Introduction
Blunt chest (thoracic) trauma (TxT) is common in
polytraumatized patients, and 20-25% of deaths in severely injured
patients treated in emergency departments are caused by chest
injuries (1). Typical
complications in these patients include disseminated intravascular
coagulation, pneumonia, acute lung injury (ALI), or its more severe
form, acute respiratory distress syndrome (ARDS) (2,3).
While the role of mechanical damage in the pathogenesis of
post-traumatic complications associated with lung injury is
undisputed, current research indicates that lung damage is driven
by local and systemic inflammatory reactions (4).
Hoth et al demonstrated increased levels of
pro-inflammatory CXCL1 in bronchoalveolar lavage fluid at 3 h
post-injury in a murine model of pulmonary contusion (5). Furthermore, the recruitment of
neutrophils to injured lung tissue, which is primarily responsible
for pulmonary dysfunction following lung contusion, has been shown
to be dependent on the expression of CXCL1, among other
pro-inflammatory factors (6).
Recently, the authors demonstrated that a 'double-hit' model of
TxT, followed by cecal ligation and puncture (CLP) as the second
hit, which was also applied in the underlying study, increased the
expression of pro-inflammatory chemokines and cytokines, including
CXCL1 (7). Thus, in line with the
two-hit hypothesis, a traumatic insult as the first hit, followed
by secondary surgeries or complications, may lead to detrimental
outcomes. Consistent with the findings of other researchers, it was
found that isolated experimental lung trauma induced a profound
inflammatory reaction; however, it was insufficient to establish
the ongoing pathological pulmonary changes associated with ALI
(7,8). However, secondary CLP following TxT,
as applied herein, reflected the human etiology of indirect lung
damage ending in ALI (7,9-11).
Another murine 'double-hit' model that includes CLP, followed by
inducing pneumonia via Pseudomonas aeruginosa 4-7 days
later, significantly improved survival when pneumonia was induced
after 7 days. Improved survival was associated with the restoration
of interferon (IFN)-γ by stimulated splenocytes (12). These findings underline the
importance of timing of the second hit, and thus also for
therapeutic interventions. IFN-γ plays a key role in the regulation
of both innate and acquired antimicrobial immunity by inter alia,
stimulating macrophage functions, such as phagocytosis, respiratory
burst activity, antigen presentation and cytokine secretion
(13,14). The functional significance of
IFN-γ in antimicrobial defense has been demonstrated by the
increased susceptibilities of IFN-γ−/− and
IFN-γ-R−/− mice to a variety of infections (15). Furthermore, IFN-γ seemed to
facilitate systemic inflammation during CLP-induced abdominal
sepsis in mice (16).
Club cell protein (CC)16 is an anti-inflammatory
protein derived from epithelial club cells in the lungs (17,18). Its systemic concentrations are
associated with the extent of lung contusion and with pulmonary
complications in traumatized patients, which underscores its
biomarker characteristics (19,20). Although its exact functions in
vivo remain unclear, CC16 plays an important protective role in
the respiratory tract against oxidative stress and inflammatory
responses due to its anti-inflammatory properties (21). With regard to the two-hit
hypothesis, the inflammatory response following trauma is essential
for host defense; however, it can cause further tissue damage if
triggered by a secondary stimulus (22,23). Since reducing inflammation
attenuates pathological injury and the survival of mice with ALI
(24), as a pulmonary
anti-inflammatory protein, CC16 may play an important regulatory
role during the development ALI following trauma. However, CC16 may
also play a pathological role in immunoparalysis with concomitant
post-traumatic infectious complications (25). This role was addressed in a recent
study by the authors, which demonstrated the anti-inflammatory
effects of CC16 on CLP-induced ALI following TxT in mice (9). While the early intrapulmonary
inhibition of CC16 following TxT reduced lung damage, the later
inhibition of CC16 deteriorated lung damage after 24 h. However, it
remains questionable whether the latter may have been followed by
reduced lung damage in a prolonged observational period. Since this
question and the one regarding the pathophysiological relevance of
these observations to survival remain unanswered, the present study
evaluated whether the local neutralization of CC16 affects
mortality after sepsis-induced ALI following blunt chest trauma in
a murine model.
Materials and methods
Animals and experimental model
All experiments were conducted in accordance with
German federal laws regarding the protection of animals. The
experiments were performed at Goethe University Hospital in
Frankfurt, Germany. The present study was approved by the
responsible government authority, the Veterinary Department of the
Regional Council in Darmstadt, Germany (Regierungspräsidium
Darmstadt; AZ: FK 1068), and the study was performed under ARRIVE
guidelines (26).
For the survival analysis, 48 male CL57BL/6N mice
(weighing 25±5 g at 6-8 weeks of age) were included, and 72 male
CL57BL/6N mice (6-8 weeks old, weighing 25±5 g) were included for
experiments with sacrifice after 6 or 24 h post-CLP. All animals
were purchased from Janvier Labs. The animals were provided with
free access to water and food ad libidum before and after
the experimental procedures. At 30 min prior to the experiment,
buprenorphine (Indivior Eu Ltd., 0.1 mg/kg body weight) was
administered to all mice subcutaneously. This process was repeated
every 12 h. For the survival analysis, buprenorphine (0.1 mg/kg
body weight) was administered every 6 h for 48 h after CLP. From
48-96 h post-CLP, buprenorphine (0.1 mg/kg body weight) was
administered every 12 h. Drinking water provided to the mice was
enriched with metamizole (Ratiopharm) to maintain adequate
analgesia during the experiment.
For TxT, mice underwent general mask anesthesia with
3% isoflurane (Baxter Deutschland GmbH), according to
partly-modified standardized protocols described previously
(7,9,10,27). Briefly, the mice were placed in
the supine position under a cylinder. The cylinder was separated by
a Mylar polyester film with a thickness of 0.05 mm [DuPont de
Nemours (Deutschland) GmbH] and placed 2.5 cm above the sternum.
Delivering compressed air to the upper part of the cylinder
ruptured the membrane and led to a standardized pressure wave. This
resulted in standardized, bilateral, blunt chest trauma. According
to their group allocation, mice received an intratracheal
application of either a CC16 antibody or a control antibody
immediately following TxT. Uteroglobin/SCGB1A1 (CC16 Ab; LS
Biosciences) or IgG control (IgG) antibody (10 µg/ml,
R&D Systems) were administered either immediately following the
induction of thoracic trauma (early application) or following CLP
(late application). For antibody administration, mice were placed
in the supine position, and the tongue was kept aside. A buttoned
cannula was placed at the beginning of the trachea, and 50
µl were carefully administered. Mice were then kept in the
reverse Trendelenburg position for 30 sec to ensure proper antibody
distribution inside the lungs. After 24 h, mice underwent
intraperitoneal anesthesia with ketamine (100 mg/kg body weight,
Zoetis Deutschland GmbH) and xylazine (10 mg/kg body weight, Bayer
Leverkusen), and depending on group allocation, the following
procedures were performed. Either median laparotomy and CLP were
performed as previously described (7,28)
or median laparotomy and eversion of the caecum without any further
manipulation was performed. Briefly, the abdominal cavity was
opened by midline laparotomy (approximately 1 cm), and the cecum
was carefully exposed. Subsequently, 7 mm of the distal caecum were
ligated using Premilene 5-0 suture (B. Braun Melsungen AG) and
perforated with a single through-and-through puncture using a 25G
cannula (BD Biosciences).
For survival experiments, a 18G cannula (BD
Biosciences) was used. After this procedure, a small amount of
feces was extruded, and the cecum was restored to the abdominal
cavity. Abdominal closure was performed by a two-layer suture.
According to group allocation, mice received an intratracheal
application of either a CC16 antibody or control IgG antibody, as
described above, immediately following thoracic trauma or following
CLP. The sham-operated (sham) control group underwent the identical
anesthesia procedures described above, but without TxT, as well as
with median laparotomy and eversion of the caecum, but without CLP.
Immediately following CLP, 1 ml of prewarmed G5% solution (B. Braun
Melsungen AG) was administered intraperitoneally. Depending on
group allocation, either 6 or 24 h post-CLP, mice received
anesthesia as described above and sacrifice was performed, or mice
were observed for 6 days after CLP and euthanized via blood
withdrawal by a puncture of the heart under general anesthesia with
3% isoflurane (Baxter Deutschland GmbH). During the survival
analysis, mice were euthanized when they reached one of the
following conditions: Permanently closed eyes, lateral position,
severe dyspnea with mouth breathing, cyanosis, body weight loss
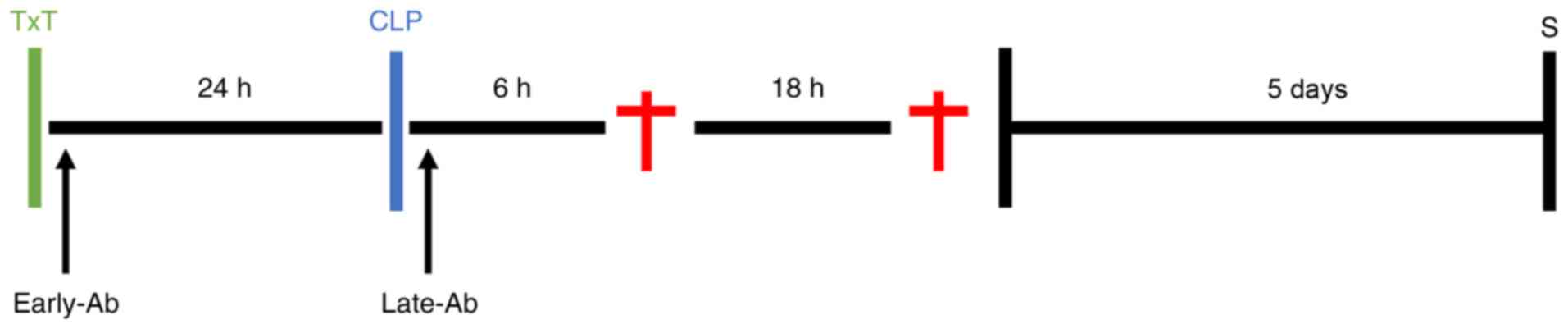
>20% or apathy. An overview of the experimental design is
presented in Fig. 1.
Group allocation
The animals were randomly allocated to experimental
groups, as described below. For survival analyses, all mice, apart
from those in the sham group received TxT followed by CLP (ALI)
after 24 h. In total, 8 mice received an intratracheal application
of CC 16 antibody, and 8 mice received an intratracheal application
of the control antibody (IgG), as described above, immediately
following TxT. A total of 16 mice received an intratracheal an
application of a CC 16 or control antibody (i.e., 8 mice received
CC 16 Ab, and 8 mice received IgG) immediately following CLP. In
total, 8 mice were allocated to the control group without any
application of antibodies, while 8 mice were allocated to the sham
group, which received median laparotomy and eversion of the caecum,
but not CLP or TxT. The same group allocation was used for the
groups of mice sacrificed at 6 and 24 h (n=6 in each group). Mice
received a CC 16 or control antibody (IgG) immediately after TxT
(early Ab) and were sacrificed after 6 h (6 h_early Ab) or 24 h (24
h_early Ab) after CLP or received a CC 16 or control antibody (IgG)
immediately after CLP (late Ab), followed by sacrifice after 6 h (6
h_late Ab) or 24 h (24 h_late Ab).
Systemic IFN-γ determination
The caval vein was punctured by a heparinized
syringe for blood withdrawal at 6 or 24 h after CLP. Subsequently,
plasma was isolated from blood samples by centrifugation (1,164 × g
for 15 min at 4°C) and stored at −80°C for further IFN-γ
measurements. IFN-γ was measured with a BD CBA Mouse Inflammation
kit (BD Biosciences) according to the manufacturer's instructions.
Briefly, 50 μl of capture beads were added to polystyrene
FACS tubes (BD Pharmingen™) to 50 μl serum.
Subsequently, 50 μl of Mouse Inflammation PE Detection
Reagent were added followed by incubating for 2 h at room
temperature. Subsequently, samples were washed with 1 ml of the
wash buffer and centrifuged at 200 × g for 5 min at room
temperature. The supernatant was discarded, and the pellets were
resuspended in 300 µl of the wash buffer. Analyses were
performed using a BD FACSCanto 2™ and FCAP Array™ Software (BD
Biosciences). Cytometric Bead Array (CBA) was performed as
previously described (29).
Quantification of CXCL1 expression levels
in the lungs
Following blood withdrawal, the animals were
perfused with 20 ml PBS via the inferior caval vein. The lungs were
then removed and snap-frozen in liquid nitrogen. Lung tissue was
homogenized in a protein lysis buffer at 4°C, followed by
centrifugation for 30 min at 4°C and 20,000 × g. Subsequently,
supernatants were stored at −80°C. CXCL1 concentrations in the
lungs were determined using a Mouse CXCL1/KC ELISA kit from R&D
Systems according to the manufacturer's instructions
(Wiesbaden-Nordenstadt). ELISA was performed using an Infinite M200
microplate reader (Tecan Deutschland GmbH).
RNA isolation and reverse transcription
(RT) semi-quantitative polymerase chain reaction (RT-qPCR)
Following sacrifice, snap-frozen specimens from the
lungs were stored at −80°C, and RNA isolation was applied using an
RNeasy-system (Qiagen GmbH), as previously described (30). Residual DNA was removed using an
RNase-free DNase kit (Qiagen GmbH). The amount and quality of RNA
were measured photometrically using a NanoDrop ND-1000 device
(NanoDrop Technologies, Inc.). Subsequently, RNA was reverse
transcribed into cDNA using an AffinityScript QPCR cDNA Synthesis
kit (Stratagene) according to the manufacturer's instructions. A
specific primer for mouse CXCL1 was used to evaluate CXCL1 gene
expression (NM_008176, UniGene no. Mm.21013; cat no. PPM03058A),
and GAPDH (NM_008084, UniGene no. Mm.343110; cat no. PPM02946E)
(both from SA Biosciences) gene expression measurements served as a
reference. According to the manufacturer's instructions, the PCR
reaction was set up in a 25 µl volume with 1X RT2
SYBR-Green/Rox qPCR Master Mix (SA Biosciences) and measured on a
Stratagene Mx3005p QPCR system (Stratagene). The two-step
amplification protocol was as follows: Initial denaturation at 95°C
for 10 min, followed by 40 cycles at 95°C with 15 sec of
denaturation and 60 sec of annealing/extension at 60°C. The
comparative threshold-cycle (CT) method (2−ΔΔCq method)
was used to calculate the target gene's relative expression
(31). Sham-operated animals
served as 100% expression reference following normalization to
GAPDH. The method was applied using a previously described protocol
(30).
Statistical analyses
Statistical analysis was performed using GraphPad
Prism 6 (GraphPad Software, Inc.). Based on the D'Agostino-Pearson
normality test, differences between the groups were analyzed using
the non-parametric Kruskal-Wallis test, followed by Dunn's post hoc
test for the correction of multiple comparisons. The data are
presented as the means ± standard error of the mean. Survival was
analyzed via the log-rank test, and the Bonferroni correction was
applied to correct for multiple comparisons. A P-value <0.05 was
considered to indicate a statistically significant difference.
Results
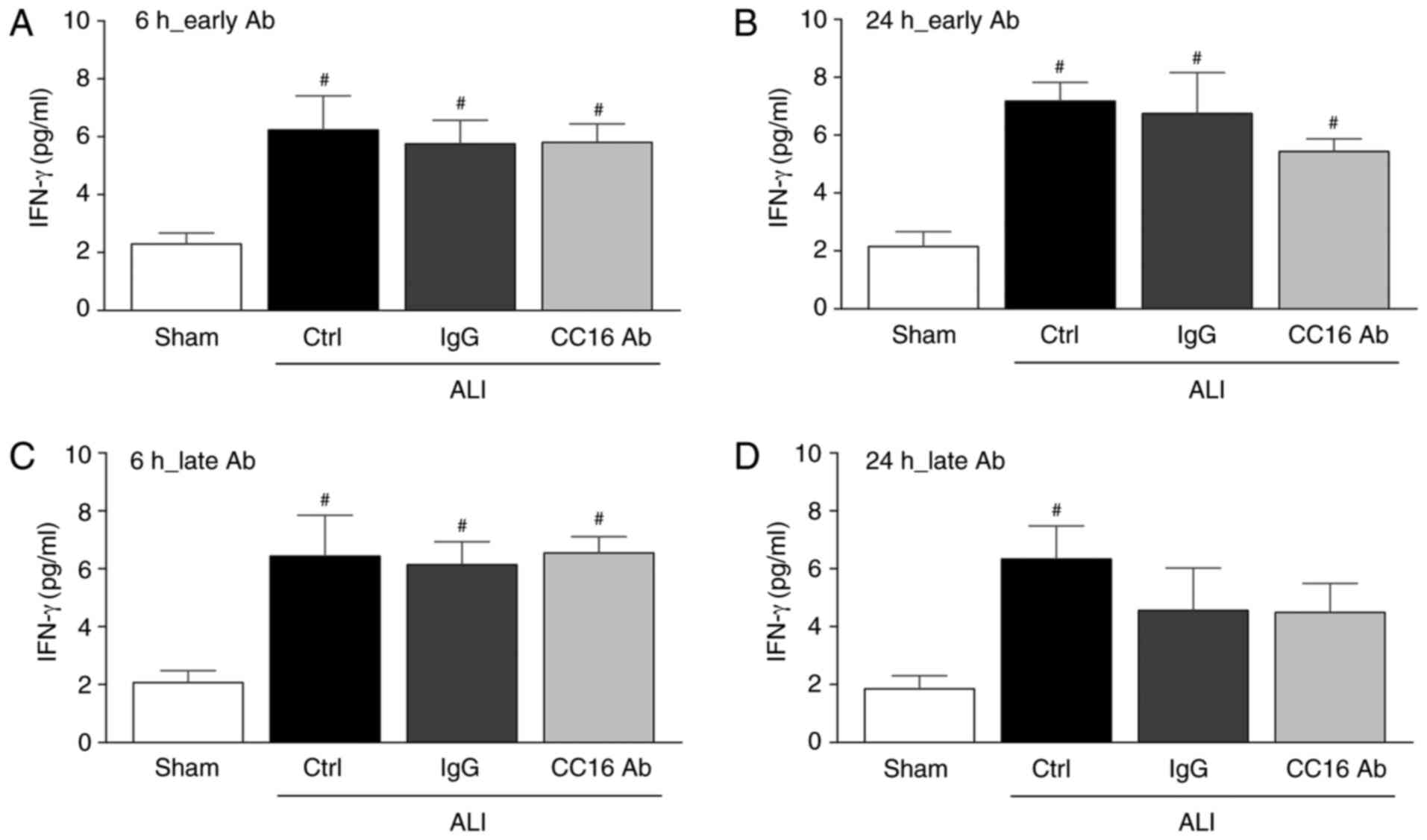
IFN-γ concentrations in the blood
At 6 or 24 h following thoracic trauma and CLP, a
significant increase in the levels of pro-inflammatory IFN-γ
compared to the sham group was detected (P<0.05; Fig. 2). Early antibody application did
not significantly alter the IFN-γ levels between the ALI groups
after 6 or 24 h (Fig. 2). Whereas
late antibody application led to comparable IFN-γ levels among all
the ALI groups after 6 h, a significant increase in the IFN-γ
levels vs. the sham group was observed only in the control group
after ALI was compared with the sham group (P<0.05; Fig. 2C and D).
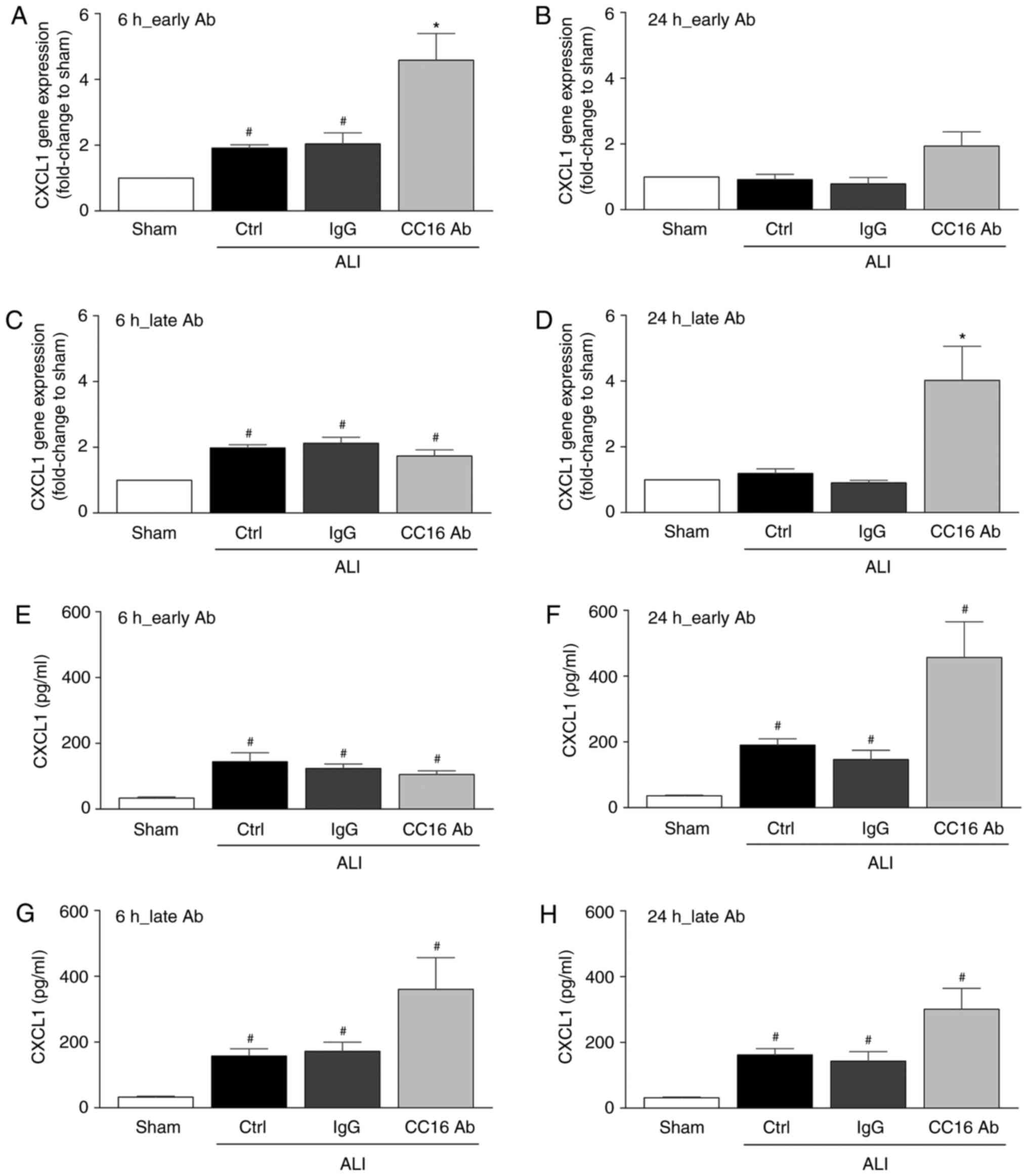
CXCL1 gene expression and protein
concentration
Although CXCL1 gene expression was significantly
elevated in the control and IgG groups at 6 h following thoracic
trauma and CLP compared to the sham group (P<0.05; Fig. 3A and C), no significant
differences were observed after 24 h among these groups (Fig. 3B and D). The early application of
the CC16 antibody significantly increased CXCL1 gene expression
after 6 h compared to all other groups (P<0.05; Fig. 3A). However, after 24 h, no
significant changes among the groups were observed (Fig. 3B). Whereas late CC16 antibody
application led to levels comparable to those of the other groups
undergoing thoracic trauma and CLP after 6 h, a significant
increase in CXCL1 gene expression following late CC16 antibody
application vs. all other groups was observed after 24 h
(P<0.05; Fig. 2D).
CXCL1 protein concentration was significantly
elevated in all 3 ALI groups at 6 and 24 h following thoracic
trauma and CLP compared to the sham group (P<0.05; Fig. 3E-H). Whereas early CC16 antibody
application led to levels comparable to those of the other groups
undergoing thoracic trauma and CLP after 6 h, a significant
increase in CXCL1 protein concentration following early CC16
antibody application vs. all the other groups was observed after 24
h (P<0.05; Fig. 3F). The late
application of the CC16 antibody following thoracic trauma and CLP
led to a slight increase in the CXLC1 protein concentrations
compared to the control and IgG groups upon ALI after 6 and 24 h;
however, this difference was insignificant (Fig. 3G and H).
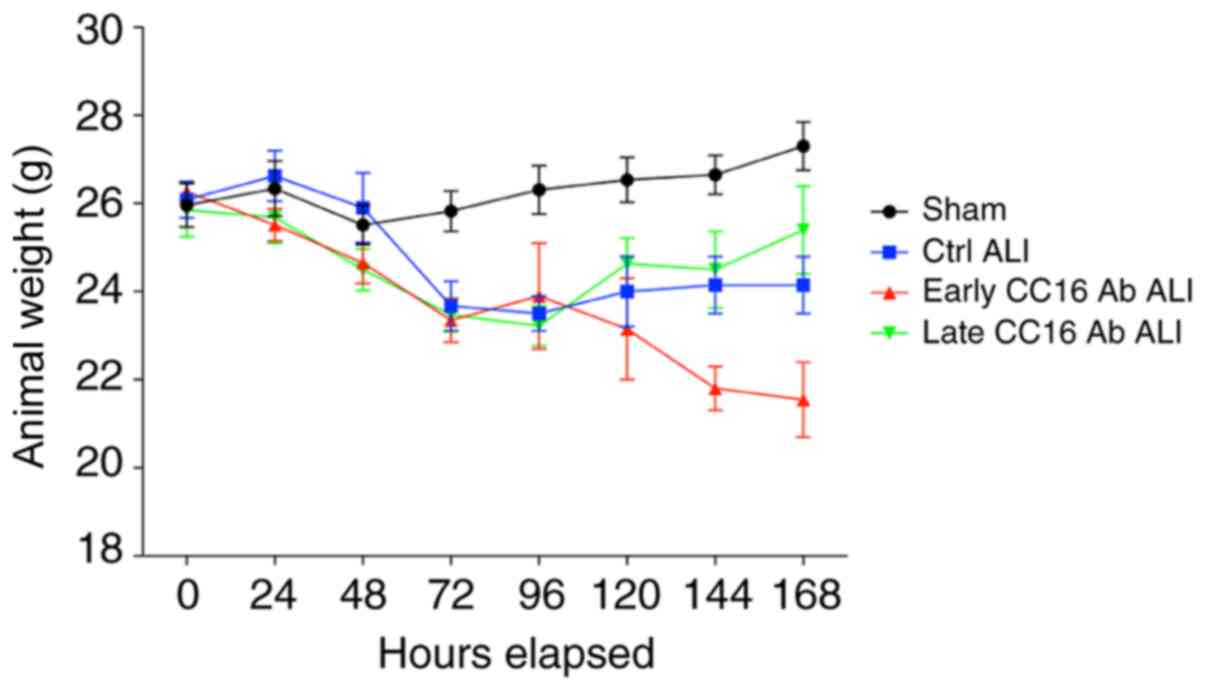
Body weight changes during the 7-day
observation period
Every 24 h for 7 days, the weight of the mice was
documented. During the first 48 h, the weight of the animals was
stable in all groups. After 48 h, all groups undergoing thoracic
trauma and CLP exhibited a decrease in weight compared with the
sham group (Fig. 4).
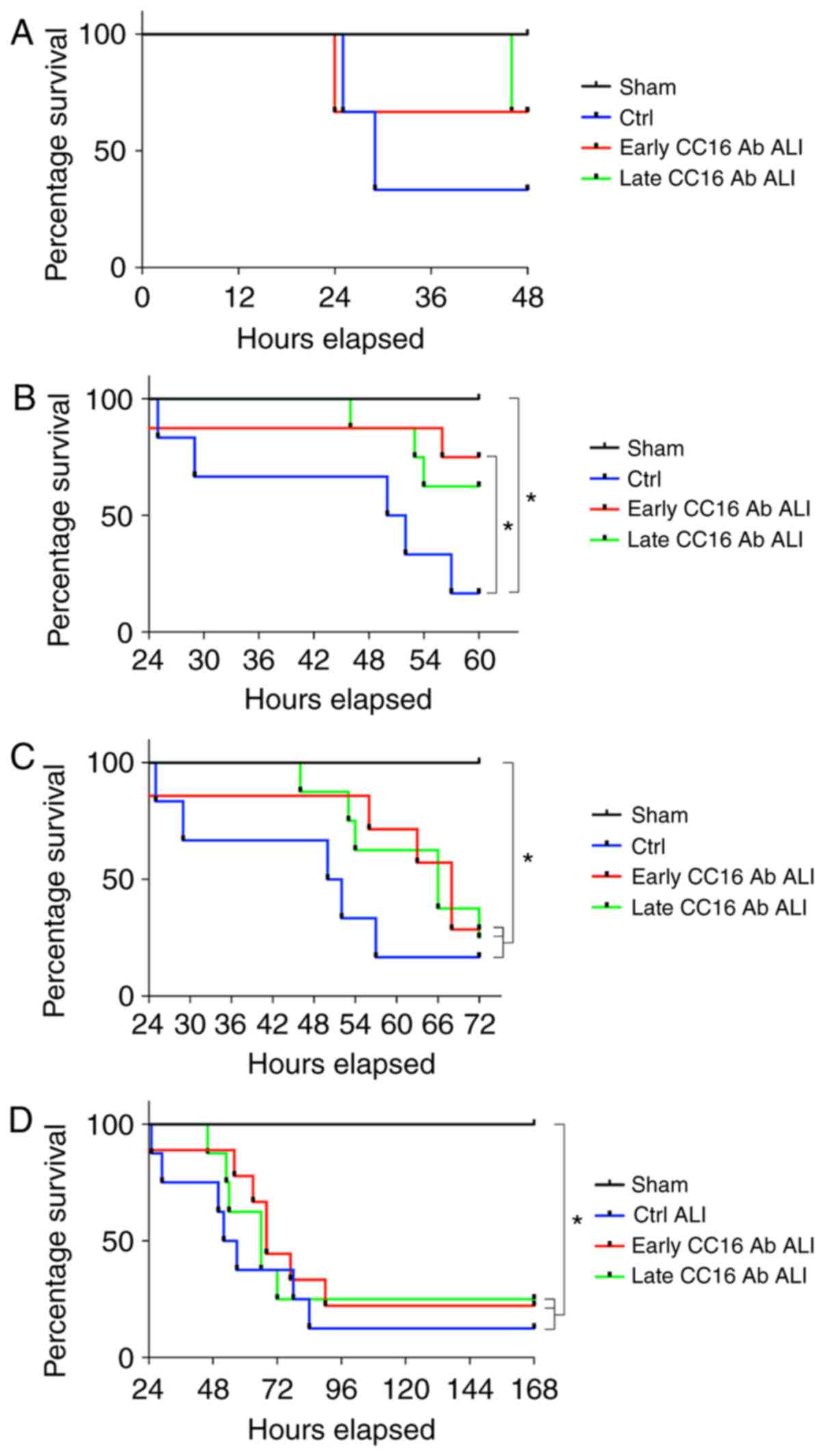
Survival rates after sepsis-induced ALI
following TxT
All animals in the sham group survived during the
observation period (Fig. 5).
Within 48 h, the survival rate decreased among the groups subjected
to TxT and CLP compared to the sham group (Fig. 5A). Within 60 h, the survival rate
in the ALI control group significantly decreased compared to the
sham group (P<0.05; Fig. 5B).
The group receiving early CC16 Ab treatment exhibited a
significantly higher survival rate compared to the control group
following TxT and CLP (P<0.05; Fig. 5B). After 72 h, no marked
differences in survival were observed between the 3 groups
undergoing TxT and CLP (Fig. 5C).
However, all ALI groups exhibited significantly decreased survival
rates compared to the sham group (P<0.05; Fig. 5C). Comparable data were observed
until the end of the observational period of 6 days post-CLP
(Fig. 5D).
Discussion
The present study investigated whether the
intratracheal neutralization of CC16 affects survival following
'double-hit' trauma consisting of TxT followed by CLP in mice.
Recently, the anti-inflammatory potential of CC16 24 h post-CLP in
this model was confirmed. The local anti-inflammatory
characteristic of CC16 is supported by the present data and by
results previously demonstrated in non-traumatic lung injury
(32,33). A recent study by the authors
demonstrated that upon the early intratracheal neutralization of
CC16 following chest trauma, reduced lung injury at 24 h post-CLP
was observed (9). Notably, the
late neutralization of CC16 following CLP aggravated lung injury
during the same observational period (9). Thus, although the anti-inflammatory
potential of CC16 in the underlying model was clearly confirmed,
previous studies did not determine whether the results are caused
by the timing of antibody application or disease kinetics or
whether the observed effects affect survival in this model. The
present study demonstrated that although the overall mortality rate
was not significantly altered upon early CC16 neutralization,
delayed mortality within 60 h after the second hit was present in
this group.
Blunt chest trauma triggers a potent inflammatory
response in lung tissue and the airways, implying the
(intrapulmonary) release of pro-inflammatory chemokines and
cytokines, which promote the activation of alveolar macrophages,
chemotaxis, and pulmonary neutrophil infiltration (34,35). Since several studies have
suggested that the pro-inflammatory response is a key driver of
lung damage during ALI, a pathologically-relevant role of the
anti-inflammatory CC16 has been postulated. Miller et al
confirmed that increased pulmonary IL-8 (CXCL8) levels in patients
suffering from ARDS were associated with an increased influx of
neutrophils, key drivers of organ damage (36). Similar findings were confirmed
in vivo via interleukin (IL)-8 neutralization, which
significantly reduced lung damage in a rabbit model of lung injury
induced by acid aspiration (37).
The recruitment of neutrophils to the lungs is a key event in the
early development of ALI and is mediated by CXCL1 (38). Reutershan et al reported
significantly reduced neutrophilic migration to the lungs in CXCL1
receptor-deficient CXCR2−/− mice in a murine model of
LPS-induced ALI (38). Similarly,
CXCL1 receptor neutralization reduced neutrophilic infiltration
and, moreover, attenuated lung damage in a mouse model of
ventilator-induced lung injury (39). Consistent with these findings, the
important role of CXCL1 in local tissue inflammation was confirmed
by its suppressed expression, which was concurrent with reduced
LPS-triggered pulmonary inflammation in mice (40). Furthermore, in sepsis-induced lung
injury, the reduced extravasation of neutrophils into alveolar
cavities was associated with reduced pulmonary CXCL1 levels
(41). Similarly, the
pathophysiologically relevant role of CXCL1 was confirmed in other
models of lung injury (42,43). The data of the present study
indicate that CC16 may have a direct effect on the pulmonary level
of CXCL1 in a 'double-hit' model of sepsis-induced ALI following
thoracic trauma. Since the early neutralization of CC16 immediately
following thoracic trauma increased CXCL1 concentrations after 24
h, this finding confirms the anti-inflammatory effect of CC16.
However, the results prompt a conflictive discussion. The
above-mentioned studies suggest that CXCL1 plays a detrimental role
in such a model, yet the early increase in CXCL1 in our study was
associated with delayed mortality. Thus, other factors must also be
pathophysiologically relevant.
It is well known that the development of
post-traumatic lung injury depends on several factors, including
damage- or pathogen-associated molecular patterns (9,22).
During post-traumatic ALI/ARDS development, numerous mediators
released from tissue-resident cells act as chemoattractants for
invading immune cells and further stimulate local cells to build a
pro-inflammatory micromilieu (44). While it is widely assumed that
this type of hyperinflammatory reaction contributes to subsequent
lung damage, Störmann et al observed less lung damage
following the early neutralization of CC16 immediately after
trauma, which was associated with an enhanced influx of
neutrophilic granulocytes (9).
This group demonstrated prolonged survival following CC16
neutralization within the early post-traumatic phase of post-blunt
chest trauma and CLP. However, survival rates will be discussed
below. Of note, while local effects have been observed, in line
with the previous study by the authors, intrapulmonary CC16
neutralization did not influence systemic inflammation, as assessed
via IFN-γ levels (9). CC16 can
function as an anti-cytokine and a natural immunosuppressor by
inhibiting the production and biological activity of IFN-γ
(45). However, the interplay
between IFN-γ and CC16 was demonstrated in the lungs, and no
systemic data were provided (46). The authors concluded that IFN-γ
may act as a potent regulator of CC16/CC10 gene expression and that
increased IFN-γ in lung epithelial cells may be modulated by
increasing CC16 production, stimulated by a regulatory feedback
loop (46,47), which potentially maintains the
balance between the local pro- and anti-inflammatory processes.
This remains to be determined in future studies.
Adjacent to its role as a biomarker for pulmonary
injury and lung complications following trauma, several studies
have demonstrated that CC16 has significant pathophysiological
effects on the development of pulmonary complications (9,20,48). Recently, the authors demonstrated
that CC16 exerts anti-inflammatory effects in sepsis-induced ALI
following thoracic trauma in vivo (9). Furthermore, early and local CC16
inhibition decreased pulmonary injury after 24 h, indicating that
CC16 may exert protective effects in early inflammation during ALI
(9). However, CC16 inhibition
after sepsis-induction aggravated lung damage after 24 h (9). Thus, although CC16 neutralization
increased early inflammation and was associated with reduced
pulmonary damage, the long-term outcome remains unclear. Therefore,
the pathological relevance of CC16 to survival in our
sepsis-induced trauma model was evaluated. In general, the approach
of influencing inflammation after traumatic insult with the aim of
preventing infectious and organ complications is highly challenging
since the post-traumatic inflammatory response, with its numerous
associated mechanisms, is very complex. Although the underlying
pathomechanisms of trauma-induced ALI are unclear, in the present
study, it was confirmed that CC16 exerts significant local
anti-inflammatory effects. Furthermore, lung inflammation induced
by cigarette smoke and adverse outcomes were ameliorated by CC16 in
a murine COPD model (32,33). Of note, in patients with pulmonary
morbidities, including COPD and asthma, CC16 plays an important
role in pathology and performs protective functions during exposure
to cigarette smoke (33,49). Although reduced lung damage
following CC16 neutralization was observed, the general mortality
rate did not change in the current model. The present study
demonstrates that early CC16 inhibition may delay mortality within
a defined timeframe after sepsis-induction; however, no evidence of
it benefitting long-term survival was observed. The highest
mortality rates were observed between 48 and 72 h in the present
model. Similar data from other studies reveal that the majority of
deaths following CLP-induced polymicrobial sepsis occur within
24-48 h after CLP or within 24-96 h (50,51). In contrast to later therapeutic
interventions, early antibody application improves survival rates
(52). Thus, the relevance of
delayed mortality to CC16 neutralization within a defined
timeframe, as shown in the present study, remains to be further
elucidated.
Considering that the timeline and protracted
inflammation following sepsis favor hospital-acquired infections
and worsen patient outcomes through immunosuppression (53), the data of this and our recent
study remain intriguing (9).
Throughout their medical care, which may include surgery, severely
injured patients are subjected to damaging endogenous or exogenous
molecules, which mediate systemic inflammation or immunosuppression
and have clinical consequences, such as infections and organ
failure (53). In a recent study
by the authors, it was discussed how, in post-traumatic
immunosuppression, it appears reasonable that the anti-inflammatory
effects of CC16 may be detrimental in the underlying model
(9). However, considering
hyperinflammation and the second-hit hypothesis, which suggests the
presence of a detrimental hyperinflammatory state following trauma,
CC16 may be expected to exert beneficial effects. The lack of
significant changes in survival rates following CC16 neutralization
for 6 days post-sepsis indicates that there are other, possibly
more decisive, factors affecting final outcomes in this model.
However, it remains to be further elaborated whether delayed
mortality observed following early anti-inflammatory therapy can be
improved in long-term outcomes. This effect could be evaluated in
future studies by alternatively supplementing the original therapy
with a second dose of either anti-CC16 Ab or recombinant CC16 at a
later timepoint.
A significant limitation of the present study
remains the lack of mechanical ventilation, as all animals were
consistently spontaneously breathing. Furthermore, fluid management
comparable to the clinical setting was not included. These two
major contributors to ALI/ARDS were not taken into account in the
present study. Moreover, by means of animal welfare, the number of
animals in each group was limited. Interspecies variability in the
innate immune response must be considered when in vivo
experimental results are applied to humans (54). Furthermore, some interesting
phenomena, such as decreased bodyweight in the early Ab treatment
group, were observed, although this decrease was insignificant
between the groups. In the present study, a sufficient explanation
cannot be provided, and it remains undetermined whether a longer
observational period may have provided evidence of further
alterations or significant differences in survival benefits between
the groups.
The present study confirmed the anti-inflammatory
effects of CC16 following CLP-induced ALI after TxT in mice. As
reported in a recent study by the authors (9), the early therapeutic strategy of
CC16 inhibition (after TxT) increased inflammation, and it was
further concomitant with delayed mortality within a defined time
window after CLP; however, no long-term survival benefits were
observed. In the previous study by the authors (9), later-stage CC16 inhibition resulted
in poor pulmonary outcomes, and no effects on short- or long-term
survival were observed. Thus, it remains to be determined whether,
in post-traumatic ALI cases, a rapid local pro-inflammatory
reaction may be necessary, which should be followed by another
therapeutic inflammation-modulating step at a later timepoint.
Acknowledgments
The authors would like to thank Katrin Jurida,
Kerstin Kontradowitz, and Alexander Schaible from the Department of
Trauma, Hand and Reconstructive Surgery, Goethe University,
Frankfurt for their outstanding technical assistance.
Funding
The present study was supported by grants from the
DFG WU 820/2-1, HI 820/5-1 and RE 3304/8-1. The funders had no role
in the design of the study, in the collection, analyses, or
interpretation of data, in writing the manuscript, or in the
decision to publish the results.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article or are available from the
corresponding author on reasonable request.
Authors' contributions
BR, SW, FH and IM were involved in the
conceptualization of the study. JTV, PS, NB and BR were involved in
the study methodology. JTV was involved in data validation. BR and
JTV were involved in the formal analysis. JTV, PS and NB were
involved in the investigative aspects of the study. JTV and PS were
involved in data curation. JTV was involved in the writing and
preparation of the original draft. JTV, SW, FH, IM and BR were
involved in the writing, reviewing and editing of the manuscript.
JTV and BR were involved in visualization of the results. BR
supervised the study. JTV and BR were involved in project
administration. BR, FH and SW were involved in funding acquisition.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All experiments were conducted in accordance with
German federal laws regarding the protection of animals. The
experiments were performed at Goethe University Hospital in
Frankfurt, Germany. The present study was approved by the
responsible government authority, the Veterinary Department of the
Regional Council in Darmstadt, Germany (Regierungspräsidium
Darmstadt, Hessen, Germany; AZ: FK 1068), and the study was
performed under ARRIVE guidelines (26).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hildebrand F, Giannoudis PV, Griensven M,
Zelle B, Ulmer B, Krettek C, Bellamy MC and Pape HC: Management of
poly-traumatized patients with associated blunt chest trauma: A
comparison of two European countries. Injury. 36:293–302. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Matthay MA, Zemans RL, Zimmerman GA, Arabi
YM, Beitler JR, Mercat A, Herridge M, Randolph AG and Calfee CS:
Acute respiratory distress syndrome. Nat Rev Dis Primers. 5:182019.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rendeki S and Molnár TF: Pulmonary
contusion. J Thorac Dis. 11(Suppl 2): S141–S151. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hoth JJ, Wells JD, Yoza BK and McCall CE:
Innate immune response to pulmonary contusion: Identification of
cell type-specific inflammatory responses. Shock. 37:385–391. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hoth JJ, Wells JD, Brownlee NA, Hiltbold
EM, Meredith JW, McCall CE and Yoza BK: Toll-like receptor
4-dependent responses to lung injury in a murine model of pulmonary
contusion. Shock. 31:376–31. 2009. View Article : Google Scholar
|
|
6
|
Hoth JJ, Wells JD, Hiltbold EM, McCall CE
and Yoza BK: Mechanism of neutrophil recruitment to the lung after
pulmonary contusion. Shock. 35:604–609. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Störmann P, Becker N, Kunnemeyer L,
Wutzler S, Vollrath JT, Lustenberger T, Hildebrand F, Marzi I and
Relja B: Contributing factors in the development of acute lung
injury in a murine double hit model. Eur J Trauma Emerg Surg.
46:21–30. 2020. View Article : Google Scholar
|
|
8
|
Proudfoot AG, McAuley DF, Griffiths MJ and
Hind M: Human models of acute lung injury. Dis Model Mech.
4:145–153. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Störmann P, Becker N, Vollrath JT, Köhler
K, Janicova A, Wutzler S, Hildebrand F, Marzi I and Relja B: Early
local inhibition of club cell protein 16 following chest trauma
reduces late sepsis-induced acute lung injury. J Clin Med.
8:8962019. View Article : Google Scholar :
|
|
10
|
Perl M, Hohmann C, Denk S, Kellermann P,
Lu D, Braumuller S, Bachem MG, Thomas J, Knöferl MW, Ayala A, et
al: Role of activated neutrophils in chest trauma-induced septic
acute lung injury. Shock. 38:98–106. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Weckbach S, Hohmann C, Denk S, Kellermann
P, Huber-Lang MS, Baumann B, Wirth T, Gebhard F, Bachem M and Perl
M: Apoptotic and inflammatory signaling via Fas and tumor necrosis
factor receptor I contribute to the development of chest
trauma-induced septic acute lung injury. J Trauma Acute Care Surg.
74:792–800. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Muenzer JT, Davis CG, Chang K, Schmidt RE,
Dunne WM, Coopersmith CM and Hotchkiss RS: Characterization and
modulation of the immunosuppressive phase of sepsis. Infect Immun.
78:1582–1592. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Boehm U, Klamp T, Groot M and Howard JC:
Cellular responses to interferon-gamma. Annu Rev Immunol.
15:749–795. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Varma TK, Lin CY, Toliver-Kinsky TE and
Sherwood ER: Endotoxin-induced gamma interferon production:
Contributing cell types and key regulatory factors. Clin Diagn Lab
Immunol. 9:530–543. 2002.PubMed/NCBI
|
|
15
|
Shtrichman R and Samuel CE: The role of
gamma interferon in antimicrobial immunity. Curr Opin Microbiol.
4:251–259. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Romero CR, Herzig DS, Etogo A, Nunez J,
Mahmoudizad R, Fang G, Murphey ED, Toliver-Kinsky T and Sherwood
ER: The role of interferon-γ in the pathogenesis of acute
intra-abdominal sepsis. J Leukoc Biol. 88:725–735. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kropski JA, Fremont RD, Calfee CS and Ware
LB: Clara cell protein (CC16), a marker of lung epithelial injury,
is decreased in plasma and pulmonary edema fluid from patients with
acute lung injury. Chest. 135:1440–1447. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Miele L, Cordella-Miele E, Mantile G, Peri
A and Mukherjee AB: Uteroglobin and uteroglobin-like proteins: The
uteroglobin family of proteins. J Endocrinol Invest. 17:679–692.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Negrin LL, Halat G, Kettner S, Gregori M,
Ristl R, Hajdu S and Heinz T: Club cell protein 16 and cytokeratin
fragment 21-1 as early predictors of pulmonary complications in
polytraumatized patients with severe chest trauma. PLoS One.
12:e01753032017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wutzler S, Lehnert T, Laurer H, Lehnert M,
Becker M, Henrich D, Vogl T and Marzi I: Circulating levels of
Clara cell protein 16 but not surfactant protein D identify and
quantify lung damage in patients with multiple injuries. J Trauma.
71:E31–E36. 2011. View Article : Google Scholar
|
|
21
|
Broeckaert F, Clippe A, Knoops B, Hermans
C and Bernard A: Clara cell secretory protein (CC16): Features as a
peripheral lung biomarker. Ann NY Acad Sci. 923:68–77. 2000.
View Article : Google Scholar
|
|
22
|
Relja B, Mörs K and Marzi I: Danger
signals in trauma. Eur J Trauma Emerg Surg. 44:301–316. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wutzler S, Lustenberger T, Relja B,
Lehnert M and Marzi I: Pathophysiology of multiple trauma:
Intensive care medicine and timing of treatment. Chirurg.
84:753–758. 2013.In German. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dong L, Zhu YH, Liu DX, Li J, Zhao PC,
Zhong YP, Chen YQ, Xu W and Zhu ZQ: Intranasal application of
budesonide attenuates lipopolysaccharide-induced acute lung injury
by suppressing nucleotide-binding oligomerization domain-like
receptor family, pyrin domain-containing 3 inflammasome activation
in mice. J Immunol Res. 2019:72643832019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Horiguchi H, Loftus TJ, Hawkins RB,
Raymond SL, Stortz JA, Hollen MK, Weiss BP, Miller ES, Bihorac A,
Larson SD, et al: Innate immunity in the persistent inflammation,
immunosuppression, and catabolism syndrome and its implications for
therapy. Front Immunol. 9:5952018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kilkenny C, Browne WJ, Cuthill IC, Emerson
M and Altman DG: Improving bioscience research reporting: The
ARRIVE guidelines for reporting animal research. PLoS Biol.
8:e10004122010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Knoferl MW, Liener UC, Seitz DH, Perl M,
Bruckner UB, Kinzl L and Gebhard F: Cardiopulmonary, histological,
and inflammatory alterations after lung contusion in a novel mouse
model of blunt chest trauma. Shock. 19:519–525. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rittirsch D, Huber-Lang MS, Flierl MA and
Ward PA: Immunodesign of experimental sepsis by cecal ligation and
puncture. Nat Protoc. 4:31–36. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Janicova A, Becker N, Xu B, Wutzler S,
Vollrath JT, Hildebrand F, Ehnert S, Marzi I, Störmann P and Relja
B: Endogenous utero-globin as intrinsic anti-inflammatory signal
modulates monocyte and macrophage subsets distribution upon sepsis
induced lung injury. Front Immunol. 10:22762019. View Article : Google Scholar
|
|
30
|
Relja B, Horstmann JP, Kontradowitz K,
Jurida K, Schaible A, Neunaber C, Oppermann E and Marzi I: Nlrp1
inflammasome is downregulated in trauma patients. J Mol Med (Berl).
93:1391–1400. 2015. View Article : Google Scholar
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
32
|
Pang M, Liu HY, Li T, Wang D, Hu XY, Zhang
XR, Yu BF, Guo R and Wang HL: Recombinant club cell protein 16
(CC16) ameliorates cigarette smoke-induced lung inflammation in a
murine disease model of COPD. Mol Med Rep. 18:2198–2206.
2018.PubMed/NCBI
|
|
33
|
Laucho-Contreras ME, Polverino F, Gupta K,
Taylor KL, Kelly E, Pinto-Plata V, Divo M, Ashfaq N, Petersen H,
Stripp B, et al: Protective role for club cell secretory protein-16
(CC16) in the development of COPD. Eur Respir J. 45:1544–1556.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Seitz DH, Perl M, Liener UC, Tauchmann B,
Braumüller ST, Brückner UB, Gebhard F and Knöferl MW: Inflammatory
alterations in a novel combination model of blunt chest trauma and
hemorrhagic shock. J Trauma. 70:189–196. 2011. View Article : Google Scholar
|
|
35
|
Reutershan J and Ley K: Bench-to-bedside
review: Acute respiratory distress syndrome-how neutrophils migrate
into the lung. Crit Care. 8:453–461. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Miller EJ, Cohen AB, Nagao S, Griffith D,
Maunder RJ, Martin TR, Weiner-Kronish JP, Sticherling M,
Christophers E and Matthay MA: Elevated levels of
NAP-1/interleukin-8 are present in the airspaces of patients with
the adult respiratory distress syndrome and are associated with
increased mortality. Am Rev Respir Dis. 146:427–432. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Folkesson HG, Matthay MA, Hebert CA and
Broaddus VC: Acid aspiration-induced lung injury in rabbits is
mediated by interleukin-8-dependent mechanisms. J Clin Invest.
96:107–116. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Reutershan J, Morris MA, Burcin TL, Smith
DF, Chang D, Saprito MS and Ley K: Critical role of endothelial
CXCR2 in LPS-induced neutrophil migration into the lung. J Clin
Invest. 116:695–702. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Belperio JA, Keane MP, Burdick MD, Londhe
V, Xue YY, Li K, Phillips RJ and Strieter RM: Critical role for
CXCR2 and CXCR2 ligands during the pathogenesis of
ventilator-induced lung injury. J Clin Invest. 110:1703–1716. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Meng J, Zou Y, Chen J, Qin F, Chen X, Chen
X and Dai S: sTLR4/sMD-2 complex alleviates LPS-induced acute lung
injury by inhibiting pro-inflammatory cytokines and chemokine CXCL1
expression. Exp Ther Med. 16:4632–4638. 2018.PubMed/NCBI
|
|
41
|
Wang J, Gong S, Wang F, Niu M, Wei G, He
Z, Gu T, Jiang Y, Liu A and Chen P: Granisetron protects
polymicrobial sepsis-induced acute lung injury in mice. Biochem
Biophys Res Commun. 508:1004–1010. 2019. View Article : Google Scholar
|
|
42
|
Zarbock A, Allegretti M and Ley K:
Therapeutic inhibition of CXCR2 by Reparixin attenuates acute lung
injury in mice. Br J Pharmacol. 155:357–364. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Dunn JLM, Kartchner LB, Stepp WH, Glenn
LI, Malfitano MM, Jones SW, Doerschuk CM, Maile R and Cairns BA:
Blocking CXCL1-dependent neutrophil recruitment prevents immune
damage and reduces pulmonary bacterial infection after inhalation
injury. Am J Physiol Lung Cell Mol Physiol. 314:L822–L834. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Niesler U, Palmer A, Radermacher P and
Huber-Lang MS: Role of alveolar macrophages in the inflammatory
response after trauma. Shock. 42:3–10. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Dierynck I, Bernard A, Roels H and De Ley
M: Potent inhibition of both human interferon-gamma production and
biologic activity by the Clara cell protein CC16. Am J Respir Cell
Mol Biol. 12:205–210. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Magdaleno SM, Wang G, Jackson KJ, Ray MK,
Welty S, Costa RH and DeMayo FJ: Interferon-gamma regulation of
Clara cell gene expression: In vivo and in vitro. Am J Physiol.
272:L1142–L1151. 1997.PubMed/NCBI
|
|
47
|
Ramsay PL, Luo Z, Magdaleno SM, Whitbourne
SK, Cao X, Park MS, Park MS, Welty SE, Yu-Lee LY and DeMayo FJ:
Transcriptional regulation of CCSP by interferon-gamma in vitro and
in vivo. Am J Physiol Lung Cell Mol Physiol. 284:L108–L118. 2003.
View Article : Google Scholar
|
|
48
|
Michel O, Murdoch R and Bernard A: Inhaled
LPS induces blood release of Clara cell specific protein (CC16) in
human beings. J Allergy Clin Immunol. 115:1143–1147. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lam DC, Kwok HH, Yu WC, Ko FW, Tam CY, Lau
AC, Fong DY and Ip MS: CC16 levels correlate with cigarette smoke
exposure in bronchial epithelial cells and with lung function
decline in smokers. BMC Pulm Med. 18:472018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Xu L, Zhang W, Kwak M, Zhang L, Lee PCW
and Jin JO: Protective effect of melatonin against polymicrobial
sepsis is mediated by the anti-bacterial effect of neutrophils.
Front Immunol. 10:13712019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chen X, Wang T, Song L and Liu X:
Activation of multiple Toll-like receptors serves different roles
in sepsis-induced acute lung injury. Exp Ther Med. 18:443–450.
2019.PubMed/NCBI
|
|
52
|
Matsuo S, Sharma A, Wang P and Yang WL:
PYR-41, a ubiq-uitin-activating enzyme E1 inhibitor, attenuates
lung injury in sepsis. Shock. 49:442–450. 2018. View Article : Google Scholar
|
|
53
|
Vourc'h M, Roquilly A and Asehnoune K:
Trauma-induced damage-associated molecular patterns-mediated remote
organ injury and immunosuppression in the acutely ill patient.
Front Immunol. 9:13302018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Matute-Bello G, Frevert CW and Martin TR:
Animal models of acute lung injury. Am J Physiol Lung Cell Mol
Physiol. 295:L379–L399. 2008. View Article : Google Scholar : PubMed/NCBI
|