Introduction
Dyschromatosis universalis hereditaria (DUH) is a
clinically heterogeneous disorder that is characterized by evident
mottled pigmentation over the entire body of affected individuals.
In 2003, similar Chinese DUH pedigrees were discovered with
dyschromatosis symmetrica hereditaria (DSH) with autosomal dominant
DUH (1) and these cases were
subsequently diagnosed as DUH rather than DSH. In the 10.2 Mb
region on chromosome 6 (6q24.2-q25.2), a c.1654 T>G (p. Tyr
551Asp, Y551D) mutation in exon 14 of SAM and SH3 domain-containing
1 (SASH1), a c.1547 T>C (p. Leu 515 Pro, L515p) mutation
in exon 13, and a c.1528 G>A (p. Glu 509 Lys, E509K) mutation in
exon 13 were previously identified in 3 pedigrees (2,3).
Further in vitro analyses indicated that the enhanced
expression of melanogenic-related partners was induced by
SASH1 mutations, and mosaic-like phenotypes of SASH1,
melanin and melanogenic enzymes were detected in the epithelial
tissues from the lesional areas of DUH-affected individuals
(2-4). Recently, novel SASH1 mutations
[c.1784T>C (p. M595T) and c.1651T>C (p. Y551H)] were found to
be associated with Chinese families with DUH (5). A c.1556 G->A (p. S519N)
heterozygous mutation in exon 13 of the SASH1 gene was
reported in familial lentigines (6). A c.1519T>G (p.Ser507Ala.)
heterozygous transition mutation in exon 13 of the SASH1
gene was also identified in a Chinese family with multiple
lentigines (7). Two novel
mutations, c.1537A>C (p. Ser513Arg) and 1527_1530dupAAGT (p.
Leu511Lysfs*21) in the SASH1 gene, were identified in 3
pediatric patients with lentiginous phenotypes, and the clinical
presentations revealed that SASH1-related phenotypes can exhibit
hyper- and hypopigmentation on the trunk and extremities (8). A homozygous missense substitution
(c.1849G>A; p. Glu617Lys) in the SASH1 gene was
identified to be associated with genodermatosis in an autosomal
recessive manner (9). These
studies indicate that SASH1 has gradually become an important gene
that mediates melanin production in the process of human skin
pigmentation in various genodermatoses related to pigment
abnormalities. SASH1 variants may cause autosomal-dominant or
autosomal-recessive genodermatosis (10).
However, the SASH1-mediated melanogenesis-molecular
signaling networks that were found in vitro and the
investigations on SASH1 gene functions reported by other
dermatologists are limited to in vitro evaluations. Further
investigations are required to verify the SASH1-involved signaling
networks and/or the SASH1 variant functions in mammals.
As the gene roles of Y551D SASH1 in the
induction of a hyperpigmentation phenotype, which were identified
in vitro, are more significant than those of E509K and L515P
SASH1, heterozygous and homozygous mouse models with a
c.1654 T>G mutation of human SASH1 were established in the
present study. The mouse models with a c.1654 T>G mutation of
SASH1 recapitulated some molecular pathological phenotypes in the
epithelial tissues of the tails of heterozygous mice; these
phenotypes were similar to those in the skin epithelial tissues
from the lesional areas of DUH-affected individuals and support the
results of the in vitro cell function experiments that have
been described previously (2-4).
Materials and methods
In vitro transcription of Cas9 mRNA and
sgRNA and construction of donor vector
The sgRNA was designed and transcribed in
vitro. The sequence of the sgRNA was as follows: 5′-CGC GGC CAT
GGA GGA GGA CG-3′ (CGG) (PAM shown in brackets). The Cas9
expression construct was linearized, transcribed in vitro
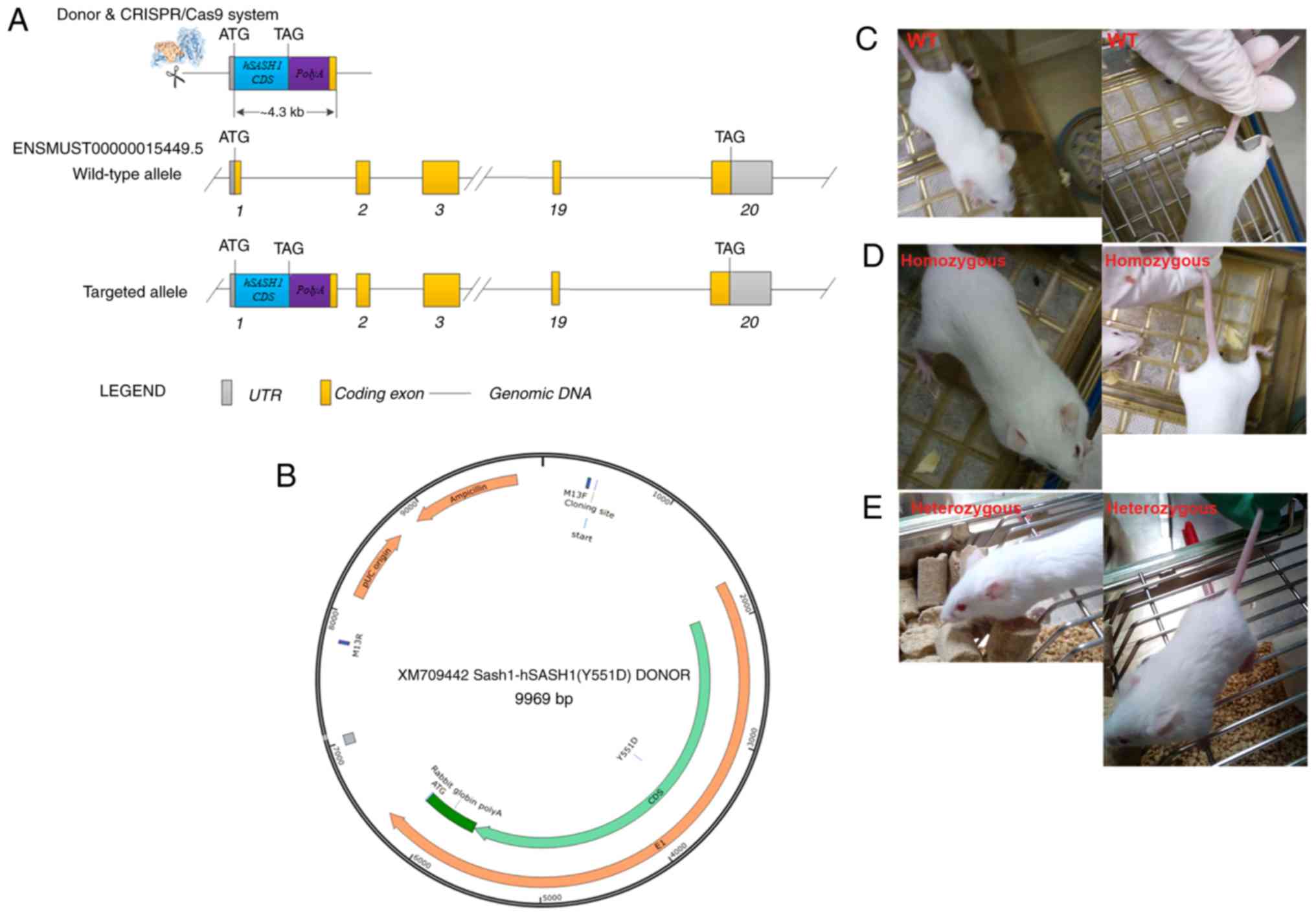
and extracted with phenol-chloroform. The donor vector [XM709442
Sash1-h SASH1(Y551D) DONOR] was designed and constructed. The
detailed mSash1-hSASH1(Y551D) Cas9-KI Targeted Genomic Sequence is
illustrated in Data S1. sgRNAs directed Cas9 endonuclease cleavage
at exon 1 near the start codon ATG to create a double-stranded
break (DSB). Such breaks were repaired, leading to the insertion of
hSASH1 (Y551D)-PolyA after the start codon, by homologous
recombination. The hSASH1 (Y551D)-PolyA cassette was placed after
the translational start codon ATG of the mouse Sash1 gene.
The strategy of generating the mSash-hSASH1(Y551D) gene knock-in
BABL/cJ mice is shown in Fig. 1A.
The structure map of the XM709442 Sash1-hSASH1 (Y551D) donor vector
is illustrated in Fig. 1B.
Animals and construction of
Sash-hSASH1(Y551D) gene knock-in BABL/cJ mice
All animal experiments were conducted according to
experimental practices and standards approved by the Ethics
Committee of the Nanjing Biomedical Institute of Nanjing University
and Guizhou Medical University (License no. 1800125). The
N-terminus of the mouse Sash1 gene (Gene ID: 70097) locus
was the knock-in site, and the knock-in fragment of hSASH1
(Y551D)-PolyA (human SASH1 Gene ID: 23328) was inserted at the ATG
start codon in exon 1 of murine Sash1. Therefore, human SASH1 and
EGFP were expressed under the control of the endogenous mouse SASH1
promoter/enhancer elements. Cas9 mRNA, sgRNAs and donor vector were
co-injected into zygotes or fertilized eggs of a total of 100
BALB/cJ mice (Nanjing Biomedical Research Institute of Nanjing
University) by microinjection. Among 54 newborns containing
hSASH1(Y551D)-PolyA cassettes on double DNA chains, 6 mice,
including 3 male BALB/cJ mice [animal strain: (T004567) BALB/cNju-h
SASH11 em1Cin(Y551D)/Nju] and 3 female mice [animal
strain: (T004567) BALB/cNju-h SASH11em1Cin(Y551D)/Nju],
were designated as the F0 generation mice, and their genotypes were
confirmed by PCR and DNA sequencing. The genotyping results of
Southern blot analysis among 8 mice are showed in Table I. These F0 generation mice were
housed and maintained under specific pathogen-free (SPF) conditions
at the Experimental Animal Center of Nanjing Biomedical Research
Institute of Nanjing University. The environment temperature was
maintained at 22-28°C; the humidity was 40-60%; the noise was lower
than 60 dB, the ammonia concentration was not >20 ppm, and the
number of air changes shall was 10-20 times/h in the SPF
experimental animal center. The sterile full nutrition granule
material sterilized by 60 Co gamma-ray was used to feed the adult
mice with doses of 3-7 g/day or the young mice with 1-3 g/day. A
small amount of sunflower seeds were fed to the mice regularly and
autoclave purified water after bottling with change frequency of
2-3 times per week was provided for drinking.
 | Table IGenotyping records of the F0
generation mice. |
Table I
Genotyping records of the F0
generation mice.
| Mouse ID no. | Sex | Southern blot
analysis results |
|---|
| 3 | Male | Negative |
| 5 | Male | Positive |
| 8 | Male | Negative |
| 25 | Male | Positive |
| 56 | Male | Positive |
| 17 | Female | Positive |
| 35 | Female | Positive |
| 54 | Female | Positive |
Mouse genotyping
All procedures were approved by the Institutional
Animal Care and Use Committee at Guizhou Medical University and
were consistent with the Guide for the Care and Use of Laboratory
Animals published by the National Institutes of Health. F1
generation mice were produced by mating F0 generation mice with a
wild-type BALB/c mouse (Nanjing Biomedical Research Institute of
Nanjing University), and F2 generation mice were produced by mating
F1 generation mice with a wild-type BALB/c mouse and F3 generation
mice were produced by in proper order. All bred mice of the F2 and
F3 generations at the age of 2 to 4 weeks were exposed to 60%
O2 plus 2% isoflurane with a 0.5 l/min isoflurane flow
and a 0.5 l/min oxygen flow in a mobile anesthesia system for small
animals (R510-46, RWD Life Science Co. Ltd.). The lower doses (the
dose of isoflurane was 1-2%) and higher doses (the dose of
isoflurane was 4-5%) of isoflurane were used to demonstrate the
effects of the non-anesthetic vs. the anesthetic dose of isoflurane
on the genotoxic effects of hyperoxia. The detailed procedure has
been previously described in the study by Kundumani-Sridharan et
al (11). Following the
anesthesia coma i.e., the limbs of the anesthetized mice did
actively contract when needling the syringe needle, the
anesthetized mice were euthanized by cervical dislocation and the
tail biopsies of the mice were cut off and collected for further
analyses. The remaining bodies of the mice were collected in
special mouse body bags for centralized processing, which were
prepared by the specific pathogen-free Experimental Animal Center
of Clinical Research Center, the Affiliated Hospital of Guizhou
Medical University. The EasyPure Genomic DNA kit (cat. no.
EE101-01, TransGen Biotech) was used to extract tissue DNA from the
mouse tails as described in the protocol provided by the
manufacturer.
The F1 generation pups of the Sash-hSASH1 (Y551D)
gene knock-in BABL/cJ mice were genotyped by PCR, followed by
sequencing and Southern blot analysis. The analyses of genotyping,
sequencing and Southern blot analysis of F1 generation mice were
performed by the Nanjing Biomedical Research Institute of Nanjing
University, and the detailed protocols of these analyses were not
provided by the Nanjing Biomedical Research Institute. The
identification strategy is presented in Fig. S1A. The primer sequences for
identification and sequencing are presented in Tables SI and SII. The F2 and F3
generation pups of the mSash-hSASH1 (Y551D) gene knock-in BABL/cJ
mice were identified by PCR that amplified the mutated knock-in
bands of the human SASH1 gene and the wild-type bands of the
mouse Sash1 gene. The PCR primer sequences and detailed
information regarding the size of the amplified bands, as well as
an illustration of how the genotype of the transgenic mice was
determined according to the amplified bands, are demonstrated in
Table SIII.
Reverse transcription PCR (RT-PCR)
Total RNA was extracted from the mouse tails using
the AxyPrep Multisource Total RNA Miniprep kit 50-prep
(AP-MN-MS-RNA-50, Corning Axgene) according to the protocol
provided by the manufacturer. Total RNA extracted from the mouse
tails was reverse transcribed into cDNA using the Easy Script
One-Step gDNA Removal and cDNA Synthesis Super Mix (AE311-02,
Transgene), and this cDNA was used as template for RT-PCR. PCR was
performed with 2X Taq Plus PCR MaterMix (KT205-01, Tiangen Biotech
Co., Ltd.) was used to amplify the cDNA and followed by nucleic
acid electrophoresis to evaluate SASH1 or Sash1
expression in the tail tissues of F2 and F3 generation. The
thermocycling conditions were as follows: 94°C, 5 min; 94°C, 30
sec, 60°C, 30 sec, 72°C, 30 sec, 30 cycles; 72°C, 5 min, 4°C
continuously. The SASH1 gene exhibits high conservation
among different species, and the human SASH1 gene exhibits a
high similarity with the mouse Sash1 gene in the whole cDNA
sequence. The fragment containing 155 bp from nucleotide 1954 to
nucleotide 2108, is located in the SASH1 SAM1 domain, was amplified
to analyze its expression in wild-type, homozygous and heterozygous
mice of the F2 and F3 generations. The following primers were used
to amplify SASH1 and GAPDH from the mouse tails, and
the sequences of the SASH1 and GAPDH primers were as follows: SASH1
forward, 5′-CCC ACT TTC CTG TTC AAT G-3′ and reverse, 5′-TGG TCG
CTG TTA CTG TCA TAC-3′; and GAPDH forward, 5′-CAC CCA CTC CTC CAC
CTT TG-3′ and reverse, 5′-ACC ACC CTG TTG CTG TAG CC-3′.
Western blot analysis and IP-WB
A total of 11 heterozygous mice of the F2 generation
and 10 heterozygous mice of the F3 generation, which harbored the
mouse Sash1-human SASH1(Y551D)-PolyA cassette in one DNA chain and
mouse Sash1 in the other DNA chain, 2 homozygous mice of the F2
generation and 1 homozygous mouse of the F3 generation, which both
harbored the mouse Sash1-human SASH1 (Y551D)-PolyA cassette in both
DNA chains, and 3 wild-type mice of the F2 generation, and 4
heterozygous mice of the F3 generation, which both contained the
mouse Sash1 gene in both DNA chains, were identified according to
the mouse genotyping results.
To identify the expression of exogenous human SASH1
in the heterozygous mice and homozygous mice, and to determine the
effects of exogenous human SASH1 on melanogenic enzymes or
melanogenic partners, mouse tail tissues from mice of the F2 and F3
generations were sheared by ultrasonic breaking, lysed with RIPA
lysis buffer and subjected to western blot analysis. The protein
concentration was quantified by BCA methods, 35 µg protein
per lane was loaded and followed by electrophoresis using 5%
concentration gel and 10% of separation gel. After electrophoresis,
the protein on the separation gel was transferred to polyvinylidene
difluoride membrane and blocked with 10% fat-free milk for 1 h at
room temperature. The primary antibodies was diluted with primary
antibody dilution buffer (P0023A, Beyotime Biotechnology) and was
incubated at 4°C for one night. The enhanced chemiluminescent (ECL)
Reagent (cat. no. KF005, Affinity Biosciences) was used to
visualize the protein bands. The densitometry of protein bands was
quantified by Quantity One software (Vision 4.6.2, the Discovery
Series) and analyzed by SPSS16.0 software (IBM, International
Business Machine).
The primary antibodies used for western blot
analysis were rabbit anti-SASH1 polyclonal antibody (1:500
dilution, cat. no. NBP-26650, Novus Biological, USA), rabbit
anti-microphthalmia-associated transcription factor (Mitf; C5)
monoclonal antibody (1:500 dilution, cat. no. NB110-10872, Novus
Biologicals, LLC) or rabbit anti-Mitf polyclonal antibody (1:500
dilution, cat. no. ab20663, Abcam), rabbit anti-GNAS polyclonal
antibody (1:500 dilution, cat. no. C2C3-2, GeneTex) and rabbit
anti-phospho-ERK1/2 (Thr202/Tyr204) monoclonal antibody (1:1,000
dilution, cat. no. 9101, Cell Signal Technology, Inc.), anti-p44/42
MAPK (Erk1/2) (137F5) rabbit mAb (1:1,000 dilution, cat. no. 4695,
Cell Signal Technology, Inc.), GFP mouse monoclonal antibody
(1:2,000 dilution, cat. no. T0005, Affinity Biosciences), mouse
anti-β-tubulin mouse monoclonal antibody 1:2,000 dilution, (cat.
no. 66240-1-Ig, Proteintech Group, Inc.) and rabbit anti-GAPDH
polyclonal antibody (1:2,000 dilution, cat. no. GTX100118,
GeneTex). The secondary antibodies used [peroxidase-conjugated
AffiniPure Goat Anti-Rabbit IgG (H+L) or peroxidase-conjugated
AffiniPure Goat Anti-Mouse IgG (H+L) (cat. no. 111-035-003, Jackson
ImmunoResearch Laboratories, Inc.] were diluted 1:10,000 and
incubated with the membranes at room temperature for 1 h.
The pEGFP-C3-SASH1 vector was introduced into 293T
cells (the Cell Bank of Chinese Academy of Sciencesa). The
transfected 293T cells were lysed with IP-WB cell lysis buffer
(P0013J, Beyotime Biotechnology). GFP-SASH1 was immunoprecipitated
with an anti-GFP antibody (1:100 dilution, cat. no. T0005, Affinity
Biosciences). The immunoprecipitates were captured by Protein A/G
plus-agarose (sc-2003, Santa Cruz Biotechnology, Inc.), and the
associated endogenous Mitf (cat. no. ab20663, Abcam) was
analyzed.
Immunohistochemical analyses
After acquiring the consents of the affected
individuals and the normal controls, the skin epithelial tissues
from the hyperpigmented lesional areas of a 28-year-old female
Y551D-affected individual and the normal skin epithelial tissues
from 2 normal controls were obtained at the Affiliated Hospital of
Guizhou Medical University on January 20, 2020. The document of
patient consent had been signed by the affected individual and 2
normal controls, and ethics approval was provided by the Ethics
Committee of the Affiliated Hospital of Guizhou Medical University.
The epithelial tissues from the tail biopsies of the mice of the F2
and F3 generations, and the skin epithelial tissues from
hyperpigmented lesional areas of Y551D-affected individuals and
normal controls were fixed in 10% formalin at 4°C for 24 h and then
embedded in paraffin. Paraffin sections (5-µm-thick) were
incubated at 60°C for 3 h and then deparaffinized and rehydrated
using xylene and an ethanol gradient. The sections were then
incubated with the rabbit anti-Mitf (C5) monoclonal antibody (1:200
dilution, cat. no. NB110-10872, Novus Biologicals, LLC), rabbit
anti-SASH1 polyclonal antibody (1:200 dilution, cat. no. NBP-26650,
Novus Biologicals, LLC), rabbit anti-Mitf (C5) monoclonal antibody
(1:200 dilution, cat. no. NB110-10872, Novus Biologicals, LLC) at
37°C for 1 h and then at 4°C for at least 8 h. After washing with
PBS 3 times, the sections were incubated with the horseradish
peroxidase-linked anti-rabbit and anti-mouse universal secondary
antibodies provided by the Immunochromogenic Kit (KIT-5006, MXB
Biotechnologies) for 1 h at 37°C. Finally, the sections were
counterstained with hematoxylin staining solution (CTS-1090, MXB
Biotechnologies at 37°C for 20 min, and photographed under a
positive position microscope BX51 (Olympus Corp.). The staining
intensity and percentage of positive cells in the sections of the 3
groups of mice, including wild-type mice, homozygous mice and
heterozygous mice of the F2 generation and F3 generation, were
calculated and scored as previously described (4). The Mitf-positive cells in the
epithelial tissues of tail biopsies from mice of the F2 and F3
generations and the Mitf-positive cells in the affected epithelial
layers from skin lesional areas of Y551D-affected patients were
examined.
Melanin staining
Melanin staining in the skin epithelial tissues of
the normal control and Y551D SASH1-affected individuals was
performed as previously described (3).
Statistical analysis
The protein densitometry values of western blot
analysis were first measured using Quantity One software (version
4.6.2) and analyzed using the homogeneity of variance test,
followed by a one-factor analysis of variance (ANOVA) with LSD
correction or Tukey's test in SPSS version 16.0 to generate the
required P-values. The data are presented as the means ± standard
error of the mean (SEM). The total scores and positively stained
cells of each visual field of the immunohistochemistry sections
were scored and analyzed using the homogeneity of variance test and
a one-factor analysis of variance (ANOVA) with LSD correction or
Tukey's test in SPSS version 16.0 to generate the required
P-values. The cartograms were made and plotted using GraphPad Prism
5 (GraphPad Software, Inc.).
Results
The human SASH1 gene and/or the mouse
Sash1 gene are both expressed in hSASH1(Y551D) gene knock-in
BABL/cJ mice of the F2 and F3 generations
A total of 6 mice (3 males and 3 females) of the F0
generation were ultimately identified as positive
Sash-hSASH1(Y551D) gene knock-in BABL/cJ mice by PCR and
sequencing. A total of 11.1% (6/54) of the live pups obtained
presented a mutational event. Mice nos. 64, 66, 68, 72, 75 and 78
of the F1 generation were identified to be positive by PCR
amplification and point mutation sequencing. The identification
strategy is shown in Fig. S1A.
The Sequencing results indicated that the human SASH1 (c.1654
T>G mutation)-PolyA cassette was successfully inserted into the
mouse genome (Fig. S1B). The
results of Southern blot analysis and point mutation sequencing are
presented in Fig. S1C-E.
Regretfully, in both the homozygous and heterozygous mice, no
hyperpigmentation spots or hypopigmentation spots were found within
the tails or other areas of the mouse bodies (Fig. 1C, D and E).
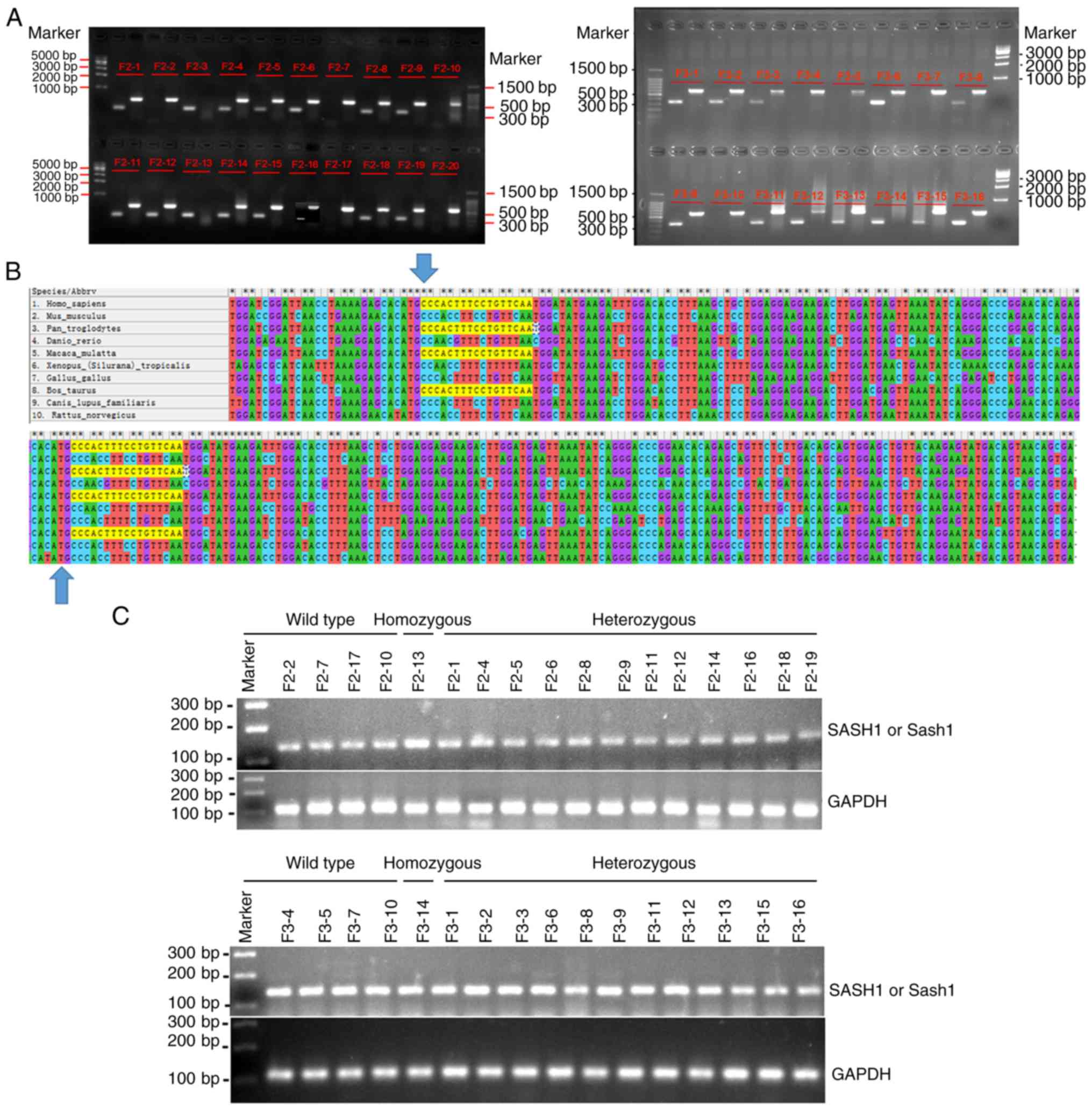
The genotypes of 16 mice of the F2 generation and 16
mice of the F3 generation were further identified by PCR. Nucleic
acid electrophoresis indicated that of the F2 generation mice, 3
mice were wild-type mice, 2 were homozygous and 11 were
heterozygous (Fig. 2A). Among the
F3 generation mice tested, 4 mice were wild-type mice, 1 was
homozygous, and 11 were heterozygous (Fig. 2A). The genotyping results are
presented in Table II. The
expression of the human SASH1 gene and/or mouse Sash1
gene was further analyzed in the wild-type, homozygous and
heterozygous mice of the F2 and F3 generations. The conservation of
the SASH1 gene in different species was also assessed, and a
high degree of conservation between mice and humans was
demonstrated (Fig. 2B).
Therefore, the expression of the human SASH1 gene and mouse
Sash1 gene at the transcriptional level was analyzed by
RT-PCR using the same pair of primers.
 | Table IIGenotyping records of the F2 and F3
generation mice. |
Table II
Genotyping records of the F2 and F3
generation mice.
| Mouse ID no. | Genotyping | Mouse ID no. | Genotyping |
|---|
| F2-2 | Wild-type | F3-4 | Wild-type |
| F2-7 | Wild-type | F3-5 | Wild-type |
| F2-17 | Wild-type | F3-7 | Wild-type |
| F2-3 | Homozygous | F3-10 | Wild-type |
| F2-13 | Homozygous | F3-14 | Homozygous |
| F2-1 | Heterozygous | F3-1 | Heterozygous |
| F2-4 | Heterozygous | F3-2 | Heterozygous |
| F2-5 | Heterozygous | F3-3 | Heterozygous |
| F2-6 | Heterozygous | F3-6 | Heterozygous |
| F2-8 | Heterozygous | F3-8 | Heterozygous |
| F2-9 | Heterozygous | F3-9 | Heterozygous |
| F2-11 | Heterozygous | F3-11 | Heterozygous |
| F2-12 | Heterozygous | F3-12 | Heterozygous |
| F2-16 | Heterozygous | F3-13 | Heterozygous |
| F2-18 | Heterozygous | F3-15 | Heterozygous |
| F2-19 | Heterozygous | F3-16 | Heterozygous |
The results of RT-PCR indicated that the human
SASH1 gene was expressed in the homozygous and heterozygous
mice, but also in the SASH1 gene wild-type and heterozygous
mice (Fig. 2C). Therefore, the
human SASH1 gene and/or mouse Sash1 gene were both
expressed in the wild-type, homozygous and heterozygous mice of the
F2 and F3 generations.
GNAS is not uniformly induced in
heterozygous hSASH1(Y551D) gene knock-in BABL/cJ mice
Upon ligand binding to G protein-coupled receptors
(GPCRs), GPCRs impart a signal to heterotrimeric G proteins, which
are composed of α-, β- and γ-subunits, resulting in the detachment
of the α-subunit from the Gβγ subunit of G proteins. The guanine
nucleotide-binding protein G(s) subunit alpha isoforms short (GNAS,
Gαs) class directly catalyzes the transformation of ATP to cAMP.
cAMP is responsible for the melanogenic actions of ligands such as
α-MSH, including the activation of tyrosinase in melanin
biosynthesis (12). The present
study first assessed the expression of the human SASH1 protein or
mouse Sash1 protein in wild-type, homozygous and heterozygous mice
using the Novus SASH1 antibody (cat. no. NBP-26650, Novus
Biologicals, LLC), which can recognize both the human SASH1 and
mouse Sash1 proteins. The results of western blot analysis
indicated that in the F2 and F3 generations, unlike the
SASH1 or Sash1 gene expressed at the transcriptional
level, SASH1 or Sash1 protein was expressed in the majority of the
heterozygous mice and wild-type mice. However, in a few
heterozygous mice and wild-type mice, SASH1 or Sash1 protein were
not detected (Figs. 3C and F, and
4A and D). The expression of
SASH1 protein in the F2 and F3 generations was compared, and
statistical analyses suggested that there were no significant
differences between the F2 and F3 generations (Fig. 4G).
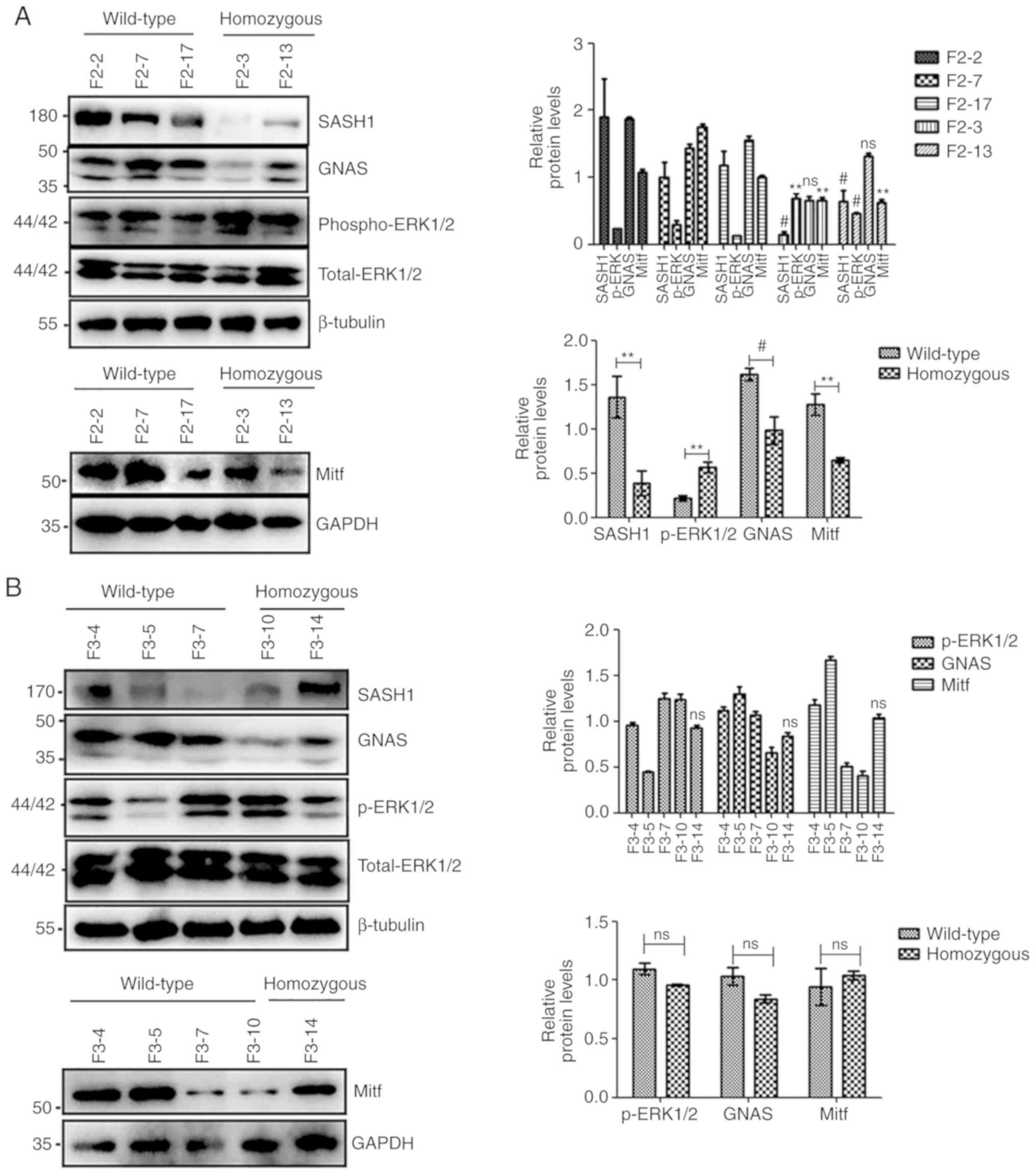 | Figure 3Mitf, but not GNAS, and
phospho-ERK1/2 were uniformly increased in heterozygous
hSASH1(Y551D) gene knock-in BABL/cJ mice compared to wild-type
mice. (A) Downregulation of SASH1 was induced by the Y551D SASH1
mutation in homozygous mice of the F2 generation compared to that
of wild-type mice. GNAS and Mitf expression was attenuated by the
downregulation of SASH1; however, phospho-ERK1/2 expression was
increased (left panel). The total densitometry values of these
proteins were also compared collectively (lower right panel). Upper
right panel: #P<0.01 vs. all 3 wild-type mice,
**P<0.001 vs. all 3 wild-type mice; ns, not
significant vs. all 3 wild-type mice. Lower right panel:
#P<0.01, homozygous mice vs. wild-type mice,
**P<0.001 vs. all 3 wild-type mice. (B) SASH1, GNAS
and Mitf expression was not attenuated by the Y551D-SASH1 mutation
in the F3 generation, as indicated by western blot and statistical
analyses. ns: No significance vs. all four wild-type mice. Mitf,
but not GNAS, and phospho-ERK1/2 were uniformly increased in
heterozygous hSASH1(Y551D) gene knock-in BABL/cJ mice compared to
wild-type mice. (C-E) Tail biopsies of wild-type mice and
heterozygous mice of the F2 generation were lysed, ultrasonicated
and subjected to western blot analysis. The results of western blot
analysis indicated that the expression of SASH1, GNAS,
phospho-ERK1/2 and Mitf was enhanced in the heterozygous
hSASH1(Y551D) gene knock-in BABL/cJ mice of the F2 generation
compared to wild-type mice. (D) The densitometry values of SASH1,
GNAS, phospho-ERK1/2 and Mitf of 11 heterozygous and 3 wild-type
mice were compared, and (E) the total densitometry values of these
proteins were also compared collectively. (D) *P<0.05
vs. all 3 wild-type mice, #P<0.01 vs. all 3 wild-type
mice, **P<0.001 vs. all 3 wild-type mice. (E)
*P<0.05 vs. wild-type mice; #P<0.01 vs.
wild-type mice, **P<0.001 vs. wild-type mice. Mitf,
but not GNAS, and phospho-ERK1/2 were uniformly increased in
heterozygous hSASH1(Y551D) gene knock-in BABL/cJ mice compared to
wild-type mice. (F-H) Western blot analysis revealed that the
expression of Mitf was enhanced in heterozygous human Y551D SASH1
gene knock-in mice of the F3 generation compared with wild-type
mice. (G) The densitometry values of SASH1, GNAS, phospho-ERK1/2
and Mitf of 10 heterozygous and 4 wild-type mice were compared, and
(H) the total densitometry values of these proteins were also
compared collectively. (G) *P<0.05 vs. all 3
wild-type mice, #P<0.01 vs. all 3 wild-type mice;
**P<0.001 vs. all 3 wild-type mice. (H)
**P<0.001 vs. wild-type mice. For all panels, ns, not
significant. |
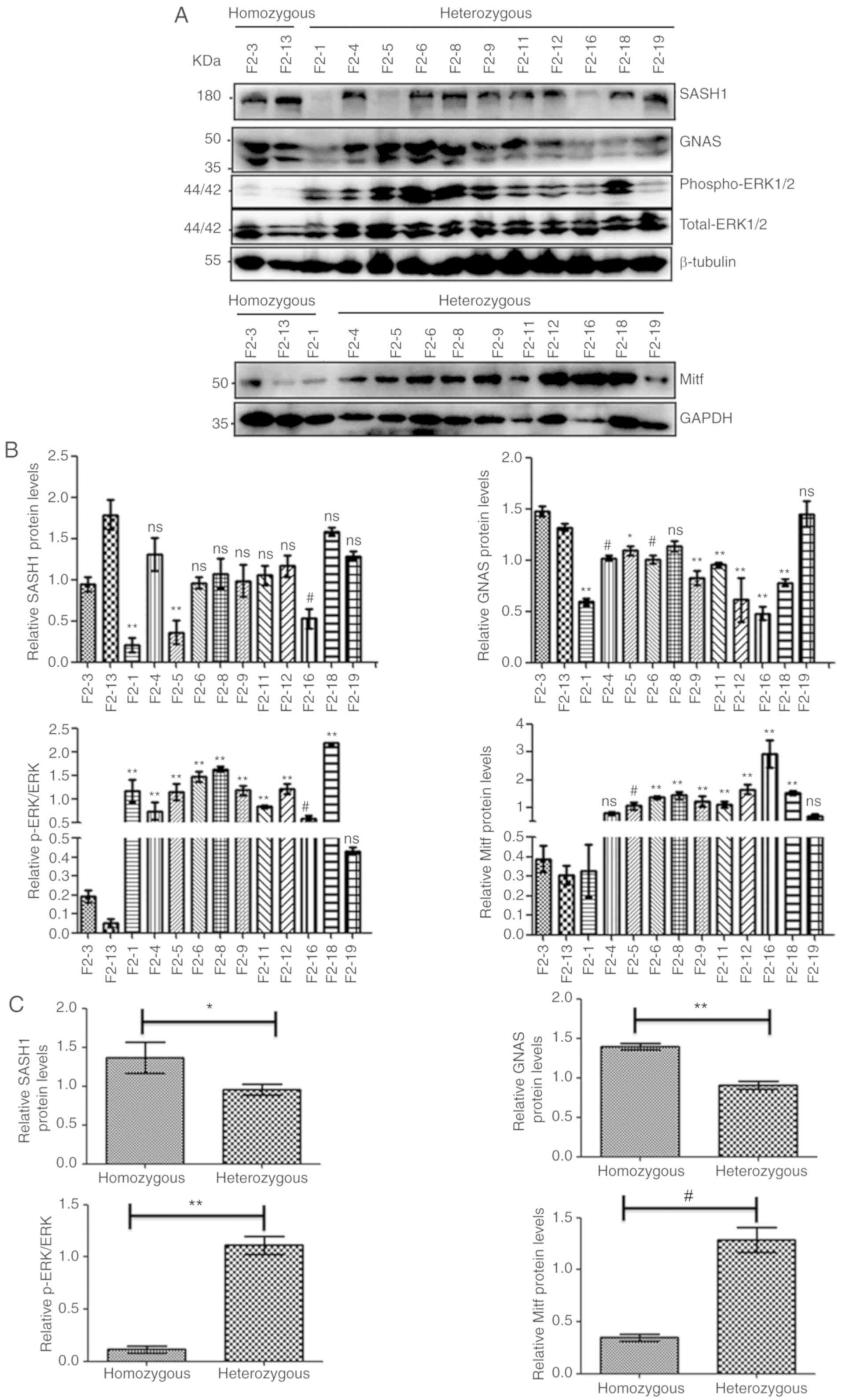 | Figure 4Mitf, but not GNAS, and
phospho-ERK1/2 expression is increased in heterozygous human Y551D
SASH1 knock-in mice compared to homozygous mice. (A) Western blot
analysis indicated that SASH1 was downregulated in heterozygous
human Y551D-SASH1- knock-in mice of the F2 generation compared to
homozygous mice. However, the protein levels of phospho-ERK1/2 and
Mitf were upregulated in heterozygous mice compared to those of
homozygous mice. (B and C) The densitometry values of SASH1, GNAS,
phospho-ERK1/2 and Mitf of 11 heterozygous and 2 homozygous mice of
the F2 generation were compared, and the total densitometry values
of these proteins were also compared collectively, respectively.
(B) *P<0.05 vs. both homozygous mice,
#P<0.01 vs. both homozygous mice;
**P<0.001 vs. both homozygous mice. (C)
#P<0.01 vs. homozygous mice, **P<0.001
vs. homozygous mice, *P<0.05 vs. homozygous mice. (D)
Western blot analysis demonstrated that the expression of Mitf and
GNAS was enhanced in heterozygous human Y551D SASH1 knock-in mice
of the F3 generation compared to homozygous mice. Mitf, but not
GNAS, and phospho-ERK1/2 expression is increased in heterozygous
human Y551D SASH1 knock-in mice compared to homozygous mice. (E and
F) The densitometry values of SASH1, GNAS, phospho-ERK1/2 and Mitf
of 11 heterozygous and 1 homozygous F3 generation mice were
compared. (F) The total gray values of these proteins were also
compared collectively. (E) *P<0.05 vs. one homozygous
mouse, respectively, #P<0.01 vs. one homozygous
mouse, respectively, **P<0.001 vs. one homozygous
mouse, respectively. (F) **P<0.001 vs. homozygous
mice. (G) The densitometry values of SASH1, Mitf, GNAS and the
ratio of p-ERK/ERK in 11 heterozygous mice of the F2 generation and
10 heterozygous mice of the F3 generation were compared. For all
panels, ns, not significant. |
In the present study, in 27.3% (3/11) of the
heterozygous mice with the Y551D-SASH1 mutation in the F2
generation, GNAS expression in the examined mice was increased
compared with that in all 3 wild-type mice (Fig. 3C and D). The total expression of
GNAS in the 11 heterozygous mice of the F2 generation was also
increased compared with that in the 3 wild-type mice of the F2
generation (Fig. 3C and E).
However, in 10 heterozygous mice with the Y551D-SASH1 mutation in
the F3 generation, GNAS expression was not induced compared with
that in 4 wild-type mice (Fig. 3F and
G). The total expression of GNAS in 10 heterozygous mice of the
F3 generation was also not induced compared with that in 4
wild-type mice (Fig. 3F and
H).
In the F2 generation, the expression of GNAS was
significantly decreased in most heterozygous mice compared with the
homozygous mice (Fig. 4A and B),
and the total expression of GNAS in the 11 heterozygous mice was
significantly decreased compared with the homozygous mice (Fig. 4A and C). In 81.8% (9/11) of the
heterozygous mice in the F3 generation, GNAS expression in the
examined mice was also upregulated compared with that in the F3-14
homozygous mouse (Fig. 4D and E).
The total expression of GNAS in the 11 heterozygous mice of the F3
generation was also upregulated compared to that in the 1
homozygous mouse (Fig. 4D and F).
In addition, obvious differences were observed in GNAS expression
between the wild-type mice and homozygous mice of the F2 generation
(Fig. 3A). However, no marked
differences were observed in GNAS expression between the F3
generation mice (Fig. 3B).
Phospho-ERK1/2 is not uniformly induced
in heterozygous hSASH1(Y551D) gene knock-in BABL/cJ mice
Mitf is phosphorylated by upstream ERK and induces
increased transcriptional activity of the tyrosinase promoter
(13-15). A previous study by the authors
demonsrtated that SASH1 was induced by the p53-POMC-MC1R signal
cascade to enhance the phosphorylation of ERK1/2 and CREB and that
mutated SASH1 alleles promoted increased protein levels of
phosphorylated ERK1/2 and CREB (4). The ratio of phospho-ERK1/2:ERK1/2
(p-ERK/ERK) was calculated when assessing the effects of expression
of human Y551D SASH1 or mouse Sash1 on that of phospho-ERK1/2 in F2
and F3 generation. The present study found that the ratio of
p-ERK/ERK was significantly increased in 90.9% (10/11) of the
heterozygous mice compared to the wild-type mice in the F2
generation (Fig. 3C and D). The
total ratio of p-ERK/ERK in the 11 heterozygous mice was also
significantly increased compared with that of the wild-type mice
(Fig. 3C and E). However, only 3
marked increases in the ratio of p-ERK/ERK among 11 heterozygous
mice were observed compared with the wild-type mice in the F3
generation, when the phospho-ERK1/2 levels were compared separately
(Fig. 3F and G).
The ratio of p-ERK/ERK was markedly enhanced in 100%
(11/11) of the heterozygous mice of the F2 generation compared with
both homozygous mice (F2-3 and F2-13) (Fig. 4A and B). Overall, the ratio of
p-ERK/ERK was significantly increased in the heterozygous mice
compared with the homozygous mice (Fig. 4A and C) of the F2 generation. In
the heterozygous mice of the F3 generation, the ratio of p-ERK/ERK
was increased only in 18.2% (2/11) of the heterozygous mice of the
F3 generation compared with the homozygous mice (Fig. 4D and E). The total ratio of
p-ERK/ERK was not markedly increased in the heterozygous mice
compared to the homozygous mice (Fig.
4D and F) of the F3 generation.
Mitf expression and Mitf-positive-stained
epithelial cells were uniformly enhanced in tail tissues of
heterozygous human SASH1(Y551D) gene-knock in BABL/cJ mice
Mitf contains both basic helix-loop-helix and
leucine zipper structural features and is a melanocyte master
transcription factor and a specific marker of melanocytes. Mitf
regulates melanocyte development and is responsible for pigment
cell-specific transcription of melanogenic enzyme genes (16). In a previous study, it was
demonstrated that the downstream melanogenic enzymes of Mitf were
induced by mutated SASH1 in the affected epithelial tissues and
normal human epithelial melanocytes (3,4).
SASH1 not only localizes to the cytoplasm as previously indicated
(2-4), but also localizes to the nucleus in
epithelial cells (10). Mitf
functions as the master regulator of the melanocytic lineage and is
constitutively nuclear or translated to the nucleus (17,18). Therefore, the present study
evaluated whether SASH1 can assemble Mitf and whether mutated SASH1
can mediate Mitf expression.
The effects of SASH1 mutations on Mitf in
hSASH1(Y551D) gene knock-in BABL/cJ mice were further identified.
In the homozygous mice of the F2 generation, a decreased Mitf
expression was induced in the homozygous human Y551D-SASH1 gene
knock-in mice compared with the wild-type mice (Fig. 3A). However, Mitf expression was
not downregulated in the homozygous mice of the F3 generation
compared with the wild-type mice (Fig. 3B). In 63.6% (7/11) of the
heterozygous mice with the Y551D-SASH1 mutation of the F2
generation, an increased Mitf expression in the examined mice was
induced compared with that in all 3 wild-type mice (Fig. 3C and D). The total expression of
Mitf in the 11 heterozygous mice of the F2 generation was also
enhanced compared to that in the 3 wild-type mice of the F2
generation (Fig. 3C and E). In
60% (6/10) of the heterozygous mice with the Y551D-SASH1 mutation
of the F3 generation, Mitf expression in the examined mice was
enhanced compared with that in the 4 wild-type mice (Fig. 3F and G). The overall expression of
Mitf in the 10 heterozygous mice of the F3 generation was also
enhanced compared to that in the 4 wild-type mice (Fig. 3F and H).
The expression of Mitf was upregulated in the
examined mice in 72.7% (8/11) of the heterozygous mice of the F2
generation compared with F2-3 and F2-13 homozygous mice (Fig. 4A and B). The expression of Mitf in
the 11 heterozygous mice of the F2 generation was also increased
compared with that in the 2 homozygous mice of the F2 generation
(Fig. 4A and C). In 60% (6/10) of
the heterozygous mice of the F3 generation, Mitf expression in the
examined mice was also upregulated compared with that in the F3-14
homozygous mouse (Fig. 4D and E).
The overall expression of Mitf in the 11 heterozygous mice of the
F3 generation was also upregulated compared with that in the 1
homozygous mouse (Fig. 4D and F).
Taken together, these data indicated that there was a fluctuating
Mitf expression between the wild-type mice and homozygous mice;
however, a significant difference in Mitf expression was observed
between the heterozygous mice and homozygous mice or the wild-type
mice. No differences in the expression of GNAS and Mitf protein, as
well as in the ratio of p-ERK/ERK were observed between the F2 and
F3 generation mice (Fig. 4G).
Immunohistochemical analyses further demonstrated
that the heterozygous mice of the F2 generation exhibited an
increased expression of Mitf and increasing numbers of Mitf
positively stained epithelial cells compared with those of the
homozygous mice (Fig. 5A and B).
The heterozygous mice also exhibited an increased expression of
Mitf and increasing numbers of Mitf positively stained epithelial
cells compared with those of wild-type mice (Fig. 5A and B). The overall comparisons
of Mitf and the numbers of Mitf-positive epithelial cells between
the wild-type and heterozygous mice, or between the homozygous and
heterozygous mice were also performed. Statistical analyses
indicated that the overall expression of Mitf and the numbers of
Mitf-positive epithelial cells of the heterozygous mice of the F2
generation were increased compared with those of the wild-type and
homozygous mice (Fig. 5B and
C).
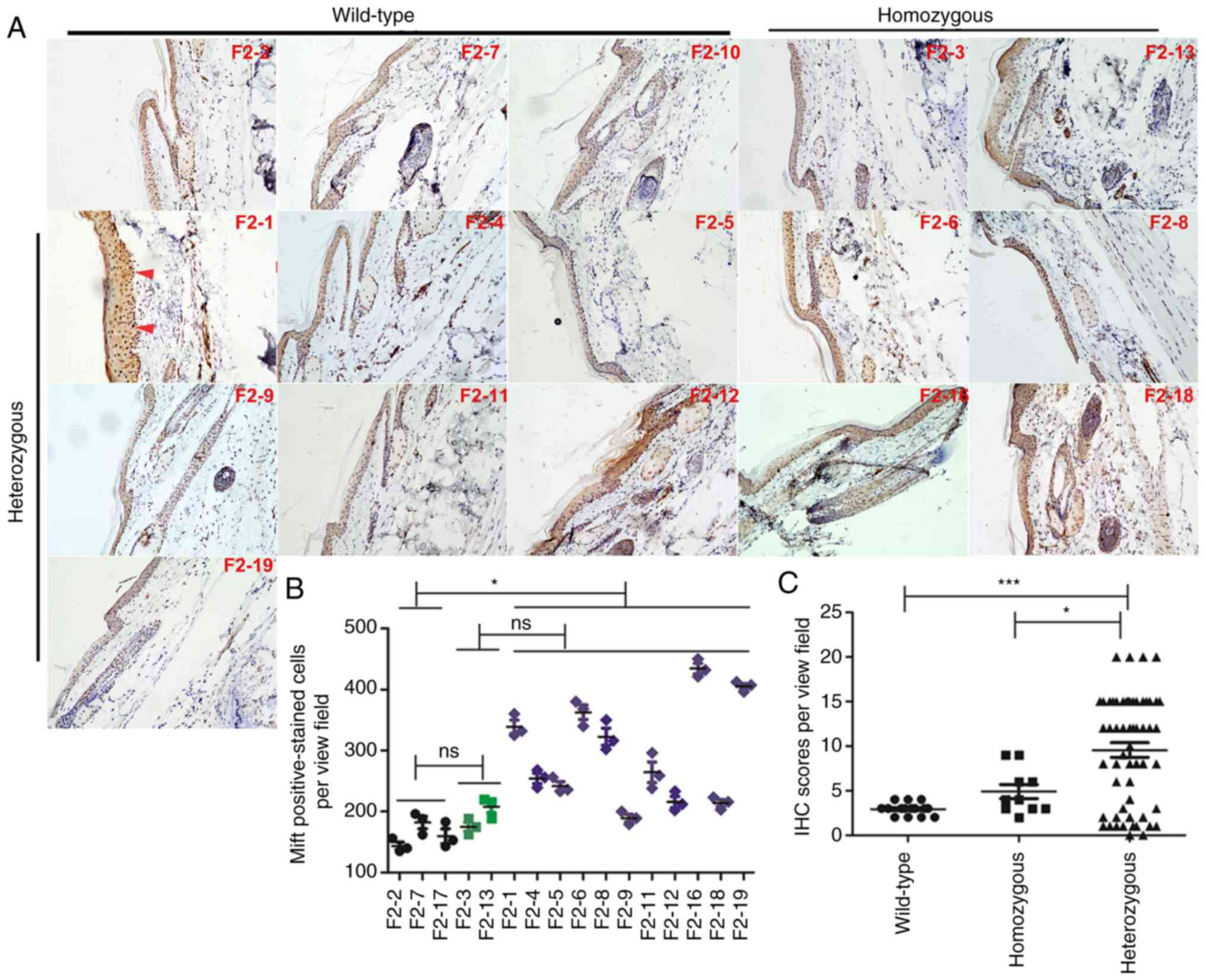 | Figure 5In the F2 generation, the number of
Mitf- and Mitf-positive epithelial cells was enhanced in
heterozygous human Y551D SASH1 knock-in mice. (A) Representative
images (magnification, ×10) of Mitf in 3 wild-type mice, 2
homozygous mice and 11 heterozygous mice. (B) A total of 5 random
visual fields in each section of 16 mice, including wild-type,
homozygous and heterozygous mice, were photographed. The
Mitf-positive epithelial cells in the tail tissues of wild-type,
homozygous and heterozygous mice were counted and analyzed
statistically. Representative Mitf-positive cells, which were
stained dark brown in the nucleus, are indicated by red arrows.
*P<0.01. (C) The staining intensity and percentage of
Mitf-positive cells per mouse were calculated, scored and analyzed
statistically. *P<0.05, ***P<0.001; ns,
not significant. |
The enhanced expression of Mitf and increasing
numbers of Mitf-positive epithelial cells were observed in the
examined heterozygous mice of the F3 generation compared with those
of the homozygous mice or wild-type mice (Fig. 6A and B). Statistical analyses
indicated that the overall expression of Mitf in the heterozygous
mice of the F3 generation was increased compared with that in the
wild-type and homozygous mice (Fig.
6B and C).
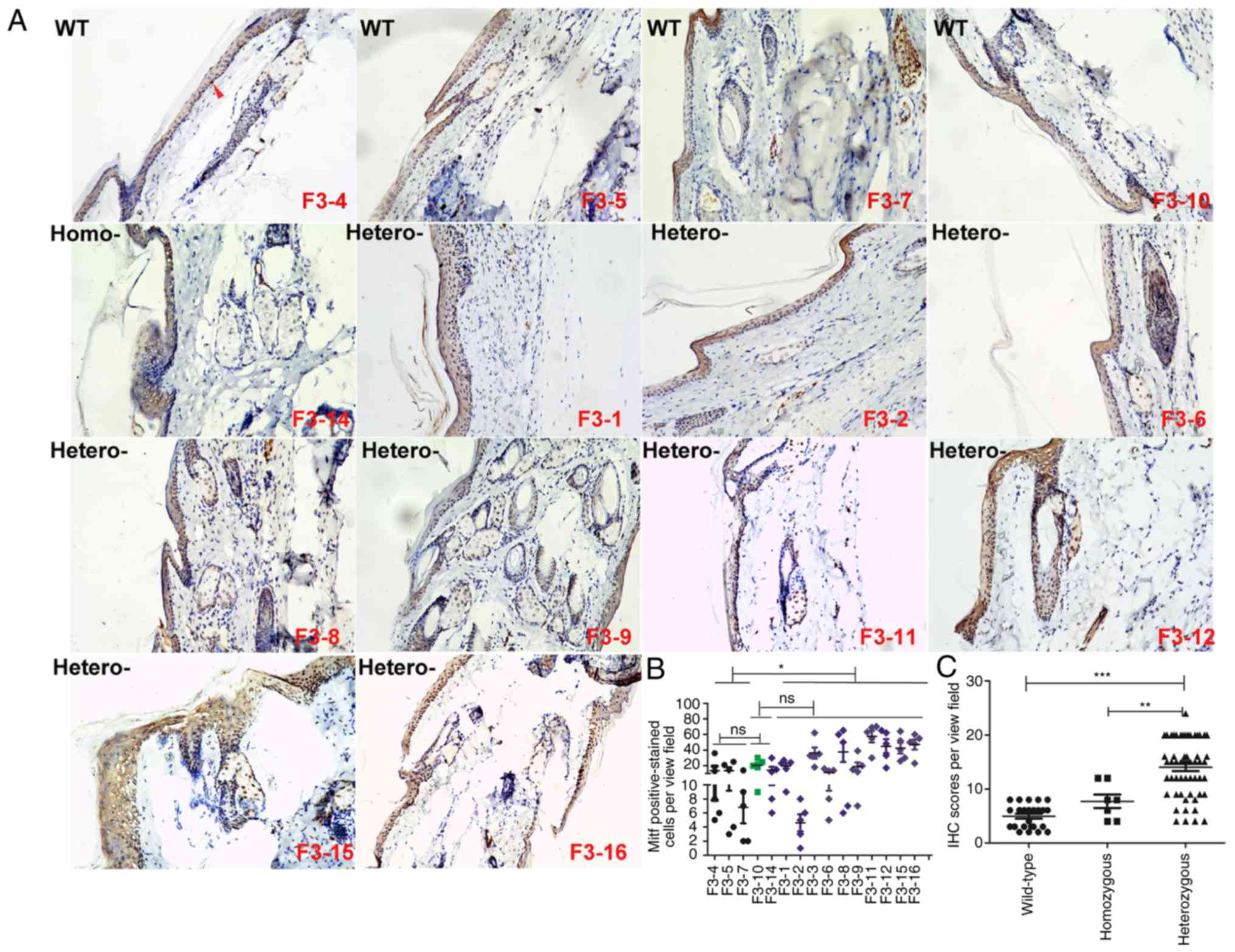 | Figure 6In the F3 generation, the number of
Mitf- and Mitf-positive epithelial cells was augmented in
heterozygous human Y551D SASH1 knock-in mice. (A) Representative
images (magnification, ×10) of Mitf in 4 wild-type mice, 1
homozygous mouse and 11 heterozygous mice. (B) A total of 51 visual
fields in each section of 15 mice, including wild-type, homozygous
and heterozygous mice, were photographed. The Mitf-positively
stained epithelial cells in the tail tissues of wild-type,
homozygous and heterozygous mice were calculated in 3 visual fields
and analyzed statistically. Representative Mitf-positive cells,
which were stained yellowish-brown in the nucleus, are indicated by
red arrows. *P<0.01. (C) Staining intensity and
percentage of Mitf-positive cells per mouse were calculated, scored
and analyzed statistically.
**P<0.01***P<0.001; ns, not
significant. |
Mitf expression and Mitf-positive
epithelial tissues are increased in the affected tissues from
Y551D-SASH1-affected patients
The above-mentioned results indicated that Mitf
expression was increased in the heterozygous human SASH1 (Y551D)
gene knock-in BABL/cJ mice. Therefore, it was hypothesized that
SASH1 may be associated with Mitf. Therefore, IP-WB analyses were
performed to identify the associations between exogenous SASH1 and
endogenous Mitf. However, IP-WB analyses indicated that GFP-SASH1
did not bind to endogenous Mitf in 293T cells (Fig. 7A). Subsequently, whether
endogenous Mitf is mediated by exogenous SASH1 was further
determined. The results of western blot analysis indicated that
endogenous Mitf expression was increased by mutated SASH1 (Fig. 7B). The induction of Mitf by
mutated SASH1 was assessed in the epithelial tissues of affected
individuals. Immunohistochemical analyses indicated that more
SASH1- and Mitf-positive epithelial cells were present in the
affected tissues than in the normal controls. A higher expression
of SASH1 and Mitf was also induced in the affected tissues
(Fig. 7C). A increased amount of
melanin was synthesized in different epithelial layers of the
affected tissues (Fig. 7D).
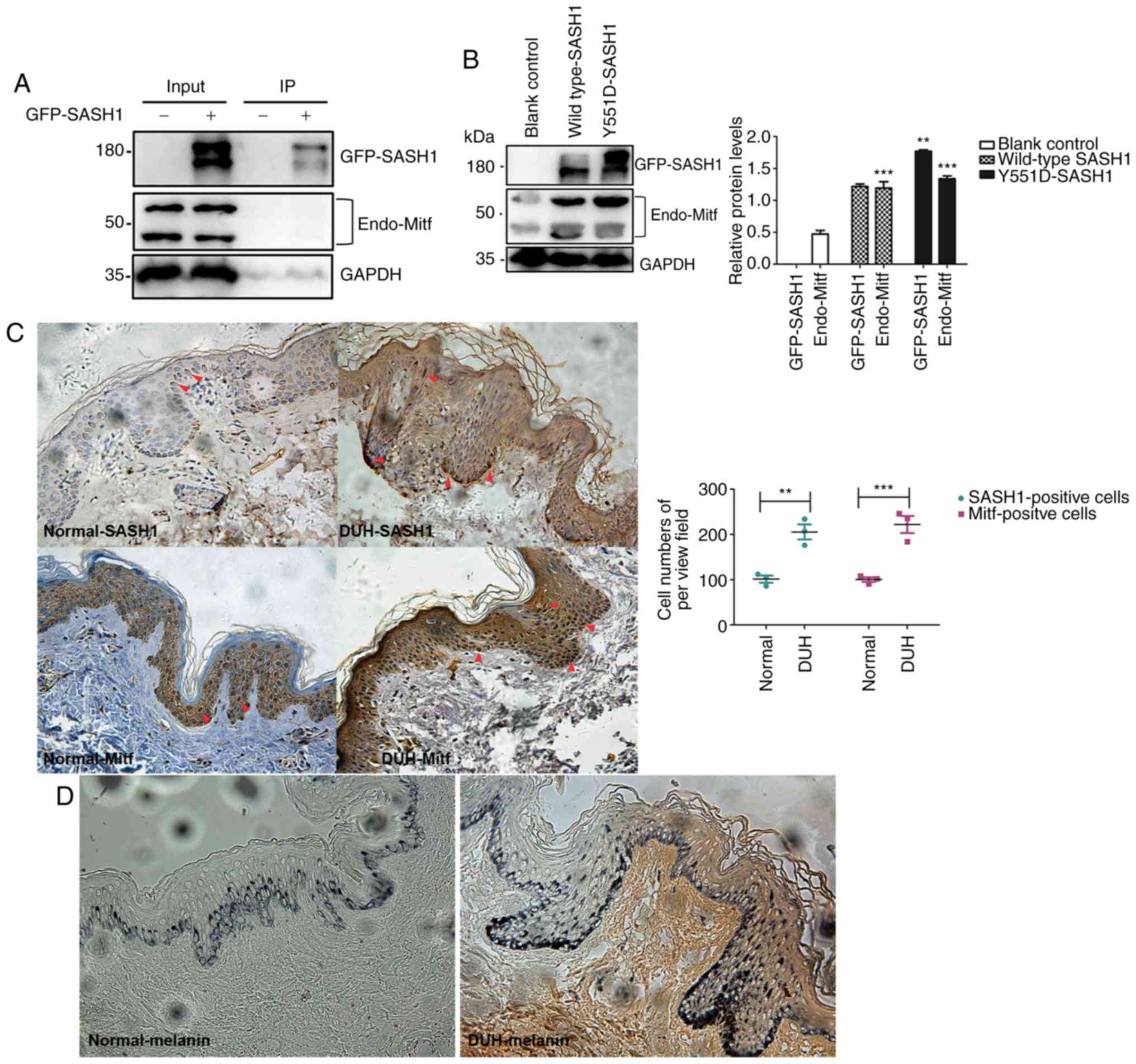 | Figure 7Mitf expression is promoted by
mutated SASH1 in vitro and in epithelial tissues affected by
the Y551D-SASH1 mutation. (A) Exogenous SASH1 is not associated
with endogenous Mitf in 293T cells. GFP-SASH1 was transfected into
293T cells. At 48 h following transfection, transfected cells were
lysed, GFP-SASH1 was immunoprecipitated, and the associated
endogenous Mitf was analyzed by IP-WB analyses. (B) Expression of
endogenous Mitf was induced by Y551D SASH1. Exogenous Y551D SASH1
and a wild-type SASH1 were introduced into 293T cells. Following
transfection, transfected cells were lysed and subjected to western
blot analyses. **P<0.001 vs. wild-type SASH1 and
***P<0.001 vs. blank control. (C) In the lesional
epithelial tissues of Y551D SASH1-affected individuals,
SASH1- and Mitf-positive cells were demonstrated in different
epithelial layers of the affected epithelial tissues and calculated
and analyzed statistically. **P<0.01,
***P<0.001. Upregulation of SASH1 and enhanced Mitf
were also induced in the affected tissues. Magnification, ×40.
Mitf-positive cells, which were stained dark brown in the nucleus,
are indicated by red arrows. (D) More melanin was synthesized and
present in different epithelial layers of the affected skin
epithelial tissues. Magnification, ×40. |
Discussion
DUH was initially described in 2 generations of 2
families in 1933 (19) and is a
group of congenital pigmentary disorders characterized by
generalized mottled hypopigmented and hyperpigmented macules
(5,20). DUH clinically overlaps with DSH
and Dowling-Degos disease and genetically exhibits heterogeneity
with at least 2 causative genes, ABCB6 and SASH1 (5,8,20).
In the present study, a heterozygous mSash-hSASH1 (Y551D)
gene knock-in BALB/cJ mouse model was established to recapitulate
some molecular pathological phenotypes of DUH. SASH1 may function
as a scaffold protein for the assembly Mitf in the nucleus,
generating a novel SASH1-Mitf signaling pathway to regulate
melanogenesis in vitro and in vivo. Y551D
SASH1 promotes Mitf expression and increases the numbers of
Mitf-positive epithelial cells in the affected human epithelial
tissues and in the tail tissues of heterozygous mice; this
phenomenon contributes to the formation of the hyperpigmentation
phenotype of DUH.
SASH1, a signaling adaptor protein of 1,247 amino
acids, is located on chromosome 6q24.3 and contains an
evolutionarily conserved SLY domain (401-555), an SH3 domain
(557-614) and two SAM domains (633-697; 1177-1241, annotation from
the UniProt database) (5). The
SASH1 gene, which functions as a potential tumor suppressor,
has been reported to be associated with the tumorigenesis of lung
cancer, breast cancer, colon cancer, cervical cancer, melanoma and
hepatocellular carcinoma cells and is inversely associated with
certain critical ultrasonographic features of breast cancer
(5,9,21-23). In the present study, the human
SASH1 gene or mouse Sash1 gene was expressed at the
transcriptional level (Fig. 2C).
However, in a few heterozygous and wild-type mice, the human SASH1
protein or mouse Sash1 protein was not detected. The underlying
reason for this may be due to the post-translational modification
of SASH1 as an RXRXXpS phosphorylated motif was identified in
SASH1; SASH1 is suggested to be phosphorylated at Ser90 (24), and multiple serine sites are
demonstrated in the PhosphoSitePlus website (https://www.genecards.org/ProductRedirect?key=fAAAAAJnAAYAAABTQVNIMQAQdAATAAAAAnYABAAAAENTVAAKcwAIYgAAAnUARAAAAGh0dHBzOi8vd3d3LnBob3NwaG9zaXRlLm9yZy91bmlwcm90QWNjQWN0aW9uLmRvP2lkPU85NDg4NSNhcHB-sZXRNc2cACm4ACmEAAA%3d%3d§ion=proteins&subsection=).
Therefore, it can be deduced that the undetectable SASH1 may be
caused by the phosphorylational degradation of SASH1.
In a previous study by the authors, it was
demonstrated that SASH1 mediated skin melanogenesis through a
cascade of p53/α-MSH/POMC/Gαs/SASH1 and novel SASH1/MAP2K2
crosstalk (3,4). Increasing numbers of SASH1 variants
have been found to be associated with human skin dyschromia,
including DUH (2-5,9),
multiple lentigines (6-8) and pigmentation defects with
palmoplantar keratoderma and skin carcinoma (9). Apart from 3 additional variants in
SASH1 that were reported, which are located in the SLY domain of
SASH1 (E509K, L515P, and Y551D), 5 of the 7 different SASH1
variants identified that result in skin dyschromia are located in
the highly conserved SLY domain (5,7,8).
All these findings suggest that the SLY domain is functionally
critical for skin pigmentation regulation and may represent a
potential mutational hotspot region (5). Recent research indicates that
endothelial SASH1 regulates alveolar epithelial cell maturation and
promotes pulmonary surfactant production through nitric oxide
signaling (25). SASH1 is
expressed in a number of human tissues and cells, including whole
skin, keratinocytes, fibroblasts and melanocytes (NCBIGene
Expression Omnibus; http://www.ncbi.nlm.nih.gov/geo/) (6). SASH1 expression has also been
detected in cultured human epidermal melanocytes (3,4),
and the exogenous human Y551D SASH1 introduced into BALB/cJ mice
did not affect the expression of endogenous Sash1 in mice as
the endogenous Sash1 protein in mice can be recognized by the SASH1
antibody (NBP1-26650, Novus Biologicals, LLC). The S519N
substitution in SASH1 was indicated to increase the number
of melanocytes and epidermal cell proliferation in skin (6). In a previous study by the authors
(4), and in the present study, it
was suggested that the Y551D SASH1 substitution not only
enhances Mitf expression, but also increases the numbers of
Mitf-positive cells in the epithelial tissue of heterozygous human
SASH1(Y551D) gene knock-in BABL/cJ mice and in Y551D affected
individuals. Mitf mediates the proliferation of melanocytes and
regulates pigment cell-specific transcription of melanogenic enzyme
genes (16). Hence, the Y551D
SASH1 substitution plays a critical role in the formation of
the hyperpigmented phenotype of DUH.
The phosphorylated active form of CREB binds and
activates Mitf (14,26-30). Mitf in turn stimulates the
transcription of key melanogenic enzymes, such as tyrosinase,
tyrosinase-related protein-1 and tyrosinase-related protein-2. Mitf
plays a central role in α-MSH-induced melanogenesis, and its
expression and activation are regulated in a complex manner
(31). In the present study, it
was found that in heterozygous mice of 2 generations and in
Y551D-affected skin epithelial tissues, Mitf was upregulated by
Y551D-SASH1 in mice and in human tissues. Although exogenous SASH1
was not associated with endogenous Mitf, endogenous Mitf was
regulated by Y551D SASH1. SASH1 serves as a novel scaffold protein
to assemble a signaling complex downstream of TLR4 (32) and oncoprotein cortactin (10). Therefore, as shown in the present
study, SASH1 may function as a scaffold molecule to assemble a
SASH1-Mitf molecular complex to regulate MITF expression in the
cell nucleus. All these findings indicate that SASH1 and Mitf may
form a novel SASH1-Mitf cascade to regulate melanogenesis.
Supplementary Data
Acknowledgments
The authors would like to thank the Clinical
Research Center at the Affiliated Hospital of Guizhou Medical
University and the Experimental Animal Center of Guizhou Medical
University for housing the animals for the experiments. The authors
would also like to thank the Nanjing Biomedical Research Institute
of Nanjing University which assisted in the creation of the models
of mSash-hSASH1(Y551D) gene knock-in BABL/cJ mice.
Funding
The present study was supported partly by the
following funds: The National Natural Science Foundation Project
(31860319 to DAZ), the Guizhou Provincial Natural Science
Foundation [grant no. Qian Ke He LH (2017) 7193 and Qian Ke He
Zhicheng (2020) 4Y125 to DAZ], the Basic Conditions Platform of
Guizhou Provincial Natural Science Foundation [grant no. Qianke
Platform (2015) 40005 to for PH] and the Guizhou Provincial Natural
Science Foundation [grant no. Qianke LH (2015) 7445 to PH].
Availability of data and materials
The datasets used and/or analyzed during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
DAZ and JZ designed the study, analyzed the data,
and wrote and revised the manuscript. ZX, DWa, DWu, JW and LC
performed the majority of the experiments. YD, JZ, YL, ZW, XW, QL
and JZ participated in some of the experiments. HH and PH provided
some suggestions as regards the construction of the mouse models
and some funds to support the projects. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
For the use of human samples, the document of
patient consent had been signed by the affected individual and 2
normal controls, and ethics approval was provided by the Ethics
Committee of the Affiliated Hospital of Guizhou Medical University.
All animal studies were conducted according to experimental
practices and standards approved by the Ethics Committee of the
Nanjing Biomedical Institute of Nanjing University and Guizhou
Medical University (License no. 1800125).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Xing QH, Wang MT, Chen XD, Feng GY, Ji HY,
Yang JD, Gao JJ, Qin W, Qian XQ, Wu SN and He L: A gene locus
responsible for dyschromatosis symmetrica hereditaria (DSH) maps to
chromosome 6q24.2-q25.2. Am J Hum Genet. 73:377–382. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhou D, Wei Z, Deng S, Wang T, Zai M, Wang
H, Guo L, Zhang J, Zhong H, He L and Xing Q: SASH1 regulates
melanocyte transepithelial migration through a novel
Gαs-SASH1-IQGAP1-E-cadherin dependent pathway. Cell Signal.
25:1526–1538. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhou D, Wei Z, Kuang Z, Luo H, Ma J, Zeng
X, Wang K, Liu B, Gong F, Wang J, et al: A novel P53/POMC/Gαs/SASH1
auto-regulatory feedback loop activates mutated SASH1 to cause
pathologic hyperpigmentation. J Cell Mol Med. 21:802–815. 2017.
View Article : Google Scholar
|
|
4
|
Zhou D, Kuang Z, Zeng X, Wang K, Ma J, Luo
H, Chen M, Li Y, Zeng J, Li S, et al: p53 regulates ERK1/2/CREB
cascade via a novel SASH1/MAP2K2 crosstalk to induce
hyperpigmentation. J Cell Mol Med. 21:2465–2480. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhong WL, Wang HJ, Lin ZM and Yang Y:
Novel mutations in SASH1 associated with dyschromatosis universalis
hereditaria. Indian J Dermatol Venereol Leprol. 85:4402019.
View Article : Google Scholar
|
|
6
|
Shellman YG, Lambert KA, Brauweiler A,
Fain P, Spritz RA, Martini M, Janssen KP, Box NF, Terzian T, Rewers
M, et al: SASH1 is involved in an autosomal dominant lentiginous
phenotype. J Invest Dermatol. 135:3192–3194. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang J, Zhang J, Li X, Wang Z, Lei D, Wang
G, Li J, Zhang S, Li Z and Li M: A novel de novo mutation of the
SASH1 gene in a chinese family with multiple lentigines. Acta Derm
Venereol. 97:530–531. 2017. View Article : Google Scholar
|
|
8
|
Zhang J, Cheng R, Liang J, Ni C, Li M and
Yao Z: Lentiginous phenotypes caused by diverse pathogenic genes
(SASH1 and PTPN11): Clinical and molecular discrimination. Clin
Genet. 90:372–377. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Courcet JB, Elalaoui SC, Duplomb L, Tajir
M, Rivière JB, Thevenon J, Gigot N, Marle N, Aral B, Duffourd Y, et
al: Autosomal-recessive SASH1 variants associated with a new
genodermatosis with pigmentation defects, palmoplantar keratoderma
and skin carcinoma. Eur J Hum Genet. 23:957–962. 2015. View Article : Google Scholar :
|
|
10
|
Martini M, Gnann A, Scheikl D, Holzmann B
and Janssen KP: The candidate tumor suppressor SASH1 interacts with
the actin cytoskeleton and stimulates cell-matrix adhesion. Int J
Biochem Cell Biol. 43:1630–1640. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kundumani-Sridharan V, Subramani J,
Raghavan S, Maiti GP, Owens C, Walker T, Wasnick J, Idell S and Das
KC: Short-duration hyperoxia causes genotoxicity in mouse lungs:
Protection by volatile anesthetic isoflurane. Am J Physiol Lung
Cell Mol Physiol. 316:L903–L917. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jiang Y, Xie X, Zhang Y, Luo X, Wang X,
Fan F, Zheng D, Wang Z and Chen Y: Regulation of G-protein
signaling by RKTG via sequestration of the G betagamma subunit to
the Golgi apparatus. Mol Cell Biol. 30:78–90. 2010. View Article : Google Scholar
|
|
13
|
Englaro W, Rezzonico R, Durand-Clément M,
Lallemand D, Ortonne JP and Ballotti R: Mitogen-activated protein
kinase pathway and AP-1 are activated during cAMP-induced
melano-genesis in B-16 melanoma cells. J Biol Chem.
270:24315–24320. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bertolotto C, Abbe P, Hemesath TJ, Bille
K, Fisher DE, Ortonne JP and Ballotti R: Microphthalmia gene
product as a signal transducer in cAMP-induced differentiation of
melanocytes. J Cell Biol. 142:827–835. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hemesath TJ, Price ER, Takemoto C,
Badalian T and Fisher DE: MAP kinase links the transcription factor
Microphthalmia to c-Kit signalling in melanocytes. Nature.
391:298–301. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Primot A, Mogha A, Corre S, Roberts K,
Debbache J, Adamski H, Dreno B, Khammari A, Lesimple T, Mereau A,
et al: ERK-regulated differential expression of the Mitf 6a/b
splicing isoforms in melanoma. Pigment Cell Melanoma Res.
23:93–102. 2010. View Article : Google Scholar
|
|
17
|
Fock V, Gudmundsson SR, Gunnlaugsson HO,
Stefansson JA, Ionasz V, Schepsky A, Viarigi J, Reynisson IE,
Pogenberg V, Wilmanns M, et al: Subcellular localization and
stability of MITF are modulated by the bHLH-Zip domain. Pigment
Cell Melanoma Res. 32:41–54. 2019. View Article : Google Scholar
|
|
18
|
Bouché V, Espinosa AP, Leone L, Sardiello
M, Ballabio A and Botas J: Drosophila Mitf regulates the V-ATPase
and the lysosomal-autophagic pathway. Autophagy. 12:484–498. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ichigawa T and Hiraga Y: A previously
undescribed anomaly of pigmentation dyschromatosis universalis
hereditaria. Jpn J Dermatol Urol. 34:360–364. 1933.In Japanese.
|
|
20
|
Zhang C, Li D, Zhang J, Chen X, Huang M,
Archacki S, Tian Y, Ren W, Mei A, Zhang Q, et al: Mutations in
ABCB6 cause dyschromatosis universalis hereditaria. J Invest
Dermatol. 133:2221–2228. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xie J, Zhang W, Zhang J, Lv QY and Luan
YF: Downregulation of SASH1 correlates with poor prognosis in
cervical cancer. Eur Rev Med Pharmacol Sci. 21:3781–3786.
2017.PubMed/NCBI
|
|
22
|
Gong X, Wu J, Wu J, Liu J, Gu H and Shen
H: Correlation of SASH1 expression and ultrasonographic features in
breast cancer. Onco Targets Ther. 10:271–276. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
He P, Zhang HX, Sun CY, Chen CY and Jiang
HQ: Overexpression of SASH1 inhibits the proliferation, invasion,
and EMT in hepatocarcinoma cells. Oncol Res. 24:25–32. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dubois F, Vandermoere F, Gernez A, Murphy
J, Toth R, Chen S, Geraghty KM, Morrice NA and MacKintosh C:
Differential 14-3-3 affinity capture reveals new downstream targets
of phosphatidylinositol 3-kinase signaling. Mol Cell Proteomics.
8:2487–2499. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Coulombe P, Paliouras GN, Clayton A,
Hussainkhel A, Fuller M, Jovanovic V, Dauphinee S, Umlandt P, Xiang
P, Kyle AH, et al: Endothelial Sash1 is required for lung
maturation through nitric oxide signaling. Cell Rep.
27:1769–1780.e4. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Price ER, Ding HF, Badalian T,
Bhattacharya S, Takemoto C, Yao TP, Hemesath TJ and Fisher DE:
Lineage-specific signaling in melanocytes. C-kit stimulation
recruits p300/CBP to microph-thalmia. J Biol Chem. 273:17983–17986.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gonzalez GA and Montminy MR: Cyclic AMP
stimulates soma-tostatin gene transcription by phosphorylation of
CREB at serine 133. Cell. 59:675–680. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jin ML, Park SY, Kim YH, Park G, Son HJ
and Lee SJ: Suppression of α-MSH and IBMX-induced melanogenesis by
cordycepin via inhibition of CREB and MITF, and activation of
PI3K/Akt and ERK-dependent mechanisms. Int J Mol Med. 29:119–124.
2012.
|
|
29
|
Kim HE, Ishihara A and Lee SG: The effects
of Caffeoylserotonin on inhibition of melanogenesis through the
downregulation of MITF via the reduction of intracellular cAMP and
acceleration of ERK activation in B16 murine melanoma cells. BMB
Rep. 45:724–729. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Saha B, Singh SK, Sarkar C, Bera R, Ratha
J, Tobin DJ and Bhadra R: Activation of the Mitf promoter by
lipid-stimulated activation of p38-stress signalling to CREB.
Pigment Cell Res. 19:595–605. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yun CY, You ST, Kim JH, Chung JH, Han SB,
Shin EY and Kim EG: p21-activated kinase 4 critically regulates
melanogenesis via activation of the CREB/MITF and β-catenin/MITF
pathways. J Invest Dermatol. 135:1385–1394. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dauphinee SM, Clayton A, Hussainkhel A,
Yang C, Park YJ, Fuller ME, Blonder J, Veenstra TD and Karsan A:
SASH1 is a scaffold molecule in endothelial TLR4 signaling. J
Immunol. 191:892–901. 2013. View Article : Google Scholar : PubMed/NCBI
|