|
1
|
Mendelson CR, Jiang B, Shelton JM,
Richardson JA and Hinshelwood MM: Transcriptional regulation of
aromatase in placenta and ovary. J Steroid Biochem Mol Biol.
95:25–33. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li J and Gibbs RB: Detection of estradiol
in rat brain tissues: Contribution of local versus systemic
production. Psychoneuroendocrinology. 102:84–94. 2019. View Article : Google Scholar
|
|
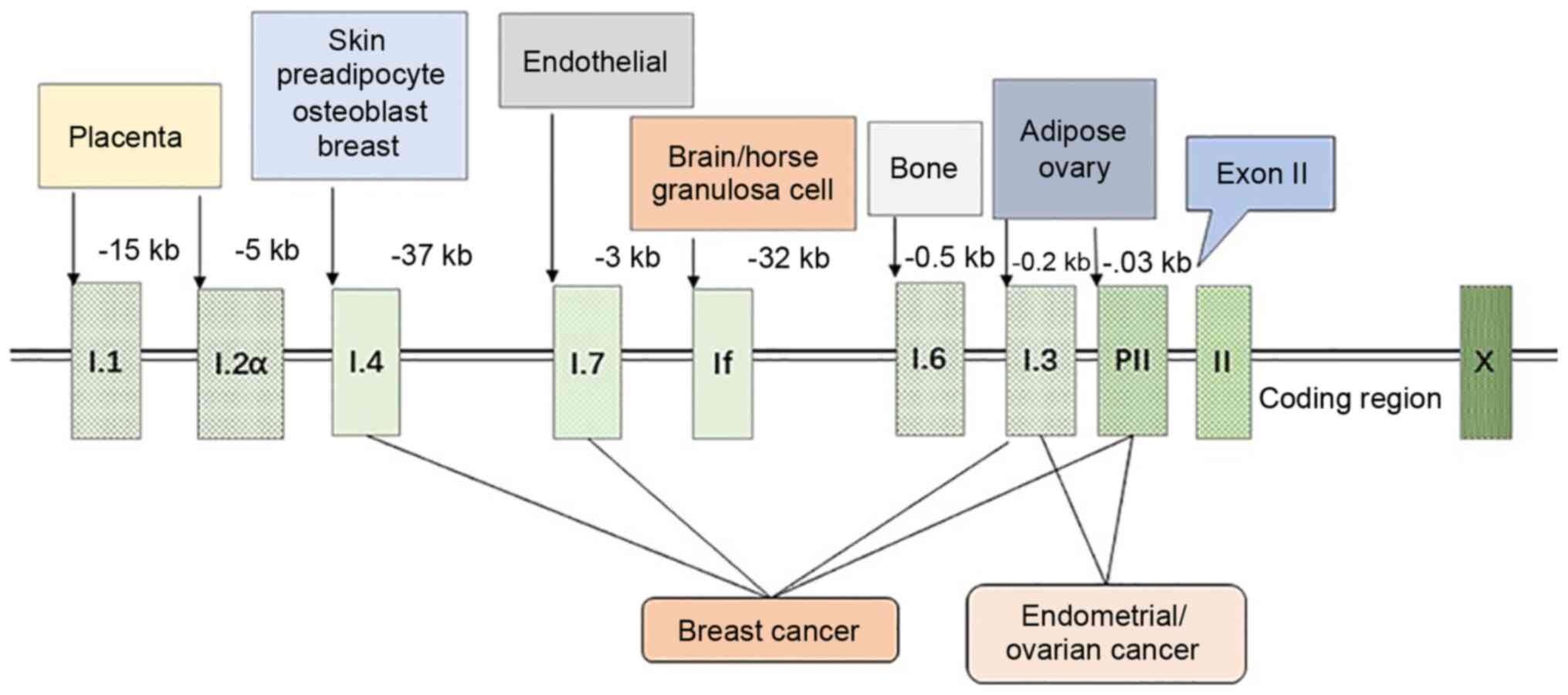
3
|
Lambard S, Silandre D, Delalande C,
Denis-Galeraud I, Bourguiba S and Carreau S: Aromatase in testis:
Expression and role in male reproduction. J Steroid Biochem Mol
Biol. 95:63–69. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mahendroo MS, Mendelson CR and Simpson ER:
Tissue-specific and hormonally controlled alternative promoters
regulate aromatase cytochrome P450 gene expression in human adipose
tissue. J Biol Chem. 268:19463–19470. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang Y, Pan P, Li X, Zhu Q, Huang T and Ge
RS: Food components and environmental chemicals of inhibiting human
placental aromatase. Food Chem Toxicol. 128:46–53. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ai A, Tang Z, Liu Y, Yu S, Li B, Huang H,
Wang X, Cao Y and Zhang W: Characterization and identification of
human immortalized granulosa cells derived from ovarian follicular
fluid. Exp Ther Med. 18:2167–2177. 2019.PubMed/NCBI
|
|
7
|
Shoham Z, Jacobs HS and Insler V:
Luteinizing hormone: Its role, mechanism of action, and detrimental
effects when hyper-secreted during the follicular phase. Fertil
Steril. 59:1153–1161. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nelson LR and Bulun SE: Estrogen
production and action. J Am Acad Dermatol. 45(Suppl 3): pp.
S116–S124. 2001, View Article : Google Scholar
|
|
9
|
Slominski A, Zbytek B, Nikolakis G, Manna
PR, Skobowiat C, Zmijewski M, Li W, Janjetovic Z, Postlethwaite A,
Zouboulis CC and Tuckey RC: Steroidogenesis in the skin:
Implications for local immune functions. J Steroid Biochem Mol
Biol. 137:107–123. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bulun SE, Chen D, Moy I, Brooks DC and
Zhao H: Aromatase, breast cancer and obesity: A complex
interaction. Trends Endocrinol Metab. 23:83–89. 2012. View Article : Google Scholar :
|
|
11
|
Zhao H, Zhou L, Shangguan AJ and Bulun SE:
Aromatase expression and regulation in breast and endometrial
cancer. J Mol Endocrinol. 57:R19–R33. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
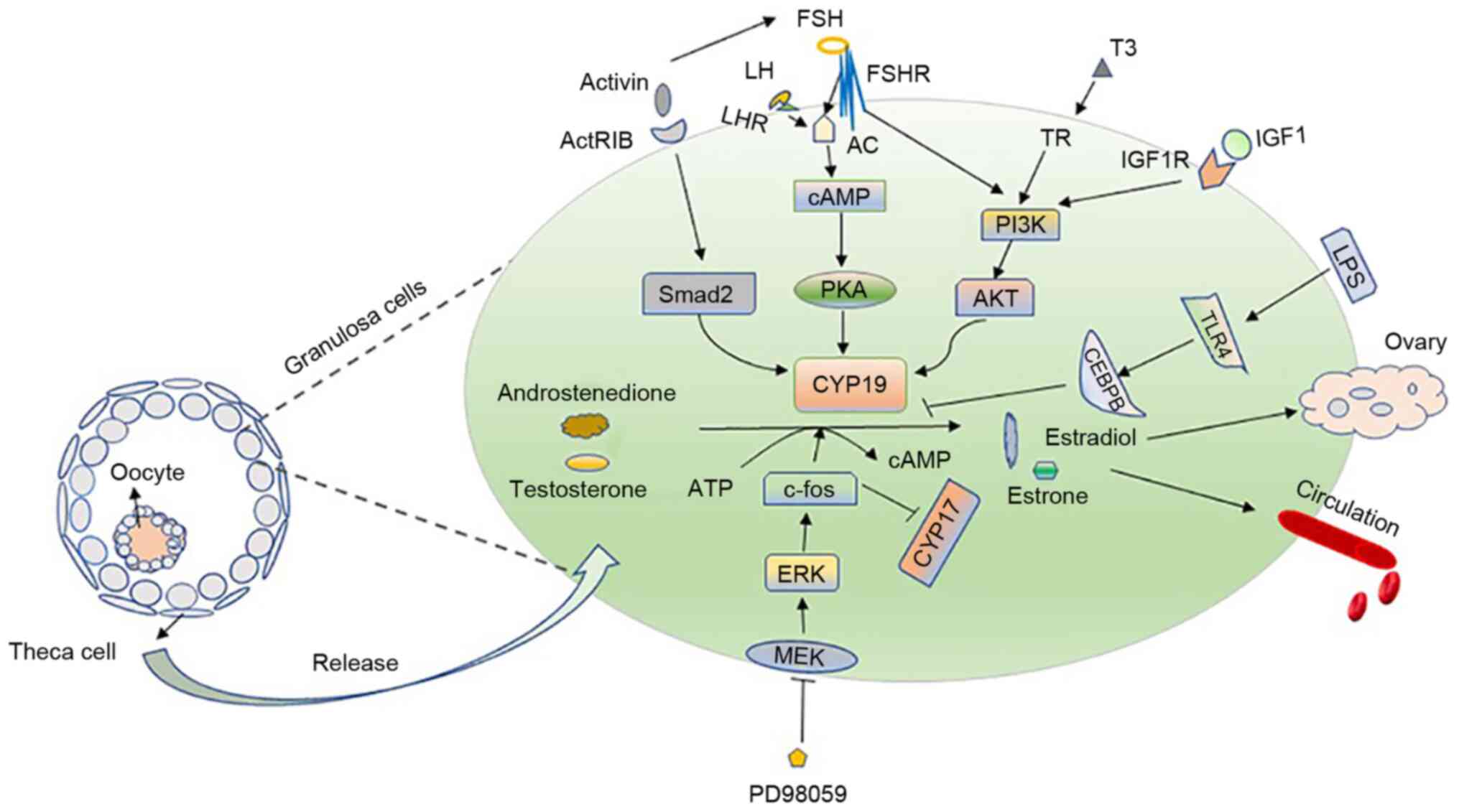
12
|
Shozu M, Zhao Y and Simpson ER: TGF-beta1
stimulates expression of the aromatase (CYP19) gene in human
osteoblast-like cells and THP-1 cells. Mol Cell Endocrinol.
160:123–133. 2000. View Article : Google Scholar : PubMed/NCBI
|
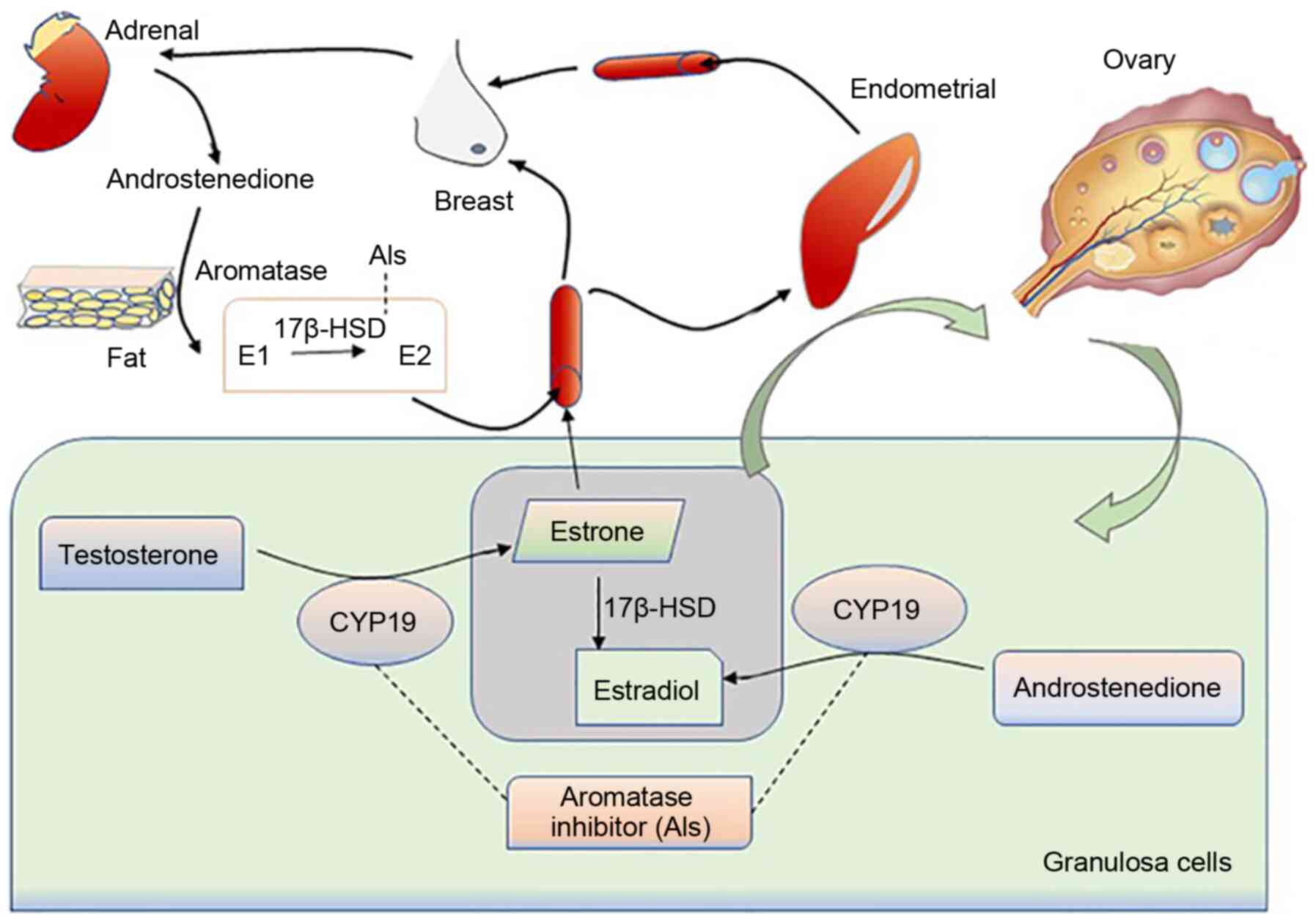
|
13
|
Stocco C: Aromatase expression in the
ovary: Hormonal and molecular regulation. Steroids. 73:473–487.
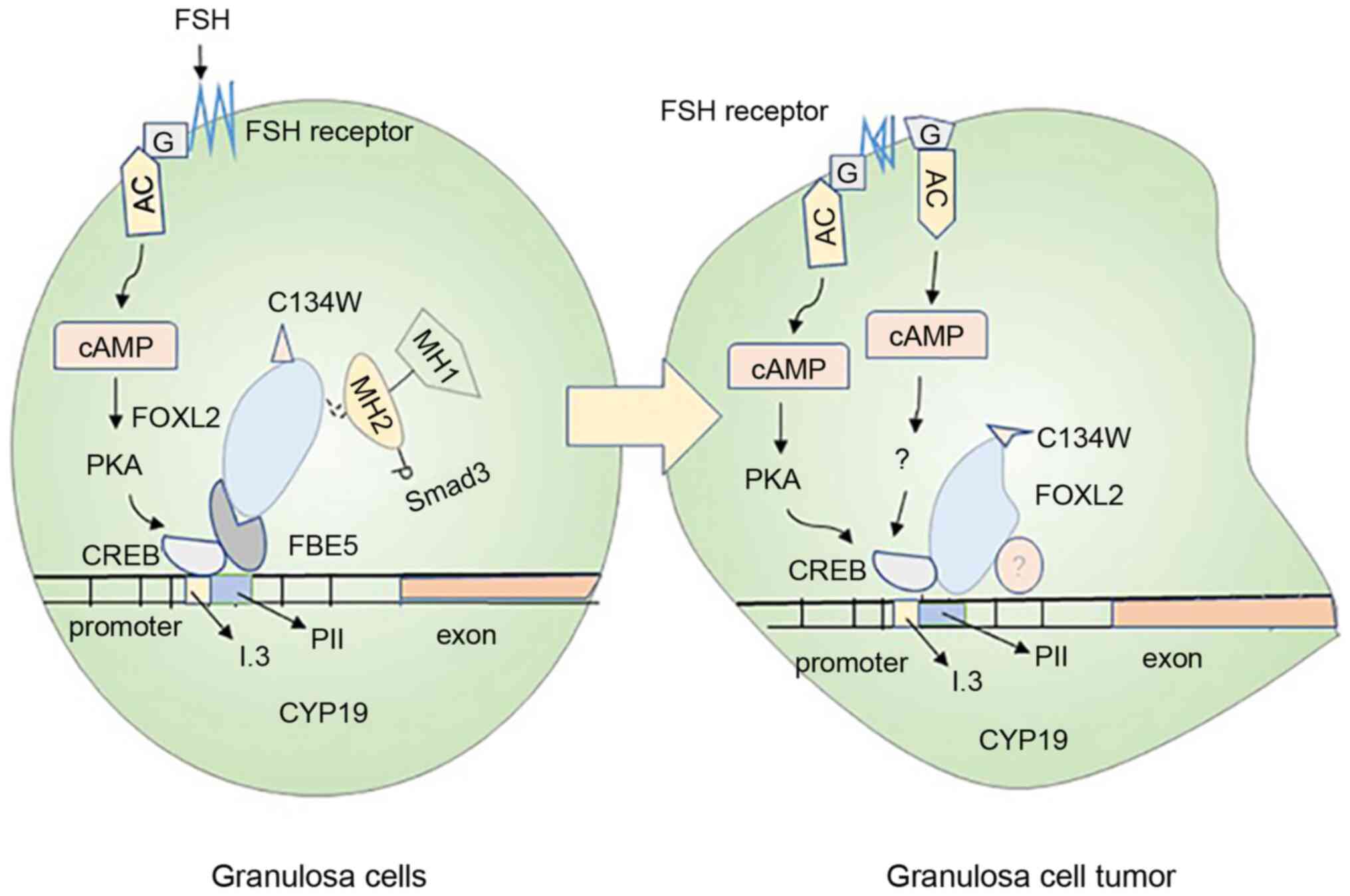
2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bulun SE, Chen D, Lu M, Zhao H, Cheng Y,
Demura M, Yilmaz B, Martin R, Utsunomiya H, Thung S, et al:
Aromatase excess in cancers of breast, endometrium and ovary. J
Steroid Biochem Mol Biol. 106:81–96. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bulun SE and Simpson ER: Aromatase
expression in women's cancers. Adv Exp Med Biol. 630:112–132. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sharma D, Ghai S and Singh D: Different
promoter usage for CYP19 gene expression in buffalo ovary and
placenta. Gen Comp Endocrinol. 162:319–328. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Solak KA, Wijnolts FMJ, Nijmeijer SM,
Blaauboer BJ, van den Berg M and van Duursen MBM: Excessive levels
of diverse phytoestrogens can modulate steroidogenesis and cell
migration of KGN human granulosa-derived tumor cells. Toxicol Rep.
1:360–372. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ghosh S, Wu Y, Li R and Hu Y: Jun proteins
modulate the ovary-specific promoter of aromatase gene in ovarian
granulosa cells via a cAMP-responsive element. Oncogene.
24:2236–2246. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li Q, Du X, Pan Z, Zhang L and Li Q: The
transcription factor SMAD4 and miR-10b contribute to E2 release and
cell apoptosis in ovarian granulosa cells by targeting CYP19A1. Mol
Cell Endocrinol. 476:84–95. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Andrieu T, Féral C, Joubert M, Benhaim A
and Mittre H: The absence of a functional nuclear receptor element
A (NREA) in the promoter II of the aromatase P450 gene in rabbit
granulosa cells. J Steroid Biochem Mol Biol. 101:127–135. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Boerboom D, Kerban A and Sirois J: Dual
regulation of promoter II- and promoter 1f-derived cytochrome P450
aromatase transcripts in equine granulosa cells during human
chorionic gonadotropin-induced ovulation: A novel model for the
study of aromatase promoter switching. Endocrinology.
140:4133–4141. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Simpson ER: Sources of estrogen and their
importance. J Steroid Biochem Mol Biol. 86:225–230. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Miyoshi T, Otsuka F and Shimasaki S: GRK-6
mediates FSH action synergistically enhanced by estrogen and the
oocyte in rat granulosa cells. Biochem Biophys Res Commun.
434:401–406. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Czajka-Oraniec I and Simpson ER: Aromatase
research and its clinical significance. Endokrynol Pol. 61:126–134.
2010.PubMed/NCBI
|
|
25
|
Velthut-Meikas A, Simm J, Tuuri T,
Tapanainen JS, Metsis M and Salumets A: Research resource: Small
RNA-seq of human granulosa cells reveals miRNAs in FSHR and
aromatase genes. Mol Endocrinol. 27:1128–1141. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mlodawska W and Slomczynska M:
Immunohistochemical localization of aromatase during the
development and atresia of ovarian follicles in prepubertal horses.
Theriogenology. 74:1707–1712. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Naganuma H, Ohtani H, Harada N and Nagura
H: Immunoelectron microscopic localization of aromatase in human
placenta and ovary using microwave fixation. J Histochem Cytochem.
38:1427–1432. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shaikh AA: Estrone and estradiol levels in
the ovarian venous blood from rats during the estrous cycle and
pregnancy. Biol Reprod. 5:297–307. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Szymańska K, Kałafut J, Przybyszewska A,
Paziewska B, Adamczuk G, Kiełbus M and Rivero-Müller A: FSHR
trans-activation and oligomerization. Front Endocrinol (Lausanne).
9:7602018. View Article : Google Scholar
|
|
30
|
Jiang C, Hou X, Wang C, May JV, Butnev VY,
Bousfield GR and Davis JS: Hypoglycosylated hFSH has greater
bioactivity than fully glycosylated recombinant hFSH in human
granulosa cells. J Clin Endocrinol Metab. 100:E852–E860. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hobeika E, Armouti M, Kala H, Fierro MA,
Winston NJ, Scoccia B, Zamah AM and Stocco C: Oocyte-secreted
factors synergize with FSH to promote aromatase expression in
primary human cumulus cells. J Clin Endocrinol Metab.
104:1667–1676. 2019. View Article : Google Scholar :
|
|
32
|
Parakh TN, Hernandez JA, Grammer JC, Weck
J, Hunzicker- Dunn M, Zeleznik AJ and Nilson JH:
Follicle-stimulating hormone/cAMP regulation of aromatase gene
expression requires beta-catenin. Proc Natl Acad Sci USA.
103:12435–12440. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kwintkiewicz J, Cai Z and Stocco C:
Follicle-stimulating hormone-induced activation of Gata4
contributes in the up-regulation of Cyp19 expression in rat
granulosa cells. Mol Endocrinol. 21:933–947. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hong Y, Li H, Yuan YC and Chen S:
Molecular characterization of aromatase. Ann N Y Acad Sci.
1155:112–120. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li Y, Gao D, Xu T, Adur MK, Zhang L, Luo
L, Zhu T, Tong X, Zhang D, Wang Y, et al: Anti-Müllerian hormone
inhibits luteinizing hormone-induced androstenedione synthesis in
porcine theca cells. Theriogenology. 142:421–432. 2020. View Article : Google Scholar
|
|
36
|
Fang Y, Wang B, Lyu S, Zhang K, Cheng Q
and Zhu Y: Virus analog decreases estradiol secretion in
FSH-treated human ovarian granulosa cells. Gynecol Endocrinol.
36:346–350. 2020. View Article : Google Scholar
|
|
37
|
Kajitani T, Liu S, Maruyama T, Uchida H,
Sakurai R, Masuda H, Nagashima T, Ono M, Arase T and Yoshimura Y:
Analysis of serum FSH bioactivity in a patient with an
FSH-secreting pituitary microadenoma and multicystic ovaries: A
case report. Hum Reprod. 23:435–439. 2008. View Article : Google Scholar
|
|
38
|
Shi J, Yoshino O, Osuga Y, Koga K, Hirota
Y, Nose E, Nishii O, Yano T and Taketani Y: Bone morphogenetic
protein-2 (BMP-2) increases gene expression of FSH receptor and
aromatase and decreases gene expression of LH receptor and StAR in
human granulosa cells. Am J Reprod Immunol. 65:421–427. 2011.
View Article : Google Scholar
|
|
39
|
Shi J, Yoshino O, Osuga Y, Koga K, Hirota
Y, Hirata T, Yano T, Nishii O and Taketani Y: Bone morphogenetic
protein-6 stimulates gene expression of follicle-stimulating
hormone receptor, inhibin/activin beta subunits, and anti-Müllerian
hormone in human granulosa cells. Fertil Steril. 92:1794–1798.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Shi J, Yoshino O, Osuga Y, Nishii O, Yano
T and Taketani Y: Bone morphogenetic protein 7 (BMP-7) increases
the expression of follicle-stimulating hormone (FSH) receptor in
human granulosa cells. Fertil Steril. 93:1273–1279. 2010.
View Article : Google Scholar
|
|
41
|
Overes HW, de Leeuw R and Kloosterboer HJ:
Regulation of aromatase activity in FSH-primed rat granulosa cells
in vitro by follicle-stimulating hormone and various amounts of
human chorionic gonadotrophin. Hum Reprod. 7:191–196. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wu Y, Ghosh S, Nishi Y, Yanase T, Nawata H
and Hu Y: The orphan nuclear receptors NURR1 and NGFI-B modulate
aromatase gene expression in ovarian granulosa cells: A possible
mechanism for repression of aromatase expression upon luteinizing
hormone surge. Endocrinology. 146:237–246. 2005. View Article : Google Scholar
|
|
43
|
Du BW, Zhang XJ, Shi N, Peng T, Gao JB,
Azimova B, Zhang R, Pu DB, Wang C, Abduvaliev A, et al:
Luteolin-7-methylether from Leonurus japonicus inhibits estrogen
biosynthesis in human ovarian granulosa cells by suppression of
aromatase (CYP19). Eur J Pharmacol. 879:1731542020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lee SY, Kang YJ, Kwon J, Nishi Y, Yanase
T, Lee KA and Koong MK: miR-4463 regulates aromatase expression and
activity for 17β-estradiol synthesis in response to
follicle-stimulating hormone. Clin Exp Reprod Med. 47:194–206.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Xu S, Linher-Melville K, Yang BB, Wu D and
Li J: Micro-RNA378 (miR-378) regulates ovarian estradiol production
by targeting aromatase. Endocrinology. 152:3941–3951. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Liu J, Li X, Yao Y and Li Q, Pan Z and Li
Q: miR-1275 controls granulosa cell apoptosis and estradiol
synthesis by impairing LRH-1/CYP19A1 axis. Biochim Biophys Acta
Gene Regul Mech. 1861:246–257. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang L, Li C, Li R, Deng Y, Tan Y, Tong C
and Qi H: MicroRNA-764-3p regulates 17β-estradiol synthesis of
mouse ovarian granulosa cells by targeting steroidogenic factor-1.
In Vitro Cell Dev Biol Anim. 52:365–373. 2016. View Article : Google Scholar
|
|
48
|
Chaurasiya V, Kumari S, Onteru SK and
Singh D: miR-326 down-regulate CYP19A1 expression and estradiol-17b
production in buffalo granulosa cells through CREB and C/EBP-β. J
Steroid Biochem Mol Biol. 199:1056082020. View Article : Google Scholar
|
|
49
|
Shi S, Zhou X, Li J, Zhang L, Hu Y, Li Y,
Yang G and Chu G: MiR-214-3p promotes proliferation and inhibits
estradiol synthesis in porcine granulosa cells. J Anim Sci
Biotechnol. 11:942020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Li Y, Liu YD, Zhou XY, Chen SL, Chen X,
Zhe J, Zhang J, Zhang QY and Chen YX: MiR-29a regulates the
proliferation, aromatase expression, and estradiol biosynthesis of
human granulosa cells in polycystic ovary syndrome. Mol Cell
Endocrinol. 498:1105402019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Al-Kawlani B, Murrieta-Coxca JM,
Chaiwangyen W, Fröhlich K, Fritzsche A, Winkler S, Markert UR and
Morales-Prieto DM: Doxorubicin induces cytotoxicity and miR-132
expression in granulosa cells. Reprod Toxicol. 96:95–101. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ogo Y, Taniuchi S, Ojima F, Hayashi S,
Murakami I, Saito Y, Takeuchi S, Kudo T and Takahashi S: IGF-1 gene
expression is differentially regulated by estrogen receptors α and
β in mouse endometrial stromal cells and ovarian granulosa cells. J
Reprod Dev. 60:216–223. 2014. View Article : Google Scholar :
|
|
53
|
Zhou J, Chin E and Bondy C: Cellular
pattern of insulin-like growth factor-I (IGF-I) and IGF-I receptor
gene expression in the developing and mature ovarian follicle.
Endocrinology. 129:3281–3288. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Mani AM, Fenwick MA, Cheng Z, Sharma MK,
Singh D and Wathes DC: IGF1 induces up-regulation of steroidogenic
and apoptotic regulatory genes via activation of
phosphatidylinositol-dependent kinase/AKT in bovine granulosa
cells. Reproduction. 139:139–151. 2010. View Article : Google Scholar
|
|
55
|
Herrmann M, Scholmerich J and Straub RH:
Influence of cytokines and growth factors on distinct steroidogenic
enzymes in vitro: A short tabular data collection. Ann NY Acad Sci.
966:166–186. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Fang L, Yu Y, Li Y, Wang S, Zhang R, Guo
Y, Li Y, Yan Y and Sun YP: Human chorionic gonadotropin-induced
amphiregulin stimulates aromatase expression in human
granulosa-lutein cells: A mechanism for estradiol production in the
luteal phase. Hum Reprod. 34:2018–2026. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Mendelson CR, Merrill JC, Steinkampf MP
and Simpson ER: Regulation of the synthesis of aromatase cytochrome
P-450 in human adipose stromal and ovarian granulosa cells.
Steroids. 50:51–59. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Mishra SR, Bharati J, Rajesh G, Chauhan
VS, Taru Sharma G, Bag S, Maurya VP, Singh G and Sarkar M:
Fibroblast growth factor 2 (FGF2) and vascular endothelial growth
factor A (VEGFA) synergistically promote steroidogenesis and
survival of cultured buffalo granulosa cells. Anim Reprod Sci.
179:88–97. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zachow RJ, Ramski BE and Lee H: Modulation
of estrogen production and 17beta-hydroxysteroid dehydrogenase-type
1, cytochrome P450 aromatase, c-met, and protein kinase Balpha
messenger ribonucleic acid content in rat ovarian granulosa cells
by hepatocyte growth factor and follicle-stimulating hormone. Biol
Reprod. 62:1851–1857. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Chen YJ, Hsiao PW, Lee MT, Mason JI, Ke FC
and Hwang JJ: Interplay of PI3K and cAMP/PKA signaling, and
rapamycin-hypersensitivity in TGFbeta1 enhancement of
FSH-stimulated steroidogenesis in rat ovarian granulosa cells. J
Endocrinol. 192:405–419. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zachow RJ, Weitsman SR and Magoffin DA:
Leptin impairs the synergistic stimulation by transforming growth
factor-beta of follicle-stimulating hormone-dependent aromatase
activity and messenger ribonucleic acid expression in rat ovarian
granulosa cells. Biol Reprod. 61:1104–1109. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Kwintkiewicz J, Nishi Y, Yanase T and
Giudice LC: Peroxisome proliferator-activated receptor-gamma
mediates bisphenol A inhibition of FSH-stimulated IGF-1, aromatase,
and estradiol in human granulosa cells. Environ Health Perspect.
118:400–406. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Bloom MS, Mok-Lin E and Fujimoto VY:
Bisphenol A and ovarian steroidogenesis. Fertil Steril.
106:857–863. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Dasmahapatra AK, Wimpee BA, Trewin AL and
Hutz RJ: 2,3,7,8-Tetrachlorodibenzo-p-dioxin increases steady-state
estrogen receptor-beta mRNA levels after CYP1A1 and CYP1B1
induction in rat granulosa cells in vitro. Mol Cell Endocrinol.
182:39–48. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Dasmahapatra AK, Wimpee BA, Trewin AL,
Wimpee CF, Ghorai JK and Hutz RJ: Demonstration of
2,3,7,8-tetrachloro-dibenzo-p-dioxin attenuation of P450
steroidogenic enzyme mRNAs in rat granulosa cell in vitro by
competitive reverse transcriptase-polymerase chain reaction assay.
Mol Cell Endocrinol. 164:5–18. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Enan E, Moran F, VandeVoort CA, Stewart
DR, Overstreet JW and Lasley BL: Mechanism of toxic action of
2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in cultured human
luteinized granulosa cells. Reprod Toxicol. 10:497–508. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Baldridge MG, Marks GT, Rawlins RG and
Hutz RJ: Very low-dose (femtomolar)
2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) disrupts steroidogenic
enzyme mRNAs and steroid secretion by human luteinizing granulosa
cells. Reprod Toxicol. 52:57–61. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Lovekamp TN and Davis BJ:
Mono-(2-ethylhexyl) phthalate suppresses aromatase transcript
levels and estradiol production in cultured rat granulosa cells.
Toxicol Appl Pharmacol. 172:217–224. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Reinsberg J, Wegener-Toper P, van der Ven
K, van der Ven H and Klingmueller D: Effect of mono-(2-ethylhexyl)
phthalate on steroid production of human granulosa cells. Toxicol
Appl Pharmacol. 239:116–123. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Davis BJ, Weaver R, Gaines LJ and Heindel
JJ: Mono-(2-ethylhexyl) phthalate suppresses estradiol production
independent of FSH-cAMP stimulation in rat granulosa cells. Toxicol
Appl Pharmacol. 128:224–228. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Simon V, Avet C, Grange-Messent V,
Wargnier R, Denoyelle C, Pierre A, Dairou J, Dupret JM and
Cohen-Tannoudji J: Carbon black nanoparticles inhibit aromatase
expression and estradiol secretion in human granulosa cells through
the ERK1/2 pathway. Endocrinology. 158:3200–3211. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Fan G, Zhang Q, Wan Y, Lv F, Chen Y, Ni Y,
Zou W, Zhang W and Wang H: Decreased levels of H3K9ac and H3K27ac
in the promotor region of ovarian P450 aromatase mediated low
estradiol synthesis in female offspring rats induced by prenatal
nicotine exposure as well as in human granulosa cells after
nicotine treatment. Food Chem Toxicol. 128:256–266. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Taupeau C, Poupon J, Treton D, Brosse A,
Richard Y and Machelon V: Lead reduces messenger RNA and protein
levels of cytochrome p450 aromatase and estrogen receptor beta in
human ovarian granulosa cells. Biol Reprod. 68:1982–1988. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Morinaga H, Yanase T, Nomura M, Okabe T,
Goto K, Harada N and Nawata H: A benzimidazole fungicide, benomyl,
and its metabolite, carbendazim, induce aromatase activity in a
human ovarian granulose-like tumor cell line (KGN). Endocrinology.
145:1860–1869. 2004. View Article : Google Scholar
|
|
75
|
Zachow R and Uzumcu M: The methoxychlor
metabolite, 2,2-bis-(p-hydroxyphenyl)-1,1,1-trichloroethane,
inhibits steroidogenesis in rat ovarian granulosa cells in vitro.
Reprod Toxicol. 22:659–665. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Rice S, Pellatt L, Ramanathan K, Whitehead
SA and Mason HD: Metformin inhibits aromatase via an extracellular
signal-regulated kinase-mediated pathway. Endocrinology.
150:4794–4801. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Fuhrmeister IP, Branchini G, Pimentel AM,
Ferreira GD, Capp E, Brum IS and von Eye Corleta H: Human granulosa
cells: Insulin and insulin-like growth factor-1 receptors and
aromatase expression modulation by metformin. Gynecol Obstet
Invest. 77:156–162. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Seto-Young D, Avtanski D, Parikh G,
Suwandhi P, Strizhevsky M, Araki T, Rosenwaks Z and Poretsky L:
Rosiglitazone and pioglitazone inhibit estrogen synthesis in human
granulosa cells by interfering with androgen binding to aromatase.
Horm Metab Res. 43:250–256. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Mu YM, Yanase T, Nishi Y, Waseda N, Oda T,
Tanaka A, Takayanagi R and Nawata H: Insulin sensitizer,
troglitazone, directly inhibits aromatase activity in human ovarian
granulosa cells. Biochem Biophys Res Commun. 271:710–713. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Gonzalez-Robayna IJ, Falender AE, Ochsner
S, Firestone GL and Richards JS: Follicle-Stimulating hormone (FSH)
stimulates phosphorylation and activation of protein kinase B
(PKB/Akt) and serum and glucocorticoid-lnduced kinase (Sgk):
Evidence for A kinase-independent signaling by FSH in granulosa
cells. Mol Endocrinol. 14:1283–1300. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Donadeu FX and Ascoli M: The differential
effects of the gonado- tropin receptors on aromatase expression in
primary cultures of immature rat granulosa cells are highly
dependent on the density of receptors expressed and the activation
of the inositol phosphate cascade. Endocrinology. 146:3907–3916.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Riccetti L, Sperduti S, Lazzaretti C,
Casarini L and Simoni M: The cAMP/PKA pathway: Steroidogenesis of
the antral follicular stage. Minerva Ginecol. 70:516–524. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Alam H, Maizels ET, Park Y, Ghaey S,
Feiger ZJ, Chandel NS and Hunzicker-Dunn M: Follicle-stimulating
hormone activation of hypoxia-inducible factor-1 by the
phosphatidylinositol 3-kinase/AKT/Ras homolog enriched in brain
(Rheb)/mammalian target of rapamycin (mTOR) pathway is necessary
for induction of select protein markers of follicular
differentiation. J Biol Chem. 279:19431–19440. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Zhou Y, Zeng C, Li X, Wu PL, Yin L, Yu XL,
Zhou YF and Xue Q: IGF-I stimulates ERβ and aromatase expression
via IGF1R/PI3K/AKT-mediated transcriptional activation in
endometriosis. J Mol Med (Berl). 94:887–897. 2016. View Article : Google Scholar
|
|
85
|
Liu J, Han Y, Tian Y, Weng X, Hu X, Liu W,
Heng D, Xu K, Yang Y and Zhang C: Regulation by
3,5,3′-tri-iodothyronine and FSH of cytochrome P450 family 19
(CYP19) expression in mouse granulosa cells. Reprod Fertil Dev.
30:1225–1233. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Cottom J, Salvador LM, Maizels ET,
Reierstad S, Park Y, Carr DW, Davare MA, Hell JW, Palmer SS, Dent
P, et al: Follicle-stimulating hormone activates extracellular
signal-regulated kinase but not extracellular signal-regulated
kinase kinase through a 100-kDa phosphotyrosine phosphatase. J Biol
Chem. 278:7167–7179. 2003. View Article : Google Scholar
|
|
87
|
Huang X, Jin J, Shen S, Xia Y, Xu P, Zou
X, Wang H, Yi L, Wang Y and Gao Q: Modulation of expression of
17-Hydroxylase/17,20 lyase (CYP17) and P450 aromatase (CYP19) by
inhibition of MEK1 in a human ovarian granulosa-like tumor cell
line. Gynecol Endocrinol. 32:201–205. 2016. View Article : Google Scholar
|
|
88
|
Findlay JK: An update on the roles of
inhibin, activin, and follistatin as local regulators of
folliculogenesis. Biol Reprod. 48:15–23. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Nomura M, Sakamoto R, Morinaga H, Wang L,
Mukasa C and Takayanagi R: Activin stimulates CYP19A gene
expression in human ovarian granulosa cell-like KGN cells via the
Smad2 signaling pathway. Biochem Biophys Res Commun. 436:443–448.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Yenuganti VR, Ravinder and Singh D:
Endotoxin induced TLR4 signaling downregulates CYP19A1 expression
through CEBPB in buffalo granulosa cells. Toxicol In Vitro.
42:93–100. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Wang Y, Lu E, Bao R, Xu P, Feng F, Wen W,
Dong Q, Hu C, Xiao L, Tang M, et al: Notch signalling regulates
steroidogenesis in mouse ovarian granulosa cells. Reprod Fertil
Dev. 31:1091–1103. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Manna PR, Molehin D and Ahmed AU:
Dysregulation of aromatase in breast, endometrial, and ovarian
cancers: An overview of therapeutic strategies. Prog Mol Biol
Transl Sci. 144:487–537. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Kato N, Uchigasaki S, Fukase M and Kurose
A: Expression of P450 aromatase in granulosa cell tumors and
sertoli-stromal cell tumors of the ovary: Which cells are
responsible for estrogenesis? Int J Gynecol Pathol. 35:41–47. 2016.
View Article : Google Scholar
|
|
94
|
Kitamura S, Abiko K, Matsumura N, Nakai H,
Akimoto Y, Tanimoto H and Konishi I: Adult granulosa cell tumors of
the ovary: A retrospective study of 30 cases with respect to the
expression of steroid synthesis enzymes. J Gynecol Oncol.
28:e312017. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Hsueh AJ, Adashi EY, Jones PB and Welsh TH
Jr: Hormonal regulation of the differentiation of cultured ovarian
granulosa cells. Endocr Rev. 5:76–127. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Cocquet J, Pailhoux E, Jaubert F, Servel
N, Xia X, Pannetier M, De Baere E, Messiaen L, Cotinot C, Fellous M
and Veitia RA: Evolution and expression of FOXL2. J Med Genet.
39:916–921. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Belli M, Iwata N, Nakamura T, Iwase A,
Stupack D and Shimasaki S: FOXL2C134W-induced CYP19 expression via
cooperation with SMAD3 in HGrC1 cells. Endocrinology.
159:1690–1703. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Fleming NI, Knower KC, Lazarus KA, Fuller
PJ, Simpson ER and Clyne CD: Aromatase is a direct target of FOXL2:
C134W in granulosa cell tumors via a single highly conserved
binding site in the ovarian specific promoter. PLoS One.
5:e143892010. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Leung K:
(S)-6-[(4-Chlorophenyl)(1H-1,2,4-triazol-1-yl)
methyl]-1-[(11)C]methyl-1H-benzotriazole. Molecular imaging and
contrast agent database (MICAD). National Center for Biotechnology
Information; Bethesda, MD: 2004
|
|
100
|
Moro F, Leombroni M, Pasciuto T,
Trivellizzi IN, Mascilini F, Ciccarone F, Zannoni GF, Fanfani F,
Scambia G and Testa AC: Synchronous primary cancers of endometrium
and ovary vs endometrial cancer with ovarian metastasis: An
observational study. Ultrasound Obstet Gynecol. 53:827–835.
2019.PubMed/NCBI
|
|
101
|
Michael MD, Kilgore MW, Morohashi K and
Simpson ER: Ad4BP/SF-1 regulates cyclic AMP-induced transcription
from the proximal promoter (PII) of the human aromatase P450
(CYP19) gene in the ovary. J Biol Chem. 270:13561–13566. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Panghiyangani R, Soeharso P, Andrijono,
Suryandari DA, Wiweko B, Kurniati M and Pujianto DA: CYP19A1 gene
expression in patients with polycystic ovarian syndrome. J Hum
Reprod Sci. 13:100–103. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Shozu M, Sumitani H, Segawa T, Yang HJ,
Murakami K, Kasai T and Inoue M: Overexpression of aromatase P450
in leiomyoma tissue is driven primarily through promoter I.4 of the
aromatase P450 gene (CYP19). J Clin Endocrinol Metab. 87:2540–2548.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Jamnongjit M and Hammes SR: Ovarian
steroids: The good, the bad, and the signals that raise them. Cell
Cycle. 5:1178–1183. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Yang F, Ruan YC, Yang YJ, Wang K, Liang
SS, Han YB, Teng XM and Yang JZ: Follicular hyperandrogenism
downregulates aromatase in luteinized granulosa cells in polycystic
ovary syndrome women. Reproduction. 150:289–296. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Dewailly D, Robin G, Peigne M, Decanter C,
Pigny P and Catteau-Jonard S: Interactions between androgens, FSH,
anti-Müllerian hormone and estradiol during folliculogenesis in the
human normal and polycystic ovary. Hum Reprod Update. 22:709–724.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Che Q, Liu M, Zhang D, Lu Y, Xu J, Lu X,
Cao X, Liu Y, Dong X and Liu S: Long noncoding RNA HUPCOS promotes
follicular fluid androgen excess in PCOS patients via aromatase
inhibition. J Clin Endocrinol Metab. 105:dgaa0602020. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Gu Y, Xu W, Zhuang B and Fu W: Role of
A-kinase anchoring protein 95 in the regulation of cytochrome P450
family 19 subfamily A member 1 (CYP19A1) in human ovarian granulosa
cells. Reprod Fertil Dev. 30:1128–1136. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Ma X, Hayes E, Prizant H, Srivastava RK,
Hammes SR and Sen A: Leptin-induced CART (cocaine- and
amphetamine-regulated transcript) is a novel intraovarian mediator
of obesity-related infertility in females. Endocrinology.
157:1248–1257. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Turkistani A and Marsh S: Pharmacogenomics
of third-generation aromatase inhibitors. Expert Opin Pharmacother.
13:1299–1307. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Kharb R, Haider K, Neha K and Yar MS:
Aromatase inhibitors: Role in postmenopausal breast cancer. Arch
Pharm (Weinheim). 353:e20000812020. View Article : Google Scholar
|
|
112
|
Usluogullari B, Duvan C and Usluogullari
C: Use of aromatase inhibitors in practice of gynecology. J Ovarian
Res. 8:42015. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Ammazzalorso A, Gallorini M, Fantacuzzi M,
Gambacorta N, De Filippis B, Giampietro L, Maccallini C, Nicolotti
O, Cataldi A and Amoroso R: Design, synthesis and biological
evaluation of imidazole and triazole-based carbamates as novel
aromatase inhibitors. Eur J Med Chem. 211:1131152021. View Article : Google Scholar
|
|
114
|
Haltia UM, Pihlajoki M, Andersson N,
Mäkinen L, Tapper J, Cervera A, Horlings HM, Turpeinen U, Anttonen
M, Bützow R, et al: Functional profiling of FSH and estradiol in
ovarian granulosa cell tumors. J Endocr Soc. 4:bvaa0342020.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Ghosh D, Lo J and Egbuta C: Recent
progress in the discovery of next generation inhibitors of
aromatase from the structure-function perspective. J Med Chem.
59:5131–5148. 2016. View Article : Google Scholar :
|
|
116
|
Steinkampf MP, Mendelson CR and Simpson
ER: Effects of epidermal growth factor and insulin-like growth
factor I on the levels of mRNA encoding aromatase cytochrome P-450
of human ovarian granulosa cells. Mol Cell Endocrinol. 59:93–99.
1988. View Article : Google Scholar : PubMed/NCBI
|