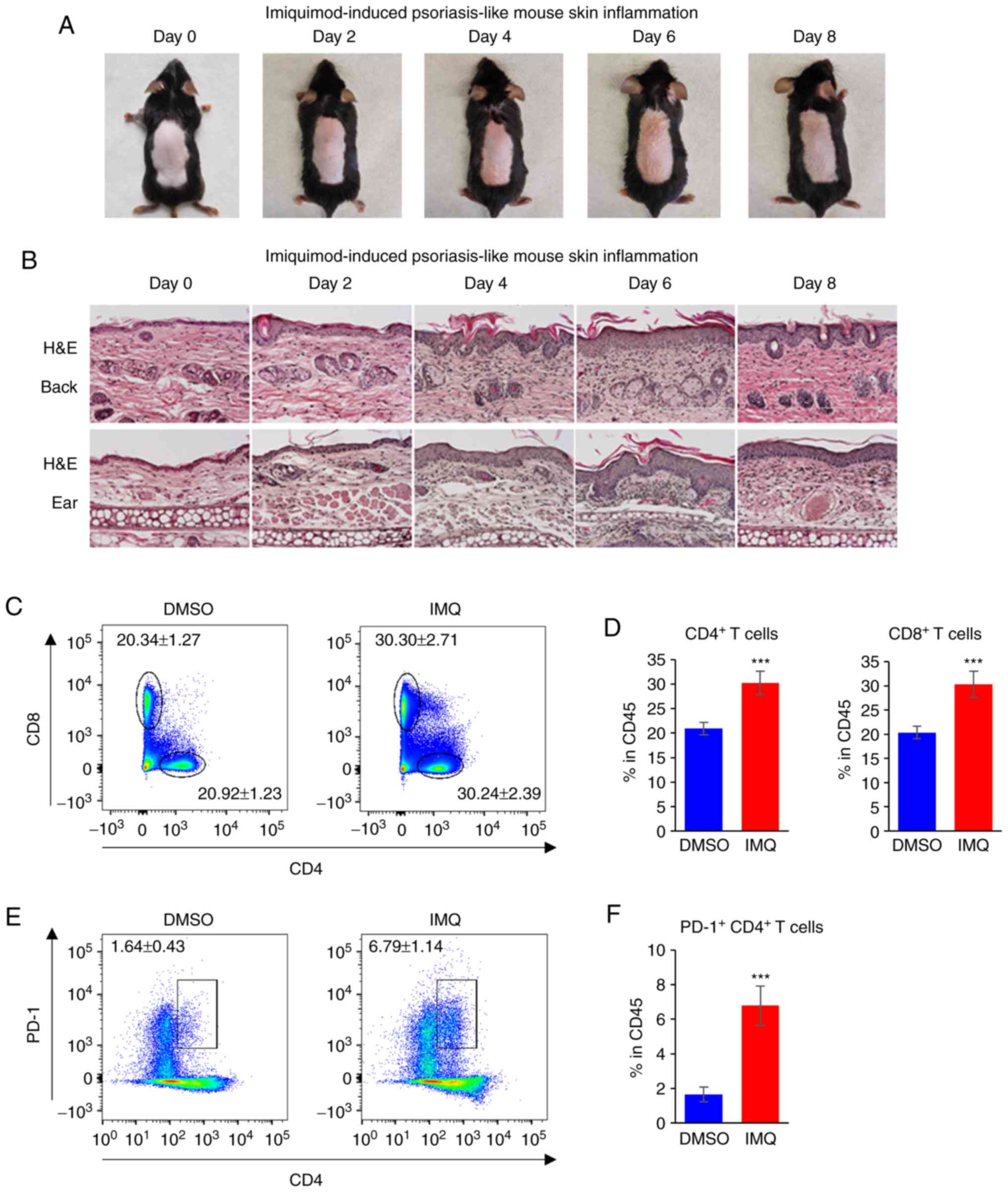
PD-1 is upregulated in IMQ-treated mouse
T cells
An IMQ-induced mouse psoriasis model was used to
determine PD-1 expression. The representative skin inflammatory
lesion was observed between days 0 and 8 (Fig. 2A). Inflammation was also indicated
by the thickness of skin determined by H&E staining of
epidermal samples from the backs and ears of the mice (Fig. 2B).
To analyze immune cell population changes,
skin-draining lymph nodes were collected to detect the
CD4+ and CD8+ T-cell populations in the
IMQ-induced psoriasis mouse model using flow cytometry. The results
demonstrated that the percentages of CD4+ and
CD8+ T cells within the CD45+ cells increased
following treatment with IMQ compared with those from mice treated
with DMSO (Fig. 2C and D). In
addition, the PD-1+ CD4+ population was
significantly increased in IMQ-treated mice compared with those
treated with DMSO (Fig. 2E and
F). These results indicated that the level of PD-1 increased in
psoriatic lesions, suggesting that PD-1 may serve a role in the
modulation of psoriatic inflammation.
Anti-PD-1 treatment enhances psoriatic
inflammation
To analyze PD-1 signaling in psoriasis progression
in vivo, a PD-1-targeting intervention was used to treat
IMQ-induced mouse psoriasis. Specifically, 200 µg/day
neutralizing mAb against PD-1 was used to treat the IMQ-induced
mice. Isotype-matched IgG was used as a negative control. PASI was
determined every day to quantify the psoriatic inflammation and
evaluate the progression of psoriasis (27). The results demonstrated that skin
inflammation increased following IMQ treatment for 6 days and
decreased after IMQ treatment for 8 days, and that
anti-PD-1-treated mice exhibited exacerbated psoriatic lesions and
higher cumulative PASI sores compared with those in the control
group (Fig. 3A and B). Epidermal
thickness for the PASI score, which indicates the level of
psoriatic inflammation, was assessed using histopathological
staining of the back skin psoriatic lesions (Fig. 3B and C). The results demonstrated
increased inflammatory immune cell populations in mice treated with
anti-PD-1 compared with those in the IgG group. In addition,
compared with mice treated with IgG, mice treated with anti-PD-1
exhibited increased percentages of CD4+ and
CD8+ T cells in the skin-draining lymph nodes on day 6
(Fig. 3D and E). The levels of
cytokines were measured in psoriatic back skin isolated from mice
treated with IgG or anti-PD-1 using RT-qPCR. The results
demonstrated that enhanced cytokine expression, including that of
IL-17, IL-22, IFN-γ and TNFα, was detected in mice with IMQ-induced
psoriasis treated with anti-PD-1 compared with that in mice treated
with IgG (Fig. 3F).
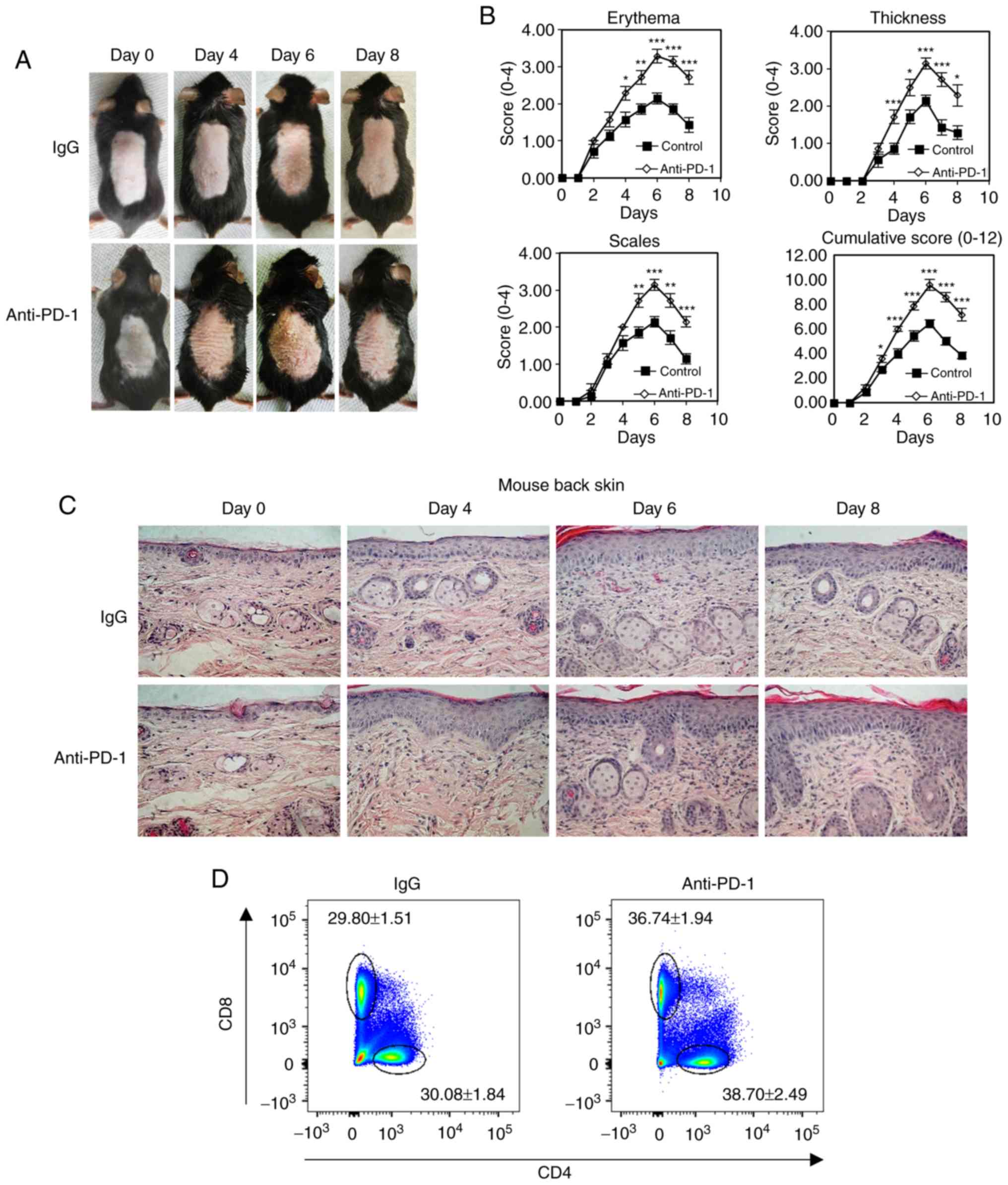 | Figure 3Anti-PD-1 treatment aggravates
psoriatic inflammation in mice with IMQ-induced psoriasis. (A)
Physical presentations of IMQ-induced mouse dorsal skin in control
IgG or anti-PD-1-treated animals. (B) erythema, thickness, scales
and cumulative disease score (mean ± SD) of five mice/group were
evaluated over time. (C) Representative histopathological staining
of IMQ-induced biopsies (N=5) harvested from mice treated with
anti-PD-1 or IgG after IMQ treatment for 0, 4, 6 and 8 days. (D and
E) Representative plots and quantification of flow cytometry
analysis of CD4+ and CD8+ T cell percentages
among the total CD45+ cells in the skin-draining lymph
nodes isolated from mice treated with IgG or anti-PD-1. (F) Reverse
transcription-quantitative PCR analysis of the expression of IL-17,
IFN-γ, IL-22 and TNF-α in mouse psoriatic skin lesions. *P<0.05,
**P<0.01, ***P<0.001 vs. IgG. PD-1, programmed cell death 1;
IMQ, imiquimod; IgG, immunoglobulin g; IL, interleukin; IFN-γ,
interferon γ; TNF-α, tumor necrosis factor α. |
PD-1-Fc inhibits inflammation in the
IMQ-induced psoriatic mouse model
The present study investigated the role of PD-1 in
psoriasis inflammation and its therapeutic potential by determining
the benefits of recombinant PD-1-Fc therapy on psoriasis
progression in IMQ-induced psoriatic mice. The recombinant PD-1-Fc
protein (50 µg per injection) exhibited a weak inflammatory
response in IMQ-treated mouse (Fig.
4A). The PASI score was measured each day, and the results
demonstrated that the PD-1-Fc-treated mice exhibited lower
cumulative PASI scores compared with those in the control group
(P<0.001 after treatment for 6 days; Fig. 4B). Histopathological examination
of skin lesions confirmed that treatment with PD-1-Fc decreased the
thickness of the epidermis of mouse back and ears (Fig. 4C). This result suggested that
treatment with PD-1-Fc reduced psoriatic inflammation. Compared
with the control mice, mice treated with PD-1-Fc exhibited
decreased percentages of CD4+ and CD8+ T
cells in the skin-draining lymph nodes (Fig. 4D and E). Cytokine levels were
detected in mouse back psoriatic skin using RT-qPCR; the results
revealed that PD-1-Fc treatment inhibited cytokine expression,
including IL-17, IL-22, IFN-γ and TNFα, in psoriatic skin compared
with that in the control mice (Fig.
4F).
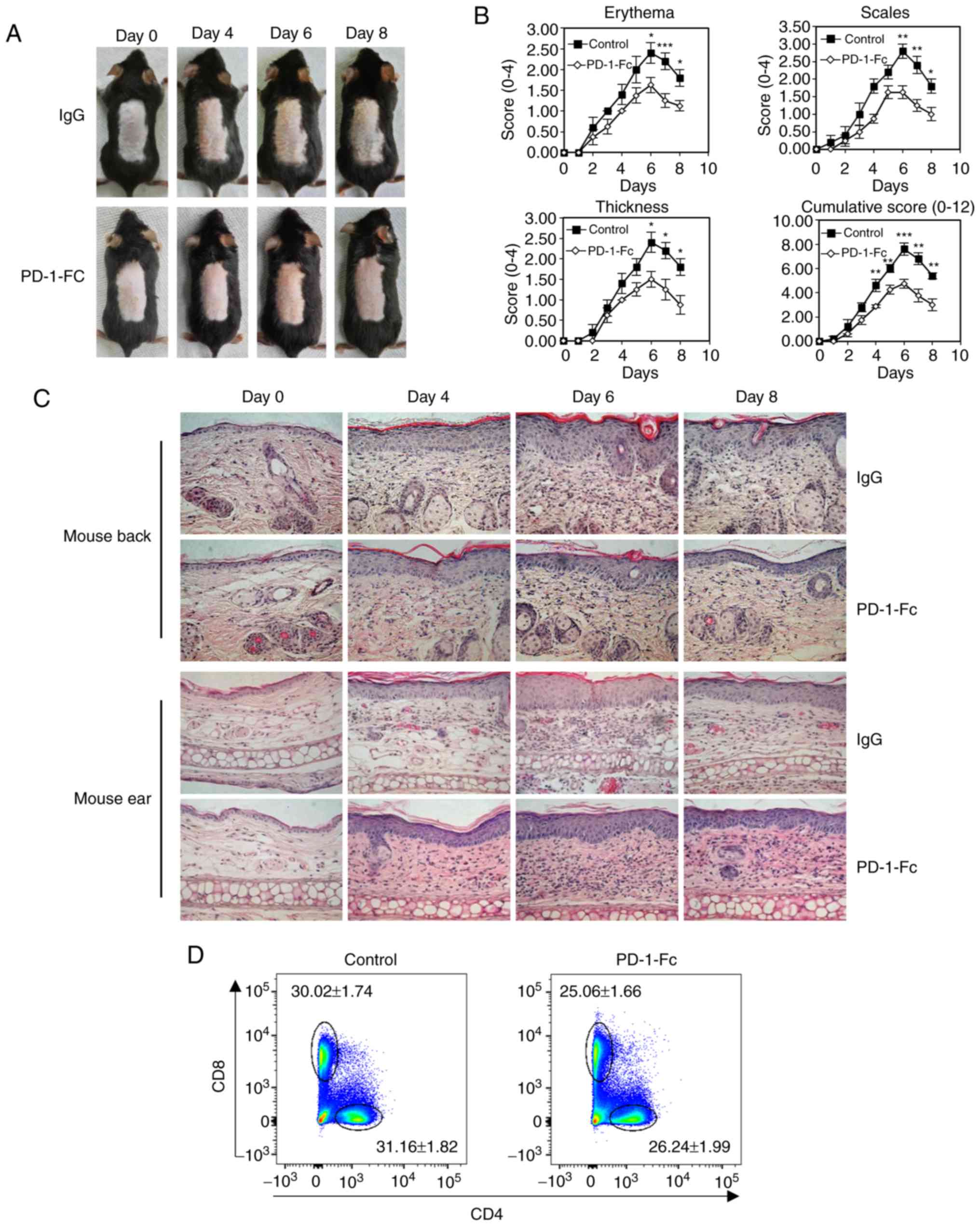 | Figure 4Recombinant PD-1-Fc fusion protein
treatment inhibits IMQ-induced psoriatic inflammation. (A-F)
IMQ-induced mice were administered PD-1-Fc protein or IgG control.
(A) Representative images of IMQ-induced psoriatic dorsal skin from
each treatment cohort. (B) Skin erythema, thickness, scales and
cumulative disease score were evaluated over time. (C)
Representative histopathological staining of IMQ-induced psoriatic
skin and ear biopsies over time. N=5. (D and E) Representative
plots and quantification of flow cytometry analysis for
CD4+ and CD8+ T cell percentages among the
total CD45+ cells in the skin-draining lymph nodes
isolated from mice treated with IgG or PD-1-Fc. (F) The mRNA
expression levels of IL-17, IFn-γ, IL-22 and TNF-α were analyzed by
reverse transcription-quantitative PCR in IMQ-induced mouse skin
treated with IgG or PD-1-Fc. *P<0.05,
**P<0.01, ***P<0.001 vs. IgG. PD-1,
programmed cell death 1; IMQ, imiquimod; IgG, immunoglobulin G; IL,
interleukin; IFN-γ, interferon γ; TNF-α, tumor necrosis factor
α. |
PD-1-Fc and anti-TNF-α exert an additive
effect to alleviate psoriatic inflammation
Anti-TNF-α therapy is used in psoriasis treatment to
reduce psoriatic inflammation (32). The IMQ-induced psoriasis model was
used to confirm the benefits of anti-TNF-α, and the resulted
demonstrated that anti-TNF-α decreased the epidermal thickness of
the mouse psoriatic ear, which is one of the key epidermal
parameters to evaluate psoriasis development (Fig. 5A). In addition, the effects of
co-treatment with anti-TNFα and PD-1-Fc were assessed. PD-1-Fc (50
µg per injection) alone or together with anti-TNFα (50
µg per injection) were administered to IMQ-treated mice with
intraperitoneal injections on days 0 and 3, starting at IMQ
treatment initiation; PD-1-Fc treatment enhanced the benefits of
anti-TNF-α therapy, and the co-treatment resulted in weaker skin
inflammation compared with that of anti-TnF-α treatment alone
(Fig. 5B and C).
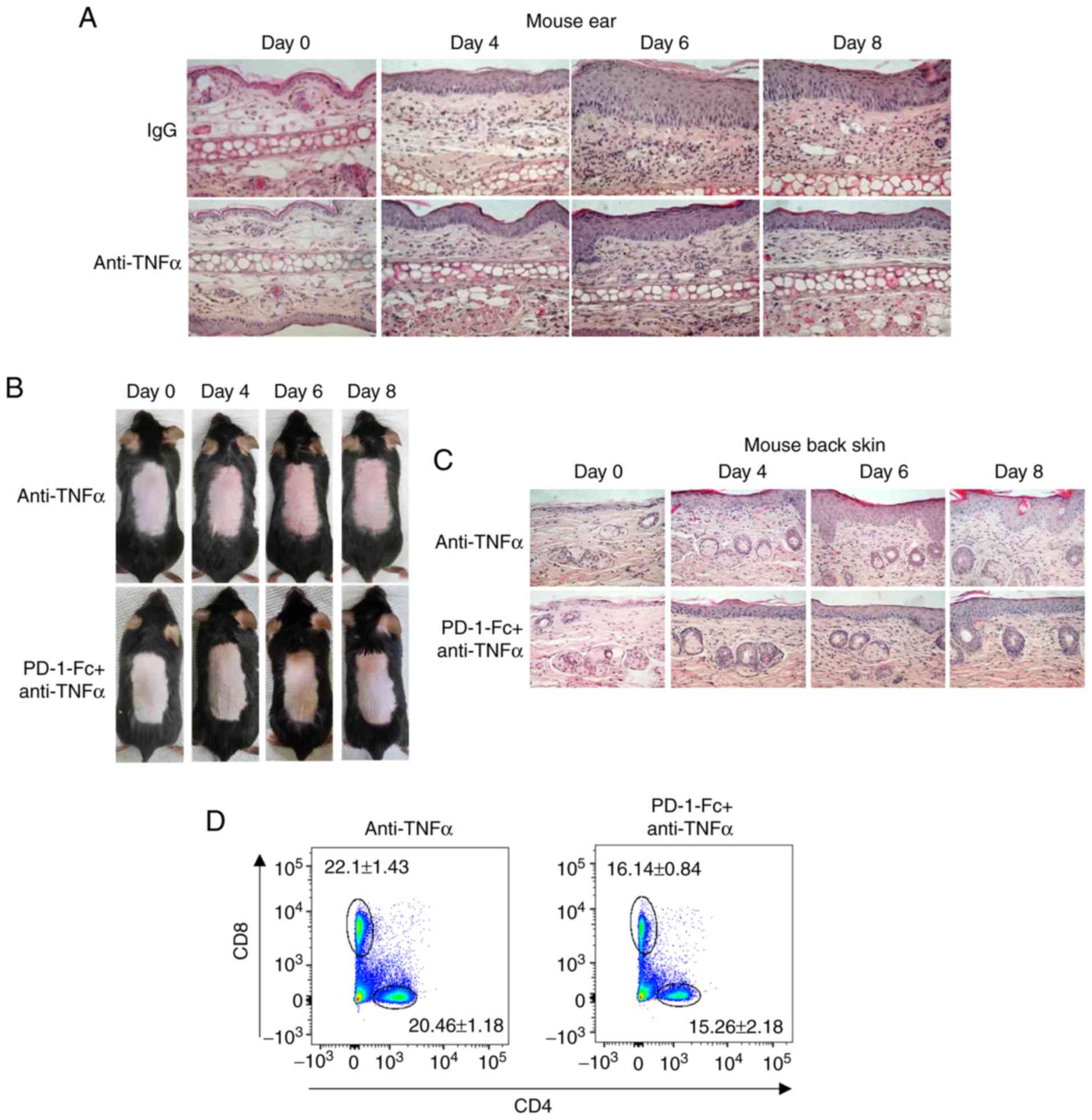 | Figure 5Recombinant PD-1-Fc fusion protein
enhances anti-TNF-α efficacy in inhibiting IMQ-induced skin
inflammation. (A) Representative histopathological staining of
anti-TNF-α-treated IMQ-induced mouse ear biopsies after IMQ
treatment for 0, 4, 6 and 8 days. N=5. (B) Representative images of
IMQ-induced mouse dorsal skin treated with anti-TNF-α or PD-1-Fc +
anti-TNF-α at the indicated time points. (C) Representative
histopathological staining of IMQ-induced mouse psoriasis treated
with anti-TNF-α or PD-1-Fc + anti-TNF-α after IMQ treatment for 0,
4, 6 and 8 days. N=5. (D and E) Representative plots and
quantification of flow cytometry analysis for CD4+ and
CD8+ T cell percentages among the total CD45+
cells in the skin-draining lymph nodes isolated from IMQ-induced
mouse model treated with anti-TNF-α or PD-1-Fc + anti-TNF-α.
*P<0.05, **P<0.01 vs. anti-TNF-α. (F)
Skin tissue from IMQ-induced mice treated with anti-TNF-α or
Pd-1-Fc + anti-TNF-α was homogenized for the western blotting assay
to detect IL-17 and IL-23 levels; β-actin was used as the loading
control. (G) IMQ-induced psoriatic mice were administered control
IgG, PD-1-Fc, anti-TNF-α or Pd-1-Fc + anti-TNF-α as indicated, and
the skin tissue was homogenized for ELISA assays to detect IL-17
and IL-23 cytokine production. N=5. ***P<0.001. PD-1,
programmed cell death 1; IMQ, imiquimod; TNF-α, tumor necrosis
factor α; IL, interleukin. |
Flow cytometry was performed to analyze the
percentage of CD4+ and CD8+ T cells in the
skin-draining lymph nodes, and the results revealed that compared
with anti-TNFα treatment alone, co-treatment with PD-1-Fc and
anti-TNFa reduced the percentages of CD4+ and
CD8+ T-cell in total CD45+ cells (Fig. 5D and E). To determine the
potential function of PD-1-Fc in microenvironmental cytokine
production, mouse back skin tissues were homogenized after 6 days
of IMQ treatment for western blotting and ELISAs. The results
demonstrated that the levels of IL-17 and IL-23 were reduced by
co-treatment with Pd-1-Fc and anti-TNF-α compared with anti-TNF-α
treatment alone (Fig. 5F). The
ELISA results revealed that PD-1-Fc or anti-TNF-α alone
significantly suppressed cytokine IL-17 and IL-23 production
(Fig. 5G), which was in agreement
with the previous conclusion. In addition, co-treatment with
PD-1-Fc and anti-TNF-α further inhibited IL-17 and IL-23 production
(Fig. 5G). These results
suggested that recombinant PD-1-Fc may be a potential candidate for
co-treatment with anti-TNFα in patients with psoriasis.
Discussion
The results of the present study identified a
potential therapeutic strategy for patients with psoriasis.
Traditional treatments are insufficient as topical agents usually
function in the short-term; phototherapy treatment has high demands
for physicians; and methotrexate, PUVA therapy, retinoids and
cyclosporine are highly toxic to patients (2). Targeted biologic therapies are
considered to be safer and more effective compared with generalized
therapies (33). Through the
analysis of clinical samples from patients with psoriasis, the
present study identified that PD-1 was expressed in human psoriatic
lesions, and upregulated PD-1 was associated with the level of
psoriatic inflammation, suggesting a potential role for PD-1 in
psoriasis development and progression. The results of the present
study also demonstrated that Pd-1-Fc treatment effectively
alleviated IMQ-induced psoriatic inflammation in mice.
T-lymphocyte activation is essential for the
maintenance of psoriasis, and multiple mechanisms are involved in
T-cell activation (34). PD-1 is
bound by its ligands PD-L1 and PD-L2 and inhibits T-cell activity
to prevent autoimmunity (23).
However, it remains unclear whether deregulation of PD-1 expression
is a hallmark of the progression of the autoimmune disease
psoriasis (23). The PD-1/PD-L1
mechanism has been extensively studied in the context of
understanding T-cell activation and immune checkpoint-targeted
therapy (35-37). Immune checkpoint inhibition with
anti-PD1 or anti-PD-L1 therapies results in psoriasis exacerbation
(38,39). PD-1 expression is detected on the
surface of several types of immune cells, including T cells,
monocytes, dendritic cells (DCs), natural killer cells, B cells and
macrophages (12,40). PD-L1 serves an important role in
the regulation of T cell-mediated immunity (41,42). DCs express both the PD-1 receptor
and PD-L1 to interact with cells expressing either PD-1 or PD-L1
(43). Pro-inflammatory cytokines
and TLR ligands induce DC activation, during which high levels of
PD-L1 expression are observed (44,45). PD-L1 is also expressed on the
surface of CD4+ and CD8+ T cells to inhibit
their activities (46). However,
there is also a co-stimulatory interaction between PD-1 and PD-L1
to promote the development of memory CD4+ T cells
(43). In addition, the function
of Pd-1 signaling in the crosstalk between DCs and other effector
cells, including γδ T cells, MDSCs and tumor-associated
macrophages, is still unclear (43). The results of the present study
demonstrated that PD-1-Fc negatively modulated psoriatic
inflammation; the specific signaling pathway involved in this
modulation needs to be explored in future studies.
Psoriasis can be induced or exacerbated by certain
drugs, such as immune checkpoint inhibitors anti-PD-1 and small
molecule TNF-α antagonists in cancer immunotherapy (47). A meta-analysis has reported that
patients with psoriasis have a high risk of cancer incidence, as
well as cancer-related death (48). The association between cancer and
psoriasis may be related to inflammation; psoriasis is a chronic
inflammatory skin disease, and chronic inflammation is associated
with increased cancer risk (49).
Immunomodulatory therapy for psoriasis treatment, which suppresses
immunity and helps to reduce psoriasis symptoms, therefore, may
decrease the risk of developing cancers (48). PD-L1 has been demonstrated to
alleviate psoriatic inflammation, and PD-L1-Fc has exhibited
promising benefits in psoriasis treatment (25). Studies have reported that PD-L1
levels serve a key role in the development of effective T cells
(41,42).
PD-1 is a T-cell regulator that belongs to the
CD28/CTLA-4 superfamily and negatively modulates T-cell activity
(7). Treatment with soluble
CTLA-4-Ig resulted in ≥50% improvement of Physician's global
Assessment in clinical studies of psoriasis vulgaris (50). The mouse psoriasis model used in
the present study was induced with IMQ, a ligand for Toll-like
receptor (TLR) 7 and TLR8, which is used to induce immune activity
and leads to mouse psoriasis; this mouse model is the most widely
used inducible psoriasis model (27,51). Using this model, a previous study
has revealed that PD-1 deficiency leads to enhanced dermal
inflammation and increased expression of inflammatory cytokines,
including IL-17 and IL-22, by innate γδ-low T cells in the
IMQ-induced psoriasis mouse model (24). The results of the present study
revealed that PD-1-Fc treatment effectively alleviated psoriatic
inflammation in the IQM-induced mouse model and has the potential
to exhibit synergistic effects with anti-TnF-α treatment.
Funding
This study was supported by the national natural
Science Foundation of China (grant nos. 81874470 and 81973860), the
national Key Research and Development Program of China (grant no.
2018yFC1705301) and the Shanghai natural Science Foundation of
China (grant no. 19ZR1458700).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SGP, BZ and XL conceived and designed the study.
SGP, MC, XYS, YQZ, TM and HJL performed experimental work, and
collected and analyzed the statistical data. SGP, BL, BZ and XL
interpreted the results. SGP, MC, CYC, BZ and XL wrote and edited
the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
This study was approved by the Institutional Review
Boards of the Yueyang Integrated Traditional Chinese and Western
Medicine Hospital (approval no. 2016-016). All mouse experiments
were performed following procedures approved by the Institutional
Animal Care and Use Committee at Beijing Chaoyang Hospital in
Capital Medical university in Beijing China (approval no.
2016-A-177).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Bowcock AM and Krueger JG: Getting under
the skin: The immunogenetics of psoriasis. Nat Rev Immunol.
5:699–711. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gottlieb AB: Psoriasis: emerging
therapeutic strategies. Nat Rev Drug Discov. 4:19–34. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nickoloff BJ, Bonish B, Huang BB and
Porcelli SA: Characterization of a T cell line bearing natural
killer receptors and capable of creating psoriasis in a SCID mouse
model system. j Dermatol Sci. 24:212–225. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chang JC, Smith LR, Froning KJ, Schwabe
BJ, Laxer JA, Caralli LL, Kurland HH, Karasek MA, Wilkinson DI,
Carlo DJ, et al: CD8+ T cells in psoriatic lesions
preferentially use T-cell receptor V beta 3 and/or V beta 13.1
genes. Proc Natl Acad Sci USA. 91:9282–9286. 1994. View Article : Google Scholar
|
|
5
|
Prinz JC, Vollmer S, Boehncke WH, Menssen
A, Laisney I and Trommler P: Selection of conserved TCR VDJ
rearrangements in chronic psoriatic plaques indicates a common
antigen in psoriasis vulgaris. Eur J Immunol. 29:3360–3368. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vollmer S, Menssen A and Prinz JC:
Dominant lesional T cell receptor rearrangements persist in
relapsing psoriasis but are absent from nonlesional skin: evidence
for a stable antigen-specific pathogenic T cell response in
psoriasis vulgaris. J Invest Dermatol. 117:1296–1301. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ishida Y, Agata Y, Shibahara K and Honjo
T: Induced expression of PD-1, a novel member of the immunoglobulin
gene superfamily, upon programmed cell death. EMBO J. 11:3887–3895.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Riley JL: PD-1 signaling in primary T
cells. Immunol Rev. 229:114–125. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Freeman GJ, Long AJ, Iwai Y, Bourque K,
Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne
MC, et al: Engagement of the PD-1 immunoinhibitory receptor by a
novel B7 family member leads to negative regulation of lymphocyte
activation. J Exp Med. 192:1027–1034. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Latchman Y, Wood CR, Chernova T, Chaudhary
D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, nunes R, et al:
PD-L2 is a second ligand for PD-1 and inhibits T cell activation.
Nat Immunol. 2:261–268. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sun X, Roudi R, Dai T, Chen S, Fan B, Li
H, Zhou Y, Zhou M, Zhu B, Yin C, et al: Immune-related adverse
events associated with programmed cell death protein-1 and
programmed cell death ligand 1 inhibitors for non-small cell lung
cancer: A PRISMA systematic review and meta-analysis. BMC Cancer.
19:5582019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Keir ME, Butte MJ, Freeman GJ and Sharpe
AH: PD-1 and its ligands in tolerance and immunity. Annu Rev
Immunol. 26:677–704. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nishimura H, Agata Y, Kawasaki A, Sato M,
Imamura S, Minato N, Yagita H, Nakano T and Honjo T:
Developmentally regulated expression of the PD-1 protein on the
surface of double-negative (CD4-CD8-) thymocytes. Int Immunol.
8:773–780. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Agata Y, Kawasaki A, Nishimura H, Ishida
Y, Tsubata T, Yagita H and Honjo T: expression of the PD-1 antigen
on the surface of stimulated mouse T and B lymphocytes. Int
Immunol. 8:765–772. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Iwai Y, Ishida M, Tanaka Y, okazaki T,
Honjo T and Minato N: Involvement of PD-L1 on tumor cells in the
escape from host immune system and tumor immunotherapy by PD-L1
blockade. Proc Natl Acad Sci USA. 99:12293–12297. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Okazaki T, Chikuma S, Iwai Y, Fagarasan S
and Honjo T: A rheostat for immune responses: The unique properties
of PD-1 and their advantages for clinical application. Nat Immunol.
14:1212–1218. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fourcade J, Sun Z, Benallaoua M, Guillaume
P, Luescher IF, Sander C, Kirkwood JM, Kuchroo V and Zarour HM:
upregulation of Tim-3 and PD-1 expression is associated with tumor
antigen-specific CD8+ T cell dysfunction in melanoma
patients. J Exp Med. 207:2175–2186. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ott PA, Hodi FS and Robert C: CTLA-4 and
PD-1/PD-L1 blockade: new immunotherapeutic modalities with durable
clinical benefit in melanoma patients. Clin Cancer Res.
19:5300–5309. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Topalian SL, Hodi FS, Brahmer JR,
Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD,
Sosman JA, Atkins MB, et al: Safety, activity, and immune
correlates of anti-PD-1 antibody in cancer. New Engl J Med.
366:2443–2454. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Brahmer JR, Tykodi SS, Chow LG, Hwu WJ,
Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, odunsi K, et al:
Safety and activity of anti-PD-L1 antibody in patients with
advanced cancer. N Engl J Med. 366:2455–2465. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang P and Wu MX: A clinical review of
phototherapy for psoriasis. Lasers Med Sci. 33:173–180. 2018.
View Article : Google Scholar :
|
|
22
|
Saurat JH, Stingl G, Dubertret L, Papp K,
Langley RG, ortonne JP, Unnebrink K, Kaul M and Camez A; CHAMPIon
Study Investigators: Efficacy and safety results from the
randomized controlled comparative study of adalimumab vs.
methotrexate vs placebo in patients with psoriasis (CHAMPIon). Br J
Dermatol. 158:558–566. 2008. View Article : Google Scholar
|
|
23
|
Francisco LM, Sage PT and Sharpe AH: The
PD-1 pathway in tolerance and autoimmunity. Immunol Rev.
236:219–242. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Imai Y, Ayithan N, Wu X, yuan Y, Wang L
and Hwang ST: Cutting edge: PD-1 regulates imiquimod-induced
psoriasiform dermatitis through inhibition of IL-17A expression by
innate gammadelta-low T cells. J Immunol. 195:421–425. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim JH, Choi YJ, Lee BH, Song My, Ban CY,
Kim J, Park J, Kim Se, Kim TG, Park SH, et al: Programmed cell
death ligand 1 alleviates psoriatic inflammation by suppressing
IL-17A production from programmed cell death 1-high T cells. J
Allergy Clin Immunol. 137:1466–1476.e3. 2016. View Article : Google Scholar
|
|
26
|
Langley RG and Ellis CN: evaluating
psoriasis with psoriasis area and severity index, psoriasis global
assessment, and lattice system physician's global assessment. J Am
Acad Dermatol. 51:563–569. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
van der Fits L, Mourits S, Voerman JS,
Kant M, Boon L, Laman JD, Cornelissen F, Mus AM, Florencia E, Prens
EP and Lubberts E: Imiquimod-induced psoriasis-like skin
inflammation in mice is mediated via the IL-23/IL-17 axis. J
Immunol. 182:5836–5845. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhu B, Zhang M, Williams EM, Keller C,
Mansoor A and Davie JK: TBX2 represses PTEN in rhabdomyosarcoma and
skeletal muscle. Oncogene. 35:4212–4224. 2016. View Article : Google Scholar :
|
|
29
|
Cui R, Widlund HR, Feige E, Lin JY,
Wilensky DL, Igras VE, D'orazio J, Fung CY, Schanbacher CF, Granter
SR and Fisher DE: Central role of p53 in the suntan response and
pathologic hyperpigmentation. Cell. 128:853–864. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
D'orazio JA, nobuhisa T, Cui R, Arya M,
Spry M, Wakamatsu K, Igras V, Kunisada T, granter SR, Nishimura EK,
et al: Topical drug rescue strategy and skin protection based on
the role of Mc1r in UV-induced tanning. Nature. 443:340–344. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Works MG, Yin F, Yin CC, Yiu Y, Shew K,
Tran TT, Dunlap N, Lam J, Mitchell T, Reader J, et al: Inhibition
of TYK2 and JAK1 ameliorates imiquimod-induced psoriasis-like
dermatitis by inhibiting IL-22 and the IL-23/IL-17 axis. J Immunol.
193:3278–3287. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lubrano E, Scriffignano S and Perrotta FM:
TNF-alpha inhibitors for the six treatment targets of psoriatic
arthritis. Expert Rev Clin Immunol. 15:1303–1312. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ronholt K and Iversen L: old and new
biological therapies for psoriasis. Int J Mol Sci. 18:E22972017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sibaud V: Dermatologic reactions to immune
checkpoint inhibitors: Skin toxicities and immunotherapy. Am J Clin
Dermatol. 19:345–361. 2018. View Article : Google Scholar
|
|
35
|
Muenst S, Soysal SD, Tzankov A and Hoeller
S: The PD-1/ PD-L1 pathway: Biological background and clinical
relevance of an emerging treatment target in immunotherapy. Expert
Opin Ther Targets. 19:201–211. 2015. View Article : Google Scholar
|
|
36
|
Okazaki T and Honjo T: The PD-1-PD-L
pathway in immunological tolerance. Trends Immunol. 27:195–201.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li X, Xiang Y, Li F, yin C, Li B and Ke X:
WnT/β-catenin signaling pathway regulating T cell-inflammation in
the tumor microenvironment. Front Immunol. 10:22932019. View Article : Google Scholar
|
|
38
|
Voudouri D, Nikolaou V, Laschos K,
Charpidou A, Soupos N, Triantafyllopoulou I, Panoutsopoulou I,
Aravantinos G, Syrigos K and Stratigos A: Anti-PD1/PDL1 induced
psoriasis. Curr Probl Cancer. 41:407–412. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
De Bock M, Hulstaert E, Kruse V and
Brochez L: Psoriasis vulgaris exacerbation during treatment with a
PD-1 checkpoint inhibitor: Case report and literature review. Case
Rep Dermatol. 10:190–197. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
He J, Hu Y, Hu M and Li B: Development of
PD-1/PD-L1 pathway in tumor immune microenvironment and treatment
for non-small cell lung cancer. Sci Rep. 5:131102015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Karunarathne DS, Horne-Debets JM, Huang
JX, Faleiro R, Leow CY, Amante F, Watkins TS, Miles JJ, Dwyer PJ,
Stacey KJ, et al: Programmed death-1 ligand 2-mediated regulation
of the PD-L1 to PD-1 axis is essential for establishing CD4(+) T
Cell Immunity. Immunity. 45:333–345. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ellis JS, guloglu FB, Tartar DM, Hoeman
CM, Haymaker CL, Cascio JA, Wan X, Dhakal M, VanMorlan A, Yahng SH
and Zaghouani H: APCs expressing high levels of programmed death
ligand 2 sustain the development of CD4 T cell memory. J Immunol.
185:3149–3157. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Versteven M, Van den Bergh JMJ, Marcq E,
Smits ELJ, Van Tendeloo VFI, Hobo W and Lion E: Dendritic cells and
programmed death-1 blockade: A joint venture to combat cancer.
Front Immunol. 9:3942018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ritprajak P and Azuma M: Intrinsic and
extrinsic control of expression of the immunoregulatory molecule
PD-L1 in epithelial cells and squamous cell carcinoma. Oral Oncol.
51:221–228. 2015. View Article : Google Scholar
|
|
45
|
Pulko V, Liu X, Krco CJ, Harris KJ,
Frigola X, Kwon ED and Dong H: TLR3-stimulated dendritic cells
up-regulate B7-H1 expression and influence the magnitude of CD8 T
cell responses to tumor vaccination. J Immunol. 183:3634–3641.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Diskin B, Adam S, Cassini MF, Sanchez G,
Liria M, Aykut B, Buttar C, Li E, Sundberg B, Salas RD, et al:
PD-L1 engagement on T cells promotes self-tolerance and suppression
of neighboring macrophages and effector T cells in cancer. Nat
Immunol. 21:442–454. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Balak DM and Hajdarbegovic E: Drug-induced
psoriasis: Clinical perspectives. Psoriasis (Auckl). 7:87–94.
2017.
|
|
48
|
Trafford AM, Parisi R, Kontopantelis E,
Griffiths CEM and Ashcroft DM: Association of psoriasis with the
risk of developing or dying of cancer: A systematic review and
meta-analysis. JAMA Dermatol. 2019.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Abrams JR, Lebwohl MG, Guzzo CA, Jegasothy
BV, Goldfarb MT, Goffe BS, Menter A, Lowe NJ, Krueger G, Brown MJ,
et al: CTLA4Ig-mediated blockade of T-cell costimulation in
patients with psoriasis vulgaris. J Clin Invest. 103:1243–1252.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
El Malki K, Karbach SH, Huppert J, Zayoud
M, Reissig S, Schüler R, Nikolaev A, Karram K, Münzel T, Kuhlmann
CR, et al: An alternative pathway of imiquimod-induced
psoriasis-like skin inflammation in the absence of interleukin-17
receptor a signaling. J Invest Dermatol. 133:441–451. 2013.
View Article : Google Scholar
|