Necroptosis, an emerging field closely related to
apoptosis, is a non-caspase-dependent cell death that has been
implicated in the pathological processes of various diseases. It is
regulated by various genes that cause regular and ordered cell
death. Through activating specific death signaling pathways, it
shares typical characteristics of necrosis, including loss of
metabolic function and subcellular changes (1,2).
Receptor-interacting protein kinase 1 (RIP1) was the first
signaling molecule identified in the necrosome (3). RIP1 and RIP3 interact with the
receptor protein, transducing death signals, and further recruiting
and phosphorylating mixed lineage kinase domain-like protein (MLKL)
(4-7). Necroptosis can be involved in the
regulation of several signaling pathways, including the
caspase-8-dependent apoptotic pathway, the mitogen-activated
protein (MAP) kinase cascade, and activation of the nuclear
factor-κB (NF-κB) pathway.
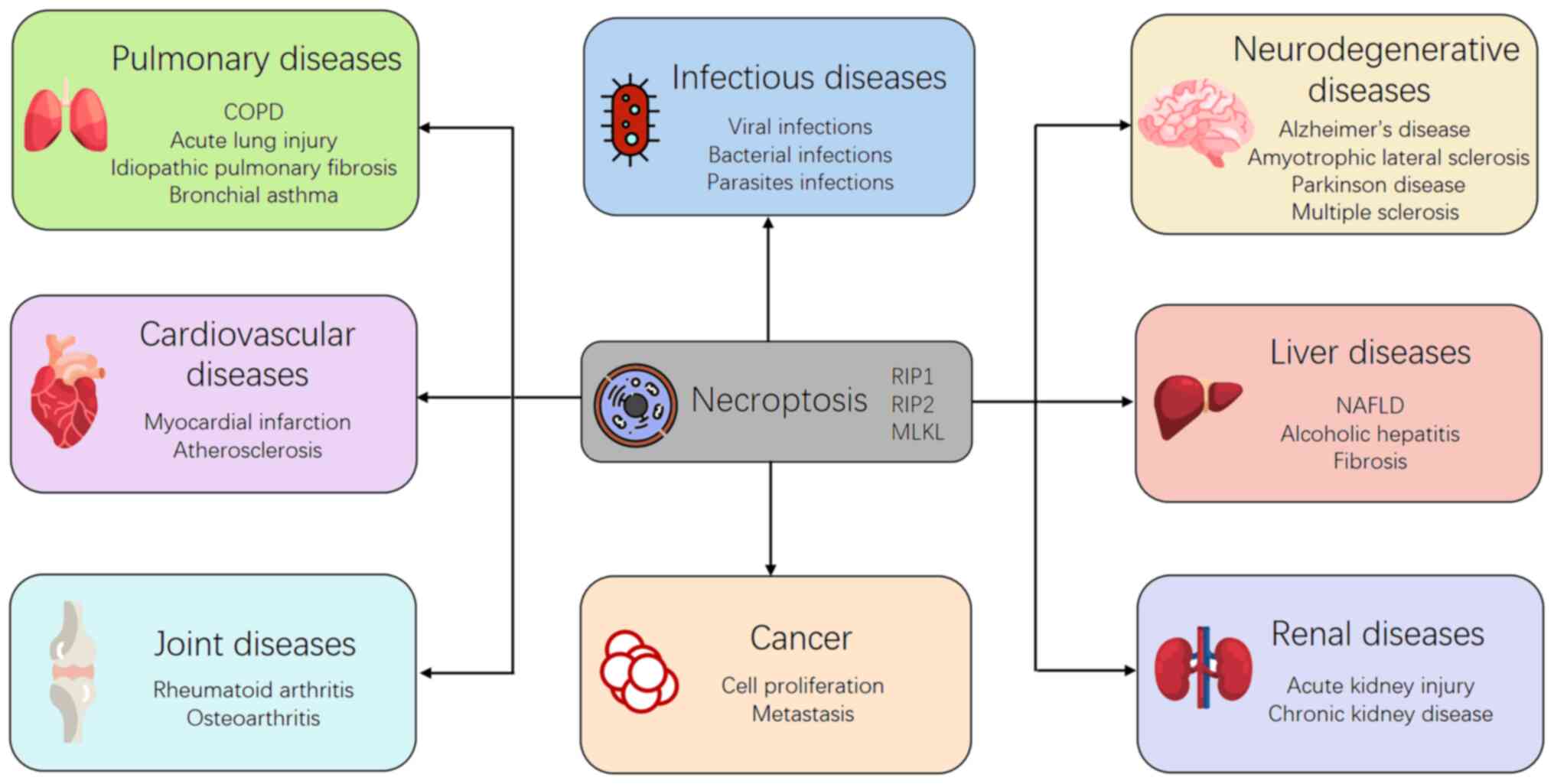
To explore the potential role of necroptosis in
human diseases, researchers have developed various methods, such as
gene knockdown and knockout, and pharmacological inhibitors. By
using these methods, it has been found that necroptosis plays an
important role in pathophysiological processes of several clinical
diseases, including infections, liver diseases, kidney injury,
neurodegenerative diseases, cardiovascular diseases, and human
tumors (8). In the current
review, we aimed to explore the potential role of necroptosis in
various clinical diseases.
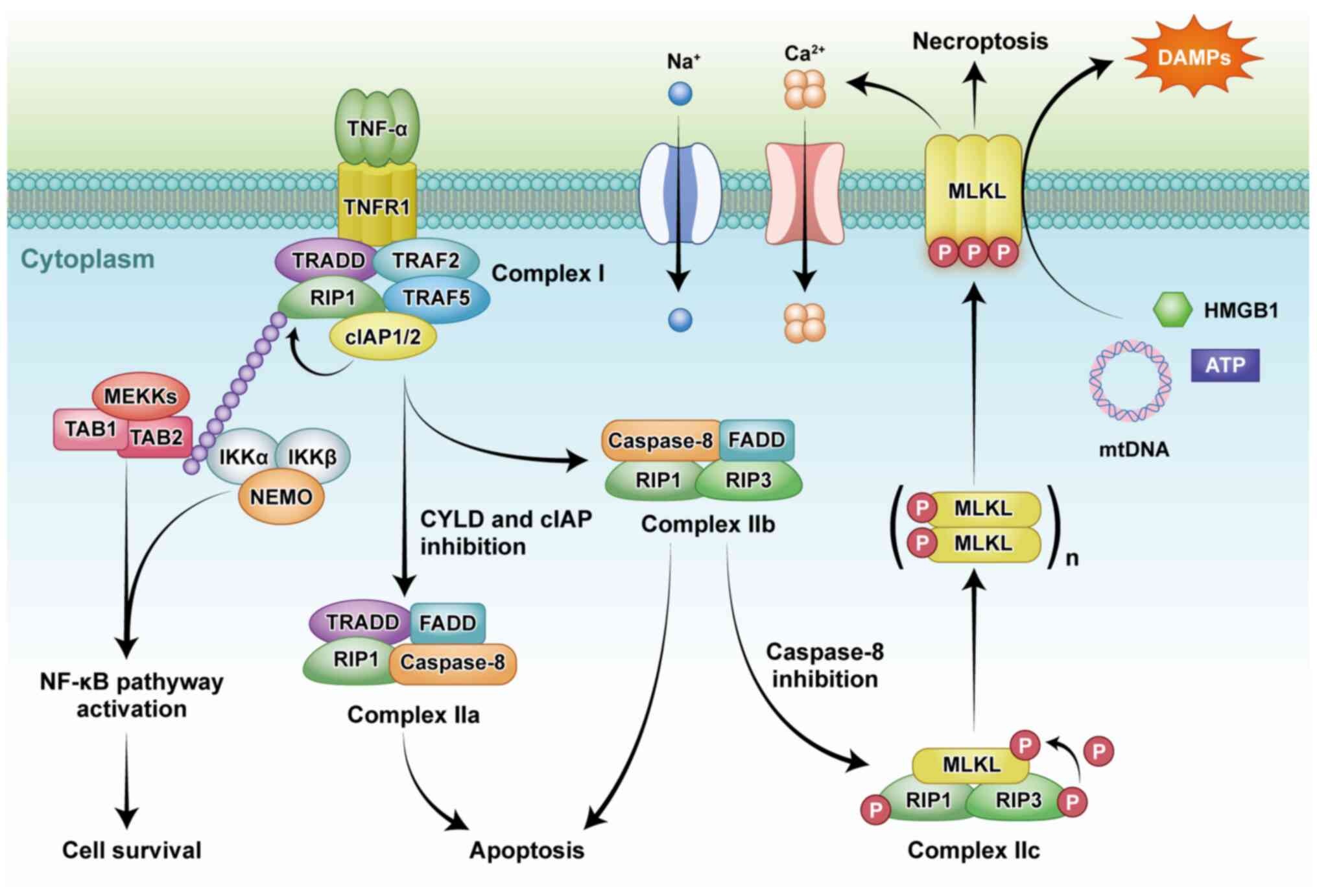
Necroptosis can be triggered by a variety of
factors, such as tumor necrosis factor receptor (TNFR) and
toll-like receptor (TLR) families, intracellular DNA and RNA
sensors, and interferon (IFN) (9-11). TNF-dependent TNFR1 stimulation
has three consequences that depend on the assembly of regulatory
proteins. These different pathways ultimately stimulated
NF-κB-dependent inflammation, caspase-8-dependent apoptosis, or
selective activation of necroptosis under caspase-8 inhibition
(Fig. 1) (12). TNF-dependent necroptosis is
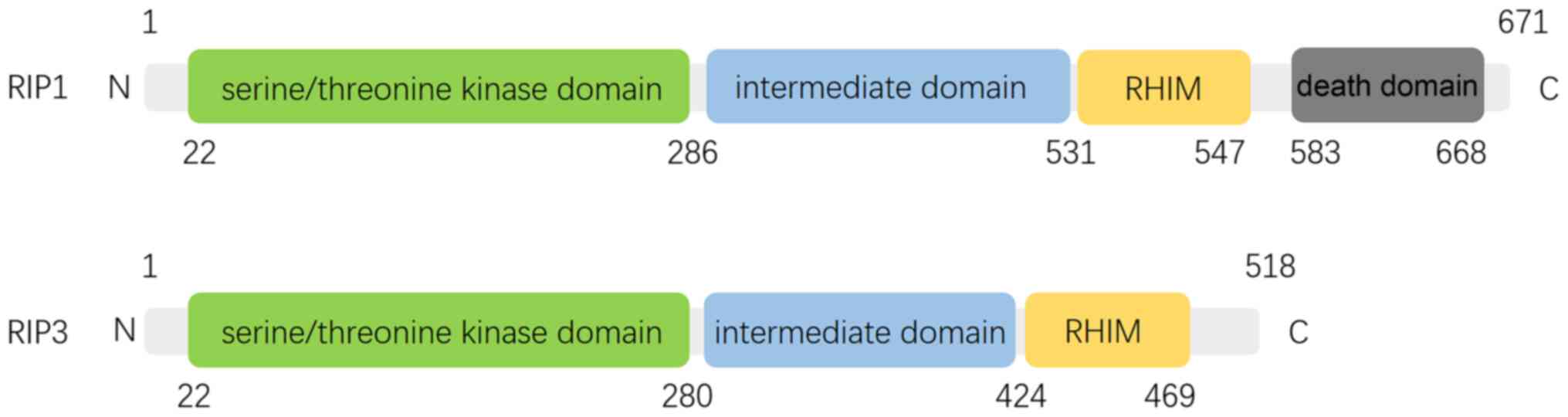
regulated by RIP1 and RIP3, which interact through unique RIP
homotypic-interacting motifs (RHIMs) (Fig. 2) (13,14).
The interaction of RIP1 and RIP3 results in
autophosphorylation, transphosphorylation, and assembly of
'necrosome' complex (5). RIP3
and MLKL are essential for necroptosis, whereas RIP1 is only
sometimes involved in this process. RIP3 and MLKL knockout mice do
not show deficiency in embryogenesis, homeostasis and development,
indicating the role of necroptosis may be not essential in
non-challenged conditions (15,16).
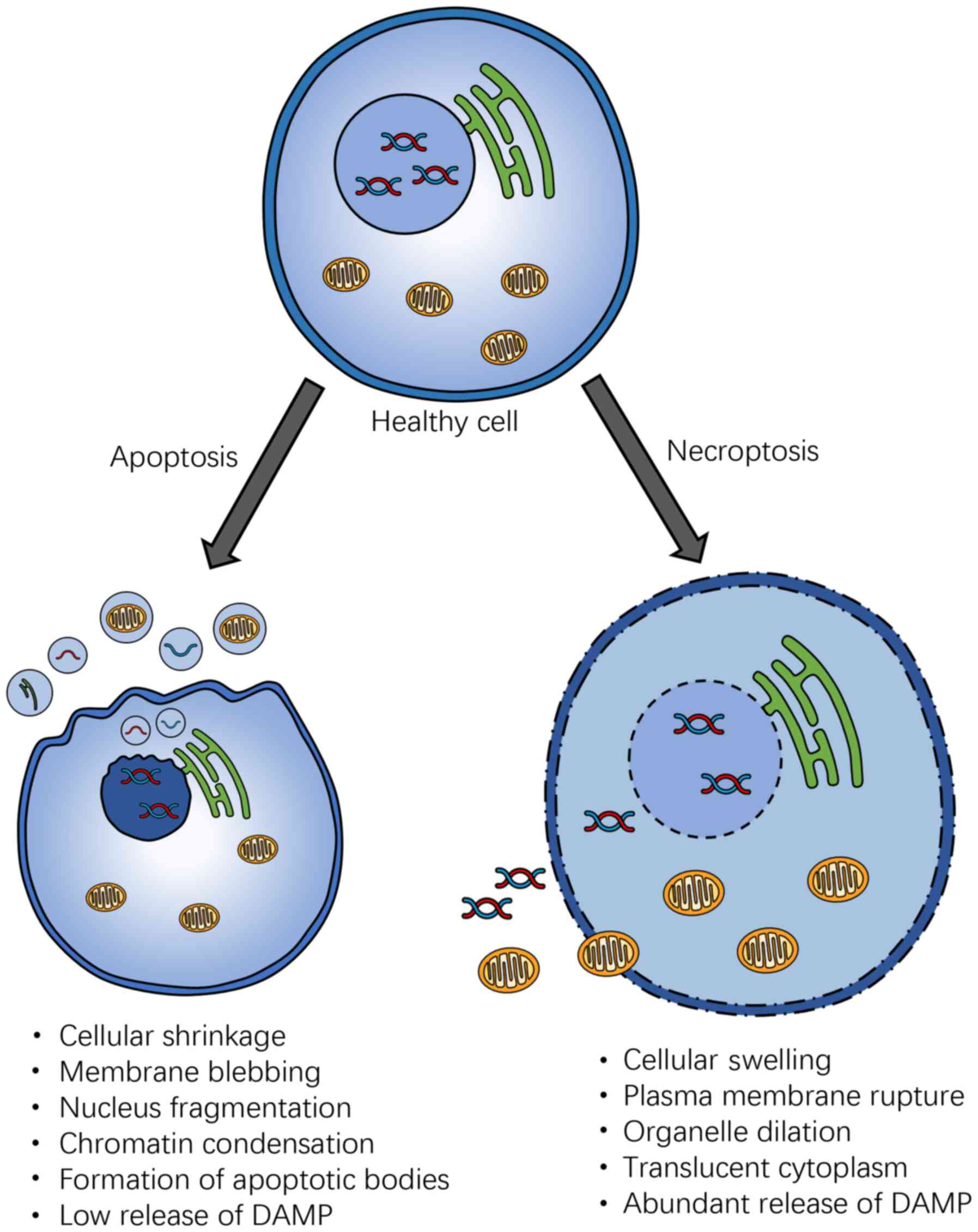
Although necroptosis is characterized by caspase
independence, the molecular pathway involved is similar to and
shares features of apoptosis. However, the immunological and
morphological consequences of necroptosis are vastly different
(Fig. 3). Necroptosis shares the
major morphological features of necrosis, such as the swelling of
organelles, gradually translucent cytoplasm, and rupture of the
cellular membrane (12). By
contrast, apoptosis is characterized by membrane blebbing, cell
shrinkage, nuclear fragmentation, and chromatin concentration
(17). The rupture of the
cellular membrane results in the release of cellular contents,
leading to the exposure of damage-associated molecular patterns
(DAMPs), triggering a strong inflammatory response in necroptosis,
suggesting necroptotic cells are more immunogenic than apoptotic
cells, which is relatively intact, with DAMP restricted to the
plasma membrane, or encapsulated in the apoptotic bodies (17). It has also been shown that
necroptosis was associated with maintenance of T-cell homeostasis,
as it has been found to be able to clear excess and abnormal T
cells in the absence of caspase-8 (18), which can prevent abnormal
proliferation of lymphocytes (19).
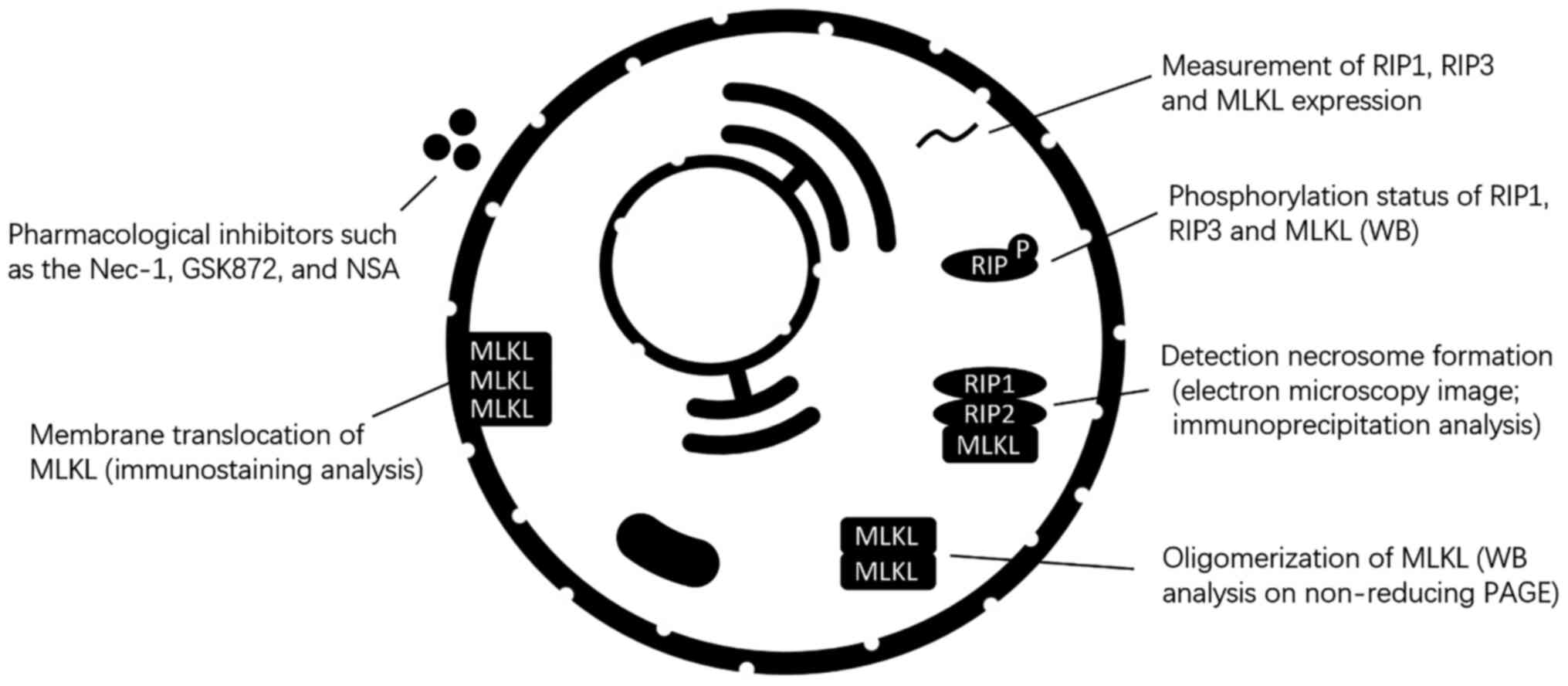
As there is currently no specific marker for
necroptosis, multiple methods are usually required to identify
necroptosis (Fig. 4). In
cultured cells, transmission electron microscopy can be used to
identify necroptotic cells (20). Detection of key molecular,
including RIP1, RIP3 and MLKL activation, necrosome formation, MLKL
oligomerization, and membrane translocation can also be used to
identify necroptosis (21).
Activation of RIP3 and MLKL can be monitored by western blot
analysis to assess phosphorylation status (22,23). Phosphorylation of MLKL at Ser358
and Thr357 and RIP3 at S227 indicates the activation of necroptosis
(24). In particular, MLKL
phosphorylation has been used as a biomarker for certain disease
diagnosis and prognosis (25).
In addition, several pharmacological inhibitors such as the
necrostatin (Nec)-1, GSK872, and necrosulfonamide (NSA) have also
been used to detect necroptosis (7,26). In vivo, the activation of
necroptosis can be identified by the elevated levels of RIP1, RIP3,
or MLKL mRNA or protein. Additionally, previous findings suggested
that RIP3 and MLKL are more specific molecular biomarkers than RIP1
for the detection of necroptosis (27).
Over the last decade, researchers have put a lot of
effort into the development of effective RIP1, RIP3, and MLKL
inhibitors, and created mouse models that lack one or more
components of the necroptotic pathway at systemic level or in
specific tissues (28). Due to
the existence of these models, the physiological function of
proteins of necroptosis have been investigated. Conditional
deletion of RIP1 in keratinocytes or intestinal epithelial cells
suggested RIP1 plays an essential role in maintaining epithelial
homeostasis (29,30). It is worth noting that the role
of RIP1 in maintaining the intestinal barrier is similar to
caspase-8 (31). In addition,
mice with Birc2, Birc3, and Xiap codeletion in the myeloid lineage
have high levels of circulating inflammatory cytokines, sterile
inflammation, and granulocytes, which can be partially corrected by
the lack of RIP1 or RIP3 (32).
Tamoxifen-induced systemic RIP1 gene knockout in adult mice
is fatal due to a surge in cell death and intestinal bone marrow
failure, which accumulates and causes fatal systemic inflammation
(33,34). Fetal hepatocytes that received
tamoxifen-induced RIP1 deletion or RIP1−/−
progenitor cells are unable to repopulate irradiated receptors.
This defect can be partially corrected by the concomitant lack of
RIP3, indicating that RIP1 plays a key role in the survival of
hematopoietic stem and progenitor cells (33,34). Furthermore, systemic inflammation
caused by RIP1−/− can be restricted in
RIP1−/−RIP3−/−Casp8−/−
hosts (34,35). These hosts show age-related
lymphoproliferative disorders similar to those developed by
RIP3−/−Casp8−/− mice (11,34,36). In addition, compared to control
animals, RIP1+/− mice, mice treated with
intravenous siRNA targeting RIP1, and Nec-1-treated mice showed a
higher rate of physiological intestinal epithelial cell
regeneration in the small intestine (37). In addition, the negative effects
of Nec-1 on the regeneration of intestinal epithelial cells are
also present in RIP3−/− mice (37). Findings of those studies suggest
that there may be a delicate balance between different cell deaths
in maintaining homeostasis in adults.
Findings have shown the crucial role of necroptosis
in inflammation during viral infection (Table I). The viruses use the host's
signaling pathways, such as anti-apoptotic proteins, to enhance
infection, thereby increasing its ability to replicate in the host
cell. It has been reported that viral encoding protein involving
the RHIM domain interacts with RIP1 and RIP3 to inhibit
virus-induced cell death (38).
Viral inhibitor of RIP activation (vRIA) disrupts the combination
of DAI and RIP3, thereby suppressing cytomegalovirus-mediated
necroptosis (9). By contrast,
human cytomegalovirus differs in protein, which does not disrupt
RIP3 binding with DAI; it works via blocking signaling downstream
of MLKL (39). Experimental
studies in mice lacking RIP3 have shown impaired virus-induced
necroptosis and increased susceptibility to viral infections such
as vaccinia virus, influenza A virus, and HSV-1 (5,25,38,40) (Fig. 5).
Necroptosis also plays an important role in the
inflammation caused by bacterial infections. Enteropathogenic E.
coli (EPEC) has been shown to synthesize and secrete large
amounts of the immunogenic effector protein NleB1 and modify the
arginine residues of the Fas-associated death domain (FADD) and
RIP1 death domains to prevent apoptosis and necroptosis (41,42). EPEC lacking NleB1 fails to
colonize intestinal epithelial cells, indicating that bacterial
necroptosis is a protective mechanism of the organism (41,42). Similarly, the absence of RIP3
sensitizes host cells to Yelsonella. Moreover, the
simultaneous knockout of FADD or caspase-8 could make cells more
sensitive (43,44). In vitro, Salmonella
typhimurium is able to escape TNFα, causing RIP1- and
RIP3-dependent necroptosis in infected macrophages. In a model of
Salmonella typhimurium venous infection, RIP3 knockout
significantly reduces splenic macrophage death, thereby reducing
bacterial numbers and prolonging mouse survival (45,46). Findings focusing on oral
Salmonella typhimurium infection have also shown that outer
protein B was downregulated during infection, which resulted in
promoting bacterial translocation, increasing macrophage
necroptosis, and exacerbating bacterial infection (Table I) (47).
Parasitic diseases such as malaria and leishmaniasis
usually cause hemolysis, anemia, and bleeding. These are due to the
release of hemoglobin (Hb) into the circulation by the rupture of
red blood cells. When Hb is oxidized, heme is generated, the Fenton
reaction starts, and peaks with the generation of reactive oxygen
species (ROS). Heme is also involved in the activation of TLR4,
causing autocrine secretion of ROS and TNF, and synergistically
activating RIP1/3-dependent necroptosis (48). In addition, it has been shown
that 10 ng/ml TNFα can induce infected human foreskin fibroblasts
egressing Toxoplasma gondii (Table I) (49).
Studies on necroptosis highlight its role in cancer
because of its necroptosis-inducing function (Table II) (50). Chen et al suggested that
necroptosis is an important cell death mechanism for blocked
apoptosis, and has been proposed as an alternative cell death
procedure to prevent cancer (20). Previous studies have shown that a
decreased expression of RIP3 or MLKL is associated with worse
prognosis and poor survival in breast cancer (51,52), colorectal cancer (53-55), acute myeloid leukemia (56,57), melanoma (58,59), head and neck squamous cell
carcinoma (60), gastric cancer
(61), ovarian cancer (62), and cervical squamous cell
carcinoma (63). However,
increased RIP3 or RIP1 expression was also correlated with cancer
development, including glioblastoma (64), lung cancer (65), and pancreatic cancer (66,67). SN38, the topoisomerase inhibitor,
was found to be able to promote necroptosis progression, inhibit
cell proliferation, and induce DNA damage accumulation in colon
cancer (68). These findings
indicate that inhibiting activities of necroptosis components may
be a strategy in the treatment of cancers.
Metastasis is the most common cause of
cancer-related death. Researchers have found that metastasis
involves a complex interaction between cancer cells and the
microenvironment. By promoting inflammation, necroptosis may be
able to promote metastasis (69). It has been shown that TNFα plays
a critical role in cancer progression. However, the exact mechanism
of this process has not been fully understood. Increased expression
of TNFα in cancer is a key characteristic in numerous malignancies
and is usually associated with a poor prognosis and decreased
survival (69). Consistent with
the pro-inflammatory properties of necroptosis and the
cancer-promoting effect of inflammation, Nec-1 was able to reduce
inflammation and colitis-related tumor formation (70), indicating that targeting
necroptosis may be a strategy for preventing cancer metastasis.
Researchers have shown that necroptosis was
activated in Parkinson's disease (PD), and may be associated with
mitochondrial defects which led to necroptosis (Table III) (71). Compared with healthy brain, the
level of necroptosis components, including RIP1, RIP3 and MLKL, was
significantly increased in the substantia nigra of PD brain
(72). Moreover, researchers
have found Nec-1 could protect PC12 cells from death in PD models
(73). This suggests that the
activation of RIP1 may be a risk factor for dopaminergic neurons
lost in PD patients. In addition, leucine-rich repeat kinase 2,
which was identified in a systematic RNAi screen, is encoded by a
gene that is frequently mutated in PD and is able to promote
activation of RIP1 (74).
Alzheimer's disease (AD) is a degenerative brain
disease featured by loss of neurons. Previous studies have found
that there were activated necroptosis in both human (75) and mouse (76) AD brain. In the AD brain, the
levels of necroptosis components, such as RIP1 and MLKL, were
significantly higher than the normal brain (Table III) (75). Treatment of AD in brain of mice
with the necroptosis inhibitor can significantly suppress
necroptosis and prevent neuronal loss (75). This indicates that targeting
necroptosis may be a new therapeutic strategy for AD treatment.
Amyotrophic lateral sclerosis (ALS) is a
neurodegenerative disease that is characterized by loss of motor
neurons. In a previous study, the ALS spinal cord was shown to have
a significant increase in necroptosis components including RIP1,
RIP3, and MLKL in the ALS mouse model compared to healthy mouse
spinal cord (77). In addition,
loss of optineurin, an ALS-related gene, resulted in susceptibility
to necroptosis. Nec-1 inhibition of RIP1 or knockout of RIP3 could
prevent demyelination and reduce axonal pathological hallmarks in
ALS mouse models (Table III)
(77). Those findings suggested
that targeting necroptosis may have potential therapeutic value in
ALS patients.
Multiple sclerosis (MS) is degenerative disease
characterized by oligodendrocyte loss and demyelination. Previous
findings have shown a significant increase in necroptosis
components including RIP1, RIP3, and MLKL in MS patients. In
addition, MLKL oligomers were significantly increased in MS
pathology samples compared with controls (Table III) (78). This suggests necroptosis is
activated in the pathogenesis of MS. In MS mouse model, oral
administration of RIP1 inhibitor can suppress oligodendrocyte
degeneration and reduce disease severity (78). Moreover, researchers have shown
that inhibition of RIP1 reduced demyelination and disease
progression in an MS model (79). Notably, MLKL was shown to be
involved in the MS process (79).
Similar to the pathologies of NAFLD, alcoholic
hepatitis is an inflammatory syndrome in liver, which can result in
high morbidity and mortality. Several studies have found that RIP3
was increased following ethanol feeding, and RIP3 deletion could
protect the liver from ethanol-mediated injury (Table IV). In addition, p-JNK was
regulated by RIP3 in a model of alcoholic hepatitis, and RIP3
deletion reduced ethanol-induced p-JNK expression (86). Another study found
pharmacological inhibition of proteasome and liver-specific PSMC1
KO mice could increase RIP3 expression, indicating RIP3 expression
was post-translationally regulated in ethanol-mediated liver injury
(87). Additionally, when RIP3
was deleted, the steatosis and inflammatory effects of ethanol in
hepatocyte could be reduced (86). However, the inflammatory and
steatosis effects of high fat diet for hepatocyte were increased
when RIP3 was deleted (80).
Hepatic fibrosis is one of the most common liver
diseases, which is closely related to liver failure and
hepatocellular cancer. RIP3 deletion reportedly aggravated hepatic
fibrosis by increasing insulin resistance (80). Furthermore, inhibition of RIP3
did not result in protective effect in carbon tetrachloride
(CCL4)-induced fibrosis (83)
(Table IV). Additionally,
curcumol suppressed serum inflammatory markers, transaminases, and
fibrosis in a dose-dependent manner by inducing necroptosis of
hepatic stellate cells in a liver fibrosis model (88). Curcumol-induced increased
necroptosis was mediated by increased expression of p-RIP3 and
p-JNK (88). Those results
indicated that pharmacotherapy which induced increased necroptosis
may be a notable strategy for the treatment of hepatic fibrosis in
the future.
Chronic obstructive pulmonary disease (COPD) is
characterized by persistent and progressive airway inflammation and
narrowing, and is a major source of the high healthcare expenditure
in the elderly (89). An
increasing number of studies have shown that necroptosis is
associated with the etiology of COPD (Table V). In addition, necroptosis of
epithelial cell is associated with COPD (90). Cigarette smoking (CS)-related
necroptosis and DAMP release could cause neutrophil inflammation in
mice, and Nec-1 could reduce the inflammation (91). In addition, researchers have
found that in airway epithelial cells, endoplasmic reticulum
chaperone protein GRP78 could promote CS-induced inflammation. This
may be due to the upregulation of necroptosis and subsequent
activation of the NF-κB pathway (92).
Acute lung injury (ALI) is one of the most common
complications in critically ill patients (93). Recent findings have shown the
involvement of RIP3-mediated necroptosis in neonatal mice with
hypoxia-induced lung injury, which can be attenuated by gene
deletions in RIP3 (94). In
additional, inhibition of RIP3 could significantly reduce
inflammatory activation and lipopolysaccharide-induced necroptosis
(95). Researchers have also
found that mice lacking RIP3 were protected from ventilator-induced
lung injury (96). Additionally,
inhibition of RIP1 can reduce systemic and pulmonary inflammation
and increase survival rate of septic neonatal mice (Table V) (97).
Idiopathic pulmonary fibrosis (IPF) is a chronic,
progressive, fibrotic lung disease characterized by the usual
interstitial pneumonia pattern at histopathologic examination
(98). In a previous study using
alveolar epithelial cells, RIP3-mediated necroptosis was associated
with IPF development by releasing DAMP (99). Additionally, RIP3 and p-MLKL
levels in the lungs of IPF patients are significantly higher than
those in healthy lungs (99).
Mice with RIP3 knockout showed a reduced cell death, with a
decrease of p-MLKL level in alveolar epithelial cells (99). RIP3 knockout could effectively
suppress the DAMP releasing, cell death, and pulmonary fibrosis
without reducing the expression of cleaved caspase-3 (Table V) (99). These indicate that inhibiting
activities of necroptosis components may be a strategy in the
treatment of IPF.
Bronchial asthma is the most common chronic
respiratory disease characterized by bronchial hyper-responsiveness
and airway obstruction (100).
Viral-induced bronchial asthma exacerbation mimicked by IFN-β
knockout mice treated with house dust mite is associated with
increased necroptosis components, including p-MLKL and LDH in the
bronchoalveolar lavage fluid (101). As a major inflammatory cytokine
in bronchial asthma, IL-33 is released in response to necroptosis
and causes eosinophil and basophil activation (102). Moreover, in a mouse model of
asthma induced by Aspergillus fumigatus extract, the
necroptosis inhibitor GW806742X can eliminate necroptosis and IL-33
response, and attenuates eosinophilia (102). Additionally, The TNFα-induced
necroptosis enhanced by mucin 1 can be reduced by Nec-1 in human
bronchial epithelial cells (103) (Table V).
Acute kidney injury (AKI) is a common and severe
clinical disease that often requires renal replacement therapy
(Table VI). Signs of an ongoing
necroptotic response have been found in AKI caused by
ischemia-reperfusion injury (IRI) (104-106), urolithiasis (107), cisplatin-based chemotherapy or
radiocontrast (104,108-111). Previous findings have shown
that compared with wild-type counterparts,
RIP3−/− and MLKL−/− mice are
less sensitive to oxalate crystal-induced AKI, and are associated
with reduced plasma creatinine levels, neutrophil infiltration, and
limited tubular injury (107,110). Moreover, the
RIP3−/− mice confer protection from mild IRI, and
the protection can be extended to severe IRIs with the deletion of
Ppif (104). Similar results
are also found when Nec-1, SfA, and 16-86 are employed alone or in
combination (104,109). The abovementioned results
suggest that inhibition of necroptosis may be a therapeutic option
for AKI treatment.
Similar to the AKI, necroptosis was also found in
chronic kidney disease (CKD) after unilateral nephrectomy (Table VI) (112). Researchers have shown that
necroptosis and the highest levels of RIP1 and RIP3 occurred 8
weeks after subtotal nephrectomy (112). Notably, the renal pathological
changes and renal function could be significantly improved after
Nec-1 treatment, and the overexpression of RIP1, RIP3, MLKL could
be significantly reduced (112). These results suggest that
necroptosis contributes to the loss of renal cells in subtotal
nephrectomized rats. Furthermore, during the AKI to CKD process,
upregulation of expression and inter-action between RIP3 and MLKL
can induce necroptosis in proximal renal tubular cells and promote
inflammasome activation under IRI conditions (113). RIP3 or MLKL knockout could
protect the renal tubular cells from necroptosis and inflammasome
activation, which prevent kidney from interstitial fibrogenesis
after IRI (113).
Myocardial infarction, characterized by regional
myocardial ischemia and hypoxia, is one of the leading causes of
death worldwide (114).
Previous findings have shown that compared to wild-type mice, the
levels of RIP1 and RIP3 were significantly higher in hearts of
ischemic mice (22,115). In an acute IRI mouse model,
RIP3 deficiency was able to protect heart from IRI-induced
necroptosis and reduce the infarct size (116). Notably, researchers have found
that Nec-1 could protect heart against short-term and long-term
effects of myocardial ischemia, including reduced necrotic cell
death and size of myocardial infarction, which helped to maintain
long-term cardiac function (22)
(Table VII).
Rheumatoid arthritis (RA), characterized by synovial
membrane inflammation, is a chronic systemic inflammatory
autoimmune disease that affects 0.5-1% of the population worldwide
(119). Previous findings have
shown a significant increase in necroptosis components including
RIP1, RIP3, and MLKL in the synovium of an arthritis mouse model
(120). Additionally,
researchers have also found in an arthritis mouse model, Nec-1
could significantly reduce these key components of necroptosis and
IL-17, IL-1β, IL-6, and TNFα (Table VIII) (121). These results suggested that
inhibiting activities of necroptosis components may be a strategy
in the treatment of RA.
Osteoarthritis (OA) is the leading cause of pain
and disability among chronic disease, which affects about 10% of
men and 18% of women older than 60 years (122). A significant increase in
necroptosis components including RIP3, and MLKL was found in highly
degenerated cartilage tissue (123). Moreover, it has been shown that
Nec-1 could significantly reduce cell death and subsequent release
proinflammatory mediators in the OA model (Table VIII) (123).
As necroptosis not only participate in the
maintenance of organismal homeostasis, but also constitute
etiological determinants of diverse human pathologies (124), at least two therapeutic
paradigms can be envisioned: i) the activation of necroptosis, as a
means to bypass the accrued resistance of most tumors to apoptosis
(125); ii) the inhibition of
necroptosis, as a strategy to limit the loss of post-mitotic cells
in pathologies such as inflammatory, ischemic, and toxic syndromes
(126). Therefore, drugs
affecting either the expression or the activity of necroptosis
mediators may have therapeutic potential (Table IX).
Several drugs have been found to upregulate the
expression of the key molecules of necroptosis, including
interferons (127), histone
deacetylase inhibitor valproic acid (128), and hypomethylating agents such
as decitabine and 5-azacytidine (51). Additionally, several traditional
Chinese medicine drugs such as shikonin (129), emodin (130), bufalin (131), and resibufogenin (132) were also found to upregulate
RIP1 and RIP3, which finally induced necroptosis. By contrast,
various drugs have been documented to downregulate necroptosis,
including immunosuppressive drug Cyclosporine A (133) and Rapamycin (134), inhibitors of the HSP90 [(G-TPP)
(135), Kongensin A (136), 17-demethoxy-reblastatin
(137), DHQ3 (137), gamitrinib (18), and geldanamycin (138)], as well as traditional Chinese
medicine such as patchouli alcohol (139).
Promising specific inhibitors are also being
developed for the central molecules of necroptosis. Currently,
several drugs with anti-necroptotic activity have been used for the
treatment of different types of cancer [Pazopanib (140), Ponatinib (140), GSK3145095 (141), Dabrafenib (26), Carfilzomib (142), and Sorafenib (143)]. Moreover, a clinically used
anti-convulsant, Phenytoin (144) as well as components found in
different plants [aucubin (145) and wogonin (146)] could inhibit RIPK1 activity. By
contrast, radiotherapy (147),
chemotherapeutic agents such as 5-fluorouracil (148), cisplatin (149), oxaliplatin (150), and anthracyclines (150), pan-BCL-2 inhibitor Obatoclax
(151), or traditional Chinese
medicines such as neoalbaconol (152) and tanshinone (153) have been documented to
upregulate necroptosis. Although these drugs did not affect the
expression of necroptotic component, these medicines may increase
the effect of the drugs affecting the expression of the necroptotic
molecules in combination therapy in cancer cells.
During the last decade, necroptosis has been
recognized as an alternative to apoptosis when cells are exposed to
various stimuli under specific conditions (154). The necrosome components, RIP1,
RIP3, and MLKL, are critical regulators of necroptotic cell death.
RIP1 functions as a traffic cop for mechanisms of cell death. MLKL
acts as the executioner of necroptosis, based on the
phosphorylation, oligomerization and membrane translocation
(155). Current understandings
demonstrated a pathway in which RIP3 activation, possibly mediated
by RIP1, induces MLKL activation, and finally results in
permeabilization of the plasma membrane and cell death.
Recent studies have revealed a complex role for
necroptosis in diverse clinical diseases, such as
ischemia-reperfusion injury, neurological and inflammatory
diseases, retinal disorders, acute kidney injury or bacterial
infections. On the one hand, it functioned as a cell-death
mechanism activated by various signal transduction cascades in the
same cell or the same tissue; on the other hand, it acted as an
inflammation inducer through the release of DAMPs. Cross-regulation
between necroptosis and other modes of cell death increase the
complexity of these pathways. The major necroptosis-regulating
proteins exert pleiotropic signaling functions that culminate in
necroptotic cell death and have cell death-independent functions,
such as regulation of inflammasome activation, mitochondrial
function and integrity, and cellular metabolic activities (96).
As necroptosis constitutes etiological determinants
of multiple human pathologies, targeting the necroptotic pathway is
a potential therapeutic approach for multiple diseases, and several
activators or inhibitors of the necroptosis pathway have been
developed, such as dabrafenib, pazopanib, and ponatinib. These
small-molecule activators or inhibitors of necroptosis may be
useful as therapeutics in a specific clinical disease. However,
most studies investigating the therapeutics targeting necroptosis
are based on in vitro experiments or animal models, thus the
feasibility of the clinical use of these compounds and agents
remains to be assessed in vivo and clinical trials.
Additionally, the off-target effects of the necroptosis-targeting
therapeutics should be scrutinized, and novel approaches that
conjugate necroptosis inducers and disease-guiding agents should be
developed to enhance selectivity and safety.
Not applicable.
YFA and WLD conceived the study; JC and XQH were
involved in data curation; XL, WLD and JC were involved in
collection of references as well as writing and editing; YFA, XQH
and JC supervised the study. All authors have read and agreed to
the published version of the manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
The authors are grateful to Dr He Xiao for his
valuable advice and English editing.
This work was supported by grants from the National Natural
Science Foundation of China (nos. 81672212, 81802153 and 81902205),
the Beijing Natural Science Foundation (nos. 7171014, 7174361 and
7182175), and the Beijing Municipal Science and Technology
Commission (no. Z171100001017085).
|
1
|
Galluzzi L, Vitale I, Abrams JM, Alnemri
ES, Baehrecke EH, Blagosklonny MV, Dawson TM, Dawson VL, El-Deiry
WS, Fulda S, et al: Molecular definitions of cell death
subroutines: Recommendations of the Nomenclature Committee on Cell
Death 2012. Cell Death Differ. 19:107–120. 2012. View Article : Google Scholar :
|
|
2
|
Moriwaki K, Balaji S, McQuade T, Malhotra
N, Kang J and Chan FK: The necroptosis adaptor RIPK3 promotes
injury-induced cytokine expression and tissue repair. Immunity.
41:567–578. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Holler N, Zaru R, Micheau O, Thome M,
Attinger A, Valitutti S, Bodmer JL, Schneider P, Seed B and Tschopp
J: Fas triggers an alternative, caspase-8-independent cell death
pathway using the kinase RIP as effector molecule. Nat Immunol.
1:489–495. 2000. View
Article : Google Scholar
|
|
4
|
He S, Wang L, Miao L, Du F, Zhao L and
Wang X: Receptor interacting protein kinase-3 determines cellular
necrotic response to TNF-alpha. Cell. 137:1100–1111. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cho YS, Challa S, Moquin D, Genga R, Ray
TD, Guildford M and Chan FK: Phosphorylation-driven assembly of the
RIP1-RIP3 complex regulates programmed necrosis and virus-induced
inflammation. Cell. 137:1112–1123. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang DW, Shao J, Lin J, Zhang N, Lu BJ,
Lin SC, Dong MQ and Han J: RIP3, an energy metabolism regulator
that switches TNF-induced cell death from apoptosis to necrosis.
Science. 325:332–336. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sun L, Wang H, Wang Z, He S, Chen S, Liao
D, Wang L, Yan J, Liu W, Lei X and Wang X: Mixed lineage kinase
domain-like protein mediates necrosis signaling downstream of RIP3
kinase. Cell. 148:213–227. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jouan-Lanhouet S, Riquet F, Duprez L,
Vanden Berghe T, Takahashi N and Vandenabeele P: Necroptosis, in
vivo detection in experimental disease models. Semin Cell Dev Biol.
35:2–13. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Upton JW, Kaiser WJ and Mocarski ES:
DAI/ZBP1/DLM-1 complexes with RIP3 to mediate virus-induced
programmed necrosis that is targeted by murine cytomegalovirus
vIRA. Cell Host Microbe. 11:290–297. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Oberst A and Green DR: It cuts both ways:
Reconciling the dual roles of caspase 8 in cell death and survival.
Nat Rev Mol Cell Biol. 12:757–763. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kaiser WJ, Upton JW, Long AB,
Livingston-Rosanoff D, Daley-Bauer LP, Hakem R, Caspary T and
Mocarski ES: RIP3 mediates the embryonic lethality of
caspase-8-deficient mice. Nature. 471:368–372. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Weinlich R, Oberst A, Beere HM and Green
DR: Necroptosis in development, inflammation and disease. Nat Rev
Mol Cell Biol. 18:127–136. 2017. View Article : Google Scholar
|
|
13
|
Sun X, Yin J, Starovasnik MA, Fairbrother
WJ and Dixit VM: Identification of a novel homotypic interaction
motif required for the phosphorylation of receptor-interacting
protein (RIP) by RIP3. J Biol Chem. 277:9505–9511. 2002. View Article : Google Scholar
|
|
14
|
Sun X, Lee J, Navas T, Baldwin DT, Stewart
TA and Dixit VM: RIP3, a novel apoptosis-inducing kinase. J Biol
Chem. 274:16871–16875. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shan B, Pan H, Najafov A and Yuan J:
Necroptosis in development and diseases. Genes Dev. 32:327–340.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dillon CP, Tummers B, Baran K and Green
DR: Developmental checkpoints guarded by regulated necrosis. Cell
Mol Life Sci. 73:2125–2136. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hacker G: The morphology of apoptosis.
Cell Tissue Res. 301:5–17. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ch'en IL, Tsau JS, Molkentin JD, Komatsu M
and Hedrick SM: Mechanisms of necroptosis in T cells. J Exp Med.
208:633–641. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lenardo M, Chan KM, Hornung F, McFarland
H, Siegel R, Wang J and Zheng L: Mature T lymphocyte
apoptosis-immune regulation in a dynamic and unpredictable
antigenic environment. Annu Rev Immunol. 17:221–253. 1999.
View Article : Google Scholar
|
|
20
|
Chen D, Yu J and Zhang L: Necroptosis: An
alternative cell death program defending against cancer. Biochim
Biophys Acta. 1865:228–236. 2016.PubMed/NCBI
|
|
21
|
He S, Huang S and Shen Z: Biomarkers for
the detection of necroptosis. Cell Mol Life Sci. 73:2177–2181.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Oerlemans MI, Liu J, Arslan F, den Ouden
K, van Middelaar BJ, Doevendans PA and Sluijter JP: Inhibition of
RIP1-dependent necrosis prevents adverse cardiac remodeling after
myocardial ischemia-reperfusion in vivo. Basic Res Cardiol.
107:2702012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dong K, Zhu H, Song Z, Gong Y, Wang F,
Wang W, Zheng Z, Yu Z, Gu Q, Xu X and Sun X: Necrostatin-1 protects
photoreceptors from cell death and improves functional outcome
after experimental retinal detachment. Am J Pathol. 181:1634–1641.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
McQuade T, Cho Y and Chan FK: Positive and
negative phosphorylation regulates RIP1- and RIP3-induced
programmed necrosis. Biochem J. 456:409–415. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang H, Sun L, Su L, Rizo J, Liu L, Wang
LF, Wang FS and Wang X: Mixed lineage kinase domain-like protein
MLKL causes necrotic membrane disruption upon phosphorylation by
RIP3. Mol Cell. 54:133–146. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li JX, Feng JM, Wang Y, Li XH, Chen XX, Su
Y, Shen YY, Chen Y, Xiong B, Yang CH, et al: The B-Raf(V600E)
inhibitor dabrafenib selectively inhibits RIP3 and alleviates
acetaminophen-induced liver injury. Cell Death Dis. 5:e12782014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kaiser WJ, Sridharan H, Huang C, Mandal P,
Upton JW, Gough PJ, Sehon CA, Marquis RW, Bertin J and Mocarski ES:
Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL. J
Biol Chem. 288:31268–31279. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Conrad M, Angeli JP, Vandenabeele P and
Stockwell BR: Regulated necrosis: Disease relevance and therapeutic
opportunities. Nat Rev Drug Discov. 15:348–366. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dannappel M, Vlantis K, Kumari S,
Polykratis A, Kim C, Wachsmuth L, Eftychi C, Lin J, Corona T,
Hermance N, et al: RIPK1 maintains epithelial homeostasis by
inhibiting apoptosis and necroptosis. Nature. 513:90–94. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Takahashi N, Vereecke L, Bertrand MJ,
Duprez L, Berger SB, Divert T, Gonçalves A, Sze M, Gilbert B,
Kourula S, et al: RIPK1 ensures intestinal homeostasis by
protecting the epithelium against apoptosis. Nature. 513:95–99.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gunther C, Martini E, Wittkopf N, Amann K,
Weigmann B, Neumann H, Waldner MJ, Hedrick SM, Tenzer S, Neurath MF
and Becker C: Caspase-8 regulates TNF-α-induced epithelial
necroptosis and terminal ileitis. Nature. 477:335–339. 2011.
View Article : Google Scholar
|
|
32
|
Wong WW, Vince JE, Lalaoui N, Lawlor KE,
Chau D, Bankovacki A, Anderton H, Metcalf D, O'Reilly L, Jost PJ,
et al: cIAPs and XIAP regulate myelopoiesis through cytokine
production in an RIPK1- and RIPK3-dependent manner. Blood.
123:2562–2572. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Roderick JE, Hermance N, Zelic M, Simmons
MJ, Polykratis A, Pasparakis M and Kelliher MA: Hematopoietic RIPK1
deficiency results in bone marrow failure caused by apoptosis and
RIPK3-mediated necroptosis. Proc Natl Acad Sci USA.
111:14436–14441. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rickard JA, O'Donnell JA, Evans JM,
Lalaoui N, Poh AR, Rogers T, Vince JE, Lawlor KE, Ninnis RL,
Anderton H, et al: RIPK1 regulates RIPK3-MLKL-driven systemic
inflammation and emergency hematopoiesis. Cell. 157:1175–1188.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dillon CP, Weinlich R, Rodriguez DA,
Cripps JG, Quarato G, Gurung P, Verbist KC, Brewer TL, Llambi F,
Gong YN, et al: RIPK1 blocks early postnatal lethality mediated by
caspase-8 and RIPK3. Cell. 157:1189–1202. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Oberst A, Dillon CP, Weinlich R, McCormick
LL, Fitzgerald P, Pop C, Hakem R, Salvesen GS and Green DR:
Catalytic activity of the caspase-8-FLIP(L) complex inhibits
RIPK3-dependent necrosis. Nature. 471:363–367. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Matsuoka Y and Tsujimoto Y: Role of RIP1
in physiological enterocyte turnover in mouse small intestine via
nonapoptotic death. Genes Cells. 20:11–28. 2015. View Article : Google Scholar
|
|
38
|
Huang Z, Wu SQ, Liang Y, Zhou X, Chen W,
Li L, Wu J, Zhuang Q, Chen C, Li J, et al: RIP1/RIP3 binding to
HSV-1 ICP6 initiates necroptosis to restrict virus propagation in
mice. Cell Host Microbe. 17:229–242. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Omoto S, Guo H, Talekar GR, Roback L,
Kaiser WJ and Mocarski ES: Suppression of RIP3-dependent
necroptosis by human cytomegalovirus. J Biol Chem. 290:11635–11648.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nogusa S, Thapa RJ, Dillon CP, Liedmann S,
Oguin TH III, Ingram JP, Rodriguez DA, Kosoff R, Sharma S, Sturm O,
et al: RIPK3 activates parallel pathways of MLKL-Driven necroptosis
and FADD-Mediated apoptosis to protect against influenza a virus.
Cell Host Microbe. 20:13–24. 2016. View Article : Google Scholar :
|
|
41
|
Pearson JS, Giogha C, Ong SY, Kennedy CL,
Kelly M, Robinson KS, Lung TW, Mansell A, Riedmaier P, Oates CV, et
al: A type III effector antagonizes death receptor signalling
during bacterial gut infection. Nature. 501:247–251. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li S, Zhang L, Yao Q, Li L, Dong N, Rong
J, Gao W, Ding X, Sun L, Chen X, et al: Pathogen blocks host death
receptor signalling by arginine GlcNAcylation of death domains.
Nature. 501:242–246. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Weng D, Marty-Roix R, Ganesan S, Proulx
MK, Vladimer GI, Kaiser WJ, Mocarski ES, Pouliot K, Chan FK,
Kelliher MA, et al: Caspase-8 and RIP kinases regulate
bacteria-induced innate immune responses and cell death. Proc Natl
Acad Sci USA. 111:7391–7396. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Philip NH, Dillon CP, Snyder AG,
Fitzgerald P, Wynosky-Dolfi MA, Zwack EE, Hu B, Fitzgerald L,
Mauldin EA, Copenhaver AM, et al: Caspase-8 mediates caspase-1
processing and innate immune defense in response to bacterial
blockade of NF-κB and MAPK signaling. Proc Natl Acad Sci USA.
111:7385–7390. 2014. View Article : Google Scholar
|
|
45
|
Robinson N, McComb S, Mulligan R, Dudani
R, Krishnan L and Sad S: Type I interferon induces necroptosis in
macrophages during infection with Salmonella enterica serovar
Typhimurium. Nat Immunol. 13:954–962. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bleriot C and Lecuit M: The interplay
between regulated necrosis and bacterial infection. Cell Mol Life
Sci. 73:2369–2378. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hu GQ, Yang YJ, Qin XX, Qi S, Zhang J, Yu
SX, Du CT and Chen W: Salmonella outer protein B suppresses colitis
development via protecting cell from necroptosis. Front Cell Infect
Microbiol. 9:872019. View Article : Google Scholar :
|
|
48
|
Fortes GB, Alves LS, de Oliveira R, Dutra
FF, Rodrigues D, Fernandez PL, Souto-Padron T, De Rosa MJ, Kelliher
M, Golenbock D, et al: Heme induces programmed necrosis on
macrophages through autocrine TNF and ROS production. Blood.
119:2368–2375. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yao Y, Liu M, Ren C, Shen J and Ji Y:
Exogenous tumor necrosis factor-alpha could induce egress of
Toxoplasma gondii from human foreskin fibroblast cells. Parasite.
24:452017. View Article : Google Scholar
|
|
50
|
Liu Y, Liu T, Lei T, Zhang D, Du S, Girani
L, Qi D, Lin C, Tong R and Wang Y: RIP1/RIP3-regulated necroptosis
as a target for multifaceted disease therapy (Review). Int J Mol
Med. 44:771–786. 2019.PubMed/NCBI
|
|
51
|
Koo GB, Morgan MJ, Lee DG, Kim WJ, Yoon
JH, Koo JS, Kim SI, Kim SJ, Son MK, Hong SS, et al:
Methylation-dependent loss of RIP3 expression in cancer represses
programmed necrosis in response to chemotherapeutics. Cell Res.
25:707–725. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Stoll G, Ma Y, Yang H, Kepp O, Zitvogel L
and Kroemer G: Pro-necrotic molecules impact local
immunosurveillance in human breast cancer. Oncoimmunology.
6:e12993022017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Feng X, Song Q, Yu A, Tang H, Peng Z and
Wang X: Receptor-interacting protein kinase 3 is a predictor of
survival and plays a tumor suppressive role in colorectal cancer.
Neoplasma. 62:592–601. 2015. View Article : Google Scholar
|
|
54
|
Moriwaki K, Bertin J, Gough PJ, Orlowski
GM and Chan FK: Differential roles of RIPK1 and RIPK3 in
TNF-induced necroptosis and chemotherapeutic agent-induced cell
death. Cell Death Dis. 6:e16362015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Li X, Guo J, Ding AP, Qi WW, Zhang PH, Lv
J, Qiu WS and Sun ZQ: Association of mixed lineage kinase
domain-like protein expression with prognosis in patients with
colon cancer. Technol Cancer Res Treat. 16:428–434. 2017.
View Article : Google Scholar :
|
|
56
|
Nugues AL, El Bouazzati H, Hetuin D,
Berthon C, Loyens A, Bertrand E, Jouy N, Idziorek T and Quesnel B:
RIP3 is downregulated in human myeloid leukemia cells and modulates
apoptosis and caspase-mediated p65/RelA cleavage. Cell Death Dis.
5:e13842014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Hockendorf U, Yabal M, Herold T,
Munkhbaatar E, Rott S, Jilg S, Kauschinger J, Magnani G, Reisinger
F, Heuser M, et al: RIPK3 restricts myeloid leukemogenesis by
promoting cell death and differentiation of leukemia initiating
cells. Cancer Cell. 30:75–91. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Geserick P, Wang J, Schilling R, Horn S,
Harris PA, Bertin J, Gough PJ, Feoktistova M and Leverkus M:
Absence of RIPK3 predicts necroptosis resistance in malignant
melanoma. Cell Death Dis. 6:e18842015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Ke H, Augustine CK, Gandham VD, Jin JY,
Tyler DS, Akiyama SK, Hall RP and Zhang JY: CYLD inhibits melanoma
growth and progression through suppression of the JNK/AP-1 and
beta1-integrin signaling pathways. J Invest Dermatol. 133:221–229.
2013. View Article : Google Scholar
|
|
60
|
McCormick KD, Ghosh A, Trivedi S, Wang L,
Coyne CB, Ferris RL and Sarkar SN: Innate immune signaling through
differential RIPK1 expression promote tumor progression in head and
neck squamous cell carcinoma. Carcinogenesis. 37:522–529. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Ertao Z, Jianhui C, Kang W, Zhijun Y, Hui
W, Chuangqi C, Changjiang Q, Sile C, Yulong H and Shirong C:
Prognostic value of mixed lineage kinase domain-like protein
expression in the survival of patients with gastric caner. Tumour
Biol. 37:13679–13685. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
He L, Peng K, Liu Y, Xiong J and Zhu FF:
Low expression of mixed lineage kinase domain-like protein is
associated with poor prognosis in ovarian cancer patients. Onco
Targets Ther. 6:1539–1543. 2013.PubMed/NCBI
|
|
63
|
Ruan J, Mei L, Zhu Q, Shi G and Wang H:
Mixed lineage kinase domain-like protein is a prognostic biomarker
for cervical squamous cell cancer. Int J Clin Exp Pathol.
8:15035–15038. 2015.
|
|
64
|
Park S, Hatanpaa KJ, Xie Y, Mickey BE,
Madden CJ, Raisanen JM, Ramnarain DB, Xiao G, Saha D, Boothman DA,
et al: The receptor interacting protein 1 inhibits p53 induction
through NF-kappaB activation and confers a worse prognosis in
glioblastoma. Cancer Res. 69:2809–2816. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Wang Q, Chen W, Xu X, Li B, He W, Padilla
MT, Jang JH, Nyunoya T, Amin S, Wang X and Lin Y: RIP1 potentiates
BPDE-induced transformation in human bronchial epithelial cells
through catalase-mediated suppression of excessive reactive oxygen
species. Carcinogenesis. 34:2119–2128. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Seifert L, Werba G, Tiwari S, Giao Ly NN,
Alothman S, Alqunaibit D, Avanzi A, Barilla R, Daley D, Greco SH,
et al: The necrosome promotes pancreatic oncogenesis via CXCL1 and
Mincle-induced immune suppression. Nature. 532:245–249. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Colbert LE, Fisher SB, Hardy CW, Hall WA,
Saka B, Shelton JW, Petrova AV, Warren MD, Pantazides BG, Gandhi K,
et al: Pronecrotic mixed lineage kinase domain-like protein
expression is a prognostic biomarker in patients with early-stage
resected pancreatic adenocarcinoma. Cancer. 119:3148–3155. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Cabal-Hierro L and O'Dwyer PJ: TNF
signaling through RIP1 kinase enhances SN38-Induced death in colon
adenocarcinoma. Mol Cancer Res. 15:395–404. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Wu Y and Zhou BP: Inflammation: A driving
force speeds cancer metastasis. Cell Cycle. 8:3267–3273. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Liu ZY, Wu B, Guo YS, Zhou YH, Fu ZG, Xu
BQ, Li JH, Jing L, Jiang JL, Tang J and Chen ZN: Necrostatin-1
reduces intestinal inflammation and colitis-associated
tumorigenesis in mice. Am J Cancer Res. 5:3174–3185.
2015.PubMed/NCBI
|
|
71
|
Exner N, Lutz AK, Haass C and Winklhofer
KF: Mitochondrial dysfunction in Parkinson's disease: Molecular
mechanisms and pathophysiological consequences. EMBO J.
31:3038–3062. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Iannielli A, Bido S, Folladori L, Segnali
A, Cancellieri C, Maresca A, Massimino L, Rubio A, Morabito G,
Caporali L, et al: Pharmacological inhibition of necroptosis
protects from dopaminergic neuronal cell death in Parkinson's
disease models. Cell Rep. 22:2066–2079. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Wu JR, Wang J, Zhou SK, Yang L, Yin JL,
Cao JP and Cheng YB: Necrostatin-1 protection of dopaminergic
neurons. Neural Regen Res. 10:1120–1124. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Amin P, Florez M, Najafov A, Pan H, Geng
J, Ofengeim D, Dziedzic SA, Wang H, Barrett VJ, Ito Y, et al:
Regulation of a distinct activated RIPK1 intermediate bridging
complex I and complex II in TNFalpha-mediated apoptosis. Proc Natl
Acad Sci USA. 115:E5944–E5953. 2018. View Article : Google Scholar
|
|
75
|
Caccamo A, Branca C, Piras IS, Ferreira E,
Huentelman MJ, Liang WS, Readhead B, Dudley JT, Spangenberg EE,
Green KN, et al: Necroptosis activation in Alzheimer's disease. Nat
Neurosci. 20:1236–1246. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Ofengeim D, Mazzitelli S, Ito Y, DeWitt
JP, Mifflin L, Zou C, Das S, Adiconis X, Chen H, Zhu H, et al:
RIPK1 mediates a disease-associated microglial response in
Alzheimer's disease. Proc Natl Acad Sci USA. 114:E8788–E8797. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Ito Y, Ofengeim D, Najafov A, Das S,
Saberi S, Li Y, Hitomi J, Zhu H, Chen H, Mayo L, et al: RIPK1
mediates axonal degeneration by promoting inflammation and
necroptosis in ALS. Science. 353:603–608. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Ofengeim D, Ito Y, Najafov A, Zhang Y,
Shan B, DeWitt JP, Ye J, Zhang X, Chang A, Vakifahmetoglu-Norberg
H, et al: Activation of necroptosis in multiple sclerosis. Cell
Rep. 10:1836–1849. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Zhang S, Su Y, Ying Z, Guo D, Pan C, Guo
J, Zou Z, Wang L, Zhang Z, Jiang Z, et al: RIP1 kinase inhibitor
halts the progression of an immune-induced demyelination disease at
the stage of monocyte elevation. Proc Natl Acad Sci USA.
116:5675–5680. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Roychowdhury S, McCullough RL, Sanz-Garcia
C, Saikia P, Alkhouri N, Matloob A, Pollard KA, McMullen MR,
Croniger CM and Nagy LE: Receptor interacting protein 3 protects
mice from high-fat diet-induced liver injury. Hepatology.
64:1518–1533. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Xu H, Du X, Liu G, Huang S, Du W, Zou S,
Tang D, Fan C, Xie Y, Wei Y, et al: The pseudokinase MLKL regulates
hepatic insulin sensitivity independently of inflammation. Mol
Metab. 23:14–23. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Saeed WK, Jun DW, Jang K, Ahn SB, Oh JH,
Chae YJ, Lee JS and Kang HT: Mismatched effects of receptor
interacting protein kinase-3 on hepatic steatosis and inflammation
in non-alcoholic fatty liver disease. World J Gastroenterol.
24:5477–5490. 2018. View Article : Google Scholar
|
|
83
|
Gautheron J, Vucur M, Reisinger F,
Cardenas DV, Roderburg C, Koppe C, Kreggenwinkel K, Schneider AT,
Bartneck M, Neumann UP, et al: A positive feedback loop between
RIP3 and JNK controls non-alcoholic steatohepatitis. EMBO Mol Med.
6:1062–1074. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Gautheron J, Vucur M, Schneider AT, Severi
I, Roderburg C, Roy S, Bartneck M, Schrammen P, Diaz MB, Ehling J,
et al: The necroptosis-inducing kinase RIPK3 dampens adipose tissue
inflammation and glucose intolerance. Nat Commun. 7:118692016.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Afonso MB, Rodrigues PM, Carvalho T,
Caridade M, Borralho P, Cortez-Pinto H, Castro RE and Rodrigues CM:
Necroptosis is a key pathogenic event in human and experimental
murine models of non-alcoholic steatohepatitis. Clin Sci (Lond).
129:721–739. 2015. View Article : Google Scholar
|
|
86
|
Roychowdhury S, McMullen MR, Pisano SG,
Liu X and Nagy LE: Absence of receptor interacting protein kinase 3
prevents ethanol-induced liver injury. Hepatology. 57:1773–1783.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Wang S, Ni HM, Dorko K, Kumer SC, Schmitt
TM, Nawabi A, Komatsu M, Huang H and Ding WX: Increased hepatic
receptor interacting protein kinase 3 expression due to impaired
proteasomal functions contributes to alcohol-induced steatosis and
liver injury. Oncotarget. 7:17681–17698. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Jia Y, Wang F, Guo Q, Li M, Wang L, Zhang
Z, Jiang S, Jin H, Chen A, Tan S, et al: Curcumol induces
RIPK1/RIPK3 complex-dependent necroptosis via JNK1/2-ROS signaling
in hepatic stellate cells. Redox Biol. 19:375–387. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Dal-Re R: Worldwide behavioral research on
major global causes of mortality. Health Educ Behav. 38:433–440.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Mizumura K, Cloonan SM, Nakahira K,
Bhashyam AR, Cervo M, Kitada T, Glass K, Owen CA, Mahmood A, Washko
GR, et al: Mitophagy-dependent necroptosis contributes to the
pathogenesis of COPD. J Clin Invest. 124:3987–4003. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Pouwels SD, Zijlstra GJ, van der Toorn M,
Hesse L, Gras R, Ten Hacken NH, Krysko DV, Vandenabeele P, de Vries
M, van Oosterhout AJ, et al: Cigarette smoke-induced necroptosis
and DAMP release trigger neutrophilic airway inflammation in mice.
Am J Physiol Lung Cell Mol Physiol. 310:L377–L386. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Wang Y, Zhou JS, Xu XC, Li ZY, Chen HP,
Ying SM, Li W, Shen HH and Chen ZH: Endoplasmic reticulum chaperone
GRP78 mediates cigarette smoke-induced necroptosis and injury in
bronchial epithelium. Int J Chron Obstruct Pulmon Dis. 13:571–581.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Rubenfeld GD, Caldwell E, Peabody E,
Weaver J, Martin DP, Neff M, Stern EJ and Hudson LD: Incidence and
outcomes of acute lung injury. N Engl J Med. 353:1685–1693. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Syed MA, Shah D, Das P, Andersson S,
Pryhuber G and Bhandari V: TREM-1 Attenuates RIPK3-mediated
necroptosis in hyperoxia-induced lung injury in neonatal mice. Am J
Respir Cell Mol Biol. 60:308–322. 2019. View Article : Google Scholar :
|
|
95
|
Chen J, Wang S, Fu R, Zhou M, Zhang T, Pan
W, Yang N and Huang Y: RIP3 dependent NLRP3 inflammasome activation
is implicated in acute lung injury in mice. J Transl Med.
16:2332018. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Siempos II, Ma KC, Imamura M, Baron RM,
Fredenburgh LE, Huh JW, Moon JS, Finkelsztein EJ, Jones DS, Lizardi
MT, et al: RIPK3 mediates pathogenesis of experimental
ventilator-induced lung injury. JCI Insight. 3:e971022018.
View Article : Google Scholar :
|
|
97
|
Bolognese AC, Yang WL, Hansen LW, Denning
NL, Nicastro JM, Coppa GF and Wang P: Inhibition of necroptosis
attenuates lung injury and improves survival in neonatal sepsis.
Surgery. Apr 27–2018.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Kobayashi K, Araya J, Minagawa S, Hara H,
Saito N, Kadota T, Sato N, Yoshida M, Tsubouchi K, Kurita Y, et al:
Involvement of PARK2-mediated mitophagy in idiopathic pulmonary
fibrosis pathogenesis. J Immunol. 197:504–516. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Lee JM, Yoshida M, Kim MS, Lee JH, Baek
AR, Jang AS, Kim DJ, Minagawa S, Chin SS, Park CS, et al:
Involvement of alveolar epithelial cell necroptosis in idiopathic
pulmonary fibrosis pathogenesis. Am J Respir Cell Mol Biol.
59:215–224. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Papi A, Brightling C, Pedersen SE and
Reddel HK: Asthma. Lancet. 391:783–800. 2018. View Article : Google Scholar
|
|
101
|
Cerps SC, Menzel M, Mahmutovic Persson I,
Bjermer L, Akbarshahi H and Uller L: Interferon-beta deficiency at
asthma exacerbation promotes MLKL mediated necroptosis. Sci Rep.
8:42482018. View Article : Google Scholar
|
|
102
|
Shlomovitz I, Erlich Z, Speir M, Zargarian
S, Baram N, Engler M, Edry-Botzer L, Munitz A, Croker BA and Gerlic
M: Necroptosis directly induces the release of full-length
biologically active IL-33 in vitro and in an inflammatory disease
model. FEBS J. 286:507–522. 2019. View Article : Google Scholar
|
|
103
|
Zhang H, Ji J, Liu Q and Xu S: MUC1
downregulation promotes TNF-α-induced necroptosis in human
bronchial epithelial cells via regulation of the RIPK1/RIPK3
pathway. J Cell Physiol. 234:15080–15088. 2019. View Article : Google Scholar :
|
|
104
|
Linkermann A, Brasen JH, Darding M, Jin
MK, Sanz AB, Heller JO, De Zen F, Weinlich R, Ortiz A, Walczak H,
et al: Two independent pathways of regulated necrosis mediate
ischemia-reperfusion injury. Proc Natl Acad Sci USA.
110:12024–12029. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Linkermann A, Skouta R, Himmerkus N, Mulay
SR, Dewitz C, De Zen F, Prokai A, Zuchtriegel G, Krombach F, Welz
PS, et al: Synchronized renal tubular cell death involves
ferroptosis. Proc Natl Acad Sci USA. 111:16836–16841. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Linkermann A, Brasen JH, Himmerkus N, Liu
S, Huber TB, Kunzendorf U and Krautwald S: Rip1
(receptor-interacting protein kinase 1) mediates necroptosis and
contributes to renal ischemia/reperfusion injury. Kidney Int.
81:751–761. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Mulay SR, Desai J, Kumar SV, Eberhard JN,
Thomasova D, Romoli S, Grigorescu M, Kulkarni OP, Popper B,
Vielhauer V, et al: Cytotoxicity of crystals involves
RIPK3-MLKL-mediated necroptosis. Nat Commun. 7:102742016.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Tristao VR, Goncalves PF, Dalboni MA,
Batista MC, Durao Mde S Jr and Monte JC: Nec-1 protects against
nonapoptotic cell death in cisplatin-induced kidney injury. Ren
Fail. 34:373–377. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Linkermann A, Heller JO, Prokai A,
Weinberg JM, De Zen F, Himmerkus N, Szabó AJ, Bräsen JH, Kunzendorf
U and Krautwald S: The RIP1-kinase inhibitor necrostatin-1 prevents
osmotic nephrosis and contrast-induced AKI in mice. J Am Soc
Nephrol. 24:1545–1557. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Xu Y, Ma H, Shao J, Wu J, Zhou L, Zhang Z,
Wang Y, Huang Z, Ren J, Liu S, et al: A role for tubular
necroptosis in Cisplatin-Induced AKI. J Am Soc Nephrol.
26:2647–2658. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Tristao VR, Pessoa EA, Nakamichi R, Reis
LA, Batista MC, Durão Junior Mde S and Monte JC: Synergistic effect
of apoptosis and necroptosis inhibitors in cisplatin-induced
nephrotoxicity. Apoptosis. 21:51–59. 2016. View Article : Google Scholar
|
|
112
|
Zhu Y, Cui H, Gan H, Xia Y, Wang L, Wang Y
and Sun Y: Necroptosis mediated by receptor interaction protein
kinase 1 and 3 aggravates chronic kidney injury of subtotal
nephrectomised rats. Biochem Biophys Res Commun. 461:575–581. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Chen H, Fang Y, Wu J, Chen H, Zou Z, Zhang
X, Shao J and Xu Y: RIPK3-MLKL-mediated necroinflammation
contributes to AKI progression to CKD. Cell Death Dis. 9:8782018.
View Article : Google Scholar : PubMed/NCBI
|
|
114
|
McManus DD, Piacentine SM, Lessard D, Gore
JM, Yarzebski J, Spencer FA and Goldberg RJ: Thirty-year (1975 to
2005) trends in the incidence rates, clinical features, treatment
practices, and short-term outcomes of patients <55 years of age
hospitalized with an initial acute myocardial infarction. Am J
Cardiol. 108:477–482. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Luedde M, Lutz M, Carter N, Sosna J,
Jacoby C, Vucur M, Gautheron J, Roderburg C, Borg N, Reisinger F,
et al: RIP3, a kinase promoting necroptotic cell death, mediates
adverse remodelling after myocardial infarction. Cardiovasc Res.
103:206–216. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Zhang T, Zhang Y, Cui M, Jin L, Wang Y, Lv
F, Liu Y, Zheng W, Shang H, Zhang J, et al: CaMKII is a RIP3
substrate mediating ischemia- and oxidative stress-induced
myocardial necroptosis. Nat Med. 22:175–182. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Lusis AJ: Atherosclerosis. Nature.
407:233–241. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Karunakaran D, Geoffrion M, Wei L, Gan W,
Richards L, Shangari P, DeKemp EM, Beanlands RA, Perisic L,
Maegdefessel L, et al: Targeting macrophage necroptosis for
therapeutic and diagnostic interventions in atherosclerosis. Sci
Adv. 2:e16002242016. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Henderson B, Revell PA and Edwards JC:
Synovial lining cell hyperplasia in rheumatoid arthritis: Dogma and
fact. Ann Rheum Dis. 47:348–349. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Lee SH, Kwon JY, Kim SY, Jung K and Cho
ML: Interferon-gamma regulates inflammatory cell death by targeting
necroptosis in experimental autoimmune arthritis. Sci Rep.
7:101332017. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Jhun J, Lee SH, Kim SY, Ryu J, Kwon JY, Na
HS, Jung K, Moon SJ, Cho ML and Min JK: RIPK1 inhibition attenuates
experimental autoimmune arthritis via suppression of
osteoclastogenesis. J Transl Med. 17:842019. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Glyn-Jones S, Palmer AJ, Agricola R, Price
AJ, Vincent TL, Weinans H and Carr AJ: Osteoarthritis. Lancet.
386:376–387. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Riegger J and Brenner RE: Evidence of
necroptosis in osteoarthritic disease: Investigation of blunt
mechanical impact as possible trigger in regulated necrosis. Cell
Death Dis. 10:6832019. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Galluzzi L, Kepp O, Chan FK and Kroemer G:
Necroptosis: Mechanisms and relevance to disease. Annu Rev Pathol.
12:103–130. 2017. View Article : Google Scholar
|
|
125
|
Della Torre L, Nebbioso A, Stunnenberg HG,
Martens JHA, Carafa V and Altucci L: The role of necroptosis:
Biological relevance and its involvement in cancer. Cancers
(Basel). 13:6842021. View Article : Google Scholar
|
|
126
|
Martens S, Hofmans S, Declercq W,
Augustyns K and Vandenabeele P: Inhibitors Targeting RIPK1/RIPK3:
Old and new drugs. Trends Pharmacol Sci. 41:209–224. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Thapa RJ, Nogusa S, Chen P, Maki JL, Lerro
A, Andrake M, Rall GF, Degterev A and Balachandran S:
Interferon-induced RIP1/RIP3-mediated necrosis requires PKR and is
licensed by FADD and caspases. Proc Natl Acad Sci USA.
110:E3109–E3118. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Bollino D, Balan I and Aurelian L:
Valproic acid induces neuronal cell death through a novel
calpain-dependent necroptosis pathway. J Neurochem. 133:174–186.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Kim HJ, Hwang KE, Park DS, Oh SH, Jun HY,
Yoon KH, Jeong ET, Kim HR and Kim YS: Shikonin-induced necroptosis
is enhanced by the inhibition of autophagy in non-small cell lung
cancer cells. J Transl Med. 15:1232017. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Zhou J, Li G, Han G, Feng S, Liu Y, Chen
J, Liu C, Zhao L and Jin F: Emodin induced necroptosis in the
glioma cell line U251 via the TNF-α/RIP1/RIP3 pathway. Invest New
Drugs. 38:50–59. 2020. View Article : Google Scholar
|
|
131
|
Li Y, Tian X, Liu X and Gong P: Bufalin
inhibits human breast cancer tumorigenesis by inducing cell death
through the ROS-mediated RIP1/RIP3/PARP-1 pathways. Carcinogenesis.
39:700–707. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Han Q, Ma Y, Wang H, Dai Y, Chen C, Liu Y,
Jing L and Sun X: Resibufogenin suppresses colorectal cancer growth
and metastasis through RIP3-mediated necroptosis. J Transl Med.
16:2012018. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Fakharnia F, Khodagholi F, Dargahi L and
Ahmadiani A: Prevention of Cyclophilin D-Mediated mPTP Opening
Using Cyclosporine-A Alleviates the Elevation of Necroptosis,
Autophagy and Apoptosis-Related Markers Following Global Cerebral
Ischemia-Reperfusion. J Mol Neurosci. 61:52–60. 2017. View Article : Google Scholar
|
|
134
|
Ding J, Yang N, Yan Y, Wang Y, Wang X, Lu
L and Dong K: Rapamycin inhibited photoreceptor necroptosis and
protected the retina by activation of autophagy in experimental
retinal detachment. Curr Eye Res. 44:739–745. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Yan C, Oh JS, Yoo SH, Lee JS, Yoon YG, Oh
YJ, Jang MS, Lee SY, Yang J, Lee SH, et al: The targeted inhibition
of mitochondrial Hsp90 overcomes the apoptosis resistance conferred
by Bcl-2 in Hep3B cells via necroptosis. Toxicol Appl Pharmacol.
266:9–18. 2013. View Article : Google Scholar
|
|
136
|
Li D, Li C, Li L, Chen S, Wang L, Li Q,
Wang X, Lei X and Shen Z: Natural Product Kongensin A is a
Non-Canonical HSP90 Inhibitor that Blocks RIP3-dependent
Necroptosis. Cell Chem Biol. 23:257–266. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Zhang Z, Li HM, Zhou C, Li Q, Ma L, Zhang
Z, Sun Y, Wang L, Zhang X, Zhu B, et al: Non-benzoquinone
geldanamycin analogs trigger various forms of death in human breast
cancer cells. J Exp Clin Cancer Res. 35:1492016. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Chen WW, Yu H, Fan HB, Zhang CC, Zhang M,
Zhang C, Cheng Y, Kong J, Liu CF, Geng D and Xu X: RIP1 mediates
the protection of geldanamycin on neuronal injury induced by
oxygen-glucose deprivation combined with zVAD in primary cortical
neurons. J Neurochem. 120:70–77. 2012. View Article : Google Scholar
|
|
139
|
Qu C, Yuan ZW, Yu XT, Huang YF, Yang GH,
Chen JN, Lai XP, Su ZR, Zeng HF, Xie Y and Zhang XJ: Patchouli
alcohol ameliorates dextran sodium sulfate-induced experimental
colitis and suppresses tryptophan catabolism. Pharmacol Res.
121:70–82. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Fauster A, Rebsamen M, Huber KV, Bigenzahn
JW, Stukalov A, Lardeau CH, Scorzoni S, Bruckner M, Gridling M,
Parapatics K, et al: A cellular screen identifies ponatinib and
pazopanib as inhibitors of necroptosis. Cell Death Dis.
6:e17672015. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Harris PA, Marinis JM, Lich JD, Berger SB,
Chirala A, Cox JA, Eidam PM, Finger JN, Gough PJ, Jeong JU, et al:
Identification of a RIP1 Kinase Inhibitor Clinical Candidate
(GSK3145095) for the treatment of pancreatic cancer. ACS Med Chem
Lett. 10:857–862. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Ali M and Mocarski ES: Proteasome
inhibition blocks necroptosis by attenuating death complex
aggregation. Cell Death Dis. 9:3462018. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Martens S, Jeong M, Tonnus W, Feldmann F,
Hofmans S, Goossens V, Takahashi N, Bräsen JH, Lee EW, Van der
Veken P, et al: Sorafenib tosylate inhibits directly necrosome
complex formation and protects in mouse models of inflammation and
tissue injury. Cell Death Dis. 8:e29042017. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
von Mässenhausen A, Tonnus W, Himmerkus N,
Parmentier S, Saleh D, Rodriguez D, Ousingsawat J, Ang RL, Weinberg
JM, Sanz AB, et al: Phenytoin inhibits necroptosis. Cell Death Dis.
9:3592018. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Wang J, Li Y, Huang WH, Zeng XC, Li XH, Li
J, Zhou J, Xiao J, Xiao B, Ouyang DS and Hu K: The protective
effect of aucubin from eucommia ulmoides against status epilepticus
by inducing autophagy and inhibiting necroptosis. Am J Chin Med.
45:557–573. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Meng XM, Li HD, Wu WF, Ming-Kuen Tang P,
Ren GL, Gao L, Li XF, Yang Y, Xu T, Ma TT, et al: Wogonin protects
against cisplatin-induced acute kidney injury by targeting
RIPK1-mediated necroptosis. Lab Invest. 98:79–94. 2018. View Article : Google Scholar
|
|
147
|
Nehs MA, Lin CI, Kozono DE, Whang EE, Cho
NL, Zhu K, Moalem J, Moore FD Jr and Ruan DT: Necroptosis is a
novel mechanism of radiation-induced cell death in anaplastic
thyroid and adrenocortical cancers. Surgery. 150:1032–1039. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Oliver Metzig M, Fuchs D, Tagscherer KE,
Gröne HJ, Schirmacher P and Roth W: Inhibition of caspases primes
colon cancer cells for 5-fluorouracil-induced TNF-α-dependent
necroptosis driven by RIP1 kinase and NF-κB. Oncogene.
35:3399–3409. 2016. View Article : Google Scholar
|
|
149
|
Choi MJ, Kang H, Lee YY, Choo OS, Jang JH,
Park SH, Moon JS, Choi SJ and Choung YH: Cisplatin-Induced
ototoxicity in rats is driven by RIP3-Dependent necroptosis. Cells.
8:4092019. View Article : Google Scholar :
|
|
150
|
Yang H, Ma Y, Chen G, Zhou H, Yamazaki T,
Klein C, Pietrocola F, Vacchelli E, Souquere S, Sauvat A, et al:
Contribution of RIP3 and MLKL to immunogenic cell death signaling
in cancer chemotherapy. Oncoimmunology. 5:e11496732016. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Basit F, Cristofanon S and Fulda S:
Obatoclax (GX15-070) triggers necroptosis by promoting the assembly
of the necrosome on autophagosomal membranes. Cell Death Differ.
20:1161–1173. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Deng Q, Yu X, Xiao L, Hu Z, Luo X, Tao Y,
Yang L, Liu X, Chen H, Ding Z, et al: Neoalbaconol induces energy
depletion and multiple cell death in cancer cells by targeting
PDK1-PI3-K/Akt signaling pathway. Cell Death Dis. 4:e8042013.
View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Lin CY, Chang TW, Hsieh WH, Hung MC, Lin
IH, Lai SC and Tzeng YJ: Simultaneous induction of apoptosis and
necroptosis by Tanshinone IIA in human hepatocellular carcinoma
HepG2 cells. Cell Death Discov. 2:160652016. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Tang D, Kang R, Berghe TV, Vandenabeele P
and Kroemer G: The molecular machinery of regulated cell death.
Cell Res. 29:347–364. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Grootjans S, Vanden Berghe T and
Vandenabeele P: Initiation and execution mechanisms of necroptosis:
An overview. Cell Death Differ. 24:1184–1195. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Upton JW, Kaiser WJ and Mocarski ES: Virus
inhibition of RIP3-dependent necrosis. Cell Host Microbe.
7:302–313. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Pan T, Wu S, He X, Luo H, Zhang Y, Fan M,
Geng G, Ruiz VC, Zhang J, Mills L, et al: Necroptosis takes place
in human immunodeficiency virus type-1 (HIV-1)-infected CD4+ T
lymphocytes. PLoS One. 9:e939442014. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Berger AK and Danthi P: Reovirus activates
a caspase-independent cell death pathway. mBio. 4:e00178–00113.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Chan FK, Shisler J, Bixby JG, Felices M,
Zheng L, Appel M, Orenstein J, Moss B and Lenardo MJ: A role for
tumor necrosis factor receptor-2 and receptor-interacting protein
in programmed necrosis and antiviral responses. J Biol Chem.
278:51613–51621. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Shrestha A, Mehdizadeh Gohari I and
McClane BA: RIP1, RIP3, and MLKL contribute to cell death caused by
clostridium perfringens enterotoxin. mBio. 10:e02985–19. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Roca FJ and Ramakrishnan L: TNF dually
mediates resistance and susceptibility to mycobacteria via
mitochondrial reactive oxygen species. Cell. 153:521–534. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Kitur K, Parker D, Nieto P, Ahn DS, Cohen
TS, Chung S, Wachtel S, Bueno S and Prince A: Toxin-induced
necroptosis is a major mechanism of Staphylococcus aureus lung
damage. PLoS Pathog. 11:e10048202015. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Roychowdhury S, Chiang DJ, Mandal P,
McMullen MR, Liu X, Cohen JI, Pollard J, Feldstein AE and Nagy LE:
Inhibition of apoptosis protects mice from ethanol-mediated
acceleration of early markers of CCl4-induced fibrosis but not
steatosis or inflammation. Alcohol Clin Exp Res. 36:1139–1147.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Lu C, Xu W, Zhang F, Shao J and Zheng S:
Nrf2 knockdown disrupts the protective effect of curcumin on
alcohol-induced hepatocyte necroptosis. Mol Pharm. 13:4043–4053.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Afonso MB, Rodrigues PM, Simao AL,
Ofengeim D, Carvalho T, Amaral JD, Gaspar MM, Cortez-Pinto H,
Castro RE, Yuan J and Rodrigues CM: Activation of necroptosis in
human and experimental cholestasis. Cell Death Dis. 7:e23902016.
View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Choi HS, Kang JW and Lee SM: Melatonin
attenuates carbon tetrachloride-induced liver fibrosis via
inhibition of necroptosis. Transl Res. 166:292–303. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Harris PA, Berger SB, Jeong JU, Nagilla R,
Bandyopadhyay D, Campobasso N, Capriotti CA, Cox JA, Dare L, Dong
X, et al: Discovery of a First-in-Class receptor interacting
protein 1 (RIP1) kinase specific clinical candidate (GSK2982772)
for the treatment of inflammatory diseases. J Med Chem.
60:1247–1261. 2017. View Article : Google Scholar : PubMed/NCBI
|