Introduction
The innate immune response is primarily elicited to
protect cells from infection or tissue injury, within minutes to
hours. However, when the onset of inflammation continues for longer
periods of time, chronic inflammation occurs and this contributes
to the pathogenesis of diseases such as arthritis, cancer,
cardiovascular and neurodegenerative diseases (1). The management of inflammatory
diseases is often combatted with the use of non-steroidal
anti-inflammatory drugs (NSAIDs), acting through the inhibition of
cyclo-oxygenase 2 (COX-2) (2,3),
corticosteroids (4), or
immunomodulatory drugs (5). The
therapeutic benefits of NSAIDs are counteracted by multiple
side-effects that arise following chronic use or high doses. The
chronic use of NSAIDs is related to cardiovascular and
gastrointestinal toxicities (6),
while high doses of these factors may cause renal dysfunction
(7). On the other hand,
prolonged corticosteroid use has several side-effects, such as
metabolic dysregulation, osteoporosis, glaucoma etc. (8), while immunomodulatory drugs cause
immunosuppression, that is often associated with infections
(9).
Taurine is a non-essential amino acid that is
abundant in all mammalian tissues (10,11) and plays a significant role in
homeostasis, since it is involved in the regulation of numerous
vital cellular processes (osmoregulation, protein phosphorylation,
membrane stability, bile acid conjugation, neuromodulation,
maintenance of calcium concentration and the detoxification of
xenobiotics) (12), thus
ensuring the proper function of various organs. Furthermore, it has
been demonstrated that taurine is effective against multiple types
of inflammatory injury (13),
including spinal cord injury (14), hepatic ischemia-reperfusion
(15), lung injury (16,17), ischemic stroke (18), lipopolysaccharide (LPS)-induced
acute lung injury (16),
2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis
(19) and dextran sodium sulfate
(DSS)-induced colitis (20,21). The anti-inflammatory effects of
taurine are usually attributed to its antioxidant effects (13), which are evidenced by the
inhibition of lipid peroxidation (22) and by its membrane-stabilizing
capacity (23).
During inflammation, taurine can be conjugated with
hypobromous acid (HOBr), generating N-bromotaurine (TauNHBr)
from either activated neutrophils or eosinophils (24,25). TauNHBr has been proven to
attenuate inflammatory conditions in a more efficient manner than
that elicited by taurine (26).
The anti-inflammatory capacity of TauNHBr is highly associated with
i) its capacity to hinder phagocyte function and to impair the
respiratory burst (26); ii) the
reduction of various pro-inflammatory mediators [tumor necrosis
factor-α (TNF-α), interleukin (IL)-6, IL-1β, IL-8 and IL-12],
nitric oxide (NO), prostaglandin E2 (PGE2)
and chemokines in both rodent and human leukocytes (27-31); iii) the inhibition of nuclear
factor κB (NF-κB) activity (27); and iv) the induction of
heme-oxygenase-1 (HO-1) expression in various cell types (3), including J774A.1 macrophages (Mφs)
(32) or rheumatoid arthritis
fibroblast-like synovial cells (33). From a clinical perspective,
TauNHBr has been proven to be beneficial in the treatment of acne
vulgaris (34), herpes zoster
(35), in the local treatment of
periodontal diseases, and in the elimination of oral biofilm
pathogens (36).
However, TauNHBr exhibits weak therapeutic
effectiveness in vivo due to its poor stability and its
rapid degradation in the blood (37). To overcome this issue, bromamine
T (BAT; chemical structure,
[CH3-C6H4-SO2-N-Br]-Na+
x 2H2O), which is a stable active bromine compound
(38), was synthesized (39). BAT, the sodium salt of
N-bromo-4-toluenesulfonamide, was the result of the reaction
of chloramine T with elemental bromine at the Institute of Hygiene
and Medical Microbiology of the Medical University of Innsbruck by
Professor Dr Waldemar Gottardi according to the method of Nair
et al (39). BAT has been
illustrated to exert anti-inflammatory and anti-microbial effects
to a similar extent as those of TauNHBr (38). In particular, BAT retains potent
anti-microbial, anti-inflammatory and anti-cancer potential in
vitro (38,40). The anti-inflammatory effect of
BAT is mediated by reducing the protein expression levels of TNF-α,
IL-6 and IL-12p40, with its most prominent effect emerging in the
inhibition of IL-12p40 protein expression, as confirmed by
enzyme-linked immunosorbent assay (ELISA) experiments (38).
BAT has been successfully used in a case of
multi-bacterial scalp infection (41). Furthermore, BAT has also been
used in patients with acne vulgaris, exerting comparable effects to
those of clindamycin, a commonly used anti-microbial (34).
The aim of the present study was to examine the
protective properties of BAT against LPS-mediated inflammation
in vitro, by using murine J774.A1 Mφς, and in vivo,
by using a murine LPS-mediated air-pouch model. Herein, the
putative effects of BAT were compared with those of taurine.
Materials and methods
Agents
Taurine (chemical structure,
C2H7NO3S or
NH2CH2CH2SO3H) was
purchased from AppliChem ITW Companies (Taurine BioChemica,
A1140,1000, Lot 3M004589). The purity was >99% and the molecular
weight was 125.15 g/mol. BAT (chemical structure,
C7H7BrNNaO2S x 2H2O or
[CH3-C6H4-SO2-N-Br]-Na+
x 2H2O) was kindly provided by the laboratories of
Professor Dr Waldemar Gottardi and Professor Dr Markus Nagl at the
Institute of Hygiene and Medical Microbiology of the Medical
University of Innsbruck (Bromamine T, Lot no 29/06/2016). BAT
(N-bromo-4-toluenesulfonamide sodium salt, BAT x
2H2O) was prepared by the reaction of chloramine T with
elemental bromine according to the method of Nair et al
(39). The specifications were a
potency of 95.8%, a bromine content of 24.83% and an effective
molecular weight of 322.02 g/mol. As a result, Nair et al
(39) demonstrated the synthesis
of BAT and Walczewska et al (38) demonstrated that BAT was a stable
active bromine compound.
Cells and cell culture
The J774.A1 murine Mφs were obtained from the
laboratories of Professor Dr Waldemar Gottardi and Professor Dr
Markus Nagl at the Institute of Hygiene and Medical Microbiology of
the Medical University of Innsbruck. All the cells were cultured in
Dulbecco's modified Eagle's medium (DMEM, 41966-029; Gibco; Thermo
Fisher Scientific, Inc.). All culture media were supplemented with
10% fetal bovine serum (FBS, 16000-044; Gibco; Thermo Fisher
Scientific, Inc.) and antibiotics (100 IU/ml penicillin and 100
μg/ml streptomycin) (full DMEM). The cells were grown in a
humidified incubator with 5% CO2 at 37°C. The J774.A1
Mφs were pre-incubated with various concentrations of the tested
agents (BAT or taurine), diluted in pure DMEM without FBS for 1.5 h
at 37°C. Subsequently, 100 ng/ml of LPS were diluted in full DMEM
and were administered to the Mφs for an additional 24 h at 37°C, as
previously described (38). Data
are presented as the means ± SEM of 3 independent experiments.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA isolation from the cultured Mφs, as well
as exudates derived from the pouches of LPS-exposed mice (model
described below), was carried out using TRIzol reagent (Invitrogen;
Thermo Fisher Scientific, Inc.). The RNA quality was confirmed,
through the presence of 28s, 18s and 5s RNA ribosomal subunits in
agarose gel electrophoresis and taking into consideration the ratio
of OD260/280 to be approximately 2 as well as the ratio of
OD260/230 to be approximately 2.2 in all samples.
Reverse transcription was performed from 1.0
μg of purified RNA using the SuperScript Reverse
Transcriptase II (Invitrogen; Thermo Fisher Scientific, Inc.)
following the manufacturer's instructions. Quantification at the
mRNA level was conducted in 96-well polymerase chain reaction (PCR)
plates using a Bio-Rad iCycler and the iQ5 Multicolor Real-Time
polymerase chain reaction (RT-PCR) detection system (Bio-Rad
Laboratories, Inc.). Each reaction contained 1X IQ SYBR-Green
Supermix (Bio-Rad Laboratories, Inc.) and 150 nmol/l of each
primer. All genes were tested in triplicate. The results were
analyzed on the iCycler software. Values were normalized against
β-actin. The relative quantification of complementary DNA (cDNA)
was performed according to the ΔΔCq method (42). Selected primers are presented in
Table SI.
Immunofluorescence
To detect the putative suppressive effect of BAT or
taurine on the nuclear translocation of phosphorylated NF-κB p65
protein (Ser 536), immunofluorescence experiments were performed.
In detail, 24-well plates were seeded overnight with
4.5x104 cells/well. The cells were pre-treated with
0.1-0.5 mM BAT or 100 mM taurine diluted in pure DMEM without FBS
for 1.5 h at 37°C. Subsequently, LPS (100 ng/ml) diluted in full
DMEM was added to each well, and the cells were incubated for 24 h
at 37°C, as previously described (38). The Mφs were washed with 1X
phosphate-buffered saline (PBS) for 5 min and fixed with 4%
paraformaldehyde (PFA) at room temperature for 10 min. The fixed
cells were permeabilized with 1x PBS/0.5% Triton X-100 for 5 min at
4°C and blocked with 1% BSA/1X PBS, diluted in 1X PBS at room
temperature. The Mφs were then incubated with the primary
anti-phospho-NF-κB-p65 monoclonal antibody (mAb; #3033; Cell
Signaling Technology, Inc.) diluted in 1% BSA/1X PBS at 4°C
overnight. The following day, the cells were incubated with (4
μg/ml) Alexa Fluor 488-labeled secondary antibody (A11008;
Thermo Fisher Scientific, Inc.) diluted in 1% BSA/1X PBS for 1 h at
room temperature, after washing with 1X PBS. Hoechst dye No. 33342
(B2261; Sigma-Aldrich Merck KGaA; 0.5 μg/ml) was used for
cellular chromatin staining. Finally, the coverslips were mounted
in Prolong Gold antifade media (Molecular Probes, Inc.) and the
cells were observed under a confocal microscope (Leica Microsystems
GmbH) with an excitation wavelength of 355 nm for Hoechst and with
an excitation wavelength of 488 nm for phosphorylated NF-κB (Ser
536). The LAS AF program was used to acquire the images.
Experiments were repeated independently 3 times and representative
images are presented.
Mice
Male black C57BL/6 mice (20 g in weight, 6 weeks of
age) were obtained from the National Hellenic Research Foundation
and housed under controlled temperature (22±2°C) and photoperiod
(12 h light; 12 h dark) with free access to water and food. All
experiments with mice were performed in the authorized animal house
of the National Hellenic Research Foundation (License no. EL 25
BIObr 031 as a breeding facility; License no. EL 25 BIOsup 032 as a
supply facility; and License no. for EL 25 BIOexp 033 as a research
installation). The experiments complied with the Protocol on the
Protection and Welfare of Animals, as obliged by the rules of the
National Hellenic Research Foundation the regulations of the
National Bioethics Committee and article 3 of the presidential
decree 160/1991 (in line with 86/609/EEC directive) regarding the
protection of experimental animals. A total of 3 mice/group were
used. The minimum number of animals was achieved according to the
3Rs (replacement, reduction and refinement), to ensure scientific
and statistical validity. The health and behaviour of the animals
were monitored daily. The mice were gradually euthanized, using
carbon dioxide (CO2). The gradual flow rate of
CO2 was as per the guidelines. The death of the animals
was confirmed through the verification of cardiac and respiratory
arrest.
Ethics approval
The present study was conducted according to the
guidelines of the Declaration of Helsinki and was approved by the
Bioethics Committee of the National Hellenic Research Foundation
(date of approval: 29/3/2020). The ethic code is PN
2-3/29-3-2020.
LPS-induced air pouch murine model of
inflammation
Air-pouches were created according to a modified
method described in the study by Sedgwick et al (43). An area of dorsal skin (4
cm2) was shaved, and 3 ml of sterile air were
subcutaneously injected to establish a single air-pouch in
6-week-old black (C57BL/6) mice. The mice were anaesthetized by an
intraperitoneal (i.p) injection of ketamine at 100 mg/kg together
with xylazine at 10 mg/kg, prior to the subcutaneous injections
during the LPS-induced air-pouch model of inflammation. The mice
weighed approximately 20 g and were housed in filtered-air
laminar-flow cabinets at a controlled temperature (22±2°C) with a
12-h light/dark cycle. A total of 3 ml of sterile air were
administered on alternate days to maintain the pouch. At 10 days
after air-pouch formation, the pouches were injected with a single
dose of 1 ml (1 μg/ml) LPS alone (positive control) or a
single dose of 1 ml (1 μg/ml) LPS plus a single dose of (3
or 6 or 9 mg/mouse) BAT concurrently or a single dose of 1 ml (1
μg/ml) LPS plus a single dose of (9 mg/mouse) taurine
concurrently, inside the air pouches of the mice for 8 h, as
previously described (44). Each
group was composed of 3 mice. This air pouch model of inflammation
has been similarly used in a wide range of studies (44-48). Inflammatory exudates were then
harvested by collecting the lavage fluids after washing the
air-pouch cavities with 2 ml PBS (1X, pH 7.4) followed by RNA
extraction. The pouch membranes were fixed in 10% (v/v) buffered
formalin for histological analysis.
Histological analysis
Ten days after air-pouch formation, substances were
administered and after 8 h, mice were euthanized and their pouches
were dissected, sampled and processed for paraffin embedding. The
protocol for paraffin embedding was as follows (total 16 h): 70%
ethanol (2 h), 80% ethanol (1 h), 95% ethanol (1 h), 100% ethanol
(4.5 h), xylene (4.5 h), paraffin (58-60°C) (4 h), embedding
tissues into paraffin blocks and trimming into the suitable 6
μm. Sections were then stained with hematoxylin (8 min) and
eosin (1 min) (H&E) at 30°C, using the following protocol: The
sections were deparaffinized, and treated with absolute alcohol (10
min), 95% alcohol (2 min), 70% alcohol (2 min) and stained with
Harris hematoxylin solution (Thermo Fisher Scientific, Inc.) (8
min) and saturated lithium carbonate (1 min) at 30°C. After
rinsing, the sections were counterstained with eosin-phloxine
solution (Thermo Fisher Scientific, Inc.) (1 min) and dehydrated
using 95% alcohol. Microphotographs were captured using a Nikon
Eclipse 80i upright microscope with CFI60 lenses, using a Leica DFC
450 C digital camera. The magnification utilized for their capture
was x200. In each section, pouch wall thickness was measured at 6
regions randomly at the upper, at the back and at the middle side
of the pouch wall. The mean of six different regions in each
section was determined. The thickness of the pouch and the area of
the corresponding region were calculated using ImageJ software
(v1.52a). The total cell number (based on nucleus count) was
calculated as cells/mm2, using the 'analyze particles'
feature of ImageJ software, in LPS-exposed mice bearing air-pouch
with or without treatment, manually. The number of PMNs was
calculated per mm2, in LPS-exposed mice bearing air
pouch with or without treatment, manually.
Statistical analysis
Data are presented as the means ± SEM. One-way
ANOVA, followed by Tukey's multiple comparison test was used to
evaluate the significance of all experiments between the LPS group
with the LPS/BAT and LPS/taurine-treated groups. Two-way ANOVA was
used to evaluate the statistical significance of weights between
the LPS group with the LPS/BAT group and LPS/taurine group.
Statistics were calculated with GraphPad Prism 6.0 (GraphPad
Software, Inc.).
Results
BAT reduces the mRNA expression levels of
pro-inflammatory mediators in LPS-stimulated J774.A1 Mφs at lower
concentrations compared to taurine
To investigate the putative inhibitory effects of
BAT and taurine on the LPS-induced inflammatory response in
vitro, the mRNA expression levels of various pro-inflammatory
cytokines secreted by J774.A1 Mφs were assessed following 1.5 h of
incubation with either BAT or taurine alone, prior to their
stimulation with LPS for 24 h. In the present study, RT-qPCR was
used to detect the mRNA expression levels of TNF-α, IL-1β, IL-18
and IL-23 following treatment of J774.A1 Mφs with either (0.1, 0.3,
0.5, 1 and 1.75 mM) BAT or (100 and 200 mM) taurine prior to their
stimulation with LPS for 24 h. Of note, BAT and taurine exerted
potent protective effects against inflammation, by reducing the
mRNA expression levels of pro-inflammatory cytokines in the
LPS-stimulated J774.A1 Mφs (Fig.
1 and Table SII). In
particular, the IL-1β mRNA levels were decreased by (51, 59, 59,
60, 81, 59 and 71%), the IL-23 mRNA levels by (68, 70, 73, 75, 81,
72 and 77%), the IL-18 mRNA levels by (15, 25, 50, 57, 81, 15 and
55%) and the TNF-α mRNA levels by (56, 79%, 83, 89, 96, 88 and 89%)
in the (0.1, 0.3, 0.5, 1 and 1.75 mM) BAT-, and in the (100 and 200
mM) taurine-treated LPS-stimulated J774.A1 Mφs compared to the
LPS-stimulated Mφs. A dose-dependent transcriptional downregulation
in the levels of pro-inflammatory cytokines was proved in the
BAT-treated LPS-stimulated J774.A1 Mφς, with the highest inhibitory
effect observed at the concentration of 1.75 mM BAT. Notably, the
highest suppression of IL-1β, IL-23 and IL-18 mRNA expression (81%)
and the greatest inhibition of TNF-α (96%) were evidenced in the
1.75 mM BAT-treated Mφs prior to the induction of LPS-mediated
inflammation. In all cases, the 1.75 mM BAT-treated LPS-stimulated
J774.A1 Mφς exhibited a greater transcriptional inhibition of all
pro-inflammatory cytokines than those of the 100 and 200 mM
taurine-treated LPS-stimulated J774.A1 Mφs. As a result, BAT
potentially exerted a more significant protective action than
taurine, by hindering the LPS inflammatory stimulus to activate
cytokine secretion from macrophages. These data were consistent
with the reduced protein expression levels of pro-inflammatory
mediators (IL-6, IL12p40, TNF-α), as previously indicated by ELISA
experiments in LPS-stimulated J774.A1 Mφs (38).
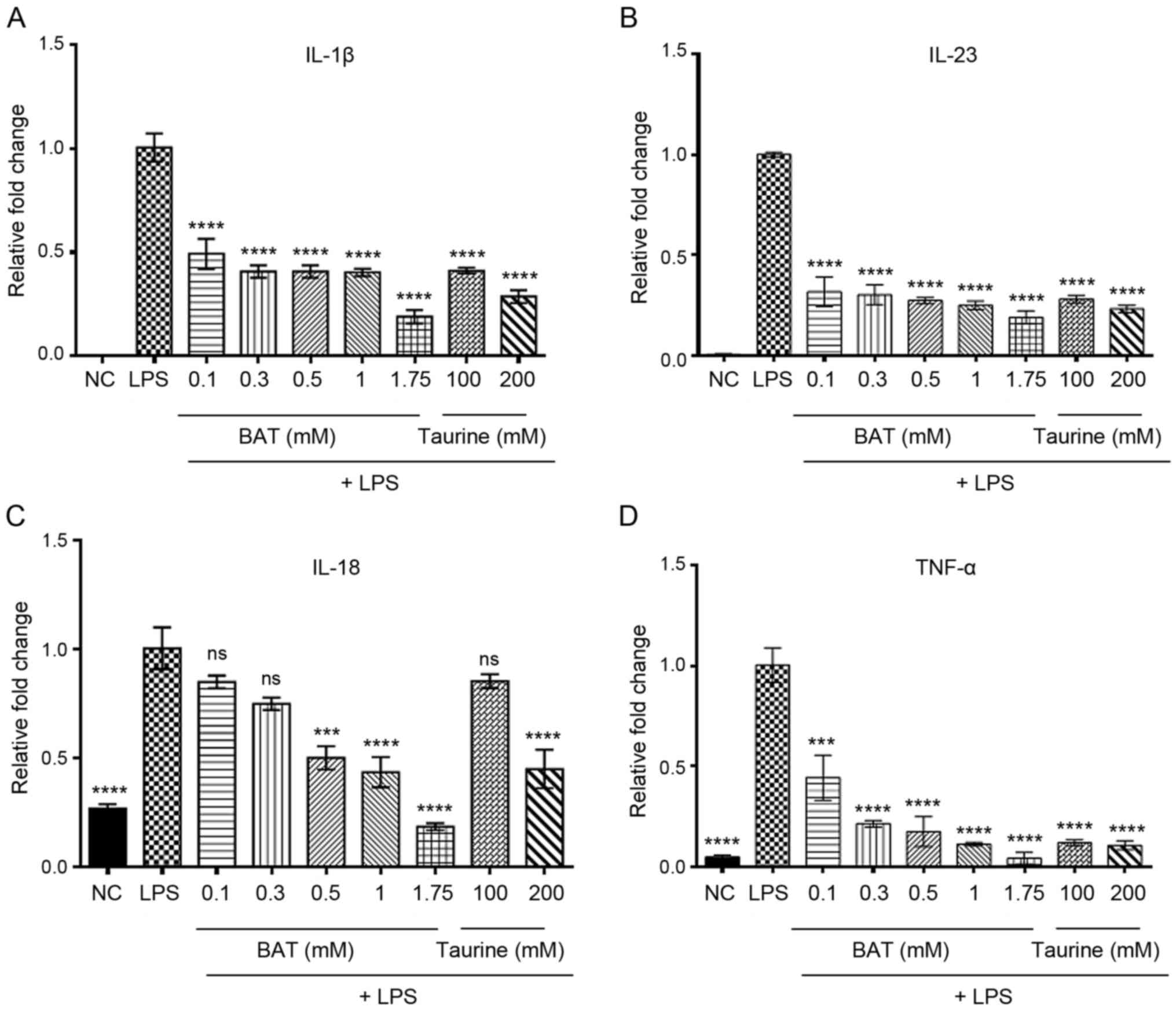 | Figure 1BAT exerts an anti-inflammatory
effect in vitro, through the transcriptional downregulation
of pro-inflammatory cytokines. RT-PCR analysis of interleukins (A)
IL-1β, (B) IL-23, (C) IL-18, (D) TNF-α mRNA levels in the
LPS-stimulated J774.A1 Mφs following (0.1-1.75 mM) BAT or (100-200
mM) taurine treatment for 1.5 h. In particular, the groups of
J774.A1 Mφς were the following: Negative control (NC), (100 ng/ml)
LPS-stimulated J774.A1 Mφs (LPS group), (100 ng/ml) LPS plus 0.1 mM
BAT-treated J774.A1 Mφs (LPS + 0.1 mM BAT group), (100 ng/ml) LPS
plus 0.3 mM BAT-treated J774.A1 Mφs (LPS + 0.3 mM BAT group), (100
ng/ml) LPS plus 0.5 mM BAT-treated J774.A1 Mφs (LPS + 0.5 mM BAT
group), (100 ng/ml) LPS plus 1 mM BAT-treated J774.A1 Mφs (LPS + 1
mM BAT group), (100 ng/ml) LPS plus 1.75 mM BAT-treated J774.A1 Mφs
(LPS + 1 mM BAT group), (100 ng/ml) LPS plus 100 mM taurine-treated
J774.A1 Mφs (LPS + 100 mM taurine group), (100 ng/ml) LPS plus 200
mM taurine-treated J774.A1 Mφs (LPS + 200 mM taurine group). Data
are presented as the means ± SEM of 3 independent experiments.
One-way ANOVA analysis followed by Tukey's multiple comparison test
revealed the statistically significant differences between LPS
alone-treated J774.A1 Mφs with LPS/BAT-treated Mφς and
LPS/taurine-treated Mφς. Statistical analysis revealed the
comparison of BAT and taurine-treated Mφs compared to LPS-treated
Mφς; ns, not significant, ***P<0.001,
****P<0.0001 vs. LPS-stimulated Mφς. Mφς,
macrophages; LPS, lipopolysaccharide; BAT, bromamine T. |
BAT inhibits the nuclear translocation of
the phosphorylated NF-κB p65 subunit in LPS-stimulated J774.A1
Mφs
To elucidate the molecular mechanisms underlying the
protective effects of BAT and taurine against
LPS-mediated-inflammation, immunofluorescence experiments were
employed to examine the exact localization of phosphorylated NF-κB
p65 subunit (Ser536) in the J774A.1 Mφs. The dynamics (serine 536
phosphorylated p65 subunit of NF-κB) were measured in BATor
taurine-treated J774.A1 Mφς, prior to their stimulation with LPS.
Following treatment with BAT or taurine, the nuclear translocation
of phospho-NF-κB p65 subunit (Ser 536) was hindered in a
dose-dependent manner, since it was mainly found in the cell
cytoplasm (Fig. 2). In
particular, the inhibition of phospho-NF-κB p65 (Ser 536) nuclear
translocation dynamics emerged in the 0.1 mM BAT-treated J774.A1
Mφς prior to their stimulation with LPS, while the most effective
inhibition of NF-κB p65 phosphorylation (Ser 536) was observed in
the 0.5 mM BAT-treated J774.A1 Mφς prior to their stimulation with
LPS, as compared to the LPS-stimulated J774A.1 Mφs. Of note, the
levels of phosphorylated NF-κB p65 in the NC J774.A1 Mφς were
similar to those of LPS-stimulated J774A.1 Mφς following BAT or
taurine treatment. As a result, BAT and taurine restored not only
the translocation, but also the levels of phosphorylated NF-κB p65
in the LPS-stimulated J774A.1 Mφς, as observed in the NC Mφς. It
was hypothesized that the sequestration of p-NF-κB p65 subunit (Ser
536) occurs in the cytoplasm of J774.A1 Mφς following BAT or
taurine treatment, prior to their stimulation with LPS, probably
due to other post-translational modifications or other mechanisms
that inhibit phospho-NF-κB p65 nuclear shutting.
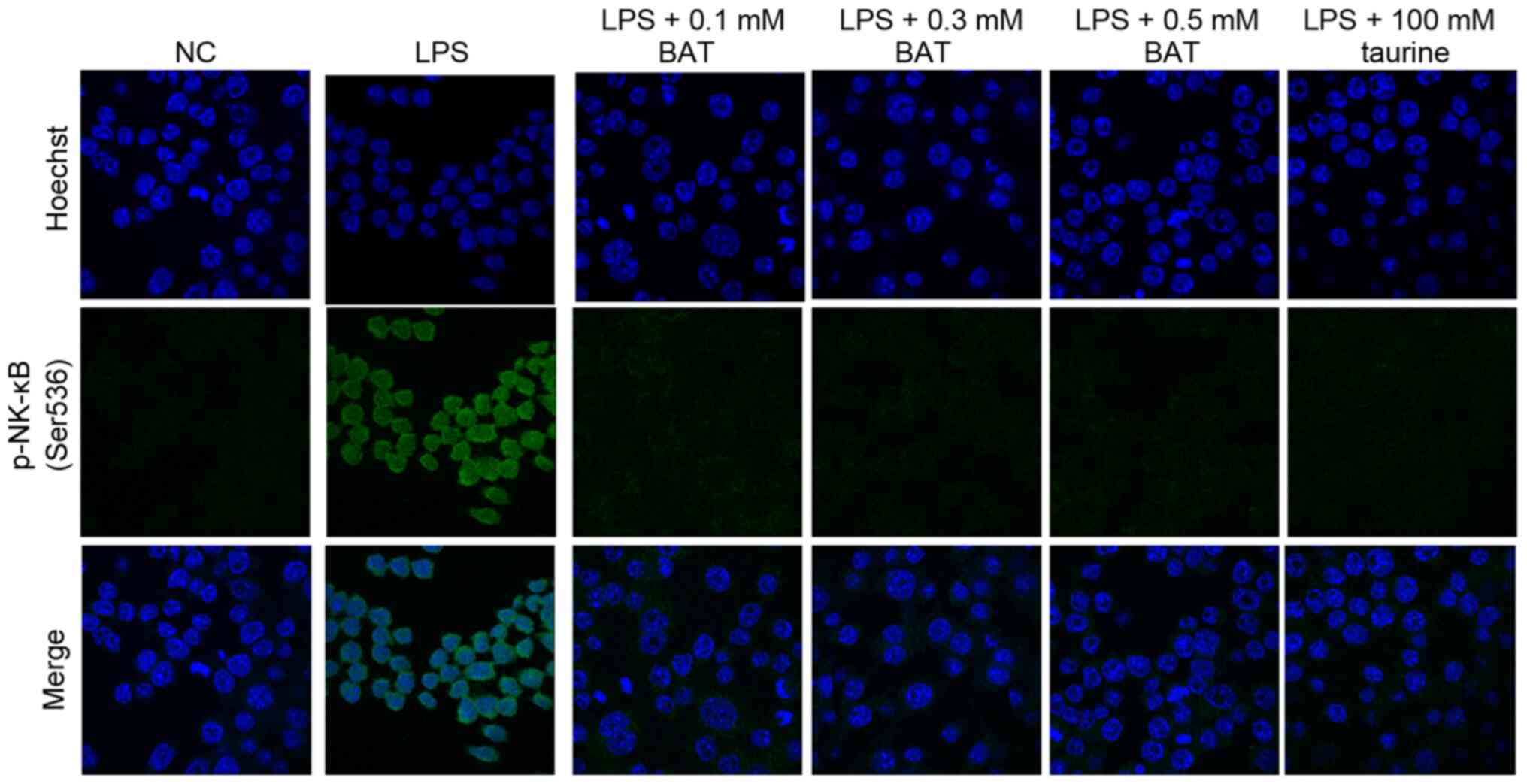 | Figure 2BAT inhibits nuclear the
translocation of phosphorylated NF-κB p65 subunit.
Immunofluorescence of phosphorylated NF-κB p65 subunit (Ser 536) in
the LPS-stimulated J774.A1 Mφs after (0.1-0.5 mM) BAT or 100 mM
taurine treatment for 1.5 h. The phosphorylated NF-κB p65 was
detected by Alexa Fluor 488-labeled immunostaining (green); nuclei
were stained with Hoechst (blue). Untreated cells were used as a
negative control (NC), while cells treated only with LPS were used
as a positive control for an inflammatory response (x630
magnification). In particular, the groups of J774.A1 Mφς were the
following: Negative control (NC), (100 ng/ml) LPS-treated J774.A1
Mφs (LPS group), (100 ng/ml) LPS plus 0.1 mM BAT-treated J774.A1
Mφs (LPS + 0.1 mM BAT group), (100 ng/ml) LPS plus 0.3 mM
BAT-treated J774.A1 Mφs (LPS + 0.3 mM BAT group), (100 ng/ml) LPS
plus 0.5 mM BAT-treated J774.A1 Mφs (LPS + 0.5 mM BAT group), (100
ng/ml) LPS plus 100 mM taurine-treated J774.A1 Mφs (LPS + 100 mM
taurine group). Mφς, macrophages; LPS, lipopolysaccharide; BAT,
bromamine T. |
BAT attenuates the LPS inflammatory
response to a greater extent than taurine
To confirm the protective effects of BAT and taurine
against LPS-mediated inflammation in vivo, the LPS-induced
air-pouch model of inflammation was used. This animal model has
been widely used to evaluate acute inflammation due to its high
sensitivity and cost-efficiency (41). In the present analysis, 4 groups
of C57BL/6 mice were subjected to the following procedure: They
were subjected to air-pouch formation subcutaneously; air
injections were administered on specific days according to the
timeline to maintain the air-pouch. At 10 days after air pouch
formation, LPS inflammatory stimulus and optionally BAT or taurine
were administered to the mice for 8 h. To elucidate the potential
protective effect of BAT against LPS-induced inflammation in
vivo, we compared its impact with that of a known
anti-inflammatory agent, namely taurine. The 4 groups of animals
were composed as follows: Group 1, mice with an air-pouch and
treated with saline; group 2, LPS-exposed mice with an air pouch;
group 3, BAT-treated LPS-exposed mice with an air pouch; and group
4, taurine-treated LPS-exposed mice with an air pouch. To ensure
that the presence of inflammation formed inside the air-pouch, the
results derived from the normal saline-negative control (NC) and
the LPS-treated group (LPS) were compared. Following the induction
of inflammation, the functional significance of either BAT or
taurine in orchestrating the distribution of inflammatory cell
populations and in determining the mRNA expression levels of
pro-inflammatory cytokines was elucidated.
Air-pouch tissues were stained with H&E to
evaluate the histological presence of various inflammatory cell
populations. The injection of (1 μg/ml) LPS into the
air-pouch on the backs of mice for 8 h resulted in increased
inflammatory cell infiltration and elevated pouch wall thickness.
All the examined parameters in the LPS group were significantly
increased compared with those in the NC group (Fig. 3 and Table SIII). Representative images with
histological changes in the pouch wall (synovial membrane and
connective tissue) in the differently treated groups compared to
the LPS group are presented in Fig.
3B. In particular, the LPS-exposed mice bearing an air pouch
treated with BAT or taurine had a fewer number of infiltrating
cells, as well as polymorphonuclear cells (PMNs) in the pouch wall
(synovial membrane and connective tissue) and thinner pouch wall
formation than those injected with LPS alone (Fig. 3 and Table SIII). Notably, pouch wall
thickness was similar between the LPS and 9 mg BAT-treated mice
bearing an air-pouch and the LPS and 9 mg taurine-treated mice
bearing an air-pouch (Fig. 3G).
The analysis of the exudates obtained from the pouches also
revealed that the inhibitory effects of BAT and taurine treatment
on the secretion of pro-inflammatory cytokines (Fig. 4 and Table SIV) were similar to those
observed in vitro (Fig. 1
and Table SI). In particular,
the LPS-exposed mice bearing an air pouch and treated with 3 or 6
or 9 mg of BAT or 9 mg of taurine exhibited decreased IL-1β mRNA
levels by 48, 81, 91 and 84%, decreased IL-23 mRNA levels by 19,
93, 94 and 39%, and decreased IL-18 mRNA levels by 26, 63, 86 and
87% respectively, as compared to the untreated LPS-exposed mice
bearing an air pouch. The IL-17 mRNA levels were reduced by 34, 51,
78 and 80% following treatment of the LPS-exposed mice bearing an
air pouch with 3, 6 and 9 mg of BAT and 9 mg of taurine,
respectively, compared to those of untreated LPS-exposed mice
bearing an air pouch. Similarly, the TNF-α mRNA levels were reduced
by 19, 53, 86 and 73% in the pouch exudates obtained from the
LPS-exposed mice and treated with 3, 6 and 9 mg of BAT or 9 mg of
taurine, respectively, as compared to those of untreated
LPS-exposed mice bearing an air pouch. Last but not least, the mRNA
levels of thymic stromal lymphopoietin (TSLP) were suppressed by
17, 61, 85 and 87% in the LPS-exposed mice with the air pouch
treated with 3, 6 and 9 mg of BAT or 9 mg of taurine, respectively,
as compared to those of untreated LPS-exposed mice bearing an air
pouch. At the transcriptional level, IL-1β, IL-23 and TNF-α were
produced at lower levels in the LPS plus 9 mg BAT-treated mice
bearing an air pouch compared to those in the LPS plus 9 mg
taurine-treated mice bearing an air pouch (Fig. 4A, B and E). Notably, the LPS plus
6 mg BAT-treated mice bearing an air pouch exhibited very low
transcriptional levels of IL-23, as opposed to the LPS plus 9 mg
taurine-treated mice bearing an air pouch (Fig. 4B). As regards the transcriptional
levels of IL-18, IL-17 and TSLP, the 2 groups of LPS plus 9 mg of
BAT and LPS plus 9 mg of taurine-treated mice bearing an air pouch
presented similar values (Fig. 4C
and F). Of note, the inhibitory effect of 9 mg BAT was the most
effective on the IL-23 mRNA levels in the LPS-exposed mice bearing
an air pouch (Fig. 4B). As a
result, the LPS plus 9 mg BAT-treated mice bearing an air pouch
exhibited a superior effect on the majority of the pro-inflammatory
cytokines tested and on cellular infiltration, compared to the
aforementioned parameters of the LPS plus 9 mg taurine-treated mice
bearing an air pouch.
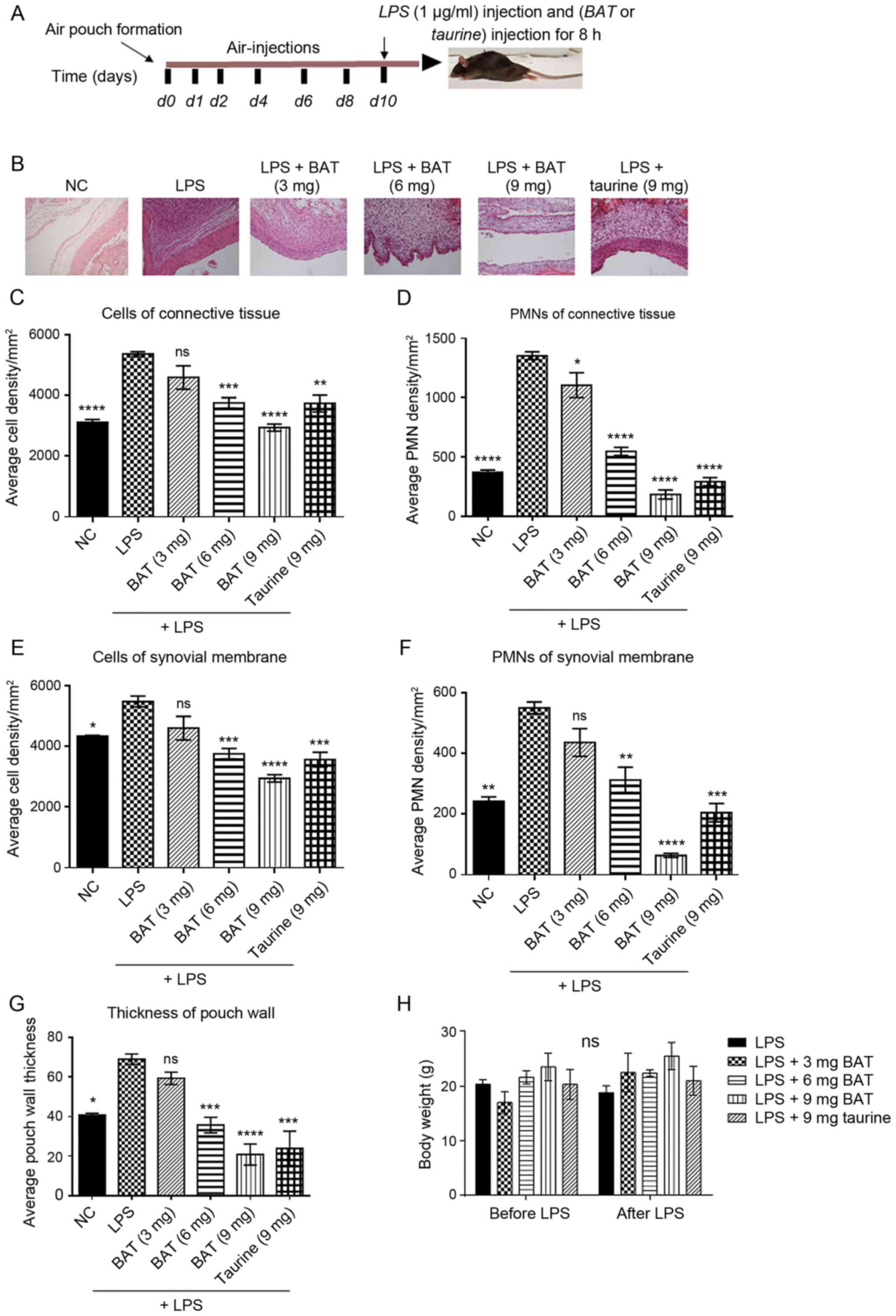 | Figure 3BAT acts as an anti-inflammatory
agent in the LPS-induced air pouch model of inflammation in
vivo, through the inhibition of total cellular infiltration and
PMN recruitment. (A) Schematic experimental design. All the groups
of mice were subjected to the following procedure: They were
subjected to air pouch formation subcutaneously, air injections
were administered on specific days according to the timeline to
mice to maintain the air pouch, and (1 μg/ml) LPS
inflammatory stimulus was administered to the mice at 10 days
following air pouch formation. The LPS group was compared with (1
μg/ml) LPS and (3 or 6 or 9 mg) BAT-treated group and with
(1 μg/ml) LPS and (9 mg) taurine-treated group for 8 h. Each
group was composed of 3 mice. (B) Representative histological
images of the pouch wall including synovial membrane and connective
tissue in all groups of mice (x200 magnification), using H&E
staining. In particular, the groups of mice were as follows:
Negative control (NC), (1 μg/ml) LPS-treated mice (LPS
group), (1 μg/ml) LPS plus (3 mg) BAT-treated mice (LPS + 3
mg BAT group), (1 μg/ml) LPS plus (6 mg) BAT-treated mice
(LPS + 6 mg BAT group), (1 μg/ml) LPS plus (9 mg)
BAT-treated mice (LPS + 9 mg BAT group), (1 μg/ml) LPS plus
(9 mg) taurine-treated mice (LPS + 9 mg taurine group). (C)
Graphical representation of the semi-quantified total cell
distribution derived from the connective tissue of experimental
groups. One-way ANOVA followed by Tukey's multiple comparison test
revealed the statistically significant differences between the
LPS-treated group with LPS/BAT-treated group and
LPS/taurine-treated group; ns, not significant,
**P<0.01, ***P<0.001,
****P<0.0001 vs. the LPS-stimulated group. (D)
Graphical representation of the semi-quantified polymorphonuclear
cell (PMNs) distribution derived from the connective tissue of
experimental groups. Each group was composed of 3 mice. One-way
ANOVA followed by Tukey's multiple comparison test revealed the
statistically significant differences between the LPS-stimulated
group with LPS/BAT-treated group and LPS/taurine-treated group;
*P<0.05, ****P<0.0001 vs. the
LPS-stimulated group. (E) Graphical representation of the
semi-quantified total cell distribution derived from the synovial
membrane of experimental groups. Each group was composed of 3 mice.
One-way ANOVA followed by Tukey's multiple comparison test revealed
the statistically significant differences between the
LPS-stimulated group with LPS/BAT-treated group and
LPS/taurine-treated group; ns, not significant,
*P<0.05, ***P<0.001,
****P<0.0001 vs. the LPS-stimulated group. (F)
Graphical representation of the semi-quantified PMN distribution
derived from the synovial membrane of experimental groups Each
group was composed of 3 mice. One-way ANOVA followed by Tukey's
multiple comparison test revealed the statistically significant
differences between the LPS-stimulated group with the
LPS/BAT-treated group and LPS/taurine-treated group; ns, not
significant, **P<0.01, ***P<0.001,
****P<0.0001 vs. the LPS-stimulated group. (G)
Quantification of pouch wall thickness on each section in all
experimental groups of mice. Random 100 μm longitudinal
pouch areas were selected to count total cells, and the mean of 6
different areas within each section was determined. LPS treatment
was used as a positive control of inflammation. The results of
untreated (NC) mice are also presented. Each group was composed of
3 mice. One-way ANOVA followed by Tukey's multiple comparison test
revealed the statistically significant differences between the
LPS-stimulated group with the LPS/BAT-treated group and
LPS/taurine-treated group; ns, not significant,
*P<0.05, ***P<0.001,
****P<0.0001 vs. the LPS-stimulated group. (H)
Graphical representation of mouse body weights, following treatment
with either BAT or taurine. BAT or taurine-treated mice did not
present any difference in body weight. Each group was composed of 3
mice. Two-way ANOVA did not reveal any statistically significant
differences between groups; ns, not significant. LPS,
lipopolysaccharide; BAT, bromamine T; PMN, polymorphonuclear
cell. |
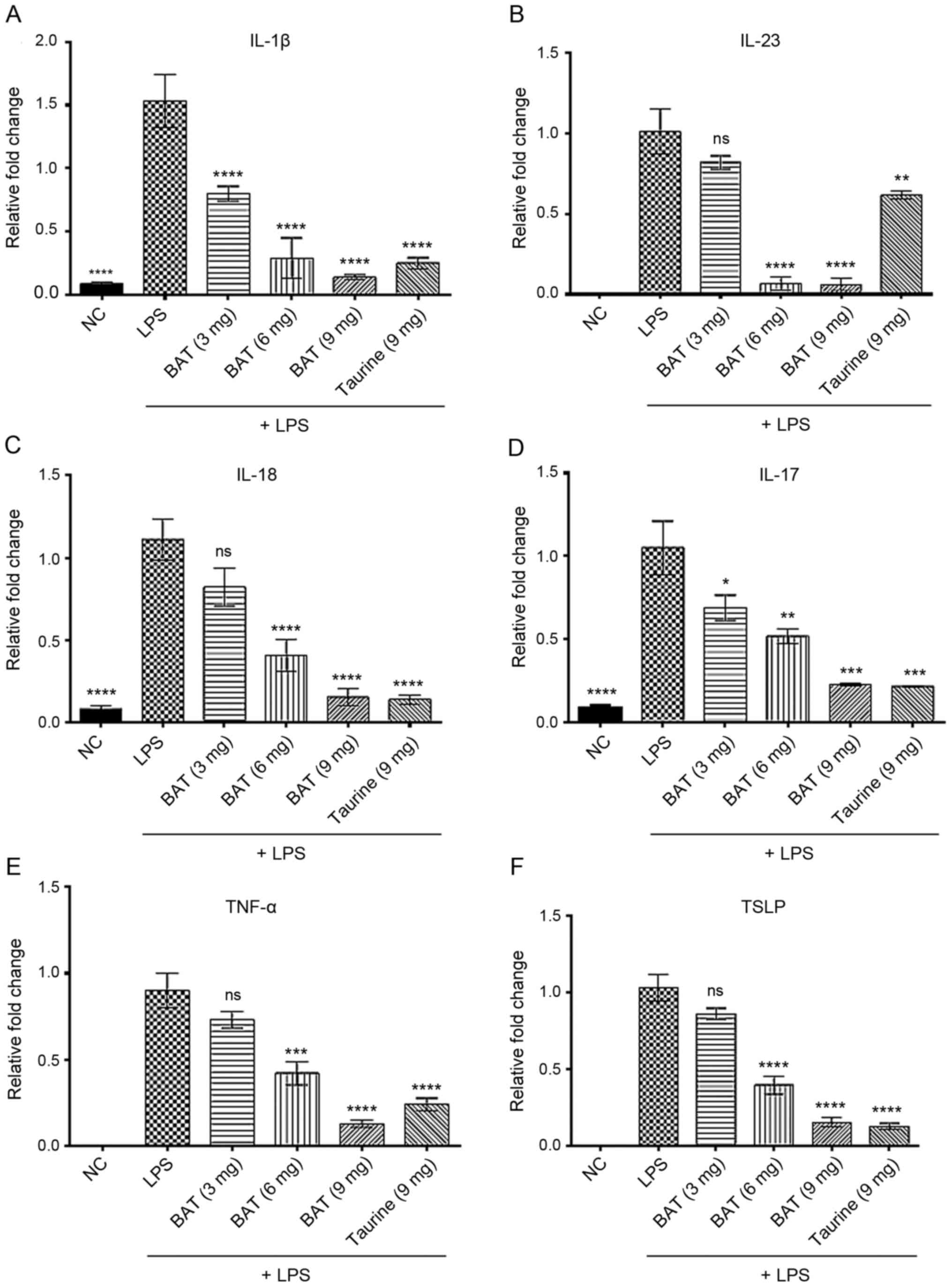 | Figure 4BAT exerts an inhibitory effect on
the transcription of pro-inflammatory cytokines in the LPS-induced
air-pouch model of inflammation in vivo. RT-PCR analysis of
(A) IL-1β, (B) IL-23, (C) IL-18, (D) IL-17, (E) TNF-α, and (F) TSLP
in (3 or 6 or 9 mg) BAT or (9 mg) taurine-treated LPS-exposed mice
with air-pouch compared to LPS-exposed mice bearing air-pouch. In
particular, the groups of mice were as follows: Negative control
(NC), (1 μg/ml) LPS-treated mice (LPS group), (1
μg/ml) LPS plus (3 mg) BAT-treated mice (LPS + 3 mg BAT
group), (1 μg/ml) LPS plus (6 mg) BAT-treated mice (LPS + 6
mg BAT group), (1 μg/ml) LPS plus (9 mg) BAT-treated mice
(LPS + 9 mg BAT group), (1 μg/ml) LPS plus (9 mg)
taurine-treated mice (LPS + 9 mg taurine group). Each group was
composed of 3 mice. One-way ANOVA analysis followed by Tukey's
multiple comparison test revealed the statistically significant
differences between the LPS-treated group with the LPS/BAT-treated
group and LPS/taurine-treated group; ns, not significant,
*P<0.05, **P<0.01,
***P<0.001, ****P<0.0001 vs. the
LPS-stimulated group. LPS, lipopolysaccharide; BAT, bromamine
T. |
Discussion
It is well established that taurine exerts a potent
anti-inflammatory effect (26);
however, its use in clinical practice is limited. This evidence has
prompted researchers to evaluate the anti-inflammatory effect of
taurine derivatives, such as TauNHBr, which comprises the reaction
product of taurine with HOBr at the inflammatory site (26). It has been reported that TauNHBr
is employed in the treatment of inflammatory and infectious
diseases, but its clinical efficacy is obstructed by high rates of
degradation (26). For this
reason, BAT was designed as the stable active bromine compound
(38) and its molecular
mechanisms in inflammation are under investigation. In the previous
study by Walczewska et al (38), the anti-inflammatory activity of
BAT was examined, starting from a concentration of 0.1 mM. Based on
those findings, the present study examined the protective
properties of BAT against LPS-induced inflammation in a
concentration range of 0.1-1.75 mM. Moreover, the biocompatibility
index of BAT (namely the ratio of the minimal concentration
exerting in vitro cytotoxicity in cell culture to the
minimal one exerting in vitro killing activity against
bacteria) ranges approximately between 1 and even 100 (38). On the other hand, in the study by
Sartori et al (49),
taurine did not exhibit any significant anti-inflammatory activity
in macrophages that were pre-treated with up to 10 mM of taurine;
thus, in the present study, a higher dose was used to examine the
potential protective activity of taurine against LPS-mediated
inflammation, given that taurine is a non-essential amino-acid in
the human setting (50).
The results of the present study indicated that the
protective effect of BAT was superior to that of taurine in
vitro using LPS-stimulated J774.A1 Mφς through the attenuation
of pro-inflammatory mediator mRNA expression. The current in
vitro observations were in accordance with the results of in
vivo experiments, as discussed below.
Initially, the results proved that BAT accounted for
the reduction of pro-inflammatory cytokines (IL-1β, IL-23, IL-18
and TNF-α) at the transcriptional level, in LPS-stimulated J774. A1
Mφς to a greater extent than taurine, through the inhibition of the
translocation of phosphorylated NF-κB p65 subunit (Ser536) in the
nuclei. Accordingly, the anti-inflammatory properties of BAT have
been observed in J774.A1 Mφς, through the suppression of the
protein expression of TNF-α, IL12p40 and PGE2 (33).
Secondly, a murine air-pouch model was used to
evaluate the protective effects of BAT on LPS-induced inflammation,
through relative qualitative and quantitative information about
inflammatory cell infiltration. Multiple inflammatory stimuli have
been used for the induction of inflammation, such as LPS, rHuPH20,
HA, Hyal type I-S and IV-S, zymosan, carrageenan (44,48,51,52). The model of LPS-induced acute
inflammation differs from other established models, due to its
greater reproducibility, the paucity of any visible side-effects
and its dependence on the activity of TNF-α, IL-1β and
myeloperoxidase (MPO) (41). In
the experiments presented herein, the potential protective action
of agents was examined 8 h following the LPS administration, since
a significant neutrophil accumulation was only observed 8 h after
the LPS injection in mice bearing an air pouch (44). A similar pattern of LPS challenge
was also used in another study (53). In the present study, pouches were
injected with a single dose of 1 ml (1 μg/ml) LPS alone
(positive control) or a single dose of 1 ml (1 μg/ml) LPS
plus a single dose of (3 or 6 or 9 mg/mouse) BAT concurrently or a
single dose of 1 ml (1 μg/ml) LPS plus a single dose of (9
mg/mouse) taurine concurrently, inside the air pouch of mice for 8
h, 10 days after air pouch formation (44). Each group was composed of 3 mice.
In the current experimental set-up, LPS-exposed mice bearing an air
pouch exhibited an increase in the inflammatory cell infiltration
and the pouch wall thickness compared to the NC mice bearing an air
pouch. To validate the putative protective role of BAT against
LPS-induced inflammation, its effects were compared with those of
taurine. The results proved that cellular density of synovial
membrane or connective tissue, pouch wall thickness and PMNs of
synovial membrane or connective tissue in BAT- or taurine-treated
groups, were significantly lower compared to the LPS group. The
highest inhibitory effect on the pro-inflammatory parameters was
observed with 9 mg of BAT and was superior to that mediated by 9 mg
of taurine on LPS-exposed mice bearing an air pouch. Notably, these
results proved the potent protective properties of BAT against
LPS-mediated inflammation in vivo, given that Mφs and
fibroblasts are accumulated, promoting the formation of granulation
tissue and subsequent collagen synthesis in the LPS-induced air
pouch model of inflammation (54). In the in vivo experiments,
higher concentrations of BAT were used relative to the in
vitro experiments. The possible presence of compounds with
amino groups (ammonium, amino acids, peptides and proteins), could
allow the mixture of bromamine compounds to be formed by transfer
of bromine, while in the presence of reducing substances (S-H
compounds, thiosulfate), BAT could be transformed to
toluenesulfonamide and, therefore, could be inactivated (personal
communication with Professor Dr M. Nagl at the Institute of Hygiene
and Medical Microbiology of the Medical University of
Innsbruck).
BAT and taurine provided a protective environment,
attenuating the possibility of inflammation caused by LPS, not only
by reducing pouch wall thickness and total cellular infiltration,
but also through the suppression of the mRNA expression of IL-1β,
IL-23, IL-18, IL-17, TNF-α and and TSLP (Fig. 4). However, the IL-6 mRNA
expression levels were not measured in the in vitro and
in vivo experiments in the present study, since protein
expression levels of IL-6 previously appeared to be reduced in the
J774A.1 Mφs following BAT treatment (38). To study the expression pattern of
specific pro-inflammatory mediators according to the applied LPS
stimulus, one of the most important pro-inflammatory mediators
(TNF-α), which is secreted from immune cells (such as Mφs, T, and B
lymphocytes) in response to LPS was evaluated (55). The present study also examined
the expression pattern of IL-1β, which is secreted by either immune
or stromal cells, constituting an important mediator of
inflammation (56). It is
well-established that the synergistic activation of TNF-α/IL-1β
mediates acute inflammatory reactions, by inducing the expression
of adhesion molecules, thus enriching the circulating leukocytes in
the inflamed site (57). The
inhibitory effect of either BAT or taurine on pro-inflammatory
cytokines (TNF-α and IL-1β) should be specially considered since
TNF-α and IL-1β have shown to play an important role in the
activation of endothelial cells by increasing the expression of
adhesion molecules, including intercellular adhesion molecule 1
(ICAM-1) and vascular cell adhesion protein 1 (VCAM-1), which are
crucial for leukocyte recruitment (58). The results of the present study
were consistent with these data, indicating that taurine is capable
of reducing the expression levels of ICAM-1 and VCAM-1 in
endothelial cells induced by hypoxia through the p38
mitogen-activated protein kinase (MAPK) pathway (59). Similarly, hyperglycemia-mediated
endothelial dysfunction is ameliorated by taurine, through
interference with adhesion molecules (60). In the present study, the analysis
of the specific cytokines was conducted only in LPS-induced
air-pouch model since TSLP is secreted by either synovial
fibroblasts (61) or bone
marrow-derived dendritic cells (DCs) (52) and IL-17 is secreted by either
CD4+ T helper (Th) cells (62) or neutrophils (63,64) in response to LPS inflammatory
stimulus. In all cases of pro-inflammatory mediators, the
protective effect of BAT was proven against LPS-mediated
inflammation. In some cases, the superiority of BAT over taurine
could be associated with the fact that BAT is an inducer of the
anti-inflammatory molecule hemo-oxygenase-1 (HO-1) (38). Furthermore, BAT reduces COX-2
levels, thus, reducing the production of inflammatory mediators
(3).
In the previous study by Walczewska et al
(38), ELISA experiments in
LPS-stimulated J774.A1 Mφs revealed reduced protein expression
levels of pro-inflammatory mediators (IL-6, IL12p40 and TNF-α)
following pre-treatment with 0.1-0.3 mM BAT. In the present study,
it was firstly demonstrated that BAT was more effective than
taurine in preventing LPS-induced inflammation through a marked
decline of mRNA expression levels of pro-inflammatory mediators
in vitro. In LPS-stimulated J774.A1 Mφs, the underlying
molecular mechanism relies on the activation of the transcription
factor NF-κB, a master regulator of pro-inflammatory cytokine
production. To the best of knowledge, this is the first study to
demonstrate that BAT prevents the mRNA expression levels of
pro-inflammatory cytokines in J774.A1 Mφs, via the inhibition of
the translocation of the phosphorylated NF-κB p65 subunit (Ser536)
in the nuclei and its subsequent cytoplasmic sequestration to
similar extent as NC Mφς. In addition, the present study provides a
comprehensive overview regarding the protective properties of BAT
or taurine against LPS-mediated inflammation in vivo. In
particular, the present study provides convincing evidence that BAT
is superior to taurine in vivo, by repressing the
transcription of the majority of pro-inflammatory cytokines to a
greater extent, and by reducing the total cell/PMN infiltration in
the air pouch of LPS-exposed mice to a greater extent. As a result,
it is demonstrated that BAT, an active bromine compound, is able to
inhibit LPS-mediated inflammation in vitro and in
vivo. The in vitro findings raise the next reasonable
challenge, whether primary macrophages derived from in vivo
experiments on LPS-inflammation can get activated and comply with
the dynamics of NF-κB signaling observed in LPS-exposed J774. A1
Mφs. The protective effect of BAT against LPS-induced inflammation
in vivo should be investigated in the future on other animal
models of acute and chronic inflammation. The data presented herein
propose the protective effect of BAT and its potential underlying
molecular mechanism, suggesting that BAT could be used as a lead
compound for the prevention of expanded acute inflammation.
Undoubtedly, further experiments are required to address the
potential anti-inflammatory effect of BAT and taurine in clinical
setting, since the majority of anti-inflammatory drugs are examined
after inflammation. Following LPS-mediated inflammation, further
experiments will shed light on the potential anti-inflammatory
effect of BAT and taurine in vitro and in vivo.
In conclusion, the present study demonstrates that
BAT and taurine inhibit the nuclear translocation of the
phosphorylated NF-κB p65 subunit (Ser536) in J774.A1 Mφs. To the
best of our knowledge, this is the first study to demonstrate the
protective properties of BAT and taurine against LPS-mediated
inflammation in vivo, using a murine LPS-mediated air pouch
model.
Supplementary Data
Funding
This study was supported by the I.K.Y. State
Scholarship Foundation for S. Baliou's Ph.D. studies. The IKY code
is 2018-050-0502-13155.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article or are available from the
corresponding author on reasonable request.
Authors' contributions
SB performed the in vitro and in vivo
experiments, analyzed the data, performed the statistical analysis
and prepared the manuscript. MS contributed to the H&E staining
and the counting of cell distribution/membrane thickness in the
samples in the in vivo experiments. SB, MG, DAS, PI and AMK
contributed to the editing of the manuscript. SB, MG, PI and VZ
contributed to the interpretation of the data. SB and PI performed
the statistical analysis of data. DAS and AMK were involved in the
conception and design of the study. VZ conceived the study, and
also contributed to the interpretation and the editing of the
manuscript. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was conducted according to the
guidelines of the Declaration of Helsinki and was approved by the
Bioethics Committee of the National Hellenic Research Foundation
(date of approval: 29/3/2020). The ethic code is PN
2-3/29-3-2020.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Editor-in-Chief for the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this article.
The other authors declare that they have no competing
interests.
Acknowledgments
The authors would like to thank Professor Dr
Waldemar Gottardi and Professor Dr Markus Nagl at the Department of
Hygiene, Microbiology and Public Health, Institute of Hygiene and
Medical Microbiology, Medical University of Innsbruck, Austria
(m.nagl@i-med.ac.at;
waldemar.gottardi@i-med.ac.at)
for the provision of bromamine T (BAT).
References
|
1
|
Lawrence T, Willoughby DA and Gilroy DW:
Anti-inflammatory lipid mediators and insights into the resolution
of inflammation. Nat Rev Immunol. 2:787–795. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Davies NM, Reynolds JK, Undeberg MR, Gates
BJ, Ohgami Y and Vega-Villa KR: Minimizing risks of NSAIDs:
Cardiovascular, gastrointestinal and renal. Expert Rev Neurother.
6:1643–1655. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kyriakopoulos AM, Nagl M, Baliou S and
Zoumpourlis V: Alleviating promotion of inflammation and cancer
induced by nonsteroidal anti-inflammatory drugs. Int J Inflam.
2017:96320182017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hardy RS, Raza K and Cooper MS:
Therapeutic glucocorticoids: Mechanisms of actions in rheumatic
diseases. Nat Rev Rheumatol. 16:133–144. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Crane E and List A: Immunomodulatory
drugs. Cancer Invest. 23:625–634. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Qandil AM: Prodrugs of nonsteroidal
anti-inflammatory drugs (NSAIDs), more than meets the eye: A
critical review. Int J Mol Sci. 13:17244–17274. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vane JR and Botting RM: Mechanism of
action of nonsteroidal anti-inflammatory drugs. Am J Med.
104:2S–8S; discussion 21S-22S. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ericson-Neilsen W and Kaye AD: Steroids:
Pharmacology, complications, and practice delivery issues. Ochsner
J. 14:203–207. 2014.PubMed/NCBI
|
|
9
|
Bascones-Martinez A, Mattila R, Gomez-Font
R and Meurman JH: Immunomodulatory drugs: Oral and systemic adverse
effects. Med Oral Patol Oral Cir Bucal. 19:e24–e31. 2014.
View Article : Google Scholar :
|
|
10
|
Leon R, Wu H, Jin Y, Wei J, Buddhala C,
Prentice H and Wu JY: Protective function of taurine in
glutamate-induced apoptosis in cultured neurons. J Neurosci Res.
87:1185–1194. 2009. View Article : Google Scholar
|
|
11
|
Chang CY, Shen CY, Kang CK, Sher YP, Sheu
WH, Chang CC and Lee TH: Taurine protects HK-2 cells from oxidized
LDL-induced cytotoxicity via the ROS-mediated mitochondrial and
p53-related apoptotic pathways. Toxicol Appl Pharmacol.
279:351–363. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schaffer S, Azuma J, Takahashi K and
Mozaffari M: Why is taurine cytoprotective? Adv Exp Med Biol.
526:307–321. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Baliou S, Kyriakopoulos AM, Spandidos DA
and Zoumpourlis V: Role of taurine, its haloamines and its lncRNA
TUG1 in both inflammation and cancer progression. On the road to
therapeutics? (Review). Int J Oncol. 57:631–664. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nakajima Y, Osuka K, Seki Y, Gupta RC,
Hara M, Takayasu M and Wakabayashi T: Taurine reduces inflammatory
responses after spinal cord injury. J Neurotrauma. 27:403–410.
2010. View Article : Google Scholar
|
|
15
|
Zhang F, Mao Y, Qiao H, Jiang H, Zhao H,
Chen X, Tong L and Sun X: Protective effects of taurine against
endotoxin-induced acute liver injury after hepatic ischemia
reperfusion. Amino Acids. 38:237–245. 2010. View Article : Google Scholar
|
|
16
|
Bhavsar TM, Cantor JO, Patel SN and
Lau-Cam CA: Attenuating effect of taurine on
lipopolysaccharide-induced acute lung injury in hamsters. Pharmacol
Res. 60:418–428. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Abdih H, Kelly CJ, Bouchier-Hayes D, Barry
M and Kearns S: Taurine prevents interleukin-2-induced acute lung
injury in rats. Eur Surg Res. 32:347–352. 2000. View Article : Google Scholar
|
|
18
|
Sun M, Zhao Y, Gu Y and Xu C:
Anti-inflammatory mechanism of taurine against ischemic stroke is
related to down-regulation of PARP and NF-κB. Amino Acids.
42:1735–1747. 2012. View Article : Google Scholar
|
|
19
|
Son MW, Ko JI, Doh HM, Kim WB, Park TS,
Shim MJ and Kim BK: Protective effect of taurine on TNBS-induced
inflammatory bowel disease in rats. Arch Pharm Res. 21:531–536.
1998. View Article : Google Scholar
|
|
20
|
Shimizu M, Zhao Z, Ishimoto Y and Satsu H:
Dietary taurine attenuates dextran sulfate sodium (DSS)-induced
experimental colitis in mice. Adv Exp Med Biol. 643:265–271. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao Z, Satsu H, Fujisawa M, Hori M,
Ishimoto Y, Totsuka M, Nambu A, Kakuta S, Ozaki H and Shimizu M:
Attenuation by dietary taurine of dextran sulfate sodium-induced
colitis in mice and of THP-1-induced damage to intestinal Caco-2
cell monolayers. Amino Acids. 35:217–224. 2008. View Article : Google Scholar
|
|
22
|
Son M, Kim HK, Kim WB, Yang J and Kim BK:
Protective effect of taurine on indomethacin-induced gastric
mucosal injury. Adv Exp Med Biol. 403:147–155. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Redmond HP, Stapleton PP, Neary P and
Bouchier-Hayes D: Immunonutrition: The role of taurine. Nutrition.
14:599–604. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Klebanoff SJ: Myeloperoxidase: Friend and
foe. J Leukoc Biol. 77:598–625. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Weiss SJ, Klein R, Slivka A and Wei M:
Chlorination of taurine by human neutrophils. Evidence for
hypochlorous acid generation. J Clin Invest. 70:598–607. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Marcinkiewicz J and Kontny E: Taurine and
inflammatory diseases. Amino Acids. 46:7–20. 2014. View Article : Google Scholar :
|
|
27
|
Marcinkiewicz J, Grabowska A, Bereta J and
Stelmaszynska T: Taurine chloramine, a product of activated
neutrophils, inhibits in vitro the generation of nitric oxide and
other macrophage inflammatory mediators. J Leukoc Biol. 58:667–674.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Marcinkiewicz J, Mak M, Bobek M, Biedroń
R, Białecka A, Koprowski M, Kontny E and Maśliński W: Is there a
role of taurine bromamine in inflammation? Interactive effects with
nitrite and hydrogen peroxide. Inflamm Res. 54:42–49. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim C, Park E, Quinn MR and Schuller-Levis
G: The production of superoxide anion and nitric oxide by cultured
murine leukocytes and the accumulation of TNF-alpha in the
conditioned media is inhibited by taurine chloramine.
Immunopharmacology. 34:89–95. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim C and Cha YN: Production of reactive
oxygen and nitrogen species in phagocytes is regulated by taurine
chloramine. Adv Exp Med Biol. 643:463–472. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Park E, Quinn MR, Wright CE and
Schuller-Levis G: Taurine chloramine inhibits the synthesis of
nitric oxide and the release of tumor necrosis factor in activated
RAW 264.7 cells. J Leukoc Biol. 54:119–124. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Olszanecki R, Kurnyta M, Biedroń R,
Chorobik P, Bereta M and Marcinkiewicz J: The role of heme
oxygenase-1 in down regulation of PGE2 production by taurine
chloramine and taurine bromamine in J774.2 macrophages. Amino
Acids. 35:359–364. 2008. View Article : Google Scholar
|
|
33
|
Kontny E, Chorąży-Massalska M, Rudnicka W,
Marcinkiewicz J and Maśliński W: Comparison of taurine chloramine
and taurine bromamine effects on rheumatoid arthritis synoviocytes.
Amino Acids. 32:447–452. 2007. View Article : Google Scholar
|
|
34
|
Marcinkiewicz J, Wojas-Pelc A, Walczewska
M, Lipko-Godlewska S, Jachowicz R, Maciejewska A, Białecka A and
Kasprowicz A: Topical taurine bromamine, a new candidate in the
treatment of moderate inflammatory acne vulgaris: A pilot study.
Eur J Dermatol. 18:433–439. 2008.PubMed/NCBI
|
|
35
|
Kyriakopoulos A, Logotheti S,
Marcinkiewicz J and Nagl M: N-chlorotaurine and N-bromotaurine
combination regimen for the cure of Valacyclovir-unresponsive
Herpes Zoster Comorbidity in a multiple Sclerosis patient. Int J
Med Pharm Case Rep. 7:1–6. 2016. View Article : Google Scholar
|
|
36
|
Pasich E, Walczewska M, Białecka A, Peruń
A, Kasprowicz A and Marcinkiewicz J: Taurine haloamines and
biofilm: II. Efficacy of taurine bromamine and chlorhexidine
against selected microorganisms of oral biofilm. Adv Exp Med Biol.
803:133–143. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Martini C, Hammerer-Lercher A, Zuck M,
Jekle A, Debabov D, Anderson M and Nagl M: Antimicrobial and
anticoagulant activities of N-Chlorotaurine, N,
N-Dichloro-2,2-dimethyltaurine, and
N-monochloro-2,2-Dimethyltaurine in human blood. Antimicrob Agents
Chemother. 56:1979–1984. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Walczewska M, Peruń A, Białecka A, Śróttek
M, Jamróz W, Dorożyński P, Jachowicz R, Kulinowski P, Nagl M,
Gottardi W and Marcinkiewicz J: Comparative analysis of
microbicidal and anti-inflammatory properties of novel taurine
bromamine derivatives and bromamine T. Adv Exp Med Biol. 975(Pt 1):
515–534. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nair CG, Lalithakumari R and Senan PI:
Bromamine-T as a new oxidimetric titrant. Talanta. 25:525–527.
1978. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Baliou S, Goulielmaki M, Ioannou P,
Cheimonidi C, Trougakos IP, Nagl M, Kyriakopoulos AM and
Zoumpourlis V: Bromamine T (BAT) exerts stronger anti-cancer
properties than taurine (Tau). Cancers (Basel). 13:1822021.
View Article : Google Scholar
|
|
41
|
Kyriakopoulos AM, Nagl M, Orth-Höller D,
Marcinkiewicz J, Baliou S and Zoumbourlis V: Successful treatment
of a unique chronic multi-bacterial scalp infection with
N-chlorotaurine, N-bromotaurine and bromamine T. Access Microbiol.
2:acmi0001262020. View Article : Google Scholar :
|
|
42
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
43
|
Sedgwick AD, Sin YM, Edwards JC and
Willoughby DA: Increased inflammatory reactivity in newly formed
lining tissue. J Pathol. 141:483–495. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Huang Z, Zhao C, Chen Y, Cowell JA, Wei G,
Kultti A, Huang L, Thompson CB, Rosengren S, Frost GI and Shepard
HM: Recombinant human hyaluronidase PH20 does not stimulate an
acute inflammatory response and inhibits lipopolysaccharide-induced
neutrophil recruitment in the air pouch model of inflammation. J
Immunol. 192:5285–5295. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Akbar M, Fraser AR, Graham GJ, Brewer JM
and Grant MH: Acute inflammatory response to cobalt chromium
orthopaedic wear debris in a rodent air-pouch model. J R Soc
Interface. 9:2109–2119. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Eteraf-Oskouei T, Mikaily Mirak S and
Najafi M: Anti-inflammatory and anti-angiogenesis effects of
verapamil on rat air pouch inflammation model. Adv Pharm Bull.
7:585–591. 2017. View Article : Google Scholar
|
|
47
|
Li B, Hu Y, Zhao Y, Cheng M, Qin H, Cheng
T, Wang Q, Peng X and Zhang X: Curcumin attenuates titanium
particle-induced inflammation by regulating macrophage polarization
in vitro and in vivo. Front Immunol. 8:552017.
|
|
48
|
Calil IL, Zarpelon AC, Guerrero AT,
Alves-Filho JC, Ferreira SH, Cunha FQ, Cunha TM and Verri WA Jr:
Lipopolysaccharide induces inflammatory hyperalgesia triggering a
TLR4/MyD88-dependent cytokine cascade in the mice paw. PLoS One.
9:e900132014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sartori T, Galvão dos Santos G,
Nogueira-Pedro A, Makiyama E, Rogero MM, Borelli P and Fock RA:
Effects of glutamine, taurine and their association on inflammatory
pathway markers in macrophages. Inflammopharmacology. 26:829–838.
2018. View Article : Google Scholar
|
|
50
|
Chesney RW: Taurine: Its biological role
and clinical implications. Adv Pediatr. 32:1–42. 1985.PubMed/NCBI
|
|
51
|
Duarte DB, Vasko MR and Fehrenbacher JC:
Models of inflammation: Carrageenan air pouch. Curr Protoc
Pharmacol. 72:5.6.1–5.6.9. 2016. View Article : Google Scholar
|
|
52
|
Zhang Y, Zhou X and Zhou B: DC-derived
TSLP promotes Th2 polarization in LPS-primed allergic airway
inflammation. Eur J Immunol. 42:1735–1743. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Segawa R, Mizuno N, Hatayama T, Jiangxu D,
Hiratsuka M, Endo Y and Hirasawa N: Lipopolysaccharide-activated
leukocytes enhance thymic stromal lymphopoietin production in a
mouse air-pouch-type inflammation model. Inflammation.
39:1527–1537. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Chen YS, Lin HH, Liu PJ, Tsai HY, Hsueh
PT, Liu HY and Chen YL: Use of 3-hydroxy fatty acid concentrations
in a murine air pouch infection model as a surrogate marker for LPS
activity: A feasibility study using environmental Burkholderia
cenocepacia isolates. J Microbiol Methods. 87:368–374. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Vassalli P: The pathophysiology of tumor
necrosis factors. Annu Rev Immunol. 10:411–452. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Germano G, Allavena P and Mantovani A:
Cytokines as a key component of cancer-related inflammation.
Cytokine. 43:374–379. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Apostolaki M, Armaka M, Victoratos P and
Kollias G: Cellular mechanisms of TNF function in models of
inflammation and autoimmunity. Curr Dir Autoimmun. 11:1–26. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Vasigar P and Batmanabane M:
Anti-inflammatoryactivity of calciumchannel blocker lercanidipine
hydrochloride. J Pharmacol Pharmacother. 4:238–242. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zhang XD, Zhao SY, Li YL, Zhu DL, Zhang R,
Sheng JJ and Wang SJ: Taurine affects expression of ICAM-1, VCAM-1
by p38 pathway in hypoxic endothelial cells. Zhongguo Zhong Yao Za
Zhi. 42:2350–2354. 2017.PubMed/NCBI
|
|
60
|
Ulrich-Merzenich G, Zeitler H, Vetter H
and Bhonde RR: Protective effects of taurine on endothelial cells
impaired by high glucose and oxidized low density lipoproteins. Eur
J Nutr. 46:431–438. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Ozawa T, Koyama K, Ando T, Ohnuma Y,
Hatsushika K, Ohba T, Sugiyama H, Hamada Y, Ogawa H, Okumura K and
Nakao A: Thymic stromal lymphopoietin secretion of synovial
fibroblasts is positively and negatively regulated by Toll-like
receptors/nuclear factor-kappaB pathway and
interferon-gamma/dexamethasone. Mod Rheumatol. 17:459–463. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zenobia C and Hajishengallis G: Basic
biology and role of interleukin-17 in immunity and inflammation.
Periodontol 2000. 69:142–159. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Eskan MA, Jotwani R, Abe T, Chmelar J, Lim
JH, Liang S, Ciero PA, Krauss JL, Li F, Rauner M, et al: The
leukocyte integrin antagonist Del-1 inhibits IL-17-mediated
inflammatory bone loss. Nat Immunol. 13:465–473. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Li L, Huang L, Vergis AL, Ye H, Bajwa A,
Narayan V, Strieter RM, Rosin DL and Okusa MD: IL-17 produced by
neutrophils regulates IFN-gamma-mediated neutrophil migration in
mouse kidney ischemia-reperfusion injury. J Clin Invest.
120:331–342. 2010. View Article : Google Scholar
|


















