Introduction
Laminectomy is commonly used in the treatment of
lumbar disc herniation and its associated conditions (1). However, up to 40% of patients suffer
from failed back surgery syndrome (FBSS), which is characterized by
recurrent and continuous pain following neurosurgical
interventions, such as lumbar laminectomy (2-5).
Extensive epidural fibrosis (EF), or scar-ring adjacent to the dura
mater, may develop following lumbar laminectomy and may lead to
clinically significant FBSS by compressing and irritating the
surrounding anatomical structures (6-8).
EF accounts for 24% of revision surgeries (9). Surgical scar excision is challenging
and extremely risky; thus, biological therapies are receiving
increasing attention (10-12).
Although the pathogenesis underlying the formation
and development of EF remains largely unknown, excessive fibroblast
proliferation and extracellular matrix (ECM) production reportedly
play key roles (13-15). A number of studies have
demonstrated that transforming growth factor-β1 (TGF-β1), the major
factor triggering EF, regulates fibroblast proliferation and ECM
production (14,16-18). Hence, efforts to inhibit
TGF-β1-associated signal transduction in order to reduce excessive
fibroblast proliferation and ECM overproduction are increasing. The
TGF-β1-induced activation of the Smad2/3 signalling pathway plays a
major role in fibroblast proliferation and ECM accumulation
(19,20). In addition, TGF-β1 stimulation
increases the levels of phosphorylated receptor-activated Smad2/3
transcription factors, which form heteromeric complexes with Smad4
and are then translocated to the nucleus. The heterocomplexes then
initiate fibrosis by recruiting various transcription factors and
binding to specific sequences in the promoter regions of target
genes (21,22). Additionally, mitogen-activated
protein kinases (MAPKs), which play important roles in
Smad-independent TGF-β1 signalling, also regulate TGF-β1-mediated
stimulation (21,23). Moreover, there is substantial
evidence to indicate that the transcriptional activity of the Smad
complex is regulated by the TGF-β1-induced activation of MAPK
(23,24). Thus, the inhibition of
TGF-β1-related pathways, such as the Smad and MAPK pathways, to
reduce the cell proliferation and ECM overproduction of fibroblasts
is regarded as a potentially crucial target for the treatment of
EF.
Honokiol
[30,5-di-(2-propenyl)-1,10-biphenyl-2,40-diol], a small polyphenol,
is a major bioactive constituent isolated from the traditional
Chinese medicine, Magnolia officinalis (25). Previous pharmacological studies
have demonstrated that honokiol exerts anti-inflammatory,
antioxidant, anti-bacterial, antitumour, anti-calmodulin and
neuroprotective effects, among others (25-29). A recent study reported that
honokiol alleviated hypertrophic scarring by inhibiting excessive
ECM deposition and hypertrophic scar-derived fibroblast
proliferation (30). Similarly,
honokiol has been reported to ameliorate fibrosis by inhibiting the
expression of pro-fibrotic factors and ECM proteins in a rat model
of renal fibrosis (31).
Additional findings provide evidence that the promising
anti-fibrotic effects of honokiol in rats with liver fibrosis are
related to the suppression of the TGF-β/Smad and MAPK signalling
path-ways (32). Based on these
findings, it was hypothesized that honokiol may attenuate the
development of EF. The present study examined its effects on the
proliferation and ECM production of TGF-β1-stimulated fibroblasts.
Moreover, the protective role of honokiol against EF was further
confirmed in a rat model post-laminectomy.
Materials and methods
Reagents and antibodies
Honokiol, purchased from Sigma-Aldrich; Merck KGaA,
was freshly dissolved in DMSO. The purity of honokiol used in the
present study was >98%. Fibronectin, connective tissue growth
factor (CTGF; cat. no. ab6992), type I collagen (cat. no.
ab260043), Smad2/3 (cat. no. ab202445), p-Smad2 (cat. no.
ab188334), p-Smad3 (cat. no. ab52903), p-p38 (cat. no. ab178867),
p-JNK (cat. no. ab124956), p-ERK (cat. no. ab201015), p38 (cat. no.
ab170099), JNK (cat. no. ab179461), ERK (cat. no. ab184699) and
GAPDH (cat. no. ab181602) antibodies, were purchased from Abcam);
primary antibody against cyclin B1 (cat. no. sc-245), cyclin D1
(cat. no. sc-8396) and cyclin E (cat. no. sc-247) were purchased
from Santa Cruz Biotechnology. Inc.; the goat anti-rabbit (cat. no.
BS13271) and anti-mouse (cat. no. BS12471) IgG-HRP were purchased
from Bioworld. Recombinant human TGF-β1 was purchased from
PeproTech Group, Inc. DMSO, carboxymethylcellulose (CMC) and
collagenase I were purchased from Sigma-Aldrich; Merck KGaA. Cell
culture reagents were purchased from Gibco; Thermo Fisher
Scientific, Inc.
Fibroblast culture
A total of 16 male Sprague-Dawley rats (3 months
old; weight, 350-400 g), were purchased from the Animal Centre of
the Chinese Academy of Sciences. Lumbar laminectomy was performed
on rats as described in a previous study (33). The rats were housed under specific
pathogen-free conditions at 25°C with a 12-h light/dark cycle and
free access to food and water. Epidural scar tissues were collected
when the rats were sacrificed. The epidural scar tissues were
placed in PBS to maintain moisture, then minced and incubated in a
solution of collagenase type I (0.1 g/l) at 37°C for 3 h to
separate the primary fibroblasts. Following enzymatic digestion,
the isolated cells were incubated in Dulbecco's modified Eagle's
medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.), supplemented
with 10% foetal bovine serum and 1% antibiotic mixture of
penicillin and streptomycin, for the culture of the cells. The
cells were incubated at 37°C with a humid atmosphere of 95% air and
5% CO2. The fibroblasts used in the present study were
at passages 2 to 5. All the cells were incubated at 37°C for 24 h
until they reached 70-80% confluence. The cells were starved in
serum-free DMEM for 24 h, followed by supplementation with 10 ng/ml
TGF-β1 and/or honokiol for 2 h prior at various concentrations (1,
2.5, 5, 10 or 20 µM), followed by incubation at 37°C for a
further 72 h.
Cell viability and proliferation
To determine the cytotoxicity of honokiol, the cells
were incubated with various concentrations (1, 2.5, 5, 10 or 20
µM) of honokiol for 72 h. For the analysis of cell
proliferation, the cells were seeded in 96-well plates for 24 h and
then stimulated with TGF-β1 and/or honokiol for 2 h prior at
various concentrations (1, 2.5, 5, 10 or 20 µM) for a
further 72 h. Subsequently, the cells were washed 3 times with
phosphate-buffered saline (PBS), and then incubated for 2 h in
CCK-8 solution (CCK-8; Dojindo, Tokyo, Japan), at 37°C. A
spectrophotometer (Thermo Fisher Scientific, Inc.) at 450 nm was
used to measure the absorbance of the cells.
EdU incorporation assay
The effects of honokiol on TGF-β1-induced
proliferation were determined using a Cell-Light EdU DNA cell kit
(Guangzhou RiboBio Co., Ltd.,), according to the manufacturer's
instructions. In brief, after being subjected to the appropriate
treatments, fibroblasts were incubated at 37°C in EdU solution (50
µmol/l) for 2 h. The cells were then fixed in 4%
formaldehyde for 30 min and incubated at room temperature with 0.5%
Triton X-100 in PBS for 10 min. After washing with PBS, Click-iT™
reaction cocktail was added to the plates for 30 min, and the cells
were then stained with Hoechst 33342 dye at room temperature for a
further 30 min. Proliferating cells were imaged under a fluorescent
microscope (Nikon Corporation) and were counted using ImageJ
software (NIH). A total of 5 fields were randomly selected for
microscopic observation.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the treated fibroblasts
and epidural scar tissues using TRIzol reagent (Invitrogen; Thermo
Fisher Scientific, Inc.), according to the manufacturer's
instructions. The concentration was deter-mined
spectrophotometrically at 260 nm (NanoDrop 2000; Thermo Fisher
Scientific, Inc.). Total RNA (1 µg) was reverse transcribed
to synthesize cDNA using PrimeScript Reverse Transcriptase (Takara
Bio, Inc.). Subsequently, 5 µl of 2X SYBR Master Mix, 0.25
µl of each primer and 4.5 µl of diluted cDNA were
used for PCR amplification. The qPCR thermo-cycling conditions were
as follows: 95°C for 3 min, 40 cycles of 95°C for 30 sec, annealing
at 60°C for 45 sec, and a final elongation step at 72°C for 20 sec.
The CFX96 Real-Time PCR System (Bio-Rad Laboratories, Inc.) was
used to conduct the reaction and detection. All mRNA quantification
data were collected and normalized to the level of the housekeeping
gene, GAPDH. The 2−ΔΔCq method (34) was used to calculate the relative
mRNA levels of each target gene. The primers used are listed in
Table I.
 | Table IPrimer sequences used in RT-qPCR. |
Table I
Primer sequences used in RT-qPCR.
| Gene | Forward primer | Reverse primer | Product size
(bp) |
|---|
| CTGF |
5′-TGGCTTGCTCAGGGTAACTG-3′ |
5′-CTGCCTCCCAAACCAGTCAT-3′ | 80 |
| Type I
collagen |
5′-CCCAGCGGTGGTTATGACTT-3′ |
5′-CGGCCACCATCTTGAGACTT-3′ | 68 |
| Fibronectin |
5′-CCCCAACTGGTTACCCTTCC-3′ |
5′-GGTGACGAAGGGGGTCTTTT-3′ | 90 |
| GAPDH |
5′-AGTGCCAGCCTCGTCTCATA-3′ |
5′-TGAACTTGCCGTGGGTAGAG-3′ | 189 |
Western blot analysis
The treated fibroblasts and epidural scar tissues
were lysed with radioimmune precipitation assay (RIPA; Beyotime
Institute of Biotechnology) buffer to extract whole-cell proteins.
Protein concentration was determined using a BCA assay kit (cat.
no. 23250; Pierce; Thermo Fisher Scientific, Inc.). Equal amounts
(30 µg each) of total proteins were subjected to
electrophoresis on sodium dodecyl sulphate polyacrylamide 10% gels
and transferred onto nitrocellulose membrane (Life Technologies;
Thermo Fisher Scientific, Inc.). After blocking, the membranes were
probed with primary antibodies against CTGF, type I collagen,
fibronectin, cyclin B1, cyclin D1, cyclin E, p-Smad2, p-Smad3,
Smad2/3, p-p38, p-JNK, p-ERK, p38, JNK, ERK and GAPDH (all 1:1,000)
overnight at 4°C. The membranes were then incubated with
appropriate secondary antibodies (1:3,000) for 2 h at room
temperature. The protein bands were visualized by
electro-chemiluminescence plus reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). The ChemiDoc™ XRS Imaging System (Bio-Rad
Laboratories, Inc.) was used to quantify the band intensity.
Immunofluorescence
Fibroblasts were plated on glass cover-slips in
24-well plates. Following stimulation with TGF-β1 (10 ng/ml) and/or
various concentrations (5, 10 or 20 µM) of honokiol for 2 h
prior, cells were fixed in 4% formaldehyde for 10 min, and
permeabilized with 0.1% Triton X-100 for 5 min, followed by
blocking with 5% normal goat serum for 5 min. The cells were then
labelled with anti-Smad3 antibody (cat. no. ab40854; 1:500)
overnight at 4°C and probed with FITC-conjugated anti-rabbit IgG
antibody (cat. no. ab6717; 1:3,000) for 1 h at room temperature and
DAPI (Roche Diagnostics GmbH). Finally, the cells were photographed
using a fluorescence microscope (Nikon Corporation).
Hepatotoxicity and survival rate
assessment
The animals were arbitrarily divided into 3 groups
(n=15), treated with 0, 10, 20 mg/kg intragastrically every other
day for 4 weeks until sacrificed. Then, the serum aspartate
aminotransferase (AST), alanine aminotransferase (ALT) and albumin
were tested colourimetrically by using commercially available kits
(Biodiagnostics, Cairo, Egypt). In addition, survival rate was
estimated using Kaplan Meier analysis.
Animal ethics statement and models
All experimental procedures for animal studies were
carried out in compliance with the principles of International
Laboratory Animal Care and the experimental protocol was approval
by the Animal Care and Use Committee at the Wenzhou Medical College
(approval no. wydw2017-0007). A total of 78 male Sprague-Dawley
rats (3 months old; weight, 350-400 g) were purchased from the
Animal Centre of the Chinese Academy of Sciences. Rats were housed
under specific pathogen-free conditions at 25°C with a 12-h
light/dark cycle and free access to food and water. The rats were
randomly assigned to the sham-operated (sham) group (n=6, treatment
without lami-nectomy), the control group (n=24), the honokiol 10
mg/kg group (n=24) and the honokiol 20 mg/kg group (n=24). The rats
in each group were weighed and injected intraperitoneally with 2%
(w/v) pentobarbital (40 mg/kg) and fixed on the operation board in
the prone position. Laminectomy was performed as described in a
previous study (33). The hair of
each rat was shaved around the first lumbar vertebra (L1), and the
exposed skin was sterilized with povidone-iodine. A dorsal midline
incision (T12-L3) was made to remove the spinous process and
vertebral plate and to expose the dura mater at the L2 level. Close
attention was paid not to traumatize the neural tissue. The rats in
the sham group were not subjected to laminectomy, but only
underwent the same surgical procedure and were exposed for 2 min.
After satisfactory haemostasis, the wound was surgically closed.
Post-operatively, the rats in the honokiol group received honokiol
(10 or 20 mg/kg) dissolved in CMC intragastrically every other day
until the rats were sacrificed, while the rats in the other 2
groups were administered the same volume of CMC. Daily monitoring
of the rats was carried out to ensure their well-being and all
animals were allowed free unrestricted activity. No animals were
found dead during the duration of the experiment. All rats were
euthanized by an overdose of pentobarbital sodium (100-150 mg/kg;
intraperitoneally injected; cat. no. B005; Nanjing Jiancheng
Bioengineering Institute) at 4 weeks post-operatively. Rat
euthanasia was confirmed prior to disposing of the animal remains
by the criteria of the AVMA euthanasia guidelines 2020 (35), including lack of pulse, breathing,
corneal reflex, response to firm toe pinch and so on. The epidural
scar and surrounding tissues were collected for subsequent
analyses.
Macroscopic assessment
Macroscopic assessment was performed at 4 weeks
post-operatively, to assess both the spaces between the dura mater
and the surrounding soft tissues. The epidural scar adhesion was
evaluated based on the Rydell classification (36) as follows: Grade 0, epidural scar
tissue was not adherent to the dura mater; grade 1, epidural scar
tissue was adherent to the dura mater, but easily dissected; grade
2, epidural scar tissue was adherent to the dura mater, and it was
difficult to dissect without disrupting the dura matter; grade 3,
epidural scar tissue was firmly adherent to the dura mater and
could not be dissected.
Determination of hydroxyproline (HPC)
content
HPC content analysis was performed to observe the
main signs of fibrosis. The HPC content of wet scar tissue obtained
from the laminectomy site was determined as described in a previous
study (37). The samples were
lyophilized, ground and hydrolysed with HCL at 110°C for 24 h. The
hydroxyproline developer (β-dimethylaminobenzaldehyde solution;
Beyotime Institute of Biotechnology, Inc.) was then used to examine
the samples and standards, and a spectrophotometer (Thermo Fisher
Scientific, Inc.) was used to evaluate the absorbance of the
solution was at 550 nm. The HPC content per milligram of scar
tissue was calculated.
Histopathological analysis
The rats from each group were sacrificed and
histopathological analysis was performed at week 4 following
surgery. The specimens, including the entire L1-L2 spinal column,
the paraspinal muscles and epidural fibrotic tissue, were fixed in
4% paraformaldehyde and decalcified, then dehydrated and embedded
in paraffin. The gross specimens were then sectioned at a thickness
of 5 µm. The slides of each disc were stained with
haematoxylin and eosin (H&E; Beyotime Institute of
Biotechnology, Inc.) and Masson's trichrome stain (Beyotime
Institute of Biotechnology, Inc.) at room temperature. Epidural
scar adhesion was evaluated under an optical microscope (Olympus
Corporation; ×40 magnification), and the number of fibroblasts was
counted under an optical microscope (Olympus Corporation; ×200
magnification).
Statistical analysis
Statistical analyses were performed using SPSS 20.0
statistical software. The experiments were performed for 3
biological replicates and 3 technical replicates. All results are
presented as the means ± SEM. The variance of ≥2 groups was
analysed using one-way analysis of variance followed by
Tukey-Kramer as post-hoc test. The Kruskal-Wallis test followed by
Dunn's multiple comparison was used to analyse non-parametric data.
Statistical significance was set at P<0.05.
Results
Honokiol inhibits the TGF-β1-induced
proliferation of fibro- blasts
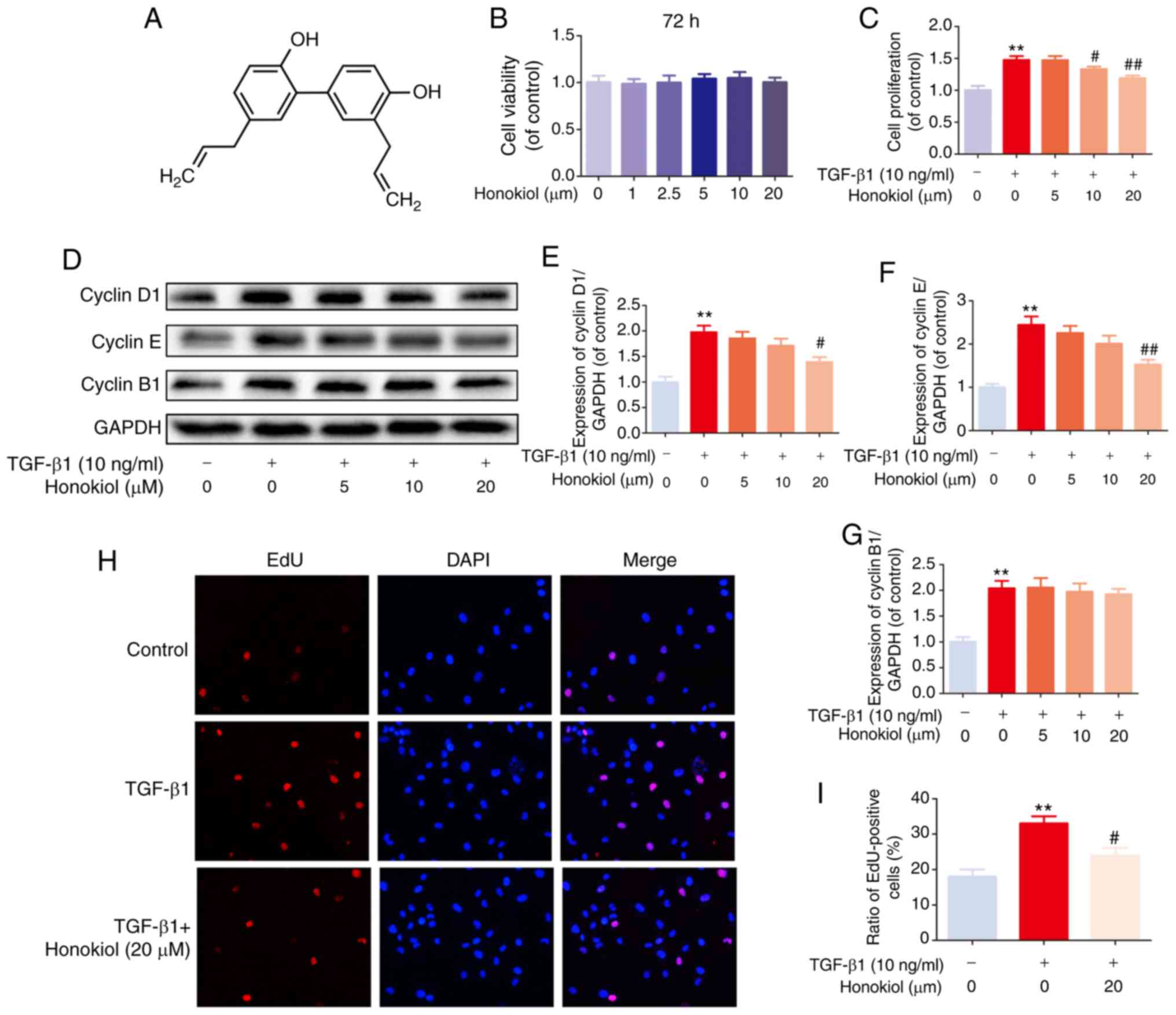
The chemical structure of honokiol is presented in
Fig. 1A. First, to ascertain the
susceptibility of fibroblasts to honokiol, a dose-response
experiment with various concentrations of honokiol (0, 1, 2.5, 5,
10 or 20 µM) was performed. Following 72 h of incubation,
cell viability was assessed by CCK-8 assay. No significant
differences were observed following 72 h of treatment with various
concentrations of honokiol (Fig.
1B). Therefore, 5, 10, or 20 µM were selected as the
appropriate concentrations of honokiol for use in subsequent
experiments. Second, to determine whether honokiol affects the
proliferation of fibroblasts, the cells were incubated with various
concentrations of honokiol in the presence of TGF-β1 (10 ng/ml) for
72 h, and cell viability was assessed by CCK-8 assay. As shown in
Fig. 1C, TGF-β1 led to an
increased fibroblast proliferation, and this was significantly
reversed by honokiol treatment in a dose-dependent manner. To
further confirm the anti-proliferative effects of honokiol, the
protein levels of cyclin D1 and cyclin E, major cyclin proteins
that are involved in the regulation of G0/G1 phase progression
(38,39), were examine by western blot
analysis. In addition, the expression of cyclin B1, which plays a
pivotal role in controlling the G2 to M phase transition and is
widely used as a marker of cell proliferation (39,40), was analysed. The results revealed
that honokiol treatment induced a significant decrease in cyclin D1
and cyclin E expression in a dose-dependent manner, whereas cyclin
B1 expression was not affected, suggesting that honokiol arrests
fibroblast proliferation during the G0/G1 phase progression by
inhibiting the expression of cyclin D1 and cyclin E (Fig. 1D-G). A similar anti-proliferative
effect was observed in the TGF-β1-stimulated fibroblasts, by EdU
fluorescence staining. Treatment with honokiol (20 µM)
significantly reduced the percentage of EdU-positive cells (red;
Fig. 1H and I). Taken together,
these results suggested that pre-treatment with honokiol suppressed
the increased fibroblast proliferation induced by TGF-β1.
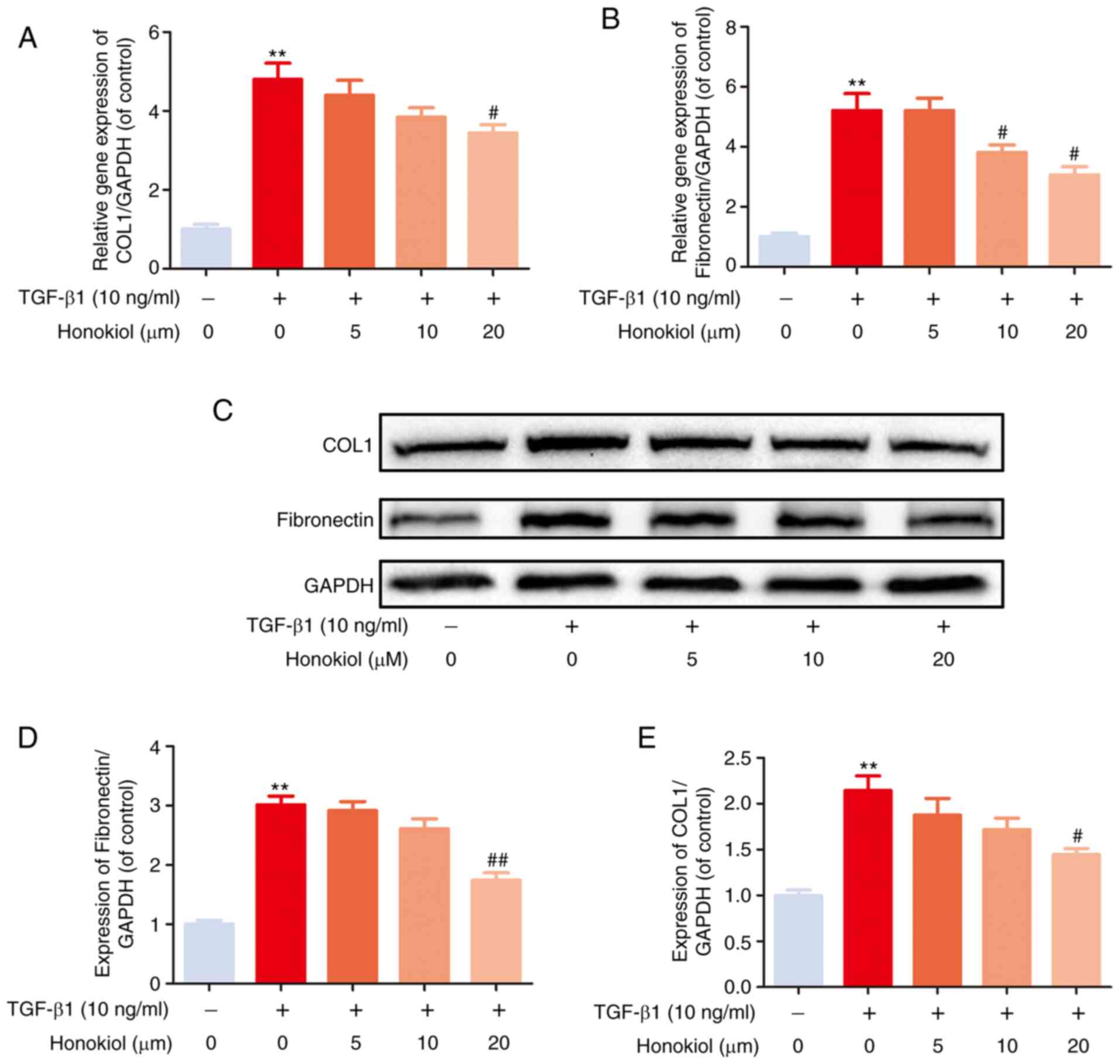
Honokiol suppresses TGF-β1-induced ECM
production
EF can trigger the increased expression of ECM
components. To examine the effects of honokiol on TGF-β1-induced
ECM production, the expression of fibronectin and type I collagen,
which are considered to be the main components of ECM, were
examined by western blot analysis. As shown in Fig. 2, the stimulation of fibroblasts
with TGF-β1 induced a noticeable increase in the expression of
fibronectin and type I collagen at both the gene and protein
levels, which was reversed by honokiol treatment in a
dose-dependent manner. These data demonstrated that honokiol
prevented the ECM overproduction induced by TGF-β1 in
fibroblasts.
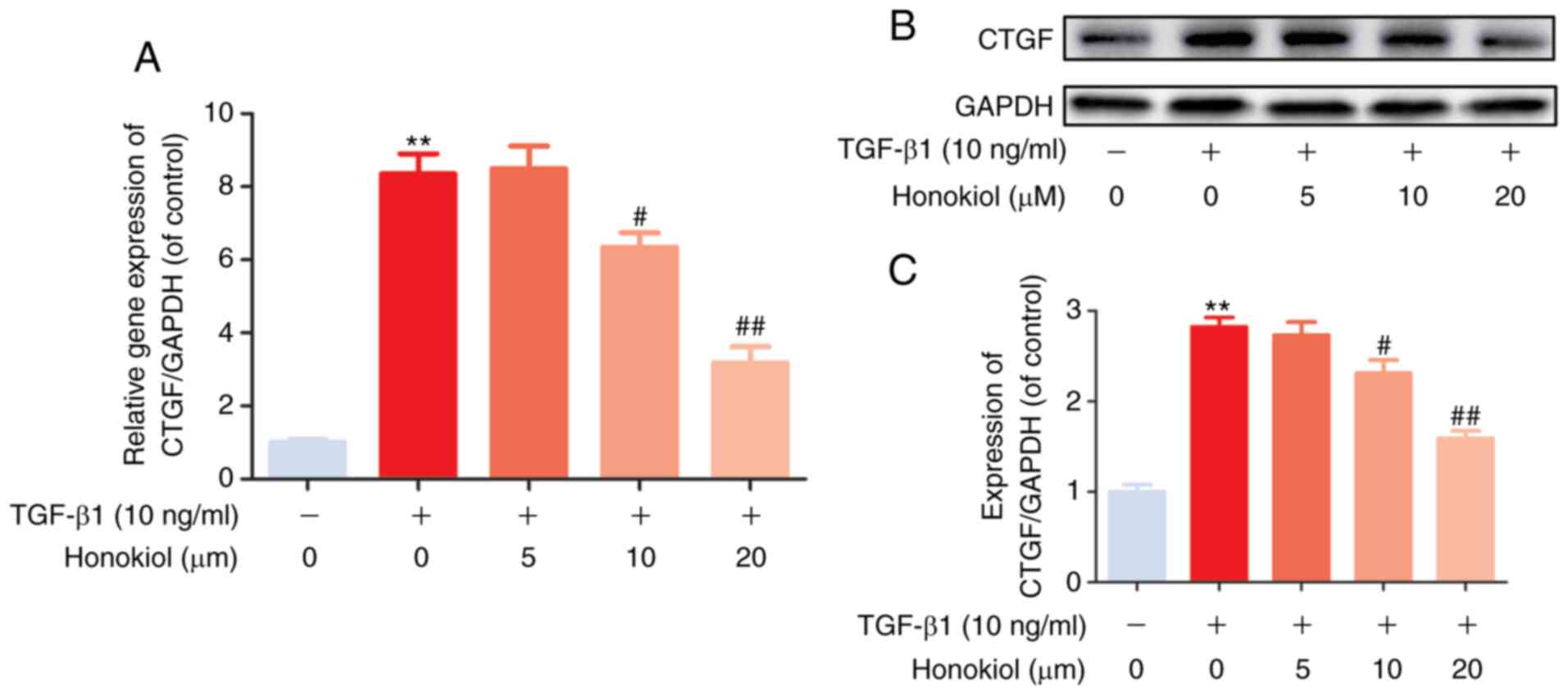
Honokiol inhibits TGF-β1-induced CTGF
expression
Previous studies have indicated that CTGF, as a
matricellular protein of the CCN family of ECM-associated proteins,
plays an important role in regulating ECM production and
proliferation in various cell types (14,17). In the present study, to further
confirm the mechanisms through which honokiol participates in
TGF-β1-induced stimulation, RT-qPCR and western blot analysis of
the gene and protein levels of CTGF were performed. As was
expected, it was found that honokiol treatment effectively
inhibited the noticeable TGF-β1-induced increase in both the mRNA
and protein levels of CTGF in a dose-dependent manner (Fig. 3). Taken together, these findings
demonstrated that the protective role of honokiol may involve the
inhibition of ECM overproduction and proliferation by suppressing
the upstream protein, CTGF.
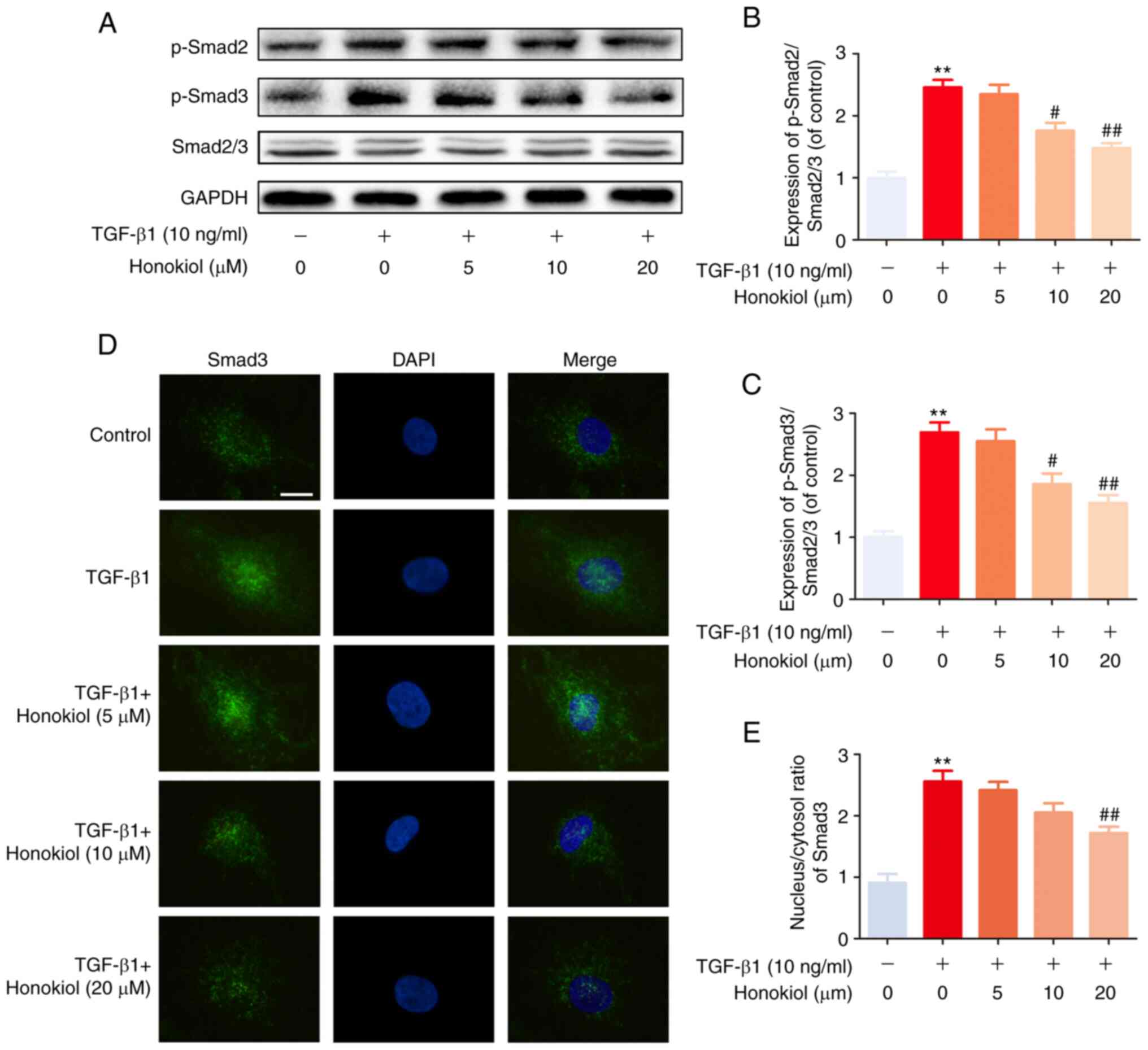
Honokiol inhibits TGF-β1-induced Smad2/3
pathway activation
To further determine the mechanisms through which
honokiol exerts its effects, the present study examined the
activation of the Smad2/3 signalling pathway, which is involved in
classic TGF-β1-induced signalling, by western blot analysis.
Regardless of the presence of honokiol, the level of phosphorylated
Smad2/3 was significantly increased following 1 h of TGF-β1
stimulation. However, honokiol treatment markedly decreased the
level of phosphorylated Smad2/3 in a dose-dependent manner
(Fig. 4A-C). Similar results were
also observed by immunofluorescence staining, which revealed the
TGF-β1-induced nuclear translocation of Smad3 (Fig. 4D and E). These results
demonstrated that honokiol suppressed TGF-β1 signalling by
inhibiting the sustained phosphorylation and nuclear translocation
of Smad2/3.
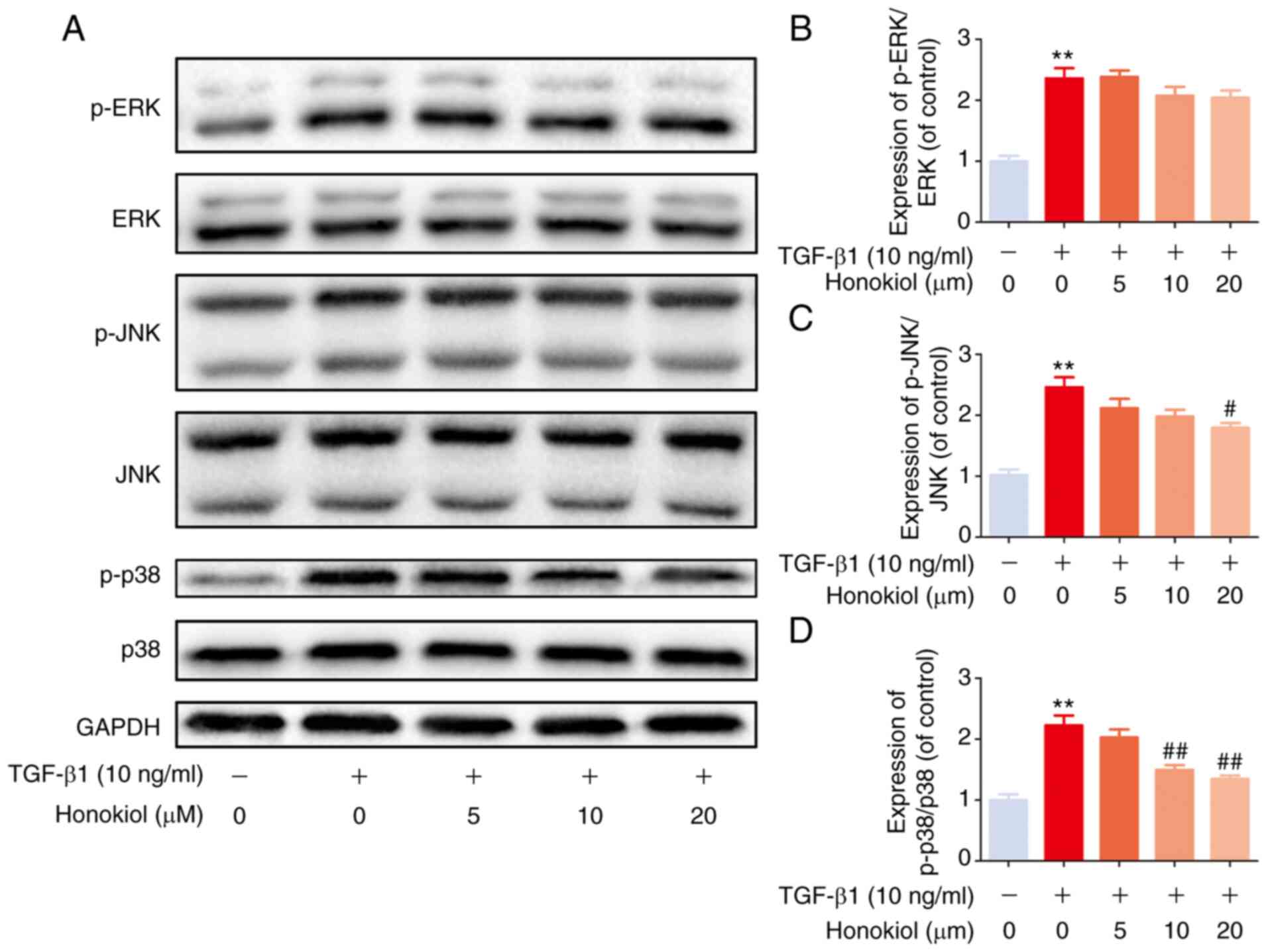
Honokiol suppresses TGF-β1-induced MAPK
pathway activation
Based on previous studies, TGF-β1 activates MAPK
signalling in a Smad2/3-independent manner (24,41). Therefore, the present study
further examined the effects of honokiol on the levels of
phosphorylated ERK, JNK and p38 by western blot analysis. Compared
with the control treatment, TGF-β1 notably increased the levels of
phosphorylated ERK, p38 and JNK, while honokiol treatment resulted
in significantly lower levels of p-p38 and p-JNK, but not p-ERK, in
a dose-dependent manner (Fig. 5).
These findings provide evidence that the protective effects of
honokiol are involved in the inhibition of the phosphorylation of
p38 and JNK MAPKs and the inhibition of the Smad2/3 pathway.
Honokiol attenuates EF in rats
post-laminectomy in vivo
To ensure the safety of the dose of honokiol (10 and
20 mg/kg) used in the present study, a toxicological anlasis was
performed. No hepatotoxicity or death were observed until the rats
were sacrificed (Table SI and Fig.
S1). To deter-mine the therapeutic effects of honokiol on EF
in vivo, a rat model of laminectomy was established. The
grade scores of epidural scar adhesion were obtained to assess the
adhesion of the EF specimens according to a previous study
(36). Macroscopically, the
epidural scar adhesions in the control group were severe and dense
around the laminectomy sites, and these epidural scar adhesions
were clearly ameliorated by honokiol treatment (Table II). Of note, in the sham group,
no scar tissue formation adjacent to the dura mater was observed
due to the intact lamina. Therefore, the samples in the sham group
were only subjected to H&E staining (data not shown). A
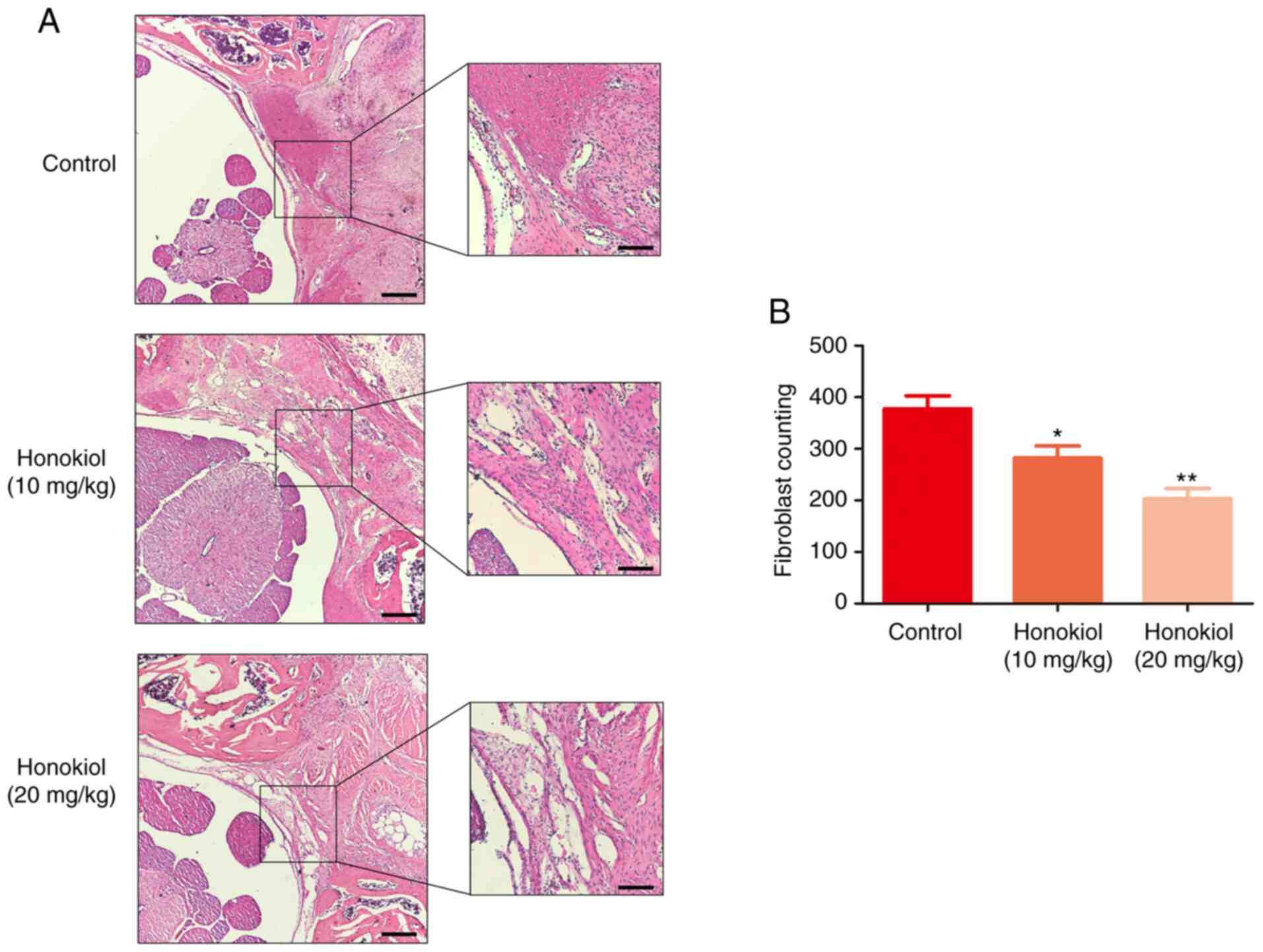
corresponding anti-EF effect was also observed in the histological
analysis. Compared with that in the control group, the degree of EF
adhesion in the honokiol group was moderate, and only loosened or
scant scar adhesion was observed in the laminectomy areas (Fig. 6A). Consistent with the
histological findings, the fibroblast counts in the H&E images
revealed that the number of fibroblasts was significantly lower in
the honokiol groups (Fig. 6B).
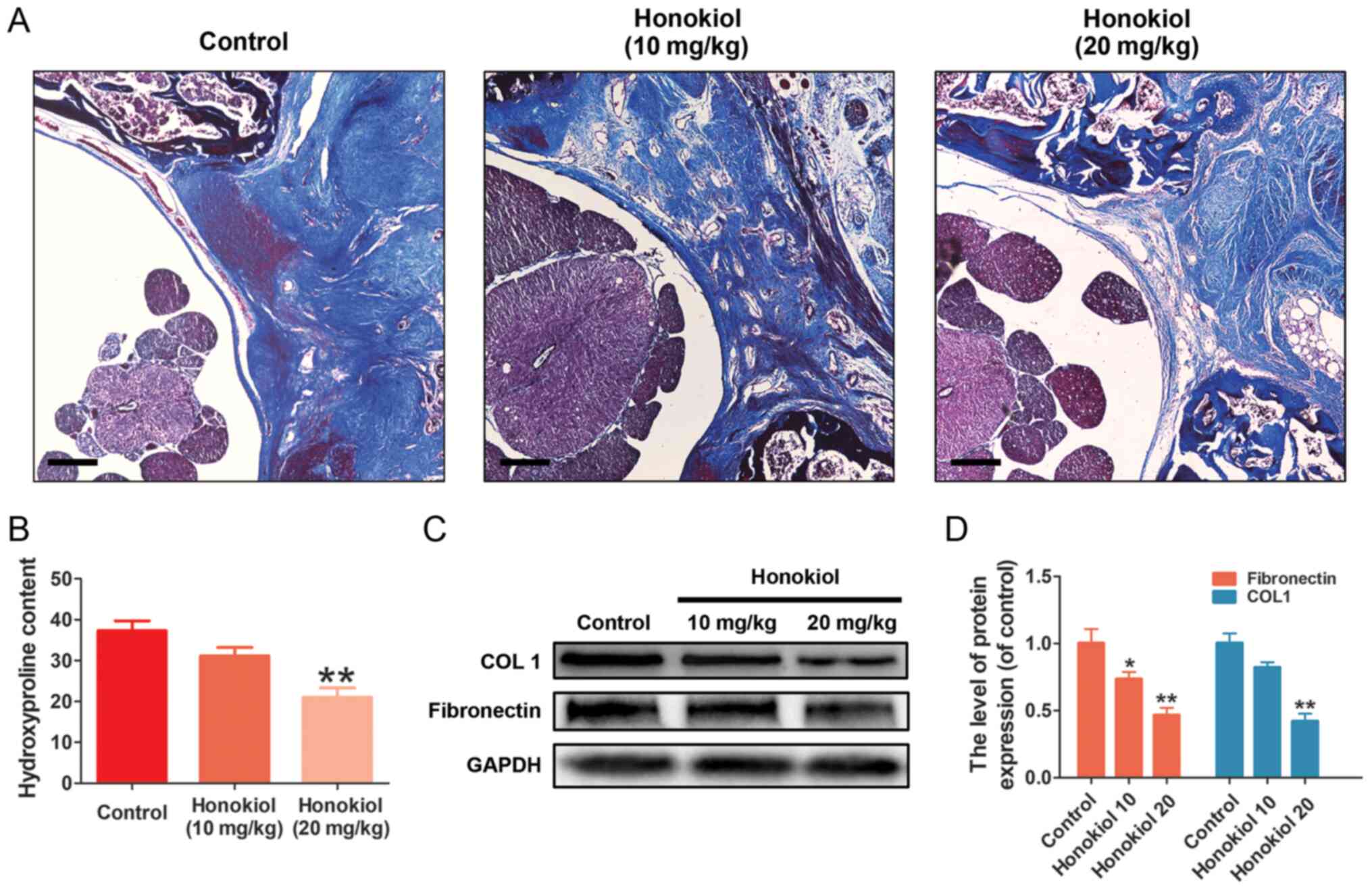
Similar results were also observed in the collagen tissues by
Masson's trichrome staining. The control group exhibited a much
higher collagen density in the epidural tissue, while the honokiol
group exhibited only scant amounts of collagen tissue (Fig. 7A), which was consistent with the
results of the HPC analysis (Fig.
7B). Moreover, the production of fibronectin and type I
collagen in the scar tissues was also decreased at the protein
level by honokiol treatment (Fig. 7C
and D). These therapeutic effects of honokiol on EF occurred in
a dose-dependent manner. Taken together, these results indicated
that honokiol ameliorated the progression of EF in vivo and
may be involved in the inhibition of excessive fibroblast
proliferation and ECM overproduction, which is consistent with the
in vitro findings.
 | Table IIDegree of epidural adhesion according
to Rydell's classification (36). |
Table II
Degree of epidural adhesion according
to Rydell's classification (36).
| Group | Grade
|
|---|
| 0 | 1 | 2 | 3 |
|---|
| Control | 0 | 0 | 2 | 4 |
| Honokiol (10
mg/kg) | 1 | 3 | 2 | 0 |
| Honokiol (20
mg/kg) | 2 | 3 | 1 | 0 |
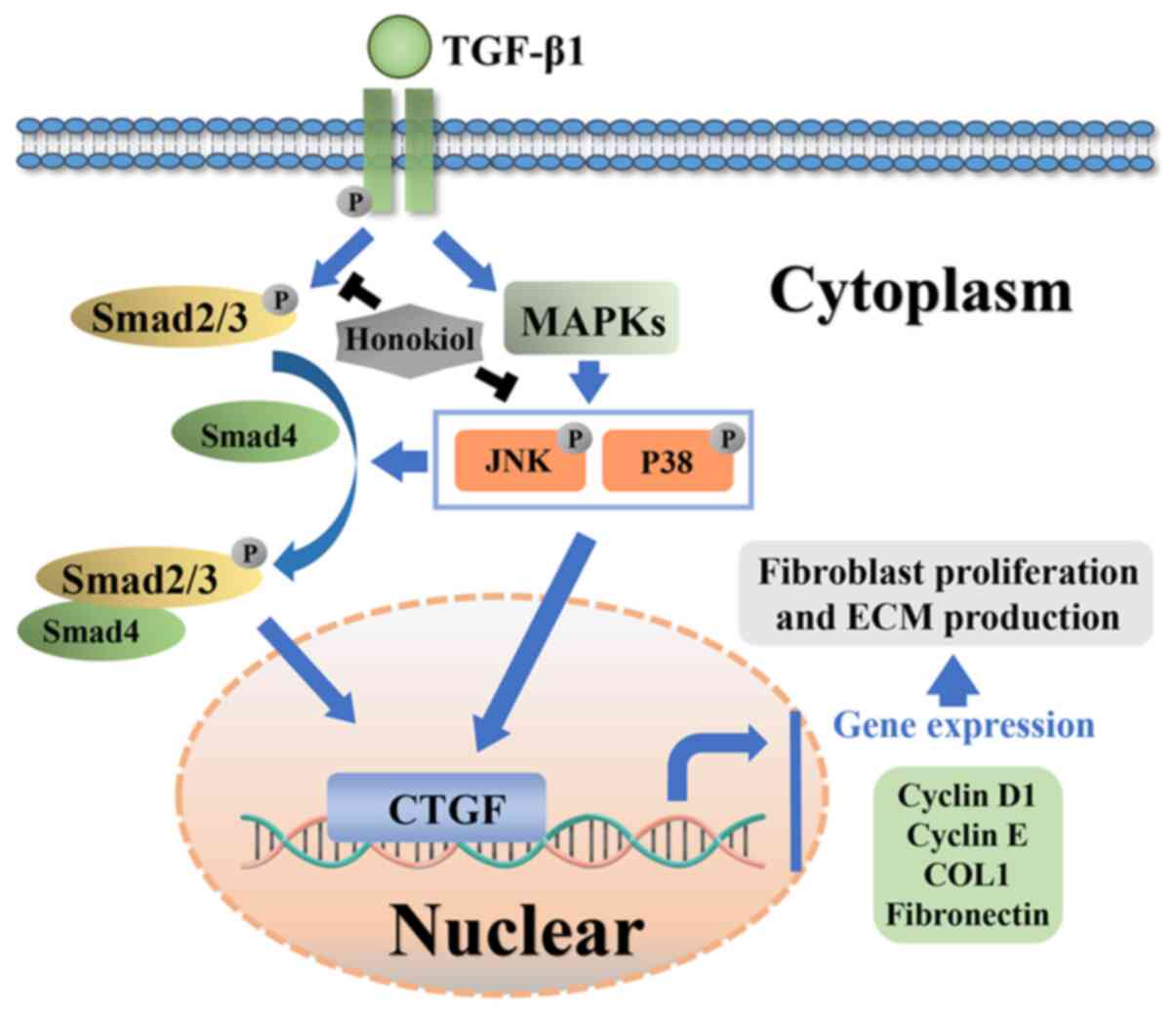
Potential molecular mechanism involved in
honokiol treatment in fibroblasts
Honokiol attenuated TGF-β1 induced excessive
fibroblast proliferation and the synthesis of ECM components in
rats post-laminectomy via suppressing Smad2/3 and MAPK signalling
pathways (Fig. 8).
Discussion
Fibrosis of the local dura mater following
laminectomy, which is not uncommon, leads to worse patient outcomes
(42,43). The current treatment options,
namely, conservative treatment and scar excision, are
unsatisfactory (44). Therefore,
biological therapies are increasingly being investigated. Honokiol,
an extract of Magnolia officinalis, exhibits potent
anti-proliferative and anti-fibrotic effects (30,31). The present study simultaneously
used primary fibroblasts and a post-laminectomy rat model to
investigate the anti-EF effect of honokiol. Consistent with the
biological activities of honokiol described in previous studies
(30,31), the present study found that
honokiol attenuated EF development by inhibiting excessive
fibroblast proliferation and ECM overproduction induced by TGF-β1
in primary fibroblasts, and the anti-EF effect was further
confirmed in a rat model post-laminectomy.
Fibroblasts are responsible for the majority of scar
tissue, and trigger the fibrosis of various tissues and organs.
During scar formation, fibroblasts rapidly proliferate and produce
large quantities of ECM components, including type collagen I and
III and fibronectin (45,46), which is widely considered to be
the predominant cause of EF following lumbar laminec-tomy (15). Therefore, accumulating evidence
emphasizes that it is crucial to suppress excessive fibroblast
proliferation and ECM production during the tissue remodelling
associated with EF (13-15). TGF-β1, a cytokine that regulates
various cellular functions, is reportedly the key initiating factor
of EF. Elevated TGF-β1 levels in scar tissue trigger aberrant
fibroblast proliferation and ECM overproduction (14,16-18). In the present study, it was found
that honokiol exerted a marked anti-proliferative effect on
TGF-β1-treated fibroblasts, which was confirmed using EdU
incorporation assays and western blot analysis of cyclin D1 and
cyclin E, major important molecules involved in proliferation.
Moreover, TGF-β1-induced fibrosis was accompanied by significant
increases in the expression levels of type I collagen and
fibronectin (at both the gene and protein levels) in primary
fibroblasts; these increases were effectively reversed by honokiol
in a dose-dependent manner. To further explore the underlying
mechanism, the effect of honokiol on CTGF was determined. CTGF, a
matricellular protein of the CCN family of ECM-associated proteins
that is minimally expressed in normal adult tissue but strongly
upregulated in fibrotic tissue (47), has been proven to participate in
TGF-β1-dependent fibrosis by regulating proliferation and ECM
production in various cell types (48-50). CTGF has been reported to mediate
the TGF-β1-induced upregulation of collagen type I in fibroblasts
(51). An increased CTGF
expression in epidural scar tissue plays a key role in EF
development by stimulating TGF-β1-induced fibroblast proliferation
and ECM production, as well as collagen and fibronectin synthesis
(13). As was expected, it was
found that TGF-β1 treatment significantly stimulated the production
of CTGF at both the gene and protein levels, which was reversed by
honokiol in a dose-dependent manner. Thus, these results suggested
that honokiol may protect against excessive TGF-β1-induced
fibroblast proliferation and ECM overproduction by inhibiting CTFG
during EF. The data of the present study are in agreement with
those of previous studies in which honokiol protected against both
hypertrophic scarring and renal fibrosis (30,31).
TGF-β1 and its downstream signalling cascades play
vital roles in initiating the pathological mechanisms under-lying
EF (51). The incubation of cells
with TGF-β leads to a long-lasting increase in CTGF (52). Subsequently, CTGF acts as an
extracellular adapter protein by binding to a transmembrane
receptor serine threonine kinase (TGF-β receptor II) through its
cysteine-rich domain, and thus helps to induce the phosphorylation
of another serine threonine kinase complex (TGF-β receptor I/ALK5)
(53). In turn, phosphorylated
ALK5 induces the phosphorylation of Smad2 and Smad3, which then
bind to Smad4 in order to form a heteromeric Smad complex to
activate the transcription of target genes, including CTGF,
triggering the transcription of genes encoding ECM proteins
(47,54-57). Notably, of the Smad transcription
factors, only Smad3 seems to be required for the regulation of
TGF-β-mediated CTGF promoter activation in primary osteoblasts
(58). Moreover, the
TGF-β1-induced activation of the Smad2/3 pathway has also been
suggested to regulate several important cell functions, including
differentiation, proliferation and ECM component synthesis
(23,24). However, TGF-β1-induced CTGF
expression can be blocked via Smad-dependent transcription
(59). It has been demonstrated
that the inhibition of the Smad2/3 pathway markedly ameliorated
keloid expression by inhibiting cell proliferation and ECM
synthesis, and keloid scarring is similar to that characterizing EF
(20,60). Therefore, the present study
explored the molecular mechanisms underlying the anti-fibrotic
effects of honokiol by suppressing Smad signal-ling. As was
expected, Smad2 and Smad3 phosphorylation were increased by TGF-β1
stimulation; honokiol significantly attenuated this effect in a
dose-dependent manner, which was further confirmed by examining the
TGF-β1-induced nuclear translocation of immunofluorescent Smad3.
The present study revealed that honokiol attenuated fibroblast
proliferation and ECM production by inhibiting Smad2/3
phosphorylation and nuclear translocation. In addition, TGF-β1 also
acts via non-canonical pathways in a cell-specific manner. The
TGF-β1-mediated induction of CTGF is further modulated by MAPK
signalling (61,62). It has been suggested that the
inhibition of any two MAPKs, namely, ERK, p38, or JNK, completely
inhibits the TGF-β1-mediated induction of CTGF in lung fibroblasts
(47). Moreover, TGF-β1-induced
MAPK activation has been reported to contribute to the modulation
of the TGF-β1-induced phosphorylation and the nuclear translocation
of Smad2/3 signalling pathway components (41,63). Although the molecular mechanisms
of the crosstalk between the MAPK and Smad signalling pathways are
not yet fully understood, interest in the important role of the
MAPK-Smad crosstalk pathway in fibrosis is growing (23). Furthermore, a recent study
demonstrated that MAPK signalling played a critical role in liver
injury and hepatic stellate cell proliferation (64). p38 MAPK signalling increases the
half-life and stability of type I collagen mRNA (65). Honokiol has also been reported to
suppress the activity of the MAPK signalling pathway by inhibiting
the phosphorylation of p38 and JNK in human epithelial cells
(66). As shown in in the present
study, TGF-β1 also activated the MAPK signalling pathway. Of note,
honokiol preconditioning significantly reduced the TGF-β1-induced
phosphorylation of p38 and JNK, but not p-ERK, which is consistent
with the findings of previous studies (32,66). Taken together, it was surmised
that the anti-EF effects of honokiol may be associated with the
inhibition of CTGF via the Smad2/3 and MAPK (p38 and JNK)
pathways.
The therapeutic effects of honokiol were further
assessed in a rat model of EF post-laminectomy. Compared to the
honokiol-treated group, the control group exhibited more severe
epidural adhesion and higher numbers of fibroblasts. Honokiol
significantly reduced collagen density, hydroxypropyl cellulose,
and fibronectin and type I collagen production by epidural scar
tissue, indicating that honokiol could ameliorate EF in
vivo. These results and those of the in vitro
experiments further demonstrated that honokiol has potential value
in preventing epidural scar adhesion.
However, the present study still has some
limitations. First, the present study did not assess the effects of
a MAPK pathway inhibitor, such as the p38 kinase inhibitor SB203580
or the JNK inhibitor SP600125. Second, in addition to fibro-blast
proliferation and ECM deposition, the lack of further mechanistic
analysis to determine the involvement of fibroblast activation,
migration and differentiation is another limitation of the present
study that will be investigated in future studies. Third, it would
be better to further confirm whether honokiol arrests cell growth
by other assays, such as flow cytometry.
In conclusion, the present study demonstrates that
honokiol may serve as a promising and effective therapeutic agent
for the treatment of EF in the future. The potential mechanisms
involved in its protective effects are the reversal of aberrant
fibroblast proliferation and ECM production by inhibiting the
Smad2/3 and MAPK pathways.
Supplementary Data
Acknowledgments
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81371988), the
Zhejiang Public service technology research program/social
development (grant no. LGF18H060008), the Science and Technology
Innovation Activity Plan for University Students in Zhejiang
Province (grant no. 2019R413007), and the Zhejiang Provincial
Public Welfare Science and Technology Project (grant no.
2017C33100).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DX, QW and HJ conceived and designed the
experiments. DX, WZ and XH performed the experiments. TQ, JS, FQ,
CL and QW analysed the data. DX, QW and HJ were involved in the
drafting of the manuscript or critically revising it for important
intellectual content. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
All experimental procedures for animal studies were
carried out in compliance with the principles of International
Laboratory Animal Care and the experimental protocol was approval
by the Animal Care and Use Committee at the Wenzhou Medical College
(approval no. wydw2017-0007).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liu P, Chen H, Yan L and Sun Y: Laminin
alpha5 modu-lates fibroblast proliferation in epidural fibrosis
through the PI3K/AKT/mTOR signaling pathway. Mol Med Rep.
21:1491–1500. 2020.PubMed/NCBI
|
|
2
|
Burton CV: Causes of failure of surgery on
the lumbar spine: Ten-Year follow-up. Mt Sinai J Med. 58:183–187.
1991.PubMed/NCBI
|
|
3
|
Burton CV, Kirkaldy-Willis WH, Yong-Hing K
and Heithoff KB: Causes of failure of surgery on the lumbar spine.
Clin Orthop Relat Res. 157:191–199. 1981.
|
|
4
|
Chen F, Zuo Z, Wang K, Zhang C, Gong H, Ye
F, Ji A and Tao H: Study on salvianolic acid B in the reduction of
epidural fibrosis in laminectomy rats. BMC Musculoskelet Disord.
15:3372014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang K, Zhao J, Su W, Lu R and Lv P:
Immunomodulatory effectiveness of licofelone in preventing epidural
fibrosis in post-laminectomy rat. Eur J Orthop Surg Traumatol.
25:S63–S68. 2015. View Article : Google Scholar
|
|
6
|
Kasimcan MO, Bakar B, Aktaş S, Alhan A and
Yilmaz M: Effectiveness of the biophysical barriers on the
peridural fibrosis of a postlaminectomy rat model: An experimental
research. Injury. 42:778–781. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu CY, Huang YH, Lee JS, Tai TW, Wu PT and
Jou IM: Efficacy of topical cross-linked hyaluronic acid hydrogel
in preventing post laminectomy/laminotomy fibrosis in a rat model.
J Orthop Res. 34:299–306. 2016. View Article : Google Scholar
|
|
8
|
Lubina ZI, Baranovic S, Karlak I, Novacic
K, Potocki-Karacic T and Lovrić D: The grading model for the
assessment of the total amount of epidural fibrosis in
postoperative lumbar spine. Eur Spine J. 22:892–897. 2013.
View Article : Google Scholar :
|
|
9
|
Ross JS, Robertson JT, Frederickson RC,
Petrie JL, Obuchowski N, Modic MT and deTribolet N: Association
between peridural scar and recurrent radicular pain after lumbar
discectomy: Magnetic resonance evaluation. ADCON-L european study
group. Neurosurgery. 38:855–861. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang H, Sun W, Fu D, Shen Y, Chen YY and
Wang LL: Update on biomaterials for prevention of epidural adhesion
after lumbar laminectomy. J Orthop Translat. 13:41–49. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brzezicki G, Jankowski R, Blok T, Klimczak
A, Szymas J, Huber J, Szukala A, Siemionow M and Nowak S:
Postlaminectomy osteo-pontin expression and associated
neurophysiological findings in rat peridural scar model. Spine
(Phila Pa 1976). 36:378–385. 2011. View Article : Google Scholar
|
|
12
|
Yakovlev AE, Timchenko AA and Parmentier
AM: Spinal cord stimulation and sacral nerve stimulation for
postlaminectomy syndrome with significant low back pain.
Neuromodulation. 17:763–765. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu H, Liu C, Sun Z, Guo X, Zhang Y, Liu M
and Li P: CCN5 attenuates profibrotic phenotypes of fibroblasts
through the smad6-CCN2 pathway: Potential role in epidural fi
brosis. Int J Mol Med. 36:123–129. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yan L, Li X, Wang J, Sun Y, Wang D, Gu J,
He J, Hu H, Chen G, Wang Q and Feng X: Immunomodulatory
effectiveness of tacrolimus in preventing epidural scar adhesion
after laminectomy in rat model. Eur J Pharmacol. 699:194–199. 2013.
View Article : Google Scholar
|
|
15
|
Zhang C, Kong X, Liu C, Liang Z, Zhao H,
Tong W, Ning G, Shen W, Yao L and Feng S: ERK2 small interfering
RNAs prevent epidural fibrosis via the efficient inhibition of
collagen expression and inflammation in laminectomy rats. Biochem
Biophys Res Commun. 444:395–400. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun HH, Wang JC, Feng XM, Zhu SL and Cai
J: Allicin inhibits proliferation and promotes apoptosis of human
epidural scar fibroblasts. World Neurosurg. 136:e460–e468. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Penn JW, Grobbelaar AO and Rolfe KJ: The
role of the TGF-β family in wound healing, burns and scarring: A
review. Int J Burns Trauma. 2:18–28. 2012.
|
|
18
|
Zhang C, Kong X, Zhou H, Liu C, Zhao X,
Zhou X, Su Y, Sharma HS and Feng S: An experimental novel study:
Angelica sinensis prevents epidural fibrosis in laminectomy rats
via down-regulation of hydroxyproline, IL-6, and TGF-β1. Evid Based
Complement Alternat Med. 2013:2918142013. View Article : Google Scholar
|
|
19
|
Lakos G, Takagawa S, Chen SJ, Ferreira AM,
Han G, Masuda K, Wang XJ, DiPietro LA and Varga J: Targeted
disruption of TGF-beta/Smad3 signaling modulates skin fibrosis in a
mouse model of scleroderma. Am J Pathol. 165:203–217. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu CS, Wu PH, Fang AH and Lan CC: FK506
inhibits the enhancing effects of transforming growth factor
(TGF)-β1 on collagen expression and TGF-β/Smad signalling in keloid
fibroblasts: Implication for new therapeutic approach. Br J
Dermatol. 167:532–541. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Massagué J and Wotton D: Transcriptional
control by the TGF-beta/smad signaling system. EMBO J.
19:1745–1754. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nakao A, Imamura T, Souchelnytskyi S,
Kawabata M, Ishisaki A, Oeda E, Tamaki K, Hanai J, Heldin CH,
Miyazono K and Dijke Pt: TGF-beta receptor-mediated signalling
through smad2, smad3 and smad4. EMBO J. 16:5353–5362. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Javelaud D and Mauviel A: Mammalian
transforming growth factor-betas: Smad signaling and
physio-pathological roles. Int J Biochem Cell Biol. 36:1161–1165.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Derynck R and Zhang YE: Smad-Dependent and
smad-independent pathways in TGF-beta family signalling. Nature.
425:577–584. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fried LE and Arbiser JL: Honokiol, a
multifunctional antiangiogenic and antitumor agent. Antioxid Redox
Signal. 11:1139–1148. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee YJ, Lee YM, Lee CK, Jung JK, Han SB
and Hong JT: Therapeutic applications of compounds in the magnolia
family. Pharmacol Ther. 130:157–176. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Woodbury A, Yu SP, Wei L and García P:
Neuro-Modulating effects of honokiol: A review. Front Neurol.
4:1302013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pan J, Lee Y, Wang Y and You M: Honokiol
targets mitochondria to halt cancer progression and metastasis. Mol
Nutr Food Res. 60:1383–1395. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shen JL, Man KM, Huang PH, Chen WC, Chen
DC, Cheng YW, Liu PL, Chou MC and Chen YH: Honokiol and magnolol as
multifunctional antioxidative molecules for dermatologic disorders.
Molecules. 15:6452–6465. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao D, Wang Y, Du C, Shan S, Zhang Y, Du
Z and Han D: Honokiol alleviates hypertrophic scar by targeting
transforming growth factor-β/smad2/3 signaling pathway. Front
Pharmacol. 8:2062017. View Article : Google Scholar
|
|
31
|
Chiang CK, Sheu ML, Lin YW, Wu CT, Yang
CC, Chen MW, Hung KY, Wu KD and Liu SH: Honokiol ameliorates renal
fibrosis by inhibiting extracellular matrix and pro-inflammatory
factors in vivo and in vitro. Br J Pharmacol. 163:586–597. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Elfeky MG, Mantawy EM, Gad AM, Fawzy HM
and El-Demerdash E: Mechanistic aspects of antifibrotic effects of
honokiol in con A-induced liver fibrosis in rats: Emphasis on
TGF-β/SMAD/MAPK signaling pathways. Life Sci. 240:1170962020.
View Article : Google Scholar
|
|
33
|
Sun Y, Wang LX, Wang L, Sun SX, Cao XJ,
Wang P and Feng L: A comparison of the effectiveness of mitomycin C
and 5-fluoro-uracil in the prevention of peridural adhesion after
laminectomy. J Neurosurg Spine. 7:423–428. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
35
|
American Veterinary Medical Association
(AVMA): AVMA Guidelines for the Euthanasia of Animals: 2020
Edition. AVMA; Schaumburg, IL: 2020, https://www.avma.org/sites/default/files/2020-01/2020-Euthanasia-Final-1-17-20.pdfurisimplehttps://www.avma.org/sites/default/files/2020-01/2020-Euthanasia-Final-1-17-20.pdf.
|
|
36
|
Rydell N: Decreased granulation tissue
reaction after installment of hyaluronic acid. Acta Orthop Scand.
41:307–311. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fukui N, Tashiro T, Hiraoka H, Oda H and
Nakamura K: Adhesion formation can be reduced by the suppression of
transforming growth factor-beta1 activity. J Orth Res. 18:212–219.
2000. View Article : Google Scholar
|
|
38
|
Möröy T and Geisen C: Cyclin E. Int J
Biochem Cell Biol. 36:1424–1439. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Maggioni D, Nicolini G, Rigolio R, Biffi
L, Pignataro L, Gaini R and Garavello W: Myricetin and naringenin
inhibit human squamous cell carcinoma proliferation and migration
in vitro. Nutr Cancer. 66:1257–1267. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Schnittger A and De Veylder L: The dual
face of cyclin B1. Trends Plant Sci. 23:475–478. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Engel ME, McDonnell MA, Law BK and Moses
HL: Interdependent SMAD and JNK signaling in transforming growth
factor-beta-mediated transcription. J Biol Chem. 274:37413–37420.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Dai J, Li X, Yan L, Chen H, He J, Wang S,
Wang J and Sun Y: The effect of suramin on inhibiting fibroblast
proliferation and preventing epidural fibrosis after laminectomy in
rats. J Orthop Surg Res. 11:1082016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jiao R, Chen H, Wan Q, Zhang X, Dai J, Li
X, Yan L and Sun Y: Apigenin inhibits fibroblast proliferation and
reduces epidural fibrosis by regulating wnt3a/β-catenin signaling
pathway. J Orthop Surg Res. 14:2582019. View Article : Google Scholar
|
|
44
|
Cruccu G, Aziz TZ, Garcia-Larrea L,
Hansson P, Jensen TS, Lefaucheur JP, Simpson BA and Taylor RS: EFNS
guidelines on neurostimulation therapy for neuropathic pain. Eur J
Neurol. 14:952–970. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Armour A, Scott PG and Tredget EE:
Cellular and molecular pathology of HTS: Basis for treatment. Wound
Repair Regen. 15:S6–S17. 2007. View Article : Google Scholar
|
|
46
|
Chrysanthopoulou A, Mitroulis I,
Apostolidou E, Arelaki S, Mikroulis D, Konstantinidis T, Sivridis
E, Koffa M, Giatromanolaki A, Boumpas DT, et al: Neutrophil
extracellular traps promote differentiation and function of
fibroblasts. J Pathol. 233:294–307. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Cicha I and Goppelt-Struebe M: Connective
tissue growth factor: Context-Dependent functions and mechanisms of
regulation. Biofactors. 35:200–208. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Fan WH, Pech M and Karnovsky MJ:
Connective tissue growth factor (CTGF) stimulates vascular smooth
muscle cell growth and migration in vitro. Eur J Cell Biol.
79:915–923. 2000. View Article : Google Scholar
|
|
49
|
Yamanaka O, Saika S, Ikeda K, Miyazaki KI,
Kitano A and Ohnishi Y: Connective tissue growth factor modulates
extracellular matrix production in human subconjunctival
fibroblasts and their proliferation and migration in vitro. Jpn J
Ophthalmol. 52:8–15. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Burns WC, Twigg SM, Forbes JM, Pete J,
Tikellis C, Thallas-Bonke V, Thomas MC, Cooper ME and Kantharidis
P: Connective tissue growth factor plays an important role in
advanced glycation end product-induced tubular
epithelial-to-mesenchymal transition: Implications for diabetic
renal disease. J Am Soc Nephrol. 17:2484–2494. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Jin H, Wang Z, Gu Z, Wu J, Bai X, Shao Z,
Miao J, Wang Q, Wang Q and Wang X: Schisandrin B attenuates
epidural fibrosis in postlaminectomy rats by inhibiting
proliferation and extracellular matrix production of fibroblasts.
Phytother Res. 33:107–116. 2019. View Article : Google Scholar
|
|
52
|
Kroening S, Solomovitch S, Sachs M,
Wullich B and Goppelt-Struebe M: Regulation of connective tissue
growth factor (CTGF) by hepatocyte growth factor in human tubular
epithelial cells. Nephrol Dial Transplant. 24:755–762. 2009.
View Article : Google Scholar
|
|
53
|
Ruiz-Ortega M, Rodríguez-Vita J,
Sanchez-Lopez E, Carvajal G and Egido J: TGF-Beta signaling in
vascular fibrosis. Cardiovasc Res. 74:196–206. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Heldin CH, Miyazono K and ten Dijke P:
TGF-Beta signal-ling from cell membrane to nucleus through SMAD
proteins. Nature. 390:465–471. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
55
|
Shi Y and Massagué J: Mechanisms of
TGF-beta signaling from cell membrane to the nucleus. Cell.
113:685–700. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Schiller M, Javelaud D and Mauviel A:
TGF-Beta-induced SMAD signaling and gene regulation: Consequences
for extra-cellular matrix remodeling and wound healing. J Dermatol
Sci. 35:83–92. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Ryer EJ, Hom RP, Sakakibara K, Nakayama
KI, Nakayama K, Faries PL, Liu B and Kent KC: PKCdelta is necessary
for smad3 expression and transforming growth factor beta-induced
fibronectin synthesis in vascular smooth muscle cells. Arterioscler
Thromb Vasc Biol. 26:780–786. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Arnott JA, Zhang X, Sanjay A, Owen TA,
Smock SL, Rehman S, DeLong WG, Safadi FF and Popoff SN: Molecular
requirements for induction of CTGF expression by TGF-beta1 in
primary osteoblasts. Bone. 42:871–885. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Lin SL, Chen RH, Chen YM, Chiang WC, Lai
CF, Wu KD and Tsai TJ: Pentoxifylline attenuates tubulointerstitial
fibrosis by blocking smad3/4-activated transcription and
profibrogenic effects of connective tissue growth factor. J Am Soc
Nephrol. 16:2702–2713. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Phan TT, Lim IJ, Chan SY, Tan EK, Lee ST
and Longaker MT: Suppression of transforming growth factor
beta/smad signaling in keloid-derived fibroblasts by quercetin:
Implications for the treatment of excessive scars. J Trauma.
57:1032–1037. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Fu M, Zhang J, Zhu X, Myles DE, Willson
TM, Liu X and Chen YE: Peroxisome proliferator-activated receptor
gamma inhibits transforming growth factor beta-induced connective
tissue growth factor expression in human aortic smooth muscle cells
by interfering with smad3. J Biol Chem. 276:45888–45894. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Kwon S, Munroe X, Crawley SC, Lee HY,
Spong S, Bradham D, Gum JR Jr, Sleisenger MH and Kim YS: Expression
of connective tissue growth factor in pancreatic cancer cell lines.
Int J Oncol. 31:693–703. 2007.PubMed/NCBI
|
|
63
|
Park JH, Yoon J, Lee KY and Park B:
Effects of geniposide on hepatocytes undergoing
epithelial-mesenchymal transition in hepatic fibrosis by targeting
TGFβ/smad and ERK-MAPK signaling pathways. Biochimie. 113:26–34.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Tang N, Zhang YP, Ying W and Yao XX:
Interleukin-1β upregu-lates matrix metalloproteinase-13 gene
expression via c-jun N-terminal kinase and p38 MAPK pathways in rat
hepatic stellate cells. Mol Med Rep. 8:1861–1865. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Tsukada S, Westwick JK, Ikejima K, Sato N
and Rippe RA: SMAD and p38 MAPK signaling pathways independently
regulate alpha1(I) collagen gene expression in unstimulated and
transforming growth factor-beta-stimulated hepatic stellate cells.
J Biol Chem. 280:10055–10064. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Tang X, Yao K, Zhang L, Yang Y and Yao H:
Honokiol inhibits H(2)O(2)-induced apoptosis in human lens
epithelial cells via inhibition of the mitogen-activated protein
kinase and akt path-ways. Eur J Pharmacol. 650:72–78. 2011.
View Article : Google Scholar
|