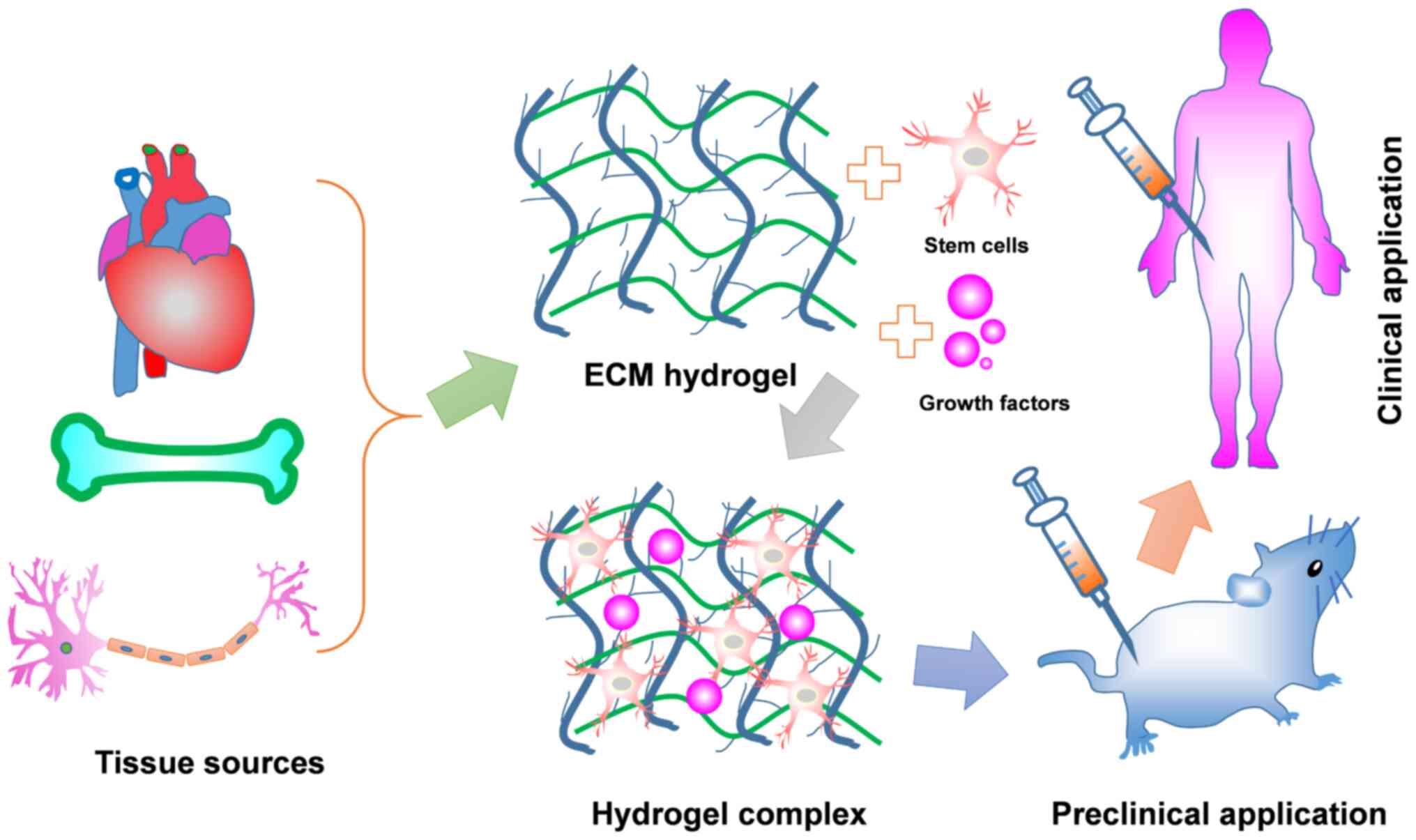
|
1
|
Bonnans C, Chou J and Werb Z: Remodelling
the extracellular matrix in development and disease. Nat Rev Mol
Cell Biol. 15:786–801. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Giobbe GG, Crowley C, Luni C, Campinoti S,
Khedr M, Kretzschmar K, De Santis MM, Zambaiti E, Michielin F,
Meran L, et al: Extracellular matrix hydrogel derived from
decellularized tissues enables endodermal organoid culture. Nat
Commun. 10:56582019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Spang MT and Christman KL: Extracellular
matrix hydrogel therapies: In vivo applications and development.
Acta Biomater. 68:1–14. 2018. View Article : Google Scholar :
|
|
4
|
Kraehenbuehl TP, Zammaretti P, Van der
Vlies AJ, Schoenmakers RG, Lutolf MP, Jaconi ME and Hubbell JA:
Three-dimensional extracellular matrix-directed cardiopro-genitor
differentiation: Systematic modulation of a synthetic
cell-responsive PEG-hydrogel. Biomaterials. 29:2757–2766. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ma Y, Ji Y, Huang G, Ling K, Zhang X and
Xu F: Bioprinting 3D cell-laden hydrogel microarray for screening
human periodontal ligament stem cell response to extracellular
matrix. Biofabrication. 7:0441052015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Aamodt JM and Grainger DW: Extracellular
matrix-based biomaterial scaffolds and the host response.
Biomaterials. 86:68–82. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vincent AT, Schiettekatte O, Goarant C,
Neela VK, Bernet E, Thibeaux R, Ismail N, Mohd Khalid MKN, Amran F,
Masuzawa T, et al: Revisiting the taxonomy and evolution of
pathogenicity of the genus Leptospira through the prism of
genomics. PLoS Negl Trop Dis. 13:e00072702019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Szalewski DA, Hinrichs VS, Zinniel DK and
Barletta RG: The pathogenicity of Aspergillus fumigatus, drug
resistance, and nanoparticle delivery. Can J Microbiol. 64:439–453.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zilelidou EA and Skandamis PN: Growth,
detection and virulence of Listeria monocytogenes in the presence
of other microorganisms: Microbial interactions from species to
strain level. Int J Food Microbiol. 277:10–25. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gilbert TW, Sellaro TL and Badylak SF:
Decellularization of tissues and organs. Biomaterials.
27:3675–3683. 2006.PubMed/NCBI
|
|
11
|
Choi JS, Yang HJ, Kim BS, Kim JD, Kim JY,
Yoo B, Park K, Lee HY and Cho YW: Human extracellular matrix (ECM)
powders for injectable cell delivery and adipose tissue
engineering. J Control Release. 139:2–7. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sackett SD, Tremmel DM, Ma F, Feeney AK,
Maguire RM, Brown ME, Zhou Y, Li X, O'Brien C, Li L, et al:
Extracellular matrix scaffold and hydrogel derived from
decellularized and delipidized human pancreas. Sci Rep.
8:104522018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lv S, Bu T, Kayser J, Bausch A and Li H:
Towards constructing extracellular matrix-mimetic hydrogels: An
elastic hydrogel constructed from tandem modular proteins
containing tenascin FnIII domains. Acta Biomater. 9:6481–6491.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rao N, Agmon G, Tierney MT, Ungerleider
JL, Braden RL, Sacco A and Christman KL: Engineering an injectable
muscle-specific microenvironment for improved cell delivery using a
nanofibrous extracellular matrix hydrogel. ACS Nano. 11:3851–3859.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Seif-Naraghi SB, Horn D, Schup-Magoffin PJ
and Christman KL: Injectable extracellular matrix derived hydrogel
provides a platform for enhanced retention and delivery of a
heparin-binding growth factor. Acta Biomater. 8:3695–3703. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Davidov T, Efraim Y, Dahan N, Baruch L and
Machluf M: Porcine arterial ECM hydrogel: Designing an in vitro
angiogenesis model for long-term high-throughput research. FASEB J.
34:7745–7758. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rosso F, Giordano A, Barbarisi M and
Barbarisi A: From cell-ECM interactions to tissue engineering. J
Cell Physiol. 199:174–180. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Engler AJ, Sen S, Sweeney HL and Discher
DE: Matrix elasticity directs stem cell lineage specification.
Cell. 126:677–689. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Divya P and Krishnan LK:
Glycosaminoglycans restrained in a fibrin matrix improve ECM
remodelling by endothelial cells grown for vascular tissue
engineering. J Tissue Eng Regen Med. 3:377–388. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim SH, Lee SH, Lee JE, Park SJ, Kim K,
Kim IS, Lee YS, Hwang NS and Kim BG: Tissue adhesive, rapid
forming, and sprayable ECM hydrogel via recombinant tyrosinase
cross-linking. Biomaterials. 178:401–412. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu J, Ding Q, Dutta A, Wang Y, Huang YH,
Weng H, Tang L and Hong Y: An injectable extracellular matrix
derived hydrogel for meniscus repair and regeneration. Acta
Biomater. 16:49–59. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tukmachev D, Forostyak S, Koci Z,
Zaviskova K, Vackova I, Vyborny K, Sandvig I, Sandvig A, Medberry
CJ, Badylak SF, et al: Injectable extracellular matrix hydrogels as
scaffolds for spinal cord injury repair. Tissue Eng Part A.
22:306–317. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ahearne M: Introduction to cell-hydrogel
mechanosensing. Interface Focus. 4:201300382014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vats K and Benoit DS: Dynamic manipulation
of hydrogels to control cell behavior: a review. Tissue Eng Part B
Rev. 19:455–469. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ghuman H, Mauney C, Donnelly J, Massensini
AR, Badylak SF and Modo M: Biodegradation of ECM hydrogel promotes
endogenous brain tissue restoration in a rat model of stroke. Acta
Biomater. 80:66–84. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Black C, Kanczler JM, de Andres MC, White
LJ, Savi FM, Bas O, Saifzadeh S, Henkel J, Zannettino A, Gronthos
S, et al: Characterisation and evaluation of the regenerative
capacity of Stro-4+ enriched bone marrow mesenchymal stromal cells
using bovine extracellular matrix hydrogel and a novel
biocompatible melt electro-written medical-grade polycaprolactone
scaffold. Biomaterials. 247:1199982020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang Y, Gallant RC and Ni H: Extracellular
matrix proteins in the regulation of thrombus formation. Curr Opin
Hematol. 23:280–287. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jiang B, Suen R, Wertheim JA and Ameer GA:
Targeting heparin to collagen within extracellular matrix
significantly reduces thrombogenicity and improves
endothelialization of decellular-ized tissues. Biomacromolecules.
17:3940–3948. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li X, Zhang C, Haggerty AE, Yan J, Lan M,
Seu M, Yang M, Marlow MM, Maldonado-Lasunció I, Cho B, et al: The
effect of a nanofiber-hydrogel composite on neural tissue repair
and regeneration in the contused spinal cord. Biomaterials.
245:1199782020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Farrokhi A, Pakyari M, Nabai L,
Pourghadiri A, Hartwell R, Jalili R and Ghahary A: Evaluation of
detergent-free and deter-gent-based methods for decellularization
of murine skin. Tissue Eng Part A. 24:955–967. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gupta SK, Mishra NC and Dhasmana A:
Decellularization methods for scaffold fabrication. Methods Mol
Biol. 1577:1–10. 2018.
|
|
32
|
Isidan A, Liu S, Li P, Lashmet M, Smith
LJ, Hara H, Cooper DKC and Ekser B: Decellularization methods for
developing porcine corneal xenografts and future perspectives.
Xenotransplantation. 26:e125642019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jackson DW, Grood ES, Arnoczky SP, Butler
DL and Simon TM: Freeze dried anterior cruciate ligament
allografts. Preliminary studies in a goat model. Am J Sports Med.
15:295–303. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jackson DW, Grood ES, Wilcox P, Butler DL,
Simon TM and Holden JP: The effects of processing techniques on the
mechanical properties of bone-anterior cruciate ligament-bone
allografts. An experimental study in goats. Am J Sports Med.
16:101–105. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mardhiyah A, Sha'ban M and Azhim A:
Evaluation of histological and biomechanical properties on
engineered meniscus tissues using sonication decellularization.
Annu Int Conf IEEE Eng Med Biol Soc. 2017:2064–2067.
2017.PubMed/NCBI
|
|
36
|
Hrebikova H, Diaz D and Mokry J: Chemical
decellularization: A promising approach for preparation of
extracellular matrix. Biomed Pap Med Fac Univ Palacky Olomouc Czech
Repub. 159:12–17. 2015. View Article : Google Scholar
|
|
37
|
Tchoukalova YD, Hintze JM, Hayden RE and
Lott DG: Tracheal decellularization using a combination of
chemical, physical and bioreactor methods. Int J Artif Organs. Sep
28–2017.Epub ahead of print. PubMed/NCBI
|
|
38
|
Jiang WC, Cheng YH, Yen MH, Chang Y, Yang
VW and Lee OK: Cryo-chemical decellularization of the whole liver
for mesenchymal stem cells-based functional hepatic tissue
engineering. Biomaterials. 35:3607–3617. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
McCrary MW, Vaughn NE, Hlavac N, Song YH,
Wachs RA and Schmidt CE: Novel sodium deoxycholate-based chemical
decellularization method for peripheral nerve. Tissue Eng Part C
Methods. 26:23–36. 2020. View Article : Google Scholar
|
|
40
|
Tebyanian H, Karami A, Motavallian E,
Aslani J, Samadikuchaksaraei A, Arjmand B and Nourani MR:
Histologic analyses of different concentrations of tritonX-100 and
Sodium dodecyl sulfate detergent in lung decellularization. Cell
Mol Biol (Noisy-le-grand). 63:46–51. 2017. View Article : Google Scholar
|
|
41
|
Vafaee T, Thomas D, Desai A, Jennings LM,
Berry H, Rooney P, Kearney J, Fisher J and Ingham E:
Decellularization of human donor aortic and pulmonary valved
conduits using low concen-tration sodium dodecyl sulfate. J Tissue
Eng Regen Med. 12:e841–e853. 2018. View Article : Google Scholar
|
|
42
|
Yu BT, Li WT, Song BQ and Wu YL:
Comparative study of the triton X-100-sodium deoxycholate method
and detergent-enzymatic digestion method for decellularization of
porcine aortic valves. Eur Rev Med Pharmacol Sci. 17:2179–2184.
2013.PubMed/NCBI
|
|
43
|
Varhac R, Robinson NC and Musatov A:
Removal of bound triton X-100 from purified bovine heart cytochrome
bc1. Anal Biochem. 395:268–270. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Dahl SL, Koh J, Prabhakar V and Niklason
LE: Decellularized native and engineered arterial scaffolds for
transplantation. Cell Transplant. 12:659–666. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chen RN, Ho HO, Tsai YT and Sheu MT:
Process development of an acellular dermal matrix (ADM) for
biomedical applications. Biomaterials. 25:2679–2686. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Goissis G, Suzigan S, Parreira DR,
Maniglia JV, Braile DM and Raymundo S: Preparation and
characterization of collagen-elastin matrices from blood vessels
intended as small diameter vascular grafts. Artif Organs.
24:217–223. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Gamba PG, Conconi MT, Lo Piccolo R, Zara
G, Spinazzi R and Parnigotto PP: Experimental abdominal wall defect
repaired with acellular matrix. Pediatr Surg Int. 18:327–331. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
McFetridge PS, Daniel JW, Bodamyali T,
Horrocks M and Chaudhuri JB: Preparation of porcine carotid
arteries for vascular tissue engineering applications. J Biomed
Mater Res A. 70:224–234. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Teebken OE, Bader A, Steinhoff G and
Haverich A: Tissue engineering of vascular grafts: Human cell
seeding of decellularised porcine matrix. Eur J Vasc Endovasc Surg.
19:381–386. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Rahman S, Griffin M, Naik A, Szarko M and
Butler PEM: Optimising the decellularization of human elastic
cartilage with trypsin for future use in ear reconstruction. Sci
Rep. 8:30972018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Warwick RM, Magee JG, Leeming JP, Graham
JC, Hannan MM, Chadwick M, Crook DW, Yearsley CP, Rayner A and
Parker R: Mycobacteria and allograft heart valve banking: An
international survey. J Hosp Infect. 68:255–261. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hensley A, Rames J, Casler V, Rood C,
Walters J, Fernandez C, Gill S and Mercuri JJ: Decellularization
and characterization of a whole intervertebral disk xenograft
scaffold. J Biomed Mater Res A. 106:2412–2423. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Crapo PM, Gilbert TW and Badylak SF: An
overview of tissue and whole organ decellularization processes.
Biomaterials. 32:3233–3243. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wong ML and Griffiths LG: Immunogenicity
in xenogeneic scaffold generation: Antigen removal vs.
Decellularization Acta Biomater. 10:1806–1816. 2014. View Article : Google Scholar
|
|
55
|
Nagata S, Hanayama R and Kawane K:
Autoimmunity and the clearance of dead cells. Cell. 140:619–630.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Dullah EC and Ongkudon CM: Current trends
in endotoxin detection and analysis of endotoxin-protein
interactions. Crit Rev Biotechnol. 37:251–261. 2017. View Article : Google Scholar
|
|
57
|
Ogikubo Y, Norimatsu M, Noda K, Takahashi
J, Inotsume M, Tsuchiya M and Tamura Y: Evaluation of the bacterial
endotoxin test for quantification of endotoxin contamination of
porcine vaccines. Biologicals. 32:88–93. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yang YG and Sykes M: Xenotransplantation:
Current status and a perspective on the future. Nat Rev Immunol.
7:519–531. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Aurora A, McCarron J, Iannotti JP and
Derwin K: Commercially available extracellular matrix materials for
rotator cuff repairs: State of the art and future trends. J
Shoulder Elbow Surg. 16(Suppl 5): S171–S178. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ercan H, Durkut S, Koc-Demir A, Elçin AE
and Elçin YM: Clinical applications of injectable biomaterials. Adv
Exp Med Biol. 1077:163–182. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Ahmadian Z, Correia A, Hasany M,
Figueiredo P, Dobakhti F, Eskandari MR, Hosseini SH, Abiri R,
Khorshid S, Hirvonen J, et al: A hydrogen-bonded extracellular
matrix-mimicking bactericidal hydrogel with radical scavenging and
hemostatic function for pH-responsive wound healing acceleration.
Adv Healthc Mater. Oct 26–2020.Epub ahead of print. View Article : Google Scholar
|
|
62
|
Ha DH, Chae S, Lee JY, Kim JY, Yoon J, Sen
T, Lee SW, Kim HJ, Cho JH and Cho DW: Therapeutic effect of
decellularized extra-cellular matrix-based hydrogel for radiation
esophagitis by 3D printed esophageal stent. Biomaterials.
266:1204772021. View Article : Google Scholar
|
|
63
|
Beachley V, Ma G, Papadimitriou C, Gibson
M, Corvelli M and Elisseeff J: Extracellular matrix
particle-glycosaminoglycan composite hydrogels for regenerative
medicine applications. J Biomed Mater Res A. 106:147–159. 2018.
View Article : Google Scholar
|
|
64
|
Lou J, Stowers R, Nam S, Xia Y and
Chaudhuri O: Stress relaxing hyaluronic acid-collagen hydrogels
promote cell spreading, fiber remodeling, and focal adhesion
formation in 3D cell culture. Biomaterials. 154:213–222. 2018.
View Article : Google Scholar
|
|
65
|
Zhang X, Li J, Ye P, Gao G, Hubbell K and
Cui X: Coculture of mesenchymal stem cells and endothelial cells
enhances host tissue integration and epidermis maturation through
AKT activation in gelatin methacryloyl hydrogel-based skin model.
Acta Biomater. 59:317–326. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Lee A, Hudson AR, Shiwarski DJ, Tashman
JW, Hinton TJ, Yerneni S, Bliley JM, Campbell PG and Feinberg AW:
3D bioprinting of collagen to rebuild components of the human
heart. Science. 365:482–487. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Jang J, Park HJ, Kim SW, Kim H, Park JY,
Na SJ, Kim HJ, Park MN, Choi SH, Park SH, et al: 3D printed complex
tissue construct using stem cell-laden decellularized extracellular
matrix bioinks for cardiac repair. Biomaterials. 112:264–274. 2017.
View Article : Google Scholar
|
|
68
|
Gjorevski N, Sachs N, Manfrin A, Giger S,
Bragina ME, Ordóñez-Morán P, Clevers H and Lutolf MP: Designer
matrices for intestinal stem cell and organoid culture. Nature.
539:560–564. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Saheli M, Sepantafar M, Pournasr B,
Farzaneh Z, Vosough M, Piryaei A and Baharvand H: Three-dimensional
liver-derived extracellular matrix hydrogel promotes liver
organoids function. J Cell Biochem. 119:4320–4333. 2018. View Article : Google Scholar
|
|
70
|
Broguiere N, Isenmann L, Hirt C, Ringel T,
Placzek S, Cavalli E, Ringnalda F, Villiger L, Züllig R, Lehmann R,
et al: Growth of epithelial organoids in a defined hydrogel. Adv
Mater. 30:e18016212018. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Augsornworawat P, Velazco-Cruz L, Song J
and Millman JR: A hydrogel platform for in vitro three dimensional
assembly of human stem cell-derived islet cells and endothelial
cells. Acta Biomater. 97:272–280. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Liu H, Wang Y, Cui K, Guo Y, Zhang X and
Qin J: Advances in hydrogels in organoids and organs-on-a-chip. Adv
Mater. 31:e19020422019. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Chuang W, Sharma A, Shukla P, Li G, Mall
M, Rajarajan K, Abilez OJ, Hamaguchi R, Wu JC, Wernig M and Wu SM:
Partial reprogramming of pluripotent stem cell-derived
cardiomyocytes into neurons. Sci Rep. 7:448402017. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Garreta E, Prado P, Tarantino C, Oria R,
Fanlo L, Martí E, Zalvidea D, Trepat X, Roca-Cusachs P,
Gavaldà-Navarro A, et al: Fine tuning the extracellular environment
accelerates the derivation of kidney organoids from human
pluripotent stem cells. Nat Mater. 18:397–405. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Gong J, Schuurmans CCL, Genderen AMV, Cao
X, Li W, Cheng F, He JJ, López A, Huerta V, Manríquez J, et al:
Complexation-induced resolution enhancement of 3D-printed hydrogel
constructs. Nat Commun. 11:12672020. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Prest TA, Yeager E, LoPresti ST, Zygelyte
E, Martin MJ, Dong L, Gibson A, Olutoye OO, Brown BN and Cheetham
J: Nerve-specific, xenogeneic extracellular matrix hydrogel
promotes recovery following peripheral nerve injury. J Biomed Mater
Res A. 106:450–459. 2018. View Article : Google Scholar
|
|
77
|
Keane TJ, DeWard A, Londono R, Saldin LT,
Castleton AA, Carey L, Nieponice A, Lagasse E and Badylak SF:
Tissue-specific effects of esophageal extracellular matrix. Tissue
Eng Part A. 21:2293–2300. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Schnellmann R and Chiquet-Ehrismann R:
Preparation and application of a decellularized extracellular
matrix for identification of ADAMTS substrates. Methods Mol Biol.
2043:275–284. 2020. View Article : Google Scholar
|
|
79
|
Li R, Li Y, Wu Y, Chen H, Yuan Y, Xu K,
Zhang H, Lu Y, Wang J, Li X, et al: Heparin-poloxamer
thermosensitive hydrogel loaded with bFGF and NGF enhances
peripheral nerve regeneration in diabetic rats. Biomaterials.
168:24–37. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Slivka PF, Dearth CL, Keane TJ, Meng FW,
Medberry CJ, Riggio RT, Reing JE and Badylak SF: Fractionation of
an ECM hydrogel into structural and soluble components reveals
distinc-tive roles in regulating macrophage behavior. Biomater Sci.
2:1521–1534. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Panorchan P, Lee JS, Kole TP, Tseng Y and
Wirtz D: Microrheology and ROCK signaling of human endothelial
cells embedded in a 3D matrix. Biophys J. 91:3499–3507. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Sjöberg J and Kanje M: The initial period
of peripheral nerve regeneration and the importance of the local
environment for the conditioning lesion effect. Brain Res.
529:79–84. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Grinsell D and Keating CP: Peripheral
nerve reconstruction after injury: A review of clinical and
experimental therapies. Biomed Res Int. 2014:6982562014. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Lin T, Liu S, Chen S, Qiu S, Rao Z, Liu J,
Zhu S, Yan L, Mao H, Zhu Q, et al: Hydrogel derived from porcine
decellularized nerve tissue as a promising biomaterial for
repairing peripheral nerve defects. Acta Biomater. 73:326–338.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Qiu S, Rao Z, He F, Wang T, Xu Y, Du Z,
Yao Z, Lin T, Yan L, Quan D, et al: Decellularized nerve matrix
hydrogel and glial-derived neurotrophic factor modifications
assisted nerve repair with decellularized nerve matrix scaffolds. J
Tissue Eng Regen Med. 14:931–943. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Ansari S, Diniz IM, Chen C, Sarrion P,
Tamayol A, Wu BM and Moshaverinia A: Human periodontal ligament-
and gingiva-derived mesenchymal stem cells promote nerve
regeneration when encapsulated in alginate/hyaluronic acid 3D
scaffold. Adv Healthc Mater. 6:102017.
|
|
87
|
Zhang L, Zhang F, Weng Z, Brown BN, Yan H,
Ma XM, Vosler PS, Badylak SF, Dixon CE, Cui XT and Chen J: Effect
of an inductive hydrogel composed of urinary bladder matrix upon
functional recovery following traumatic brain injury. Tissue Eng
Part A. 19:1909–1918. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Wang JY, Liou A, Ren ZH, Zhang L, Brown
BN, Cui XT, Badylak SF, Cai YN, Guan YQ, Leak RK, et al:
Neurorestorative effect of urinary bladder matrix-mediated neural
stem cell trans-plantation following traumatic brain injury in
rats. CNS Neurol Disord Drug Targets. 12:413–425. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Buckenmeyer MJ, Meder TJ, Prest TA and
Brown BN: Decellularization techniques and their applications for
the repair and regeneration of the nervous system. Methods.
171:41–61. 2020. View Article : Google Scholar
|
|
90
|
Hong LT, Kim YM, Park HH, Hwang DH, Cui Y,
Lee EM, Yahn S, Lee JK, Song SC and Kim BG: An injectable hydrogel
enhances tissue repair after spinal cord injury by promoting
extracellular matrix remodeling. Nat Commun. 8:5332017. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Jiang X, Yang Z and Dong M: Cardiac repair
in a murine model of myocardial infarction with human induced
pluripotent stem cell-derived cardiomyocytes. Stem Cell Res Ther.
11:2972020. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Farnebo S, Woon CY, Schmitt T, Joubert LM,
Kim M, Pham H and Chang J: Design and characterization of an
injectable tendon hydrogel: A novel scaffold for guided tissue
regeneration in the musculoskeletal system. Tissue Eng Part A.
20:1550–1561. 2014. View Article : Google Scholar
|
|
93
|
Curley CJ, Dolan EB, Otten M, Hinderer S,
Duffy GP and Murphy BP: An injectable alginate/extra cellular
matrix (ECM) hydrogel towards acellular treatment of heart failure.
Drug Deliv Transl Res. 9:1–13. 2019. View Article : Google Scholar
|
|
94
|
Grover GN, Rao N and Christman KL:
Myocardial matrix-poly-ethylene glycol hybrid hydrogels for tissue
engineering. Nanotechnology. 25:0140112014. View Article : Google Scholar
|
|
95
|
Efraim Y, Sarig H, Cohen Anavy N, Sarig U,
de Berardinis E, Chaw SY, Krishnamoorthi M, Kalifa J, Bogireddi H,
Duc TV, et al: Biohybrid cardiac ECM-based hydrogels improve long
term cardiac function post myocardial infarction. Acta Biomater.
50:220–233. 2017. View Article : Google Scholar
|
|
96
|
Waters R, Alam P, Pacelli S, Chakravarti
AR, Ahmed RP and Paul A: Stem cell-inspired secretome-rich
injectable hydrogel to repair injured cardiac tissue. Acta
Biomater. 69:95–106. 2018. View Article : Google Scholar :
|
|
97
|
Guruswamy Damodaran R and Vermette P:
Tissue and organ decellularization in regenerative medicine.
Biotechnol Prog. 34:1494–1505. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Seif-Naraghi SB, Salvatore MA,
Schup-Magoffin PJ, Hu DP and Christman KL: Design and
characterization of an injectable pericardial matrix gel: A
potentially autologous scaffold for cardiac tissue engineering.
Tissue Eng Part A. 16:2017–2027. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Karabekmez FE, Duymaz A and Moran SL:
Early clinical outcomes with the use of decellularized nerve
allograft for repair of sensory defects within the hand. Hand (NY).
4:245–249. 2009. View Article : Google Scholar
|
|
100
|
Pipino G, Risitano S, Alviano F, Wu EJ,
Bonsi L, Vaccarisi DC and Indelli PF: Microfractures and hydrogel
scaffolds in the treatment of osteochondral knee defects: A
clinical and histological evaluation. J Clin Orthop Trauma.
10:67–75. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Fitzpatrick LE and McDevitt TC:
Cell-derived matrices for tissue engineering and regenerative
medicine applications. Biomater Sci. 3:12–24. 2015. View Article : Google Scholar
|
|
102
|
Loh QL and Choong C: Three-dimensional
scaffolds for tissue engineering applications: Role of porosity and
pore size. Tissue Eng Part B Rev. 19:485–502. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Defrere J and Franckart A: Freeze-dried
fascia lata allografts in the reconstruction of anterior cruciate
ligament defects. A two- to seven-year follow-up study. Clin Orthop
Relat Res. 303:56–66. 1994.
|
|
104
|
Mahirogullari M, Ferguson CM, Whitlock PW,
Stabile KJ and Poehling GG: Freeze-dried allografts for anterior
cruciate ligament reconstruction. Clin Sports Med. 26:625–637.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Jackson DW, Grood ES, Cohn BT, Arnoczky
SP, Simon TM and Cummings JF: The effects of in situ freezing on
the anterior cruciate ligament. An experimental study in goats. J
Bone Joint Surg Am. 73:201–213. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Freytes DO, Badylak SF, Webster TJ, Geddes
LA and Rundell AE: Biaxial strength of multilaminated extracellular
matrix scaffolds. Biomaterials. 25:2353–2361. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Lin P, Chan WC, Badylak SF and Bhatia SN:
Assessing porcine liver-derived biomatrix for hepatic tissue
engineering. Tissue Eng. 10:1046–1053. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Schenke-Layland K, Vasilevski O, Opitz F,
Opitz F, König K, Riemann I, Halbhuber KJ, Wahlers T and Stock UA:
Impact of decellularization of xenogeneic tissue on extracellular
matrix integrity for tissue engineering of heart valves. J Struct
Biol. 143:201–208. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Ott HC, Matthiesen TS, Goh SK, Black LD,
Kren SM, Netoff TI and Taylor DA: Perfusion-decellularized matrix:
Using nature's platform to engineer a bioartificial heart. Nat Med.
14:213–221. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
110
|
Uygun BE, Soto-Gutierrez A, Yagi H, Izamis
ML, Guzzardi MA, Shulman C, Milwid J, Kobayashi N, Tilles A,
Berthiaume F, et al: Organ reengineering through development of a
transplantable recellularized liver graft using decellularized
liver matrix. Nat Med. 16:814–820. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Brown BN, Valentin JE, Stewart-Akers AM,
McCabe GP and Badylak SF: Macrophage phenotype and remodeling
outcomes in response to biologic scaffolds with and without a
cellular component. Biomaterials. 30:1482–1491. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Reing JE, Brown BN, Daly KA, Freund JM,
Gilbert TW, Hsiong SX, Huber A, Kullas KE, Tottey S, Wolf MT and
Badylak SF: The effects of processing methods upon mechanical and
biologic properties of porcine dermal extracellular matrix
scaffolds. Biomaterials. 31:8626–8633. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Elder BD, Kim DH and Athanasiou KA:
Developing an articular cartilage decellularization process toward
facet joint cartilage replacement. Neurosurgery. 66:722–727. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Woods T and Gratzer PF: Effectiveness of
three extraction techniques in the development of a decellularized
bone-anterior cruciate ligament-bone graft. Biomaterials.
26:7339–7349. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Nakamura N, Saito K, Kimura T and Kishida
A: Recellularization of decellularized cancellous bone scaffolds
using low-temperature cell seeding. Tissue Cell. 66:1013852020.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Hudson TW, Zawko S, Deister C, Lundy S, Hu
CY, Lee K and Schmidt CE: Optimized acellular nerve graft is
immunologically tolerated and supports regeneration. Tissue Eng.
10:1641–1651. 2004. View Article : Google Scholar
|
|
117
|
Conconi MT, De Coppi P, Bellini S, Zara G,
Sabatti M, Marzaro M, Zanon GF, Gamba PG, Parnigotto PP and
Nussdorfer GG: Homologous muscle acellular matrix seeded with
autologous myoblasts as a tissue-engineering approach to abdominal
wall-defect repair. Biomaterials. 26:2567–2574. 2005. View Article : Google Scholar
|
|
118
|
Poon CJ, Pereira E, Cotta MV, Sinha S,
Palmer JA, Woods AA, Morrison WA and Abberton KM: Preparation of an
adipogenic hydrogel from subcutaneous adipose tissue. Acta
Biomater. 9:5609–5620. 2013. View Article : Google Scholar
|
|
119
|
Mendibil U, Ruiz-Hernandez R,
Retegi-Carrion S, Garcia-Urquia N, Olalde-Graells B and Abarrategi
A: Tissue-specific decellularization methods: Rationale and
strategies to achieve regenerative compounds. Int J Mol Sci.
21:54472020. View Article : Google Scholar :
|
|
120
|
Flynn LE: The use of decellularized
adipose tissue to provide an inductive microenvironment for the
adipogenic differentiation of human adipose-derived stem cells.
Biomaterials. 31:4715–4724. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Brown BN, Freund JM, Han L, Rubin JP,
Reing JE, Jeffries EM, Wolf MT, Tottey S, Barnes CA, Ratner BD and
Badylak SF: Comparison of three methods for the derivation of a
biologic scaffold composed of adipose tissue extracellular matrix.
Tissue Eng Part C Methods. 17:411–421. 2011. View Article : Google Scholar :
|
|
122
|
Gilbert TW: Strategies for tissue and
organ decellularization. J Cell Biochem. 113:2217–2222. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Petersen TH, Calle EA, Zhao L, Lee EJ, Gui
L, Raredon MB, Gavrilov K, Yi T, Zhuang ZW, Breuer C, et al:
Tissue-engineered lungs for in vivo implantation. Science.
329:538–541. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Dong X, Wei X, Yi W, Gu C, Kang X, Liu Y,
Li Q and Yi D: RGD-modified acellular bovine pericardium as a
bioprosthetic scaffold for tissue engineering. J Mater Sci Mater
Med. 20:2327–2336. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Hodgson MJ, Knutson CC, Momtahan N and
Cook AD: Extracellular matrix from whole porcine heart
decellularization for cardiac tissue engineering. Methods Mol Biol.
1577:95–102. 2018. View Article : Google Scholar
|
|
126
|
Obata T, Tsuchiya T, Akita S, Kawahara T,
Matsumoto K, Miyazaki T, Masumoto H, Kobayashi E, Niklason LE and
Nagayasu T: Utilization of natural detergent potassium laurate for
decellularization in lung bioengineering. Tissue Eng Part C
Methods. 25:459–471. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Shirakigawa N and Ijima H:
Decellularization of liver and organogenesis in rats. Methods Mol
Biol. 1577:271–281. 2018. View Article : Google Scholar
|
|
128
|
Song JJ, Guyette JP, Gilpin SE, Gonzalez
G, Vacanti JP and Ott HC: Regeneration and experimental orthotopic
transplantation of a bioengineered kidney. Nat Med. 19:646–651.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Piccoli M, Trevisan C, Maghin E, Franzin C
and Pozzobon M: Mouse skeletal muscle decellularization. Methods
Mol Biol. 1577:87–93. 2018. View Article : Google Scholar
|