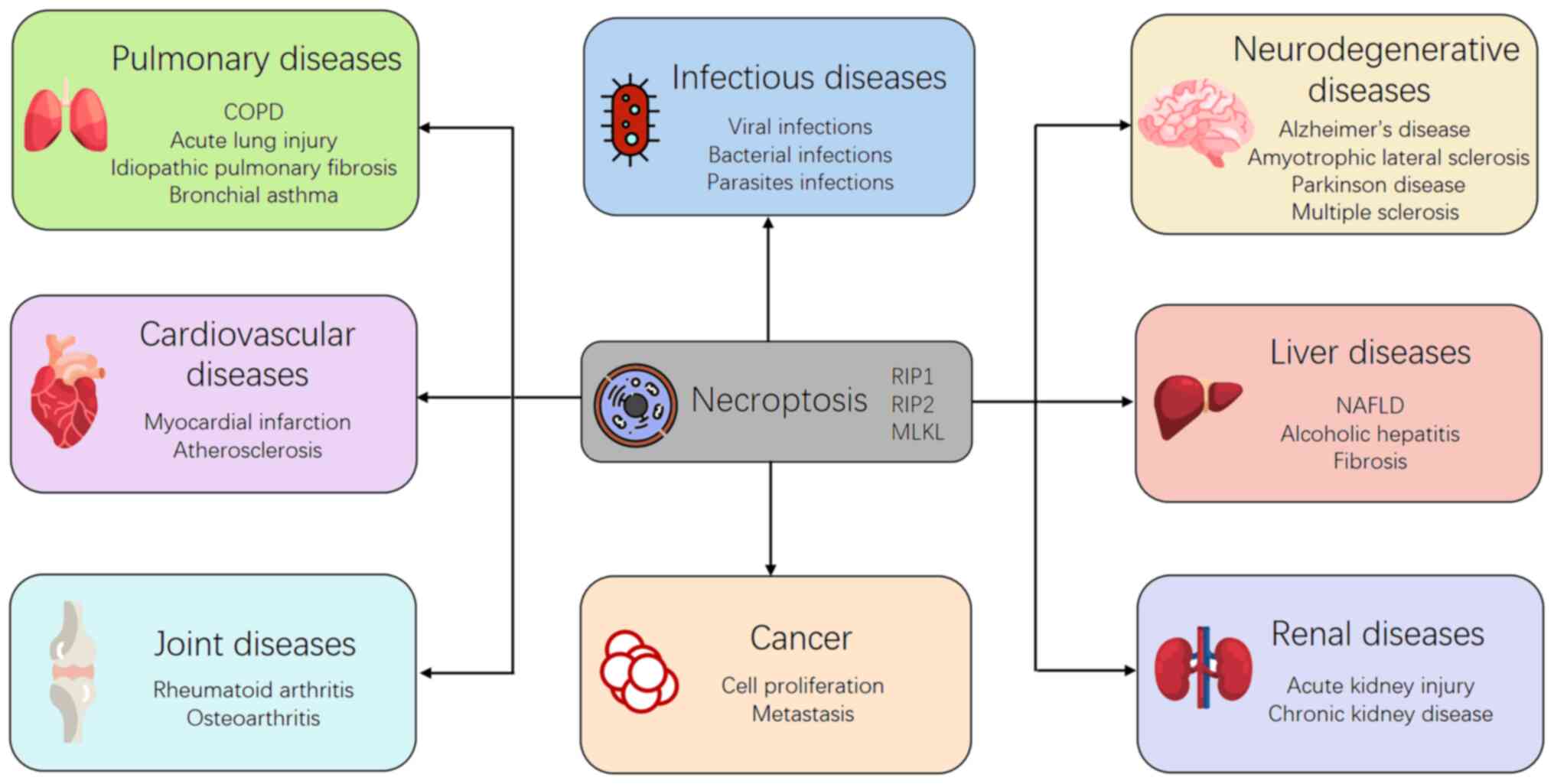
|
1
|
Galluzzi L, Vitale I, Abrams JM, Alnemri
ES, Baehrecke EH, Blagosklonny MV, Dawson TM, Dawson VL, El-Deiry
WS, Fulda S, et al: Molecular definitions of cell death
subroutines: Recommendations of the Nomenclature Committee on Cell
Death 2012. Cell Death Differ. 19:107–120. 2012. View Article : Google Scholar :
|
|
2
|
Moriwaki K, Balaji S, McQuade T, Malhotra
N, Kang J and Chan FK: The necroptosis adaptor RIPK3 promotes
injury-induced cytokine expression and tissue repair. Immunity.
41:567–578. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Holler N, Zaru R, Micheau O, Thome M,
Attinger A, Valitutti S, Bodmer JL, Schneider P, Seed B and Tschopp
J: Fas triggers an alternative, caspase-8-independent cell death
pathway using the kinase RIP as effector molecule. Nat Immunol.
1:489–495. 2000. View
Article : Google Scholar
|
|
4
|
He S, Wang L, Miao L, Du F, Zhao L and
Wang X: Receptor interacting protein kinase-3 determines cellular
necrotic response to TNF-alpha. Cell. 137:1100–1111. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cho YS, Challa S, Moquin D, Genga R, Ray
TD, Guildford M and Chan FK: Phosphorylation-driven assembly of the
RIP1-RIP3 complex regulates programmed necrosis and virus-induced
inflammation. Cell. 137:1112–1123. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang DW, Shao J, Lin J, Zhang N, Lu BJ,
Lin SC, Dong MQ and Han J: RIP3, an energy metabolism regulator
that switches TNF-induced cell death from apoptosis to necrosis.
Science. 325:332–336. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sun L, Wang H, Wang Z, He S, Chen S, Liao
D, Wang L, Yan J, Liu W, Lei X and Wang X: Mixed lineage kinase
domain-like protein mediates necrosis signaling downstream of RIP3
kinase. Cell. 148:213–227. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jouan-Lanhouet S, Riquet F, Duprez L,
Vanden Berghe T, Takahashi N and Vandenabeele P: Necroptosis, in
vivo detection in experimental disease models. Semin Cell Dev Biol.
35:2–13. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Upton JW, Kaiser WJ and Mocarski ES:
DAI/ZBP1/DLM-1 complexes with RIP3 to mediate virus-induced
programmed necrosis that is targeted by murine cytomegalovirus
vIRA. Cell Host Microbe. 11:290–297. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Oberst A and Green DR: It cuts both ways:
Reconciling the dual roles of caspase 8 in cell death and survival.
Nat Rev Mol Cell Biol. 12:757–763. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kaiser WJ, Upton JW, Long AB,
Livingston-Rosanoff D, Daley-Bauer LP, Hakem R, Caspary T and
Mocarski ES: RIP3 mediates the embryonic lethality of
caspase-8-deficient mice. Nature. 471:368–372. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Weinlich R, Oberst A, Beere HM and Green
DR: Necroptosis in development, inflammation and disease. Nat Rev
Mol Cell Biol. 18:127–136. 2017. View Article : Google Scholar
|
|
13
|
Sun X, Yin J, Starovasnik MA, Fairbrother
WJ and Dixit VM: Identification of a novel homotypic interaction
motif required for the phosphorylation of receptor-interacting
protein (RIP) by RIP3. J Biol Chem. 277:9505–9511. 2002. View Article : Google Scholar
|
|
14
|
Sun X, Lee J, Navas T, Baldwin DT, Stewart
TA and Dixit VM: RIP3, a novel apoptosis-inducing kinase. J Biol
Chem. 274:16871–16875. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shan B, Pan H, Najafov A and Yuan J:
Necroptosis in development and diseases. Genes Dev. 32:327–340.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dillon CP, Tummers B, Baran K and Green
DR: Developmental checkpoints guarded by regulated necrosis. Cell
Mol Life Sci. 73:2125–2136. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hacker G: The morphology of apoptosis.
Cell Tissue Res. 301:5–17. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ch'en IL, Tsau JS, Molkentin JD, Komatsu M
and Hedrick SM: Mechanisms of necroptosis in T cells. J Exp Med.
208:633–641. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lenardo M, Chan KM, Hornung F, McFarland
H, Siegel R, Wang J and Zheng L: Mature T lymphocyte
apoptosis-immune regulation in a dynamic and unpredictable
antigenic environment. Annu Rev Immunol. 17:221–253. 1999.
View Article : Google Scholar
|
|
20
|
Chen D, Yu J and Zhang L: Necroptosis: An
alternative cell death program defending against cancer. Biochim
Biophys Acta. 1865:228–236. 2016.PubMed/NCBI
|
|
21
|
He S, Huang S and Shen Z: Biomarkers for
the detection of necroptosis. Cell Mol Life Sci. 73:2177–2181.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Oerlemans MI, Liu J, Arslan F, den Ouden
K, van Middelaar BJ, Doevendans PA and Sluijter JP: Inhibition of
RIP1-dependent necrosis prevents adverse cardiac remodeling after
myocardial ischemia-reperfusion in vivo. Basic Res Cardiol.
107:2702012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dong K, Zhu H, Song Z, Gong Y, Wang F,
Wang W, Zheng Z, Yu Z, Gu Q, Xu X and Sun X: Necrostatin-1 protects
photoreceptors from cell death and improves functional outcome
after experimental retinal detachment. Am J Pathol. 181:1634–1641.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
McQuade T, Cho Y and Chan FK: Positive and
negative phosphorylation regulates RIP1- and RIP3-induced
programmed necrosis. Biochem J. 456:409–415. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang H, Sun L, Su L, Rizo J, Liu L, Wang
LF, Wang FS and Wang X: Mixed lineage kinase domain-like protein
MLKL causes necrotic membrane disruption upon phosphorylation by
RIP3. Mol Cell. 54:133–146. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li JX, Feng JM, Wang Y, Li XH, Chen XX, Su
Y, Shen YY, Chen Y, Xiong B, Yang CH, et al: The B-Raf(V600E)
inhibitor dabrafenib selectively inhibits RIP3 and alleviates
acetaminophen-induced liver injury. Cell Death Dis. 5:e12782014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kaiser WJ, Sridharan H, Huang C, Mandal P,
Upton JW, Gough PJ, Sehon CA, Marquis RW, Bertin J and Mocarski ES:
Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL. J
Biol Chem. 288:31268–31279. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Conrad M, Angeli JP, Vandenabeele P and
Stockwell BR: Regulated necrosis: Disease relevance and therapeutic
opportunities. Nat Rev Drug Discov. 15:348–366. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dannappel M, Vlantis K, Kumari S,
Polykratis A, Kim C, Wachsmuth L, Eftychi C, Lin J, Corona T,
Hermance N, et al: RIPK1 maintains epithelial homeostasis by
inhibiting apoptosis and necroptosis. Nature. 513:90–94. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Takahashi N, Vereecke L, Bertrand MJ,
Duprez L, Berger SB, Divert T, Gonçalves A, Sze M, Gilbert B,
Kourula S, et al: RIPK1 ensures intestinal homeostasis by
protecting the epithelium against apoptosis. Nature. 513:95–99.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gunther C, Martini E, Wittkopf N, Amann K,
Weigmann B, Neumann H, Waldner MJ, Hedrick SM, Tenzer S, Neurath MF
and Becker C: Caspase-8 regulates TNF-α-induced epithelial
necroptosis and terminal ileitis. Nature. 477:335–339. 2011.
View Article : Google Scholar
|
|
32
|
Wong WW, Vince JE, Lalaoui N, Lawlor KE,
Chau D, Bankovacki A, Anderton H, Metcalf D, O'Reilly L, Jost PJ,
et al: cIAPs and XIAP regulate myelopoiesis through cytokine
production in an RIPK1- and RIPK3-dependent manner. Blood.
123:2562–2572. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Roderick JE, Hermance N, Zelic M, Simmons
MJ, Polykratis A, Pasparakis M and Kelliher MA: Hematopoietic RIPK1
deficiency results in bone marrow failure caused by apoptosis and
RIPK3-mediated necroptosis. Proc Natl Acad Sci USA.
111:14436–14441. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rickard JA, O'Donnell JA, Evans JM,
Lalaoui N, Poh AR, Rogers T, Vince JE, Lawlor KE, Ninnis RL,
Anderton H, et al: RIPK1 regulates RIPK3-MLKL-driven systemic
inflammation and emergency hematopoiesis. Cell. 157:1175–1188.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dillon CP, Weinlich R, Rodriguez DA,
Cripps JG, Quarato G, Gurung P, Verbist KC, Brewer TL, Llambi F,
Gong YN, et al: RIPK1 blocks early postnatal lethality mediated by
caspase-8 and RIPK3. Cell. 157:1189–1202. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Oberst A, Dillon CP, Weinlich R, McCormick
LL, Fitzgerald P, Pop C, Hakem R, Salvesen GS and Green DR:
Catalytic activity of the caspase-8-FLIP(L) complex inhibits
RIPK3-dependent necrosis. Nature. 471:363–367. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Matsuoka Y and Tsujimoto Y: Role of RIP1
in physiological enterocyte turnover in mouse small intestine via
nonapoptotic death. Genes Cells. 20:11–28. 2015. View Article : Google Scholar
|
|
38
|
Huang Z, Wu SQ, Liang Y, Zhou X, Chen W,
Li L, Wu J, Zhuang Q, Chen C, Li J, et al: RIP1/RIP3 binding to
HSV-1 ICP6 initiates necroptosis to restrict virus propagation in
mice. Cell Host Microbe. 17:229–242. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Omoto S, Guo H, Talekar GR, Roback L,
Kaiser WJ and Mocarski ES: Suppression of RIP3-dependent
necroptosis by human cytomegalovirus. J Biol Chem. 290:11635–11648.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nogusa S, Thapa RJ, Dillon CP, Liedmann S,
Oguin TH III, Ingram JP, Rodriguez DA, Kosoff R, Sharma S, Sturm O,
et al: RIPK3 activates parallel pathways of MLKL-Driven necroptosis
and FADD-Mediated apoptosis to protect against influenza a virus.
Cell Host Microbe. 20:13–24. 2016. View Article : Google Scholar :
|
|
41
|
Pearson JS, Giogha C, Ong SY, Kennedy CL,
Kelly M, Robinson KS, Lung TW, Mansell A, Riedmaier P, Oates CV, et
al: A type III effector antagonizes death receptor signalling
during bacterial gut infection. Nature. 501:247–251. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li S, Zhang L, Yao Q, Li L, Dong N, Rong
J, Gao W, Ding X, Sun L, Chen X, et al: Pathogen blocks host death
receptor signalling by arginine GlcNAcylation of death domains.
Nature. 501:242–246. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Weng D, Marty-Roix R, Ganesan S, Proulx
MK, Vladimer GI, Kaiser WJ, Mocarski ES, Pouliot K, Chan FK,
Kelliher MA, et al: Caspase-8 and RIP kinases regulate
bacteria-induced innate immune responses and cell death. Proc Natl
Acad Sci USA. 111:7391–7396. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Philip NH, Dillon CP, Snyder AG,
Fitzgerald P, Wynosky-Dolfi MA, Zwack EE, Hu B, Fitzgerald L,
Mauldin EA, Copenhaver AM, et al: Caspase-8 mediates caspase-1
processing and innate immune defense in response to bacterial
blockade of NF-κB and MAPK signaling. Proc Natl Acad Sci USA.
111:7385–7390. 2014. View Article : Google Scholar
|
|
45
|
Robinson N, McComb S, Mulligan R, Dudani
R, Krishnan L and Sad S: Type I interferon induces necroptosis in
macrophages during infection with Salmonella enterica serovar
Typhimurium. Nat Immunol. 13:954–962. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bleriot C and Lecuit M: The interplay
between regulated necrosis and bacterial infection. Cell Mol Life
Sci. 73:2369–2378. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hu GQ, Yang YJ, Qin XX, Qi S, Zhang J, Yu
SX, Du CT and Chen W: Salmonella outer protein B suppresses colitis
development via protecting cell from necroptosis. Front Cell Infect
Microbiol. 9:872019. View Article : Google Scholar :
|
|
48
|
Fortes GB, Alves LS, de Oliveira R, Dutra
FF, Rodrigues D, Fernandez PL, Souto-Padron T, De Rosa MJ, Kelliher
M, Golenbock D, et al: Heme induces programmed necrosis on
macrophages through autocrine TNF and ROS production. Blood.
119:2368–2375. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yao Y, Liu M, Ren C, Shen J and Ji Y:
Exogenous tumor necrosis factor-alpha could induce egress of
Toxoplasma gondii from human foreskin fibroblast cells. Parasite.
24:452017. View Article : Google Scholar
|
|
50
|
Liu Y, Liu T, Lei T, Zhang D, Du S, Girani
L, Qi D, Lin C, Tong R and Wang Y: RIP1/RIP3-regulated necroptosis
as a target for multifaceted disease therapy (Review). Int J Mol
Med. 44:771–786. 2019.PubMed/NCBI
|
|
51
|
Koo GB, Morgan MJ, Lee DG, Kim WJ, Yoon
JH, Koo JS, Kim SI, Kim SJ, Son MK, Hong SS, et al:
Methylation-dependent loss of RIP3 expression in cancer represses
programmed necrosis in response to chemotherapeutics. Cell Res.
25:707–725. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Stoll G, Ma Y, Yang H, Kepp O, Zitvogel L
and Kroemer G: Pro-necrotic molecules impact local
immunosurveillance in human breast cancer. Oncoimmunology.
6:e12993022017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Feng X, Song Q, Yu A, Tang H, Peng Z and
Wang X: Receptor-interacting protein kinase 3 is a predictor of
survival and plays a tumor suppressive role in colorectal cancer.
Neoplasma. 62:592–601. 2015. View Article : Google Scholar
|
|
54
|
Moriwaki K, Bertin J, Gough PJ, Orlowski
GM and Chan FK: Differential roles of RIPK1 and RIPK3 in
TNF-induced necroptosis and chemotherapeutic agent-induced cell
death. Cell Death Dis. 6:e16362015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Li X, Guo J, Ding AP, Qi WW, Zhang PH, Lv
J, Qiu WS and Sun ZQ: Association of mixed lineage kinase
domain-like protein expression with prognosis in patients with
colon cancer. Technol Cancer Res Treat. 16:428–434. 2017.
View Article : Google Scholar :
|
|
56
|
Nugues AL, El Bouazzati H, Hetuin D,
Berthon C, Loyens A, Bertrand E, Jouy N, Idziorek T and Quesnel B:
RIP3 is downregulated in human myeloid leukemia cells and modulates
apoptosis and caspase-mediated p65/RelA cleavage. Cell Death Dis.
5:e13842014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Hockendorf U, Yabal M, Herold T,
Munkhbaatar E, Rott S, Jilg S, Kauschinger J, Magnani G, Reisinger
F, Heuser M, et al: RIPK3 restricts myeloid leukemogenesis by
promoting cell death and differentiation of leukemia initiating
cells. Cancer Cell. 30:75–91. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Geserick P, Wang J, Schilling R, Horn S,
Harris PA, Bertin J, Gough PJ, Feoktistova M and Leverkus M:
Absence of RIPK3 predicts necroptosis resistance in malignant
melanoma. Cell Death Dis. 6:e18842015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Ke H, Augustine CK, Gandham VD, Jin JY,
Tyler DS, Akiyama SK, Hall RP and Zhang JY: CYLD inhibits melanoma
growth and progression through suppression of the JNK/AP-1 and
beta1-integrin signaling pathways. J Invest Dermatol. 133:221–229.
2013. View Article : Google Scholar
|
|
60
|
McCormick KD, Ghosh A, Trivedi S, Wang L,
Coyne CB, Ferris RL and Sarkar SN: Innate immune signaling through
differential RIPK1 expression promote tumor progression in head and
neck squamous cell carcinoma. Carcinogenesis. 37:522–529. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Ertao Z, Jianhui C, Kang W, Zhijun Y, Hui
W, Chuangqi C, Changjiang Q, Sile C, Yulong H and Shirong C:
Prognostic value of mixed lineage kinase domain-like protein
expression in the survival of patients with gastric caner. Tumour
Biol. 37:13679–13685. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
He L, Peng K, Liu Y, Xiong J and Zhu FF:
Low expression of mixed lineage kinase domain-like protein is
associated with poor prognosis in ovarian cancer patients. Onco
Targets Ther. 6:1539–1543. 2013.PubMed/NCBI
|
|
63
|
Ruan J, Mei L, Zhu Q, Shi G and Wang H:
Mixed lineage kinase domain-like protein is a prognostic biomarker
for cervical squamous cell cancer. Int J Clin Exp Pathol.
8:15035–15038. 2015.
|
|
64
|
Park S, Hatanpaa KJ, Xie Y, Mickey BE,
Madden CJ, Raisanen JM, Ramnarain DB, Xiao G, Saha D, Boothman DA,
et al: The receptor interacting protein 1 inhibits p53 induction
through NF-kappaB activation and confers a worse prognosis in
glioblastoma. Cancer Res. 69:2809–2816. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Wang Q, Chen W, Xu X, Li B, He W, Padilla
MT, Jang JH, Nyunoya T, Amin S, Wang X and Lin Y: RIP1 potentiates
BPDE-induced transformation in human bronchial epithelial cells
through catalase-mediated suppression of excessive reactive oxygen
species. Carcinogenesis. 34:2119–2128. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Seifert L, Werba G, Tiwari S, Giao Ly NN,
Alothman S, Alqunaibit D, Avanzi A, Barilla R, Daley D, Greco SH,
et al: The necrosome promotes pancreatic oncogenesis via CXCL1 and
Mincle-induced immune suppression. Nature. 532:245–249. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Colbert LE, Fisher SB, Hardy CW, Hall WA,
Saka B, Shelton JW, Petrova AV, Warren MD, Pantazides BG, Gandhi K,
et al: Pronecrotic mixed lineage kinase domain-like protein
expression is a prognostic biomarker in patients with early-stage
resected pancreatic adenocarcinoma. Cancer. 119:3148–3155. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Cabal-Hierro L and O'Dwyer PJ: TNF
signaling through RIP1 kinase enhances SN38-Induced death in colon
adenocarcinoma. Mol Cancer Res. 15:395–404. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Wu Y and Zhou BP: Inflammation: A driving
force speeds cancer metastasis. Cell Cycle. 8:3267–3273. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Liu ZY, Wu B, Guo YS, Zhou YH, Fu ZG, Xu
BQ, Li JH, Jing L, Jiang JL, Tang J and Chen ZN: Necrostatin-1
reduces intestinal inflammation and colitis-associated
tumorigenesis in mice. Am J Cancer Res. 5:3174–3185.
2015.PubMed/NCBI
|
|
71
|
Exner N, Lutz AK, Haass C and Winklhofer
KF: Mitochondrial dysfunction in Parkinson's disease: Molecular
mechanisms and pathophysiological consequences. EMBO J.
31:3038–3062. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Iannielli A, Bido S, Folladori L, Segnali
A, Cancellieri C, Maresca A, Massimino L, Rubio A, Morabito G,
Caporali L, et al: Pharmacological inhibition of necroptosis
protects from dopaminergic neuronal cell death in Parkinson's
disease models. Cell Rep. 22:2066–2079. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Wu JR, Wang J, Zhou SK, Yang L, Yin JL,
Cao JP and Cheng YB: Necrostatin-1 protection of dopaminergic
neurons. Neural Regen Res. 10:1120–1124. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Amin P, Florez M, Najafov A, Pan H, Geng
J, Ofengeim D, Dziedzic SA, Wang H, Barrett VJ, Ito Y, et al:
Regulation of a distinct activated RIPK1 intermediate bridging
complex I and complex II in TNFalpha-mediated apoptosis. Proc Natl
Acad Sci USA. 115:E5944–E5953. 2018. View Article : Google Scholar
|
|
75
|
Caccamo A, Branca C, Piras IS, Ferreira E,
Huentelman MJ, Liang WS, Readhead B, Dudley JT, Spangenberg EE,
Green KN, et al: Necroptosis activation in Alzheimer's disease. Nat
Neurosci. 20:1236–1246. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Ofengeim D, Mazzitelli S, Ito Y, DeWitt
JP, Mifflin L, Zou C, Das S, Adiconis X, Chen H, Zhu H, et al:
RIPK1 mediates a disease-associated microglial response in
Alzheimer's disease. Proc Natl Acad Sci USA. 114:E8788–E8797. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Ito Y, Ofengeim D, Najafov A, Das S,
Saberi S, Li Y, Hitomi J, Zhu H, Chen H, Mayo L, et al: RIPK1
mediates axonal degeneration by promoting inflammation and
necroptosis in ALS. Science. 353:603–608. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Ofengeim D, Ito Y, Najafov A, Zhang Y,
Shan B, DeWitt JP, Ye J, Zhang X, Chang A, Vakifahmetoglu-Norberg
H, et al: Activation of necroptosis in multiple sclerosis. Cell
Rep. 10:1836–1849. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Zhang S, Su Y, Ying Z, Guo D, Pan C, Guo
J, Zou Z, Wang L, Zhang Z, Jiang Z, et al: RIP1 kinase inhibitor
halts the progression of an immune-induced demyelination disease at
the stage of monocyte elevation. Proc Natl Acad Sci USA.
116:5675–5680. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Roychowdhury S, McCullough RL, Sanz-Garcia
C, Saikia P, Alkhouri N, Matloob A, Pollard KA, McMullen MR,
Croniger CM and Nagy LE: Receptor interacting protein 3 protects
mice from high-fat diet-induced liver injury. Hepatology.
64:1518–1533. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Xu H, Du X, Liu G, Huang S, Du W, Zou S,
Tang D, Fan C, Xie Y, Wei Y, et al: The pseudokinase MLKL regulates
hepatic insulin sensitivity independently of inflammation. Mol
Metab. 23:14–23. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Saeed WK, Jun DW, Jang K, Ahn SB, Oh JH,
Chae YJ, Lee JS and Kang HT: Mismatched effects of receptor
interacting protein kinase-3 on hepatic steatosis and inflammation
in non-alcoholic fatty liver disease. World J Gastroenterol.
24:5477–5490. 2018. View Article : Google Scholar
|
|
83
|
Gautheron J, Vucur M, Reisinger F,
Cardenas DV, Roderburg C, Koppe C, Kreggenwinkel K, Schneider AT,
Bartneck M, Neumann UP, et al: A positive feedback loop between
RIP3 and JNK controls non-alcoholic steatohepatitis. EMBO Mol Med.
6:1062–1074. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Gautheron J, Vucur M, Schneider AT, Severi
I, Roderburg C, Roy S, Bartneck M, Schrammen P, Diaz MB, Ehling J,
et al: The necroptosis-inducing kinase RIPK3 dampens adipose tissue
inflammation and glucose intolerance. Nat Commun. 7:118692016.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Afonso MB, Rodrigues PM, Carvalho T,
Caridade M, Borralho P, Cortez-Pinto H, Castro RE and Rodrigues CM:
Necroptosis is a key pathogenic event in human and experimental
murine models of non-alcoholic steatohepatitis. Clin Sci (Lond).
129:721–739. 2015. View Article : Google Scholar
|
|
86
|
Roychowdhury S, McMullen MR, Pisano SG,
Liu X and Nagy LE: Absence of receptor interacting protein kinase 3
prevents ethanol-induced liver injury. Hepatology. 57:1773–1783.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Wang S, Ni HM, Dorko K, Kumer SC, Schmitt
TM, Nawabi A, Komatsu M, Huang H and Ding WX: Increased hepatic
receptor interacting protein kinase 3 expression due to impaired
proteasomal functions contributes to alcohol-induced steatosis and
liver injury. Oncotarget. 7:17681–17698. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Jia Y, Wang F, Guo Q, Li M, Wang L, Zhang
Z, Jiang S, Jin H, Chen A, Tan S, et al: Curcumol induces
RIPK1/RIPK3 complex-dependent necroptosis via JNK1/2-ROS signaling
in hepatic stellate cells. Redox Biol. 19:375–387. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Dal-Re R: Worldwide behavioral research on
major global causes of mortality. Health Educ Behav. 38:433–440.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Mizumura K, Cloonan SM, Nakahira K,
Bhashyam AR, Cervo M, Kitada T, Glass K, Owen CA, Mahmood A, Washko
GR, et al: Mitophagy-dependent necroptosis contributes to the
pathogenesis of COPD. J Clin Invest. 124:3987–4003. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Pouwels SD, Zijlstra GJ, van der Toorn M,
Hesse L, Gras R, Ten Hacken NH, Krysko DV, Vandenabeele P, de Vries
M, van Oosterhout AJ, et al: Cigarette smoke-induced necroptosis
and DAMP release trigger neutrophilic airway inflammation in mice.
Am J Physiol Lung Cell Mol Physiol. 310:L377–L386. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Wang Y, Zhou JS, Xu XC, Li ZY, Chen HP,
Ying SM, Li W, Shen HH and Chen ZH: Endoplasmic reticulum chaperone
GRP78 mediates cigarette smoke-induced necroptosis and injury in
bronchial epithelium. Int J Chron Obstruct Pulmon Dis. 13:571–581.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Rubenfeld GD, Caldwell E, Peabody E,
Weaver J, Martin DP, Neff M, Stern EJ and Hudson LD: Incidence and
outcomes of acute lung injury. N Engl J Med. 353:1685–1693. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Syed MA, Shah D, Das P, Andersson S,
Pryhuber G and Bhandari V: TREM-1 Attenuates RIPK3-mediated
necroptosis in hyperoxia-induced lung injury in neonatal mice. Am J
Respir Cell Mol Biol. 60:308–322. 2019. View Article : Google Scholar :
|
|
95
|
Chen J, Wang S, Fu R, Zhou M, Zhang T, Pan
W, Yang N and Huang Y: RIP3 dependent NLRP3 inflammasome activation
is implicated in acute lung injury in mice. J Transl Med.
16:2332018. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Siempos II, Ma KC, Imamura M, Baron RM,
Fredenburgh LE, Huh JW, Moon JS, Finkelsztein EJ, Jones DS, Lizardi
MT, et al: RIPK3 mediates pathogenesis of experimental
ventilator-induced lung injury. JCI Insight. 3:e971022018.
View Article : Google Scholar :
|
|
97
|
Bolognese AC, Yang WL, Hansen LW, Denning
NL, Nicastro JM, Coppa GF and Wang P: Inhibition of necroptosis
attenuates lung injury and improves survival in neonatal sepsis.
Surgery. Apr 27–2018.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Kobayashi K, Araya J, Minagawa S, Hara H,
Saito N, Kadota T, Sato N, Yoshida M, Tsubouchi K, Kurita Y, et al:
Involvement of PARK2-mediated mitophagy in idiopathic pulmonary
fibrosis pathogenesis. J Immunol. 197:504–516. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Lee JM, Yoshida M, Kim MS, Lee JH, Baek
AR, Jang AS, Kim DJ, Minagawa S, Chin SS, Park CS, et al:
Involvement of alveolar epithelial cell necroptosis in idiopathic
pulmonary fibrosis pathogenesis. Am J Respir Cell Mol Biol.
59:215–224. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Papi A, Brightling C, Pedersen SE and
Reddel HK: Asthma. Lancet. 391:783–800. 2018. View Article : Google Scholar
|
|
101
|
Cerps SC, Menzel M, Mahmutovic Persson I,
Bjermer L, Akbarshahi H and Uller L: Interferon-beta deficiency at
asthma exacerbation promotes MLKL mediated necroptosis. Sci Rep.
8:42482018. View Article : Google Scholar
|
|
102
|
Shlomovitz I, Erlich Z, Speir M, Zargarian
S, Baram N, Engler M, Edry-Botzer L, Munitz A, Croker BA and Gerlic
M: Necroptosis directly induces the release of full-length
biologically active IL-33 in vitro and in an inflammatory disease
model. FEBS J. 286:507–522. 2019. View Article : Google Scholar
|
|
103
|
Zhang H, Ji J, Liu Q and Xu S: MUC1
downregulation promotes TNF-α-induced necroptosis in human
bronchial epithelial cells via regulation of the RIPK1/RIPK3
pathway. J Cell Physiol. 234:15080–15088. 2019. View Article : Google Scholar :
|
|
104
|
Linkermann A, Brasen JH, Darding M, Jin
MK, Sanz AB, Heller JO, De Zen F, Weinlich R, Ortiz A, Walczak H,
et al: Two independent pathways of regulated necrosis mediate
ischemia-reperfusion injury. Proc Natl Acad Sci USA.
110:12024–12029. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Linkermann A, Skouta R, Himmerkus N, Mulay
SR, Dewitz C, De Zen F, Prokai A, Zuchtriegel G, Krombach F, Welz
PS, et al: Synchronized renal tubular cell death involves
ferroptosis. Proc Natl Acad Sci USA. 111:16836–16841. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Linkermann A, Brasen JH, Himmerkus N, Liu
S, Huber TB, Kunzendorf U and Krautwald S: Rip1
(receptor-interacting protein kinase 1) mediates necroptosis and
contributes to renal ischemia/reperfusion injury. Kidney Int.
81:751–761. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Mulay SR, Desai J, Kumar SV, Eberhard JN,
Thomasova D, Romoli S, Grigorescu M, Kulkarni OP, Popper B,
Vielhauer V, et al: Cytotoxicity of crystals involves
RIPK3-MLKL-mediated necroptosis. Nat Commun. 7:102742016.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Tristao VR, Goncalves PF, Dalboni MA,
Batista MC, Durao Mde S Jr and Monte JC: Nec-1 protects against
nonapoptotic cell death in cisplatin-induced kidney injury. Ren
Fail. 34:373–377. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Linkermann A, Heller JO, Prokai A,
Weinberg JM, De Zen F, Himmerkus N, Szabó AJ, Bräsen JH, Kunzendorf
U and Krautwald S: The RIP1-kinase inhibitor necrostatin-1 prevents
osmotic nephrosis and contrast-induced AKI in mice. J Am Soc
Nephrol. 24:1545–1557. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Xu Y, Ma H, Shao J, Wu J, Zhou L, Zhang Z,
Wang Y, Huang Z, Ren J, Liu S, et al: A role for tubular
necroptosis in Cisplatin-Induced AKI. J Am Soc Nephrol.
26:2647–2658. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Tristao VR, Pessoa EA, Nakamichi R, Reis
LA, Batista MC, Durão Junior Mde S and Monte JC: Synergistic effect
of apoptosis and necroptosis inhibitors in cisplatin-induced
nephrotoxicity. Apoptosis. 21:51–59. 2016. View Article : Google Scholar
|
|
112
|
Zhu Y, Cui H, Gan H, Xia Y, Wang L, Wang Y
and Sun Y: Necroptosis mediated by receptor interaction protein
kinase 1 and 3 aggravates chronic kidney injury of subtotal
nephrectomised rats. Biochem Biophys Res Commun. 461:575–581. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Chen H, Fang Y, Wu J, Chen H, Zou Z, Zhang
X, Shao J and Xu Y: RIPK3-MLKL-mediated necroinflammation
contributes to AKI progression to CKD. Cell Death Dis. 9:8782018.
View Article : Google Scholar : PubMed/NCBI
|
|
114
|
McManus DD, Piacentine SM, Lessard D, Gore
JM, Yarzebski J, Spencer FA and Goldberg RJ: Thirty-year (1975 to
2005) trends in the incidence rates, clinical features, treatment
practices, and short-term outcomes of patients <55 years of age
hospitalized with an initial acute myocardial infarction. Am J
Cardiol. 108:477–482. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Luedde M, Lutz M, Carter N, Sosna J,
Jacoby C, Vucur M, Gautheron J, Roderburg C, Borg N, Reisinger F,
et al: RIP3, a kinase promoting necroptotic cell death, mediates
adverse remodelling after myocardial infarction. Cardiovasc Res.
103:206–216. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Zhang T, Zhang Y, Cui M, Jin L, Wang Y, Lv
F, Liu Y, Zheng W, Shang H, Zhang J, et al: CaMKII is a RIP3
substrate mediating ischemia- and oxidative stress-induced
myocardial necroptosis. Nat Med. 22:175–182. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Lusis AJ: Atherosclerosis. Nature.
407:233–241. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Karunakaran D, Geoffrion M, Wei L, Gan W,
Richards L, Shangari P, DeKemp EM, Beanlands RA, Perisic L,
Maegdefessel L, et al: Targeting macrophage necroptosis for
therapeutic and diagnostic interventions in atherosclerosis. Sci
Adv. 2:e16002242016. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Henderson B, Revell PA and Edwards JC:
Synovial lining cell hyperplasia in rheumatoid arthritis: Dogma and
fact. Ann Rheum Dis. 47:348–349. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Lee SH, Kwon JY, Kim SY, Jung K and Cho
ML: Interferon-gamma regulates inflammatory cell death by targeting
necroptosis in experimental autoimmune arthritis. Sci Rep.
7:101332017. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Jhun J, Lee SH, Kim SY, Ryu J, Kwon JY, Na
HS, Jung K, Moon SJ, Cho ML and Min JK: RIPK1 inhibition attenuates
experimental autoimmune arthritis via suppression of
osteoclastogenesis. J Transl Med. 17:842019. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Glyn-Jones S, Palmer AJ, Agricola R, Price
AJ, Vincent TL, Weinans H and Carr AJ: Osteoarthritis. Lancet.
386:376–387. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Riegger J and Brenner RE: Evidence of
necroptosis in osteoarthritic disease: Investigation of blunt
mechanical impact as possible trigger in regulated necrosis. Cell
Death Dis. 10:6832019. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Galluzzi L, Kepp O, Chan FK and Kroemer G:
Necroptosis: Mechanisms and relevance to disease. Annu Rev Pathol.
12:103–130. 2017. View Article : Google Scholar
|
|
125
|
Della Torre L, Nebbioso A, Stunnenberg HG,
Martens JHA, Carafa V and Altucci L: The role of necroptosis:
Biological relevance and its involvement in cancer. Cancers
(Basel). 13:6842021. View Article : Google Scholar
|
|
126
|
Martens S, Hofmans S, Declercq W,
Augustyns K and Vandenabeele P: Inhibitors Targeting RIPK1/RIPK3:
Old and new drugs. Trends Pharmacol Sci. 41:209–224. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Thapa RJ, Nogusa S, Chen P, Maki JL, Lerro
A, Andrake M, Rall GF, Degterev A and Balachandran S:
Interferon-induced RIP1/RIP3-mediated necrosis requires PKR and is
licensed by FADD and caspases. Proc Natl Acad Sci USA.
110:E3109–E3118. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Bollino D, Balan I and Aurelian L:
Valproic acid induces neuronal cell death through a novel
calpain-dependent necroptosis pathway. J Neurochem. 133:174–186.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Kim HJ, Hwang KE, Park DS, Oh SH, Jun HY,
Yoon KH, Jeong ET, Kim HR and Kim YS: Shikonin-induced necroptosis
is enhanced by the inhibition of autophagy in non-small cell lung
cancer cells. J Transl Med. 15:1232017. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Zhou J, Li G, Han G, Feng S, Liu Y, Chen
J, Liu C, Zhao L and Jin F: Emodin induced necroptosis in the
glioma cell line U251 via the TNF-α/RIP1/RIP3 pathway. Invest New
Drugs. 38:50–59. 2020. View Article : Google Scholar
|
|
131
|
Li Y, Tian X, Liu X and Gong P: Bufalin
inhibits human breast cancer tumorigenesis by inducing cell death
through the ROS-mediated RIP1/RIP3/PARP-1 pathways. Carcinogenesis.
39:700–707. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Han Q, Ma Y, Wang H, Dai Y, Chen C, Liu Y,
Jing L and Sun X: Resibufogenin suppresses colorectal cancer growth
and metastasis through RIP3-mediated necroptosis. J Transl Med.
16:2012018. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Fakharnia F, Khodagholi F, Dargahi L and
Ahmadiani A: Prevention of Cyclophilin D-Mediated mPTP Opening
Using Cyclosporine-A Alleviates the Elevation of Necroptosis,
Autophagy and Apoptosis-Related Markers Following Global Cerebral
Ischemia-Reperfusion. J Mol Neurosci. 61:52–60. 2017. View Article : Google Scholar
|
|
134
|
Ding J, Yang N, Yan Y, Wang Y, Wang X, Lu
L and Dong K: Rapamycin inhibited photoreceptor necroptosis and
protected the retina by activation of autophagy in experimental
retinal detachment. Curr Eye Res. 44:739–745. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Yan C, Oh JS, Yoo SH, Lee JS, Yoon YG, Oh
YJ, Jang MS, Lee SY, Yang J, Lee SH, et al: The targeted inhibition
of mitochondrial Hsp90 overcomes the apoptosis resistance conferred
by Bcl-2 in Hep3B cells via necroptosis. Toxicol Appl Pharmacol.
266:9–18. 2013. View Article : Google Scholar
|
|
136
|
Li D, Li C, Li L, Chen S, Wang L, Li Q,
Wang X, Lei X and Shen Z: Natural Product Kongensin A is a
Non-Canonical HSP90 Inhibitor that Blocks RIP3-dependent
Necroptosis. Cell Chem Biol. 23:257–266. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Zhang Z, Li HM, Zhou C, Li Q, Ma L, Zhang
Z, Sun Y, Wang L, Zhang X, Zhu B, et al: Non-benzoquinone
geldanamycin analogs trigger various forms of death in human breast
cancer cells. J Exp Clin Cancer Res. 35:1492016. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Chen WW, Yu H, Fan HB, Zhang CC, Zhang M,
Zhang C, Cheng Y, Kong J, Liu CF, Geng D and Xu X: RIP1 mediates
the protection of geldanamycin on neuronal injury induced by
oxygen-glucose deprivation combined with zVAD in primary cortical
neurons. J Neurochem. 120:70–77. 2012. View Article : Google Scholar
|
|
139
|
Qu C, Yuan ZW, Yu XT, Huang YF, Yang GH,
Chen JN, Lai XP, Su ZR, Zeng HF, Xie Y and Zhang XJ: Patchouli
alcohol ameliorates dextran sodium sulfate-induced experimental
colitis and suppresses tryptophan catabolism. Pharmacol Res.
121:70–82. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Fauster A, Rebsamen M, Huber KV, Bigenzahn
JW, Stukalov A, Lardeau CH, Scorzoni S, Bruckner M, Gridling M,
Parapatics K, et al: A cellular screen identifies ponatinib and
pazopanib as inhibitors of necroptosis. Cell Death Dis.
6:e17672015. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Harris PA, Marinis JM, Lich JD, Berger SB,
Chirala A, Cox JA, Eidam PM, Finger JN, Gough PJ, Jeong JU, et al:
Identification of a RIP1 Kinase Inhibitor Clinical Candidate
(GSK3145095) for the treatment of pancreatic cancer. ACS Med Chem
Lett. 10:857–862. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Ali M and Mocarski ES: Proteasome
inhibition blocks necroptosis by attenuating death complex
aggregation. Cell Death Dis. 9:3462018. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Martens S, Jeong M, Tonnus W, Feldmann F,
Hofmans S, Goossens V, Takahashi N, Bräsen JH, Lee EW, Van der
Veken P, et al: Sorafenib tosylate inhibits directly necrosome
complex formation and protects in mouse models of inflammation and
tissue injury. Cell Death Dis. 8:e29042017. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
von Mässenhausen A, Tonnus W, Himmerkus N,
Parmentier S, Saleh D, Rodriguez D, Ousingsawat J, Ang RL, Weinberg
JM, Sanz AB, et al: Phenytoin inhibits necroptosis. Cell Death Dis.
9:3592018. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Wang J, Li Y, Huang WH, Zeng XC, Li XH, Li
J, Zhou J, Xiao J, Xiao B, Ouyang DS and Hu K: The protective
effect of aucubin from eucommia ulmoides against status epilepticus
by inducing autophagy and inhibiting necroptosis. Am J Chin Med.
45:557–573. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Meng XM, Li HD, Wu WF, Ming-Kuen Tang P,
Ren GL, Gao L, Li XF, Yang Y, Xu T, Ma TT, et al: Wogonin protects
against cisplatin-induced acute kidney injury by targeting
RIPK1-mediated necroptosis. Lab Invest. 98:79–94. 2018. View Article : Google Scholar
|
|
147
|
Nehs MA, Lin CI, Kozono DE, Whang EE, Cho
NL, Zhu K, Moalem J, Moore FD Jr and Ruan DT: Necroptosis is a
novel mechanism of radiation-induced cell death in anaplastic
thyroid and adrenocortical cancers. Surgery. 150:1032–1039. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Oliver Metzig M, Fuchs D, Tagscherer KE,
Gröne HJ, Schirmacher P and Roth W: Inhibition of caspases primes
colon cancer cells for 5-fluorouracil-induced TNF-α-dependent
necroptosis driven by RIP1 kinase and NF-κB. Oncogene.
35:3399–3409. 2016. View Article : Google Scholar
|
|
149
|
Choi MJ, Kang H, Lee YY, Choo OS, Jang JH,
Park SH, Moon JS, Choi SJ and Choung YH: Cisplatin-Induced
ototoxicity in rats is driven by RIP3-Dependent necroptosis. Cells.
8:4092019. View Article : Google Scholar :
|
|
150
|
Yang H, Ma Y, Chen G, Zhou H, Yamazaki T,
Klein C, Pietrocola F, Vacchelli E, Souquere S, Sauvat A, et al:
Contribution of RIP3 and MLKL to immunogenic cell death signaling
in cancer chemotherapy. Oncoimmunology. 5:e11496732016. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Basit F, Cristofanon S and Fulda S:
Obatoclax (GX15-070) triggers necroptosis by promoting the assembly
of the necrosome on autophagosomal membranes. Cell Death Differ.
20:1161–1173. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Deng Q, Yu X, Xiao L, Hu Z, Luo X, Tao Y,
Yang L, Liu X, Chen H, Ding Z, et al: Neoalbaconol induces energy
depletion and multiple cell death in cancer cells by targeting
PDK1-PI3-K/Akt signaling pathway. Cell Death Dis. 4:e8042013.
View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Lin CY, Chang TW, Hsieh WH, Hung MC, Lin
IH, Lai SC and Tzeng YJ: Simultaneous induction of apoptosis and
necroptosis by Tanshinone IIA in human hepatocellular carcinoma
HepG2 cells. Cell Death Discov. 2:160652016. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Tang D, Kang R, Berghe TV, Vandenabeele P
and Kroemer G: The molecular machinery of regulated cell death.
Cell Res. 29:347–364. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Grootjans S, Vanden Berghe T and
Vandenabeele P: Initiation and execution mechanisms of necroptosis:
An overview. Cell Death Differ. 24:1184–1195. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Upton JW, Kaiser WJ and Mocarski ES: Virus
inhibition of RIP3-dependent necrosis. Cell Host Microbe.
7:302–313. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Pan T, Wu S, He X, Luo H, Zhang Y, Fan M,
Geng G, Ruiz VC, Zhang J, Mills L, et al: Necroptosis takes place
in human immunodeficiency virus type-1 (HIV-1)-infected CD4+ T
lymphocytes. PLoS One. 9:e939442014. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Berger AK and Danthi P: Reovirus activates
a caspase-independent cell death pathway. mBio. 4:e00178–00113.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Chan FK, Shisler J, Bixby JG, Felices M,
Zheng L, Appel M, Orenstein J, Moss B and Lenardo MJ: A role for
tumor necrosis factor receptor-2 and receptor-interacting protein
in programmed necrosis and antiviral responses. J Biol Chem.
278:51613–51621. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Shrestha A, Mehdizadeh Gohari I and
McClane BA: RIP1, RIP3, and MLKL contribute to cell death caused by
clostridium perfringens enterotoxin. mBio. 10:e02985–19. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Roca FJ and Ramakrishnan L: TNF dually
mediates resistance and susceptibility to mycobacteria via
mitochondrial reactive oxygen species. Cell. 153:521–534. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Kitur K, Parker D, Nieto P, Ahn DS, Cohen
TS, Chung S, Wachtel S, Bueno S and Prince A: Toxin-induced
necroptosis is a major mechanism of Staphylococcus aureus lung
damage. PLoS Pathog. 11:e10048202015. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Roychowdhury S, Chiang DJ, Mandal P,
McMullen MR, Liu X, Cohen JI, Pollard J, Feldstein AE and Nagy LE:
Inhibition of apoptosis protects mice from ethanol-mediated
acceleration of early markers of CCl4-induced fibrosis but not
steatosis or inflammation. Alcohol Clin Exp Res. 36:1139–1147.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Lu C, Xu W, Zhang F, Shao J and Zheng S:
Nrf2 knockdown disrupts the protective effect of curcumin on
alcohol-induced hepatocyte necroptosis. Mol Pharm. 13:4043–4053.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Afonso MB, Rodrigues PM, Simao AL,
Ofengeim D, Carvalho T, Amaral JD, Gaspar MM, Cortez-Pinto H,
Castro RE, Yuan J and Rodrigues CM: Activation of necroptosis in
human and experimental cholestasis. Cell Death Dis. 7:e23902016.
View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Choi HS, Kang JW and Lee SM: Melatonin
attenuates carbon tetrachloride-induced liver fibrosis via
inhibition of necroptosis. Transl Res. 166:292–303. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Harris PA, Berger SB, Jeong JU, Nagilla R,
Bandyopadhyay D, Campobasso N, Capriotti CA, Cox JA, Dare L, Dong
X, et al: Discovery of a First-in-Class receptor interacting
protein 1 (RIP1) kinase specific clinical candidate (GSK2982772)
for the treatment of inflammatory diseases. J Med Chem.
60:1247–1261. 2017. View Article : Google Scholar : PubMed/NCBI
|