Introduction
Osteocalcin (OC) is a multipurpose bone-derived
hormone (1), which can be
γ-carboxylated at one or more of its glutamic acid residues
(2). Therefore, OC can be
present as one of 2 forms, carboxylated and undercarboxylated OC.
Uncarboxylated osteocalcin (GluOC) is the form with no
γ-carboxylated glutamic acid residu (3). GluOC is not only an index used to
assess the health of bones, but also a biologically active molecule
directly secreted into the blood to mediate glucose and energy
metabolism (4,5). GluOC can affect the role of glucose
by promoting the proliferation of β cells, increasing the secretion
of insulin and improving the sensitivity of insulin target tissues,
such as muscle and adipogenic tissue (6,7).
However, the precise mechanisms through which GluOC improves the
effects of glucose on osteoblasts have not yet been fully
elucidated, at least to the best of our knowledge.
Osteoporosis is considered a serious skeletal
disease, characterized by abnormal bone structure and low bone
mineral density (8,9). The imbalance between osteoblast and
adipocyte differentiation of bone marrow-derived stromal cells
(BMSCs) is a major cause of osteoporosis (10-12). Diabetic osteoporosis is a severe
diabetic complication affecting the bones. The number of patients
with diabetic osteoporosis is increasing, and the disease is thus
gaining increasing attention (13-15). Previous studies have suggested
that diabetic patients have a lower bone quality primarily due to
hyperglycemia (16,17). In a previous study on primary rat
osteoblasts, the authors demonstrated that high glucose induced
oxidative stress and resulted in the activation of the
phosphoinositide 3-kinase (PI3K)/Akt pathway to induce adipogenic
differentiation (18). Moreover,
it was demonstrated that high glucose conditions activated the
cyclic AMP (cAMP)/protein kinase A(PKA) pathway to stimulate the
adipocytic differentiation of MG63 cells (19). GluOC has been shown to inhibit
the high glucose-induced inhibition of cellular proliferation, as
well as the osteogenic differentiation of MC3T3E1 cells (20). However, the corresponding
receptors and signaling pathways through which GluOC exerted its
effects on osteoblasts have not yet been fully elucidated.
G-protein coupled receptor, class C, group 6,
subtype A (GPRC6A), a relatively recently discovered
G-protein-coupled receptor, has been identified in several tissues
and organs in humans, mice and rats, including skeletal muscle,
pancreas, kidneys, heart, liver, lungs and brain (21). It has been reported that GPRC6A
can bind to a variety of ligands, such as L-α amino acids, calcium,
magnesium, testosterone and osteocalcin (22,23). Pi et al (24) demonstrated that 293 cells
transfected with full-length GPRC6A cDNA exhibited a dose-dependent
response to treatment with GluOC; GluOC activated the extracellular
signal-regulated kinase (ERK) pathway via the GPRC6A/phospholipase
C (PLC)/protein kinase C (PKC) signaling pathway. Otani et
al (25) found that in 3T3L1
cells, GluOC was assumed to bind to GPRC6A, and this resulted in
the increased intracellular accumulation of cAMP, and thus, in the
subsequent activation of PKA to promote the synthesis and secretion
of adiponectin. Karsenty and Oury (26) also found that GluOC upregulated
cAMP levels via GPRC6A to promote the synthesis and secretion of
testosterone in testicular mesenchymal cells. In another study, in
TC-6 cells, GluOC stimulated PKD1 in a dose-dependent manner via
GPRC6A, suggesting the active participation of GPRC6A in the
maintenance of glucose homeostasis (27). However, whether GPRC6A acts as a
GluOC receptor in MC3T3E1 has not yet been confirmed, at least to
the best of our knowledge.
The present study assessed whether GPRC6A functions
as a receptor of GluOC in the high glucose-induced inhibition of
the osteogenic differentiation of MC3T3E1 cells. To the best of our
knowledge, the present study is the first to demonstrate that GluOC
activates the GPRC6A/cAMP/PKA/AMP-activated protein kinase (AMPK)
signaling pathway to reverse the high glucose-induced inhibition of
the osteogenic differentiation of MC3T3E1 cells. Thus, the results
of the present study add to a growing body of evidence indicating
that in MC3T3E1 cells, GPRC6A plays a significant role in
osteogenic differentiation promoted by GluOC. Additionally, these
results also highlight a potentially novel means for the prevention
or treatment of diabetic osteoporosis.
Materials and methods
Materials
Alizarin Red S and Oil Red O were obtained from
Sigma-Aldrich; Merck KGaA. Small interfering (si) RNAs were
synthesized by Shanghai Gima Corp. Inhibitors, including SQ22536
[adenylate cyclase (AC) inhibitor], U73122 (PLC inhibitor), H-89
(PKA inhibitor) and BML-275 (AMPK inhibitor) were obtained from
TargetMol. Antibodies against PKA (cat. no. 4782), AMPKα (cat. no.
5831), phospho-PKA (cat. no. 5661) and phospho-AMPKα (cat. no.
2535) were purchased from Cell Signaling Technology, Inc.
Antibodies against cyclin D1 (cat. no. ab134175), proliferating
cell nuclear antigen (PCNA; cat. no. ab29) and β-actin (cat. no.
ab8226) were purchased from Abcam.
Cells and cell culture
MC3T3E1 cells (cat. no. GNM15) were obtained from
The Cell Bank of Type Culture Collection of The Chinese Academy of
Sciences. Initially, the cells were grown in α-MEM (HyClone;
Cytiva) supplemented with 10% FBS (Gibco; Thermo Fisher Scientific,
Inc.) with 5% CO2 at 37°C. The concentration of FBS in
α-MEM was lowered to 4% when treating the cells, and the medium was
replaced every day during the treatments. The control group was
exposed to a glucose concentration of 5.5 mM.
Cell proliferation assay
A Cell Counting kit-8 (cat. no. CK04; Dojindo
Molecular Technologies, Inc.) was used to detect the proliferation
of the MC3T3E1 cells. MC3T3E1 cells were plated in 96-well plates
(3×103 cells/well) for 24 h, after which the cells were
cultured in 4% FBS α-MEM with various concentrations of GluOC (0,
0.1, 1, 3 or 10 ng/ml) and high glucose (25.5 mM) for 1, 2 or 3
days, as previously described (20). Subsequently, 100 μl fresh
medium and 10 μl CCK-solution 8 were added to each well, and
the cells were cultured for 30 min in a humidified incubator with
5% CO2 at 37°C. An automated microplate reader (Synergy
H1; Biotek Instruments, Inc.) at 450 nm was used to measure the
absorbance.
Mineralization assay
The MC3T3E1 cells were grown overnight at a density
of 1×106 cells/well in 6-well plates, and then cultured
in osteoblastic differentiation medium (HyClone; Cytiva) (α-MEM, 4%
FBS, 100 nM dexamethasone, 10 mM β-glycerophosphate disodium, 50
mg/l vitamin C) containing high glucose (25.5 mM) or GluOC (3
ng/ml) for 28 days. The cells were fixed at room temperature with
10% formaldehyde for 30 min and calcium nodules were stained using
Alizarin Red S. The staining was imaged using a microscope
(magnification, ×100, DM750; Leica Microsystems, Inc.) and the red
areas represented calcified nodules. For quantitative detection,
0.1 M cetylpyridinium chloride was used to dissolve the calcium
nodules for 15 min. Following a 20-fold dilution, the absorbance
value was measured at 570 nm by an automated microplate reader
(Synergy H1; Biotek Instruments, Inc.).
Assay of lipid droplets
The MC3T3E1 cells were grown overnight at a density
of 1×106 cells/well in 6-well plates and then cultured
in adipocytic differentiation medium (α-MEM, 4% FBS, 1 mM
dexamethasone, 10 mg/l insulin) containing high glucose (25.5 mM)
or GluOC (3 ng/ml) for 16 days. The cells were fixed at room
temperature with 10% formaldehyde for 30 min and Oil Red O was used
to stain the lipid droplets. The staining was imaged using a
microscope (magnification, ×500, DM750; Leica Microsystems, Inc.),
and the red areas represented lipid droplets. For quantitative
detection, isopropanol was used to dissolve lipid droplets for 10
min and the absorbance value was measured at 510 nm by an automated
microplate reader (Synergy H1; Biotek Instruments, Inc.).
Alkaline phosphatase (ALP) assay
The MC3T3E1 cells were seeded into 6-well plates
(1×106 cells/well) and cultured in 4% FBS α-MEM
containing high glucose (25.5 mM), siRNAs (100 pmol), inhibitors
(10 μM) or GluOC (3 ng/ml) for 7 days. A total of 200
μl pre-cooled PBS was added to collect the cells, and the
supernatant was retained to measure ALP activity, using an Alkaline
Phosphatase assay kit (cat. no. A059-2; Nanjing Jiancheng
Bioengineering Institute) according to the manufacturer's
protocol.
Type I collagen (COLI) assay
The secretion of COLI was performed as described
above for the measurement of ALP activity. The secretion of COLI
was quantified using a specific ELISA kit (cat. no. SEA571Mu;
Cloud-Clone Corp.), according to the manufacturer's protocol.
Reverse transcription-quantitative PCR
(RT-qPCR)
The MC3T3E1 cells in 6-well plates (1×106
cells/well) were treated with or without high glucose (25.5 mM),
siRNAs (100 pmol), inhibitors (10 μM) or GluOC (3 ng/ml).
Total RNA extraction from the MC3T3E1 cells was performed using a
Total RNA kit (cat. no. DP419; Tiangen Biotech, Co., Ltd.). A total
of 2 μg RNA was reverse transcribed into cDNA using the
TransScript One-Step gDNA Removal and cDNA Synthesis SuperMix (cat.
no. A T311-03; TransGen Biotech.) and quantitative PCR (qPCR) was
performed in a 20 μl reaction volume with the TransStart Top
Green qPCR SuperMix (+Dye II) (cat. no. AQ132-24; TransGen
Biotech.). The following thermocycling conditions were used: 40
cycles at 94°C for 5 sec, 60°C for 15 sec and 72°C for 10 sec. To
quantify GPRC6A expression, qPCR was performed after 24 h. The
expression of the adipogenic genes, peroxisome
proliferator-activated receptor γ (PPARγ) and FAS, was assessed
after 3 days. The expression of the osteogenic genes, Runt-related
transcription factor 2 (Runx2) and osterix (Osx), was assessed
after 5 days. The sequences of the primers used are listed in
Table I. Expression data were
normalized to β-actin, which was used as the internal standard.
 | Table IPrimer sequences designed for
RT-qPCR. |
Table I
Primer sequences designed for
RT-qPCR.
| Gene | Forward primers
(5′→3′) | Reverse primers
(5′→3′) |
|---|
| Runx2 | GCCTTCAAGGTGGTAGCCC
C |
GTTACCCGCCATGACAGTA |
| Osx |
ACTGGCTAGGTGGTGGTCAG |
GGTAGGGAGCTGGGTTAAGG |
| PPARγ |
GCATGGTGCCTTCGCTGA |
TGGCATCTCTGTGTCAACCATG |
| FAS |
GGCTGCAGTGAATGAATTTG |
TTCGTACCTCCTTGGCAAAC |
| GPRC6A |
TCCGGAGTCAAGCTGGGATA |
CCCTTGGCATGTAGCTGGAA |
| β-actin |
GCTCTTTTCCAGCCTTCCTT |
AGGTCTTTACGGATGTCAACG |
siRNAs and transfection
siRNA-1 (siRNA.m1638, CCA ACA CAG CTG TTG CTA T) and
siRNA-2 (siRNA.m2553, GCAGAAGACTAACACCAAA), which were specific for
GPRC6A were used in the present study. GPRC6A (GenBank accession
no. NM_153071) was used as a template for synthesizing the siRNAs.
Cells at a density of 2×105 cells/well in 24-well plates
were cultured overnight in 10% FBS α-MEM, and then transiently
transfected with 100 pmol siRNA and 5 μl
Lipofectamine® 2000 (cat. no. 11668-019, Invitrogen;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocol. RT-qPCR analysis was used to quantify GPRC6A expression
after 24 h. Subsequently, the medium was changed to 4% FBS α-MEM
containing 25.5 mM glucose or 3 ng/ml GluOC. Scrambled siRNA (UUC
UCC GAA CGU GUC ACG UTT) was used as a negative control.
Radioimmunoassay for cAMP
MC3T3E1 cells at a density of 2×105
cells/well in 24-well plates were cultured in 4% FBS α-MEM and
divided into a control group, high glucose group, GluOC group and a
SQ22536 group. The cAMP assay kit (cat. no. ab133051; Abcam) was
used to measure the intracellular accumulation of cAMP after 24 h,
according to the manufacturer's protocol.
Western blot analysis
MC3T3E1 cells in 6-well plates (1×106
cells/well) were treated either with or without glucose (25.5 mM),
siRNAs (100 pmol), inhibitors (10 μM) and GluOC (3 ng/ml).
The cold cell lysates were acquired by ultrasonic disruption with
RIPA lysis buffer on ice after two ice-cold rinses with PBS. Cell
lysates were centrifuged (10,000 × g) at 4°C for 20 min, and the
protein concentrations of the extracted proteins were measured
using a BCA Detection kit (cat. no. B5000; Beijing Lablead Biotech,
Co., Ltd.). A total of 10 μg proteins (for each lane) in
cell lysates were loaded and separated by 12% SDS-PAGE, and then
transferred to PVDF membranes. The membranes were blocked in TBS-5%
Tween-20 (TBST) containing 5% non-fat milk at room temperature for
2 h and incubated overnight at 4°C with rabbit-derived antibodies,
including anti-cyclin D1 (1:10,000), anti-PCNA (1:2,000),
anti-β-actin (1:1,000), anti-PKA (1:1,000), anti-phosphorylated
(p-)PKA (1:1,000), anti-AMPKα (1:1,000), anti-p-AMPKα (1:1,000).
The membranes were then washed 3 times with TBST (10 min each),
followed by incubation with horseradish peroxidase-conjugated
secondary antibody (1:10,000; cat. no. ab6721; Abcam) at 25°C for 2
h. The protein bands were visualized using an ECL kit (Biomiga). To
quantify the expression of proteins, the intensity of bands was
normalized to the respective β-actin control, and the bands of the
phospho-proteins were normalized to the total levels of that
specific protein. ImageJ version 6 (National Institutes of Health)
was used for densitometric analysis.
Statistical analysis
Data are presented as the means ± standard deviation
of at least 3 independent repeats. Statistical analysis was
performed using GraphPad Prism version 6.0 (GraphPad Software,
Inc.). Differences between the groups were compared using a one-way
ANOVA followed by a Tukey's post hoc test. P<0.05 was considered
to indicate a statistically significant difference.
Results
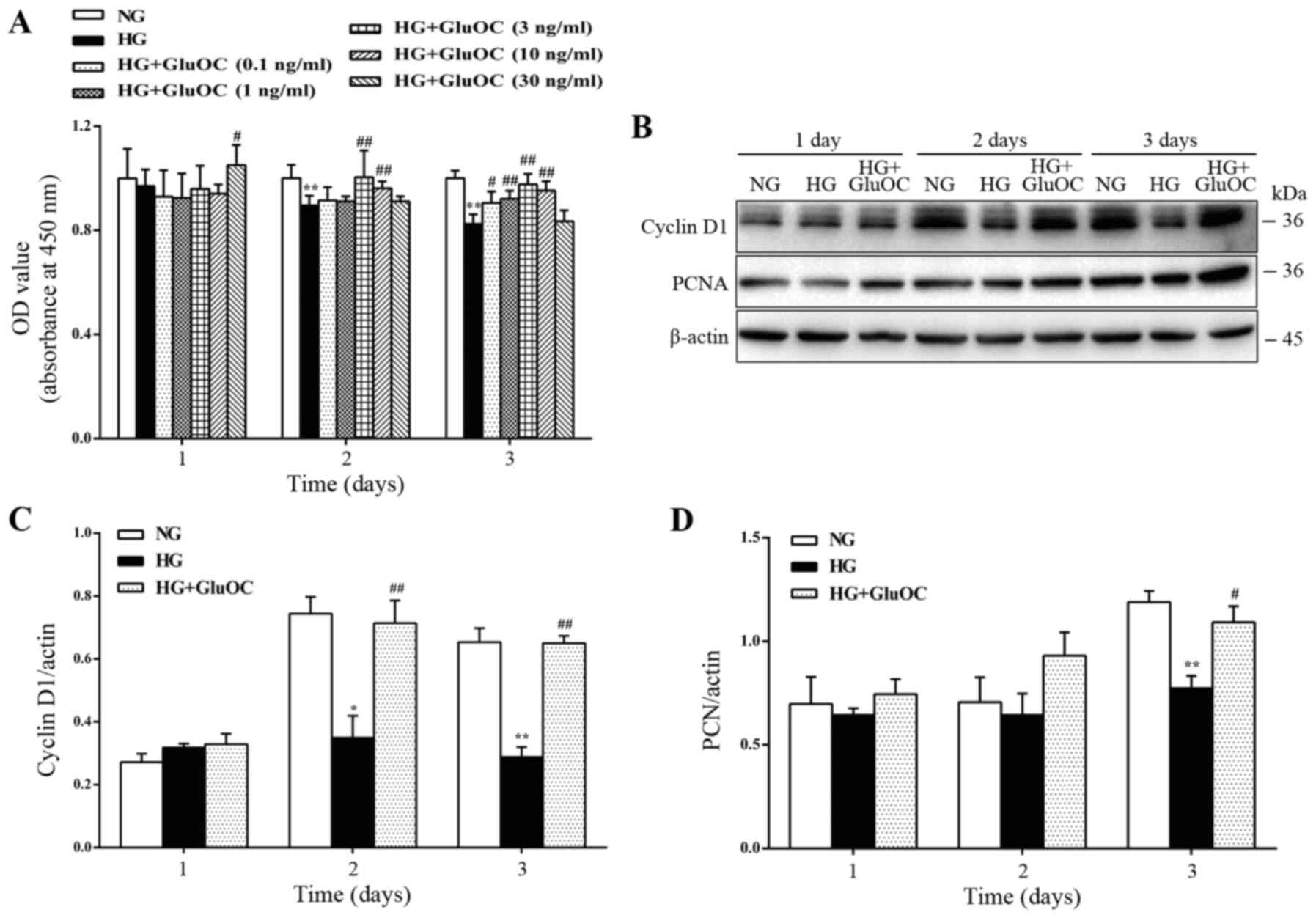
GluOC promotes the proliferation of
MC3T3E1 cells under high glucose conditions
First, the MC3T3E1 cells were treated with high
glucose or various concentrations of GluOC (0.1, 1, 3, 10 and 30
ng/ml) to confirm whether GluOC promotes the proliferation of
MC3T3E1 cells under high glucose conditions. High glucose inhibited
cell proliferation, whereas treatment with 3 ng/ml GluOC
significantly alleviated the inhibitory effects of high glucose on
the proliferation of the MC3T3E1 cells (Fig. 1A). Thus, in all subsequent
experiments, 3 ng/ml GluOC was used as the working dose to treat
the MC3T3E1 cells. Similarly, the expression levels of cyclin D1
and PCNA were reduced by high glucose, whereas the addition of
GluOC reversed the inhibitory effects of high glucose on cyclin D1
and PCNA expression (Fig. 1B-D).
These results suggest that GluOC reversed the high glucose-induced
inhibition of the proliferation of MC3T3E1 cells.
GluOC promotes the osteogenic
differentiation and inhibits the adipogenic differentiation of
MC3T3E1 cells under high glucose conditions GluOC promotes the
mineralization and inhibits the high glucose-induced formation of
lipid droplets in MC3T3E1 cells
It has been reported that MC3T3E1 cells generate
calcium nodules during mineralization, and these calcium nodules
are considered as an osteoblastic parameter (28). Alizarin Red S staining of calcium
nodules indicated that cells treated with GluOC generated
significantly more calcium nodules, whereas fewer calcium nodules
were observed in the high glucose group (Fig. 2A). Fewer lipid droplets stained
by Oil Red O were observed in the GluOC-treated cells (Fig. 2B). GluOC did not affect the cells
in the normal glucose group. Both of the images and the results of
quantitative analysis indicated that GluOC promoted the
mineralization and inhibited the high glucose-induced formation of
lipid droplets in MC3T3E1 cells.
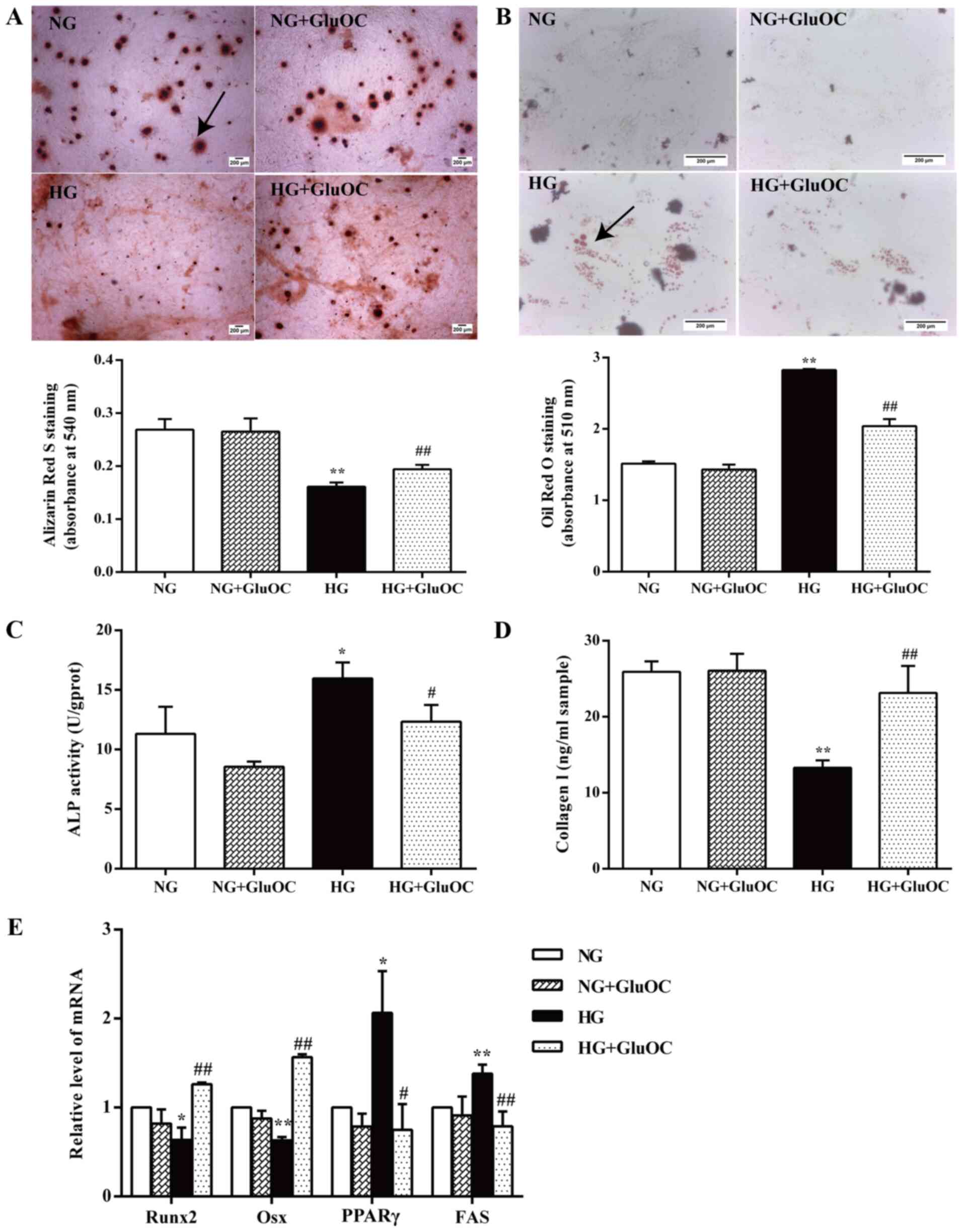 | Figure 2GluOC promotes the osteogenic
differentiation and inhibits the adipogenic differentiation of
MC3T3E1 cells under high glucose conditions. Cells were cultured in
inductive α-MEM containing 5.5 mM glucose (NG group) or 25.5 mM
glucose (HG group), with or without 3 ng/ml GluOC (NG + GluOC group
and HG + GluOC group, respectively). (A) Representative images of
Alizarin Red S staining of calcium nodules and quantitative
analysis. Scale bar, 200 μm. (B) Representative images of
Oil Red O staining images of lipid droplets and the quantitative
analysis. Scale bar, 200 μm. (C) ALP activity and (D) COLI
expression in the different groups. (E) Expression of the
osteogenic-specific genes (Runx2 and Osx), as well as the
adipogenic-specific genes (PPARγ and FAS) in MC3T3E1 cells in the
different groups. *P<0.05, **P<0.01 vs.
NG group; #P<0.05, ##P<0.01 vs. HG
group. GluOC, uncarboxylated osteocalcin; COLI, type I collagen;
Runx2, Runt-related transcription factor 2; Osx, osterix; PPARγ,
peroxisome proliferator-activated receptor γ; NG, normal glucose;
HG, high glucose. |
GluOC promotes the expression of COLI and
inhibits high glucose-induced ALP activity in MC3T3E1 cells
Osteoblasts express various phenotypic markers
including ALP and collagenous bone matrix protein-COLI (29,30). Thus, ALP activity and the COLI
secretion of MC3T3E1 cells cultured with or without GluOC for 7
days under high glucose conditions were examined in the present
study. As shown in Fig. 2C, high
glucose upregulated ALP activity, whereas treatment with GluOC
reversed this effect. Conversely, an increased COLI production was
observed in the cells treated with GluOC (Fig. 2D). GluOC did not affect the cells
in the normal glucose group. These findings suggested that GluOC
increased COLI expression and decreased the high glucose-induced
ALP activity in MC3T3E1 cells.
GluOC promotes the expression of
osteogenic genes and inhibits the high glucose-induced expression
of adipogenic genes in MC3T3E1 cells
RT-qPCR was used to further confirm the effects of
GluOC on the expression of relevant genes. As shown in Fig. 2E, the gene expression levels of
the osteogenic markers (Runx2 and Osx) in the high glucose group
were significantly decreased, whereas the expression of adipogenic
genes (PPARγ and FAS) was increased. The effects of high glucose
were reversed by GluOC; however, GluOC did not affect the cells in
the normal glucose group. These results demonstrated that GluOC
significantly affected the upregulation of osteogenic gene
expression and downregulated adipogenic gene expression in MC3T3E1
cells. Thus, GluOC reversed the inhibitory effects of high glucose
on the osteogenic differentiation of MC3T3E1 cells.
GluOC reverses the high glucose-induced
inhibition of osteogenic differentiation via GPRC6A in MC3T3E1
cells GluOC increases the expression of GPRC6A in MC3T3E1
cells
The expression of GPRC6A, which was hypothesized to
be a GluOC receptor in MC3T3E1 cells, was detected to determine
whether GPRC6A is the receptor of GluOC. Confluent cells were
cultured in 6-well plates with 4% FBS and treated with high glucose
or GluOC for 6, 12 or 24 h, respectively. RT-qPCR analysis
indicated that treatment with GluOC significantly increased the
expression of GPRC6A in MC3T3E1 cells (Fig. 3A).
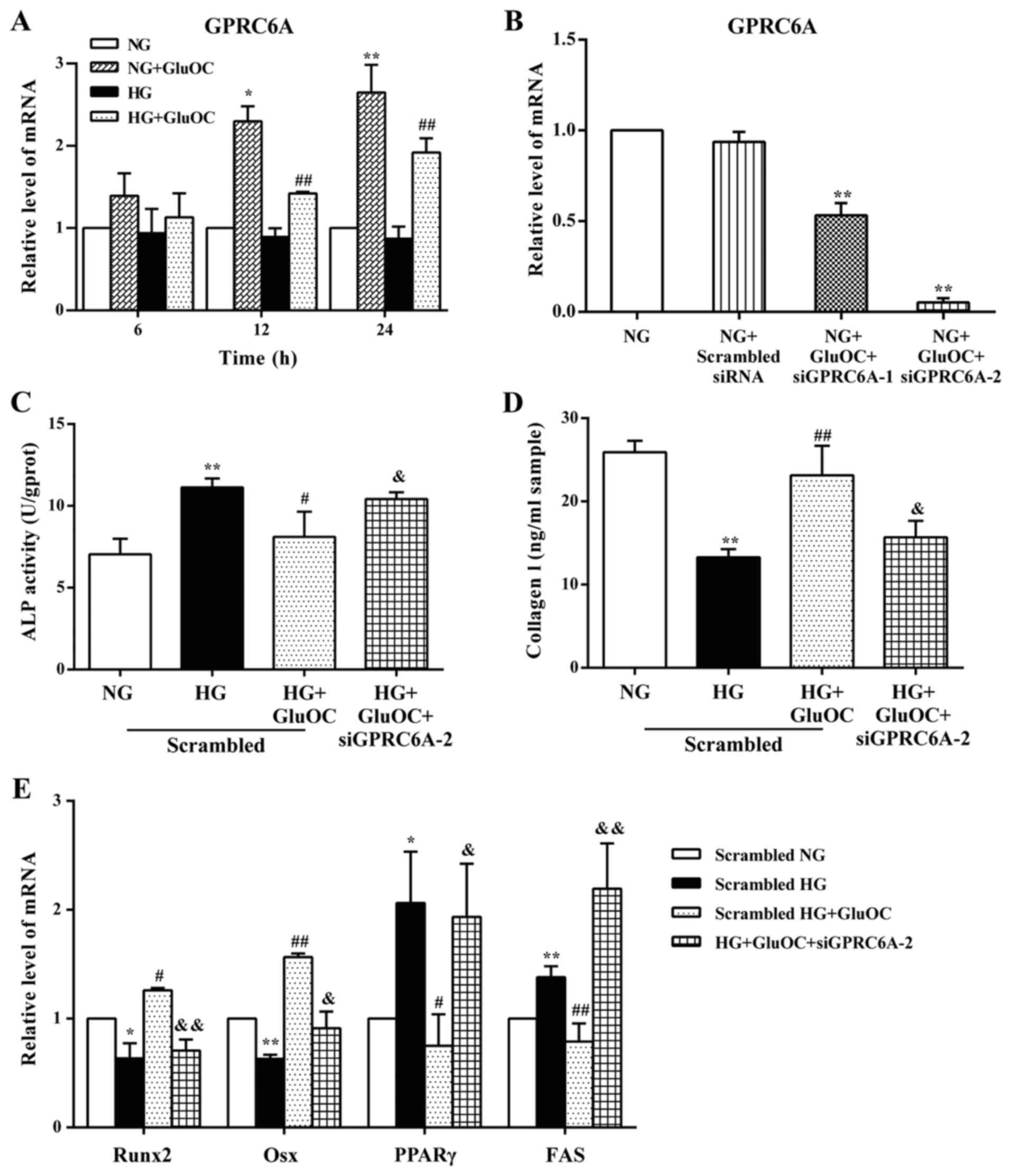 | Figure 3GPRC6A functions as a receptor of
GluOC in MC3T3E1 cells. (A) GluOC promoted the expression of
GPRC6A. The expression of GPRC6A was detected after treatment with
high glucose and GluOC for 6, 12 and 24 h. (B) GPRC6A mRNA levels
were analyzed following transfection with GPCR6A targeting siRNAs
after 24 h. (C-E) GPRC6A knockdown abrogated the GluOC-mediated
increase in osteogenic differentiation, and promoted adipogenic
differentiation. (C) ALP activity and (D) COLI expression was
determined in the different groups. (E) Expression of the
osteogenic-specific genes (Runx2 and Osx) and adipogenic-specific
genes (PPARγ and FAS). *P<0.05,
**P<0.01 vs. NG group; #P<0.05,
##P<0.01 vs. HG group; &P<0.05,
&&P<0.01 vs. HG + GluOC group. GPRC6A, GPCR
class C group 6 subtype A receptor; GluOC, uncarboxylated
osteocalcin; COLI, type I collagen; Runx2, Runt-related
transcription factor 2; Osx, osterix; PPARγ, peroxisome
proliferator-activated receptor γ; NG, normal glucose; HG, high
glucose. |
GPRC6A functions as a receptor of GluOC
in MC3T3E1 cells
To further confirm whether GluOC stimulates the
osteogenic differentiation of MC3T3E1 cells under high glucose
conditions via GPRC6A, siRNAs were used to knockdown GPRC6A
expression. RT-qPCR analysis indicated that GPR6CA siRNA-2
significantly reduced the expression of GPRC6A by 90% (Fig. 3B). Based on these results, GPR6CA
siRNA-2 was selected to knockdown GPRC6A expression in all
subsequent experiments. To determine the effects of GPRC6A on the
osteoblast differentiation of MC3T3E1 cells, the present study
examined the activity of ALP and the expression of COLI, as well as
the expression of the aforementioned genes (Runx2, Osx, PPARγ and
FAS) following GPRC6A knockdown. The promoting effects of GluOC on
the osteogenic differentiation of MC3T3E1 cells were reversed by
the knockdown of GPRC6A (the knockdown of GPRC6A increased ALP
activity, decreased COLI production, decreased Runx2 and Osx
expression, and increased PPARγ and FAS expression), and the
results were similar to those in the HG group (Fig. 3C-E). Taken together, these
results suggest that GPRC6A functions as a receptor of GluOC in
MC3T3E1 cells.
GluOC reverses the high glucose-induced
inhibition of osteogenic differentiation via the
GPRC6A/cAMP/PKA/AMPK signaling pathway in MC3T3E1 cells GluOC
promotes the osteogenic differentiation of MC3T3E1 cells by
activating AC through GPRC6A
The intracellular signaling pathways activated by
the GluOC-mediated activation of GPRC6A in MC3T3E1 cells were
determined. The results of western blot analysis revealed that
GluOC reversed the high glucose-induced decrease in the
phosphorylation of PKA and AMPK, and that this effect was abrogated
by the knockdown of GPR6CA or SQ22536, an AC inhibitor.
Additionally, it was confirmed that the addition of U73122, a PLC
inhibitor, did not affect the effects of GluOC on PKA and AMPK
phosphorylation under high glucose conditions (Fig. 4A-F). These findings suggest that
GluOC activated downstream PKA and AMPK through GPRC6A-AC rather
than GPRC6A-PLC. As shown in Fig.
4G, GluOC increased the intracellular cAMP concentrations,
reversing the decrease observed in the HG group; the addition of
SQ22536 attenuated this effect, suggesting that GluOC promoted the
production of cAMP through AC. Additionally, SQ22536 reversed the
increase in the expression of COLI, Runx2 and Osx induced by GluOC,
whereas ALP activity, and the expression of PPARγ and FAS were
significantly increased under high glucose conditions. U73122, a
PLC inhibitor, did not affect the effects of GluOC under high
glucose conditions (Fig. 4H-J).
These findings suggest that the activation of AC rather than PLC
through GPRC6A is the mechanism through which GluOC reverses the
inhibitory effects of high glucose on the osteogenic
differentiation of MC3T3E1 cells.
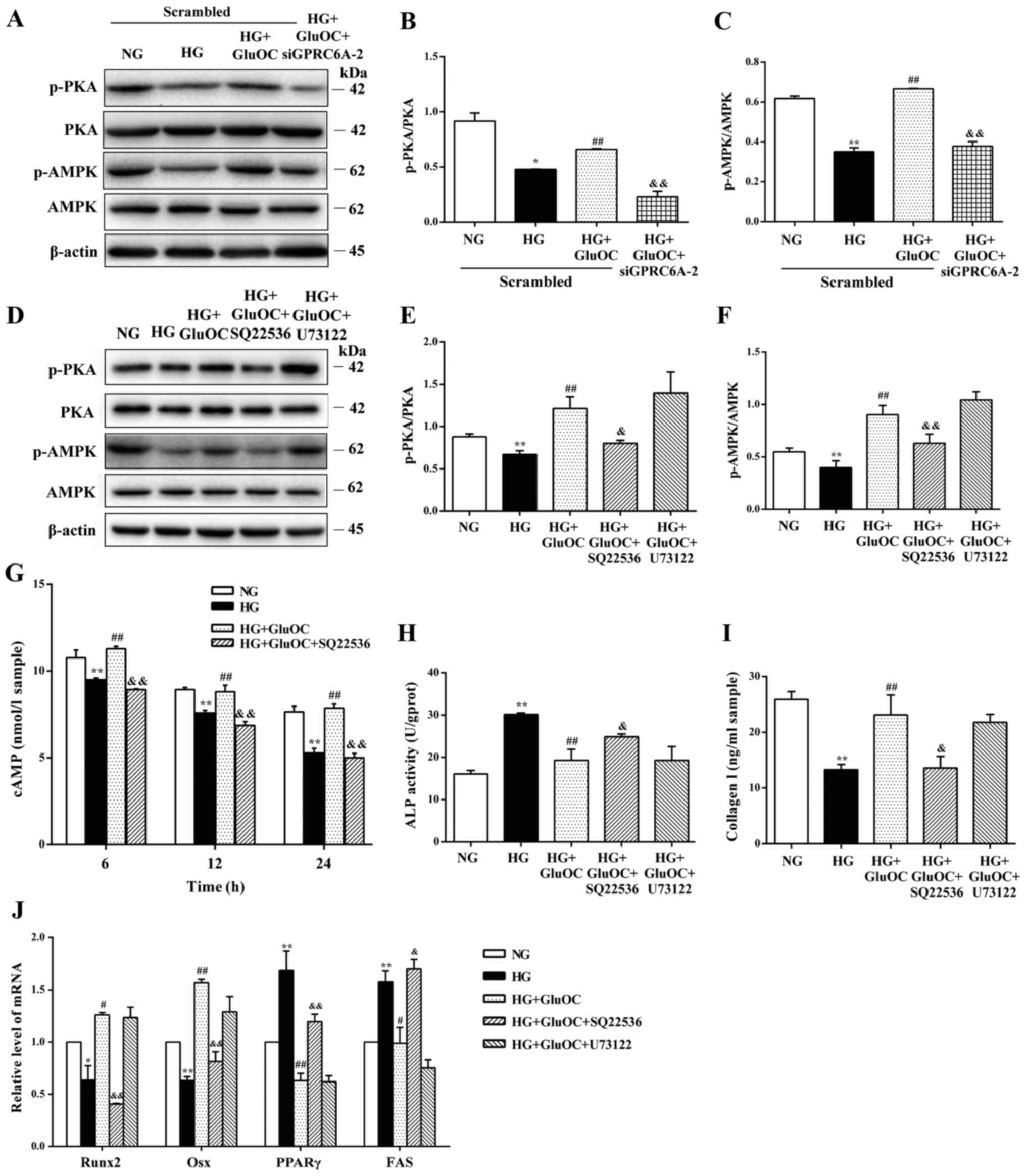 | Figure 4GluOC promotes osteogenic
differentiation of MC3T3E1 cells under high glucose conditions via
GPRC6A-AC. MC3T3-E1 cells were treated with GluOC (3 ng/ml) and a
GPCR6A targeting siRNA, SQ22536 (adenylate cyclase inhibitor), or
U73122 (phospholipase C inhibitor). (A-C) Western blot analysis of
PKA, p-PKA, AMPK and p-AMPK expression following the siRNA-mediated
knockdown of GPCR6A in cells. (D-F) Protein expression levels of
PKA, p-PKA, AMPK and p-AMPK treated with or without SQ22536 and
U73122. (G) Accumulation of intracellular cAMP following the above
treatments. (H-J) GluOC promoted osteogenic differentiation of
MC3T3E1 cells via GPRC6A-AC rather than GPRC6A-PLC. (H) ALP
activity and (I) the levels of COLI in cells following the above
treatments. (J) Expression of osteogenic-specific genes (Runx2 and
Osx) and adipogenic-specific genes (PPARγ and FAS).
*P<0.05, **P<0.01 vs. NG group;
#P<0.05, ##P<0.01 vs. HG group;
&P<0.05, &&P<0.01 vs. HG +
GluOC group. GPRC6A, GPCR class C group 6 subtype A receptor;
GluOC, uncarboxylated osteocalcin; COLI, type I collagen; Runx2,
Runt-related transcription factor 2; Osx, osterix; PPARγ,
peroxisome proliferator-activated receptor γ; PKA, protein kinase
A; AMPK, AMP-activated protein kinase; NG, normal glucose; HG, high
glucose. |
GluOC phosphorylates PKA and AMPK to
promote the osteogenic differentiation of MC3T3E1 cells
Finally, the crucial functions of PKA and AMPK on
the GluOC-mediated promotion of the osteogenic differentiation of
MC3T3E1 cells were determined. The addition of H-89 inhibited the
phosphorylation of PKA and AMPK, and treatment with BML-275 did not
affect the phosphorylation of PKA, but only inhibited the
phosphorylation of AMPK, suggesting that AMPK was a downstream
factor of GPRC6A/cAMP/PKA (Fig.
5A-C). As shown in Fig.
5D-F, the expression of COLI, Runx2 and Osx induced by GluOC
was suppressed by H-89 and BML-275, whereas ALP activity, and the
expression of PPARγ and FAS were significantly increased similar to
the results in the HG group. These results demonstrated that the
sequential activation of PKA and AMPK was the mechanism through
which GluOC reversed the inhibitory effects of high glucose on the
osteogenic differentiation of MC3T3E1 cells. Taken together, GluOC
activated AC and PKA through GPRC6A, and the effects of GluOC were
AMPK phosphorylation-dependent, which further promoted the
osteogenic differentiation and inhibited the adipogenic
differentiation of MC3T3E1 cells under high glucose conditions
(Fig. 6).
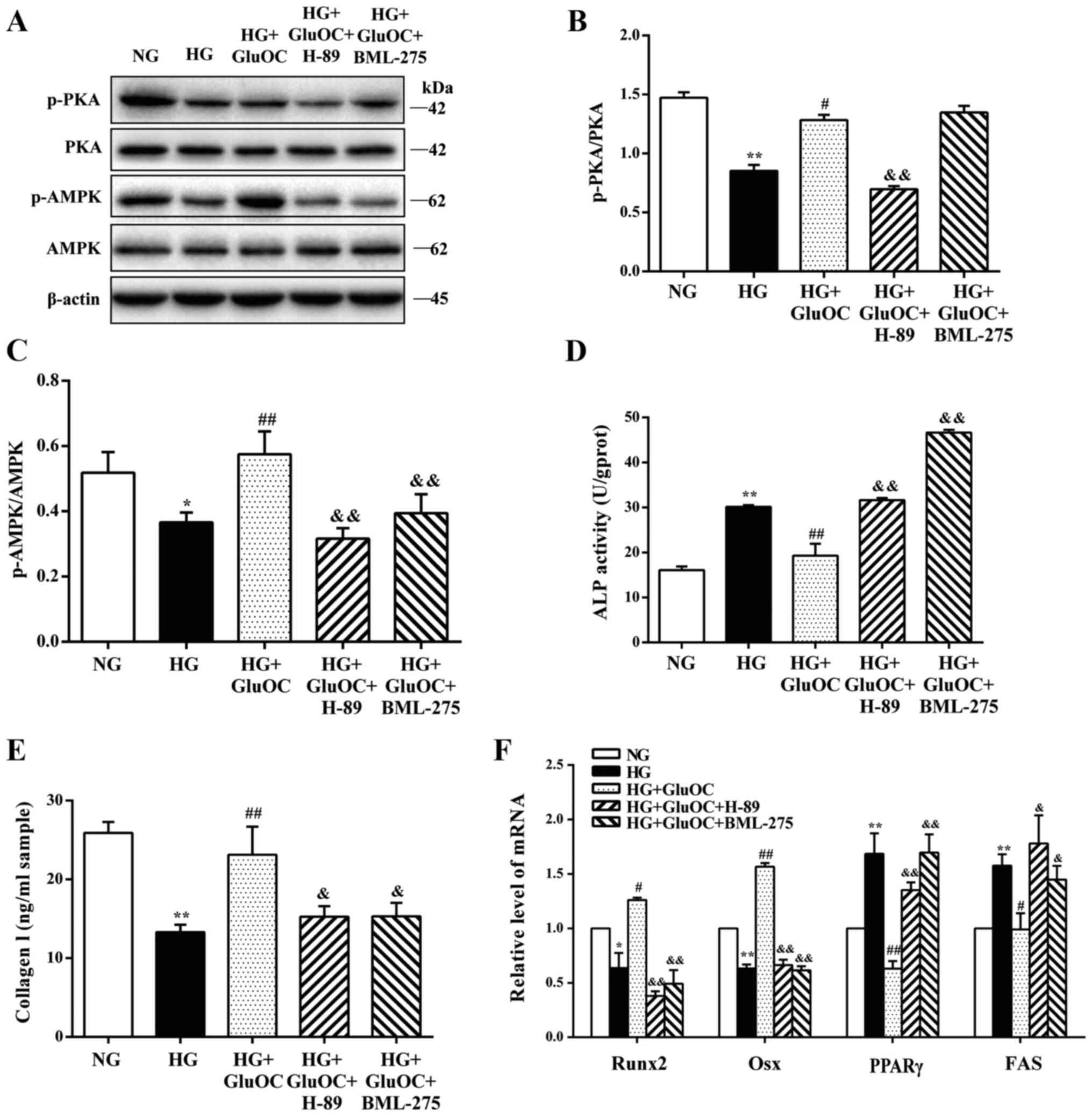 | Figure 5GluOC promotes the osteogenic
differentiation of MC3T3E1 cells under high glucose conditions via
the PKA/AMPK signaling pathway. MC3T3-E1 cells were treated with
GluOC (3 ng/ml) combined with H-89 or BML-275. (A-C) Western blot
analysis of PKA, p-PKA, AMPK and p-AMPK expression. (D-F) GluOC
promoted the osteogenic differentiation of MC3T3E1 cells via the
PKA/AMPK signaling pathway. (D) ALP activity and (E) the levels of
COLI in cells following the above treatments. (F) Expression of
osteogenic-specific genes (Runx2 and Osx) and adipogenic-specific
genes (PPARγ and FAS). *P<0.05,
**P<0.01 vs. NG group; #P<0.05,
##P<0.01 vs. HG group; &P<0.05,
&&P<0.01 vs. HG + GluOC group. GPRC6A, GPCR
class C group 6 subtype A receptor; GluOC, uncarboxylated
osteocalcin; COLI, type I collagen; Runx2, Runt-related
transcription factor 2; Osx, osterix; PPARγ, peroxisome
proliferator-activated receptor γ; PKA, protein kinase A; AMPK,
AMP-activated protein kinase; NG, normal glucose; HG, high
glucose. |
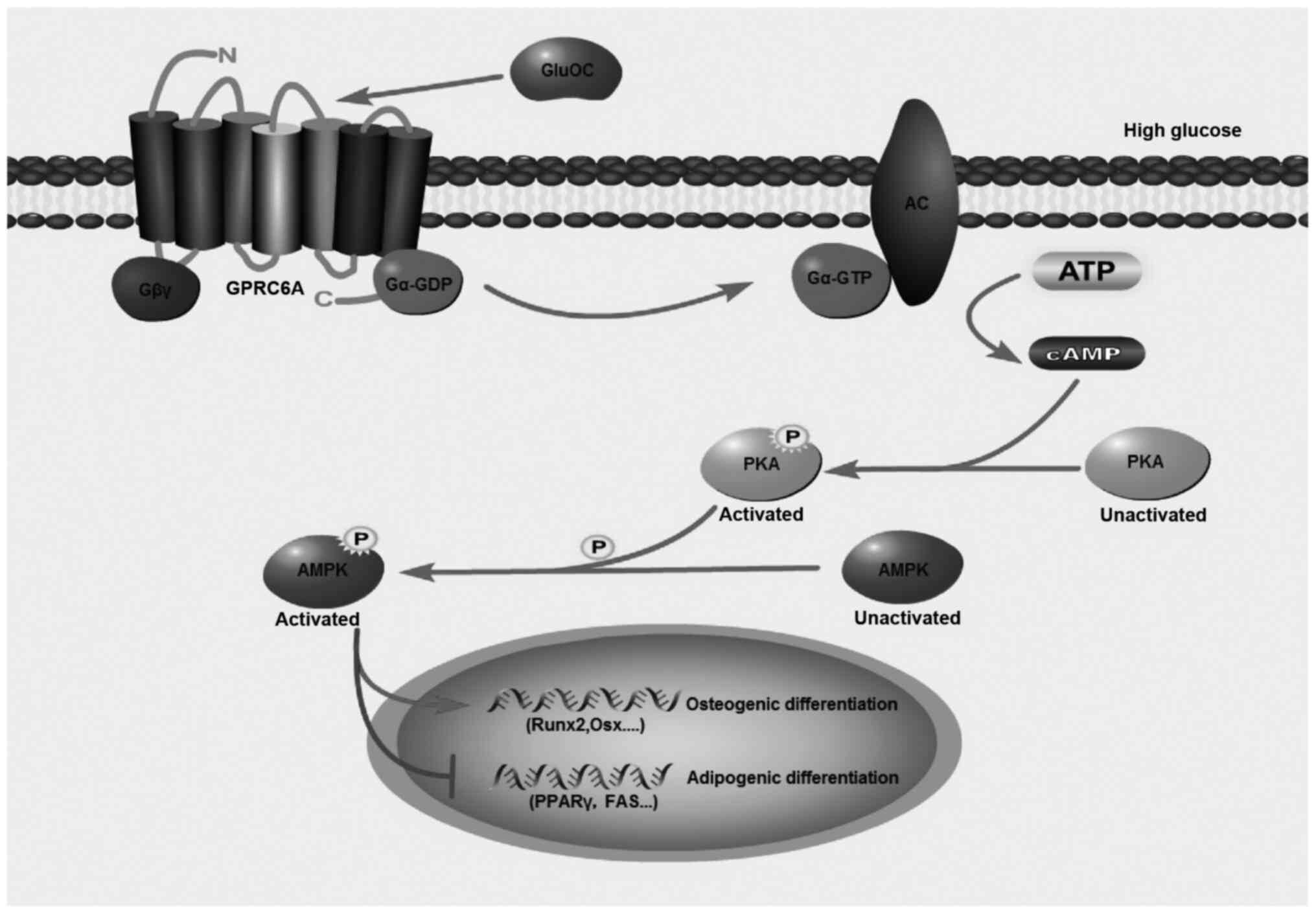 | Figure 6Model for the effects of GluOC on the
osteogenic differentiation of MC3T3E1 cells under high glucose
conditions. GluOC activates AC and PKA, and the effects of GluOC
are AMPK phosphorylation-dependent, which further promotes
osteogenic differentiation and inhibits adipogenic differentiation
in MC3T3E1 cells under high glucose conditions. GPRC6A, GPCR class
C group 6 subtype A receptor; GluOC, uncarboxylated osteocalcin;
COLI, type I collagen; Runx2, Runt-related transcription factor 2;
Osx, osterix; PPARγ, peroxisome proliferator-activated receptor γ;
PKA, protein kinase A; AMPK, AMP-activated protein kinase. |
Discussion
In the present study, it was found that GluOC
reversed high glucose-induced inhibition of osteogenic
differentiation of MC3T3E1 cells via the GPRC6A/cAMP/PKA/AMPK
signaling pathway, manifested by an increase in COLI protein
expression and a decrease in ALP activity, as well as the increased
expression of osteoblast-specific genes (Runx2 and Osx), and the
decreased expression of adipogenic genes (PPARγ and FAS). The
results of the present study provide novel insight into the role of
GluOC and the molecular mechanisms underlying the inhibition of the
high glucose-induced inhibition of osteogenic differentiation of
MC3T3E1 cells.
Diabetic osteoporosis is a severe complication of
bones in patients with diabetes, and is becoming an ever-increasing
public health concern (31). In
a previous study by the authors, it was demonstrated that high
glucose, a major cause of diabetic osteoporosis, resulted in
abnormal structural changes and a decrease in bone density,
specifically of the bone trabecula in KK-Ay mice (32). Additionally, in the present
study, it was confirmed that high glucose exerted an inhibitory
effect on the osteoblastic differentiation of MC3T3E1 cells. Thus,
the identifying factors that inhibit the adipogenic differentiation
of osteoblasts induced by high glucose has potentially wide
prospects for the prevention of diabetic osteoporosis.
In recent years, bone has been considered as not
only a structural organ, but also as an endocrine organ that can
secrete protein factors into the circulation (33). GluOC, the active form of OC, is
crucial for the regulation of energy metabolism and glucose
metabolism (34,35). GluOC relieves diabetes by
increasing insulin synthesis and secretion. GluOC can also act on
adipocytes and Leydig cells to promote the production of
adiponectin and testosterone, respectively (36,37). Studies have demonstrated that
GluOC functions by binding to its putative receptor, GPRC6A. GPRC6A
is a relatively recently discovered GluOC receptor. A growing
number of studies have confirmed that GPRC6A is expressed by β
cells, and GluOC directly activates GPRC6A to regulate β-cell
proliferation and insulin secretion (38,39). GluOC activates GPRC6A to
stimulate mammalian target of rapamycin (mTOR) phosphorylation in
myocytes (40) and stimulates
Leydig cells to produce vitamin D via the activation of GPRC6A
(41). In a previous study, the
authors demonstrated that GluOC ameliorated hepatic glucose and
lipid metabolism in KK-Ay mice (42). Moreover, GluOC has been shown to
exert a positive effect on the osteogenic differentiation of BMSCs
(43). The conclusion that GluOC
promotes the osteogenic differentiation of MC3T3E1 cells was
confirmed in the present study. However, the receptor of GluOC in
osteoblasts has not yet been identified. To the best of our
knowledge, the present study demonstrated, for the first time, that
GPRC6A functioned as a receptor of GluOC in MC3T3E1 cells. GluOC
promoted the expression of GPRC6A, irrespective of the glucose
concentration. Following transfection with GPR6CA siRNA, the
inhibitory effects of GluOC on ALP activity and adipogenic gene
expression were abrogated, as well as the effect on the increase in
COLI and osteogenic gene expression. These results demonstrated
that GPRC6A functioned as a receptor of GluOC in MC3T3E1 cells.
The signaling pathways that are mediated via GPRC6A
vary. It has been shown that GluOC activates GPCR6A, thereby
increasing the expression of testosterone via the cAMP/CREB pathway
(44). GluOC binding to GPRC6A
also increases the cAMP concentrations and consequently, PKA
phosphorylation in 3T3L1 adipocytes, thereby promoting the
production of adiponectin (5).
In the present study, it was found that GluOC subsequently
activated the GPRC6A/cAMP/PKA/AMPK signaling pathway to reverse the
high glucose-induced inhibition of osteoblastic differentiation of
MC3T3E1 cells. As shown in Fig.
4, the addition of SQ22536, an inhibitor of AC, reduced the
phosphorylation levels of PKA and AMPK compared with the GluOC
group, intracellular CAMP accumulation and the expression of
osteogenic-specific markers decreased and the expression of
adipogenic-specific markers increased. However, the addition of the
PLC inhibitor, U73122, did not affect the results, indicating that
GluOC activated AC, rather than PLC after binding to GPRC6A, and
that AC mediated the high glucose-induced inhibition of
osteoblastic differentiation of MC3T3E1 cells. Blocking the
cAMP/PKA/AMPK signal transduction pathway with H-89 or BML-275 also
abrogated the effects of GluOC on osteoblastic differentiation, as
evidenced by the increased expression of adipogenic gene expression
and the increased activity of ALP, as well as the reduced
production of COLI and reduced osteogenic gene expression. The
above-mentioned results suggest that the GPRC6A/cAMP/PKA/AMPK
signaling pathway was responsible for GluOC mediated inhibition of
the high glucose-induced inhibition of osteoblastic differentiation
of MC3T3E1 cells.
In conclusion, to the best of our knowledge, the
present study is the first to demonstrate that GluOC reverses high
glucose-induced inhibition of osteogenic differentiation by
activating the GPRC6A/cAMP/PKA/AMPK signaling pathway in MC3T3E1
cells (Fig. 6). These findings
further clarify the pathogenesis of diabetic osteoporosis at the
cellular level, indicating that the GluOC/GPRC6A signaling pathway
may potentially serve as a therapeutic target for the prevention or
treatment of diabetic osteoporosis.
Availability of data and materials
All analyses and results obtained from the current
experiments are included in the present study.
Authors' contributions
All authors (LM, FG, JX and JY) contributed to the
conception and design of the entire study. FG and JX performed an
abundant search of relevant literature on osteocalcin. LM performed
the experiments and wrote the manuscript. LM and JY were
responsible for modifying the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they no competing
interests.
Acknowledgements
Not applicable.
Funding
No funding was received.
References
|
1
|
Khrimian L, Obri A, Ramos-Brossier M,
Rousseaud A, Moriceau S, Nicot AS, Mera P, Kosmidis S, Karnavas T,
Saudou F, et al: Gpr158 mediates osteocalcin's regulation of
cognition. J Exp Med. 214:2859–2873. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen Y, Li J, Liao J, Hu Y, Zhang H, Yang
X, Wang Q, Mo Z and Cheng J: Potential protective effect of
osteocalcin in middle-aged men with erectile dysfunction: Evidence
from the FAMHES project. Sci Rep. 8:67212018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liang Y, Tan A, Liang D, Yang X, Liao M,
Gao Y, Jiang Y, Yao Z, Lin X, Lu Z, et al: Low osteocalcin level is
a risk factor for impaired glucose metabolism in a Chinese male
population. J Diabetes Investig. 7:522–528. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lin X, Brennan-Speranza TC, Levinger I and
Yeap BB: Undercarboxylated osteocalcin: Experimental and human
evidence for a role in glucose homeostasis and muscle regulation of
insulin sensitivity. Nutrients. 10:8472018. View Article : Google Scholar :
|
|
5
|
Otani T, Matsuda M, Mizokami A, Kitagawa
N, Takeuchi H, Jimi E, Inai T and Hirata M: Osteocalcin triggers
Fas/FasL-mediated necroptosis in adipocytes via activation of p300.
Cell Death Dis. 9:11942018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fernandes TAP, Gonçalves LML and Brito
JAA: Relationships between bone turnover and energy metabolism. J
Diabetes Res. 2017:90213142017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zoch ML, Clemens TL and Riddle RC: New
insights into the biology of osteocalcin. Bone. 82:42–49. 2016.
View Article : Google Scholar
|
|
8
|
Edwards BJ: Osteoporosis risk calculators.
J Clin Densitom. 20:379–388. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Golob AL and Laya MB: Osteoporosis:
Screening, prevention, and management. Med Clin North Am.
99:587–606. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ivanova S, Vasileva L, Ivanova S, Peikova
L and Obreshkova D: Osteoporosis: Therapeutic options. Folia Med
(Plovdiv). 57:181–190. 2015. View Article : Google Scholar
|
|
11
|
Wang C, Meng H, Wang X, Zhao C, Peng J and
Wang Y: Differentiation of bone marrow mesenchymal stem cells in
osteoblasts and adipocytes and its role in treatment of
osteoporosis. Med Sci Monit. 22:226–233. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guo Q, Chen Y, Guo L, Jiang T and Lin Z:
miR-23a/b regulates the balance between osteoblast and adipocyte
differentiation in bone marrow mesenchymal stem cells. Bone Res.
4:160222016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ma R, Zhu R, Wang L, Guo Y, Liu C, Liu H,
Liu F, Li H, Li Y, Fu M and Zhang D: Diabetic osteoporosis: A
review of its traditional Chinese medicinal use and clinical and
preclinical research. Evid Based Complement Alternat Med.
2016:32183132016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Roy B: Biomolecular basis of the role of
diabetes mellitus in osteoporosis and bone fractures. World J
Diabetes. 4:101–113. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Qi J, Hu KS and Yang HL: Roles of TNF-α,
GSK-3β and RANKL in the occurrence and development of diabetic
osteoporosis. Int J Clin Exp Pathol. 8:11995–12004. 2015.
|
|
16
|
Bahrambeigi S, Yousefi B, Rahimi M and
Shafiei-Irannejad V: Metformin; an old antidiabetic drug with new
potentials in bone disorders. Biomed Pharmacother. 109:1593–1601.
2019. View Article : Google Scholar
|
|
17
|
Takagi S, Miura T, Yamashita T, Ando N,
Nakao H, Ishihara E and Ishida T: Characteristics of diabetic
osteopenia in KK-Ay diabetic mice. Biol Pharm Bull. 35:438–443.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang Y and Yang JH: Activation of the
PI3K/Akt pathway by oxidative stress mediates high glucose-induced
increase of adipogenic differentiation in primary rat osteoblasts.
J Cell Biochem. 114:2595–2602. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang W, Zhang X, Zheng J and Yang J: High
glucose stimulates adipogenic and inhibits osteogenic
differentiation in MG-63 cells through cAMP/protein kinase
A/extracellular signal-regulated kinase pathway. Mol Cell Biochem.
338:115–122. 2010. View Article : Google Scholar
|
|
20
|
Liu J and Yang J: Uncarboxylated
osteocalcin inhibits high glucose-induced ROS production and
stimulates osteoblastic differentiation by preventing the
activation of PI3K/Akt in MC3T3-E1 cells. Int J Mol Med.
37:173–181. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Clemmensen C, Smajilovic S, Wellendorph P
and Brauner-Osborne H: The GPCR, class C, group 6, subtype A
(GPRC6A) receptor: From cloning to physiological function. Br J
Pharmacol. 171:1129–1141. 2014. View Article : Google Scholar :
|
|
22
|
Pi M, Xu F, Ye R, Nishimoto SK, Kesterson
RA, Williams RW, Lu L and Quarles LD: Humanized GPRC6A(KGKY) is a
gain-of-function polymorphism in mice. Sci Rep. 10:111432020.
View Article : Google Scholar
|
|
23
|
Pi M, Nishimoto SK and Quarles LD: GPRC6A:
Jack of all metabolism (or master of none). Mol Metab. 6:185–193.
2016. View Article : Google Scholar
|
|
24
|
Pi M, Wu Y and Quarles LD: GPRC6A mediates
responses to osteocalcin in beta-cells in vitro and pancreas in
vivo. J Bone Miner Res. 26:1680–1683. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Otani T, Mizokami A, Hayashi Y, Gao J,
Mori Y, Nakamura S, Takeuchi H and Hirata M: Signaling pathway for
adiponectin expression in adipocytes by osteocalcin. Cell Signal.
27:532–544. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Karsenty G and Oury F: Regulation of male
fertility by the bone-derived hormone osteocalcin. Mol Cell
Endocrinol. 382:521–526. 2014. View Article : Google Scholar
|
|
27
|
Smajilovic S, Clemmensen C, Johansen LD,
Wellendorph P, Holst JJ, Thams PG, Ogo E and Bräuner-Osborne H: The
L-α-amino acid receptor GPRC6A is expressed in the islets of
Langerhans but is not involved in L-arginine-induced insulin
release. Amino Acids. 44:383–390. 2013. View Article : Google Scholar
|
|
28
|
Chen JH, Lin X, Bu C and Zhang X: Role of
advanced glycation end products in mobility and considerations in
possible dietary and nutritional intervention strategies. Nutr
Metab (Lond). 15:722018. View Article : Google Scholar
|
|
29
|
Deng A, Zhang H, Hu M, Liu S, Gao Q, Wang
Y and Guo C: Knockdown of Indian hedgehog protein induces an
inhibition of cell growth and differentiation in osteoblast
MC3T3-E1 cells. Mol Med Rep. 16:7987–7992. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Smilic TN, Novakovic TR,
Markovic-Jovanovic SR, Smilic LLJ, Mitic JS and Radunovic ML: The
relevance of osteoclastic and osteoblastic activity markers
follow-up in patients on antiresorptive osteoporosis treatment. J
Clin Densitom. 21:322–328. 2018. View Article : Google Scholar
|
|
31
|
Khosla S and Hofbauer LC: Osteoporosis
treatment: Recent developments and ongoing challenges. Lancet
Diabetes Endocrinol. 5:898–907. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fu C, Zhang X, Ye F and Yang J: High
insulin levels in KK-Ay diabetic mice cause increased cortical bone
mass and impaired trabecular micro-structure. Int J Mol Sci.
16:8213–8226. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Verma H and Garg R: Comment on 'bone
regulates glucose metabolism as an endocrine organ through
osteocalcin'. Int J Endocrinol. 2016:97249292016. View Article : Google Scholar
|
|
34
|
Wang J, Yan DD, Hou XH, Bao YQ, Hu C,
Zhang ZL and Jia WP: Association of bone turnover markers with
glucose metabolism in Chinese population. Acta Pharmacol Sin.
38:1611–1617. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu JM, Rosen CJ, Ducy P, Kousteni S and
Karsenty G: Regulation of glucose handling by the skeleton:
Insights from mouse and human studies. Diabetes. 65:3225–3232.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mizokami A, Kawakubo-Yasukochi T and
Hirata M: Osteocalcin and its endocrine functions. Biochem
Pharmacol. 132:1–8. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tao SC and Guo SC: Extracellular vesicles
in bone: 'Dogrobbers' in the 'eternal battle field'. Cell Commun
Signal. 17:62019. View Article : Google Scholar
|
|
38
|
Pi M, Kapoor K, Ye R, Nishimoto SK, Smith
JC, Baudry J and Quarles LD: Evidence for osteocalcin binding and
activation of GPRC6A in β-cells. Endocrinology. 157:1866–1880.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dumitru N, Carsote M, Cocolos A, Petrova
E, Olaru M, Dumitrache C and Ghemigian A: The link between bone
osteocalcin and energy metabolism in a group of postmenopausal
women. Curr Health Sci J. 45:47–51. 2019.PubMed/NCBI
|
|
40
|
Ye R, Pi M, Cox JV, Nishimoto SK and
Quarles LD: CRISPR/Cas9 targeting of GPRC6A suppresses prostate
cancer tumorigenesis in a human xenograft model. J Exp Clin Cancer
Res. 36:902017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
De Toni L, De Filippis V, Tescari S,
Ferigo M, Ferlin A, Scattolini V, Avogaro A, Vettor R and Foresta
C: Uncarboxylated osteocalcin stimulates 25-hydroxy vitamin D
production in Leydig cell line through a GPRC6a-dependent pathway.
Endocrinology. 155:4266–4274. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang XL, Wang YN, Ma LY, Liu ZS, Ye F and
Yang JH: Uncarboxylated osteocalcin ameliorates hepatic glucose and
lipid metabolism in KKAy mice via activating insulin signaling
pathway. Acta Pharmacol Sin. 41:383–393. 2020. View Article : Google Scholar :
|
|
43
|
Liu Z and Yang J: Uncarboxylated
osteocalcin promotes osteogenic differentiation of mouse bone
marrow-derived mesenchymal stem cells by activating the
Erk-Smad/β-catenin signalling pathways. Cell Biochem Funct.
38:87–96. 2020. View Article : Google Scholar
|
|
44
|
Camerino C, Conte E, Cannone M, Caloiero
R, Fonzino A and Tricarico D: Nerve growth factor, brain-derived
neurotrophic factor and osteocalcin gene relationship in energy
regulation, bone homeostasis and reproductive organs analyzed by
mRNA quantitative evaluation and linear correlation analysis. Front
Physiol. 7:4562016. View Article : Google Scholar : PubMed/NCBI
|