1. Introduction
Interleukin-6 (IL-6) was first identified as a
factor derived from T-helper type 2 (Th2) lymphocytes >40 years
ago (1). On the basis of the
biological abilities of IL-6 to stimulate B-cell differentiation,
the interleukin was categorised among the B-cell stimulating
factors (BSFs) and B-cell differentiation factors (BCDFs) (2). As a member of the BSFs, IL-6 was
named BSF-2 and grouped together with BSF1 and interleukin-4
(2). IL-6 was included in the
group of BCDFs due to its capacity to stimulate the secretion of
IgM and IgG in B cells (3).
After the nomenclature meeting held in New York at the end of 1988,
BCDF/BSF-2 was finally referred to as IL-6 (4), as the biochemical properties of
this factor showed an isoelectric point between 5 and 6 (2).
Over the last 40 years, several molecular features
of IL-6 have been identified. Furthermore, new abilities of IL-6
have prompted its use as a target in medical practice for infective
and cancerous diseases, including COVID-19. The present review
highlights the current knowledge on the molecular and nanomolecular
structures involved in active IL-6 signalling. By examining both
inflammatory and cognitive IL-6 models, new properties of the IL-6
cytokine have been evaluated. Specifically, the cytological and
histological locations of IL-6 signalling have been analysed
together with serum concentrations of IL-6 in order to distinguish
between the classic and trans-signalling IL-6 pathways.
2. Three-dimensional shapes of chains
involved in nanomolecular IL-6 signalling
Molecular analysis reveals that the human IL-6 gene
is localized on the short arm of chromosome 7 (5). Depending on the genetic approach,
IL-6 has been mapped to either the p21 or p15.3 region of the
chromosome (6). By expanding
from 22,725,889 to 22,732,002 base pairs, four introns and five
exons were cloned in both human and mouse IL-6 genes (https://ghr.nlm.nih.gov/gene/IL6#location) (7). A polymorphic locus, Rs1800796, has
been identified in the IL-6 promoter region (8). The recognition of genetic mutations
in this genomic sequence has been used to assess human cancer risk
(8).
In the 212-amino acid human IL-6 glycoprotein, 28
amino acids are linked to peptide signalling (https://www.genecards.org). The molecular mass of IL-6
is 23,718 Da, ranging between 21 and 30 kDa (https://www.genecards.org) (9-11).
The IL-6 topology is composed of a secondary
structure that includes helicoidal motives related to four long
α-chains. Via a bundle model, these α-chains are structured in
three-dimensions to achieve the tertiary shape (9,12-14).
The bundle helicoidal complex is also observed also
in a number of human cytokines such as IL-11, oncostatin M (OSM),
ciliary neurotrophic (CNTF), leukemic inhibitory (LIF),
cardiotrophin-like factor 1 (CT-1), erythropoietin, granulocyte
colony-stimulating factor, IL-12, growth hormone, prolactin, IL-10,
interferon and leptin (12).
However, despite these factors adopting structures similar to the
IL-6 bundle prototype model, they show little identity with the
IL-6 amino acid sequence (12).
By contrast, a viral homolog to the human IL-6 protein has been
identified in herpesvirus 8 associated with Kaposi's sarcoma
(15). This amino acid sequence
is capable of arranging itself in a bundle helicoidal structure.
This association is the reason that the viral protein is named
viral IL-6 (15).
IL-6 works through interaction with the complex
peptide IL-6 receptor (IL-6R). This receptor includes two
transmembrane glycoproteins referred to as the IL-6Rα and
gp130/IL6ß (gp130) subunits (16,17). Two different active genes
contribute to the generation of human IL-6R.
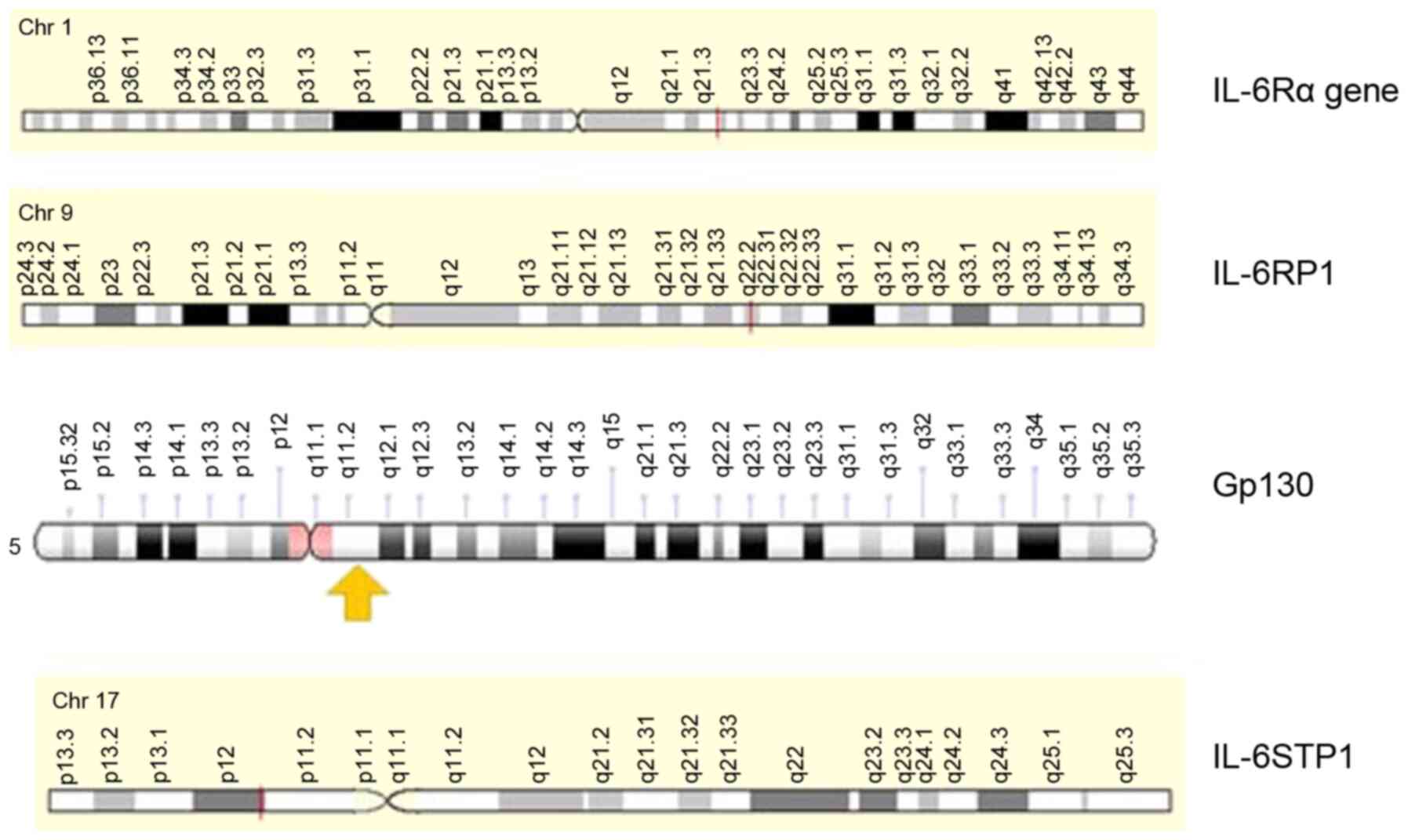
Originally, the IL-6Rα gene was mapped on the long
arm of chromosome 1 in the q21.3 cytoband (18) (Fig. 1). By counting 13 exons, this was
cloned in 64,258 bases (https://www.
genecards.org). The gene that codes for Gp130 is located on
chromosome 5 at band q11 (19)
(Fig. 1).
The IL-6Rα transcript encodes a modular glycoprotein
made up of one α-chain with a size of 80 kDa, also known as IL6Q,
gp80, CD126, IL6RA, IL6RQ, IL-6RA or IL-6R-1 (18). Specifically, IL-6Rα acts by
binding the IL-6 ligand; however, this activity is not enough to
transduce any signal (18). By
contrast, the gp130 glycoprotein chain is unable to directly bind
IL-6, but is capable of IL-6 signal transduction (19).
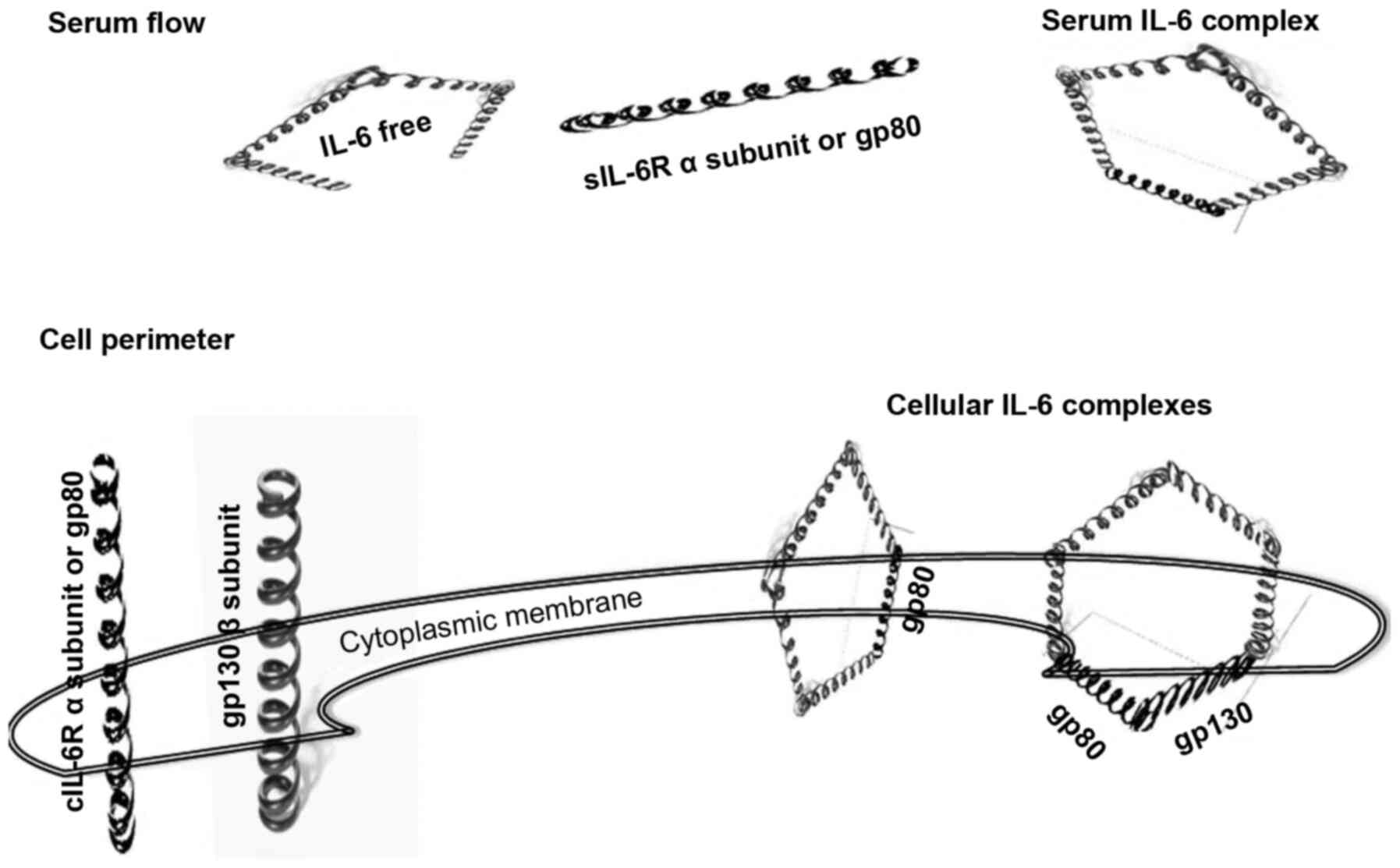
The assembly of IL-6 with its respective receptors
occurs through a unique two-phase process. First, the four α-chains
of IL-6 capture the α-chain of IL-6R with a dissociation constant
(Kd) of ~1 Nm (20) (Fig. 2). In this stage, IL-6, composed
of a dimeric structure, does not perform any signalling activity
(21,22). The next assembly step is the
construction of a hexameric cluster, where the complex of five
α-chains binds gp130 with a Kd of ~10 pM (17,20) (Fig. 2). The previous binding of IL-6
with IL-6R occurs with lower affinity than that with the complex
and gp130. In this second stage, the IL-6 complex is composed of a
trimeric structure that, like in the first step, does not perform
any signalling. To begin signalling, the IL-6/IL-6Rα/gp130 trimer
proceeds through a homodimerization process that forms a hexameric
complex (22).
These data suggest that the transition from low to
high affinity IL-6 binding occurs due to phenomena pertaining to
nanoparticle (NP) spheres. In fact, the geometric shape of the
complex becomes crucial for the efficiency of binding at the
nanometre scale (21,23). The five α-chains of the
IL-6/IL-6Rα complex are only suitable for binding in a pentameric
orientation. The hexamer is the competent form for energetic
transition leading to the dimerization of the IL-6/IL-6Rα/gp130
cluster. Notably, the pentameric complex of α-chains, corresponding
with the dimeric form of the IL-6/IL-6Rα structure, may appear
either in the serum or in cellular compartments (Fig. 2). By contrast, the clusters of
IL-6/IL-6Rα/gp130 are closed in cellular structures. The
IL-6/IL-6Rα/gp130 complex is found in both the non-signalling
trimer and signalling hexamer forms (Fig. 2) (23).
The trimeric model of IL-6 signalling is replicated
in several other cytokines, including IL-11, LIF, OSM, CNTF and
CT-1. This is due to their ability to bind gp130 on the cellular
surface to elicit signal transduction (24). For these reasons, the
physiological responses of these cytokines could occur
simultaneously. These cytokines have been included in the group of
L-6-type cytokine receptor mediators (24-26).
There are two main ideas that are inspired by the
assessment of the nanomolecular structure of IL-6 signalling:
Firstly, the nanomolecular shapes of the IL-6 system are largely
independent of genetic composition. Therefore, genetic
investigation has to be associated with nanomolecular evidence to
completely track the physiological and pathological signals of
IL-6. Secondly, the efficacy of IL-6 therapeutic targets is also
dependent on the geometric shape of IL-6 signalling structures.
Therefore, prior to determining the clinical benefits of IL-6
therapy, the nanomolecular conformations of IL-6 signalling have to
be estimated.
3. Genes, pseudogenes and competitive
endogenous RNA (ceRNA) for molecular control of IL-6
signalling
In contrast to the parental genes composing IL-6R, a
distinct pseudogene was demonstrated for IL-6Rα and the gp130 gene
(https://www.genecards.org) (19) (Fig. 2). These pseudogenes share much of
their sequences with their corresponding parental genes (19). Conversely, pseudogene transcripts
are equivalent to non-coding RNA or to antisense RNA and therefore
are unable to produce biologically active proteins (27).
The IL-6Rα pseudogene, IL-6RP1, is found on the long
arm of chromosome 9 at the locus q22.2 (Fig. 2). A repetition of gp130 sequence,
IL-6STP1, is detected on the short arm of chromosome 17 and
assigned at the p11 region (Fig.
2); this corresponds with a gp130 non-transcribed pseudogene
(19).
IL6-STP1 transcripts have been shown to be involved
in microbial defence processes (28). Via activation of the IL-6 family
cytokines, IL6-STP1 stimulates inflammatory cells to secrete
acute-phase proteins (APP), including fibrinogen, a1-antitrypsin
and hepcidin (28). In a mouse
polymicrobial infection model, removal of IL-6STP1 caused
inhibition of APP production associated with the dysregulation of
the inflammatory response and an increase in mortality (28).
Pseudogene RNAs are part of an intricate network of
competitive endogenous RNAs (ceRNAs) where all non-coding RNAs are
assigned to two large groups: microRNAs (miRNAs) and long
non-coding RNAs (27,29,30). miRNAs have <200 nucleotides,
while long non-coding RNAs are >200 molecular bases. However, by
using the same code, the full set of ceRNAs competes with parental
mRNA (31).
ceRNAs absorb mRNA as a 'sponge' to dynamically
balance mRNA levels for protein transcription efficiency (27,32). As a consequence, the ceRNA matrix
serves to regulate protein expression (27,32). It is likely that ceRNAs mimic an
ancient anti-viral defence biomechanism that appeared during the
evolutionary scale of eukaryotic species such as those in plants
(33). For these reasons, ceRNAs
are the cornerstone of recent molecular strategies that use
'silencing' genes to test cancerous biological aggressiveness, as
well as to develop innovative molecular therapeutic approaches for
cancer (27,34-37).
Data on the role of miRNA in the
post-transcriptional regulation of IL-6 expression are gradually
increasing (38). In line with
the algorithms of miRanda, MicroCosm v5 and the TargetScan v7.1
database (http://multimir.org), 15 miRNAs profiles
have been recorded to have potential involvement with IL-6
expression (38,39) (Table I).
 | Table IData source for mioRNA profiles
involved in the regulation of interleukin 6 expression. |
Table I
Data source for mioRNA profiles
involved in the regulation of interleukin 6 expression.
| miRNA | (Refs.) |
|---|
| hsa-let-7a | (38) |
| hsa-let-7d | (38) |
| hsa-let-7e | (38) |
| hsa-let-7f | (38) |
| hsa-let-7g | (38,39) |
| hsa-let-7i | (38) |
| hsa-miR-23a | (38) |
| hsa-miR-23b | (38) |
| hsa-miR-26a | (38) |
| hsa-miR-26b | (38) |
| hsa-miR-126 | (38) |
| hsa-miR-132 | (38) |
| hsa-miR-155 | (38) |
| hsa-miR-142-3p | (38) |
| hsa-miR-146-a | (38) |
In non-cancerous cellular models of
polymorphonuclear leukocytes (PMNs) taken from cord blood and adult
blood, post-transcriptional regulation of IL-6 expression was
demonstrated by lethal 7g (let-7g) and miR-142-3p modulation
(38). The let-7g gene has been
located on chromosome 3 at loci p21 (40,41). This is a genomic region involved
in carcinogenic processes of the lung (40,41). miR-142-3p has been associated
with colorectal cancer and has been recorded on chromosome 17 at
loci q22 (41). Let-7g and
miR-142-3p have been found to be related with IL-6 expression, as
both downregulate the production of IL-6 mRNA as well as protein
(38). The role of miR-142-3p
with regard to the endogenous expression of IL-6 has been
previously predicted by mouse models (42).
In a cancerous cellular model and in normal
controls, IL-6 overexpression was combined with decreases in
let-7c, let-7d, let-7f, let-7g and mir-98 (43). Furthermore, all of these miRNAs
were expressed in higher concentrations in the normal controls than
in the cancerous cells (43).
Finally, the let precursor let-7c exhibited a similar effect to
let-7g in normal PMNs, as let-7c downregulated the mRNA and protein
expression of IL-6 in the cancerous cells and controls (38,43).
In summary, the ceRNAs involved in IL-6 signalling
play roles in the regulation of IL-6 expression in physiological
processes and even in cancerous transformation of cells.
4. Inflammatory and cognitive models of IL-6
cytokines
Since the identification of IL-6 in stromal,
epithelial and muscle cells, new roles and functions have been
attributed to this cytokine. It is clear that IL-6 could operate
through paracrine, autocrine and endocrine mechanisms (44,45). As long as IL-6 signalling is
detected in the endocrine and nervous systems, the model of
function for the IL-6 inflammatory cytokine is changed; IL-6 may
have roles in the regulation of endocrine secretion and in nervous
impulse propagation (46,47).
IL-6 inflammatory model
IL-6 controls the inflammatory response primarily
through orchestration of pro-inflammatory and anti-inflammatory
effects (48,49). This is due to the activation of
two different IL-6 pathways. The first is known as classic
signalling, which operates in support of anti-inflammatory effects.
Gp80 and gp130 are triggered through serum-derived free IL-6 in the
cellular compartment (49). This
pathway is dependent on cellular expression of the IL-6R components
and the concentration of free IL-6 in the serum (Fig. 1) (49). The second trans-signalling
pathway, promotes pro-inflammatory activities via IL-6 (49). In the serum compartment, free
IL-6 recruits gp80 and the gp80/IL-6 complex activates cellular
gp130 (Fig. 1) (49). Therefore, during trans-signalling
with IL-6, a balance is maintained between the amount of serum
gp80/IL-6 complex and the cellular expression of gp130 (49).
Expression of IL-6 associated with cognate receptor
IL-6-Rα has been detected in cancerous and autoimmune endocrine
diseases of the thyroid such as Grave's disease (GD) and
Hashimoto's thyroiditis (HT) (50-53). In ex vivo pathological
tissue, thyroid follicular cells exhibited intracellular IL-6 and
IL-6-Rα. Greater immunoexpression of IL-6 and IL-6R were reported
in cancerous follicular cells, as well as in HT and GD cases with
high lymphoid infiltration (50-53). Simultaneous expression of
receptor and ligand was not observed in normal follicular thyroid
cells (50-53). To characterize classic and
trans-signalling IL-6 in HT patients, free IL-6 and bound gp80/IL-6
complex levels were measured in the serum (48,49). Both IL-6 pathways appeared to be
involved in the development and progression of HT. This was due to
an increase in bound gp80/IL-6 in the serum of HT patients compared
with that of healthy controls (48). Furthermore, free IL-6 was a
candidate for an early diagnostic marker for the development of
autoimmune disease in the HIV-seropositive (HIV+)
population (49). This was due
to a high concentration of free IL-6 in the serum, which was
correlated with the occurrence of autoimmune disease in
HIV+ subjects.
The inflammatory IL-6 model is frequently used to
explain IL-6 modulation of the response to toxicity of
nanomaterials (54). In
vitro and in vivo studies have underlined the
pro-inflammatory effects of several natural and synthetic NPs. Due
to their capacity for internalization, inhalation of NPs actives
several pathways that, in addition to causing apoptosis, fibrosis,
genotoxicity and tumourigenesis, also causes strong inflammation at
the lung level and beyond (55-59). Briefly, following phagocytosis by
macrophages, the NPs trigger the response of other immune cells.
The macrophages also release the inflammasome NLRP3, a multiprotein
complex whose activation is prompted by numerous different signals,
including pathogen-associated molecular patterns and
danger-associated molecular patterns (60,61). NLRP3 is activated by reactive
oxygen species (ROS) overproduction and the inflammatory cascade
continues since NLRP3, in turn, induces the expression of IL-6,
IL-1β and TNF-α genes (62).
In the central nervous system, the NP induction of
microglia activation causes the onset and progression of chronic
brain inflammation, leading to a loss of neuronal cells and an
increase in white matter abnormalities; this is associated with an
increased risk for autism spectrum disorders, a lower IQ in
children, neurodegenerative diseases, such as Parkinson's disease
(PD), Alzheimer's disease and multiple sclerosis, and strokes
(63). As demonstrated by
Visalli et al (57) in
the differentiated SH-SY5Y neuronal model, the exposure to
synthetic NPs significantly increased transcript levels of IL-6,
IL-1β and TNF-α, confirmed by the measurement of cytokine levels
detected in the cell supernatants. The role of neuroinflammation
and microglia activation in neurotoxicity was detected in an in
vivo study using cortical stereotactic injection of
carbon-based NPs into the mouse brain (64). According to Bussy et al
(65) the brain region-specific
sensitivity to NP exposure is most likely related to the number of
microglial cells in the different brain regions.
IL-6 cognitive model
Two main sources have contributed to set the
cognitive model of IL-6 cytokines: The cellular distribution and
histological localization of IL-6/IL-6R signalling in brain tissue;
and the different neurological responses to IL-6 serum
concentrations due to activation of either the classic or
trans-signalling pathway (66,67).
Normal neurons and microglia are able to secrete
IL-6 polypeptide, as well as transcribe genomic IL-6-Rα mRNA
(66). IL-6 has been implicated
in the pathogenesis and cure of PD. Environmental exposure to
pesticides also causes degeneration of dopaminergic neurons by
activation of inflammatory cytokines such as IL-6 (68,69). The alteration of dopaminergic
transmission produces a complex symptomology due to impairment of
motor and cognitive performance. Several new medicinal plant
extracts and phytochemicals, such as ellagic acid, are potentially
suitable for use to alleviate PD symptoms due of their ability to
decrease the anti-inflammatory activities of cytokines in cellular
models (70,71)
Conversely, IL-6-Rα sequences have uniquely been
detected in microglia and remain undetected in oligodendrocytes and
astrocytes (66,72). In human and rat brain tissues,
IL-6 was mainly localized in the hippocampal region (67,73,74). Several reports have associated
the grey matter of the human brain with cognitive processes such as
memory consolidation and learning, appearance of depression,
post-traumatic stress and childhood maltreatment disorders
(74-76). Under physiological conditions,
the left hippocampus showed an association between decreased grey
matter volume and an increase in serum IL-6 (74). An increase in right hippocampal
volumes involving the head, parahippocampal gyrus and dorsal parts
of the amygdala were associated with the IL-6 polymorphism
rs1800795 (76).
In neurons and oligodendrocytes, the IL-6 response
was mediated by trans-signalling (66). In microglia, the IL-6 classic and
trans-signalling pathways were observed (67). In target brain cells neurons,
IL-6 trans-signalling promoted neuronal degeneration (77,78). By contrast, the regeneration of
neural tissue was mediated through IL-6 classic signalling via the
involvement of microglia cells (66,78).
In summary, the classic and trans-signaling pathways
are the basis of two models, the inflammatory and cognitive models,
with cellular expression and serum levels of IL-6 used to
distinguish between them.
5. Role of IL-6 in COVID-19
IL-6 is a cytokine with a number of different
functions that plays a role in the host acute response to
inflammation; it modulates host defence through numerous different
mechanisms and actions directed towards monocytes, B cells and
controlling homeostasis between Th1 and Th2 activity (75-79).
Severe acute respiratory syndrome coronavirus 2
(SARS-CoV-2; also known as COVID-19) primarily attacks airway and
alveolar epithelial cells, vascular endothelial cells and
macrophages in the lung, where there is expression of its host
target receptor, angiotensin-converting enzyme 2 (80). Since IL-6 is relevant during
infection that affects the mucosal sites, particularly at the upper
and lower respiratory tract levels, it represents one of most
important cytokines involved in the host response to SARS-CoV-2
infection (81). Severe
COVID-19-induced pneumonia is marked by hyperactivation of the
immune system and especially by excessive production of IL-6
(82,83). In this regard, several studies
have revealed a strong correlation between high systemic levels of
IL-6 and respiratory failure in severely affected patients with
COVID-19 (84). According to
these studies, a 2.9-fold higher mean IL-6 serum concentration was
observed in patients with complicated COVID-19 compared with that
in patients presenting with non-complicated disease. The strong
correlation between IL-6 and COVID-19 has led to the consideration
of serum IL-6 levels as potential diagnostic markers, disease
severity stratification indicators and prognostic indexes (85,86). Moderately elevated IL-6 levels
(>80 pg/ml) were, in fact, sufficient to identify
COVID-19-infected patients and to predict progression towards
respiratory failure. In addition, IL-6 has emerged as the most
significant predictor of mortality in patients with COVID-19
(87).
The pathophysiological role of IL-6 in COVID-19 is
well documented by several studies (83,88). IL-6 is generally considered the
key driver of the hyperinflammatory process in COVID-19; it exerts
effects on numerous different cellular targets that express a
functional IL-6R, including T cells, B cells, vascular endothelial
cells, monocytes and hepatocytes (79). By means of these actions on such
a wide array of cellular targets, IL-6 mediates key effects on
cellular immunity, exerting, at the same time, pro-inflammatory and
anti-inflammatory functions (83,88).
The relevance of IL-6 is also demonstrated by the
numerous studies that have explored its potential utility as a
potential therapeutic target (89-93). The efficacy of a number of
targeted monoclonal antibodies, directed against IL-6 or its
receptor, is currently under investigation in several different
countries. Numerous interventional clinical trials are, in fact,
currently ongoing, using drugs that target IL-6, such as
tocilizumab (Actemra) (38 interventional clinical trials, none of
them yet with final and published results; https://www.clinicaltrials.gov/ct2/results?cond=COVID-19+and+tocilizumab&age_v=&gndr==&type=Intr&rslt==&Search=Apply;
accessed on 28 march 2021), sarilumab (Kevzara) (9 interventional
clinical trials, none of them yet with final and published results;
https://www.clinicaltrials.gov/ct2/results?recrs=&cond=COVID-19+and+Sarilumab&term==&cntry=&state==&city=&dist=;
accessed on 28 March 2021), siltuximab (Sylvant) (1 interventional
clinical trial; ClinicalTrials.gov identifier, NCT04329650) and
clazakizumab (formerly ALD518 and BMS-945429) (6 interventional
clinical trials, none of them yet with final and published results;
https://www.clinicaltrials.gov/ct2/results?cond=COVID-19+and+Clazakizumab&age_v=&gndr=&type=Intr&rslt=&Search=Apply;
accessed on 28 March 2021). These drugs act by inhibiting both
classical and trans IL-6 pathways, and represent promising
therapeutic options for the treatment of the most severe forms of
COVID-19 (94-103).
6. Conclusion
In the present review, the molecular and
nanomolecular structures involved in active IL-6 signalling were
examined. Specially, the review reported that energetic transition
from low to high affinity of IL-6 binding has to occur at the
nanometre scale through changes of geometric orientation by
conversion from the pentamer to hexamer shape.
The role played by miRNA in the post-transcriptional
regulation of IL-6 expression has been evaluated through genes,
pseudogenes and ceRNA of IL-6.
In addition, classic and trans-signaling pathways
were analysed to evaluate the role of IL-6 in inflammatory and
cognitive processes through anatomical localization and serum
levels of active compounds in both pathways. Finally, the analysis
of the pathogenic, diagnostic, prognostic and therapeutic roles of
IL-6 in SARS-CoV-2 infection clearly demonstrates the central role
of IL-6 in the ongoing global COVID-19 pandemic.
Availability of data and materials
Not applicable.
Authors' contributions
MT, SS, AF, AV, GV and AP provided substantial
contributions to the conception or design of the work, or the
acquisition, analysis or interpretation of data. MT, SS, GV and AD
were rewponsible for drafting the work or revising it critically
for important intellectual content. All authors revised the
manuscript and provided important intellectual contributions. All
authors have read and approved the final manuscript. All authors
agree to be accountable for all aspects of the work in ensuring
that questions related to the accuracy or integrity of any part of
the work are appropriately investigated and resolved. Data
authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
No funding was received.
References
|
1
|
Schimpl A and Wecker E: Replacement of T
cell function by a T cell product. Nat New Biol. 237:15–17. 1972.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hirano T: Revisiting the 1986 molecular
cloning of interleukin 6. Front Immunol. 5:4562014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hirano T, Taga T, Nakano N, Yasukawa K,
Kashiwamura S, Shimizu K, Nakajima K, Pyun KH and Kishimoto T:
Purification to homogeneity and characterization of human B-cell
differentiation factor (BCDF or BSFp-2). Proc Natl Acad Sci USA.
82:5490–5494. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sehgal PB, Grieninger G and Tosato G:
Regulation of the acute phase and immune responses: Interleukin-6.
Ann N Y Acad Sci. 557:1–583. 1989.
|
|
5
|
Sehgal PB, Zilberstein A, Ruggieri RM, May
LT, Ferguson-Smith A, Slate DL, Revel M and Ruddle FH: Human
chromosome 7 carries the beta 2 interferon gene. Proc Natl Acad Sci
USA. 83:5219–5222. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sutherland GR, Baker E, Callen DF, Hyland
VJ, Wong G, Clark S, Jones SS, Eglinton LK, Shannon MF, Lopez AF,
et al: Interleukin 4 is at 5q31 and interleukin 6 is at 7p15. Hum
Genet. 79:335–337. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Somers W, Stahl M and Seehra JS: 1.9 A
crystal structure of interleukin 6: Implications for a novel mode
of receptor dimerization and signaling. EMBO J. 16:989–997. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou L, Zheng Y, Tian T, Liu K, Wang M,
Lin S, Deng Y, Dai C, Xu P, Hao Q, et al: Associations of
interleukin-6 gene polymorphisms with cancer risk: Evidence based
on 49,408 cancer cases and 61,790 controls. Gene. 670:136–147.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Simpson RJ, Hammacher A, Smith DK,
Matthews JM and Ward LD: Interleukin-6: Structure-function
relationships. Protein Sci. 6:929–955. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hirano T: Interleukin 6 and its receptor:
Ten years later. Int Rev Immunol. 16:249–284. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hunter CA and Jones SA: IL-6 as a keystone
cytokine in health and disease. Nat Immunol. 16:448–457. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sumikawa H, Fukuhara K, Suzuki E, Matsuo Y
and Nishikawa K: Tertiary structural models for human interleukin-6
and evaluation by a sequence-structure compatibility method and NMR
experimental information. FEBS Lett. 404:234–240. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Heinrich PC, Castell JV and Andus T:
Interleukin-6 and the acute phase response. Biochem J. 265:621–636.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bazan JF: Structural design and molecular
evolution of a cytokine receptor superfamily. Proc Natl Acad Sci
USA. 87:6934–6938. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sakakibara S and Tosato G: Viral
Interleukin-6: Role in Kaposi's sarcoma-associated herpesvirus:
Associated malignancies. J Interferon Cytokine Res. 31:791–801.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yamasaki K, Taga T, Hirata Y, Yawata H,
Kawanishi Y, Seed B, Taniguchi T, Hirano T and Kishimoto T: Cloning
and expression of the human interleukin-6 (BSF-2/IFN beta 2)
receptor. Science. 241:825–828. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hibi M, Murakami M, Saito M, Hirano T,
Taga T and Kishimoto T: Molecular cloning and expression of an IL-6
signal transducer, gp130. Cell. 63:1149–1157. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kluck PM, Wiegant J, Jansen RP, Bolk MW,
Raap AK, Willemze R and Landegent JE: The human interleukin-6
receptor alpha chain gene is localized on chromosome 1 band q21.
Hum Genet. 90:542–544. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rodriguez C, Grosgeorge J, Nguyen VC,
Gaudray P and Theillet C: Human gp130 transducer chain gene (IL6ST)
is localized to chromosome band 5q11 and possesses a pseudogene on
chromosome band 17p11. Cytogenet Cell Genet. 70:64–67. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Taga T, Hibi M, Hirata Y, Yamasaki K,
Yasukawa K, Matsuda T, Hirano T and Kishimoto T: Interleukin-6
triggers the association of its receptor with a possible signal
transducer, gp130. Cell. 58:573–581. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Boulanger MJ, Chow DC, Brevnova EE and
Garcia KC: Hexameric structure and assembly of the
interleukin-6/IL-6 alpha-receptor/gp130 complex. Science.
300:2101–2104. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lacroix M, Rousseau F, Guilhot F, Malinge
P, Magistrelli G, Herren S, Jones SA, Jones GW, Scheller J,
Lissilaa R, et al: Novel insights into interleukin 6 (IL-6) Cis-
and trans-signaling pathways by differentially manipulating the
assembly of the IL-6 signaling complex. J Biol Chem.
290:26943–26953. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Trovato MC, Andronico D, Sciacchitano S,
Ruggeri RM, Picerno I, Di Pietro A and Visalli G: Nanostructures:
Between natural environment and medical practice. Rev Environ
Health. 33:295–307. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Weidle UH, Klostermann S, Eggle D and
Krüger A: Interleukin 6/interleukin 6 receptor interaction and its
role as a therapeutic target for treatment of cachexia and cancer.
Cancer Genomics Proteomics. 7:287–302. 2010.PubMed/NCBI
|
|
25
|
Heinrich PC, Behrmann I, Haan S, Hermanns
HM, Müller-Newen G and Schaper F: Principles of interleukin
(IL)-6-type cytokine signalling and its regulation. Biochem J.
374:1–20. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Scheller J, Chalaris A, Schmidt-Arras D
and Rose-John S: The pro- and anti-inflammatory properties of the
cytokine inter-leukin-6. Biochim Biophys Acta. 1813:878–888. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
An Y, Furber KL and Ji S: Pseudogenes
regulate parental gene expression via ceRNA network. J Cell Mol
Med. 21:185–192. 2017. View Article : Google Scholar
|
|
28
|
Kuscuoglu D, Janciauskiene S, Hamesch K,
Haybaeck J, Trautwein C and Strnad P: Liver-master and servant of
serum proteome. J Hepatol. 69:512–524. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tuck AC and Tollervey D: A
transcriptome-wide atlas of RNP composition reveals diverse classes
of mRNAs and lncRNAs. Cell. 154:996–1009. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
St Laurent G, Wahlestedt C and Kapranov P:
The landscape of long noncoding RNA classification. Trends Genet.
31:239–251. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pink RC, Wicks K, Caley DP, Punch EK,
Jacobs L and Carter DR: Pseudogenes: Pseudo-functional or key
regulators in health and disease? RNA. 17:792–798. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Salmena L, Poliseno L, Tay Y, Kats L and
Pandolfi PP: A ceRNA hypothesis: The Rosetta stone of a hidden RNA
language? Cell. 146:353–358. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
van Rij RP and Andino R: The silent
treatment: RNAi as a defense against virus infection in mammals.
Trends Biotechnol. 24:186–193. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lavra L, Ulivieri A, Dominici R, Trovato
MC, Bartolazzi A, Soddu S and Sciacchitano S: Analysis of the role
of p53 and galectin-3 in proliferation and apoptosis of thyroid
carcinoma cell lines by specific RNA interference experiments.
Biomed Pharmacother. 60:4912006. View Article : Google Scholar
|
|
35
|
Cecchinelli B, Lavra L, Rinaldo C,
Iacovelli S, Gurtner A, Gasbarri A, Ulivieri A, Del Prete F,
Trovato M, Piaggio G, et al: Repression of the antiapoptotic
molecule Galectin-3 by homeodomain-interacting protein kinase
2-activated p53 is required for p53-Induced apoptosis. Mol Cell
Biol. 26:4746–4757. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bautista RR, Gómez AO, Miranda AH, Dehesa
AZ, Villarreal-Garza C, Ávila-Moreno F and Arrieta O: Correction
to: Long non-coding RNAs: Implications in targeted diagnoses,
prognosis, and improved therapeutic strategies in human non- and
triple-negative breast cancer. Clin Epigenetics. 10:1062018.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hosseinahli N, Aghapour M, Duijf PHG and
Baradaran B: Treating cancer with microRNA replacement therapy: A
literature review. J Cell Physiol. 233:5574–5588. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Huang HC, Yu HR, Hsu TY, Chen IL, Huang
HC, Chang JC and Yang KD: MicroRNA-142-3p and let-7g negatively
regulates augmented IL-6 production in neonatal polymorphonuclear
leukocytes. Int J Biol Sci. 13:690–700. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liao YC, Wang YS, Guo YC, Lin WL, Chang MH
and Juo SH: Let-7g improves multiple endothelial functions through
targeting transforming growth factor-beta and SIRT-1 signaling. J
Am Coll Cardiol. 63:1685–1694. 2014. View Article : Google Scholar
|
|
40
|
Johnson CD, Esquela-Kerscher A, Stefani G,
Byrom M, Kelnar K, Ovcharenko D, Wilson M, Wang X, Shelton J,
Shingara J, et al: The let-7 MicroRNA represses cell proliferation
pathways in human cells. Cancer Res. 67:7713–7722. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gao X, Xu W, Lu T, Zhou J, Ge X and Hua D:
MicroRNA-142-3p promotes cellular invasion of colorectal cancer
cells by activation of RAC1. Technol Cancer Res Treat.
17:15330338187905082018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sun Y, Varambally S, Maher CA, Cao Q,
Chockley P, Toubai T, Malter C, Nieves E, Tawara I, Wang Y, et al:
Targeting of microRNA-142-3p in dendritic cells regulates
endotoxin-induced mortality. Blood. 117:6172–6183. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sung SY, Liao CH, Wu HP, Hsiao WC, Wu IH,
Jinpu Yu, Lin SH and Hsieh CL: Loss of let-7 microRNA upregulates
IL-6 in bone marrow-derived mesenchymal stem cells triggering a
reactive stromal response to prostate cancer. PLoS One.
8:e716372013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Selzner N, Selzner M, Odermatt B, Tian Y,
Van Rooijen N and Clavien PA: ICAM-1 triggers liver regeneration
through leukocyte recruitment and Kupffer cell-dependent release of
TNF-alpha/IL-6 in mice. Gastroenterology. 124:692–700. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yoshiya S, Shirabe K, Imai D, Toshima T,
Yamashita Y, Ikegami T, Okano S, Yoshizumi T, Kawanaka H and
Maehara Y: Blockade of the apelin-APJ system promotes mouse liver
regeneration by activating Kupffer cells after partial hepatectomy.
J Gastroenterol. 50:573–582. 2015. View Article : Google Scholar
|
|
46
|
Kishimoto T: Interleukin-6: From basic
science to medicine-40 years in immunology. Annu Rev Immunol.
23:1–21. 2005. View Article : Google Scholar
|
|
47
|
Papanicolaou DA and Vgontzas AN:
Interleukin-6: The endocrine cytokine. J Clin Endocrinol Metab.
85:1331–1333. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ruggeri RM, Sciacchitano S, Vitale A,
Cardelli P, Galletti M, Vitarelli E, Barresi G, Benvenga S,
Trimarchi F and Trovato M: Serum hepatocyte growth factor is
increased in hashimoto's thyroiditis whether or not associated with
nodular goiter as compared with healthy non goitrous individuals. J
Endocrinol Invest. 32:465–469. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Trovato M, Ruggeri RM, Sciacchitano S,
Vicchio TM, Picerno I, Pellicanò G, Valenti A and Visalli G: Serum
interleukin-6 levels are increased in HIV-infected patients that
develop autoimmune disease during long-term follow-up.
Immunobiology. 223:264–268. 2018. View Article : Google Scholar
|
|
50
|
Ruggeri RM, Villari D, Simone A, Scarfi R,
Attard M, Orlandi F, Barresi G, Trimarchi F, Trovato M and Benvenga
S: Co-expression of interleukin-6 (IL-6) and interleukin-6 receptor
(IL-6R) in thyroid nodules is associated with co-expression of CD30
ligand/CD30 receptor. J Endocrinol Invest. 25:959–966. 2002.
View Article : Google Scholar
|
|
51
|
Trovato M, Grosso M, Vitarelli E, Ruggeri
RM, Alesci S, Trimarchi F, Barresi G and Benvenga S: Distinctive
expression of STAT3 in papillary thyroid carcinomas and a subset of
follicular adenomas. Histol Histopathol. 18:393–399.
2003.PubMed/NCBI
|
|
52
|
Ruggeri RM, Barresi G, Sciacchitano S,
Trimarchi F, Benvenga S and Trovato M: Immunoexpression of the CD30
ligand/CD30 and IL-6/IL-6R signals in thyroid autoimmune diseases.
Histol Histopathol. 21:249–256. 2006.
|
|
53
|
Trovato M: A historical excursus of
diagnostic methods for Hashimoto thyroiditis and Graves' disease.
Gazz Med Ital Arch Sci Med. 179:479–485. 2020. View Article : Google Scholar
|
|
54
|
Elsabahy M and Wooley KL: Cytokines as
biomarkers of nanoparticle immunotoxicity. Chem Soc Rev.
42:5552–5576. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Visalli G, Baluce B, Bertuccio M, Picerno
I and Di Pietro A: Mitochondrial-Mediated apoptosis pathway in
alveolar epithelial cells exposed to the metals in
Combustion-Generated particulate matter. J Toxicol Environ Health
A. 78:697–709. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Visalli G, Facciolà A, Iannazzo D, Piperno
A, Pistone A and Di Pietro A: The role of the iron catalyst in the
toxicity of multi-walled carbon nanotubes (MWCNTs). J Trace Elem
Med Biol. 43:153–160. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Visalli G, Currò M, Iannazzo D, Pistone A,
Pruiti Ciarello M, Acri G, Testagrossa B, Bertuccio MP, Squeri R
and Di Pietro A: In vitro assessment of neurotoxicity and
neuroinflammation of homemade MWCNTs. Environ Toxicol Pharmacol.
56:121–128. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Visalli G, Facciolà A, Currò M, Laganà P,
La Fauci V, Iannazzo D, Pistone A and Di Pietro A: Mitochondrial
impairment induced by sub-chronic exposure to multi-walled carbon
nanotubes. Int J Environ Res Public Health. 16:7922019. View Article : Google Scholar :
|
|
59
|
Facciolà A, Visalli G, La Maestra S,
Ceccarelli M, D'Aleo F, Nunnari G, Pellicanò GF and Di Pietro A:
Carbon nanotubes and central nervous system: Environmental risks,
toxicological aspects and future perspectives. Environ Toxicol
Pharmacol. 65:23–30. 2019. View Article : Google Scholar
|
|
60
|
Palomäki J, Välimäki E, Sund J, Vippola M,
Clausen PA, Jensen KA, Savolainen K, Matikainen S and Alenius H:
Long, needle-like carbon nanotubes and asbestos activate the NLRP3
inflammasome through a similar mechanism. ACS Nano. 5:6861–6870.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Neagu M, Constantin C, Popescu ID, Zipeto
D, Tzanakakis G, Nikitovic D, Fenga C, Stratakis CA, Spandidos DA
and Tsatsakis AM: Inflammation and metabolism in cancer
cell-mitochondria key player. Front Oncol. 9:3482019. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Arnoldussen YJ, Skogstad A, Skaug V, Kasem
M, Haugen A, Benker N, Weinbruch S, Apte RN and Zienolddiny S:
Involvement of IL-1 genes in the cellular responses to carbon
nanotube exposure. Cytokine. 73:128–137. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Migliore L, Uboldi C, Di Bucchianico S and
Coppedè F: Nanomaterials and neurodegeneration. Environ Mol
Mutagen. 56:149–170. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Bardi G, Nunes A, Gherardini L, Bates K,
Al-Jamal KT, Gaillard C, Prato M, Bianco A, Pizzorusso T and
Kostarelos K: Functionalized carbon nanotubes in the brain:
Cellular internalization and neuroinflammatory responses. PLoS One.
8:e809642013. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Bussy C, Al-Jamal KT, Boczkowski J, Lanone
S, Prato M, Bianco A and Kostarelos K: Microglia determine brain
region-specific neurotoxic responses to chemically functionalized
carbon nanotubes. ACS Nano. 9:7815–7830. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Rothaug M, Becker-Pauly C and Rose-John S:
The role of inter-leukin-6 signaling in nervous tissue. Biochim
Biophys Acta. 1863:1218–1227. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Hu J, Feng X, Valdearcos M, Lutrin D,
Uchida Y, Koliwad SK and Maze M: Interleukin-6 is both necessary
and sufficient to produce perioperative neurocognitive disorder in
mice. Br J Anaesth. 120:537–545. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Fenga C, Gangemi S, Di Salvatore V,
Falzone L and Libra M: Immunological effects of occupational
exposure to lead (Review). Mol Med Rep. 15:3355–3360. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Gangemi S, Gofita E, Costa C, Teodoro M,
Briguglio G, Nikitovic D, Tzanakakis G, Tsatsakis AM, Wilks MF,
Spandidos DA and Fenga C: Occupational and environmental exposure
to pesticides and cytokine pathways in chronic diseases (Review).
Int J Mol Med. 38:1012–1020. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Shahpiri Z, Bahramsoltani R, Hosein
Farzaei M, Farzaei F and Rahimi R: Phytochemicals as future drugs
for Parkinson's disease: A comprehensive review. Rev Neurosci.
27:651–668. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Ardah MT, Bharathan G, Kitada T and Haque
ME: Ellagic acid prevents dopamine neuron degeneration from
oxidative stress and neuroinflammation in MPTP Model of Parkinson's
disease. Biomolecules. 10:15192020. View Article : Google Scholar
|
|
72
|
Gadient RA and Otten U: Expression of
interleukin-6 (IL-6) and interleukin-6 receptor (IL-6R) mRNAs in
rat brain during postnatal development. Brain Res. 637:10–14. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Marsland AL, Gianaros PJ, Abramowitch SM,
Manuck SB and Hariri AR: Interleukin-6 covaries inversely with
hippocampal grey matter volume in middle-aged adults. Biol
Psychiatry. 64:484–490. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
MacQueen GM, Campbell S, McEwen BS,
Macdonald K, Amano S, Joffe RT, Nahmias C and Young LT: Course of
illness, hippocampal function, and hippocampal volume in major
depression. Proc Natl Acad Sci USA. 100:1387–1392. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Baune BT, Konrad C, Grotegerd D, Suslow T,
Birosova E, Ohrmann P, Bauer J, Arolt V, Heindel W, Domschke K, et
al: Interleukin-6 gene (IL-6): A possible role in brain morphology
in the healthy adult brain. J Neuroinflammation. 9:1252012.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Campbell IL, Abraham CR, Masliah E, Kemper
P, Inglis JD, Oldstone MB and Mucke L: Neurologic disease induced
in transgenic mice by cerebral overexpression of interleukin 6.
Proc Natl Acad Sci USA. 90:10061–10065. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Campbell IL, Erta M, Lim SL, Frausto R,
May U, Rose-John S, Scheller J and Hidalgo J: Trans-signaling is a
dominant mechanism for the pathogenic actions of interleukin-6 in
the brain. J Neurosci. 34:2503–2513. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Chucair-Elliott AJ, Conrady C, Zheng M,
Kroll CM, Lane TE and Carr DJ: Microglia-induced IL-6 protects
against neuronal loss following HSV-1 infection of neural
progenitor cells. Glia. 62:1418–1434. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Chomarat P, Banchereau J, Davoust J and
Palucka AK: IL-6 switches the differentiation of monocytes from
dendritic cells to macrophages. Nat Immunol. 1:510–514. 2000.
View Article : Google Scholar
|
|
80
|
Urashima M, Chauhan D, Hatziyanni M, Ogata
A, Hollenbaugh D, Aruffo A and Anderson KC: CD40 ligand triggers
interleukin-6 mediated B cell differentiation. Leuk Res.
20:507–515. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Yang R, Masters AR, Fortner KA, Champagne
DP, Yanguas-Casás N, Silberger DJ, Weaver CT, Haynes L and Rincon
M: IL-6 promotes the differentiation of a subset of naive CD8+ T
cells into IL-21-producing B helper CD8+ T cells. J Exp Med.
213:2281–2291. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Diehl S and Rincón M: The two faces of
IL-6 on Th1/Th2 differentiation. Mol Immunol. 39:531–536. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Gubernatorova EO, Gorshkova EA, Polinova
AI and Drutskaya MS: IL-6: Relevance for immunopathology of
SARS-CoV-2. Cytokine Growth Factor Rev. 53:13–24. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Xu H, Zhong L, Deng J, Peng J, Dan H, Zeng
X, Li T and Chen Q: High expression of ACE2 receptor of 2019-nCoV
on the epithelial cells of oral mucosa. Int J Oral Sci. 12:82020.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Rose-John S, Winthrop K and Calabrese L:
The role of IL-6 in host defence against infections: Immunobiology
and clinical implications. Nat Rev Rheumatol. 13:399–409. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Chen G, Wu D, Guo W, Cao Y, Huang D, Wang
H, Wang T, Zhang X, Chen H, Yu H, et al: Clinical and immunological
features of severe and moderate coronavirus disease 2019. J Clin
Invest. 130:2620–2629. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Ruan Q, Yang K, Wang W, Jiang L and Song
J: Clinical predictors of mortality due to COVID-19 based on an
analysis of data of 150 patients from Wuhan, China. Intensive Care
Med. 46:846–848. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Abbasifard M and Khorramdelazad H: The
bio-mission of interleukin-6 in the pathogenesis of COVID-19: A
brief look at potential therapeutic tactics. Life Sci.
257:1180972020. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Coomes EA and Haghbayan H: Interleukin-6
in Covid-19: A systematic review and meta-analysis. Rev Med Virol.
30:1–9. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Zhang J, Hao Y, Ou W, Ming F, Liang G,
Qian Y, Cai Q, Dong S, Hu S, Wang W and Wei S: Serum interleukin-6
is an indicator for severity in 901 patients with SARS-CoV-2
infection: A cohort study. J Transl Med. 18:4062020. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Herold T, Jurinovic V, Arnreich C,
Hellmuth JC, von Bergwelt-Baildon M, Klein M and Weinberger T:
Level of IL-6 predicts respiratory failure in hospitalized
symptomatic COVID-19 patients. medRxiv. https://doi.org/10.1101/2020.04.01.20047381.
Accessed April 27, 2020.
|
|
92
|
Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z,
Xiang J, Wang Y, Song B, Gu X, et al: Clinical course and risk
factors for mortality of adult inpatients with COVID-19 in Wuhan,
China: A retrospective cohort study. Lancet. 395:1054–1062. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Atal S and Fatima Z: IL-6 Inhibitors in
the treatment of serious COVID-19: A promising therapy? Pharmaceut
Med. 34:223–231. 2020.PubMed/NCBI
|
|
94
|
Xu X, Han M, Li T, Sun W, Wang D, Fu B,
Zhou Y, Zheng X, Yang Y, Li X, et al: Effective treatment of severe
COVID-19 patients with tocilizumab. Proc Natl Acad Sci USA.
117:10970–10975. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Fu B, Xu X and Wei H: Why tocilizumab
could be an effective treatment for severe COVID-19? J Transl Med.
18:1642020. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Toniati P, Piva S, Cattalini M, Garrafa E,
Regola F, Castelli F, Franceschini F, Airò P, Bazzani C, Beindorf
EA, et al: Tocilizumab for the treatment of severe COVID-19
pneumonia with hyperinflammatory syndrome and acute respiratory
failure: A single center study of 100 patients in Brescia, Italy.
Autoimmun Rev. 19:1025682020. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Issa N, Dumery M, Guisset O, Mourissoux G,
Bonnet F and Camou F: Feasibility of tocilizumab in ICU patients
with COVID-19. J Med Virol. 93:46–47. 2021. View Article : Google Scholar
|
|
98
|
Alattar R, Ibrahim TBH, Shaar SH, Abdalla
S, Shukri K, Daghfal JN, Khatib MY, Aboukamar M, Abukhattab M,
Alsoub HA, et al: Tocilizumab for the treatment of severe
coronavirus disease 2019. J Med Virol. 92:2042–2049. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Della-Torre E, Campochiaro C, Cavalli G,
De Luca G, Napolitano A, La Marca S, Boffini N, Da Prat V, Di
Terlizzi G, Lanzillotta M, et al: Interleukin-6 blockade with
sarilumab in severe COVID-19 pneumonia with systemic
hyperinflammation: An open-label cohort study. Ann Rheum Dis.
79:1277–1285. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Benucci M, Giannasi G, Cecchini P, Gobbi
FL, Damiani A, Grossi V, Infantino M and Manfredi M: COVID-19
pneumonia treated with sarilumab: A clinical series of eight
patients. J Med Virol. 92:2368–2370. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Palanques-Pastor T, López-Briz E and
Poveda Andrés JL: Involvement of interleukin 6 in SARS-CoV-2
infection: Siltuximab as a therapeutic option against COVID-19. Eur
J Hosp Pharm. 27:297–298. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Gritti G, Raimondi F, Ripamonti D, Riva I,
Landi F, Alborghetti L, Frigeni M, Damiani M, Micò C, Fagiuoli S,
et al: Use of siltuximab in patients with COVID-19 pneumonia
requiring ventilatory support. medRxiv. https://doi.org/10.1101/2020.04.01.20048561.
|
|
103
|
Vaidya G, Czer LSC, Kobashigawa J,
Kittleson M, Patel J, Chang D, Kransdorf E, Shikhare A, Tran H, Vo
A, et al: Successful treatment of severe COVID-19 pneumonia with
clazakizumab in a heart transplant recipient: A case report.
Transplant Proc. 52:2711–2714. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Stelzer G, Rosen R, Plaschkes I, Zimmerman
S, Twik M, Fishilevich S, Stein TI, Nudel R, Lieder I, Mazor Y, et
al: The GeneCards suite: From gene data mining to disease genome
sequence analysis. Curr Protoc Bioinformatics. 54:1.30.1–1.30.33.
2016. View
Article : Google Scholar
|
|
105
|
Hunt SE, McLaren W, Gil L, Thormann A,
Schuilenburg H, Sheppard D, Parton A, Armean IM, Trevanion SJ,
Flicek P and Cunningham F: Ensembl variation resources. Database
(Oxford). 2018:bay1192018. View Article : Google Scholar
|