Introduction
Sepsis, which is a highly complex syndrome, is one
of the leading causes of mortality in patients suffering from
trauma, burns and critical surgery (1). Furthermore, severe sepsis develops
into acute lung injury (ALI) in 50% of patients. In septic
conditions, the excessive inflammatory response and apoptosis leads
to the disruption of alveolar epithelial cells, an increase in
epithelial permeability, and the influx of edema fluid into the
alveolar space, finally leading to ALI (2). Thus, the control of aberrant
inflammation and apoptosis contributes substantially toward
improving the prognosis of patients with ALI. However, there is no
current effective therapeutic approach for sepsis, and its
treatment involves significant healthcare costs (3).
The overwhelming inflammatory response that occurs
in ALI can lead to the release of a large number of
pro-inflammatory cytokines in the lungs, such as interleukin
(IL)-6, tumor necrosis factor-α (TNF-α) and IL-1β, that ultimately
lead to alveolar epithelial cell death (4,5).
However, the factors that play a role in the transmission of
inflammatory signals associated with ALI have not yet been fully
elucidated. Nuclear factor-κB (NF-κB) has been recognized as an
important inflammatory mediator in a number of diseases, including
lupus nephritis, cancers (6,7)
and ALI (8,9). Once NF-κB dimers are activated in
response to certain stimuli, they enter the nucleus and lead to
inflammatory cytokine expression. It has been reported that the
degree of activation of NF-κB is associated with disease
complications and a more severe outcome in patients with ALI
(10,11). Transforming growth factor-β
(TGF-β)-activated kinase 1 (TAK1, also known as MAP3K7) is a
serine/threonine protein kinase that is critically involved in the
activation of NF-κB. Activated TAK1 can induce the phosphorylation
of inhibitor of IκB kinase (IKK) and lead to IKK-induced NF-κB
activation, which then leads to the synthesis and release of
pro-inflammatory mediators (12). All these findings indicate that
the targeting of TAK1/NF-κB pathway may provide novel treatment
strategies for patients with ALI.
MicroRNAs (miRNAs/miRs) are a family of short,
small, non-coding RNAs that negatively regulate target gene
expression through either translation repression or RNA degradation
(13). Increasing evidence has
demonstrated that miRNAs are effective regulators of the
inflammatory response and apoptosis in various types of organ
injuries (14-16). For example, Li et al
(17) found that an increase in
miR-129 levels leads to the amelioration of neuroinflammation and
damage to the blood-spinal cord barrier after ischemia-reperfusion
(I/R), via the inhibition of high mobility group box-1 (HMGB1) and
the TLR3-cytokine pathway. miR-129 was also reported to alleviate
nerve injury and inflammatory response in Alzheimer's disease via
the downregulation of SOX6 (18). Furthermore, Chen et al
(19) demonstrated the
protective effect of miR-129 on myocardial I/R injury through the
suppression of cardiomyocyte apoptosis via targeting HMGB1 in rats.
Based on these previous findings, it is possible that miR-129 plays
a role in inflammation and apoptosis in ALI. However, the role of
miR-129 in the pathogenesis of ALI is largely unknown.
The aim of the present study was to investigate the
role of miRNAs in the pathogenesis of ALI. To this end, several
differentially expressed miRNAs deposited in a public dataset,
namely, the Gene Expression Omnibus (GEO) database (GSE133733),
were evaluated concerning their potential role in ALI. Based on an
analysis of these miRNAs, miR-129 was selected for further
analysis. Using a mouse model of ALI, the functional role and
underlying mechanisms of miR-129 were investigated. The findings
presented herein may prove useful for the development of novel
research strategies for the molecular therapeutic targeting of
ALI.
Materials and methods
Animals and treatment
Male BALB/c mice (age, 6 weeks; weight, 18-22 g)
were purchased from Shanghai SLAC Laboratory Animal Co., Ltd. All
mice were housed under standard conditions (12-h light-dark cycle
at 25-27°C and ~40% humidity) with free access to food and
water.
The mice were randomly divided into four groups (10
mice per group) as follows: i) The control group: 1.5 mg/kg normal
saline (NS) was administered; ii) lipopolysaccharide (LPS) group:
0.2 ml of LPS (10 mg/kg, Escherichia coli 055:B5;
Sigma-Aldrich; Merck KGaA) was intravenously administered via the
tail vein; iii) LPS + agomir-129 group; and iv) LPS + agomir
negative control (NC) group. In the mice in the
agomir-129/agomir-NC group, in addition to LPS, 0.2 ml
agomir-129/agomir-NC (8 mg/kg) was intravenously administered via
the tail vein (20). Agomir-129
or agomir-NC were injected intravenously via the tail vein every
third day until ALI was induced. Pentobarbital sodium (50 mg/kg,
intraperitoneal injection) was used for anesthesia before each
operation, and all efforts were made to minimize animal suffering.
No mice were found dead during the anesthesia process. If an animal
reached the defined humane endpoints [a >15% body weight loss in
1-2 days or an overall >20% loss in body weight or displayed
obvious signs of suffering (lethargy, squinted eyes, dehydration or
hunched back), it was humanely euthanized. Sacrifice was performed
by an intraperitoneal injection of pentobarbital sodium (50 mg/kg)
followed by cervical dislocation, and the death of the mice was
confirmed by the cessation of respiration and heartbeat (21). In the survival experiment, the
mice were observed for 7 days after the LPS injection, while other
mice were observed for 3 days after the LPS injection. All mice
were humanely sacrificed when they reached the humane endpoint or
the set experimental endpoint. The lungs were then excised, intact.
Subsequently, the trachea was lavaged with 1 ml of sterile ice-cold
PBS and bronchoalveolar lavage fluid (BALF) was collected. BALF was
pooled and centrifuged at 200 × g for 10 min at 4°C, and the
harvested supernatant was stored at −80°C until mediator
analysis.
In addition, the survival experiments were performed
in 4 groups of mice (n=10/group) (LPS, Control, LPS + agomir-129,
LPS + agomir-NC). The survival rate was defined from 0 to 7 days
following treatment and analyzed with the Kaplan-Meier survival
analysis and the log rank test. All animal experiments were
approved by the Animal Care and Use Committee of Henan Provincial
People's Hospital (approval no. 2019-00112).
miRNA expression profile data from
GEO
miRNA data (accession no. GSE133733) were downloaded
from open microarray datasets, deposited in NCBI's GEO database
(http://www.ncbi.nlm.nih.gov/geo/). The
miRNA array expression data were analyzed with the Qlucore Omics
Explorer (QOE 3.1) bioinformatics software (http://www.qlucore.com/).
Cell culture and transfection
RAW 264.7 cells (cat. no. ATCC® TIB-71)
were obtained from ATCC and cultured in DMEM/F12 supplemented with
10% FBS (Abcam) at 37°C and in a 5% CO2 atmosphere.
RAW264.7 cells (2×105 cells) were seeded in a 6-well
plate and transfected with 100 nM of miR-129 mimics/inhibitor at
37°C for 48 h using Lipofectamine® 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.). miR-129 mimic/inhibitor and their
NC oligonucleotides (NC mimic or NC inhibitor) were obtained from
Shanghai GenePharma Co., Ltd. The sequences were as follows:
miR-129 mimic, 3′-CUUUUUGCGGUCUGGGCUUGC-5′; miR-129 inhibitor,
3′-GCAAGCCCAGACCGCAAAAAG-5′; mimic NC,
5′-UUUGUACUACACAAAAGUACUG-3′; inhibitor NC,
5′-UUCUCCGAACGUGUCACGUTT-3′. The cells were harvested at 48 h
following transfection for testing.
Transfection of pcDNA-TAK1 and
si-TAK1
A series of ALI cell models have been established to
simulate the status of ALI caused by diverse pathogeneses. The
human type II lung epithelial A549 cell line is usually used to
establish cell apoptosis models in LPS-induced ALI (22,23). Among these, RAW 264.7 macrophages
are usually used to establish cell inflammation responses models in
LPS-induced ALI (24,25). Therefore, RAW cells were selected
for use in ex vivo experiments as the focus of the present
study was on the inflammatory responses in ALI. For this purpose,
RAW 264.7 cells were co-transfected with miR-129 mimics (100
nmol/l), pcDNA-TAK1 (2 μg), or si-TAK1 (100 nmol/l) for 48 h
using Lipofectamine 3000® (Invitrogen; Thermo Fisher
Scientific, Inc.) at 37°C in 5% CO2, followed by LPS
stimulation (100 ng/ml) for a further 6 h, as previously described
(26). Subsequently, they were
cultured in complete medium for 48 h, in order to perform the
subsequent experiments. A TAK1 expression vector was constructed
through the insertion of the full sequence of TAK1 into the pcDNA
3.1 vector (Invitrogen; Thermo Fisher Scientific, Inc.). Specific
small interfering RNAs (siRNAs) for TAK1
(5′-CATCCAGTGCCAAGCAGCTCATATT-3′) and si-Scramble
(5′-CATGAGTCCAACGGATCACTCCATT-3′) were purchased from Shanghai
GenePharma Co., Ltd. RAW 264.7 cells co-transfected with the
pcDNA-vector and si-Scramble were used as a control group.
Agomir-129 and agomir-NC were synthesized by GenePharma Co., Ltd.
The agomiR-129 and agomiR-NC, whose sequence was the same as the
miR-129 mimics and mimics NC, were modified with methylation,
cholesterol addition and thiophosphorylation, in order to improve
the stability and transfection efficiency and to simulate miRNA
activity in the animal body (27).
Reverse transcription-quantitative PCR
(RT-qPCR) analysis
Total RNA was isolated from the lung tissues using
the RNeasy Mini kit (Qiagen GmbH). Reverse transcripts of miR-129
and TAK1 were synthesized using the MicroRNA Reverse Transcription
kit (Thermo Fisher Scientific, Inc.) and the PrimeScript RT reagent
kit (Takara Bio, Inc.), respectively. miR-129 and TAK1 expression
was measured with SYBR-Green (Beijing Solarbio Science &
Technology Co., Ltd.) on a Light Cycler instrument (Bio-Rad
Laboratories, Inc.). The following primers were used for RT-qPCR
analysis: miR-129 forward, 5′-GTTGGGGAGATTTAGTTTGTT-3′ and reverse,
5′-CCTACTCCAATTCCCCCTATAATAC-3′; U6 forward,
5′-CTCGCTTCGGCAGCACA-3′ and reverse, 5′-AACGCTTCACGAATTTGCGT-3′;
TAK1 forward, 5′-GATATCCTGTCGACAGCCTCCGC-3′ and reverse,
5′-AACGTAACGGGCCCAGAGAA-3′; GAPDH forward,
5′-GTGGTGAAGACGCCAGTGGA-3′ and reverse, 5′-CGAGCCACATCGCTCAGACA-3′.
The thermocycling conditions were as follows: 50°C for 2 min and
95°C for 10 min, followed by 40 cycles of 95°C for 15 sec and 60°C
for 10 min. Relative miRNA expression was analyzed using the
2−ΔΔCq method (28).
Lung histology
Mouse lungs from all groups were fixed with 10%
formalin and micro-sectioned to a thickness of 5 μm using a
Leica SM2010R microtome (Leica Microsystems, Inc.). The tissues
were embedded in paraffin and then subjected to hematoxylin and
eosin (H&E) staining. Images were viewed and captured by a
Nikon E-800M microscope (Nikon Corporation) at ×200 magnification.
Inflammatory cell infiltration, bleeding and interstitial and
alveolar edema were observed under a light microscope, according to
the following lung injury score: 0, normal pulmonary appearance; 1,
slight injury, mild interstitial congestion and neutrophil
leukocyte infiltrations; 2, moderate injury: Moderate interstitial
congestion and neutrophil leukocyte infiltrations; 3, severe
injury, perivascular edema formation, partial leukocyte
infiltration, moderate neutrophil leukocyte infiltration; 4, very
severe histological injury: Severe destruction of the lung
architecture and massive neutrophil leukocyte infiltration.
Lung permeability
The Evans blue (EB) (Sigma-Aldrich; Merck KGaA) dye
extravasation method was used to assess pulmonary permeability, as
previously described (29). The
dye concentration was reported as the absorbance relative to the
weight of dry lung tissue (μg/100 mg dry tissue).
Lung wet/dry ratio
Following a 72-h LPS challenge, the mice were
sacrificed. The lungs were excised, and the right lungs were
weighed and dried in an incubator at 55°C. After 60 h, the lungs
were weighed again to calculate the wet-to-dry (W/D) ratio.
Measurement of pro-inflammatory cytokine
levels
Murine cytokine-specific Quantikine ELISA kits
(R&D Systems Europe, Ltd.) were used to detect the IL-6, IL-1β,
TNF-α, Cxcl2, JE (the murine homolog of human CCL2) and KC (the
murine homolog of human IL-8) levels in BALF, as previously
described (14).
TUNEL staining
The lung tissue sections were deparaffinized with
xylene, rehydrated with ethanol at graded concentrations of 70-100%
(v/v) and washed with water. The sections were then treated with
100 μl proteinase K (20 μg/ml, Roche Diagnostics) for
15 min, at room temperature. After the sections were washed three
times with PBS, they were stained with prepared terminal
deoxynucleotidyl transferase-mediated dUTP-biotin nick-end labeling
(TUNEL) solution, using the in situ Cell Death Detection kit
(Roche Diagnostics). Cell quantification was performed under an
inverted fluorescence microscope (DP73; Olympus Corporation) at
×200 magnification. TUNEL-positive cells were counted in three
fields of view per section.
Bioinformatics analysis
The miRNA target prediction tools PicTar version
2007 (https://pictar.mdc-berlin.de/) and
TargetScan Release 7.0 (http://targetscan.org/) were used to search for
putative targets of miR-153.
Luciferase assay
The 3′-UTR of TAK1 and its mutated sequence were
inserted into the pGL3 control vector (Promega Corporation) to
construct the wild-type (wt) TAK1-3′-UTR vector and mutant
TAK1-3′-UTR vector, respectively. RAW 264.7 cells were seeded in
24-well plates (5.0×105/well) and were transfected with
either the wild-type or mutant reporter vector (2 μg),
combined with the miR-129 mimics/inhibitor (100 nM), using
Lipofectamine 2000® (Invitrogen, Thermo Fisher
Scientific Inc.). The Renilla luciferase expression vector
pRL-TK (Promega Corporation) was employed as the reference. At 48 h
post-transfection, luciferase activity was detected using the dual
luciferase reporter kit (Beyotime Institute of Biotechnology).
Immunohistochemistry
The expression of cleaved caspase-3, TAK1 and p-p65
in the lung sections was evaluated by immunohistochemical staining,
as previously described (30),
with the following primary antibodies diluted to 1:200: Cleaved
caspase-3 (cat no. 9664, Cell Signaling Technology, Inc.), TAK1
(cat no. 5206, Cell Signaling Technology, Inc.), and p-p65 (cat no.
3036, Cell Signaling Technology, Inc.). Samples were photographed
under a Leica DMD 108 light microscope. Immunohistochemical
staining was independently evaluated by two observers, and the
immunohistochemistry images of lung tissue samples were scored as
previously described (31).
Western blot analysis
Total protein was extracted using the RIPA buffer.
The extraction and isolation of nuclear and cytoplasmic proteins
were performed with the Cytoplasmic and Nuclear Protein Extraction
kit (cat no. 78833, Thermo Fisher Scientific, Inc.), according to
the manufacturer's instructions. The protein concentration was
determined using the bicinchoninic acid assay (Pierce, Thermo
Fisher Scientific Inc.), following centrifugation of the protein
fraction at 10,000 × g for at 4°C for 15 min. Proteins (20-50
μg) were separated by 12% SDS-PAGE (w/v) and transferred
onto PVDF membranes (EMD Millipore). The membranes were then
blocked with 1% BSA for 2 h at room temperature, and incubated with
the following primary antibodies overnight at 4°C: TAK1 (cat no.
5206, 1:1,000), Bax (cat no. 5023, 1:1,000), Bcl-2 (cat no. 3498,
1:1,000), nuclear p-p65 (cat no. 3036, 1:1,000), p-IκB-α (cat no.
2859, 1:1,000), IκB-α (cat no. 4814, 1:1,000), histone H3 (cat no.
9728, 1:1,000) and β-actin (cat no. 4970, 1:1,000). Subsequently,
the membranes were incubated with HRP-linked anti-rabbit IgG
antibody (cat no. 7074, 1:2,000) and incubated with ECL reagent (GE
Healthcare) for the detection of protein expression. All antibodies
were obtained from Cell Signaling Technology, Inc. The gray value
of the analyzed proteins was determined using the ImageJ software
(version 1.46; Rawak Software Inc.).
Statistical analysis
GraphPad Prism 5.0 (GraphPad Software, Inc.) was
used to perform the statistical analyses. Statistically significant
differences were analyzed using an unpaired Student's t-test or
one-way analysis of variance followed by Tukey's post hoc test. The
correlation between TAK1 and miR-129 expression was analyzed using
Pearson's correlation analysis. The differences in overall survival
were assessed by Kaplan-Meier survival analysis and the log-rank
test. All data are presented as the mean ± SD, and P<0.05 was
considered to indicate a statistically significant difference.
Results
Downregulation of miR-129 in mice with
ALI
The analysis of the expression of miRNAs included in
the miRNA array expression profile dataset (GSE133733) from GEO
revealed that there was a significant difference between the
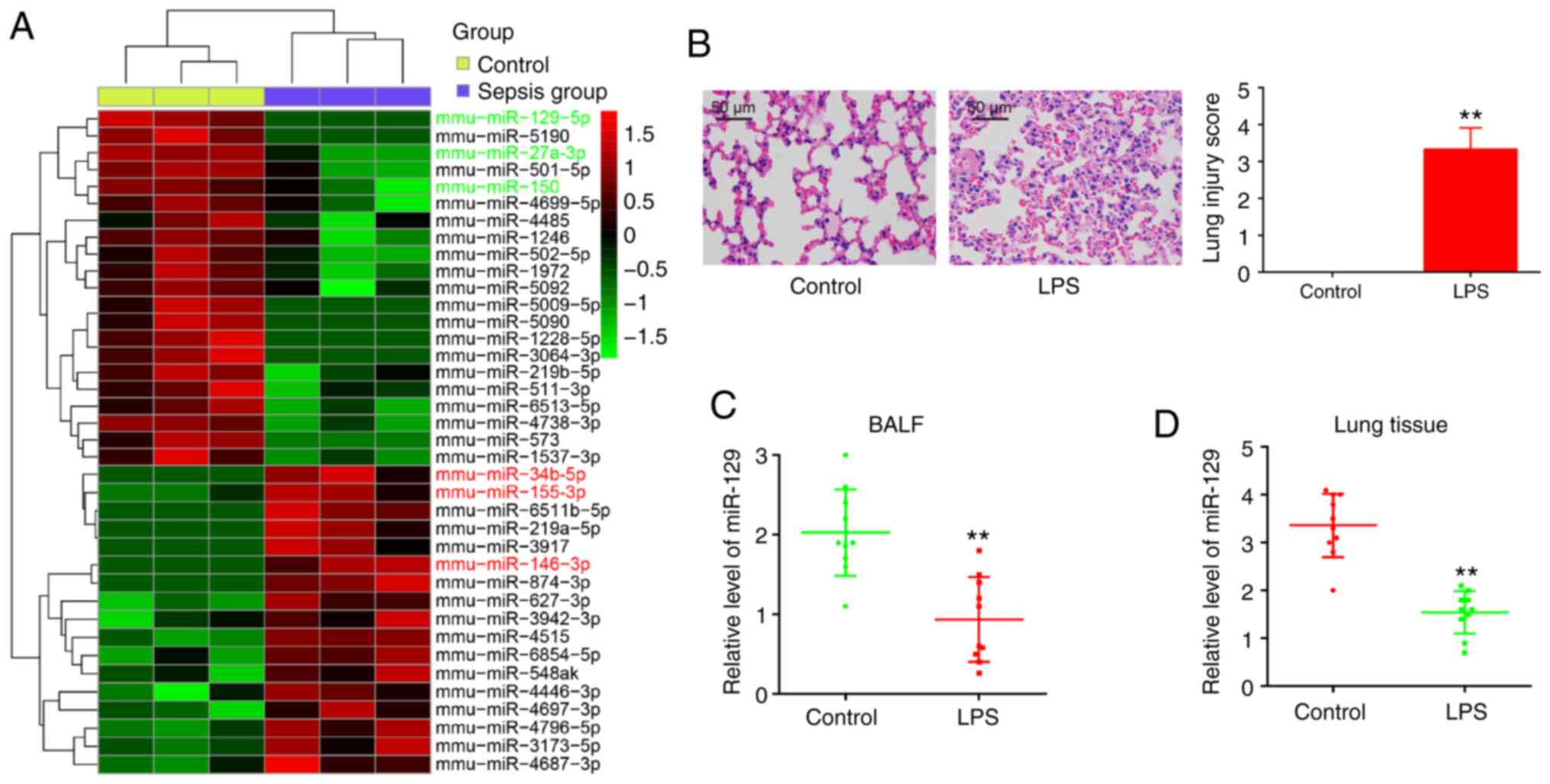
control group and the sepsis group (Fig. 1A). Out of the 38 miRNAs with a
notable difference in relative expression levels, 21 were
downregulated and 17 were upregulated in the sepsis group, compared
with the control group: The expression of miR-129-5p (32), miR-27a (33), and miR-150 (34) was decreased, while the expression
of miR-34b-5p (35), miR-155-3p
(36) and miR-146-3p (37) was increased. This finding is
consistent with previous reports and indicates the reliability of
the microarray results in the present study. However, miR-129 was
selected for further investigation as it exhibited the most
statistically significant difference between the two groups and as
previous studies have discussed the role of miR-129 in regulating
injury and inflammatory response in different organ injury models
(19,38,39).
To evaluate the therapeutic effects of miR-129, a
model of LPS-induced lung injury was used, as previously described
(40), as LPS administration is
one of the most widely used murine experimental models for lung
injury (41,42). As depicted in Fig. 1B, the administration of LPS alone
resulted in the destruction of the lung architecture and in
inflammation. The lung injury scores based on histopathological
changes were significantly higher in the LPS group than in the
control group, indicating that the ALI model was successfully
established. At 72 h after the LPS challenge, lung tissue and BALF
were harvested, and RT-qPCR was performed in order to measure the
miR-129 expression levels. As was expected, miR-129 expression was
notably decreased in response to the LPS challenge in BALF and lung
tissues (Fig. 1C and D). These
findings indicate that miR-129 may be involved in the development
of ALI.
Alleviation of LPS-induced lung injury
via miR-129 upregulation
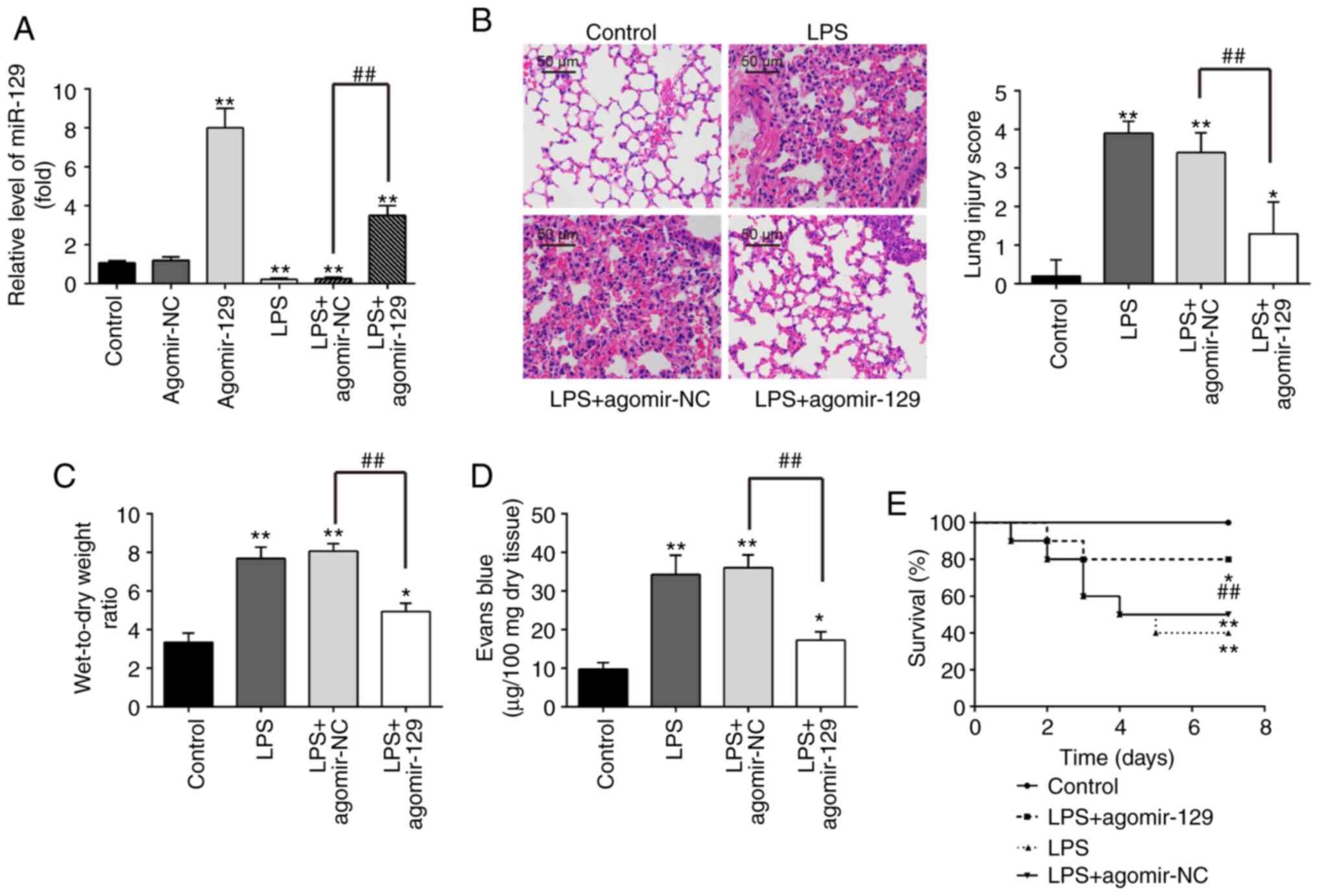
Gain-function experiments were performed through the
administration of agomiR-129, in order to investigate the potential
protective effects of miR-129 against LPS-induced ALI. The
expression levels of miR-129 in lung tissues was significantly
elevated following the agomiR-129 injection in comparison with the
control group expression levels (Fig. 2A), suggesting miR-129
overexpression has been successfully induced in mice, following
agomiR-129 administration. Moreover, the miR-129 expression levels
were significantly decreased in the LPS group as compared with the
control group, whereas in the LPS + agomiR-129 group, the level of
miR-129 was significantly increased compared with the LPS +
agomiR-NC group (Fig. 2A).
Histopathological analysis revealed that agomiR-129 administration
attenuated the severity of lung injury caused by LPS, as indicated
by the decrease in lung injury scores (Fig. 2B). The lung W/D ratio was
calculated as an indicator of lung edema, which is an important
feature of ALI (43). These
results demonstrated that the lung W/D ratio was markedly increased
by the LPS challenge, whereas it was significantly decreased
following agomiR-129 administration in mice with LPS-induced ALI
(Fig. 2C). In addition, the
evaluation of lung microvascular permeability with the EB
extravasation method revealed that the LPS challenge led to a
significant increase in EB extravasation, which is considered as an
indicator of lung vascular permeability, as compared with the
control group EB extravasation (Fig.
2D). As was expected, agomiR-129 administration effectively
reduced the LPS-induced increase in EB extravasation. Furthermore,
agomiR-129 administration resulted in a significant increase in the
survival rate (P<0.01; Fig.
2E). Taken together, these findings suggest that miR-129 may
alleviate the pathological effects of ALI.
Alleviation of LPS-induced inflammation
in BALF via miR-129 upregulation
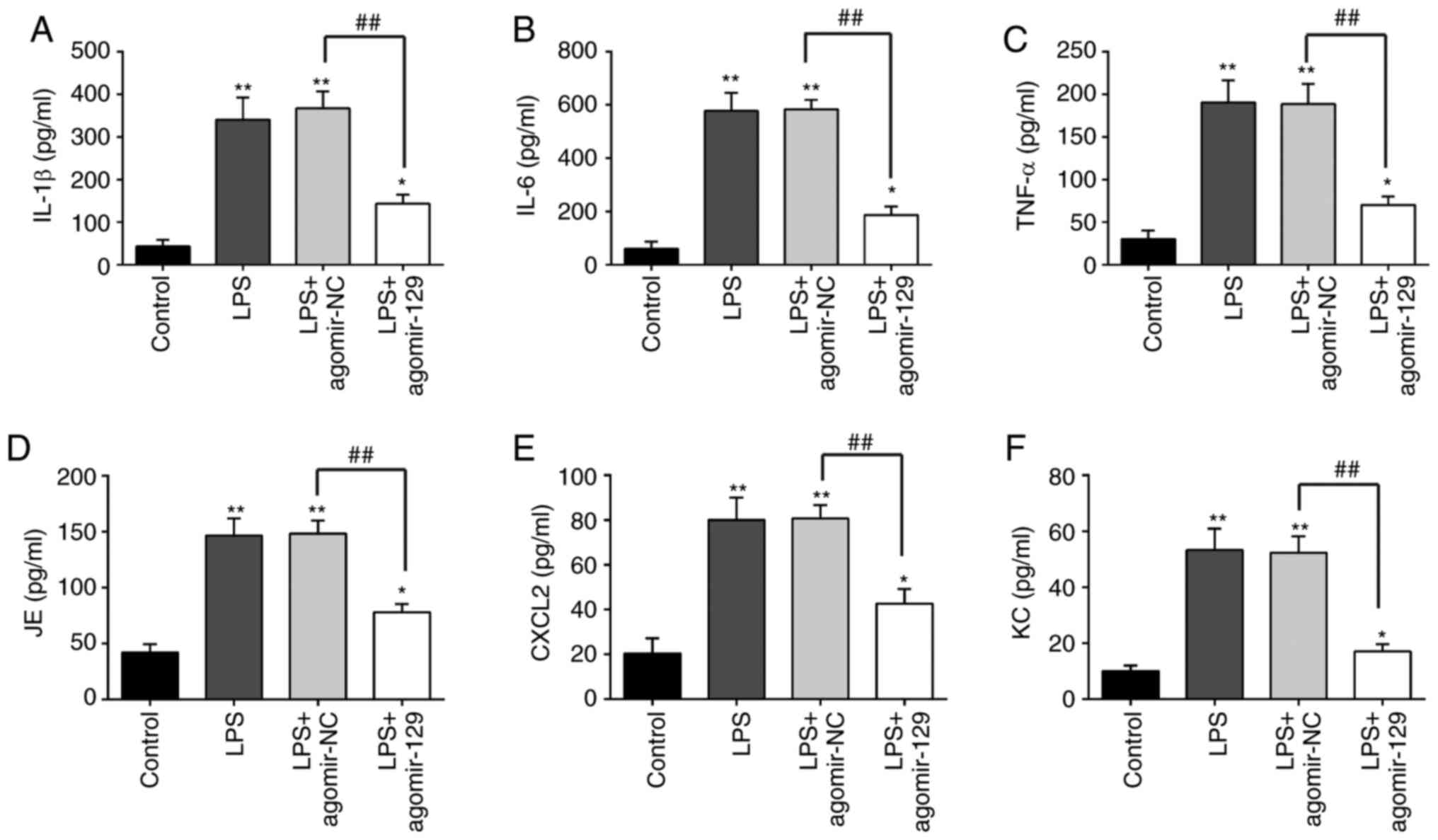
The effects of miR-129 on the inflammatory response
in ALI were then examined. The measurement of the levels of
pro-inflammatory cytokines and chemokines in BALF from mice with
ALI revealed that the concentrations of the inflammatory cytokines,
IL-1β, IL-6 and TNF-α, and the chemokines, JE, Cxcl2 and KC, were
markedly higher in the LPS group than in the control group.
However, agomiR-129 administration inhibited the LPS-induced
inflammatory cytokine and chemokine production (Fig. 3). These results indicate that
miR-129 upregulation alleviates LPS-induced inflammatory response
in ALI.
Alleviation of LPS-induced apoptosis by
miR-129 upregulation
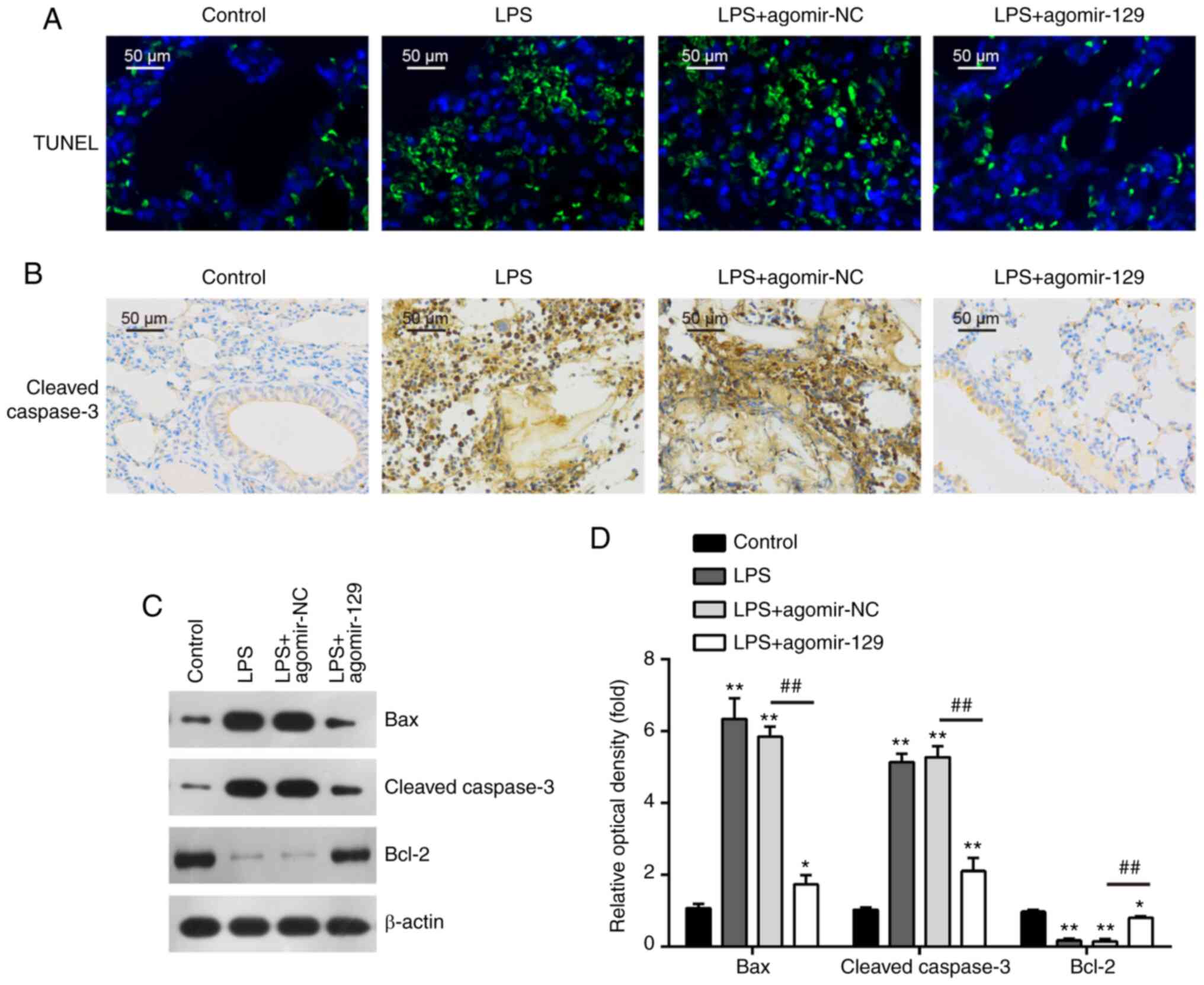
Since alveolar epithelial cells apoptosis is another
important feature of ALI, lung tissue apoptosis was evaluated by
TUNEL assay. The number of TUNEL-positive cells was markedly
increased in the mouse lungs following LPS administration; however,
the number of TUNEL-positive cells was markedly decreased in the
lungs of the mice that were administered agomir-129 after the LPS
administration (Fig. 4A). In
addition, the relative levels of important proteins involved in
apoptotic pathways were measured. Immunohistochemistry assay
demonstrated that the expression levels of cleaved caspase-3 were
markedly higher in the LPS group than in the control group, whereas
agomiR-129 administration markedly inhibited the LPS-induced
increase in caspase-3 expression levels (Fig. 4B). In addition, western blot
analysis revealed that in comparison with the control group, the
protein levels of the pro-apoptotic proteins, cleaved caspase-3 and
Bax, were significantly increased, while the protein levels of the
anti-apoptotic protein, Bcl-2, were decreased in the LPS group.
AgomiR-129 administration markedly attenuated the effects of LPS on
the apoptosis-related protein levels (Fig. 4C and D). Collectively, these
results suggest that miR-129 upregulation inhibits LPS-induced lung
cell apoptosis in mice.
miR-129-induced inhibition of TAK1 under
in vitro and in vivo conditions
In order to elucidate the molecular mechanisms
instigating the miR-129 protective effects against LPS-induced
apoptosis and the inflammatory response in mice with ALI, the
potential target genes of miR-129 were analyzed using PicTar
version 2007 (https://pictar.mdc-berlin.de/) and TargetScan Release
7.0 (http://targetscan.org/). Bioinformatics
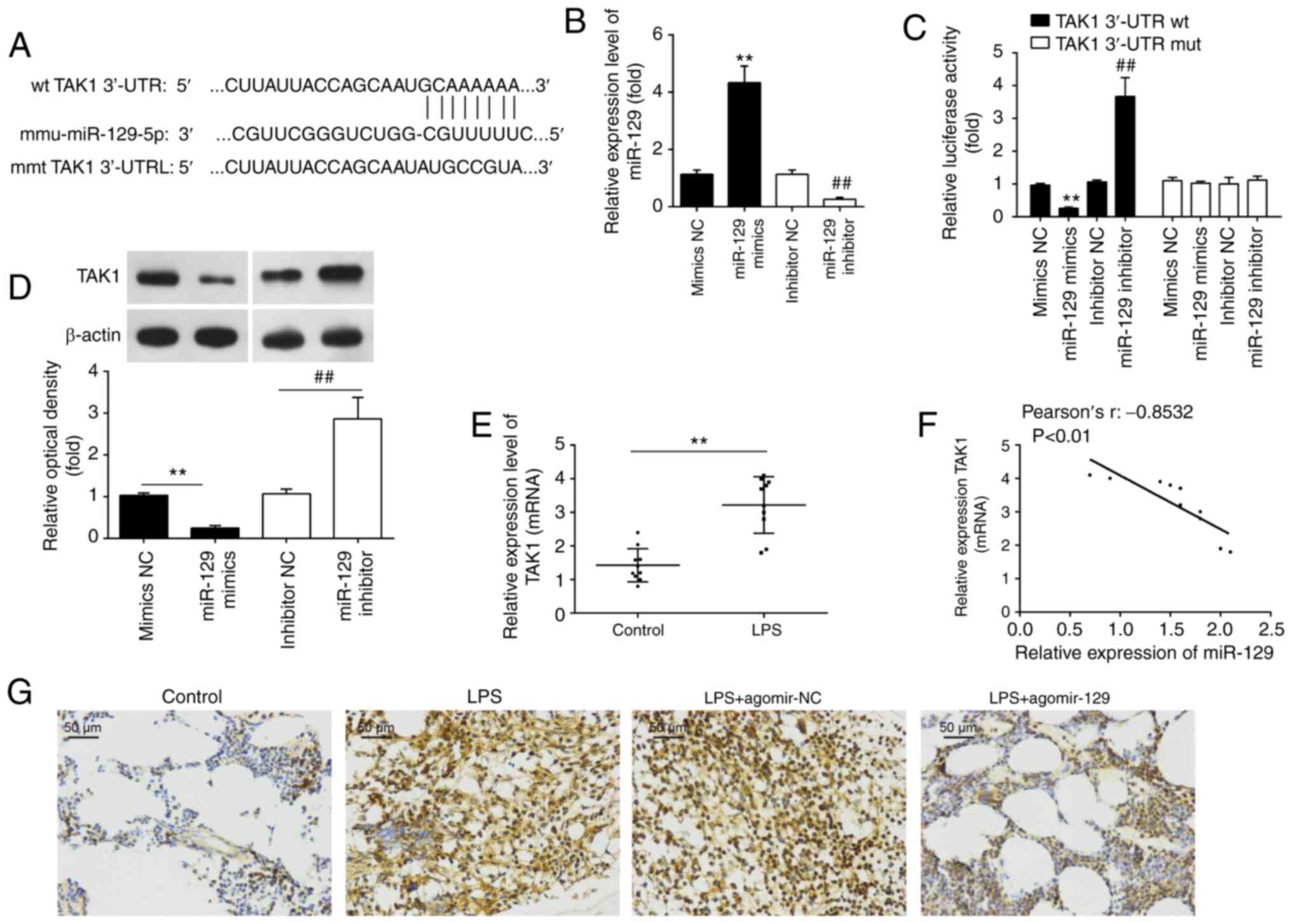
analysis indicated that TAK1 was a miR-129 potential target
(Fig. 5A). To verify this
association, a luciferase reporter assay was performed. To this
end, the transfection efficiency of miR-129 mimics/inhibitor was
first evaluated by RT-qPCR. The results revealed that miR-129
relative expression was significantly upregulated/downregulated as
compared with the mir-NC group (Fig.
5B). Subsequently, it was found that the luciferase activity of
wt-TAK1 3′UTR was significantly decreased after miR-129 mimics
transfection and increased after the miR-129 inhibitor
transfection. However, this effect was not observed, when mut-TAK1
3′UTR was used (Fig. 5C). It was
also observed that miR-129 overexpression resulted in a decrease in
TAK1 expression in RAW 264.7 cells, while miR-129 knockdown
resulted in a significant increase in TAK1 protein expression
levels (Fig. 5D).
In order to determine whether miR-129 also regulates
TAK1 expression in vivo, TAK1 expression was detected in the
lungs of mice with ALI. The results revealed that TAK1 expression
was significantly increased following the LPS challenge (Fig. 5E). Furthermore, miR-129
expression was found to inversely correlate with TAK1 protein
expression levels in lung tissues (Fig. 5F). As also observed in
vitro, the increase in TAK1 protein expression due to LPS
administration was markedly attenuated by the agomiR-129
administration (Fig. 5G). On the
whole, these results indicate that TAK1 is a functional target of
miR-129 in ALI.
TAK1-mediated protective effect of
miR-129 against LPS-induced apoptosis and inflammatory response in
RAW264.7 cells
Considering the important role of TAK1 in the
inflammatory response following ALI, the present study attempted to
determine whether TAK1 mediated the protective effects of miR-129
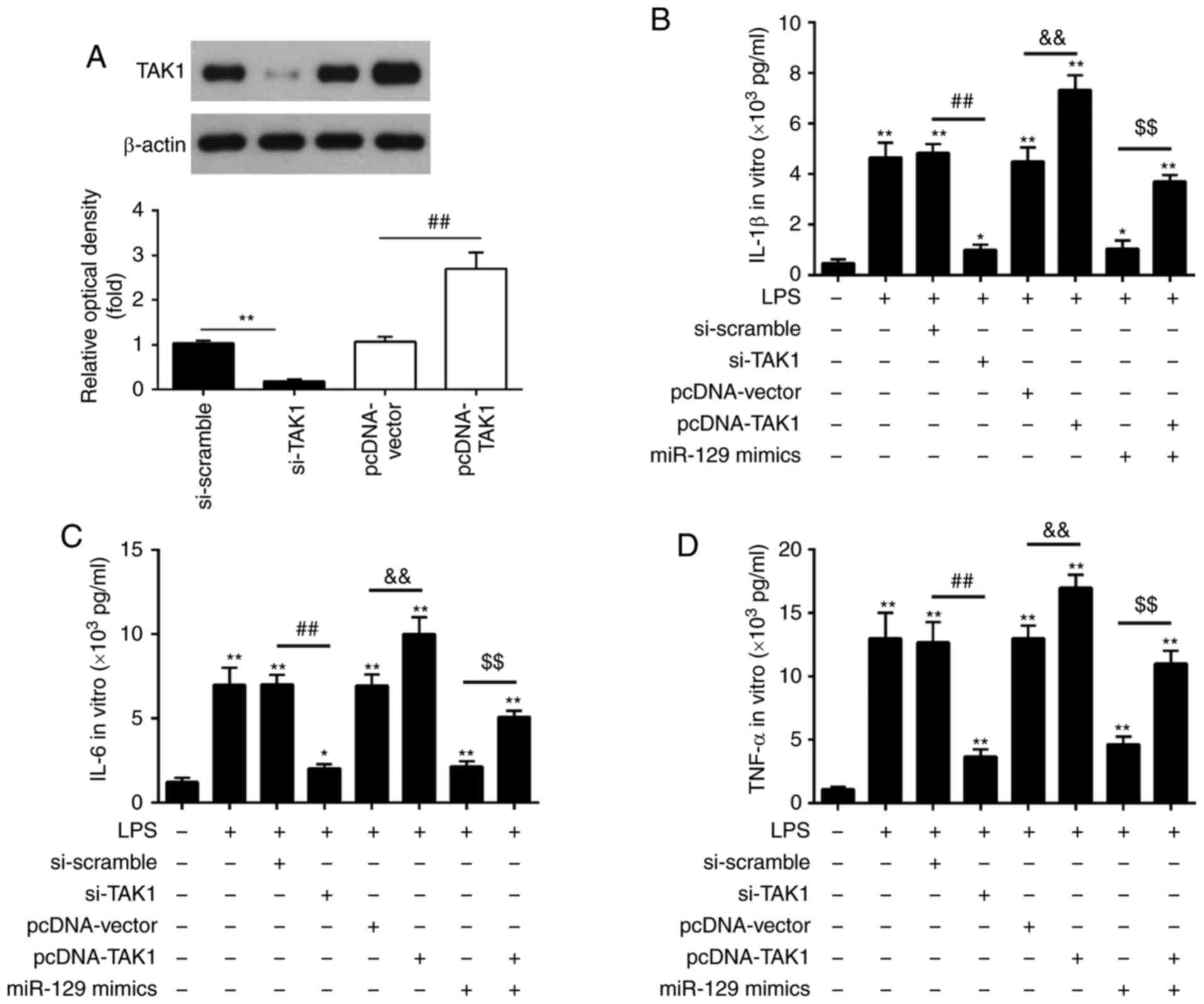
against ALI-associated inflammation (44). A TAK1 expression vector,
pcDNA-TAK1, or si-TAK1, together with miR-129 mimics, was
transfected into RAW264.7 cells 6 h prior to LPS stimulation. The
results demonstrated that pcDNA-TAK1 caused a significant increase
in TAK1 protein expression levels, while si-TAK1 transfection
resulted in a marked decrease in TAK1 protein expression levels
(Fig. 6A). The production of the
inflammatory cytokines, IL-1β, IL-6 and TNF-α was evaluated using
ELISA in LPS-stimulated RAW264.7 cells co-transfected with
pcDNA-TAK1 and miR-129 mimics or si-TAK1 and miR-129 mimics. As
depicted in Fig. 6B-D, LPS
stimulation significantly increased the secretion of IL-1β, IL-6
and TNF-α, which was suppressed by si-TAK1, whereas it was enhanced
by TAK1 overexpression. In LPS-stimulated RAW264.7 cells,
transfection with si-scramble or pcDNA-vector alone had no
significant effect on the inflammatory cytokine levels when
compared with the LPS group. Notably, the inhibitory effects of
miR-129 mimics on the production of the inflammatory cytokines were
reversed by TAK1 overexpression in LPS-stimulated RAW264.7 cells
Thus, these findings imply that miR-129 protects RAW264.7 cells
from LPS-induced inflammatory response by targeting TAK1.
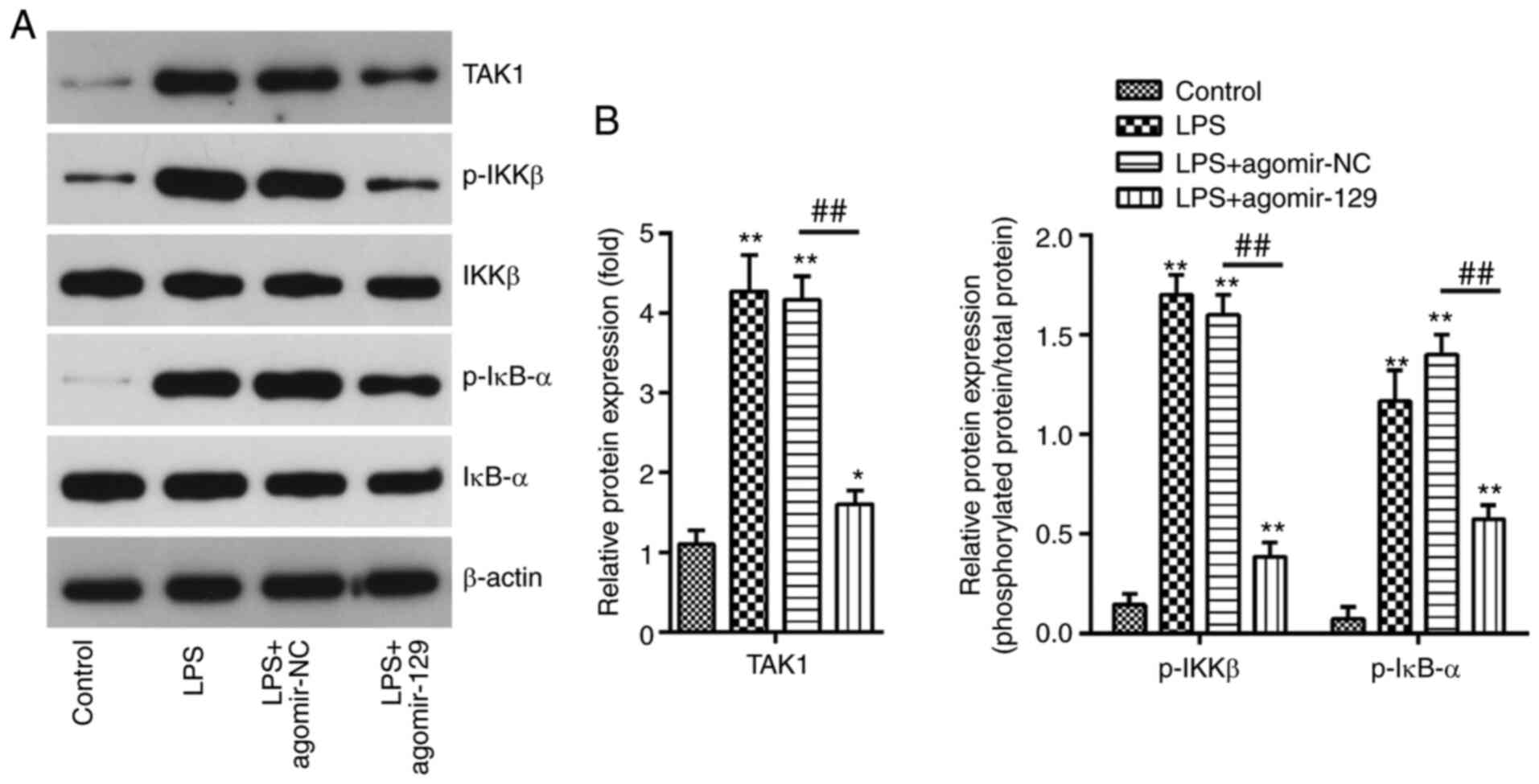
Inhibition of TAK1/NF-κB pathway
activation by miR-129 upregulation
TAK1 has been shown to activate the NF-κB pathway
(45), and the inhibition of the
TAK1/NF-κB signaling pathway has been found to reduce the
production of the pro-inflammatory cytokines, IL-1β, IL-6 and TNF-α
(46). In the present study, in
order to investigate the effects of miR-129 on TAK1/NF-κB pathway
activation, the p-IKKβ, IKKβ, p-IκB-α, and IκB-α expression levels
of were examined by western blot analysis. It was found that the
TAK1, p-IKKβ and p-IκB-α protein expression levels were
significantly increased after the LPS challenge, and that these
effects were attenuated by agomiR-129 administration (Fig. 7). To confirm that miR-129 blocks
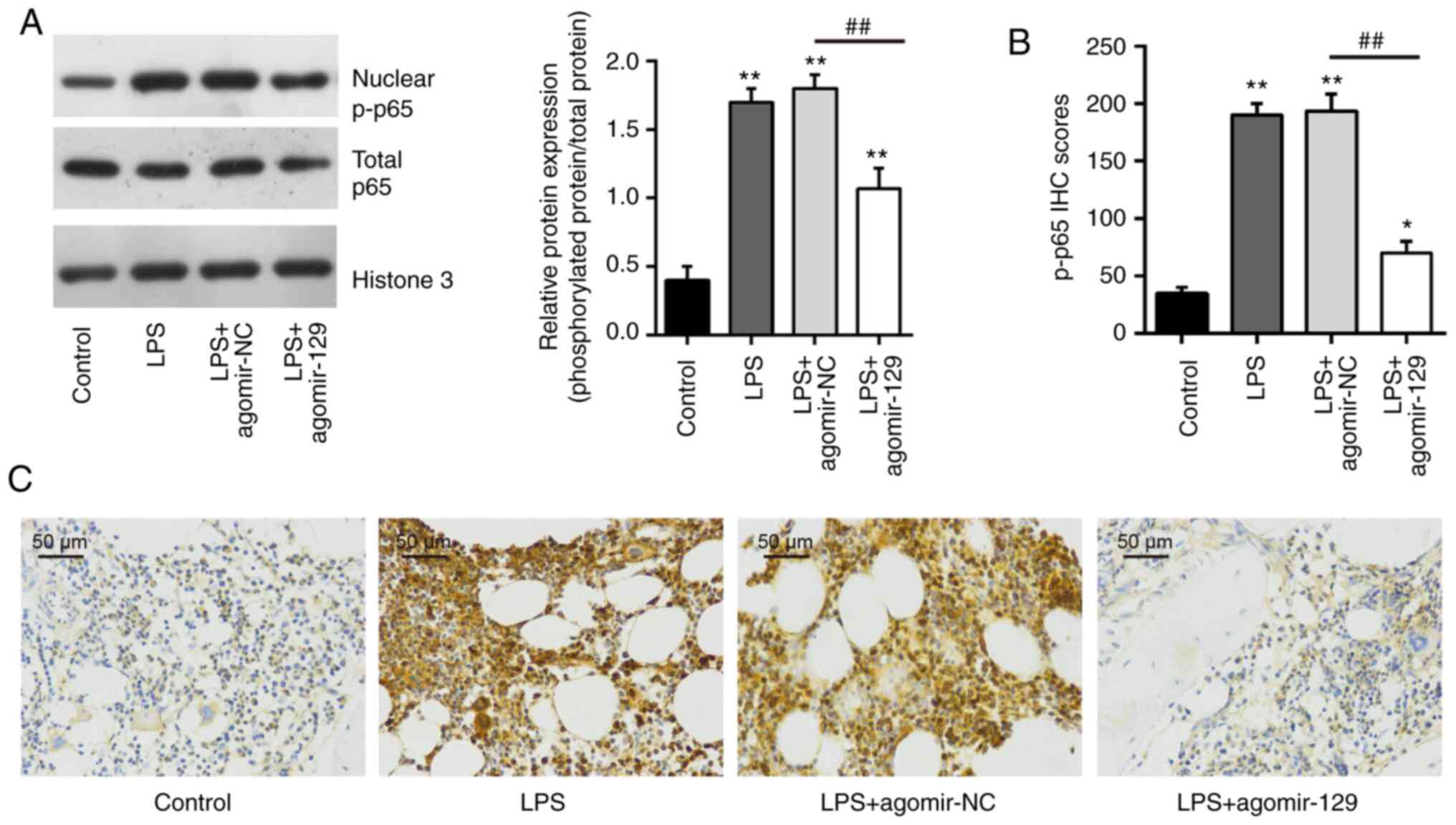
the NF-κB pathway, the expression of nuclear p-p65 was examined by
western blot analysis and immunohistochemistry. As was expected,
the increased expression of nuclear p-p65 induced by LPS was
reduced after agomiR-129 administration (Fig. 8). All these data indicate that
miR-129 upregulation may block TAK1-mediated activation of the
NF-κB pathway in mice with ALI.
Discussion
In the present study, the potential role of miR-129
in the pathogenic mechanisms of ALI was investigated. According to
the results obtained, miR-129 was downregulated in lung tissues and
BALF from mice with ALI; the subsequent upregulation of miR-129
alleviated LPS-induced ALI by suppressing pulmonary inflammation
and apoptosis in mice. Additionally, it was demonstrated that TAK1
was a target of miR-129, that the miR-129 mimic agomiR-129 blocked
LPS-induced TAK1 upregulation and, that it blocked activation of
the NF-κB pathway. These findings suggest that miR-129 upregulation
may be a promising therapeutic strategy for the prevention and
treatment of ALI in future clinical trials.
A large body of evidence has revealed that miRNAs
are aberrantly expressed in ALI, and may influence the
pathophysiological mechanisms of ALI, such as inflammation and
apoptosis (40,47). For example, Wang et al
(26) demonstrated that miR-326
suppressed sepsis-induced inflammation and oxidative stress in
macrophages via downregulation of TLR4. Additionally, Zhang et
al (48) found that miR-146a
upregulation alleviated inflammatory reaction and lung tissue
injury in mice with sepsis-induced ALI, and Cao et al
(49) demonstrated that miR-145
could ameliorate sepsis-induced lung injury by inhibiting the
TGFBR2 signaling pathway. Thus, understanding the functions of
miRNAs that are abnormally expressed in sepsis-induced ALI may help
to improve therapeutic approaches for ALI. In the present study,
the analysis of the microarray dataset (GSE133733) retrieved from
GEO revealed that large numbers of miRNAs were significantly
deregulated; in particular, miR-129 exhibited the highest level of
downregulation. The ALI-associated decrease in its expression was
confirmed by RT-qPCR analysis in lung tissue and BALF from mice
with ALI. All these findings strongly indicate that miR-129 may be
involved in sepsis-induced ALI.
The release of inflammatory factors and cell
apoptosis in alveolar epithelial cells are considered be the
underlying pathogenic mechanisms of ALI (50). In particular, the disruption of
epithelial cell function and increased alveolar epithelial cell
apoptosis have been observed in sepsis-induced ALI (51). Furthermore, apoptotic signaling
pathways were found to be significantly more active in patients
with ALI (52). In addition, the
in vivo administration of apoptotic protease inhibitors has
been found to reduce the mortality of mice with ALI (53). Therefore, the inhibition of
apoptosis may be an effective strategy with which to decrease the
incidence of ALI and ultimately provide a cure.
Previous studies have demonstrated that miR-129
plays protective roles in multiple injury models. For example, Yang
et al (54) demonstrated
that miR-129 overexpression improved neurological function by
reducing tissue loss and cell apoptosis in a rat spinal cord injury
model. Furthermore, Zeng et al (18) found that the upregulation of
miR-129 reduced nerve injury and inflammatory response in rats with
Alzheimer's disease via the downregulation of SOX6, and Ma et
al (38) reported that
miR-129 alleviated myocardial injury induced by I/R both under
in vitro and in vivo conditions by targeting
suppressor of cytokine signaling 2. Chen et al (19) also found that miR-129 played a
protective role in myocardial I/R injury by regulating HMGB1
expression. Importantly, a recent study reported that the
expression of miR-129 was decreased in mice with sepsis, and an
enhanced miR-129 expression markedly improved sepsis-induced
intestinal injury (55).
However, the role of miR-129 in sepsis-induced lung injury has not
yet been reported, at least to the best of our knowledge. In the
present study, it was demonstrated that miR-129 upregulation
markedly reduced the severity of lung injury and alleviated lung
edema and lung permeability, accompanied by a significant reduction
in the inflammatory response and apoptosis in mice with ALI. Taken
together, the aforementioned findings indicate that the
upregulation of miR-129 alleviated in vivo LPS-induced lung
injury by suppressing inflammation and apoptosis. However, the
underlying molecular mechanisms involved in miR-129-mediated
inflammation and apoptosis suppression remain unclear.
TAK1, which is a member of the mitogen-activated
protein kinase family, has received attention in inflammatory
diseases including ALI (44,56). For instance, the inhibition of
TAK1 was previously shown to mediate the potent protective effect
of 4′-hydroxywogonin against LPS-induced in mice with ALI (57). Another study also mentioned that
methyl salicylate 2-O-β-d-lactoside, a natural derivative of
salicylate extracted from Gaultheria yunnanensis, exerted a
therapeutic effect against LPS-induced ALI by inhibiting TAK1/NF-κB
in mice (12). In the present
study, bioinformatics analysis and luciferase assay indicated that
TAK1 was a direct target of miR-129. More importantly, TAK1 was
significantly upregulated and inversely correlated with miR-129
levels in lung tissues from ALI mice. Furthermore, it was found
that TAK1 overexpression reversed miR-129 inhibitory effects on
LPS-induced inflammatory response and apoptosis in RAW264.7 cells,
whereas TAK1 inhibition aggravated LPS-induced inflammatory
response and apoptosis. All these data indicate that miR-129
upregulation improves LPS-induced inflammation and apoptosis
through TAK1 targeting.
It is well known that NF-κB is a downstream
signaling factor of TAK1 (45),
and the TAK1/NF-κB axis has been demonstrated to play important
roles in a variety of biological processes, such as inflammation
(58). Of note, a previous study
demonstrated that miR-129 ameliorated intestinal inflammation in
trinitrobenzene sulphonic acid-induced colitis mice through
inhibition of the NF-κB signaling pathway (59). Another study demonstrated that
miR-129 improved neuronal injury of the hippocampus by suppressing
the inflammation response via inhibition of the NF-kB signaling
pathway (60). Given the
interaction between TAK1 and miR-129, it was further determined
whether miR-129 affects the NF-κB pathway by regulating TAK1
expression in sepsis-induced ALI. As was expected, miR-129
upregulation significantly decreased NF-κB pathway-related core
factor (nuclear p-p65, p-IKKβ, and p-IκB-α) relative expression
levels in ALI mice. These results indicate that miR-129 suppressed
the inflammatory response and apoptosis in ALI through the
TAK1/NF-κB signaling pathway.
miR-129 may also exert its protective effects in ALI
via the regulation of other downstream targets. However,
investigating additional miR-129 targets was beyond the scope of
the present study, due to current laboratory limitations.
Therefore, further future research is required in order to
investigate and identify other alternative miR-129 targets taking
part in ALI development. In addition, the present study
investigated the roles of miR-129 and its underlying mechanisms
in vitro and in vivo model of ALI; however, data from
clinical trials are required to validate the preliminary in
vitro and in vivo result obtained. Therefore, the
functions of miR-129 warrant further investigation in clinical
trials data in the future.
In conclusion, the present study demonstrated that
miR-129 ameliorated sepsis-induced ALI, via the suppression of
inflammatory response and apoptosis, by targeting the TAK1/NF-κB
pathway. Thus, the miR-129/TAK1/NF-κB axis may serve as a potential
therapeutic target for ALI and holds promise for its therapeutic
management.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WY, LX, XJ and SL performed the experiments,
contributed to data analysis, and wrote the manuscript. WY, LX, XJ
and SL analyzed the data. LW conceptualized the study design and
contributed to data analysis and experimental materials. WY and LW
confirm the authenticity of all the raw data. All authors have read
and approved the final version of the manuscript.
Ethics approval and consent to
participate
The animal experiments were approved by the Animal
Care and Use Committee of Henan Provincial People's Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
The present study was supported by the 23456 Talent Project of
Henan Provincial People's Hospital and Medical Service Capacity
Improvement Project in Henan Provincial Medical Institutions.
References
|
1
|
Hoesel LM, Neff TA, Neff SB, Younger JG,
Olle EW, Gao H, Pianko MJ, Bernacki KD, Sarma JV and Ward PA:
Harmful and protective roles of neutrophils in sepsis. Shock.
24:40–47. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Villar J, Blanco J, Añón JM, Santos-Bouza
A, Blanch L, Ambrós A, Gandía F, Carriedo D, Mosteiro F, Basaldúa
S, et al: The ALIEN study: Incidence and outcome of acute
respiratory distress syndrome in the era of lung protective
ventilation. Intensive Care Med. 37:1932–1941. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gill SE, Taneja R, Rohan M, Wang L and
Mehta S: Pulmonary microvascular albumin leak is associated with
endothelial cell death in murine sepsis-induced lung injury in
vivo. PLoS One. 9:e885012014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jiang C, Ting AT and Seed B: PPAR-gamma
agonists inhibit production of monocyte inflammatory cytokines.
Nature. 391:82–86. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Strieter RM, Belperio JA and Keane MP:
Cytokines in innate host defense in the lung. J Clin Invest.
109:699–705. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hoesel B and Schmid JA: The complexity of
NF-κB signaling in inflammation and cancer. Mol Cancer. 12:862013.
View Article : Google Scholar
|
|
7
|
Jiang T, Tian F, Zheng H, Whitman SA, Lin
Y, Zhang Z, Zhang N and Zhang DD: Nrf2 suppresses lupus nephritis
through inhibition of oxidative injury and the NF-κB-mediated
inflammatory response. Kidney Int. 85:333–343. 2014. View Article : Google Scholar
|
|
8
|
Gross CM, Kellner M, Wang T, Lu Q, Sun X,
Zemskov EA, Noonepalle S, Kangath A, Kumar S, Gonzalez-Garay M, et
al: LPS-induced acute lung injury involves NF-κB-mediated down-
regulation of SOX18. Am J Respir Cell Mol Biol. 58:614–624. 2018.
View Article : Google Scholar :
|
|
9
|
Yang H, Lv H, Li H, Ci X and Peng L:
Oridonin protects LPS-induced acute lung injury by modulating
Nrf2-mediated oxidative stress and Nrf2-independent NLRP3 and NF-κB
pathways. Cell Commun Signal. 17:622019. View Article : Google Scholar
|
|
10
|
Yang KY, Arcaroli JJ and Abraham E: Early
alterations in neutrophil activation are associated with outcome in
acute lung injury. Am J Respir Crit Care Med. 167:1567–1574. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schwartz MD, Moore EE, Moore FA, Shenkar
R, Moine P, Haenel JB and Abraham E: Nuclear factor-kappa B is
activated in alveolar macrophages from patients with acute
respiratory distress syndrome. Crit Care Med. 24:1285–1292. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang S, Yu Z, Yuan T, Wang L, Wang X, Yang
H, Sun L, Wang Y and Du G: Therapeutic effect of methyl salicylate
2-O-β-d-lactoside on LPS-induced acute lung injury by inhibiting
TAK1/NF-kappaB phosphorylation and NLRP3 expression. Int
Immunopharmacol. 40:219–228. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guo Z, Gu Y, Wang C, Zhang J, Shan S, Gu
X, Wang K, Han Y and Ren T: Enforced expression of miR-125b
attenuates LPS-induced acute lung injury. Immunol Lett. 162:18–26.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang Y, Xie Y, Zhang L and Zhao H:
MicroRNA-155 participates in smoke-inhalation-induced acute lung
injury through inhibition of SOCS-1. Molecules. 25:10222020.
View Article : Google Scholar :
|
|
16
|
Wan G, An Y, Tao J, Wang Y, Zhou Q, Yang R
and Liang Q: MicroRNA-129-5p alleviates spinal cord injury in mice
via suppressing the apoptosis and inflammatory response through
HMGB1/TLR4/NF-κB pathway. Biosci Rep. 40:BSR201933152020.
View Article : Google Scholar
|
|
17
|
Li XQ, Chen FS, Tan WF, Fang B, Zhang ZL
and Ma H: Elevated microRNA-129-5p level ameliorates
neuroinflammation and blood-spinal cord barrier damage after
ischemia-reperfusion by inhibiting HMGB1 and the TLR3-cytokine
pathway. J Neuroinflammation. 14:2052017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zeng Z, Liu Y, Zheng W, Liu L, Yin H,
Zhang S, Bai H, Hua L, Wang S, Wang Z, et al: MicroRNA-129-5p
alleviates nerve injury and inflammatory response of Alzheimer's
disease via downregulating SOX6. Cell cycle. 18:3095–3110. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen ZX, He D, Mo QW, Xie LP, Liang JR,
Liu L and Fu WJ: MiR-129-5p protects against myocardial
ischemia-reperfusion injury via targeting HMGB1. Eur Rev Med
Pharmacol Sci. 24:4440–4450. 2020.PubMed/NCBI
|
|
20
|
Xu Z, Zhang C, Cheng L, Hu M, Tao H and
Song L: The microRNA miR-17 regulates lung FoxA1 expression during
lipopolysaccharide-induced acute lung injury. Biochem Biophys Res
Commun. 445:48–53. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Carbone L, Carbone ET, Yi EM, Bauer DB,
Lindstrom KA, Parker JM, Austin JA, Seo Y, Gandhi AD and Wilkerson
JD: Assessing cervical dislocation as a humane euthanasia method in
mice. J Am Assoc Lab Anim Sci. 51:352–356. 2012.PubMed/NCBI
|
|
22
|
Li C, Yang D, Cao X, Wang F, Jiang H, Guo
H, Du L, Guo Q and Yin X: LFG-500, a newly synthesized flavonoid,
attenuates lipopolysaccharide-induced acute lung injury and
inflammation in mice. Biochem Pharmacol. 113:57–69. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lei C, Jiao Y, He B, Wang G, Wang Q and
Wang J: RIP140 down-regulation alleviates acute lung injury via the
inhibition of LPS-induced PPARγ promoter methylation. Pulm
Pharmacol Ther. 37:57–64. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ayaz G, Halici Z, Albayrak A, Karakus E
and Cadirci E: Evaluation of 5-HT7 receptor trafficking on in vivo
and in vitro model of lipopolysaccharide (LPS)-induced inflammatory
cell injury in rats and LPS-treated A549 cells. Biochem Genet.
55:34–47. 2017. View Article : Google Scholar
|
|
25
|
Shao L, Meng D, Yang F, Song H and Tang D:
Irisin-mediated protective effect on LPS-induced acute lung injury
via suppressing inflammation and apoptosis of alveolar epithelial
cells. Biochem Biophys Res Commun. 487:194–200. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang Z, Yan J, Yang F, Wang D, Lu Y and
Liu L: MicroRNA-326 prevents sepsis-induced acute lung injury via
targeting TLR4. Free Radic Res. 54:408–418. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bian B, Yu XF, Wang GQ and Teng TM: Role
of miRNA-1 in regulating connexin 43 in ischemia-reperfusion heart
injury: A rat model. Cardiovasc Pathol. 27:37–42. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
29
|
Moitra J, Sammani S and Garcia JG:
Re-evaluation of evans blue dye as a marker of albumin clearance in
murine models of acute lung injury. Transl Res. 150:253–265. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Villar J, Cabrera-Benitez NE, Valladares
F, García-Hernández S, Ramos-Nuez Á, Martín-Barrasa JL, Muros M,
Kacmarek RM and Slutsky AS: Tryptase is involved in the development
of early ventilator-induced pulmonary fibrosis in sepsis-induced
lung injury. Crit Care. 19:1382015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Varghese F, Bukhari AB, Malhotra R and De
A: IHC Profiler: An open source plugin for the quantitative
evaluation and automated scoring of immunohistochemistry images of
human tissue samples. PLoS One. 9:e968012014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wu G, Li X, Li M and Zhang Z: Long
non-coding RNA MALAT1 promotes the proliferation and migration of
Schwann cells by elevating BDNF through sponging miR-129-5p. Exp
Cell Res. 390:1119372020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ju M, Liu B, He H, Gu Z, Liu Y, Su Y, Zhu
D, Cang J and Luo Z: MicroRNA-27a alleviates LPS-induced acute lung
injury in mice via inhibiting inflammation and apoptosis through
modulating TLR4/MyD88/NF-κB pathway. Cell Cycle. 17:2001–2018.
2018. View Article : Google Scholar :
|
|
34
|
Li P, Yao Y, Ma Y and Chen Y: MiR-150
attenuates LPS-induced acute lung injury via targeting AKT3. Int
Immunopharmacol. 75:1057942019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xie W, Lu Q, Wang K, Lu J, Gu X, Zhu D,
Liu F and Guo Z: miR-34b-5p inhibition attenuates lung inflammation
and apoptosis in an LPS-induced acute lung injury mouse model by
targeting progranulin. J Cell Physiol. 233:6615–6631. 2018.
View Article : Google Scholar :
|
|
36
|
Yan Y, Lou Y and Kong J: MiR-155 expressed
in bone marrow-derived lymphocytes promoted
lipopolysaccharide-induced acute lung injury through Ang-2-Tie-2
pathway. Biochem Biophys Res Commun. 510:352–357. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Vaporidi K, Vergadi E, Kaniaris E,
Hatziapostolou M, Lagoudaki E, Georgopoulos D, Zapol WM, Bloch KD
and Iliopoulos D: Pulmonary microRNA profiling in a mouse model of
ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol.
303:L199–L207. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ma R, Chen X, Ma Y, Bai G and Li DS:
MiR-129-5p alleviates myocardial injury by targeting suppressor of
cytokine signaling 2 after ischemia/reperfusion. Kaohsiung J Med
Sci. 36:599–606. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Huang X, Hou X, Chuan L, Wei S, Wang J,
Yang X and Ru J: miR-129-5p alleviates LPS-induced acute kidney
injury via targeting HMGB1/TLRs/NF-kappaB pathway. Int
Immunopharmacol. 89:1070162020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fang Y, Gao F, Hao J and Liu Z:
microRNA-1246 mediates lipopolysaccharide-induced pulmonary
endothelial cell apoptosis and acute lung injury by targeting
angiotensin-converting enzyme 2. Am J Transl Res. 9:1287–1296.
2017.PubMed/NCBI
|
|
41
|
Tianzhu Z and Shumin W: Esculin inhibits
the inflammation of LPS-induced acute lung injury in mice via
regulation of TLR/NF-κB pathways. Inflammation. 38:1529–1536. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mei SH, McCarter SD, Deng Y, Parker CH,
Liles WC and Stewart DJ: Prevention of LPS-induced acute lung
injury in mice by mesenchymal stem cells overexpressing
angiopoietin 1. PLoS Med. 4:e2692007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cheng KT, Xiong S, Ye Z, Hong Z, Di A,
Tsang KM, Gao X, An S, Mittal M, Vogel SM, et al:
Caspase-11-mediated endothelial pyroptosis underlies
endotoxemia-induced lung injury. J Clin Invest. 127:4124–4135.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Liu H, Lin Z and Ma Y: Suppression of Fpr2
expression protects against endotoxin-induced acute lung injury by
interacting with Nrf2-regulated TAK1 activation. Biomed
Pharmacother. 125:1099432020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gao D, Wang R, Li B, Yang Y, Zhai Z and
Chen DY: WDR34 is a novel TAK1-associated suppressor of the
IL-1R/TLR3/TLR4-induced NF-kappaB activation pathway. Cell Mol Life
Sci. 66:2573–2584. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chen Q, Wu S, Wu Y, Chen L and Pang Q:
MiR-149 suppresses the inflammatory response of chondrocytes in
osteoarthritis by down-regulating the activation of TAK1/NF-κB.
Biomed Pharmacother. 101:763–768. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Nan CC, Zhang N, Cheung KCP, Zhang HD, Li
W, Hong CY, Chen HS, Liu XY, Li N and Cheng L: Knockdown of lncRNA
MALAT1 alleviates LPS-induced acute lung injury via inhibiting
apoptosis through the miR-194-5p/FOXP2 axis. Front Cell Dev Biol.
8:5868692020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhang J, Ding C, Shao Q, Liu F, Zeng Z,
Nie C and Qian K: The protective effects of transfected
microRNA-146a on mice with sepsis-induced acute lung injury in
vivo. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 27:591–594. 2015.In
Chinese. PubMed/NCBI
|
|
49
|
Cao X, Zhang C, Zhang X, Chen Y and Zhang
H: MiR-145 negatively regulates TGFBR2 signaling responsible for
sepsis-induced acute lung injury. Biomed Pharmacother. 111:852–858.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Li L, Wu W, Huang W, Hu G, Yuan W and Li
W: NF-κB RNAi decreases the Bax/Bcl-2 ratio and inhibits
TNF-α-induced apoptosis in human alveolar epithelial cells. Inflamm
Res. 62:387–397. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Gong Y, Lan H, Yu Z, Wang M, Wang S, Chen
Y, Rao H, Li J, Sheng Z and Shao J: Blockage of glycolysis by
targeting PFKFB3 alleviates sepsis-related acute lung injury via
suppressing inflammation and apoptosis of alveolar epithelial
cells. Biochem Biophys Res Commun. 491:522–529. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Albertine KH, Soulier MF, Wang Z, Ishizaka
A, Hashimoto S, Zimmerman GA, Matthay MA and Ware LB: Fas and fas
ligand are up-regulated in pulmonary edema fluid and lung tissue of
patients with acute lung injury and the acute respiratory distress
syndrome. Am J Pathol. 161:1783–1796. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kawasaki M, Kuwano K, Hagimoto N, Matsuba
T, Kunitake R, Tanaka T, Maeyama T and Hara N: Protection from
lethal apoptosis in lipopolysaccharide-induced acute lung injury in
mice by a caspase inhibitor. Am J Pathol. 157:597–603. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yang R, Cai X, Li J, Liu F and Sun T:
Protective effects of MiR-129-5p on acute spinal cord injury rats.
Med Sci Monit. 25:8281–8288. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Du X, Tian D, Wei J, Yan C, Hu P, Wu X and
Yang W: MEG3 alleviated LPS-induced intestinal injury in sepsis by
modulating miR-129-5p and surfactant protein D. Mediators Inflamm.
2020:82327342020. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Chen Z, Zhang D, Li M and Wang B:
Costunolide ameliorates lipoteichoic acid-induced acute lung injury
via attenuating MAPK signaling pathway. Int Immunopharmacol.
61:283–289. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Fan C, Wu LH, Zhang GF, Xu F, Zhang S,
Zhang X, Sun L, Yu Y, Zhang Y and Ye RD: 4′-Hydroxywogonin
suppresses lipopolysaccharide-induced inflammatory responses in RAW
264.7 macrophages and acute lung injury mice. PLoS One.
12:e01811912017. View Article : Google Scholar
|
|
58
|
Cai PC, Shi L, Liu VW, Tang HW, Liu IJ,
Leung TH, Chan KK, Yam JW, Yao KM, Ngan HY and Chan DW: Elevated
TAK1 augments tumor growth and metastatic capacities of ovarian
cancer cells through activation of NF-κB signaling. Oncotarget.
5:7549–7562. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Meng Q, Wu W, Pei T, Xue J, Xiao P, Sun L,
Li L and Liang D: miRNA-129/FBW7/NF-κB, a novel regulatory pathway
in inflammatory bowel disease. Mol Ther Nucleic Acids. 19:731–740.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Liu AH, Wu YT and Wang YP: MicroRNA-129-5p
inhibits the development of autoimmune encephalomyelitis-related
epilepsy by targeting HMGB1 through the TLR4/NF-kB signaling
pathway. Brain Res Bull. 132:139–149. 2017. View Article : Google Scholar : PubMed/NCBI
|