Introduction
Atopic dermatitis (AD) is the most common chronic
inflammatory skin disease. This condition generally occurs in
infancy and is characterized by pruritus, a chronically relapsing
course of eczematous lesions and elevated serum IgE levels
(1). AD is considered to be
triggered by a complex combination of multiple factors. Risk
factors for AD consist of environmental changes, genetic
susceptibility, epidermal barrier abnormalities, the dysbiosis of
skin microbiota and T cell-driven skin inflammation (2). All of the above-mentioned factors
can lead to the clinical heterogeneity observed among patients with
AD.
Interleukin (IL)-18 is generally expressed in AD.
IL-18 respectively enhances Th1 and Th2 responses, including
allergic inflammation under different surrounding cytokine
environments (3). IL-18 was
originally considered a Th1 cytokine through its ability to induce
interferon (IFN)-γ production (4). The activation of caspase-1 and
cleaved pro-IL-1β and pro-IL18 result in the active forms of IL-1β
and IL18, respectively. These active forms can then stimulate
inflammatory responses (5). IL-18
has been reported to induce the production of IgE and Th2
cytokines, such as IL-4 and IL-13 (6). IL-18 has also been shown to be
associated with the severity of AD (7). In addition, the scoring of AD
(SCORAD), a recommended outcome assessment for the symptoms of AD,
has been shown to be positively associated with serum IL-18 levels
in patients with AD (8,9). However, the biological activities of
IL-18, associate with a number of skin diseases, are not yet fully
understood.
Thymic stromal lymphopoietin (TSLP) is a cytokine
produced by keratinocytes, fibroblasts and mast cells upon exposure
to allergens, viral infections, trauma and other cytokines. It is
highly expressed in acute and chronic lesions of AD and is
considered the 'master-switch of allergic inflammation' due to its
central role in evoking Th2 responses via dendritic cell activation
(10,11). In general, AD is a complex,
chronic inflammatory disease associated with environmental
influences, underlying defects in the epidermal barrier and
cytokine dysregulation.
The pathogenic role of IL-18, as regards AD, remains
to be determined. One approach to examine this association involves
the use of animal models of AD. Such a model can be induced in mice
through the topic application of calcipotriol (MC903), a
low-calcemic analog of vitamin D3 (12,13). In the present study, the effects
of exposure to MC903 were examined in IL-18 knockout (KO) mice in
order to investigate the effects of IL-18 on the development of
AD-like lesions.
Materials and methods
Mice and treatment
Female wild-type (WT) and IL-18 KO C57BL/6 mice
(12-15 weeks old, weight range, 22-25 g; n=20) were purchased from
Jackson Laboratory (stock no. C57BL/6:000664-JAX; IL-18 KO:
004130-JAX). Female mice were used as in general, female mice are
considered less likely to exhibit aggressive behavior, which can
lead to unnecessary skin injuries, which may have impeded the
research purposes of the present study. According to
Moosbrugger-Martinz et al (12), AD-like symptoms induced by MC903
were not dependent on mouse sex or on genetic background. An
AD-like mouse model established using female mice is widely used by
a number of researchers (13-15). In the present study, all mice were
housed individually in ventilated cages under specific
pathogen-free conditions at 22±2̊C with a 12-h light/dark cycle.
All experimental protocols were strictly approved by the guide for
the care and use of laboratory animals (NIH Publication, 8th
Edition, 2011) (16) and were
conducted according to the guidelines provided and approved by the
Institutional Animal Care and Use Committee at China Medical
University (IACUC no. 16008M). The mice were divided into 4 groups
(n=5/group) as follows: i) The WT control; ii) IL-18 KO control;
iii) MC903-treated WT mice; and iv) MC903-treated IL-18 KO
mice.
To establish an AD-like mouse model, all mice were
shaved on their dorsal skin surface to expose a 2x3 cm area. MC903
(4 nmol; Sigma-Aldrich; Merck KGaA) diluted in ethanol was applied
once a day topically to the exposed skin for 15 consecutive days
according to previously published protocols (12,14,17). The mice in the control groups
received an identical volume of ethanol. Skin samples were
collected from all mice on day 16.
Scoring the severity of skin
inflammation
To evaluate the severity of AD, the scoring of
dermatitis was conducted in each group on days 0, 1, 3, 5, 7, 9,
11, 13 and 15. The scoring of dermatitis (SCORAD) represents an
established method with which to assess the signs of AD (9,18).
This method consists of scoring the following factors on a scale
from 0-3: Erythema, papule/edema, exudation/crust, excoriation,
fissures and lichenification. The scores were classified as
follows: 0 (none), 1 (mild), 2 (moderate) and 3 (severe) and were
recorded independently by 2 investigators on every two 2 prior to
the daily application of MC903.
Sample collection
The mice were anesthetized by 3-4% isoflurane for
the induction and 1.5-2% for the maintenance of anesthesia to
finish daily application of MC903. On day 16, all mice were
euthanized by raising to 10% isoflurane for 5 min following the
induction of anesthesia. Then they were transferred to a closed
container with significantly high dose isoflurane for sample
collection. When the mice were fully sedated (by the lack of active
paw reflex, and the termination of breathing for >2 min), blood
samples were then obtained by cardiac puncture. Serum was collected
after centrifugation and stored at -80̊C. Treated skin areas were
obtained and then divided into 2 sections. One section was stored
at -80̊C immediately for the extraction of total RNA and the second
was fixed with 4% paraformaldehyde for histopathological analysis
and immunohistochemistry.
Histopathological examination
The collected tissues fixed with 4% paraformaldehyde
were embedded in paraffin and cut into 5-µm-thick sections.
Deparaffinized tissue sections were stained with hematoxylin and
eosin (Solarbio, G1121) and Toluidine blue (Solarbio, G3663) using
standard procedures at room temperature. The staining duration for
staining with hematoxylin, eosin and toluidine blue was 5, 1 and 10
min, respectively. All sections were examined by 2 independent
pathologists with use of an Olympus CHB213 light microscope
(Olympus Corporation) at x400 magnification to measure the
thickness of the epidermis and to count the number of neutrophils,
eosinophils, histocytes and mast cells per 0.025 mm2 of dermis. The distance from the
stratum corneum to the basement membrane zone were measured as the
thickness of the epidermis. The average maximum thickness at 3 high
magnification fields was obtained by 2 independent individuals. The
number of neutrophils, eosinophils, histocytes and mast cells per
0.025 mm2 of dermis were
counted.
Immunohistochemistry
Tissue sections were incubated overnight at 4̊C with
IL-1b polyclonal rabbit antibody (dilution, 1:100; cat. no. ab9722,
Abcam), mouse Anti-filaggrin (FLG) rabbit polyclonal antibody
(dilution, 1:100; cat. no. TA323075, Origene) or signal transducer
and activator of transcription (STAT)3 rabbit monoclonal antibody
(dilution, 1:300; cat. no. ab31370, Abcam). The EliVision Super Kit
(Maixin) was then used for immunostaining. A total of 5 views were
selected in the center of each tissue slide for evaluation. The
mean integral optic density (MIOD) was used to analyze overall
protein expression as calculated by dividing the integral optic
density (IOD) value by area obtained with the use of Image-Pro Plus
6.0 software (Media Cybernetics, Inc.).
Determination of total serum IgE levels
and TSLP
Serum levels of total IgE and TSLP were measured
with the use of mouse serum enzyme-linked immunosorbent assay kits
(Thermo Fisher Scientific, Inc. and R&D Systems, Inc.)
according to the manufacturers' protocols.
RNA isolation and reverse
transcription-quantitative PCR
Total RNA was extracted from the treated skin of
each mouse with the use of an miRNeasy mini kit (Qiagen GmbH)
according to the manufacturers' instructions and quantified with a
ND1000 Nanometer. Complementary DNA (cDNA) was synthesized by
utilizing 1 µg mRNA extracted as templates with the GoScript
Reverse Transcription kit (Promega Corp.) according to the
protocols supplied. qPCR was performed in 384-well plates with use
of RT2 SYBR-Green qPCR Mastermix (Promega Corp.) and the 7900 HT
Fast Real-Time PCR system (Applied Biosystems). The real-time qPCR
mix contained 0.2 µl of each primer, 0.1 µl CXR Reference Dye, 5 µl
qPCR Master Mix and 1 µl of cDNA. Nuclease-free water was added to
achieve a final reaction volume of 10 µl. The real-time PCR
reaction conditions were set to 95°C for 2 min, followed by 40
cycles at 95°C prolongation for 15 sec and 60°C for 1 min each. A
melting curve was then calculated for each PCR product to confirm
synthesis specificity. The Ct value was calculated with use of the
RQ Manager software (Applied Biosystems). Glyceraldehyde
3-phosphate dehydrogenase (GAPDH) was used as the internal control
and expression levels of target genes were calculated by applying
the 2-ΔΔCq method (19). The experiments were repeated 3
times. The primer sequences for GAPDH IL-1β, IL-4, IL-9, STAT3,
corticotropin-releasing hormone receptor (CRHR2) TSLP and caspase-1
were designed using Primer-Premier 6.0 software. The sequences of
the primers were as follows: GAPDH sense, 5′-GTG CTC TCT GCT CCT
CCC TGT-3′ and antisense, 5′-CGG CCA AAT CCG TTC ACA CCG-3′; IL-1β
sense, 5′-CAC TAC AGG CTC CGA GAT GAA-3′ and antisense, 5′-TGT CGT
TGC TTG GTT CTC CT-3′; IL-4 sense, 5′-GAA CTC TAG TGT TCT CAT GGA
GC-3′ and antisense, 5′-AGT GAT GTG GAC TTG GAC TCA T-3′; IL-9
sense, 5′-CAA CCT GCT GAC ATT CTA CAG AG-3′ and antisense, 5′-CCT
GAC ATC GCT CCA GAG ATT T-3′; STAT3 sense, 5′-AGG ACA TCA GTG GCA
AGA CC-3′ and antisense, 5′-GTA GAG GTA GAC AAG TGG AGA CA-3′;
CRHR2 sense, 5′-GAC CAC GGG AAG TGA G TT A-3′ and antisense, 5′-TCC
CAG GCA TAC TCT GAT TT-3′; caspase-1 sense, 5′-CGT GGAG AGA AAC AAG
GAG TG-3′ and antisense, 5′-TGG TGT TGA AGA GCA GAA AGC-3′.
Statistical analysis
Data are expressed as the mean and the standard
error of mean (SEM) and statistically significant differences
between 2 groups were determined using the Student's t-test.
Results were considered statistically significant when P<0.05.
GraphPad Prism 5 (GraphPad Software, Inc.) was used for statistical
analysis and for plotting the graphs.
Results
IL-18 is associated with the severity of
MC903-induced AD-like lesions
An IL-18 deficiency alleviated the AD-like
inflammatory symptoms induced by MC903 in mice. More severe degrees
of erythema, edema, scaling, exudation and crusts were observed in
the MC903-treated skin samples of the WT vs. the IL-18 KO mice. By
contrast, all mice in the control groups exhibited mild erythema
(Fig. 1A). The SCORAD values in
the MC903-treated mice were significantly increased in the WT as
compared with the IL-18 KO mice (WT mice, 8.4±0.5099; KO mice,
3.4±0.5099; P=0.0001; Fig. 1B and
C). These results are very similar with those reported
previously (8).
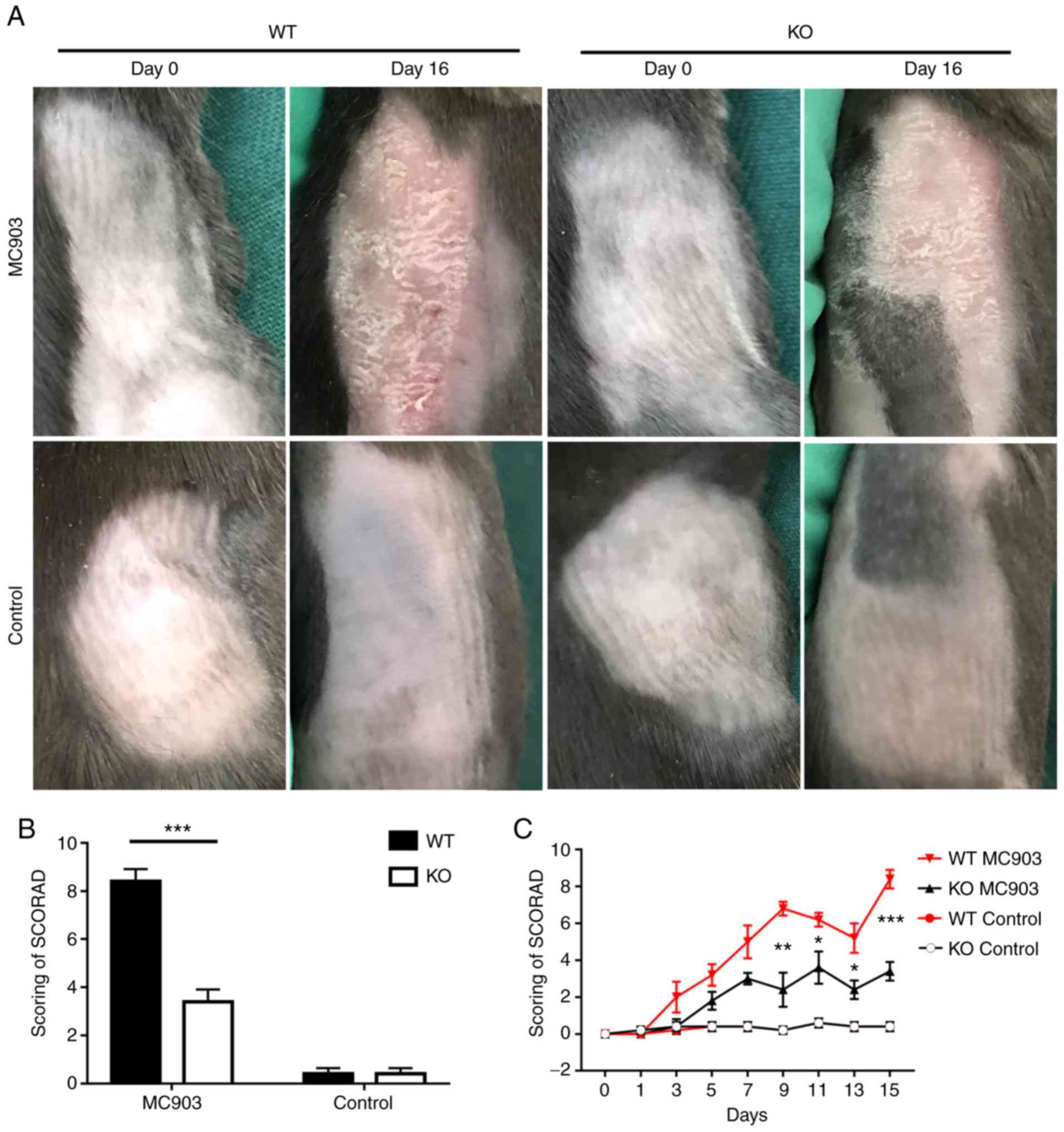 | Figure 1(A) Topical application of MC903
induces AD-like lesions in WT and KO mice, (B) Scoring of
dermatitis (SCORAD) revealed higher scores in WT mice vs. KO mice
treated with MC903 (WT mice, 8.4±0.5099; KO mice, 3.4±0.5099,
***P=0.0001). No statistically significant differences
were observed between WT mice and KO controls (WT mice, 1.8±0.3742;
KO mice, 1.4±0.2449, P>0.05). Data are expressed as the means ±
SEM. (C) SCORAD was conducted on days 0, 1, 3, 5, 7, 9, 11, 13 and
15 following MC903 treatment. *P<0.05,
**P<0.01, ***P<0.001. AD, atopic
dermatitis; WT, wild-type; KO, IL-18 knockout. |
IL-18 deficiency attenuates AD-like
inflammation induced by MC903 in mice
Compared to the control groups, histopathological
examination of the skin samples of the MC903-treated mice revealed
acanthosis, hyperkeratosis and parakeratosis. An increased number
of inflammatory cells infiltrated the dermis, including
neutrophils, histocytes, eosinophils, mast cells and lymphocytes,
along with dermal fibrosis. Lymphocytes occasionally extended into
the epidermis (Fig. 2A). These
results are consistent with those reported previously (13,20). In the MC903-induced AD-like
lesions, IL-18 deficiency resulted in lower numbers of total
inflammatory cell infiltration than that observed in the WT mice
(WT mice, 28.24±1.996 cells per 0.025 mm2; KO mice, 20.8±2.274 cells per 0.025
mm2; P<0.05; Fig. 2B).
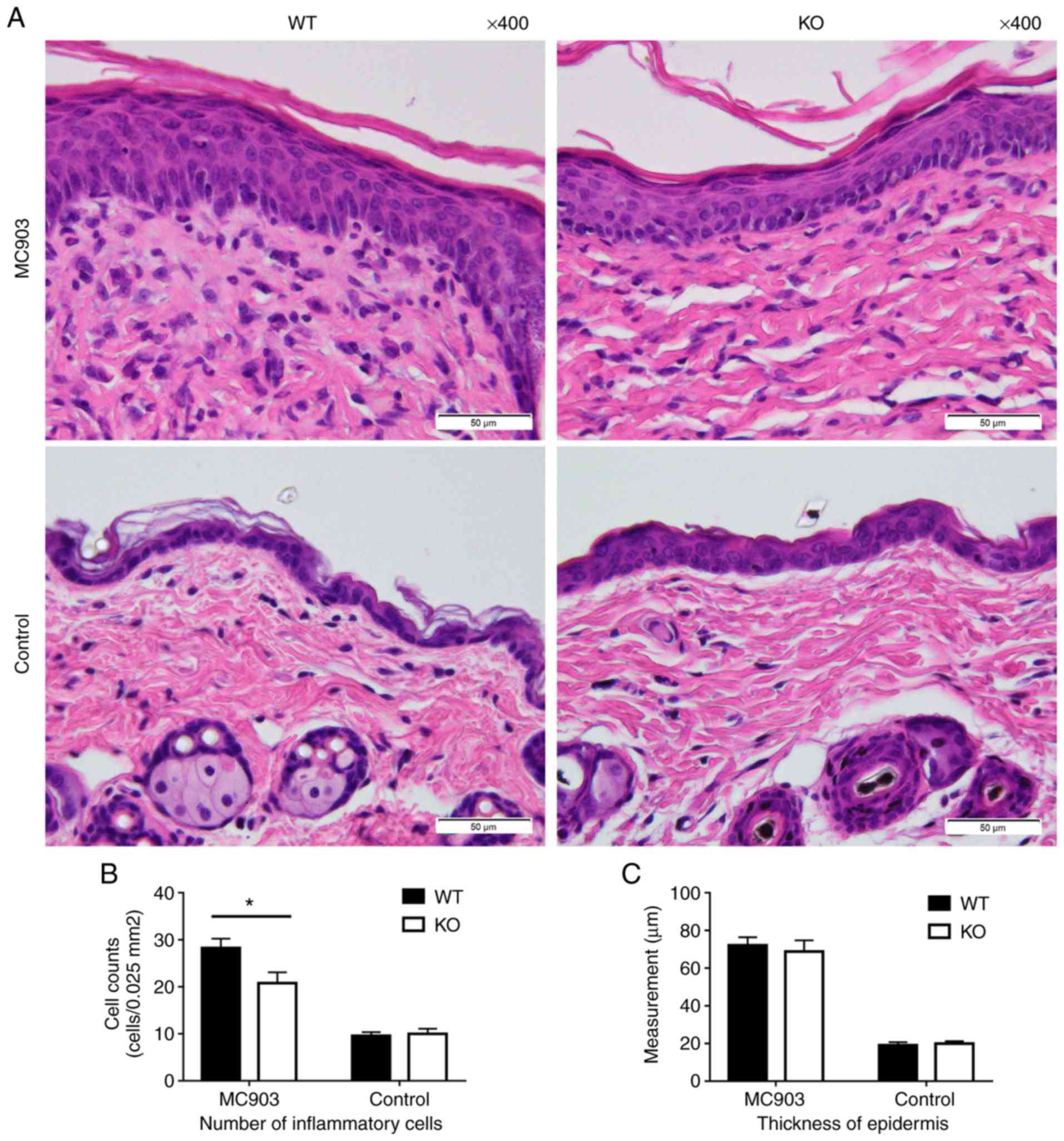 | Figure 2(A) Histopathological examination of
treated skin as assessed by H&E staining. MC903-treated skin
exhibited clear evidence of inflammatory cell infiltration. (B)
Counts of total inflammatory cells in the dermis. Data are
expressed as the means ± SEM of cells per 0.025 mm2 (MC903: WT mice, 28.24±1.996; KO mice,
20.8±2.274, *P<0.05; controls: WT mice, 9.55±0.7915;
KO mice, 5.9.95±1.141, P>0.05). (C) Mean ± SEM of epidermal
thickness in µm (MC903: WT mice, 72.22±4.212; KO mice, 68.77±5.982,
P>0.05; controls: WT mice, 19.07±1.615; KO mice, 19.99±1.247,
P>0.05). WT, wild-type; KO, IL-18 knockout. |
However, IL-18 deficiency failed to affect epidermal
thickness in the MC903-induced AD-like skin lesions (control: WT
mice, 19.07±1.615 µm vs. KO mice, 19.99±1.247 µm, P>0.05;
MC903-treated mice: WT mice, 72.22±4.212 µm vs. KO mice,
68.77±5.982 µm, P>0.05; Fig.
2C).
Mast cells labeled with Toluidine blue were used as
a means to assess MC903-induced AD-like lesions in the mouse model.
Significant differences were found between the WT and KO mice in
the AD-like lesions induced by MC903. Mast cell counts in the WT
mice were significantly higher than those in the KO mice (WT mice,
9.76±0.6882 cells per 0.025 mm2;
KO mice, 6.68±0.989 cells per 0.025 mm2; P<0.05; Fig. 3A and B). No statistically
significant differences were observed between the number of
eosinophils in the WT vs. KO AD-like lesions (WT mice, 1.04±0.2926
cells per 0.025 mm2; KO mice,
1.28±0.3072 cells per 0.025 mm2;
P>0.05; Fig. 3C).
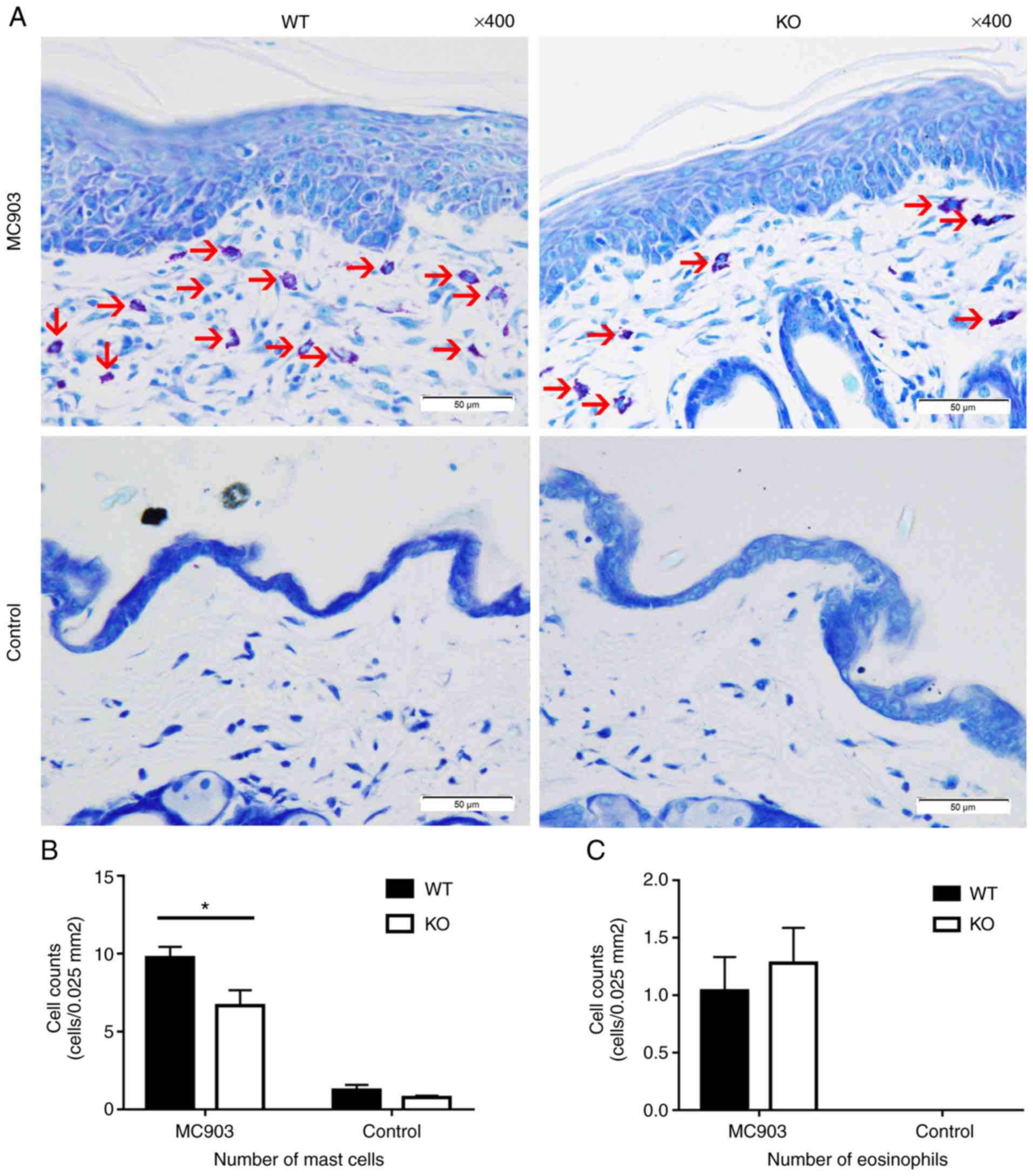 | Figure 3(A) Mast cells stained with Toluidine
blue within the dermis of each group (mast cells are indicated by
red arrows). (B) Counts of mast cells in the dermis; data are
expressed as the means ± SEM, cells per 0.025 mm2 (MC903: WT mice, 9.76±0.6882; KO mice,
6.68±0.989, *P<0.05; controls: WT mice, 1.24±0.337;
KO mice, 0.76±0.1166, P>0.05). (C) Counts of eosinophils in the
dermis of MC903 treated WT and KO mice; data are expressed as the
means ± SEM, cells per 0.025 mm2
(WT mice, 1.04±0.2926; KO mice, 1.28±0.3072, P>0.05). No
eosinophils were observed in the skin samples of the controls. WT,
wild-type; KO, IL-18 knockout. |
IL-18 deficiency exerts no effects on
TSLP levels, but upregu- lates serum IgE levels
The serum levels of TSLP and total IgE within both
WT and IL-18 KO mice were markedly increased in response to MC903
treatment. However, no significant differences were observed for
the serum levels of TSLP between the WT and IL-18 KO mice (MC903
group: WT mice, 192±50.29 pg/ml; KO mice, 128.3±13.94 pg/ml,
P>0.05; control group: WT mice, 5.879±0.47 pg/ml; KO mice,
6.504±0.68 pg/ml, P>0.05; Fig.
4A). As such, the mRNA levels of TSLP within the skin tissue
also exhibited no significant differences (P>0.05, Fig. 4C). However, the serum levels of
total IgE in the WT mice were significantly decreased as compared
with those in the IL-18 KO mice for both the MC903-treated and
control mice (MC903 group: WT mice, 518.4±42.82; KO mice,
637.4±28.22, P<0.05; control group: WT mice, 41.07±3.856; KO
mice, 59.21±4.666, P<0.05; Fig.
4B).
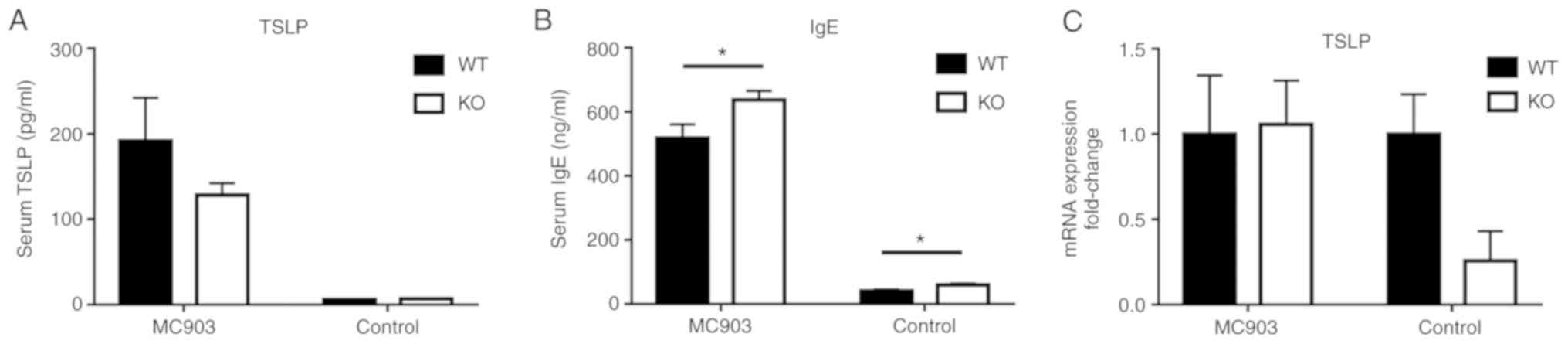 | Figure 4(A) Serum levels of TSLP; data are
expressed as the means ± SEM, pg/ml (MC903: WT mice, 192±50.29; KO
mice, 128.3±13.94, P>0.05; controls: WT mice, 5.879±0.47; KO
mice, 6.504±0.68, P>0.05). (B) Serum levels of total IgE; data
are expressed as the means ± SEM, ng/ml. (MC903: WT mice,
518.4±42.82; KO mice, 637.4±28.22, *P<0.05; controls:
WT mice, 41.07±3.856; KO mice, 59.21±4.666, *P<0.05).
(C) No statistically significant differences were observed in the
skin tissue mRNA expression of TSLP were obtained between WT and KO
mice (P>0.05). WT, wild-type; KO, IL-18 knockout; TSLP, thymic
stromal lymphopoietin. |
IL-18 partially upregulates the mRNA
levels of pro- inflammatory Th2 type cytokines in MC903-induced
AD-like lesions
Considering the crucial role of inflammatory
cytokines in the development of AD, the expression levels of IL-1β,
IL-4, IL-9, STAT3, TSLP and caspase-1 in the skin lesions were
assessed. As shown in Fig. 6, in
both the MC903-treated and control IL-18 KO mice, the relative mRNA
expression levels of IL-1β, IL-9, STAT3 and caspase-1 in the WT
mice did not differ significantly (P>0.05, Fig. 6). Similar results were obtained by
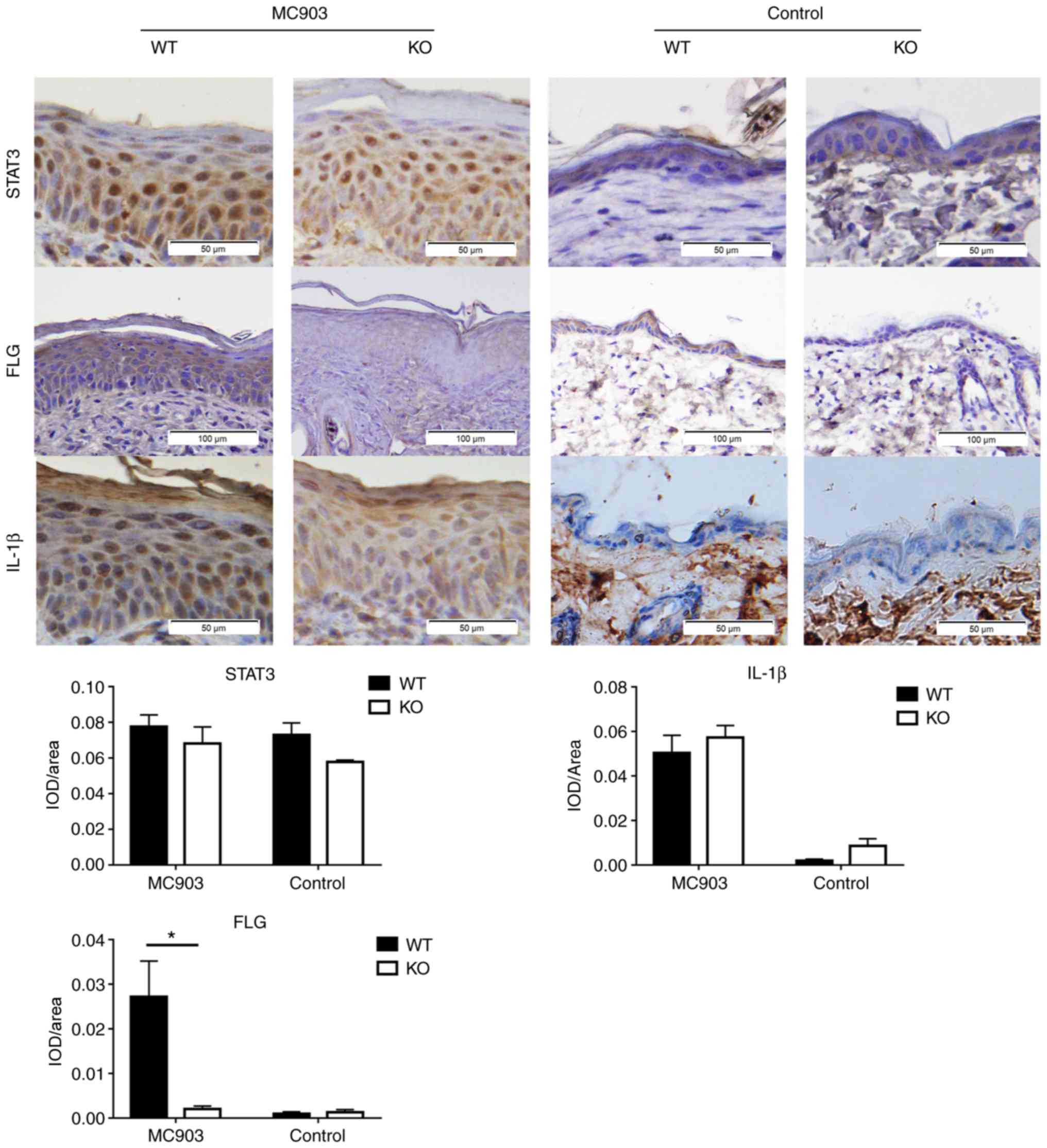
immunohistochemistry for IL-1β and STAT3 (Fig. 5). However, IL-18 significantly
upregulated the mRNA expression levels of IL-4 and CRHR2 in the
MC903-induced AD-like lesions, as shown in the MC903-treated WT
mice (P<0.05 and P<0.01, respectively, Fig. 6), whereas the mRNA expression of
CRHR1 exhibited no statistically significant difference (P>0.05,
data not shown).
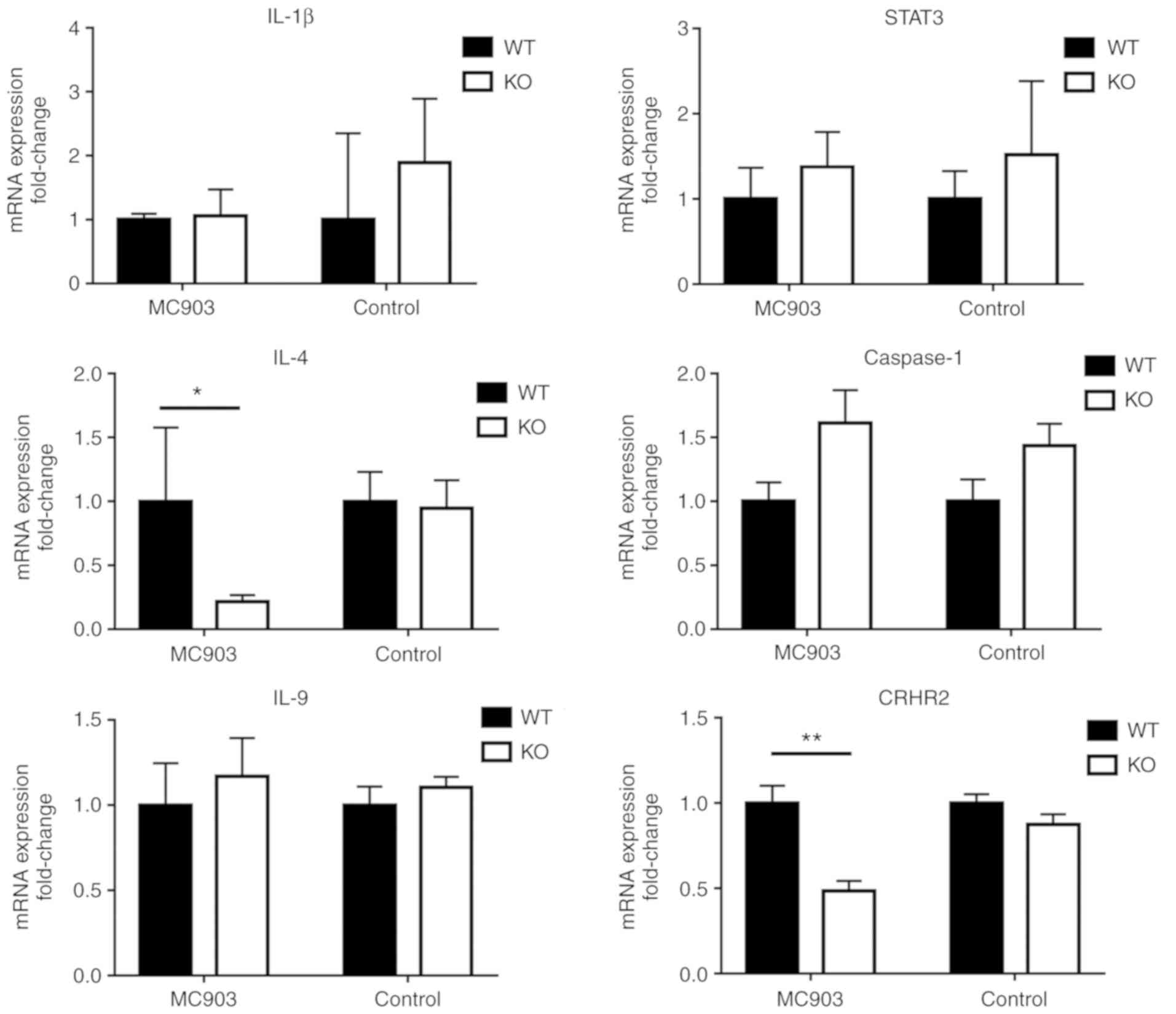 | Figure 6No statistically significant
differences were found between WT and KO control or MC903 treated
mice were obtained for IL-1β, IL-9, STAT3 and caspase-1 mRNA
expression (P>0.05). In response to MC903 treatment, IL-4 and
CRHR2 mRNA expression in WT mice was significantly upregulated as
compared with that observed in KO mice (*P<0.05 and
**P<0.01, respectively), while no statistically
significant differences were obtained between the WT and KO
controls (P>0.05). WT, wild-type; KO, IL-18 knockout; IL,
interleukin; CRHR2, corticotropin-releasing hormone receptor 2;
STAT3, signal transducer and activator of transcription 3 |
IL-18 upregulates the expression of FLG
in the epidermis of MC903-induced AD-like lesions
FLG is a keratin filament-aggregating protein that
serves as a major structural component of the stratum corneum. When
FLG is broken down, its products such as histidine contribute to
epidermal hydration, acid mantle formation, lipid processing, and
barrier function (21,22). In the present study, the results
from immunohistochemistry revealed that a lower expression of FLG
was present in the epidermis of the MC903-treated IL-18 KO mice vs.
the MC903-treated WT mice (P<0.01; Fig. 5). By contrast, no differences were
observed in FLG expression between the WT and IL-18 KO control mice
(P>0.05; Fig. 5).
Discussion
In the present study, IL-18 KO reduced the
production of pro-inflammatory cytokines and chemokines, and the
infiltration of mast cells in AD. An imbalance between Th1 and Th2
immune functions has been proven to be a critical factor in the
development of AD. Th2 cytokines are known to signal through
JAK-dependent pathways in immune cells (23). The Th2 cytokines, IL-4 and IL-13,
are essential for disease pathogenesis and the eventual development
of the chronic itch associated with AD, as sensory neurons are
directly activated by IL-4 and IL-13 along itch-sensory pathways in
mice (24). Activated STAT3 and
STAT5 are required for mast cell function, which then participate
in the pathogenesis of AD by producing pro-inflammatory cytokines
and chemokines (25). The present
study demonstrated that IL-18 KO reduced mRNA expression of IL-4 in
AD-like lesions (Fig. 6).
Moreover, in the mouse model of MC903-induced AD-like skin lesions,
it was found that the numbers of mast cells in the WT mice were
significantly increased as compared with the KO mice (Fig. 3A and B). Thus, degranulation is
decreased and lower amounts of chemotaxis resulting from mast cells
appear to contribute to an alleviation in the development of
AD-like lesions.
Generally, IL-18 is involved in the phosphorylation
of STAT3 and promotes cell proliferation (26,27). However, the present study
demonstrated IL-18 KO did not affect STAT3 expression in the
process of AD-like lesions. STAT3 is required for Th2 cytokine
production, transcription factor expression and Th2 cell-mediated
allergic inflammation. In the presence of activated STAT6, STAT3
promotes Th2 cell differentiation and cytokine production (28). Serum levels of STAT3 have been
shown to be increased in childhood AD and exhibit a significant
positive association with SCORAD indices (29). STAT3 also plays an important role
in the proximal signaling involved in IgE-dependent mast cell
degranulation (30). In the
present study, the expression of STAT3 exhibited no significant
differences between the WT and KO mice during the development of
AD-like lesions (Fig. 6). By
contrast, the serum IgE levels were significantly increased and
differed significantly between the WT and KO mice in this mouse
model of AD (Fig. 4B). As a
result, the capacity for Th2 induction of the JAK-STAT3 pathway was
partially affected by IL-18 deficiency during the process of the
development of AD-like lesions.
IL-18 KO may alleviate the inflammation of AD like
lesions by reducing CRHR2 expression. Acute stress leads to
increased skin vascular permeability and inflammation, through mast
cell activation by CRH (31,32). CRHR1 and CRHR2 have been
identified within the skin, and are expressed by epidermal
keratinocytes, mast cells, melanocytes, peripheral lymphocytes and
dermal blood vessel endothelial cells (33). CRH increases vascular permeability
through the degranulation of mast cells via CRHR1 receptors
(34) and can also directly lead
to vasodilation mediated by CRHR2 receptors via a nitric
oxide-cGMP-dependent pathway (35). A previous study (36) indicated lower levels of CRHR1 gene
expression within the skin of AD patients as compared with the
controls, while serum CRH levels in patients with AD were higher
than those in the controls. In the present study, however, the
mouse model of MC903-induced AD-like lesions exhibited a lower
expression of CRHR2 (Fig. 6), as
well as decreased amounts of mast cell infiltration (Fig. 3A and B). In the absence of IL-18,
the expression of CRHR1 exhibited no marked differences in the skin
samples from WT vs. KO mice (data not shown).
IL-18 may exert a negative effect on serum IgE
levels and acts as a protective factor to the function of FLG. A
previous study revealed that the knockdown of FLG increased the
production of IL-18 in stratified human keratinocytes (37). It was uncertain however, whether
FLG dysfunction affected the outcome of the AD process. FLG
dysfunction mutations represent the most well-known genetic risk
factor for AD. FLG expression is affected by intragenic copy number
variation and reduced by increased local pH, protease activity, and
Th2 cytokine levels (1). Of note,
IgE represents an established factor associated with autoantibodies
and disease severity (38,39)
and patients with lower serum IgE levels exhibit a significantly
lower incidence of FLG mutations and a higher percentage of
IFNγ-producing Th1 cells (40).
In the present study, a higher expression of FLG was found in the
epidermis of MC903-treated WT mice vs. MC903-treated KO mice
(Fig. 5). As noted previously,
serum IgE levels of WT mice were significantly decreased as
compared to KO mice (Fig. 4B). It
seems likely that IL-18 may act as a protective factor in epidermal
barrier dysfunction resulting from AD lesions.
In conclusion, during the process of AD-like lesion
development, IL-18 deficiency partially alleviates Th2-induced
inflammation by reducing the expression of the Th2 cytokine, IL-4.
In addition, an IL-18 deficiency also reduces the infiltration of
mast cells. On the other hand, IL-18 helped protect the epidermal
barrier under normal FLG and lower serum IgE. As a result, in IL-18
KO mice, MC903-induced AD lesions exhibited a partial improvement
as regards skin inflammation. Thus, the present study revealed that
IL-18 may function as a pleiotropic cytokine and may be beneficial
factor in AD development.
The mechanisms of action of AD however, warrant
further in-depth investigations. A limitation of the present study
is that it did not determine whether IL-18 plays a key role in the
interaction between the serum levels of IgE and FLG mutation.
Moreover, IL-18 KO seemed to exert a significant advantage by
improving AD-like lesions.
It is true that the phosphorylation of STAT3 in
IL-18 KO mice was inhibited (41). In addition, caspase-1 has cleaved
and inactive forms, and they cannot be distinguished by RT-qPCR.
The measurement of the phosphorylation of STAT3 and caspase-1 is
necessary for the investigation of the mechanisms of IL-18 in the
pathogenesis of AD. The authors aim to perform further studies in
the future to more deeply investigate the functions of IL-8, and
the associated phosphorylation of STAT3 and caspase-1 in AD-like
skin lesions.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81673070 and
81872538).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article or are available from the
corresponding author on reasonable request.
Authors' contributions
JLC and RQQ made substantial contributions to the
conception and design of the study. JLC performed the model
established, histological examination of the skin, and was a major
contributor in writing the manuscript. XLN and YLG performed the
RT-qPCR analysis. LM was involved in data acquisition, analysis and
interpretation. HDC and XHG were involved in the conception and
design of this study. All authors read and approved the final
manuscript and agree to be accountable for all aspects of the work
in ensuring that questions related to the accuracy or integrity of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
All experimental protocols were strictly approved by
the guide for the care and use of laboratory animals (NIH
Publication, 8th Edition, 2011) and were conducted according to the
guidelines provided by the Institutional Animal Care and Use
Committee at China Medical University (IACUC no. 16008M).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Bolognia J, Schaffer J and Cerroni L:
Dermatology. 4th edition. Elsevier Ltd; 2018
|
|
2
|
Weidinger S, Beck LA, Bieber T, Kabashima
K and Irvine AD: Atopic dermatitis. Nat Rev Dis Primers. 4:12018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee JH, Cho DH and Park HJ: IL-18 and
cutaneous inflammatory diseases. Int J Mol Sci. 16:29357–29369.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Okamura H, Tsutsi H, Komatsu T, Yutsudo M,
Hakura A, Tanimoto T, Torigoe K, Okura T, Nukada Y, Hattori K, et
al: Cloning of a new cytokine that induces IFN-gamma production by
T cells. Nature. 378:88–91. 1995. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sá DC and Festa CN: Inflammasomes and
dermatology. An Bras Dermatol. 91:566–578. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wild JS, Sigounas A, Sur N, Siddiqui MS,
Alam R, Kurimoto M and Sur S: IFN-gamma-inducing factor (IL-18)
increases allergic sensitization, serum IgE, Th2 cytokines, and
airway eosinophilia in a mouse model of allergic asthma. J Immunol.
164:2701–2710. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hon KL, Tsang KY, Kung JS, Leung TF, Lam
CW and Wong CK: Clinical signs, staphylococcus and atopic
eczema-related seromarkers. Molecules. 22:2912017. View Article : Google Scholar
|
|
8
|
Zedan K, Rasheed Z, Farouk Y, Alzolibani
AA, Bin Saif G, Ismail HA and Al Robaee AA: Immunoglobulin E,
interleukin-18 and interleukin-12 in patients with atopic
dermatitis: Correlation with disease activity. J Clin Diagn Res.
9:WC01–WC05. 2015.PubMed/NCBI
|
|
9
|
Chopra R, Vakharia PP, Sacotte R, Patel N,
Immaneni S, White T, Kantor R, Hsu DY and Silverberg JI: Severity
strata for eczema area and severity index (EASI), modified EASI,
scoring atopic dermatitis (SCORAD), objective SCORAD, atopic
dermatitis severity index and body surface area in adolescents and
adults with atopic dermatitis. Br J Dermatol. 177:1316–1321. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ziegler SF and Artis D: Sensing the
outside world: TSLP regulates barrier immunity. Nat Immunol.
11:289–293. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Eyerich K and Novak N: Immunology of
atopic eczema: Overcoming the Th1/Th2 paradigm. Allergy.
68:974–982. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Moosbrugger-Martinz V, Schmuth M and
Dubrac S: A mouse model for atopic dermatitis using topical
application of vitamin D3 or of its analog MC903. Methods Mol Biol.
1559:91–106. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Choi J, Kim JR, Kim H, Kim YA, Lee HJ, Kim
J and Lee KW: The atopic dermatitis-like symptoms induced by MC903
were alleviated in JNK1 knockout mice. Toxicol Sci. 136:443–449.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu XJ, Mu ZL, Zhao Y and Zhang JZ:
Topical tetracycline improves MC903-induced atopic dermatitis in
mice through inhibition of inflammatory cytokines and thymic
stromal lymphopoietin expression. Chin Med J (Engl). 129:1483–1490.
2016. View Article : Google Scholar
|
|
15
|
Li M, Hener P, Zhang Z, Kato S, Metzger D
and Chambon P: Topical vitamin D3 and low-calcemic analogs induce
thymic stromal lymphopoietin in mouse keratinocytes and trigger an
atopic dermatitis. Proc Natl Acad Sci USA. 103:11736–11741. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
National Research Council (US) Committee
for the Update of the Guide for the Care and use of Laboratory
Animals: Guide for the Care and Use of Laboratory Animals. 8th
edition. National Academies Press (US); Washington, DC: 2011
|
|
17
|
Yu J, Luo Y, Zhu Z, Zhou Y, Sun L, Gao J,
Sun J, Wang G, Yao X and Li W: A tryptophan metabolite of the skin
microbiota attenuates inflammation in patients with atopic
dermatitis through the aryl hydrocarbon receptor. J Allergy Clin
Immunol. 143:2108–2119.e12. 2019. View Article : Google Scholar
|
|
18
|
Nomura T and Kabashima K: Advances in
atopic dermatitis in 2015. J Allergy Clin Immunol. 138:1548–1555.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
20
|
Turner MJ, Dasilva-Arnold SC, Yi Q,
Mehrotra P, Kaplan MH and Travers JB: Topical application of a
vitamin D analogue exacerbates atopic dermatitis and induces the
atopic dermatitis-like phenotype in Stat6VT mice. Pediatr Dermatol.
30:574–578. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Irvine AD, McLean WH and Leung DY:
Filaggrin mutations associated with skin and allergic diseases. N
Engl J Med. 365:1315–1327. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Brown SJ and McLean WH: One remarkable
molecule: Filaggrin. J Invest Dermatol. 132(3 Pt 2): 751–762. 2012.
View Article : Google Scholar :
|
|
23
|
Kelly-Welch AE, Hanson EM, Boothby MR and
Keegan AD: Interleukin-4 and interleukin-13 signaling connections
maps. Science. 300:1527–1528. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Oetjen LK, Mack MR, Feng J, Whelan TM, Niu
H, Guo CJ, Chen S, Trier AM, Xu AZ, Tripathi SV, et al: Sensory
neurons Co-opt classical immune signaling pathways to mediate
chronic itch. Cell. 171:217–228.e13. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Morales JK, Falanga YT, Depcrynski A,
Fernando J and Ryan JJ: Mast cell homeostasis and the JAK-STAT
pathway. Genes Immun. 11:599–608. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
El-Darawish Y, Li W, Yamanishi K, Pencheva
M, Oka N, Yamanishi H, Matsuyama T, Tanaka Y, Minato N and Okamura
H: Frontline science: IL-18 primes murine NK cells for
proliferation by promoting protein synthesis, survival, and
autophagy. J Leukoc Biol. 104:253–264. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Alboni S, Montanari C, Benatti C,
Sanchez-Alavez M, Rigillo G, Blom JM, Brunello N, Conti B, Pariante
MC and Tascedda F: Interleukin 18 activates MAPKs and STAT3 but not
NF-κB in hippocampal HT-22 cells. Brain Behav Immun. 40:85–94.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Stritesky GL, Muthukrishnan R, Sehra S,
Goswami R, Pham D, Travers J, Nguyen ET, Levy DE and Kaplan MH: The
transcription factor STAT3 is required for T helper 2 cell
development. Immunity. 34:39–49. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lyu Y, Zhu L, Qi R, Xu J, Di Z, Chen H and
Gao X: Serum levels of suppressor of cytokine signaling 3 and
signal transducer and activator of transcription 3 in childhood
atopic dermatitis. Chin Med J (Engl). 127:2389–2391. 2014.
|
|
30
|
Siegel AM, Stone KD, Cruse G, Lawrence MG,
Olivera A, Jung MY, Barber JS, Freeman AF, Holland SM, O'Brien M,
et al: Diminished allergic disease in patients with STAT3 mutations
reveals a role for STAT3 signaling in mast cell degranulation. J
Allergy Clin Immunol. 132:1388–1396. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Theoharides TC, Singh LK, Boucher W, Pang
X, Letourneau R, Webster E and Chrousos G: Corticotropin-releasing
hormone induces skin mast cell degranulation and increased vascular
permeability, a possible explanation for its proinflammatory
effects. Endocrinology. 139:403–413. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Crompton R, Clifton VL, Bisits AT, Read
MA, Smith R and Wright IM: Corticotropin-releasing hormone causes
vasodilation in human skin via mast cell-dependent pathways. J Clin
Endocrinol Metab. 88:5427–5432. 2033. View Article : Google Scholar
|
|
33
|
Ganceviciene R, Graziene V, Fimmel S and
Zouboulis CC: Involvement of the corticotropin-releasing hormone
system in the pathogenesis of acne vulgaris. Br J Dermatol.
160:345–352. 2009. View Article : Google Scholar
|
|
34
|
Singh LK, Boucher W, Pang X, Letourneau R,
Seretakis D, Green M and Theoharides TC: Potent mast cell
degranulation and vascular permeability triggered by urocortin
through activation of corticotropin-releasing hormone receptors. J
Pharmacol Exp Ther. 288:1349–1356. 1999.PubMed/NCBI
|
|
35
|
Clifton VL, Read MA, Leitch IM, Giles WB,
Boura AL, Robinson PJ and Smith R: Corticotropin-releasing
hormone-induced vasodilatation in the human fetal-placental
circulation: Involvement of the nitric oxide-cyclic guanosine
3′,5′-monophosphate-mediated pathway. J Clin Endocrinol Metab.
80:2888–2893. 1995.PubMed/NCBI
|
|
36
|
Vasiadi M, Therianou A, Sideri K,
Smyrnioti M, Sismanopoulos N, Delivanis DA, Asadi S,
Katsarou-Katsari A, Petrakopoulou T, Theoharides A, et al:
Increased serum CRH levels with decreased skin CRHR-1 gene
expression in psoriasis and atopic dermatitis. J Allergy Clin
Immunol. 129:1410–1413. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sakai T, Hatano Y, Zhang W, Fujiwara S and
Nishiyori R: Knockdown of either filaggrin or loricrin increases
the productions of interleukin (IL)-1α, IL-8, IL-18 and granulocyte
macrophage colony-stimulating factor in stratified human
keratinocytes. J Dermatol Sci. 80:158–160. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Holmes J, Fairclough LC and Todd I: Atopic
dermatitis and autoimmunity: The occurrence of autoantibodies and
their association with disease severity. Arch Dermatol Res.
311:141–162. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jin JJ, Zou YX and Zeng SW: Risk factors
for and expression of immune and inflammatory factors in atopic
dermatitis in Chinese population: A birth cohort study. Mol Cell
Probes. 30:168–173. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kabashima-Kubo R, Nakamura M, Sakabe J,
Sugita K, Hino R, Mori T, Kobayashi M, Bito T, Kabashima K,
Ogasawara K, et al: A group of atopic dermatitis without IgE
elevation or barrier impairment shows a high Th1 frequency:
Possible immunological state of the intrinsic type. J Dermatol Sci.
67:37–43. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Netea MG, Joosten LA, Lewis E, Jensen DR,
Voshol PJ, Kullberg BJ, Tack CJ, van Krieken H, Kim SH, Stalenhoef
AF, et al: Deficiency of interleukin-18 in mice leads to
hyperphagia, obesity and insulin resistance. Nat Med. 12:650–656.
2006. View
Article : Google Scholar : PubMed/NCBI
|