1. Introduction
Ovarian cancer ranks as the third leading type of
cancer among all the gynecological cancers, with a very high
mortality rate according to the latest report by the International
Agency for Research on Cancer (IARC) (1,2).
The majority of patients are diagnosed at an advanced stage and
there is no curative therapy (3).
The most common therapy for advanced stage disease includes maximal
cytoreductive surgery and platinum/taxane-based chemotherapy, which
always attains an initial response (4,5).
Over the past 20 years, this combination has become the standard of
care for patients with ovarian cancer (6). Although 70% of ovarian cancer
patients exhibit an effective response to the first application of
platinum chemo-therapy (7), the
majority (80%) will relapse within 6 months, and this has been
classified as 'platinum resistance' (8,9),
and is one of the major reasons for the high clinical mortality
rate of patients with ovarian cancer (10). Therefore, chemoresistance has
become an urgent issue that needs to be resolved in clinical
practice, encouraging the identification of more effective drugs.
Poly ADP ribose polymer (PARP) inhibitors play an antitumor role by
inhibiting DNA repair and promoting tumor cell apoptosis (11). At present, some clinical trials
have found that PARP combined with other anticancer agents, such as
carboplatin and paclitaxel, can improve the prognosis of patients
with recurrent drug-resistant ovarian cancer (12).
Apoptosis is critical for regulating cellular
homeostasis. The dysfunction of apoptosis is one of the
characteristics of cancer and other diseases (13). Therefore, most conventional
chemotherapeutic agents, such as cisplatin, confer an antitumor
effects by mainly relying on the activation of the apoptosis of
cancer cells (14). As
significant regulators of apoptosis, the B-cell lymphoma-2 (Bcl-2)
family is closely related to the chemoresistance of ovarian cancer.
Additionally, cancer cells can evade apoptosis in multiple pathways
to achieve immortalization and the evasion of apoptosis is a
significant mechanism of the chemoresistance of cancer cells
(15,16). BH3 profiling is a functional assay
that can measure how primed a cell is to execute apoptosis by
detecting the release of pro-apoptotic factors. This assay was
proposed to solve the clinical issue of neoplastic hematological
disorders due to the high heterogeneity and chemoresistance rate of
the cells (17) and may be of
assistance in overcoming chemoresistance in ovarian cancer.
In the following chapters, the central role of Bcl-2
family members in the apoptotic pathway and the mechanisms through
which they regulate apoptosis in ovarian cancer in response to
antitumor agents, such as platinum, will be discussed. The present
review also discusses the clinical function of a novel assay, BH3
profiling.
2. Central role of the Bcl-2 family in the
apoptotic pathway
Apoptosis, also known as programmed cell death, is a
programmed biological activity with distinct genetic and epigenetic
pathways that involves a form or cellular suicide without
triggering inflammatory responses, which is the main characteristic
differentiating the process from necrosis (18,19). From a molecular mechanistic
aspect, apoptosis can be activated by 3 main signaling pathways:
The extrinsic pathway, intrinsic apoptosis and endoplasmic
reticulum (ER) stress-induced apoptosis (20). Additionally, some other apoptotic
pathways have been found, such as the granzyme B pathway (21). It seems that these pathways are
irrelevant; however, they can actually be linked through Bcl-2
family proteins. Research on the mechanisms of apoptosis in cells
has shed light on the fact that the Bcl-2 family plays a central
role in the apoptotic pathway (22) (Fig.
1). The threshold of cell fate is mediated by the balance
between pro-survival and pro-apoptotic Bcl-2 family members
(23). This basic interaction is
conserved from sponges to humans (24,25). The specific regulatory mechanisms
of these apoptotic signaling pathways differ from body to body.
However, all of these mechanisms eventually return to the loss of
pro-survival activity and the gain of pro-apoptotic activity, which
is why the interactions of this family are explored and their
regulatory function network is clarified (26).
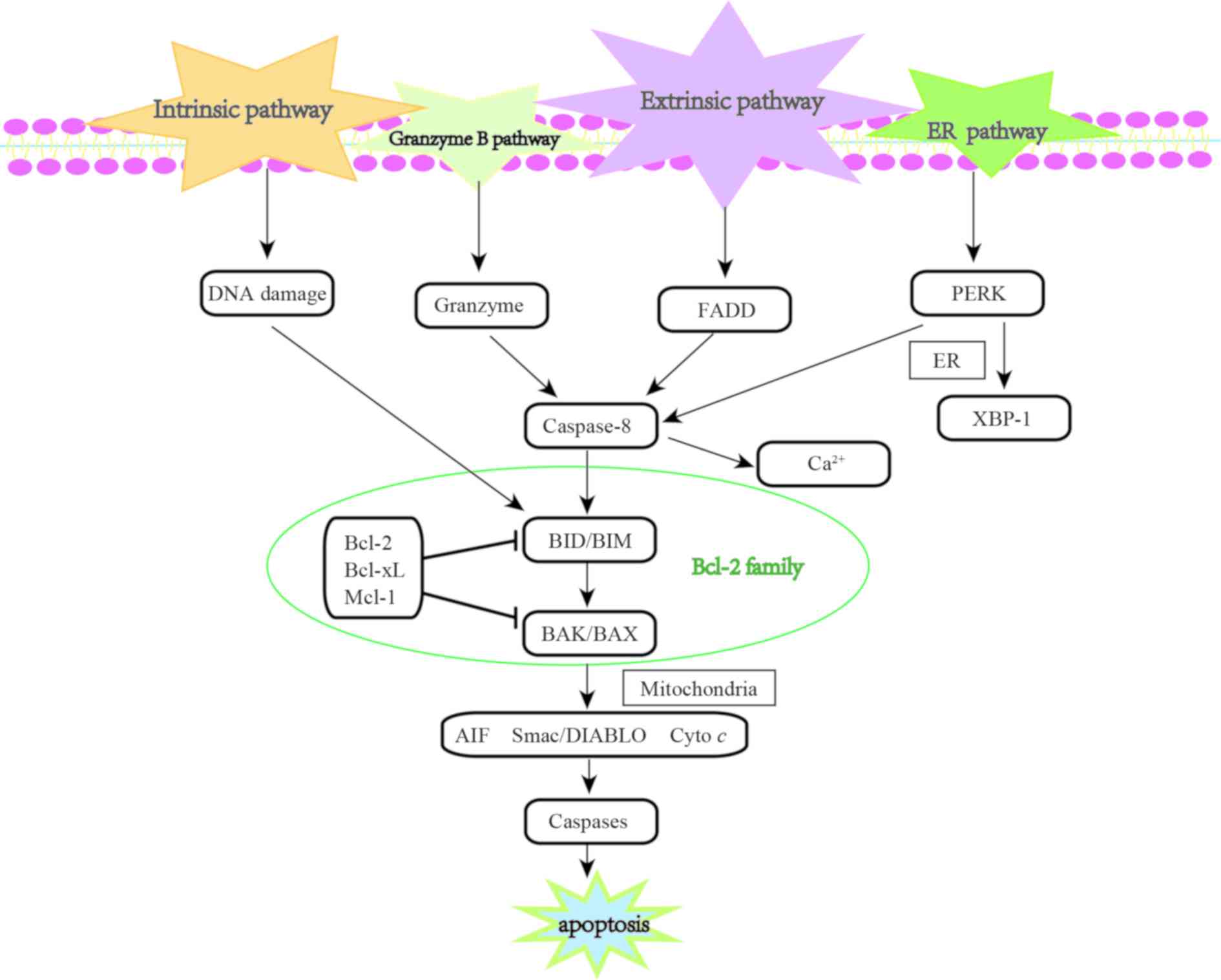 | Figure 1Overview of the signaling pathway of
apoptosis. The diagram illustrates the main proteins and 4
signaling pathways mediating cell apoptosis. These are: i) The
intrinsic pathway; ii) the granzyme B pathway; iii) the extrinsic
pathway; and iv) the endoplasmic reticulum (ER) stress-induced
apoptotic pathway. The 4 pathways converge on the mitochondrion,
and Bcl-2 family members play a central in regulating apoptosis
among all the four pathways. ER, endoplasmic reticulum; FADD,
fas-associating protein with a novel death domain; PERK, protein
kinase R-like ER kinase; XBP-1, X-box binding protein 1; AIF,
apoptosis-inducing factor; Smac/DIABLO, Second mitochondrial
activator of caspases/direct IAP-binding protein with low PI; Cyto
c, cytochrome c; BID, BH3-interacting domain; BIM,
Bcl-2-interacting protein; BAK, Bcl-2 antagonist killer 1; BAX,
Bcl-2 associated X Protein; Bcl-2, B cell lymphoma protein 2;
Bcl-xL, Bcl-2-related protein long form of Bcl-x; Mcl-1, myeloid
cell leukaemia-1. |
To date, at least 16 members of this family that
contain Bcl-2 homology (BH) domains have been identified; these
proteins can be further sorted into 3 major groups (Table I). The first group is the proteins
that contain only one BH domain, termed BH3-only proteins, such as
BID and BIM, which function as apoptotic promoters. BH3-only
proteins are activated earlier than any of other proteins involved
in this pathway; thus, they act as molecular sentinels. The
existence of the BH3 motif is a key feature of pro-apoptotic
proteins, and it is necessary for pro-apoptotic activity (27,28). The second group is composed of the
pro-apoptotic proteins that contain 3 BH domains (BH1, BH2 and
BH3), such as BAX and BAK. The third group includes anti-apoptotic
proteins, such as Bcl-2, Bcl-xL and Mcl-1, which contains 4 BH
domains (BH1, BH2, BH3 and BH4). They can protect cells from
apoptosis by sequestering their pro-apoptotic counterparts
(19,29-32).
 | Table IBcl-2 family members. |
Table I
Bcl-2 family members.
| Name | BH domain | Members | Function |
|---|
| BH3-only
proteins | BH3 | BID, BIM, NOXA,
PUMA, BIK, BAD, BF, HRK | Initiators of
apoptosis: Proteins that is first activated during the initiation
of apoptosis. |
| Pro-apoptotic
proteins | BH1, BH2, BH3 | BAX, BAK, BOK | Executors of
apoptosis: Proteins that can change the permeability of
mitochondrial membrane by aggregating into the outer membrane of
mitochondria to form oligomers. |
| Anti-apoptotic
proteins | BH1, BH2, BH3,
BH4 | Bcl-2, Bcl-xL,
Mcl-1, Bcl-w, A1/BFL-1 | Antagonists of
apoptosis: Proteins that inhibit the aggregation of proapoptotic
proteins to prevent the initiation of apoptosis. |
After a variety of stimuli reach cells to induce
apoptosis, the Bcl-2 family is eventually activated as multiple
proteins and pathways converge upon the Bcl-2 family (29). It has been widely postulated that
the majority of Bcl-2 family proteins are located in the nuclear
envelope, the ER and the outer mitochondrial membrane (OMM). These
characteristic locations are consistent with the function of Bcl-2
family proteins. However, the role of the Bcl-2 family in OMM is
the core mechanism of intrinsic apoptosis (26). Exactly as is described, mutant
Bcl-2 family proteins that are disabled to anchor the membrane have
been found to be less effective at preventing apoptosis in some
systems (31).
As molecular sentinels, BH3-only proteins, such as
BID and BIM are the first members to be activated. There is
evidence to suggest that BH3-only proteins can directly interact
with and activate BAX/BAK (33,34). There is an activation priority
existing between these proteins: BID first activates BAK, while BIM
first activates BAX (35).
Activated pro-apoptotic proteins translocate to the mitochondrion
and disrupt the integrity of the OMM in a process termed
mitochondrial outer membrane permeabilization (MOMP). The exact
mechanism of this process remains unclear (36). Certain studies have suggested an
interesting hypothesis in which activated BAX undergoes
oligomerization at the mitochondrial membrane, forming rings, lines
and incomplete rings or arc structures of different shapes and
sizes, resulting in the mitochondrial inner membrane remodeling to
facilitate the release of cytochrome c (37,38), and subsequently activating the
caspase cascade (26). Another
possible mechanism is that Bcl-2 family pro-apoptotic proteins can
break the stable state of the Ca2+ level within the ER
and then increase the Ca2+ transfer to the
mitochondrion, which leads to mitochondrial swelling and then to
the perturbation or rupture of the OMM, resulting in mitochondrial
dysfunction and the release of pro-apoptotic molecules into the
cytosol (39).
A crucial component that cannot be ignored is the
mitochondrion intermembrane space (IMS), where the majority of
pro-apoptotic factors are located (32). Once the OMM is disrupted,
pro-apoptotic proteins release from the IMS into the cytoplasm in
response to apoptotic signals, such as DNA damage, oxidative
stress, ER stress and other events (31). As soon as these pro-apoptotic
factors are released into the cytosol, they can initiate different
cascades, leading to apoptosis and eventually causing cell death.
All of the functional factors, such as cytochrome c,
apoptosis protease activating factor 1 (Apaf-1), dATP and
procaspase-9, assemble together and interact with each other,
forming the apoptosome. This structure favors procaspase-9 rapidly
converting into caspase-9, thereby eventually killing the cell
(31).
3. Concise mechanisms of platinum-based
chemotherapy
Platinum has been an indispensable antitumor agent
for almost 40 years, since it was first approved in the USA by the
Food and Drug Administration (FDA) in 1978 for the treatment of
testicular, bladder and ovarian cancers (40). As platinum plays an increasing
role in the treatment of cancers, such as ovarian cancer, the
elucidation of the mechanisms through which platinum and its
analogues function in normal and cancer cells has become
imperative. However, the exact functional mechanisms of
chemotherapeutic drugs have not been fully elucidated.
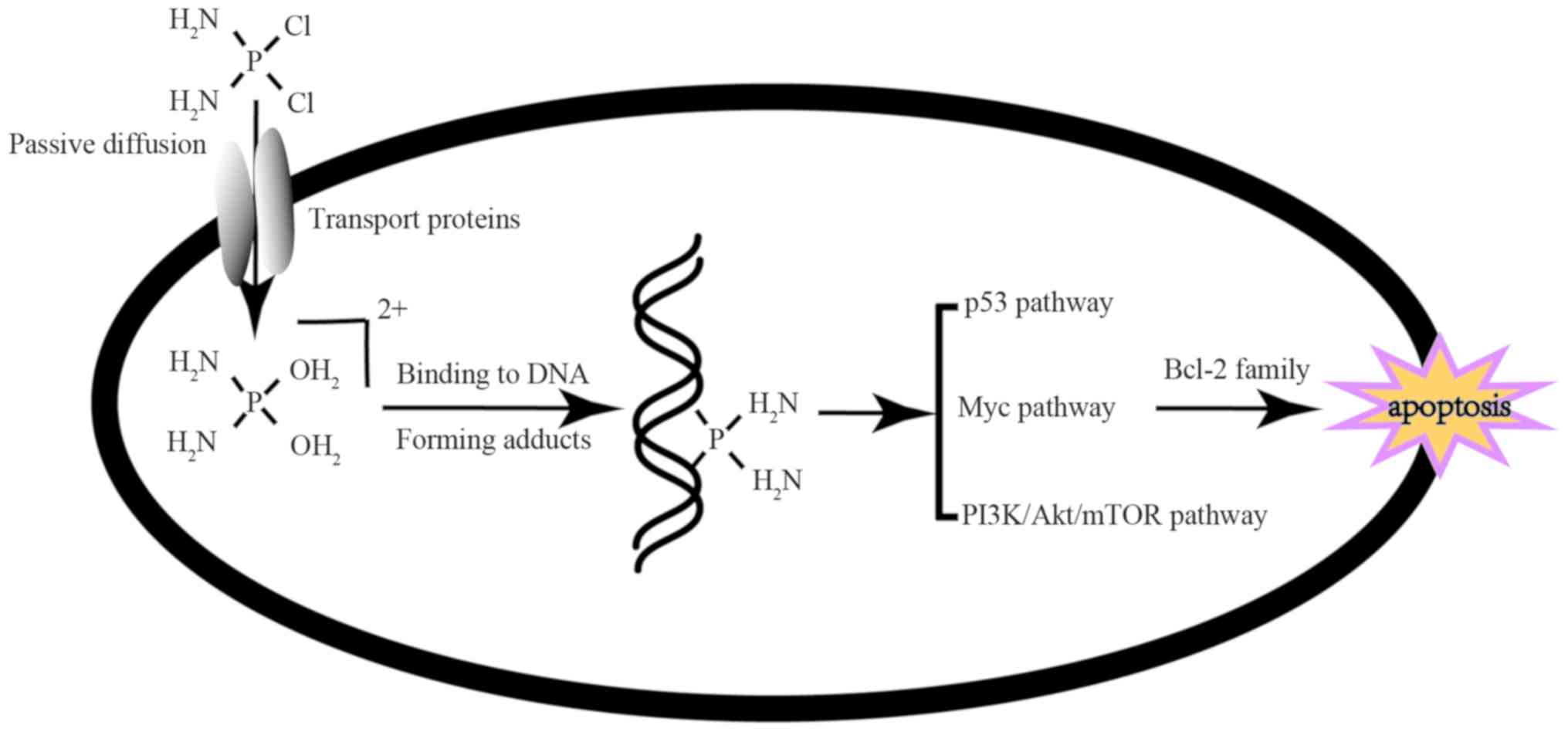
Briefly, there are 4 steps that platinum undergoes
to function as an antitumor agent. First, drugs need to enter to
cells through passive diffusion or active transport. In this step,
some transporters involved in the cellular uptake of platinum and
its derivatives have been identified, such as copper transporter 1,
which is considered as the major influx transporter for platinum
drugs (41). Pan et al
found that theaflavin-3,3′-digallate (TF3), a black tea polyphenol,
upregulated the expression of copper transporter 1 to enhance the
cytotoxicity of platinum in ovarian cancer (42). Once entering the cell, platinum
will undergo a substitution reaction in which a water molecule
replaces the chloride as a ligand, which is conducive to platinum
binding to DNA and causing DNA damage by forming stable Pt-DNA
crosslinks, which will destabilize the structure and function of
the DNA. Due to the DNA repair system, however, cells can survive
from agent attack. Alternatively, cells that cannot repair the DNA
lesion will activate several signaling pathways, including the
p53-mediated signaling pathway (43,44), the Myc gene-mediated signaling
pathway (45,46) and the PI3K/Akt/mTOR signaling
pathway (47,48). The activation of these signaling
pathways will affect the levels of Bcl-2 family proteins and
ultimately lead to apoptosis, which functions as the terminal event
in chemotherapy (Fig. 2).
Chemoresistance serves as a crucial reason for a poor prognosis and
a high mortality of ovarian cancer. However, these factors
mentioned above do not completely describe the full biological
repertoire of the resistance mechanism to platinum in ovarian
cancer.
4. Bcl-2 family members in ovarian
cancer
As the induction of cell apoptosis is the terminal
event in platinum-based chemotherapy, resistance to apoptosis is
one of the crucial labels of resistance to chemotherapy (49). There are a number of cancer cells
that evade apoptosis. Preventing cancer cells from evading
apoptosis is a promising strategy with which to reverse resistance
to chemotherapy. The Bcl-2 family, as a central part of the
apoptotic signaling network, plays a vital role in the resistance
of ovarian cancer to chemo-therapy. The Bcl-2 family members were
initially discovered to be hallmarks of follicular lymphomas
(50). There is a sophisticated
interaction network between Bcl-2 family members that has yet to be
completely known (51). However,
in cancer cells, the interaction among Bcl-2 family members may not
be the same as in normal cells and there is ample evidence to
indicate that a number of cancer cell lines present a disrupted
Bcl-2 protein expression that is associated with cancer survival
and chemoresistance.
Bcl-2 pro-survival proteins
Ovarian cancer, similar to other tumors, has been
shown to overexpress Bcl-2 and its family members (52-55). Of note, although Bcl-2
pro-survival proteins can prolong the survival period of tumor
cells in hematological tumors, playing an important role in
tumori-genesis (54), the truth
seems to be opposite in ovarian cancer cell lines. Although the
expression of Bcl-2 family proteins in ovarian cancer cells and
tissues has not yet been fully elucidated, studies have confirmed
that Bcl-2 family proteins play a key role in ovarian cancer
chemotherapy (56,57). Bcl-2 pro-survival proteins are
listed below:
i) Bcl-2 protein
Bcl-2 was the first discovered member of the Bcl-2
family (30). The expression of
Bcl-2 is intricate in ovarian cancer cells and tissues. In a
previous study, by detecting Bcl-2 in a panel of 12 parent human
ovarian carcinoma cell lines, the expression level of Bcl-2 was
found to be negatively associated with cisplatin sensitivity. Bcl-2
protein expression was relatively low in ovarian cancer resistant
cell lines (such as SKOV-3, 59M, OVCAR-3), but high in sensitive
cell lines (such as 41M and CH1). In addition, the expression
pattern of Bcl-2 also differed in various cell lines. For example,
in parent cells and the cisplatin-resistant cell line, A2780, Bcl-2
was almost undetectable (52).
However, another study investigating the expression of Bcl-2
protein in ovarian cancer tissues revealed a different pattern in
which Bcl-2 protein expression was evidently increased in lymphatic
metastasis and post-operative recurrence tissue, and was associated
with tumor stage (58). Bcl-2
knockdown by siRNA in chemoresistant multicellular spheroids of
ovarian cancer has also revealed enhanced apoptosis (59). For this abnormal expression
phenomenon, it is considered that apoptosis occurs due to the
protein-protein interaction between pro-apoptotic factors and
pro-survival factors. BAX, as a pro-apoptotic factor, can form
heterodimers with pro-survival factors, such as Bcl-2 or homodimers
with itself. The trend of apoptosis depends on the ratio of the two
dimers (53). Therefore, the
occurrence of apoptosis is not a single effect produced by one
certain molecule, but a result of the participation of all
molecules.
ii) Mcl-1
Mcl-1 is, a pro-survival protein in the Bcl-2
family. It has long been found that its upregulated expression is
associated with a poor prognosis in ovarian cancer. Due to its
short half-life of ~30 min and an unstable nature, it can respond
to stress conditions of cells (60). The ubiquitination of Mcl-1 is an
important strategy with which to inactivate Mcl-1; thus, several
ubiquitinases have been found to play a role in the stability of
Mcl-1. In ovarian cancer cells, ubiquitinating enzymes (DUBs) can
protect Mcl-1 from ubiquitination to maintain the stability of
Mcl-1, At the cell and tissue level of ovarian cancer, the
expression levels of Mcl-1 and DUBs have been shown to be
positively associated with chemoresistance, indicating that this
signal axis is attributed to chemoresistance in ovarian cancer
(61). Additionally, Usp13,
another ubiquitination enzyme, can also be used to stabilize Mcl-1
through ubiquitination (62).
Moreover, the use of the Mcl-1 antagonist, MIM1, can effectively
increase the sensitivity of ovarian cancer cells to paclitaxel
(63). The increased stability of
Mcl-1 seems to be the main mechanism of chemoresistance in ovarian
cancer. Additionally, Mcl-1 has been found to be the direct target
of some novel drugs. For example, as calcium is a universal second
messenger and a significant component in ER-induced apoptosis, when
inhibiting the calcium signal in ovarian cancer cells, it was found
that the Mcl-1 was down-regulated by calcium signal through a
calmodulin-mediated pathway (64). Moreover, the overexpression of
Mcl-1 was shown to reverse the apoptosis induced by ABT737,
indicating a crucial hurdle of Mcl-1 in chemosensitivity (64).
iii) Bcl-xL
Soon after the Bcl-2 gene was cloned, Bcl-xL, with a
very similar structure to Bcl-2 was identified (30). In early previous studies, Bcl-xL
was found to mediate cisplatin resistance in ovarian cancer cells
(65). Later studies indicated
that Bcl-xL contributes to the chemotherapeutic resistance of
ovarian cancer stem cells to a greater extent than Bcl-2, as in
chemotherapy-resistant ovarian cancer cells that preferentially
express Bcl-xL, the 40% knockdown of Bcl-xL expression is
sufficient to fully activate caspases (49), indicating that the acquired
chemoresistance of ovarian cancer is related to the abnormal
increase of Bcl-xL expression.
Pro-apoptotic proteins
Pro-apoptotic proteins can be divided into 2
subgroups: The executors of apoptosis and the initiators of
apoptosis, which are mainly responsible for activating the
executors. The coordinated expression of both endows ovarian cancer
cells with chemosensitivity. The pro-apoptotic proteins are listed
below:
i) BH3-only proteins
BH3-only proteins have been assumed to be initiators
of apoptosis; thus, they have to be sensitive to diverse types of
cell insults. The loss of BIK deceases the anti-tumor effect of
cisplatin by blocking the process of apoptosis in ovarian cancer
(66). PUMA, another BH3-only
protein, is considered to be a transcription target of p53 and an
effective apoptosis-inducing factor of a number of tumor cells in
recent years. It has been found that the high expression of PUMA
can effectively induce the apoptosis of ovarian cancer cells by
reducing the threshold set by pro-survival Bcl-xL and Mcl-1, and
then increase the chemosensitivity of resistant A2780 and SKOV3
cells to cisplatin, suggesting that PUMA is an important target
which may be used to overcome the chemoresistance of ovarian cancer
(67). BID is considered to be
the most effective initiator among BH3-only proteins. When
constructed into tumor-specific oncolytic adenoviruses, the
overexpression of BID exhibited great antitumor activation and
enhanced the chemosensitivity to cisplatin in 9 ovarian cancer cell
lines, consistent with the effect in a subcutaneous tumor xenograft
model (68).
ii) BAX, BAK and BOK
BAX, as the main effector of apoptosis, is also an
important mediator of cell apoptosis. BAK exerts similar effects on
the induction of apoptosis as BAX, and BOK has been possibly less
extensively studied in ovarian cancer. BAX, BAK and BOK directly
integrate into the OMM using their insertion domains, affecting
membrane permeability, eventually causing the downstream events of
apoptosis (69). It has been
demonstrated that the phosphorylation of BAX will alter its
pro-apoptosis activity into pro-survival, leading to the drug
resistance of ovarian cancer cells, suggesting that the change in
apoptotic molecule activity is an important reason for the drug
resistance of tumor cells (70).
Another study found that BAX conducts the Hsp70-mediated apoptosis
in ovarian cancer. Hsp70 protein can protect cells from apoptosis
and decrease the activation of BAX, which can significantly
attenuate the apoptosis induced by cisplatin in both resistant and
sensitive cell lines. Using immunoprecipitation, it was found that
Hsp70 co-immunoprecipitated with BAX in the resistant cell lines
C13 and A2780cp, but not in sensitive cells (71). According to a study on ovarian
cancer cells, no significant increase in the drug sensitivity of
tumor cells was observed in BAX-overexpressing cells (52). However, the upregulation of BAX by
BAX-expressing vectors in the ovarian cancer cell lines, SKOV3ip
and DOV13, induced significant cell death (72). At the tissue level, upon analyzing
the expression of BAX in 45 patients with epithelial ovarian
cancer, patients with high levels of BAX in tumor tissues achieved
complete sensitivity to chemotherapy, while patients with low
levels of BAX did not (73).
Recently, strong evidence has suggested that mitochondrial porin
voltage-dependent anion channel 2 (VDAC2) is essential for BAX
translocating to mitochondria-specific membranes (74,75). BAK, another pro-apoptotic factor,
also revealed a significant interaction with VDAC2. VDAC2
maintained BAK in an inactive conformation by interacting with its
hydrophobic tail, while the absence of VDAC2 did not result in BAK
activation though the threshold of apoptosis decreased (76). Some studies have demonstrated that
VDAC2 plays a complex role in regulating the interaction of BAX and
BAK with the mitochondria, suggesting that VDAC2 may be a promising
target for regulating the activation of pro-apoptotic proteins
(77,78).
5. Bcl-protein inhibitors and ovarian
cancer
The exact mechanisms of platinum therapeutic
resistance remain unclear and the development of novel platinum
analogues is tedious; these pose two significant issues in the
clinical treatment of ovarian cancer. Platinum drugs activate a
series of signaling pathways, such as p53, inducing DNA damage
(79). An important target of p53
is BH3-only proteins, including Puma and Noxa (80). However, tumor cells neutralize the
pro-apoptotic effects of these drugs with their high expression of
Bcl-2 pro-survival proteins (81), which functions as an inhibitor of
pro-apoptotic proteins. Edlich et al found that the
pro-survival proteins, Bcl-xL, Bcl-2 and Mcl-1, interacted with BAX
to promote BAX retrotranslocation from the mitochondria into the
cytoplasm and then prevent apoptosis (82). Therefore, it is of great clinical
significance to study the drugs that directly target Bcl-2
pro-survival protein. Over the past decades, the identification of
a variety of small molecular inhibitors of Bcl-2 pro-survival
proteins has promoted their development and application in clinical
practice (81). They have similar
structural and functional characteristics to BH3 only proteins,
thus, they are also termed 'BH3 mimetics' (83,84) (Table II).
 | Table IIPromising drugs that have an impact
on improving chemoresistance in ovarian cancers. |
Table II
Promising drugs that have an impact
on improving chemoresistance in ovarian cancers.
| Drugs | Targeting
molecules | Whether tested in
ovarian cancer | Combination
therapy | Side-effects | (Refs.) |
|---|
| ABT737 | Bcl-2, Bcl-xL,
Bcl-w | Yes (in
vitro and ex vivo) | Carboplatin,
Cisplatin, | Failing to be
orally available | (89,91,92) |
| ABT263 | Bcl-2, Bcl-xL,
Bcl-w | Yes (in
vitro and in vivo) | Carboplatin,
paclitaxel, PARP inhibitor |
Thrombocytopenia | (94-96) |
| AT101 | Pan-proteins of
anti-apoptotic Bcl-2 family members | Yes (in
vitro) | Cisplatin | | (100,101) |
| ABT199 | Bcl-2 | Yes (in
vitro) | Carboplatin | | (103,104) |
| S63845 | Mcl-1 | No | | | (105,106) |
| TW-37 | Pan-proteins of
anti-apoptotic Bcl-2 family members | Yes (in
vitro) | Cisplatin | | (107) |
ABT-737
ABT-737 was developed through nuclear magnetic
resonance (NMR)-based fragment screening and is the first classical
BH3-mimetic that can target the 3 pro-survival proteins, Bcl-xL,
Bcl-2 and Bcl-w (85). In ovarian
cancer, ABT-737 induces apoptosis more potently in
cisplatin-resistant ovarian cancer cells than in
cisplatin-sensitive ovarian cancer cells and exerts a synergistic
effect on ovarian cancer cells, indicating that ABT-737 may
represent a promising therapeutic strategy in treating patients
with cisplatin-resistant ovarian cancer (86). ABT-737 binds to Bcl-2 protein,
resulting in the release of BAX/BAK, which can promote tumor cell
apoptosis (87). The mechanisms
through which ABT-737 induces apoptosis have been further studied
(88). ABT-737 increases the
level of DRP-1 in the mitochondria of ovarian cancer cells
(88), and subsequently induces
mitochondrial fission, resulting in cytochrome c release,
while another mechanism is that it reverses cisplatin resistance by
inducing Ca2+ transfer from the ER to the mitochondrion
and cytosol, resulting in the enhancement of ER- and
mitochondrion-induced apoptosis (89). Furthermore, the combination
therapy of cisplatin and ABT-737, can significantly increase cell
death in chemo-resistant ovarian cancer cell lines (87). Most notably, previously, fresh
specimens from 25 patients with advanced serous ovarian cancer
(HGSOC) were exposed to ABT-737 with or without carboplatin in
vitro. An antitumor effect of ABT-737 was observed, although it
was not enhanced by carboplatin, indicating that the mechanism
through which ABT-737 induces apoptosis in ovarian cancers in
vivo is not the same as that in vitro (90) and further investigations are
required.
ABT-263
Although ABT-737 exerts a significant promoting
effect on cisplatin-induced apoptosis, it is difficult to implement
in clinical applications as it is not orally available. Lately, a
closely related drug, ABT-263 (Navitoclax), was identified that has
been shown to enhance chemosensitivity in clinical testing
(85,91,92) and to reduce chemoresistance in
ovarian cancer (93). In 3 HGSOC
cell lines, OVCAR3, OVCAR8 and OV90, the effects of ABT-263
combined with PARP inhibitors were evaluated by detecting DNA
damage accumulation, cell cycle progression, apoptosis induction
and Bcl-2 family protein expression levels of tumor cells. ABT-263
alone can increase the expression of BIM, and its combination with
PARP inhibitors can induce the apoptosis of tumor cells to a
greater extent (94). Of note,
when combined with carboplatin and paclitaxel, ABT-263 exhibited
additive activity in inducing apoptosis in vitro, which
provided a rationale for the treatment of ovarian cancer with
ABT-263 (91). Recently, a study
found that the combination of MEK inhibitor and ABT-263
significantly inhibited tumor growth in vivo and in
vitro in ovarian cancer (95).
AT101
As the toxicity of Navitoclax was observed with the
occurrence of dose-limiting thrombocytopenia (96) in clinical trials, there are other
BH3-only mimetics, such as AT101, which is a natural product from
cottonseed with a BH3-mimetic structure (97). AT101 is clinically safe and has
entered phase II clinical trials. A previous study demonstrated
that although AT101 can activate BAX in the induction of apoptosis,
the knockdown of BAX did not completely inhibit the activation of
caspase-3, while the downregulation of Smac greatly impacted the
apoptosis induced by AT101, indicating that Smac release is a
determinant of events in AT101-induced apoptosis, but not dependent
on BAX activation (98) in
ovarian cancer. In addition, in a study combining AT101 with
cisplatin in the treatment of ovarian cancer cells, combination
treatment evidently decreased the expression of some pro-survival
proteins, such as Bcl-2, and increased the expression of
pro-apoptotic proteins, indicating great potential for overcoming
chemoresistance (99).
ABT-199
The highly selective Bcl-2 inhibitor, ABT-199, which
was approved by the FDA for chronic lymphocytic leukemia (CLL) with
17p deletion, has been shown to inhibit the cancer cell growth in
several of cancer types (100),
including ovarian cancer cells (101). Although thrombocytopenia can be
avoided with the use of ABT-199, in a study characterizing the
ability of ABT199, ABT-737 and other roles in ovarian cancer,
ABT-737 successfully augmented carboplatin-induced tumor cell
apoptosis, while ABT-199 failed to do so (102). This may be due to the
phosphorylation of Bcl-2 protein induced by chemotherapeutic drugs,
which prevents the targeting of phosphorylated Bcl-2 by ABT199 and
the induction of the apoptosis of the tumor cells (101). These findings remind us that
efforts to explore the functional mechanisms of drugs are equally
important as their discovery.
In addition, Mcl-1, functions not only as a
pro-survival protein, but also as the cause of embryonic lethality
in Mcl-1 knockout mice. S63845 is a specific molecular inhibitor of
Mcl-1 and only affect the pro-survival function of Mcl-1 by binding
to the BH3 domain (103). It has
been reported that S63845 has a high safety dose for the treatment
of cancers in mice, and is more effective in killing multiple
myeloma cell lines than ABT-263 and ABT-199, while sparing the
normal tissues at efficacious doses (104). TW-37 is a small-molecule
inhibitor of Bcl-2 family proteins. Treatment of ovarian cancer
cells with TW-37 alone or combined with cisplatin has been shown to
result in the inhibition of growth and the induction ofapoptosis by
downregulating the expression of Bcl-2, suggesting that TW-37 may
be an efficient agent for treating ovarian cancer (105).
At present, molecular-targeted drugs that
selectively target Bcl-2 family proteins have been examined in
clinical trials. As early as April 2016, the selective Bcl-2
inhibitor, venetoclax (AbbVie), was approved by the FDA to be used
to treat CLL due to its positive clinical results. In terms of the
importance of the Bcl-2 family in chemotherapy, targeted Bcl-2
family proteins also provide hope for the treatment of ovarian
cancer (106).
6. BH3 profiling and ovarian cancer
Recognizing how Bcl-2 family proteins function in
the apoptosis of cancer cells induced by chemotherapeutic drugs,
Letai's research team (please see below) investigated an analytical
technique based on Bcl-2 family proteins. Cancer cells have
different expression codes among Bcl-2 family proteins. BH3-only
proteins decide whether cancer cells are primed for death. In other
words, BH3-only proteins can be effective predictive biomarkers,
which is the rationale for BH3 profiling and dynamic BH3 profiling
(DBP) (107,108).
BH3 profiling and Bcl-2 family
members
Bcl-2 family proteins are crucial regulators of
apoptosis. Thus, a delicate balance between members of the Bcl-2
family determines whether the cancer cell will live or die under
stress conditions (30).
Pro-apoptotic proteins bind with anti-apoptotic proteins to form
complexes and inhibit apoptosis. BH3-only protein is first
activated as a 'guard'. Activated BH3-only protein can release
pro-apoptotic proteins by binding with anti-apoptotic proteins, or
directly activate (109), such
as BAX and BAK, which aggregate together and then induce apoptosis
(51). The interaction of Bcl-2
family proteins determines whether cells have the potential to go
through apoptosis, indicating that the apoptotic state of cancer
cells depends on not just one molecule, but the comprehensive
effect of the interaction of many components. Thus, BH3 profiling
technology has become an important and innovative method. Upon
extracting mitochondria from cancer cells of interest and then
exposing them to a BH3 peptide that promotes apoptosis, cytochrome
c release can be measured to determine MOMP. If the
permeability of mitochondrial membrane in vitro increases
significantly, the mitochondria are considered to be prone to
apoptosis (33,107,110). The term 'priming' refers to the
critical condition for mitochondria to reach the apoptotic
threshold. The BH3 profiling technique can be used to detect the
threshold for apoptosis in specific cells (19). The level of the threshold
determines how difficult it would be for the cells to go through
apoptosis.
Performing a BH3 profiling assay requires the
extraction of mitochondria from cells and purifying enough
mitochondria requires a relatively large number of cells as
experimental materials (107-109 cells),
which, to a certain extent, decreases the clinical operability and
application. Therefore, Ryan and Letai further improved the
experimental technology, based on the rationale of the BH3
profiling assay and proposed the dynamic BH3 profiling assay
(111). The main improvement is
that this method omits the procedure of extracting mitochondria,
replaced by treatment with a low concentration of digoxin to not
only improve the permeability of cytomembranes, but also to
maintain their integrity. BH3 mimetic peptide synthesized in
vitro can enter into cells and successfully interact with
mitochondria. JC-1, an ideal fluorescent probe, is widely used to
detect mitochondrial membrane potential (112). The degree of mitochondrial
apoptosis can be evaluated by detecting the color changing of the
fluorescent probe. The dynamic BH3 profiling assay had the same
effect compared to BH3 profiling through comparative analysis
(113). For example, Montero
et al discovered that DBP successfully predicts sensitivity
to chemotherapy in non-small cell lung cancer and breast cancer
cell lines, and helps to select the optimal treatment strategy for
patients (108).
BH3 profiling for ovarian cancer
therapy
BH3 profiling and DBP assay provide novel insight
into the individualized therapy for ovarian cancer. Both functional
assays can be used to detect drug toxicity, predict the clinical
response of ovarian cancer patients to a certain treatment, and
find the best combination between drugs (108). If the response of patients to
drugs can be predicted and the most optimized treatment strategy
can be selected, this would greatly reduce treatment-related pain
and the economic burden to patients. Fortunately, the emergence of
the BH3 profiling assay may break this deadlock. It can be used to
detect the mitochondrion priming towards antitumor drugs and
predict the clinical responses to certain agents, only requiring 4
h from the blood draw to the profiling results (114).
It has been suggested that a number of agents may
have some ability to reverse chemoresistance or induce apoptosis in
ovarian cancer; however, it becomes more challenging to identify
the right agents for the right patient (115). BH3 profiling and DBP assay
provide practicable methods to measure how primed a cell is when
treated with an antitumor agent. BH3 profiling was proposed to
detect the proper drugs for treating patients with tumors, while
DBP extends the clinical utilization of the BH3 profiling (115). Patients with ovarian cancer
exhibit different responses to different drugs. DBP can also
predict the chemosensitivity of ovarian cancer. For example, when
using DBP to detect the priming of single-cell suspensions of
samples from 16 primary ovarian adenocarcinomas and the results are
consistent with the carboplatin response in ovarian cancer
(116), indicating that DBP can
be used in predicting the susceptibility of ovarian cancer to
antitumor agents and select the most effective drugs.
Some researchers have found that the apoptotic
priming of cancer cells is positively associated with the clinical
response of patients to chemotherapeutic drugs. The stronger the
priming, the better the prognosis. Ni et al followed 14
patients with ovarian cancer and tested their carbohydrate antigen
125 (CA125) levels to determine their response to chemotherapy and
found that individuals with high apoptotic priming of mitochondria
measured prior to chemotherapy had a better final response to
chemotherapy (116).
BH3 profiling can have great application prospects
for searching for novel biomarkers in future experimental research
on ovarian cancer. For example, by detecting the apoptotic priming
in cancer cells, the researchers found an association between the
level of the OMM scaffold protein Sab in cells and the
chemosensitivity of ovarian cancer cells, suggesting that Sab may
be a biomarker for predicting the prognosis of ovarian cancer after
chemotherapy (117). Thus, DBP
or BH3 profiling can predict the chemosensitivity of patients with
ovarian cancer and have great potential in medical research and
individualized treatments.
7. Conclusion
In conclusion, the high rate of chemoresistance of
ovarian cancer renders this type of cancer the most lethal among
the cancers affecting women, and the dysregulation of apoptosis is
one of the important mechanisms. Bcl-2 family members have a
complicated interaction network and play a central role in
regulating apoptosis. Recent findings revealed novel inter-action
relationships among Bcl-2 family members. Further research of the
intricate molecular events regulating cell apoptosis would be
extremely beneficial for cancer therapy, providing promising
targets for overcoming the chemoresistance. Although a number of
targeted drugs have entered the clinical trial stage, there are
some prospective drugs that are still at the cell level, and few
animal models have been established. BH3 profiling or DBP can test
the the chemosensitivity of cancer cells. In the future, the
combination of BH3 profiling or DBP and drugs targeting Bcl-2
family proteins may help to promote individualized treatments for
patients with ovarian cancer.
Funding
The present study was supported by the project of
Hunan Provincial Natural Science Foundation of China (grant no.
2018JJ3782), the China Scholarship Fund, CSC (grant no.
201806375049), the Postgraduate Independent Exploration And
Innovation Project Of Central South University (grant nos.
2018zzts937, 2018zzts952, 2019zzts1055) and the Project of Degree
and Postgraduate Education and Teaching Reform in Hunan Province
(grant no. JG2018A003).
Availability of data and materials
Not applicable.
Authors' contributions
JY, HL, XJ and DZ reviewed the literature and wrote
the article. SX reviewed the literature and revised the article.
All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Webb PM and Jordan SJ: Epidemiology of
epithelial ovarian cancer. Best Pract Res Clin Obstet Gynaecol.
41:3–14. 2017. View Article : Google Scholar
|
|
3
|
Davis A, Tinker AV and Friedlander M:
'Platinum resistant' ovarian cancer: What is it, who to treat and
how to measure benefit? Gynecol Oncol. 133:624–631. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
International Collaborative Ovarian and
Neoplasm Group: Paclitaxel plus carboplatin versus standard
chemotherapy with either single-agent carboplatin or
cyclophosphamide, doxorubicin, and cisplatin in women with ovarian
cancer: The ICON3 randomised trial. Lancet. 360:505–515. 2002.
View Article : Google Scholar
|
|
5
|
Boussios S, Karihtala P, Moschetta M,
Abson C, Karathanasi A, Zakynthinakis-Kyriakou N, Ryan JE, Sheriff
M, Rassy E and Pavlidis N: Veliparib in ovarian cancer: A new
synthetically lethal therapeutic approach. Invest New Drugs.
38:181–193. 2020. View Article : Google Scholar
|
|
6
|
Markman M: Optimizing primary chemotherapy
in ovarian cancer. Hematol Oncol Clin North Am. 17:957–968. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bast RC Jr, Hennessy B and Mills GB: The
biology of ovarian cancer: New opportunities for translation. Nat
Rev Cancer. 9:415–428. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lindemann K, Gao B, Mapagu C, Fereday S,
Emmanuel C, Alsop K, Traficante N; Australian Ovarian Cancer Study
Group; Harnett PR, Bowtell DDL and DeFazio A: Response rates to
second-line platinum-based therapy in ovarian cancer patients
challenge the clinical definition of platinum resistance. Gynecol
Oncol. 150:239–246. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Matulonis UA, Sood AK, Fallowfield L,
Howitt BE, Sehouli J and Karlan BY: Ovarian cancer. Nat Rev Dis
Primers. 2:160612016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bowtell DD, Bohm S, Ahmed AA, Aspuria PJ,
Bast RC Jr, Beral V, Berek JS, Birrer MJ, Blagden S, Bookman MA, et
al: Rethinking ovarian cancer II: Reducing mortality from
high-grade serous ovarian cancer. Nat Rev Cancer. 15:668–679. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xie H, Wang W, Xia B, Jin W and Lou G:
Therapeutic applications of PARP inhibitors in ovarian cancer.
Biomed Pharmacother. 127:1102042020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Boussios S, Karihtala P, Moschetta M,
Karathanasi A, Sadauskaite A, Rassy E and Pavlidis N: Combined
strategies with poly (ADP-Ribose) polymerase (PARP) inhibitors for
the treatment of ovarian cancer: A literature review. Diagnostics
(Basel). 9:872019. View Article : Google Scholar
|
|
13
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Matsuura K, Huang NJ, Cocce K, Zhang L and
Kornbluth S: Downregulation of the proapoptotic protein MOAP-1 by
the UBR5 ubiquitin ligase and its role in ovarian cancer resistance
to cisplatin. Oncogene. 36:1698–1706. 2017. View Article : Google Scholar :
|
|
15
|
Fernald K and Kurokawa M: Evading
apoptosis in cancer. Trends Cell Biol. 23:620–633. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hassan M, Watari H, AbuAlmaaty A, Ohba Y
and Sakuragi N: Apoptosis and molecular targeting therapy in
cancer. Biomed Res Int. 2014:1508452014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Valentin R, Grabow S and Davids MS: The
rise of apoptosis: Targeting apoptosis in hematologic malignancies.
Blood. 132:1248–1264. 2018. View Article : Google Scholar
|
|
18
|
Kerr JF, Wyllie AH and Currie AR:
Apoptosis: A basic biological phenomenon with wide-ranging
implications in tissue kinetics. Br J Cancer. 26:239–257. 1972.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chung C: Restoring the switch for cancer
cell death: Targeting the apoptosis signaling pathway. Am J Health
Syst Pharm. 75:945–952. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Green DR and Llambi F: Cell death
signaling. Cold Spring Harb Perspect Biol. 7:a0060802015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Martinvalet D: Mitochondrial entry of
cytotoxic proteases: A new insight into the granzyme B cell death
pathway. Oxid Med Cell Longev. 2019:91652142019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Solano-Gálvez SG, Abadi-Chiriti J,
Gutiérrez-Velez L, Rodríguez-Puente E, Konstat-Korzenny E,
Álvarez-Hernández DA, Franyuti-Kelly G, Gutiérrez-Kobeh L and
Vázquez-López R: Apoptosis: Activation and inhibition in health and
disease. Med Sci (Basel). 6. pp. 542018
|
|
23
|
Shamas-Din A, Kale J, Leber B and Andrews
DW: Mechanisms of action of Bcl-2 family proteins. Cold Spring Harb
Perspect Biol. 5:a87142013. View Article : Google Scholar
|
|
24
|
Caria S, Hinds MG and Kvansakul M:
Structural insight into an evolutionarily ancient programmed cell
death regulator- the crystal structure of marine sponge BHP2 bound
to LB-Bak-2. Cell Death Dis. 8:e25432017. View Article : Google Scholar
|
|
25
|
Kvansakul M and Hinds MG: The Bcl-2
family: Structures, interactions and targets for drug discovery.
Apoptosis. 20:136–150. 2015. View Article : Google Scholar
|
|
26
|
Banjara S, Suraweera CD, Hinds MG and
Kvansakul M: The Bcl-2 family: Ancient origins, conserved
structures, and diver-gent mechanisms. Biomolecules. 10:1282020.
View Article : Google Scholar
|
|
27
|
Kvansakul M and Hinds MG: The structural
biology of BH3-only proteins. Methods Enzymol. 544:49–74. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang DC and Strasser A: BH3-Only
proteins-essential initiators of apoptotic cell death. Cell.
103:839–842. 2000. View Article : Google Scholar
|
|
29
|
Elkholi R, Renault TT, Serasinghe MN and
Chipuk JE: Putting the pieces together: How is the mitochondrial
pathway of apoptosis regulated in cancer and chemotherapy? Cancer
Metab. 2:162014. View Article : Google Scholar
|
|
30
|
Adams CM, Clark-Garvey S, Porcu P and
Eischen CM: Targeting the Bcl-2 family in B cell lymphoma. Front
Oncol. 8:6362019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Estaquier J, Vallette F, Vayssiere JL and
Mignotte B: The mitochondrial pathways of apoptosis. Adv Exp Med
Biol. 942:157–183. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Martinou JC and Youle RJ: Mitochondria in
apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev
Cell. 21:92–101. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Letai A, Bassik MC, Walensky LD,
Sorcinelli MD, Weiler S and Korsmeyer SJ: Distinct BH3 domains
either sensitize or activate mitochondrial apoptosis, serving as
prototype cancer therapeutics. Cancer Cell. 2:183–192. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mérino D, Giam M, Hughes PD, Siggs OM,
Heger K, O'Reilly LA, Adams JM, Strasser A, Lee EF, Fairlie WD and
Bouillet P: The role of BH3-only protein bim extends beyond
inhibiting Bcl-2-like prosurvival proteins. J Cell Biol.
186:355–362. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sarosiek KA, Chi X, Bachman JA, Sims JJ,
Montero J, Patel L, Flanagan A, Andrews DW, Sorger P and Letai A:
BID prefer-entially activates BAK while BIM preferentially
activates BAX, affecting chemotherapy response. Mol Cell.
51:751–765. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Scorrano L, Oakes SA, Opferman JT, Cheng
EH, Sorcinelli MD, Pozzan T and Korsmeyer SJ: BAX and BAK
regulation of endoplasmic reticulum Ca2+: A control point for
apoptosis. Science. 300:135–139. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Große L, Wurm CA, Brüser C, Neumann D,
Jans DC and Jakobs S: Bax assembles into large ring-like structures
remodeling the mitochondrial outer membrane in apoptosis. EMBO J.
35:402–413. 2016. View Article : Google Scholar
|
|
38
|
Salvador-Gallego R, Mund M, Cosentino K,
Schneider J, Unsay J, Schraermeyer U, Engelhardt J, Ries J and
García-Sáez AJ: Bax assembly into rings and arcs in apoptotic
mitochondria is linked to membrane pores. EMBO J. 35:389–401. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Smaili SS, Hsu YT, Youle RJ and Russell
JT: Mitochondria in Ca2+ signaling and apoptosis. J Bioenerg
Biomembr. 32:35–46. 2000. View Article : Google Scholar
|
|
40
|
van Zyl B, Tang D and Bowden NA:
Biomarkers of platinum resistance in ovarian cancer: What can we
use to improve treatment. Endocr Relat Cancer. 25:R303–R318. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Larson CA, Blair BG, Safaei R and Howell
SB: The role of the mammalian copper transporter 1 in the cellular
accumulation of platinum-based drugs. Mol Pharmacol. 75:324–330.
2009. View Article : Google Scholar :
|
|
42
|
Pan H, Kim E, Rankin GO, Rojanasakul Y, Tu
Y and Chen YC: Theaflavin-3,3'-digallate enhances the inhibitory
effect of cisplatin by regulating the copper transporter 1 and
glutathione in human ovarian cancer cells. Int J Mol Sci.
19:1172018. View Article : Google Scholar
|
|
43
|
Vousden KH and Lane DP: p53 in health and
disease. Nat Rev Mol Cell Biol. 8:275–283. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Miyashita T and Reed JC: Tumor suppressor
p53 is a direct transcriptional activator of the human bax gene.
Cell. 80:293–299. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Conacci-Sorrell M, McFerrin L and Eisenman
RN: An overview of MYC and its interactome. Cold Spring Harb
Perspect Med. 4:a143572014. View Article : Google Scholar
|
|
46
|
Cao X, Bennett RL and May WS: c-Myc and
caspase-2 are involved in activating Bax during cytotoxic
drug-induced apoptosis. J Biol Chem. 283:14490–14496. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hahne JC, Honig A, Meyer SR, Gambaryan S,
Walter U, Wischhusen J, Häussler SF, Segerer SE, Fujita N, Dietl J
and Engel JB: Downregulation of AKT reverses platinum resistance of
human ovarian cancers in vitro. Oncol Rep. 28:2023–2028. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Cheaib B, Auguste A and Leary A: The
PI3K/Akt/mTOR pathway in ovarian cancer: Therapeutic opportunities
and challenges. Chin J Cancer. 34:4–16. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Cardenas C, Montagna MK, Pitruzzello M,
Lima E, Mor G and Alvero AB: Adipocyte microenvironment promotes
Bclxl expression and confers chemoresistance in ovarian cancer
cells. Apoptosis. 22:558–569. 2017. View Article : Google Scholar
|
|
50
|
Adams JM and Cory S: The Bcl-2 apoptotic
switch in cancer development and therapy. Oncogene. 26:1324–1337.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Leibowitz B and Yu J: Mitochondrial
signaling in cell death via the Bcl-2 family. Cancer Biol Ther.
9:417–422. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Beale PJ, Rogers P, Boxall F, Sharp SY and
Kelland LR: BCL-2 family protein expression and platinum drug
resistance in ovarian carcinoma. Br J Cancer. 82:436–440. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Marx D and Meden H: Differential
expression of apoptosis-associated genes Bax and Bcl-2 in ovarian
cancer. Methods Mol Med. 39:687–691. 2001.PubMed/NCBI
|
|
54
|
Fauvet R, Dufournet C, Poncelet C, Uzan C,
Hugol D and Daraï E: Expression of pro-apoptotic (p53, p21, bax,
bak and fas) and anti-apoptotic (Bcl-2 and Bcl-x) proteins in
serous versus mucinous borderline ovarian tumours. J Surg Oncol.
92:337–343. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Palmer JE, Sant Cassia LJ, Irwin CJ,
Morris AG and Rollason TP: P53 and bcl-2 assessment in serous
ovarian carcinoma. Int J Gynecol Cancer. 18:241–248. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Chaudhry P, Srinivasan R and Patel FD:
Expression of the major fas family and Bcl-2 family of proteins in
epithelial ovarian cancer (EOC) and their correlation to
chemotherapeutic response and outcome. Oncol Res. 18:549–559. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Binju M, Amaya-Padilla MA, Wan G,
Gunosewoyo H, Suryo Rahmanto Y and Yu Y: Therapeutic inducers of
apoptosis in ovarian cancer. Cancers (Basel). 11:17862019.
View Article : Google Scholar
|
|
58
|
Liang M and Zhao J: Protein expressions of
AIB1, p53 and Bcl-2 in epithelial ovarian cancer and their
correlations with the clinical pathological features and prognosis.
Eur Rev Med Pharmacol Sci. 22:5134–5139. 2018.PubMed/NCBI
|
|
59
|
Yang Y, Li S, Sun Y, Zhang D, Zhao Z and
Liu L: Reversing platinum resistance in ovarian cancer
multicellular spheroids by targeting Bcl-2. Onco Targets Ther.
12:897–906. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Inuzuka H, Shaik S, Onoyama I, Gao D,
Tseng A, Maser RS, Zhai B, Wan L, Gutierrez A, Lau AW, et al:
SCF(FBW7) regulates cellular apoptosis by targeting MCL1 for
ubiquitylation and destruction. Nature. 471:104–109. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Wu X, Luo Q, Zhao P, Chang W, Wang Y, Shu
T, Ding F, Li B and Liu Z: MGMT-activated DUB3 stabilizes MCL1 and
drives chemoresistance in ovarian cancer. Proc Natl Acad Sci USA.
116:2961–2966. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zhang S, Zhang M, Jing Y, Yin X, Ma P,
Zhang Z, Wang X, Di W and Zhuang G: Deubiquitinase USP13 dictates
MCL1 stability and sensitivity to BH3 mimetic inhibitors. Nat
Commun. 9:2152018. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Habata S, Iwasaki M, Sugio A, Suzuki M,
Tamate M, Satohisa S, Tanaka R and Saito T: BAG3-mediated Mcl-1
stabilization contributes to drug resistance via interaction with
USP9X in ovarian cancer. Int J Oncol. 49:402–410. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Bonnefond ML, Lambert B, Giffard F,
Abeilard E, Brotin E, Louis MH, Gueye MS, Gauduchon P, Poulain L
and N'Diaye M: Calcium signals inhibition sensitizes ovarian
carcinoma cells to anti-Bcl-xL strategies through Mcl-1
down-regulation. Apoptosis. 20:535–550. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Liu JR, Fletcher B, Page C, Hu C, Nunez G
and Baker V: Bcl-xL is expressed in ovarian carcinoma and modulates
chemo-therapy-induced apoptosis. Gynecol Oncol. 70:398–403. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Nawrocki ST, Kelly KR, Smith PG, Espitia
CM, Possemato A, Beausoleil SA, Milhollen M, Blakemore S, Thomas M,
Berger A and Carew JS: Disrupting protein NEDDylation with MLN4924
is a novel strategy to target cisplatin resistance in ovarian
cancer. Clin Cancer Res. 19:3577–3590. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Yuan Z, Cao K, Lin C, Li L, Liu HY, Zhao
XY, Liu L, Deng HX, Li J, Nie CL and Wei YQ: The p53 upregulated
modulator of apoptosis (PUMA) chemosensitizes intrinsically
resistant ovarian cancer cells to cisplatin by lowering the
threshold set by Bcl-x(L) and Mcl-1. Mol Med. 17:1262–1274. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Dai Y, Zhao XJ, Li F, Yuan Y, Yan DM, Cao
H, Huang XY, Hu Z, Ma D and Gao QL: Truncated Bid regulates
cisplatin response via activation of mitochondrial apoptosis
pathway in ovarian cancer. Hum Gene Ther. 31:325–338. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Yamaguchi H, Bhalla K and Wang HG: Bax
plays a pivotal role in thapsigargin-induced apoptosis of human
colon cancer HCT116 cells by controlling Smac/Diablo and Omi/HtrA2
release from mitochondria. Cancer Res. 63:1483–1489.
2003.PubMed/NCBI
|
|
70
|
Kale J, Kutuk O, Brito GC, Andrews TS,
Leber B, Letai A and Andrews DW: Phosphorylation switches Bax from
promoting to inhibiting apoptosis thereby increasing drug
resistance. EMBO Rep. 19:e452352018. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Yang X, Wang J, Zhou Y, Wang Y, Wang S and
Zhang W: Hsp70 promotes chemoresistance by blocking Bax
mitochondrial translocation in ovarian cancer cells. Cancer Lett.
321:137–143. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Huang X, Lin T, Gu J, Zhang L, Roth JA,
Stephens LC, Yu Y, Liu J and Fang B: Combined TRAIL and Bax gene
therapy prolonged survival in mice with ovarian cancer xenograft.
Gene Ther. 9:1379–1386. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Tai YT, Lee S, Niloff E, Weisman C,
Strobel T and Cannistra SA: BAX protein expression and clinical
outcome in epithelial ovarian cancer. J Clin Oncol. 16:2583–2590.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Lauterwasser J, Todt F, Zerbes RM, Nguyen
TN, Craigen W, Lazarou M, van der Laan M and Edlich F: The porin
VDAC2 is the mitochondrial platform for Bax retrotranslocation. Sci
Rep. 6:329942016. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Edlich F: BCL-2 proteins and apoptosis:
Recent insights and unknowns. Biochem Biophys Res Commun.
500:26–34. 2018. View Article : Google Scholar
|
|
76
|
Lazarou M, Stojanovski D, Frazier AE,
Kotevski A, Dewson G, Craigen WJ, Kluck RM, Vaux DL and Ryan MT:
Inhibition of Bak activation by VDAC2 is dependent on the Bak
transmembrane anchor. J Biol Chem. 285:36876–36883. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Shoshan-Barmatz V, Keinan N and Zaid H:
Uncovering the role of VDAC in the regulation of cell life and
death. J Bioenerg Biomembr. 40:183–191. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Shimizu S, Narita M and Tsujimoto Y: Bcl-2
family proteins regulate the release of apoptogenic cytochrome c by
the mitochondrial channel VDAC. Nature. 399:483–487. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Boulikas T and Vougiouka M: Cisplatin and
platinum drugs at the molecular level (Review). Oncol Rep.
10:1663–1682. 2003.PubMed/NCBI
|
|
80
|
Li J, Lee B and Lee AS: Endoplasmic
reticulum stress-induced apoptosis: Multiple pathways and
activation of p53-up-regulated modulator of apoptosis (PUMA) and
NOXA by p53. J Biol Chem. 281:7260–7270. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Campbell KJ and Tait S: Targeting BCL-2
regulated apoptosis in cancer. Open Biol. 8:1800022018. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Edlich F, Banerjee S, Suzuki M, Cleland
MM, Arnoult D, Wang C, Neutzner A, Tjandra N and Youle RJ: Bcl-x(L)
retrotranslocates Bax from the mitochondria into the cytosol. Cell.
145:104–116. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Delbridge AR and Strasser A: The BCL-2
protein family, BH3-mimetics and cancer therapy. Cell Death Differ.
22:1071–1080. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Billard C: BH3 mimetics: Status of the
field and new developments. Mol Cancer Ther. 12:1691–1700. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Oltersdorf T, Elmore SW, Shoemaker AR,
Armstrong RC, Augeri DJ, Belli BA, Bruncko M, Deckwerth TL, Dinges
J, Hajduk PJ, et al: An inhibitor of Bcl-2 family proteins induces
regression of solid tumours. Nature. 435:677–681. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Xu Y, Gao W, Zhang Y, Wu S, Liu Y, Deng X,
Xie L, Yang J, Yu H, Su J and Sun L: ABT737 reverses cisplatin
resistance by targeting glucose metabolism of human ovarian cancer
cells. Int J Oncol. 53:1055–1068. 2018.PubMed/NCBI
|
|
87
|
Dai Y, Jin S, Li X and Wang D: The
involvement of Bcl-2 family proteins in AKT-regulated cell survival
in cisplatin resistant epithelial ovarian cancer. Oncotarget.
8:1354–1368. 2017. View Article : Google Scholar :
|
|
88
|
Yu Y, Xu L, Qi L, Wang C, Xu N, Liu S, Li
S, Tian H, Liu W, Xu Y and Li Z: ABT737 induces mitochondrial
pathway apoptosis and mitophagy by regulating DRP1-dependent
mitochondrial fission in human ovarian cancer cells. Biomed
Pharmacother. 96:22–29. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Xie Q, Su J, Jiao B, Shen L, Ma L, Qu X,
Yu C, Jiang X, Xu Y and Sun L: ABT737 reverses cisplatin resistance
by regulating ER-mitochondria Ca2+ signal transduction in human
ovarian cancer cells. Int J Oncol. 49:2507–2519. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Lheureux S, N'Diaye M, Blanc-Fournier C,
Dugué AE, Clarisse B, Dutoit S, Giffard F, Abeilard E, Briand M,
Labiche A, et al: Identification of predictive factors of response
to the BH3-mimetic molecule ABT-737: An ex vivo experiment in human
serous ovarian carcinoma. Int J Cancer. 136:E340–E350. 2015.
View Article : Google Scholar
|
|
91
|
Stamelos VA, Robinson E, Redman CW and
Richardson A: Navitoclax augments the activity of carboplatin and
paclitaxel combinations in ovarian cancer cells. Gynecol Oncol.
128:377–382. 2013. View Article : Google Scholar
|
|
92
|
Tse C, Shoemaker AR, Adickes J, Anderson
MG, Chen J, Jin S, Johnson EF, Marsh KC, Mitten MJ, Nimmer P, et
al: ABT-263: A potent and orally bioavailable Bcl-2 family
inhibitor. Cancer Res. 68:3421–3428. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Wong M, Tan N, Zha J, Peale FV, Yue P,
Fairbrother WJ and Belmont LD: Navitoclax (ABT-263) reduces
Bcl-x(L)-mediated chemoresistance in ovarian cancer models. Mol
Cancer Ther. 11:1026–1035. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Yokoyama T, Kohn EC, Brill E and Lee JM:
Apoptosis is augmented in high-grade serous ovarian cancer by the
combined inhibition of Bcl-2/Bcl-xL and PARP. Int J Oncol.
50:1064–1074. 2017. View Article : Google Scholar :
|
|
95
|
Iavarone C, Zervantonakis IK, Selfors LM,
Palakurthi S, Liu JF, Drapkin R, Matulonis UA, Hallberg D,
Velculescu VE, Leverson JD, et al: Combined MEK and BCL-2/XL
inhibition is effective in high-grade serous ovarian cancer
patient-derived xenograft models and bim levels are predictive of
responsiveness. Mol Cancer Ther. 18:642–655. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Wilson WH, O'Connor OA, Czuczman MS,
LaCasce AS, Gerecitano JF, Leonard JP, Tulpule A, Dunleavy K, Xiong
H, Chiu YL, et al: Navitoclax, a targeted high-affinity inhibitor
of BCL-2, in lymphoid malignancies: A phase 1 dose-escalation study
of safety, pharmacokinetics, pharmacodynamics, and antitumour
activity. Lancet Oncol. 11:1149–1159. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Meng Y, Tang W, Dai Y, Wu X, Liu M, Ji Q,
Ji M, Pienta K, Lawrence T and Xu L: Natural BH3 mimetic
(-)-gossypol chemosensitizes human prostate cancer via Bcl-xL
inhibition accompanied by increase of Puma and Noxa. Mol Cancer
Ther. 7:2192–2202. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Hu W, Wang F, Tang J, Liu X, Yuan Z, Nie C
and Wei Y: Proapoptotic protein Smac mediates apoptosis in
cisplatin-resistant ovarian cancer cells when treated with the
anti-tumor agent AT101. J Biol Chem. 287:68–80. 2012. View Article : Google Scholar :
|
|
99
|
Karaca B, Atmaca H, Bozkurt E, Kisim A,
Uzunoglu S, Karabulut B, Sezgin C, Sanli UA and Uslu R: Combination
of AT-101/cisplatin overcomes chemoresistance by inducing apoptosis
and modulating epigenetics in human ovarian cancer cells. Mol Biol
Rep. 40:3925–3933. 2013. View Article : Google Scholar
|
|
100
|
Touzeau C, Dousset C, Le Gouill S, Sampath
D, Leverson JD, Souers AJ, Maïga S, Béné MC, Moreau P,
Pellat-Deceunynck C and Amiot M: The Bcl-2 specific BH3 mimetic
ABT-199: A promising targeted therapy for t(11;14) multiple
myeloma. Leukemia. 28:210–212. 2014. View Article : Google Scholar :
|
|
101
|
Song T, Zhang M, Liu P, Xue Z, Fan Y and
Zhang Z: Identification of JNK1 as a predicting biomarker for
ABT-199 and paclitaxel combination treatment. Biochem Pharmacol.
155:102–109. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Abed MN, Abdullah MI and Richardson A:
Antagonism of Bcl-XL is necessary for synergy between carboplatin
and BH3 mimetics in ovarian cancer cells. J Ovarian Res. 9:252016.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Yamaguchi R, Lartigue L and Perkins G:
Targeting Mcl-1 and other Bcl-2 family member proteins in cancer
therapy. Pharmacol Ther. 195:13–20. 2019. View Article : Google Scholar
|
|
104
|
Kotschy A, Szlavik Z, Murray J, Davidson
J, Maragno AL, Toumelin-Braizat GL, Chanrion M, Kelly GL, Gong JN,
Moujalled DM, et al: The MCL1 inhibitor S63845 is tolerable and
effective in diverse cancer models. Nature. 538:477–482. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Wang H, Zhang Z, Wei X and Dai R:
Small-molecule inhibitor of Bcl-2 (TW-37) suppresses growth and
enhances cisplatin-induced apoptosis in ovarian cancer cells. J
Ovarian Res. 8:32015. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Deng J: How to unleash mitochondrial
apoptotic blockades to kill cancers? Acta Pharm Sin B. 7:18–26.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Certo M, Del Gaizo Moore V, Nishino M, Wei
G, Korsmeyer S, Armstrong SA and Letai A: Mitochondria primed by
death signals determine cellular addiction to antiapoptotic BCL-2
family members. Cancer Cell. 9:351–365. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Montero J, Sarosiek KA, DeAngelo JD,
Maertens O, Ryan J, Ercan D, Piao H, Horowitz NS, Berkowitz RS,
Matulonis U, et al: Drug-induced death signaling strategy rapidly
predicts cancer response to chemotherapy. Cell. 160:977–989. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Kim H, Tu HC, Ren D, Takeuchi O, Jeffers
JR, Zambetti GP, Hsieh JJD and Cheng EHY: Stepwise activation of
BAX and BAK by tBID, BIM, and PUMA initiates mitochondrial
apoptosis. Mol Cell. 36:487–499. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Deng J, Carlson N, Takeyama K, Dal Cin P,
Shipp M and Letai A: BH3 profiling identifies three distinct
classes of apoptotic blocks to predict response to ABT-737 and
conventional chemotherapeutic agents. Cancer Cell. 12:171–185.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Ryan J and Letai A: BH3 profiling in whole
cells by fluorimeter or FACS. Methods. 61:156–164. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Elefantova K, Lakatos B, Kubickova J,
Sulova Z and Breier A: Detection of the mitochondrial membrane
potential by the cationic dye JC-1 in L1210 cells with massive
overexpression of the plasma membrane ABCB1 drug transporter. Int J
Mol Sci. 19:19852018. View Article : Google Scholar :
|
|
113
|
Ryan J, Montero J, Rocco J and Letai A:
iBH3: Simple, fixable BH3 profiling to determine apoptotic priming
in primary tissue by flow cytometry. Biol Chem. 397:671–678. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Del Gaizo Moore V and Letai A:
rofiling-measuring integrated function of the mitochondrial
apoptotic pathway to predict cell fate decisions. Cancer Lett.
332:202–205. 2013. View Article : Google Scholar
|
|
115
|
Montero J and Letai A: Dynamic BH3
profiling-poking cancer cells with a stick. Mol Cell Oncol.
3:e10401442016. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Ni CT, Sarosiek KA, Vo TT, Ryan JA,
Tammareddi A, Del Gaizo Moore V, Deng J, Anderson KC, Richardson P,
Tai YT, et al: Pretreatment mitochondrial priming correlates with
clinical response to cytotoxic chemotherapy. Science.
334:1129–1133. 2011. View Article : Google Scholar
|
|
117
|
Paudel I, Hernandez SM, Portalatin GM,
Chambers TP and Chambers JW: Sab concentrations indicate
chemotherapeutic susceptibility in ovarian cancer cell lines.
Biochem J. 475:3471–3492. 2018. View Article : Google Scholar : PubMed/NCBI
|