1. Introduction
Acute myocardial infarction (AMI) is the leading
cause of morbidity and mortality worldwide (1). Timely reperfusion is crucial for
salvaging the ischemic myocardium. However, the rapid restoration
of blood flow into the myocardial tissue following a period of
ischemia may lead to additional tissue damage, termed myocardial
ischemia-reperfusion (IR) injury, resulting in an increase in the
myocardial infarct size and a decrease in cardiac function
(2). The mechanisms responsible
for myocardial IR injury are intricate, involving an excessive
inflammatory response, endoplasmic reticulum stress, calcium
overload, oxidative stress and cardiomyocyte death (e.g.,
autophagy, apoptosis and necroptosis) (3).
Neuregulin-1 (NRG-1), a stress-mediated growth
factor that binds cardiomyocyte tyrosine kinase receptors of the
erythroblastic leukemia viral oncogene (e.g., ErbB2 and ErbB4), is
mainly synthesized and secreted by endocardial and microvasculature
endothelial cells in the heart (4). Upon ligand binding, activated
NRG-1/ErbB signaling directs cell fates (e.g., survival, migration,
differentiation and proliferation) (5). Currently, the therapeutic potential
of NRG-1 in cardiovascular diseases is gradually being revealed.
Numerous studies have demonstrated that NRG-1 protects myocardial
cells from injury and restores cardiac functions, such as
protection against doxorubicin (DOX)-induced cardiomyocyte toxicity
(6) and anti-fibrotic and
anti-remodeling effects in heart failure models (7). Notably, the administration of
recombinant human NRG-1 has been shown to significantly improve
cardiac function in phase I and II clinical trials of heart failure
(HF) (8). Given the beneficial
effects of NRG-1 on cellular survival and cardiac function, the
role of NRG-1 in myocardial IR injury should be of interest to
researchers. Furthermore, the protective mechanisms of NRG-1 share
common features with myocardial IR injury. On the other hand,
myocardial IR injury is one of the major pathophysiological
conditions in the pathogenesis of HF, and effective treatments with
IR injury could hamper the onset and development of HF.
2. Overview of NRG-1
Structure and isoforms of NRG-1
As a member of the Neuregulin family of growth
factors, NRG-1, encoded by a gene spanning 2.4 million base pairs
in mice and 2.6 million base pairs in humans and rats, is located
on chromosome 8 in humans and mice, and on chromosome 16 in rats
(9,10). The 6 known types of NRG-1 proteins
(types I-VI) are classified by 6 different transcriptional
initiation sites, and alternative splicing of the NRG-1 gene
produces 33 different isoforms in humans (11,12). The 6 types of NRG-1 are defined by
differences at the N-terminus; more specifically, these differences
lay in the linker region between the transmembrane domain and the
EGF-like domain encoded by exons (13,14). All 6 isoforms have an EGF-like
domain, which is necessary and sufficient for the activation of
ErbB receptors. However, only type I, II, IV and V isoforms have an
additional immunoglobulin (Ig)-like domain, which is located
between the N-terminal sequence and the EGF-like domain (Fig. 1A), while NRG-1 types III and VI
are characterized by an N-terminal region connected directly to the
EGF-like domain (Fig. 1B)
(10,14). Of note, the N-terminal region of
NRG-1 type III consists of a cysteine-rich domain (CRD) and an
additional N-terminal transmembrane domain (TM), and this unique
structure limits the functional fragment release (Fig. 1C) (14). NRG-1 protein is functionally shed
by proteolytic cleavage of its membrane-bound precursor (pro-NRG-1)
(15). A bioactive extracellular
NRG1 fragment is released by type I transmembrane domain proteases
[e.g., beta-secretase (BACE), a disintegrin and metallopeptidase
domain (ADAM)17, ADAM19 and ADAM10], termed mature NRG-1, acting in
a juxtacrine or paracrine manner, while mature NRG-1 type III is
anchored by CRD rather than shedding from the membrane (16).
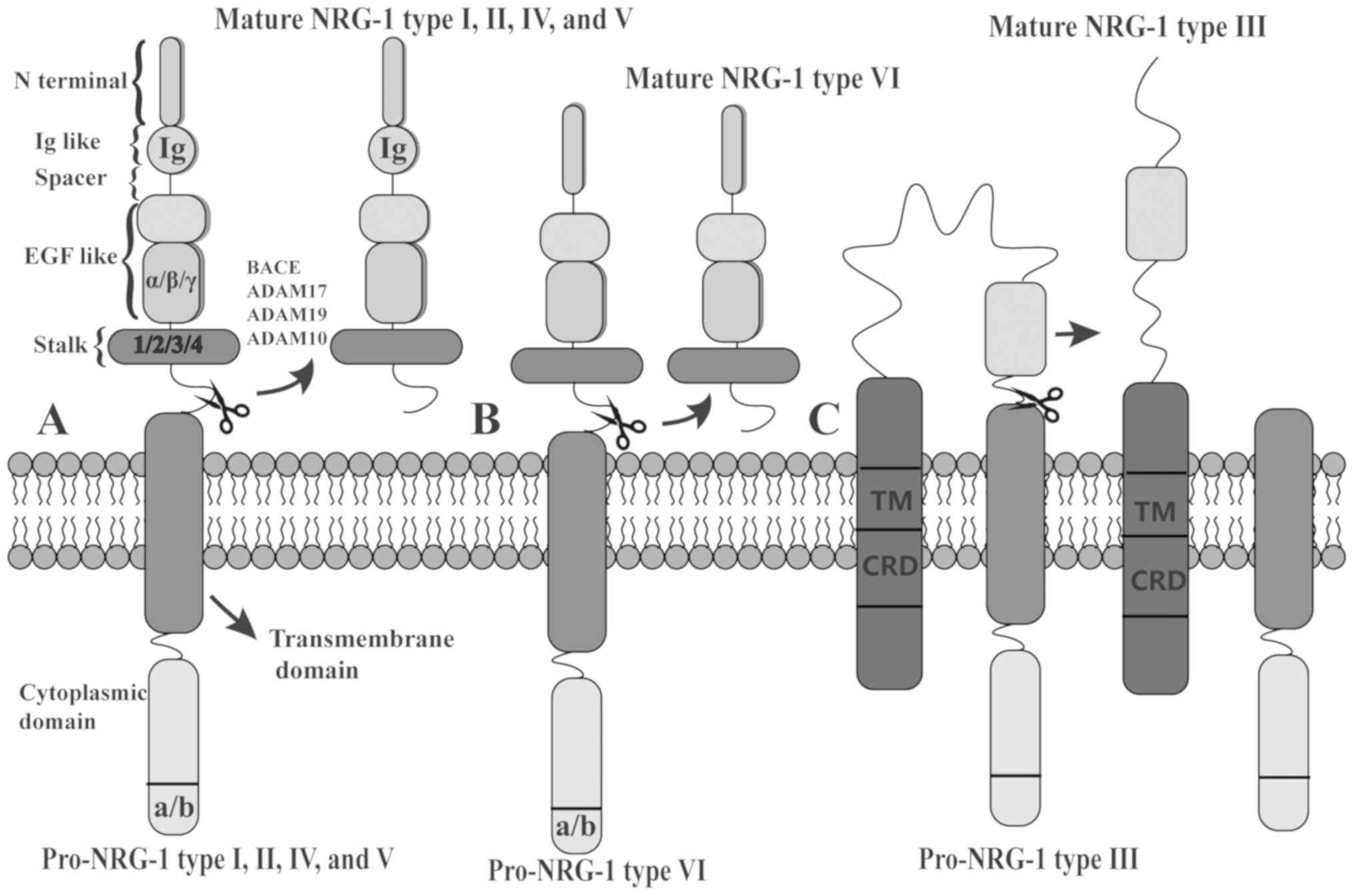 | Figure 1Structure of NRG-1 and
characteristics of the 6 types. (A) NRG-1 types I, II, IV and V
isoforms have an additional immunoglobulin (Ig)-like domain, while
the (B) N-terminal region of types III and VI are connected
directly to the EGF-like domain. Of note, (C) type III contains a
cysteine-rich domain (CRD) and an additional N-terminal
transmembrane domain (TM). Pro-NRG-1 undergoes type I transmembrane
domain proteolysis (e.g., BACE, ADAM17, ADAM19 and ADAM10) to
release the functional fragment, termed mature NRG-1, except in the
case of NRG-1 type III. NRG-1, neuregulin-1. |
ErbB receptor and associated signaling
pathways
To exert vital biological functions, NRG-1 must bind
to a family of ErbB to activate tyrosine kinase receptor proteins.
The ErbB family encompasses four transmembrane tyrosine kinase
receptors: ErbB1 [also termed epidermal growth factor receptor
(EGFR)], ErbB2, ErbB3 and ErbB4 (10,13). Upon ligand binding, an ErbB
receptor encounters structural modifications in the juxta-membrane
domain (JMD) (17), which leads
to the homodimerization or heterodimerization of ErbB receptors due
to differences in receptor affinity. NRG-1 ligand binding triggers
the homodimerization of ErbB4 and the heterodimerization of
ErbB-2/3, ErbB-2/4 and ErbB-3/4 (18). Compared with NRG-1, EGF has more
potent affinity to the binding site of ErbB1 (19). Among the ErbB receptors, only
ErbB4 can form functional homodimers upon NRG-1 binding, as it
consists of both ligand binding and active kinase domains (15). On the other hand, ErbB2 lacks the
ligand binding pocket, while ErbB3 only has a pseudokinase domain.
The absence of sufficient signal transmission requires the
heterodimerization of both receptors to exert their function
(13).
The dimerization of ErbB receptors (either homo- or
heterodimerization) activates the tyrosine kinase domain and
induces the phosphorylation of the cytoplasmic region of the ErbB
partner (20). Subsequently, the
phosphorylated tyrosine residues recruit various adaptors/effectors
and trigger a switch in signal transmission. The main activated
downstream signaling molecules are the phosphoinositide 3-kinase
(PI3K)/Akt and Raf/MEK/extracellular regulated kinase (ERK)
pathways (21), which are both
important reperfusion injury salvage kinases (RISK). Other
downstream kinases known to be activated by NRG-1 include Pyk2,
c-Abl, JNK, Fyn and CDK5 (10).
In the cardiovascular system, these pathways affect cell survival,
migration, adhesion and differentiation properties and
proliferation (22), and they
play an indispensable role in maintaining cardiovascular
homeostasis when cardiomyocytes encounter stimuli or insults, such
as hypoxia, acidosis and oxidative stress, that contribute to
myocardial IR injury.
3. NRG-1: Related mechanisms involved in
myocardial IR injury
The NRG-1/ErbB pathway is considered a compensatory
protective mechanism of cardiac insult (23). It has been reported that the
cardiac NRG-1/ErbB pathway is upregulated following myocardial IR.
Fang et al observed the significant upregulation of NRG-1 at
both the mRNA and protein levels, which was accompanied by a marked
increase in the phosphorylation of the ErbB4 receptor in the
IR-affected myocardium (24).
Morano et al also found that the expression of ErbB3 was
upregulated upon ischemic injury (25). It is generally recognized that
excessive reactive oxygen species (ROS) are important mediators of
reperfusion injury. Accumulated ROS has been shown to upregulate
the NRG-1 secretion and the phosphorylation of ErbB (26), which can also be induced by
hypoxia (27).
Thus, myocardial ischemia appears to be involved in
the upregulation of NRG-1/ErbB in the heart following IR, and the
core part of this upregulation may be the accelerated generation of
ROS, which is attributed to adenosine triphosphate (ATP)
deficiency, hypoxia, mitochondrial permeability transition pore
(mPTP) opening and mitochondrial damage. Since the attributions of
IR injury are not individual segments but complex networks, and the
association between NRG-1 and IR injury is not a single
unidirectional link, in the present review, the effects of NRG-1
via the pathways from related triggers of ROS to eventual cell
death during IR are illustrated (Fig.
2).
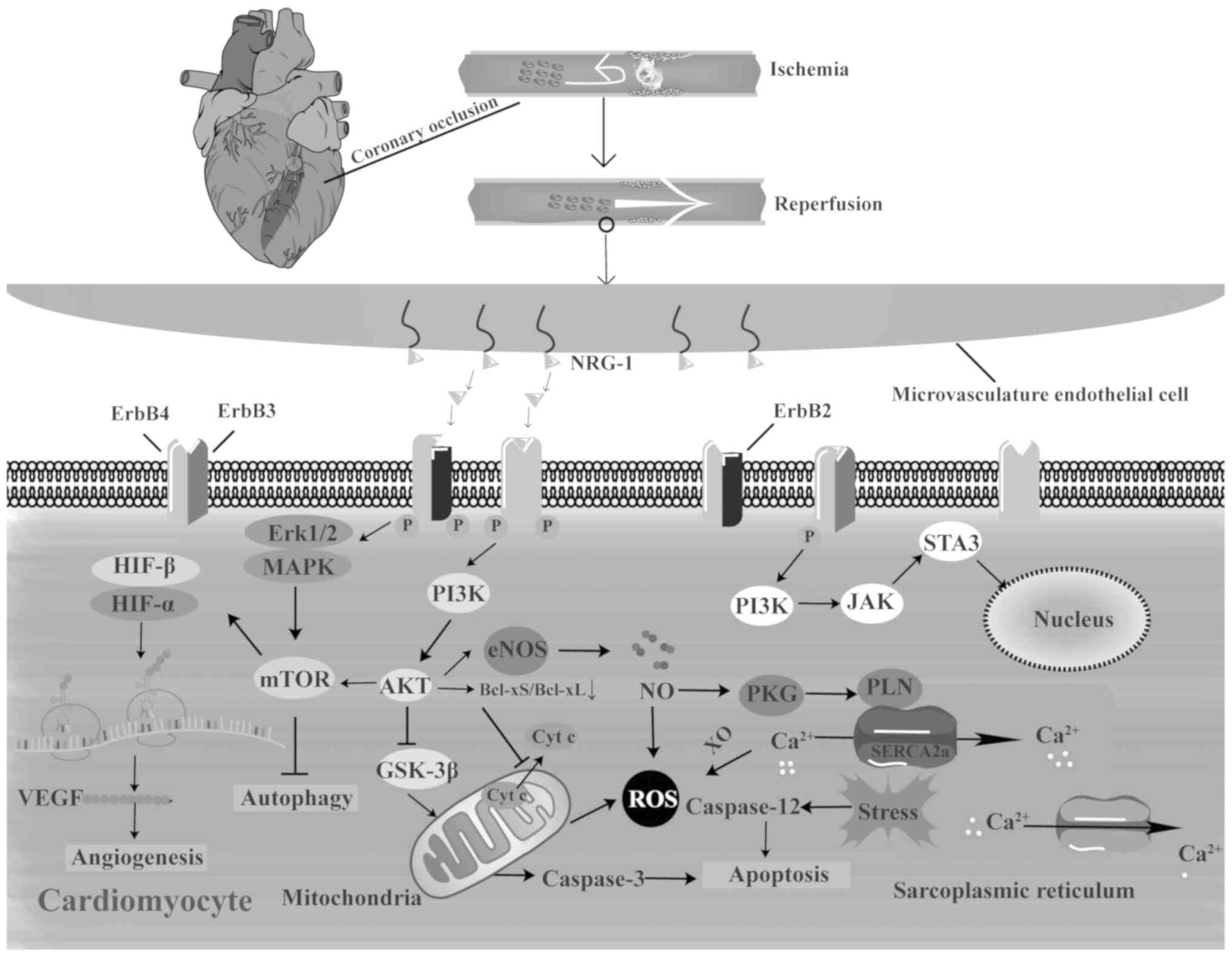 | Figure 2Mechanisms of action of NRG-1 in
myocardial IR injury. NRG-1 is upregulated during myocardial IR
injury and is released from the microvas-culature endothelium by
specific proteases. Upon ligand binding, NRG-1/ErbB activates
several signaling pathways to protect against myocardial IR injury
involved in endoplasmic reticulum stress, calcium overload,
oxidative stress and cell death. The main signaling pathways
include PI3K/Akt and Erk/MAPK, which are both important reperfusion
injury salvage kinases. Cyt c, cytochrome; HIF,
hypoxia-inducible factor; NO, nitric oxide; NRG-1, neuregulin-1;
ROS, reactive oxygen species; SERCA2a, sarcoplasmic reticulum
Ca2+-ATPase; VEGF, vascular endothelial growth factor;
XO, xanthine oxidase. |
Excessive inflammatory response
With the initial activation of inflammation
accompanied by the release of pro-inflammatory cytokines, as well
as inflammatory leukocyte recruitment and infiltration, the heart
attempts to defend itself against deleterious stimuli during IR.
However, excessive inflammation in the endangered myocardial region
can lead to exacerbated myocyte death via pro-apoptotic signaling
pathways and further cardiac remodeling (28,29). Toll-like receptors (TLRs) and
nuclear factor-κB (NF-κB) are the primary pathways associated with
IR-induced inflammation (3). TLRs
are expressed by inflammatory cells, endothelial cells and
cardiomyocytes. In addition to defending against microorganisms,
TLRs, particularly TLR-4, play a critical role in
inflammation-induced apoptosis during IR (30). NF-κB pathways can be divided into
canonical and alternative/non-canonical pathways. The canonical
NF-κB pathway involves the phosphorylation and degradation of
inhibitory κB (IκB), resulting in the nuclear translocation of
p65/p50 NF-κB heterodimers and ultimately triggering the production
of pro-inflammatory molecules (31). By contrast, the alternative NF-κB
pathway promotes the generation of anti-apoptotic and
anti-inflammatory molecules through the enhanced translocation of
RelB/p52 NF-κB heterodimers by the activation of IKB kinase-α
(IKK-α) (32,33).
Studies have demonstrated the anti-inflammatory
effects of NRG-1 in the setting of sepsis-induced cardiac injury
and ischemic stroke (34,35). NRG-1 alleviates the inflammatory
response by both the inhibition of canonical NF-κB and the
activation of alternative NF-κB. Consistent with the alleviation of
the inflammatory response, the pro-inflammatory factors [e.g.,
tumor necrosis factor (TNF)-α, interleukin (IL)-6 and IL-1β] are
downregulated and anti-inflammatory factors [e.g.,
granulocyte-colony stimulating factor (G-CSF) and IL-9] are
upregulated (35). Furthermore,
NRG-1/ErbB4 can inhibit the action of macrophages through the
PI3K/AKT pathway, attenuating myocardium inflammation and fibrosis
(36). Although there is limited
evidence available to indicate that NRG-1 exerts its
anti-inflammatory effects during IR to protect the heart,
inflammation is a ubiquitous stress-response involving the entire
body, and ischemic stroke shares similar features as myocardial
infarction. Consequently, it can be inferred that NRG-1 modulates
inflammation by regulating the NF-κB pathway and suppressing
macrophage activation during IR injury, thus salvaging at-risk
myocytes. On the other hand, repressing the maladaptive organelle
response attributed to inflammation (e.g., oxidative stress,
endoplasmic reticulum stress and calcium overload) may also be an
approach to exert anti-inflammatory effects.
Endoplasmic reticulum (ER) stress
ER stress, as a transient adaptive response aimed at
reducing the level of unfolded proteins and returning the ER to
homeostasis, following the upregulation of molecular chaperones,
ultimately increases the ability of the ER to regulate calcium
content and protect myocardial cells (37,38). However, prolonged or severe ER
stress, attributed to persistent hypoxia, oxidative stress and
calcium overload, can abandon its pro-survival efforts and instead
trigger apoptotic cell death by activating caspase-12 (an ER
stress-specific proapoptotic molecule) and c-JUN N-terminal kinase
and upregulating CCAAT/enhancer binding protein homologous protein
(CHOP) (39). Increasing evidence
has indicated that ER stress plays a crucial role in the
pathogenesis of myocardial IR injury (40,41). It has been demonstrated that
NRG-1/ErbB protects against cardiac IR injury by suppressing
cardiac ER stress through the PI3K/Akt pathway and directly
inhibits the upregulation of ER stress-related markers [e.g.,
glucose-regulated protein (GRP)78, CHOP and cleaved caspase-12] in
both neonatal and adult ventricular myocytes to delay the onset of
ER stress, reducing caspase-12-related apoptosis (39). On the other hand, NRG-1 also
hinders the onset of ER stress by alleviating oxidative stress and
calcium overload, which will be discussed in detail below.
Calcium overload and nitric oxide
(NO)
The disequilibrium of intracellular Ca2+
homeostasis plays a significant role during the pathogenesis of IR
injury and contributes to the impaired cardiac contractile function
(3). ER stress-induced calcium
overload is the upstream signal for IR injury (42). The increased level of calcium
activates Ca2+-dependent xanthine oxidase (XO), which
results in ROS generation and cellular apoptosis (43).
NO, recognized as an inorganic free radical gas, is
synthesized from the amino acid L-arginine by nitric oxide
synthases (NOSs), such as neuronal NOS (nNOS or NOS I), inducible
NOS (iNOS or NOS II), and endothelial NOS (eNOS or NOS III)
(44). NO functions as an
important biological molecule attenuating myocardial IR injury via
the regulation of cardiac contractility and vasodilation (45). Under calcium overload, NRG-1
rapidly enhances the level of NO in adult ventricular myocytes
through the activation of the PI3K/Akt/eNOS pathway. The increase
in NO generation leads to PKG activation with the phosphorylation
of phospholamban, which promotes sarcoplasmic reticulum calcium
ATPase (SERCA2a) activity as well as calcium uptake by the
sarcoplasmic reticulum (46). It
has been shown that NRG-1 regulates calcium through the production
of NO. The generation of NO also enhances the open probability of
mitochondrial adenosine triphosphate-sensitive K+
(mitoKATP) channels, but reduces the mPTP open probability.
Notably, accumulated NO may exert a detrimental effect through the
formation of peroxynitrite (a byproduct of NO degradation due to
decreased NO bioavailability) (47), which aggravates oxidative stress
and activates the apoptotic signaling pathway. It has been
demonstrated that the hyperactivation of eNOS induced by NRG-1 can
lead to an increase in ROS production (48), resulting in an increased MI size.
The theory of eNOS uncoupling may explain this phenomenon. Briefly,
under substrate (L-arginine) or cofactor deficiency, eNOS would
transfer electrons to molecular oxygen rather than to substrate,
resulting in the burst of ROS (49). It seems paradoxical due to the
dual role of NO; however, its combination with L-arginine can
reverse this deleterious effect.
Oxidative stress and HIF-1α
As previously mentioned, a surge of ROS has been
shown to stimulate NRG-1 secretion. Moreover, NRG-1/ErbB can defend
against oxidative stress during IR. Morano et al revealed
that the expression of ErbB3 receptor in H9c2 cells increased the
cell survival rate and enhanced mitochondrial resistance to
oxidative stress by maintaining mitochondrial membrane potential
(25), which inhibits the release
of ROS and cytochrome c through the opening of mitoKATP
channels. In addition to inhibiting the network of maladaptive
organelle responses that aggravate oxidative stress, NRG-1 also
induces adaptations of transcription factors involved in redox
regulation, including 11 factors [catalase (CAT), Cu-Zn-dismutase
(SOD1), thioredoxin (TXN), TXNRD1, protein disulfide-isomerase
(PDI)A1, PDIA4, PDIA3, glutathione S-transferase Pi 1 (GSTP1),
glutathione peroxidase 1 (GPX1), glutamate-cysteine ligase
catalytic subunit (GCLC) and thiosulfate sulfurtransferase (TST)],
to exert its radical scavenging effects (50). Furthermore, NRG-1 increases
glutathione reductase mRNA, whose translation products are known as
important antioxidants (51).
Hypoxia-inducible factor-1 (HIF-1), a heterodimeric
transcription factor consisting of a constitutively expressed β
subunit and an oxygen-regulated α subunit, regulates angiogenesis,
proliferation and cellular metabolism, assisting cells in the
adaption to hypoxic environments (52,53). Under anoxic conditions, HIF-1α
becomes stable and is accompanied by an upregulated transcriptional
response to ROS, inflammatory mediators and somatotropic hormone
(54,55). Increased levels of HIF-1α and the
enhanced transcriptional activity of key HIF-1 target genes, such
as vascular endothelial growth factor (VEGF), play a critical role
in myocardial IR injury. The activated HIF-1α/VEGF signaling
pathway promotes the proliferation of cardiac microvasculature
endothelial cells, which leads to NRG-1 generation and confers
cardio-protective effects against anoxia injury during IR (56,57). It has previously been demonstrated
that hypoxia can induce NRG-1 secretion and the phosphorylation of
ErbB (27), and it has been shown
that ErbB3 is upregulated by HIF-1α (58). Thus, it can be inferred that
HIF-1α may upregulate NRG-1 via unidentified pathways. Of note,
NRG-1 can in turn mediate HIF-1α expression. It has been
demonstrated that the PI3K/Akt/mammalian target of rapamycin (mTOR)
pathway activated by NRG-1 can lead to HIF-1α activation and can
regulate angiogenesis (59).
Collectively, the interactions between HIF-1α and NRG-1 mediate
angiogenesis by triggering an increase in the proliferation,
migration and invasion of endothelial cells, as well as in the
formation of a capillary-like tubular structure network. This helps
cardiomyocytes to survive hypoxic conditions and under subsequent
reperfusion injury.
Alleviation of cardiomyocyte death
The eventual and principal target of all therapeutic
interventions is aimed at reducing the MI size and salvaging the
myocardium. Thus, the prevention of cardiomyocyte death is crucial
for cardiac function recovery and the estimation of intervention
effects. Studies have demonstrated that the caspase-8-dependent
Fas/FasL extrinsic death receptor pathway, caspase-9-related
mitochondrial apoptosis and caspase-12-involved ER stress are
associated with cardio-myocyte death in response to IR injury
(60-62). Notably, NRG-1 has the ability to
inhibit cell death by improving mitochondrial membrane potential
(63), suppressing calcium
overload (64), suppressing
endoplasmic reticulum stress (38), alleviating the inflammatory
response (35) and ultimately
maintaining cellular viability during myocardial IR injury.
Apoptotic death
Apoptosis is an ATP-consuming form of programmed
cell death characterized by chromatin accumulation, DNA
fragmentation and apoptotic body formation, typically without an
inflammatory response or membrane stability changes (2,65).
Apoptosis can be initiated extrinsically by the activation of
sarcolemma receptors (e.g., Fas and TNF-α) or intrinsically by the
mitochondrial release of cytochrome c, which initiates a
cascade of caspase activation and ultimately, intracellular
proteolysis (66,67). NRG-1 has been demonstrated to
directly suppress cardiomyocyte apoptosis by inhibiting mPTP
opening, cytochrome c release and caspase-3 activation
through PI3K/Akt signaling. Furthermore, the inhibition of ErbB2
and ErbB4 receptors leads to the induction of Bcl-xL splicing
toward its pro-apoptotic protein Bcl-xS, thus activating
mitochondrial dysfunction and apoptosis (68-70). This indirectly confirms the role
of NRG-1/ErbB in myocyte apoptosis. Moreover, alleviating ER stress
can inhibit the release of caspase-12, further reducing apoptotic
death.
Autophagic death
Autophagy denotes a regulated process of lysosomal
degradation and the recycling of cytoplasm or mitochondrial
proteins (mitophagy), characterized by the formation of
double-membrane vesicles (autophagosomes) and elevated levels of
light chain 3, beclin-1, autophagy-related gene (ATG) 5-12 complex,
p62 and parkin (71). It has been
demonstrated that autophagy acts as a 'double-edged sword' in the
pathology of IR injury. To the best of our knowledge, autophagy may
exert beneficial effects during the early period of ischemia, but
detrimental effects during the late period of ischemia and
reperfusion (72). The present
review focuses on the prevention of the detrimental effects induced
by autophagy. It has been illustrated that the PI3K/PKB/mTOR and
mitogen-activated protein kinase (MAPK)/ERK1/2/mTOR pathways
activated by NRG-1/ErbB via phosphorylation of phosphatidylinositol
are involved in the negative regulation of autophagy (73,74). Moreover, the Akt-mediated
reduction of ROS (Akt/ROS signaling) results in the upregulation of
Bcl-2, which plays a role in the anti-autophagy effects of NRG-1
(73).
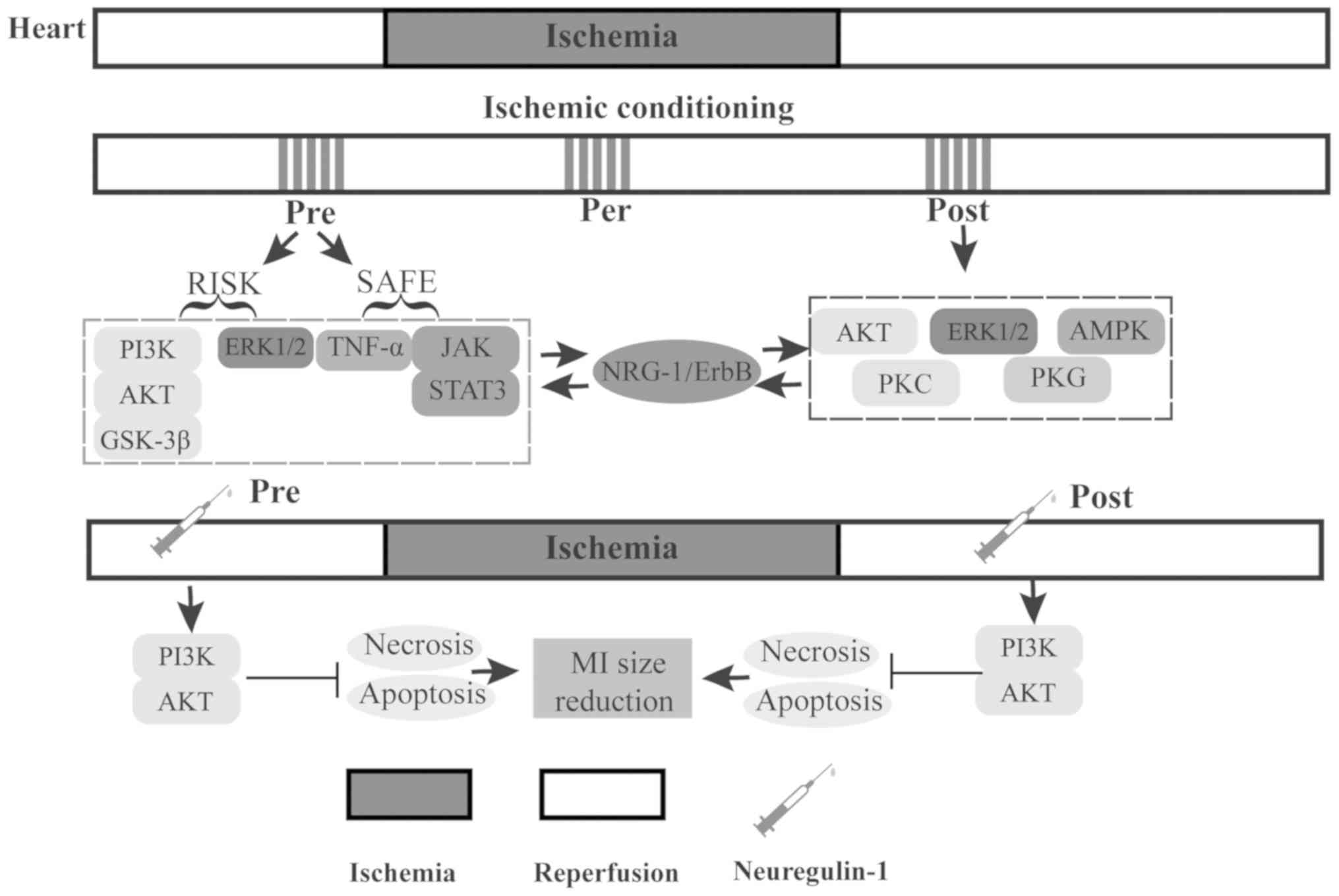
4. Involvement of NRG-1 in conditioning
against myocardial IR injury
Conditioning is a practice of applying brief
episodes of intermittent nonlethal stimulus, which confers
protection against myocardial IR injury (75). Considering the temporal
association between the stimulus and ischemia, conditioning can be
classified into 3 types, including preconditioning, perconditioning
and postconditioning (76,77).
More recently, some experimental studies have explored the
association between NRG-1 and different types of conditioning
(Table I). Although the
mechanisms of NRG-1 involved in conditioning remain poorly
understood, the present review focused on the NRG-1-induced
cardioprotective effects against IR injury in terms of
preconditioning and postconditioning (Fig. 3).
 | Table IInvolvement of NRG-1 in
conditioning. |
Table I
Involvement of NRG-1 in
conditioning.
| Author/(Refs.) | Year | Model | NRG-1
treatment | Timepoint | Species | Ischemia | Reperfusion |
|---|
| Fang et al
(24) | 2010 | LAD ligature and
reopening | 4 µg/kg,
IV | 20 min before the
hearts were subjected to IR | Rats | 45 min | 3 h |
| Ebner et al
(49) | 2013 | LAD ligature and
reopening | 8 µg/kg,
IP | 5 min before
reopening of the ligature | Mice | 45 min | 30 min |
| Wang et al
(88) | 2018 | LAD ligature and
reopening | 3 µg/kg,
IV | At the onset of
reperfusion | Rats | 45 min | 24 h |
| Langendorff
isolated heart | 20 ng/ml, perfused
for 20 min | At the onset of
reperfusion | Rats | 30 min | 2 h |
Preconditioning
Preconditioning is the protective stimulus applied
prior to the onset of a sustained episode of isch-emia (78). Generally, preconditioning
comprises two temporal windows, as well as protection peaks. The
first window (acute form) occurs several minutes following the
stimulus and lasts for 1-3 h, while the second window (delay form)
begins a few hours (peak at 24 h) after the stimulus and lasts
several days but no longer than 72 h (79). The major interventions of
preconditioning include ischemic conditioning (IPC),
pharmacological preconditioning, remote ischemic preconditioning
(RIPC) and physical preconditioning, which have been shown to
reduce the MI size (78,80). Hereinafter, the role of NRG-1 in
IPC and pharmacological preconditioning is described.
IPC refers to a process of repetitive non-lethal
ischemia prior to sustained lethal myocardial ischemia, which
increases cardiac resistance against IR injury (81). It has been shown that the
reperfusion injury salvage kinase [e.g., the PI3K/AKT/glycogen
synthase kinase (GSK)-3β and ERK1/2 pathways] and the survivor
activator factor enhancement pathways (e.g., TNF-α, JAK2/STAT3
pathway) are the main pathways involved in IPC-induced
cardioprotection (75,82). Notably, NRG-1/ErbB shares the same
signaling pathways with IPC as above, and NRG-1 is rapidly
upregulated during IPC and myocardial IR injury. This indicates
that NRG-1 may at least partially play an important role in
IPC-induced cardioprotection.
Pharmacological preconditioning is characterized by
pre-treatment with drugs (78).
In vivo, NRG-1 preconditioning protects the heart against IR
injury by reducing myocardial necrosis and apoptosis mediated by a
PI3K/Akt-dependent mechanism. Consistent with MI size limitations,
NRG-1 preconditioning also decreases the level of plasma CK and LDH
after 45 min of regional myocardial ischemia and 180 min of
reperfusion (24). Thus, NRG-1
preconditioning is effective at reducing myocardium damage and
represents a novel cardiac protective strategy that can be used in
the setting of elective myocardial IR, as encountered during
cardiac surgery and acute myocardial infarction.
Postconditioning
Postconditioning, defined as cardioprotective
intervention applied at the onset of reperfusion following
sustained ischemia (83), can be
achieved by short repeated occlusions of the vessel prior to
permanent reperfusion [ischemic postconditioning (IP)] or by
pharmacological interventions [pharmacological postconditioning
(pPC)], which both have recently been shown to have potential as
novel cardioprotective interventions against IR injury (77).
For conferring protection against IR injury, IP
plays a key role in the alleviation of oxidative stress,
inflammation and apoptosis through NO production and mitoKATP
channels opening by salvage kinase pathways, including AKT, ERK1/2,
5'AMP-activated protein kinase (AMPK), protein kinase (PK)C and PKG
(84-86). Of note, in vivo, IP
promotes NRG-1 protein expression, as well as the
upregulation/activation of ErbB3 and ErbB4, indicating that the
cardioprotection may be mediated by the NRG-1/ErbB3 and ErbB4
signaling pathways, which has also recently been confirmed in
ischemic local postconditioning (87).
pPC with NRG-1 concurrently with reperfusion has
been shown to inhibit apoptosis and reduce MI size in a IR rat
model or isolated murine heart (25), exerting a cardioprotective effect
via the PI3K/Akt pathway (87,88). Therefore, pPC with NRG-1 may be an
effective treatment following timely reperfusion, such as
thrombolytic therapy or primary percutaneous coronary intervention
(PPCI).
5. Therapeutic potential of NRG-1 against
myocardial IR injury
As accumulating evidence has indicated that the
enhanced activation of the NRG-1/ErbB axis primarily contributes to
attenuating myocardial IR injury, NRG-1 has emerged as a novel
promising therapeutic alternative for reperfusion injury.
Hereinafter, studies regarding other therapeutic potentials of
NRG-1 in myocardial IR injury are mentioned and discussed.
Stem cell-based therapies
Recently, stem cell-based therapies have provided
great promise for interventions in ischemic heart diseases
(89,90). Among the forms of ischemic heart
diseases, stem cells have been explored, particularly in the
setting of myocardial infarction (91). In this connection, it has been
reported that NRG-1 is a pivotal target of stem cell regulation,
both in cultured cardiomyocytes and in whole embryos (5). In addition to mediating ventricular
myocyte proliferation, NRG-1/ErbB has been found to be involved in
embryonic stem cell (ESC) differentiation into cardiac myocyte
lineages (92) and further, in
the induction of cardiac conduction system cell differentiation.
Moreover, the differentiation of stem cells into working-type
cardiomyocytes can be modulated by NRG-1 (93). These effects can be interpreted as
the upregulation of connexin with NRG-1 administration in
ESC-derived cardiomyocytes, such as connexin 40 (Cx40) and
connextin-45 (Cx45) (94). As
intracellular channel proteins, connexins bridge gaps with
cardiomyocytes to achieve synchronized contraction of the heart
(95). The upregulated expression
of connexins induced by NRG-1 also helps ESC differentiate into
cardiac myocytes via MEK/ERK, and different cell lineages may be
attributed to the different expression of connexin (94). It has been reported that treatment
with cardiosphere-derived cells (CDCs) improves ventricular
function in children with single ventricle physiology (96), which indicates the potential of
cardiac self-repair. Since myocardial IR injury is inevitable in
MI, further attention should be paid to restituting and restoring
functional and structural components of the heart; stem cell
transplantation administered with NRG-1 may be a strategic
option.
Gene-based therapy
Gene-based therapy utilizes gene delivery systems
(e.g., viral and non-viral vectors) to modulate gene expression at
the cellular level to treat pathological conditions (97). As regards IR injury, it has been
reported that lentivirus-mediated hNRG-1 gene transduction
establishes a stable expression system in infarcted hearts of rats
and further activates the PI3K/Akt/eNOS pathway to promote
neovascularization and angiogenesis, as manifested by enhanced
expression of VEGF. In addition, the overexpression of hNRG-1
alleviates myocyte apoptosis through the Bcl-2/Bax signaling
pathway (98). Collectively, the
gene-based therapy of NRG-1 helps attenuate IR injury and
eventually improves cardiac function. Although the application is
still limited to animal experiments, and gene-based therapy has not
yet been popularized, the significant protective effects suggest
that gene delivery can be an alternative approach for
NRG-1-dependent therapeutic strategies against myocardial IR
injury.
NRG-1-loaded microparticles
The widespread clinical use of cardiovascular
protein treatment may be hampered due to the limited stability and
rapid degradation of protein, and novel formulation strategies that
take into account sustained drug bioavailability in the infarcted
border zone are urgently required (99,100). The application of microparticles
(MPs) through catheter-based intramyocardial injection, with
minimally invasive methods, may be a desirable approach for the
clinical translation of cardiac regenerative medicine of MI
(101). Briefly, cardiovascular
protein molecules, such as NRG-1, are encapsulated into delicate
bioresorbable scaffolds (PLGA) to form MPs and injected into target
cardiac tissue with the guidance of visual cardiac mapping to
achieve precise treatment (102). It has previously been
demonstrated that NRG-1 plays critical roles in cardiac remodeling
and MI size limitation through RISK and survivor activating factor
enhancement (SAFE) pathways (103), and these benefits can be
maximally utilized in the target infarcted zone. NRG-1-loaded MPs
have been previously applied in a porcine model of IR over a period
of months without severe side-effects; additionally, a prolonged
and effective angiogenic stimulus was provided to the ischemic
myocardium due to the sustained release, which failed to achieve
success in clinical trials by applying pro-angiogenic factors
(104). Notably, the
transplantation of adipose-derived stem cells combined with
NRG-1-loaded MPs stimulated cardiomyocyte proliferation and
provided more complete healing in a rat myocardial infarction
(105), suggesting that 'the
whole is greater than the sum of its parts.' With the growing
morbidity of AMI and the progress being made in precision medicine,
NRG-1-loaded MPs may be a promising treatment for patients with MI
by enhancing patient compliance and curative effects.
Cardiac transplantation
Cardiac transplantation, considered as the only
effective therapy for end-stage heart failure, requires appropriate
storage conditions for donor hearts to attenuate IR injury and
preserve heart function during reperfusion (106). It has been demonstrated that
rhNRG-1 mitigates left ventricular remodeling and sarcomere
disorganization by upregulation of the RISK pathway (107,108). Notably, by combining
organ-storage solution (Celsior) with rhNRG-1 in an isolated
working rat heart model, additional cardiac preservation was
observed after hypothermic storage, as evidenced by the reduction
of myocyte apoptosis and necrosis during transplantation. Moreover,
this recovery function could be enhanced by combination with other
cardioprotective agents (e.g., glyceryl trinitrate and cariporide)
(109). Accompanied by increased
steady-state level of phosphorylated kinases [e.g., p-Akt,
p-ERK1/2, p-signal transducer and activator of transcription 3
(STAT3) and p-GSK-3β] and reduced cleaved caspase-3, NRG-1 may
exert these benefits by activating downstream pathways, including
Akt, Ekr1/2 and JAK/STAT3, and involving caspase-3 related
apoptosis (109,110). With rhNRG-1 supplementation, the
goals of a longer storage time and higher cardiac vitality can be
achieved during transplantation, and the success rate of surgery
can be enhanced due to attenuation of IR injury. This suggests that
NRG-1 may partially mitigate the contradiction between wanting
donor hearts and growing clinical needs via the potential benefit
against myocardial IR injury.
6. Conclusion and future perspectives
Recently, increasing evidence has gradually revealed
the potential cardiac benefits of NRG-1 against myocardial IR
injury. In this regard, it has been demonstrated that NRG-1
modulates several endocellular transcripts (e.g., SOD1 and TXN),
important mediators (e.g., NO and HIF-1α) and signaling pathways
(e.g., PI3K/Akt and MAPK/ERK1/2 pathways), thereby forming a
complicated network that contributes to straining the inflammatory
response, alleviating ER stress, suppressing calcium overload,
inhibiting oxidative stress and repressing cellular death (e.g.,
apoptosis and autophagy) in cardiomyocytes during IR injury.
Endogenous NRG-1 is a potential cardioprotective
mediator of conditioning, and preconditioning or postconditioning
with exogenous NRG-1 also confers cardioprotective effects against
IR injury. However, the mechanisms underlying the involvement of
NRG-1 in conditioning are not yet clear and warrant more in-depth
investigation. Significantly, several therapeutic potentials of
NRG-1 have been revealed (e.g., the application of NRG-1 in stem
cell-based therapies, gene transduction, microparticle delivery of
hNRG-1 and cardiac transplantation), which may assist in expanding
the therapeutic strategies of NRG-1 against myocardial IR injury in
clinical practice.
Finally, for future research perspectives, the
further directions of NRG-1 research in myocardial IR are as
follows: i) NRG-1 promotes glucose uptake independently of insulin
in the liver and cardiomyocytes via the PI3Kα/Akt/AS160 pathway and
GLUT4 translocation (111,112), which illuminates possible
research of diabetic patients with acute coronary syndrome, such as
the application of neuregulin-1 in myocardial IR rats with
diabetes; ii) the neuregulin-1/ErbB pathway enhances leptin levels
and improves behavior against obesity, enlightening the possible
approach of exploring underlying the mechanisms of action of NRG-1
in a myocardial IR model with obesity or a high-fat diet (113,114); iii) crosstalk between NRG-1 and
HIF-1 remains poorly understood and warrants further in-depth
exploration, such as the level of change of HIF-1 in
hypoxia/reoxygenation (H/R) cardiomyocytes accompanied by NRG-1
treatment.
In conclusion, the NRG-1/ErbB network is a critical
modulator of IR injury, and NRG-1 may be a promising therapeutic
target in the future.
Acknowledgments
Not applicable.
Funding
The present study was supported by the Natural
Science Foundation of Guangdong Province (nos. 2015A030310478 and
2017A030313703).
Availability of data and materials
Not applicable.
Authors' contributions
YL reviewed the related science literature and wrote
the manuscript. HL supported YL in the revisions of the manuscript
and processing of the figures. XW and HL conceived the study and
supervised the writing of the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that there are no competing
interests.
References
|
1
|
Chan SH, Hung CH, Shih JY, Chu PM, Cheng
YH, Lin HC and Tsai KL: SIRT1 inhibition causes oxidative stress
and inflammation in patients with coronary artery disease. Redox
Biol. 13:301–309. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ibáñez B, Heusch G, Ovize M and Van de
Werf F: Evolving therapies for myocardial ischemia/reperfusion
injury. J Am Coll Cardiol. 65:1454–1471. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhou H, Ma Q, Zhu P, Ren J, Reiter RJ and
Chen Y: Protective role of melatonin in cardiac
ischemia-reperfusion injury: From pathogenesis to targeted therapy.
J Pineal Res. 64:2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mendes-Ferreira P, De Keulenaer GW,
Leite-Moreira AF and Brás-Silva C: Therapeutic potential of
neuregulin-1 in cardiovascular disease. Drug Discov Today.
18:836–842. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rupert CE and Coulombe KL: The roles of
neuregulin-1 in cardiac development, homeostasis, and disease.
Biomarker Insights. 10(Suppl 1): S1–S9. 2015.
|
|
6
|
Liu YQ, Yang M, Duan CH, Su GB, Wang JH,
Liu YF and Zhang J: Protective role of neuregulin-1 toward
doxorubicin-induced myocardial toxicity. Genet Mol Res.
13:4627–4634. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Galindo CL, Kasasbeh E, Murphy A, Ryzhov
S, Lenihan S, Ahmad FA, Williams P, Nunnally A, Adcock J, Song Y,
et al: Anti-remodeling and anti-fibrotic effects of the
neuregulin-1beta glial growth factor 2 in a large animal model of
heart failure. J Am Heart Assoc. 3:e0007732014. View Article : Google Scholar
|
|
8
|
Miao J, Huang S, Su YR, Lenneman CA,
Wright M, Harrell FE, Sawyer DB and Lenihan DJ: Effects of
endogenous serum neuregulin-1β on morbidity and mortality in
patients with heart failure and left ventricular systolic
dysfunction. Biomarkers. 23:704–708. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chou CF and Ozaki M: In silico analysis of
neuregulin 1 evolution in vertebrates. Biosci Rep. 30:267–275.
2010. View Article : Google Scholar
|
|
10
|
Kataria H, Alizadeh A and
Karimi-Abdolrezaee S: Neuregulin-1/ErbB network: An emerging
modulator of nervous system injury and repair. Prog Neurobiol.
180:1016432019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu X, Bates R, Yin DM, Shen C, Wang F, Su
N, Kirov SA, Luo Y, Wang JZ, Xiong WC and Mei L: Specific
regulation of NRG1 isoform expression by neuronal activity. J
Neurosci. 31:8491–8501. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tan W, Wang Y, Gold B, Chen J, Dean M,
Harrison PJ, Weinberger DR and Law AJ: Molecular cloning of a
brain-specific, developmentally regulated neuregulin 1 (NRG1)
isoform and identification of a functional promoter variant
associated with schizophrenia. J Biol Chem. 282:24343–24351. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hu X, Fan Q, Hou H and Yan R: Neurological
dysfunctions associated with altered BACE1-dependent Neuregulin-1
signaling. J Neurochem. 136:234–249. 2016. View Article : Google Scholar :
|
|
14
|
Zhang Z, Huang J, Shen Y and Li R:
BACE1-dependent Neuregulin-1 signaling: An implication for
schizophrenia. Front Mol Neurosci. 10:3022017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Willem M: Proteolytic processing of
Neuregulin-1. Brain Res Bull. 126:178–182. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mostaid MS, Lloyd D, Liberg B, Sundram S,
Pereira A, Pantelis C, Karl T, Weickert CS, Everall IP and Bousman
CA: Neuregulin-1 and schizophrenia in the genome-wide association
study era. Neurosci Biobehav Rev. 68:387–409. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nagano T, Namba H, Abe Y, Aoki H, Takei N
and Nawa H: In vivo administration of epidermal growth factor and
its homologue attenuates developmental maturation of functional
excitatory synapses in cortical GABAergic neurons. Eur J Neurosci.
25:380–390. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Olayioye MA, Neve RM, Lane HA and Hynes
NE: The ErbB signaling network: Receptor heterodimerization in
development and cancer. EMBO J. 19:3159–3167. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schlessinger J: Ligand-induced,
receptor-mediated dimerization and activation of EGF receptor.
Cell. 110:669–672. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
D'Uva G and Lauriola M: Towards the
emerging crosstalk: ERBB family and steroid hormones. Semin Cell
Dev Biol. 50:143–152. 2016. View Article : Google Scholar
|
|
21
|
Li KX, Lu YM, Xu ZH, Zhang J, Zhu JM,
Zhang JM, Cao SX, Chen XJ, Chen Z, Luo JH, et al: Neuregulin 1
regulates excitability of fast-spiking neurons through Kv1.1 and
acts in epilepsy. Nat Neurosci. 15:267–273. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Falls D: Neuregulins: Functions, forms,
and signaling strategies. Exp Cell Res. 284:14–30. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Odiete O, Hill MF and Sawyer DB:
Neuregulin in cardiovascular development and disease. Circ Res.
111:1376–1385. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fang SJ, Wu XS, Han ZH, Zhang XX, Wang CM,
Li XY, Lu LQ and Zhang JL: Neuregulin-1 preconditioning protects
the heart against ischemia/reperfusion injury through a
PI3K/Akt-dependent mechanism. Chin Med J (Engl). 123:3597–3604.
2010.
|
|
25
|
Morano M, Angotti C, Tullio F, Gambarotta
G, Penna C, Pagliaro P and Geuna S: Myocardial ischemia/reperfusion
upregulates the transcription of the Neuregulin1 receptor ErbB3,
but only postconditioning preserves protein translation: Role in
oxidative stress. Int J Cardiol. 233:73–79. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kuramochi Y, Cote GM, Guo X, Lebrasseur
NK, Cui L, Liao R and Sawyer DB: Cardiac endothelial cells regulate
reactive oxygen species-induced cardiomyocyte apoptosis through
neuregulin-1beta/erbB4 signaling. J Biol Chem. 279:51141–51147.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Iivanainen E, Paatero I, Heikkinen SM,
Junttila TT, Cao R, Klint P, Jaakkola PM, Cao Y and Elenius K:
Intra- and extracellular signaling by endothelial neuregulin-1. Exp
Cell Res. 313:2896–2909. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Griffiths HR, Gao D and Pararasa C: Redox
regulation in metabolic programming and inflammation. Redox Biol.
12:50–57. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nahrendorf M, Pittet MJ and Swirski FK:
Monocytes: Protagonists of infarct inflammation and repair after
myocardial infarction. Circulation. 121:2437–2445. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vilahur G and Badimon L:
Ischemia/reperfusion activates myocardial innate immune response:
The key role of the toll-like receptor. Front Physiol. 5:4962014.
View Article : Google Scholar
|
|
31
|
Hoesel B and Schmid JA: The complexity of
NF-κB signaling in inflammation and cancer. Mol Cancer. 12:862013.
View Article : Google Scholar
|
|
32
|
Sun SC: Non-canonical NF-κB signaling
pathway. Cell Res. 21:71–85. 2011. View Article : Google Scholar
|
|
33
|
Sun SC: The non-canonical NF-κB pathway in
immunity and inflammation. Nat Rev Immunol. 17:545–558. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhou Q, Wang L, Wang X, Xiong Q, Wei Y,
Dang S and Zhong L: Effect of neuregulin-1 on heart function and
inflammatory mediators in rats with sepsis. Zhonghua Wei Zhong Bing
Ji Jiu Yi Xue. 30:140–144. 2018.In Chinese. PubMed/NCBI
|
|
35
|
Simmons LJ, Surles-Zeigler MC, Li Y, Ford
GD, Newman GD and Ford BD: Regulation of inflammatory responses by
neureg-ulin-1 in brain ischemia and microglial cells in vitro
involves the NF-kappa B pathway. J Neuroinflammation. 13:2372016.
View Article : Google Scholar
|
|
36
|
Vermeulen Z, Hervent AS, Dugaucquier L,
Vandekerckhove L, Rombouts M, Beyens M, Schrijvers DM, De Meyer
GRY, Maudsley S, De Keulenaer GW and Segers VFM: Inhibitory actions
of the NRG-1/ErbB4 pathway in macrophages during tissue fibrosis in
the heart, skin, and lung. Am J Physiol Heart Circ Physiol.
313:H934–H945. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu MQ, Chen Z and Chen LX: Endoplasmic
reticulum stress: A novel mechanism and therapeutic target for
cardiovascular diseases. Acta Pharmacol Sin. 37:425–443. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xu M, Wu X, Jie B, Zhang X, Zhang J, Xin Y
and Guo Y: Neuregulin-1 protects myocardial cells against
H2O2-induced apoptosis by regulating
endoplasmic reticulum stress. Cell biochemistry and function.
32:464–469. 2014. View Article : Google Scholar
|
|
39
|
Fang SJ, Li PY, Wang CM, Xin Y, Lu WW,
Zhang XX, Zuo S, Ma CS, Tang CS, Nie SP and Qi YF: Inhibition of
endoplasmic reticulum stress by neuregulin-1 protects against
myocardial ischemia/reperfusion injury. Peptides. 88:196–207. 2017.
View Article : Google Scholar
|
|
40
|
Groenendyk J, Agellon LB and Michalak M:
Coping with endoplasmic reticulum stress in the cardiovascular
system. Annu Rev Physiol. 75:49–67. 2013. View Article : Google Scholar
|
|
41
|
Wu H, Ye M, Yang J, Ding J, Yang J, Dong W
and Wang X: Nicorandil protects the heart from ischemia/reperfusion
injury by attenuating endoplasmic reticulum response-induced
apoptosis through PI3K/Akt signaling pathway. Cell Physiol Biochem.
35:2320–2332. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhu H, Jin Q, Li Y, Ma Q, Wang J, Li D,
Zhou H and Chen Y: Melatonin protected cardiac microvascular
endothelial cells against oxidative stress injury via suppression
of IP3R-[Ca2+] c/VDAC-[Ca2+]m axis by
activation of MAPK/ERK signaling pathway. Cell Stress Chaperones.
23:101–113. 2018. View Article : Google Scholar
|
|
43
|
Zhang Y, Zhou H, Wu W, Shi C, Hu S, Yin T,
Ma Q, Han T, Zhang Y, Tian F and Chen Y: Liraglutide protects
cardiac microvascular endothelial cells against
hypoxia/reoxygenation injury through the suppression of the
SR-Ca(2+)-XO-ROS axis via activation of the
GLP-1R/PI3K/Akt/survivin pathways. Free Radic Biol Med. 95:278–292.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Förstermann U and Sessa WC: Nitric oxide
synthases: Regulation and function. Eur Heart J. 33:829–837.
837a–837d. 2012. View Article : Google Scholar :
|
|
45
|
Yu X, Ge L, Niu L, Lian X, Ma H and Pang
L: The dual role of inducible nitric oxide synthase in myocardial
ischemia/reperfu-sion injury: Friend or foe? Oxid Med Cell Longev.
2018:83648482018. View Article : Google Scholar
|
|
46
|
Brero A, Ramella R, Fitou A, Dati C,
Alloatti G, Gallo MP and Levi R: Neuregulin-1beta1 rapidly
modulates nitric oxide synthesis and calcium handling in rat
cardiomyocytes. Cardiovasc Research. 88:443–452. 2010. View Article : Google Scholar
|
|
47
|
Cadenas S: ROS and redox signaling in
myocardial ischemia-reperfusion injury and cardioprotection. Free
Radic Biol Med. 117:76–89. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lemmens K, Fransen P, Sys SU, Brutsaert DL
and De Keulenaer GW: Neuregulin-1 induces a negative inotropic
effect in cardiac muscle: Role of nitric oxide synthase.
Circulation. 109:324–326. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ebner B, Lange SA, Eckert T, Wischniowski
C, Ebner A, Braun-Dullaeus RC, Weinbrenner C, Wunderlich C, Simonis
G and Strasser RH: Uncoupled eNOS annihilates neuregulin-1β-induced
cardioprotection: A novel mechanism in pharmacological
postconditioning in myocardial infarction. Mol Cell Biochem.
373:115–123. 2013. View Article : Google Scholar
|
|
50
|
Giraud MN, Fluck M, Zuppinger C and Suter
TM: Expressional reprogramming of survival pathways in rat
cardiocytes by neuregulin-1beta. J Appl Physiol (1985). 99:313–322.
2005. View Article : Google Scholar
|
|
51
|
Timolati F, Ott D, Pentassuglia L, Giraud
MN, Perriard JC, Suter TM and Zuppinger C: Neuregulin-1 beta
attenuates doxorubicin-induced alterations of
excitation-contraction coupling and reduces oxidative stress in
adult rat cardiomyocytes. J Mol Cell Cardiol. 41:845–854. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Greer SN, Metcalf JL, Wang Y and Ohh M:
The updated biology of hypoxia-inducible factor. EMBO J.
31:2448–2460. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zimna A and Kurpisz M: Hypoxia-inducible
factor-1 in physiological and pathophysiological angiogenesis:
Applications and therapies. Biomed Res Int. 2015:5494122015.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wang W, Xu B, Xuan H, Ge Y, Wang Y, Wang
L, Huang J, Fu W, Michie SA and Dalman R: Hypoxia-inducible factor
1 in clinical and experimental aortic aneurysm disease. J Vasc
Surg. 68:1538–1550.e2. 2018. View Article : Google Scholar
|
|
55
|
Movafagh S, Crook S and Vo K: Regulation
of hypoxia-inducible factor-1a by reactive oxygen species: New
developments in an old debate. J Cell Biochem. 116:696–703. 2015.
View Article : Google Scholar
|
|
56
|
Wang J, Zhou J, Wang Y, Yang C, Fu M,
Zhang J, Han X, Li Z, Hu K and Ge J: Qiliqiangxin protects against
anoxic injury in cardiac microvascular endothelial cells via
NRG-1/ErbB-PI3K/Akt/mTOR pathway. J Cell Mol Med. 21:1905–1914.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Lim CS, Kiriakidis S, Sandison A, Paleolog
EM and Davies AH: Hypoxia-inducible factor pathway and diseases of
the vascular wall. J Vasc Surg. 58:219–230. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Humtsoe JO, Pham E, Louie RJ, Chan DA and
Kramer RH: ErbB3 upregulation by the HNSCC 3D microenvironment
modulates cell survival and growth. Oncogene. 35:1554–1564. 2016.
View Article : Google Scholar
|
|
59
|
Karar J and Maity A: PI3K/AKT/mTOR pathway
in angiogenesis. Front Mol Neurosci. 4:512011. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Kozlov AV, Lancaster JR Jr, Meszaros AT
and Weidinger A: Mitochondria-meditated pathways of organ failure
upon inflammation. Redox Biol. 13:170–181. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Liu XM, Yang ZM and Liu XK: Fas/FasL
induces myocardial cell apoptosis in myocardial
ischemia-reperfusion rat model. Eur Rev Med Pharmaco. 21:2913–2918.
2017.
|
|
62
|
Groenendyk J, Sreenivasaiah PK, Kim DH,
Agellon LB and Michalak M: Biology of endoplasmic reticulum stress
in the heart. Circ Res. 107:1185–1197. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Zhang XX, Wu XS, Mi SH, Fang SJ, Liu S,
Xin Y and Zhao QM: Neuregulin-1 promotes mitochondrial biogenesis,
attenuates mitochondrial dysfunction, and prevents
hypoxia/reoxygenation injury in neonatal cardiomyocytes. Cell
Biochem Funct. Feb;10:2020.Epub ahead of print.
|
|
64
|
Wang X, Zhuo X, Gao J, Liu H, Lin F and Ma
A: Neuregulin-1β partially improves cardiac function in
volume-overload heart failure through regulation of abnormal
calcium handling. Front Pharmacol. 10:6162019. View Article : Google Scholar
|
|
65
|
Badalzadeh R, Mokhtari B and Yavari R:
Contribution of apoptosis in myocardial reperfusion injury and loss
of cardio-protection in diabetes mellitus. J Physiol Sci.
65:201–215. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Orogo AM and Gustafsson AB: Cell death in
the myocardium: My heart won't go on. IUBMB life. 65:651–656. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Kleinbongard P, Schulz R and Heusch G:
TNFα in myocardial ischemia/reperfusion, remodeling and heart
failure. Heart Fail Rev. 16:49–69. 2011. View Article : Google Scholar
|
|
68
|
Rohrbach S, Muller-Werdan U, Werdan K,
Koch S, Gellerich NF and Holtz J: Apoptosis-modulating interaction
of the neuregulin/erbB pathway with anthracyclines in regulating
BclxS and Bcl-xL in cardiomyocytes. J Mol Cell Cardiol. 38:485–493.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Kuramochi Y, Lim CC, Guo X, Colucci WS,
Liao R and Sawyer DB: Myocyte contractile activity modulates
norepi-nephrine cytotoxicity and survival effects of
neuregulin-1beta. Am J Physiol Cell Physiol. 286:C222–C229. 2004.
View Article : Google Scholar
|
|
70
|
Fukazawa R, Miller TA, Kuramochi Y, Frantz
S, Kim YD, Marchionni MA, Kelly RA and Sawyer DB: Neuregulin-1
protects ventricular myocytes from anthracycline-induced apoptosis
via erbB4-dependent activation of PI3-kinase/Akt. J Mol Cell
Cardiol. 35:1473–1479. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Dong Y, Undyala VV, Gottlieb RA, Mentzer
RM Jr and Przyklenk K: Autophagy: Definition, molecular machinery,
and potential role in myocardial ischemia-reperfusion injury. J
Cardiovasc Pharmacol Ther. 15:220–230. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Aghaei M, Motallebnezhad M, Ghorghanlu S,
Jabbari A, Enayati A, Rajaei M, Pourabouk M, Moradi A, Alizadeh AM
and Khori V: Targeting autophagy in cardiac ischemia/reperfu-sion
injury: A novel therapeutic strategy. J Cell Physiol.
234:16768–16778. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
An T, Huang Y, Zhou Q, Wei BQ, Zhang RC,
Yin SJ, Zou CH, Zhang YH and Zhang J: Neuregulin-1 attenuates
doxorubicin-induced autophagy in neonatal rat cardiomyocytes. J
Cardiovasc Pharmacol. 62:130–137. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Surviladze Z, Sterk RT, DeHaro SA and
Ozbun MA: Cellular entry of human papillomavirus type 16 involves
activation of the phosphatidylinositol 3-kinase/Akt/mTOR pathway
and inhibition of autophagy. J Virol. 87:2508–2517. 2013.
View Article : Google Scholar :
|
|
75
|
Sanada S, Komuro I and Kitakaze M:
Pathophysiology of myocardial reperfusion injury: Preconditioning,
postconditioning, and translational aspects of protective measures.
Am J Physiol Heart Circ Physiol. 301:H1723–H1741. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Hausenloy DJ and Yellon DM: The
therapeutic potential of ischemic conditioning: An update. Nat Rev
Cardiol. 8:619–629. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Heusch G: Treatment of myocardial
ischemia/reperfusion injury by ischemic and pharmacological
postconditioning. Compr Physiol. 5:1123–1145. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Hausenloy DJ and Yellon DM: Ischaemic
conditioning and reperfusion injury. Nat Rev Cardiol. 13:193–209.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Heusch G: Molecular basis of
cardioprotection: Signal transduction in ischemic pre-, post-, and
remote conditioning. Circ Res. 116:674–699. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Stokfisz K, Ledakowicz-Polak A, Zagorski M
and Zielinska M: Ischaemic preconditioning-current knowledge and
potential future applications after 30 years of experience. Adv Med
Sci. 62:307–316. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Diaz RJ and Wilson GJ: Studying ischemic
preconditioning in isolated cardiomyocyte models. Cardiovasc Res.
70:286–296. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Rossello X and Yellon DM: The RISK pathway
and beyond. Basic Res Cardiol. 113:22018. View Article : Google Scholar
|
|
83
|
Ovize M, Baxter GF, Di Lisa F, Ferdinandy
P, Garcia-Dorado D, Hausenloy DJ, Heusch G, Vinten-Johansen J,
Yellon DM and Schulz R; Working Group of Cellular Biology of Heart
of European Society of Cardiology: Postconditioning and protection
from reperfusion injury: Where do we stand? Position paper from the
Working Group of Cellular Biology of the Heart of the European
Society of Cardiology. Cardiovasc Res. 87:406–423. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Hao M, Zhu S, Hu L, Zhu H, Wu X and Li Q:
Myocardial ischemic postconditioning promotes autophagy against
ischemia reperfusion injury via the activation of the
nNOS/AMPK/mTOR pathway. Int J Mol Sci. 18:6142017. View Article : Google Scholar :
|
|
85
|
Jivraj N, Liew F and Marber M: Ischaemic
postconditioning: Cardiac protection after the event. Anaesthesia.
70:598–612. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Bice JS and Baxter GF: Postconditioning
signalling in the heart: Mechanisms and translatability. Br J
Pharmacol. 172:1933–1946. 2015. View Article : Google Scholar :
|
|
87
|
Pilz PM, Hamza O, Gidlöf O, Gonçalves IF,
Tretter EV, Trojanek S, Abraham D, Heber S, Haller PM, Podesser BK
and Kiss A: Remote ischemic perconditioning attenuates adverse
cardiac remodeling and preserves left ventricular function in a rat
model of reperfused myocardial infarction. Int J Cardiol.
285:72–79. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Wang F, Wang H, Liu X, Yu H, Zuo B, Song
Z, Wang N, Huang W and Wang G: Pharmacological postconditioning
with Neuregulin-1 mimics the cardioprotective effects of ischaemic
postconditioning via ErbB4-dependent activation of reperfusion
injury salvage kinase pathway. Mol Med. 24:392018. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Khanabdali R, Rosdah AA, Dusting GJ and
Lim SY: Harnessing the secretome of cardiac stem cells as therapy
for ischemic heart disease. Biochem Pharmacol. 113:1–11. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Yu H, Lu K, Zhu J and Wang J: Stem cell
therapy for ischemic heart diseases. Br Med Bull. 121:135–154.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Barzegar M, Kaur G, Gavins FNE, Wang Y,
Boyer CJ and Alexander JS: Potential therapeutic roles of stem
cells in ischemia-reperfusion injury. Stem Cell Res. 37:1014212019.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Sun M, Yan X, Bian Y, Caggiano AO and
Morgan JP: Improving murine embryonic stem cell differentiation
into cardiomyocytes with neuregulin-1: Differential expression of
microRNA. Am J Physiol Cell Physiol. 301:C21–C30. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Zhu WZ, Xie Y, Moyes KW, Gold JD, Askari B
and Laflamme MA: Neuregulin/ErbB signaling regulates cardiac
subtype specification in differentiating human embryonic stem
cells. Circ Res. 107:776–786. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Wang Z and Huang J: Neuregulin-1 increases
connexin-40 and connexin-45 expression in embryonic stem
cell-derived cardio-myocytes. Appl Biochem Biotechnol. 174:483–493.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Schulz R, Gorge PM, Gorbe A, Ferdinandy P,
Lampe PD and Leybaert L: Connexin 43 is an emerging therapeutic
target in ischemia/reperfusion injury, cardioprotection and
neuroprotection. Pharmacol Ther. 153:90–106. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Ummarino D: Heart failure: Recombinant
neuregulin for HF treatment. Nat Rev Cardiol. 14:1282017.PubMed/NCBI
|
|
97
|
Cao Y, Tan YF, Wong YS, Liew MWJ and
Venkatraman S: Recent advances in chitosan-based carriers for gene
delivery. Mar Drugs. 17:3812019. View Article : Google Scholar :
|
|
98
|
Xiao J, Li B, Zheng Z, Wang M, Peng J, Li
Y and Li Z: Therapeutic effects of neuregulin-1 gene transduction
in rats with myocardial infarction. Coron Artery Dis. 23:460–468.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Xiao S and Shaw RM: Cardiomyocyte protein
trafficking: Relevance to heart disease and opportunities for
therapeutic intervention. Trends Cardiovasc Med. 25:379–389. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Wang ZV and Hill JA: Protein quality
control and metabolism: Bidirectional control in the heart. Cell
Metab. 21:215–226. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Pascual-Gil S, Abizanda G, Iglesias E,
Garbayo E, Prósper F and Blanco-Prieto MJ: NRG1 PLGA MP locally
induce macrophage polarisation toward a regenerative phenotype in
the heart after acute myocardial infarction. J Drug Target.
27:573–581. 2019. View Article : Google Scholar
|
|
102
|
Pascual-Gil S, Simon-Yarza T, Garbayo E,
Prosper F and Blanco-Prieto MJ: Cytokine-loaded PLGA and PEG-PLGA
microparticles showed similar heart regeneration in a rat
myocardial infarction model. Int J Pharm. 523:531–533. 2017.
View Article : Google Scholar
|
|
103
|
Kirabo A, Ryzhov S, Gupte M, Sengsayadeth
S, Gumina RJ, Sawyer DB and Galindo CL: Neuregulin-1β induces
proliferation, survival and paracrine signaling in normal human
cardiac ventricular fibroblasts. J Mol Cell Cardiol. 105:59–69.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Garbayo E, Gavira JJ, de Yebenes MG,
Pelacho B, Abizanda G, Lana H, Blanco-Prieto MJ and Prosper F:
Catheter-based intra-myocardial injection of FGF1 or NRG1-loaded
MPs improves cardiac function in a preclinical model of
ischemia-reperfusion. Sci Rep. 6:259322016. View Article : Google Scholar
|
|
105
|
Díaz-Herráez P, Saludas L, Pascual-Gil S,
Simón-Yarza T, Abizanda G, Prósper F, Garbayo E and Blanco-Prieto
MJ: Transplantation of adipose-derived stem cells combined with
neuregulin-microparticles promotes efficient cardiac repair in a
rat myocardial infarction model. J Control Release. 249:23–31.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Bhagra SK, Pettit S and Parameshwar J:
Cardiac transplantation: Indications, eligibility and current
outcomes. Heart. 105:252–260. 2019. View Article : Google Scholar
|
|
107
|
Liu X, Gu X, Li Z, Li X, Li H, Chang J,
Chen P, Jin J, Xi B, Chen D, et al: Neuregulin-1/erbB-activation
improves cardiac function and survival in models of ischemic,
dilated, and viral cardiomyopathy. J Am Coll Cardiol. 48:1438–1447.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Gao R, Zhang J, Cheng L, Wu X, Dong W,
Yang X, Li T, Liu X, Xu Y, Li X and Zhou M: A Phase II, randomized,
double-blind, multicenter, based on standard therapy,
placebo-controlled study of the efficacy and safety of recombinant
human neuregulin-1 in patients with chronic heart failure. J Am
Coll Cardiol. 55:1907–1914. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Jabbour A, Gao L, Kwan J, Watson A, Sun L,
Qiu MR, Liu X, Zhou MD, Graham RM, Hicks M and MacDonald PS: A
recombinant human neuregulin-1 peptide improves preservation of the
rodent heart after prolonged hypothermic storage. Transplantation.
91:961–967. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Harvey RP, Wystub-Lis K, del Monte-Nieto
G, Graham RM and Tzahor E: Cardiac regeneration therapies-targeting
neuregulin 1 signalling. Heart Lung Circ. 25:4–7. 2016. View Article : Google Scholar
|
|
111
|
Caillaud K, Boisseau N, Ennequin G,
Chavanelle V, Etienne M, Li X, Denis P, Dardevet D, Lacampagne A
and Sirvent P: Neuregulin 1 improves glucose tolerance in adult and
old rats. Diabetes Metab. 42:96–104. 2016. View Article : Google Scholar
|
|
112
|
Pentassuglia L, Heim P, Lebboukh S,
Morandi C, Xu L and Brink M: Neuregulin-1β promotes glucose uptake
via PI3K/Akt in neonatal rat cardiomyocytes. Am J Physiol
Endocrinol Metab. 310:E782–E794. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Ennequin G, Boisseau N, Caillaud K,
Chavanelle V, Gerbaix M, Metz L, Etienne M, Walrand S, Masgrau A,
Guillet C, et al: Exercise training and return to a well-balanced
diet activate the neuregulin 1/ErbB pathway in skeletal muscle of
obese rats. J Physiol. 593:2665–2677. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Ennequin G, Boisseau N, Caillaud K,
Chavanelle V, Etienne M, Li X, Montaurier C and Sirvent P:
Neuregulin 1 affects leptin levels, food intake and weight gain in
normal-weight, but not obese, db/db mice. Diabetes Metab.
41:168–172. 2015. View Article : Google Scholar : PubMed/NCBI
|