Introduction
Acute ischemic stroke (AIS) is the most frequent
cause of permanent disability in adults worldwide (1). Due to its high incidence, disability
and recurrence rate, as well as complications, AIS has become a
severe threat to human health and quality of life (2). The most effective treatment for AIS
is intravascular recanalization within a certain time window
(3-5). Recanalization following AIS is
likely to cause cerebral ischemia-reperfusion injury (CIRI), during
which excess free radicals attack cells in the tissues that regain
blood supply (6,7). CIRI leads to neuronal cell necrosis
and apoptosis through a series of pathological processes, such as
energy metabolism disorders, excitotoxicity, neuroinflammation,
oxidative damage and calcium overload (8). Early treatment with neuroprotective
drugs can reduce apoptosis and the release of superoxide free
radicals (9).
Apoptosis is one of the most important mechanisms of
CIRI. In the core of the ischemic region, the severe death of
neurons occurs rapidly, although neurons in the ischemic penumbra
can be saved with timely and effective treatment (9,10).
Previous studies have confirmed that the number of apoptotic
neurons in the ischemic penumbra determines the size of the
infarction volume and the severity of the neurological defects
(11-13). Neuronal apoptosis is crucial to
the treatment and prognosis of CIRI (14,15); the primary task of CIRI prevention
and treatment is to inhibit neuronal apoptosis.
Calcium overload is an important link between
apoptosis and neuronal necrosis (16,17). A number of studies have
demonstrated that extracellular calcium can be absorbed and
transported into the mitochondria during ischemia-reperfusion (I/R)
injury, leading to increased mitochondrial membrane permeability
and promoting oxidative reaction and apoptosis (18-20). Calcium-sensing receptor (CaSR) is
a member of the G-protein coupled receptor superfamily and is
sensitive to the extracellular application of Ca2+,
Gd3+ and other polycationic agonists (21), as well as antagonists such as
NPS-2390 and NPS-2143 (22-24). CaSR maintains calcium homeostasis,
and its activity serves an important role in increased
intracellular calcium levels during I/R injury (17,25,26). Previous studies have reported that
the activation of CaSR aggravates myocardial and renal I/R injury
and mouse CIRI (19,23,27,28). However, it remains unclear whether
increased CaSR expression may induce apoptosis by increasing
intracellular calcium overload during CIRI, and whether
Astragaloside IV may reduce CaSR expression to alleviate
intracellular calcium overload and further inhibit CIRI-induced
apoptosis.
Traditional Chinese medicines, such as Buyang Huanwu
decoction, have been widely used in the treatment of AIS (29,30). Astragaloside IV
(C14H68O14) is one of the main
active chemicals and the quality-control marker of the traditional
Chinese medicine Astragalus membranaceus, which is the main
ingredient of Buyang Huanwu decoction, as recorded in the book
'Correction on the Errors of Medical Works' written by Qingren Wang
in the Qing Dynasty (31,32). Numerous studies have demonstrated
that Astragaloside IV has anti-inflammatory and antiapoptotic
properties, is involved in the regulation of vascular remodeling
and energy metabolism, and exerts a neuroprotective effect on CIRI
(16,18,27,33-37). Our previous study has demonstrated
that Astragaloside IV alleviates CIRI by inhibiting neuronal
apoptosis and confirmed that it exerts its role by regulating
autophagy (9). In addition,
Astragaloside IV has been reported to reduce myocardial injury
induced by anoxia/reoxygenation through the inhibition of
intracellular calcium overload (27).
Based on the above information, we hypothesized that
Astragaloside IV may serve a neuroprotective role by inhibiting
apoptosis via the reduction of the expression of CaSR.
Materials and methods
Chemicals and reagents
Dulbecco's modified Eagle's medium (DMEM) and 0.25%
Trypsin (without EDTA) were obtained from Thermo Fisher Scientific,
Inc. Fetal bovine serum (FBS) was purchased from Zhejiang Tianhang
Biotechnology Co., Ltd. Earle's balanced salt solution (1X EBSS
without calcium, magnesium or sugar) was obtained from Lea Gene
Biotechnology Co., Ltd. Astragaloside IV (purity >98%) was
purchased from Shanghai Yuan Ye Bio-Technology Co. Ltd. NPS-2143
was provided by MedChemExpress. GdCl3 (purity
>99.99%) was purchased from Shanghai Aladdin Biotechnology Co.,
Ltd. Cell Counting Kit-8 (CCK-8) kit was obtained from Beijing
Zoman Biotechnology Co., Ltd. A calcium assay kit (cat. no.
C004-2-1) was obtained from the Nanjing Jiancheng Bioengineering
Institute. An Annexin V-FITC apoptosis detection kit was obtained
from Vazyme Biotech Co., Ltd. A total protein extraction kit (cat.
no. BC3640), BCA protein quantitative kit (cat. no. PC0020) and
Antifading Mounting Medium (with DAPI; cat. no. S2110) were
purchased from Beijing Solarbio Science & Technology Co., Ltd.
Antibodies against B-cell lymphoma 2 (Bcl-2; cat. no. ab201566),
cleaved caspase-3 (cat. no. ab49822), CaSR (cat. no. ab19347),
β-actin (cat. no. ab8224), GAPDH (cat. no. ab9485),
apoptosis-inducing factor (AIF; cat. no. ab2086) and neuronal
nuclei (NeuN; cat. no. ab190195) were purchased from Abcam.
Antibody against Bcl-2-associated X protein (Bax; cat. no. GB1107)
and 2,3,5-triphenyltetrazolium chloride (TTC) dye solution were
obtained from Wuhan Servicebio Technology Co., Ltd. Horseradish
peroxidase (HRP)-conjugated goat anti-mouse/rabbit immunoglobulin G
(IgG) antibodies (cat. nos. bs-0296G-HRP and bs-0295G-HRP) were
purchased from Beijing Biosynthesis Biotechnology Co., Ltd. Alexa
Fluor 488-conjugated goat anti-mouse (cat. no. GB25303) and
Cy3-conjugated donkey anti-rabbit (cat. no. GB21403) IgG antibodies
were obtained from Wuhan Servicebio Technology Co., Ltd.
Middle cerebral artery (MCA)
occlusion/reperfusion (MACO/R) model
A total of 40 adult Sprague Dawley rats (male; age,
7-8 weeks; weight, 270±20 g) were provided by Beijing Vital River
Laboratory Animal Technology Co., Ltd. (permit no. SCXK;
2016-0006). All experimental protocols and animal handling
procedures were performed in accordance with the National
Institutes of Health (NIH) Guide for the Care and Use of Laboratory
Animals 1996. The experimental protocols were approved by the
Committee of Experimental Animals of the Hebei University of
Chinese Medicine (Shijiazhuang, China; approval no.
DWLL2018032).
The animals were maintained at 23±1°C with 50±10%
relative humidity in the Experimental Animal Center for 1 week. The
animals were allowed free access to food and water in a 12-h
light/dark cycle and randomly divided into four groups: i) Sham;
ii) middle cerebral artery occlusion/reperfusion (MCAO/R); iii)
MCAO/R + Astragaloside IV (AST-IV); and iv) MCAO/R + CaSR
antagonist (NPS-2143). All rats under-went MCAO/R with the
exception of the Sham group. Once the MCAO/R rat model was
established, the rats were assigned into groups randomly.
The rats were fasted from food for 12 h and water
for 4 h and anesthetized by intraperitoneal injection with 2%
pentobarbital sodium (40 mg/kg bodyweight) before surgery. The
anesthetized rats were placed in a supine position on the operating
table and shaved with sterilization around the incision site.
Cerebral ischemia/reperfusion was induced by transient occlusion of
the left MCA with a nylon monofilament for 2 h and reperfusion for
24 h as previously described (38). Briefly, a midline incision was
made in the neck. The left common carotid artery (CCA), left
external carotid artery (ECA) and left internal carotid artery
(ICA) were isolated and tied at the origin of the ECA and at the
distal end of the ECA. The left CCA and ICA were temporarily
occluded. Subsequently, a nylon suture was introduced into the ECA
and pushed up the ICA until resistance was felt. The filament was
inserted 18.5±0.5 mm from the carotid bifurcation, effectively
blocking the MCA. After 2 h, the suture was removed, and the ECA
was permanently tied. In the Sham group, the arteries were exposed
and isolated, but not disturbed. Throughout the experiment, the
rats were fixed on a small multi-functional animal laboratory bench
that detected the anal temperature of the rats and maintained the
temperature at a minimum of 37°C. Based on our previous study, rats
in the AST-IV group were intraperitoneally injected with 20 mg/kg
Astragaloside IV during reperfusion (9). Rats in the NPS-2143 group were
intraperitoneally injected with 10 µmol/kg NPS-2143 during
reperfusion (23,24,28), and rats in the Sham group received
an equal volume of physiological saline. Room temperature was
maintained at 28-32°C throughout the surgery. A total of 36 rats
entered further experiments, with 9 rats per group; each group was
divided into three sub-groups for further experiments, including
TTC, hematoxylin and eosin (H&E) or double immunofluorescence
staining and western blotting.
Neurological scoring
Neurological scores were evaluated at 24 h
post-reperfusion by a blinded observer using the Zea Longa 5-point
scoring system to evaluate the sensorimotor deficits: 0 points,
normal performance with no neurological deficits; 1 point,
contralateral forepaws could not fully extend; 2 points, circling
to the opposite side when walking; 3 points, falling to the
opposite side when walking; 4 points, no spontaneous walking and
loss of consciousness (39).
MCAO/R-treated rats that scored 1-3 points were selected as the
experimental subjects. According to the neurological score, three
rats were removed: One in the MCAO/R group (dead), one in AST-IV
group (scored 4), and one in NPS-2143 group (scored 4). To ensure
an equal number of animals in each group, one rat was randomly
removed from the Sham group. Finally, 36 rats remained with 9
rats/group. These rats were anesthetized by an intraperitoneal
injection of pentobarbital sodium (150 mg/kg bodyweight). After
deep anesthesia was confirmed by the lack of response to stimuli
such as the toe and tail pinching, the rats were sacrificed by
cervical dislocation, and the brain tissues were rapidly removed
for experiments.
Measurement of infarct volume
Brains (n=3 per group) were removed at 24 h
post-reperfusion and gently frozen at −20°C for 15 min to keep the
morphology intact during slicing. Briefly, the brains were sliced
into five serial 2-mm coronal sections, incubated in a 2% TTC dye
solution for 15 min at 37°C in the dark and transferred to 4%
paraformaldehyde in phosphate buffer for fixation at 4°C overnight.
The extent of ischemic infarction was traced and the integrated
volume was calculated manually by an analyst who was blind to the
experimental conditions using Image-Pro Plus 6.0 image analysis
software (Media Cybernetics, Inc.). The relative infarction volume
was expressed as a percentage of the corrected infarct volume in
the whole brain volume as follows: Infarct volume (%)=total infarct
volume/total brain volume ×100%.
H&E staining
At 24 h post-reperfusion, the rats (n=3 per group)
were sacrificed, and the intact brain tissue was carefully removed
and placed in a buffer (cat. no. G1101; Wuhan Servicebio Technology
Co., Ltd.) containing 4% formaldehyde. Following fixation,
dehydration and clearing, the samples were embedded in paraffin and
cut into 5-µm serial coronal sections, mounted on slides and
used for H&E staining. The sections were stained with H&E
according to the standard protocol (40,41) to observe the morphological changes
of injured neurons in the cerebral cortex and the hippocampus
following MCAO/R surgery. Images of stained slides were acquired
using an Olympus DP72 optical microscope (Olympus Corporation). To
quantify the data of brain injury, three randomly selected
high-magnification (×400) fields per slide were selected in both
the cortex and the hippocampus, and the mean value of each slide
was calculated for image analysis.
Double immunofluorescence staining
At 24 h post-reperfusion, the rats (n=3 per group)
were sacrificed, and 5-µm serial coronal sections were
prepared as aforementioned. The sections were dewaxed with xylene
for 15 min, twice, hydrated in a decreasing series of ethanol (100,
100, 85 and 75%, followed by distilled water) at room temperature
and treated with an Antigen retrieval solution (Wuhan Servicebio
Technology Co., Ltd.) for 10 min at 100°C. Following gentle
cooling, the sections were sealed with 3% BSA (cat. no. G5001;
Wuhan Servicebio Technology Co., Ltd.) at room temperature. For
double immunofluorescence histochemistry, the sections were
incubated at 4°C overnight with a mixture of anti-CaSR (1:80) and
anti-NeuN (1:50) antibodies. Antibody staining was visualized using
Alexa Fluor 488-conjugated goat anti-mouse (1:100) and
Cy3-conjugated donkey anti-rabbit secondary antibodies (1:100) for
1 h at room temperature in the dark. Counterstaining of cell nuclei
was performed by mounting the sections with Antifading Mounting
Medium (with DAPI). Images of the stained slides were captured
under a confocal microscope. Three high-magnification (×400) fields
were randomly selected for image analysis.
Cell culture
Highly differentiated PC12 pheochromocytoma cells
were gifted by Professor Shun-Jiang Xu (The First Hospital of Hebei
Medical University, Shijiazhuang, China) (originally purchased from
Procell Life Science & Technology Co., Ltd.; cat. no. CL-0481;
cell vitality, >95%; cell density, >75%). PC12 cells were
cultured in complete culture medium (DMEM supplemented with 10%
FBS) at 37°C in a humidified atmosphere containing 5%
CO2; the medium was changed every 2 days (42,43). The cultured PC12 cells were used
to establish an in vitro model of CIRI.
In vitro model of oxygen-glucose
deprivation/reoxygenation (OGD/R)
The OGD/R model has been commonly recognized as the
in vitro model simulating CIRI (44-46). First, the complete culture medium
was discarded, and the cells were washed twice with 1X PBS. PC12
cells were then added to EBSS to simulate the ischemic state and
placed into the tri-gas incubator, which was set to maintain the
inside environment at 1% O2, 94% N2 and 5%
CO2 at 37°C for 2 h. Subsequently, PC12 cells were
washed twice with 1X PBS, and the complete culture medium was
added. Finally, PC12 cells were cultured in normal conditions for
reoxygenation for 24 h. During the reoxygenation period, 100
µmol/l Astragaloside IV, 25 µmol/l CaSR antagonist
NPS-2143 or 300 µmol/l CaSR agonist GdCl3 was
added to the PC12 cells.
CCK-8 assay
CCK-8 assay was used to detect the viability of PC12
cells. Cells from one bottle were inoculated into 96-well plates at
a density of 3-5×104 cells/well for 24 h and exposed to
OGD/R. At 24 h post-reoxygenation, 10 µl/well CCK-8 reagent
was added to the cells, followed by 1-h incubation at 37°C. The
optical density (OD) was measured using a Varioskan™ LUX
multi-function microplate reader (Thermo Fisher Scientific, Inc.)
at 450 nm, and cell viability was calculated as follows: Cell
viability=(OD value of experimental group-OD value of blank
group)/(OD value of control group-OD value of blank group) ×100%.
Each group was tested four times in parallel for statistical
analysis.
Flow cytometry
PC12 cells were dissociated by 0.25% trypsin
(without EDTA) and washed twice with 1X PBS at 4°C. The cells were
then centrifuged at 300 × g at 4°C for 5 min and resuspended in 100
µl 1X binding buffer. Subsequently, 5 µl FITC and PI
were added to the cells and mixed gently avoiding light for 15 min
at room temperature. Finally, 400 µl 1X binding buffer was
added to the cells, and the apoptotic rate was measured by an FC
500 MCL flow cytometer (Beckman Coulter, Inc.) and analyzed CXP
software (version 2.1; Beckman Coulter, Inc.).
Western blotting
Total protein extraction from the ischemic penumbra
area of brain tissue of the CIRI model rats (n=3) or PC12 cells was
performed using a total protein extraction kit. The protein
concentration was measured using the BCA method. Equal amounts of
total protein extracts (30 µg/lane) were separated by 10%
SDS-PAGE and transferred to polyvinylidene difluoride membranes by
the semi-dry transfer method. The membranes were blocked with 5%
nonfat milk in TBS with 0.1% Tween 20 for 1.5 h at room temperature
and subsequently incubated overnight at 4°C with anti-CaSR (1:400),
anti-cleaved caspase-3 (1:1,000), anti-β-actin (1:1,000),
anti-Bcl-2 (1:800) and anti-Bax (1:800) antibodies, followed by
incubation with HRP-conjugated goat anti-mouse IgG (1:3,000) or
anti-rabbit IgG (1:3,000) antibodies at room temperature for 1 h.
The labeled proteins were detected using the enhanced luminol-based
ECL reagent kit (Wuhan Servicebio Technology Co., Ltd.) and the FX5
Spectra Imaging System, and the optical density of the bands was
measured by ImageJ software (version 1.8.0; National Institute of
Health). The in vitro experiment was repeated three
times.
Calcium assay
Calcium concentration in the culture medium and PC12
cells was measured using a commercial kit according to the
manufacturer's instructions. After 24 h of OGD/R, the cell culture
medium of each group was collected and centrifuged at 4,025 × g at
4°C for 5 min, and the supernatant liquid was collected. The
working fluid was prepared according to the manufacturer's
instructions. The supernatant liquid and all reagents were added to
a 96-well plate at the required proportions and mixed. Finally, the
OD values of each well were detected at 610 nm using a microplate
reader. Each set of experiments was performed in triplicate. The
calcium concentration in the culture medium was calculated as
follows: Calcium concentration=(OD value of experimental group-OD
value of blank group)/(OD value of standard well-OD value of blank
group).
To determine the calcium concentration in PC12
cells, cells from each group were collected and lysed with
deionized water at a ratio of 1:9 to extract the total cell
protein. The BCA method was used to quantify the proteins in each
group of cells. The subsequent steps were the same as those used
for the determination of the calcium concentration in the cell
culture medium. Each set of experiments was performed in
triplicate. The calcium concentration in PC12 cells was calculated
as follows: Calcium concentration=(OD value of experimental
group-OD value of blank group)/(OD value of standard well-OD value
of blank group)/protein concentration.
Statistical analysis
Data are presented as the mean ± SD from ≥3
experimental repeats. All data were analyzed by SPSS 21.0 software
(IBM Corp.). One-way analysis of variance followed by Bonferroni's
post hoc test was used to compare data among the groups. P<0.05
was considered to indicate a statistically significant
difference.
Results
In the present study, the neuroprotective effects of
Astragaloside IV against CIRI were first observed in vivo
and in vitro. Models of CIRI were established by MCAO/R in
rats and by OGD/R in PC12 cells. Secondly, a series of experiments
were performed to study the protective mechanism of Astragaloside
IV.
Astragaloside IV inhibits MCAO/R-induced
cerebral damage in rats
To assess the neuroprotective effects of
Astragaloside IV, NFS, TTC and H&E staining were evaluated in a
MCAO/R rat model. Rats were administered Astragaloside IV, NPS-2143
or an equal volume of physiological saline for 24 h after
reperfusion, and neurological deficits were observed using the Zea
Longa Neurological Score, which can effectively evaluate the
neurological deficits of MCAO/R rats (39). Rats in the Sham group presented
with no neurological deficits; by contrast, a notable increase in
neurological deficits was observed in rats of the MCAO/R group
(P<0.05 vs. Sham). Treatment with Astragaloside IV and the CaSR
inhibitor NPS-2143 significantly decreased the neurological score
of MCAO/R rats (P<0.05 vs. MCAO/R; Fig. 1A).
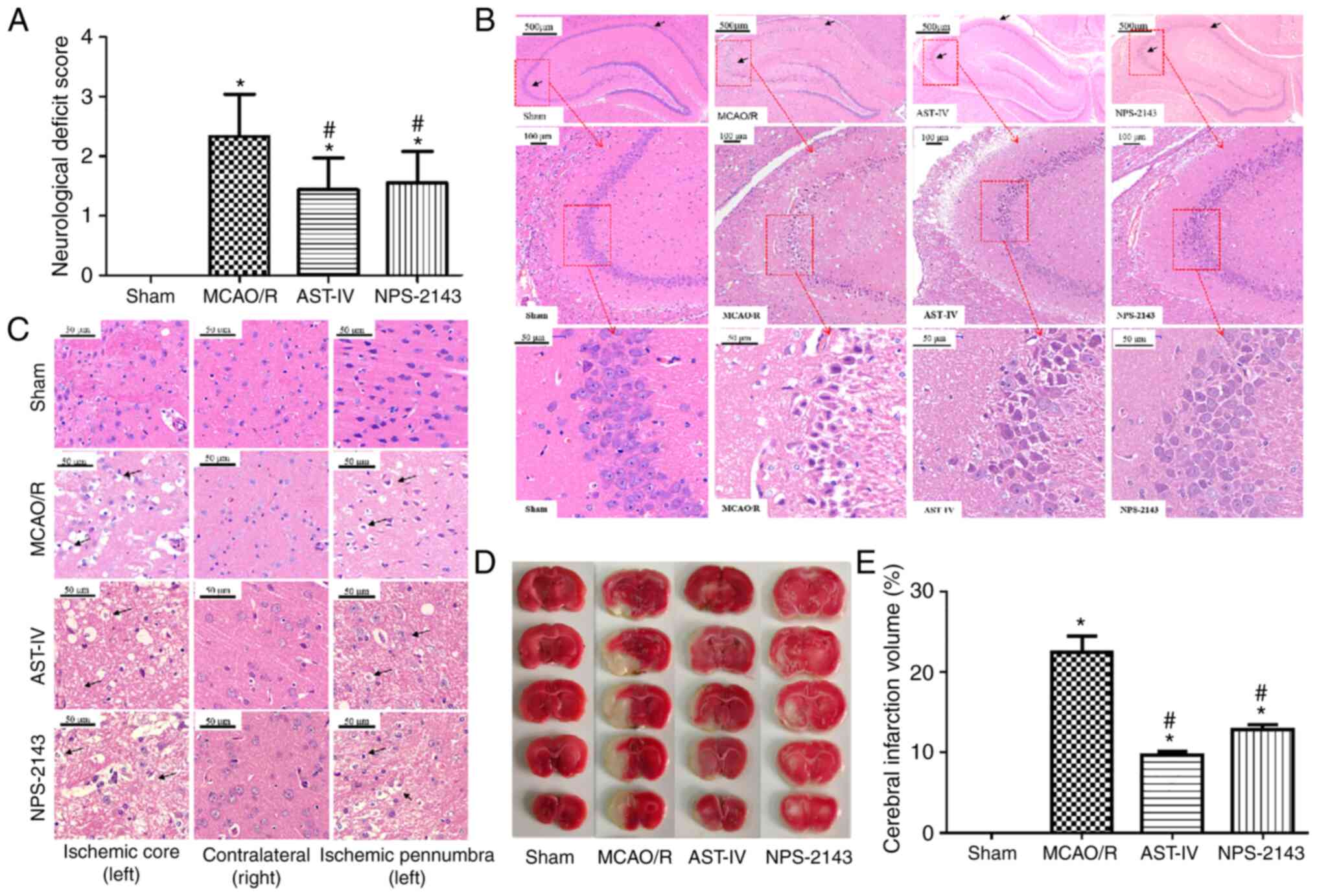 | Figure 1Neuroprotective effects of
Astragaloside IV on a MCAO/R rat model. (A) Neurological function
scores of rats at 24 h after post-MCAO/R. n=9. (B) Representative
images of H&E staining in the rat hippocampus following CIRI.
Magnification, ×50 (top), ×100 (middle) and ×400 (bottom). (C)
Representative images of H&E staining in the rat cerebral
cortex following CIRI. Magnification, ×400. (D) Representative
images of 2,3,5-triphenyltetra-zolium chloride-stained serial
coronal brain sections. (E) Infarction volume of rats in different
groups at 24 h post-MCAO/R. n=3. *P<0.05 vs. Sham;
#P<0.05 vs. MCAO/R. MCAO/R, middle cerebral artery
occlusion/reperfusion; CaSR, calcium-sensing receptor; AST-IV,
MCAO/R + Astragaloside IV; NPS-2143, MCAO/R + CaSR antagonist;
CIRI, cerebral ischemia-reperfusion injury. |
H&E staining was used to observe the
morphological changes of rat brain tissue and determine the success
of model establishment (Fig. 1B and
C). Neurons in the hippocampus (Fig. 1B) and the cortex (Fig. 1C) of the rats in the Sham group
presented a normal structure, which was similar to that of the
nonischemic brain tissue of the MCAO/R rats. The cells exhibited a
clear shape, compact structure and clear nucleoli. However, edema
and necrosis were observed in the ischemic cortex and hippocampus
of the MCAO/R rats: The numbers of neurons decreased, the gaps
around neurons increased, and the neurons were swollen or shrunk
with disappeared nucleoli and a dissolved nuclear membrane. The
pathomorphological changes of the ischemic side brain tissues in
the AST-IV and NPS-2143 groups were alleviated compared with those
in the MCAO/R group, with fewer apoptotic cells as observed by the
cell morphology.
TTC staining revealed no cerebral infarction among
rats in the Sham group, whereas the infarction volume in rats of
the MCAO/R group was increased (P<0.05 vs. Sham). Astragaloside
IV and NPS-2143 significantly reduced the infarction volume of
MCAO/R rats (P<0.05 vs. MCAO/R; Fig. 1D and E).
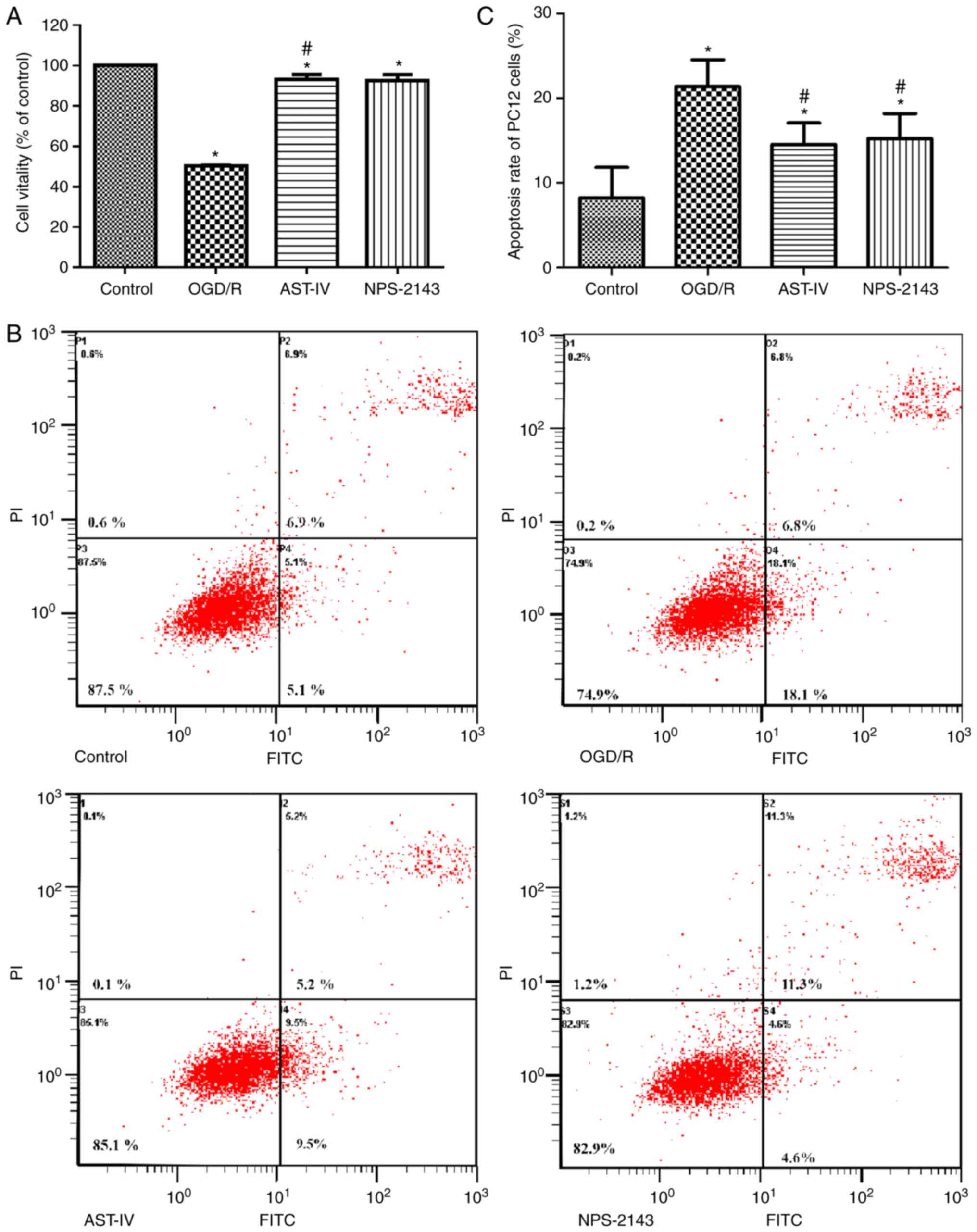
Astragaloside IV increases the viability
of PC12 cells and decreases the apoptotic rate following OGD/R
To study the therapeutic potential of Astragaloside
IV against OGD/R, the cell viability and apoptotic rates were
determined by CCK-8 and flow cytometry assay, respectively. The
results demonstrated that the cell viability decreased and the
apoptotic rate increased significantly following OGD/R (P<0.05
vs. Control), whereas treatment with Astragaloside IV or the CaSR
inhibitor NPS-2143 immediately following reoxygenation improved
cell viability and inhibited the apoptotic rate, respectively,
compared with those in the OGD/R group (Fig. 2). These results suggested that
Astragaloside IV exerted a protective effect on OGD/R PC12 cells,
which was similar to that of NPS-2143, suggesting that the
protective effect of Astragaloside IV on CIRI maybe associated with
CaSR.
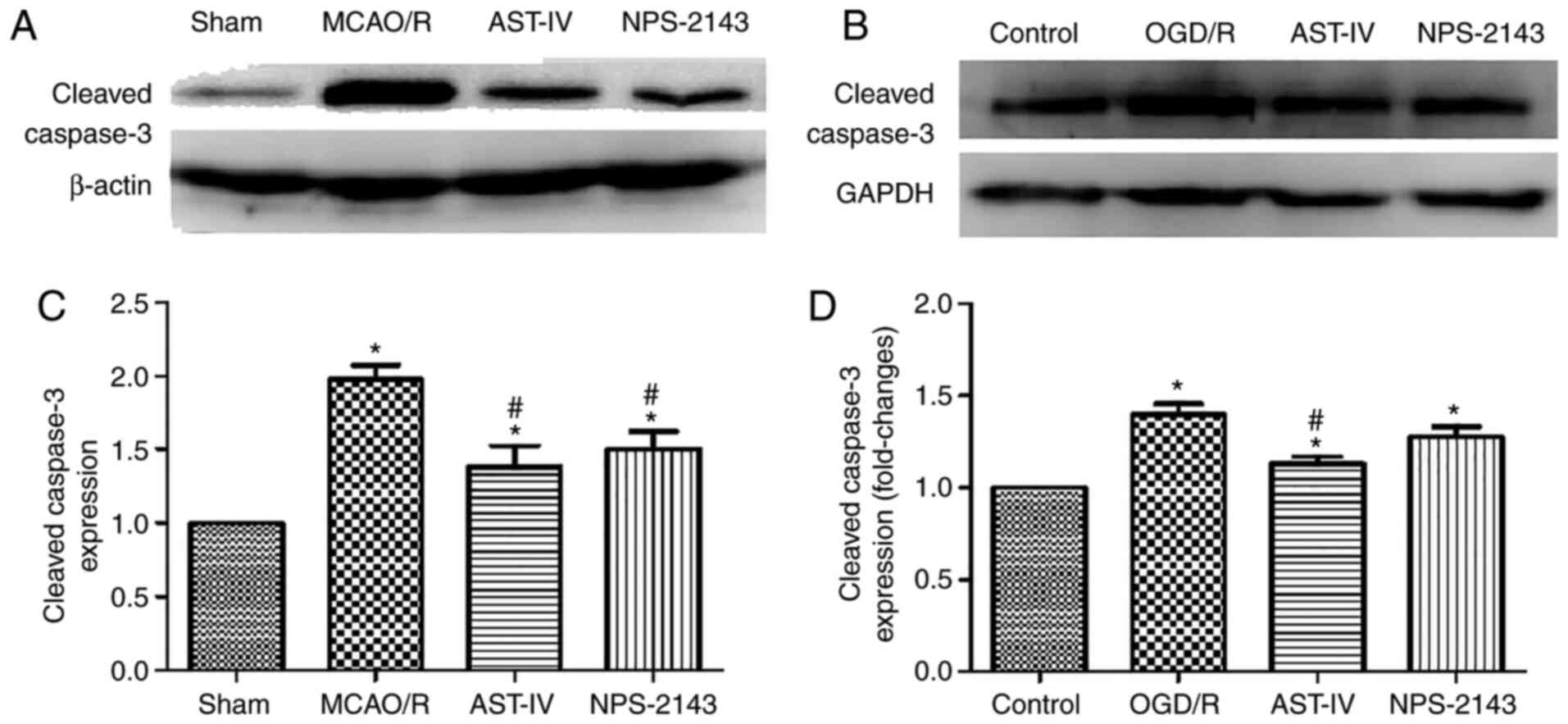
Astragaloside IV reduces the protein
expression of cleaved caspase-3
To determine the antiapoptotic effects of
Astragaloside IV on OGD/R PC12 cells and MCAO/R rats, the protein
expression levels of cleaved caspase-3 were detected as an index of
apoptosis by western blotting. The results demonstrated that MCAO/R
significantly increased the protein expression levels of cleaved
caspase-3 in the rat brain tissue compared with those in the Sham
group, and OGD/R had the same effect on PC12 cells (both
P<0.05). Compared with that in the OGD/R and MCAO/R groups,
treatment with Astragaloside IV or NPS-2143 reduced the levels of
cleaved caspase-3 expression (P<0.05). These results suggested
that Astragaloside IV may inhibit apoptosis to exert its cerebral
anti-ischemic effects. The similar effects of the CaSR inhibitor
NPS-2143 to those of Astragaloside IV indicated that the
anti-apoptotic effect of Astragaloside IV may be associated with
CaSR (Fig. 3).
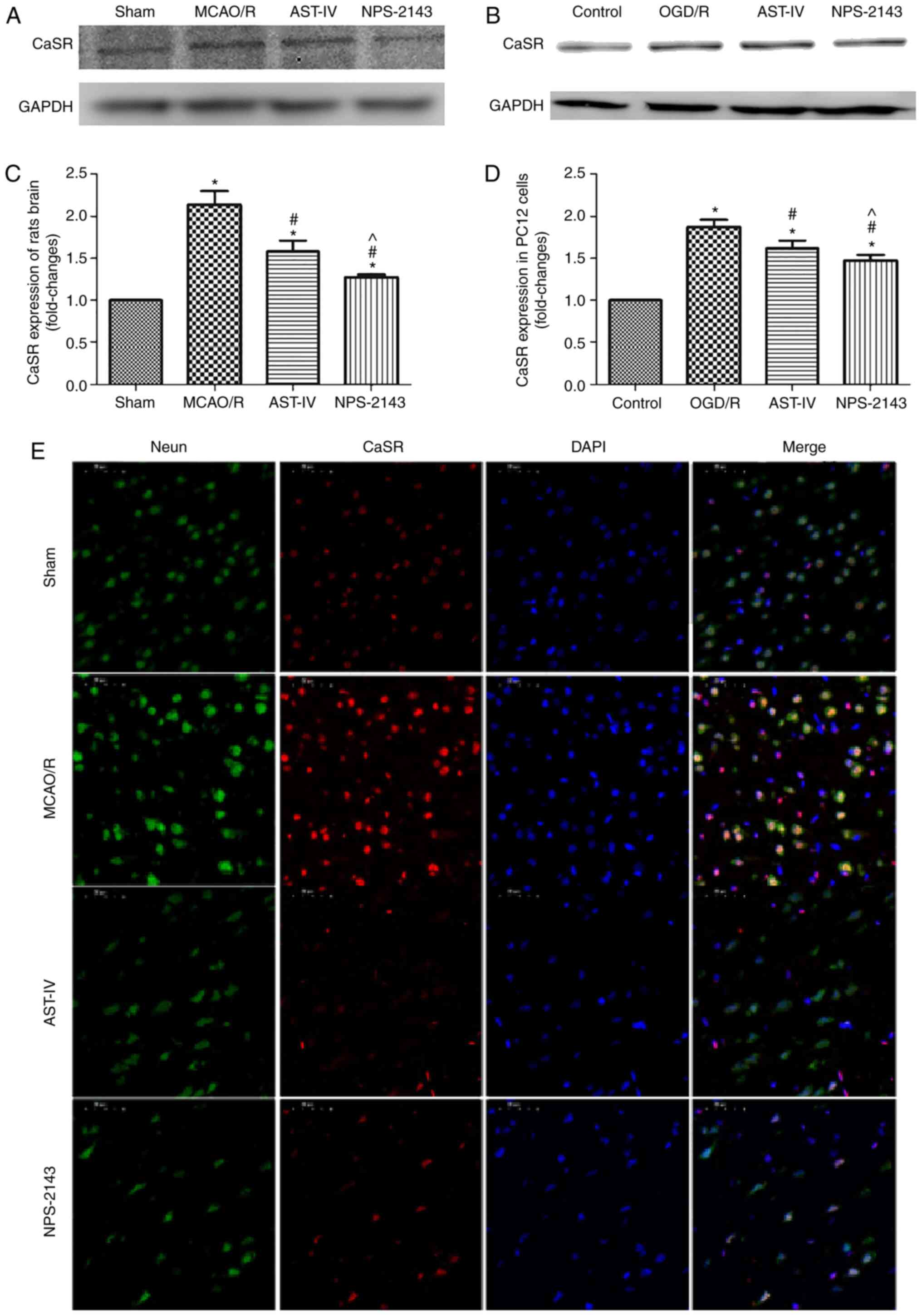
Astragaloside IV decreases the protein
expression of CaSR
As aforementioned, Astragaloside IV alleviated CIRI
in the rat and PC12 cell models, and its neuroprotective roles were
associated with the inhibition of apoptosis and the reduction of
the calcium flow into PC12 cells during OGD/R. The possible
underlying mechanisms were further investigated. Western blotting
results demonstrated that the CaSR protein expression levels in
MCAO/R rat brain tissue and OGD/R PC12 cells were increased
compared with those in the Sham and Control group, respectively
(both P<0.05). Treatment with Astragaloside IV decreased the
protein expression levels of CaSR in the MCAO/R brain tissue and
OGD/R PC12 cells compared with those in the respective model groups
(both P<0.05). In addition, CaSR antagonist NPS-2143 also
reduced the expression of CaSR in the brain tissue of MCAO/R rats
and in OGD/R PC12 cells (both P<0.05; Fig. 4A-D).
Double immunofluorescence staining revealed that the
number of neurons in the brain tissue was high, whereas that of
CaSR-positive cells was low in the Sham group. The expression of
CaSR on neurons in the MCAO/R group appeared to be increased
compared with that in the Sham group. Compared with that in the
MCAO/R group, the expression of CaSR on neurons in the AST-IV and
NPS-2143 groups appeared to be downregulated (Fig. 4E).
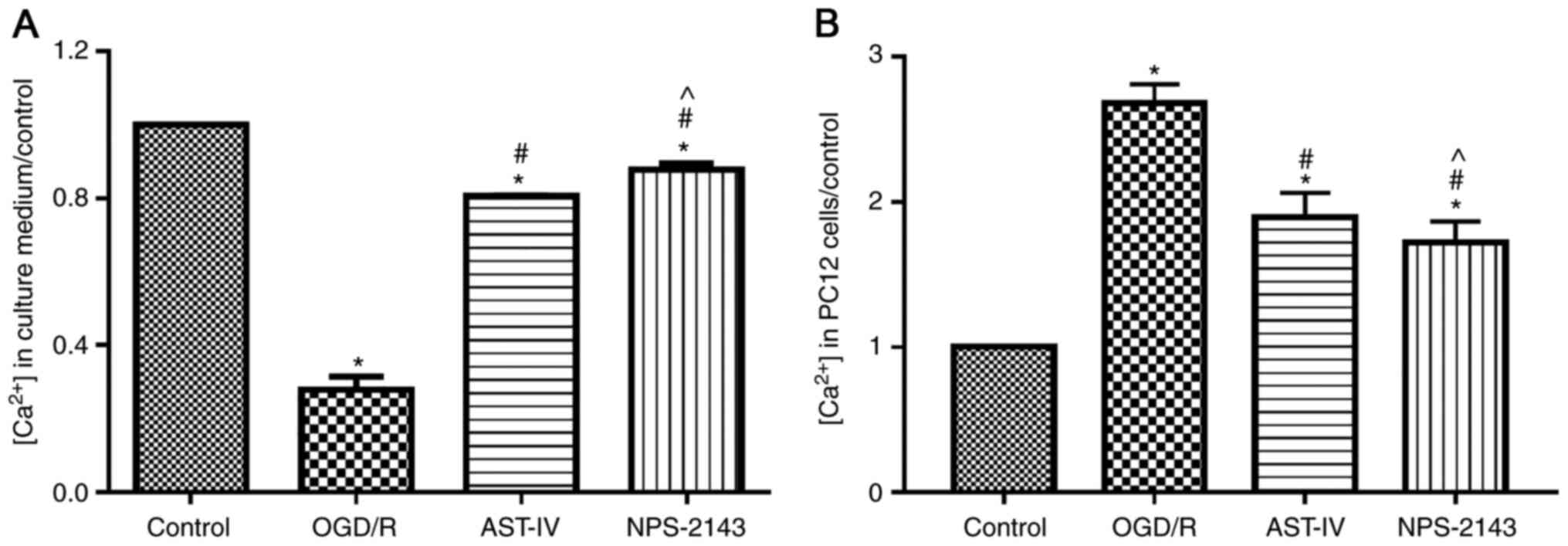
Astragaloside IV inhibits OGD/R-induced
calcium flow into PC12 cells
To determine whether Astragaloside IV inhibited
calcium overload, the concentration of calcium in the culture
medium and PC12 cells was tested using a commercial kit. The
results demonstrated that OGD/R promoted the flow of calcium ions
into PC12 cells and increased the intracellular calcium
concentration compared with that in the control group (P<0.05).
By contrast, Astragaloside IV or the CaSR antagonist NPS-2143
reduced the calcium flow into cells and inhibited calcium overload
induced by OGD/R (both P<0.05 vs. OGD/R; Fig. 5).
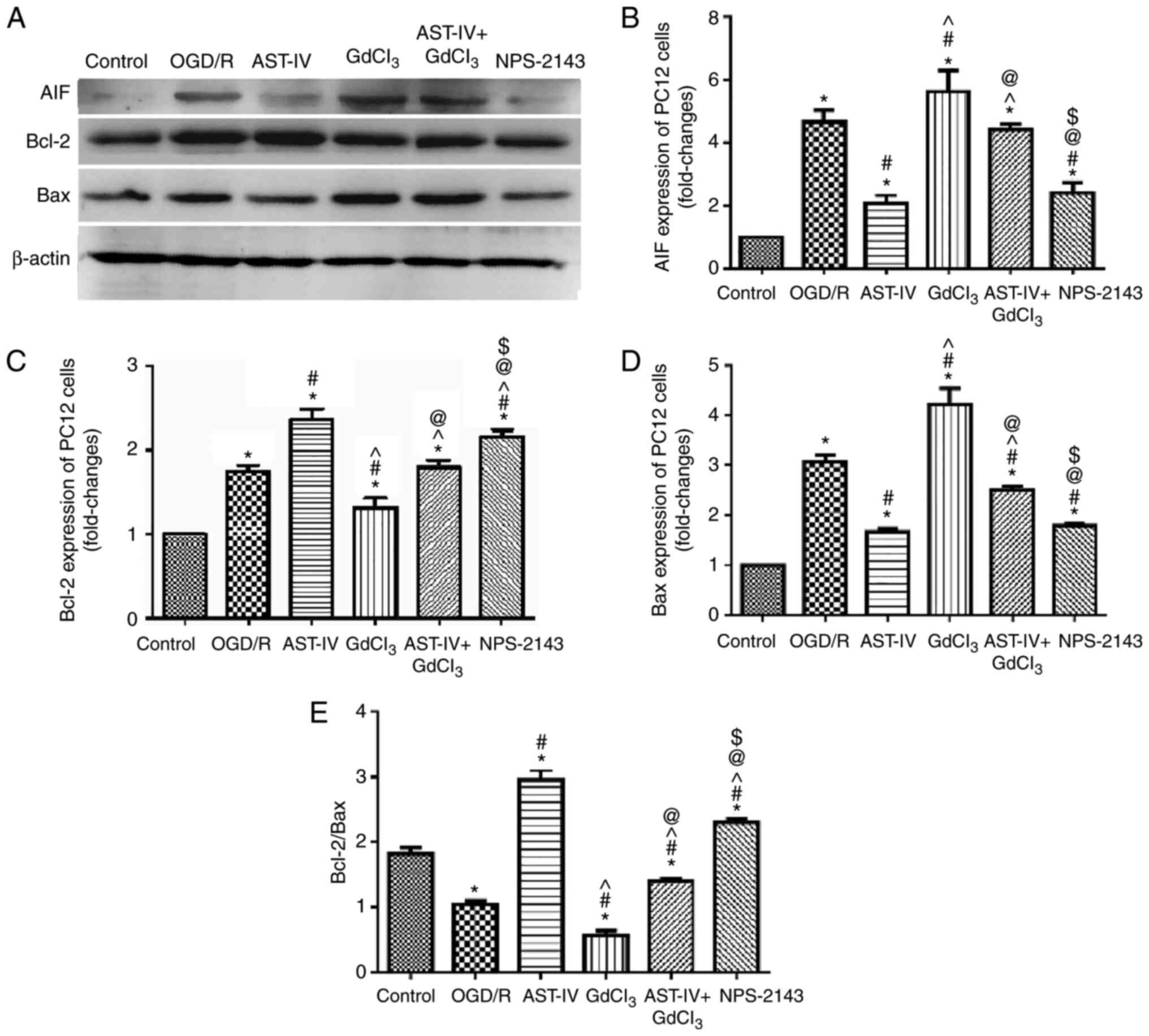
Astragaloside IV decreases the protein
expression of AIF and increases the ratio of Bcl-2/Bax during
OGD/R
To determine the inhibitory effects of Astragaloside
IV on the OGD/R-induced apoptosis of PC12 cells, the expression
levels of the proapoptotic proteins AIF and Bax and the
antiapoptotic protein Bcl-2 were detected by western blot-ting.
OGD/R PC12 cells were treated with Astragaloside IV, the CaSR
agonist GdCl3, Astragaloside IV + GdCl3 or
the CaSR antagonist NPS-2143. The results demonstrated notable
changes in the protein expression levels of AIF, Bax and Bcl-2
among the groups. The expression levels of AIF and Bax increased,
whereas those of Bcl-2 decreased significantly in PC12 cells
following OGD/R (P<0.05 vs. Control). Treatment with
Astragaloside IV decreased the expression levels of AIF and Bax and
increased those of Bcl-2 in PC12 cells following OGD/R (P<0.05
vs. OGD/R); the effects of GdCl3 were the opposite to
those of Astragaloside IV (P<0.05 vs. OGD/R). These results also
revealed that the effects of Astragaloside IV were partly offset by
GdCl3 (P<0.05 vs. AST-IV). In addition, the effects
of NPS-2143 treatment were similar to those of Astragaloside IV
(Fig. 6).
The changes in the Bcl-2/Bax ratio in the PC12 cells
following different treatments were also analyzed; the Bcl-2/Bax
ratio was significantly decreased by OGD/R (P<0.05 vs. Control).
Both Astragaloside IV and NPS-2143 increased the Bcl-2/Bax ratio in
PC12 cells following OGD/R (P<0.05 vs. OGD/R). However,
treatment with GdCl3 decreased the Bcl-2/Bax ratio in
PC12 cells following OGD/R (P<0.05 vs. OGD/R) and offset the
protective effect of Astragaloside IV (P<0.05 vs.
GdCl3).
Overall, these results demonstrated that
Astragaloside IV and NPS-2143 inhibited the expression levels of
pro-apoptotic proteins and increased those of anti-apoptotic
proteins, whereas GdCl3 exerted the opposite effects,
which may be partly offset by Astragaloside IV. These results
suggested that CaSR may be involved in the protective effects of
Astragaloside IV over CIRI.
Discussion
Stroke is the most frequent cause of permanent
disability in adults worldwide (47,48). Previous studies have demonstrated
that brain ischemic injury secondary to arterial occlusion is
characterized by neuronal apoptosis (49,50). CIRI triggers complex pathological
pathways of the ischemia and reperfusion cascade and ultimately
causes irreversible neuronal injury in the ischemic core (51). However, neurons in the penumbra,
surrounding the ischemic core, can be salvaged, offering the
possibility of rescuing brain tissue following CIRI and reducing
post-CIRI disability (15,52).
Thus, the use of neuroprotective drugs following reperfusion after
ischemic stroke is necessary to reduce apoptosis. The results of
the present study demonstrated that Astragaloside IV improved NFS
and cerebral infarct volume and significantly reduced the
blood-brain barrier permeability in a rat model of MCAO/R through
its antiapoptotic effects. The present study also performed further
investigation into the neuroprotective mechanism of Astragaloside
IV to provide a theoretical basis for its application in patients
with stroke.
Apoptosis is one of the main pathological mechanisms
of CIRI that begins shortly after cerebral ischemia, is accelerated
by reperfusion and partially leads to cerebral infarction (47). The timely and effective inhibition
of the apoptosis process can prevent the loss of neuronal cells,
reduce the damage to brain tissue caused by ischemia and
reperfusion, and reduce the symptoms of neurological defects
(5). Our previous study
demonstrated that Astragaloside IV attenuated CIRI by promoting the
degree of autophagy and downregulating apoptosis in a rat model of
MCAO/R and an HT22 cell model of OGD/R (9). The results of the present study
revealed that Astragaloside IV relieved brain injury induced by
CIRI, reduced apoptosis, inhibited the calcium flow into PC12
cells, decreased CaSR expression and increased the ratio of
Bcl-2/Bax compared with those in the corresponding control groups.
These results indicated the underlying mechanisms of the
neuroprotective effect of Astragaloside IV and demonstrated the
pivotal role of CaSR in CIRI.
CaSR is a G protein-coupled receptor that is present
throughout the central nervous system (53,54). An increasing number of studies
have reported that CaSR is involved in modulating various cellular
functions, including cell proliferation, differentiation and
apoptosis in the central nervous system by sensing changes in the
extracellular calcium concentration (55-58). CaSR can be activated by
extracellular calcium, magnesium and CaSR agonists to increase the
intracellular calcium concentration (19,57); increased intracellular calcium
caused by CaSR contributes to CIRI-induced brain injury. The
results of the present study revealed that in PC12 cells subjected
to OGD/R, cell viability was decreased and the expression of
cleaved caspase-3 was significantly increased compared with those
in the control group, accompanied by the upregulation of CaSR,
which was reversed by Astragaloside IV and NPS-2143. Similarly, the
injury of rat brain tissue induced by MCAO/R was alleviated by
treatment with Astragaloside IV or NPS-2143, along with the
inhibition of CaSR. These results suggested that Astragaloside IV
alleviated CIRI, and that the underlying mechanism may be
associated with CaSR.
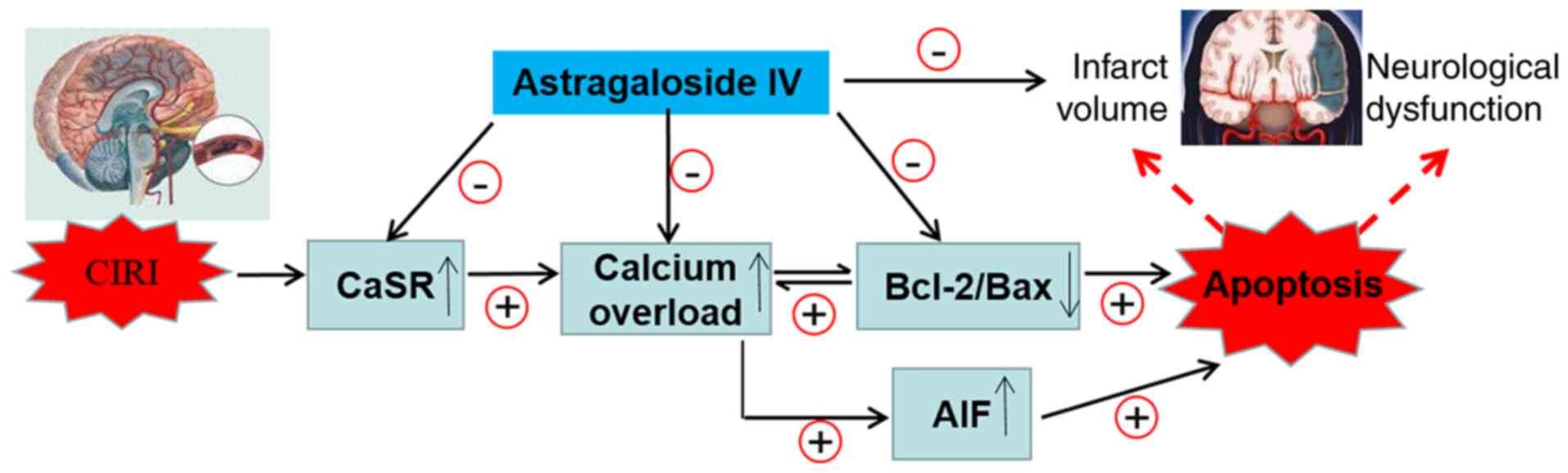
Upregulation of CaSR leads to changes in the
expression of AIF, Bcl-2 and Bax that serve a key role in
regulating the mitochondrial pathway of apoptosis (59,60). To gain additional insights into
the association between apoptosis and the activation of CaSR during
CIRI, the intracellular and extracellular concentration of calcium
was detected in OGD/R PC12 cells subjected to Astragaloside IV,
GdCl3 (a specific CaSR agonist), NPS-2143 (a specific
CaSR antagonist) or a combination of Astragaloside IV and
GdCl3 treatments, and the protein expression of AIF,
Bcl-2 and Bax in vitro was analyzed. The results
demonstrated that Astragaloside IV inhibited the calcium flow into
the cells, and that the increase in the intracellular calcium
concentration in PC12 cells induced the expression of Bcl-2, as
well as decreased the protein expression levels of AIF, Bax and
CaSR compared with those in the control cells. These effects of
Astragaloside IV were similar to those obtained with the CaSR
inhibitor NPS-2143 and were partly abolished by the CaSR agonist
GdCl3. Therefore, the downregulation of CaSR may
contribute to the protective effect of Astragaloside IV on CIRI. In
addition, the changes in CaSR expression induced by MCAO/R, the
CaSR inhibitor NPS-2143 and the CaSR agonist GdCl3
demonstrated that the decrease in AIF and Bcl-2/Bax may contribute
to the mechanism of CaSR in promoting CIRI. These results may help
further understand the molecular mechanisms of Astragaloside
IV-induced neuroprotection (Fig.
7).
The results of the present study demonstrated that
Astragaloside IV alleviated CIRI in a rat model and decreased the
protein expression level of CaSRs. The CaSR inhibitor NPS-2143
exhibited similar effects to those of Astragaloside IV; thus, the
effects of the CaSR agonist GdCl3 and Astragaloside IV +
GdCl3 on OGD/R-induced injury were further investigated
in vitro on PC12 cells. The in vitro experimental
results demonstrated that the CaSR agonist GdCl3
aggravated cell injury induced by OGD/R and promoted apoptosis,
whereas Astragaloside IV reduced the damage of CaSR agonists.
Therefore, Astragaloside IV may inhibit apoptosis by decreasing the
protein expression of CaSRs, thus reducing CIRI.
Traditional Chinese medicine glycosides exhibit a
wide range of pharmacological activities. Certain glycosides, such
as ginsenoside Rg1 and oleuropein, serve an important role in the
treatment of central nervous system diseases; however, their
blood-brain barrier permeability is low (61,62). By contrast, the small molecular
weight of Astragaloside IV (784.97 Da) and the destruction of the
blood-brain barrier following CIRI leads to high permeability
(63). A previous study on
cellular and animal models has demonstrated that Astragaloside IV
possesses potent protective effects on the brain (9), which was consistent with the results
of the present study. In the present study, the animal sample size
was small, as a large number of previous experiments have confirmed
the protective effects of Astragaloside IV on CIRI (9); to ensure adherence to animal ethics
requirements, a small sample size was used to achieve the
experimental purpose. Follow-up experiments with a larger sample
size will be performed to further study the neuroprotective
mechanism of Astragaloside IV on CIRI, as well as the role of
calcium-sensitive receptors in CIRI.
The PC12 cell line used in the present study is a
type of highly differentiated PC12-derived neuron-like cell induced
by neurotrophic factor (Fig. 8C).
Different morphology can be observed in highly differentiated
(Fig. 8C), poorly differentiated
(Fig. 8B) and undifferentiated
(Fig. 8A) PC12 cells. PC12 is a
stable cell line that can be directly cultured and passed down, and
it has been widely used to study diseases of the nervous system
in vitro (43,46,64). Therefore, in the present study,
the highly differentiated PC12 cells were used to determine the
effects and mechanism of Astragaloside IV on OGD/R-induced
apoptosis. Future studies should use primary cultured rat neurons
and brain specimens of MCAO/R rats to further study the action of
Astragaloside IV on apoptosis in CIRI and explore other potential
neuroprotective pathways of Astragaloside IV.
The results of the present study demonstrated the
inhibition of apoptosis may protect neurons by downregulating the
expression of CaSR. Astragaloside IV alleviated cerebral injury in
MCAO/R rats, improved the viability of PC12 cells following OGD/R,
reduced the expression levels of CaSR and inhibited apoptosis
compared with those in the corresponding control groups.
Astragaloside IV may exert its neuroprotective effect by inhibiting
apoptosis through the prevention of the increase of CIRI-induced
CaSR expression.
Funding
This study was supported by the Science Foundation
of Hebei Province (grant no. H2019423074), Science and Technology
Ability Improvement Project of the Hebei University of Chinese
Medicine (grant no. KTY2019049), Basic Research Project of
Outstanding Young Teachers of the University of Chinese Medicine
(grant no. YQ2019007), Hebei Province Graduate Innovative Ability
Training Project (grant no. CXZZBS2019156), Research Project of
Hebei Administration of Traditional Chinese Medicine (grant no.
2018111) and the Science Foundation for the Higher Education
Institutions of Hebei Province (grant no. ZD2018002).
Availability of data and materials
The datasets used and analyzed in the current study
are available from the corresponding authors on reasonable
request.
Authors' contributions
WJG conceived the study. JLT and YZ performed data
analysis. SJD and WJG acquired funding. SJD and YMZ performed the
experiments. XHZ was responsible for project administration. YJD
established the experimental animal model. WJG supervised the
study. XHZ performed the pathomorphology experiments. SJD and YZ
wrote the original draft of the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
All experiments were approved by the Institutional
Animal Care and Use Committee of Hebei University of Chinese
Medicine (Shijiazhuang, China; approval no. DWLL2018032).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors would like to thank Professor Shun-Jiang
Xu (The First Hospital of Hebei Medical University, Shijiazhuang,
China) for providing PC12 cells.
References
|
1
|
Hankey GJ: Stroke. Lancet. 389:641–654.
2017. View Article : Google Scholar
|
|
2
|
Writing Group Members; Mozaffarian D,
Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de
Ferranti S, Després JP, et al: Heart disease and stroke
statistics-2016 update: A report from the American heart
association. Circulation. 133:e38–e360. 2016.
|
|
3
|
Powers WJ, Rabinstein AA, Ackerson T,
Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk
BM, Hoh B, et al: Guidelines for the early management of patients
with acute ischemic stroke: 2019 update to the 2018 guidelines for
the early management of acute ischemic stroke: A guideline for
healthcare professionals from the American heart
association/American stroke association. Stroke. 50:e344–e418.
2019. View Article : Google Scholar
|
|
4
|
Qureshi AI, Singh B, Huang W, Du Z,
Lobanova I, Liaqat J and Siddiq F: Mechanical thrombectomy in acute
ischemic stroke patients performed within and outside clinical
trials in the United States. Neurosurgery. 86:E2–E8. 2020.
|
|
5
|
Chen YW, Sung SF, Chen CH, Tang SC, Tsai
LK, Lin HJ, Huang HY, Po HL, Sun Y, Chen PL, et al: Intravenous
thrombolysis administration 3-4.5 h after acute ischemic stroke: A
retrospective, multicenter study. Front Neurol. 10:10382019.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen CH and Hsieh CL: Effect of
acupuncture on oxidative stress iduced by cerebral
ischemia-reperfusion injury. Antioxidants (Basel). 9:2482020.
View Article : Google Scholar
|
|
7
|
Wu L, Xiong X, Wu X, Ye Y, Jian Z, Zhi Z
and Gu L: Targeting oxidative stress and inflammation to prevent
ischemia-reperfusion injury. Front Mol Neurosci. 13:282020.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Khandelwal P, Yavagal DR and Sacco RL:
Acute ischemic stroke intervention. J Am Coll Cardiol.
67:2631–2644. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang Y, Zhang Y, Jin XF, Zhou XH, Dong
XH, Yu WT and Gao WJ: The role of astragaloside IV against cerebral
ischemia/reperfusion injury: Suppression of apoptosis via promotion
of P62-LC3-autophagy. Molecules. 24:18382019. View Article : Google Scholar :
|
|
10
|
Liu D, Gu Y, Wang W and Chen W: Astragalin
alleviates ischemia/reperfusion-induced brain injury via
suppression of endoplasmic reticulum stress. Mol Med Rep.
22:4070–4078. 2020.PubMed/NCBI
|
|
11
|
Liu Y, Yang H, Jia G, Li L, Chen H, Bi J
and Wang C: The synergistic neuroprotective effects of combined
rosuvastatin and resveratrol pretreatment against cerebral
ischemia/reperfusion injury. J Stroke Cerebrovasc Dis.
27:1697–1704. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gu JH, Ge JB, Li M, Wu F, Zhang W and Qin
ZH: Inhibition of NF-κB activation is associated with
anti-inflammatory and anti-apoptotic effects of ginkgolide B in a
mouse model of cerebral ischemia/reperfusion injury. Eur J Pharm
Sci. 47:652–660. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou H, Yang WS, Li Y, Ren T, Peng L, Guo
H, Liu JF, Zhou Y, Zhao Y, Yang LC and Jin X: Oleoylethanolamide
attenuates apoptosis by inhibiting the TLR4/NF-κB and ERK1/2
signaling pathways in mice with acute ischemic stroke. Naunyn
Schmiedebergs Arch Pharmacol. 390:77–84. 2017. View Article : Google Scholar
|
|
14
|
Naito MG, Xu D, Amin P, Lee J, Wang H, Li
W, Kelliher M, Pasparakis M and Yuan J: Sequential activation of
necroptosis and apoptosis cooperates to mediate vascular and neural
pathology in stroke. Proc Natl Acad Sci USA. 117:4959–4970. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bai X, Tan TY, Li YX, Li Y, Chen YF, Ma R,
Wang SY, Li Q and Liu ZQ: The protective effect of cordyceps
sinensis extract on cerebral ischemic injury via modulating the
mitochondrial respiratory chain and inhibiting the mitochondrial
apoptotic pathway. Biomed Pharmacother. 124:1098342020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang HL, Zhou QH, Xu MB, Zhou XL and Zheng
GQ: Astragaloside IV for experimental focal cerebral ischemia:
Preclinical evidence and possible mechanisms. Oxid Med Cell Longev.
2017:84243262017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lu FH, Tian Z, Zhang WH, Zhao YJ, Li HL,
Ren H, Zheng HS, Liu C, Hu GX, Tian Y, et al: Calcium-sensing
receptors regulate cardiomyocyte Ca2+ signaling via the
sarcoplasmic reticulum-mitochondrion interface during
hypoxia/reoxygenation. J Biomed Sci. 17:502010. View Article : Google Scholar
|
|
18
|
Yin F, Zhou H, Fang Y, Li C, He Y, Yu L,
Wan H and Yang J: Astragaloside IV alleviates ischemia
reperfusion-induced apoptosis by inhibiting the activation of key
factors in death receptor pathway and mitochondrial pathway. J
Ethnopharmacol. 248:1123192020. View Article : Google Scholar
|
|
19
|
Yin B, Hou XW and Lu ML: Astragaloside IV
attenuates myocardial ischemia/reperfusion injury in rats via
inhibition of calcium-sensing receptor-mediated apoptotic signaling
path-ways. Acta Pharmacol Sin. 40:599–607. 2019. View Article : Google Scholar
|
|
20
|
Zhang Y, Qiao L, Xu W, Wang X, Li H, Xu W,
Chu K and Lin Y: Paeoniflorin attenuates cerebral ischemia-induced
injury by regulating Ca2+/CaMKII/CREB signaling pathway.
Molecules. 22:3592017. View Article : Google Scholar
|
|
21
|
Yan L, Zhu T, Sun T, Wang L, Pan S, Tao Z,
Yang Z and Cao K: Activation of calcium-sensing receptors is
associated with apoptosis in a model of simulated cardiomyocytes
ischemia/reperfusion. J Biomed Res. 24:301–307. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kienitz MC, Niemeyer A, Konig GM, Kostenis
E, Pott L and Rinne A: Biased signaling of Ca2+-sensing
receptors in cardiac myocytes regulates GIRK channel activity. J
Mol Cell Cardiol. 130:107–121. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhen Y, Ding C, Sun J, Wang Y, Li S and
Dong L: Activation of the calcium-sensing receptor promotes
apoptosis by modulating the JNK/p38 MAPK pathway in focal cerebral
ischemia-reperfusion in mice. Am J Transl Res. 8:911–921.
2016.PubMed/NCBI
|
|
24
|
Zhang ZL, Li ZR, Li JS and Wang SR:
Calcium-sensing receptor antagonist NPS-2143 suppresses
proliferation and invasion of gastric cancer cells. Cancer Gene
Ther. 27:548–557. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pearce SH and Thakker RV: The calcium
sensing receptor: Insights into extracellular calcium homeostasis
in health and disease. J Endocrinol. 154:371–378. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chattopadhyay N, Vassilev PM and Brown EW:
Calcium-sensing receptor: Roles in and beyond systemic calcium
homeostasis. Biol Chem. 378:759–768. 1997.PubMed/NCBI
|
|
27
|
Meng D, Chen XJ, Bian YY, Li P, Yang D and
Zhang JN: Effect of astragalosides on intracellular calcium
overload in cultured cardiac myocytes of neonatal rats. Am J Chin
Med. 33:11–20. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hu B, Tong F, Xu L, Shen Z, Yan L, Xu G
and Shen R: Role of calcium sensing receptor in
streptozotocin-induced diabetic rats exposed to renal ischemia
reperfusion injury. Kidney Blood Press Res. 43:276–286. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pan R, Cai J, Zhan L, Guo Y, Huang RY, Li
X, Zhou M, Xu D, Zhan J and Chen H: Buyang Huanwu decoction
facilitates neuro-rehabilitation through an improvement of synaptic
plasticity in cerebral ischemic rats. BMC Complement Altern Med.
17:1732017. View Article : Google Scholar
|
|
30
|
Zheng XW, Shan CS, Xu QQ, Wang Y, Shi YH,
Wang Y and Zheng GQ: Buyang Huanwu decoction targets SIRT1/VEGF
pathway to promote angiogenesis after cerebral ischemia/reperfusion
injury. Front Neurosci. 12:9112018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Qi LW, Yu QT, Yi L, Ren MT, Wen XD, Wang
YX and Li P: Simultaneous determination of 15 marker constituents
in various radix Astragali preparations by solid-phase extraction
and high-performance liquid chromatography. J Sep Sci. 31:97–106.
2008. View Article : Google Scholar
|
|
32
|
Sun WX, Zhang ZF, Xie J, He Y, Cheng Y,
Ding LS, Luo P and Qing LS: Determination of an astragaloside IV
derivative LS-102 in plasma by ultra-performance liquid
chromatography-tandem mass spectrometry in dog plasma and its
application in a pharmacokinetic study. Phytomedicine. 53:243–251.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Qu YZ, Li M, Zhao YL, Zhao ZW, Wei XY, Liu
JP, Gao L and Gao GD: Astragaloside IV attenuates cerebral
ischemia-reperfusion-induced increase in permeability of the
blood-brain barrier in rats. Eur J Pharmacol. 606:137–141. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang J, Li J, Lu J, Zhang Y, Zhu Z and Wan
H: Synergistic protective effect of astragaloside
IV-tetramethylpyrazine against cerebral ischemic-reperfusion injury
induced by transient focal ischemia. J Ethnopharmacol. 140:64–72.
2012. View Article : Google Scholar
|
|
35
|
Yin YY, Li WP, Gong HL, Zhu FF, Li WZ and
Wu GC: Protective effect of astragaloside on focal cerebral
ischemia/reperfusion injury in rats. Am J Chin Med. 38:517–527.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li M, Ma RN, Li LH, Qu YZ and Gao GD:
Astragaloside IV reduces cerebral edema post-ischemia/reperfusion
correlating the suppression of MMP-9 and AQP4. Eur J Pharmacol.
715:189–195. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li M, Qu YZ, Zhao ZW, Wu SX, Liu YY, Wei
XY, Gao L and Gao GD: Astragaloside IV protects against focal
cerebral ischemia/reperfusion injury correlating to suppression of
neutrophils adhesion-related molecules. Neurochem Int. 60:458–465.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu P, Tang YY, Yang XS, Dai J, Yang M,
Zhang H, Liu Y, Yan H and Song XY: Validation of a preclinical
animal model to assess brain recovery after acute stroke. Eur J
Pharmacol. 835:75–81. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Longa EZ, Weinstein PR, Carlson S and
Cummins R: Reversible middle cerebral artery occlusion without
craniectomy in rats. Stroke. 20:84–91. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhou P, Lu S, Luo Y, Wang S, Yang K, Zhai
Y, Sun G and Sun X: Attenuation of TNF-α-induced inflammatory
injury in endothelial cells by ginsenoside Rb1 via inhibiting
NF-κB, JNK and p38 signaling pathways. Front Pharmacol. 8:4642017.
View Article : Google Scholar
|
|
41
|
Peng L, Yin J, Wang S, Ge M, Han Z, Wang
Y, Zhang M, Xie L and Li Y: TGF-β2/Smad3 signaling pathway
activation through enhancing VEGF and CD34 ameliorates cerebral
ischemia/reperfusion injury after isoflurane post-conditioning in
rats. Neurochem Res. 44:2606–2618. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang Y, Li B and Zhang X: Scutellaria
barbata D: Don (SBD) protects oxygen glucose
deprivation/reperfusion-induced injuries of PC12 cells by
up-regulating Nrf2. Artif Cells Nanomed Biotechnol. 47:1797–1807.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhao DY, Yu DD, Ren L and Bi GR:
Ligustilide protects PC12 cells from oxygen-glucose
deprivation/reoxygenation-induced apoptosis via the LKB1-AMPK-mTOR
signaling pathway. Neural Regen Res. 15:473–481. 2020. View Article : Google Scholar
|
|
44
|
Meng X, Xie W, Xu Q, Liang T, Xu X, Sun G
and Sun X: Neuroprotective effects of radix scrophulariae on
cerebral ischemia and reperfusion injury via MAPK pathways.
Molecules. 23:24012018. View Article : Google Scholar :
|
|
45
|
Li ZR, Yang L, Zhen J, Zhao Y and Lu ZN:
Nobiletin protects PC12 cells from ERS-induced apoptosis in OGD/R
injury via activation of the PI3K/AKT pathway. Exp Ther Med.
16:1470–1476. 2018.PubMed/NCBI
|
|
46
|
Wang G, Wang T, Zhang Y, Li F, Yu B and
Kou J: Schizandrin protects against OGD/R-induced neuronal injury
by suppressing autophagy: Involvement of the AMPK/mTOR pathway.
Molecules. 24:36242019. View Article : Google Scholar :
|
|
47
|
Donnan GA, Fisher M, Macleod M and Davis
SM: Stroke. Lancet. 371:1612–1623. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Georgiopoulos G, Ntaios G, Stamatelopoulos
K, Manios E, Korompoki E, Vemmou E, Milionis H, Masi S, Lip GYH and
Vemmos K: Comparison of risk scores for the prediction of the
overall cardiovascular risk in patients with ischemic stroke: The
athens stroke registry. J Stroke Cerebrovasc Dis. 28:1044152019.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Li D and Ai Y: Hydrogen saline suppresses
neuronal cell apoptosis and inhibits the p38 mitogenactivated
protein kinase-caspase3 signaling pathway following cerebral
ischemia-reperfusion injury. Mol Med Rep. 16:5321–5325. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Tan XF, Qin T, Li N, Yang YG, Zheng JH,
Xie L and Chen MH: High-potassium preconditioning enhances
tolerance to focal cerebral ischemia-reperfusion injury through
anti-apoptotic effects in male rats. J Neurosci Res. 97:1253–1265.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Dirnagl U, Iadecola C and Moskowitz MA:
Pathobiology of ischaemic stroke: An integrated view. Trends
Neurosci. 22:391–397. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ren Q, Hu Z, Jiang Y, Tan X, Botchway BOA,
Amin N, Lin G, Geng Y and Fang M: SIRT1 protects against apoptosis
by promoting autophagy in the oxygen glucose
deprivation/reperfusion-induced injury. Front Neurol. 10:12892019.
View Article : Google Scholar
|
|
53
|
Rogers KV, Dunn CK, Hebert SC and Brown
EM: Localization of calcium receptor mRNA in adult rat central
nervous system by in situ hybridization. Brain Res. 744:47–56.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ruat M, Molliver ME, Snowman AM and Snyder
SH: Calcium sensing receptor: Molecular cloning in rat and
localization to nerve terminals. Proc Natl Acad Sci USA.
92:3161–3165. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Bandyopadhyay S, Tfelt-Hansen J and
Chattopadhyay N: Diverse roles of extracellular calcium-sensing
receptor in the central nervous system. J Neurosci Res.
88:2073–2082. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ruat M and Traiffort E: Roles of the
calcium sensing receptor in the central nervous system. Best Pract
Res Clin Endocrinol Metab. 27:429–442. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Sun YH, Li YQ, Feng SL, Li BX, Pan ZW, Xu
CQ, Li TT and Yang BF: Calcium-sensing receptor activation
contributed to apoptosis stimulates TRPC6 channel in rat neonatal
ventricular myocytes. Biochem Biophys Res Commun. 394:955–961.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Tfelt-Hansen J, Hansen JL, Smajilovic S,
Terwilliger EF, Haunso S and Sheikh SP: Calcium receptor is
functionally expressed in rat neonatal ventricular cardiomyocytes.
Am J Physiol Heart Circ Physiol. 290:H1165–H1171. 2006. View Article : Google Scholar
|
|
59
|
Zhang G, Zhang T, Wu L, Zhou X, Gu J, Li
C, Liu W, Long C, Yang X, Shan L, et al: Neuroprotective effect and
mechanism of action of tetramethylpyrazine nitrone for ischemic
stroke therapy. Neuromolecular Med. 20:97–111. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Lončarević-Vasiljković N, Milanović D,
Pešić V, Tešić V, Brkić M, Lazić D, Avramović V and Kanazir S:
Dietary restriction suppresses apoptotic cell death, promotes Bcl-2
and Bcl-xl mRNA expression and increases the Bcl-2/Bax protein
ratio in the rat cortex after cortical injury. Neurochem Int.
96:69–76. 2016. View Article : Google Scholar
|
|
61
|
Zheng T, Jiang H, Jin R, Zhao Y, Bai Y, Xu
H and Chen Y: Ginsenoside Rg1 attenuates protein aggregation and
inflammatory response following cerebral ischemia and reperfusion
injury. Eur J Pharmacol. 853:65–73. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Yu H, Liu P, Tang H, Jing J, Lv X, Chen L,
Jiang L, Xu J and Li J: Oleuropein, a natural extract from plants,
offers neuroprotection in focal cerebral ischemia/reperfusion
injury in mice. Eur J Pharmacol. 775:113–119. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Wang Y, Luo J and Li SY: Nano-curcumin
simultaneously protects the blood-brain barrier and reduces M1
microglial activation during cerebral ischemia-reperfusion injury.
ACS Appl Mater Interfaces. 11:3763–3770. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Hou Y, Wang J and Feng J: The
neuroprotective effects of curcumin are associated with the
regulation of the reciprocal function between autophagy and HIF-1α
in cerebral ischemia-reperfusion injury. Drug Des Devel Ther.
13:1135–1144. 2019. View Article : Google Scholar :
|