|
1
|
World Health Organization (WHO):
Coronavirus disease (COVID-19) pandemic. https://www.who.int/emergencies/diseases/novel-coronavirus-2019urisimplehttps://www.who.int/emergencies/diseases/novel-coronavirus-2019.
Accessed November 2, 2020.
|
|
2
|
Liu YC, Kuo RL and Shih SR: COVID-19: The
first documented coronavirus pandemic in history. Biomed J.
43:328–333. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tsay SF, Kao CC, Wang HH and Lin CC:
Nursing's response to COVID-19: Lessons learned from SARS in
Taiwan. Int J Nurs Stud. 108:1035872020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chang CL and McAleer M: Alternative global
health security indexes for risk analysis of COVID-19. Int J
Environ Res Public Health. 17:31612020. View Article : Google Scholar :
|
|
5
|
Hsu YC, Liu YA, Lin MH, Lee HW, Chen TJ,
Chou LF and Hwang SJ: Visiting policies of hospice wards during the
COVID-19 pandemic: An environmental scan in Taiwan. Int J Environ
Res Public Health. 17:28572020. View Article : Google Scholar :
|
|
6
|
Schwartz J, King CC and Yen MY: Protecting
healthcare workers during the coronavirus disease 2019 (COVID-19)
outbreak: lessons from Taiwan's severe acute respiratory syndrome
response. Clin Infect Dis. 71:858–860. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu YC, Chen CS and Chan YJ: The outbreak
of COVID-19: An overview. J Chin Med Assoc. 83:217–220. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
U.S. Food & Drug Administration (FDA):
Coronavirus Disease 2019 (COVID-19). https://www.fda.gov/news-events/press-announcements/covid-19-update-fda-broadens-emergency-use-authorization-veklury-remdesivir-include-all-hospitalizedurisimplehttps://www.fda.gov/news-events/press-announcements/covid-19-update-fda-broadens-emergency-use-authorization-veklury-remdesivir-include-all-hospitalized.
Accessed August 28, 2020.
|
|
9
|
Park SE: Epidemiology, virology, and
clinical features of severe acute respiratory syndrome
-coronavirus-2 (SARS-CoV-2; Coronavirus Disease-19). Clin Exp
Pediatr. 63:119–124. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li Q, Guan X, Wu P, Wang X, Zhou L, Tong
Y, Ren R, Leung KSM, Lau EHY, Wong JY, et al: Early transmission
dynamics in Wuhan, China, of novel coronavirus-infected pneumonia.
N Engl J Med. 382:1199–1207. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Day M: Covid-19: Identifying and isolating
asymptomatic people helped eliminate virus in Italian village. BMJ.
3:m11652020. View Article : Google Scholar
|
|
12
|
Chang TH, Wu JL and Chang LY: Clinical
characteristics and diagnostic challenges of pediatric COVID-19: A
systematic review and meta-analysis. J Formos Med Assoc.
119:982–989. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z,
Xiang J, Wang Y, Song B, Gu X, et al: Clinical course and risk
factors for mortality of adult inpatients with COVID-19 in Wuhan,
China: A retrospective cohort study. Lancet. 395:1054–1062. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lin CT, Bookman K, Sieja A, Markley K,
Altman RL, Sippel J, Perica K, Reece L, Davis C, Horowitz E, et al:
Clinical informatics accelerates health system adaptation to the
COVID-19 pandemic: Examples from Colorado. J Am Med Inform Assoc.
Jul 20–2020.Epub ahead of print. View Article : Google Scholar
|
|
15
|
Liu X, Zhang R and He G: Hematological
findings in corona-virus disease 2019: Indications of progression
of disease. Ann Hematol. 99:1421–1428. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ippolito D, Maino C, Pecorelli A,
Allegranza P, Cangiotti C, Capodaglio C, Mariani I, Giandola T,
Gandola D, Bianco I, et al: Chest X-ray features of SARS-CoV-2 in
the emergency department: A multicenter experience from northern
Italian hospitals. Respir Med. 170:1060362020. View Article : Google Scholar : PubMed/NCBI
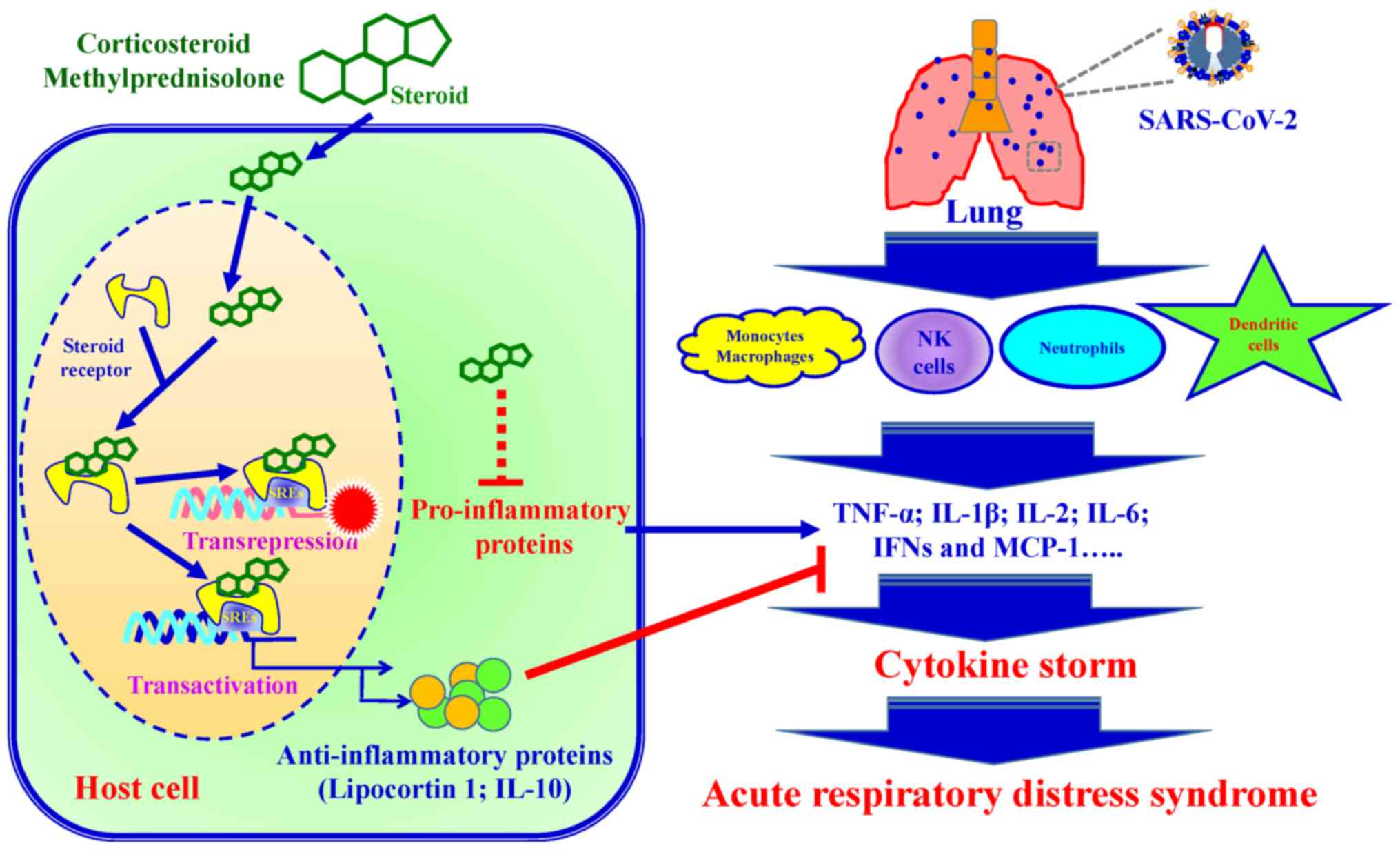
|
|
17
|
Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J,
Wang B, Xiang H, Cheng Z, Xiong Y, et al: Clinical characteristics
of 138 hospitalized patients with 2019 novel coronavirus-infected
pneumonia in Wuhan, China. JAMA. 323:1061–1069. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tu YF, Chien CS, Yarmishyn AA, Lin YY, Luo
YH, Lin YT, Lai WY, Yang DM, Chou SJ, Yang YP, et al: A review of
SARS-CoV-2 and the Ongoing Clinical Trials. Int J Mol Sci.
21:26572020. View Article : Google Scholar :
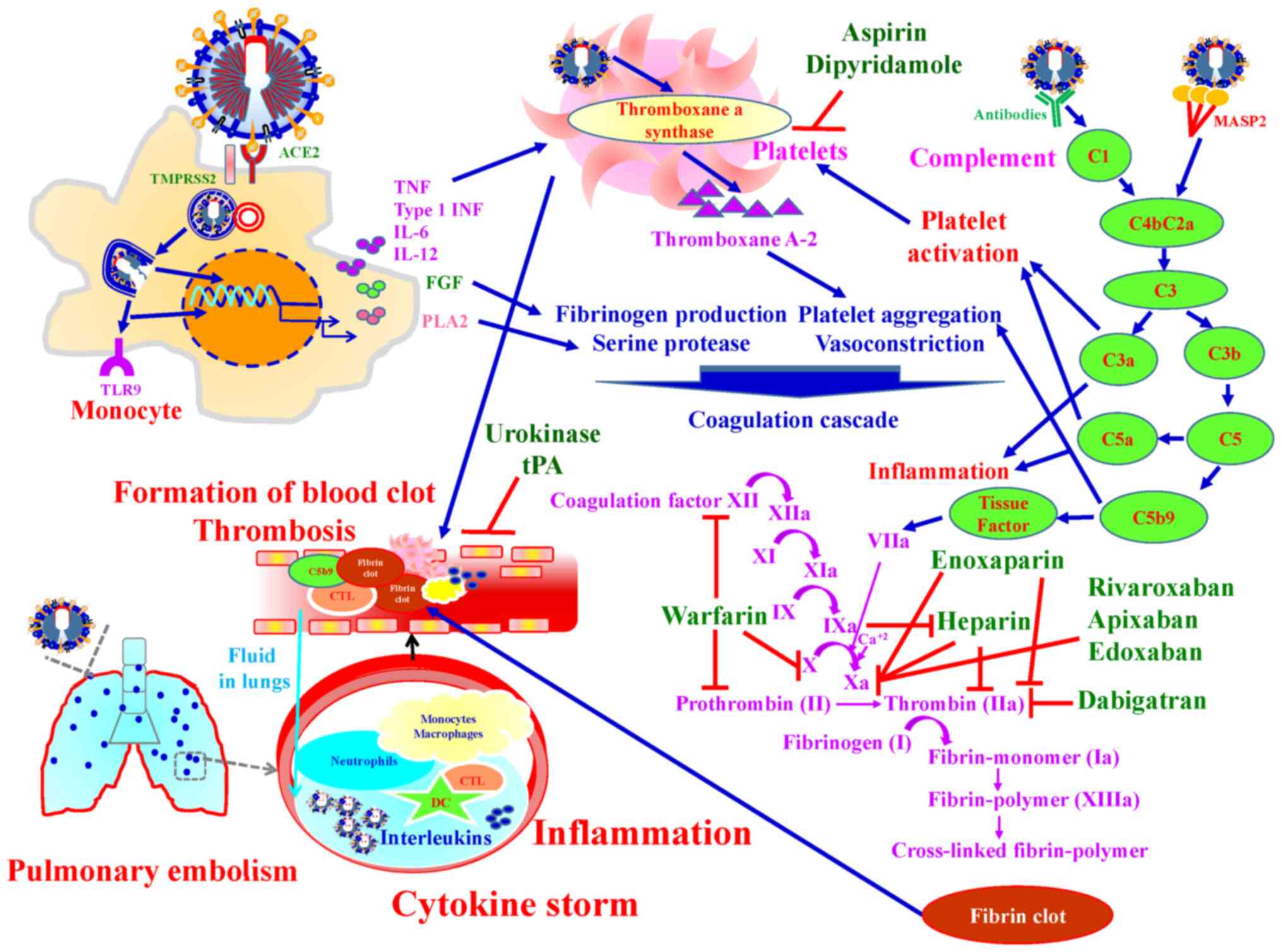
|
|
19
|
Davis B, Rothrock AN, Swetland S, Andris
H, Davis P and Rothrock SG: Viral and atypical respiratory
co-infections in COVID-19: A systematic review and meta-analysis. J
Am Coll Emerg Physicians Open. 1:533–548. 2020. View Article : Google Scholar
|
|
20
|
Oliva A, Siccardi G, Migliarini A,
Cancelli F, Carnevalini M, D'Andria M, Attilia I, Danese VC,
Cecchetti V, Romiti R, et al: Co-infection of SARS-CoV-2 with
Chlamydia or Mycoplasma pneumoniae: A case series and review of the
literature. Infection. Jul 28–2020.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Assiri A, Al-Tawfiq JA, Al-Rabeeah AA,
Al-Rabiah FA, Al-Hajjar S, Al-Barrak A, Flemban H, Al-Nassir WN,
Balkhy HH, Al-Hakeem RF, et al: Epidemiological, demographic, and
clinical characteristics of 47 cases of Middle East respiratory
syndrome coronavirus disease from Saudi Arabia: A descriptive
study. Lancet Infect Dis. 13:752–761. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Phan LT, Nguyen TV, Luong QC, Nguyen TV,
Nguyen HT, Le HQ, Nguyen TT, Cao TM and Pham QD: Importation and
human-to-human transmission of a novel coronavirus in Vietnam. N
Engl J Med. 382:872–874. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rothan HA and Byrareddy SN: The
epidemiology and pathogenesis of coronavirus disease (COVID-19)
outbreak. J Autoimmun. 109:1024332020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tan L, Wang Q, Zhang D, Ding J, Huang Q,
Tang YQ, Wang Q and Miao H: Lymphopenia predicts disease severity
of COVID-19: A descriptive and predictive study. Signal Transduct
Target Ther. 5:332020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Su YJ and Lai YC: Comparison of clinical
characteristics of coronavirus disease (COVID-19) and severe acute
respiratory syndrome (SARS) as experienced in Taiwan. Travel Med
Infect Dis. 36:1016252020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sung YY, Wu YC, Li CY, Hsu CY, Hsu CY,
Liang ST, Huang WC, Pan KY, Tsai JH and Yen YH: Interim Guidelines
for Clinical Management of SARS-CoV-2 Infection (5th edition)
Ministry of Health and Welfare. Taiwan: Centers for Disease
Control; 2020, https://www.cdc.gov.tw/File/Get/-ewtg9-RCAetCPKR4_rnCwurisimplehttps://www.cdc.gov.tw/File/Get/-ewtg9-RCAetCPKR4_rnCw.
Accessed March 26, 2020.
|
|
27
|
Wu A, Peng Y, Huang B, Ding X, Wang X, Niu
P, Meng J, Zhu Z, Zhang Z, Wang J, et al: Genome composition and
divergence of the novel coronavirus (2019-nCoV) originating in
China. Cell Host Microbe. 27:325–328. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Singhal T: A Review of coronavirus
disease-2019 (COVID-19). Indian J Pediatr. 87:281–286. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li G and De Clercq E: Therapeutic options
for the 2019 novel coronavirus (2019-nCoV). Nat Rev Drug Discov.
19:149–150. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
National Institutes of Health (NIH),
National Center for Biotechnology Information (NCBI): NCBI
SARS-CoV-2 Resources. https://www.ncbi.nlm.nih.gov/Structure/SARS-CoV-2.htmlurisimplehttps://www.ncbi.nlm.nih.gov/Structure/SARS-CoV-2.html.
Accessed October 27, 2020.
|
|
31
|
Hoffmann M, Kleine-Weber H and Pöhlmann S:
A multibasic cleavage site in the spike protein of SARS-CoV-2 is
essential for infection of human lung cells. Mol Cell.
78:779–784.e5. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schoeman D and Fielding BC: Is there a
link between the pathogenic human coronavirus envelope protein and
immunopathology? A review of the literature. Front Microbiol.
11:20862020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Malik YA: Properties of coronavirus and
SARS-CoV-2. Malays J Pathol. 42:3–11. 2020.PubMed/NCBI
|
|
34
|
Mu J, Xu J, Zhang L, Shu T, Wu D, Huang M,
Ren Y, Li X, Geng Q, Xu Y, et al: SARS-CoV-2-encoded nucleocapsid
protein acts as a viral suppressor of RNA interference in cells.
Sci China Life Sci. 63:1–4. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Baez-Santos YM, Mielech AM, Deng X, Baker
S and Mesecar AD: Catalytic function and substrate specificity of
the papain-like protease domain of nsp3 from the Middle East
respiratory syndrome coronavirus. J Virol. 88:12511–12527. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sakai Y, Kawachi K, Terada Y, Omori H,
Matsuura Y and Kamitani W: Two-amino acids change in the nsp4 of
SARS coronavirus abolishes viral replication. Virology.
510:165–174. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Angelini MM, Akhlaghpour M, Neuman BW and
Buchmeier MJ: Severe acute respiratory syndrome coronavirus
nonstructural proteins 3, 4, and 6 induce double-membrane vesicles.
mBio. 4:e00524–13. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Snijder EJ, Decroly E and Ziebuhr J: The
nonstructural proteins directing coronavirus RNA synthesis and
processing. Adv Virus Res. 96:59–126. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rathnasinghe R, Karlicek RF, Schotsaert M,
Koffas MA, Arduini B, Jangra S, Wang B, Davis JL, Alnaggar M, Costa
A, et al: Scalable, effective, and rapid decontamination of
SARS-CoV-2 contaminated N95 respirators using germicidal
ultra-violet C (UVC) irradiation device. medRxiv. View Article : Google Scholar
|
|
40
|
Boopathi S, Poma AB and Kolandaivel P:
Novel 2019 corona-virus structure, mechanism of action, antiviral
drug promises and rule out against its treatment. J Biomol Struct
Dyn. Apr 30–2020.Epub ahead of print. View Article : Google Scholar
|
|
41
|
Verdecchia P, Cavallini C, Spanevello A
and Angeli F: The pivotal link between ACE2 deficiency and
SARS-CoV-2 infection. Eur J Intern Med. 76:14–20. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen WH, Strych U, Hotez PJ and Bottazzi
ME: The SARS-CoV-2 vaccine pipeline: An overview. Curr Trop Med
Rep. Mar 3–2020.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chan VS, Chan KY, Chen Y, Poon LL, Cheung
AN, Zheng B, Chan KH, Mak W, Ngan HY, Xu X, et al: Homozygous
L-SIGN (CLEC4M) plays a protective role in SARS coronavirus
infection. Nat Genet. 38:38–46. 2006. View
Article : Google Scholar
|
|
44
|
Jeffers SA, Tusell SM, Gillim-Ross L,
Hemmila EM, Achenbach JE, Babcock GJ, Thomas WD Jr, Thackray LB,
Young MD, Mason RJ, et al: CD209L (L-SIGN) is a receptor for severe
acute respiratory syndrome coronavirus. Proc Natl Acad Sci USA.
101:15748–15753. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
McKee DL, Sternberg A, Stange U, Laufer S
and Naujokat C: Candidate drugs against SARS-CoV-2 and COVID-19.
Pharmacol Res. 157:1048592020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Stahlmann R and Lode H: Medication for
COVID-19-an over-view of approaches currently under study. Dtsch
Arztebl Int. 117:213–219. 2020.PubMed/NCBI
|
|
47
|
Kupferschmidt K and Cohen J: Race to find
COVID-19 treatments accelerates. Science. 367:1412–1413. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yip CC, Ho CC, Chan JF, To KK, Chan HS,
Wong SC, Leung KH, Fung AY, Ng AC, Zou Z, et al: Development of a
novel, genome subtraction-derived, SARS-CoV-2-specific
COVID-19-nsp2 real-time RT-PCR assay and its evaluation using
clinical specimens. Int J Mol Sci. 21:25742020. View Article : Google Scholar :
|
|
49
|
Yan C, Cui J, Huang L, Du B, Chen L, Xue
G, Li S, Zhang W, Zhao L, Sun Y, et al: Rapid and visual detection
of 2019 novel coronavirus (SARS-CoV-2) by a reverse transcription
loop-mediated isothermal amplification assay. Clin Microbiol
Infect. 26:773–779. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Rahman H, Carter I, Basile K, Donovan L,
Kumar S, Tran T, Ko D, Alderson S, Sivaruban T, Eden JS, et al:
Interpret with caution: An evaluation of the commercial
AusDiagnostics versus in-house developed assays for the detection
of SARS-CoV-2 virus. J Clin Virol. 127:1043742020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Pujadas E, Ibeh N, Hernandez MM, Waluszko
A, Sidorenko T, Flores V, Shiffrin B, Chiu N, Young-Francois A,
Nowak MD, et al: Comparison of SARS-CoV-2 detection from
nasopharyngeal swab samples by the Roche cobas(R) 6800 SARS-CoV-2
test and a laboratory-developed real-time RT-PCR test. J Med Virol.
May 8–2020.Epub ahead of print. View Article : Google Scholar
|
|
52
|
Montesinos I, Gruson D, Kabamba B, Dahma
H, Van den Wijngaert S, Reza S, Carbone V, Vandenberg O, Gulbis B,
Wolff F and Rodriguez-Villalobos H: Evaluation of two automated and
three rapid lateral flow immunoassays for the detection of
anti-SARS-CoV-2 antibodies. J Clin Virol. 128:1044132020.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Thabet L, Mhalla S, Naija H, Jaoua MA,
Hannachi N, Fki-Berrajah L, Toumi A and Karray-Hakim H: SARS-CoV-2
infection virological diagnosis. Tunis Med. 98:304–308.
2020.PubMed/NCBI
|
|
54
|
Vásárhelyi B, Kristóf K, Ostorházi E,
Szabó D, Prohászka Z and Merkely B: The diagnostic value of rapid
anti IgM and IgG detecting tests in the identification of patients
with SARS CoV-2 virus infection. Orv Hetil. 161:807–812. 2020.In
Hungarian. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Green K, Graziadio S, Turner P, Fanshawe T
and Allen J: Molecular and antibody point-of-care tests to support
the screening, diagnosis and monitoring of COVID-19. Centre for
Evidence- Based Medicine (CEBM); Oxford; 2020, https://www.cebm.net/covid-19/molecular-and-antibody-point-of-care-tests-to-support-the-screening-diagnosis-and-monitoring-of-covid-19/urisimplehttps://www.cebm.net/covid-19/molecular-and-antibody-point-of-care-tests-to-support-the-screening-diagnosis-and-monitoring-of-covid-19/.
Accessed April 7, 2020.
|
|
56
|
Xue X, Zhu C, Huang S, Pan L, Xu J and Li
W: Effect of heat inactivation of blood samples on the efficacy of
three detection methods of SARS-CoV-2 antibodies. Nan Fang Yi Ke Da
Xue Xue Bao. 40:316–320. 2020.In Chinese. PubMed/NCBI
|
|
57
|
Huang P, Liu T, Huang L, Liu H, Lei M, Xu
W, Hu X, Chen J and Liu B: Use of chest CT in combination with
negative RT-PCR assay for the 2019 novel coronavirus but high
clinical suspicion. Radiology. 295:22–23. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wang P: Combination of serological total
antibody and RT-PCR test for detection of SARS-COV-2 infections. J
Virol Methods. 283:1139192020. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Lange C, Wolf J, Auw-Haedrich C, Schlecht
A, Boneva S, Lapp T, Horres R, Agostini H, Martin G, Reinhard T and
Schlunck G: Expression of the COVID-19 receptor ACE2 in the human
conjunctiva. J Med Virol. May 6–2020.Epub ahead of print.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zhou J, Li C, Liu X, Chiu MC, Zhao X, Wang
D, Wei Y, Lee A, Zhang AJ, Chu H, et al: Infection of bat and human
intestinal organoids by SARS-CoV-2. Nat Med. 26:1077–1083. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Ziegler CGK, Allon SJ, Nyquist SK, Mbano
IM, Miao VN, Tzouanas CN, Cao Y, Yousif AS, Bals J, Hauser BM, et
al: SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in
human airway epithelial cells and is detected in specific cell
subsets across tissues. Cell. 181:1016–1035.e19. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Rahman N, Basharat Z, Yousuf M, Castaldo
G, Rastrelli L and Khan H: Virtual screening of natural products
against type II transmembrane serine protease (TMPRSS2), the
priming agent of coronavirus 2 (SARS-CoV-2). Molecules.
25:22712020. View Article : Google Scholar :
|
|
63
|
Huang J, Song W, Huang H and Sun Q:
Pharmacological therapeutics targeting RNA-dependent RNA
polymerase, proteinase and spike protein: From mechanistic studies
to clinical trials for COVID-19. J Clin Med. 9:11312020. View Article : Google Scholar :
|
|
64
|
ClinicalTrials.gov: NUnlom: COVID-19.
https://clinicaltrials.gov/ct2/results?cond=covid-19urisimplehttps://clinicaltrials.gov/ct2/results?cond=covid-19.
Accessed August 17, 2020.
|
|
65
|
Amawi H, Abu Deiab GI, A Aljabali AA, Dua
K and Tambuwala MM: COVID-19 pandemic: An overview of epidemiology,
pathogenesis, diagnostics and potential vaccines and therapeutics.
Ther Deliv. 11:245–268. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Vankadari N: Arbidol: A potential
antiviral drug for the treatment of SARS-CoV-2 by blocking
trimerization of the spike glycoprotein. Int J Antimicrob Agents.
56:1059982020. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Zhu Z, Lu Z, Xu T, Chen C, Yang G, Zha T,
Lu J and Xue Y: Arbidol monotherapy is superior to
lopinavir/ritonavir in treating COVID-19. J Infect. 81:e21–e23.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Deng L, Li C, Zeng Q, Liu X, Li X, Zhang
H, Hong Z and Xia J: Arbidol combined with LPV/r versus LPV/r alone
against Corona Virus Disease 2019: A retrospective cohort study. J
Infect. 81:e1–e5. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Wang X, Cao R, Zhang H, Liu J, Xu M, Hu H,
Li Y, Zhao L, Li W, Sun X, et al: The anti-influenza virus drug,
arbidol is an efficient inhibitor of SARS-CoV-2 in vitro. Cell
Discov. 6:282020. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Dong L, Hu S and Gao J: Discovering drugs
to treat coronavirus disease 2019 (COVID-19). Drug Discov Ther.
14:58–60. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Hulseberg CE, Fénéant L, Szymańska-de Wijs
KM, Kessler NP, Nelson EA, Shoemaker CJ, Schmaljohn CS, Polyak SJ
and White JM: Arbidol and other low-molecular-weight drugs that
inhibit lassa and Ebola viruses. J Virol. 93:e02185–18. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Kadam RU and Wilson IA: Structural basis
of influenza virus fusion inhibition by the antiviral drug Arbidol.
Proc Natl Acad Sci USA. 114:206–214. 2017. View Article : Google Scholar
|
|
73
|
Zeng LY, Yang J and Liu S: Investigational
hemagglutinin-targeted influenza virus inhibitors. Expert Opin
Investig Drugs. 26:63–73. 2017. View Article : Google Scholar
|
|
74
|
Roshanravan N, Ghaffari S and Hedayati M:
Angiotensin converting enzyme-2 as therapeutic target in COVID-19.
Diabetes Metab Syndr. 14:637–639. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Hamming I, Timens W, Bulthuis ML, Lely AT,
Navis G and van Goor H: Tissue distribution of ACE2 protein, the
functional receptor for SARS coronavirus. A first step in
understanding SARS pathogenesis. J Pathol. 203:631–637. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Xu J and Lazartigues E: Expression of ACE2
in human neurons supports the neuro-invasive potential of COVID-19
virus. Cell Mol Neurobiol. Jul 4–2020.Epub ahead of print.
View Article : Google Scholar :
|
|
77
|
Wedell J, Banzhaf G, Meier zu Eissen P and
Schlageter M: Experiences with a subcutaneous, fully resorbable
bridge in construction a double loop ileo- and colostomy. Chirurg.
61:36–38. 1990.In German. PubMed/NCBI
|
|
78
|
Li MY, Li L, Zhang Y and Wang XS:
Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide
variety of human tissues. Infect Dis Poverty. 9:452020. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Zhang X, Zheng J, Yan Y, Ruan Z, Su Y,
Wang J, Huang H, Zhang Y, Wang W, Gao J, et al:
Angiotensin-converting enzyme 2 regulates autophagy in acute lung
injury through AMPK/mTOR signaling. Arch Biochem Biophys.
672:1080612019. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
de Moraes PL, Kangussu LM, Castro CH,
Almeida AP, Santos RAS and Ferreira AJ: Vasodilator effect of
angiotensin-(17) on vascular coronary bed of rats: Role of Mas, ACE
and ACE2. Protein Pept Lett. 24:869–875. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Basu R, Poglitsch M, Yogasundaram H,
Thomas J, Rowe BH and Oudit GY: Roles of angiotensin peptides and
recombinant human ACE2 in heart failure. J Am Coll Cardiol.
69:805–819. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Qiao B and Olvera de la Cruz M: Enhanced
binding of SARS-CoV-2 spike protein to receptor by distal polybasic
cleavage sites. ACS Nano. 14:10616–10623. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Kruse RL: Therapeutic strategies in an
outbreak scenario to treat the novel coronavirus originating in
Wuhan, China. F1000Res. 9:722020. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Zhang H, Penninger JM, Li Y, Zhong N and
Slutsky AS: Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2
receptor: Molecular mechanisms and potential therapeutic target.
Intensive Care Med. 46:586–590. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Mejia Torres RE, Banegas EI, Mendoza M,
Diaz C, Bucheli ST, Fontecha GA, Alam MT, Goldman I, Udhayakumar V
and Zambrano JO: Efficacy of chloroquine for the treatment of
uncomplicated Plasmodium falciparum malaria in Honduras. Am J Trop
Med Hyg. 88:850–854. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Taherian E, Rao A, Malemud CJ and Askari
AD: The biological and clinical activity of anti-malarial drugs in
autoimmune disorders. Curr Rheumatol Rev. 9:45–62. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Pastick KA, Okafor EC, Wang F, Lofgren SM,
Skipper CP, Nicol MR, Pullen MF, Rajasingham R, McDonald EG, Lee
TC, et al: Review: Hydroxychloroquine and chloroquine for treatment
of SARS-CoV-2 (COVID-19). Open Forum Infect Dis. 7:ofaa1302020.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Piszczatoski CR and Powell J: Emergency
approval of chloroquine and hydroxychloroquine for treatment of
COVID-19. Ann Pharmacother. 54:827–831. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Shukla AM, Archibald LK, Shukla AW, Mehta
HJ and Cherabuddi K: Chloroquine and hydroxychloroquine in the
context of COVID-19. Drugs Context. 9(2020)–4. –5. 2020. View Article : Google Scholar
|
|
90
|
Sturrock BR and Chevassut TJ: Chloroquine
and COVID-19-a potential game changer? Clin Med (Lond). 20:278–281.
2020. View Article : Google Scholar
|
|
91
|
Colson P, Rolain JM, Lagier JC, Brouqui P
and Raoult D: Chloroquine and hydroxychloroquine as available
weapons to fight COVID-19. Int J Antimicrob Agents. 55:1059322020.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Annangi S: Chloroquine and
hydroxychloroquine for COVID-19: A word of caution. Respirology.
25:683–684. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Ferner RE and Aronson JK: Chloroquine and
hydroxychloroquine in covid-19. BMJ. 369:m14322020. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Badyal DK and Mahajan R: Chloroquine: Can
it be a novel drug for COVID-19. Int J Appl Basic Med Res.
10:128–130. 2020.PubMed/NCBI
|
|
95
|
Hu TY, Frieman M and Wolfram J: Insights
from nano-medicine into chloroquine efficacy against COVID-19. Nat
Nanotechnol. 15:247–249. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Ren Y, Yao MC, Huo XQ, Gu Y, Zhu WX, Qiao
YJ and Zhang YL: Study on treatment of 'cytokine storm' by
anti-2019-nCoV prescriptions based on arachidonic acid metabolic
pathway. Zhongguo Zhong Yao Za Zhi. 45:1225–1231. 2020.In Chinese.
PubMed/NCBI
|
|
97
|
McGonagle D, Sharif K, O'Regan A and
Bridgewood C: The role of cytokines including interleukin-6 in
COVID-19 induced pneumonia and macrophage activation syndrome-like
disease. Autoimmun Rev. 19:1025372020. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Lagunas-Rangel FA and Chávez-Valencia V:
High IL-6/IFN-γ ratio could be associated with severe disease in
COVID-19 patients. J Med Virol. Apr 16–2020.Epub ahead of print.
View Article : Google Scholar
|
|
99
|
Aizawa T, Imaizumi T, Hirono K, Watanabe
S, Tsugawa K and Tanaka H: Chloroquine attenuates TLR3-mediated
plasminogen activator inhibitor-1 expression in cultured human
glomerular endothelial cells. Clin Exp Nephrol. 23:448–454. 2019.
View Article : Google Scholar
|
|
100
|
Clancy RM, Markham AJ, Reed JH, Blumenberg
M, Halushka MK and Buyon JP: Targeting downstream transcription
factors and epigenetic modifications following Toll-like receptor
7/8 ligation to forestall tissue injury in anti-Ro60 associated
heart block. J Autoimmun. 67:36–45. 2016. View Article : Google Scholar :
|
|
101
|
Mahase E: Hydroxychloroquine for covid-19:
The end of the line? BMJ. 369:m23782020. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Wu R, Wang L, Kuo HD, Shannar A, Peter R,
Chou PJ, Li S, Hudlikar R, Liu X, Liu Z, et al: An update on
current therapeutic drugs treating COVID-19. Curr Pharmacol Rep.
May 11–2020.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Ahsan W, Javed S, Bratty MA, Alhazmi HA
and Najmi A: Treatment of SARS-CoV-2: How far have we reached? Drug
Discov Ther. 14:67–72. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Du YX and Chen XP: Favipiravir:
Pharmacokinetics and concerns about clinical trials for 2019-nCoV
infection. Clin Pharmacol Ther. 108:242–247. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Reina J: Remdesivir, the antiviral hope
against SARS-CoV-2. Rev Esp Quimioter. 33:176–179. 2020.In Spanish.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Cao YC, Deng QX and Dai SX: Remdesivir for
severe acute respiratory syndrome coronavirus 2 causing COVID-19:
An evaluation of the evidence. Travel Med Infect Dis.
35:1016472020. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Augustin M, Hallek M and Nitschmann S:
Remdesivir for patients with severe COVID-19. Internist (Berl).
61:644–645. 2020.In German. View Article : Google Scholar
|
|
108
|
Li Z, Wang X, Cao D, Sun R, Li C and Li G:
Rapid review for the anti-coronavirus effect of remdesivir. Drug
Discov Ther. 14:73–76. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Jean SS, Lee PI and Hsueh PR: Treatment
options for COVID-19: The reality and challenges. J Microbiol
Immunol Infect. 53:436–443. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Lu CC, Chen MY, Lee WS and Chang YL:
Potential therapeutic agents against COVID-19: What we know so far.
J Chin Med Assoc. 83:534–536. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Simsek Yavuz S and Ünal S: Antiviral
treatment of COVID-19. Turk J Med Sci. 50:611–619. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Choy KT, Wong AY, Kaewpreedee P, Sia SF,
Chen D, Hui KPY, Chu DKW, Chan MCW, Cheung PP, Huang X, et al:
Remdesivir, lopinavir, emetine, and homoharringtonine inhibit
SARS-CoV-2 replication in vitro. Antiviral Res. 178:1047862020.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
U.S. Food & Drug Administration (FDA):
Coronavirus (COVID-19) Update: FDA issues emergency use
authorization for potential COVID-19 treatment. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-issues-emergency-use-authorization-potential-covid-19-treatmenturisimplehttps://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-issues-emergency-use-authorization-potential-covid-19-treatment.
Accessed May 1, 2020.
|
|
114
|
Chan KW, Wong VT and Tang SCW: COVID-19:
An update on the epidemiological, clinical, preventive and
therapeutic evidence and Guidelines of integrative Chinese-Western
medicine for the management of 2019 novel coronavirus disease. Am J
Chin Med. 48:737–762. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Martinez MA: Compounds with therapeutic
potential against novel respiratory 2019 coronavirus. Antimicrob
Agents Chemother. 64:e0039920–2020. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Guzik TJ, Mohiddin SA, Dimarco A, Patel V,
Savvatis K, Marelli-Berg FM, Madhur MS, Tomaszewski M, Maffia P,
D'Acquisto F, et al: COVID-19 and the cardiovascular system:
Implications for risk assessment, diagnosis, and treatment options.
Cardiovasc Res. 116:1666–1687. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Elfiky AA: Ribavirin, remdesivir,
sofosbuvir, galidesivir, and tenofovir against SARS-CoV-2 RNA
dependent RNA polymerase (RdRp): A molecular docking study. Life
Sci. 253:1175922020. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Elfiky AA: Anti-HCV, nucleotide
inhibitors, repurposing against COVID-19. Life Sci. 248:1174772020.
View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Costanzo M, De Giglio MAR and Roviello GN:
SARS-CoV-2: Recent reports on antiviral therapies based on
lopinavir/rito-navir, darunavir/umifenovir, hydroxychloroquine,
remdesivir, favipiravir and other drugs for the treatment of the
new coronavirus. Curr Med Chem. 27:4536–4541. 2020. View Article : Google Scholar
|
|
120
|
Ye XT, Luo YL, Xia SC, Sun QF, Ding JG,
Zhou Y, Chen W, Wang XF, Zhang WW, Du WJ, et al: Clinical efficacy
of lopinavir/ritonavir in the treatment of Coronavirus disease
2019. Eur Rev Med Pharmacol Sci. 24:3390–3396. 2020.PubMed/NCBI
|
|
121
|
Sallard E, Lescure FX, Yazdanpanah Y,
Mentre F and Peiffer-Smadja N: Type 1 interferons as a potential
treatment against COVID-19. Antiviral Res. 178:1047912020.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Sauñe PM, Bryce-Alberti M,
Portmann-Baracco AS and Accinelli RA: Methylprednisolone pulse
therapy: An alternative management of severe COVID-19. Respir Med
Case Rep. 31:1012212020.PubMed/NCBI
|
|
123
|
Yang JW, Yang L, Luo RG and Xu JF:
Corticosteroid administration for viral pneumonia: COVID-19 and
beyond. Clin Microbiol Infect. 26:1171–1177. 2020. View Article : Google Scholar :
|
|
124
|
Li SF, Gong MJ, Zhao FR, Shao JJ, Xie YL,
Zhang YG and Chang HY: Type I interferons: Distinct biological
activities and current applications for viral infection. Cell
Physiol Biochem. 51:2377–2396. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Andreakos E and Tsiodras S: COVID-19:
Lambda interferon against viral load and hyperinflammation. EMBO
Mol Med. 12:e124652020. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Du B, Qiu HB, Zhan X, Wang YS, Kang HYJ,
Li XY, Wang F, Sun B and Tong ZH: Pharmacotherapeutics for the New
Coronavirus Pneumonia. Zhonghua Jie He He Hu Xi Za Zhi.
43:E0122020.In Chinese.
|
|
127
|
Vidal P: Interferon α in cancer
immunoediting: From elimination to escape. Scand J Immunol.
91:e128632020. View Article : Google Scholar
|
|
128
|
Nelemans T and Kikkert M: Viral innate
immune evasion and the pathogenesis of emerging RNA virus
infections. Viruses. 11:9612019. View Article : Google Scholar :
|
|
129
|
Abdul-Sater AA, Majoros A, Plumlee CR,
Perry S, Gu AD, Lee C, Shresta S, Decker T and Schindler C:
Different STAT transcription complexes drive early and delayed
responses to type I IFNs. J Immunol. 195:210–216. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Langevin C, Aleksejeva E, Passoni G, Palha
N, Levraud JP and Boudinot P: The antiviral innate immune response
in fish: Evolution and conservation of the IFN system. J Mol Biol.
425:4904–4920. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Zhou Q, Wei XS, Xiang X, Wang X, Wang ZH,
Chen V, Shannon CP, Tebbutt SJ, Kollmann TR and Fish EN:
Interferon-a2b treatment for COVID-19. medRxiv. https://www.101101/2020040620042580urisimplehttps://101101/2020040620042580.
|
|
132
|
Morgenstern B, Michaelis M, Baer PC, Doerr
HW and Cinatl J Jr: Ribavirin and interferon-beta synergistically
inhibit SARS-associated coronavirus replication in animal and human
cell lines. Biochem Biophys Res Commun. 326:905–908. 2005.
View Article : Google Scholar
|
|
133
|
Mantlo E, Bukreyeva N, Maruyama J,
Paessler S and Huang C: Antiviral activities of type I interferons
to SARS-CoV-2 infection. Antiviral Res. 179:1048112020. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Young MJ, Clyne CD and Chapman KE:
Endocrine aspects of ACE2 regulation: RAAS, steroid hormones and
SARS-CoV-2. J Endocrinol. 247:R45–R62. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Xavier AM, Anunciato AK, Rosenstock TR and
Glezer I: Gene expression control by glucocorticoid receptors
during innate immune responses. Front Endocrinol (Lausanne).
7:312016. View Article : Google Scholar
|
|
136
|
Hardy RS, Raza K and Cooper MS:
Therapeutic glucocorticoids: Mechanisms of actions in rheumatic
diseases. Nat Rev Rheumatol. 16:133–144. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Song P, Li W, Xie J, Hou Y and You C:
Cytokine storm induced by SARS-CoV-2. Clin Chim Acta. 509:280–287.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Komiyama M and Hasegawa K: Anticoagulant
therapy for patients with coronavirus disease 2019: Urgent need for
enhanced awareness. Eur Cardiol. 15:e582020. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Merrill JT, Erkan D, Winakur J and James
JA: Emerging evidence of a COVID-19 thrombotic syndrome has
treatment implications. Nat Rev Rheumatol. 16:581–589. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Fletcher-Sandersjöö A and Bellander BM: Is
COVID-19 associated thrombosis caused by overactivation of the
complement cascade? A literature review. Thromb Res. 194:36–41.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Conway EM and Pryzdial ELG: Is the
COVID-19 thrombotic catastrophe complement-connected? J Thromb
Haemost. Aug 6–2020.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Furie B and Furie BC: Mechanisms of
thrombus formation. N Engl J Med. 359:938–949. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Giannis D, Ziogas IA and Gianni P:
Coagulation disorders in coronavirus infected patients: COVID-19,
SARS-CoV-1, MERS-CoV and lessons from the past. J Clin Virol.
127:1043622020. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Bikdeli B, Madhavan MV, Jimenez D, Chuich
T, Dreyfus I, Driggin E, Nigoghossian C, Ageno W, Madjid M, Guo Y,
et al: COVID-19 and thrombotic or thromboembolic disease:
Implications for prevention, antithrombotic therapy, and follow-up:
JACC State-of-the-Art review. J Am Coll Cardiol. 75:2950–2973.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Liu J, Li J, Arnold K, Pawlinski R and Key
NS: Using heparin molecules to manage COVID-2019. Res Pract Thromb
Haemost. 4:518–523. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Panka BA, de Grooth HJ, Spoelstra-de Man
AM, Looney MR and Tuinman PR: Prevention or treatment of ards with
aspirin: A review of preclinical models and meta-analysis of
clinical studies. Shock. 47:13–21. 2017. View Article : Google Scholar
|
|
147
|
Chen W, Janz DR, Bastarache JA, May AK,
O'Neal HR Jr, Bernard GR and Ware LB: Prehospital aspirin use is
associated with reduced risk of acute respiratory distress syndrome
in critically ill patients: A propensity-adjusted analysis. Crit
Care Med. 43:801–807. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Li Z, Li X, Huang YY, Wu Y, Liu R, Zhou L,
Lin Y, Wu D, Zhang L, Liu H, et al: Identify potent SARS-CoV-2 main
protease inhibitors via accelerated free energy perturbation-based
virtual screening of existing drugs. Proc Natl Acad Sci USA.
117:27381–27387. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Whyte CS, Morrow GB, Mitchell JL, Chowdary
P and Mutch NJ: Fibrinolytic abnormalities in acute respiratory
distress syndrome (ARDS) and versatility of thrombolytic drugs to
treat COVID-19. J Thromb Haemost. 18:1548–1555. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Ju B, Zhang Q, Ge J, Wang R, Sun J, Ge X,
Yu J, Shan S, Zhou B, Song S, et al: Human neutralizing antibodies
elicited by SARS-CoV-2 infection. Nature. 584:115–119. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Graham BS: Rapid COVID-19 vaccine
development. Science. 368:945–946. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Shepard HM, Phillips GL, D Thanos C and
Feldmann M: Developments in therapy with monoclonal antibodies and
related proteins. Clin Med (Lond). 17:220–232. 2017. View Article : Google Scholar
|
|
153
|
Thanh Le T, Andreadakis Z, Kumar A, Gómez
Román R, Tollefsen S, Saville M and Mayhew S: The COVID-19 vaccine
development landscape. Nat Rev Drug Discov. 19:305–306. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Corey L, Mascola JR, Fauci AS and Collins
FS: A strategic approach to COVID-19 vaccine R&D. Science.
368:948–950. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Zhao Z, Li Y, Zhou L, Zhou X, Xie B, Zhang
W and Sun J: Prevention and treatment of COVID-19 using Traditional
Chinese Medicine: A review. Phytomedicine. 153308:2020.
|
|
156
|
Zhang YS, Cong WH, Zhang JJ, Guo FF and Li
HM: Research progress of intervention of Chinese herbal medicine
and its active components on human coronavirus. Zhongguo Zhong Yao
Za Zhi. 45:1263–1271. 2020.In Chinese. PubMed/NCBI
|
|
157
|
McKimm-Breschkin JL, Jiang S, Hui DS,
Beigel JH, Govorkova EA and Lee N: Prevention and treatment of
respiratory viral infections: Presentations on antivirals,
traditional therapies and host-directed interventions at the 5th
ISIRV Antiviral Group conference. Antiviral Res. 149:118–142. 2018.
View Article : Google Scholar
|
|
158
|
Teschke R, Larrey D, Melchart D and Danan
G: Traditional Chinese Medicine (TCM) and herbal hepatotoxicity:
RUCAM and the role of novel diagnostic biomarkers such as
MicroRNAs. Medicines (Basel). 3. pp. 182016, View Article : Google Scholar
|
|
159
|
Hu XY, Logue M and Robinson N:
Antimicrobial resistance is a global problem - a UK perspective.
Eur J Integr Med. 36:1011362020. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Ho LTF, Chan KKH, Chung VCH and Leung TH:
Highlights of traditional Chinese medicine frontline expert advice
in the China national guideline for COVID-19. Eur J Integr Med.
36:1011162020. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Porte L, Legarraga P, Vollrath V, Aguilera
X, Munita JM, Araos R, Pizarro G, Vial P, Iruretagoyena M, Dittrich
S and Weitzel T: Evaluation of a novel antigen-based rapid
detection test for the diagnosis of SARS-CoV-2 in respiratory
samples. Int J Infect Dis. 99:328–333. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Mirijello A, D'Errico MM, Lamarca A,
Piscitelli P and De Cosmo S: Comment on Matricardi PM et al: The
first, holistic immunological model of COVID-19: Implications for
prevention, diagnosis, and public health measures. Pediatr Allergy
Immunol. May 17–2020.Epub ahead of print. View Article : Google Scholar
|