Introduction
Diabetic retinopathy (DR) is a primary microvascular
complication of diabetes mellitus. DR is characterized by
inflammation and neuronal dysfunction associated with concomitant
abnormal angiogenesis that leads to inevitable and permanent visual
deficits (1). Epidemiological and
clinical research has revealed that the global diabetic population
in 2011 was approximately 350 million and this is estimated to
increase to approximately 500 million by the year 2030 (2), one-third of which will suffer from
DR (3). Thus, numerous therapies
have been proposed to alleviate retinopathy induced by
hyperglycemia, including vitrectomy, laser photocoagulation and the
intravitreal injection of anti-vascular endothelial growth factor
(VEGF) agents (4). Although the
aforementioned interventions have exhibited notable therapeutic
effects in the treatment of DR, there are some inevitable
side-effects, such as peripheral vision loss, decrease in night
vision and the loss of visual sensitivity (5).
Mesenchymal stem cells (MSCs) are stromal cells that
have the ability to self-renew and also exhibit multilineage
differentiation potential (6).
Owing to their high expandability in vitro and their
excellent ability to differentiate, MSCs are considered to be a
promising resource for stem cell-based therapy (7). Recent studies have demonstrated that
MSC transplantation, or a combination with other factors, has broad
application prospects in the treatment of DR (8,9).
The results of animal experiments have also demonstrated that the
transplantation of MSCs into the retina of DR-affected rats
effectively delayed retinal degeneration through neuroprotection
(10). Moreover, MSCs have been
shown to improve the integrity of the blood-retinal barrier by
differentiating into glial-like and photoreceptor cells in the
retina, resulting in alleviated DR in rats with streptozotocin
(STZ)-induced diabetes (11).
Nevertheless, the exact role of MSCs in the treatment of DR has not
yet been fully determined, and the mechanisms of retinal repair at
the molecular level remain unclear.
With the advantage of procurement, storage and
transplantation compared to other stem cells, umbilical
cord-derived MSCs (UC-MSCs) have attracted increasing attention in
clinical settings (12,13). A previous study suggested that MSC
transplantation ameliorated STZ-induced DR in rats, although the
reduced viability of MSCs attenuated the therapeutic effect
(14). According to previous
studies, all-trans retinoic acid (ATRA), a metabolic product of
vitamin A (15), and the retinoid
signaling pathway are involved in several biological processes,
leading to neuronal proliferation and differentiation (16). Additionally, ATRA induces the
in vitro differentiation of stem cells into several types of
neural cells, such as neuroblastoma (17), embryonic stem (18) and embryonic carcinoma cells
(19). It also enhances the
migration and proliferation of MSCs, and induces UC-MSC
differentiation into neuron-like cells (20,21). Therefore, in the present study,
UC-MSCs with or without ATRA treatment were transplanted into the
vitreous body of rats subjected to STZ-induced DR. Retinal
pathology was observed in terms of inflammation and angiogenesis,
which are known to play essential roles in the pathogenesis of DR
(22,23). The levels of related cytokines
were determined to preliminarily examine the effects and mechanisms
of UC-MSCs on STZ-induced DR. The findings of the present study may
provide a theoretical basis for and new insight into the
application of MSCs in the treatment of DR.
Materials and methods
Extraction and treatment of UC-MSCs
Sprague-Dawley rats, purchased from the Laboratory
Animal Centre, Huazhong Agricultural University, were artificially
caged at a male-female ratio of 1:2 to obtain rats at 19 days of
gestation. Caesarean section was performed after the rats were
euthanized with an intraperitoneal injection of pentobarbital
sodium in 150 mg/kg to collect the umbilical cord tissue, and
vascular tissue was subsequently removed. The remaining Wharton's
jelly tissue was then cut up and digested with Dulbecco's modified
Eagle's medium (DMEM, HyClone; GE Healthcare Life Sciences)
containing 0.1% type IV collagenase (Gibco; Thermo Fisher
Scientific, Inc.) for 10 min, followed by 0.25% trypsinization for
20 min at 37°C. Following purification, the cells were resuspended
in DMEM containing 1% penicillin-streptomycin-amphotericin B
solution (Bioswamp, Myhalic Biotechnology Co., Ltd.) and 10% fetal
bovine serum (FBS, Gibco; Thermo Fisher Scientific, Inc.) at a
concentration of 2×105 cells/ml,
seeded into a culture flask pre-coated with polylysine
(Sigma-Aldrich; Merck KGaA) and incubated under constant humidity
conditions at 37°C in an atmosphere containing 5% CO2.
The culture medium was changed the following day and every 3 days
thereafter. Passaging was carried out after the cells reached 85%
confluence. UC-MSCs in the third passage were examined for CD29,
CD44, CD73, CD105, CD90, CD34, CD45 and HLA-DR expression by flow
cytometry (Beckman Coulter, Inc.) and observed under an inverted
fluorescence microscope (Leica Microsystems GmbH). The obtained
UC-MSCs were then treated with 0.5 μM ATRA (Shanghai Aladdin
Biochemical Technology Co., Ltd.) for 12, 24 or 48 h, as previously
described (21). Untreated
UC-MSCs served as the control (CON).
3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide
(MTT) assay
MTT assay was performed to evaluate the viability of
the ATRA-treated UC-MSCs. Briefly, 180 μl of cells in the
logarithmic phase were plated into 96-well plates at a
concentration of 5×103 cells/well
and incubated at constant humidity at 37°C in an atmosphere
containing 5% CO2 overnight. Following treatment with
ATRA (0.5 μM) for 12, 24 or 48 h, the cells were treated
with 20 μl of MTT (5 mg/ml; Bioswamp, Myhalic Biotechnology
Co., Ltd.) for 4 h, followed by the addition of 150 μl of
dimethyl sulfoxide. The absorbance was measured using a microplate
reader (Thermo Fisher Scientific, Inc.) at 490 nm after 10 min of
shaking.
Flow cytometry
Flow cytometry was carried out to identify the
UC-MSCs and evaluate the apoptosis of UC-MSCs with or without ATRA
treatment. For cell identification, the cells were collected at a
concentration of 1×106 cell/ml
into 8 Eppendorf tubes, with 100 μl of cells in each tube,
wherein 2 μl of CD29-fluorescein isothiocyanate (FITC)
(85-11-0291-80; eBioscience, Inc.), CD44-FITC (MA5-16906;
eBioscience, Inc.), CD90-FITC (554894, BD Biosciences), CD34-FITC
(MA1-10204; eBioscience, Inc.), CD45-FITC (11-0460-82; eBioscience,
Inc.), CD73-FITC (PAB45829; Bioswamp, Myhalic Biotechnology Co.,
Ltd.), CD105-FITC (MA1-19231; Invitrogen; Thermo Fisher Scientific,
Inc.), or 1 μl of HLA-DR antibody (ab175085, Abcam) were
added followed by incubation for 45 min in the dark (one tube acted
as the control). After washing with phosphate-buffered saline (PBS,
Bioswamp, Myhalic Biotechnology Co., Ltd.), the cells were
resuspended with 100 μl flow cytometry staining buffer and
incubated with 2 μl FITC-conjugated Affinipure goat
anti-rabbit IgG(H+L) (PAB160016; Bioswamp, Myhalic Biotechnology
Co., Ltd.) for 45 min in the dark, 400 μl of flow cytometry
staining buffer was added. The cells were analyzed, and data were
acquired using a flow cytometer (Beckman Coulter, Inc.). For the
evaluation of apoptosis, cells were harvested by trypsin,
resuspended in a binding buffer at a concentration of 1×105 cells/ml, labeled with 10 μl of
Annexin V-FITC and 10 μl of propidium iodide (PI) for 30 min
at room temperature, and analyzed by flow cytometry. A minimum of
2×104 cells was acquired for each
sample.
Immunofluorescence
Following treatment with ATRA, the cell status was
detected by immunofluorescence. Incubated cells were washed twice
with PBS (pH 7.4) and fixed in 4% paraformaldehyde at room
temperature for 30 min, followed by 3 washes in PBS. The cells were
then permeabilized using 0.5% Triton X-100 (Bioswamp, Myhalic
Biotechnology Co., Ltd.) in PBS for 20 min and blocked in 5% bovine
serum albumin (BSA) for 1 h. The cells were subsequently incubated
with primary antibodies against Nestin (1:200, ab92391, Abcam),
neuron-specific enolase (NSE; 1:200, ab53025, Abcam) and glial
fibrous acidic protein (GFAP; 1:200, ab16997, Abcam) for 1 h at
room temperature, and thereafter with AlexaFluor 488-conjugated
Affinipure goat anti-rabbit secondary antibody (1:10,000, SAB43742,
Bioswamp, Myhalic Biotechnology Co., Ltd.) for 1 h at room
ttemperature. The nuclei were stained with DAPI (Bioswamp, Myhalic
Biotechnology Co., Ltd.) and stained cells were examined under a
fluorescence microscope (Leica, Microsystems GmbH).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The mRNA expression levels of Nestin, NSE and GFAP
in UC-MSCs following treatment with ATRA for 24 h were evaluated by
RT-qPCR. Total RNA was extracted using TRIzol reagent (Ambion) and
reversed transcribed into cDNA according to the following system:
Total RNA (500 ng) combination with 4 μl 5X PrimeScript II
buffer (Takara), 2 μl, 10 mM of dNTP Mix (Solarbio), 1
μl Oligo DT18 primer (Takara), 1 μl, 40 U/μl
of Recombinant RNase Inhibitor (Takara), 1 μl, 200
U/μl of PrimeScript II RTase (Takara) and 11 μl
ddH2O, followed by amplification using SYBR FAST qPCR
Master Mix (KM4101, Kapa Biosystems). The thermocycling conditions
are as follows: 95°C for 3 min; 40 cycles of denaturation at 95°C
for 50 sec, annealing at 56°C for 10 sec, and extension at 72°C for
20 sec; and final extension from 65°C to 95°C at the rate of 5
sec/0.5°C. The primer sequences were as follows: NSE forward,
5′-GCCTCTACGGGCATCT-3′ and reverse, 5′-ATC AGG TTG TCC AGT TTC-3′;
GFAP forward, 5′-GAC GCA TCA CCT CCG CT-3′ and reverse, 5′-CGC CTT
GTT TTG CTG TTC-3′; Nestin forward, 5′-AGG GGC AGA CAT CAT TGG-3′
and reverse, 5′-CAG AGA CTA GCG GCA TTC C-3′; GAPDH forward, 5′-CCA
CTC CTC CAC CTT TG-3′ and reverse, 5′-CAC CAC CCT GTT GCT GT-3′.
The 2−ΔΔCq method was utilized to calculate the relative
mRNA expression levels (24).
GAPDH served as an internal reference gene.
Animal model
A total of 40 Sprague-Dawley rats (weighing 120±7.0
g, 7 weeks old) were obtained from the Laboratory Animal Centre,
Huazhong Agricultural University, and raised in a
specific-pathogen-free room with a humidity of 50-60% and a
temperature of 23±2°C with standard water and diet. Diabetes was
induced with a single intraperitoneal injection of freshly prepared
STZ (Sigma-Aldrich; Merck KGaA) solution in citrate buffer (pH 4.5,
Sigma-Aldrich; Merck KGaA) at 60 mg/kg as previously described
(22). The rats were confirmed to
be diabetic when the fasting blood glucose levels were measured to
be ≥16.7 mmol/l at 1 week after the injection, as previously
described (25). Diabetic rats
were fed a standard diet and unlimited water for 2 months. The
fasting blood glucose levels were continuously monitored each week
to ensure the models were successful. Insulin was not used during
the study for the treatment of any extreme glycemic levels. All
animal protocols were approved by the Institutional Review Board of
Wuhan Myhalic Biotechnology Co., Ltd. based on the ethical
Guidelines for Animal Care and Use of the Model Animal Research
Institute (HLK-20180802-01) and were performed in accordance with
the Association for Research in Vision and Ophthalmology Statement
for the Use of Animals in Ophthalmic and Vision Research.
Intravitreal injection of UC-MSCs in
diabetic rats
After 2 months, 30 diabetic rats with lens opacities
(milky-white colored lens) were selected to establish the DR model
and were randomly divided into 3 groups (n=10 per group) as
follows: The model (MOD), ATRA and UC-MSC group. The rats in the
MOD group were intravitreally injected with 2 μl of saline.
Those in the ATRA and UC-MSC groups were administered an
intravitreal injection of ATRA-treated or normal UC-MSCs (2
μl, 1×105-6 cells), respectively (12). The control rats (CON group, n=10)
were not subjected to STZ induction. Prior to the injection,
UC-MSCs with or without ATRA treatment were labeled with CM-Dil
(Invitrogen; Thermo Fisher Scientific, Inc.) solution. After 8
weeks of treatment, the lenses of the rats were observed, and the
rats were then euthanized with an intraperitoneal injection of a
lethal dose of pentobarbital sodium at 150 mg/kg; death was
confirmed with no heartbeat and respiration. The eyeballs were
collected and fixed with 4% paraformaldehyde for 24 h. The
harvested retinal tissues were immersed in 10% formalin buffer.
Immunofluorescence evaluation of the
status of transplanted cells
The status of transplanted cells in the ATRA and
UC-MSCs groups was evaluated by immunofluorescence staining.
Following fixation in 10% formalin buffer for 48 h, the retinal
tissues were embedded in paraffin and sliced at a thickness of 4
μm, followed by dewaxing with water. The tissues were then
stained with 4′,6-diamidino-2-phenylindole (DAPI), and examined
under a fluorescence microscope (Leica, Microsystems GmbH).
Hematoxylin and eosin (H&E)
staining
Following fixation in 10% formalin buffer for 48 h,
the retinal tissues were embedded in paraffin and sliced at a
thickness of 4 μm, followed by dewaxing with water. The
sections were stained in hematoxylin solution (Bioswamp, Myhalic
Biotechnology Co., Ltd.) for 3 min and subsequently in eosin
solution (Bioswamp, Myhalic Biotechnology Co., Ltd.) for 3 min at
room temperature. Following dehydration, the tissues were cleared
with xylene and mounted with neutral balsam. After staining, the
sections were observed under an upright microscope (Leica,
Microsystems GmbH), and Leica Application Suite was used to collect
and analyze the relevant parts of the samples.
Terminal deoxynucleotidyl transferase
dUTP nick-end labeling (TUNEL) staining
Qualitative analysis of apoptosis in retinal tissues
was performed using a TUNEL kit (Roche Diagnosticis) according to
the manufacturer's instructions. Following fixation in 10% formalin
buffer for 48 h, the retinal tissues were embedded in paraffin and
sliced at a thickness of 2-3 μm, followed by dewaxing with
water. The tissues were treated with protease K at room temperature
for 30 min, 50 μl of TUNEL reaction mixture in a dark
humidified chamber at 37°C for 1 h, and 50 μl of
converter-POD for 1 h. Finally, diaminobenzidine was added, and the
core was counter-stained with hematoxylin. The sections were
observed under an upright microscope (Leica, Microsystems GmbH).
The obtained images were quantified using Image Pro-Plus 6.0 (Media
Cybernetics, Inc.).
Western blot analysis
The protein expression of CTGF, tissue plasminogen
activator (t-PA), plasminogen activator inhibitor (PAI),
transforming growth factor-β2 (TGFβ2), and VEGF in retinal tissues
was measured by western blot analysis. The collected retinas were
homogenized in radioimmunoprecipitation assay lysis buffer
(Bioswamp, Myhalic Biotechnology Co., Ltd.) and centrifuged at
12,000 × g for 15 min. The concentration of proteins was detected
using a bicinchoninic acid protein assay kit (Bioswamp, Myhalic
Biotechnology Co., Ltd.). Total proteins (20 μg) were
separated by 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis and transferred onto polyvinylidene fluoride
membranes (MilliporeSigma). The membranes were blocked with 5% skim
milk for 2 h at room temperature in Tris-buffered saline and
incubated with primary antibodies against CTGF (1:1,000, ab6992,
Abcam), t-PA (1:2,000, ab227069, Abcam), PAI (1:1,000, ab66705,
Abcam), TGFβ2 (1:1,000, ab113670, Abcam), VEGF (1:2,000, ab32152,
Abcam), or GAPDH (1:1,000, 2118, Cell Signaling Technology, Inc.)
for 1 h at room temperature, followed by incubation with goat
anti-rabbit IgG secondary antibody (1:10,000, SAB43714, Bioswamp,
Myhalic Biotechnology Co., Ltd.) for 2 h at room temperature.
Immunoreactivity was visualized by colorimetric reaction using an
enhanced chemiluminescence substrate buffer (MilliporeSigma).
Membranes were scanned with a Tanon-5200 imager (Shanghai Tianneng
Technology Co., Ltd.) and analyzed using IQTL 8.1 software.
Enzyme-linked immunosorbent assay
(ELISA)
The levels of interleukin (IL)-1 (RA20108), IL-6
(RA20607) and IL-10 (RA20090) in the serum of retinas were detected
using ELISA kits (Bioswamp, Myhalic Biotechnology Co., Ltd.)
according to the manufacturer's instructions. The absorbance was
measured at 450 nm using a microplate reader (Labsystems Multiskan
MS, Finland).
Statistical analysis
The data of the present study are expressed as the
means ± standard deviation (SD). Statistical analysis was performed
using IBM SPSS statistics 19.0. The statistical significance of
differences between >2 groups was evaluated by one-way analysis
of variance followed by Bonferroni's test and significant
differences between 2 groups were analyzed using a t-test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Successful extraction of UC-MSCs
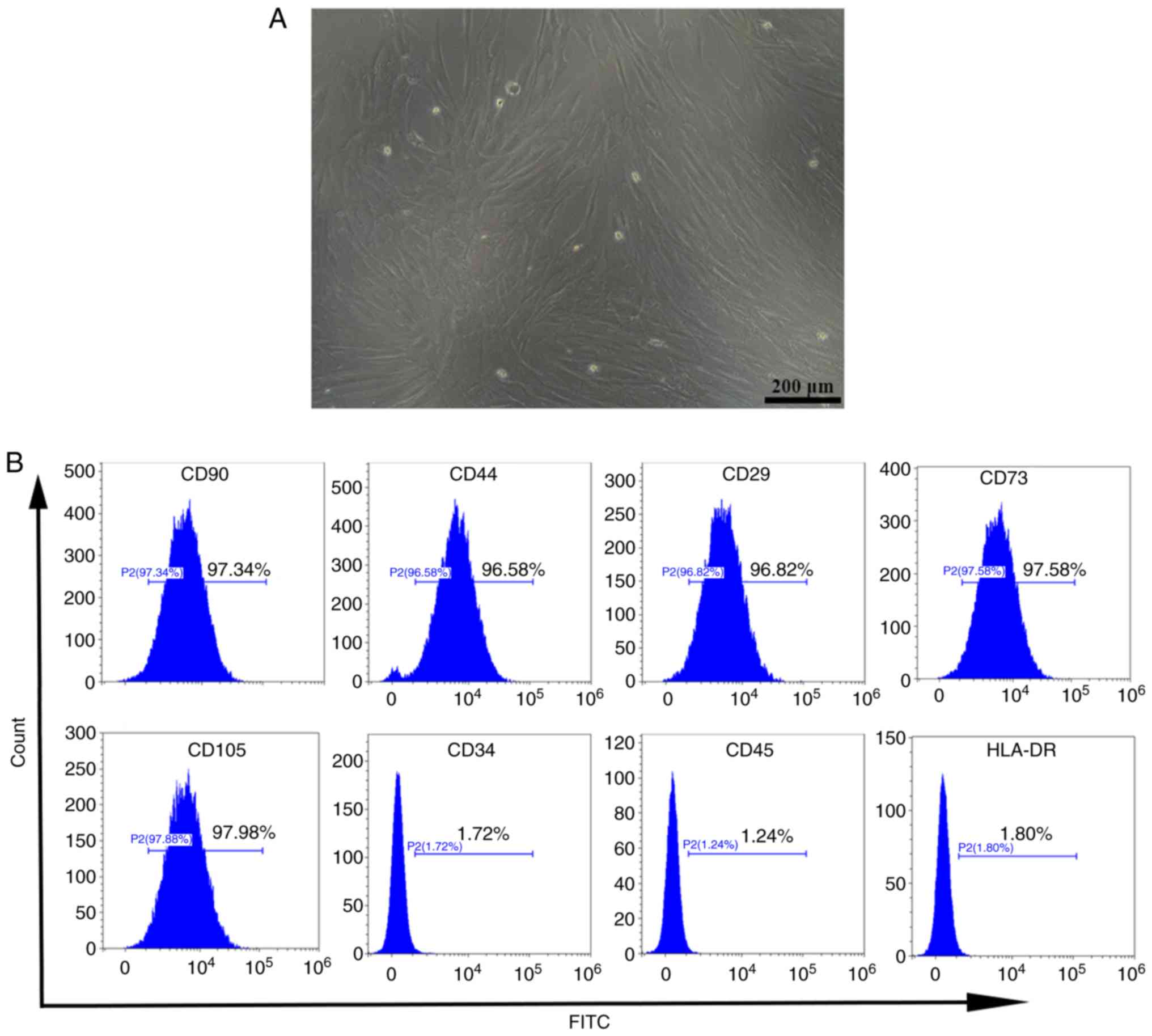
To confirm that UC-MSCs were successfully extracted,
cells were visually evaluated using a microscope, whereby the
typical spindle-shaped fibroblast-like morphology of UC-MSCs was
observed (Fig. 1A). Flow
cytometry was then carried out to detect the expression of surface
antigens of UC-MSCs. As shown in Fig.
1B, the extracted cells strongly expressed the mesenchymal cell
markers, CD90 (97.37%), CD44 (96.58%), CD29 (96.82%), CD73 (97.58%)
and CD105 (97.98%), while the hematopoietic markers, HLA-DR
(1.80%), CD45 (1.24%), and CD34, (1.72%) were absent (20,21). These findings indicate that
UC-MSCs were successfully isolated without the inclusion of other
cell populations.
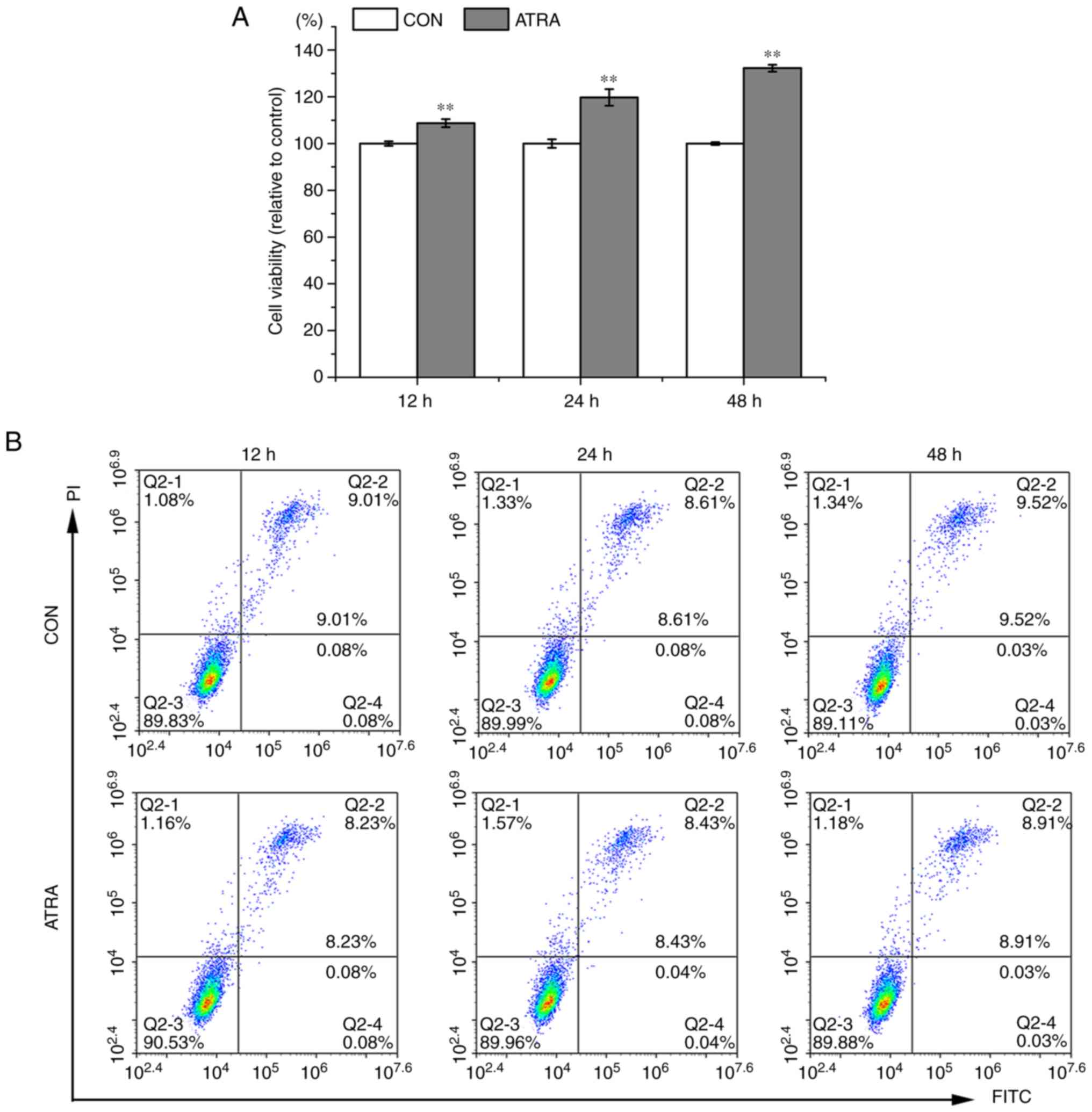
ATRA promotes UC-MSC proliferation and
differentiation
Following treatment of the UC-MSCs with ATRA for 24,
48 and 72 h, cell proliferation and differentiation were
determined. Compared to the untreated cells, the viability of the
ATRA-treated cells significantly increased (P<0.01, Fig. 2A). In addition, ATRA exerted
minimal effects on the apoptosis of UC-MSCs (Fig. 2B). Immunofluorescence staining was
performed to examine the differentiation of UC-MSCs treated with
ATRA. Strong positive staining was observed for the glial cell
marker, GFAP, the nerve cell marker, Nestin, and the
neuron-specific marker, NSE (26), in the ATRA-treated UC-MSCs
compared to that of signals in the untreated UC-MSCs (Fig. 2C). In addition, the mRNA
expression levels of Nestin, NSE and GFAP in the untreated UC-MSCs
were lower than those in the ATRA-treated UC-MSCs (Fig. 2D), suggesting that ATRA promoted
UC-MSC differentiation into different types of nerve cells.
Moreover, reticular neuron-like cells were observed (red boxes)
after the UC-MSCs were treated with ATRA for 24 h (Fig. 2C). Thus, 24 h of ATRA treatment
was selected as the final treatment duration for UC-MSCs in the
transplantation experiment.
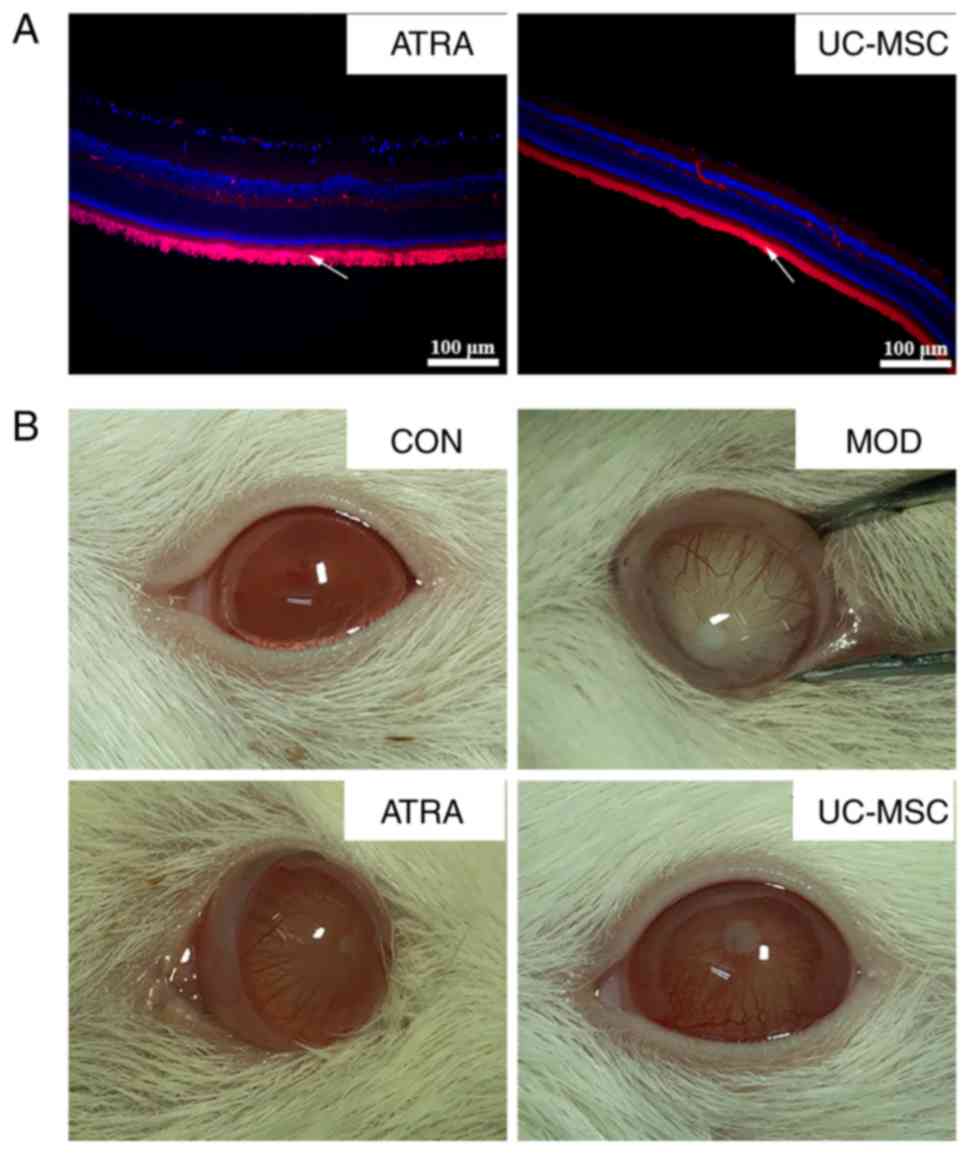
Transplantation of ATRA-treated or
untreated UC-MSCs attenuates STZ-induced DR in rats
The blood glucose levels of all selected rats with
STZ-induced DR were >16.7 mmol/l (data not shown). At 8 weeks
following transplantation with CM-Dil-labeled UC-MSCs, the UC-MSCs
in retinal tissue were visualized by immunofluorescence. A shown in
Fig. 3A, retinal tissues
contained a large number of well-distributed UC-MSCs (white arrow
pointing towards red area). The transplantation of untreated or
ATRA-treated UC-MSCs improved the lens opacity of rats with DR
macroscopically (Fig. 3B).
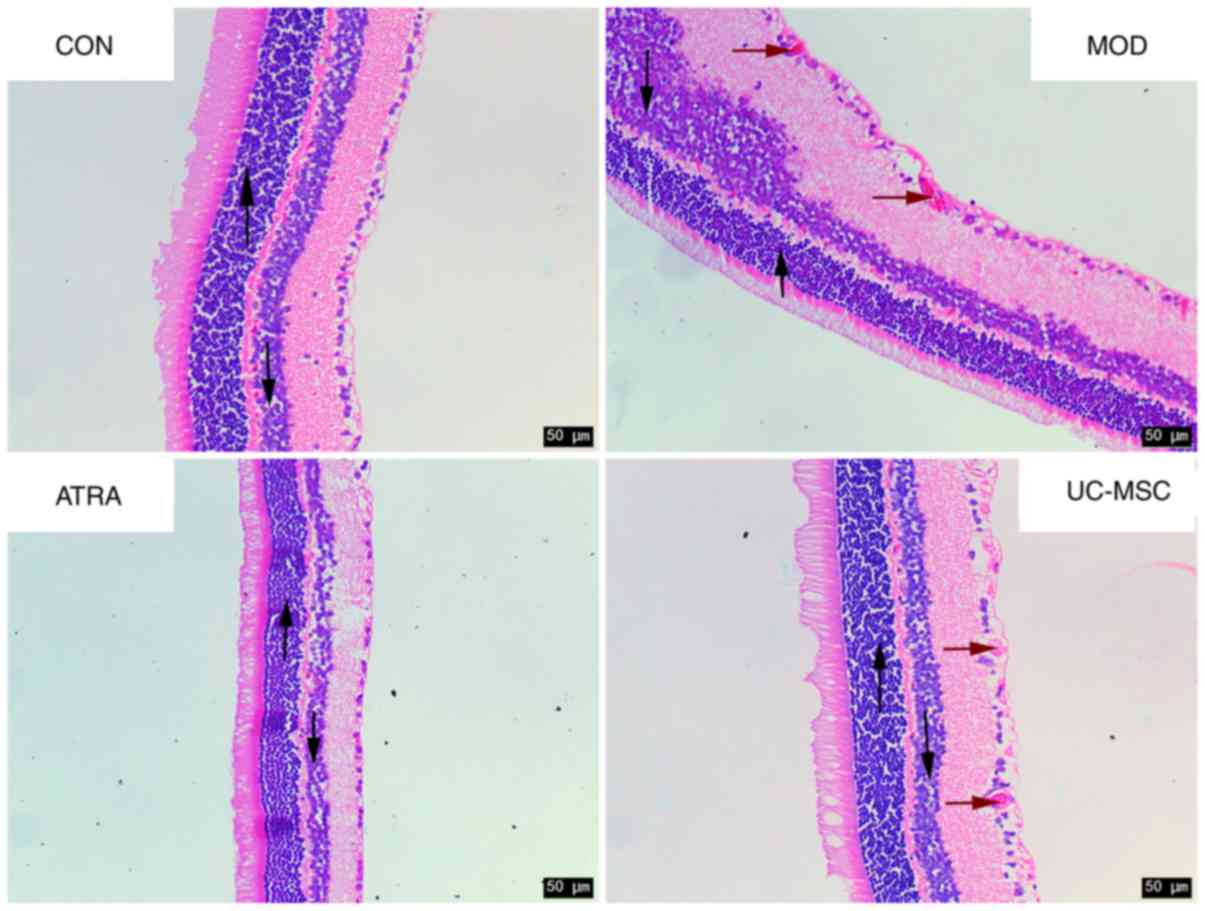
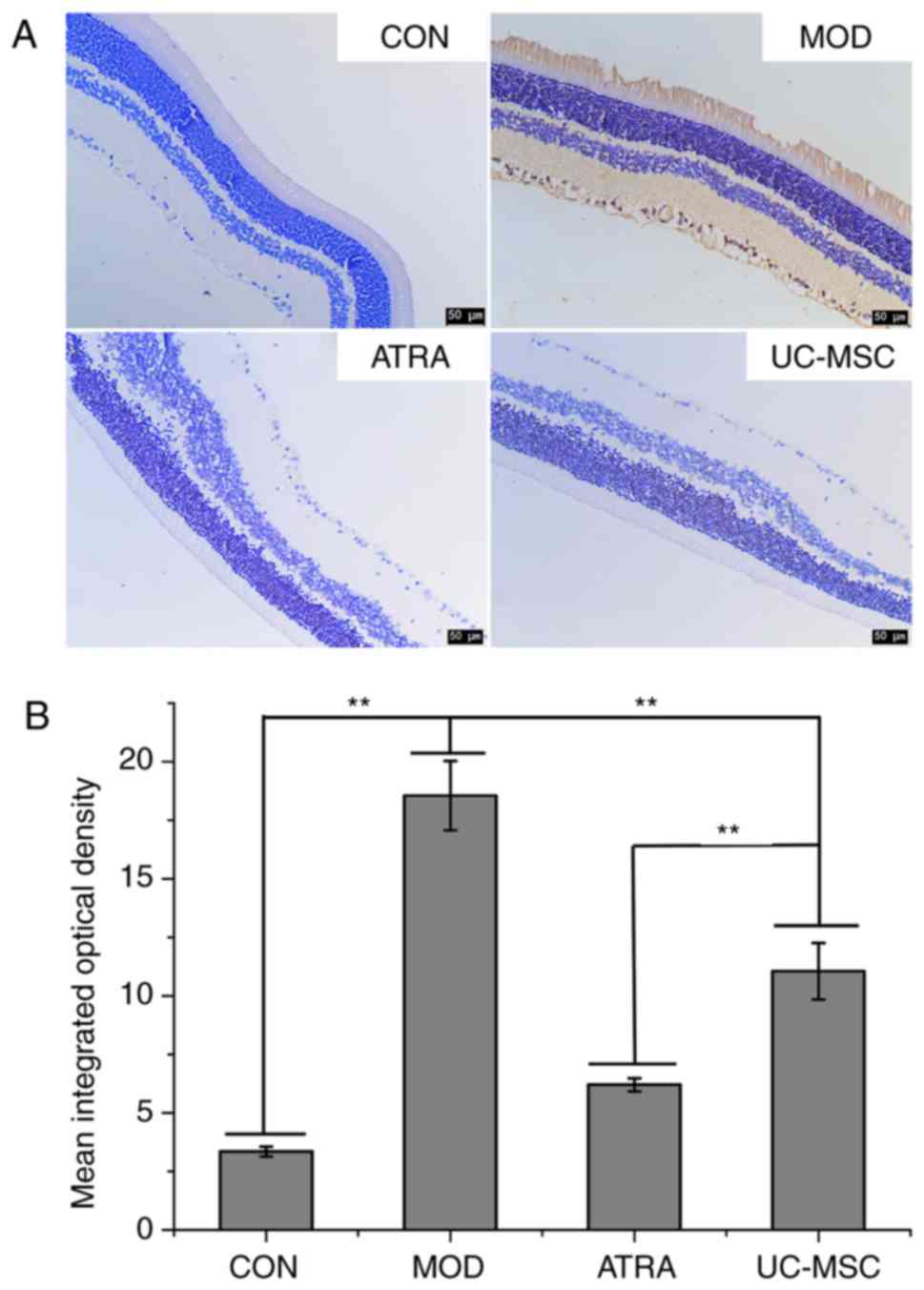
Furthermore, the pathological morphology of retinal
tissues was investigated by H&E staining (Fig. 4). The surface of the retina was
smooth, and cells at the outer nuclear layer (ONL, upward arrow),
inner nuclear layer (INL, downward arrow) and retinal ganglion
cells (RGCs) were all regularly arranged in the CON group. However,
cells at the ONL and INL appeared in irregular arrangements in the
MOD group. Additionally, RGCs protruded through the inner limiting
membrane with a rough retinal surface (red arrows). With UC-MSC
treatment, the cells at the ONL and INL exhibited a regular
arrangement. This was accompanied by greater RGC protrusion, which
was even more prominent in the ATRA group and closely resembled
that of the CON group. These findings suggest that UC-MSC
transplantation attenuated DR in rats and that ATRA further
accentuated the effects of UC-MSCs.
Transplantation of untreated or
ATRA-treated UC-MSCs attenuates apoptosis in retinal tissue of rats
with DR
TUNEL assay was performed to detect apoptosis in
retinal tissues (Fig. 5). The
number of TUNEL-positive cells (brown) was significantly higher in
the MOD group compared to the CON group (P<0.01). However, the
number of TUNEL-positive cells in the UC-MSC group decreased
compared to the MOD group (P<0.01) and further decreased in the
ATRA group (P<0.01).
Transplantation of untreated or
ATRA-treated UC-MSCs inhibits neovascularization and imminent
fibrosis in retinal tissue of rats with DR
The expression levels of the angiogenesis-related
proteins, VEGF, t-PA and PAI, and that of the fibrosis-associated
proteins, CTGF and TGFβ2, were assessed by western blot analysis.
As shown in Fig. 6, the levels of
all the above-mentioned proteins were upregulated in the MOD group
compared to those in the CON group (P<0.01). Comparatively,
UC-MSC transplantation decreased the expression of these proteins
(P<0.01) and this decrease was further enhanced in the ATRA
group compared to the UC-MSC group (P<0.01, apart from t-PA).
Therefore, UC-MSC transplantation inhibited the neovascularization
of retinal tissues in rats with DR, and treatment of UC-MSCs with
ATRA further promoted this inhibition.
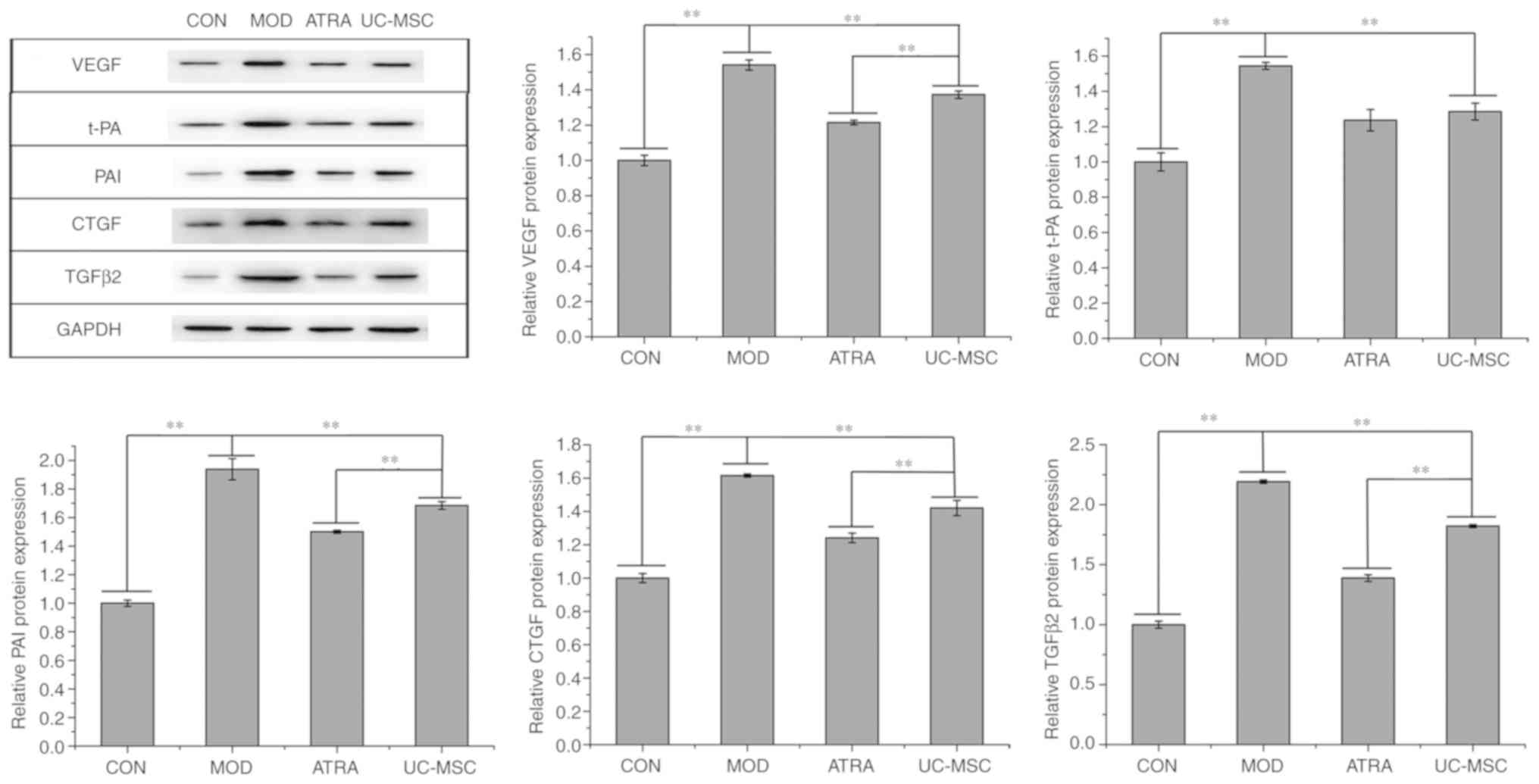 | Figure 6Angiogenesis and fibrosis in retinal
tissues. Expression and quantification of proteins related to
angiogenesis- and fibrosis-related proteins in retinal tissue. Data
represent the means ± SD (n=3), **P<0.01. VEGF,
vascular endothelial growth factor; t-PA, tissue plasminogen
activator; PAI, plasminogen activator inhibitor; TGFβ2,
transforming growth factor β2; CTGF, connective tissue growth
factor; UC-MSCs, umbilical cord-derived mesenchymal stem cells
(untreated); DR, diabetic retinopathy; ATRA, UC-MSCs treated with
all-trans retinoic acid; MOD, model group with DR; CON,
control. |
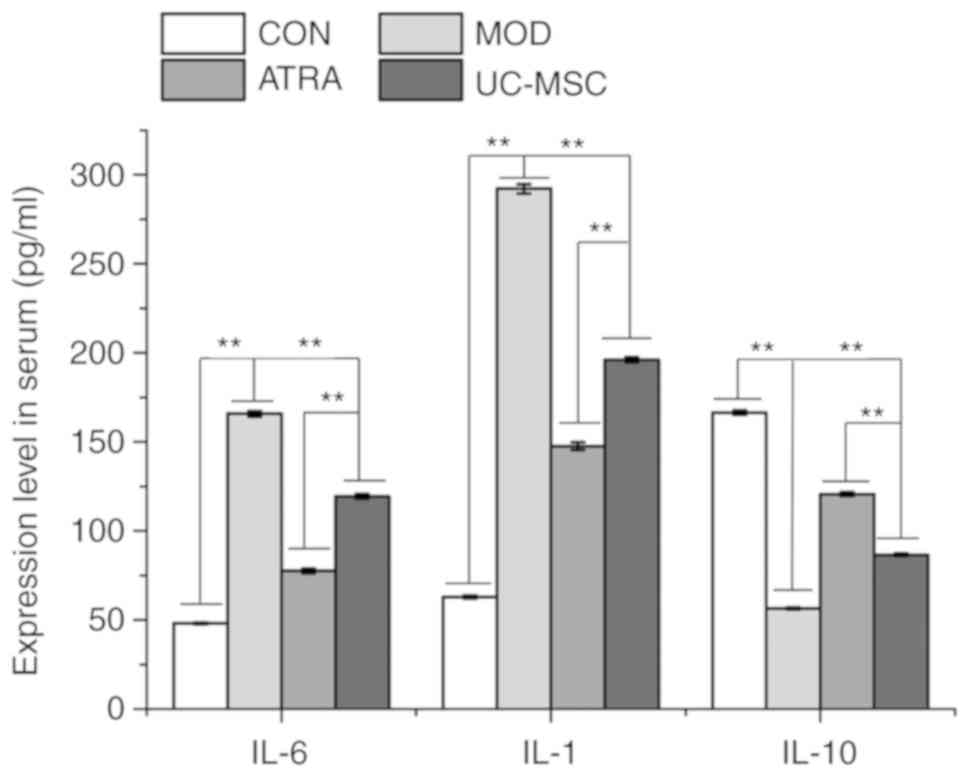
Transplantation of untreated or
ATRA-treated UC-MSCs attenuates inflammation in retinal tissue of
rats with DR
As shown in Fig.
7, compared to the CON group, the secretion levels of IL-6 and
IL-1 in the serum of retinas were notably increased in the MOD
group (P<0.01). In addition, compared to the MOD group, the
levels of IL-6 and IL-1 were markedly decreased in the UC-MSC group
(P<0.01) and were even further decreased in the ATRA group
(P<0.01). The expression IL-10 exhibited an opposite trend to
that of IL-6 and IL-1, indicating that UC-MSC transplantation
attenuated inflammation in the retinal tissue of rats with DR,
which was further suppressed by treatment of the UC-MSCs with
ATRA.
Discussion
MSC transplantation is considered a promising
intervention therapy for DR (1,27).
Differentiation into nerve cells is one of the vital functions of
MSCs in DR treatment (11,13,28).
Neural stem cells derived from UC-MSCs have been shown to exert a
neuroprotective effect and to notably attenuate the progression of
DR (13). Nevertheless, research
into the role of MSCs in the treatment of DR remains incomplete,
and the mechanisms of retinal repair at the molecular level remain
unclear. The present study demonstrated that treatment with ATRA
promoted the proliferation of UC-MSCs and their differentiation
into nerve cells. The transplantation of UC-MSCs with or without
ATRA treatment visually ameliorated DR in rats with STZ-induced
diabetes, alleviated retinal tissue damage and apoptosis, inhibited
angiogenesis and attenuated inflammation in the retina via the
regulation of various cytokines. Moreover, UC-MSCs treated with
ATRA exerted a more prominent therapeutic effect than the
transplantation of untreated UC-MSCs.
Several experimental and clinical studies have
identified abnormal ocular angiogenesis as a critical
pathophysiologic mediator of DR (29-31); for example, uncontrolled ocular
angiogenesis leads to a leaky and fragile vasculature. The
subsequent accumulation of fluids and protein exudates in ocular
cavities results in the enhancement of corneal opacity, which
eventually leads to blindness, a devastating feature of the final
stage of the disease (32). VEGF,
a recognized pro-angiogenic cytokine, is overexpressed in response
to a maintained hyperglycemic environment (33,34), resulting in retinal
neovascularization, which is involved in the pathogenesis of DR
(35). Anti-VEGF therapy has been
considered safe and promising for the management of DR (35). Apart from activating retinal
angiogenesis, VEGF participates in activating several biologically
active substances, such as t-PA and PAI, which are involved in
neovascularization (36). T-PA
and PAI are highly expressed in DR and are associated with their
occurrence and development (36,37). The present study demonstrated that
VEGF, t-PA and PAI were highly expressed in rats with DR, but were
downregulated after UC-MSC transplantation, resulting in the
attenuation of DR. In addition, UC-MSCs treated with ATRA exhibited
a better efficiency than untreated UC-MSCs. Retinal fibrosis is
another important factor in the pathogenesis of DR. It has been
reported that elevated levels of CTGF and TGFβ2 are positively
associated with the formation of retinal fibrosis (38-40), and that fibrosis is accelerated by
the upregulation of CTGF and TGFβ2 in DR (41). The intravitreal injection of
anti-CTGF shRNA has been shown to reduce the level of CTGF in
diabetic mice, leading to decreased injury in the retinal
microvascular structure (42).
The present study demonstrated that UC-MSC transplantation reduced
the expressionω of CTGF and TGFβ2 in rats with DR.
Additionally, diabetic-induced inflammation plays a
vital role in the pathogenesis of DR, which may induce
leukocyte-endothelial interactions and eventually cause damage to
the retinal microvasculature (22,43). MSCs possess immunomodulatory
functions and are considered to be promising alternative agents in
the treatment of inflammatory diseases (44). MSCs infected with C-X-C chemokine
receptor type 4 have been shown to attenuate the progression of DR
by inhibiting the expression of inflammation-regulating cytokines,
such as IL-6 and tumor necrosis factor-α (8). The present study demonstrated that
UC-MSC transplantation reduced the levels of pro-inflammatory IL-1
and IL-6 (45), while increasing
an anti-inflammatory response (46) in rats with STZ-induced DR.
Moreover, the ATRA-treated UC-MSCs exhibited a better efficiency
than the untreated UC-MSCs.
Overall, the present study suggested that UC-MSCs
attenuated STZ-induced DR in rats by regulating cytokines related
to angiogenesis and inflammation at the molecular level. The effect
of angiogenesis and anti-apoptosis may be associated with the
differentiation of MSCs into neural cells, although the effect may
also be in conjunction with the differentiation of MSCs into neural
cells. The transplantation of UC-MSCs treated with ATRA displayed a
more prominent therapeutic effect than untreated UC-MSCs, as
demonstrated by its better regulation of angiogenesis and
inflammation in rats with STZ-induced DR, and the proliferation and
differentiation of MSCs. These findings provide a theoretical basis
for and new insight into the application of MSCs in the treatment
of DR. However, due to neglect, the weights of the rats were not
recorded in the present study. The authors aim to certainly record
the weight changes of rats in follow-up studies, which will explore
the specific signaling pathways associated with angiogenesis and
the inflammatory response in rats with DR treated with UC-MSCs.
Funding
This study was supported by the Health Commission of
Hubei Province Scientific Research Project (grant no.
WJ2009F038).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XX and ZC were responsible for the conception and
design of the study. KZ, JL, GD, PW and HX performed the
experiments. KZ and JL analyzed the data and drafted the
manuscript. XX and ZC revised the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
All animal protocols were approved by the
Institutional Review Board of Wuhan Myhalic Biotechnology Co., Ltd.
based on the ethical Guidelines for Animal Care and Use of the
Model Animal Research Institute (HLK-20180802-01).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Abbreviations:
|
DR
|
diabetic retinopathy
|
|
UC-MSCs
|
umbilical cord-derived mesenchymal
stem cells
|
|
ATRA
|
all-trans retinoic acid
|
|
STZ
|
streptozocin
|
|
NES
|
neuron-specific enolase
|
|
GFAP
|
glial fibrous acidic protein
|
|
CTGF
|
connective tissue growth factor
|
|
t-PA
|
tissue plasminogen activator
|
|
PAI
|
plasminogen activator inhibitor
|
|
TGFβ2
|
transforming growth factor β2
|
|
VEGF
|
vascular endothelial growth factor
|
|
IL-1
|
interleukin-1
|
Acknowledgments
Not applicable.
References
|
1
|
Fiori A, Terlizzi V, Kremer H, Gebauer J,
Hammes HP, Harmsen MC and Bieback K: Mesenchymal stromal/stem cells
as potential therapy in diabetic retinopathy. Immunobiology.
223:729–743. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Whiting DR, Guariguata L, Weil C and Shaw
J: IDF diabetes atlas: Global estimates of the prevalence of
diabetes for 2011 and 2030. Diabetes Res Clin Pract. 94:311–321.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yau JW, Rogers SL, Kawasaki R, Lamoureux
EL, Kowalski JW, Bek T, Chen SJ, Dekker JM, Fletcher A, Grauslund
J, et al: Global prevalence and major risk factors of diabetic
retinopathy. Diabetes Care. 35:556–564. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Selvaraj K, Gowthamarajan K, Karri VV,
Barauah UK, Ravisankar V and Jojo GM: Current treatment strategies
and nanocarrier based approaches for the treatment and management
of diabetic retinopathy. J Drug Target. 25:386–405. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang C, Xu Y, Tan HY, Li S, Wang N, Zhang
Y and Feng Y: Neuroprotective effect of He-Ying-Qing-Re formula on
retinal ganglion cell in diabetic retinopathy. J Ethnopharmacol.
214:179–189. 2018. View Article : Google Scholar
|
|
6
|
Ding DC, Shyu WC and Lin SZ: Mesenchymal
stem cells. Cell Transplant. 20:5–14. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nagamura-Inoue T and He H: Umbilical
cord-derived mesenchymal stem cells: Their advantages and potential
clinical utility. World J Stem Cells. 6:195–202. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gu X, Yu X, Zhao C, Duan P, Zhao T, Liu Y,
Li S, Yang Z, Li Y, Qian C, et al: Efficacy and safety of
autologous bone marrow mesenchymal stem cell transplantation in
patients with diabetic retinopathy. Cell Physiol Biochem. 49:40–52.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang J, Zhang W, He GH, Wu B and Chen S:
Transfection with CXCR4 potentiates homing of mesenchymal stem
cells in vitro and therapy of diabetic retinopathy in vivo. Int J
Ophthalmol. 11:766–772. 2018.
|
|
10
|
Scalinci SZ, Scorolli L, Corradetti G,
Domanico D, Vingolo EM, Meduri A, Bifani M and Siravo D: Potential
role of intravitreal human placental stem cell implants in
inhibiting progression of diabetic retinopathy in type 2 diabetes:
Neuroprotective growth factors in the vitreous. Clin Ophthalmol.
5:691–696. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang Z, Li K, Yan X, Dong F and Zhao C:
Amelioration of diabetic retinopathy by engrafted human
adipose-derived mesenchymal stem cells in streptozotocin diabetic
rats. Graefes Arch Clin Exp Ophthalmol. 248:1415–1422. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hsieh JY, Wang HW, Chang SJ, Liao KH, Lee
IH, Lin WS, Wu CH, Lin WY and Cheng SM: Mesenchymal stem cells from
human umbilical cord express preferentially secreted factors
related to neuroprotection, neurogenesis, and angiogenesis. PLoS
One. 8:e726042013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang W, Wang Y, Kong J, Dong M, Duan H
and Chen S: Therapeutic efficacy of neural stem cells originating
from umbilical cord-derived mesenchymal stem cells in diabetic
retinopathy. Sci Rep. 7:4082017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mottaghi S, Larijani B and Sharifi AM:
Atorvastatin: An efficient step forward in mesenchymal stem cell
therapy of diabetic retinopathy. Cytotherapy. 15:263–266. 2013.
View Article : Google Scholar
|
|
15
|
Siddikuzzaman, Guruvayoorappan C and
Berlin Grace VM: All trans retinoic acid and cancer.
Immunopharmacol Immunotoxicol. 33:241–249. 2011. View Article : Google Scholar
|
|
16
|
Gong M, Bi Y, Jiang W, Zhang Y, Chen L,
Hou N, Chen J and Li T: Retinoic acid receptor beta mediates
all-trans retinoic acid facilitation of mesenchymal stem cells
neuronal differentiation. Int J Biochem Cell Biol. 45:866–875.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tanaka K, Tamiya-Koizumi K, Hagiwara K,
Ito H, Takagi A, Kojima T, Suzuki M, Iwaki S, Fujii S, Nakamura M,
et al: Role of down-regulated neutral ceramidase during all-trans
retinoic acid-induced neuronal differentiation in SH-SY5Y
neuroblastoma cells. J Biochem. 151:611–620. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Murashov AK, Pak ES, Hendricks WA, Owensby
JP, Sierpinski PL, Tatko LM and Fletcher PL: Directed
differentiation of embryonic stem cells into dorsal interneurons.
FASEB J. 19:252–254. 2005. View Article : Google Scholar
|
|
19
|
Xi J and Yang Z: Expression of RALDHs
(ALDH1As) and CYP26s in human tissues and during the neural
differentiation of P19 embryonal carcinoma stem cell. Gene Expr
Patterns. 8:438–442. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pourjafar M, Saidijam M, Etemadi K and
Najafi R: All-trans retinoic acid enhances in vitro mesenchymal
stem cells migration by targeting matrix metalloproteinases 2 and
9. Biotechnol Lett. 39:1263–1268. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jin W, Xu YP, Yang AH and Xing YQ: In
vitro induction and differentiation of umbilical cord mesenchymal
stem cells into neuron-like cells by all-trans retinoic acid. Int J
Ophthalmol. 8:250–256. 2015.PubMed/NCBI
|
|
22
|
Yin Y, Chen F, Wang W, Wang H and Zhang X:
Resolvin D1 inhibits inflammatory response in STZ-induced diabetic
retinopathy rats: Possible involvement of NLRP3 inflammasome and
NF-κB signaling pathway. Mol Vis. 23:242–250. 2017.
|
|
23
|
Jenkins AJ, Joglekar MV, Hardikar AA,
Keech AC, O'Neal DN and Januszewski AS: Biomarkers in diabetic
retinopathy. Rev Diabet Stud. 12:159–195. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
25
|
Xie X, Peng J, Huang K, Huang J, Shen X,
Liu P and Huang H: Polydatin ameliorates experimental
diabetes-induced fibronectin through inhibiting the activation of
NF-κB signaling pathway in rat glomerular mesangial cells. Mol Cell
Endocrinol. 362:183–193. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fu X, Li S, Zhou S, Wu Q, Jin F and Shi J:
Stimulatory effect of icariin on the proliferation of neural stem
cells from rat hippo-campus. BMC Complement Altern Med. 18:342018.
View Article : Google Scholar
|
|
27
|
Ding SSL, Subbiah SK, Khan MSA, Farhana A
and Mok PL: Empowering mesenchymal stem cells for ocular
degenerative disorders. Int J Mol Sci. 20:pii: E1784. 2019.
View Article : Google Scholar
|
|
28
|
Yue X, Zhifeng G, Biyu S, Guofeng X,
Tianqiu Z, Jinxia J, Jing X, Suzhe L, Man L, Wei T, et al: Roles of
Wnt/β-catenin signaling in retinal neuron-like differentiation of
bone marrow mesenchymal stem cells from nonobese diabetic mice. J
Mol Neurosci. 49:250–261. 2013. View Article : Google Scholar
|
|
29
|
Wu W, Lei H, Shen J and Tang L: The role
of netrin-1 in angiogenesis and diabetic retinopathy: A promising
therapeutic strategy. Discov Med. 23:315–323. 2017.PubMed/NCBI
|
|
30
|
Gardlik R and Fusekova I: Pharmacologic
therapy for diabetic retinopathy. Semin Ophthalmol. 30:252–263.
2015. View Article : Google Scholar
|
|
31
|
Khan AA, Rahmani AH and Aldebasi YH:
Diabetic retinopathy: Recent updates on different biomarkers and
some therapeutic agents. Curr Diabetes Rev. 14:523–533. 2018.
View Article : Google Scholar
|
|
32
|
Zhang SX and Ma JX: Ocular
neovascularization: Implication of endogenous angiogenic inhibitors
and potential therapy. Prog Retin Eye Res. 26:1–37. 2007.
View Article : Google Scholar
|
|
33
|
Boyer DS, Hopkins JJ, Sorof J and Ehrlich
JS: Anti-vascular endothelial growth factor therapy for diabetic
macular edema. Ther Adv Endocrinol Metab. 4:151–169. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Stewart MW: Anti-vascular endothelial
growth factor drug treatment of diabetic macular edema: The
evolution continues. Curr Diabetes Rev. 8:237–246. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bahrami B, Hong T, Gilles MC and Chang A:
Anti-VEGF therapy for diabetic eye diseases. Asia Pac J Ophthalmol
(Phila). 6:535–545. 2017.
|
|
36
|
Wu SL, Zhan DM, Xi SH and He XL: Roles of
tissue plasminogen activator and its inhibitor in proliferative
diabetic retinopathy. Int J Ophthalmol. 7:764–767. 2014.PubMed/NCBI
|
|
37
|
Wu S and He X: The correlation study of
the expression of VEGF with t-PA and PAI expression in plasma and
intraocular tissue in proliferative diabetic retinopathy. Zhonghua
Yan Ke Za Zh. 50:448–453. 2014.In Chinese.
|
|
38
|
Zhang M, Chu S, Zeng F and Xu H:
Bevacizumab modulates the process of fibrosis in vitro. Clin Exp
Ophthalmol. 43:173–179. 2015. View Article : Google Scholar
|
|
39
|
Van Geest RJ, Lesnik-Oberstein SY, Tan HS,
Mura M, Goldschmeding R, Van Noorden CJ, Klaassen I and
Schlingemann RO: A shift in the balance of vascular endothelial
growth factor and connective tissue growth factor by bevacizumab
causes the angiofibrotic switch in proliferative diabetic
retinopathy. Br J Ophthalmol. 96:587–590. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kuiper EJ, Van Nieuwenhoven FA, de Smet
MD, van Meurs JC, Tanck MW, Oliver N, Klaassen I, Van Noorden CJ,
Goldschmeding R and Schlingemann RO: The angio-fibrotic switch of
VEGF and CTGF in proliferative diabetic retinopathy. PLoS One.
3:e26752008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang Q, Qi Y, Chen L, Shi X, Bai Y, Huang
L, Yu W, Jiang Y, Zhao M and Li X: The relationship between
anti-vascular endothelial growth factor and fibrosis in
proliferative retinopathy: Clinical and laboratory evidence. Br J
Ophthalmol. 100:1443–1450. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hu B, Zhang Y, Zeng Q, Han Q, Zhang L, Liu
M and Li X: Intravitreal injection of ranibizumab and CTGF shRNA
improves retinal gene expression and microvessel ultrastructure in
a rodent model of diabetes. Int J Mol Sci. 15:1606–1624. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cheung N, Mitchell P and Wong TY: Diabetic
retinopathy. Lancet. 376:124–136. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Regmi S, Pathak S, Kim JO, Yong CS and
Jeong JH: Mesenchymal stem cell therapy for the treatment of
inflammatory diseases: Challenges, opportunities, and future
perspectives. Eur J Cell Biol. 98:1510412019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Navarro-Gonzalez JF and Mora-Fernandez C:
The role of inflammatory cytokines in diabetic nephropathy. J Am
Soc Nephrol. 19:433–442. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Petersen AM and Pedersen BK: The
anti-inflammatory effect of exercise. J Appl Physiol (1985).
98:1154–1162. 2005. View Article : Google Scholar
|