Introduction
Among all types of cancers worldwide, lung cancer is
associated with the highest mortality and morbidity, with more than
1.5 million new cases diagnosed worldwide, as reported in 2014
(1). Approximately 85% of all
lung cancer cases are non-small cell lung cancer (NSCLC) (2). Currently, chemotherapy, radiotherapy
and surgical resection remain the primary treatment options for
NSCLC (1,2). Despite progress in the development
of targeted therapies and the improved efficacy of chemotherapy
drugs, the 5-year survival rate of patients with NSCLC remains low
(1,2). The low survival rate of patients
with NSCLC is due to their high chance of tumour metastasis, which
is caused by a number of different factors, such as tumour cell
invasion/migration, epithelial-mesenchymal transition (EMT) and
angiogenesis (3-8). In addition, activation of the C-X-C
chemokine receptor type 4 (CXCR4)/stromal cell-derived factor 1
(SDF1) pathway plays an essential role in the metastasis of NSCLC
(9,10).
Hesperidin (5,7,3-trihydroxy-4-methoxy-flavanone
7-rhamnoglucoside), a type of glycoside flavonoid, has been shown
to exert anticancer effects by inhibiting proliferation and
promoting apoptosis in numerous types of cancer, including lung
cancer and breast cancer (11-13). It has also been reported that
hesperidin inhibits the growth of NSCLC cells in vitro by
regulating the activation of pathways related to immune responses
and apoptosis (14,15). Therefore, hesperidin may be used
as a novel anti-proliferative agent in the treatment of NSCLC.
As a type of short non-coding RNA, with a length of
~22 nucleotides, microRNAs (miRNAs or miRs) can regulate the
expression of their target mRNAs by binding to the 3′-untranslated
region (3′-UTR) (16,17). As miRNAs act as a negative
regulators of gene expression, they serve an essential role in
regulating the growth, migration, invasion and metastasis of cancer
cells (18-20).
In a previous study, it was shown that miR-132
expression is downregulated in clinical samples and cell lines of
metastatic lung cancer, and miR-132 could target the regulator of
ETM, zinc finger E-box binding homeobox 2 (ZEB2), indicating that
miR-132 may act as a tumour suppressor (21). miR-132 can also suppress the
metastasis and EMT of NSCLC cells by inhibiting the function of
ZEB2. Notably, it was observed that two downstream effectors of
ZEB2, vimentin and E-cadherin, are also regulated by miR-132,
suggesting that miR-132 may be implicated in the invasion and
migration of NSCLC cells by regulating the EMT process (22).
The dysregulated miR-132 expression is thought to
play an essential role in the pathogenesis of mental disorders
(22). In fact, growing evidence
has demonstrated that miR-132 can affect the functions of
inflammatory and neurotrophic systems, both of which are implicated
in the pathogenesis of depression (23,24). A previous study demonstrated that
hesperidin increases miR-132 expression in the prefrontal cortex of
lipopolysaccharide-treated mice, suggesting that miR-132 is
implicated in the function of hesperidin as an antidepressant
(25). Other studies have
speculated that the increased expression of miR-132 may protect the
brain by stimulating the synthesis of neurotrophin and by blocking
the onset of neuroinflammation (26,27).
Yi et al (26) reported that the overexpression of
ZEB2 is associated with a poor prognosis of NSCLC and shorter
survival. By contrast, the knockdown of ZEB2 increased the
sensitivity of cancer cells to chemotherapeutic agents by inducing
cell cycle arrest in the S phase and by inducing cell apoptosis.
These findings indicated that ZEB2 may act as an oncogene in NSCLC.
In another study, the knockdown of ZEB2 inhibited the EMT, and the
invasion and migration of NSCLC, which is similar to the effect of
miR-200c overexpression (28).
It has been reported that the administration of
hesperidin can upregulate the expression of miR-132, which is a
potential regulator of haematological and neurological expressed 1
(HN1), TGF-β1 and ZEB2 (25,28-30). In addition, HN1, TGF-β1 and ZEB2
have been shown to be involved in the pathogenesis of NSCLC
(31-34). The present study investigated the
effect of hesperidin on the proliferation and apoptosis of NSCLC
cells.
Materials and methods
Animals and treatment
A total of 24 adult Sprague-Dawley (SD) male rats
(weight, 328-365 g), which were obtained from the Experimental
Animal Centre of Yantai Hospital of Traditional Chinese Medicine,
were used in this study. All rats were placed in an environment
with 50-60% humidity and a temperature of 22-24°C. The rats were
given free access to water and food, and a 12 h light-dark cycle
was applied. Following 7 days of environmental adaptation, the rats
were randomly assigned into two groups; a negative control (NC)
group and a hesperidin group, with 12 rats in each group. In the NC
group, the rats were transplanted with NSCLC cells. In the
hesperidin group, the rats were transplanted with NSCLC cells and
treated with 60 mg/kg/day hesperidin at the same time to
investigate the effect of hesperidin on NSCLC. The NSCLC cells were
transplanted into the right lung using a 28G1/2 needle. At the end
of the experiment, all rats were sacrificed by sodium pentobarbital
(100 mg/kg i.p.) and the tumour tissues were isolated and measured.
Death was confirmed by combined factors, including lack of pulse,
breathing, corneal reflex and response to toe pinch; inability to
hear respiratory sounds and heartbeat; and failure of syringe and
needle to move after percutaneous cardiac puncture following
unconsciousness. The weight of SD male rats at the start of the
study was 259±35 and 263±38 g in the NC and hesperidin groups,
respectively, while the weight of the rats at the end of the study
was 342±52 and 322±48 g in the NC and hesperidin groups,
respectively. The tumour volume was measured using callipers and
was calculated as the height × width ×2. All animal experiments in
the present study were approved by the Animal Ethics Committee of
Yantai Hospital of Traditional Chinese Medicine.
RNA isolation and reverse
transcription-quantitative PCR (RT-qPCR)
A TRIzol reagent kit (Invitrogen; Thermo Fisher
Scientific, Inc.) was used to extract the total RNA from isolated
tissue samples or cultured A549 and H460 cells. The concentration
of isolated RNA was measured using a NanoDrop 3000
spectrophotometer (Thermo Fisher Scientific, Inc.). Subsequently,
isolated RNA was reverse transcribed into cDNA using a PrimeScript
RT reagent kit (Takara Bio, Inc.) and oligo primers (Takara Bio,
Inc.). The conditions of RT were as follows: 38°C for 15 min and
85°C for 5 sec. Subsequently, qPCR was performed using the Power
SYBR Green PCR Master mix (Takara Bio, Inc.). During qPCR, U6 RNA
was used as the internal control to normalize the mRNA expression
levels of HN1, ZEB2 and miR-132. The primer sequences were as
follows: HN1 forward, 5′-CGC AGG CCC TAA ACT ACC AG-3′ and reverse,
5′-TGC TAC TGT CGA TGT GGA CC-3′; ZEB2 forward, 5′-GCA GTG AGC ATC
GAA GAG TAC C-3′ and reverse, 5′-GGC AAA AGC ATC TGG AGT TCC AG-3′;
miR-132 forward, 5′-ACC GTG GCT TTC GAT TG TT-3′ and reverse,
5′-GAA CAT GTC TGC GTA TCT C-3′; and U6 forward, 5′-GTG CTC GCT TCG
GCA GCA-3′ and reverse, 5′-CAA ATA TGG AAC GCT TC-3′. The
conditions of qPCR were as follows: Pre-denaturation at 95°C for 30
sec, 35 cycles of denaturation at 94°C for 15 sec, annealing at
56°C for 45 sec and extension at 72°C for 45 sec. At the end of
each qPCR cycle, the cycle threshold (Cq) value was acquired. The
relative mRNA expression of HN1, ZEB2 and miR-132 was calculated
using the 2−ΔΔCq method (35). Three independent experiments were
performed.
Cell culture and transfection
A549 and H460 cells were purchased from American
Type Culture Collection and incubated at 37°C with 5%
CO2. The culture medium was Dulbecco's modified Eagle's
medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.), supplemented
with 10% foetal bovine serum (FBS; Gibco; Thermo Fisher Scientific,
Inc.).
For hesperidin treatment experiments, the cells were
seeded into 96-well plates at a density of 1×105
cells/well and incubated at 37°C and 5% CO2 for 12 h,
followed by treatment with 1 or 2.5 µM hesperidin for 48 h
at room temperature. The cells were harvested after 48 h of
treatment and analysed. For transfection experiments, the cells
were seeded into 6-well plates at a density of 1×104
cells/well and incubated at 37°C and 5% CO2 overnight
until the cells were 70-80% confluent. On the following day, the
cells were transfected with miR-132 mimic, HN1 small interfering
RNA (siRNA), ZEB2 siRNA, miRNA negative controls (NCs) or siRNA
control (all from Santa Cruz Biotechnology, Inc.) using
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. After 48 h of
transfection, the cells were collected to analyse the expression of
target genes. All experiments were performed in triplicate.
Cytotoxicity assay
Transfected A549 and H460 cells were seeded into
48-well plates and cultured in DMEM overnight. Subsequently, 5
mg/ml MTT (Sigma-Aldrich; Merck KGaA) was added to each well, and
the incubation was continued for a further 4 h. Subsequently, DMSO
(Sigma-Aldrich; Merck KGaA) was added to each well, and the plates
were placed on a shaker for 10 min. Finally, the optical density in
each well was measured using a spectrophotometer at a wavelength of
490 nm. All experiments were run in triplicate.
Colony formation assay
After 48 h of transfection, the cells were detached
with 0.25% trypsin and resuspended in 2X DMEM (containing 20% FBS,
Gibco; Thermo Fisher Scientific, Inc.) to adjust the cell
concentration to 4×104 cells/ml. Hard agar medium (lower
layer) and soft agar medium (upper layer) were then prepared by
mixing a 1.0% soft agar solution and a 0.7% soft agar solution with
the 2X DMEM medium in a 1:1 ratio, respectively. Subsequently, the
hard agar medium was added into a six-well plate (1.5 ml/well) and
cooled to room temperature, followed by the addition of the soft
agar medium on top of the basal medium to form a double agar layer.
Following the solidification of the upper agar layer, the plate was
incubated at 37°C with 5% CO2 for 14-21 days, with
medium added at regular intervals to maintain the moisture on the
agar surface. The formation of the cell colonies was observed under
an inverted microscope (magnification ×200). The stained colonies
were photographed, and the number of clones was counted and
analysed using Image-Pro Plus 6.0 software (Media Cybernetics,
Inc.). All experiments were run in triplicate.
Vector construction, mutagenesis and
luciferase assay
A combination of bioinformatics tools, including
TargetScan (www.targetscan.org) and the miRNA
database miRBase (www.mirbase.org), were used to identify the potential
regulatory relationship between miR-132 and HN1, as well as the
regulatory relationship between miR-132 and ZEB2. After PCR was
carried out to amplify the 3′-UTR of HN1 and ZEB2 containing the
binding sites of miR-132, the PCR products were inserted into pcDNA
vectors (Promega Corporation) using a TA cloning kit (Invitrogen;
Thermo Fisher Scientific, Inc.) to generate the expression
constructs of wild-type HN1 and ZEB2. In addition, the Quick Change
XL site-directed mutagenesis kit (Stratagene; Merck KGaA) was used
to mutate the miR-132 binding sites in the 3′-UTR of HN1 and ZEB2.
The mutated sequences were also amplified by PCR and inserted into
pcDNA vectors to generate the expression constructs of mutant HN1
and ZEB2. Subsequently, A549 and H460 cells were co-transfected
with miR-132 and wild-type/mutant ZEB2 or miR-132 and
wild-type/mutant HN1 using Lipofectamine 2000, following the
manufacturer's instructions. At 48 h post-transfection, the
luciferase activity was measured using a Dual-Luciferase Reporter
assay system (Promega Corporation) on a luminometer. The activity
of Renilla luciferase was used as an internal control. Each
experiment was repeated three times.
Western blot analysis
Following two washes with PBS, NSCLC tissues and
transfected cells were ground in liquid nitrogen and homogenized
with the addition of lysis buffer (BioSharp, Inc.). Subsequently,
sample tissues were centrifuged at 241.49 × g for 30 min at 4°C to
remove debris. The supernatant was collected to measure the total
protein concentration using a bicinchoninic acid kit (BioTeke
Corporation), according to the manufacturer's protocol. Protein (50
µg) from each sample was then mixed with 2X SDS loading
buffer and boiled at 100°C for 5 min. following separation by 10%
SDS-PAGE, the protein was transferred onto a polyvinylidene
fluoride membrane. The membrane was then blocked with 5% skimmed
milk for 1 h at room temperature and incubated overnight at 4°C
with diluted (1:100) primary antibodies against HN1 (cat. no.
ab126705; Abcam), ZEB2 (cat. no. ab223688; Abcam) and GAPDH (cat.
no. ab8245; Abcam). Subsequently, the membrane was washed three
times with TBST and incubated for 1 h at room temperature with
rabbit anti-mouse horseradish peroxidase-labelled secondary
antibodies (1:2,000; cat. no. ab6728; Abcam). The membrane was then
developed using an enhanced chemiluminescence reagent (Biomiga,
Inc.) and visualized using an X-ray apparatus. GAPDH served as the
internal control to quantify the relative protein expression levels
of HN1 and ZEB2. ImageJ software (version 1.41; National Institutes
of Health) was used to quantify the western blots. Each experiment
was repeated three times.
Apoptosis analysis by flow cytometry and
TUNEL staining
FITC Annexin V Apoptosis Detection kit I (cat. no.
556547; BD Biosciences) was used according to the manufacture's
protocol to analyse the apoptosis of transfected A549 and H460
cells, with a flow cytometer (Thermo Fisher Scientific, Inc.). In
addition, apoptosis was assessed using a TUNEL Alexa Fluor™ 488
Imaging Assay kit (cat. no. C10245; Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. Each
experiment was repeated three times.
Statistical analysis
All data are presented as the mean ± standard error
of the mean. One-way ANOVA and an unpaired Student's t-test were
utilized to compare results among multiple groups and between two
groups, respectively. All statistical analysis was performed using
SPSS version 19.0 (IBM Corp.). P<0.05 was considered to indicate
a statistically significant difference.
Results
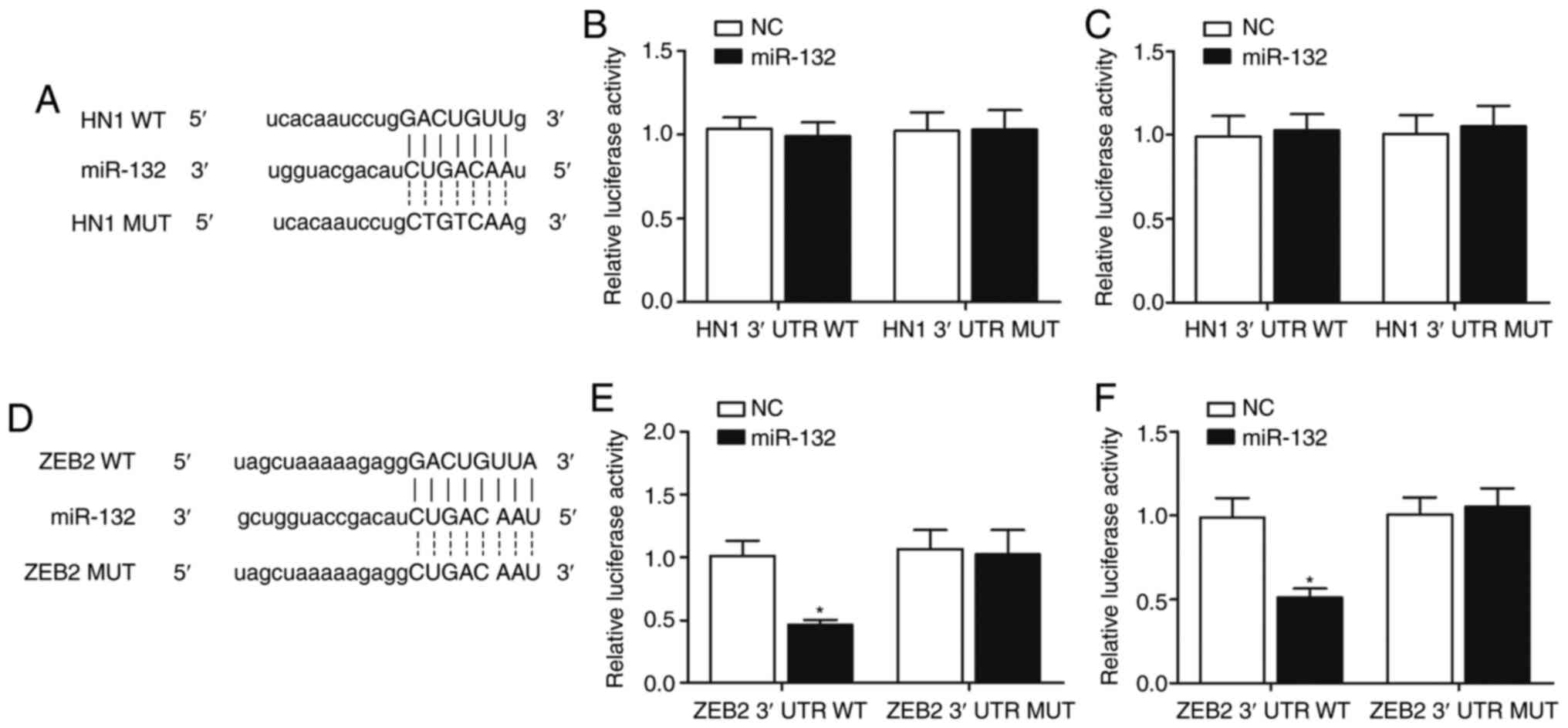
ZEB2 is a candidate target gene of
miR-132
By performing computational analysis using
TargetScan and the miRNA database miRBase, HN1 and ZEB2 were
identified as possible target genes of miR-132, with both 3'-UTRs
of HN1 (Fig. 1A) and ZEB2
(Fig. 1D) containing a potential
binding site for miR-132. To further investigate whether miR-132
targets HN1 and ZEB2 directly, luciferase reporter vectors
containing wild-type or mutant 3′-UTRs of HN1 or ZEB2 were created
and co-transfected into A549 and H460 cells with miR-132 mimic or
NC. The luciferase activity of wild-type or mutant HN1 3′-UTR was
not significantly different in A549 (Fig. 1B) and H460 (Fig. 1C) cells co-transfected with
miR-132 mimic compared with the NC. By contrast, co-transfection of
A549 (Fig. 1E) and H460 (Fig. 1F) cells with miR-132 mimic and
wild-type ZEB2 3′-UTR significantly reduced the luciferase activity
of the cells, suggesting that ZEB2 is a target gene of miR-132.
Effect of miR-132 and hesperidin on ZEB2
expression
To further investigate the effect of miR-132 on ZEB2
and HN1 expression, the expression levels of ZEB2 and HN1 were
measured in A549 cells (Fig.
2A-J) and H460 cells (Fig.
2K-T) transfected with miR-132 or ZEB2/HN1 siRNA. The
successful transfections of miR-132 mimic (Fig. S1A and D), HN1 siRNA (Fig. S1B and E) and ZEB2 siRNA (Fig. S1C and F) in A549 and H460 cells
were validated by RT-qPCR. In addition, the cells were treated with
different concentrations of hesperidin before measuring the
expression of ZEB2 and HN1. As presented in Fig. 2, miR-132 mimic had no effect on
the mRNA (Fig. 2A and K) and
protein (Fig. 2C, D, M and N)
expression levels of HN1, while transfection with HN1 siRNA
significantly decreased the expression of HN1 mRNA (Fig. 2A and K) and protein (Fig. 2C, D, M and N) in A549 and H460
cells. By contrast, the transfection of A549 and H460 cells with
miR-132 mimic or ZEB2 siRNA significantly reduced the expression of
ZEB2 mRNA (Fig. 2B and L) and
protein (Fig. 2C, E, M and O). In
addition, treatment of cells with various doses of hesperidin
demonstrated no effect on the expression of HN1 mRNA (Fig. 2F and P) and protein (Fig. 2H, I, R and S), while hesperidin
treatment significantly reduced the expression of ZEB2 mRNA
(Fig. 2G and Q) and protein
(Fig. 2H, J, R and T) in a
concentration-dependent manner. In summary, these results indicate
that miR-132 and hesperidin inhibit ZEB2 expression.
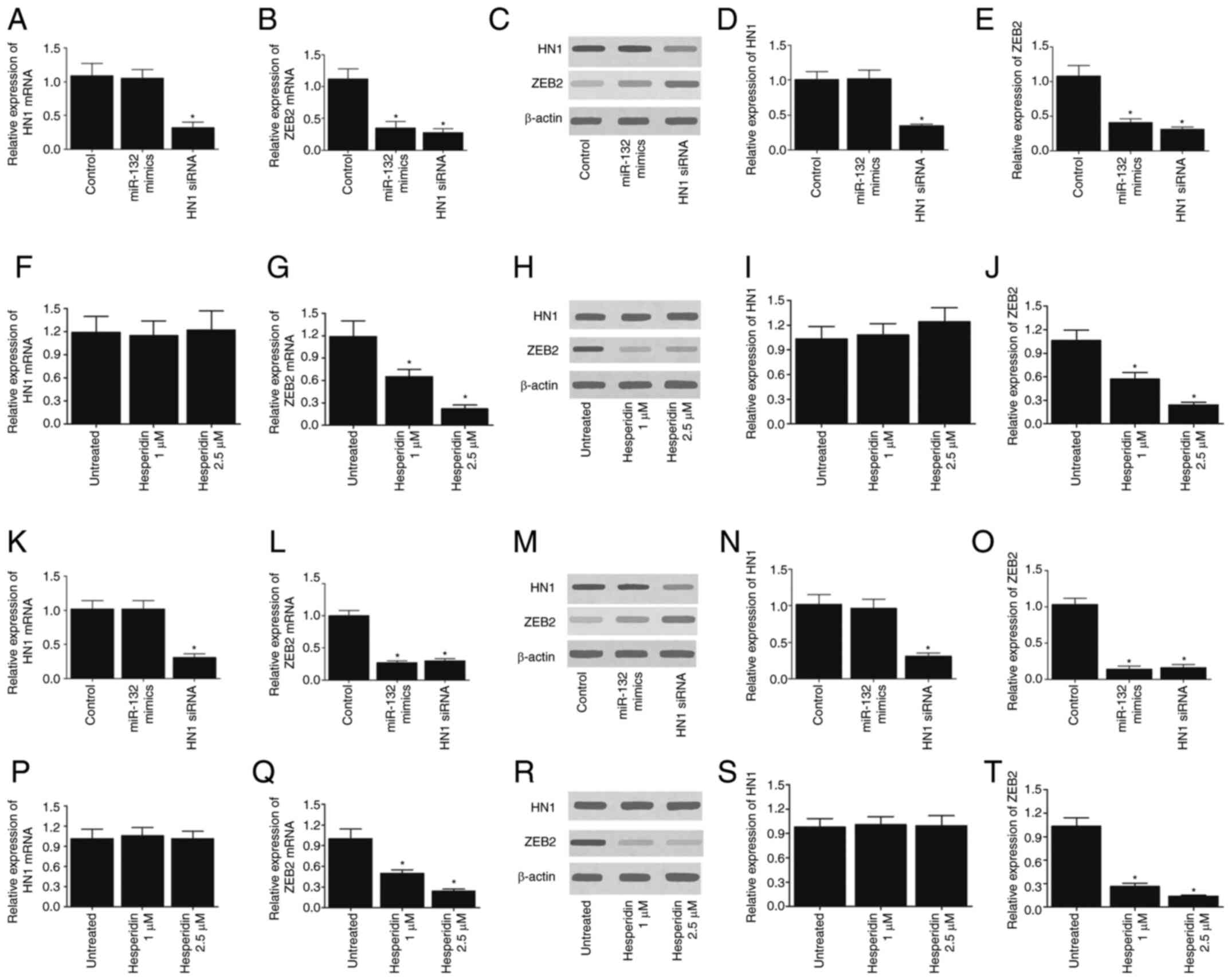 | Figure 2Effect of miR-132 and hesperidin on
ZEB2 expression in A549 and H460 cells. (A) In A549 cells, miR-132
mimic demonstrated no effect on the mRNA expression of HN1, whereas
HN1 siRNA reduced the mRNA expression of HN1. (B) In A549 cells,
miR-132 mimic and ZEB2 siRNA both decreased the mRNA expression of
ZEB2. (C and D) In A549 cells, miR-132 mimic demonstrated no effect
on the protein expression of HN1, but reduced the protein
expression of ZEB2. (E) In A549 cells, miR-132 mimic and ZEB2 siRNA
both decreased the protein expression of ZEB2. (F) Treatment with
hesperidin exhibited no effect on the mRNA expression of HN1 in
A549 cells. (G) Treatment with hesperidin decreased the mRNA
expression of ZEB2 in a concentration-dependent manner in A549
cells. (H) Western blot results of HN1 and ZEB2 protein in A549
cells treated with different concentrations of hesperidin. (I)
Treatment with hesperidin demonstrated no effect on the protein
expression of HN1 in A549 cells. (J) Treatment with hesperidin
decreased the protein expression of ZEB2 in a
concentration-dependent manner in A549 cells. (K) In H460 cells,
miR-132 mimic had no effect on the mRNA expression of HN1, whereas
HN1 siRNA reduced the mRNA expression of HN1. (L) In H460 cells,
miR-132 mimic and ZEB2 siRNA both decreased the mRNA expression of
ZEB2. (M and N) In H460 cells, miR-132 mimic exhibited no effect on
the protein expression of HN1, but HN1 siRNA reduced the
expression. (O) In H460 cells, miR-132 mimic and ZEB2 siRNA both
decreased the protein expression of ZEB2. (P) Treatment with
hesperidin demonstrated no effect on the mRNA expression of HN1 in
H460 cells. (Q) Treatment with hesperidin decreased the mRNA
expression of ZEB2 in a concentration-dependent manner in H460
cells. (R) Western blot results of HN1 and ZEB2 protein in H460
cells treated with different concentrations of hesperidin. (S)
Treatment with hesperidin had no effect on the protein expression
of HN1, (T) but decreased the protein expression of ZEB2 in a
concentration-dependent manner in H460 cells. *P<0.05
vs. control/untreated group. ZEB2, zinc finger E-box binding
homeobox 2; miR-132, microRNA-132; HN1, neurological expressed 1;
siRNA, small interfering RNA. |
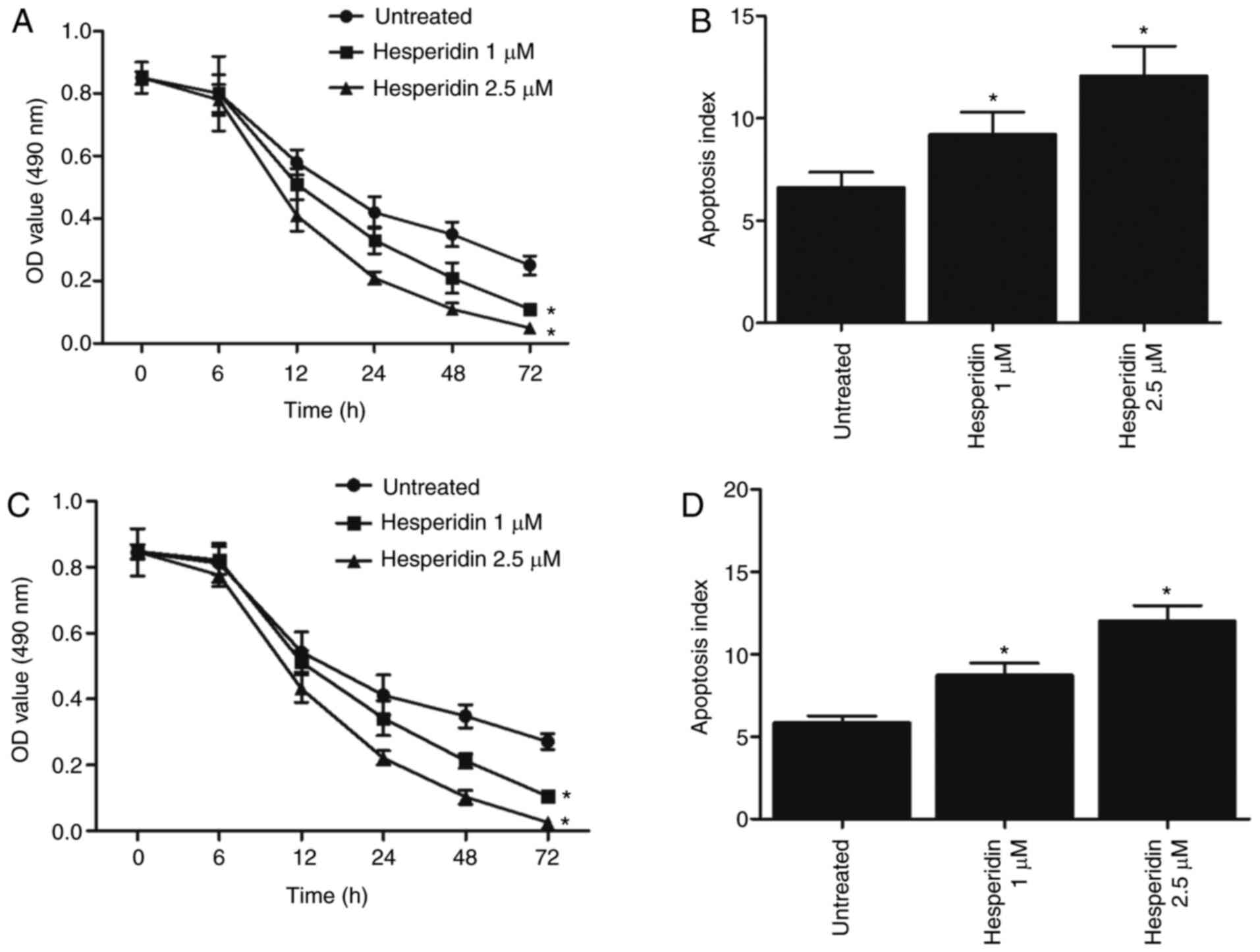
Effect of hesperidin on cell
proliferation and apoptosis
MTT assay and flow cytometry analysis were used to
evaluate the effect of hesperidin (1 and 2.5 µM) on the
viability and apoptosis of A549 and H460 cells. The results
demonstrated that treatment with hesperidin significantly inhibited
the proliferation of A549 (Fig.
3A) and H460 (Fig. 3C) cells,
and significantly promoted apoptosis (Fig. 3B and D) in a dose-dependent
manner.
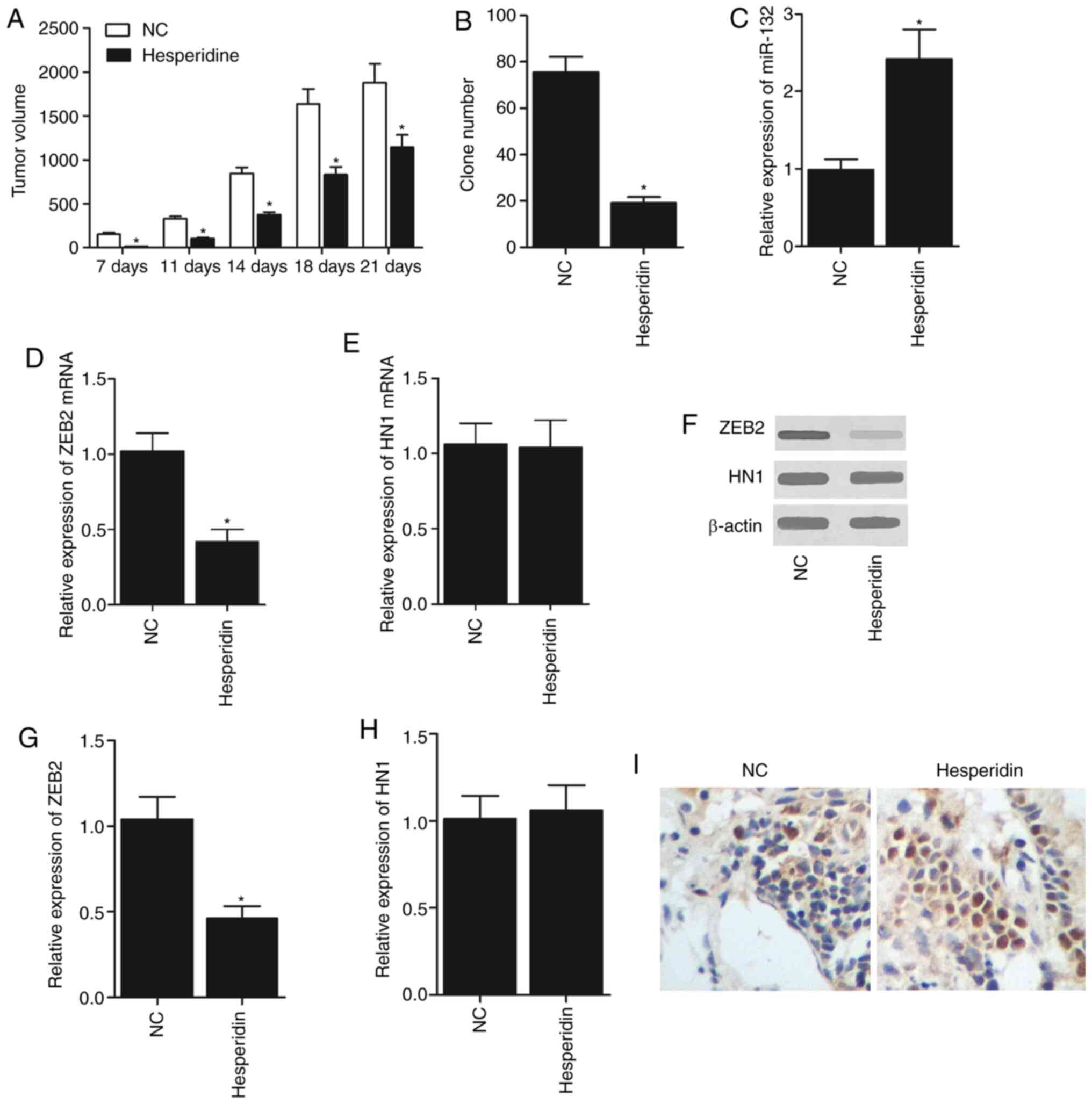
Effect of hesperidin on NSCLC
To investigate the effect of hesperidin on NSCLC,
experimental rats were randomly assigned to two groups. In one
group, the rats were trans-planted with NSCLC cells. In another
group, the rats were transplanted with NSCLC cells and treated with
hesperidin at the same time. As presented in Fig. 4, the rats trans-planted with NSCLC
cells and treated with hesperidin had significantly smaller tumour
volumes compared with the rats that only received NSCLC cell
transplantation (Fig. 4A). In
addition, treatment with hesperidin significantly decreased the
colony formation efficiency of NSCLC cells (Fig. 4B), and the TUNEL staining also
revealed more apoptotic NSCLC cells upon the administration of
hesperidin compared with the untreated NSCLC cells (Fig. 4I). Furthermore, RT-qPCR, western
blotting and IHC were used to detect the levels of miR-132, HN1 and
ZEB2 in the rats from the two groups. The results demonstrated that
hesperidin treatment significantly increased miR-132 expression
(Fig. 4C), and significantly
decreased the ZEB2 mRNA (Fig. 4D)
and protein (Fig. 4F and G)
expression levels in rats transplanted with NSCLC cells.
Furthermore, it was identified that the mRNA (Fig. 4E) and protein (Fig. 4F and H) expression levels of HN1
were similar in the two groups of rats.
Discussion
Hesperidin is a member of the flavanone family, and
flavanones are mainly produced in citrus fruits. Due to its effects
on disease treatment and prevention, hesperidin has become a topic
of interest in recent years (36). In particular, the anticancer
properties of hesperidin have been studied extensively (36). Hesperidin also exerts other
biological effects, including anti-inflammatory, antioxidant and
anti-mutagenic effects (37,38). In addition, hesperidin can inhibit
the proliferation of cancer cells in oral cancer (39). Furthermore, hesperidin can
suppress cancer cell invasion by inhibiting the expression of
proteins participating in EMT and members of the matrix
metallopeptidase (MMP) protein family (13,40,41). A previous study demonstrated that
hesperidin induces the apoptosis of A549 cells by activating the
proteins that participate in mitochondrial apoptosis and by
regulating the expression of molecules associated with cell cycle
progression (15). Another study
indicated that SDF-1/CXCR-4 signalling can block the role of
hesperidin in suppressing the invasion and migration of A549 cells
(14). Based on these results,
hesperidin may be a promising agent in the treatment of NSCLC.
Balakrishnan and Menon (40)
demonstrated that hesperidin induces the apoptosis of colon cancer
cells by increasing the activity of caspase-9 and caspase-3.
Furthermore, it has been reported that hesperidin induces the
apoptosis of HepG2 cells by activating ERK1/2 signalling (42). The present study investigated the
effect of hesperidin on cell proliferation and apoptosis using an
MTT assay and flow cytometry analysis. The results demonstrated
that hesperidin treatment repressed cell proliferation and promoted
cell apoptosis in a dose-dependent manner. Furthermore, RT-qPCR and
western blot analysis were performed to detect the effect of
miR-132 and hesperidin on the expression of HN1 and ZEB2. The
results indicated that both miR-132 and hesperidin could
significantly decrease the expression of ZEB2, while showing no
effect on HN1 expression.
It has previously been reported that hesperidin can
suppress the growth and induce the apoptosis of cancer cells
(14). In particular, hesperidin
triggers apoptotic signal-ling in NCI-H358 and A549 cells (14,43). In addition, hesperidin exerts
minimal cytotoxicity in MRC-5 cells, a type of normal lung
fibroblast (43). Hesperidin has
been shown to increase the expression of caspase-3 enzyme and
reduce the level of MMP, which is closely associated apoptosis
(44). The results of
FITC-Annexin V/PI staining in a recent study also demonstrated the
pro-apoptotic effect of hesperidin in NSCLC cells (15).
miRNA-132 has been reported to regulate the in
vivo expression of proteins associated with inflammation. A
previous study demonstrated that miRNA-132 enhances the signalling
of the cholinergic anti-inflammatory pathway (45). It was demonstrated that miRNA-132
antagonists not only reduced the efficacy of hesperidin in the
treatment of depression but also blocked its anti-inflammatory
effects. According to the data published in previous studies, it
was hypothesized that hesperidin can restore the negative feedback
between pro-inflammatory cytokines and miRNA-132 (46,47). The present study transplanted
NSCLC cells into rats and treated some rats with hesperidin at the
same time to investigate the effect of hesperidin on NSCLC. The
results demonstrated that treatment with hesperidin decreased the
tumour volume and colony formation efficiency of NSCLC cells by
increasing miR-132 expression and decreasing ZEB2 expression.
Furthermore, among multiple targets for miR-132 in cancer, ZEB2, as
an inhibitor of E-cadherin transcription, was reported to play an
essential role in EMT and acts as a direct target of miR-132, and
the ectopic expression of miR-132 significantly reduced the
invasiveness of CRC cells and inhibited their EMT process (21).
It is worth noting that there were limitations of
the present study. Due to the fact that an overdose of hesperidin
may kill animals, while a low dose of hesperidin may exhibit no
signifi-cant therapeutic effect on the animals, a preliminary test
was performed to determine the dose to treat the animals (data not
shown). However, only two animals in each group were used to
determine the dose in the preliminary test, which was an
inappropriate sample size for statistical analysis. Furthermore,
the present findings were not validated with human subjects. It
would be necessary to recruit human subjects and validate the
administration of hesperidin in future studies.
In conclusion, the present study demonstrated that
the administration of hesperidin upregulates the expression of
miR-132, a potential regulator of HN1, TGF-β1 and ZEB2. Since HN1,
TGF-β1 and ZEB2 have been reported to be involved in the
pathogenesis of NSCLC, the administration of hesperidin may
alleviate NSCLC by inhibiting the proliferation and promoting the
apoptosis of NSCLC cells via the miR-132/ZEB2 pathway.
Supplementary Data
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analysed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
ST and LD designed the study. AH supervised the
study. PT and WL collected the references. ST, LD, YM and PT
collected the experimental data, AH, WL and YM collected the
patient data. AH, JW, WL and XH analysed the data. ST, YM and JW
wrote the manuscript. AH improved the manuscript. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments in the present study were
approved by the Animal Ethics Committee of Yantai Hospital of
Traditional Chinese Medicine (approval no. YTYX16X316).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
DeSantis CE, Lin CC, Mariotto AB, Siegel
RL, Stein KD, Kramer JL, Alteri R, Robbins AS and Jemal A: Cancer
treatment and survivorship statistics, 2014. CA Cancer J Clin.
64:252–271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
He J, Shen J, Yang C, Jiang L, Liang W,
Shi X, Xu X and He J: Adjuvant chemotherapy for the completely
resected stage IB nonsmall cell lung cancer: A Systematic review
and meta-analysis. Medicine (Baltimore). 94:e9032015. View Article : Google Scholar
|
|
3
|
Lai WY, Wang WY, Chang YC, Chang CJ, Yang
PC and Peck K: Synergistic inhibition of lung cancer cell invasion,
tumor growth and angiogenesis using aptamer-siRNA chimeras.
Biomaterials. 35:2905–2914. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hong S, Tan M, Wang S, Luo S, Chen Y and
Zhang L: Efficacy and safety of angiogenesis inhibitors in advanced
non-small cell lung cancer: A systematic review and meta-analysis.
J Cancer Res Clin Oncol. 141:909–921. 2015. View Article : Google Scholar
|
|
5
|
Qin Y, Zhang Q, Lee S, Zhong WL, Liu YR,
Liu HJ, Zhao D, Chen S, Xiao T, Meng J, et al: Doxycycline reverses
epithelial-to-mesenchymal transition and suppresses the
proliferation and metastasis of lung cancer cells. Oncotarget.
6:40667–40679. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fischer KR, Durrans A, Lee S, Sheng J, Li
F, Wong ST, Choi H, El Rayes T, Ryu S, Troeger J, et al:
Epithelial-to-mesenchymal transition is not required for lung
metastasis but contributes to chemoresistance. Nature. 527:472–476.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li Y, Chao Y, Fang Y, Wang J, Wang M,
Zhang H, Ying M, Zhu X and Wang H: MTA1 promotes the invasion and
migration of non-small cell lung cancer cells by downregulating
miR-125b. J Exp Clin Cancer Res. 32:332013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen L, Gibbons DL, Goswami S, Cortez MA,
Ahn YH, Byers LA, Zhang X, Yi X, Dwyer D, Lin W, et al: Metastasis
is regulated via microRNA-200/ZEB1 axis control of tumour cell
PD-L1 expression and intratumoral immunosuppression. Nat Commun.
5:52412014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li H, Chen Y, Xu N, Yu M, Tu X, Chen Z,
Lin M, Xie B, Fu J and Han L: AMD3100 inhibits brain-specific
metastasis in lung cancer via suppressing the SDF-1/CXCR4 axis and
protecting blood-brain barrier. Am J Transl Res. 9:5259–5274.
2017.
|
|
10
|
Xie S, Zeng W, Fan G, Huang J, Kang G,
Geng Q, Cheng B, Wang W and Dong P: Effect of CXCL12/CXCR4 on
increasing the metastatic potential of non-small cell lung cancer
in vitro is inhibited through the downregulation of CXCR4 chemokine
receptor expression. Oncol Lett. 7:941–947. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kamaraj S, Ramakrishnan G, Anandakumar P,
Jagan S and Devaki T: Antioxidant and anticancer efficacy of
hesperidin in benzo(a)pyrene induced lung carcinogenesis in mice.
Invest New Drugs. 27:214–22. 2009. View Article : Google Scholar
|
|
12
|
Khamis AAA, Ali EMM, El-Moneim MAA,
Abd-Alhaseeb MM, El-Magd MA and Salim EI: Hesperidin, piperine and
bee venom synergistically potentiate the anticancer effect of
tamoxifen against breast cancer cells. Biomed Pharmacother.
105:1335–1343. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Roohbakhsh A, Parhiz H, Soltani F, Rezaee
R and Iranshahi M: Molecular mechanisms behind the biological
effects of hesperidin and hesperetin for the prevention of cancer
and cardiovascular diseases. Life Sci. 124:64–74. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xia R, Xu G, Huang Y, Sheng X, Xu X and Lu
H: Hesperidin suppresses the migration and invasion of non-small
cell lung cancer cells by inhibiting the SDF-1/CXCR-4 pathway. Life
Sci. 201:111–120. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xia R, Sheng X, Xu X, Yu C and Lu H:
Hesperidin induces apoptosis and G0/G1 arrest in human non-small
cell lung cancer A549 cells. Int J Mol Med. 1:464–472. 2017.
|
|
16
|
Kataoka M and Wang DZ: Non-coding RNAs
including miRNAs and lncRNAs in cardiovascular biology and disease.
Cells. 3:883–898. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Moreno-Moya JM, Vilella F and Simon C:
MicroRNA: Key gene expression regulators. Fertil Steril.
101:1516–1523. 2014. View Article : Google Scholar
|
|
18
|
Ell B and Kang Y: MicroRNAs as regulators
of bone homeostasis and bone metastasis. Bonekey Rep. 3:5492014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Janaki Ramaiah M, Lavanya A, Honarpisheh
M, Zarea M, Bhadra U and Bhadra MP: MiR-15/16 complex targets p70S6
kinase 1 and controls cell proliferation in MDA-MB-231 breast
cancer cells. Gene. 552:255–264. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li W, Zang W, Liu P, Wang Y, Du Y, Chen X,
Deng M, Sun W, Wang L, Zhao G and Zhai B: MicroRNA-124 inhibits
cellular proliferation and invasion by targeting Ets-1 in breast
cancer. Tumour Biol. 35:10897–10904. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
You J, Li Y, Fang N, Liu B, Zu L, Chang R,
Li X and Zhou Q: MiR-132 suppresses the migration and invasion of
lung cancer cells via targeting the EMT regulator ZEB2. PLoS One.
9:e918272014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hansen KF, Karelina K, Sakamoto K, Wayman
GA, Impey S and Obrietan K: miRNA-132: A dynamic regulator of
cognitive capacity. Brain Struct Funct. 218:817–831. 2013.
View Article : Google Scholar
|
|
23
|
Marler KJ, Suetterlin P, Dopplapudi A,
Rubikaite A, Adnan J, Maiorano NA, Lowe AS, Thompson ID, Pathania
M, Bordey A, et al: BDNF promotes axon branching of retinal
ganglion cells via miRNA-132 and p250GAP. J Neurosci. 34:969–979.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Marques-Rocha JL, Samblas M, Milagro FI,
Bressan J, Martínez JA and Marti A: Noncoding RNAs, cytokines, and
inflammation-related diseases. FASEB J. 29:3595–3611. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li M, Shao H, Zhang X and Qin B:
Hesperidin alleviates lipopoly-saccharide-induced neuroinflammation
in mice by promoting the miRNA-132 pathway. Inflammation.
39:1681–1689. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yi LT, Li J, Liu BB, Luo L, Liu Q and Geng
D: BDNF-ERK-CREB signalling mediates the role of miR-132 in the
regulation of the effects of oleanolic acid in male mice. J
Psychiatry Neurosci. 39:348–359. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pan B and Liu Y: Effects of duloxetine on
microRNA expression profile in frontal lobe and hippocampus in a
mouse model of depression. Int J Clin Exp Pathol. 8:15454–15461.
2015.
|
|
28
|
You J, Li Y, Fang N, Liu B, Zu L, Chang R,
Li X and Zhou Q: MiR-132 suppresses the migration and invasion of
lung cancer cells via targeting the EMT regulator ZEB2. PLoS One.
9:e918272014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang B, Lu L and Zhang X, Ye W, Wu J, Xi
Q and Zhang X: Hsa-miR-132 regulates apoptosis in non-small cell
lung cancer independent of acetylcholinesterase. J Mol Neurosci.
53:335–344. 2014. View Article : Google Scholar
|
|
30
|
Zhang JX, Zhai JF, Yang XT and Wang J:
MicroRNA-132 inhibits migration, invasion and
epithelial-mesenchymal transition by regulating TGFβ1/Smad2 in
human non-small cell lung cancer. Eur Rev Med Pharmacol Sci.
20:3793–3801. 2016.PubMed/NCBI
|
|
31
|
Chen YK, Wang HC, Ho CT, Chen HY, Li S,
Chan HL, Chung TW, Tan KT, Li YR and Lin CC: 5-demethylnobiletin
promotes the formation of polymerized tubulin, leads to G2/M phase
arrest and induces autophagy via JNK activation in human lung
cancer cells. J Nutr Biochem. 26:484–504. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jayaprakasha GK, Mandadi KK, Poulose SM,
Jadegoud Y, Nagana Gowda GA and Patil BS: Novel triterpenoid from
Citrus aurantium L. possesses chemopreventive properties against
human colon cancer cells. Bioorg Med Chem. 16:5939–5951. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee DH, Park KI, Park HS, Kang SR,
Nagappan A, Kim JA, Kim EH, Lee WS, Hah YS, Chung HJ, et al:
Flavonoids isolated from Korea Citrus aurantium L. induce G2/M
phase arrest and apoptosis in human gastric cancer AGS cells. Evid
Based Complement Alternat Med. 2012:5159012012.
|
|
34
|
Leclere L, Fransolet M, Cote F, Cambier P,
Arnould T, Van Cutsem P and Michiels C: Heat-modified citrus pectin
induces apoptosis-like cell death and autophagy in HepG2 and A549
cancer cells. PLoS One. 10:e01158312015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
36
|
Yang T, Wan Z, Liu Z, Li H, Wang H, Lu N,
Chen Z, Mei X and Ren X: In situ mineralization of anticancer drug
into calcium carbonate monodisperse nanospheres and their
pH-responsive release property. Mater Sci Eng C Mater Biol Appl.
63:384–392. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Galati EM, Monforte MT, Kirjavainen S,
Forestieri AM, Trovato A and Tripodo MM: Biological effects of
hesperidin, a citrus flavonoid. (Note I): Antiinflammatory and
analgesic activity. Farmaco. 40:709–712. 1994.PubMed/NCBI
|
|
38
|
Huang MT, Wood AW, Newmark HL, Sayer JM,
Yagi H, Jerina DM and Conney AH: Inhibition of the mutagenicity of
bay-region diolepoxides of polycyclic aromatic hydrocarbons by
phenolic plant flavonoids. Carcinogenesis. 4:1631–1637. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tanaka T, Makita H, Ohnishi M, Mori H,
Satoh K, Hara A, Sumida T, Fukutani K, Tanaka T and Ogawa H:
Chemoprevention of 4-nitroquinoline 1-oxide-induced oral
carcinogenesis in rats by flavonoids diosmin and hesperidin, each
alone and in combi-nation. Cancer Res. 57:246–252. 1997.PubMed/NCBI
|
|
40
|
Balakrishnan A and Menon VP: Effect of
hesperidin on matrix metalloproteinases and antioxidant status
during nico-tine-induced toxicity. Toxicology. 238:90–98. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kamaraj S, Anandakumar P, Jagan S,
Ramakrishnan G and Devaki T: Modulatory effect of hesperidin on
benzo(a)pyrene induced experimental lung carcinogenesis with
reference to COX-2, MMP-2 and MMP-9. Eur J Pharmacol. 649:320–327.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yumnam S, Park HS, Kim MK, Nagappan A,
Hong GE, Lee HJ, Lee WS, Kim EH, Cho JH, Shin SC and Kim GS:
Hesperidin induces paraptosis like cell death in hepatoblastoma,
HepG2 Cells: Involvement of ERK1/2 MAPK [corrected]. PLoS One.
9:e1013212014. View Article : Google Scholar
|
|
43
|
Birsu Cincin Z, Unlu M, Kiran B, Sinem
Bireller E, Baran Y and Cakmakoglu B: Anti-proliferative, apoptotic
and signal transduction effects of hesperidin in non-small cell
lung cancer cells. Cell Oncol (Dordr). 38:195–204. 2015. View Article : Google Scholar
|
|
44
|
Cincin ZB, Kiran B, Baran Y and Cakmakoglu
B: Hesperidin promotes programmed cell death by downregulation of
nonge-nomic estrogen receptor signalling pathway in endometrial
cancer cells. Biomed Pharmaco. 103:336–345. 2018. View Article : Google Scholar
|
|
45
|
Shaked I, Meerson A, Wolf Y, Avni R,
Greenberg D, Gilboa-Geffen A and Soreq H: MicroRNA-132 potentiates
cholinergic anti-inflammatory signaling by targeting
acetylcho-linesterase. Immunity. 31:965–973. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Liu F, Li Y, Jiang R, Nie C, Zeng Z, Zhao
N, Huang C, Shao Q, Ding C, Qing C, et al: miR-132 inhibits
lipopolysaccharide-induced inflammation in alveolar macrophages by
the cholinergic anti-inflammatory pathway. Exp Lung Res.
41:261–269. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kong H, Yin F, He F, Omran A, Li L, Wu T,
Wang Y and Peng J: The effect of miR-132, miR-146a, and miR-155 on
MRP8/TLR4-induced astrocyte-related inflammation. J Mol Neurosci.
57:28–37. 2015. View Article : Google Scholar : PubMed/NCBI
|