1. Introduction
According to global statistical reports, 17.5
million individuals succumb to cardiovascular diseases (CVDs)
annually worldwide (1). In recent
years it has been found that dysfunctional microflora-dependent
intestinal metabolism is a risk factor for CVDs and is relevant to
the development of various angiocardiopathies, including stroke,
hypertension, atherosclerosis and myocardial dysfunction (2). With modern-day changes in living
conditions, CVDs are no longer considered geriatric diseases, as
they also affect younger age groups (3).
The pathogenic mechanisms underlying the development
of CVDs have both genetic and environmental elements. CVD is not a
single-factor disease, as it is often affected by multiple factors,
such as hyperglycemia (4),
hyperlipidemia (5) and hormonal
dysfunction (6), further
complicating the understanding of this disease. The clinical
methods for diagnosing and treating CVDs are continuously improved
and updated, and the association between intestinal flora and CVD
has become a research hotspot in recent years, with researchers
finding that anaerobic bacteria constitute the main intestinal
flora, with 500-1,000 recognized species to date (7). It has been found that imbalances in
intestinal flora are associated with various CVDs in humans, and
there is evidence that metabolites regulate the development of CVDs
through inflammation, apoptosis and other signaling pathways
(8,9).
Intestinal flora produces a number of metabolites,
but trimethylamine (TMA) and trimethylamine N-oxide (TMAO)
(10), bile acids (BAs) (11), short-chain fatty acids (SCFAs)
(12) and aromatic amino acids
(AAAs) (13) are considered the
most important for cardiac and vascular function in humans
(Fig. 1).
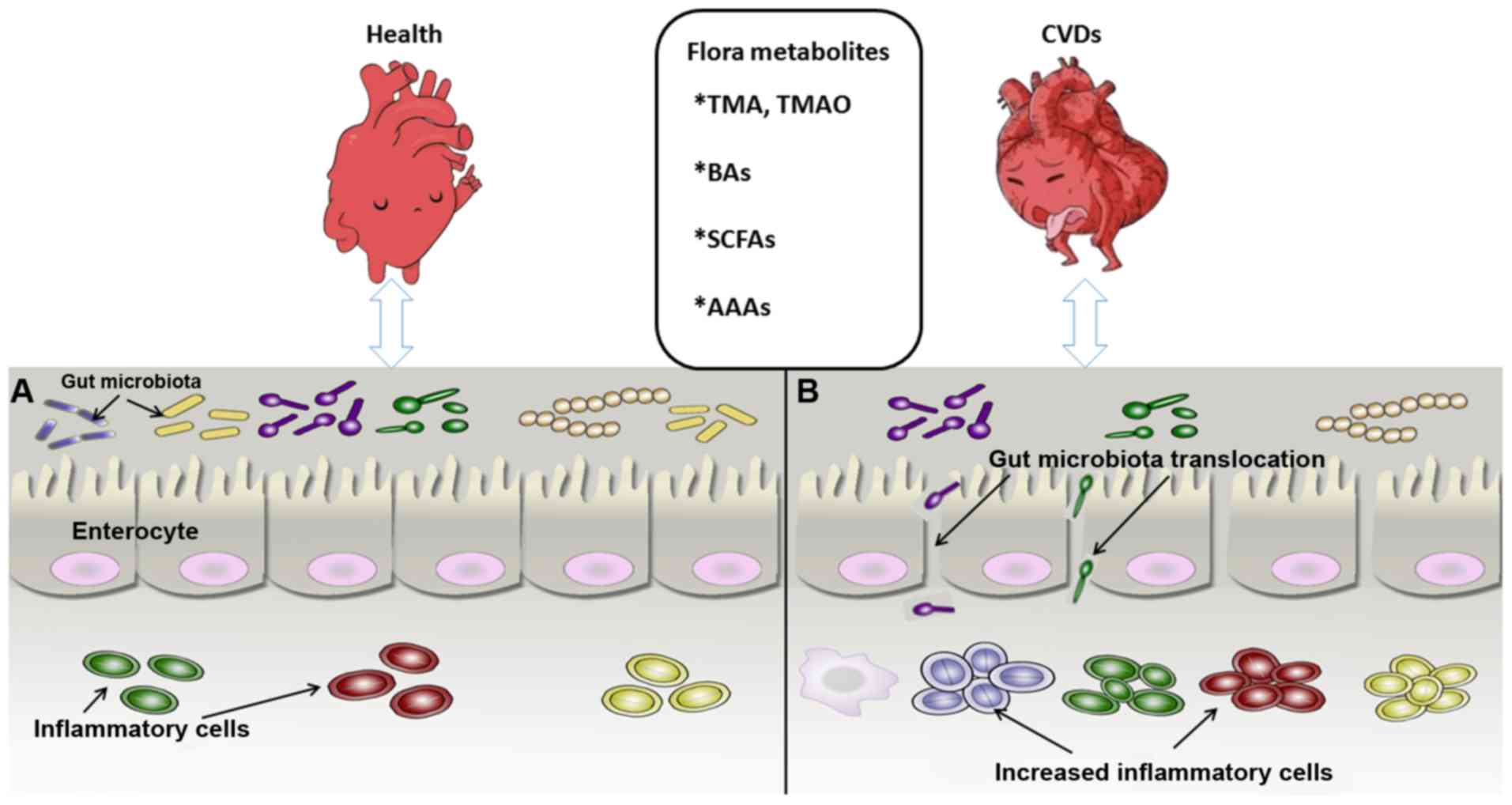 | Figure 1Association between intestinal flora
metabolites and CVDs. (A) Gut microbiota and cardiovascular system
in the healthy state. The microbial biomass increases, with the
inflammatory cells maintaining a normal defense function and the
intestinal epithelial cells remining tightly connected to maintain
the mucosal barrier. (B) Intestinal flora state under CVD
condition. The microbial biomass is reduced significantly and the
intestinal epithelium permeability increases, leading to
translocation of intestinal bacteria and marked proliferation of
inflammatory cells, which impairs intestinal mucosal barrier
function and may causes CVDs and metabolic syndrome, among others.
CVDs, cardiovascular diseases; BAs, bile acids; SCFAs, short-chain
fatty acids; AAAs, aromatic amino acids; TMA, trimethylamine; TMAO,
trimethylamine N-oxide. |
2. Association between gut microbiota
metabolites and angiocardiopathy
Angiocardiopathies, which include coronary
atherosclerotic cardiopathy, diabetic cardiomyopathy, heart
failure, stroke, hypertension and peripheral vascular disease, are
a group of diseases affecting the heart and blood vessels (14). Over the last few decades, dietary
and lifestyle modifications have been focused on lowering or
eliminating modifiable angiocardiopathy risk factors, such as
hypercholesterolemia, hyperlipidemia, hypertension, type 2 diabetes
mellitus, or smoking (15).
Consequently, although overall mortality from CVDs has declined
over this time period, angiocardiopathy remains the main cause of
death across the globe (16).
The digestive tract is colonized to different
degrees by intestinal microbes that play a key role in digestive
physiology and intestinal homeostasis in humans and other mammals,
and their role in metabolism is associated with carbohydrate and
protein digestion (17). The gut
microbiome functions like an endocrine organ, with colonic
microorganisms mediating the final stages of the digestive process,
which may impact host physiology (18). The intestinal tract continuously
absorbs the low-molecular-weight metabolites produced by intestinal
flora, which are transported to the liver for processing and
release into the systemic circulation. These metabolites act on
several sites in the body and may be beneficial and/or harmful to
the host by, for example, promoting or reducing the risk of CVDs
(19). Intestinal homeostasis
imbalance, immune dysfunction, environmental and other factors may
cause a number of diseases, and intestinal microflora plays an
important role in maintaining a healthy intestinal balance. Recent
studies indicated that gut dysbacteriosis may be associated with
the occurrence and development of atherosclerosis, myocardial
infarction, hypertension, diabetes and hyperlipidemia, and that
microbiota metabolites may play a protective or aggravating role in
angiocardiopathies (18,20). The majority of studies in the
field of metabolic diseases has been focused on the role of chronic
inflammation (21). Wang et
al (22) found that
intestinal flora participates in the etiopathogenesis of
cardiovascular and metabolic diseases, suggesting that the
microbiome may represent a target for the treatment of metabolic
diseases. However, the mechanisms and impact of intestinal flora in
the pathogenesis of disease and its complications have yet to be
fully elucidated.
3. Targeted regulation of CVDs by several
prime metabolites from gut microbiota
The gut flora may be subdivided into three broad
categories: Beneficial, harmful and neutral bacteria (23). The gut microbiota participates in
host metabolism by interacting with host signaling pathways, such
as the TMA or TMAO, SCFA, primary and secondary BA and
phosphatidylcholine pathways (10,24). The metabolites mentioned above may
be pro-inflammatory, or protective and anti-inflammatory, or play a
largely unknown biological role. Therefore, it is important to
identify new factors implicated in the occurrence and development
of diseases associated with the effect of gut microbiota on such
pathways. Hence, the focus of the present review was our current
knowledge on the three most extensively investigated metabolites
produced by intestinal flora.
Associations between choline, TMA, TMAO
and cardiovascular risk factors
Choline is the precursor of phosphatidylcholine,
sphingomyelin, acetylcholine and betaine, and also participates in
signaling and lipid transport, one-carbon metabolism,
neurotransmission and membrane structure (25). Of note, choline,
phosphatidylcholine and carnitine are metabolized by intestinal
flora to produce TMA, which is transformed in the liver into TMAO
by flavin monooxygenase 3 (FMO3) (26). TMAO, a small quaternary amine that
directly induces conformational changes in proteins, stabilizes
protein folding and acts as a small molecular protein chaperone
(27), whereas TMA can affect
signal transduction by directly interacting with a family of GPRs.
More importantly, it can upregulate the scavenger receptors CD36
and SRA on the surface of macrophages, thereby promoting the
accumulation of cytoplasmic cholesterol and accelerating the
development of atherosclerosis (28,29). TMAO may accelerate platelet
activation and inflammation, the levels of which are increased in
atherosclerosis and associated complex CVDs (30).
Brown and Hazen discovered that
atherosclerosis-prone mice fed choline- and TMAO-rich diets
exhibited accelerated development of atherosclerosis and
cholesterol metabolism disorders (31). The main mechanism by which TMAO
promotes atherosclerosis is the reverse transport of cholesterol
and its catabolism, and atherosclerotic plaques containing large
amounts of bacterial DNA have been confirmed by autopsy (32,33). This evidence suggests that the
chronic inflammation caused by intestinal flora promotes the
formation of atherosclerotic plaques. Clinical cohort studies have
demonstrated that in vivo choline, TMAO and betaine levels
may be used as predictors of short- and long-term malignant
cardiovascular events (31).
However, Winther et al (34) analyzed 1,159 patients with type 1
diabetes and found that high plasma TMAO concentrations increased
the risk of cardiovascular events, renal vascular disease and
mortality in these patients, indicating that TMAO damages the
micro- and macrovasculature, leading to renal failure (22). In recent years, researchers have
come to realize that several of the pathways involved in
inflammation, apoptosis, pyroptosis and autophagy are involved in
regulating intestinal flora and its metabolites, which may have a
positive or negative impact on the body. In mouse experiments, Li
et al observed that TMAO can activate the toll-like receptor
4 (TLR4)-NLR family pyrin domain containing 3 (NLRP3)-transforming
growth factor-β (TGF-β) signaling pathway in vivo as well as
in vitro, leading to an increase in the production of
reactive oxygen species, inflammatory cytokines and collagen
deposition, and resulting in severe cardiac fibrosis and cardiac
dysfunction (35). TMAO also
induces proliferation, migration and collagen synthesis in cardiac
fibroblasts. Possible mechanisms underlying the role of TMAO in
angiocardiopathy were proposed recently: TMAO may be involved in
homeostatic balance in the endoplasmic reticulum, as shown by an
experiment where overexpression of the green fluorescent
protein-tagged iodide transporter pendrin caused endoplasmic
reticulum perturbation (36). The
endoplasmic reticulum stress kinase (PERK) is recognized by TMAO
and stimulates the PERK embranchment of the unfolded protein
response, causing dysfunction of glucose metabolism; hence,
targeting the TMAO-PERK pathway may improve metabolic disorders
(37). Moreover, TMAO has been
shown to accelerate endothelial cell dysfunction by activating the
protein kinase C (PKC)/nuclear factor-κB (NF-κB)/vascular cell
adhesion molecule 1 (VCAM-1) pathway during the pathological
process of atherosclerosis (38).
Therefore, altering the abundance of intestinal flora and
inhibiting the production of TMAO may be of value in preventing and
treating CVDs.
Interaction of gut microflora with BA
metabolism and its impact on disease states
BAs are synthesized in the liver from cholesterol
via the classic and alternative pathways (39) (Fig.
2), and they are recycled mainly through the enterohepatic
circulatory system (40). The
classic pathway undergoes a series of reactions to produce diols
and triols, which are carboxylated by mitochondrial CYP27A1 to
produce cholic acid (3α,7α,12α-trihydroxy-5β-cholic acid; CA) and
chenodeoxycholic acid (3α,7α-dihydroxy-5β-cholic acid; CDCA), the
latter of which is the primary BA formed in the human body. These
two primary BAs combine with taurine or glycine to form secondary
BAs, which are subsequently excreted into the bile. Alternating
hydroxylation reactions produce primary BAs with different
structures, particularly α-, β- and γ-polyphenolic acids.
Therefore, under the action of intestinal flora, the chemical
diversity of the BA pool in the body increases, and CA and CDCA
produce two major secondary BAs, deoxycholic acid
(3α,12α-dihydroxy-5β-cholic acid; DCA) and lithocholic acid
(3α-hydroxy-5β-cholic acid; LCA), respectively. CYP7A1, a
microsomal cytochrome P450 isozyme, catalyzes the first and
rate-limiting steps of this pathway. Nuclear receptors and genes
regulated by BAs/oxysterol are potential targets for the treatment
of cardiovascular and liver diseases and the reduction of
triglyceride and cholesterol levels (40,41). The bile stored in the gallbladder
is stimulated for release into the intestine postprandially
(42). Intestinal flora
modulation of BAs is pivotal to producing DCA and LCA (43). Most BAs are reabsorbed at the end
of the ileum and upper colon by the apical sodium-dependent BA
transporter and the ileal BA transporter in the intestinal cells
(44). BAs play vital roles in
lipid and glucose metabolism and energy consumption, and have also
emerged as newly identified metabolic regulators of signaling
molecules (45).
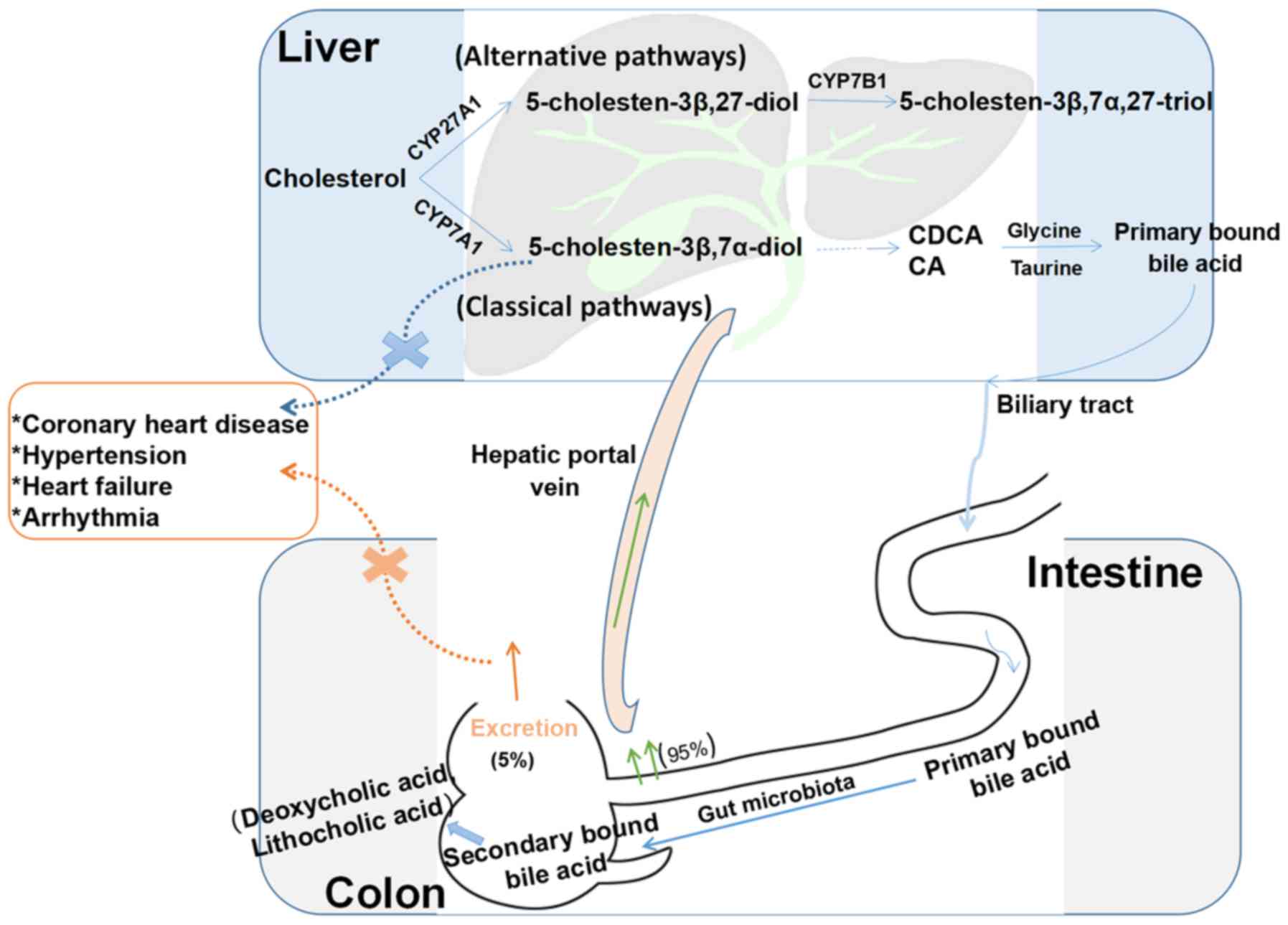 | Figure 2Enterohepatic circulation of BAs. BAs
are synthesized from cholesterol in the liver through classic and
alternative pathways. The classic pathway includes a series of
reactions to produce diols and triols, which are carboxylated by
mitochondrial CYP27A1 to produce the primary BAs CA and CDCA. These
two BAs combine with taurine or glycine to form primary conjugated
BAs, which are then secreted from the biliary tract into the
intestinal cavity and metabolized by the intestinal flora to form
secondary BAs (deoxycholic acid and lithocholic acid).
Approximately 95% of BAs are reabsorbed in the terminal ileum and
ascending colon, and approximately 5% are excreted in the feces.
The alternative pathway includes a series of enzymatic reactions to
form 5-cholesten-3β,7α,27-triol. When 5-cholesten-3β,7α-diol
production disorders or secondary BA excretion disorders occur,
they may lead to coronary heart disease, hypertension and other
cardiovascular diseases. BAs, bile acids; CA, cholic acid; CDCA,
chenodeoxycholic acid. |
Gut bacteria affect BA synthesis by regulating the
expression of the farnesoid X receptor (FXR) and G protein-coupled
receptors (GPRs) (46). Numerous
experiments have confirmed that FXR is a BA receptor that is
expressed in the aorta and cardiomyocytes. BAs may compromise cell
membrane and DNA integrity, and/or induce protein degeneration and
inactivation (47). BAs inhibit
the growth of intestinal flora through the FXR target system
(48), and FXR plays a major role
in protecting the distal small intestine from bacterial invasion.
Activation of FXR may prevent translocation of intestinal flora. It
was previously reported that the total BA (TBA) levels in the serum
of patients with hypertension are directly proportional to the
severity of the disease (41).
BAs may be involved in the occurrence and progression of
hypertension through 11β-hydroxysteroid dehydrogenase (11β-HSD)
(49). This enzyme can rapidly
inactivate cortisol and prevent excessive cortisol and aldosterone
from competing for mineralocorticoid receptors. However, when the
expression of 11β-HSD is deficient or suppressed, blood pressure
(BP) may increase (50). For
patients with coronary atherosclerotic disease caused by a high
cholesterol diet (51), BA
excretion disorders may significantly reduce the excretion of TBAs,
DCA and LCA (52). GPR5 is an
intracellular receptor for BAs, and intestinal flora can inhibit
the synthesis of BAs by activating GPR5 expression. Therefore, BAs
and their derivatives activate the anti-atherosclerotic effect of
FXR. Thus, the BA content in plasma may be used as a predictive
factor for CVD risk (24). In
vitro electrophysiological experiments have confirmed that
different BAs exert various effects on cardiomyocytes, mainly by
altering the concentrations of sodium, potassium and calcium.
Hydrophilic BAs (e.g., ursodeoxycholic acid) may play a role in
stabilizing the cell membrane potential and preventing arrhythmia
(53). Lipophilic BAs readily
induce changes in membrane potential, exert toxic
electrophysiological effects on cell membranes, and facilitate the
occurrence of arrhythmias (54).
It was also found that cardiomyocyte apoptosis occurs after free
BAs activate FXR and caspase-9/caspase-3, regulate BCL-2/BAX
expression, cause mitochondrial dysfunction, and ultimately lead to
ischemia-reperfusion injury in myocardial cells (55). Certain antibodies can reduce TMAO
synthesis by blocking the choline-TMA pathway, thereby reducing the
incidence of atherosclerosis (56). The interaction between BAs and gut
intestinal flora exerts important regulatory effects on CVDs, and
may prove useful in guiding treatment.
Crosstalk between gut microbiota and
SCFAs, and modulation of the mechanisms involved in CVDs
Fatty acids (FAs), which are carboxyl-containing,
long-chain aliphatic hydrocarbons, are a staple component of
glycolipids, phospholipids and neutral fats. Obesity caused by a
high-fat diet (HFD) increases the risk of CVD; however, the type of
FA consumed has a more pronounced effect on the disease (56). In fact, the levels of eight
metabolites in the intestinal flora are known to undergo
significant changes with such diets; these include
ethylmethylacetic acid, butyric acid, valeric acid, palmitic acid,
stearic acid, arachidonic acid, indole and indoleacetic acid, the
latter of which increases sputum production. Stearic and palmitic
acids, which are the main saturated FAs, are believed to stimulate
inflammatory signaling by macrophages and readily cause chronic
vascular inflammation in vivo and in vitro (51,57).
According to the data released in 2015 relating to
medical office BP measurements, hypertension has a global
prevalence of 1.13 billion and 150 million cases in Central and
Eastern Europe, respectively. In recent years, studies (58) have found that dysbiosis of the
intestinal flora affects BP stability. Metabolites from microbial
flora, such as SCFAs and TMAO, are implicated in the pathogenesis
of CVD. Therefore, it has been suggested that the metabolic
products from microflora may be used as targets to treat
hypertension (58). SCFAs, which
are mainly produced by anaerobic bacteria, such as
Lactobacillus and Bifidobacteria, are an important
energy source for intestinal microflora and the intestinal
epithelial cells of the host, and can inhibit the growth of harmful
bacteria, maintain the acid-base balance in the intestinal tract,
reduce inflammatory responses, and regulate the host's intestinal
immunity. Butyric acid, acetic acid and propionic acid constitute
90% of the intestinal SCFAs (59). They are absorbed into the blood
circulation, providing energy for the heart, brain, muscles and
kidneys, and affect the transport and metabolism of epithelial
cells (60). Two studies have
reported that some Anaerostipes and other eubacterial
species utilize D- and L-lactate to generate acetate, propionate
and butyrate (15,17). Experiments have revealed that
SCFAs can regulate the immune response by activating GPRs and
inhibiting histone deacetylases (HDACs), as well as partially
suppressing inflammation. GPR is expressed in almost all immune
cells, and SCFAs mainly activate GPR41, GPR43 and GPR109 (61). It was recently discovered that, in
type 2 diabetes, the intestinal metabolite sodium butyrate (NaB)
maintains blood glucose homeostasis and expedites glycogen
metabolism by adjusting the GPR43-protein kinase (Akt)-glycogen
synthase kinase (GSK) 3 signaling pathway, and the results from
experiments in db/db mice have shown that NaB administration can
reduce glycogen staining in cells and improve hepatic lobular
fibrosis and steatosis, thereby decreasing the damage to systemic
blood vessels caused by hyperglycemia (15). HDACs are a group of proteins
involved in modifying chromosome structure and regulating gene
expression. The degree of deacetylation of Foxp3 is affected by
HDAC9, which is prone to Foxp3 degradation. SCFAs may also be used
by the body through HDAC inhibition to enhance histone acetylation
of Foxp3, promote the differentiation of T cells into effector and
regulatory T cells, and inhibit the inflammatory response (62). Moreover, SCFAs have been shown to
affect olfactory receptors in blood vessels and kidneys, thereby
affecting BP (63). A
cross-sectional study on BP and intestinal flora in hypertensive
subjects revealed that some SCFA-producing microbes
(Lachnospiraceae, Bacteroides plebeius and
Bacteroides coprocola) were more highly abundant, while
others (Roseburia hominis, Faecalibacterium
prausnitzii, Ruminococcaceae NK4A214,
Christensenellaceae_R-7 and Ruminococcaceae_UCG-010)
were less abundant (58). These
species are positively correlated with systolic and diastolic BP.
The same study reported that the level of SCFAs in the plasma was
low, whereas the level of fecal SCFAs was significantly higher,
indicating that the intestinal SCFA absorption rate is low
(58). The SCFA content is
positively correlated with Clostridium, Lactobacillus
and Blautia XlVa levels, whereas acetic acid, propionic acid
and butyric acid levels are inversely proportional to the abundance
of Streptococci, Enterococci and other bacteria
(22).
Thus, it appears that SCFAs can enhance the
abundance of intestinal flora and improve the numbers of beneficial
flora, so as to maintain the metabolic balance of sugars, lipids
and proteins and reduce the occurrence of CVDs, diabetes and
hypertension.
Gut microbiota-derived AAAs and types of
angiocardiopathy
AAAs, such as tyrosine (Tyr), tryptophan (Trp) and
phenylalanine (Phe), are benzene ring-containing amino acids. Among
them, Trp and Phe must be obtained from the diet, whereas Tyr may
be obtained by Phe hydroxylation in the human kidney and liver
(64). Dietary proteins, such as
fish, beef, pork and chicken, are the main sources of AAAs
(65). Tyr and Trp are the
precursors of a large number of bioactive molecules: Dopamine,
epinephrine, norepinephrine and thyroid hormone are derived from
the former, and serotonin, melatonin and niacin from the latter
(66,67).
Dodd et al (45) characterized a pathway whereby the
metabolites of AAAs were generated by the gut symbiont
Clostridium sporogenes, and all three AAAs were used as
substrates in this pathway. The authors demonstrated that
Clostridium sporogenes, a type of intestinal bacteria from
the Firmicutes phylum, can regulate AAA metabolism through
the fldC gene and metabolize dietary Trp into indolepropionic acid
(IPA), which accumulates in the serum and exhibits wide differences
between individuals. IPA affects intestinal epithelial permeability
and systemic immunity by acting directly on the progesterone X
receptor (45).
Shishehbor et al determined the levels of
3-nitrotyrosine in the plasma from patients with coronary heart
disease via stable isotope-dilution high-performance liquid
chromatography and on-line electrospray ionization tandem mass
spectrometry (67). They found
that modified apolipoprotein A-1, apolipoprotein B-100, fibrinogen
and nitrated Tyr residues promote atherosclerosis, which may be a
risk factor for thrombosis (68).
Metabolites from AAAs (such as Trp) inhibit inflammation by
upregulating the aryl hydrocarbon receptor (AhR) in astrocytes or
T-cells; however, excessive intake of foods containing long-chain
FAs is likely to affect the production of metabolites (69). AhR is a hydrocarbon with a benzene
ring structure and, as a ligand-induced transcription factor, it is
expressed in both immune and epithelial cells (70). It is known that Trp is an
essential amino acid that it is metabolized by intestinal flora to
produce immunoregulatory products that can bind to AhR. Among
these, Trp-positive bacterial species of Lactobacillus can
produce various indole metabolites from dietary Trp, activating AhR
and inhibiting inflammation (71). Indoleamine 2,3-dioxygenase is an
enzyme induced in numerous types of immune cells (e.g.,
macrophagocytes) in response to inflammatory stimuli, thereby
facilitating the degradation of Trp along the kynurenine pathway;
however, when the activity of this enzyme is high, it may worsen
angiocardiopathy and accelerate vascular inflammation and
atherosclerosis (72).
Additionally, obesity is known to be associated with an increase in
enteric canal indoleamine 2,3-dioxygenase activity (73). When this enzyme is inhibited or
the gene encoding it is knocked out, insulin sensitivity improves,
the intestinal mucosal barrier is preserved, and endotoxemia and
chronic inflammation improve, while lipid metabolism in adipose
tissues and the liver is regulated (73). Therefore, the products of AAA
catabolism extracted from the intestinal flora may be used as
biomarkers for imbalances in intestinal flora, an may also serve as
targets for intestinal flora development (74). Hoyles et al performed
rodent and human hepatocyte experiments to combine metagenomic
sequences with metabolomics data to identify the mechanism(s)
through which AAAs exert their biological effects. A direct role
for gut-derived degradation of AAAs from microorganisms in
peripheral arterial disease into phenylacetic acid has been shown
to involve increased branched-chain amino acid (BCAA) utilization
and AAA metabolism to promote steatosis. Circulating fat metabolism
indicators support BCAA metabolism and microbial metabolism of AAAs
such as Phe, Tyr and Trp in liver steatosis (75).
4. Treatment of CVDs based on intestinal
microflora and their metabolites
Intestinal microbes are used to study the
pathogenesis and determine the direction of treatment for CVDs.
Research in this area has focused on fecal transplantation, the
antibacterial effect of antibiotics, probiotics and prebiotics, but
systematic research in this area remains relatively scarce. The
treatment options and methods focusing on physical activity and
dietary regulation are shown in Fig.
3.
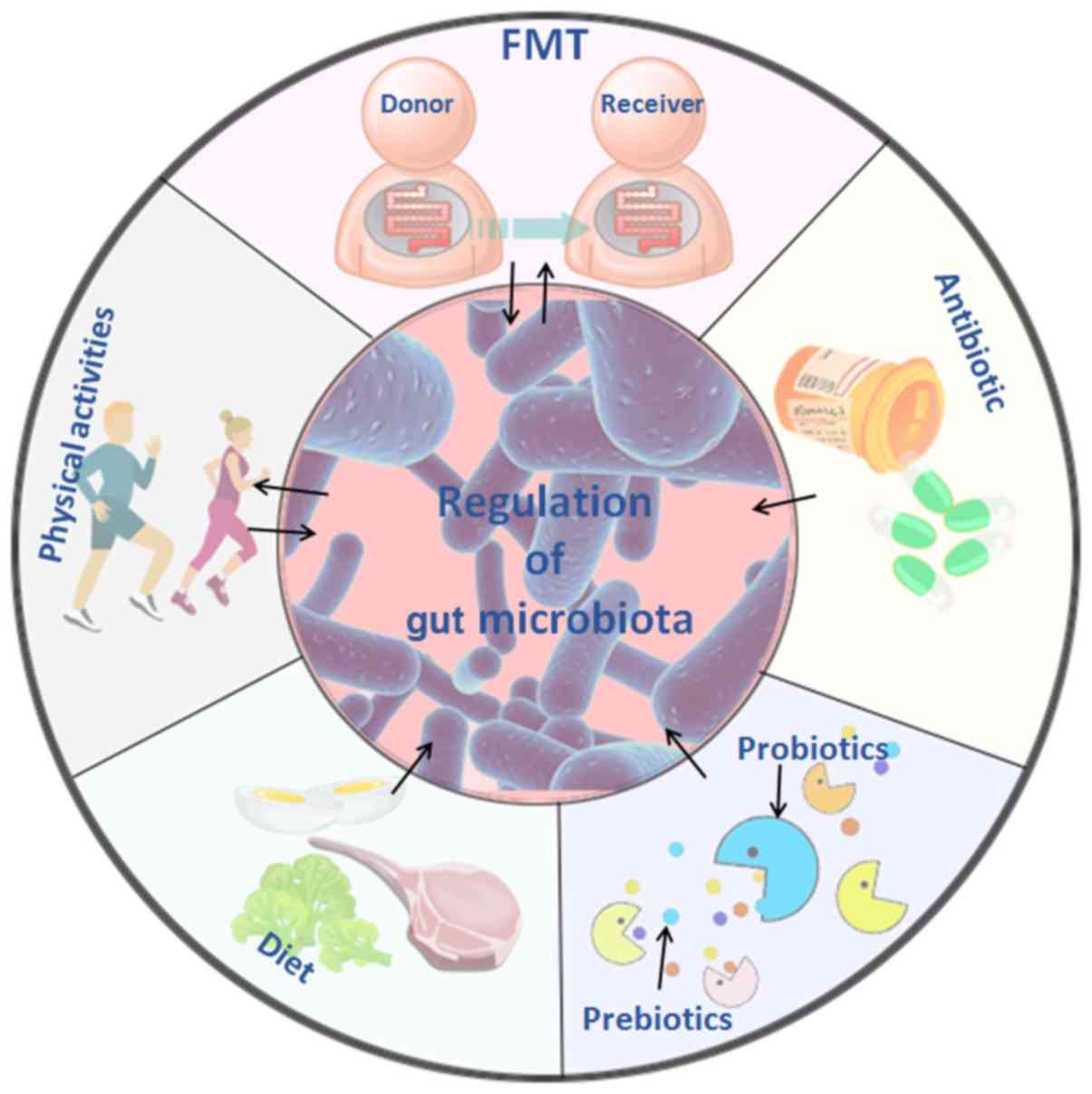 | Figure 3Treatment of cardiovascular diseases
based on intestinal microflora and its metabolites. Five treatment
methods that are closely associated with intestinal flora are
depicted: FMT involves transplanting the intestinal flora of
healthy individuals into the patient, and it may delay or prevent
developing the disease through the effects of the flora or its
metabolites. The use of antibiotics may alter the structure and
abundance of intestinal flora, inhibit the proliferation of harmful
flora, and improve the progression and prognosis of cardiovascular
diseases to a certain extent. Probiotics, such as
Bifidobacterium species and lactic acid bacteria species
temporarily colonize the intestine, and change the intestinal
environment by affecting cardiovascular disease-related pathways
and metabolites; prebiotics can selectively stimulate the growth of
predominant bacteria in the intestine, improve glucose and lipid
metabolism and cooperate with probiotics improve the gut
microbiome. Changes in diet composition and habits lead to changes
in the metabolic function of intestinal flora, and the reduction of
harmful metabolites, such as TMAO, may reduce the incidence of
cardiovascular disease. Physical activities of different types and
intensities affect the abundance and metabolites of intestinal
flora, increase the abundance of beneficial flora, and reduce the
high-risk factors that cause cardiovascular disease. TMAO,
trimethylamine N-oxide; FMT, fecal microbiota transplantation. |
Approaches and rationale for the use of
fecal microbiota transplantation (FMT)
FMT has been a hot research topic in recent years,
and new discoveries have been made in disease treatment and the
mechanisms regarding its use. FMT refers to the transfer of
microbial communities from healthy donors to patients and has
become an effective treatment option for chronic diseases (76). Studies have shown that the
composition of the flora in the intestine may be associated with
CVDs such as atherosclerosis, hypertension and heart failure. An
imbalance in intestinal flora may cause metabolic disorders and the
release of endotoxins, which may ultimately exacerbate the
occurrence and development of CVDs (77).
In a cohort study using FMT from patients to
germ-free mice (78), it was
observed that, compared with healthy controls, microbial richness
and diversity were markedly decreased, and that the
Prevotella genus dominated the gut enterotype, whereas
reduced numbers of Prevotella bacteria were associated with
a healthy status, and overgrowth of bacteria such as
Prevotella and Klebsiella was observed in metagenomic
and metabolomic analyses (79). A
number of microbial CVD-specific biomarkers have been identified,
and fecal microbiome-targeted strategies are currently considered
as a powerful tool for early diagnosis and treatment of different
diseases. More importantly, through fecal transfer experimentation
and gut microbiota remodeling, the intestinal microbiome has been
confirmed to be involved in the pathogenesis of multiple diseases,
such as obesity, depressive disorders, chronic ileal inflammation,
liver diseases and atherosclerosis (63). A previous study indicated that
there are clinical and mechanistic links between atherosclerotic
heart disease and the microbial metabolism of certain dietary
nutrients producing intestinal TMAO, and verified that intestinal
microbial transplantation may enhance choline-induced TMAO
production and susceptibility to atherosclerosis (78). The findings mentioned above
indicate that, when intestinal flora from a subject with CVD is
transplanted into a healthy organism, it may result in the
development of angiocardiopathy, thus confirming that alterations
in intestinal microflora play a pivotal role in driving the
development of CVDs (21,63). FMT from patients with type 2
diabetes to germ-free mice revealed that the microbiome-induced
modulation of the dipeptidyl peptidase-4 inhibitor (DPP-4i)
contributed to its hypoglycemic effect, and that the DPP-4i-altered
microbiome improved glucose tolerance in the colonized mice, while
acarbose did not (80). Moreover,
DPP-4i increased the abundance of Bacteroidetes, and also
promoted a functional shift in the gut microbiome, particularly by
increasing the production of succinate (80). This suggests that transplanting
the gut flora from a diseased into a healthy organism may promote
disease development and that, in turn, transplanting the gut flora
from a healthy into a non-healthy organism may alleviate or even
cure the disease.
Antibiotics influence the effect of
intestinal flora and related metabolites on CVD
Antibiotics have been used clinically to treat
microbial infections, and their long-term use, misuse, or abuse are
associated with a variety of side effects in the recipients. Wan
et al (81) reported that
the early administration of antibiotics or FMT may reduce the
oxidative catabolism of FAs and amino acids in newborn piglets. The
synthesis of such acids may represent a point of reference for
regulating host metabolism.
Lam et al detected intestinal flora
metabolites by mass spectrometry after feeding rats with
broad-spectrum antibiotics, and found that the catabolism of AAAs
was affected the most. Furthermore, antibiotic use has been shown
to reduce the incidence of myocardial infarction in vivo,
indicating that the intestinal flora affects the severity of
myocardial infarction in rats. In addition, vancomycin protects the
heart by activating Janus kinase-2, Akt/phosphoinositide 3-kinase,
p44/42 mitogen-activated protein kinase (MAPK) and p38 MAPK
pathways and KATP channels (19). Antibiotics can inhibit the
metabolism of L-carnitine into TMA in intestinal flora, thereby
reducing the synthesis of TMAO, reducing its stimulation of
macrophages and the vascular endothelium, and decreasing the
incidence of atherosclerosis (82).
A direct association between intestinal flora and
the severity of myocardial infarction induced in rats has been
highlighted by statistical analysis (83). In addition, Le Roy et al
(84) used spectral antibiotics
to inhibit the intestinal flora in ApoE−/− mice with
hypercholesterolemia. The authors found that intestinal flora in
the mice exhibited a significant correlation with cholesterol
regulation. Furthermore, samples of intestinal flora from patients
with hypercholesterolemia were transplanted into experimental mice,
which induced a plasma cholesterol phenotype in these mice. Some
researchers have evaluated the role of intestinal flora
transplantation as a complementary therapy for obesity, and found
that it may reverse the effects of antibiotics and re-establish
balance in the microbiome, thus restoring normal function and
improving the diversity of the microbiome (85). Experiments have shown that
controlling plasma cholesterol levels is key to preventing CVDs,
and that gut flora can determine the levels of circulating
cholesterol. This may provide a new method for supporting health
and dietary habits as a first choice for maintaining homeostasis in
the microbiome (86). In another
study, antibiotic therapy has been shown to alleviate glucose
intolerance, inflammation and liver steatosis caused by HFD, along
with reduced liver lipogenesis and increased heat production in
subcutaneous white adipose tissue (43). The diversity of intestinal flora
in HFD mice and mice treated with antibiotics in their drinking
water was significantly reduced, altering the function of the flora
in the host (79).
Of note, antibiotic treatment may change the
absorption rate, BA pool and bioavailability of drugs (87). The use of antibiotics to treat
CVDs remains controversial due to their long-term side effects, and
their use may decrease intestinal flora abundance and promote
antibiotic resistance.
The cardiovascular system, probiotics,
prebiotics and intestinal flora
Probiotics are a class of active microorganisms that
are beneficial to the host. After colonizing the human body, they
alter the composition of the flora in the host to a certain degree
(88) by promoting nutrient
absorption and maintaining intestinal health by regulating host
mucosal and systemic immune function or by regulating the balance
of the intestinal flora (89).
Probiotics include bacteria and yeast that act in the small and
large intestine. Most probiotics include certain strains of
Escherichia coli, Bifidobacteria species,
Lactobacillus species, Lactococcus lactis,
Streptococcus species and some Enterococcus species,
with Saccharomyces boulardii being the most commonly used
type of yeast. Based on the fecal persistence of the ingested
strain, probiotics appear to temporarily settle in the gut and act
by altering the colonic environment. Probiotics work in different
ways through direct or indirect regulation in the host. First, they
enhance the barrier function of the gastrointestinal tract through
the tight junction proteins of the intestinal epithelium and the
mucins secreted by goblet cells. Second, some probiotics can
produce 'bacteriocin', SCFAs and other antimicrobial factors that
inhibit pathogen growth. Third, probiotics regulate the phenotype
and activity of T cells, natural killer cells and macrophages, and
reduce proinflammatory factor release by regulating the NF-κB
pathway (90).
Prebiotics are non-digestible but fermentable
dietary supplements that can improve the host's health by
selectively stimulating the growth and activity of one or more
dominant colonies already present in the colon, but are not limited
to Lactobacillus and Bifidobacterium (91). A separate meta-analysis of a
randomized clinical trial demonstrated that the intake of
probiotics and prebiotics in foods or supplements significantly
improved blood glucose levels, insulin levels and insulin
resistance (92). Resveratrol
(RSV) has been found to significantly regulate the growth of
certain intestinal flora, including increasing the ratio of
Bacteroidetes to Firmicutes, as well as the growth of
Bacteroides, Lactobacillus and
Bifidobacterium. This indicates that RSV may be a good
candidate as a prebiotic that may be used to promote the growth of
flora that can confer health benefits to the host (56). Galactooligosaccharides (GOS),
which are common prebiotics, may participate in the regulation of
lipid metabolism and are beneficial to the intestinal flora. In
experimental studies on Sprague-Dawley rats, administration of GOS
and fucoidan (a complex polysaccharide) improved dyslipidemia and
exerted a positive effect on total cholesterol and triglyceride
levels in overweight adults, and reduced hepatic steatosis and
aortic arch atheroma formation (93). GOS may also reduce total serum
cholesterol by regulating the microbiome in infants (93). Guar gum, which is also a type of
prebiotic, may change the composition of intestinal flora in rodent
models of HFD, thereby reducing diet-induced obesity and improving
glucose tolerance, but it may worsen the liver phenotype, leading
to increased inflammation and fibrosis in a HFD model (23). Probiotics or prebiotics may be
used as therapeutic agents for BA-related metabolic disorders,
suggesting that microbiome-based therapies may be effective in
preventing and/or treating intestinal-related diseases, including
atherosclerosis (94).
Probiotics and prebiotics also improve the
accumulation of toxins produced by harmful intestinal bacteria in
the body by regulating the abundance of intestinal bacteria and the
interactions between symbiotic bacteria. In the clinical setting,
there are few reliable statistical analyses on the safety and side
effects associated with the use of probiotics and prebiotics;
therefore, the medicinal value of microbial therapy requires
further investigation.
Physical activity may positively modulate
gut microbiota in CVDs
Proper physical activity is known to boost
metabolism and immunity. However, with the increasing scope of
research in the field of intestinal flora, it is suspected that
exercise affects the regulation of intestinal flora and the extent
to which intestinal flora impacts disease prevention and
progression. Maintaining homeostatic balance in the intestinal
flora is now considered to support the health of the host. It has
been demonstrated that physical activity can independently reduce
the risk of coronary heart disease and other CVDs (94), such as hypertension,
hyperlipidemia, obesity and diabetes (95,96). Vascular wall shear stress during
exercise improves endothelial cell function and can lower serum
C-reactive protein levels (97).
Aerobic capacity, which is expressed as peak oxygen
consumption (VO2 peak), is the gold standard used for
predicting cardiovascular health and all-cause mortality, even for
patients who already have various CVDs and coronary risk factors
(98). Cardiopulmonary fitness is
associated with increased intestinal flora diversity in healthy
individuals, and the intestinal flora of hypertensive patients is
significantly imbalanced with a reduced abundance of certain flora.
Among these microbes, opportunistic genera, such as
Streptococcus and Klebsiella, are common, whereas
others, such as Clostridium tenella, are more common in
healthy individuals. Although the intrinsic mechanism through which
intestinal flora regulate BP remains unclear, SCFAs play a key role
in the pathogenesis of hypertension. SCFAs produced by the
intestinal flora regulate BP through Olfr78 (an olfactory receptor)
and Gpr41 (99). High-throughput
16S rDNA sequencing analysis of intestinal flora uncovered that,
when healthy individuals exercised, their Bacteroides and
Bifidobacteria levels and SCFAs increased significantly
(83), helping to maintain the
dynamic balance of the intestinal immune system and, to a certain
extent, maintaining BP stability (95).
Different exercise intensities differ in their
effects on intestinal flora. Bernardo et al used moderate
exercise intensity to study the intestinal flora of obese and
hypertensive rats. Fecal samples from the rats were collected
before and after exercise for 16S rRNA detection, and the results
revealed that exercise changed the composition and diversity of all
the intestinal microorganisms at the genus level.
Streptococcus and Aggregatibacter were more abundant
before exercise, whereas Allobaculum, Pseudomonas and
Lactobacillus were more abundant after exercise, among which
Lactobacillus was the most abundant (100). Therefore, it is not surprising
that diet restriction and increased physical activity as an
intervention for obese individuals resulted in a greater abundance
of intestinal Bacteroides after weight loss (101). Controlling intestinal flora
diversity may be another approach to reducing obesity.
There are differences in the intestinal flora
between those subjected to voluntary or forced exercise, and
exercise intensity may be a variable that affects the function of
intestinal flora. High-intensity intermittent exercise increases
the diversity of microorganisms in the distal intestine and in the
feces and the ratio of Bacteroides to thick-walled flora,
while also enhancing the tricarboxylic acid metabolic loop during
diet-induced obesity (100). In
a previous study on the effect of intestinal flora on exercise
capacity, serum-free glutathione peroxidase levels in the livers of
mice without specific pathogens were higher compared with those in
sterile mice, and the time of endurance swimming was longer
compared with that of sterile mice (102,103). Other studies have demonstrated
that different amounts of exercise differ in their effects, and
exercise intensity may alter the composition of the intestinal
flora, such that regular physical exercise exerts an
anti-inflammatory effect, leading to patterns of increased
anti-inflammatory cytokine and/or decreased pro-inflammatory
cytokine levels (96,104,105).
Exercise, an independent factor affecting the
composition and diversity of intestinal flora among external
environmental stressors, improves the body's metabolism and immune
system. At present, there are few systematic studies on the effects
of specific factors, such as exercise intensity, quantity, time and
mode, on the intestinal flora. The specific regulatory mechanism of
exercise on the intestinal flora awaits further study.
Effects of dietary habits on risk factors
for CVD
The improvement in living standards has been
accompanied by poor eating habits, such as the excessive intake of
high-fat, high-cholesterol and high-salt diets, as well as tobacco
and alcohol consumption, which are known causes of angiocardiopathy
(106). It has been demonstrated
that the benefits of a healthy diet are enhanced by the
anti-inflammatory effects of SCFAs and other bioactive products
produced by the intestinal flora (51). A healthy diet is conducive to the
optimal functioning of the gastrointestinal tract and the
composition of its microbial flora (107). Epidemiological investigations
have demonstrated that Western diets are high in sugar and fat and
lack sufficient dietary fiber, all of which are associated with an
increased risk of obesity and CVDs (108). In addition, recent research from
Tindall et al indicated that consuming FAs, bioactive
compounds and the fiber in walnuts improved cardiovascular risk
factors by regulating the abundance of intestinal flora (109). Moreover, it was reported that
diindolylmethane and indole-3-methanol from cruciferous plants can
reduce plasma TMAO levels by inhibiting flavin-containing
monooxygenase, thereby preventing the development of CVDs.
Therefore, the consumption of purple cabbage and broccoli may
reduce TMAO levels and alleviate its adverse effects on the body
(36). The evidence mentioned
above supports the concept that a healthy diet containing fruits
and vegetables may greatly reduce the risk factors for CVD.
5. Conclusion
Under normal conditions, intestinal flora is
dynamically balanced to maintain health (Fig. 4). When the bodily environment,
external environment and dietary factors change, causing an
imbalanced flora, disease development may occur. As mentioned in
this review, TMA, choline and L-carnitine are metabolites produced
by intestinal flora via oxidation of FMO3 and TMAO; however,
increased plasma TMA and TMAO levels exert a stimulatory effect on
vascular endothelial and inflammatory cells, particularly TMAO, as
it can further activate the TLR4-NLRP3-TGF-β pathway, resulting in
prominent release of inflammatory factors and collagen, eventually
leading to myocardial fibrosis and cardiac dysfunction. TMAO
promotes vascular endothelial cell dysfunction and induces
atherosclerosis by activating the PKC/NF-κB/VCAM-1 pathway. In
addition, TMAO can stimulate macrophages to upregulate the CD36 and
SRA scavenger receptors. When excessive lipid is deposited in the
cytoplasm and exceeds the cell's own metabolic capacity, foam cells
form and further accelerate the development of atherosclerosis. In
addition to the inflammatory cells and cholesterol crystals present
in atherosclerotic plaques, some autopsy reports show that such
plaques contain bacterial DNA, making it likely that intestinal
flora is translocated into the circulatory system where it
participates in the disease process. BAs participate in glucose and
lipid metabolism and energy consumption in the body through the
enterohepatic circulation. BAs act on FXR to inhibit the abnormal
proliferation of intestinal flora and to regulate the expression of
BCL-2/BAX, which causes cardiomyocyte apoptosis. Through 11β-HSD,
BAs also regulate cortisol and aldosterone levels and maintain BP
stability. Furthermore, BAs and their derivatives play an
anti-atherosclerosis role after activating FXR. Hydrophilic BAs and
the main ions inside and outside the cell membrane exert an
anti-arrhythmic effect, while lipophilic BAs cause arrhythmias
through changes in the membrane potential. SCFAs, which mainly
comprise butyric acid, acetic acid and propionic acid, participate
in the regulation of immune function by activating GPR and HDAC.
The NaB metabolite from the flora activates the GPR43-Akt-GSK3
signaling pathway to regulate blood glucose homeostasis and reduce
damage to the systemic blood vessels from hyperglycemia. Fecal SCFA
levels in hypertensive patients are significantly higher compared
with those in the plasma, suggesting that the intestinal SCFA
absorption rate is low and indicating a potential target for
pathogenesis and BP-lowering treatments. When AAAs undergo
nitrification, halogenation, sulfonation, alkylation and acylation
reactions through GPRs, they can produce substances that damage the
myocardial and vascular endothelia, promoting the development of
CVDs. Additionally, nitroAAAs, the metabolites formed by aromatic
electrophilic substitution reactions such as acetylation, can
accumulate in the body and affect the normal metabolism of cells
and organ function, and the intestinal flora associated with a
Trp-rich diet may produce a variety of indole metabolites, which in
turn activate AhR, inhibit inflammation and reduce CVD risk
factors.
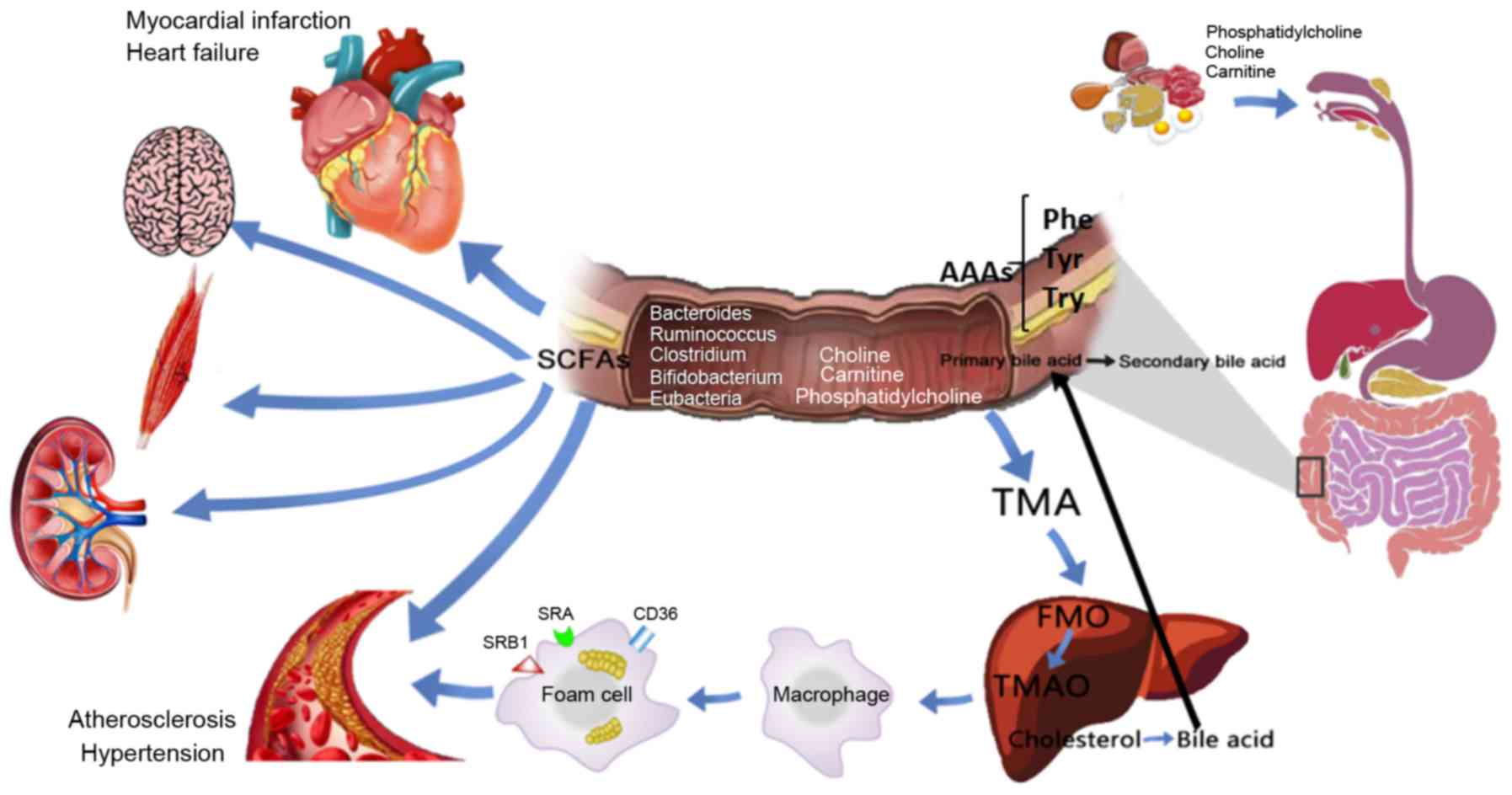 | Figure 4Potential role and mechanism of
action of gut microbiota metabolites in CVD development. Dietary
choline, phosphatidylcholine and carnitine produce TMA through
decay and metabolism of the intestinal flora, and the absorbed TMA
reaches the liver and is oxidized by FMO3 to form TMAO, which
stimulates macrophages to upregulate scavenger receptors (such as
CD36, SRA and SRB1), resulting in foam cell formation and
accelerating the progression of chronic vascular inflammation, such
as atherosclerosis. BAs enter the enterohepatic circulation through
the intestinal flora and related enzymes. SCFAs are important for
maintaining the normal function of the large intestine and the
morphology and function of colonic epithelial cells. They can also
promote the absorption of sodium and the aggregation of bacteria.
Dietary proteins are metabolized by intestinal flora to produce
AAAs, including Phe, Tyr and Trp, which participate in various
metabolic processes. CVDs, cardiovascular diseases; TMA,
trimethylamine; TMAO, trimethylamine N-oxide; FMO3, flavin
monooxygenase 3; SCFAs, short-chain fatty acids; AAAs, aromatic
amino acids; BAs, bile acids; Phe, phenylalanine; Trp, tryptophan;
Tyr, tyrosine. |
In light of the current scientific knowledge and
our improved understanding of intestinal flora, researchers have
proposed a number of treatments that fit into what is referred to
as the 'enteric-cardiac theory', such as intestinal flora
transplantation, antibiotic therapy, dietary supplementation with
probiotics and prebiotics, exercise and dietary adjustments. Among
them, exercise and dietary changes are feasible methods for
improving overall health, whereas intestinal flora transplantation
may cause long-term adverse reactions. Methods for maintaining the
homeostasis of the intestinal flora and improving the abundance of
beneficial bacteria is a new research direction for the prevention
and treatment of CVDs. Macrogenomic analysis of gut microflora in
humans has revealed significant differences in the intestinal
microbial structure between healthy individuals and patients with
CVDs. The pathway of intestinal microbial metabolites may be used
as an entry point for the study of treatment methods, and for
in-depth research on the pathogenesis of CDVs. As the disruption of
the microbial homeostatic balance is strongly associated with
disease, researchers have investigated whether intestinal flora
transplantation, drug interventions, dietary regulation and
exercise can maintain a healthy balance of intestinal flora.
Studying the pathogenesis of CVDs, such as atherosclerosis,
hypertension and myocardial infarction, in the context of the
microbiome may lead to the identification of new drugs and methods
with few side effects, cost-effectiveness and good curative
efficacy.
Acknowledgments
Not applicable.
Funding
The present study was supported by National Natural
Science Foundation of China (grant nos. 81970056, 81700269 and
81741129), Southern Marine Science and Engineering Guangdong
Laboratory Zhanjiang (grant no. ZJW-2019-007), Natural Science
Foundation of Guangdong Province (grant no. 2019A1515011925), and
Science and technology plan project of Zhanjiang (grant no.
2019A01002).
Availability of data and materials
Not applicable.
Authors' contributions
WL and HL contributed to the conception and design
of the study. YZ, XS and ZH performed the literature searches and
wrote the manuscript. YZ prepared the figures. WL, ZL, YH, YQ and
CC wrote and revised the manuscript. All the authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
GBD 2015 Mortality and Causes of Death
Collaborators: Global, regional, and national life expectancy,
all-cause mortality, and cause-specific mortality for 249 causes of
death, 1980-2015: A systematic analysis for the global burden of
disease study 2015. Lancet. 388:1459–1544. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Drouin N, Kloots T, Schappler J, Rudaz S,
Kohler I, Harms A, Lindenburg PW and Hankemeier T: Electromembrane
extraction of highly polar compounds: Analysis of cardiovascular
biomarkers in plasma. Metabolites. 10:42019. View Article : Google Scholar
|
|
3
|
Andersson C and Vasan RS: Epidemiology of
cardiovascular disease in young individuals. Nat Rev Cardiol.
15:230–240. 2018. View Article : Google Scholar
|
|
4
|
Maqbool M, Cooper ME and Jandeleit-Dahm
KAM: Cardiovascular disease and diabetic kidney disease. Semin
Nephrol. 38:217–232. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Defesche JC, Gidding SS, Harada-Shiba M,
Hegele RA, Santos RD and Wierzbicki AS: Familial
hypercholesterolaemia. Nat Rev Dis Primers. 3:170932017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jabbar A, Pingitore A, Pearce SH, Zaman A,
Iervasi G and Razvi S: Thyroid hormones and cardiovascular disease.
Nat Rev Cardiol. 14:39–55. 2017. View Article : Google Scholar
|
|
7
|
Backhed F, Ding H, Wang T, Hooper LV, Koh
GY, Nagy A, Semenkovich CF and Gordon JI: The gut microbiota as an
environmental factor that regulates fat storage. Proc Natl Acad Sci
USA. 101:15718–15723. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhu W, Gregory JC, Org E, Buffa JA, Gupta
N, Wang Z, Li L, Fu X, Wu Y, Mehrabian M, et al: Gut microbial
metabolite TMAO enhances platelet hyperreactivity and thrombosis
risk. Cell. 165:111–124. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Perez NB, Dorsen C and Squires A:
Dysbiosis of the gut micro-biome: A concept analysis. J Holist
Nurs. 8980101198795272019.Epub ahead of print.
|
|
10
|
Skye SM, Zhu W, Romano KA, Guo CJ, Wang Z,
Jia X, Kirsop J, Haag B, Lang JM, DiDonato JA, et al: Microbial
transplantation with human gut commensals containing CutCis
sufficient to transmit enhanced platelet reactivity and thrombosis
potential. Circ Res. 123:1164–1176. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fu J and Kuipers F: Systems genetics
approach reveals cross-talk between bile acids and intestinal
microbes. PLoS Genet. 15:e10083072019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ohira H, Tsutsui W and Fujioka Y: Are
short chain fatty acids in gut microbiota defensive players for
inflammation and atherosclerosis? J Atheroscler Thromb. 24:660–672.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Devlin AS, Marcobal A, Dodd D, Nayfach S,
Plummer N, Meyer T, Pollard KS, Sonnenburg JL and Fischbach MA:
Modulation of a circulating uremic solute via rational genetic
manipulation of the gut microbiota. Cell Host Microbe. 20:709–715.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yao C, Chen BH, Joehanes R, Otlu B, Zhang
X, Liu C, Huan T, Tastan O, Cupples LA, Meigs JB, et al: Integromic
analysis of genetic variation and gene expression identifies
networks for cardiovascular disease phenotypes. Circulation.
131:536–549. 2015. View Article : Google Scholar :
|
|
15
|
Zhang WQ, Zhao TT, Gui DK, Gao CL, Gu JL,
Gan WJ, Huang W, Xu Y, Zhou H, Chen WN, et al: Sodium butyrate
improves liver glycogen metabolism in Type 2 diabetes mellitus. J
Agric Food Chem. 67:7694–7705. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Odegaard AO, Koh WP, Gross MD, Yuan JM and
Pereira MA: Combined lifestyle factors and cardiovascular disease
mortality in Chinese men and women: The Singapore Chinese health
study. Circulation. 124:2847–2854. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Macfarlane GT and Macfarlane S: Bacteria,
colonic fermentation, and gastrointestinal health. J AOAC Int.
95:50–60. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tang WH, Kitai T and Hazen SL: Gut
microbiota in cardiovascular health and disease. Circ Res.
120:1183–1196. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lam V, Su J, Hsu A, Gross GJ, Salzman NH
and Baker JE: Intestinal microbial metabolites are linked to
severity of myocardial infarction in rats. PLoS One.
11:e01608402016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Caro-Gomez E, Sierra JA, Escobar JS,
Alvarez-Quintero R, Naranjo M, Medina S, Velasquez-Mejia EP,
Tabares-Guevara JH, Jaramillo JC, Leon-Varela YM, et al: Green
coffee extract improves cardiometabolic parameters and modulates
gut micro-biota in High-Fat-Diet-Fed ApoE−/− Mice.
Nutrients. 11:4972019. View Article : Google Scholar
|
|
21
|
Tran HQ, Ley RE, Gewirtz AT and Chassaing
B: Flagellin-elicited adaptive immunity suppresses flagellated
microbiota and vaccinates against chronic inflammatory diseases.
Nat Commun. 10:56502019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang Z, Roberts AB, Buffa JA, Levison BS,
Zhu W, Org E, Gu X, Huang Y, Zamanian-Daryoush M, Culley MK, et al:
Non-lethal inhibition of gut microbial trimethylamine production
for the treatment of atherosclerosis. Cell. 163:1585–1595. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Grabherr F, Grander C, Effenberger M,
Adolph TE and Tilg H: Gut dysfunction and non-alcoholic fatty liver
disease. Front Endocrinol (Lausanne). 10:6112019. View Article : Google Scholar
|
|
24
|
Ramirez-Perez O, Cruz-Ramon V,
Chinchilla-Lopez P and Mendez-Sanchez N: The role of the gut
microbiota in bile acid metabolism. Ann Hepato. 16(Suppl. 1:
S3-105): S21–S20. 2017. View Article : Google Scholar
|
|
25
|
Chen H, Peng L, Perez de Nanclares M,
Trudeau MP, Yao D, Cheng Z, Urriola PE, Mydland LT, Shurson GC,
Overland M and Chen C: Identification of sinapine-derived choline
from a rapeseed diet as a source of serum Trimethylamine N-Oxide in
pigs. J Agric Food Chem. 67:7748–7754. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang Z, Klipfell E, Bennett BJ, Koeth R,
Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, et al:
Gut flora metabolism of phosphatidylcholine promotes cardiovascular
disease. Nature. 472:57–63. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ufnal M, Zadlo A and Ostaszewski R: TMAO:
A small molecule of great expectations. Nutrition. 31:1317–1323.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Koeth RA, Wang Z, Levison BS, Buffa JA,
Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, et al: Intestinal
microbiota metabolism of L-carnitine, a nutrient in red meat,
promotes atherosclerosis. Nat Med. 19:576–585. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Suzuki H, Kurihara Y, Takeya M, Kamada N,
Kataoka M, Jishage K, Ueda O, Sakaguchi H, Higashi T, Suzuki T, et
al: A role for macrophage scavenger receptors in atherosclerosis
and susceptibility to infection. Nature. 386:292–296. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Brown JM and Hazen SL: The gut microbial
endocrine organ: Bacterially derived signals driving
cardiometabolic diseases. Annu Rev Med. 66:343–359. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li XS, Obeid S, Klingenberg R, Gencer B,
Mach F, Raber L, Windecker S, Rodondi N, Nanchen D, Muller O, et
al: Gut microbiota-dependent trimethylamine N-oxide in acute
coronary syndromes: A prognostic marker for incident cardiovascular
events beyond traditional risk factors. Eur Heart J. 38:814–824.
2017.PubMed/NCBI
|
|
32
|
Liu TX, Niu HT and Zhang SY: Intestinal
microbiota metabolism and atherosclerosis. Chin Med J (Engl).
128:2805–2811. 2015. View Article : Google Scholar
|
|
33
|
Ott SJ, El Mokhtari NE, Musfeldt M,
Hellmig S, Freitag S, Rehman A, Kuhbacher T, Nikolaus S, Namsolleck
P, Blaut M, et al: Detection of diverse bacterial signatures in
atherosclerotic lesions of patients with coronary heart disease.
Circulation. 113:929–937. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Winther SA, Ollgaard JC, Tofte N, Tarnow
L, Wang Z, Ahluwalia TS, Jorsal A, Theilade S, Parving HH, Hansen
TW, et al: Utility of plasma concentration of Trimethylamine
N-Oxide in predicting cardiovascular and renal complications in
individuals with type 1 diabetes. Diabetes Care. 42:1512–1520.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li X, Geng J, Zhao J, Ni Q, Zhao C, Zheng
Y, Chen X and Wang L: Trimethylamine N-Oxide exacerbates cardiac
fibrosis via activating the NLRP3 inflammasome. Front Physiol.
10:8662019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shepshelovich J, Goldstein-Magal L,
Globerson A, Yen PM, Rotman-Pikielny P and Hirschberg K: Protein
synthesis inhibitors and the chemical chaperone TMAO reverse
endoplasmic reticulum perturbation induced by overexpression of the
iodide transporter pendrin. J Cell Sci. 118(Pt 8): 1577–1586. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen S, Henderson A, Petriello MC, Romano
KA, Gearing M, Miao J, Schell M, Sandoval-Espinola WJ, Tao J, Sha
B, et al: Trimethylamine N-Oxide binds and activates PERK to
promote metabolic dysfunction. Cell Metab. 30:1141–1151.e5. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ma G, Pan B, Chen Y, Guo C, Zhao M, Zheng
L and Chen B: Trimethylamine N-oxide in atherogenesis: Impairing
endothelial self-repair capacity and enhancing monocyte adhesion.
Biosci Rep. 37:BSR201602442017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shiffka SJ, Kane MA and Swaan PW: Planar
bile acids in health and disease. Biochim Biophys Acta Biomembr.
1859:2269–2276. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chiang JY: Bile acid regulation of gene
expression: Roles of nuclear hormone receptors. Endocr Rev.
23:443–463. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lefebvre P, Cariou B, Lien F, Kuipers F
and Staels B: Role of bile acids and bile acid receptors in
metabolic regulation. Physiol Rev. 89:147–191. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hoving LR, Katiraei S, Heijink M, Pronk A,
van der Wee-Pals L, Streefland T, Giera M, Willems van Dijk K and
van Harmelen V: Dietary mannan oligosaccharides modulate gut
microbiota, increase fecal bile acid excretion, and decrease plasma
cholesterol and atherosclerosis development. Mol Nutr Food Res.
62:e17009422018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sun L, Pang Y, Wang X, Wu Q, Liu H, Liu B,
Liu G, Ye M, Kong W and Jiang C: Ablation of gut microbiota
alleviates obesity-induced hepatic steatosis and glucose
intolerance by modulating bile acid metabolism in hamsters. Acta
Pharm Sin B. 9:702–710. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tsuei J, Chau T, Mills D and Wan YJ: Bile
acid dysregulation, gut dysbiosis, and gastrointestinal cancer. Exp
Biol Med (Maywood). 239:1489–1504. 2014. View Article : Google Scholar
|
|
45
|
Dodd D, Spitzer MH, Van Treuren W, Merrill
BD, Hryckowian AJ, Higginbottom SK, Le A, Cowan TM, Nolan GP,
Fischbach MA and Sonnenburg JL: A gut bacterial pathway metabolizes
aromatic amino acids into nine circulating metabolites. Nature.
551:648–652. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Jia ET, Liu ZY, Pan M, Lu JF and Ge QY:
Regulation of bile acid metabolism-related signaling pathways by
gut microbiota in diseases. J Zhejiang Univ Sci B. 20:781–792.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Merritt ME and Donaldson JR: Effect of
bile salts on the DNA and membrane integrity of enteric bacteria. J
Med Microbiol. 58(Pt 12): 1533–1541. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lin S, Yang X, Yuan P, Yang J, Wang P,
Zhong H, Zhang X, Che L, Feng B, Li J, et al: Undernutrition shapes
the gut microbiota and bile acid profile in association with
altered gut-liver FXR signaling in weaning pigs. J Agric Food Chem.
67:3691–3701. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wu P, Zhang Y, Liu Y, Wang X, Guo Z, Zhang
Y, Liang X and Lai W: Effects of cholic acid on blood pressure and
production of vascular aldosterone and corticosterone. Steroids.
64:291–295. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Valdivia C, Carvajal CA, Campino C,
Allende F, Martinez-Aguayo A, Baudrand R, Vecchiola A, Lagos CF,
Tapia-Castillo A, Fuentes CA, et al: Citosine-adenine-repeat
microsatellite of 11β-hydroxysteroid dehydrogenase 2 gene in
hypertensive children. Am J Hypertens. 29:25–32. 2016. View Article : Google Scholar
|
|
51
|
Wan Y, Wang F, Yuan J, Li J, Jiang D,
Zhang J, Li H, Wang R, Tang J, Huang T, et al: Effects of dietary
fat on gut microbiota and faecal metabolites, and their
relationship with cardiometabolic risk factors: A 6-month
randomised controlled-feeding trial. Gut. 68:1417–1429. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Charach G, Karniel E, Novikov I, Galin L,
Vons S, Grosskopf I and Charach L: Reduced bile acid excretion is
an independent risk factor for stroke and mortality: A prospective
follow-up study. Atherosclerosis. 293:79–85. 2020. View Article : Google Scholar
|
|
53
|
Fedorova OV, Zernetkina VI, Shilova VY,
Grigorova YN, Juhasz O, Wei W, Marshall CA, Lakatta EG and Bagrov
AY: Synthesis of an Endogenous steroidal Na Pump inhibitor
mari-nobufagenin, implicated in human cardiovascular diseases, is
initiated by CYP27A1 via Bile Acid pathway. Circ Cardiovasc Genet.
8:736–745. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Rainer PP, Primessnig U, Harenkamp S,
Doleschal B, Wallner M, Fauler G, Stojakovic T, Wachter R, Yates A,
Groschner K, et al: Bile acids induce arrhythmias in human atrial
myocardium-implications for altered serum bile acid composition in
patients with atrial fibrillation. Heart. 99:1685–1692. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Pu J, Yuan A, Shan P, Gao E, Wang X, Wang
Y, Lau WB, Koch W, Ma XL and He B: Cardiomyocyte-expressed
farnesoid-X-receptor is a novel apoptosis mediator and contributes
to myocardial ischaemia/reperfusion injury. Eur Heart J.
34:1834–1845. 2013. View Article : Google Scholar :
|
|
56
|
Chen ML, Yi L, Zhang Y, Zhou X, Ran L,
Yang JN, Zhu JD, Zhang QY and Mi MT: Resveratrol attenuates
Trimethylamine-N-Oxide (TMAO)-Induced atherosclerosis by regulating
TMAO Synthesis and bile acid metabolism via remodeling of the Gut
microbiota. mBio. 7:e02210–15. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Nguyen MT, Favelyukis S, Nguyen AK,
Reichart D, Scott PA, Jenn A, Liu-Bryan R, Glass CK, Neels JG and
Olefsky JM: A subpopulation of macrophages infiltrates hypertrophic
adipose tissue and is activated by free fatty acids via Toll-like
receptors 2 and 4 and JNK-dependent pathways. J Biol Chem.
282:35279–35292. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Calderon-Perez L, Gosalbes MJ, Yuste S,
Valls RM, Pedret A, Llaurado E, Jimenez-Hernandez N, Artacho A,
Pla-Paga L, Companys J, et al: Gut metagenomic and short chain
fatty acids signature in hypertension: A cross-sectional study. Sci
Rep. 10:64362020. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Morrison DJ and Preston T: Formation of
short chain fatty acids by the gut microbiota and their impact on
human metabolism. Gut Microbes. 7:189–200. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Manrique Vergara D and González Sánchez
ME: Short chain fatty acids (butyric acid) and intestinal diseases.
Nutr Hosp. 34(Suppl 4): S58–S61. 2017.In Spanish.
|
|
61
|
Sun M, Wu W, Liu Z and Cong Y: Microbiota
metabolite short chain fatty acids, GPCR, and inflammatory bowel
diseases. J Gastroenterol. 52:1–8. 2017. View Article : Google Scholar
|
|
62
|
Smith PM, Howitt MR, Panikov N, Michaud M,
Gallini CA, Bohlooly-Y M, Glickman JN and Garrett WS: The microbial
metabolites, short-chain fatty acids, regulate colonic Treg cell
homeostasis. Science. 341:569–573. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Li J, Zhao F, Wang Y, Chen J, Tao J, Tian
G, Wu S, Liu W, Cui Q, Geng B, et al: Gut microbiota dysbiosis
contributes to the development of hypertension. Microbiome.
5:142017. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Le B, Bůžková P, Robbins JA, Fink HA,
Raiford M, Isales CM, Shikany JM, Coughlin SS and Carbone LD: The
association of aromatic amino acids with incident hip fracture,
aBMD, and body composition from the cardiovascular health study.
Calcif Tissue Int. 105:161–172. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Tessari P, Lante A and Mosca G: Essential
amino acids: Master regulators of nutrition and environmental
footprint? Sci Rep. 6:260742016. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Fernstrom JD and Fernstrom MH: Tyrosine,
phenylalanine, and catecholamine synthesis and function in the
brain. J Nutr. 137(6 Suppl 1): 1539S–1547S; discussion 1548S. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Shishehbor MH, Aviles RJ, Brennan ML, Fu
X, Goormastic M, Pearce GL, Gokce N, Keaney JF Jr, Penn MS,
Sprecher DL, et al: Association of nitrotyrosine levels with
cardiovascular disease and modulation by statin therapy. JAMA.
289:1675–1680. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Thomson L: 3-nitrotyrosine modified
proteins in atherosclerosis. Dis Markers. 2015:7082822015.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Haase S, Haghikia A, Wilck N, Müller DN
and Linker RA: Impacts of microbiome metabolites on immune
regulation and autoimmunity. Immunology. 154:230–238. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Brawner KM, Yeramilli VA, Duck LW, Van Der
Pol W, Smythies LE, Morrow CD, Elson CO and Martin CA: Depletion of
dietary aryl hydrocarbon receptor ligands alters microbiota
composition and function. Sci Rep. 9:147242019. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Zelante T, Iannitti RG, Cunha C, De Luca
A, Giovannini G, Pieraccini G, Zecchi R, D'Angelo C,
Massi-Benedetti C, Fallarino F, et al: Tryptophan catabolites from
microbiota engage aryl hydrocarbon receptor and balance mucosal
reactivity via interleukin-22. Immunity. 39:372–385. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Metghalchi S, Ponnuswamy P, Simon T,
Haddad Y, Laurans L, Clément M, Dalloz M, Romain M, Esposito B,
Koropoulis V, et al: Indoleamine 2,3-dioxygenase fine-tunes immune
homeostasis in atherosclerosis and colitis through repression of
interleukin-10 production. Cell Metab. 22:460–471. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Laurans L, Venteclef N, Haddad Y,
Chajadine M, Alzaid F, Metghalchi S, Sovran B, Denis RGP, Dairou J,
Cardellini M, et al: Genetic deficiency of indoleamine
2,3-dioxygenase promotes gut microbiota-mediated metabolic health.
Nat Med. 24:1113–1120. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Lamas B, Richard ML, Leducq V, Pham HP,
Michel ML, Da Costa G, Bridonneau C, Jegou S, Hoffmann TW,
Natividad JM, et al: CARD9 impacts colitis by altering gut
microbiota metabolism of tryptophan into aryl hydrocarbon receptor
ligands. Nat Med. 22:598–605. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Hoyles L, Fernández-Real JM, Federici M,
Serino M, Abbott J, Charpentier J, Heymes C, Luque JL, Anthony E,
Barton RH, et al: Molecular phenomics and metagenomics of hepatic
steatosis in non-diabetic obese women. Nat Med. 24:1070–1080. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Kakihana K, Fujioka Y, Suda W, Najima Y,
Kuwata G, Sasajima S, Mimura I, Morita H, Sugiyama D, Nishikawa H,
et al: Fecal microbiota transplantation for patients with
steroid-resistant acute graft-versus-host disease of the gut.
Blood. 128:2083–2088. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Moludi J, Maleki V, Jafari-Vayghyan H,
Vaghef-Mehrabany E and Alizadeh M: Metabolic endotoxemia and
cardiovascular disease: A systematic review about potential roles
of prebiotics and probiotics. Clin Exp Pharmacol Physiol.
47:927–939. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Gregory JC, Buffa JA, Org E, Wang Z,
Levison BS, Zhu W, Wagner MA, Bennett BJ, Li L, DiDonato JA, et al:
Transmission of atherosclerosis susceptibility with gut microbial
transplantation. J Biol Chem. 290:5647–5660. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Lu F, Liu F, Zhou Q, Hu X and Zhang Y:
Effects of grape pomace and seed polyphenol extracts on the
recovery of gut microbiota after antibiotic treatment in high-fat
diet-fed mice. Food Sci Nutr. 7:2897–2906. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Liao X, Song L, Zeng B, Liu B, Qiu Y, Qu
H, Zheng Y, Long M, Zhou H, Wang Y, et al: Alteration of gut
microbiota induced by DPP-4i treatment improves glucose
homeostasis. EBioMedicine. 44:665–674. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Wan JJ, Lin CH, Ren ED, Su Y and Zhu WY:
Effects of early intervention with maternal fecal bacteria and
antibiotics on liver metabolome and transcription in neonatal pigs.
Front Physiol. 10:1712019. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Janeiro MH, Ramirez MJ, Milagro FI,
Martinez JA and Solas M: Implication of trimethylamine N-Oxide
(TMAO) in disease: Potential biomarker or new therapeutic target.
Nutrients. 10:13982018. View Article : Google Scholar :
|
|
83
|
Queipo-Ortuño MI, Seoane LM, Murri M,
Pardo M, Gomez-Zumaquero JM, Cardona F, Casanueva F and Tinahones
FJ: Gut microbiota composition in male rat models under different
nutritional status and physical activity and its association with
serum leptin and ghrelin levels. PLoS One. 8:e654652013. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Roy Le T, Lécuyer E, Chassaing B, Rhimi M,
Lhomme M, Boudebbouze S, Ichou F, Haro Barceló J, Huby T, Guerin M,
et al: The intestinal microbiota regulates host cholesterol
homeostasis. BMC Biol. 17:942019. View Article : Google Scholar
|
|
85
|
Heymsfield SB and Wadden TA: Mechanisms,
pathophysiology, and management of obesity. N Engl J Med.
376:254–266. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Guirro M, Costa A, Gual-Grau A, Herrero P,
Torrell H, Canela N and Arola L: Effects from diet-induced gut
microbiota dysbiosis and obesity can be ameliorated by fecal
microbiota transplantation: A multiomics approach. PLoS One.
14:e02181432019. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Enright EF, Joyce SA, Gahan CG and Griffin
BT: Impact of gut microbiota-mediated bile acid metabolism on the
solubilization capacity of bile salt micelles and drug solubility.
Mol Pharm. 14:1251–1263. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Langlands SJ, Hopkins MJ, Coleman N and
Cummings JH: Prebiotic carbohydrates modify the mucosa associated
micro-flora of the human large bowel. Gut. 53:1610–1616. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Hill C, Guarner F, Reid G, Gibson GR,
Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S,
et al: Expert consensus document. The International Scientific
Association for Probiotics and Prebiotics consensus statement on
the scope and appropriate use of the term probiotic. Nat Rev
Gastroenterol Hepatol. 11:506–514. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Gallo A, Passaro G, Gasbarrini A, Landolfi
R and Montalto M: Modulation of microbiota as treatment for
intestinal inflammatory disorders: An uptodate. World J
Gastroenterol. 22:7186–7202. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Quigley EM: Prebiotics and probiotics:
Their role in the management of gastrointestinal disorders in
adults. Nutr Clin Pract. 27:195–200. 2012. View Article : Google Scholar
|
|
92
|
Mozaffarian D: Dairy foods, obesity, and
metabolic health: The role of the food matrix compared with single
nutrients. Adv Nutr. 10:917S–923S. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Chen Q, Liu M, Zhang P, Fan S, Huang J, Yu
S, Zhang C and Li H: Fucoidan and galactooligosaccharides
ameliorate high-fat diet-induced dyslipidemia in rats by modulating
the gut micro-biota and bile acid metabolism. Nutrition. 65:50–59.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Francavilla G, Abrignani MG, Braschi A,
Sciacca R, Francavilla VC, Caracciolo MM, Renda N, Riccio C,
Scaglione A and Braschi G: Physical exercise and sport activities
in patients with and without coronary heart disease. Monaldi Arch
Chest Dis. 68:87–95. 2007.In Italian. PubMed/NCBI
|
|
95
|
Cheng YJ, Zhao XJ, Zeng W, Xu MC, Ma YC
and Wang M: Effect of intradialytic exercise on physical
performance and cardiovascular risk factors in patients receiving
maintenance hemodialysis: A pilot and feasibility study. Blood
Purif. 1–10. 2019.Epub ahead of print.
|
|
96
|
Codella R, Luzi L and Terruzzi I: Exercise
has the guts: How physical activity may positively modulate gut
microbiota in chronic and immune-based diseases. Dig Liver Dis.
50:331–341. 2018. View Article : Google Scholar
|
|
97
|
Chen J, Guo Y, Gui Y and Xu D: Physical
exercise, gut, gut microbiota, and atherosclerotic cardiovascular
diseases. Lipids Health Dis. 17:172018. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Ito S: High-intensity interval training
for health benefits and care of cardiac diseases-The key to an
efficient exercise protocol. World J Cardiol. 11:171–188. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Kang Y and Cai Y: Gut microbiota and
hypertension: From pathogenesis to new therapeutic strategies. Clin
Res Hepatol Gastroenterol. 42:110–117. 2018. View Article : Google Scholar
|
|
100
|
Petriz BA, Castro AP, Almeida JA, Gomes
CP, Fernandes GR, Kruger RH, Pereira RW and Franco OL: Exercise
induction of gut microbiota modifications in obese, non-obese and
hypertensive rats. BMC Genomics. 15:5112014. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Verdam FJ, Fuentes S, de Jonge C,
Zoetendal EG, Erbil R, Greve JW, Buurman WA, de Vos WM and Rensen
SS: Human intestinal microbiota composition is associated with
local and systemic inflammation in obesity. Obesity (Silver
Spring). 21:E607–E615. 2013. View Article : Google Scholar
|
|
102
|
Denou E, Marcinko K, Surette MG, Steinberg
GR and Schertzer JD: High-intensity exercise training increases the
diversity and metabolic capacity of the mouse distal gut
micro-biota during diet-induced obesity. Am J Physiol Endocrinol
Metab. 310:E982–E993. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Hsu YJ, Chiu CC, Li YP, Huang WC, Huang
YT, Huang CC and Chuang HL: Effect of intestinal microbiota on
exercise performance in mice. J Strength Cond Res. 29:552–558.
2015. View Article : Google Scholar
|
|
104
|
Starkie R, Ostrowski SR, Jauffred S,
Febbraio M and Pedersen BK: Exercise and IL-6 infusion inhibit
endotoxin-induced TNF-alpha production in humans. FASEB J.
17:884–886. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Pedersen BK and Saltin B: Exercise as
medicine-evidence for prescribing exercise as therapy in 26
different chronic diseases. Scand J Med Sci Sports. 25(Suppl 3):
S1–S72. 2015. View Article : Google Scholar
|
|
106
|
Fung TT, Rexrode KM, Mantzoros CS, Manson
JE, Willett WC and Hu FB: Mediterranean diet and incidence of and
mortality from coronary heart disease and stroke in women.
Circulation. 119:1093–1100. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Holscher HD, Guetterman HM, Swanson KS, An
R, Matthan NR, Lichtenstein AH, Novotny JA and Baer DJ: Walnut
consumption alters the gastrointestinal microbiota, microbially
derived secondary bile acids, and health markers in healthy adults:
A randomized controlled trial. J Nutr. 148:861–867. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Psaltopoulou T, Hatzis G, Papageorgiou N,
Androulakis E, Briasoulis A and Tousoulis D: Socioeconomic status
and risk factors for cardiovascular disease: Impact of dietary
mediators. Hellenic J Cardiol. 58:32–42. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Tindall AM, Mclimans CJ, Petersen KS,
Kris-Etherton PM and Lamendella R: Walnuts and vegetable oils
containing oleic acid differentially affect the gut microbiota and
associations with cardiovascular risk factors: Follow-up of a
randomized, controlled, feeding trial in adults at risk for
cardiovascular disease. J Nutr. 150:806–817. 2020. View Article : Google Scholar :
|


















