Introduction
Lung cancer is the most commonly diagnosed type of
cancer, accounting for 11.6% of all cases, and is the leading cause
of cancer-associated mortality, accounting for 18.4% of all
cancer-associated deaths worldwide in 2018 (1). Lung cancer is classified into two
main categories, non-small cell lung cancer (NSCLC) and small cell
lung cancer, of which the former accounts for ~85% of all cases
(2). NSCLC is further subdivided
into three major pathological types, squamous cell carcinoma,
adenocarcinoma and large cell carcinoma (3). With the increase in the use of
low-dose computed-tomography screening and the overall improvement
in public health awareness, the detection of early lung cancer is
increasing annually, along with a decrease in mortality rates
(4); however, lung cancer is
often initially diagnosed as advanced or locally advanced,
particularly in less economically developed regions (5). Therefore, although significant
progress has been made in the diagnosis and clinical treatment of
lung cancer, the 5-year overall survival rate remains at ~19% in
2019 (6). Identifying novel
candidate molecules involved in lung cancer or effective drugs for
clinical treatment to improve the overall survival of lung cancer
is therefore urgently required.
Traditional Chinese herbal medicine has gained
increasing attention as novel anticancer drugs or novel clinical
adjuvants to improve chemotherapy, or to relieve the related side
effects (7). Liriope
platyphylla (LP), a medical plant predominantly found in China,
Korea and Japan, has been used to treat persistent coughs,
neurodegenerative diseases and asthma (8), due to its anti-bacterial and
anti-inflammatory effects (9,10),
as well as its ability to improve memory (11). In addition, steroidal saponins,
the primary bioactive constituents of various medical plants,
including LP, exhibit anticancer effects against several types of
cancer cells, including breast, colorectal, and prostate cancer
cells (12-15). Furthermore, it has been reported
that the abundance of steroidal saponins in LP contributes to the
biological properties of LP (16,17). Liriopesides B (LPB), a traditional
herb isolated from the roots of LP, exhibits antitumor activity in
human ovarian cancer cells (18).
However, to the best of our knowledge, the potential therapeutic
effects of LPB in NSCLC remain unknown. Due to their lower toxicity
and better tolerability compared with unnatural products, natural
products may be ideal candidates for cancer treatment, and this may
explain the widespread interest among researchers worldwide
(7).
In the present study, the antitumor effects of LPB
on H460 and H1975 cells were assessed. LPB inhibited proliferation,
induced apoptosis, increased autophagy and caused G1/S phase
arrest. Furthermore, LPB also downregulated the expression of PD-L1
both at the transcriptional and translational levels. These results
highlight the potent antitumor effects of LPB, suggesting that LPB
may serve as a novel strategy for the clinical treatment of
NSCLC.
Materials and methods
Cell lines and cell culture
H460 and H1975 cells, which have relatively high
expression of PD-L1, were selected to be used in the present study.
Both cell lines were purchased from The Cell Bank of Type Culture
Collection of the Chinese Academy of Sciences. Both cell lines were
cultured in RPmI-1640 medium (Corning, Inc.) with 10% FBS (Corning,
Inc.), penicillin (100 IU/ml) and streptomycin (100 µg/ml)
in an incubator with 5% Co2 at 37°C.
Antibodies and reagents
The following antibodies were purchased from Cell
Signaling Technology, Inc.: Anti-poly (ADP-ribose) polymerase
(PARP; cat. no. 9532; 1:1,000), anti-cleaved PARP (cat. no. 5625;
1:1,000), anti-caspase-3 (cat. no. 9662; 1:1,000), anti-cleaved
caspase-3 (cat. no. 9664; 1:1,000), anti-caspase-9 (cat. no. 9502;
1:1,000), anti-cleaved caspase-9 (cat. no. 9501; 1:1,000),
anti-cleaved caspase-8 (cat. no. 9496; 1:1,000), anti-Survivin
(cat. no. 2808; 1:1,000), anti-Bax (cat. no. 5023; 1:1,000),
anti-Bcl-2 (cat. no. 4223; 1:1,000), anti-Bid (cat. no. 2002;
1:1,000), anti-Bcl-xl (cat. no. 2764; 1:1,000), anti-cytochrome c
(cat. no. 4280; 1:1,000), anti-cox Iv (cat. no. 4850; 1:1,000),
anti-programmed death-ligand 1 (PD-L1; cat. no. 78701; 1:1,000),
anti-phosphorylated (p)-eRK1/2 (Thr202/Tyr204; cat. no. 4370;
1:1,000), anti-eRK1/2 (cat. no. 4695; 1:1,000), anti-p-JNK
(Thr183/Tyr185; cat. no. 9251; 1:1,000), anti-JNK (cat. no. 9252;
1:1,000), anti-p-P38 (Thr180/Tyr182; cat. no. 9211; 1:1,000),
anti-P38 (cat. no. 9212; 1:1,000), anti-p-AKT (Ser473, cat. no.
4060; 1:1,000), anti-AKT (cat. no. 9272; 1:1,000), anti-β-actin
(cat. no. 3700; 1:2,000), anti-p-retinoblastoma (Rb; Ser807/811;
cat. no. 8516; 1:1,000), anti-Rb (cat. no. 9313; 1:1,000), anti-P21
(cat. no. 2947; 1:1,000), anti-CDK6 (cat. no. 3136; 1:1,000),
anti-cyclin D1 (cat. no. 2978; 1:1,000), anti-cyclin D3 (cat. no.
2936; 1:1,000), anti-GAPDH (cat. no. 97166; 1:2,000),
anti-AmP-activated protein kinase α (AMPKα; cat. no. 5831;
1:1,000), anti-unc-51 like autophagy activating kinase (uLK1; cat.
no. 8054; 1:1,000), anti-p-uLK1 (Ser555; cat. no. 5869; 1:1,000),
anti-p-AMPKα (Thr172; cat. no. 50081; 1:1,000), anti-mToR (cat. no.
2983; 1:1,000) and anti-p-mToR (Ser2448; cat. no. 5536; 1:1,000).
Anti-tBid (cat. no. ab10640; Abcam; 1:1,000), anti-CD274 (cat. no.
557924; BD Pharmingen; BD Biosciences), anti-IgG (cat. no. 556650;
BD Pharmingen; BD Biosciences) and Fc block (cat. no. 564765; BD
Pharmingen; BD Biosciences) were also purchased.
Cell Counting Kit-8 (CCK-8) assay
Cell viability was determined using a CCK-8 assay
(Beyotime Institute of Biotechnology) assay. Cells were seeded in
96-well plates (5×103 per well) and incubated with LPB
(purity ≥98%; Chengdu must Bio-Technology Co., Ltd.) at 37°C for 24
or 48 h at different concentrations (0, 10, 20, 30, 40, 50 and 60
µm). Subsequently, 10 µl CCK-8 solution was added to
the cells, and further incubated at 37°C for 1 h. The absorbance
was measured at 450 nm using a microplate spectrophotometer. A
total of five wells were used for each experimental condition.
Colony formation assay
H460 and H1975 cells were seeded into 6-well plates
at a density of 1×103 cells per well. Following
treatment with LPB (0, 20, 40 and 60 µm) for ~2 weeks at
37°C, cells were washed with PBS twice, then fixed in 4%
paraformaldehyde for 30 min at 4°C, and stained with 0.5% crystal
violet for 20 min at room temperature. Visualized colonies were
then imaged and counted.
Annexin V-FITC/PI double staining
Apoptosis was quantified using a FITC Annexin v
Apoptosis Detection kit I (cat. no. 556547; BD Pharmingen; BD
Biosciences) according to the manufacturer's protocol. Cells were
treated with the indicated concentrations (0, 20, 40 and 60
µm) of LPB for 24 h at 37°C. Subsequently, cells were
collected and washed twice with PBS. Subsequently, 1×106
cells were resuspended in 100 µl 1x binding buffer (diluted
with ddH2o), and 5 µl FITC and 5 µl PI
were added to each tube and incubated for 30 min at room
temperature in the dark. After staining, 500 µl 1x binding
buffer was added to each tube before analysis using a flow
cytometer and FlowJo v10.4 software (FlowJo LLC).
Hoechst 33342 staining
Characteristic apoptotic morphological changes were
assessed by fluorescence microscopy using Hoechst 33342 (cat. no.
145331; Sigma-Aldrich; merck KGaA). Briefly, the cells were seeded
in 6-well plates at a density of 1×106 per well,
followed by treatment with LPB (0, 20, 40 and 60 µm) for 24
h at 37°C. Cells were washed with PBS twice and then stained with 5
µg/ml Hoechst 33342 for 15 min at room temperature in the
dark. Following staining, cells were observed with a fluorescence
microscope (magnification, ×200).
Western blot analysis
Cell lysates were prepared using RIPA lysis buffer
containing protease inhibitor (cat. no. 5892970001; Roche
Diagnostics) and phosphatase inhibitor (cat. no. 524629; EMD
Millipore). Following quantitation using a BCA Protein assay kit
(Pierce; Thermo Fisher Scientific, Inc.), equal quantities of
proteins (20-30 µg/lane) were loaded on a 12% SDS-PAGE gel,
followed by transfer to PVDF membranes. The membrane was blocked
with 3% BSA (cat. no. A600332-0100; Sangon Biotech Co., Ltd.) for 1
h at room temperature, and washed with TBS and 0.1% Tween-20
(TBST). Subsequently, the membrane was incubated with the relevant
primary antibodies at room temperature for 2 h, washed three times
with TBST, and then incubated with horseradish peroxidase
(HRP)-conjugated anti-rabbit IgG (cat. no. 7074; 1:2,000; Cell
Signaling Technology, Inc.) and HRP-conjugated goat anti-mouse IgG
(cat. no. ab6789; 1:5,000; Abcam) secondary antibodies for 1 h at
room temperature. After washing in TBST three times, signals were
visualized using an enhanced chemiluminescence assay (Biosharp Life
Sciences).
Mitochondrial membrane potential
analysis
The loss of mitochondrial membrane potential in
LPB-treated H460 and H1975 cells was analyzed using a mitochondrial
membrane Potential Detection JC-1 kit (cat. no. 551302; BD
Pharmingen; Biosciences). H460 and H1975 cells (1×106
cells) were cultured in 6-well plates for 24 h followed by LPB
treatment with the indicated concentrations (0, 20, 40 and 60
µm) for 24 h at 37°C. The cells were harvested and washed
with 1x assay buffer and stained with JC-1 detection reagent for 15
min at 37°C. The stained cells were then analyzed using a flow
cytometer (BD FACSCanto™ II Flow cytometer; BD Biosciences) and
FlowJo v10.4 software (FlowJo LLC), and the fluorescence intensity
in the FITC and Pe channels were assessed.
Mitochondrial isolation
The mitochondrial fraction was isolated from cell
lysates using a mitochondria Isolation kit (cat. no. 89874; Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocol.
Briefly, 800 µl Reagent A was added to 2×107 H460
and H1975 cells, vortexed at 1,500 × g for 5 sec, followed by
incubation on ice for 2 min. Subsequently, 10 µl Reagent B
was added to the tubes and vortexed at 3,000 × g for 5 sec. Next,
800 µl Reagent C was added to each tube and inverted several
times, followed by centrifugation at 700 × g for 10 min at 4°C. The
supernatant was transferred to a 2 ml tube and centrifuged at
12,000 × g for 15 min at 4°C. The supernatant was then collected as
cytoplasmic protein and the precipitate was centrifuged at 12,000 ×
g for 5 min at 4°C following addition of 500 µl reagent C.
Finally, the mitochondrial pellets were lysed using RIPA lysis
buffer on ice for 30 min. Western blot analysis was used to detect
the expression of proteins in the mitochondrial fraction.
Detection of membrane-bound PD-L1
PD-L1 expressed at the membrane of H460 and H1975
cells was evaluated by flow cytometry. Briefly, single-cell
suspensions were collected, and 2.5 µl Fc block was added
and incubated for 10 min at room temperature. Subsequently, 20
µl Pe-conjugated anti-CD274 or anti-IgG (isotype control)
was added and incubated for 30 min at room temperature. There was
no washing step between the two staining steps. After staining,
cells were washed twice in PBS and then used for flow cytometry
analysis with FlowJo v10.4 software (FlowJo LLC).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from cells using TRIzol
reagent (cat. no. 15596-026; Ambion; Thermo Fisher Scientific,
Inc.). RT was performed using HiScript II Select RT Supermix for
qPCR (cat. no. R232-01; vazyme Biotech Co., Ltd.) and qPCR analysis
was performed using SYBR qPCR master mix (cat. no. Q311-02; vazyme
Biotech Co., Ltd.). The RT reaction conditions were as follows:
50°C for 15 min and 85°C for 5 sec, followed by preservation at
4°C. The qPCR thermocycling conditions were as follows:
Pretreatment at 95°C for 30 sec; followed by 40 cycles at 95°C for
10 sec, 60°C for 30 sec and then preservation at 4°C. The sequences
of the primers used were: PD-L1 forward, 5′-CCT ACT GGC ATT TGC TGA
ACG CAT -3′ and reverse, 5′-ACC ATA GCT GAT CAT GCA GCG GTA -3′;
β-actin forward, 5′-ATC TGG CAC CAC ACC T-3′ and reverse, 5′-CGT
CAT ACT CCT GCT T-3′. The 2−ΔΔCq method (19) was used to analyze the expression
of mRNA. Relative abundance was expressed as the ratio of the
analyzed gene to β-actin.
Cell cycle staining
Following treatment with LPB (0, 20, 40 and 60
µM) for 24 h at 37°C, cells were collected by centrifugation
at 500 × g for 5 min at room temperature. The cells were
resuspended in 1 ml PBS and 3 ml absolute ethanol was added; the
tube was gently vortexed whilst adding ethanol, and subsequently,
the cells were left to fix at -20°C overnight. The fixed cells were
centrifuged at 500 × g for 10 min at room temperature, the ethanol
was discarded, and 1-2 ml PBS was added and left at room
temperature for 10 min. Following centrifugation at 500 × g for 5
min at room temperature, 1 ml DNA staining solution (cat. no.
CCS012; multiSciences Biotech Co., Ltd.) was added to the tube, and
incubated for 30 min at room temperature in the dark. A flow
cytometer was used to detect cell cycle distribution and FlowJo
v10.4 software (FlowJo LLC) was used for analysis.
Lentiviral transfection
A total of 5×105 cells were seeded into a
6-well plate before transfection. Following incubation overnight,
50 µl GFP-RFP-LC3 (Hanbio Biotechnology Co., Ltd.) was added
to the plate along with 5 µg/ml polybrene (Hanbio
Biotechnology Co., Ltd.). Subsequently, cells were centrifuged at
1,000 × g for 1 h at room temperature. Following transfection for
48 h, successfully transfected cells were used to detect and
analyze autophagy. Images were captured with a fluorescence
microscope (magnification, ×200).
Statistical analysis
All experiments were repeated independently at least
three times. Data are expressed as the mean ± standard deviation.
Significant differences between the control and treatment groups
were assessed using one-way ANOVA followed by Dunnett's post hoc
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Liriopesides B suppresses the viability
and growth of human NSCLC cells in a dose-dependent manner
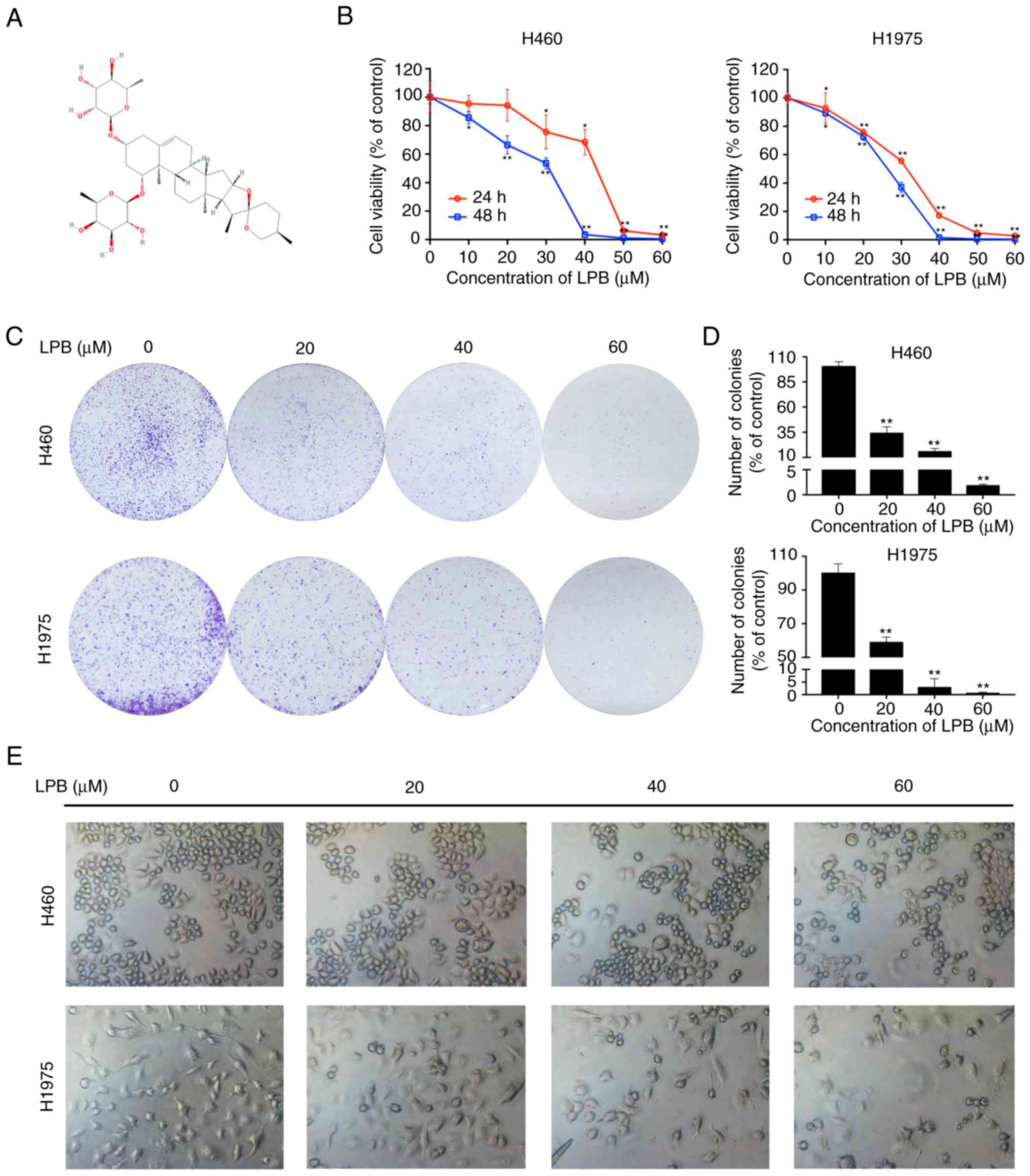
The chemical structure of LPB obtained from National
Center for Biotechnology Information (www.ncbi.nlm.nih.gov/pccom-pound/) is presented in
Fig. 1A. To investigate whether
LPB reduces cell viability of human NSCLC cells, a CCK-8 assay was
used to detect the viability of H460 and H1975 cells treated with
LPB for 24 and 48 h (Fig. 1B).
The IC50 values of LPB treatment for 24 h in H460 and H1975 cells
were 42.62 and 32.25 µM, respectively. Compared with
untreated cells, 60 µm LPB significantly reduced cell
viability of H460 and H1975 cells after 24 h treatment. Thus,
whether treatment with LPB affected clonal growth of human lung
cancer cells was assessed. A colony formation assay was performed,
and the results demonstrated that LPB suppressed the clonogenic
growth of H460 and H1975 cells in a dose-dependent manner (Fig. 1C and D). Additionally, the
morphological changes and numbers of H460 and H1975 cells treated
with the indicated concentrations were observed using a
fluorescence microscope under bright-field (Fig. 1e). These results suggest that LPB
inhibits the growth of H460 and H1975 cells.
Liriopesides B induces apoptosis of NSCLC
cells
Flow cytometry was used to detect apoptosis of H460
and H1975 cells following treatment with LPB. LPB significantly
increased the proportion of apoptotic cells in both H460 and H1975
cells (Fig. 2A). The percentage
of apoptotic H460 and H1975 cells after treatment with 60 µm
LPB was 80.1 and 60.9%, respectively, and in the control, it was
12.7 and 8.3%, respectively. Additionally, the fluorescence images
revealed the changes in H460 and H1975 cells stained with Hoechst
33342 following treatment with the indicated concentrations of LPB
(0, 20, 40 or 60 µm) for 24 h. Chromatin condensation, DNA
fragmentation and cell shrinkage, which are all features of
apoptotic cells, was observed under a fluorescence microscope
(Fig. 2B). To further investigate
the mechanism underlying this phenomenon, expression levels of
apoptosis-associated proteins were determined by western blotting
(Fig. 2C). The levels of cleaved
caspase-3, -8, -9 and PARP increased after 24 h treatment with the
indicated concentrations of LPB both in H460 and H1975 cells,
whereas the levels of Survivin decreased, suggesting that LPB
induced apoptosis by activating caspases.
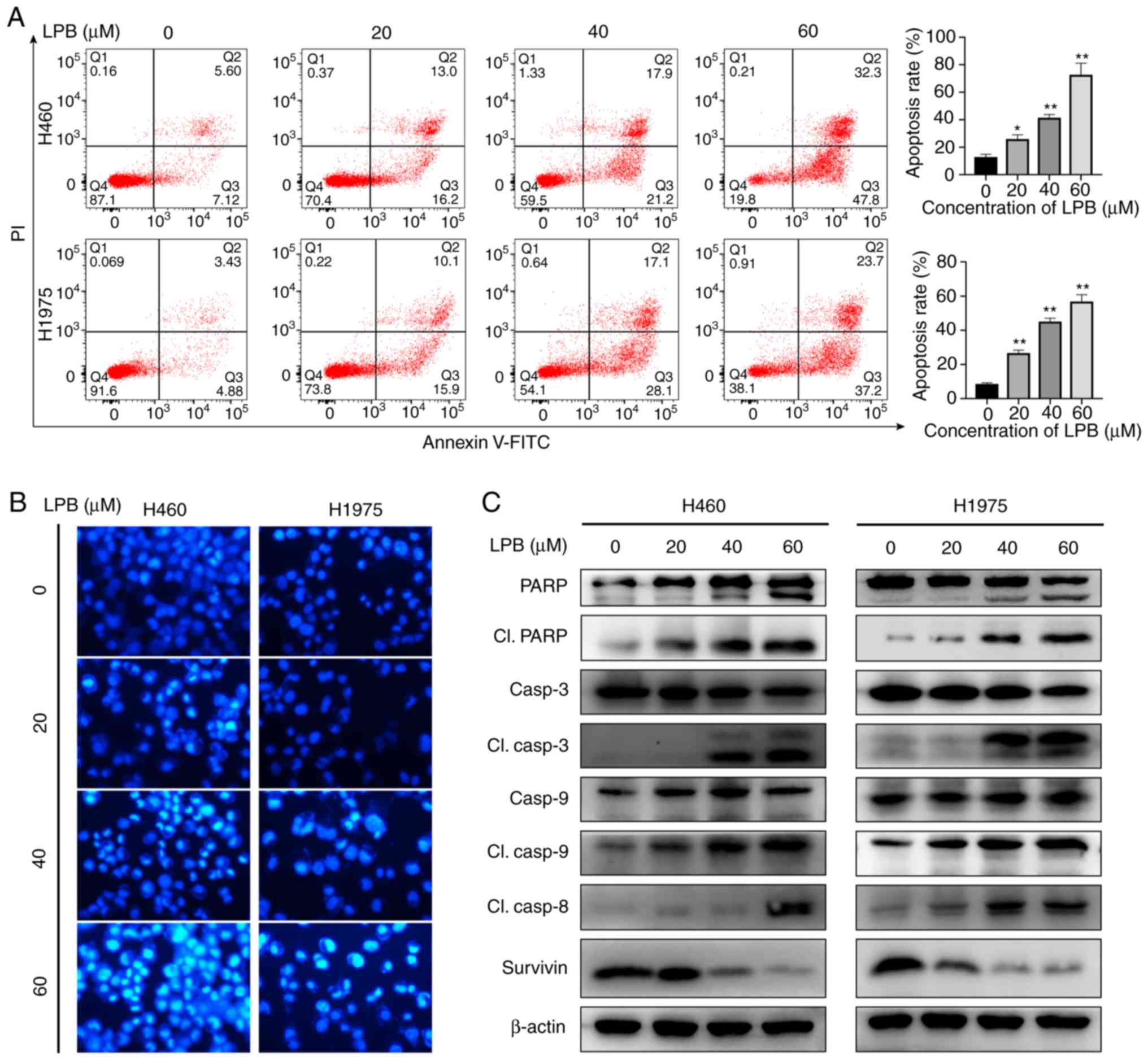 | Figure 2LPB induces apoptosis of H460 and
H1975 cells. (A) Annexin V-FITC and PI double staining were used to
detect apoptosis induced by different concentrations of LPB for 24
h. *P<0.05, **P<0.001 vs. 0 µm
LPB. (B) Morphological changes in apoptotic cells treated with LPB
are shown by Hoechst 33342 staining using fluorescence microscopy.
Magnification, ×200. (C) Expression levels of PARP, cleaved PARP,
caspase-3, cleaved caspase-3, caspase-9, cleaved caspase-9, cleaved
caspase-8 and Survivin were examined by western blotting in H460
and H1975 treated with LPB for 24 h at different concentrations.
Results are representative of three independent experiments. LPB,
liriopesides B; PARP, poly (ADP-ribose) polymerase. |
Liriopesides B initiates the
mitochondrial apoptosis pathway in H460 and H1975 cells
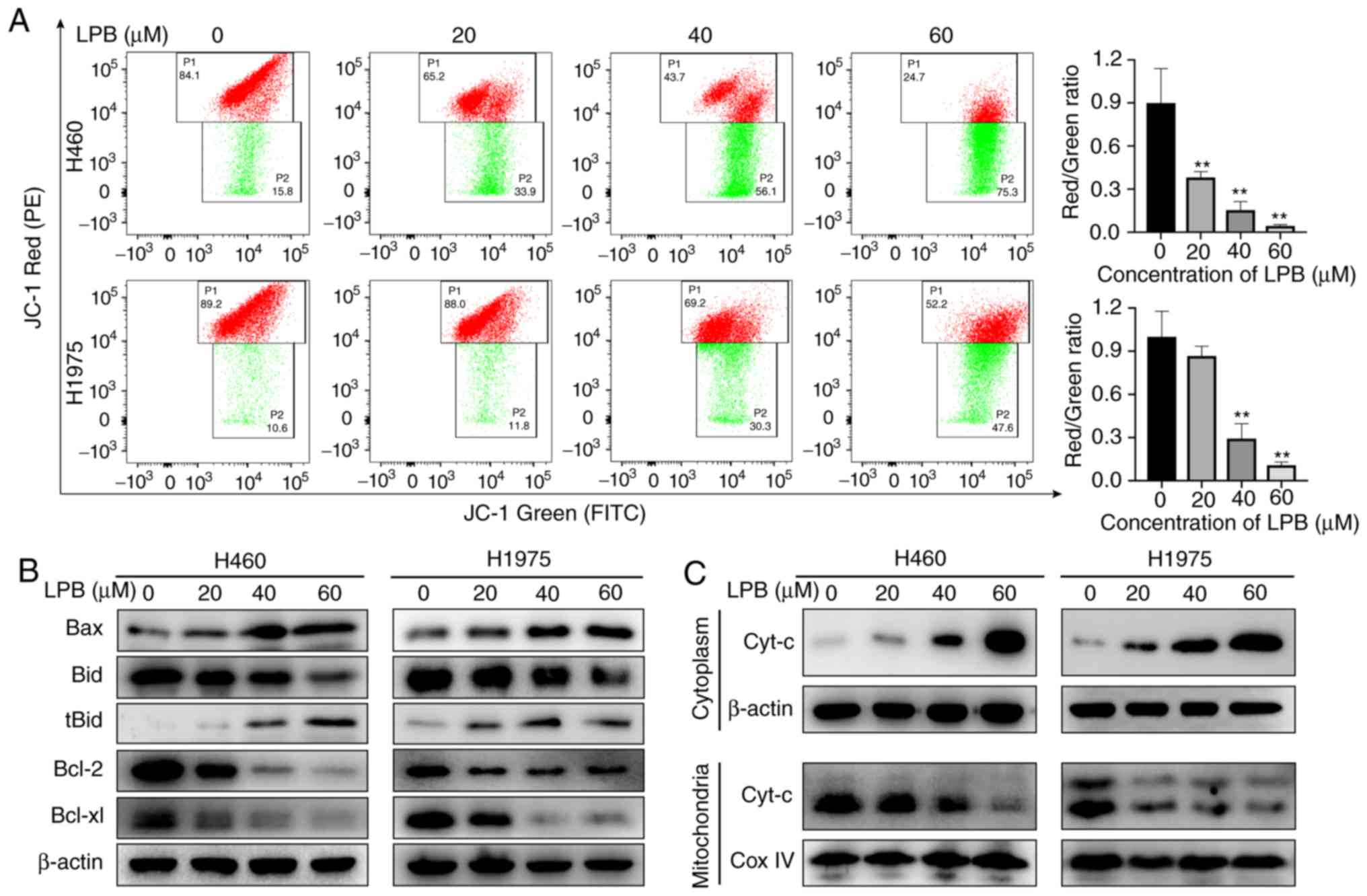
To determine whether the mitochondrial apoptosis
pathway is involved in apoptosis, a JC-1 staining assay was
performed to detect the mitochondrial membrane potential. The JC-1
probe displayed red fluorescence under normal conditions as the J
aggregates translocated to the inner mitochondrial membrane.
However, red fluorescence changed as J monomers formed in apoptotic
cells, which appeared green. These changes indicated a decrease in
mitochondrial membrane potential. LPB significantly decreased the
mitochondrial membrane potential in both H460 and H1975 cells
(Fig. 3A). Due to their function
in mediating mitochondrial membrane permeability, western blot
analysis was used to examine the levels of the apoptosis-related
proteins (Fig. 3B). The results
demonstrated that the expression levels of Bax and tBid, known as
pro-apoptotic proteins, were increased, whereas the expression
levels of the anti-apoptotic proteins, Bcl-2 and Bcl-xl, were
decreased after treatment with LPB. Furthermore, western blotting
revealed that the expression of cytochrome c decreased in the
mitochondria and increased in the cytoplasm following treatment
with LPB, suggesting that cytochrome c was released from the
mitochondria into the cytoplasm to initiate apoptosis. Taken
together, these results suggest the mitochondrial apoptosis pathway
is involved in LPB-induced apoptosis.
Liriopesides B induces G1/S arrest in
NSCLC cells
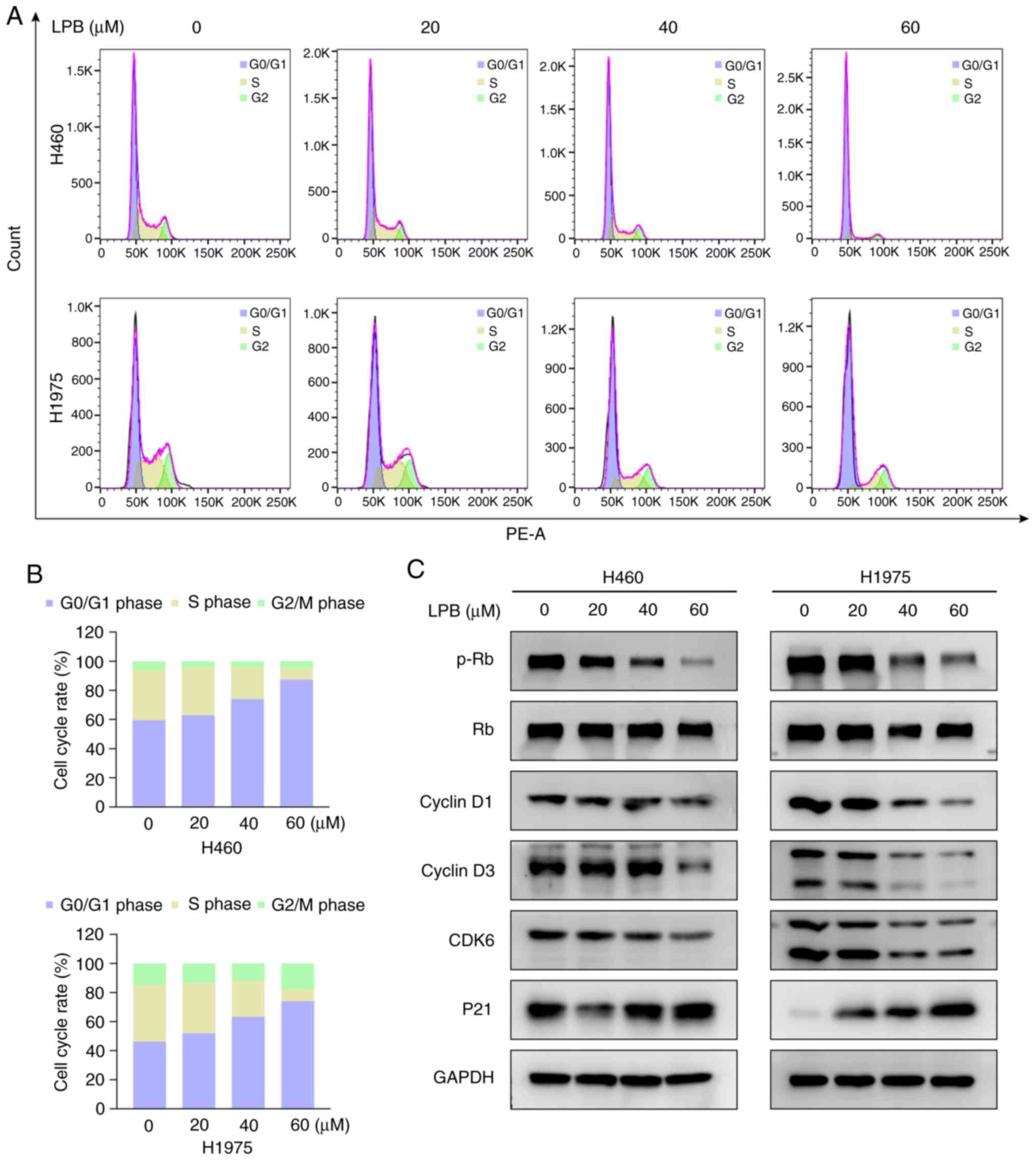
Cell cycle arrest is a frequently observed antitumor
mechanism of several other traditional Chinese medicines (20,21). As LPB suppressed NSCLC cell
growth, its effects on cell cycle progression were assessed. H460
and H1975 cells were treated with LPB for 24 h, and analyzed by
flow cytometry. LPB induced G1/S phase arrest in a
concentration-dependent manner (Fig.
4A), with the percentage of cells in the G1 phase increasing
from 59.5 to 87.4% in H460, and increasing from 46.2 to 74.0% in
H1975 cells in untreated and 60 µm group, respectively
(Fig. 4B). To determine the
underlying mechanism, the expression levels of proteins associated
with the G1/S phase checkpoint were assessed, demonstrating that
LPB decreased the phosphorylation of Rb, and the total expression
of Cyclin D1, Cyclin D3 and CDK6 in both H460 and H1975 cells,
whilst upregulating the expression of P21 only in H1975 cells
(Fig. 4C). The atypical
expression of P21 in H460 cells indicates that there may be some
complex regulatory relationship that is not yet clear, therefore
further experimental investigations are required. Taken together,
these results indicate that LPB induced G1/S phase arrest via a
P21-Cyclin D/CDK6 signaling pathway.
LPB activates autophagy via an AMPKα-mTOR
signaling pathway in H460 and H1975 cells
Autophagy is a catalytic process that causes
autophagic lysosome-mediated degradation of the main contents of
the cytoplasm, abnormal protein aggregation and damaged organelles,
and this serves an important role in tumor development (22). In the present study, there was a
notable aggregation of LC3 puncta observed following LPB treatment,
and the degree of aggregation increased in a
concentration0dependent manner (Fig.
5A). It has been reported that the AMPK-mTOR signaling pathway
may regulate autophagy (23), and
thus the effect of LPB on this pathway was assessed in NSCLC cells.
Western blotting demonstrated that LPB reduced the levels of
p-mTOR, and increased phosphorylation of AMPKα and ULK (Fig. 5B). The expression levels of LC3
were also increased following treatment with LPB for 24 h in both
H460 and H1975 cells (Fig. 5B).
Taken together, LPB may induce autophagy in H460 and H1975 cells
via an AMPKα-mTOR signaling pathway.
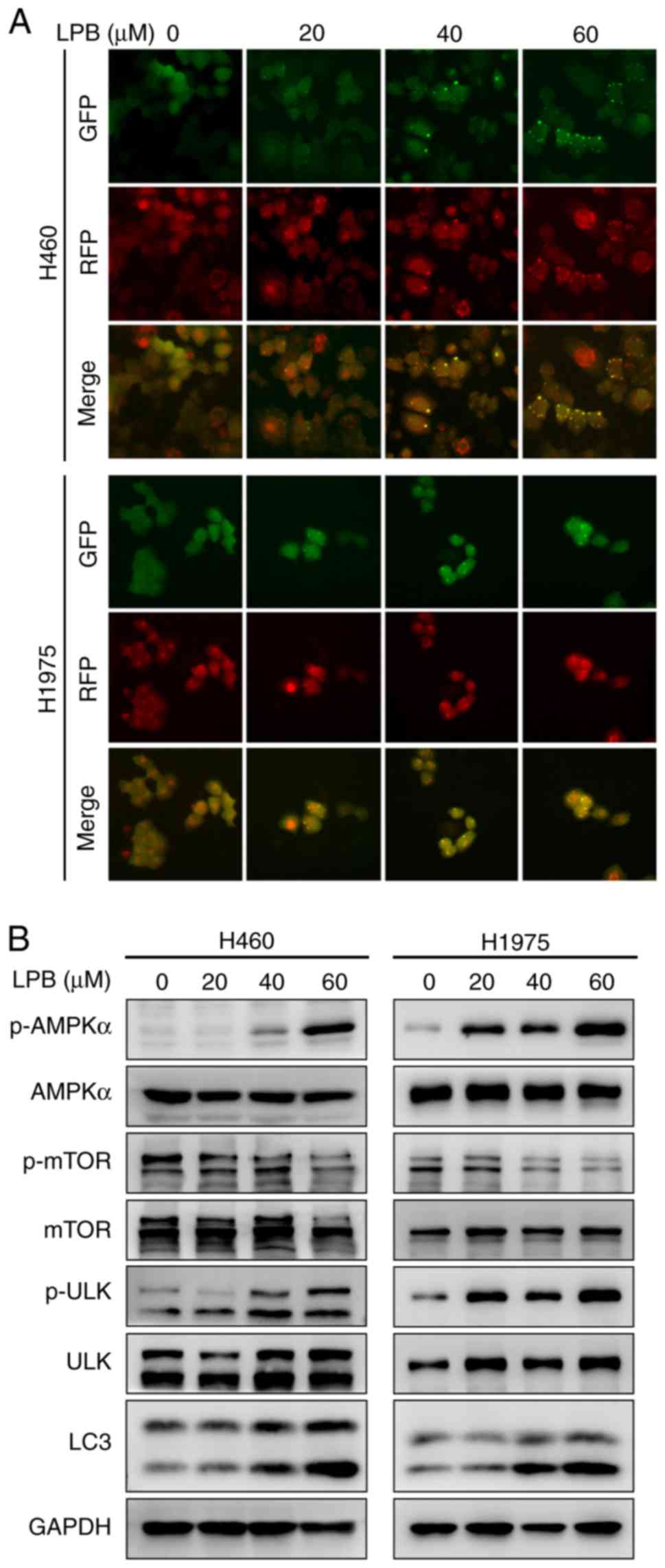 | Figure 5LPB induces autophagy via an
AMPKα-mToR signaling pathway in H460 and H1975 cells. (A) H460 and
H1975 cells, stably expressing GFP-RFP-LC3, were treated with LPB.
Representative images of LC3 puncta captured using a fluorescence
microscope are presented. Magnification, ×200. (B) AMPKα, p-AMPKα,
p-mTOR, mTOR, p-ULK, ULK, and LC3 expression was detected using
western blotting in H460 and H1975 cells treated with LPB. LPB,
liriopesides B; p-, phosphorylated; AMPKα, AMP-activated protein
kinase α; uLK, unc-51 like autophagy activating kinase. |
Liriopesides B inhibits mitogen-activated
protein kinase (MAPK) and AKT signaling pathways in NSCLC
cells
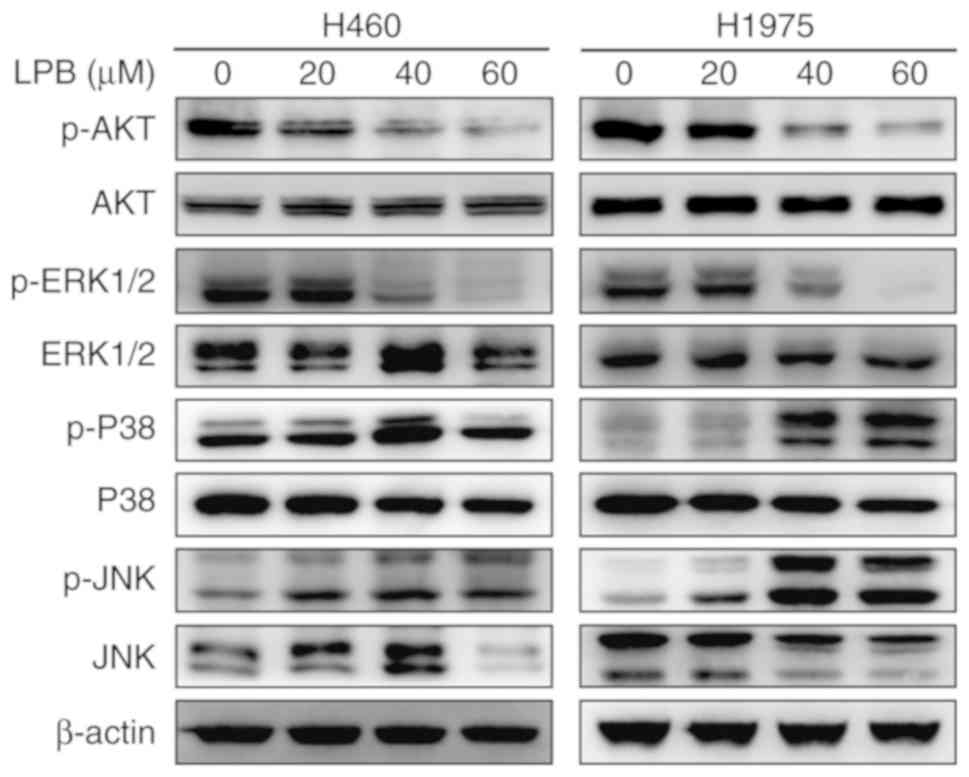
The MAPK signaling pathway is involved in several
aspects of maintaining cell survival (24). To investigate the mechanism by
which LPB suppresses the proliferation of NSCLC cells, the MAPK
signaling pathway was assessed by western blotting. Three classical
pathways, eRK1/2, p38/MAPK and c-JNK, are involved in the MAPK
signaling pathway (25). The
activation of protein kinases was evaluated by western blot
analysis to determine the effect of LPB on them. LPB decreased
eRK1/2 phosphorylation in a dose-dependent manner in both H460 and
H1975 cells, and increased p38/MAPK and JNK phosphorylation
(Fig. 6). Similar to the MAPK
signaling pathway, the level of phosphorylated AKT was reduced by
LPB.
Liriopesides B decreases the expression
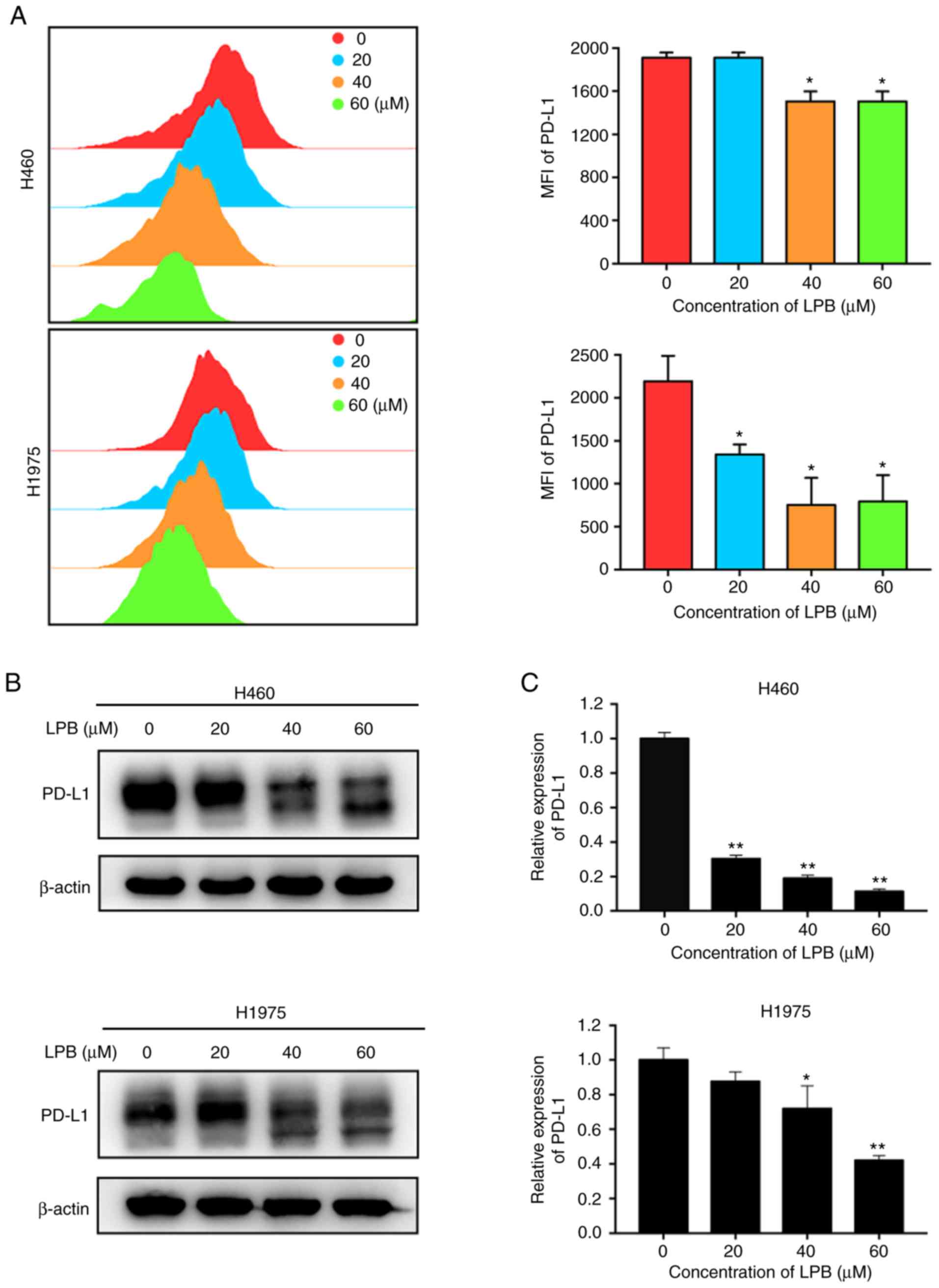
of PD-L1 in H460 and H1975 cells
It has been reported that PD-L1 translocates to the
tumor cell membrane and binds to PD-1, which is expressed on the
T-cell membrane to exert immune escape (26). Thus, the expression of PD-L1 on
the surface of H460 and H1975 cells was assessed by flow cytometry
in the present study. The levels of PD-L1 expressed on the cell
membrane of H460 and H1975 significantly decreased following
treatment with LPB (Fig. 7A). The
total PD-L1 expression levels were determined using western
blotting (Fig. 7B) and RT-qPCR
(Fig. 7C). The results
demonstrated that PD-L1 expression was suppressed by LPB in a
dose-dependent manner. These results indicate LPB reduced both the
total expression of PD-L1 and the expression of PD-L1 at the cell
membrane of H460 and H1975 cells.
Discussion
Cancer is the second leading cause of death
worldwide, accounting for ~10 million deaths in 2018 (1). In addition, lung cancer is the most
commonly diagnosed cancer and the leading cause of
cancer-associated mortality (1).
Although cancer diagnoses and treatment techniques have improved
significantly, a notable proportion of patients are diagnosed in
the first instance with advanced stage cancer (27). In addition, chemotherapy remains
the primary treatment for people with advanced stage or relapsed
tumors (28). Due to the various
adverse reactions and/or the development of multi-drug resistance
with current chemotherapy regimens, a safe and effective adjuvant
therapy or reagent is required. Natural compounds, isolated from
medicinal plants, are potential resources for the development of
novel chemotherapeutic reagents including steroidal saponins, which
exhibit antitumor functions (29,30). Whilst the exact mechanisms
underlying the effects of these compounds are not yet fully
understood, some reagents have been used to treat cancer patients
with fewer side effects than established treatments (31). The anti-bacterial activity, memory
enhancement effect and anti-inflammatory effects of spicatoside A
(SPA) have been shown in several studies (11,32,33). It has also been reported that SPA
may suppress the proliferation of colon cancer cells via cell cycle
regulation and apoptosis (34).
Therefore, in the present study, the effects of LPB, which contains
a similar active compound to SPA, on NSCLC cells were assessed.
The CCK-8 assay, colony formation assay and
phase-contrast microscopy demonstrated that LPB significantly
reduced cell viability and clonogenic growth of H460 and H1975
cells in a dose-dependent manner. Apoptosis, also referred to as
programmed cell death I, is a desirable outcome of traditional
cancer therapy, including chemotherapy and radiotherapy (35). The results suggested that LPB
increased cleavage of caspase-8 into its active form, and cleaved
caspase-8 shears Bid to tBid, which is then translocated to the
mitochondrial outer membrane and interacts with Bax/Bcl-2 (36) to alter the mitochondrial membrane
potential (37). These changes
eventually contribute to the increase in permeability of the
mitochondrial membrane, leading to the release of cytochrome c from
the mitochondria into the cytoplasm. Cytochrome c is a
well-conserved electron-transport protein and is part of the
respiratory chain localized in the mitochondrial intermembrane
space (38). Cytochrome c,
released from the mitochondria, can integrate with
pro-caspase-9/apoptotic protease activating factor 1 in the
cytoplasm and this complex cleaves caspase-9 from an inactive
proenzyme to its active form, cleaved caspase-9 (39). This event further triggers the
activation of caspase-3, and finally, PARP is cleaved by the
activated caspases, leading to nuclear condensation and apoptotic
cell death (40).
Additionally, LPB induced G1/S phase arrest, as
detected by flow cytometry, by decreasing the expression of Cyclin
D1, Cyclin D3 and CDK6, whilst increasing the expression of P21.
These results suggest that cell cycle arrest also serves a role in
the antitumor effects of LPB. Notably, it was identified that LPB
could cause cell cycle arrest in the G1 phase at low
concentrations, while apoptotic cells appeared when the
concentration was increased to a certain level. The possible
explanation of this phenomenon is that following stimulation by
LPB, the cell cycle paused to repair DNA damage, but as the
compound concentration increases, the outcome for cells that cannot
repair the damage goes to death, including apoptosis. Furthermore,
LPB may significantly induce autophagy via the AMPK-mToR signaling
pathway in H460 and H1975 cells, as notable LC3 puncta were
observed. LPB was thus shown to exhibit significant effects on
apoptosis, cell cycle arrest and autophagy. However, it is unknown
how these effects interact and affect each other, and further
investigation is required.
Furthermore, it has been reported that the MAPK
pathway serves a vital role in the development and progression of
several types of cancer (41).
The eRK1/2 signaling pathway serves a critical role in survival
(42), and JNK is associated with
proapoptotic activities in several tumor cell lines (43-45). Compared with eRK1/2 or JNK, the
p38/MAPK signaling pathway displays a relatively complex function.
It has been reported that p38/MAPK may function as a tumor
suppressor by increasing apoptosis (46). However, under certain conditions,
the p38/MAPK signaling pathway may also initiate resistance to
apoptosis (47,48). In the present study, LPB treatment
increased p38/MAPK and JNK phosphorylation and decreased eRK1/2
phosphorylation. Therefore, it was hypothesized that LPB induced
apoptosis in H460 and H1975 cells via the MAPK signaling
pathway.
PD-L1, also known as CD274, has gained increasing
attention. When PD-L1 binds with PD-1, it promotes T-cell tolerance
and thus immune escape (49).
Furthermore, it has been reported that the overexpression of PD-L1
is associated with a poor prognosis in several types of human
cancer (50). In the present
study, it was shown that LPB may significantly inhibit the
expression of PD-L1, both at the transcriptional and translational
levels. This suggests that LPB may potentially reduce the
expression of PD-L1, and thereby suppress immune escape of tumor
cells and exert antitumor effects. However, the PD-L1 inhibition of
LPB and its subsequent effects need to be confirmed in
vivo.
In conclusion, the antitumor effects of LPB in NSCLC
cells were shown for the first time in the present study, to the
best of our knowledge. Five novel findings may account for the
antitumor effects of LPB: i) Activation of caspases; ii) decrease
in mitochondrial membrane potential; iii) induction of G1/S phase
arrest; iv) induction of autophagy; and v) inhibition of PD-L1
expression. The effects of LPB on NSCLC cells may be a potential
therapeutic strategy, which may circumvent the adverse side effects
and drug resistance that are frequently associated with current
NSCLC chemotherapy. The promising anticancer mechanisms of LPB
should be further studied to establish an efficacious and safe
therapeutic strategy for the treatment of NSCLC. In terms of the
limitation of this research, whether LPB acts on the cell surface
or penetrates into the cell needs to be confirmed by radio-labeling
or a fluorescent labeling method. Furthermore, we are currently
unable to track the specific effective location or target of our
compound in selected cells, which has become a limitation of the
present study. This part of the work will be further improved in
future research. In addition, further in vivo
pharmacological and clinical investigations are required. The
results of the present study suggest that LPB may be a promising
therapeutic for the treatment of NSCLC.
Acknowledgments
Not applicable.
Funding
This study was financially supported by the National
Key R&D Program of China (grant no. 2017YFC0113500), major
Science and Technology Projects of Zhejiang Province (grant no.
2014C03032), Zhejiang Lung Cancer Diagnosis and Treatment
Technology Research Center (grant no. JBZx-202007), Zhejiang
Provincial Key Discipline of Traditional Chinese medicine (grant
no. 2017-xK-A33), Natural Science Foundation of Zhejiang Province
(grant no. LY18H160021), Natural Science Foundation of Zhejiang
Province (grant no. LQ20H160050), Zhejiang Traditional Chinese
medicine Scientific Research Fund Program (grant nos. 2016ZA125 and
2018ZB073) and Zhejiang medical General Research Program (grant no.
2015KYB140).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HS made substantial contributions to the data
collection, analysis and interpretation. JHu and WL designed the
study. LZ and JHa were mainly responsible for experiments related
to flow cytometry and prepared the figures. LW and ZW were
responsible for experimental data collection and drafting the
article. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLoBoCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Govindan R, Page N, Morgensztern D, Read
W, Tierney R, Vlahiotis A, Spitznagel EL and Piccirillo J: Changing
epidemiology of small-cell lung cancer in the united States over
the last 30 years: Analysis of the surveillance, epidemiologic, and
end results database. J Clin Oncol. 24:4539–4544. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Oser MG, Niederst MJ, Sequist LV and
Engelman JA: Transformation from non-small-cell lung cancer to
small-cell lung cancer: molecular drivers and cells of origin.
Lancet Oncol. 16:e165–e172. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nawa T: Low-dose CT screening for lung
cancer reduced lung cancer mortality in Hitachi City. Int J Radiat
Biol. 95:1441–1446. 2019. View Article : Google Scholar
|
|
5
|
Hoffman PC, Mauer AM and Vokes EE: Lung
cancer. Lancet. 355:479–485. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ashraf-Uz-Zaman M, Bhalerao A, Mikelis CM,
Cucullo L and German NA: Assessing the current state of lung cancer
chemo-prevention: A comprehensive overview. Cancers (Basel).
12:e12652020. View Article : Google Scholar
|
|
8
|
Hur J, Lee P, Moon E, Kang I, Kim SH, Oh
MS and Kim SY: Neurite outgrowth induced by spicatoside A, a
steroidal saponin, via the tyrosine kinase A receptor pathway. Eur
J Pharmacol. 620:9–15. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee YC, Lee JC, Seo YB and Kook YB:
Liriopis tuber inhibit OVA-induced airway inflammation and
bronchial hyperresponsiveness in murine model of asthma. J
Ethnopharmacol. 101:144–152. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim SW, Chang IM and Oh KB: Inhibition of
the bacterial surface protein anchoring transpeptidase sortase by
medicinal plants. Biosci Biotechnol Biochem. 66:2751–2754. 2014.
View Article : Google Scholar
|
|
11
|
Kwon G, Lee HE, Lee DH, Woo H, Park SJ,
Gao Q, Ahn YJ, Son KH and Ryu JH: Spicatoside A enhances memory
consolidation through the brain-derived neurotrophic factor in
mice. Neurosci Lett. 572:58–62. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chan JY, Koon JC, Liu X, Detmar M, Yu B,
Kong SK and Fung KP: Polyphyllin D, A steroidal saponin from Paris
polyphylla, inhibits endothelial cell functions in vitro and
angiogenesis in zebrafish embryos in vivo. J Ethnopharmacol.
137:64–69. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sy LK, Yan SC, Lok CN, Man RYK and Che CM:
Timosaponin A-III induces autophagy preceding mitochondria-mediated
apoptosis in HeLa cancer cells. Cancer Res. 68:10229–10237. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang Y, Tang Q, Jiang S, Li M and Wang X:
Anti-colorectal cancer activity of macrostemonoside A mediated by
reactive oxygen species. Biochem Biophys Res Commun. 441:825–830.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang Y, Che CM, Chiu JF and He QY: Dioscin
(saponin)-induced generation of reactive oxygen species through
mitochondria dysfunction: A proteomic-based study. J Proteome Res.
6:4703–4710. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Neychev VK, Nikolova E, Zhelev N and Mitev
VI: Saponins from Tribulus terrestris L are less toxic for normal
human fibroblasts than for many cancer lines: Influence on
apoptosis and proliferation. Exp Biol Med (Maywood). 232:126–133.
2007.
|
|
17
|
Naveed MA, Riaz N, Saleem M, Jabeen B,
Ashraf M, Ismail T and Jabbar A: Longipetalosides A-C, new
steroidal saponins from Tribulus longipetalus. Steroids. 83:45–51.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang H, Yu H, Sun Y, Zhao H, Guo Z and Yu
B: Liriopesides B inhibited cell growth and decreased CA125 level
in human ovarian cancer A2780 cells. Nat Prod Res. 31:2198–2202.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
20
|
Fan L, Li L, Yu X, Liang Z, Cai T, Chen Y,
Xu Y, Hu T, Wu L and Lin L: Jianpiyifei II granules suppress
apoptosis of bronchial epithelial cells in chronic obstructive
pulmonary disease via inhibition of the reactive oxygen
species-endoplasmic reticulum stress-Ca(2+) signaling pathway.
Front Pharmacol. 11:5812020. View Article : Google Scholar
|
|
21
|
Zhang D, Zhang Q, Zheng Y and Lu J:
Anti-breast cancer and toxicity studies of total secondary saponin
from Anemone raddeana Rhizome on mCF-7 cells via RoS generation and
PI3K/AKT/mTOR inactivation. J Ethnopharmacol. 1129842020.
View Article : Google Scholar
|
|
22
|
Mercer CA, Kalippan A and Dennis PB: A
Novel, human Atg13 binding protein, Atg101, interacts with uLK1 and
is essential for macroautophagy. Autophaghy. 5:649–662. 2009.
View Article : Google Scholar
|
|
23
|
Gu X, Li Y, Chen K, Wang X, Wang Z, Lian
H, Lin Y, Rong X, Chu M, Lin J and Guo X: Exosomes derived from
umbilical cord mesenchymal stem cells alleviate viral myocarditis
through activating AMPK/mTOR-mediated autophagy flux pathway. J
Cell Mol Med. May 18–2020.Epub ahead of print. View Article : Google Scholar
|
|
24
|
Ohguchi H, Harada T, Sagawa M, Kikuchi S,
Tai YT, Richardson PG, Hideshima T and Anderson KC: KDm6B modulates
MAPK pathway mediating multiple myeloma cell growth and survival.
Leukemia. 31:2661–2669. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fang JY and Richardson BC: The MAPK
signalling pathways and colorectal cancer. Lancet Oncol. 6:322–327.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dong W, Wu X, Ma S, Wang Y, Nalin AP, Zhu
Z, Zhang J, Benson DM, He K, Caligiuri MA and Yu J: The mechanism
of anti-PD-L1 antibody efficacy against PD-L1-negative tumors
identifies NK cells expressing PD-L1 as a cytolytic effector.
Cancer Discov. 9:1422–1437. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
de Koning HJ, van der Aalst Cm, de Jong
PA, Scholten ET, Nackaerts K, Heuvelmans MA, Lammers JJ, Weenink C,
Yousaf-Khan U, Horeweg N, et al: Reduced lung-cancer mortality with
volume CT screening in a randomized trial. N Engl J Med.
382:503–513. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Arbour KC and Riely GJ: Systemic therapy
for locally advanced and metastatic non-small cell lung cancer: A
review. JAMA. 322:764–774. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Awang K, Azmi MN, Aun LI, Aziz AN, Ibrahim
H and Nagoor NH: The apoptotic effect of 1′s-1′-acetoxychavicol
acetate from Alpinia conchigera on human cancer cells. Molecules.
15:8048–8059. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yeh CC, Tseng CN, Yang JI, Huang HW, Fang
Y, Tang JY, Chang FR and Chang HW: Antiproliferation and induction
of apoptosis in Ca9-22 oral cancer cells by ethanolic extract of
Gracilaria tenuistipitata. Molecules. 17:10916–10927. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Normile D: Asian medicine. The new face of
traditional chinese medicine. Science. 299:188–190. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lim H, Min DS, Kang Y, Kim HW, Son KH and
Kim HP: Inhibition of matrix metalloproteinase-13 expression in
IL-1β-treated articular chondrocytes by a steroidal saponin,
spicatoside A, and its cellular mechanisms of action. Arch Pharm
Res. 38:1108–1116. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Park SH, Lee HJ, Ryu J, Son KH, Kwon SY,
Lee SK, Kim YS, Hong JH, Seok JH and Lee CJ: effects of
ophiopogonin D and spicatoside A derived from Liriope Tuber on
secretion and production of mucin from airway epithelial cells.
Phytomedicine. 21:172–176. 2014. View Article : Google Scholar
|
|
34
|
Kim WK, Pyee Y, Chung HJ, Park HJ, Hong
JY, Son KH and Lee SK: Antitumor activity of spicatoside A by
modulation of autophagy and apoptosis in human colorectal cancer
cells. J Nat Prod. 79:1097–1104. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pistritto G, Trisciuoglio D, Ceci C,
Garufi A and D'orazi G: Apoptosis as anticancer mechanism: Function
and dysfunction of its modulators and targeted therapeutic
strategies. Aging (Albany NY). 8:603–619. 2016. View Article : Google Scholar
|
|
36
|
Luo X, Budihardjo I, Zou H, Slaughter C
and Wang X: Bid, a Bcl2 interacting protein, mediates cytochrome c
release from mitochondria in response to activation of cell surface
death receptors. Cell. 94:481–490. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li H, Zhu H, Xu CJ and Yuan J: Cleavage of
BID by caspase 8 mediates the mitochondrial damage in the Fas
pathway of apoptosis. Cell. 94:491–501. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Schägger H: Respiratory chain
supercomplexes of mitochondria and bacteria. Biochim Biophys Acta.
1555:154–159. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li P, Nijhawan D, Budihardjo I,
Srinivasula SM, Ahmad M, Alnemri ES and Wang X: Cytochrome c and
dATP-dependent formation of Apaf-1/caspase-9 complex initiates an
apoptotic protease cascade. Cell. 91:479–489. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu X, Kim CN, Yang J, Jemmerson R and
Wang X: Induction of apoptotic program in cell-free extracts:
Requirement for dATP and cytochrome c. Cell. 86:147–157. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Khavari TA and Rinn J: Ras/Erk MAPK
signaling in epidermal homeostasis and neoplasia. Cell Cycle.
6:2928–2931. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Burotto M, Chiou VL, Lee JM and Kohn EC:
The MAPK pathway across different malignancies: A new perspective.
Cancer. 120:3446–3456. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li Y, Zhao L, Sun H, Yu J, Li N, Liang J,
Wang Y, He M, Bai X, Yu Z, et al: Gene silencing of FANCF
potentiates the sensitivity to mitoxantrone through activation of
JNK and p38 signal pathways in breast cancer cells. PLoS one.
7:e442542012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mansouri A, Ridgway LD, Korapati AL, Zhang
Q, Tian L, Wang Y, Siddik ZH, Mills GB and Claret Fx: Sustained
activation of JNK/p38 MAPK pathways in response to cisplatin leads
to Fas ligand induction and cell death in ovarian carcinoma cells.
J Biol Chem. 278:19245–19256. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sau A, Filomeni G, Pezzola S, D'Aguanno S,
Tregno FP, Urbani A, Serra M, Pasello M, Picci P, Federici G and
Caccuri Am: Targeting GSTP1-1 induces JNK activation and leads to
apoptosis in cisplatin-sensitive and -resistant human osteosarcoma
cell lines. Mol Biosyst. 8:994–1006. 2012. View Article : Google Scholar
|
|
46
|
Deacon K, Mistry P, Chernoff J, Blank J
and Patel R: p38 mitogen-activated protein kinase mediates cell
death and p21-activated kinase mediates cell survival during
chemotherapeutic drug-induced mitotic arrest. Mol Biol Cell.
14:2071–2087. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Salim H, Akbar N, Zong D, Vaculova AH,
Lewensohn R, Moshfegh A, Viktorsson K and Zhivotovsky B: miRNA-214
modulates radiotherapy response of non-small cell lung cancer cells
through regulation of p38MAPK, apoptosis and senescence. Br J
Cancer. 107:1361–1373. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chen SF, Nieh S, Jao SW, Liu CL, Wu CH,
Chang YC, Yang CY and Lin YS: Quercetin suppresses drug-resistant
spheres via the p38 MAPK-Hsp27 apoptotic pathway in oral cancer
cells. PLoS one. 7:e492752012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Keir ME, Butte MJ, Freeman GJ and Sharpe
AH: PD-1 and its ligands in tolerance and immunity. Annu Rev
Immunol. 26:677–704. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Konishi J, Yamazaki K, Azuma M, Kinoshita
I, Dosaka-Akita H and Nishimura M: B7-H1 expression on non-small
cell lung cancer cells and its relationship with tumor-infiltrating
lymphocytes and their PD-1 expression. Clin Cancer Res.
10:5094–6100. 2004. View Article : Google Scholar : PubMed/NCBI
|