Introduction
Alcoholic liver disease is associated with high
morbidity rates worldwide. Alcohol consumption accounts for 3.8% of
annual global mortality worldwide, and the majority of these deaths
are due to alcoholic liver disease (1). Furthermore, alcoholic liver injury
(ALI) is a major cause of morbidity and mortality in industrialized
and developing countries, especially China (2). ALI causes a series of changes,
progressing from steatosis to hepatitis, fibrosis, cirrhosis and
ultimately hepatocellular carcinoma (3). Heavy alcohol consumption (>60
g/day) causes acute ALI (AALI) in the short term (4). Moreover, AALI cannot be diagnosed
clinically, thus patients are diagnosed in advanced stages of the
disease (5). Currently, there are
few treatments, and the most common steroid treatment,
corticosteroids, is not satisfactory (6). In addition, cytokine therapy is
expensive and difficult to perform (7,8).
Therefore, there is an urgent need to develop novel safe and
effective drugs.
There are several unknown bioactive peptides in the
human daily diet, such as milk and corn, which researchers have
extracted from food and have shown to be beneficial to humans
(9). Bioactive peptides derived
from proteins are beneficial to human health, and were reported to
possess numerous biological activities, such as anti-oxidation,
anti-hypertension, anti-diabetes and immune regulation, and also
play important roles in regulating immune responses and certain
physiological functions in vivo (10-12). Previous studies revealed that
bioactive peptides play an important role in early ALI and chronic
alcoholic injury in mice (13,14), and can promote alcohol clearance
and bile acid metabolism, as well as reduce serum the levels or
activities of total cholesterol (TC), triglyceride (TG), alanine
aminotransferase (ALT) and aspartate aminotransferase (AST)
(14).
PGPIPN (Pro-Gly-Pro-Ile-Pro-Asn) originates from
β-casein (residues 63-68) in bovine milk (15). Generally, short peptides (≤7 amino
acids) can be absorbed directly via the digestive tract into the
blood (16). PGPIPN-containing
three prolines can resist hydrolysis by digestive enzymes in the
gastrointestinal tract (17).
PGPIPN has been reported to have immunoregulation and anticancer
effects (15,18-20). For example, PGPIPN inhibited cell
proliferation and induced cell apoptosis in the human ovarian
cancer cell line SKOV 3 in vitro and reduced tumor growth
rates in mice (21). Recent
studies showed that PGPIPN can alleviate alcoholic fatty liver
disease (13). Therefore, the aim
of the present study was to investigate whether PGPIPN can
alleviate AALI in mice. The results suggested that PGPIPN may be
used as a potential treatment for AALI.
Materials and methods
Reagents
PGPIPN (purity >99.5%, confirmed by reversed
phase-high-performance liquid chromatography) was supplied by
Sangon Biotech Co., Ltd. Hematoxylin solution was purchased from
Sigma-Aldrich (Merck KGaA). The 78 kDa glucose-regulated protein
(GRP78; cat. no. ab108615), C/EBP homologous protein (CHOP; cat.
no. ab11419), caspase-3 (cat. no. ab4051), cleaved caspase-3 (cat.
no. ab49822) and β-actin (cat. no. ab8227) antibodies were
purchased from Abcam. Protein kinase R-like (PKR) endoplasmic
reticulum kinase (PERK; cat. no. 3192), phosphorylated (p)-PERK
(Thr980; cat. no. 3179), p-eukaryotic initiation factor 2α
(p-eIF-2α; Ser51; cat. no. 3398), eIF-2α (cat. no. 5324),
inositol-requiring enzyme 1α (IRE-1α; cat. no. 3294) and spliced
X-box binding protein 1 (XBP-1s; cat. no. 82914) antibodies were
purchased from Cell Signaling Technology, Inc. Secondary antibodies
[horseradish peroxidase (HRP)-conjugated goat anti-mouse
immunoglobulin G (IgG), cat. no. GAM-HRP; HRP-conjugated goat
anti-rabbit IgG, cat. no. GAR-HRP] and Super Signal West Pico kit
(ECL Chromogenic kit) was purchased from Thermo Fisher Scientific,
Inc.
Alcohol-induced animal models and
pharmacological intervention
A total of 60 healthy male Kunming mice (weight,
18-22 g; 6-8 weeks old) were purchased from Anhui Medical
Experimental Animal Center (batch no. 0000469). All animal
experiments were performed under procedures approved by the
Institutional Animal Care and Use Committee of Anhui Medical
University (approval no. LLSC20180132). All methods and protocols
used in the relevant studies, including animal and related studies
in vivo, were conducted in accordance with the relevant
guidelines and regulations of the Committee's protocol. All mice
were housed in a specific pathogen-free sterile rooms at 22±2°C,
40-60% relative humidity and a 12-h light/dark cycle in the Anhui
Medical Laboratory Animal Center. Animal suffering in experiments
was alleviated as much as possible. All mice, with free access to
food and drinking water, were acclimatized for 1 week, then divided
into six groups (n=10/group): Control, model, glutathione (GSH),
low-dose PGPIPN (PGPIPN L), moderate-dose PGPIPN (PGPIPN M) and
high-dose PGPIPN (PGPIPN H). The test period was 10 days. PGPIPN L,
PGPIPN M and PGPIPN H groups were treated with PGPIPN at 0.04, 0.4
and 4 mg/kg body weight via oral gavage each day, respectively. The
control and model groups were treated with the same volume of
saline (250 µl) instead of PGPIPN. The GSH group was treated
with GSH instead of PGPIPN as a positive control at 20 mg/kg body
weight. During the last 3 days, the model, PGPIPN L, PGPIPN M,
PGPIPN H and GSH groups were administered with 9.5 mol/l ethanol at
15 ml/kg body weight for oral gavage 2 h after the aforementioned
peptide infusion, and the control group was administered the same
volume of saline. At the end of the experiment, all mice were
anesthetized, and serum and liver samples were weighed, collected
and stored. The liver index was calculated, liver index (%)=liver
weight/body weight. Some liver tissues were fixed in paraffin after
10% neutral buffered formalin, and the remaining tissues were
cryopreserved in liquid nitrogen for subsequent experiments.
Morphological observation and calculation
of liver injury and hepatocyte apoptosis
Mice liver tissues were fixed with 4%
paraformaldehyde solution at room temperature for 24 h, dehydrated
with alcohol, embedded in paraffin and prepared into tissue
sections. Liver tissues stained with hematoxylin and eosin
(H&E) were observed and analyzed under an optical microscope at
×200 magnification. Hepatocyte apoptosis of liver tissues was
analyzed with a TUNEL assay according to the manufacturer's
instructions (cat. no. 12156792910; Roche Diagnostics GmbH). Mice
liver tissues were fixed with 4% paraformaldehyde solution at room
temperature for 20 min. Subsequently, paraffin sections were made,
followed by dewaxing, hydration and penetration, then stained with
TUNEL reagent (TdT + fluorescein-labeled dUTP) at 37°C for 1 h in
the dark and humidified atmosphere. The nuclei were stained with
methyl green at room temperature for a few seconds. The number of
fields of view observed by fluorescent microscopy was 200-500
cells. Apoptosis was quantified using ImageJ software (version
1.44; National Institutes of Health). The apoptosis index (AI) was
calculated as following formula: AI=(number apoptotic cells/total
number of cells) ×100%.
Analysis of biochemical materials,
cytokines and enzyme activities related to lipid metabolism,
oxidation and liver injury in serum and/or liver tissues of animal
models
Serum TG, TC, ALT and AST concentrations or
activities were determined using a Roche Cobas automated
biochemical analyzer (Roche Diagnostics). A 10% w/v liver
homogenate was prepared from fresh liver tissue. The TG. (cat. no.
F001-1-1), malondialdehyde (MDA; cat. no. A003-1-1), superoxide
dismutase (SOD; cat. no. A001-1-1) and glutathione peroxidase
(GSH-PX; cat. no. A005-1-2) concentrations or activities in liver
were quantified using the aforementioned commercially available
kits (Nanjing Jiancheng Bioengineering Institute) according to the
manufacturer's instructions. Tumor necrosis factor-α (TNF-α; cat.
no. XFFM1870), interleukin (IL)-1β (cat. no. RIA-127) and IL-6
(cat. no. XEFM028D) were quantified by radioimmunoassay using
commercially available kits (Shanghai Xinfan Biological Technology
Co., Ltd.) according to the manufacturer's instructions.
Reverse transcription-quantitative PCR
(RT-qPCR)
At the end of the experiment, all mice liver tissues
were harvested, in which the total RNAs were extracted with
TRIzol® reagent and RNA extraction buffer according to
the manufacturer's instructions (cat. no. 15596026; Invitrogen;
Thermo Fisher Scientific, Inc.). RNA purity and concentration were
determined by ultraviolet spectrophotometry. According to the RNA
template and primer [Oligo(dT)], the first strand cDNA was
synthesized in a reverse transcription reaction including buffers,
dNTPs, AMV reverse transcriptase, recombinant RNasin and total RNA,
according to the manufacturer's instructions (Revert Aid First
Strand cDNA Synthesis kit; cat. no. K1621; Thermo Fisher
Scientific, Inc.). The reverse transcription reaction conditions
were 42°C for 1 h and 70°C for 5 min. Hepatic mRNA expression
levels of caspase-3, XBP-1s and CHOP were measured by RT-qPCR using
the Applied Biosystems 7500 Real-Time PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The following primer
pairs (synthesized by Sangon Biotech Co., Ltd.) were used for the
qPCR: Caspase-3 forward, 5′-ATG GAG AAC AAC AAA ACC TCA GT-3′ and
reverse, 5′-TTG CTC CCA TGT ATG GTC TTT AC-3′; XBP-1s forward,
5′-TGC TGA GTC CGC AGC AGG TG-3′ and reverse, 5′-GCT GGC AGG CTC
TGG GGA AG-3′; CHOP forward, 5′-CTG GAA GCC TGG TAT GAG GAT-3′ and
reverse, 5′-CAG GGT CAA GAG TAG TGA AGG T-3′ and β-actin forward,
5′-GAA ATC GTG CGT GAC ATC AAA G-3′ and reverse, 5′-TGT AGT TTC ATG
GAT GCC ACA G-3′. β-actin was used as the housekeeping gene.
RT-qPCR was performed using TB Green Premix Ex Taq
(cat. no. RR420L; Takara Bio Inc.) according to the manufacturer's
instructions. The following thermocycling conditions were used for
the qPCR: 95°C for 30 sec and 40 cycles of 95°C for 5 sec and 60°C
for 34 sec. At the end of PCR cycling steps, data for each sample
were displayed as a melting curve. The specificity of the amplified
products was confirmed using melting curve analysis. For each
target gene, mRNA expression levels were calculated using the
2−ΔΔCq method (ΔCq=target gene Cq - β-actin Cq value)
(22). All reactions were
performed in triplicate, and a mixture lacking a complementary DNA
template was used as the negative control. In total, two
independent experiments were run.
Western blotting
The liver tissues of the mice were lysed using RIPA
buffer (cat. no. R0010; Beijing Solarbio Science & Technology
Co., Ltd.) with 1% phenylmethanesulfonyl fluoride. Protein
concentration was measured using a bicinchoninic acid protein assay
kit (cat. no. P0012; Beyotime Institute of Biotechnology). Proteins
isolated from liver tissues were separated by SDS-PAGE [5% stacking
gel, 10 or 12% lower gel (w/v); 22.5 µg protein in 15
µl loaded per lane], and subsequently transferred to PVDF
membranes. Following blocking with 5% (w/v) dry skim milk for 2 h,
then immunoblots were performed using a standard protocol (23). The membranes were incubated with
primary antibodies and later incubated with HRP-conjugated
secondary antibodies. The operating procedures were undertaken in
biochemical incubator at room temperature (22°C). The following
primary antibodies were used: Rabbit or mouse monoclonal antibodies
[GRP78, PERK, p-PERK (Thr980), eIF-2α, p-eIF-2α (Ser51), IRE-1α,
XBP-1s and CHOP; 1:1,000] and rabbit polyclonal antibodies
(caspase-3 and cleaved caspase-3; 1:500; β-actin; 1:1,000).
Secondary antibodies used were goat anti-mouse and goat anti-rabbit
IgG, diluted at 1:10,000. Proteins were detected using the ECL
system and exposed in a chemiluminescent imaging system (Clinx
Science Instruments Co., Ltd.), and obtained images were
quantitatively analyzed using ImageJ software (version1.48u;
National Institutes of Health). β-actin was used as a control. All
reactions were performed in triplicate, and two independent
experiments were run.
Statistical analysis
Data are presented as the mean ± SD. Statistical
analyses were performed with SPSS version 20.0 (IBM Corp.) using
one-way ANOVA followed by Tukey's post hoc test. P<0.05 was
considered to indicate a statistically significant different.
Results
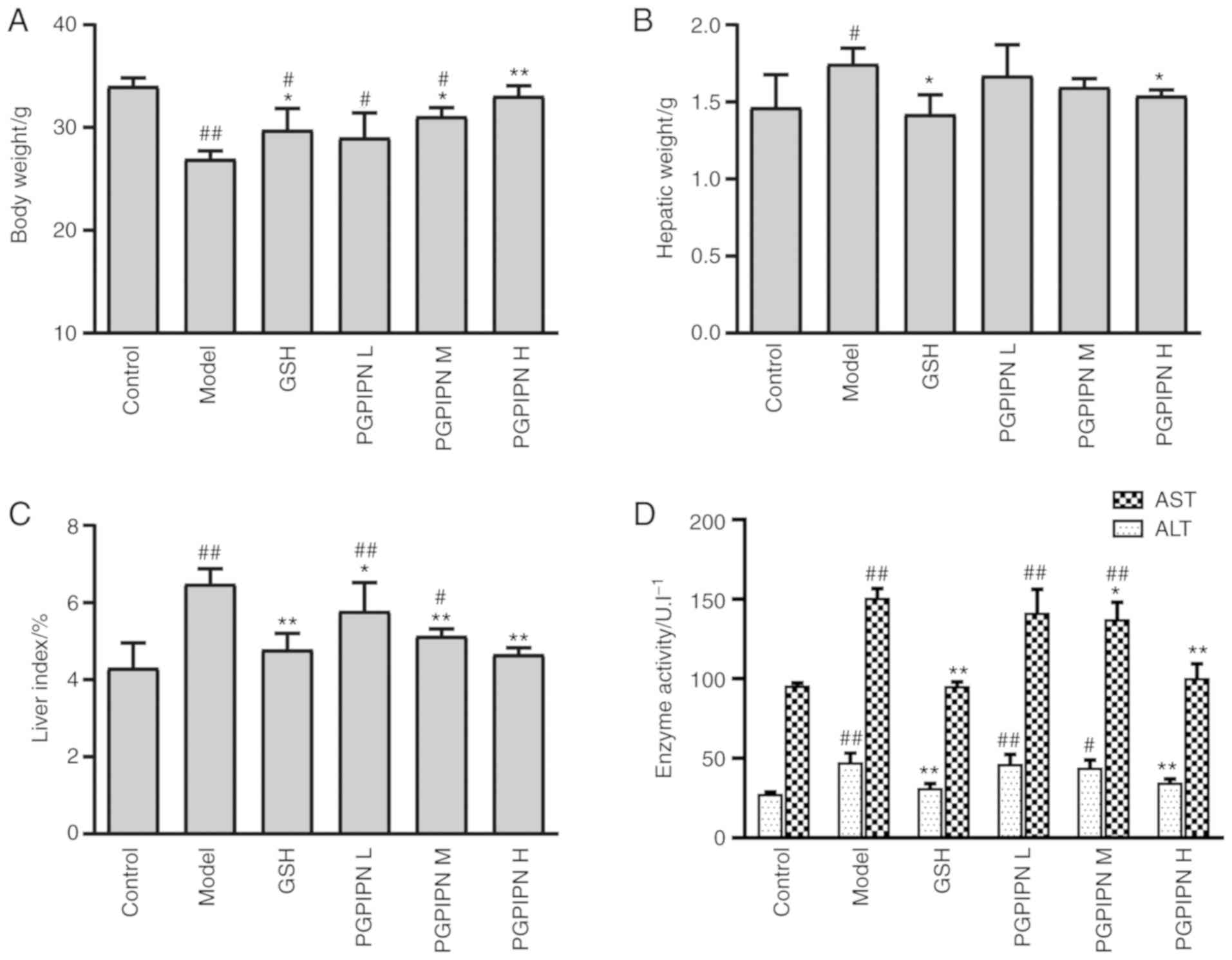
PGPIPN prevents and reduces AALI in model
animals
To investigate the specific role of PGPIPN in the
treatment of AALI, a mice model of AALI was successfully
established. The body weights of mice in the model group were
significantly lower compared with the control group, but the
hepatic weights and liver indexes were significantly higher
compared with the control group (Fig.
1A-C). Therefore, AALI resulted in body weight loss and
increased hepatic weight and liver index. However, PGPIPN relieved
AALI symptoms and antagonized the effects of alcohol in a
dose-dependent manner, which was similar to that of GSH as a
positive control (Fig. 1A-C).
Compared with the control group, the activities of ALT and AST in
the mice serums of the model group were significantly higher
(Fig. 1D). Compared with the
model group, PGPIPN significantly decreased the activities of ALT
and AST in a dose-dependent manner. This suggested that PGPIPN
effectively reduced liver inflammation.
The effect of PGPIPN on mouse liver was analyzed via
histological observation of liver tissues stained with H&E
under light microscopy. It was found that the cells of control
group were arranged in a radial line, the structures of hepatic
lobule were intact, the hepatocyte nucleus and cell boundaries were
clear, and no necrosis was observed (Fig. 2A). In the model group, the hepatic
cord arrangements were disordered and there were inflammatory cell
infiltrations in the hepatocyte spaces (Fig. 2B). Furthermore, there were several
swollen hepatocytes and vacuolization of the cytoplasm, as well as
partial atrophied hepatocyte nuclei, in which these nuclei even
disappeared and partial necrosis (Fig. 2B). In low- and medium-dose PGPIPN
groups, hepatocyte swellings were alleviated, inflammatory cell
infiltrations were reduced, and hepatocyte nuclear atrophy,
disappearance and vacuolization were observed (Fig. 2D and E). In the high-dose PGPIPN
group, the hepatic cord arrangements and hepatocyte structures were
normal, and no obvious inflammatory cell infiltrations and
cytoplasmic vacuolization were observed (Fig. 2F), which was similar to the GSH
group (Fig. 2C).
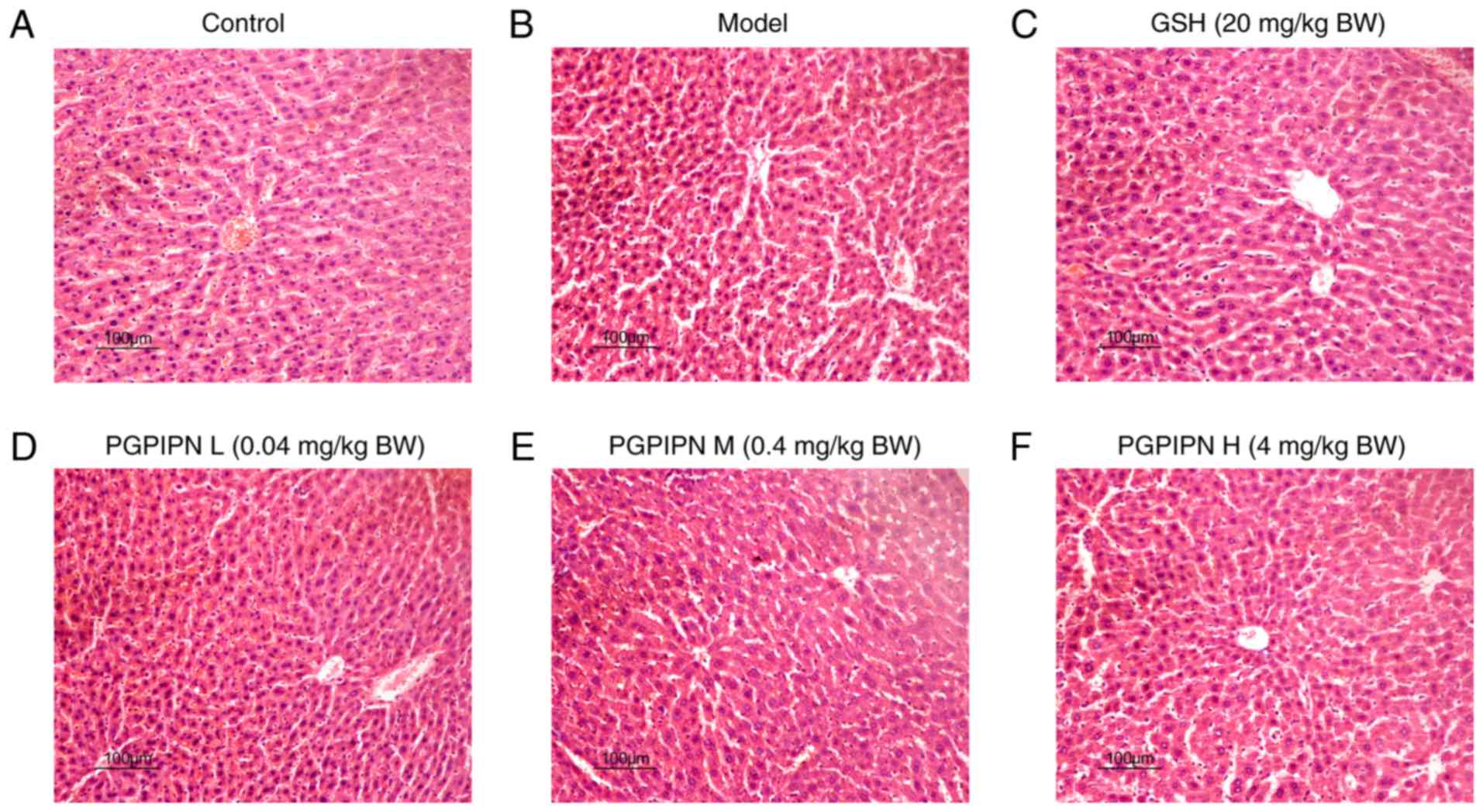 | Figure 2PGPIPN attenuates the pathological
changes of mice liver tissues in acute alcoholic liver injury.
Hematoxylin and eosin staining in (A) Control, (B) model, (C) GSH,
(D) PGPIPN L, (E) PGPIPN M and (F) PGPIPN H groups. Magnification,
×200. PGPIPN, Pro-Gly-Pro-Ile-Pro-Asn; L, low dose; M, medium dose;
H, high dose; GSH, glutathione; BW, body weight. |
A TUNEL assay was performed on the liver tissues of
mice. TUNEL-positive cells in the liver tissues of the model group
(Fig. 3Ab) were markedly
increased compared with the control group (Fig. 3Aa). Compared with the model group,
PGPIPN decreased TUNEL-positive cells in the liver tissues
(Fig. 3Ad-f), of which moderate-
and high-dose PGPIPN groups reached significant levels, and the
decrease in high-dose PGPIPN group was greater compared with the
GSH group (positive control; Fig.
3Ac). The AI was also significantly increased in the model
group compared with control group (Fig. 3B). Compared with the model group,
PGPIPN decreased hepatocyte apoptosis in a dose-dependent manner,
and the effect was the most obvious in the PGPIPN H group (Fig. 3B).
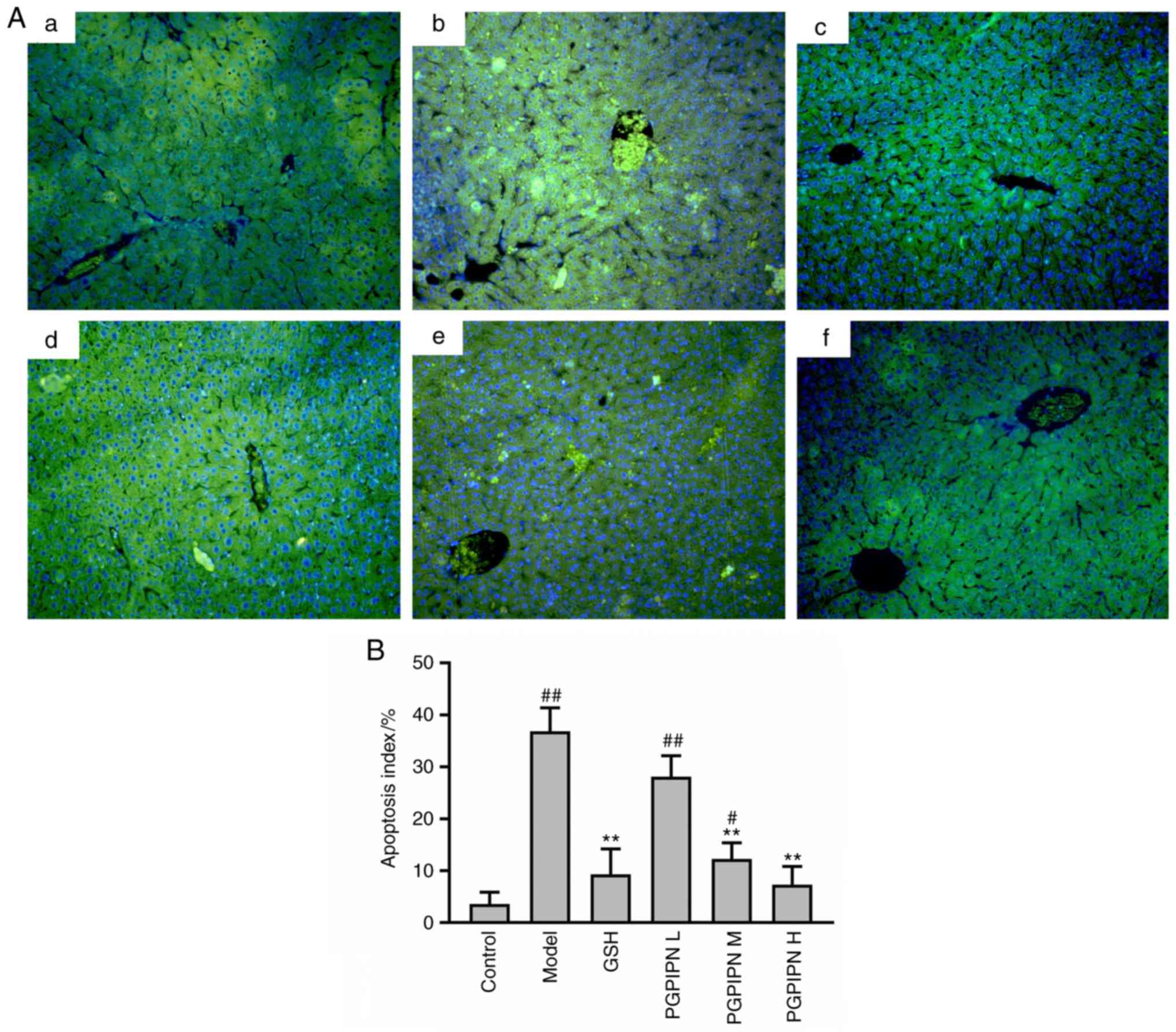 | Figure 3PGPIPN attenuates hepatocyte
apoptosis of mice induced with alcohol-intake. (A) Liver tissues
stained with a TUNEL kit (fluorescein-dUTP) and observed under an
inverted fluorescence microscope. a, Control group; b, Model group;
c, GSH group; d, PGPIPN L group; e, PGPIPN M group; f, PGPIPN H
group. Magnification, ×200. (B) Quantification of apoptosis using
ImageJ software. Data are presented as the mean ± standard
deviation of ten mice in each group. **P<0.01 vs.
model. #P<0.05 and ##P<0.01 vs.
control. PGPIPN, Pro-Gly-Pro-Ile-Pro-Asn; L, low dose; M, medium
dose; H, high dose; GSH, glutathione; BW, body weight. |
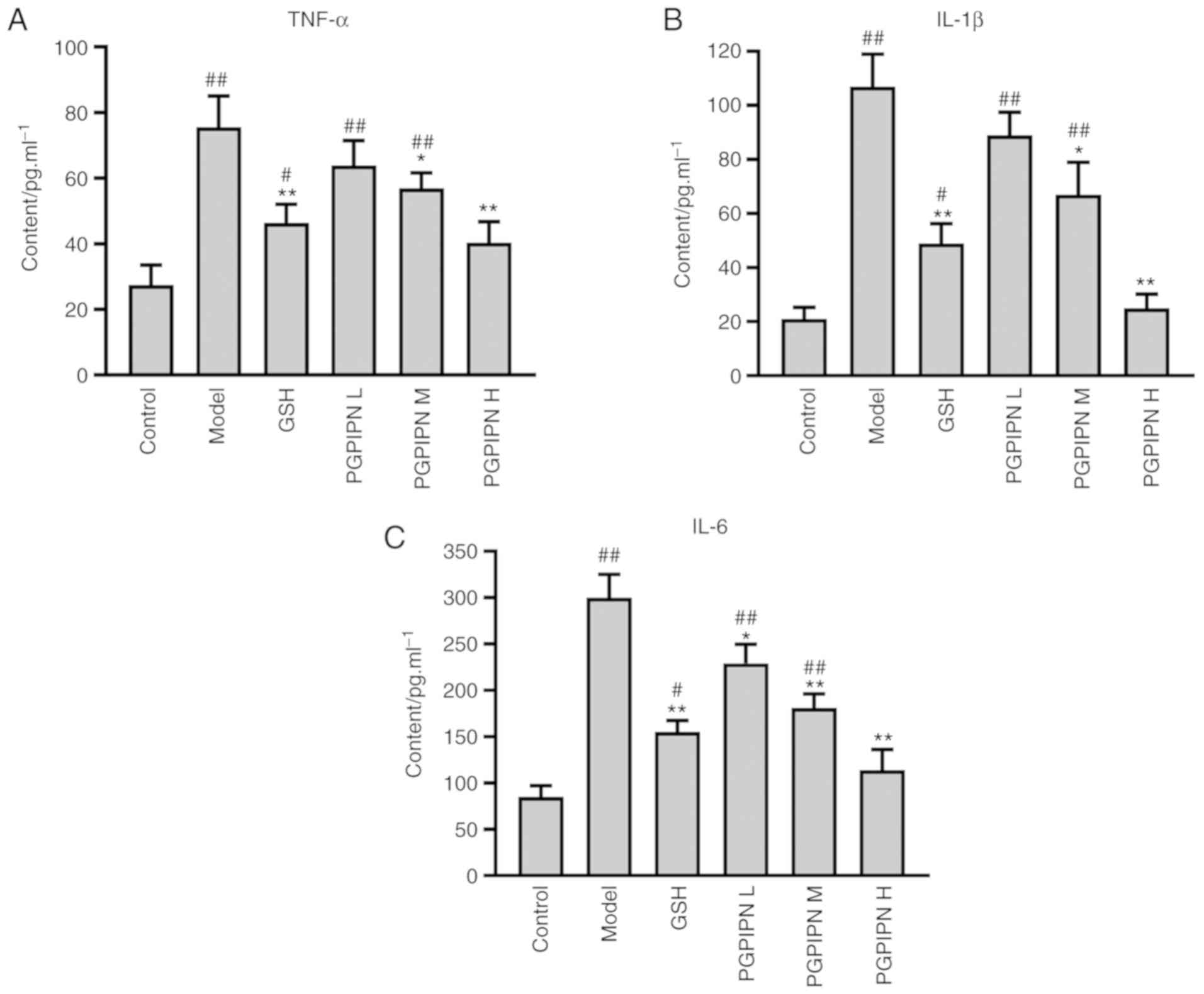
PGPIPN decreases the levels of mouse
pro-inflammatory cytokines
The levels of TNF-α, IL-1β and IL-6 were determined
in mouse liver homogenates by radioimmunoassay. The results
indicated that the TNF-α, IL-1β and IL-6 levels of model group mice
were significantly increased compared with the control group. In
addition, compared with the model group, PGPIPN significantly
reduced the levels TNF-α, IL-1β and IL-6 contents, similar to
levels found the GSH group (Fig.
4).
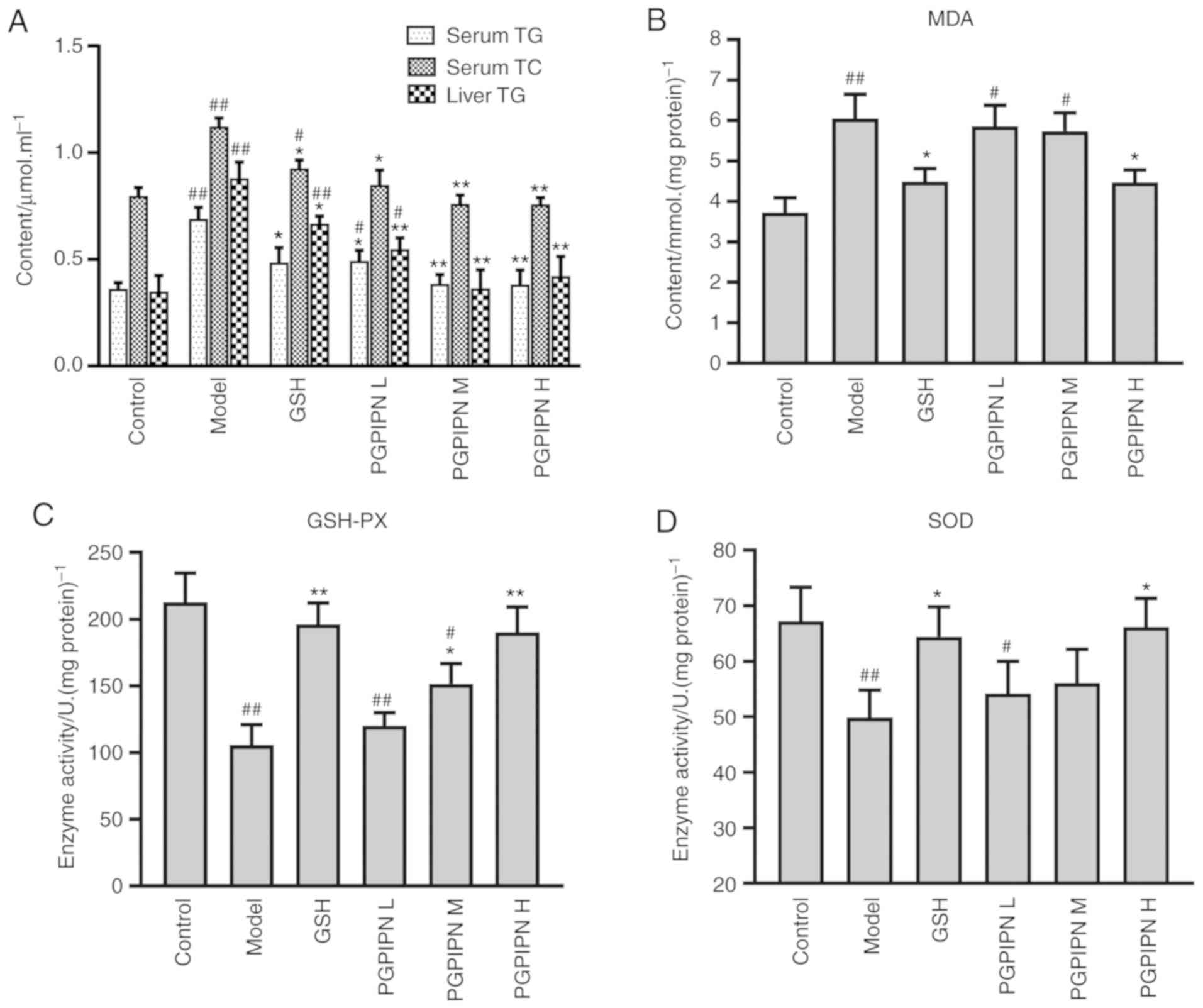
PGPIPN attenuates alcohol-induced lipid
metabolism and oxidative stress in mouse liver
The TG and TC in serum and liver homogenates of
model group mice were significantly higher compared with the
control group (Fig. 5A). Compared
with the model group, PGPIPN significantly reduced the TG and TC
levels in mice serum and liver, reductions of which were higher
compared with the GSH group (positive control; Fig. 5A). Even at a low dose, PGPIPN had
a significant effect on reducing aforementioned TG and TC levels.
Thus, it was speculated that PGPIPN may attenuate liver damage by
regulating lipid metabolism. The results also indicated that MDA
levels in the liver tissues of the model group was significantly
increased, and the activities of GSH-PX and SOD were significantly
decreased compared with the control group (Fig. 5B-D). Furthermore, PGPIPN in medium
and high doses significantly reduced MDA levels and increased of
GSH-Px and SOD activity in mice liver tissues, effects of which
were similar to that of GSH group as a positive control. However,
PGPIPN in low doses had no significant effect on these three
indicators.
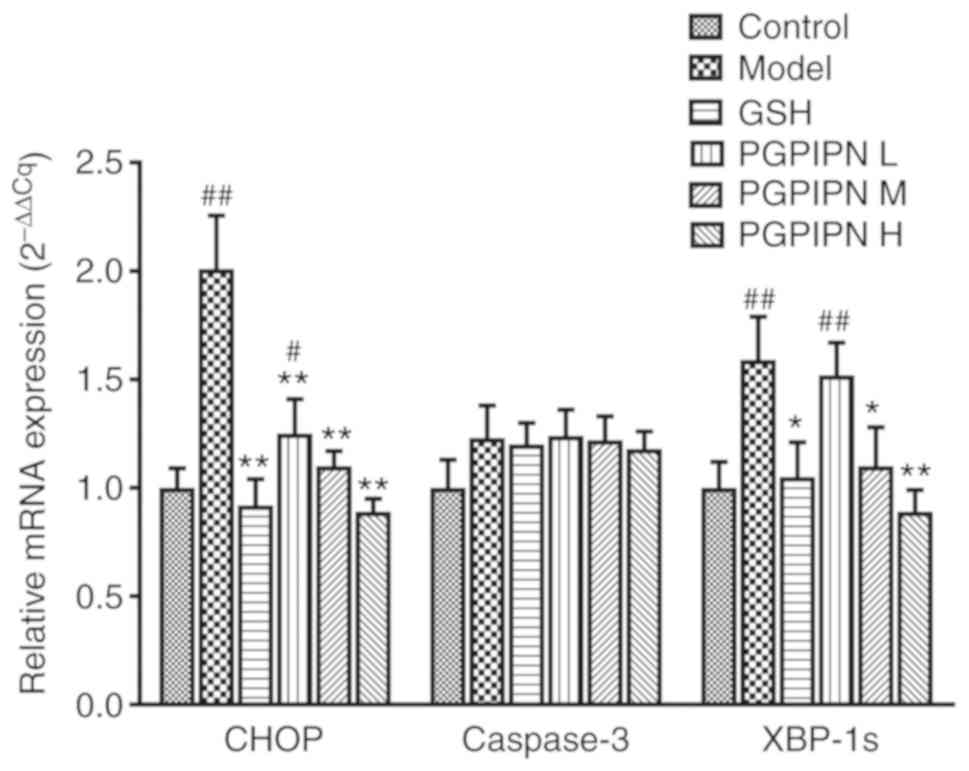
PGPIPN regulates the expression of genes
associated with endoplasmic reticulum stress (ERS) in
hepatocytes
The present study examined the mRNA expression
levels of XBP-1s, CHOP and caspase-3 by RT-qPCR, which are three
key indicators of ERS (24).
Compared with control group mice, the mRNA expression levels of
XBP-1s and CHOP were significantly increased in alcohol-induced
model mice (Fig. 6). PGPIPN
significantly decreased the expression levels of XBP-1s and CHOP
compared with the model group (Fig.
6). The effects of PGPIPN on mRNA expression levels of XBP-1s
and CHOP genes were dose-dependent. However, the mRNA expression of
caspase-3 did not change significantly between the six groups,
although the control group showed markedly lower expression
(Fig. 6).
Western blotting was used to analyze GRP78, PERK,
p-PERK, eIF-2α, p-eIF-2α, IRE-1α, XBP-1s, CHOP, caspase-3 and
cleaved caspase-3 proteins, which are related to ERS in liver
tissues of mice (24). The
protein expression levels of p-PERK, p-eIF-2α, XBP-1s, CHOP,
caspase-3 and cleaved caspase-3 in the model group were
significantly higher compared with the control group, but the
expression levels of GRP78, PERK, eIF-2α and IRE-1α did show any
significant changes (Fig. 7A, B and
D). PGPIPN reduced the expression levels of p-PERK, p-eIF-2α,
XBP-1s, CHOP, caspase-3 and cleaved caspase-3 proteins compared
with the model group in a dose-dependent manner (Fig. 7A, B and D). However, the peptide
had little effect on GRP78, PERK, eIF-2α, IRE-1α and caspase-3
protein expression levels, although the peptide at high doses could
affect GRP78 and caspase-3 protein expression levels. The results
showed that the phosphorylation levels of p-PERK/PERK and
p-eIF-2α/eIF-2α in model group were significantly higher compared
with the control group, while PGPIPN reduced the phosphorylation of
PERK and eIF-2α (Fig. 7A and C).
Consequently, PGPIPN had the most significant effects on the
phosphorylation of PERK/eIF pathway, spliced XBP-1 and cleaved
caspase-3 proteins (Fig. 7).
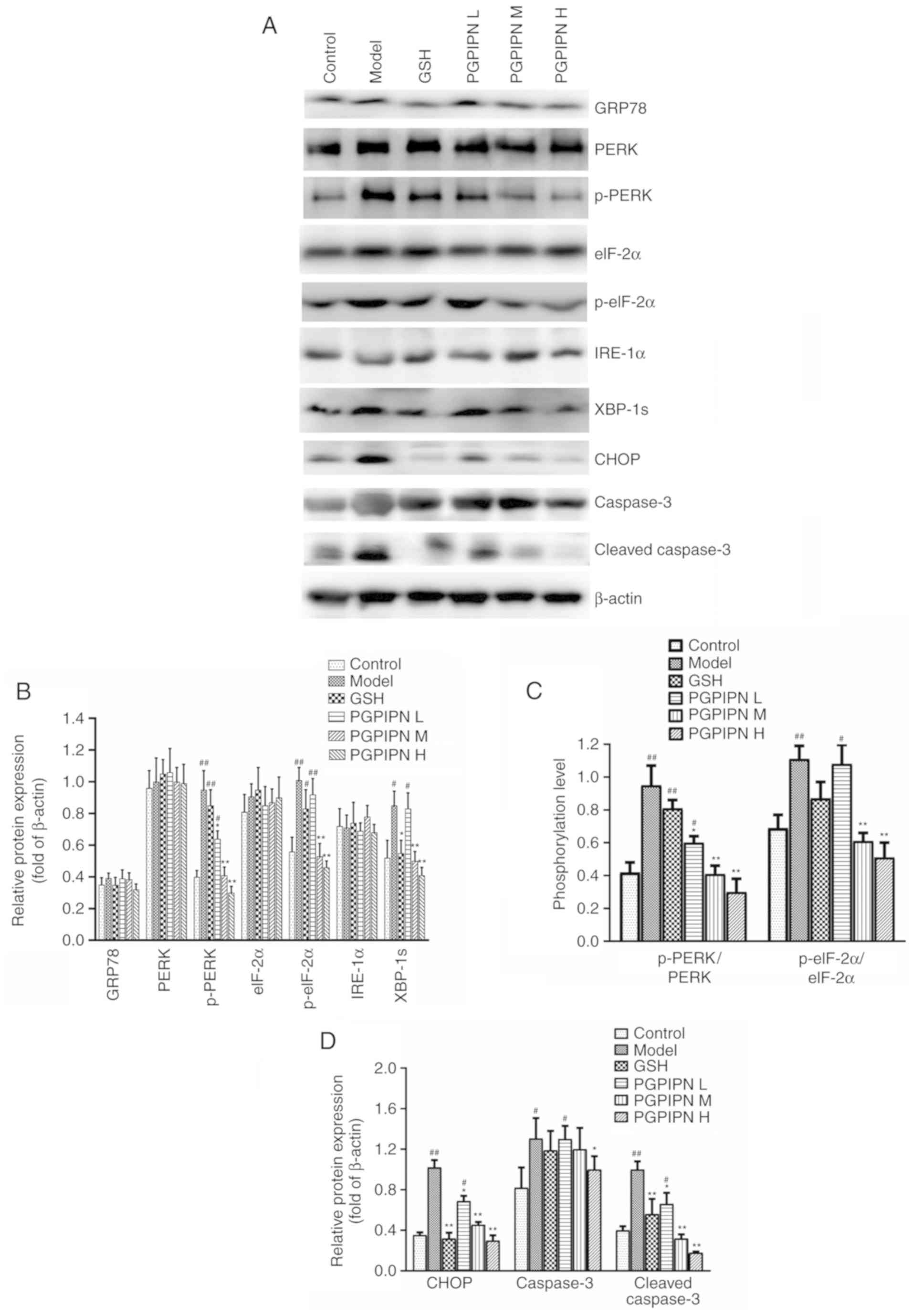 | Figure 7PGPIPN regulates the contents and/or
activities of proteins associated with endoplasmic reticulum stress
in hepatocytes. (A) GRP78, PERK, p-PERK, eIF-2α, p-eIF-2α, IRE-1α,
XBP-1s, CHOP, caspase-3 and cleaved caspase-3 in mice liver tissues
in different groups were detected via western blotting. β-actin was
used as the internal reference gene. (B) Relative protein band
intensities of GRP78, PERK, p-PERK, eIF-2α, p-eIF-2α, IRE-1α and
XBP-1s. (C) Phosphorylation levels of p-PERK/PERK and
p-eIF-2α/eIF-2α from relative intensities of protein bands in (A)
and normalized to β-actin. (D) Relative protein band intensities of
CHOP, caspase-3 and cleaved caspase-3. n=10. Data are presented as
the mean ± standard deviation of ten mice in each group.
*P<0.05 and **P<0.01 vs. model.
#P<0.05 and ##P<0.01 vs. control.
PGPIPN, Pro-Gly-Pro-Ile-Pro-Asn; CHOP, C/EBP homologous protein;
XBP-1s, spliced X-box binding protein 1; p-, phosphorylated; GRP78,
78 kDa glucose-regulated protein; IRE-1α, inositol-requiring enzyme
1α; eIF-2α, eukaryotic initiation factor 2α; L, low dose; M, medium
dose; H, high dose; GSH, glutathione. |
Discussion
Bioactive peptides can be obtained in natural
resources such as milk protein or synthesized by rational design
and have been shown to be potential therapeutic agents for a
variety of human diseases, including cancer (25). Bioactive peptides have been
described as food ingredients that also exert physiological
effects, in addition to nutritional value (26). Moreover, peptides are natural
amino acids and have less potential toxicity (27). The present results indicated that
PGPIPN plays a significant role in alleviating acute alcohol
intake-induced liver injury in vivo, suggesting that PGPIPN
has a protective effect on AALI.
In the early stages of AALI, TG accumulates in liver
cells, leading to the development of fatty liver (28). Oxidative stress and inflammatory
response are the main causes of alcoholic liver disease, which
increases the severity of fatty liver, oxidizes unsaturated fatty
acids, increases the production of lipid peroxidation products and
ultimately leads to liver fibrosis by activating stellate cells
(29-31). The main cause of AALI is a large
intake of alcohol in a short period of time (32). The main metabolism of alcohol
(~90%) occurs in the liver, and the liver is injured after heavy
drinking, which results in an increase in hepatocyte permeability
due to cellular oxidative stress or damage (33). ALT and AST are mainly present in
the mitochondria of hepatocytes (34). When hepatocytes are broken due to
AALI, ALT and AST of hepatocytes are rapidly released into the
blood, resulting in an increase in serum (35,36). Moreover, alcohol metabolism
produces a large amount of acetaldehyde, which induces cell
peroxidation to produce MDA (37). The levels of MDA in vivo
are an indicator of lipid peroxidation in the body, which can
reflect the degree of oxidative stress damage caused by free
radical attack (38). The present
results demonstrated that PGPIPN effectively reduced MDA levels,
enhanced GSH-PX and SOD activities and inhibited alcohol-induced
liver oxidative stress damage.
TNF-α is a pro-inflammatory cytokine produced by
immune cells (mainly T lymphocytes), which belongs to a family of
both soluble and cell-bound cytokines that has a wide range of
biological functions, such as induction of inflammation, apoptosis
and lymphatic development (39).
IL-1β and IL-6 are two types of cytokines produced by fibroblast,
immunocyte and epithelial cells in response to infection and
inflammation (40). Furthermore,
IL-1β and IL-6 are involved in inflammatory and heat-generating
reactions in liver tissues (41).
It was reported that decreases in TNF-α, IL-1β and IL-6 levels
could reduce inflammation, oxidative stress and apoptosis (42,43). Previous studies showed that
pro-inflammatory cytokines such as TNF-α, IL-1β and IL-6 play a key
role in the development and progression of alcoholic hepatitis
(32,44). Moreover, pro-inflammatory
cytokines such as TNF-α, IL-1β and IL-6 may cause hepatocyte damage
(30,45). In the present study, it was found
that PGPIPN effectively reduced the expression levels of TNF-α,
IL-1β and IL-6, and then reduced inflammation and hepatocyte
damage.
Since ERS often causes unfolded or misfolded
proteins to accumulate in the endoplasmic reticulum, the
characteristic molecules involved in unfolded protein responses are
commonly used to indicate ERS (46). GRP78, as an endoplasmic reticulum
resident and chaperone molecule, is a major regulator of ERS
response (47). GRP78 regulates
the activation of three ER transmembrane binding sensors, IRE1α,
cyclic AMP-dependent transcription factor ATF-6 α (ATF6) and PERK,
via PERK/eIF-2α and IRE1α or ATF6/XBP-1s axes, which are two
branches of the unfolded protein response (UPR), affecting the
expression of CHOP (48). CHOP
was revealed to regulate several pro- and anti-apoptotic genes
including Bcl-2, tribbles related protein 3 (TRB3) and growth
arrest and DNA damage-inducible protein 34 (GADD34), which are
target genes of CHOP (49). The
expression of CHOP in normal cells is extremely low; however, CHOP
expression and its accumulation in the nucleus are upregulated
during hepatocyte apoptosis induced by ERS (50). Moreover, CHOP does not directly
induce apoptosis, but activates caspase-3 to initiate the apoptosis
pathway (49). Activated
caspase-3 (cleaved caspase-3) specifically cuts proteins associated
with cellular activity and triggers the apoptosis cascade reaction,
resulting in cell apoptosis (51). The present results suggested that
PGPIPN significantly decreased the phosphorylation of the PERK/eIF
pathway and levels of spliced XBP-1, resulting in a significant
decrease in CHOP. The reduction of CHOP could decrease the
activation of caspase-3 in liver tissues of mice, thus alleviating
ERS. According to the changes in CHOP and cleaved caspase-3 levels,
PGPIPN could simultaneously downregulate ERS and attenuate acute
alcoholic liver cell damage in mice.
ERS regulates proinflammatory cytokine mature and
secretion, such as TNF-α and IL-6, the main mechanism of which may
be achieved by acting on several signaling pathways, such as the
STAT3 pathway, and activating nucleotide-binding oligomerization
domain-like receptors (52,53). However, several proinflammatory
cytokines can also promote ERS and ERS-related gene expression
(54). Our previous studies also
showed that PGPIPN exerted immune and antioxidant functions both
in vitro and in vivo (data not shown). Fiedorowicz
et al (55) reported that
some bioactive peptides from bovine caseins could bind the
µ-opioid receptor on the cytomembrane to influence the
proliferation and cytokine secretion of human peripheral blood
mononuclear cells. The changes in the immune response can affect
the UPR and some cytokines, especially the expression levels of UPR
target genes such as IRE1α and ATF6. Therefore, it was speculated
that PGPIPN may regulate cell signal transduction and alter the
expression level or activity of proteins associated with ERS in
hepatocytes; however, this requires further investigation.
Moreover, the present results suggested that PGPIPN may be a novel
safe therapeutic agent for the treatment or prevention of AALI and
its related complications.
Acknowledgments
The authors thank Mr Guanjun Chen of the Center for
Scientific Research of Anhui Medical University for valuable help
in our experiment. The authors would like to thank Mr Hao Li of the
First Affiliated Hospital of Anhui Medical University for help in
the assessment, sampling and pathological examination of alcoholic
liver injury from liver tissues of model mice.
Funding
The study and the preparation of the paper were
funded by the National Natural Science Foundation of China (grant
nos. 81472448 and 81601107) and the National College Students
Innovation and Entrepreneurship Training Program of China (grant
no. 201910366004).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YQ conceived the idea, designed the study,
contributed reagents and materials and wrote the manuscript. QX, HX
and XC performed research and analyzed data. PW, JL, WW and FG
performed research. YX performed statistical analyses and revised
the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All animal experiments were performed under
procedures approved by the Institutional Animal Care and Use
Committee of Anhui Medical University (approval no.
LLSC20180132).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Marroni CA, Fleck AM Jr, Fernandes SA,
Galant LH, Mucenic M, de Mattos Meine MH, Mariante-Neto G and
Brandão ABM: Liver transplantation and alcoholic liver disease:
History, controversies, and considerations. World J Gastroenterol.
24:2785–2805. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jiang Z, Wang J, Xue H, Wang M, Jiang H,
Liang Y, Dias AC, Gregory M, Chen C and Zhang X: Protective effect
of wild Corni fructus methanolic extract against acute alcoholic
liver injury in mice. Redox Rep. 22:338–345. 2017. View Article : Google Scholar
|
|
3
|
Louvet A and Mathurin P: Alcoholic liver
disease: Mechanisms of injury and targeted treatment. Nat Rev
Gastroenterol Hepatol. 12:231–242. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
O'Shea RS, Dasarathy S and McCullough AJ;
Practice Guideline Committee of the American Association for the
Study of Liver Diseases; Practice Parameters Committee of the
American College of Gastroenterology: Alcoholic liver disease.
Hepatology. 51:307–328. 2010. View Article : Google Scholar
|
|
5
|
Torruellas C, French SW and Medici V:
Diagnosis of alcoholic liver disease. World J Gastroentero.
20:11684–11699. 2014. View Article : Google Scholar
|
|
6
|
Stickel F, Datz C, Hampe J and Bataller R:
Erratum: Pathophysiology and management of alcoholic liver disease:
Update 2016. Gut Liver. 11:4472017. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu Y, Chen X, Qiu M, Chen W, Zeng Z and
Chen Y: Emodin ameliorates ethanol-induced fatty liver injury in
mice. Pharmacology. 94:71–77. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Stickel F, Datz C, Hampe J and Bataller R:
Pathophysiology and management of alcoholic liver disease: Update
2016. Gut Liver. 11:173–188. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Marcone S, Haughton K, Simpson PJ, Belton
O and Fitzgerald DJ: Milk-derived bioactive peptides inhibit human
endothelial-monocyte interactions via PPAR-γ dependent regulation
of NF-κB. J Inflamm (Lond). 12:12015. View Article : Google Scholar
|
|
10
|
Saadi S, Saari N, Anwar F, Abdul Hamid A
and Ghazali HM: Recent advances in food biopeptides: Production,
biological functionalities and therapeutic applications.
Biotechnology Advances. 33:80–116. 2015. View Article : Google Scholar
|
|
11
|
Gokhale AS and Satyanarayanajois S:
Peptides and peptidomimetics as immunomodulators. Immunotherapy.
6:755–774. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lonnerdal B: Nutritional and physiologic
significance of human milk proteins. Am J Clin Nutr.
77:1537S–1543S. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Qi N, Liu C, Yang H, Shi W, Wang S, Zhou
Y, Wei C, Gu F and Qin Y: Therapeutic hexapeptide (PGPIPN) prevents
and cures alcoholic fatty liver disease by affecting the
expressions of genes related with lipid metabolism and oxidative
stress. Oncotarget. 8:88079–88093. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu Y, Pan X, Zhang S, Wang W, Cai M, Li Y,
Yang F and Guo H: Protective effect of corn peptides against
alcoholic liver injury in men with chronic alcohol consumption: A
randomized double-blind placebo-controlled study. Lipids Health
Dis. 13:1922014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Meisel H: Biochemical properties of
regulatory peptides derived from milk proteins. Biopolymers.
43:119–128. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gevaert B, Veryser L, Verbeke F,
Wynendaele E and De Spiegeleer B: Fish hydrolysates: A regulatory
perspective of bioactive peptides. Protein Pept Lett. 23:1052–1060.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kanwar JR, Kanwar RK, Sun X, Punj V, Matta
H, Morley SM, Parratt A, Puri M and Sehgal R: Molecular and
biotechnological advances in milk proteins in relation to human
health. Curr Protein Pept Sci. 10:308–338. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gill HS, Doull F, Rutherfurd KJ and Cross
ML: Immunoregulatory peptides in bovine milk. Br J Nutr. 84(Suppl
1): S111–S117. 2000. View Article : Google Scholar
|
|
19
|
Fiat AM, Migliore-Samour D, Jolles P,
Drouet L, Bal dit Sollier C and Caen J: Biologically active
peptides from milk proteins with emphasis on two examples
concerning antithrombotic and immunomodulating activities. J Dairy
Sci. 76:301–310. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Meisel H and FitzGerald RJ: Biofunctional
peptides from milk proteins: Mineral binding and cytomodulatory
effects. Curr Pharm Des. 9:1289–1295. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao M, Wei C, Yang X, Zhou J, Wang J, Gu
F, Lei T and Qin Y: The milk-derived hexapeptide PGPIPN inhibits
the invasion and migration of human ovarian cancer cells by
regulating the expression of MTA1 and NM23H1 genes. Int J Oncol.
48:1721–1729. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
23
|
Green MR and Sambrook J and Sambrook J:
Molecular cloning: A laboratory manual. Cold Spring Harbor
Laboratory Press; Cold Spring Harbor, NY: 2012
|
|
24
|
Lv SX and Qiao X: Isovitexin (IV) induces
apoptosis and autophagy in liver cancer cells through endoplasmic
reticulum stress. Biochem Biophys Res Commun. 496:1047–1054. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang W, Gu F, Wei C, Tang Y, Zheng X, Ren
M and Qin Y: PGPIPN, a therapeutic hexapeptide, suppressed human
ovarian cancer growth by targeting BCL2. PLoS One. 8:e607012013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Phelan M and Kerins D: The potential role
of milk-derived peptides in cardiovascular disease. Food Funct.
2:153–167. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kreider RB, Iosia M, Cooke M, Hudson G,
Rasmussen C, Chen H, Mollstedt O and Tsai MH: Bioactive properties
and clinical safety of a novel milk protein peptide. Nutr J.
10:992011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Purohit V, Gao B and Song BJ: Molecular
mechanisms of alcoholic fatty liver. Alcohol Clin Exp Res.
33:191–205. 2009. View Article : Google Scholar :
|
|
29
|
You M, Jogasuria A, Taylor C and Wu J:
Sirtuin 1 signaling and alcoholic fatty liver disease.
Hepatobiliary Surg Nutr. 4:88–100. 2015.PubMed/NCBI
|
|
30
|
Qu BG, Wang H, Jia YG, Su JL, Wang ZD,
Wang YF, Han XH, Liu YX, Pan JD and Ren GY: Changes in tumor
necrosis factor-alpha, heat shock protein 70, malondialdehyde, and
super-oxide dismutase in patients with different severities of
alcoholic fatty liver disease: A prospective observational study.
Medicine Baltimore. 94:e6432015. View Article : Google Scholar
|
|
31
|
Abdelmegeed MA, Banerjee A, Jang S, Yoo
SH, Yun JW, Gonzalez FJ, Keshavarzian A and Song BJ: CYP2E1
potentiates binge alcohol-induced gut leakiness, steatohepatitis,
and apop-tosis. Free Radic Biol Med. 65:1238–1245. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhou JY, Jiang ZA, Zhao CY, Zhen Z, Wang W
and Nanji AA: Long-term binge and escalating ethanol exposure
causes necro-inflammation and fibrosis in rat liver. Alcohol Clin
Exp Res. 37:213–222. 2013. View Article : Google Scholar
|
|
33
|
Cederbaum AI: Alcohol metabolism. Clin
Liver Dis. 16:667–685. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ding H and Wen Z: Overexpression of C-sis
inhibits H2O2-induced Buffalo rat liver cell apoptosis in vitro and
alleviates liver injury in a rat model of fulminant hepatic
failure. Int J Mol Med. 42:873–882. 2018.PubMed/NCBI
|
|
35
|
Xing H, Jia K, He J, Shi C, Fang M, Song
L, Zhang P, Zhao Y, Fu J and Li S: Establishment of the tree shrew
as an alcohol-induced fatty liver model for the study of alcoholic
liver diseases. PLoS One. 10:e01282532015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
He Y, Liu Q, Li Y, Yang X, Wang W, Li T,
Zhang W, Cui Y, Wang C and Lin R: Protective effects of
hydroxysafflor yellow A (HSYA) on alcohol-induced liver injury in
rats. J Physiol Biochem. 71:69–78. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kimura M, Yokoyama A and Higuchi S:
Aldehyde dehydro-genase-2 as a therapeutic target. Expert Opin Ther
Targets. 23:955–966. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ding RB, Tian K, Cao YW, Bao JL, Wang M,
He C, Hu Y, Su H and Wan JB: Protective effect of panax notoginseng
saponins on acute ethanol-induced liver injury is associated with
ameliorating hepatic lipid accumulation and reducing
ethanol-mediated oxidative stress. J Agric Food Chem. 63:2413–2422.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mehaffey E and Majid DSA: Tumor necrosis
factor-α, kidney function, and hypertension. Am J Physiol Renal
Physiol. 313:F1005–F1008. 2017. View Article : Google Scholar
|
|
40
|
Slaats J, Ten Oever J, van de Veerdonk FL
and Netea MG: IL-1β/IL-6/CRP and IL-18/ferritin: Distinct
inflammatory programs in infections. PLoS Pathog. 12:e10059732016.
View Article : Google Scholar
|
|
41
|
Unver N and McAllister F: IL-6 family
cytokines: Key inflammatory mediators as biomarkers and potential
therapeutic targets. Cytokine Growth Factor Rev. 41:10–17. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang H, Li J, Gai Z, Kullak-Ublick GA and
Liu Z: TNF-α deficiency prevents renal inflammation and oxidative
stress in obese mice. Kidney Blood Press Res. 42:416–427. 2017.
View Article : Google Scholar
|
|
43
|
Yang L, Sun YY, Liu YR, Yin NN, Bu FT, Yu
HX, Du XS, Li J and Huang C: PTP1B promotes macrophage activation
by regulating the NF-KB pathway in alcoholic liver injury. Toxicol
Lett. 319:11–21. 2020. View Article : Google Scholar
|
|
44
|
Lee JS, An Y, Yoon CJ, Kim JY, Kim KH,
Freeman AF, Yim JJ, Shin EC, Holland SM, Lee EY and Ju YS: Germline
gain-of-function mutation of STAT1 rescued by somatic mosaicism in
immune dysregulation-polyendocrinopathy-enteropathy-X-linked-like
disorder. J Allergy Clin Immunol. 145:1017–1021. 2020. View Article : Google Scholar
|
|
45
|
Starkel P, Schnabl B, Leclercq S, Komuta
M, Bataller R, Argemi J, Palma E, Chokshi S, Hellerbrand C,
Maccioni L, et al: Deficient IL-6/Stat3 signaling, high TLR7, and
type I interferons in early human alcoholic liver disease: A triad
for liver damage and fibrosis. Hepatol Commun. 3:867–882.
2019.PubMed/NCBI
|
|
46
|
Senft D and Ronai ZA: UPR, autophagy, and
mitochondria crosstalk underlies the ER stress response. Trends
Biochem Sci. 40:141–148. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Shimizu A, Kaira K, Yasuda M, Asao T and
Ishikawa O: Clinical and pathological significance of er stress
marker (BiP/GRP78 and PERK) expression in malignant melanoma.
Pathol Oncol Res. 23:111–116. 2017. View Article : Google Scholar
|
|
48
|
Kouznetsova VL, Hu H, Teigen K, Zanetti M
and Tsigelny IF: Cripto stabilizes GRP78 on the cell membrane.
Protein Sci. 27:653–661. 2018. View Article : Google Scholar :
|
|
49
|
Li Y, Guo Y, Tang J, Jiang J and Chen Z:
New insights into the roles of CHOP-induced apoptosis in ER stress.
Acta Biochim Biophys Sin (Shanghai). 46:629–640. 2014. View Article : Google Scholar
|
|
50
|
Malhi H and Kaufman RJ: Endoplasmic
reticulum stress in liver disease. J Hepatol. 54:795–809. 2011.
View Article : Google Scholar
|
|
51
|
Jo HJ, Yang JW, Park JH, Choi ES, Lim CS,
Lee S and Han CY: Endoplasmic reticulum stress increases DUSP5
expression via PERK-CHOP pathway, leading to hepatocyte death. Int
J Mol Sci. 20:43692019. View Article : Google Scholar :
|
|
52
|
Yakin M, Seo B and Rich A:
Tunicamycin-induced endoplasmic reticulum stress up-regulates
tumour-promoting cytokines in oral squamous cell carcinoma.
Cytokine. 120:130–143. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Chen HP, Zhou Y, Qin XF, Wang L, Lin XF,
Chen H and Hu YB: Endoplasmic reticulum stress regulates autophagy
and tumor necrosis factor-α secretion of RAW264.7 cells induced by
silica. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 38:91–95.
2020.In Chinese. PubMed/NCBI
|
|
54
|
Pinto AP, da Rocha AL, Cabrera EMB,
Marafon BB, Kohama EB, Rovina RL, Simabuco FM, Bueno Junior CR, de
Moura LP, Pauli JR, et al: Role of interleukin-6 in inhibiting
hepatic autophagy markers in exercised mice. Cytokine.
130:1550852020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Fiedorowicz E, Jarmolowska B, Iwan M,
Kostyra E, Obuchowicz R and Obuchowicz M: The influence of µ-opioid
receptor agonist and antagonist peptides on peripheral blood
mononuclear cells (PBMCs). Peptides. 32:707–712. 2011. View Article : Google Scholar
|