1. Introduction
Coronaviruses (CoVs) belong to one of the four
genera of the Coronaviridae family characterized by a
positive-sense single-stranded RNA genome of ~30 kb (1). Previously, CoVs were considered
serious pathogens in animals with low influence on human health
(1-3). However, at the beginning of the
21st century, the outbreak of the severe acute respiratory syndrome
(SARS)-CoV emerged, followed by the Middle East respiratory
syndrome (MERS)-CoV a decade later, confirming the animal-to-human
and human-to-human transmission of CoVs (1,4,5).
Currently, the world is under a pandemic of the third wave of CoV,
initially identified as 2019-novel CoV that emerged in December
2019 in Wuhan, Hubei Province, China (6); following phylogeny analysis and
taxonomy, and based on the established naming practice for viruses
in this genus, the Coronaviridae Study Group of the
International Committee on Taxonomy Viruses termed the novel virus
SARS-CoV-2 (7). Subsequently, on
February 11, 2020, the World Health Organization (WHO) reported
that the disease caused by SARS-CoV-2 would be named coronavirus
disease 2019 (COVID-19) (8).
This disease is characterized by a respiratory syndrome that can
progress to severe interstitial pneumonia and acute respiratory
distress syndrome (ARDS) (9-11). The causative agent of COVID-19,
SARS-CoV-2, is transmitted mainly between individuals through
contact, respiratory droplets and aerosols, allowing the virus to
spread rapidly (8,12). The transmission rate of
SARS-CoV-2 has been reported to be higher compared with that of
SARS-CoV (rq=5.7 vs. ~3.0) (13,14). The mechanism of infection of
SARS-CoV-2 involves the spike protein of SARS-CoV-2 binding to the
angiotensin-converting enzyme 2 (ACE2) in the host lung epithelial
cells to enter the cell and initiate infection (15,16). ACE2 expression levels are high in
the intestine, heart, and kidneys; therefore, this virus
compromises various organs (17).
The outbreak and rapid spread of SARS-CoV-2 are a
health threat with unprecedented consequences worldwide. On August
3, 2020, the Johns Hopkins University dashboard reported 18,282,208
confirmed cases and 693,694 deaths worldwide due to COVID-19
(18). On the same date, five
Latin American countries (Brazil, Mexico, Peru, Chile and Colombia)
were among the top 10 countries with the highest number of
confirmed cases, and three countries in this region (Brazil, Mexico
and Peru) were in the top 10 countries with the highest number of
deaths (18). To date, various
factors have been identified as predisposing for an aggressive
phenotype of COVID-19, including the male sex, age >65 years,
smoking and comorbidities such as diabetes, hypertension and
cardiovascular disease (19).
The majority of these comorbidities are associated with a sedentary
lifestyle and an unhealthy diet that is commonly characterized by a
high intake of saturated fats, salt, sugars, refined grains and
processed meats (20,21).
A healthy diet is characterized by appropriate
consumption of macronutrients and micronutrients, and is necessary
for growth, development and adequate physiological functioning
(21). Nutrition is also
essential for the function of the immune system; this relationship
is currently being studied. Particularly, it has been reported that
the Mediterranean diet, as well as nutrients and active food
components can modulate the immune response through the inhibition
of pro-inflammatory mediators, production of anti-inflammatory
functions and participation in the communication between the innate
and adaptive immune system (22). For example, the Mediterranean
diet (23), vitamin D (24), and polyunsaturated fatty acids
(PUFAs) (25) have demonstrated
promising effects on chronic inflammation and autoimmune diseases,
whereas vitamin E (26), zinc
(27) and probiotics (28) exhibit effects in reducing
infections. Immunonutrition is defined as the provision of
nutrients in amounts greater than those typically recommended in a
diet that modulates the immune system activity; immunonutrients
include amino acids, PUFAs, short-chain fatty acids, vitamins and
trace elements (29,30). In particular, vitamin D is a
crucial immunonutrient that can be obtained through the diet;
however, it is produced mostly (80%) endogenously by induction of
ultraviolet-B (UV-B) rays in the skin (31). Although the primary function of
vitamin D appears to be calcium homeostasis, this vitamin also
serves immunomodulatory functions and may have protective effects
against respiratory infections (32).
Vitamin D deficiency is considered a public health
problem worldwide; it is estimated that one billion individuals are
deficient in vitamin D, and that insufficiency affects ~50% of the
population (33). Various
factors influence vitamin D deficiency, such as age, geographic
latitude and skin pigmentation (34). In Latin American countries,
vitamin D insufficiency has been suggested to be a potential public
health problem; however, no representative data are available from
this region, and the magnitude of the problem cannot be established
(35).
Based on the aforementioned information, the
restoration of adequate serum levels of vitamin D through
supplementation has demonstrated a protective effect against
respiratory infections (32). In
addition, considering the lack of effective therapies for the
prevention and treatment of COVID-19, it is essential to propose
novel therapeutic options. Therefore, the present review aims to
overview the potential immunomodulatory effects of vitamin D in the
prevention of COVID-19 and to establish guideline recommendations
for vitamin D supplementation for the Latin American
population.
2. SARS-CoV-2 infection
Until late December 2019, only six CoV species had
been identified with implications for human health, of which four
(229E, OC43, NL63, and HKU1) can cause mild symptoms such as the
common cold (6). Currently,
SARS-CoV-2, in addition to the other two remaining CoV strains
(SARS-CoV and MERS-CoV), can cause fatal outcomes (6). The genome of SARS-CoV-2 shares 79%
similarity with that of SARS-CoV (36). The CoV genome encodes four main
proteins: Spike, membrane, nucleocapsid and envelope (1,9).
The spike protein of the virus is responsible for the viral entry
to the host cells by recognizing and binding to the ACE2 receptor,
which is highly expressed in various types of cells, including type
II alveolar and myocardial cells, as well as the proximal tubule
cells of the kidney (17,37,38).
The virus spike protein binding with the ACE2 receptor is
proteolytically processed by the transmembrane serine protease 2,
which causes the cleavage of ACE2 and the activation of the virus
spike protein to facilitate its entry to the target cell (16,39). Once inside the cell, the viral
RNA genome is released into the cytoplasm to begin its replication
process (40).
ARDS is one of the features of the severe COVID-19
as the virus can negatively regulate the expression of ACE2,
causing the upregulation of angiotensin II (Ang II), which
interacts with the Ang II type 1 receptor (AT1R) to modulate the
nuclear factor-KB (NF-KB) signaling pathway, as well as macrophage
activation that leads to the excessive production of
pro-inflammatory cytokines (41). This exacerbated cytokine
production is commonly referred to as a cytokine storm, which, in
addition to contributing to ARDS, triggers a pathogenic
inflammatory immune response that leads to multiple organ failure
and death in severe COVID-19 (41-43).
3. Latin America: A vulnerable region
On March 11, 2020, the WHO classified COVID-19 as a
pandemic due to the alarming worldwide spread of the virus and
governments' inaction to prevent infection (8). A number of Latin American countries
are currently among the countries with the highest number of
confirmed cases and deaths associated with COVID-19 (18). Vulnerability to COVID-19 in Latin
America is caused by various factors such as precarious health
systems, housing conditions, high rates of non-communicable
diseases, income inequality, and poverty levels (44,45). Although some countries in this
region have already started vaccinating their population (Costa
Rica, Argentina, Mexico, and Chile) (46), accessibility and individual
factors (socioeconomic, education, religious and cultural) may
affect vaccine coverage (47).
Clinical trials have been conducted to identify a
treatment for COVID-19; however, limited effective therapies are
available for the prevention or treatment of this disease (48-52). Furthermore, despite the current
availability of vaccines, their distribution may not be equitable
since during the 2009 H1N1 swine flu pandemic, countries with the
highest economic position left the poorer countries with limited
supplies (53,54). In addition, although health
services in Latin America have notably improved since 1950, there
are still deficiencies and inequity in health care (55). Therefore, it is important to
propose host-directed therapeutic alternatives of easy access such
as immunonutrients that may modulate the immune response to
minimize the mortality rate of SARS-CoV-2 infection until a
universal and effective solution is identified (56). One of the immunonutrients that
has received the most interest is vitamin D; the sufficiency in
serum levels of this vitamin in individuals may lead to a less
severe course of COVID-19 and a faster recovery compared with that
in individuals with vitamin D deficiency by helping prevent the
cytokine storm, as well as ARDS, which is one of the leading causes
of mortality among patients infected with SARS-CoV-2 (57).
4. Vitamin D
V itamin D is a fat-soluble vitamin present in two
main isoforms; vitamin D2 (ergocalciferol) which is
mainly present in mushrooms, and vitamin D3
(cholecalciferol), which is abundant in fish, egg yolk and liver
(58). Chylomicrons support
intestinal absorption of both vitamin D isoforms; however, vitamin
D3 is more easily absorbed compared with vitamin
D2 (33,58). However, achieving the recommended
vitamin D dose from food sources may be impossible for a large part
of the population (31,58).
As aforementioned, the major source of vitamin D for
physiological functions is through synthesis in the epidermis from
a cholesterol precursor (7-dehydrocholesterol) following exposure
to UV-B radiation (290-320 nm) from the sun (31,59). This process induces to the
formation of pre-vitamin D3, which isomerizes to vitamin
D3 in a thermo-sensitive process (60). Dietary or skin-synthesized
vitamin D3 binds to the vitamin D-binding protein (DBP),
which transports it to the liver, where it is metabolized mainly by
the enzyme vitamin D-25-hydroxylase to form calcidiol, also termed
25-hydroxyvitamin D [25(OH)D] (59). Subsequently, 25(OH)D is
transformed in the kidneys by cytochrome P450 family 27 subfamily B
member 1 (CYP27B1, also termed 25-hydroxyvitamin D-1α-hydroxylase)
to obtain 1,25-dihydroxyvitamin D [1,25(OH)2D], also
termed calcitriol, which is the main active form of vitamin D
responsible for its physiological functions (59,60). In the regulation of calcitriol
production, parathyroid hormone (PTH) has the ability to stimulate
renal calcitriol production by activating CYP27B1, whereas
fibroblast growth factor 23 (FGF-23) and calcitriol itself inhibit
CYP27B1 (60,61). Similarly, high serum calcium and
calcitriol concentrations inhibit CYP27B1 indirectly by suppressing
PTH, and high serum phosphate concentration suppresses renal
calcitriol production through the stimulation of FGF-23 (61). Excess 1,25(OH)2D is
excreted through the bile or urine as calcitroic acid (Fig. 1) (31,62).
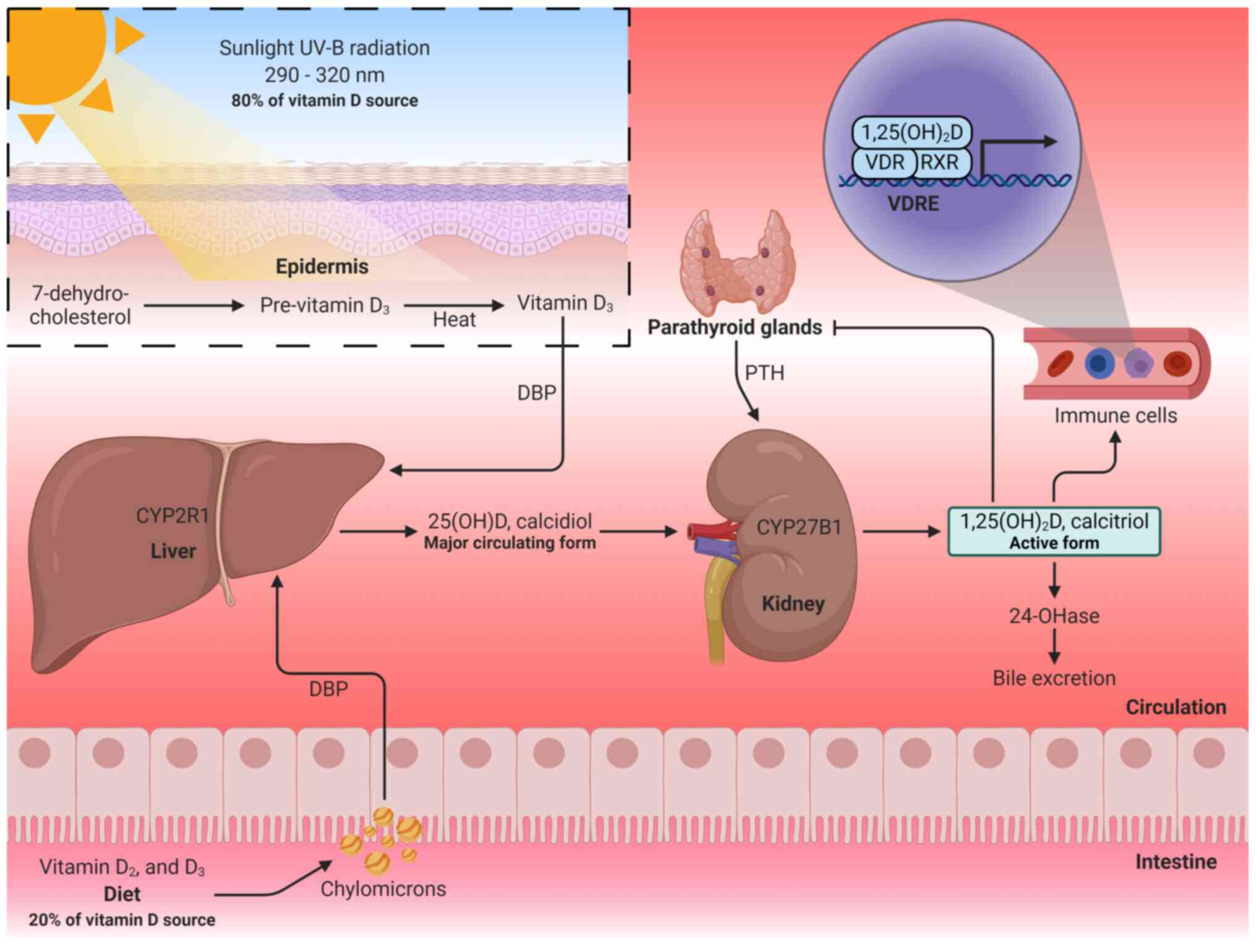 | Figure 1Vitamin D metabolism. Vitamin D is
obtained through diet or skin synthesis by sun exposure and is
converted to calcidiol in the liver. Subsequently, a second
hydroxylation in the kidneys transforms calcidiol into calcitriol
(active form of vitamin D). Calcitriol binds to the VDR and forms a
complex with the RXR to regulate gene transcription. 25(OH)D,
25-hydroxyvitamin D; 1,25(OH)2D, 1,25-dihydroxyvitamin D; VDR,
vitamin D receptor; RXR, retinoid-X receptor; VDREs, vitamin D
response elements; PTH, parathyroid hormone; 24-OHase,
25-hydroxyvitamin D-24-hydroxylase. The figure was created using
BioRender (https://biorender.com/). |
Calcitriol exerts its genomic functions through the
vitamin D receptor (VDR), which acts as a transcription factor that
forms a complex with the retinoid-X receptor (RXR); this complex
recruits transcriptional coactivators or corepressors to regulate
gene transcription by binding to vitamin D response elements
(VDREs) in the DNA (59,60). In addition to the endocrine
function of calcitriol in regulating calcium levels for bone
remodeling, extrarenal hydroxylation occurs to form calcitriol,
exerting paracrine and autocrine effects (61,63). Extrarenal hydroxylation by
CYP27B1 occurs in the prostate, brain, placenta, lungs and immune
cells (63). In particular, VDR
activation by locally produced calcitriol has been reported to
mediate the immune response (62-64), as discussed in the following
sections.
5. Vitamin D and respiratory infection
Among the most common viruses that affect human
health through respiratory tract infections are influenza viruses,
classified into four types. Influenza type A is the most common in
seasonal epidemics and pandemics, and is the primary cause of a
severe illness that is associated with high mortality rates in
high-risk populations (adults >65 years, individuals with
chronic diseases or immunosuppression, pregnant women, individuals
with obesity and infants ≤6 months) (65). The influenza viruses exhibit
typical winter infection peaks in temperate zones (66), which correspond to November to
April and May to October in the northern and southern hemisphere,
respectively (65). By contrast,
in tropical zones, the seasonality of influenza infections appears
to be poorly defined, although it is assumed that it can occur
throughout the year (65,67).
In 1981, Hope-Simpson (68) was the first to describe the
association between influenza infection peaks and temperate
latitudes in the winter. He proposed the existence of a seasonal
stimulus associated with the seasonality of epidemic influenza, and
that the decrease in solar radiation during winter influenced the
presence of the seasonal stimulus. The study also suggested that,
in the tropical regions, although UV-B radiation is less seasonal,
influenza outbreaks are more severe during the rainy seasons. Thus,
Hope-Simpson described that latitude determined the time of
epidemics in the annual cycle, since solar radiation may act
positively or negatively on the virus, the host or their
interaction (68). After >20
years, Cannell et al (69) proposed that vitamin D was a
likely candidate to be the seasonal stimulus described by
Hope-Simpson. Since vitamin D3 is obtained by sun
exposure, the serum levels of 25(OH)D are lower in people who live
in temperate latitudes, and because vitamin D has modulating
effects on the immune system (69).
Based on the aforementioned theories, it has been
reported that the levels of UV-B radiation in countries outside the
40° N and 40° S latitude range are insufficient to produce vitamin
D in the skin during winter (70). The average concentration of
25(OH)D in European countries during winter has been reported to be
11.6 ng/ml (29 nmol/l) (71),
14.3 ng/ml (35.75 nmol/l) (72)
and 13.3 ng/ml (33.25 nmol/l) (73). Similarly, in the northern and
central regions of the United States of America, the concentration
of 25(OH)D during winter is ~21 ng/ml (52.5 nmol/l), whereas in the
summer, it is ~28 ng/ml (70 nmol/l) (74). Canada has reported that between
December and January (winter), there is a peak in the prevalence of
25(OH)D insufficiency/deficiency in its population (75). Although the influence of solar
radiation on vitamin D deficiency is evident, several additional
factors impact its deficiency, which will be discussed in
subsequent sections. These factors can also affect the tropical
zone population, such as that in Latin America, although the
intensity of the sun rays in this region is greater (70). Other factors associated with the
seasonality of respiratory infections include the congregation
indoors during winter, which increases the probability of
contagion, as well as the cold and dry conditions that contribute
to the influenza transmission (69,76,77). A recent meta-analysis of 14
observational studies has reported that a low serum concentration
of 25(OH)D is a risk factor for acute respiratory tract infection
(OR=1.83; 95% CI, 1.42-2.37; P-value for heterogeneity, <0.001)
(78). Similarly, in a
sub-analysis of four studies, a low serum concentration of 25(OH)D
was associated with high mortality from acute respiratory tract
infection (OR=3.00; 95% CI, 1.89-4.78; P-value for heterogeneity,
0.029). Notably, the funnel plot in the aforementioned study
identified evidence of publication bias (78). By contrast, a meta-analysis of 25
randomized controlled trials has reported that vitamin D
supplementation is associated with a lower risk of acute
respiratory tract infections (OR=0.88; 95% CI, 0.81-0.96; P=0.003;
P-value for heterogeneity, <0.001) (32).
6. Vitamin D and COVID-19
CoVs cause respiratory infections ranging from the
common cold to severe conditions such as pneumonia and ARDS
(1,6,10). Therefore, the immunoregulatory
effects of vitamin D are being discussed due to its potential
beneficial effects for clinical outcomes in SARS-CoV-2 infection.
In the international platforms for the registration of clinical
trials, a number of studies evaluating the effects of vitamin D
supplementation on COVID-19 have been registered, although the
majority of these studies have not yet reported any results.
Despite this temporal limitation, numerous studies support the
association between vitamin D and the clinical outcomes of
COVID-19.
Among the observational studies published in the
first semester of 2020, significant associations were reported
between latitudes and mortality from COVID-19, as well as between
25(OH)D deficiency and SARS-CoV-2 infection (57,79-84). These studies are summarized in
Table I.
 | Table IObservational studies of the
association between vitamin D and COVID-19. |
Table I
Observational studies of the
association between vitamin D and COVID-19.
| First author,
year | Study design | Subjects or
countries | Main outcome | Conclusion | (Refs.) |
|---|
| Rhodes et
al, 2020 | Ecological | 120 countries | Correlation of
latitude degrees with mortality from COVID-19 per million in
different countries: rho=0.53; P≤0.0001 | Mortality per
million is higher in countries with a latitude above 35° n; above
this latitude, people do not receive sufficient sunlight to retain
adequate vitamin D levels during winter | (57) |
| Ilie et al,
2020 | Ecological | 20 countries | Correlation of
mortality from COVID-19 per million with a mean 25(OH)D
concentration in different countries: r=-0.43; P=0.05 | The association may
explain the possible protection of vitamin D from the negative
consequences of SARS-CoV-2 infection | (79) |
| Hastie et
al, 2020 | Ecological | 1,474 subjects | Association of
25(OH)D concentration with positive COVID-19: OR=1.00; 95% CI,
0.998-1.01; P=0.208 | The results do not
support the potential of 25(OH)D concentration for susceptibility
to COVID-19 infection | (80) |
| D'Avolio et
al, 2020 | Retrospective | 107 subjects | Difference of
25(OH)D concentration in the positive and negative SARS-CoV-2
groups: 11.1 ng/mla (IQR,
8.2-21.0) vs. 24.6 ng/mla
(IQR, 8.9-30.5); P=0.004 | Low concentration
of 25(OH)D may represent a risk factor for infection with
SARS-CoV-2 | (81) |
| Meltzer et
al, 2020 | Retrospective | 499 subjects | Association of
25(OH)D deficiency with a positive test for COVID-19: RR=1.77;
P=0.015 | Individuals with
vitamin D deficiency have a higher risk of a positive test for
COVID-19 compared with those with sufficiency | (82) |
| Whittemore,
2020 | Ecological | 88 countries | Correlation of
latitude with death rates per million from COVID-19 in different
countries: r=0.40; P≤0.00005 | Mortality per
million is lower in populations closest to the Equator. The
correlation supports a possible association between latitude,
sunlight exposure, vitamin D and COVID-19 mortality | (83) |
| Panagiotou et
al, 2020 | retrospective | 134 subjects | Difference in the
prevalence of 25(OH)D sufficiency between ICU and non-ICU patients:
19 vs. 39.1%; P=0.02 | 25(OH)D deficiency
was more prevalent in patients who required admission to the ICu
than those who only needed management in medical wards; therefore,
vitamin D could be a determinant of the severity of the disease.
There was no significant association between 25(OH)D concentration
and mortality | (84) |
Studies from the second half of 2020 that include
observational, quasi-experimental studies and clinical trials have
also reported valuable information on the association between
vitamin D and COVID-19. A number of these studies are described
below.
Merzon et al (85) evaluated the association between
low levels of 25(OH)D and the risk of infection by COVID-19 through
an ecological study (secondary data analysis from population
databases). The mean serum of 25(OH)D concentration was lower in
the positive compared with the negative COVID-19 cases (P=0.026).
In addition, an association was demonstrated between 25(OH)D <30
ng/ml (<75 nmol/l) and the risk of infection by SARS-CoV-2.
Therefore, it was concluded that suboptimal plasma levels of
25(OH)D may be a potential risk factor for COVID-19. Another study
identified that the GT rs7041 genotype of the DBP gene may
confer susceptibility to COVID-19, whereas the TT rs7041 genotype
may exert a protective effect (86). The authors of the aforementioned
study considered that polymorphisms in the DBP gene may
alter the affinity of DBP for vitamin D metabolites, which may
affect COVID-19 prevalence and mortality.
In a retrospective study, Carpagnano et al
(87) reported that patients
with 25(OH)D concentration <10 ng/ml (25 nmol/l) had a 50%
probability of mortality, whereas the risk for those with a
concentration >10 ng/ml was only 5% (P=0.019). Therefore, the
authors concluded that vitamin D deficiency may be a risk factor
for mortality in patients with COVID-19 (87). Similar results were obtained in
other studies, which reported that vitamin D deficiency
significantly increased the risk of mortality from COVID-19
(88,89). By contrast, Ling et al
(90) reported no significant
association between serum concentrations of 25(OH)D and COVID-19
mortality. However, multivariate analysis revealed that treatment
with high-dose vitamin D3 booster therapy reduced the
risk of mortality (90).
Therefore, Ling et al (90) consider that vitamin
D3, due to its low cost, may be a potential therapeutic
option for COVID-19 worldwide.
A cross-sectional study reported vitamin D
deficiency (<12 ng/ml; <30 nmol/l) in 15.6% of the samples
from individuals with symptoms suggestive of COVID-19, and the
presence of antibodies to the SARS-CoV-2 virus was higher in
subjects with vitamin D deficiency compared with that in
individuals with higher levels of 25(OH)D (P=0.003) (91). In addition, vitamin D deficiency
was identified as an independent factor for seropositivity to
SARS-CoV-2 in subjects with COVID-19 symptoms (91). Therefore, Faniyi et al
(91) conclude that
supplementation with vitamin D may be an adequate therapeutic
strategy for preventing or alleviating COVID-19.
In a case-control study by Ye et al (92), it was reported that patients with
COVID-19 presented with lower levels of 25(OH)D compared with those
in healthy control subjects (P<0.05). A subsequent sub-group
analysis comparing mild and severe COVID-19 cases identified a
significant association between vitamin D deficiency and COVID-19
severity (92).
A prospective cohort study comparing asymptomatic
individuals (group A) vs. patients with severe COVID-19 (group B)
reported a lower concentration of 25(OH)D in group B (P=0.0001).
The incidence of vitamin D deficiency (<20 ng/ml; <50 nmol/l)
and mortality were higher in group B compared with those in group A
(93). In a sub-analysis,
patients with 25(OH)D concentration <20 ng/ml (50 nmol/l)
presented with significantly higher serum concentrations of IL-6
(P=0.0300) and ferritin (P=0.0003) compared with those in patients
with a concentration ≥20 ng/ml; thus, the authors concluded that
vitamin D deficiency may increase the inflammatory status and the
possibility of a severe COVID-19 phenotype (93). By contrast, another prospective
cohort study (94) reported no
associations between vitamin D deficiency and clinical features of
patients with COVID-19; however, the authors suggested that this
result may be due to the evaluated population mainly comprising
comorbid elderly patients.
In a quasi-experimental study by Annweiler et
al (95), 66 elderly
patients (mean age, 87.7+9.0 years) with COVID-19 residing in a
nursing home were evaluated. The intervention group included
patients who received an oral bolus of 80,000 IU (2,000 ^g) vitamin
D3 as part of the routine maintenance treatment; the
control group did not receive vitamin D3
supplementation. The results demonstrated that vitamin
D3 had a protective effect on mortality, as the survival
analysis revealed a shorter survival time among residents who did
not receive vitamin D supplementation (P=0.002). Similar results
were reported in another quasi-experimental study by Annweiler
et al (96).
Additionally, it was suggested that long-term regular vitamin D
supplementation may protect against infections such as SARS-CoV-2
more effectively compared with oral bolus administered after
COVID-19 diagnosis (96).
An open-label clinical trial evaluated whether
calcifediol treatment may reduce the need for admission of patients
with COVID-19 to the intensive care unit (ICU) (97). The intervention group received
calcifediol and standard treatment (azithromycin and
hydroxychloroquine); the control group only received the standard
treatment. The patients were followed-up until they were admitted
to the ICU, discharged or succumbed to the disease. The probability
of admission to the ICU was significantly lower in the intervention
group (2%) compared with that in the control group (50%)
(P<0.001) (97). Therefore,
Entrenas Castillo et al (97) concluded that calcifediol may
improve the clinical outcome of subjects with COVID-19.
Rastogi et al (98) conducted a clinical trial to
evaluate the effects of high-dose vitamin D3 on
SARS-CoV-2 viral clearance. Patients with mild symptoms or
asymptomatic individuals positive for SARS-CoV-2 infection and with
vitamin D deficiency were randomly assigned to the intervention
group to receive 60,000 IU (1,500 ^g) of vitamin D3
daily, or the control group, who received a placebo. The
intervention was performed daily for 7 days. Patients who reached a
concentration >50 ng/ml of 25(OH)D received only one additional
dose of 60,000 IU, whereas those who did not reach the desired
25(OH)D levels received the same daily dose until day 14. The
patients were evaluated periodically until day 21 or virus
negativity. In the intervention group, ~63% of the subjects had a
negative result for SARS-CoV-2, whereas only 20.8% of those in the
control group had this outcome (P=0.018). In addition, the
intervention group presented with a more pronounced decrease in
fibrinogen levels compared with that in the control group
(P=0.001). No episodes of hypercalcemia were observed in the
evaluated population (98).
Therefore, Rastogi et al (98) considered that vitamin D may
reduce the transmission rates of SARS-CoV-2 infection.
A recent meta-analysis has reported that
insufficient vitamin D levels increase the rates of hospitalization
and mortality among patients with COVID-19 (99). Severe cases have a higher
probability of vitamin D deficiency (OR=1.64; 95% CI, 1.30-2.09;
I2=35.7%). Thus, vitamin D intervention as an adjunctive
treatment may be crucial in severe cases of COVID-19 with low
25(OH)D levels. Similarly, supplementation in therapeutic doses may
be useful for the prevention of SARS-CoV-2 infection, according to
D'Avolio et al (81),
Meltzer et al (82),
Merzon et al (85) and
Faniyi et al (91), since
the active metabolite of vitamin D exerts biological activities in
the innate immune system, in particular, through the maintenance of
the integrity of physical barriers and the promotion of
antimicrobial peptides (64). In
addition, another meta-analysis has reported an association between
low 25(OH)D levels and the risk of SARS-CoV-2 infection (OR=1.43;
95% CI, 1.00-2.05) (100).
However, these results should be interpreted with caution due to
the heterogeneity of the included studies
(I2=64.9%; P=0.036) and the risk of publication
bias. Finally, according to the meta-analysis by Martineau et
al (32), vitamin D
supplementation did not significantly affect any types of adverse
events. However, the individual recommendatios of vitamin
D3 for preventive or adjuvant treatment should be
evaluated by a physician. Similarly, a consensus is considered
necessary to propose public health policies for supplementation
with this vitamin in risk groups.
7. Immunomodulatory mechanisms of vitamin
D
As aforementioned, vitamin D in its active form
[1,25(OH)2D or calcitriol] binds to the VDR and RXR to
regulate gene transcription. The classic functions of vitamin D are
the regulation of calcium absorption, homeostasis, bone metabolism,
cell growth and division (61).
In addition, VDR is expressed in immune cells such as macrophages,
dendritic cells (Dcs), B and T lymphocytes, and neutrophils,
suggesting that vitamin D may be an important regulator of the
immune system (101,102).
Physical barriers
Physical barriers are the first line of defense
against infection. Currently, the prevention and treatment of
diseases focus on the preservation and restoration of the proper
functioning of epithelial cells (103). In the pulmonary epithelium, the
severity of acute lung injury is associated with its barrier
dysfunction (104). To maintain
the integrity of the alveolar wall, which forms a physical barrier
against the external environment, the integrity of tight junctions
(TJs) and adherens junctions (AJs) between the alveolar epithelial
cells is essential (105).
Epithelial TJs create a barrier that regulates the paracellular
permeability of small molecules (106). The composition of TJs includes
occludin, claudins and zonula occludens (ZO) proteins (105). AJs mainly comprise
transmembrane proteins such as E-cadherin, as well as intracellular
components (β-catenin and a-catenin), which regulate the adhesion
of cells to their neighbors (105,106). A recent study has reported that
in VDR−/− mice, the mRNA levels of claudins 2, 4, 10,
12, 15, and 18, as well as the protein levels of claudins 2, 4, 12,
and 18 were significantly decreased compared with those in
wild-type (WT) mice (107). In
addition, another study reported a significant decrease in mRNA and
protein levels of ZO-1 and occludin in VDR−/− compared
with WT mice (108). In both
studies, VDR−/− mice exhibited significant increases in
the levels of inflammatory mediators compared with those in WT
mice; therefore, these results suggest that VDR may serve a crucial
role in maintaining lung permeability (Fig. 2A) (107,108).
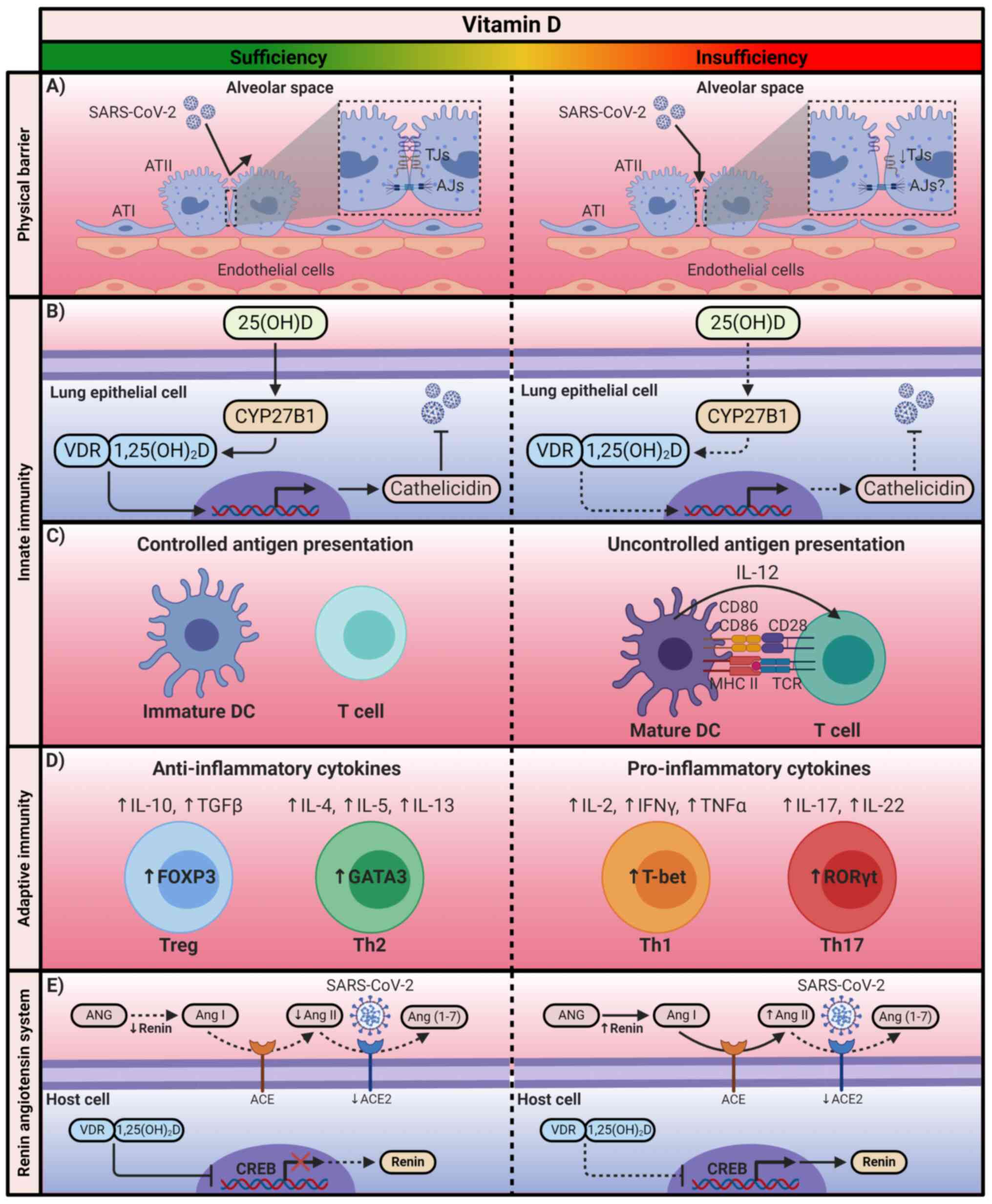 | Figure 2The immunomodulatory mechanism of
Vitamin D. Calcitriol exerts its immunomodulatory effects through
the positive or negative regulation of the transcription of the
genes associated with the immune system and the renin-angiotensin
system. SARS-CoV-2, severe acute respiratory syndrome coronavirus
2; 25(OH)D, 25-hydroxyvitamin D; 1,25(OH)2D, 1,25-dihydroxyvitamin
D; VDR, vitamin D receptor; ATI, alveolar type I cell; ATII,
alveolar type II cell; TJs, tight junctions; AJs, adherens
junctions; CYP27B1, cytochrome P450 family 27 subfamily B member 1;
DCs, dendritic cells; MCH II, major histocompatibility complex
class II; TCR, T-cell receptor; FOXP3, forkhead box P3; GATA3,
GATA-binding protein 3; T-bet, T-box transcription factor TBX21;
RORγt, Retinoic acid receptor-related orphan receptor γt; ANG,
angiotensinogen; Ang, angiotensin; ACE, angiotensin-converting
enzyme; CREB, cAMP response element-binding protein. The figure was
created using BioRender (https://biorender.com/). |
Innate immunity
The innate immune response is responsible for the
early recognition of invading pathogens to prevent infection
(109). This action is mainly
carried out by pathogen-recognition receptors, among which the
Toll-like receptors (TLRs) are prominent (110). In monocytes, the heterodimer
TLR2/1 interaction with pathogens induces antimicrobial peptides
such as β-defensins and human cathelicidin (LL-37), as well as the
activation of autophagy and phagolysosomal fusion (109,110). These antimicrobial peptides
neutralize infection by disturbing the pathogen membrane
homeostasis and promoting autophagy (109,111). Cathelicidin and autophagy
complement each other to enhance pathogen clearance (110). TLR activation induces the
expression of CYP27B1 and VDR in monocytes; subsequently, locally
produced 1,25(OH)2D exerts its function through VDRs to
upregulate the expression of p-defensins and LL-37 (112). This mechanism can also occur in
epithelial cells of the intestine and the lungs, as well as in
keratinocytes (111,113,114). By contrast, it has been
reported that the induction of cathelicidin in lung epithelial
cells is independent of TLRs (111). Previous studies have
demonstrated that the antimicrobial activity of the innate immune
response is partially dependent on vitamin D levels (Fig. 2B) (115-117).
Calcitriol can also alter the functioning of
antigen-presenting cells, which are responsible for the initiation
of the adaptive immune response. In particular, it has been
described that 1,25(OH)2D can preserve an immature
phenotype of DCs by reducing the expression of MHC class II
molecules, as well as co-stimulatory molecules (CD80, CD86), which
also results in a decline of IL-12 secretion (Fig. 2C) (118-121).
Adaptive immunity
Vitamin D also exerts immunomodulatory effects on
the adaptive immune response. Calcitriol has been reported to
suppress the activity of type 1 T-helper (Th1) cells, achieving the
repression of pro-inflammatory cytokine production, including IL-2
and IFNγ (111). The repression
of IL-2 production is mediated by the 1,25(OH)2D-VDR-RXR
complex, which blocks the formation of the nuclear factor of
activated T-cells (NFAT) and activator protein 1 complex (122). The
1,25(OH)2D-VDR-RXR complex binds to the IL-2 promoter to
interrupt the function of NFAT (111). By contrast, it has been
reported that 1,25(OH)2D-VDR-RXR binds to the IFNy
promoter to interfere with its activation (123). In addition, calcitriol affects
the regulation of IL-17 in Th17 cells (124). Although its mechanism is not
yet fully understood, it has been suggested that
1,25(OH)2D inhibits the master regulator of Th17 cells.
Additionally, the 1,25(OH)2D-VDR-RXR complex competes
with NFAT for the IL-17A promoter; once the complex is bound, it
recruits histone deacetylases to limit the transcription of this
cytokine (124-126). Calcitriol has also been
demonstrated to inhibit NF-κB via upregulation of IκB expression or
by interfering with the binding of NF-κB to DNA (Fig. 2D) (127-129).
By contrast, vitamin D favors the differentiation
of Th2 cells and the subsequent production of anti-inflammatory
cytokines IL-4, IL-5 and IL-13 (130,131). 1,25(OH)2D
upregulates GATA-binding protein 3 (GATA3), which is recognized as
the master regulator of Th2 cells (130,132). This upregulation is mediated by
the activation of STAT6, which acts upstream of GATA3 transcription
(109,130,133). In addition, vitamin D increases
the differentiation of regulatory T cells (Tregs) through the
upregulation of the transcription factor FOXP3 and CTLA-4
expression; Treg differentiation contributes to the production of
anti-inflammatory cytokines, such as IL-10 (Fig. 2D) (125,134-136).
Renin-angiotensin system
The renin-angiotensin system (RAS) comprises renin,
angiotensinogen (ANG), Ang I, Ang-converting enzyme (ACE) and Ang
II (41,137). This system acts as a cascade
where renin degrades ANG to produce Ang I; subsequently, the ACE
transforms Ang I to II (138).
Ang II regulates blood pressure and electrolyte balance (137). As aforementioned, the
maintained interaction of Ang II with AT1R contributes to the
production of pro-inflammatory cytokines by activating NF-κB and
macrophages (41). The crucial
role of ACE2 in this cascade is its ability to maintain a balance,
since ACE2 degrades Ang II to produce Ang (1-7)
with vasodilator, antiproliferative, antithrombotic and
anti-inflammatory effects (139). However, in SARS-CoV-2
infection, a negative regulation of ACE2 has been demonstrated,
which leads to the subsequent cytokine storm and, therefore, severe
COVID-19 (41,139).
1,25(OH)2D has been reported to be an
essential regulator of RAS since it suppresses the activity of
renin; although the underlying mechanism is currently unclear, it
has been suggested that 1,25(OH)2D suppresses renin
expression by blocking the binding of the cAMP response
element-binding protein with its response elements in the renin
gene promoter (Fig. 2E)
(140-142).
8. Causes of vitamin D deficiency
Vitamin D insufficiency affects ~50% of the world's
population. The high prevalence of this vitamin insufficiency is
considered a public health problem (33), since hypovitaminosis D has been
identified as an independent risk factor for all-cause mortality in
the general population (143).
Vitamin D is commonly referred to as the 'sunshine vitamin', as
most of the endogenous vitamin D is synthesized during exposure to
solar UV-B rays, which causes the formation of vitamin
D3 in the skin (33,70). Therefore, its deficiency is
generally attributed to latitude or insufficient solar radiation;
however, several additional factors contribute to low serum levels
of 25(OH)D, which is a reliable marker of vitamin D status
(144). These additional
factors that predispose individuals to vitamin D deficiency impact
tropical countries despite sufficient solar radiation intensity
(70). For example, skin
pigmentation and the use of sunscreens affect the synthesis of
vitamin D3 in the skin (145-147), low vitamin D intake and obesity
decrease the bioavailability of 25(OH)D (58,148), whereas liver failure and
nephrotic syndrome alter its synthesis and excretion, respectively
(149-151). Similarly, certain factors
impact the catabolism and synthesis of 1,25(OH)D (152-156). These and other factors are
described in detail in Table II
(157-162).
 | Table IICauses of vitamin D deficiency. |
Table II
Causes of vitamin D deficiency.
| Cause | Effect | (Refs.) |
|---|
Reduced synthesis
in the skin
Sunscreen: Use of sunscreens to prevent sunburn and skin
cancer
Skin pigmentation: Melanin reduces the penetration of UV-B
rays
Time of day: The more oblique the zenith angle, the fewer UV-B
photons reach the Earth's surface
Aging: Decreased concentration of 7-dehydrocholesterol in the
epidermis
Other factors: Clothing habits, cloud cover, pollution, season and
latitude | May reduce vitamin
D3 synthesis under strictly controlled
conditions
Reduced effectiveness of vitamin D3 synthesis in the
skin
The production of vitamin D3 in the skin is absent in
the early hours or late in the day 50% decrease in vitamin
D3 production in old age
Decrease or absence of vitamin D3 synthesis in the
skin | (145-147,159,160) |
Decreased
bioavailability
Diet: Limited intake of natural and fortified foods with vitamin D,
lack of supplementation
Malabsorption: Intestinal malabsorption syndromes (cystic fibrosis,
celiac disease, inflammatory bowel disease and short bowel
syndrome); bile acid sequestrants (colestipol and cholestyramine)
and lipase inhibitors (orlistat)
Obesity: Volumetric dilution of vitamin D in the compartments that
are increased in obesity (serum, muscle, liver and adipose
tissue) | Decreased
bioavailability of 25(OH)D
Decreased ability to absorb vitamin D
Decreased bioavailability of 25(OH)D | (58,148,161,162) |
Increased
catabolism
Drugs: Glucocorticoid and antiepileptic treatment |
1,25(OH)2D degradation due to
increased
24-OHase activity. These drugs may increase the expression of
24-OHase through the activation of the pregnane X receptor | (152-154) |
Decreased synthesis
of 25(OH)D
Liver failure: Chronic liver disease | Decreased
hydroxylation of vitamin D resulting in low levels of 25(OH)D | (149) |
Increased urinary
loss of 25(OH)D
Nephrotic syndrome: 25(OH)D bound to DBP is lost in the urine | Significant loss of
25(OH)D in urine due to proteinuria | (150,151) |
Decreased synthesis
of 1,25(OH)2D
Chronic kidney disease: Decreased renal mass limits the amount of
CYP27B1; decreased glomerular filtration rate may limit delivery of
substrate to the CYP27B1 | Progressive
decrease in 1,25(OH)2D during the course of kidney
disease | (155,156) |
Genetic
polymorphisms
Single nucleotide polymorphisms at DHCR7 (rs7944926),
GC (rs2282679), CYP2R1 (rs10741657), CYP27B1
(rs10877012) and VDR (rs2228570) | Low serum 25(OH)D
concentrations and less effective transcriptional activation of
VDR | (157,158) |
9. Vitamin D deficiency in Latin America: A
paradox of the tropical zone
As aforementioned, in addition to latitude, various
factors influence vitamin D levels in the human body (62). Therefore, the Latin American
population, the vast majority of which resides in the tropical
zone, is not exempt from vitamin D deficiency (70). Vitamin D insufficiency in this
region may be a public health problem; however, despite studies
that report 25(OH)D deficiencies in the Latin American population,
it is impossible to establish the magnitude of the problem due to
the lack of nationally representative data (35). Table III summarizes the most recent
reports on the status of 25(OH)D in the population of Latin
American countries that are among the top 10 countries by confirmed
cases and deaths from COVID-19 globally (18,163-167). Although the definition of serum
25(OH)D status varies among the studies, the majority of these
reports align with the recommendations of the Institute of Medicine
(IOM) (168) and the Endocrine
Society (169): Vitamin D
deficiency is defined as serum levels of 25(OH)D <20 ng/ml
(<50 nmol/l), and vitamin D insufficiency is defined as serum
levels between 21 ng/ml (52.5 nmol/l) and 29 ng/ml (72.5 nmol/l).
Of note, the IOM suggests that 25(OH)D levels >20 ng/ml (>50
nmol/l) are sufficient to meet the needs of ~98% of the population;
however, this recommendation mainly considers the maintenance of
bone health (168).
 | Table IIISerum concentration of 25(OH)D in
some Latin American countries. |
Table III
Serum concentration of 25(OH)D in
some Latin American countries.
| Country | Age group | Age (years) | N | Mean 25(OH)D,
ng/ml | (Refs.) |
|---|
| Brazil | Adults | 39.8±10.9 | 572 | 23.2±5.9 | (163) |
| Mexico | Adults | 57.8±16.6 | 117 | 18.4±7.2 | (164) |
| Peru | Adolescents | 14.9±0.8 | 1,441 | 25.3 (rangeb, 73.1) | (165) |
| Chile | Adult
women
Older women | 35.4±8.5
73.6±6.6 | 1,245
686 | 20.2±8.0
18±8.5 | (166) |
| Colombia | Adults | 57a (rangeb, 24.0) | 1,339 | 32.3a(rangeb, 23.2) | (167) |
10. Vitamin D supplementation for infection
prevention
Various factors cause a high prevalence of vitamin
D deficiency. However, although latitude and season are key
factors, certain countries with long winters report lower rates of
deficiency compared with those in countries in the tropical zone;
this may be due to food fortification, high consumption of fatty
fish and supplementation (170). Therefore, although the
recommendations for restoring the levels of vitamin D include the
consumption of foods rich in vitamin D and increased sun exposure,
considering the difficulty of generalized access to foods such as
fish, clothing habits and the avoidance of sunlight,
supplementation may represent an effective strategy (58). Furthermore, the recommendation of
vitamin D supplementation may not only help prevent low
concentrations of 25(OH)D and improve bone health, but may also be
useful for the prevention of complications following infection with
SARS-CoV-2 (57).
Regarding the recommendations for vitamin D intake,
these may vary between populations. The IOM recommends daily
consumption of 600 IU (15 μg) for children >1 year and
adults ≤70 years (168).
Similarly, the European Food Safety Authority recommends a daily
intake of 600 IU (15 μg), with a maximum intake of 4,000
IU/day (100 μg) in healthy adults (171,172). The UK Scientific Advisory
Committee on Nutrition recommends vitamin D intake of 400 IU/day
(10 μg) for everyone in the general population >4 years
(173), whereas the European
Food Safety Agency has reported that vitamin D doses of ≤10,000
IU/day (≤250 μg) are safe if there are no comorbidities
(171,172). Other reports indicate that
doses ≤6,000 IU/day (≤150 μg) are necessary to achieve serum
25(OH)D concentrations >40 ng/ml (100 nmol/l). Doses of ≤15,000
IU/day (≤375 μg) have also been reported to be safe and
effective for rapidly increasing 25(OH)D concentrations (174). The Brazilian Society of
Endocrinology and Metabology (175) and the Ministry of Health of the
Government of Chile (176)
adhere to the recommendations indicated by the IOM. In colombia,
the colombian consensus on Vitamin D recommends an intake of ≤2,000
IU/day (≤50 μg) in cases of insufficiency, and ≤6,000 IU/day
(≤150 μg) in deficiency (177). The suggested daily intake in
Mexico is only 224 IU (5.6 μg), as there are currently no
studies demonstrating the need to supplement vitamin D in the
Mexican population (178).
Supplementation with vitamin D may be necessary for
individuals with deficiency to achieve a sufficient concentration
of 25(OH)D, which is >30 ng/ml (75 nmol/l), even with
consumption of fortified foods, since it is difficult to maintain
this concentration with food alone (179). An international consensus for
the recommended daily intake for vitamin D would be useful;
however, considering that vitamin D deficiency is an undoubted
global problem, that there is a lack of clinical trials that
accurately indicate the appropriate dose of vitamin D
supplementation. Due to the need to establish accessible strategies
for the prevention of complications from COVID-19, the authors of
the present review agree with the current proposal of Grant et
al (180), who suggested
that individuals with low levels of 25(OH)D should be supplemented
for a month with 10,000 IU/day (250 μg) of vitamin
D3 for the rapid restoration of the desired
concentrations between 40 and 60 ng/ml (100 and 150 nmol/l). For
maintenance, this should be followed by daily supplementation of
5,000 IU (125 μg).
Notably, that baseline monitoring of 25(OH)D
concentrations should be considered. In addition, avoiding high
doses of calcium and assessing the consumption of magnesium and
vitamin K2 should be considered for the prevention of
long-term adverse effects of high doses of vitamin D (180,181).
11. Conclusions
The lack of effective therapies and the uncertainty
of universal access to possible vaccines for COVID-19 demand
alternatives with potential immunomodulatory effects such as
vitamin D supplementation, which may contribute to the prevention
of respiratory infections and their complications. However, it is
necessary to await the results of the undergoing clinical trials
and to continue with the execution of further studies to determine
the effects of vitamin D supplementation on COVID-19 and establish
the ideal dosage. Observational studies appear to demonstrate an
association between low vitamin D concentrations and susceptibility
to SARS-CoV-2 infection. However, it is also vital to carry out
national and international studies to determine the prevalence of
vitamin D deficiency in Latin America. The authors of the present
study call on the corresponding authorities to assess the
fortification with vitamin D of foods for daily consumption, since
supplementation may represent a difficulty for individuals with a
low income.
Funding
This research was funded by the National Council of
Science and Technology (CONACYT Ciencia Básica; grant no.
A1-S-8774) and Universidad de Guadalajara through the
'Fortalecimiento de la Investigatión y el Posgrado 2020' fund.
Availability of data and materials
Not applicable.
Authors' contributions
FJTH, and JFMV conceived, drafted, and finalized
the manuscript. JFMV, GASZ, GGE, JHB, and GMO critically reviewed
the manuscript for important intellectual content. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
da Costa VG, Moreli ML and Saivish MV: The
emergence of SARS, MERS and novel SARS-2 coronaviruses in the 21st
century. Arch Virol. 165:1517–1526. 2020. View Article : Google Scholar
|
|
2
|
Lee C: Porcine epidemic diarrhea virus: An
emerging and re-emerging epizootic swine virus. Virol J.
12:1932015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bande F, Arshad SS, Bejo MH, Moeini H and
Omar AR: Progress and challenges toward the development of vaccines
against avian infectious bronchitis. J Immunol Res.
2015:4248602015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fouchier RA, Kuiken T, Schutten M, van
Amerongen G, van Doornum GJ, van den Hoogen BG, Peiris M, Lim W,
Stohr K and Osterhaus AD: Aetiology: Koch's postulates fulfilled
for SARS virus. Nature. 423:2402003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zaki AM, van Boheemen S, Bestebroer TM,
Osterhaus ADME and Fouchier RAM: Isolation of a novel coronavirus
from a man with pneumonia in Saudi Arabia. N Engl J Med.
367:1814–1820. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhu N, Zhang D, Wang W, Li X, Yang B, Song
J, Zhao X, Huang B, Shi W, Lu R, et al: A novel coronavirus from
patients with pneumonia in China, 2019. N Engl J Med. 382:727–733.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Coronaviridae Study Group of the
International Committee on Taxonomy of Viruses: The species Severe
acute respiratory syndrome-related coronavirus: Classifying
2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 5:536–544. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
World Health Organization: Timeline of
WHO's response to COVID-19. 2020.
|
|
9
|
Petrosillo N, Viceconte G, Ergonul O,
Ippolito G and Petersen E: COVID-19, SARS and MERS: Are they
closely related? Clin Microbiol Infect. 26:729–734. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen N, Zhou M, Dong X, Qu J, Gong F, Han
Y, Qiu Y, Wang J, Liu Y, Wei Y, et al: Epidemiological and clinical
characteristics of 99 cases of 2019 novel coronavirus pneumonia in
Wuhan, China: A descriptive study. Lancet. 395:507–513. 2020.
View Article : Google Scholar :
|
|
11
|
Liu K, Fang YY, Deng Y, Liu W, Wang MF, Ma
JP, Xiao W, Wang YN, Zhong MH, Li CH, et al: Clinical
characteristics of novel coronavirus cases in tertiary hospitals in
Hubei Province. Chin Med J (Engl). 133:1025–1031. 2020. View Article : Google Scholar
|
|
12
|
Tang S, Mao Y, Jones RM, Tan Q, Ji JS, Li
N, Shen J, LV Y, Pan L, Ding P, et al: Aerosol transmission of
SARS-CoV-2? Evidence, prevention and control. Environ Int.
144:1060392020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sanche S, Lin YT, Xu C, Romero-Severson E,
Hengartner N and KE R: High contagiousness and rapid spread of
severe acute respiratory syndrome coronavirus 2. Emerg Infect Dis.
26:1470–1477. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bauch CT, Lloyd-Smith JO, Coffee MP and
Galvani AP: Dynamically modeling SARS and other newly emerging
respiratory illnesses: Past, present, and future. Epidemiology.
16:791–801. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Walls AC, Park YJ, Tortorici MA, Wall A,
McGuire AT and Veesler D: Structure, function, and antigenicity of
the SARS-CoV-2 spike glycoprotein. Cell. 181:281–292.e6. 2020.
View Article : Google Scholar :
|
|
16
|
Hoffmann M, Kleine-Weber H, Schroeder S,
Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH,
Nitsche A, et al: SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2
and is blocked by a clinically proven protease inhibitor. Cell.
181:271–280.e8. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zou X, Chen K, Zou J, Han P, Hao J and Han
Z: Single-cell RNA-seq data analysis on the receptor ACE2
expression reveals the potential risk of different human organs
vulnerable to 2019-nCoV infection. Front Med. 14:185–192. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Johns Hopkins University Coronavirus
Resource Center: COVID-19 Dashboard by the Center for Systems
Science and Engineering (CSSE) at Johns Hopkins University (JHU).
2020.
|
|
19
|
Zheng Z, Peng F, Xu B, Zhao J, Liu H, Peng
J, Li Q, Jiang C, Zhou Y, Liu S, et al: Risk factors of critical
& mortal COVID-19 cases. A systematic literature review and
meta-analysis. J Infect. 81:e16–e25. 2020. View Article : Google Scholar
|
|
20
|
Peters R, Ee N, Peters J, Beckett N, Booth
A, Rockwood K and Anstey KJ: Common risk factors for major
noncommunicable disease, a systematic overview of reviews and
commentary. The implied potential for targeted risk reduction. Ther
Adv Chronic Dis. Oct 15–2019.Epub ahead of print. View Article : Google Scholar
|
|
21
|
Cena H and Calder PC: Defining a healthy
diet: Evidence for the role of contemporary dietary patterns in
health and disease. Nutrients. 12:3342020. View Article : Google Scholar :
|
|
22
|
Wu D, Lewis ED, Pae M and Meydani SN:
Nutritional modulation of immune function. Analysis of evidence,
mechanisms, and clinical relevance. Front Immunol. 9:31602019.
View Article : Google Scholar
|
|
23
|
Forsyth C, Kouvari M, D'Cunha NM,
Georgousopoulou EN, Panagiotakos DB, Mellor DD, Kellett J and
Naumovski N: The effects of the Mediterranean diet on rheumatoid
arthritis prevention and treatment. A systematic review of human
prospective studies. Rheumatol Int. 38:737–747. 2018. View Article : Google Scholar
|
|
24
|
Zheng R, Gonzalez A, Yue J, Wu X, Qiu M,
Gui L, Zhu S and Huang L: Efficacy and safety of vitamin D
supplementation in patients with systemic lupus erythematosus. A
meta-analysis of randomized controlled trials. Am J Med Sci.
358:104–114. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee KR, Midgette Y and Shah R: Fish oil
derived omega 3 fatty acids suppress adipose NLRP3 inflammasome
signaling in human obesity. J Endocr Soc. 3:504–515. 2018.
View Article : Google Scholar
|
|
26
|
Hussain MI, Ahmed W, Nasir M, Mushtaq MH,
Sheikh AA, Shaheen AY and Mahmood A: Immune boosting role of
vitamin E against pulmonary tuberculosis. Pak J Pharm Sci. 32(Suppl
1): S269–S276. 2019.
|
|
27
|
Martinez-Estevez NS, Alvarez-Guevara AN
and Rodriguez-Martinez CE: Effects of zinc supplementation in the
prevention of respiratory tract infections and diarrheal disease in
Colombian children. A 12-month randomised controlled trial.
Allergol Immunopathol (Madr). 44:368–375. 2016. View Article : Google Scholar
|
|
28
|
Zhang H, Yeh C, Jin Z, Ding L, Liu BY,
Zhang L and Dannelly HK: Prospective study of probiotic
supplementation results in immune stimulation and improvement of
upper respiratory infection rate. Synth Syst Biotechnol. 3:113–120.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
McCarthy MS and Martindale RG:
Immunonutrition in critical illness. What is the role? Nutr Clin
Pract. 33:348–358. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chow O and Barbul A: Immunonutrition. Role
in wound healing and tissue regeneration. Adv Wound Care (New
Rochelle). 3:46–53. 2014. View Article : Google Scholar
|
|
31
|
Pilz S, Zittermann A, Trummer C,
Theiler-Schwetz V, Lerchbaum E, Keppel MH, Grübler MR, März W and
Pandis M: Vitamin D testing and treatment. A narrative review of
current evidence. Endocr Connect. 8:R27–R43. 2019. View Article : Google Scholar
|
|
32
|
Martineau AR, Jolliffe DA, Hooper RL,
Greenberg L, Aloia JF, Bergman P, Dubnov-Raz G, Esposito S, Ganmaa
D, Ginde AA, et al: Vitamin D supplementation to prevent acute
respiratory tract infections. Systematic review and meta-analysis
of individual participant data. BMJ. 356:i65832017. View Article : Google Scholar
|
|
33
|
Nair R, Maseeh A and Vitamin D: The
'sunshine' vitamin. J Pharmacol Pharmacother. 3:118–126.
2012.PubMed/NCBI
|
|
34
|
Roth DE, Abrams SA, Aloia J, Bergeron G,
Bourassa MW, Brown KH, Calvo MS, Cashman KD, Combs G, De-Regil LM,
et al: Global prevalence and disease burden of vitamin D
deficiency: A roadmap for action in low- and middle-income
countries. Ann NY Acad Sci. 1430:44–79. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Brito A, Cori H, Olivares M, Fernanda
Mujica M, Cediel G and Lopez de Romana D: Less than adequate
vitamin D status and intake in Latin America and the Caribbean. A
problem of unknown magnitude. Food Nutr Bull. 34:52–64. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ren LL, Wang YM, Wu ZQ, Xiang ZC, Guo L,
Xu T, Jiang YZ, Xiong Y, Li YJ, Li XW, et al: Identification of a
novel coronavirus causing severe pneumonia in human: A descriptive
study. Chin Med J (Engl). 133:1015–1024. 2020. View Article : Google Scholar
|
|
37
|
Li W, Moore M J, Vasilieva N, Sui J, Wong
S K, Berne M A, Somasundaran M, Sullivan JL, Luzuriaga K, Greenough
TC, et al: Angiotensin-converting enzyme 2 is a functional receptor
for the SARS coronavirus. Nature. 426:450–454. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jia HP, Look DC, Shi L, Hickey M, Pewe L,
Netland J, Farzan M, Wohlford-Lenane C, Perlman S and McCray PB Jr:
ACE2 receptor expression and severe acute respiratory syndrome
coronavirus infection depend on differentiation of human airway
epithelia. J Virol. 79:14614–14621. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rabi FA, Al Zoubi MS, Kasasbeh GA, Salameh
DM and Al-Nasser AD: SARS-CoV-2 and coronavirus disease 2019: What
we know so far. Pathogens. 9:2312020. View Article : Google Scholar :
|
|
40
|
Yuki K, Fujiogi M and Koutsogiannaki S:
COVID-19 pathophysiology: A review. Clin Immunol. 215:1084272020.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Banu N, Panikar SS, Leal LR and Leal AR:
Protective role of ACE2 and its downregulation in SARS-CoV-2
infection leading to macrophage activation syndrome: Therapeutic
implications. Life Sci. 256:1179052020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li X, Geng M, Peng Y, Meng L and Lu S:
Molecular immune pathogenesis and diagnosis of COVID-19. J Pharm
Anal. 10:102–108. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Nile SH, Nile A, Qiu J, Li L, Jia X and
Kai G: COVID-19: Pathogenesis, cytokine storm and therapeutic
potential of interferons. Cytokine Growth Factor Rev. 53:66–70.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Burki T: COVID-19 in Latin America. Lancet
Infect Dis. 20:547–548. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bolano-Ortiz TR, Camargo-Caicedo Y,
Puliafito SE, Ruggeri MF, Bolano-Diaz S, Pascual-Flores R, Saturno
J, Ibarra-Espinosa S, Mayol-Bracero OL, Torres-Delgado E and
Cereceda-Balic F: Spread of SARS-CoV-2 through Latin America and
the Caribbean region: A look from its economic conditions, climate
and air pollution indicators. Environ Res. 191:1099382020.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
World Health Organization: Our World in
Data: Coronavirus (COVID-19) Vaccinations. 2021.
|
|
47
|
Guzman-Holst A, DeAntonio R, Prado-Cohrs D
and Juliao P: Barriers to vaccination in Latin America: A
systematic literature review. Vaccine. 38:470–481. 2020. View Article : Google Scholar
|
|
48
|
Pathak DSK, Salunke DAA, Thivari DP,
Pandey A, Nandy DK, Harish VK, Ratna D, Pandey DS, Chawla DJ,
Mujawar DJ, Dhanwate DA and Menon DV: No benefit of
hydroxychloroquine in COVID-19: Results of systematic review and
meta-analysis of randomized controlled trials'. Diabetes Metab
Syndr. 14:1673–1680. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Cao B, Wang Y, Wen D, Liu W, Wang J, Fan
G, Ruan L, Song B, Cai Y, Wei M, et al: A trial of
lopinavir-ritonavir in adults hospitalized with severe covid-19. N
Engl J Med. 382:1787–1799. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Novartis: Novartis provides update on
CAN-COVID trial in hospitalized patients with COVID-19 pneumonia
and cytokine release syndrome (CRS). 2020.
|
|
51
|
Sanofi: Sanofi provides update on Kevzara®
(sarilumab) Phase 3 trial in severe and critically ill COVID-19
patients outside the U.S. 2020.
|
|
52
|
AstraZeneca: Update on CALAVI phase II
trials for calquence in patients hospitalised with respiratory
symptoms of COVID-19. 2020.
|
|
53
|
Iserson KV: SARS-CoV-2 (COVID-19) vaccine
development and production: An ethical way forward. Camb Q Healthc
Ethics. 30:59–68. 2021. View Article : Google Scholar
|
|
54
|
Bollyky TJ, Gostin LO and Hamburg MA: The
equitable distribution of COVID-19 therapeutics and vaccines. JAMA.
323:2462–2463. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Barreto SM, Miranda JJ, Figueroa JP,
Schmidt MI, Munoz S, Kuri-Morales PP and Silva JB Jr: Epidemiology
in Latin America and the Caribbean: Current situation and
challenges. Int J Epidemiol. 41:557–571. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zumla A, Hui DS, Azhar EI, Memish ZA and
Maeurer M: Reducing mortality from 2019-nCoV: Host-directed
therapies should be an option. Lancet. 395:e35–e36. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Rhodes JM, Subramanian S, Laird E and
Kenny RA: Editorial: Low population mortality from COVID-19 in
countries south of latitude 35 degrees North supports vitamin D as
a factor determining severity. Aliment Pharmacol Ther.
51:1434–1437. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Lamberg-Allardt C: Vitamin D in foods and
as supplements. Prog Biophys Mol Biol. 92:33–38. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Jeon SM and Shin EA: Exploring vitamin D
metabolism and function in cancer. Exp Mol Med. 50:202018.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Bikle DD: Vitamin D metabolism, mechanism
of action, and clinical applications. Chem Biol. 21:319–329. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Christakos S, Dhawan P, Verstuyf A,
Verlinden L and Carmeliet G: Vitamin D: Metabolism, molecular
mechanism of action, and pleiotropic effects. Physiol Rev.
96:365–408. 2016. View Article : Google Scholar :
|
|
62
|
Holick MF: Vitamin D deficiency. N Engl J
Med. 357:266–281. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Bivona G, Agnello L and Ciaccio M: The
immunological implication of the new vitamin D metabolism. Cent J
Immunol. 43:331–334. 2018. View Article : Google Scholar
|
|
64
|
Charoenngam N and Holick MF: Immunologic
effects of vitamin d on human health and disease. Nutrients.
12:20972020. View Article : Google Scholar :
|
|
65
|
Ghebrehewet S, MacPherson P and Ho A:
Influenza. BMJ. 355:i62582016. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Tamerius JD, Shaman J, Alonso WJ,
Bloom-Feshbach K, Uejio CK, Comrie A and Viboud C: Environmental
predictors of seasonal influenza epidemics across temperate and
tropical climates. PLoS Pathog. 9:e10031942013. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Arbeitskreis Blut, Untergruppe 'Bewertung
Blutassoziierter Krankheitserreger': Influenza virus. Transfus Med
Hemother. 36:32–39. 2009. View Article : Google Scholar
|
|
68
|
Hope-simpson RE: The role of season in the
epidemiology of influenza. J Hyg (Lond). 86:35–47. 1981. View Article : Google Scholar
|
|
69
|
Cannell JJ, Vieth R, Umhau JC, Holick M F,
Grant WB, Madronich S, Garland CF and Giovannucci E: Epidemic
influenza and vitamin D. Epidemiol Infect. 134:1129–1140. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Mendes MM, Hart KH, Botelho PB and
Lanham-New SA: Vitamin D status in the tropics: Is sunlight
exposure the main determinant? Nutr Bull. 43:428–434. 2018.
View Article : Google Scholar
|
|
71
|
Huotari A and Herzig KH: Vitamin D and
living in northern latitudes-an endemic risk area for vitamin D
deficiency. Int J Circumpolar Health. 67:164–178. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Kmiec P, Zmijewski M, Waszak P, Sworczak K
and Lizakowska-Kmiec M: Vitamin D deficiency during winter months
among an adult, predominantly urban, population in Northern Poland.
Endokrynol Pol. 65:105–113. 2014.PubMed/NCBI
|
|
73
|
Kmiec P, Zmijewski M, Lizakowska-Kmiec M
and Sworczak K: Widespread vitamin D deficiency among adults from
northern Poland (54°N) after months of low and high natural UVB
radiation. Endokrynol Pol. 66:30–38. 2015.
|
|
74
|
Kroll MH, Bi C, Garber CC, Kaufman HW, Liu
D, Caston-Balderrama A, Zhang K, Clarke N, Xie M, Reitz RE, et al:
Temporal relationship between vitamin D status and parathyroid
hormone in the United States. PLoS One. 10:e01181082015. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Greene-Finestone LS, Berger C, de Groh M,
Hanley DA, Hidiroglou N, Sarafin K, Poliquin S, Krieger J, Richards
JB and Goltzman D; CaMos Research Group: 25-Hydroxyvitamin D in
canadian adults: Biological, environmental, and behavioral
correlates. Osteoporos Int. 22:1389–1399. 2011. View Article : Google Scholar
|
|
76
|
Lowen AC, Mubareka S, Steel J and Palese
P: Influenza virus transmission is dependent on relative humidity
and temperature. PLoS Pathog. 3:1470–1476. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Lowen Ac and Steel J: Roles of humidity
and temperature in shaping influenza seasonality. J Virol.
88:7692–7695. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Pham H, Rahman A, Majidi A, Waterhouse M
and Neale RE: Acute respiratory tract infection and
25-hydroxyvitamin D concentration: A systematic review and
meta-analysis. Int J Environ Res Public Health. 16:30202019.
View Article : Google Scholar :
|
|
79
|
Ilie PC, Stefanescu S and Smith L: The
role of vitamin D in the prevention of coronavirus disease 2019
infection and mortality. Aging clin Exp Res. 32:1195–1198. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Hastie CE, Mackay DF, Ho F, Celis-Morales
CA, Katikireddi SV, Niedzwiedz CL, Jani BD, Welsh P, Mair FS, Gray
SR, et al: Vitamin D concentrations and COVID-19 infection in UK
Biobank. Diabetes Metab Syndr. 14:561–565. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
D'Avolio A, Avataneo V, Manca A, Cusato J,
De Nicolo A, Lucchini R, Keller F and Cantù M: 25-Hydroxyvitamin D
concentrations are lower in patients with positive PcR for
SARS-coV-2. Nutrients. 12:13592020. View Article : Google Scholar :
|
|
82
|
Meltzer DO, Best TJ, Zhang H, Vokes T,
Arora V and Solway J: Association of vitamin D deficiency and
treatment with cOVID-19 incidence. MedRxiv. 2020.05.08.20095893.
2020.
|
|
83
|
Whittemore PB: COVID-19 fatalities,
latitude, sunlight, and vitamin D. Am J Infect control.
48:1042–1044. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Panagiotou G, Tee SA, Ihsan Y, Athar W,
Marchitelli G, Kelly D, Boot CS, Stock N, Macfarlane J, Martineau
AR, et al: Low serum 25-hydroxyvitamin D (25[OH]D) levels in
patients hospitalized with cOVID-19 are associated with greater
disease severity. Clin Endocrinol (Oxf). 93:508–511. 2020.
View Article : Google Scholar
|
|
85
|
Merzon E, Tworowski D, Gorohovski A,
Vinker S, Golan Cohen A, Green I and Frenkel Morgenstern M: Low
plasma 25(OH) vitamin D level is associated with increased risk of
COVID-19 infection: An Israeli population-based study. FEBS J.
287:3693–3702. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Batur LK and Hekim N: The role of DBP gene
polymorphisms in the prevalence of new coronavirus disease 2019
infection and mortality rate. J Med Virol. Aug 8–2020.Epub ahead of
print.
|
|
87
|
Carpagnano GE, Di Lecce V, Quaranta VN,
Zito A, Buonamico E, Capozza E, Palumbo A, Di Gioia G, Valerio VN
and Resta O: Vitamin D deficiency as a predictor of poor prognosis
in patients with acute respiratory failure due to COVID-19. J
Endocrinol Invest. Aug 9–2020.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Abrishami A, Dalili N, Mohammadi Torbati
P, Asgari R, Arab-Ahmadi M, Behnam B and Sanei-Taheri M: Possible
association of vitamin D status with lung involvement and outcome
in patients with COVID-19: A retrospective study. Eur J Nutr. Oct
30–2020.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
De Smet D, De Smet K, Herroelen P,
Gryspeerdt S and Martens GA: Serum 25(OH)D level on hospital
admission associated with COVID-19 stage and mortality. Am J Clin
Pathol. Nov 25–2020.Epub ahead of print. PubMed/NCBI
|
|
90
|
Ling SF, Broad E, Murphy R, Pappachan JM,
Pardesi-Newton S, Kong MF and Jude EB: High-dose cholecalciferol
booster therapy is associated with a reduced risk of mortality in
patients with COVID-19: A cross-sectional multi-centre
observational study. Nutrients. 12:37992020. View Article : Google Scholar :
|
|
91
|
Faniyi AA, Lugg ST, Faustini SE, Webster
C, Duffy JE, Hewison M, Shields A, Nightingale P, Richter AG and
Thickett DR: Vitamin D status and seroconversion for COVID-19 in UK
healthcare workers. Eur Respir J. Dec 10–2020.Epub ahead of print.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Ye K, Tang F, Liao X, Shaw B A, Deng M,
Huang G, Qin Z, Peng X, Xiao H, Chen C, et al: Does serum vitamin D
level affect COVID-19 infection and its severity?-A case-control
study. J Am Coll Nutr. Oct 13–2020.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Jain A, Chaurasia R, Sengar NS, Singh M,
Mahor S and Narain S: Analysis of vitamin D level among
asymptomatic and critically ill COVID-19 patients and its
correlation with inflammatory markers. Sci Rep. 10:201912020.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Cereda E, Bogliolo L, Klersy C, Lobascio
F, Masi S, Crotti S, De Stefano L, Bruno R, Corsico AG, Di Sabatino
A, et al: Vitamin D 25OH deficiency in COVID-19 patients admitted
to a tertiary referral hospital. Clin Nutr. Nov 2–2020.Epub ahead
of print. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Annweiler C, Hanotte B, Grandin de
l'Eprevier C, Sabatier JM, Lafaie L and Célarier T: Vitamin D and
survival in COVID-19 patients: A quasi-experimental study. J
Steroid Biochem Mol Biol. 204:1057712020. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Annweiler G, Corvaisier M, Gautier J,
Dubée V, Legrand E, Sacco G and Annweiler C: Vitamin D
supplementation associated to better survival in hospitalized frail
elderly COVID-19 patients: The GERIA-COVID Quasi-experimental
study. Nutrients. 12:33772020. View Article : Google Scholar :
|
|
97
|
Entrenas Castillo M, Entrenas Costa LM,
Vaquero Barrios JM, Alcala Diaz JF, Lopez Miranda J, Bouillon R and
Quesada Gomez JM: 'Effect of calcifediol treatment and best
available therapy versus best available therapy on intensive care
unit admission and mortality among patients hospitalized for
COVID-19: A pilot randomized clinical study'. J Steroid Biochem Mol
Biol. 203:1057512020. View Article : Google Scholar
|
|
98
|
Rastogi A, Bhansali A, Khare N, Suri V,
Yaddanapudi N, Sachdeva N, Puri GD and Malhotra P: Short term,
high-dose vitamin D supplementation for COVID-19 disease: A
randomised, placebo-controlled, study (SHADE study). Postgrad Med
J. Nov 12–2020.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Pereira M, Dantas Damascena A, Galvâo
Azevedo LM, de Almeida Oliveira T and da Mota Santana J: Vitamin D
deficiency aggravates COVID-19: Systematic review and
meta-analysis. Crit Rev Food Sci Nutr. Nov 4–2020.Epub ahead of
print. View Article : Google Scholar
|
|
100
|
Liu N, Sun J, Wang X, Zhang T, Zhao M and
Li H: Low vitamin D status is associated with coronavirus disease
2019 outcomes: A systematic review and meta-analysis. Int J Infect
Dis. 104:58–64. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Shoenfeld Y, Giacomelli R, Azrielant S,
Berardicurti O, Reynolds JA and Bruce IN: Vitamin D and systemic
lupus erythe-matosus-the hype and the hope. Autoimmun Rev.
17:19–23. 2018. View Article : Google Scholar
|
|
102
|
Cantorna MT, Snyder L, Lin YD and Yang L:
Vitamin D and 1,25(OH)2D regulation of T cells. Nutrients.
7:3011–3021. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Zhang YG, Wu S and Sun J: Vitamin D,
vitamin D receptor, and tissue barriers. Tissue Barriers.
1:e231182013. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Ware LB and Matthay MA: Alveolar fluid
clearance is impaired in the majority of patients with acute lung
injury and the acute respiratory distress syndrome. Am J Respir Git
care Med. 163:1376–1383. 2001. View Article : Google Scholar
|
|
105
|
Matthay MA, Zemans RL, Zimmerman GA, Arabi
YM, Beitler JR, Mercat A, Herridge M, Randolph AG and Calfee Cs:
Acute respiratory distress syndrome. Nat Rev Dis Primers. 5:182019.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Campbell HK, Maiers JL and DeMali KA:
Interplay between tight junctions & adherens junctions. Exp
cell Res. 358:39–44. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Chen H, Lu R, Zhang YG and Sun J: Vitamin
D receptor deletion leads to the destruction of tight and adherens
junctions in lungs. Tissue Barriers. 6:1–13. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Shi YY, Liu TJ, Fu JH, Xu W, Wu LL, Hou AN
and Xue XD: Vitamin D/VDR signaling attenuates
lipopolysaccharide-induced acute lung injury by maintaining the
integrity of the pulmonary epithelial barrier. Mol Med Rep.
13:1186–1194. 2016. View Article : Google Scholar :
|
|
109
|
Sassi F, Tamone C and D'Amelio P: Vitamin
D: Nutrient, hormone, and immunomodulator. Nutrients. 10:16562018.
View Article : Google Scholar :
|
|
110
|
Hewison M: Antibacterial effects of
vitamin D. Nat Rev Endocrinol. 7:337–345. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Wei R and Christakos S: Mechanisms
underlying the regulation of innate and adaptive immunity by
vitamin D. Nutrients. 7:8251–8260. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Chung C, Silwal P, Kim I, Modlin RL and Jo
EK: Vitamin D-cathelicidin axis: At the crossroads between
protective immunity and pathological inflammation during infection.
Immune Netw. 20:e122020. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Svensson D, Nebel D, Voss U, Ekblad E and
Nilsson BO: Vitamin D-induced up-regulation of human keratinocyte
cathelicidin anti-microbial peptide expression involves retinoid X
receptor a. cell Tissue Res. 366:353–362. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Schrumpf JA, van Sterkenburg MA, Verhoosel
RM, Zuyderduyn S and Hiemstra PS: Interleukin 13 exposure enhances
vitamin D-mediated expression of the human cathelicidin
antimicrobial peptide 18/LL-37 in bronchial epithelial cells.
Infect Immun. 80:4485–4494. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Kim EW, Teles RMB, Haile S, Liu PT and
Modlin RL: Vitamin D status contributes to the antimicrobial
activity of macrophages against mycobacterium leprae. PLoS Negl
Trop Dis. 12:e00066082018. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Fabri M, Stenger S, Shin DM, Yuk JM, Liu
PT, Realegeno S, Lee HM, Krutzik SR, Schenk M, Sieling PA, et al:
Vitamin D is required for IFN-gamma-mediated antimicrobial activity
of human macrophages. Sci Transl Med. 3:104ra1022011. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Martineau AR, Wilkinson KA, Newton SM,
Floto RA, Norman AW, Skolimowska K, Davidson RN, Sørensen OE,
Kampmann B, Griffiths CJ and Wilkinson RJ: IFN-gamma- and
TNF-independent vitamin D-inducible human suppression of
mycobacteria: The role of cathelicidin LL-37. J Immunol.
178:7190–7198. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Piemonti L, Monti P, Sironi M, Fraticelli
P, Leone BE, Dal cin E, Allavena P and Di carlo V: Vitamin D3
affects differentiation, maturation, and function of human
monocyte-derived dendritic cells. J Immunol. 164:4443–4451. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Széles L, Keresztes G, Töröcsik D,
Balajthy Z, Krenacs L, Poliska S, Steinmeyer A, Zuegel U, Pruenster
M, Rot A and Nagy L: 1,25-dihydroxyvitamin D3 is an autonomous
regulator of the transcriptional changes leading to a tolerogenic
dendritic cell phenotype. J Immunol. 182:2074–2083. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Aranow C: Vitamin D and the immune system.
J Investig Med. 59:881–886. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Prietl B, Treiber G, Pieber TR and Amrein
K: Vitamin D and immune function. Nutrients. 5:2502–2521. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Alroy I, Towers TL and Freedman LP:
Transcriptional repression of the interleukin-2 gene by vitamin D3:
Direct inhibition of NFATp/AP-1 complex formation by a nuclear
hormone receptor. Mol Cell Biol. 15:5789–5799. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Cippitelli M and Santoni A: Vitamin D3: A
transcriptional modulator of the interferon-gamma gene. Eur J
Immunol. 28:3017–3030. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Palmer MT, Lee YK, Maynard CL, Oliver JR,
Bikle DD, Jetten AM and Weaver CT: Lineage-specific effects of
1.25-dihydroxyvitamin D(3) on the development of effector CD4 T
cells. J Biol Chem. 286:997–1004. 2011. View Article : Google Scholar
|
|
125
|
Dankers W, Colin EM, van Hamburg JP and
Lubberts E: Vitamin D in autoimmunity: molecular mechanisms and
therapeutic potential. Front Immunol. 7:6972017. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Tang J, Zhou R, Luger D, Zhu W, Silver PB,
Grajewski RS, Su SB, Chan CC, Adorini L and Caspi RR: Calcitriol
suppresses antiretinal autoimmunity through inhibitory effects on
the Th17 effector response. J Immunol. 182:4624–4632. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Wöbke TK, Sorg BL and Steinhilber D:
Vitamin D in inflammatory diseases. Front Physiol.
5:2442014.PubMed/NCBI
|
|
128
|
Cohen-Lahav M, Shany S, Tobvin D,
Chaimovitz C and Douvdevani A: Vitamin D decreases NFkappaB
activity by increasing IkappaBalpha levels. Nephrol Dial
Transplant. 21:889–897. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Harant H, Wolff B and Lindleyl J:
1Alpha,25-dihydroxyvitaminD3 decreases DNA binding of nuclear
factor-kappa B in human fibroblasts. FEBS Lett. 436:329–334. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Sloka S, Silva C, Wang J and Yong VW:
Predominance of Th2 polarization by vitamin D through a
STAT6-dependent mechanism. J Neuroinflammation. 8:562011.
View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Calton EK, Keane KN, Newsholme P and
Soares MJ: The impact of vitamin D levels on inflammatory status: A
systematic review of immune cell studies. PLoS One.
10:e01417702015. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Ho IC, Tai TS and Pai SY: GATA3 and the
T-cell lineage: Essential functions before and after
T-helper-2-cell differentiation. Nat Rev Immunol. 9:125–135. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Baeke F, Takiishi T, Korf H, Gysemans C
and Mathieu C: Vitamin D: Modulator of the immune system. Curr Opin
Pharmacol. 10:482–496. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Joshi S, Pantalena LC, Liu XK, Gaffen SL,
Liu H, Rohowsky-Kochan C, Ichiyama K, Yoshimura A, Steinman L,
Christakos S and Youssef S: 1,25-dihydroxyvitamin D(3) ameliorates
Th17 autoimmunity via transcriptional modulation of
interleukin-17A. Mol Cell Biol. 31:3653–3669. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Jeffery LE, Burke F, Mura M, Zheng Y,
Qureshi OS, Hewison M, Walker LS, Lammas DA, Raza K and Sansom DM:
1.25-Dihydroxyvitamin D3 and IL-2 combine to inhibit T cell
production of inflammatory cytokines and promote development of
regulatory T cells expressing CTLA-4 and FoxP3. J Immunol.
183:5458–5467. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Nanduri R, Mahajan S, Bhagyaraj E, Sethi
K, Kalra R, Chandra V and Gupta P: The active form of vitamin D
transcriptionally represses Smad7 signaling and activates
extracellular signal-regulated kinase (ERK) to inhibit the
differentiation of a inflammatory T helper cell subset and suppress
experimental autoimmune encephalomyelitis. J Biol Chem.
290:12222–12236. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Li YC: Vitamin D regulation of the
renin-angiotensin system. J Cell Biochem. 88:327–331. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Fountain JH and Lappin SL: Physiology,
Renin Angiotensin System. StatPearls Publishing; Treasure Island,
FL: 2020
|
|
139
|
Mahmudpour M, Roozbeh J, Keshavarz M,
Farrokhi S and Nabipour I: COVID-19 cytokine storm: The anger of
inflammation. Cytokine. 133:1551512020. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Aygun H: Vitamin D can prevent COVID-19
infection-induced multiple organ damage. Naunyn Schmiedebergs Arch
Pharmacol. 393:1157–1160. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Ajabshir S, Asif A and Nayer A: The
effects of vitamin D on the renin-angiotensin system. J
Nephropathol. 3:41–43. 2014.PubMed/NCBI
|
|
142
|
Yuan W, Pan W, Kong J, Zheng W, Szeto FL,
Wong KE, Cohen R, Klopot A, Zhang Z and Li YC:
1,25-dihydroxyvitamin D3 suppresses renin gene transcription by
blocking the activity of the cyclic AMP response element in the
renin gene promoter. J Biol Chem. 282:29821–29830. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Melamed ML, Michos ED, Post W and Astor B:
25-hydroxyvitamin D levels and the risk of mortality in the general
population. Arch Intern Med. 168:1629–1637. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Cashman KD, van den Heuvel EG, Schoemaker
RJ, Preveraud DP, Macdonald HM and Arcot J: 25-Hydroxyvitamin D as
a biomarker of vitamin D status and its modeling to inform
strategies for prevention of vitamin D deficiency within the
population. Adv Nutr. 8:947–957. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Norval M and Wulf HC: Does chronic
sunscreen use reduce vitamin D production to insufficient levels?
Br J Dermatol. 161:732–736. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Neale RE, Khan SR, Lucas RM, Waterhouse M,
Whiteman DC and Olsen CM: The effect of sunscreen on vitamin D: A
review. Br J Dermatol. 181:907–915. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Brenner M and Hearing VJ: The protective
role of melanin against UV damage in human skin. Photochem
Photobiol. 84:539–549. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Vranic L, Mikolasevic I and Milic S:
Vitamin D deficiency: Consequence or cause of obesity? Medicina
(Kaunas). 55:5412019. View Article : Google Scholar
|
|
149
|
Iruzubieta P, Teran A, Crespo J and
Fabrega E: Vitamin D deficiency in chronic liver disease. World J
Hepatol. 6:901–915. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Goldstein DA, Haldimann B, Sherman D,
Norman AW and Massry SG: Vitamin D metabolites and calcium
metabolism in patients with nephrotic syndrome and normal renal
function. J clin Endocrinol Metab. 52:116–121. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Banerjee S, Basu S, Akhtar S, Sinha R, Sen
A and Sengupta J: Free vitamin D levels in steroid-sensitive
nephrotic syndrome and healthy controls. Pediatr Nephrol.
35:447–454. 2020. View Article : Google Scholar
|
|
152
|
Skversky AL, Kumar J, Abramowitz MK,
Kaskel FJ and Melamed ML: Association of glucocorticoid use and low
25-hydroxyvitamin D levels: Results from the national health and
nutrition examination survey (NHANEs): 2001-2006. J Clin Endocrinol
Metab. 96:3838–3845. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Fernandez H, Mohammed HT and Patel T:
Vitamin D supplementation for bone health in adults with epilepsy:
A systematic review. Epilepsia. 59:885–896. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Zhou C, Assem M, Tay JC, Watkins PB,
Blumberg B, Schuetz EG and Thummel KE: steroid and xenobiotic
receptor and vitamin D receptor crosstalk mediates cYP24 expression
and drug-induced osteomalacia. J Clin Invest. 116:1703–1712. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Al-Badr W and Martin KJ: Vitamin D and
Kidney Disease. Clin J Am Soc Nephrol. 3:1555–1560. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Chung S, Kim M, Koh ES, Hwang HS, Chang
YK, Park CW, Kim SY, Chang YS and Hong YA: serum
1,25-dihydroxyvitamin D better reflects renal parameters than
25-hydoxyvitamin D in patients with glomerular diseases. Int J Med
Sci. 14:1080–1087. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Dastani Z, Li R and Richards B: Genetic
regulation of vitamin D levels. Calcif Tissue Int. 92:106–117.
2013. View Article : Google Scholar
|
|
158
|
Wang TJ, Zhang F, Richards JB, Kestenbaum
B, van Meurs JB, Berry D, Kiel DP, streeten EA, Ohlsson C, Koller
DL, et al: Common genetic determinants of vitamin D insufficiency:
A genome-wide association study. Lancet. 376:180–188. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Alshahrani FM, Almalki MH, Aljohani N,
Alzahrani A, Alsaleh Y and Holick MF: Vitamin D: Light side and
best time of sunshine in Riyadh, audi Arabia. Dermatoendocrinol.
5:177–180. 2013. View Article : Google Scholar
|
|
160
|
Gallagher JC: Vitamin D and aging.
Endocrinol Metab Clin North Am. 42:319–332. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Margulies SL, Kurian D, Elliott MS and Han
Z: Vitamin D deficiency in patients with intestinal malabsorption
syndromes-think in and outside the gut. J Dig Dis. 16:617–633.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Robien K, Oppeneer SJ, Kelly JA and
Hamilton-Reeves JM: Drug-vitamin D interactions: A systematic
review of the literature. Nutr Clin Pract. 28:194–208. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Cembranel F, D'Orsi E, Jakovljevic Pudla
Wagner K, Weber Corseuil Giehl M, Moreno YMF and Gonzalez-Chica DA:
Obesity and 25(OH)D serum concentration are more important than
vitamin D intake for changes in nutritional status indicators: A
population-based longitudinal study in a state capital city in
Southern Brazil. Nutrients. 11:23662019. View Article : Google Scholar :
|
|
164
|
Martrnez-Zavala N, Löpez-Sanchez GN,
Vergara-Lopez A, Chavez-Tapia NC, Uribe M and Nuno-Lambarri N:
Vitamin D deficiency in Mexicans have a high prevalence: A
cross-sectional analysis of the patients from the centro Médico
Nacional 20 de Noviembre. Arch Osteoporos. 15:882020. View Article : Google Scholar
|
|
165
|
Tomaino K, Romero KM, Robinson CL, Baumann
LM, Hansel NN, Pollard SL, Gilman RH, Mougey E, Lima JJ and
Checkley W; PURA study investigators: Association between serum
25-hydroxy vitamin D levels and blood pressure among adolescents in
two resource-limited settings in Peru. Am J Hypertens.
28:1017–1023. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Solis-Urra P, Cristi-Montero C,
Romero-Parra J, Zavala-Crichton JP, Saez-Lara MJ and Plaza-Diaz J:
Passive commuting and higher sedentary time is associated with
vitamin D deficiency in adult and older women: Results from Chilean
national health survey 2016-2017. Nutrients. 11:3002019. View Article : Google Scholar
|
|
167
|
Hernando VU, Andry MM, Maria Virginia PF
and Valentina A: Vitamin D nutritional status in the adult
population in Colombia-an analytical cross-sectional study.
Heliyon. 6:e034792020. View Article : Google Scholar
|
|
168
|
Ross AC, Manson JE, Abrams SA, Aloia JF,
Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL,
Jones G, et al: The 2011 report on dietary reference intakes for
calcium and vitamin D from the institute of medicine What
clinicians need to know. J Clin Endocrinol Metab. 96:53–58. 2011.
View Article : Google Scholar
|
|
169
|
Holick MF, Binkley NC, Bischoff-Ferrari
HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM and
Endocrine Society: Evaluation, treatment, and prevention of vitamin
D deficiency: An endocrine society clinical practice guideline. J
Clin Endocrinol Metab. 96:1911–1930. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Palacios C and Gonzalez L: Is vitamin D
deficiency a major global public health problem? J Steroid Biochem
Mol Biol. 144:138–145. 2014. View Article : Google Scholar
|
|
171
|
Dobson R, Cock HR, Brex P and Giovannoni
G: Vitamin D supplementation. Pract Neurol. 18:35–42. 2018.
View Article : Google Scholar
|
|
172
|
EFSA Panel on Dietetic Products Nutrition
and Allergies (NDA): Scientific opinion on the tolerable upper
intake level of vitamin D. EFSA J. 10:28132012.
|
|
173
|
Scientific Advisory Committee on
Nutrition: Vitamin D and Health. 2016.
|
|
174
|
Ebadi M and Montano-Loza AJ: Perspective:
Improving vitamin D status in the management of COVID-19. Eur J
Clin Nutr. 74:856–859. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Maeda SS, Borba VZ, Camargo MB, Silva DM,
Borges JL, Bandeira F and Lazaretti-Castro M: Recomendaçôes da
Sociedade Brasileira de Endocrinologia e Metabologia (SBEM) para o
diagnostico e tratamento da hipovitaminose D. Arq Bras Endocrinol
Metabol. 58:411–433. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Ministerio de Salud del Gobierno de Chile:
Estudio para revision y actualization de las guias alimentarias
para la poblacion chilena. 2013.
|
|
177
|
Vasquez-Awad D, Cano-Gutiérrez CA,
Gomez-Ortiz A, Gonzalez MA, Guzman-Moreno R, Martinez-Reyes JI,
Rosero-Olarte O, Rueda-Beltz C and Acosta-Reyes JL: Vitamina D.
Consenso colombiano de expertos. Medicina. 39:140–157. 2017.
|
|
178
|
Lopez-Gonzalez D, Méndez-Sanchez L,
Guagnelli MA and Clark P: Deficiencia de vitamina D en la edad
pediatrica. una oportunidad de prevencion. Bol Med Hosp Infant Mex.
72:225–234. 2015.
|
|
179
|
Pludowski P, Holick MF, Grant WB,
Konstantynowicz J, Mascarenhas MR, Haq A, Povoroznyuk V, Balatska
N, Barbosa AP, Karonova T, et al: Vitamin D supplementation
guidelines. J Steroid Biochem Mol Biol. 175:125–135. 2018.
View Article : Google Scholar
|
|
180
|
Grant WB, Lahore H, McDonnell SL, Baggerly
CA, French CB, Aliano JL and Bhattoa HP: Evidence that vitamin D
supplementation could reduce risk of influenza and COVID-19
infections and deaths. Nutrients. 12:9882020. View Article : Google Scholar :
|
|
181
|
Goddek S: Vitamin D3 and K2 and their
potential contribution to reducing the COVID-19 mortality rate. Int
J Infect Dis. 99:286–290. 2020. View Article : Google Scholar : PubMed/NCBI
|
















