Introduction
The skin is a complex tissue composed of two
different compartments: The epidermis, comprised mostly of
keratinocytes, and the underlying dermal matrix, with fibroblasts
as its major cellular component (1). Cutaneous squamous cell carcinoma
(cSCC) is characterized by the abnormal proliferation of
keratinocytes, which leads to the development of tumors,
principally of the scalp, face and the back of the hand (2). It is the most widespread type of
skin cancer and is followed by basal cell carcinoma (3). Over the last decades, the incidence
of cSCC has increased by 10% per year (4), mostly among the young population
(5). Although this type of skin
cancer is characterized by an intricate etiology, which often leads
to misdiagnosis, exposure to ultraviolet (UV) radiation may be the
primary cause of cSCC, resulting in DNA damage (6). cSCC is associated with a high
degree of malignancy and an elevated level of invasion (7). Indeed, recurrent cSCC is associated
with a higher risk of aesthetic co-morbidity, the development of
distant metastasis and mortality (8).
Radiation therapy, chemotherapy, biological
therapies and surgery are conventional treatments for cSCC
(9,10). The primary treatment regimen
selected by oncologists is early surgical intervention. However,
the invasiveness of the procedure causes marked esthetical changes
that often cause high psychological distress to the patients
(11). In addition, several
side-effects of long-term chemotherapy have been reported,
including chemoresistance, fibrosis, necrosis and secondary tumor
development (12). On the other
hand, the radiotherapy regimen can cause skin complications, such
as exudation, dermatitis, peeling and ulcers resulting from the
inflammation caused by this type of treatment (13). Therefore, the development of
potential alternative therapies is necessary for cSCC.
Cisplatinum [cis-diammine-dichloroplatinum (II)] is
a potent chemotherapeutic agent widely used in the treatment of
skin cancer (14-16), among a wide variety of tumor
types, including testicular (17,18), ovarian (19,20), cervical (21-24), head and neck (25-27) and lung (28,29) cancer. It has been observed that
cisplatinum induces DNA damage and blocks cell cycle progression at
the specific G2/M checkpoint (30), leading to the induction of
apoptosis of proliferating cells (31). However, it has a relatively high
cytotoxic activity and, consequently, its application must be
limited to the prevention of side-effects, such as ototoxicity and
neurotoxicity (32), which are
considered dose-limiting effects (33). Clinicians have therefore
developed a strategy with which to restrict the cytotoxicity of
cisplatinum, which includes its combination with non-platinum-based
drugs, such as topoisomerase inhibitors, antimetabolites and
taxanes (34,35). However, numerous pre-clinical and
clinical studies conducted on these combined therapies have yielded
contradictory results, which seemed to depend on the treatment
regimen and tumor cell features (12,34,35).
The primary beneficial effects of light-emitting
diode (LED) application on human health were found by the National
Aeronautics and Space Administration, with the development of LEDs
that produce a narrow spectrum of light in a non-coherent manner,
delivering the appropriate and required wavelength and intensity
(36). Over the past 15 years,
LED technology has continuously improved (36,37). Current LED therapy has the great
advantage of high flexibility and adaptability that allows for the
treatment of a wide variety of skin conditions exhibiting different
biological effects. Its mechanism of action, namely
photo-biomodulation, consists of the collective effects of 3
elements (a photosensitizing agent, a light source and oxygen)
interacting contemporaneously (38). The source emits a light whose
energy and wavelength emission activate the photosensitizer
(38). In addition to the amount
of energy, photo-biomodulation also depends on the irradiation time
(36). LED therapy has been
approved by the US Food and Drug Administration for its use by
aestheticians, due to its capacity to increase ATP and
transcription factor production, regulate oxidative stress and
modulate collagen synthesis (37).
Therapeutic LED application has been improved and
its use has increased in the treatment of several clinical
conditions, such as lung-related diseases (39,40), age-associated macula
deterioration (41) and
different types of solid tumors (37,42). LEDs can generate different
wavelengths, which lead to different biological activities.
However, the effects induced by the blue light are not yet fully
understood. It has been observed that, in the skin and retina, blue
light induces suspected mediators of skin aging and age-related
macular degeneration (36). The
disruption of key cellular processes, such as mitosis and
mitochondrial activity as a consequence of blue-light application
(43) and the maintenance of DNA
integrity (44) have been widely
reported. The latest analyses focused on the modulation of cell
signaling pathways by light energy (45,46), which includes UV wavelengths or
energies belonging to the visible light range (37,47,48). Numerous studies have suggested
that UVA light (320-400 nm) may stimulate anti-inflammatory and
antioxidative cytoprotective pathways (49,50), playing a key anti-tumorigenic
role (51). Previous in
vitro and in vivo investigations on skin cancer have
revealed that blue LED induces the apoptosis of cancer cells
(52) and a reduction of tumor
growth in mice (51). In
addition, it has been demonstrated that blue LED irradiation
triggers apoptotic cell death through the mitochondria-mediated
intrinsic pathway and shortens the early stage of tumor growth in
melanoma cells (36).
Accordingly, the expression of numerous genes associated with
tumorigenesis and cell metastasis is inhibited following exposure
to blue LED (36).
The combination of standard therapeutic approaches
has been recently attracting the attention of researchers and
physicians for cancer treatment (53). On the other hand, light therapy
and its combination with chemical drugs have also been shown to
have a high therapeutic efficacy (12,54). This is an important advantage,
since the dose of single drugs can be reduced, leading to a
reduction in adverse side-effects, while also maintaining treatment
efficacy (55).
In the present study, the photobiological effects of
blue and red light associated with the cisplatinum treatment of
A431 and HaCaT skin cell lines were investigated to determine
whether a combined treatment can increase the apoptotic rate, as
compared with single cisplatinum or light treatment. If that is
found to be the case, combination treatment could be proven to be a
promising treatment for skin cancer.
Materials and methods
Cells lines and culture conditions
The A431 epidermoid carcinoma cell line (ECACC
85090402) was purchased from Merck Life Sciences (Merck Life
Science). The HaCaT non-tumorigenic keratinocyte cell line was used
as a control and was acquired from the Experimental Zooprophylactic
Institute of Lombardia and Emilia Romagna (Brescia, Italy). The
HaCaT cell line can be used as non-tumor control of the A431 cells
(56-60) and it is considered a reliable
model for skin diseases and for in vitro carcinogenesis of
human skin keratinocytes (61,62). The HaCaT and A431 cell lines were
cultured under the same culture conditions to prevent differences
in phototoxicity related to different growth mediums. The cell
lines were cultured in high-glucose DMEM (Euroclone S.p.A.)
supplemented with 10% fetal bovine serum (FBS; Euroclone S.p.A.)
and 1% penicillin/streptomycin antibiotics (Euroclone S.p.A.) at
37°C in an atmosphere containing 5% CO2. Both cell lines
were sub-cultured every 3-4 days. Both cell lines were
mycoplasma-free.
Light exposure
Prior to light exposure, the cells were gradually
starved to synchronize the cell cycle. Both cell lines were first
cultured with low-glucose DMEM (Euroclone S.p.A.) supplemented with
0.1% FBS and 1% penicillin/streptomycin for 24 h and then DMEM
without phenol red (Lonza Group, Ltd.), also complemented with 0.1%
FBS and 1% penicillin/streptomycin for a further 24 h.
Samples were exposed to sham, blue, or red
single-color LEDs in a specific incubator at 37°C and 5%
CO2 for 3 days. Constant darkness was considered the
sham light source, while light exposures were performed in a 12-h
light/dark cycle (12L:12D). High-power blue and red LEDs (LD W5AM
and LH W5AM Golden DRAGON® Plus, respectively; Osram)
were used as the light sources. The LED viewing angle was 170° and
the cells were placed at 14 cm above the light sources. The
homogenous distribution of light and the spectrum of emission of
each monochromatic LED were previously verified using an
illuminance meter (CL-70; Konica Minolta Sensing, Inc.). The
dominant wavelength was 465 nm for blue and 658 nm for red LEDs.
Irradiance at peak wavelength at the cell surface was 0.84
W/m2 for blue and 1.10 W/m2 for red LEDs,
corresponding to the same total spectrum irradiance of 28.50
W/m2 for both light sources. The light energy
transferred each day to cells was 1.23 J/mm2. The light
exposure was set to reproduce the solar radiation as precisely as
possible (63). Blue and red
lights were specifically selected to test the opposite sides of the
spectrum of visible light. Interference between light sources was
prevented by using black curtains; sham exposure was additionally
ensured by wrapping the plate with aluminum foil. LEDs in the
incubator were fixed on an aluminum tank by thermal conductive
paste, and the water circulation inside the tank (Amersham
Multitemp III; GE Healthcare) extracted the heat generated by the
LEDs, so they could work at a constant temperature. These
conditions assured a constant electric current and therefore a
constant emitted energy. Air circulation inside the incubator was
ensured using a fan. To exclude any thermal effects, the
temperature at the cell level was verified and constantly measured
during experiments with Thermochron iButton DS1922L (Maxim
Integrated). On the fourth day from the time of the start of sham
or light exposure, the cells were transferred to the previous
incubator and started the cisplatinum treatment.
Cisplatinum treatment
Cisplatinum (Merck KGaA) was dissolved in
physiological solution at a final stock concentration of 3 mM and
stored at −20°C until use. The appropriate concentration of
cisplatinum in distilled water was assessed for each cell line
through the estimation of the half-maximal inhibitory concentration
(IC50) resulting after 24 h in 18 μM for A431
cells and 30 μM for HaCaT cells (data not shown). Each
group, following light exposure, was divided into 2 subgroups,
treated or not treated with the half-maximal inhibitory
concentration (IC50) of cisplatinum for 24 h at 37°C and
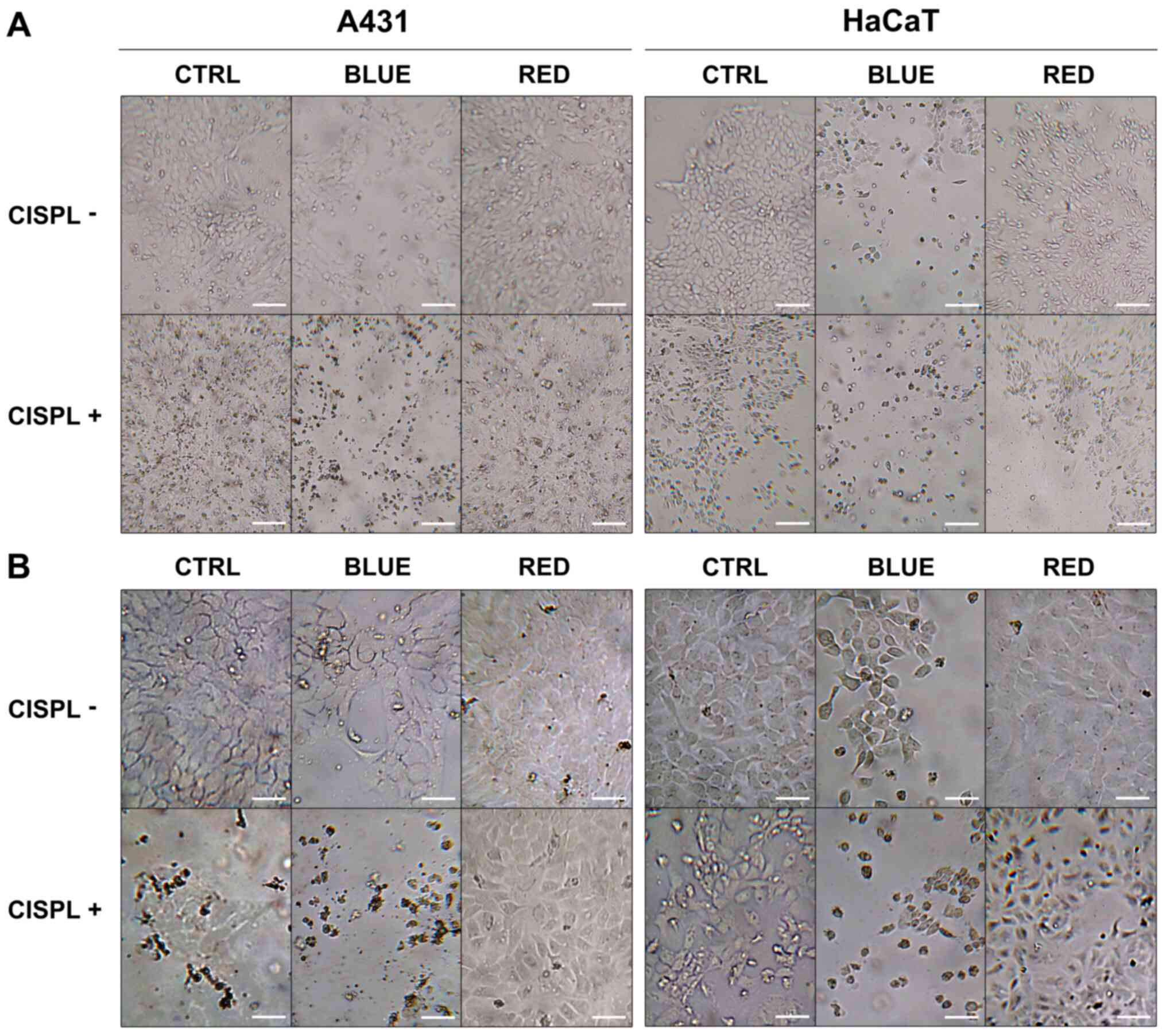
5% of CO2, and were then analyzed. Images of the cells
were acquired on an inverted microscope (Leitz Fluovert; Leica
Microsystems, Inc.) equipped with a digital camera (Canon EOS M50;
Canon, Inc.).
5-Fluorouracil (5-FU) treatment
5-FU (Merck KGaA), which was used for the data shown
in the supplementary figures, was dissolved in physiological
solution at a final stock concentration of 3.84 mM. The appropriate
concentration of 5-FU in distilled water was assessed for each cell
line by estimating the IC50 after 72 h in 75 μM
for A431 cells and 100 μM for HaCaT cells. Each group,
following light exposure, was divided into 2 subgroups, treated or
not treated with the IC50 of 5-FU for 72 h at 37°C and
5% of CO2; cell viability assay was then performed.
Cell viability assay
To assess the effects of cisplatinum and light on
cell viability, an XTT assay was performed, following the
manufacturer's instructions (Cell Proliferation Kit II XTT; Merck
KGaA). Briefly, the A431 and HaCaT cells were seeded in 96-well
plates (Corning Inc.) at a final concentration of
1.6×104 cells/well and exposed to a cycle of 12L:12D
(blue or red light) for 3 days; constant darkness was used as
control. Subsequently, the appropriate concentration of cisplatinum
for each cell line was added. At 24 h, 50 μl XTT
(2,3-bis-(2-met
hoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide)
solution was added to each well, followed by incubation for 2 h at
37°C and 5% CO2. Finally, the absorbance at 450 nm with
650 nm as the reference wavelength was measured using an absorbance
microplate ELISA plate reader (Sunrise™ Absorbance Reader; Tecan
Group Ltd.).
Apoptosis analysis
The apoptotic rate of both cell lines was evaluated
using an Annexin V/Propidium Iodide (PI) apoptosis detection kit
(eBioscience™ Annexin V-FITC Apoptosis Detection kit; Thermo Fisher
Scientific, Inc.), according to the manufacturer's instructions.
Cells were seeded in a 6-well plate (Corning Inc.) at a
concentration of 2×105 cells/ml. Following treatment
with light and cisplatinum, cells were harvested, centrifuged (770
× g for 5 min at 4°C), and washed with phosphate-buffered saline
(PBS; Euroclone). The cells were then resuspended in binding buffer
plus Annexin V following 10 min of incubation at room temperature
in the dark. Finally, cells were stained with PI and assessed using
the FACSCalibur flow cytometer (BD Biosciences) equipped with Cell
Quest software set on a logarithmic scale (BD Biosciences). A
minimum of 20,000 cells was acquired for each sample. Data were
analyzed using FlowJo™ Software (FlowJo™ Software for Windows
version 7.6.1.; FlowJo LLC).
Cell cycle analysis
The A431 and HaCaT cells were seeded in a 6-well
plate (Corning Inc.) at a concentration of 2×105
cells/ml. Following treatments, the cells were harvested and
centrifuged at 300 × g for 6 min at room temperature, fixed with
4.5 ml cold ethanol solution (70% in PBS) and kept in ice at 4°C
for at least 2 h. The cells were then washed twice with PBS before
being resuspended in PI staining solution with 0.1% Triton X-100
(Santa Cruz Biotechnology, Inc.), RNAse 0.2 mg/ml (Merck KGaA) and
PI 2 mg/ml (Merck KGaA) and incubated at 37°C for 15 min. Flow
cytometric analysis was performed using a FACSCalibur™ flow
cytometer (BD Biosciences) equipped with CellQuest software set on
a linear scale (BD Pharmingen). A minimum of 20,000 cells was
acquired for each sample. Data were analyzed using FlowJo™
Software.
Western blot analysis
To analyze protein expression, western blot analysis
was performed. Proteins were extracted with RIPA buffer (50 mM
Tris-HCl pH 7.4, 1% NP-40, 0.1% SDS, 150 mM NaCl, and 2 mM EDTA +
protease inhibitors) as follows: Cells were lysed and maintained in
ice for 30 min, with vortexing every 10 min. The cells were then
centrifuged (Eppendorf® Microcentrifuge 5415; Merck
KGaA) at 16,000 × g for 20 min at 4°C. Supernatants were collected
and protein concentrations were measured using a Bradford assay
(Merck KGaA). Protein (30 μg) was treated with LDS Sample
Buffer (Thermo Fisher Scientific, Inc.) and a Sample Reducing Agent
(Thermo Fisher Scientific, Inc.). The tubes were then boiled at
100°C for 5 min. Finally, equal amounts of protein (30 μg)
were resolved on precast 4-12% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (Bolt 4-12% Bis-Tris
Plus; Thermo Fisher Scientific, Inc.) in MES SDS running buffer
(Thermo Fisher Scientific, Inc.) at 100 V and 35 mA, before
entering the gel, and at 165 V and 60 mA after entering the gel. A
protein ladder (SeeBlue™ Plus2 Prestained Standard; Thermo Fisher
Scientific, Inc.) was used as a reference for protein size.
Following running, proteins were transferred to a nitrocellulose
membrane (NC; Amersham™ Protran™ Nitrocellulose Blotting Membrane;
GE Healthcare) using a semi-dry method in transfer buffer (Tris 25
mM, 0.2 M glycine, 20% methanol). The transfer was performed at 30
V and an amperage calculated based on the membrane's measurements
(w × h × 0.8). The protein transfer was verified by immersing the
membrane in red Ponceau [Ponceau S solution for electrophoresis
(0.2%); SERVA Electrophoresis GmbH] and then washed 3 times for 5
min with PBS-Tween-20 (PBST) 0.1%. Subsequently, the membrane was
blocked with 5% milk-PBST (non-fat dried milk; Euroclone S.p.A.)
for 1 h at room temperature and then incubated overnight at 4°C
with pertinent primary antibodies: Rabbit polyclonal anti-human
anti-Gapdh antibody (dilution, 1:1,000; cat. no. A300-639A-M;
Bethyl Laboratories Inc.), rabbit polyclonal anti-human
anti-caspase-9 (Casp-9; dilution, 1:1,000; cat. no. 9502; Cell
Signaling Technology, Inc.), mouse monoclonal anti-human
anti-caspase-8 (Casp-8; dilution, 1:1,000; cat. no. 9746; Cell
Signaling Technology, Inc.), mouse monoclonal anti-human
anti-caspase-3 (Casp-3; dilution, 1:500; cat. no. sc-7272; Santa
Cruz Biotechnology, Inc.), rabbit polyclonal anti-human anti-BH3
interacting domain death agonist (Bid; dilution, 1:1,000; cat. no.
2002; Cell Signaling Technology, Inc.), rabbit polyclonal
anti-human anti-Bcl-2-associated X protein (Bax; dilution, 1:1,000;
cat. no. 2772; Cell Signaling Technology, Inc.), mouse monoclonal
anti-human anti-cytochrome c (Cyt c; dilution,
1:1,000; cat. no. sc-13156; Santa Cruz Biotechnology, Inc.), rabbit
monoclonal anti-human anti-p53 (dilution, 1:1,000; cat. no. 2527;
Cell Signaling Technology, Inc.) and rabbit polyclonal anti-human
anti-apoptosis independent factor (Aif; dilution, 1:1,000; cat. no.
4642; Cell Signaling Technology, Inc.). The following day, the NC
membrane was washed 3 times with PBST 0.1% and incubated with
corresponding horseradish peroxidase (HRP)-conjugated secondary
antibodies at room temperature for 3 h: Goat anti-rabbit IgG (H+I)
peroxidase/HRP-conjugated (dilution, 1:3,000; cat. no. E-AB-K1813;
Elabscience Biotechnology, Inc.) or goat anti-mouse IgG (H+I) FITC
conjugated (dilution, 1:10,000; cat. no. A90-116F; Bethyl
Laboratories, Inc.). Finally, another 3 5-min washes with PBST were
performed prior to membrane development. For signal
chemiluminescent detection, the membrane was incubated for 2 min in
the dark at room temperature with SuperSignal™ West Femto Maximum
Sensitivity Substrate (Thermo Fisher Scientific, Inc.) and then
developed in Alliance Mini (UVITEC Cambridge) equipped with
NineAlliance Software (UVITEC Cambridge). Band quantification was
carried out using the same software. Membrane stripping was
performed prior to the addition of Gapdh where similar molecular
weight proteins were previously assessed.
Statistical analysis
Experiments were conducted in triplicate, and the
results were reported as the means ± standard deviation (SD) from 3
independent experiments. Graphpad Prism software (version 7.00 for
Windows; GraphPad Software, Inc.) was used for all statistical
analysis. One-way ANOVA and Tukey's HSD post hoc test was used to
evaluate statistical significance. P<0.05 was considered to
indicate a statistically significant difference.
Results
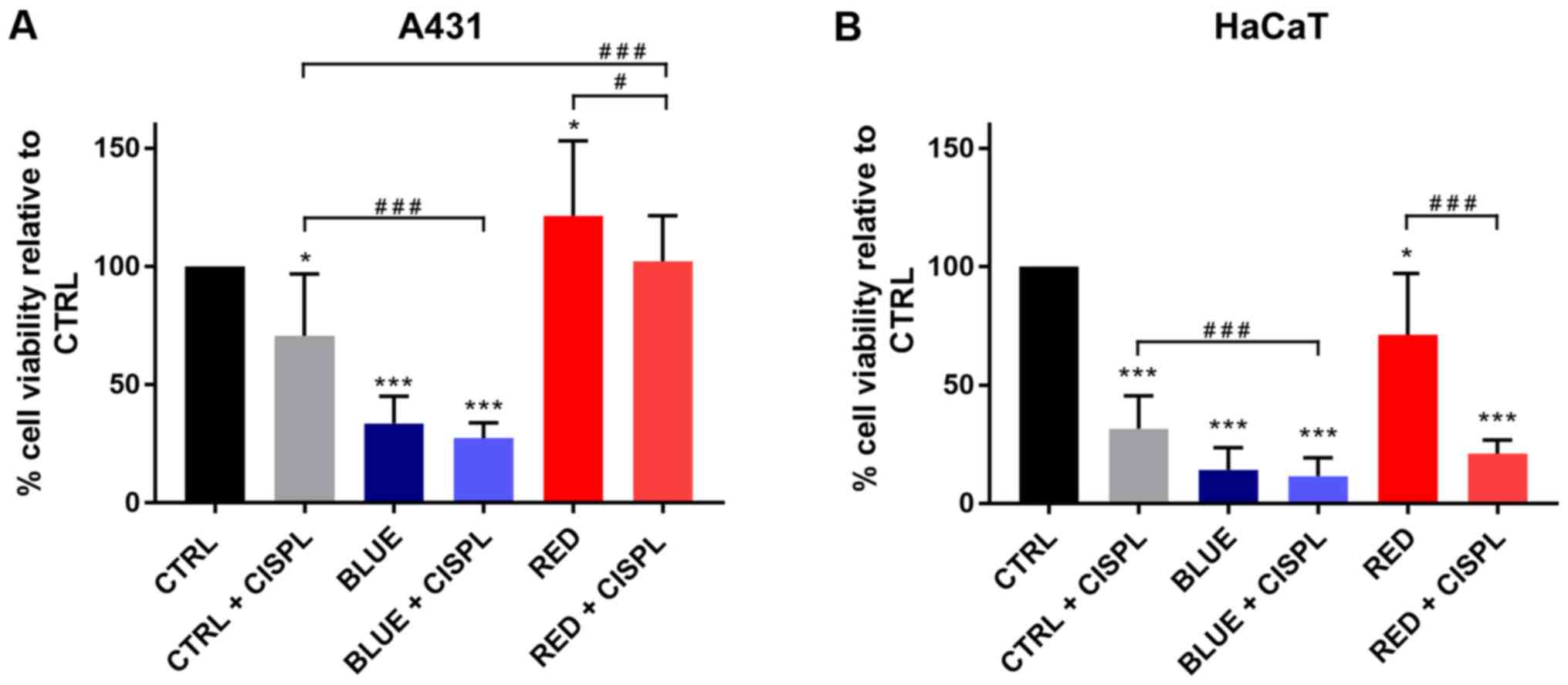
Combined treatment with blue light and
cisplatinum is more effective in reducing cell viability than
single treatments
The A431 and HaCaT cells were treated to assess
whether the combination of cisplatinum and different light spectra
decreased cell viability, as compared to the single treatments. The
viability of the tumor cells was markedly inhibited following
exposure to blue light (BLUE), as compared to the untreated cells
(CTRL) (P<0.001; Figs. 1 and
2A). The higher percentage of
inhibition in viable cells was obtained with blue light and
cisplatinum (BLUE + CISPL), as compared to the CTRL group and CTRL
+ CISPL group (P<0.001). On the contrary, neither irradiation
with red light alone (RED) nor treatment with red light and
cisplatinum (RED + CISPL) led to a marked reduction in tumor cell
viability, when compared with the untreated cells. Treatment with
red light and cisplatinum increased A431 cell viability, as
compared to the CTRL + CISPL group (P<0.001). The results
obtained for the RED + CISPL group were similar to those of the
CTRL, but lower than those of the RED group (P<0.05).
The HaCaT cells (Figs. 1 and 2B) displayed a notable decrease in
viability in the BLUE and BLUE + CISPL groups compared to the CTRL
group (P<0.001). In addition, cell viability was reduced in the
BLUE + CISPL group, as compared to CTRL + CISPL group (P<0.001).
With regards to the RED group, the results were different than
those obtained from the A431 cells, since the RED + CISPL exhibited
a lower cell viability (P<0.001 vs. CTRL; P<0.001 vs.
RED).
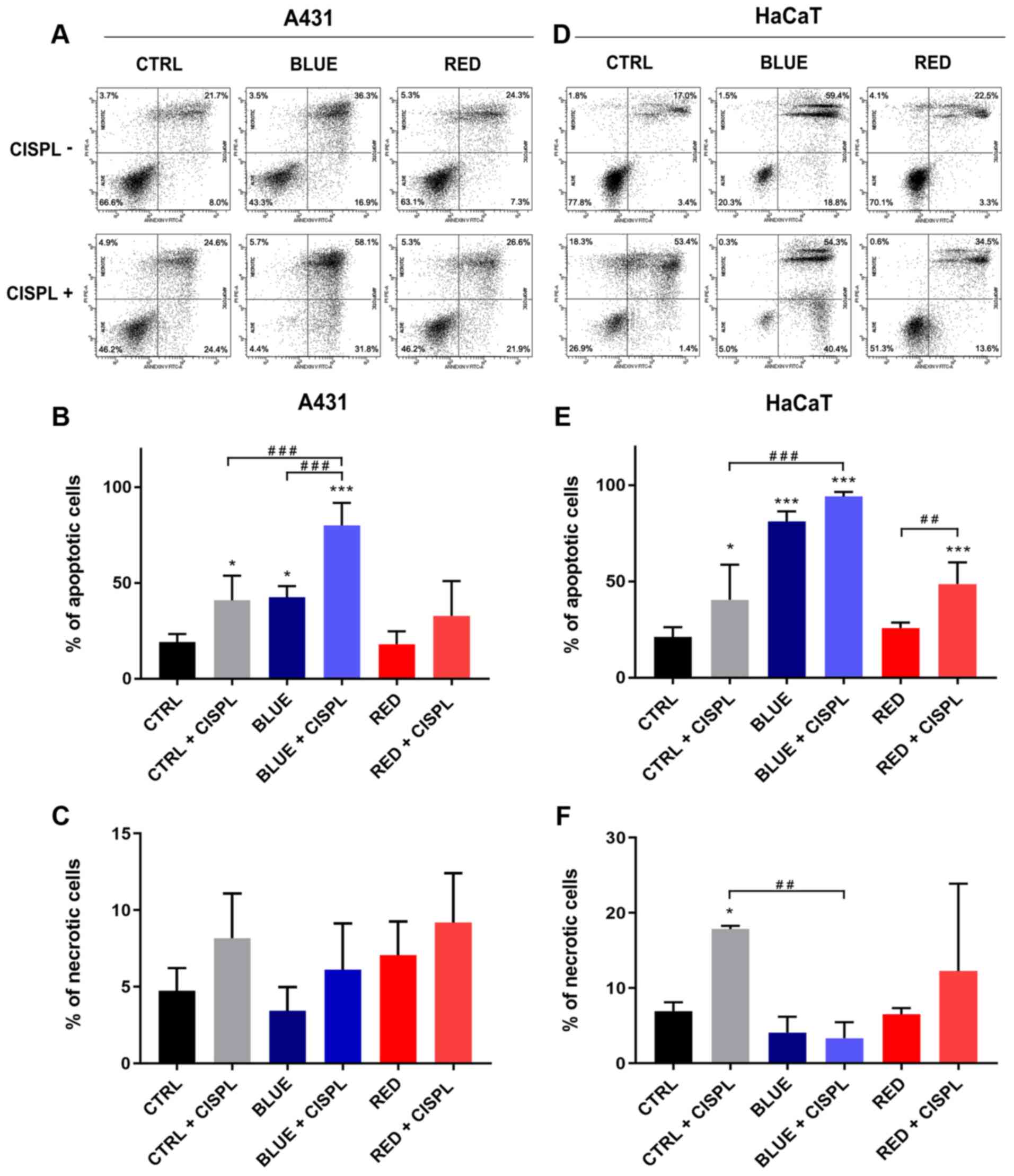
Combined treatment with blue light and
cisplatinum yields a higher percentage of apoptotic cells than
single treatments
Flow cytometry confirmed that CTRL + CISPL, BLUE and
BLUE + CISPL caused a significant increase in the apoptotic rate
(Annexin V-positive cells), as compared to the CTRL in both cell
lines (Fig. 3A, B and E). These
results revealed that the BLUE + CISPL group exhibited an increase
in the number of apoptotic A431 cells (mean, 80.1%), as compared to
the CTRL + CISPL (mean, 40.5%; P<0.001) or BLUE (mean, 42.6%;
P<0.001) groups. The HaCaT cell line displayed an increased
apoptotic rate in the BLUE + CISPL group, as compared to the CTRL
group (P<0.001) (Fig. 3D and
E). On the other hand, a high quantity of HaCaT cells
undergoing apoptosis was also observed, even when treated with blue
light alone (P<0.001 vs. CTRL) (Fig. 3D and E). The RED and RED + CISPL
groups exhibited a percentage of apoptotic and necrotic A431 cells
similar to the CTRL (Fig. 3A, B and
C). With regards to the HaCaT cells, the RED + CISPL group
exhibited a significant increase in the percentage of apoptotic
cells with respect to the CTRL and RED groups (P<0.001 and
P<0.01 respectively; Fig. 3D and
E).
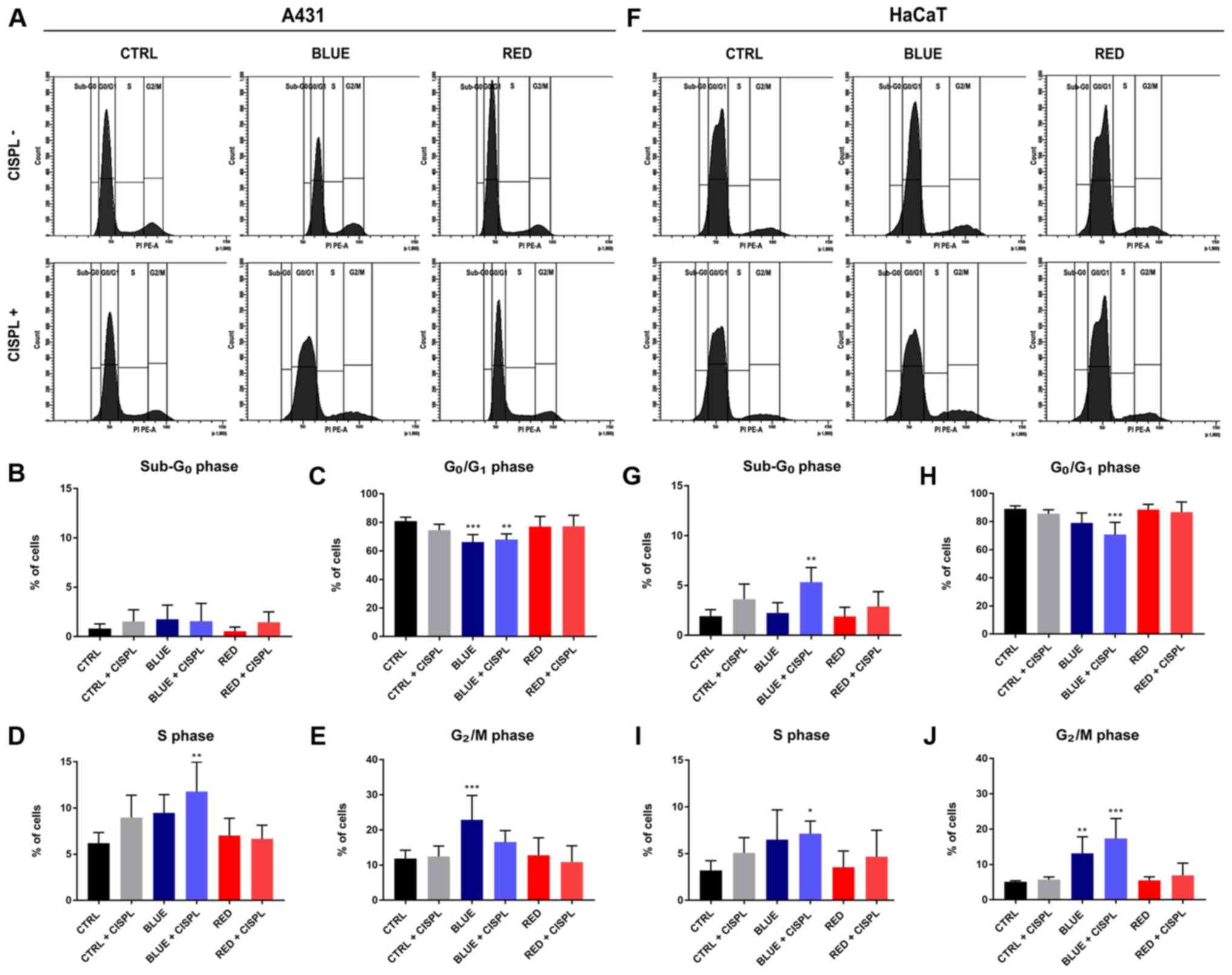
Combined treatment with blue light and
cisplatinum leads to S and G2/M cell cycle arrest
Cell cycle analysis indicated that the A431 cells
treated with BLUE + CISPL were arrested at the S phase (P<0.01;
Fig. 4A and D), while the
percentage of cells in the G0/G1 checkpoint was significantly
lower, as compared to the CTRL (P<0.01; Fig. 4A and C). The BLUE group exhibited
lower percentages of cells in the G0/G1 and
higher ones in the G2/M phase, as compared to the CTRL
(P<0.001; Fig. 4A, C and E).
With regards to the HaCaT cell line, cell cycle analysis indicated
that the number of cells in the BLUE + CISPL group was
significantly higher in the S phase and in the G2/M
phase (P<0.05 and P<0.001; Fig. 4F, I and J) and lower in the
G0/G1 phase (P<0.001; Fig. 4A and H) compared with the CTRL.
Furthermore, by observing the sub-G0 phase (Fig. 4A and G), the cells in the BLUE +
CISPL exhibited a higher percentage of cells in this phase, as
compared to the CTRL group (P<0.01).
Crucial role of apoptotic key factors in
the fate of A431 and HaCaT cells following treatment with light and
cisplatinum
The results described above led to the investigation
of the molecular mechanisms underlying the effects of the
treatments used. Hence, the expression levels of cell death-related
proteins, such as Casp-9, Casp-8, Casp-3, Bid, Bax, Cyt c, p53 and
Aif were analyzed by western blot analysis (representative protein
bands are shown in Fig.
S1).
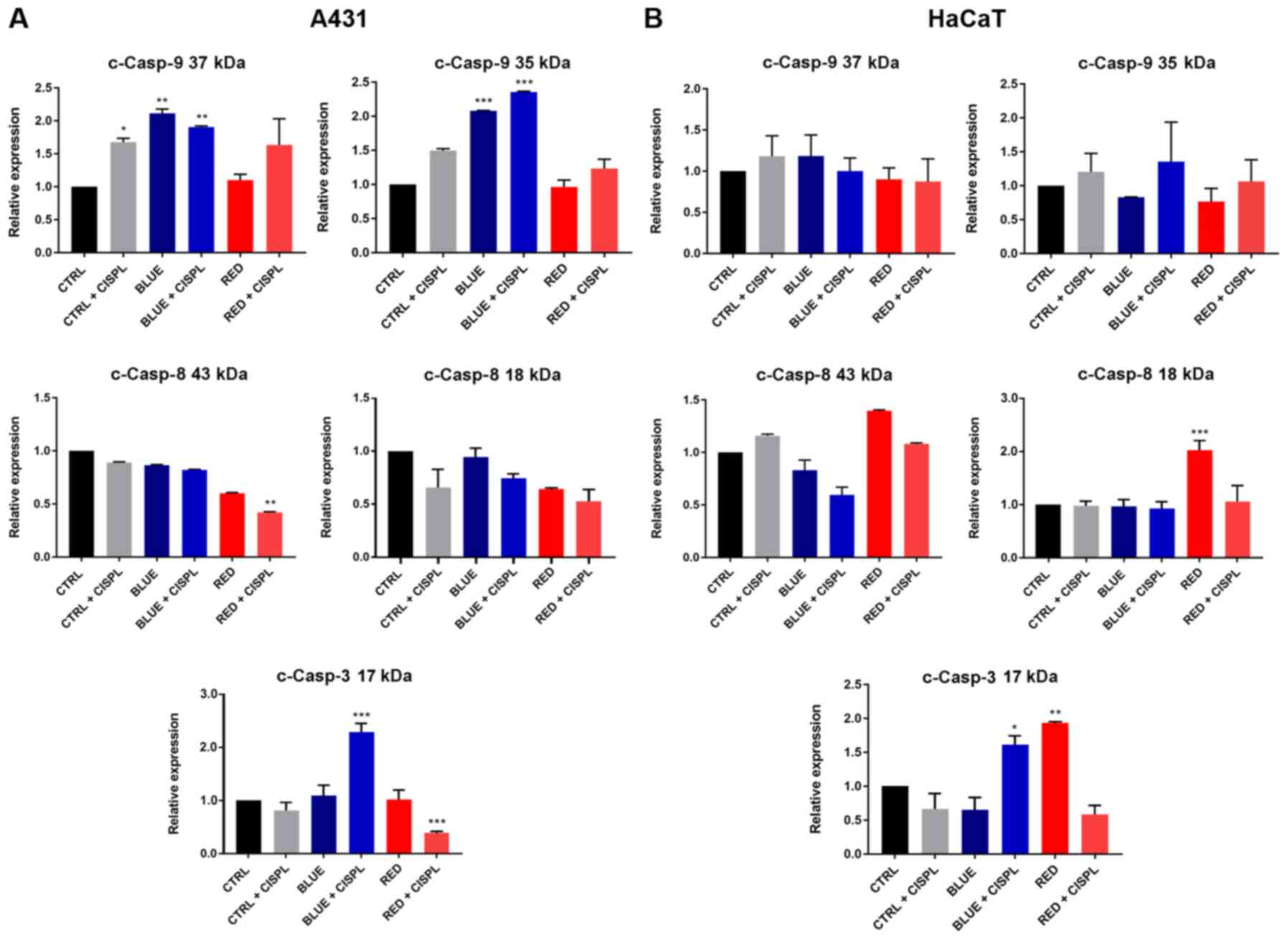
The protein expression of cleaved Casp-9 in the A431
cells was in line with the results obtained from the apoptosis and
cell cycle analysis, since the two cleaved forms of the protein
(cleaved at 37 and 35 kDa) increased upon treatment with BLUE and
BLUE + CISPL, as compared to the CTRL (Fig. 5A). With regards to the HaCaT cell
line, on the other hand, no significant changes in either 37 KD or
in 35 kDa cleaved Casp-9 were identified (Fig. 5B). Casp-8 expression was also
analyzed. Pro-caspase 8 expression was not altered among the
treatments (data not shown), while the results on the Casp-8
cleaved fragments exhibited a decreasing trend in 43 and 18 kDa
protein expression in the A431 tumor cells in all treatments, as
compared to the CTRL group (Fig.
5A). However, it is important to note the higher reduction of
Casp-8 43 kDa upon RED + CISPL treatment (P<0.01 vs. CTRL). In
the HaCaT cells, the cleaved Casp-8 fragment (18 kDa) was
significantly increased only in the RED group (P<0.05 vs. CTRL;
Fig. 5B).
The key apoptotic factor, Casp-3, in the A431 cell
line at the 17 kDa subunit level exhibited a significant increase
in its expression in the BLUE + CISPL group as compared to the CTRL
group (P<0.001; Fig. 5A). The
same cell line instead exhibited a decrease in the expression of
Casp-3 17 kDa in the RED + CISPL group (P<0.001). In the HaCaT
cells, the level of Casp-3 17 kDa in the BLUE + CISPL and RED
groups was increased (P<0.05 and P<0.01, respectively;
Fig. 5B).
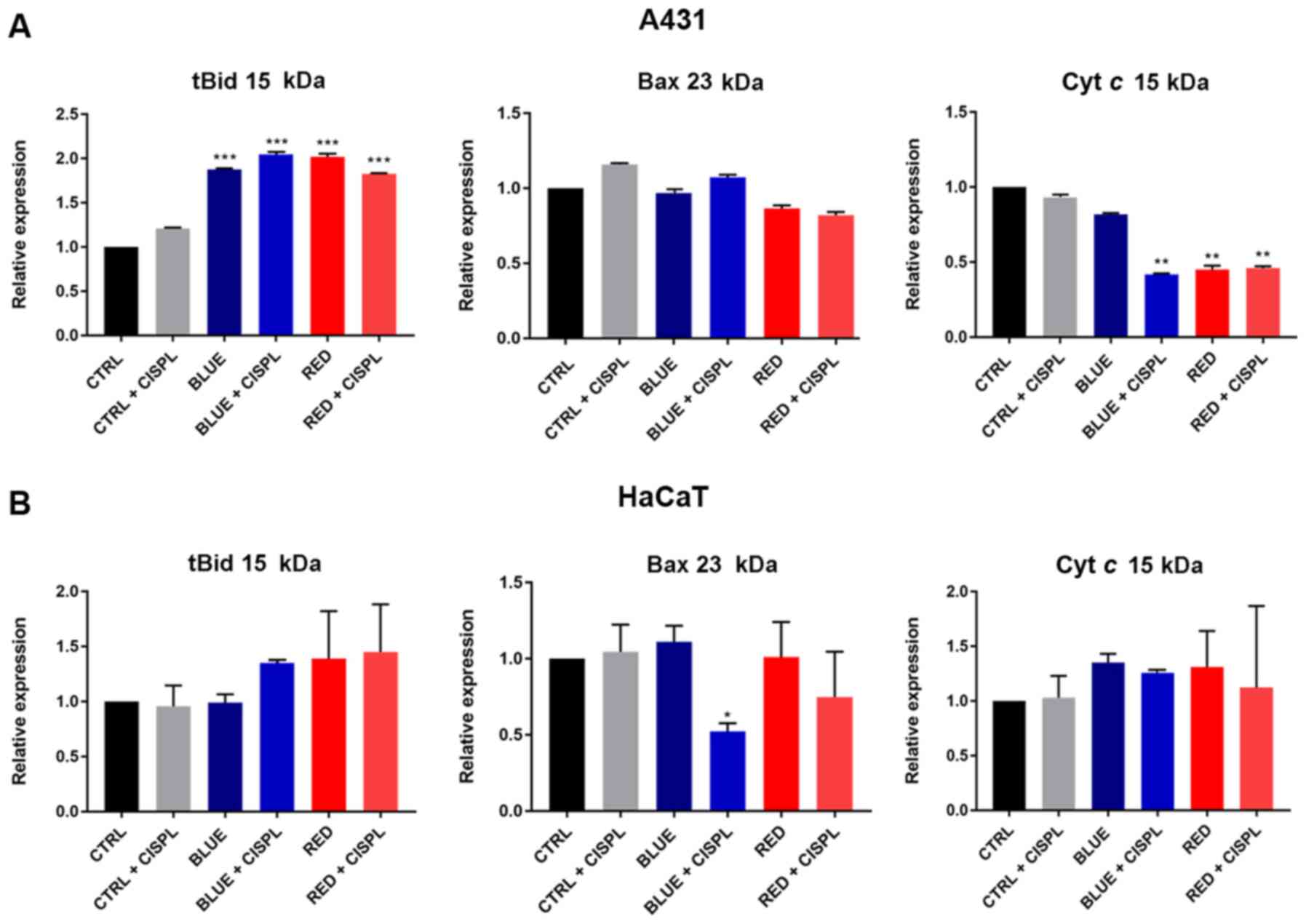
Bid protein was assessed by analyzing the expression
of its active cleaved form with a molecular weight of 15 kDa
(tBid). A significant increase was observed in its expression in
the A431 cells in all groups treated with light alone and light
combined with cisplatinum, as compared to the CTRL group
(P<0.001; Fig. 6A). No
significant differences in tBID expression were observed among the
groups in the HaCaT cells (Fig.
6B).
Bax protein expression in the A431 cells was not
significantly altered among the groups (Fig. 6A), while in the HaCaT cells, its
expression was markedly decreased in the BLUE + CISPL group
(P<0.05 vs. CTRL; Fig.
6B).
Furthermore, lower values of cytosolic Cyt c
were observed in the A431 cells treated with blue light combined
with cisplatinum, as compared to the CTRL group (P<0.01;
Fig. 6A).
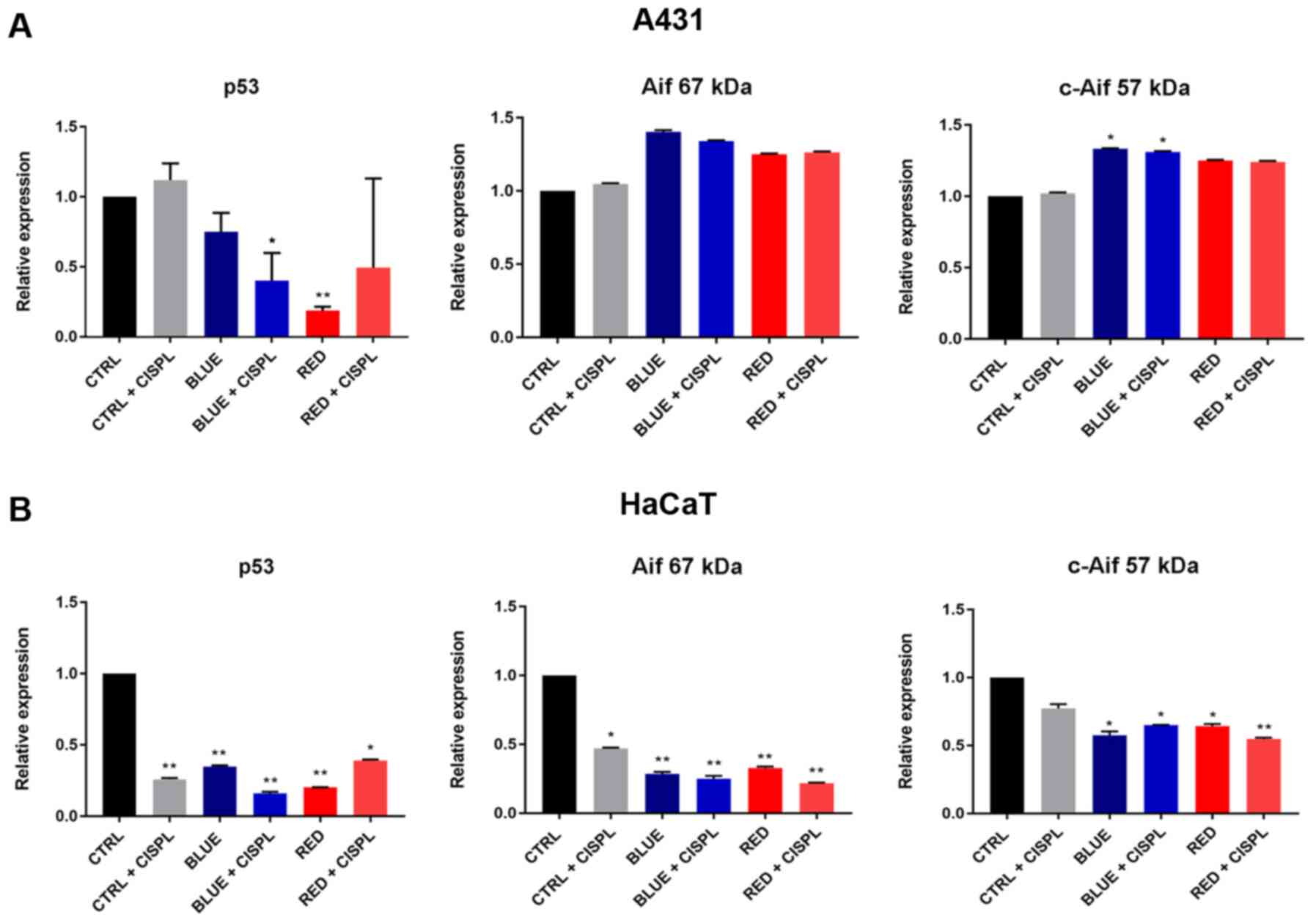
These results demonstrated that combined treatment
with blue light and cisplatinum (BLUE + CISPL) effectively induced
apoptosis, which was associated with a decrease in the p53 protein
expression level in the A431 (P<0.05 vs. CTRL; Fig. 7A) and HaCaT cell lines (P<0.01
vs. CTRL; Fig. 7B). In the HaCaT
cells, all treatments led to a significant decrease in p53
expression (Fig. 7B).
With regards to the analysis of expression of the
necroptosis-related protein, Aif, the data revealed a marked
increase in its cleaved form (57 kDa) in the A431 cells following
exposure to blue light (P<0.05 BLUE vs. CTRL; Fig. 7A) and blue light combined with
cisplatinum (P<0.05 BLUE + CISPL vs. CTRL). Conversely, the
HaCaT cells exhibited a significant decrease in the 57 kDa-cleaved
protein expression following exposure to light alone (P<0.05
BLUE vs. CTRL and RED vs. CTRL; Fig.
7B) and to light followed by the addition of cisplatinum
(P<0.05 BLUE + CISPL vs. CTRL; P<0.01 RED + CISPL vs.
CTRL).
Discussion
CSCC is a type of malignant skin neoplasia with a
high morbidity risk (10);
hence, it is fundamental for the development of novel alternative
therapies to increase tumor vulnerability. Alternative therapies
using LEDs (phototherapy) in combination with chemotherapeutic
agents, including cisplatinum, have recently been proposed as a
different approach to tumor treatment (12,16,32). In the present study, the effects
of LED spectra and cisplatinum, used alone or in combination, were
assessed on normal keratinocytes (HaCaT) and cSCC cancer cells
(A431).
The results revealed that combined treatment was
more effective on the A431 cells than single treatments,
significantly decreasing cell viability and increasing the
apoptotic rate. A higher effectiveness of BLUE + CISPL combined
treatment was observed when A431 cells were exposed to both agents,
which induced a significant increase in the percentage of apoptotic
A431 cells (~2-fold the effect of blue light as used alone). The
apoptotic effects of blue light were observed in other in
vitro and in vivo studies, where blue irradiation caused
a decrease in cellular growth and induced cell death in skin cancer
cell lines and animal models (51). A higher efficacy of blue light
combined with cisplatinum on inducing apoptosis was observed in
some cancer cell lines and xenograft mouse models (32,36,51). The present data suggested that
blue light can enhance the cytotoxic effects of cisplatinum on A431
cells, exerting a more prominent effect on apoptosis when used in
combination. It was also observed that cells were more sensitive to
blue light exposure alone, as compared to red light exposure alone,
suggesting that the blue light is more cytotoxic than the red one.
Indeed, the A431 cells displayed a high viability rate when exposed
to the red light and showed no changes in the number of apoptotic
cells, even under combined treatment with cisplatinum. It was thus
hypothesized that somehow, red light confers to A431 cells a type
of resistance to cisplatinum, which was not observed when the cells
were treated with blue light and cisplatinum. This could be related
to the tumorigenic features of A431 cells as HaCaT cells were
otherwise sensitive to red light combined with cisplatinum.
Given current insights into the association between
apoptosis and cell cycle progression, the present study then sought
to determine whether there were any differences in cell cycle
progression upon single or combined light and cisplatinum
treatment. Focusing on BLUE + CISPL treatment, a significant
increase in the percentage of cells in the S and/or G2/M
phases was observed in both normal keratinocytes and skin cancer
cells, suggesting that this treatment also exerted a cytostatic
effect. This was in agreement with studies describing that HaCaT
cells have a defective G1 checkpoint, responding to UVB
radiation by arresting cells in the S and G2 phases
(64). The same effect was
reported when HaCaT cells were exposed to blue and red LEDs
together with curcumin (64).
Apoptosis is the main programmed-cell death pathway
that cells form under any cytotoxic stimulus (52). There are two controlled pathways
involved in apoptosis: The death receptorregulated or extrinsic
pathway that activates Casp-8, and the mitochondria-controlled or
intrinsic pathway that activates Casp-9 (16,65). There are other apoptosis-related
proteins associated with the different pathways (intrinsic,
extrinsic, or both), including Bax (pro-apoptotic), tBid
(anti-apoptotic) and Cyt c, which are associated with the
intrinsic pathway (52,66), and p53 and Casp-3 proteins. Apart
from apoptosis, other cell death mechanisms have been described,
including necroptosis, in which the Aif protein plays a central
role (67). To further elucidate
the molecular mechanisms underlying the apoptotic process that A431
and HaCaT cells undergo following different treatments, the
expression of diverse cell death-related proteins was analyzed.
The data obtained revealed several outcomes. The
cytofluorimetric analysis indicated that cells underwent an
apoptotic process since Annexin V was bound to phosphatidylserine
molecules expressed in the outer membrane of apoptotic cells. It
was therefore then evaluated whether the intrinsic or extrinsic
apoptotic pathway was activated.
The activation of Casp-9, rather than Casp-8, in the
A431 cells confirmed that cells treated with blue light and blue
light combined with cisplatinum were involved in the intrinsic
apoptotic pathway. The blue light activated the initiator Casp-9
but not the effector Casp-3; these results were associated with the
low apoptotic rate of this treatment The advantage of using
combined treatment (BLUE + CISPL) was associated with an increased
expression of active effector Casp-3 subunit. However, following
the further evaluation of Cyt c activated by cleaved Casp-9
(52,65,66), low expression values were
observed. In addition, the tBid protein (associated with the
extrinsic pathway) exhibited high levels in all treatments with
light alone and light combined with cisplatinum. These
contradictory outcomes were also observed in a study on cervical
carcinoma cells, which demonstrated that Casp-8 formed a complex
with Bid, leading to its cleavage into t-Bid and to the subsequent
activation of type II extrinsic apoptosis (65). However, that study also reported
the formation of the Casp-8/Bid complex in cells that were
undergoing mitochondrial-independent apoptosis (65). In a previous study, p53
expression, a key apoptosis-related protein, was analyzed (68). A decrease in the expression of
the total protein was found in A431 cells that had undergone BLUE +
CISPL combined treatment and red light exposure alone. Previous
studies on the behavior of p53 against different stresses have
revealed a high temporal dynamism in its expression levels. In
particular, it has been observed that cells exposed to UV (69,70) and cisplatinum (71) exhibited important variations of
p53 protein expression. It was hypothesized that the low p53
expression identified in the cells in the present study was linked
to the blue light and cisplatinum treatment, which differently
modulated the p53 expression profile over time. Based on these
conflicting data, it was hypothesized that cells were undergoing
another type of cell death. Therefore, the expression levels of the
necroptosis-related protein, Aif, were analyzed. Increased
expression levels of cleaved (c-Aif) proteins were observed in the
A431 cells treated with blue light alone and blue light combined
with cisplatinum. These data suggest that tumor cells were
undergoing necroptosis triggered by the light, given that single
treatment with cisplatinum did not yield any significant increase
in c-Aif expression values. In light of these results, it cannot be
affirmed that A431 cells have one particular cell death mechanism,
since protein expression analysis showed that, indeed, tumor cells
also follow the intrinsic and extrinsic apoptotic pathways, and the
necroptotic cell death mechanism. This could be explained by a
possible phosphatidylserine re-localization, which has been
described in other skin cancer cells under pro-apoptotic stimuli
and has been speculated to induce smaller cross-reactive proteins
that might lead to different cell death mechanisms working
simultaneously (72).
In the HaCaT cell line, expression analysis of the
same proteins did not yield well-defined results, with regards to
the cell death mechanism. In fact, caspase analysis suggested the
activation of the extrinsic apoptotic pathway, since cleaved-Casp-9
(37 and 35 kDa) expression levels were not significant at all.
However, the expression of the active 18 kDa subunit of Casp-8 and
the active 17 kDa subunit of Casp-3 were increased following red
light treatment. It is therefore likely that the HaCaT cells
underwent extrinsic apoptosis cell death only following red light
treatment. Treatment with blue light combined with cisplatinum
caused only the increase of the active fragment of Casp-3; further
investigations of the mechanism of cell death in HaCaT treated are
necessary. Furthermore, p53 expression levels were low in all
groups, potentially due to the expression dynamism displayed by
p53, depending on the type and intensity of the stress (69-72). The results of the expression
analysis of necroptosis-related protein Aif ruled out this cell
death mechanism, since both full-length proteins and cleaved
fragments were very lowly expressed in all groups.
The mechanisms leading to cell death differed in
each of the analyzed cell lines. However, it can be affirmed that
combined treatment, particularly BLUE + CISPL, promoted the
activation of programmed cell death in both cell lines, although
the exact mechanisms were not clarified. The fact that both
apoptotic pathways were active in both cell lines has been
supported by a recent study by Laubach et al (73) on HaCaT and A431 cells undergoing
treatment with curcumin and light irradiation. That study theorized
a switch in the apoptotic mechanism related to changes in cellular
redox balance and a mechanism of apoptotic stimulation independent
from classical death receptors. Keratinocytes and skin tumor cells
were also reported to undergo different apoptotic processes, due to
the different expression of apoptotic regulators and apoptotic
responses involved in reaching the diverse functional needs of the
skin (73).
The combination of different treatments for the
targeting of cSCC has several advantages: Targeting of different
key signal transduction pathways; more efficient damage caused in
tumor cells; increased therapeutic efficacy; additive or even
synergistic effects that allow for the dose of the most toxic
component can be reduced to eliminate or lessen noxious
side-effects (32). The results
of the present study indicated that the combination of blue light
radiation at 465 nm followed by cisplatinum could be used as a
potential treatment for cSCC and recommended further in vivo
tests and clinical trials. In particular, the use of light combined
with a local cisplatinum-based treatment could be investigated in
non-metastatic cSCC, or when the conventional surgical approach is
not an option. In addition, given the availability of local
treatments containing 5-FU, the effect of blue radiation combined
with 5-FU was investigated. Preliminary viability data (Fig. S2) revealed an effect of blue
light radiation combined with 5-FU similar to that obtained with
cisplatinum, but less significant. Since novel therapeutic
strategies are being developed for cSCC (74-77), the apoptotic effect of blue-light
treatment can also be investigated in combination with the new
treatments for cSCC, such as immune checkpoint agents or
electrochemotherapy. A limitation of the present study is the use
of only one skin cancer cell line making results about A431
referred only to cSCC and not to other types of skin cancer. The
use of HaCaT cells instead of primary human keratinocytes is
another limitation, although the selection was based on the use of
the same culture conditions. As regards experiments with light, it
is known that the culture conditions, in particular the medium
utilized, may influence the results (78,79). The use of normal human epidermal
keratinocytes-adult cells (NHEK cells) was attempted (data not
shown). In the experiments in the present study, the NHEK cells
exposed to light in the KGM-Gold medium showed a change in
morphology, while in the same mediums used for A431, the NHEK cells
showed extremely slow growth and a clear change in morphology (data
not shown). Rather, the HaCaT cells growing in the same conditions
of A431 cells made the results more comparable.
In conclusion, the data of the present study
suggested that the combination of blue light and cisplatinum
reduced the survival rate of A431 cells and triggered the apoptotic
death of A431 cells. Further studies are required to fully
elucidate the exact molecular mechanisms through which combined
treatments of blue light and cisplatinum mediate apoptotic cell
death, and to understand its overall mechanism in skin cancer.
Supplementary Data
Funding
The present study was supported by a generous
donation from iGuzzini illuminazione S.p.A. (Recanati, Italy). The
funder had no role in the design of the study, the collection,
analysis or interpretation of data, the writing of the manuscript,
or the decision to publish the results.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article or are available from the
corresponding author on reasonable request.
Authors' contributions
MB, MFT, MEZ and LS conceived and designed the
study. MB, MFT, MEZ, RL and EMB performed the experiments. FP, CL,
VR and AP were responsible for the methodology. MFT and MEZ
organized and wrote the manuscript. RL, CL, VR and MB reviewed and
edited the manuscript. MB and LS supervised the study. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Barolet D, Roberge CJ, Auger FA, Boucher A
and Germain L: Regulation of skin collagen metabolism in vitro
using a pulsed 660 nm LED light source: Clinical correlation with a
single-blinded study. J Invest Dermatol. 129:2751–2759. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mateus C: Cutaneous squamous cell
carcinoma. Rev Prat. 64:45–52. 2014.PubMed/NCBI
|
|
3
|
Parekh V and Seykora JT: Cutaneous
squamous cell carcinoma. Clin Lab Med. 37:503–525. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Smoller BR: Squamous cell carcinoma: From
precursor lesions to high-risk variants. Mod Pathol. 19(Suppl 2):
S88–S92. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Palme CE, MacKay SG, Kalnins I, Morgan GJ
and Veness MJ: The need for a better prognostic staging system in
patients with metastatic cutaneous squamous cell carcinoma of the
head and neck. Curr Opin Otolaryngol Head Neck Surg. 15:103–106.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Garcia-Zuazaga J and Olbricht SM:
Cutaneous squamous cell carcinoma. Adv Dermatol. 24:33–57. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kyrgidis A, Tzellos TG, Kechagias N,
Patrikidou A, Xirou P, Kitikidou K, Bourlidou E, Vahtsevanos K and
Antoniades K: Cutaneous squamous cell carcinoma (SCC) of the head
and neck: Risk factors of overall and recurrence-free survival. Eur
J Cancer. 46:1563–1572. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Clayman GL, Lee JJ, Holsinger FC, Zhou X,
Duvic M, El-Naggar AK, Prieto VG, Altamirano E, Tucker SL, Strom
SS, et al: Mortality risk from squamous cell skin cancer. J Clin
Oncol. 23:759–765. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sreedhar A, Li J and Zhao Y: Next-gen
therapeutics for skin cancer: Nutraceuticals. Nutr Cancer.
70:697–709. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Burton KA, Ashack KA and Khachemoune A:
Cutaneous squamous cell carcinoma: A review of high-risk and
metastatic disease. Am J Clin Dermatol. 17:491–508. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Que SKT, Zwald FO and Schmults CD:
Cutaneous squamous cell carcinoma: Management of advanced and
high-stage tumors. J Am Acad Dermatol. 78:249–261. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Compagnin C, Mognato M, Celotti L, Canti
G, Palumbo G and Reddi E: Cell proliferation and cell cycle
alterations in oesophageal p53-mutated cancer cells treated with
cisplatin in combination with photodynamic therapy. Cell Prolif.
43:262–274. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bray FN, Simmons BJ, Wolfson AH and Nouri
K: Acute and chronic cutaneous reactions to ionizing radiation
therapy. Dermatol Ther (Heidelb). 6:185–206. 2016. View Article : Google Scholar
|
|
14
|
Ando N, Kato H, Igaki H, Shinoda M, Ozawa
S, Shimizu H, Nakamura T, Yabusaki H, Aoyama N, Kurita A, et al: A
randomized trial comparing postoperative adjuvant chemotherapy with
cisplatin and 5-fluorouracil versus preoperative chemotherapy for
localized advanced squamous cell carcinoma of the thoracic
esophagus (JCOG9907). Ann Surg Oncol. 19:68–74. 2012. View Article : Google Scholar
|
|
15
|
Salman M and Naseem I: Riboflavin as
adjuvant with cisplatin: Study in mouse skin cancer model. Front
Biosci (Elite Ed). 7:242–254. 2015.
|
|
16
|
Dasari S and Tchounwou PB: Cisplatin in
cancer therapy: Molecular mechanisms of action. Eur J Pharmacol.
740:364–378. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mego M, Svetlovska D, Miskovska V,
Obertova J, Palacka P, Rajec J, Sycova-Mila Z, Chovanec M,
Rejlekova K, Zuzák P, et al: Phase II study of everolimus in
refractory testicular germ cell tumors. Urol Oncol.
34:122.e17–122.e22. 2016. View Article : Google Scholar
|
|
18
|
Terenziani M, De Pasquale MD, Bisogno G,
Biasoni D, Boldrini R, Collini P, Conte M, Dall'Igna P, Inserra A,
Melchionda F, et al: Malignant testicular germ cell tumors in
children and adolescents: The AIEOP (Associazione Italiana
Ematologia Oncologia Pediatrica) protocol. Urol Oncol.
36:502.e7–502.e13. 2018. View Article : Google Scholar
|
|
19
|
Shi T, Jiang R, Yu J, Yang H, Tu D, Dai Z,
Shen Y, Zhang Y, Cheng X, Jia H, et al: Addition of intraperitoneal
cisplatin and etoposide to first-line chemotherapy for advanced
ovarian cancer: A randomised, phase 2 trial. Br J Cancer.
119:12–18. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tempfer CB, Giger-Pabst U, Seebacher V,
Petersen M, Dogan A and Rezniczek GA: A phase I, single-arm,
open-label, dose escalation study of intraperitoneal cisplatin and
doxorubicin in patients with recurrent ovarian cancer and
peritoneal carcinomatosis. Gynecol Oncol. 150:23–30. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gupta S, Maheshwari A, Parab P,
Mahantshetty U, Hawaldar R, Sastri Chopra S, Kerkar R, Engineer R,
Tongaonkar H, Ghosh J, et al: Neoadjuvant chemotherapy followed by
radical surgery versus concomitant chemotherapy and radiotherapy in
patients with stage IB2, IIA, or IIB squamous cervical cancer: A
randomized controlled trial. J Clin Oncol. 36:1548–1555. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kitagawa R, Katsumata N, Shibata T, Kamura
T, Kasamatsu T, Nakanishi T, Nishimura S, Ushijima K, Takano M,
Satoh T and Yoshikawa H: Paclitaxel Plus carboplatin versus
paclitaxel plus cisplatin in metastatic or recurrent cervical
cancer: The open-label randomized phase III trial JCOG0505. J Clin
Oncol. 33:2129–2135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rosen VM, Guerra I, McCormack M,
Nogueira-Rodrigues A, Sasse A, Munk VC and Shang A: Systematic
review and network meta-analysis of bevacizumab plus first-line
topotecan-paclitaxel or cisplatin-paclitaxel versus
non-bevacizumab-containing therapies in persistent, recurrent, or
metastatic cervical cancer. Int J Gynecol Cancer. 27:1237–1246.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Small W Jr, Bacon MA, Bajaj A, Chuang LT,
Fisher BJ, Harkenrider MM, Jhingran A, Kitchener HC, Mileshkin LR,
Viswanathan AN and Gaffney DK: Cervical cancer: A global health
crisis. Cancer. 123:2404–2412. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Noronha V, Joshi A, Patil VM, Agarwal J,
Ghosh-Laskar S, Budrukkar A, Murthy V, Gupta T, D'Cruz AK, Banavali
S, et al: Once-a-week versus once-every-3-weeks cisplatin
chemoradiation for locally advanced head and neck cancer: A phase
III randomized noninferiority trial. J Clin Oncol. 36:1064–1072.
2018. View Article : Google Scholar
|
|
26
|
Strojan P, Vermorken JB, Beitler JJ, Saba
NF, Haigentz M Jr, Bossi P, Worden FP, Langendijk JA, Eisbruch A,
Mendenhall WM, et al: Cumulative cisplatin dose in concurrent
chemoradiotherapy for head and neck cancer: A systematic review.
Head Neck. 38(Suppl 1): E2151–E2158. 2016. View Article : Google Scholar
|
|
27
|
Szturz P, Wouters K, Kiyota N, Tahara M,
Prabhash K, Noronha V, Castro A, Licitra L, Adelstein D and
Vermorken JB: Weekly low-dose versus three-weekly high-dose
cisplatin for concurrent chemoradiation in locoregionally advanced
non-nasopharyngeal head and neck cancer: A systematic review and
meta-analysis of aggregate data. Oncologist. 22:1056–1066. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gridelli C, Morabito A, Cavanna L, Luciani
A, Maione P, Bonanno L, Filipazzi V, Leo S, Cinieri S, Ciardiello
F, et al: Cisplatin-based first-line treatment of elderly patients
with advanced non-small-cell lung cancer: Joint analysis of MILES-3
and MILES-4 phase III trials. J Clin Oncol. 36:2585–2592. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rossi A and Di Maio M: Platinum-based
chemotherapy in advanced non-small-cell lung cancer: Optimal number
of treatment cycles. Expert Rev Anticancer Ther. 16:653–660. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sarin N, Engel F, Kalayda GV, Mannewitz M,
Cinatl J Jr, Rothweiler F, Michaelis M, Saafan H, Ritter CA, Jaehde
U and Frötschl R: Cisplatin resistance in non-small cell lung
cancer cells is associated with an abrogation of cisplatin-induced
G2/M cell cycle arrest. PLoS One. 12:e01810812017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu L, Fan J, Ai G, Liu J, Luo N, Li C and
Cheng Z: Berberine in combination with cisplatin induces
necroptosis and apoptosis in ovarian cancer cells. Biol Res.
52:372019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
de Freitas LM, Soares CP and Fontana CR:
Synergistic effect of photodynamic therapy and cisplatin: A novel
approach for cervical cancer. J Photochem Photobiol B. 140:365–373.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Oun R, Moussa YE and Wheate NJ: The side
effects of platinum-based chemotherapy drugs: A review for
chemists. Dalton Trans. 47:6645–6653. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Crul M, van Waardenburg RC, Beijnen JH and
Schellens JH: DNA-based drug interactions of cisplatin. Cancer
Treat Rev. 28:291–303. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fuertes MA, Alonso C and Pérez JM:
Biochemical modulation of cisplatin mechanisms of action:
Enhancement of antitumor activity and circumvention of drug
resistance. Chem Rev. 103:645–662. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Oh PS and Jeong HJ: Therapeutic
application of light emitting diode: Photo-oncomic approach. J
Photochem Photobiol B. 192:1–7. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sorbellini E, Rucco M and Rinaldi F:
Photodynamic and photobiological effects of light-emitting diode
(LED) therapy in dermatological disease: An update. Lasers Med Sci.
33:1431–1439. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Brancaleon L and Moseley H: Laser and
non-laser light sources for photodynamic therapy. Lasers Med Sci.
17:173–186. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ost D: Photodynamic therapy in lung
cancer. A review Methods Mol Med. 75:507–526. 2003.
|
|
40
|
Sutedja TG and Postmus PE: Photodynamic
therapy in lung cancer. A review. J Photochem Photobiol B.
36:199–204. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Silva JN, Filipe P, Morlière P, Mazière
JC, Freitas JP, Gomes MM and Santus R: Photodynamic therapy:
Dermatology and ophthalmology as main fields of current
applications in clinic. Biomed Mater Eng. 18:319–327.
2008.PubMed/NCBI
|
|
42
|
Breskey JD, Lacey SE, Vesper BJ, Paradise
WA, Radosevich JA and Colvard MD: Photodynamic therapy:
Occupational hazards and preventative recommendations for clinical
administration by healthcare providers. Photomed Laser Surg.
31:398–407. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
del Olmo-Aguado S, Manso AG and Osborne
NN: Light might directly affect retinal ganglion cell mitochondria
to potentially influence function. Photochem Photobiol.
88:1346–1355. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chui C, Hiratsuka K, Aoki A, Takeuchi Y,
Abiko Y and Izumi Y: Blue LED inhibits the growth of Porphyromonas
gingivalis by suppressing the expression of genes associated with
DNA replication and cell division. Lasers Surg Med. 44:856–864.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Rybchyn MS, De Silva WGM, Sequeira VB,
McCarthy BY, Dilley AV, Dixon KM, Halliday GM and Mason RS:
Enhanced repair of UV-induced DNA damage by 1,25-dihydroxyvitamin
D3 in skin is linked to pathways that control cellular energy. J
Invest Dermatol. 138:1146–1156. 2018. View Article : Google Scholar
|
|
46
|
Suh SS, Lee SG, Youn UJ, Han SJ, Kim IC
and Kim S: Comprehensive expression profiling and functional
network analysis of porphyra-334, one mycosporine-like amino acid
(MAA), in human keratinocyte exposed with UV-radiation. Mar Drugs.
15:1962017. View Article : Google Scholar :
|
|
47
|
Muhammad S, Qasid SH, Rehman S and Rai AB:
Visible light communication applications in healthcare. Technol
Health Care. 24:135–138. 2016. View Article : Google Scholar
|
|
48
|
Tsibadze A, Chikvaidze E, Katsitadze A,
Kvachadze I, Tskhvediani N and Chikviladze A: Visible light and
human skin (Review). Georgian Med News. 46–53. 2015.In Russian.
|
|
49
|
Gegotek A, Atalay S, Domingues P and
Skrzydlewska E: The differences in the proteome profile of
cannabidiol-treated skin fibroblasts following UVA or UVB
irradiation in 2D and 3D cell cultures. Cells. 8:9952019.
View Article : Google Scholar :
|
|
50
|
Zhang C, Yuchi H, Sun L, Zhou X and Lin J:
Human amnion-derived mesenchymal stem cells protect against UVA
irradiation-induced human dermal fibroblast senescence, in vitro.
Mol Med Rep. 16:2016–2022. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Patel AD, Rotenberg S, Messer RL, Wataha
JC, Ogbureke KU, McCloud VV, Lockwood P, Hsu S and Lewis JB: Blue
light activates phase 2 response proteins and slows growth of a431
epidermoid carcinoma xenografts. Anticancer Res. 34:6305–6313.
2014.PubMed/NCBI
|
|
52
|
Niu T, Tian Y, Wang G, Guo G, Tong Y and
Shi Y: Inhibition of ROS-NF-κB-dependent autophagy enhances
hypocrellin A united LED red light-induced apoptosis in squamous
carcinoma A431 cells. Cell Signal. 69:1095502020. View Article : Google Scholar
|
|
53
|
Meulemans J, Delaere P and Vander Poorten
V: Photodynamic therapy in head and neck cancer: Indications,
outcomes, and future prospects. Curr Opin Otolaryngol Head Neck
Surg. 27:136–141. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kerr C, Adhikary G, Grun D, George N and
Eckert RL: Combination cisplatin and sulforaphane treatment reduces
proliferation, invasion, and tumor formation in epidermal squamous
cell carcinoma. Mol Carcinog. 57:3–11. 2018. View Article : Google Scholar
|
|
55
|
Hwang H, Biswas R, Chung PS and Ahn JC:
Modulation of EGFR and ROS induced cytochrome c release by
combination of photodynamic therapy and carboplatin in human
cultured head and neck cancer cells and tumor xenograft in nude
mice. J Photochem Photobiol B. 128:70–77. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhang X, Liu X, Kang S, Liu C and Hao Y:
Resveratrol enhances the effects of ALA-PDT on skin squamous cells
A431 through p38/MAPK signaling pathway. Cancer Biomark.
21:797–803. 2018. View Article : Google Scholar
|
|
57
|
Tampucci S, Carpi S, Digiacomo M, Polini
B, Fogli S, Burgalassi S, Macchia M, Nieri P, Manera C and Monti D:
Diclofenac-derived hybrids for treatment of actinic keratosis and
squamous cell carcinoma. Molecules. 24:17932019. View Article : Google Scholar :
|
|
58
|
Carbone C, Martins-Gomes C, Pepe V, Silva
AM, Musumeci T, Puglisi G, Furneri PM and Souto EB: Repurposing
itraconazole to the benefit of skin cancer treatment: A combined
azole-DDAB nanoencapsulation strategy. Colloids Surf B
Biointerfaces. 167:337–344. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Chen H, Pan J, Zhang L, Chen L, Qi H,
Zhong M, Shi X, Du J and Li Q: Downregulation of estrogen-related
receptor alpha inhibits human cutaneous squamous cell carcinoma
cell proliferation and migration by regulating EMT via fibronectin
and STAT3 signaling pathways. Eur J Pharmacol. 825:133–142. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ou C, Liu H, Ding Z and Zhou L:
Chloroquine promotes gefitinib-induced apoptosis by inhibiting
protective autophagy in cutaneous squamous cell carcinoma. Mol Med
Rep. 20:4855–4866. 2019.PubMed/NCBI
|
|
61
|
Fusenig NE and Boukamp P: Multiple stages
and genetic alterations in immortalization, malignant
transformation, and tumor progression of human skin keratinocytes.
Mol Carcinog. 23:144–158. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Colombo I, Sangiovanni E, Maggio R,
Mattozzi C, Zava S, Corbett Y, Fumagalli M, Carlino C, Corsetto PA,
Scaccabarozzi D, et al: HaCaT cells as a reliable in vitro
differentiation model to dissect the inflammatory/repair response
of human keratinocytes. Mediators Inflamm. 2017:74356212017.
View Article : Google Scholar
|
|
63
|
Cordero RR, Damiani A, Seckmeyer G,
Jorquera J, Caballero M, Rowe P, Ferrer J, Mubarak R, Carrasco J,
Rondanelli R, et al: The solar spectrum in the atacama det. Sci
Rep. 6:224572016. View Article : Google Scholar
|
|
64
|
Faurschou A, Gniadecki R, Calay D and Wulf
HC: TNF-alpha impairs the S-G2/M cell cycle checkpoint and
cyclobutane pyrimidine dimer repair in premalignant skin cells:
Role of the PI3K-Akt pathway. J Invest Dermatol. 128:2069–2077.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Schug ZT, Gonzalvez F, Houtkooper RH, Vaz
FM and Gottlieb E: BID is cleaved by caspase-8 within a native
complex on the mitochondrial membrane. Cell Death Differ.
18:538–548. 2011. View Article : Google Scholar :
|
|
66
|
Huang C and Yu Y: Synergistic cytotoxicity
of β-elemene and cisplatin in gingival squamous cell carcinoma by
inhibition of STAT3 signaling pathway. Med Sci Monit. 23:1507–1513.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Tao JX, Zhou WC and Zhu XG: Mitochondria
as potential targets and initiators of the blue light hazard to the
retina. Oxid Med Cell Longev. 2019:64353642019. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Hientz K, Mohr A, Bhakta-Guha D and
Efferth T: The role of p53 in cancer drug resistance and targeted
chemotherapy. Oncotarget. 8:8921–8946. 2017. View Article : Google Scholar :
|
|
69
|
Batchelor E and Loewer A: Recent progress
and open challenges in modeling p53 dynamics in single cells. Curr
Opin Syst Biol. 3:54–59. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Batchelor E, Loewer A, Mock C and Lahav G:
Stimulus-dependent dynamics of p53 in single cells. Mol Syst Biol.
7:4882011. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Paek AL, Liu JC, Loewer A, Forrester WC
and Lahav G: Cell-to-cell variation in p53 dynamics leads to
fractional killing. Cell. 165:631–642. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Bowen AR, Hanks AN, Allen SM, Alexander A,
Diedrich MJ and Grossman D: Apoptosis regulators and responses in
human melanocytic and keratinocytic cells. J Invest Dermatol.
120:48–55. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Laubach V, Kaufmann R, Bernd A,
Kippenberger S and Zöller N: Extrinsic or intrinsic apoptosis by
curcumin and light: Still a mystery. Int J Mol Sci. 20:9052019.
View Article : Google Scholar :
|
|
74
|
Cives M, Mannavola F, Lospalluti L, Sergi
MC, Cazzato G, Filoni E, Cavallo F, Giudice G, Stucci LS, Porta C
and Tucci M: Non-melanoma skin cancers: Biological and clinical
features. Int J Mol Sci. 21:53942020. View Article : Google Scholar :
|
|
75
|
Gargiulo M, Papa A, Capasso P, Moio M,
Cubicciotti E and Parascandolo S: Electrochemotherapy for
non-melanoma head and neck cancers: Clinical outcomes in 25
patients. Ann Surg. 255:1158–1164. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Goggins CA and Khachemoune A: The use of
electrochemotherapy in combination with immunotherapy in the
treatment of metastatic melanoma: A focused review. Int J Dermatol.
58:865–870. 2019. View Article : Google Scholar
|
|
77
|
Montuori M, Santurro L, Feliziani A, DE
Sanctis F, Ricciardi E, Gaudio D, Campione E, Bianchi L, Silvi MB
and Rossi P: Electrochemotherapy for basocellular and
squamocellular head and neck cancer: preliminary experience in day
surgery unit. G Ital Dermatol Venereol. 153:19–25. 2018.
|
|
78
|
Grzelak A, Rychlik B and Bartosz G:
Light-dependent generation of reactive oxygen species in cell
culture media. Free Radic Biol Med. 30:1418–1425. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Stockley JH, Evans K, Matthey M, Volbracht
K, Agathou S, Mukanowa J, Burrone J and Káradóttir RT: Surpassing
light-induced cell damage in vitro with novel cell culture media.
Sci Rep. 7:8492017. View Article : Google Scholar : PubMed/NCBI
|