A large number of bacteria live in various parts of
the human body (skin, oral cavity, pharynx, upper respiratory
tract, stomach, small intestine, colon). The gastrointestinal tract
contains ~100 trillion intestinal bacteria of ~1,000 species
(weighing ~1.5 kg), which live in symbiosis with humans. The
majority of intestinal bacteria are found in the colon (1-4).
Dysbiosis, a qualitative and quantitative aberrancy of gut
micro-biota, has attracted marked attention. At present, advances
in molecular biological techniques have rendered it possible to
analyze gut microbiota at the DNA and RNA levels without culturing,
and methods such as 16S ribosomal RNA (16S rRNA) targeting analysis
and metagenomic analysis (analysis of the entire genetic
information of bacteria that constitute the gut microbiota) using
next-generation sequencers have been developed (5). The
relationship between gut microbiota and various diseases has been
extensively reported (1-4).
Gut microbiota has been revealed to play important
roles not only in digestion but also in immunity and metabolism.
Gut microbiota is essential for the immune system, energy intake
and fat storage, and humans use them to build complex immune
regulatory mechanisms and to obtain energy from food (1-4). With
proper diet, the gut microbiota can trigger changes in the balance
of short-chain fatty acids, which are used as an energy source (3).
Gut microbiota can be said to be an organ in itself. More than 99%
of gut microbiota belong to four phylums: Firmicutes phylum
(gram-positive bacteria), Bacteroidetes phylum
(gram-negative bacteria), Proteobacteria phylum (such as
Escherichia coli, Salmonella, Vibrio and Helicobacter), and
Actinobacteria phylum (such as Bifidobacteria) (6).
The composition of gut microbiota markedly changes with aging (7).
There are several patterns of aging-related changes in the gut
microbiota, including a group that decreases with aging
(Actinobacteria), a group that increases with aging
(Bacteroidetes), a group that is more prevalent only in
adults (Firmicutes), and a group that is more prevalent in
infants and the elderly (Proteobacteria) (7). The
composition of gut microbiota can also change with food intake (8).
The intestines of people who regularly consume an abundance of
vegetables, fish, and fiber are likely to be rich in bacteria that
help reduce inflammation, while the intestines of meat-lovers are
likely to be rich in bacteria that promote inflammation (8).
Long-term improvement of eating habits can improve the balance of
intestinal microflora.
Rigorous research in recent years has revealed that
the composition of the gut microbiota is altered and the diversity
of bacteria is reduced (dysbiosis) in obesity, inflammatory bowel
diseases, and liver diseases compared with healthy individuals
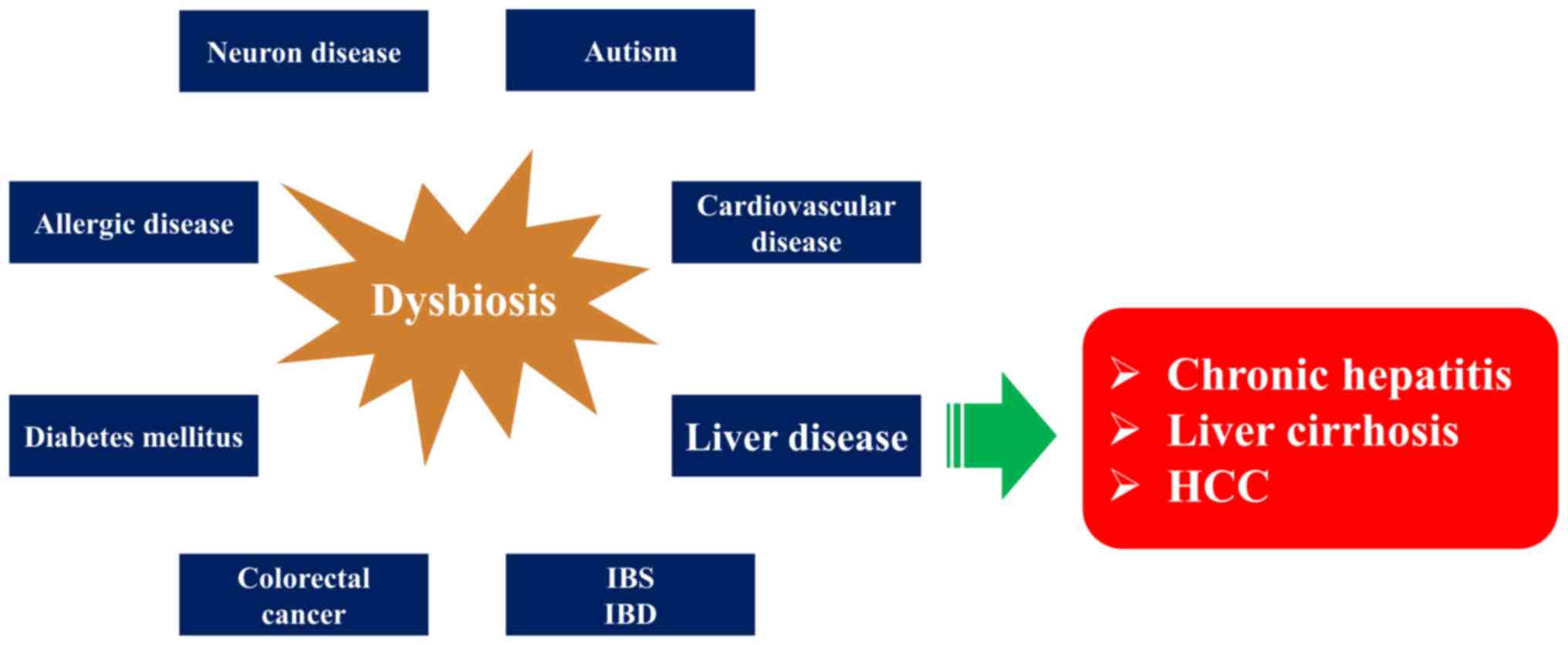
(2,4). At present, such changes (i.e., dysbiosis) have been noted
in colorectal cancer (9), type 2 diabetes (10), irritable bowel
syndrome (11), atherosclerotic heart diseases (12,13), allergic
diseases (14), autism (15), and even neurological diseases (16)
(Fig. 1). Thus, aberrancies in
the balance of the gut microbiota can disrupt host homeostasis and
lead to a variety of diseases. The liver is the first organ to be
nourished by the portal blood flow of intestinal origin, and liver
diseases are considered to be strongly influenced by various
factors of intestinal origin, such as intestinal bacteria,
bacterial components, and intestinal bacterial metabolites (17).
Various factors, including pathogen-associated molecular patterns
(PAMPs), which are transported to the liver by portal vein blood
flow, have attracted particular attention and their significance
has been extensively investigated (17).
Hepatic stellate cells (HSCs) and hepatic
macrophages are important cells that are affected by intestinal
bacteria and metabolites in the development of chronic liver
diseases (CLDs). Firstly, HSCs become activated under chronic liver
injury or in vitro culture conditions, and change to a
myofibroblast-like cell morphology with high expression of α-smooth
muscle actin (α-SMA), and induce liver fibrosis by producing high
levels of extracellular matrix such as collagen (18). In addition,
HSCs can be activated by various stimuli such as reactive oxygen
species (ROS), damage associated molecular patterns (DAMPs),
cytokines, and chemokines (18). Activated HSCs persistently express
signals related to cell proliferation, fibrosis, and growth
factors. Therefore, it is known that they play an important role in
the formation of the cancer micro-environment, which supports the
growth and development of cancer cells by inducing angiogenesis and
fibrosis (19). Secondly, liver macrophages include Kupffer cells
and monocyte-derived macrophages: during inflammation, activated
liver macrophages produce various secretory factors and induce
influx of bone marrow-derived monocytes and neutrophils, and also
activate HSCs to induce liver fibrosis (19). In addition, activated
liver macrophages produce matrix metalloprotease, an extracellular
matrix-degrading enzyme, and express tumor necrosis factor-related
apoptosis inducing ligand (TRAIL), which induces apoptosis in liver
parenchymal cells. Therefore, it is attracting attention as a
therapeutic target for liver fibrosis (20).
This review outlined the relationship between gut
microbiota and liver diseases: hepatitis virus-related liver
diseases, non-alcoholic fatty liver disease (NAFLD) and
non-alcoholic steatohepatitis (NASH), autoimmune liver diseases,
alcoholic liver disease, liver cirrhosis (LC) and hepatocellular
carcinoma (HCC), and also outlined the treatments of dysbiosis
(antibiotics, prebiotics, probiotics and fecal microbiota
transplantation) in liver disease. The present review included
mainly original studies and review articles regarding dysbiosis and
liver disease between 1995-2021. In total 113 studies were
included.
A decrease in the diversity of gut microbiota has
been reported in the intestinal microflora of patients with chronic
hepatitis C virus (HCV) infection (21,22). The gut bacteria of
patients with HCV infection exhibit an increase in harmful
bacteria, a decrease in beneficial bacteria, and a decrease in
bacterial species (21,22). Changes in the gut microbiota in
patients with HCV are common and are caused by antibody-producing
cells derived from B lymphocytes (22). According to the analysis of
the gut microbiota of chronic hepatitis C patients in Egypt, where
HCV infection is the highest in the world, Prevotella,
Faecalibacterium, Acinetobacter, Veillonella
and Phascolarctobacterium are increased in the intestinal
microflora (21). As the disease progresses, changes in the gut
microbiota become clearer, and it has been reported that patients
with chronic HCV infection along with LC have clearly lower
diversity of gut microbiota than those without LC (23,24).
Furthermore, Inoue et al analyzed the gut microbiota of
hepatitis C patients by fibrosis progression and reported that: i)
changes in gut microbiota were already observed even in HCV
carriers with normal liver function [persistent normalized alanine
aminotransferase, (PNALT)], ii) as the disease condition worsens
from PNALT, chronic hepatitis, LC, and HCC, the occupancy rate of
indigenous bacteria in the intestinal flora decreases, the number
of bacterial species comprising the flora decreases, and the pH of
the stool increases, making it easier to develop dysbiosis at a
high rate with the progression of liver fibrosis and iii) as
hepatitis C progresses, there is an aberrant increase in
Streptococcus salivarius in the intestinal flora, and these
bacteria can degrade urea in the intestinal tract to produce
ammonia, resulting in a high pH of the stool (24). Thus, in
patients with HCV, close correlation between the degree of liver
fibrosis and gut microbiota changes has been identified, however,
correlation between HCV viral load and gut microbiota changes is
unknown.
Only ~5-10% of adults develop chronic hepatitis B
infection from acute hepatitis B virus (HBV) infection, but 90% of
newborns and 30-50% of children aged 1-5 years fail to eliminate
HBV from their bodies (25,26). In addition to the maturation of the
immune system, the gut microbiota has also been implicated in the
age-related differences in HBV viral elimination capacity (27). As
aforementioned, the composition of gut microbiota markedly changes
with aging (7). Adult mice with stable gut microbiota can eliminate
HBV virus within 6 weeks after infection, but when dysbiosis is
induced by anti-biotics, viral elimination becomes impossible,
suggesting the importance of anti-HBV activity by regulation of the
immune system through gut microbiota (28).
The gut microbiota of patients with chronic HBV
infection and HBV-related LC have been reported to be characterized
by a decrease in Bifidobacteria and lactic acid-producing
bacteria and an increase in Enterococcus and
Enterobacteriaceae (29,30). Wei et al reported a
decrease in Bacteroidetes (4 vs. 53%) and an increase in
Proteobacteria (43 vs. 4%) in a comparison of the gut
microbiota of patients with HBV-related LC and healthy subjects
(31). In a recent study, gut microbiota composition in the three
different stages (i.e., chronic hepatitis B, LC and HCC) of
HBV-related CLD patients and healthy individuals was compared (32).
The β-diversity (diversity differences between the two samples)
demonstrated a separate clustering of healthy individuals and
HBV-CLD patients, and gut microbiota of healthy individuals was
more consistent, whereas those of chronic hepatitis B, LC and HCC
varied substantially (32). The abundance of Firmicutes was
lower, and that of Bacteroidetes was higher in patients with
chronic hepatitis B, LC and HCC than in healthy individuals.
Metagenomic analysis of microbial communities demonstrated an
increase in glycan biosynthesis and metabolism-related genes in
HBV-CLD compared with healthy individuals. Their results denoted
that HBV-CLD can be associated with gut dysbiosis, with features
including an increase in potential harmful bacteria
(Bacteroidetes) or related genes and a decrease in potential
beneficial bacteria (Firmicutes) or related genes (32).
Autoimmune hepatitis (AIH) is a typical autoimmune
disease frequently observed in middle-aged or older women, and its
association with gut microbiota has received marked attention (33).
A recent study in mice revealed that Enterococcus
gallinarum, an intestinal bacterium, causes AIH when it
migrates from the intestine to the liver (34). In humans,
Enterococcus gallinarum was detected in the liver of AIH
patients, but not in healthy controls. Manfredo Vieira et al
used fluorescence to track bacteria in mice and identified that
Enterococcus gallinarum was present in lymph nodes, liver
and spleen in AIH patients (35). Interestingly, the secretion of
immune signals associated with AIH such as the induction of TH17
cells was increased by Enterococcus gallinarum in these
organs, but the presence of other types of bacteria in these organs
did not cause AIH (34). It has also been reported that the
diversity of gut microbiota is decreased in AIH patients (36).
Bifidobacterium, which is associated with disease activity
in AIH, has been reported to be decreased (37). Gut microbiota has
also been revealed to be involved in AIH exacerbations. The
exacerbation of AIH is triggered by interleukin (IL)-18, which is
induced by TLR ligands derived from gut microbiota (38,39).
Primary biliary cholangitis (PBC) is an autoimmune
liver disease characterized by progressive destruction of the
intrahepatic bile ducts, leading to bile stasis, LC, and liver
failure. CD4+ and CD8+ T lymphocytes directly
target bile duct epithelial cells (40-42). The involvement of
microorganisms such as Escherichia coli in the etiology or
pathogenesis of PBC has been known for a long time, and vaginal or
urinary tract infections in particular have been cited as risk
factors for PBC (40-42). The major corresponding antigen of
anti-mitochondrial antibodies (AMAs) is pyruvate dehydrogenase
complex E2 component (PDC-E2), and as AMAs and autoreactive T cells
of PBC patients cross-react with PDC-E2 derived from enteric
bacteria such as Escherichia coli, autoimmunity by molecular
homology has been postulated as a mechanism of PBC development
(40-42). One of the histological features of PBC is granuloma
formation, which is a tissue reaction caused by immune response to
foreign antigens including microorganisms. Molecular biological
identification of microorganisms in granulomas by PBC revealed
genes derived from enteric bacteria such as Propionibacterium
acnes (43). PBC, as well as AIH, has been indicated to be
associated with dysbiosis (44), and although intestinal bacterial
diversity is decreased in patients with PBC, it improves with
ursodeoxycholic acid (UDCA), the standard treatment for PBC (44).
Dysbiosis can be a poor prognostic factor for PBC (45).
Primary sclerosing cholangitis (PSC), an intractable
autoimmune disease for which there are few effective treatments
other than liver transplantation, is often associated with
inflammatory bowel disease such as ulcerative colitis, and the
post-transplant recurrence rate was lower in patients who underwent
total colectomy before liver transplantation (46). It has also been
reported that oral administration of vancomycin to PSC patients
resulted in improvement in hepatobiliary enzyme levels and liver
histological findings (47). These findings indicated that
inflammation of the gastrointestinal tract and gut microbiota may
be involved in the pathogenesis and prognosis of PSC (48,49).
Nakamoto et al found that three species of enteric bacteria
(Klebsiella pneumoniae, Proteus mirabilis and
Enterococcus gallinarum), which cause activation of Th17
cells in the liver, were present in the stool of PSC patients with
a high probability in the mesenteric lymph nodes (50). Th17 cells
are closely associated with chronic inflammation in autoimmune
diseases (51). Moreover, it was revealed that Klebsiella
disrupts the intestinal barrier in mice, migrates to lymph nodes
outside the intestinal tract, and induces an excessive immune
response in the liver (50). Furthermore, the Th17 immune response
in the mouse liver was attenuated to ~30% by the elimination of
Klebsiella by antibiotics. These findings may lead to the
development of new therapeutic and diagnostic agents against PSC
targeting gut microbiota (50).
In alcoholic liver injury, alterations in gut
microbiota have been recognized as an important risk factor for
disease progression, along with alcohol consumption and genetic
factors (52,53). Alcohol has been revealed to induce dysbiosis in
animal models and humans (54), and alcohol and its degradation
products disrupt tight junctions in the intestinal epithelium,
increasing intestinal permeability (i.e., leaky gut) and
inflammatory responses (55). In humans, a decrease in
butyrate-producing Clostridiales and an increase in
inflammation-inducing Enterobacteriaceae have been observed
with alcohol consumption, and in patients who progress to
cirrhosis, an increase in oral indigenous bacteria and a decrease
in numerous bacteria such as Bacteroidales in the intestine
have been reported (56). It has been reported that intestinal
bacteria-derived PAMPs such as lipopolysaccharide (LPS) are
increased after heavy alcohol intake (57). Chronic alcohol intake
also alters the production of short-chain fatty acids (SCFAs) as an
energy source. A decrease in SCFAs has been observed in the
intestinal tract of rats after alcohol intake (58). In a previous
study using a rat model of alcoholic liver injury, it was reported
that antibiotics suppressed alcoholic liver injury by inhibiting
LPS (59).
NAFLD is currently one of the most important issues
in liver disease, with a prevalence of 25% worldwide. NAFLD is
recognized as one of the major risk factors for HCC and is expected
to become the most common indication for liver transplantation in
the near future (60,61). A total of ~20% of patients with NAFLD may
progress to NASH with chronic inflammation, and then to LC and HCC
(61,62). The histological picture of NASH is predominantly
neutrophilic, and the involvement of endotoxins derived from
gram-negative bacteria has been considered for its pathological
development. Obesity induces dysbiosis of gut microbiota, leading
to a decrease in diversity and an increase in the Firmicutes
to Bacteroidetes ratio (63). The increased Firmicutes
to Bacteroidetes ratio is also observed in diabetic patients
(10). Dysbiosis in NAFLD and NASH patients increases intestinal
permeability and causes stress on the liver by various gut
microbiota-derived PAMPs (64).
It has been revealed that LPS in portal blood
reaches the liver and increases TNF-α production in Kupffer cells
via TLR4 signaling enhancement. Using mouse models, it has been
revealed that Kupffer cell-derived TNF-α signaling also plays an
important role in the pathogenesis of NASH (65,66). Furthermore,
leptin, is a hormone secreted by adipocytes, and its main function
is to suppress appetite by acting on the appetite center in the
hypothalamus of the brain (67). Obesity in NAFLD is often
accompanied by hyperleptinemia. Leptin-signal transducer and
activator of transcription 3 (STAT3) signaling enhances CD14
expression in Kupffer cells, and the resulting increased
sensitivity of Kupffer cells to LPS is one of the mechanisms of
NAFLD pathogenesis (68). In addition, alcohol-producing bacteria
are increased in NASH patients, and blood ethanol levels are
predominantly elevated, causing oxidative stress and inflammation
to the liver (69).
Liver fibrosis progression and gut microbiota in
NAFLD patients have also been studied (aforementioned in the
Introduction section). Gut microbiota-derived LPS activates TLR4
signaling in HSCs in addition to Kupffer cells, and decreases
downstream transforming growth factor (TGF)-β pseudo-receptor Bambi
expression, which enhances the sensitivity of HSCs to TGF-β,
resulting in their activation and development of hepatic fibrosis
(70). This hepatic fibrosis was inhibited by the suppression of LPS
from the intestinal tract by intestinal treatment with antibiotics
(70). NAFLD patients often consume high-fat and high-cholesterol
diets, which accumulate free cholesterol in HSCs of NAFLD livers,
resulting in further enhancement of LPS/TLR4 signaling in HSCs and
exacerbation of NAFLD fibrosis (70,71). Furthermore, inflammation
of the intestinal tract causes increased intestinal permeability.
When NAFLD mice with a high-fat diet (HFD) were treated with
dextran sulfate sodium to induce colitis, inflammation and fibrosis
of the NAFLD liver deteriorated, along with an increase in blood
endotoxin levels (72).
Ammonia is mainly produced in the intestinal tract
as a byproduct of protein digestion and intestinal bacterial
metabolism, flows into the portal vein, and is metabolized as urea
in the liver through the urea cycle. In advanced cirrhosis, the
function of the urea cycle is impaired, and ammonia enters the
systemic circulation as a result of inadequate metabolism. Ammonia
removal beyond the metabolic capacity of the liver depends on the
kidney, brain, and skeletal muscle (78). In the brain, astrocytes
detoxify ammonia by producing glutamine from ammonia and glutamate
via the glutamine synthesis pathway. The swelling of astrocytes by
the glutamine produced in this process is one of the causes of
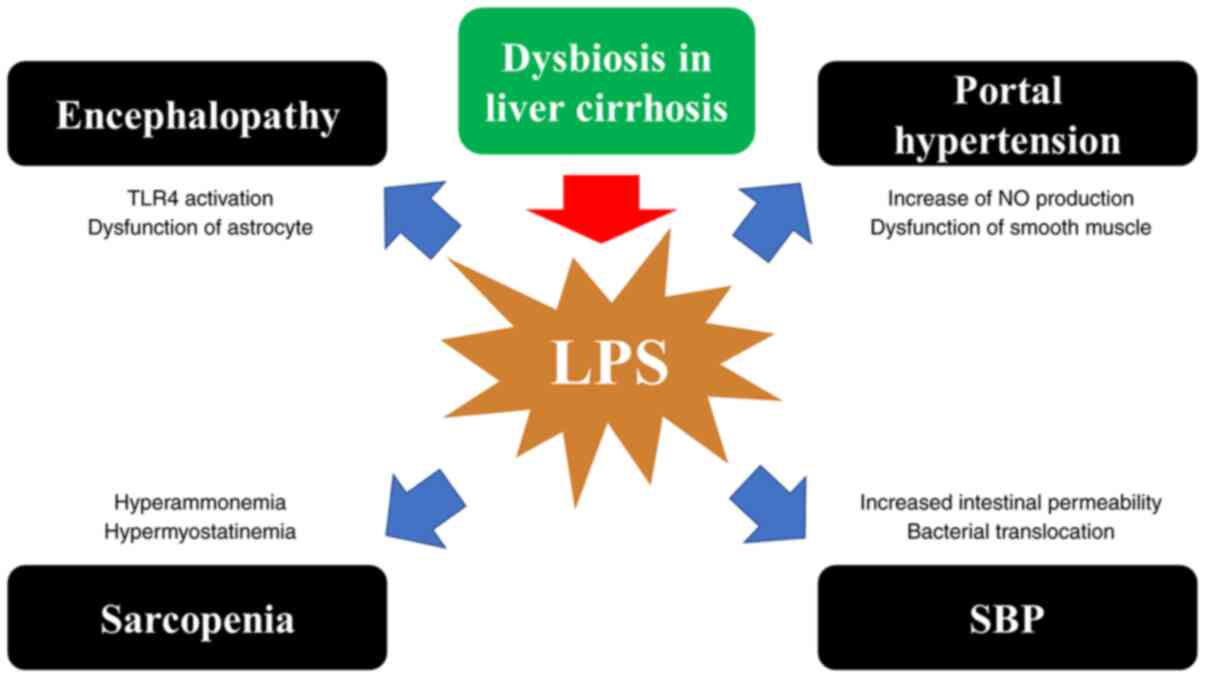
brain edema and encephalopathy (79). Gut dysbiosis can be
associated with the incidence and severity of neuroinflammation and
encephalopathy (79). LC patients with dysbiosis are prone to
sarcopenia with high levels of myostatin (a myokine that inhibits
muscle protein synthesis) in muscle caused by hyperammonemia due to
harmful bacteria in the intestine (80-82). In LC patients, LPS
causes swelling and dysfunction of astrocytes from activation of
TLR4 in microglia and endothelial cells, inducing hepatic
encephalopathy (83,84). Dysbiosis may also cause neuroinflammation,
leading to encephalopathy (85). LPS exacerbates portal hypertension
from increased NO production (increased NO production increases
portal pressure while increasing hepatic portal blood flow) and
vascular smooth muscle dysfunction (86). LPS also increases
intestinal permeability, predisposes to bacterial translocation,
and causes spontaneous bacterial peritonitis (SBP) (87) (Fig. 2).
Similar to hepatitis virus-related HCC, NASH-related
HCC in most cases develops through chronic hepatitis, liver
fibrosis, and LC. However, some cases have been reported to develop
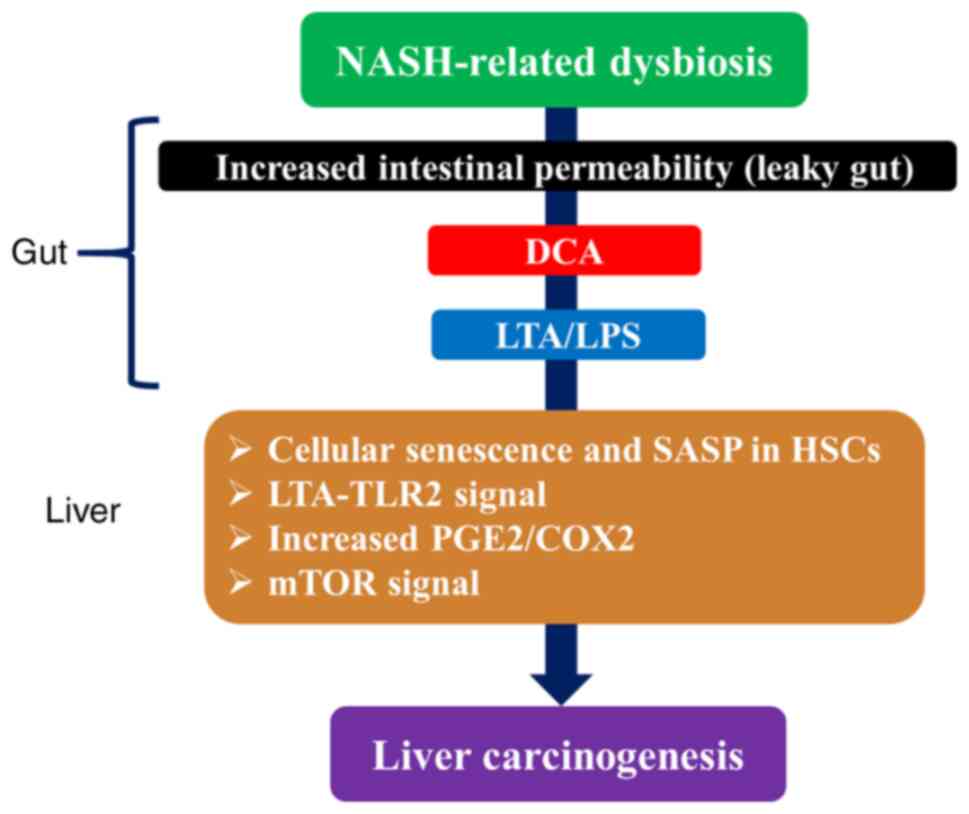
HCC without LC (88). Deoxycholate (DCA), a secondary bile acid
converted by gut microbiota, was reported to be important in the
formation of this non-cirrhotic NASH-related HCC (89,90). In obese
mice treated with the carcinogen DMBA at birth and with HFD, HCC
was revealed to develop in all mice. It was revealed that DCA,
which increased with obesity, created a microenvironment for the
development of HCC by inducing cellular senescence and
senescence-associated secretory phenotype (SASP; a phenomenon in
which senescent cells that accumulate in the body with aging are
highly expressed and secrete a variety of inflammatory proteins) in
HSCs through gut-liver circulation (89,90). It has recently been
revealed that senescent cells secrete pro-inflammatory cytokines,
and the accumulation of senescent cells with aging is considered to
be a trigger for the functional decline of organs and tissues,
resulting in various aging-related diseases (91,92). In addition,
long-term HFD treatment alters the gut microbiota, induces the
growth of gram-positive bacteria such as Clostridium and
excessive DCA production, and induces the translocation of
lipoteichoic acid (LTA; a component of gram-positive bacteria) into
the liver due to the breakdown of the intestinal barrier, thereby
promoting the progression of HCC through the activation of LTA/TLR2
signaling. LTA, along with DCA, enhances SASP production in HSCs
and increases COX-2-mediated production of prostaglandin
E2 (PGE2) and expression of TLR2 (89,90). It
has been reported that DCA levels in the blood of NASH patients are
elevated (93). Furthermore, high expression of COX-2 and excessive
PGE2 production were observed in HSCs in patients with
non-cirrhotic NASH-related HCC, indicating that a similar mechanism
functions in humans (89). Conversely, it has also been suggested
that DCA promotes the progression of HCC by activating mTOR
signaling (94) (Fig. 3). DCA was
revealed to activate mTOR and act in a phosphoinositide 3-kinase
(PI3K)-dependent manner (94). It is well known that alterations in
the PI3K/Akt/mTOR pathway are an important contributor to
tumorigenesis.
As an example of anti-biotic therapy, antibiotics
such as rifaximin have been revealed to be effective in the
treatment of liver diseases associated with the small intestine
bacterial overgrowth (SIBO) (95,96). Dysbiosis caused by severe
alcoholic hepatitis can be reversed by rifaximin therapy by
reducing Veillonella (97). In addition, rifaximin may not
affect systemic inflammation (98). Rifaximin therapy can ameliorate
endotoxemia and encephalopathy without affecting gut microbiota in
decompensated LC subjects (99). Prebiotics contain food components
that are not easily digested and absorbed in the upper part of the
gastrointestinal tract, and they promote intestinal peristalsis and
the growth of specific intestinal bacteria (100). Pectin, as one of
the prebiotics, has been revealed to prevent liver diseases by
promoting the growth of Bacteroides and inhibiting the
decrease of Bacteroides caused by alcohol consumption, and
is expected to be applied as a therapeutic agent (101). Probiotics
refer to living microorganisms that provide health benefits to
humans, and the anti-obesity effects of Bifidobacterium
breve administration have been reported in mice and humans
(102,103). This mechanism is considered to include the possibility
that Bifidobacterium breve promotes fatty acid degradation
by inducing β-oxidation in the liver and inhibiting the reduction
of intestinal barrier function caused by a HFD (104). Probiotics
are expected to be most effective against CLDs by strongly
affecting the gut-liver axis. A meta-analysis of the effects of
probiotics on NAFLD and NASH reported that probiotic therapy
lowered alanine aminotransferase (ALT), total cholesterol, and
TNF-α and improved insulin resistance in patients with NAFLD and
NASH (105). In addition, when probiotics and prebiotics were
combined in patients with NAFLD, ALT level and fatty liver were
greatly improved (106,107). In a study using an aflatoxin-induced
HCC rat model, it was reported that probiotic fermented milk and
chlorophyllin revealed tumor growth by suppressing the expression
of c-Myc, Bcl-2, cyclin D1, and Ras p21 (108). Dapito et al
also reported that inactivation of TLR4 by anti-biotics reduced HCC
by 80-90% (75). Thus, animal models indicated that regulation of
the gut microbiota may be a preventive strategy for HCC.
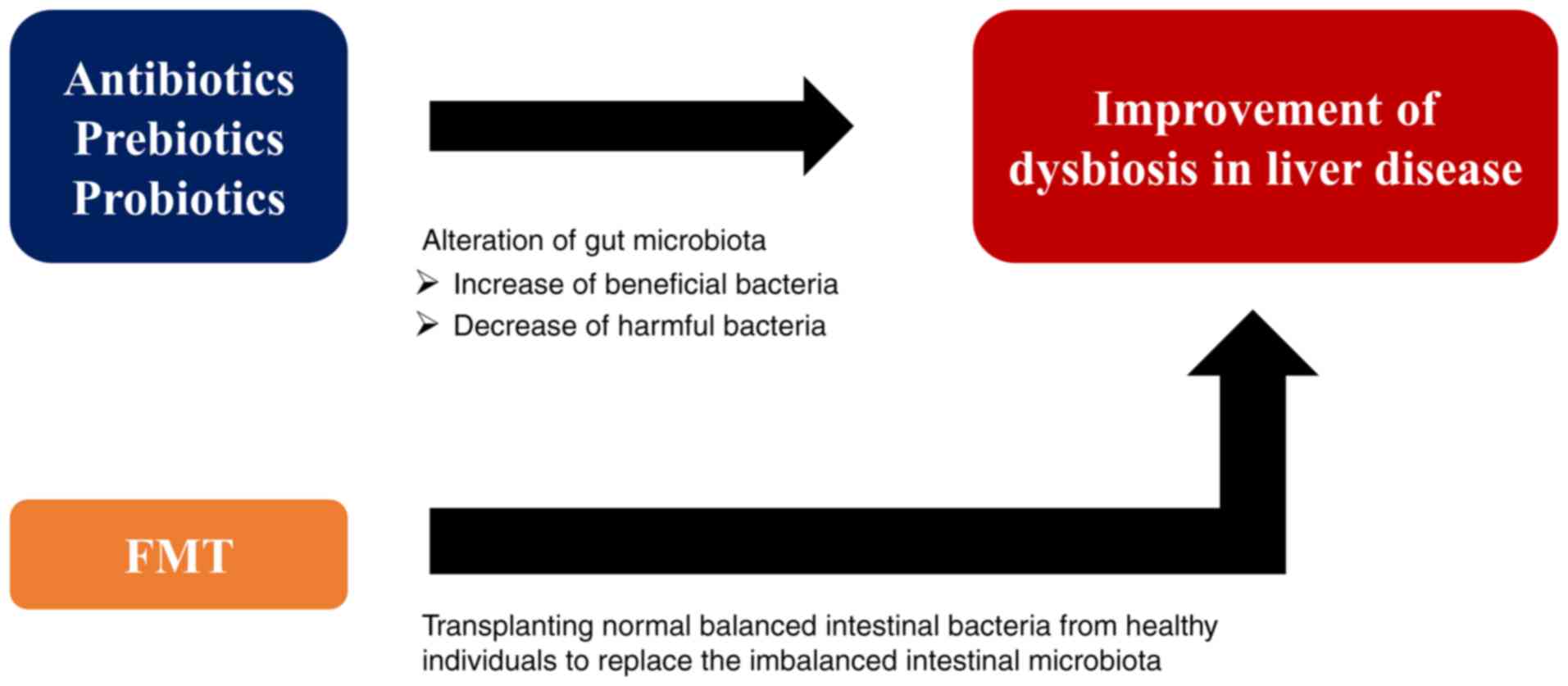
Fecal microbiota transplantation (FMT) is a method
of attempting to treat various diseases by transplanting normally
balanced intestinal bacteria from healthy individuals to replace
the imbalanced intestinal microbiota. In 2013, van Nood et
al reported a randomized controlled trial (RCT) of FMT for
recurrent Clostridium diffcile infection, and since then,
the clinical application of FMT has been attracting attention.
According to RCTs and systematic reviews of recurrent
Clostridium difficile, 60-90% of patients were cured without
recurrence by single FMT (109-111).
There are several studies on FMT in liver diseases.
FMT altered the gut microbiota of mice with high sensitivity to
ethanol and improved alcoholic liver injury (3). FMT can also
improve cirrhosis-related neuroinflammation in mice (112). In
humans, a pilot study was conducted in 8 male patients with severe
alcoholic hepatitis. The results revealed that FMT was effective
and safe in treating hepatic damage within 1 week after FMT, and
eventually exhibited improvement in severe hepatic damage and
survival even after 1 year (61). In addition, in a previous study
of FMT vs. standard therapy in 20 male LC patients associated with
recurrent hepatic encephalopathy, FMT from donors was associated
with improvement of dysbiosis, improved cognitive function and
shorter hospital stay in the recipients compared with the standard
therapy group (113). Further clinical trials are underway to
determine whether FMT can be safely used to treat CLDs.
In recent years, it has become clear that gut
microbiota is closely related to the pathogenesis of various liver
diseases, and research on the mechanism of promotion or suppression
of HCC via the gut-liver axis has become a fascinating topic. The
components of gut microbiota such as LPS and LTA are associated
with liver fibrosis and HCC progression. In addition,
gut-microbiota-derived metabolites such as secondary bile acids and
fatty acids, cellular senescence and SASP are also closely related
to liver pathology. Elucidation of the detailed molecular
mechanisms of the effects of gut microbiota-derived substances via
the gut-liver axis will lead to the development of advanced methods
for the treatment and prevention of liver diseases. FMT is gaining
attention as a treatment that can improve dysbiosis as well as
antibiotics, prebiotics and probiotics (Fig. 4). However, numerous issues remain
to be clarified, such as the administration method, long-term
benefits, and side effects of FMT. It is anticipated that more
evidence will be generated in the future.
Not applicable.
HN wrote the article. SF, AA, KY, HO, SN and KH
edited and reviewed the article. Literature research was performed
by all the authors. All authors read and approved the final
manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
|
1
|
Cani PD: Human gut microbiome: Hopes,
threats and promises. Gut. 67:1716–1725. 2018. View Article : Google Scholar
|
|
2
|
Albhaisi SAM, Bajaj JS and Sanyal AJ: Role
of gut microbiota in liver disease. Am J Physiol Gastrointest Liver
Physiol. 318:G84–G98. 2020. View Article : Google Scholar
|
|
3
|
Gomaa EZ: Human gut microbiota/microbiome
in health and diseases: A review. Antonie Van Leeuwenhoek.
113:2019–2040. 2020. View Article : Google Scholar
|
|
4
|
Cox AJ, West NP and Cripps AW: Obesity,
inflammation, and the gut microbiota. Lancet Diabetes Endocrinol.
3:207–215. 2015. View Article : Google Scholar
|
|
5
|
Schriefer AE, Cliften PF, Hibberd MC,
Sawyer C, Brown-Kennerly V, Burcea L, Klotz E, Crosby SD, Gordon JI
and Head RD: A multi-amplicon 16S rRNA sequencing and analysis
method for improved taxonomic profiling of bacterial communities. J
Microbiol Methods. 154:6–13. 2018. View Article : Google Scholar
|
|
6
|
Hills RD Jr, Pontefract BA, Mishcon HR,
Black CA, Sutton SC and Theberge CR: Gut microbiome: Profound
implications for diet and disease. Nutrients. 11:16132019.
View Article : Google Scholar
|
|
7
|
Odamaki T, Kato K, Sugahara H, Hashikura
N, Takahashi S, Xiao JZ, Abe F and Osawa R: Age-related changes in
gut microbiota composition from newborn to centenarian: A
cross-sectional study. BMC Microbiol. 16:902016. View Article : Google Scholar
|
|
8
|
Bolte LA, Vich Vila A, Imhann F, Collij V,
Gacesa R, Peters V, Wijmenga C, Kurilshikov A, Campmans-Kuijpers
MJE, Fu J, et al: Long-term dietary patterns are associated with
pro-inflammatory and anti-inflammatory features of the gut
microbiome. Gut. 70:1287–1298. 2021. View Article : Google Scholar
|
|
9
|
Koliarakis I, Messaritakis I, Nikolouzakis
TK, Hamilos G, Souglakos J and Tsiaoussis J: Oral bacteria and
intestinal dysbiosis in colorectal cancer. Int J Mol Sci.
20:41462019. View Article : Google Scholar
|
|
10
|
Yang G, Wei J, Liu P, Zhang Q, Tian Y, Hou
G, Meng L, Xin Y and Jiang X: Role of the gut microbiota in type 2
diabetes and related diseases. Metabolism. 117:1547122021.
View Article : Google Scholar
|
|
11
|
El-Salhy M, Hatlebakk JG and Hausken T:
Diet in irritable bowel syndrome (IBS): Interaction with gut
microbiota and gut hormones. Nutrients. 11:18242019. View Article : Google Scholar
|
|
12
|
Li DY and Tang WHW: Gut microbiota and
atherosclerosis. Curr Atheroscler Rep. 19:392017. View Article : Google Scholar
|
|
13
|
Ahmad AF, Dwivedi G, O'Gara F,
Caparros-Martin J and Ward NC: The gut microbiome and
cardiovascular disease: Current knowledge and clinical potential.
Am J Physiol Heart Circ Physiol. 317:H923–H938. 2019. View Article : Google Scholar
|
|
14
|
McKenzie C, Tan J, Macia L and Mackay CR:
The nutrition-gut microbiome-physiology axis and allergic diseases.
Immunol Rev. 278:277–295. 2017. View Article : Google Scholar
|
|
15
|
Hughes HK, Rose D and Ashwood P: The gut
microbiota and dysbiosis in autism spectrum disorders. Curr Neurol
Neurosci Rep. 18:812018. View Article : Google Scholar
|
|
16
|
Sanchez JMS, DePaula-Silva AB, Libbey JE
and Fujinami RS: Role of diet in regulating the gut microbiota and
multiple sclerosis. Clin Immunol. 1083792020.Online ahead of print.
View Article : Google Scholar
|
|
17
|
Zindel J and Kubes P: DAMPs, PAMPs, and
LAMPs in immunity and sterile inflammation. Annu Rev Pathol.
15:493–518. 2020. View Article : Google Scholar
|
|
18
|
Puche JE, Saiman Y and Friedman SL:
Hepatic stellate cells and liver fibrosis. Compr Physiol.
3:1473–1492. 2013. View Article : Google Scholar
|
|
19
|
Barry AE, Baldeosingh R, Lamm R, Patel K,
Zhang K, Dominguez DA, Kirton KJ, Shah AP and Dang H: Hepatic
stellate cells and hepatocarcinogenesis. Front Cell Dev Biol.
8:7092020. View Article : Google Scholar
|
|
20
|
Ju C and Tacke F: Hepatic macrophages in
homeostasis and liver diseases: From pathogenesis to novel
therapeutic strategies. Cell Mol Immunol. 13:316–327. 2016.
View Article : Google Scholar
|
|
21
|
Aly AM, Adel A, El-Gendy AO, Essam TM and
Aziz RK: Gut microbiome alterations in patients with stage 4
hepatitis C. Gut Pathog. 8:422016. View Article : Google Scholar
|
|
22
|
Preveden T, Scarpellini E, Milić N, Luzza
F and Abenavoli L: Gut microbiota changes and chronic hepatitis C
virus infection. Expert Rev Gastroenterol Hepatol. 11:813–819.
2017. View Article : Google Scholar
|
|
23
|
Heidrich B, Vital M, Plumeier I, Döscher
N, Kahl S, Kirschner J, Ziegert S, Solbach P, Lenzen H, Potthoff A,
et al: Intestinal microbiota in patients with chronic hepatitis C
with and without cirrhosis compared with healthy controls. Liver
Int. 38:50–58. 2018. View Article : Google Scholar
|
|
24
|
Inoue T, Nakayama J, Moriya K, Kawaratani
H, Momoda R, Ito K, Iio E, Nojiri S, Fujiwara K, Yoneda M, et al:
Gut dysbiosis associated with hepatitis C virus infection. Clin
Infect Dis. 67:869–877. 2018. View Article : Google Scholar
|
|
25
|
Trépo C, Chan HL and Lok A: Hepatitis B
virus infection. Lancet. 384:2053–2063. 2014. View Article : Google Scholar
|
|
26
|
Batsis ID, Wasuwanich P and Karnsakul WW:
The management of hepatitis B and hepatitis C in children. Minerva
Pediatr. 71:59–75. 2019.
|
|
27
|
Yang R, Xu Y, Dai Z, Lin X and Wang H: the
immunologic role of gut microbiota in patients with chronic HBV
infection. J Immunol Res. 2018:23619632018. View Article : Google Scholar
|
|
28
|
Chou HH, Chien WH, Wu LL, Cheng CH, Chung
CH, Horng JH, Ni YH, Tseng HT, Wu D, Lu X, et al: Age-related
immune clearance of hepatitis B virus infection requires the
establishment of gut microbiota. Proc Natl Acad Sci USA.
112:2175–2180. 2015. View Article : Google Scholar
|
|
29
|
Xu M, Wang B, Fu Y, Chen Y, Yang F, Lu H,
Chen Y, Xu J and Li L: Changes of fecal Bifidobacterium species in
adult patients with hepatitis B virus-induced chronic liver
disease. Microb Ecol. 63:304–313. 2012. View Article : Google Scholar
|
|
30
|
Lu H, Wu Z, Xu W, Yang J, Chen Y and Li L:
Intestinal microbiota was assessed in cirrhotic patients with
hepatitis B virus infection. Intestinal microbiota of HBV cirrhotic
patients. Microb Ecol. 61:693–703. 2011. View Article : Google Scholar
|
|
31
|
Wei X, Yan X, Zou D, Yang Z, Wang X, Liu
W, Wang S, Li X, Han J, Huang L and Yuan J: Abnormal fecal
microbiota community and functions in patients with hepatitis B
liver cirrhosis as revealed by a metagenomic approach. BMC
Gastroenterol. 13:1752013. View Article : Google Scholar
|
|
32
|
Zeng Y, Chen S, Fu Y, Wu W, Chen T, Chen
J, Yang B and Ou Q: Gut microbiota dysbiosis in patients with
hepatitis B virus-induced chronic liver disease covering chronic
hepatitis, liver cirrhosis and hepatocellular carcinoma. J Viral
Hepat. 27:143–155. 2020. View Article : Google Scholar
|
|
33
|
Czaja AJ: Examining pathogenic concepts of
autoimmune hepatitis for cues to future investigations and
interventions. World J Gastroenterol. 25:6579–6606. 2019.
View Article : Google Scholar
|
|
34
|
Bogdanos DP and Sakkas LI: Enterococcus
gallinarum as a component of the autoinfectome: The
gut-liver-autoimmune rheumatic disease axis is alive and kicking.
Mediterr J Rheumatol. 29:187–189. 2018. View Article : Google Scholar
|
|
35
|
Manfredo Vieira S, Hiltensperger M, Kumar
V, Zegarra-Ruiz D, Dehner C, Khan N, Costa FRC, Tiniakou E,
Greiling T, Ruff W, et al: Translocation of a gut pathobiont drives
autoimmunity in mice and humans. Science. 359:1156–1161. 2018.
View Article : Google Scholar
|
|
36
|
Wei Y, Li Y, Yan L, Sun C, Miao Q, Wang Q,
Xiao X, Lian M, Li B, Chen Y, et al: Alterations of gut microbiome
in autoimmune hepatitis. Gut. 69:569–577. 2020. View Article : Google Scholar
|
|
37
|
Liwinski T, Casar C, Ruehlemann MC, Bang
C, Sebode M, Hohenester S, Denk G, Lieb W, Lohse AW, Franke A and
Schramm C: A disease-specific decline of the relative abundance of
Bifidobacterium in patients with autoimmune hepatitis. Aliment
Pharmacol Ther. 51:1417–1428. 2020. View Article : Google Scholar
|
|
38
|
Kido M, Watanabe N, Okazaki T, Akamatsu T,
Tanaka J, Saga K, Nishio A, Honjo T and Chiba T: Fatal autoimmune
hepatitis induced by concurrent loss of naturally arising
regulatory T cells and PD-1-mediated signaling. Gastroenterology.
135:1333–1343. 2008. View Article : Google Scholar
|
|
39
|
Ikeda A, Aoki N, Kido M, Iwamoto S,
Nishiura H, Maruoka R, Chiba T and Watanabe N: Progression of
autoimmune hepatitis is mediated by IL-18-producing dendritic cells
and hepatic CXCL9 expression in mice. Hepatology. 60:224–236. 2014.
View Article : Google Scholar
|
|
40
|
Lleo A, Wang GQ, Gershwin ME and
Hirschfield GM: Primary biliary cholangitis. Lancet. 396:1915–1926.
2020. View Article : Google Scholar
|
|
41
|
Lleo A, Leung PSC, Hirschfield GM and
Gershwin EM: The pathogenesis of primary biliary cholangitis: A
comprehensive review. Semin Liver Dis. 40:34–48. 2020. View Article : Google Scholar
|
|
42
|
Gulamhusein AF and Hirschfield GM: Primary
biliary cholangitis: Pathogenesis and therapeutic opportunities.
Nat Rev Gastroenterol Hepatol. 17:93–110. 2020. View Article : Google Scholar
|
|
43
|
Harada K, Tsuneyama K, Sudo Y, Masuda S
and Nakanuma Y: Molecular identification of bacterial 16S ribosomal
RNA gene in liver tissue of primary biliary cirrhosis: Is
Propionibacterium acnes involved in granuloma formation?
Hepatology. 33:530–536. 2001. View Article : Google Scholar
|
|
44
|
Tang R, Wei Y, Li Y, Chen W, Chen H, Wang
Q, Yang F, Miao Q, Xiao X, Zhang H, et al: Gut microbial profile is
altered in primary biliary cholangitis and partially restored after
UDCA therapy. Gut. 67:534–541. 2018. View Article : Google Scholar
|
|
45
|
Furukawa M, Moriya K, Nakayama J, Inoue T,
Momoda R, Kawaratani H, Namisaki T, Sato S, Douhara A, Kaji K, et
al: Gut dysbiosis associated with clinical prognosis of patients
with primary biliary cholangitis. Hepatol Res. 50:840–852. 2020.
View Article : Google Scholar
|
|
46
|
Buchholz BM, Lykoudis PM, Ravikumar R,
Pollok JM and Fusai GK: Role of colectomy in preventing recurrent
primary sclerosing cholangitis in liver transplant recipients.
World J Gastroenterol. 24:3171–3180. 2018. View Article : Google Scholar
|
|
47
|
Shah A, Crawford D, Burger D, Martin N,
Walker M, Talley NJ, Tallis C, Jones M, Stuart K, Keely S, et al:
Effects of antibiotic therapy in primary sclerosing cholangitis
with and without inflammatory bowel disease: A systematic review
and meta-analysis. Semin Liver Dis. 39:432–441. 2019. View Article : Google Scholar
|
|
48
|
Little R, Wine E, Kamath BM, Griffiths AM
and Ricciuto A: Gut microbiome in primary sclerosing cholangitis: A
review. World J Gastroenterol. 26:2768–2780. 2020. View Article : Google Scholar
|
|
49
|
Prokopič M and Beuers U: Management of
primary sclerosing cholangitis and its complications: An
algorithmic approach. Hepatol Int. 15:6–20. 2021. View Article : Google Scholar
|
|
50
|
Nakamoto N, Sasaki N, Aoki R, Miyamoto K,
Suda W, Teratani T, Suzuki T, Koda Y, Chu PS, Taniki N, et al: Gut
pathobionts underlie intestinal barrier dysfunction and liver T
helper 17 cell immune response in primary sclerosing cholangitis.
Nat Microbiol. 4:492–503. 2019. View Article : Google Scholar
|
|
51
|
Yasuda K, Takeuchi Y and Hirota K: The
pathogenicity of Th17 cells in autoimmune diseases. Semin
Immunopathol. 41:283–297. 2019. View Article : Google Scholar
|
|
52
|
Bajaj JS: Alcohol, liver disease and the
gut microbiota. Nat Rev Gastroenterol Hepatol. 16:235–246. 2019.
View Article : Google Scholar
|
|
53
|
Kobayashi M, Asai A, Ito I, Suzuki S,
Higuchi K and Suzuki F: Short-term alcohol abstinence improves
antibacterial defenses of chronic alcohol-consuming mice against
gut bacteria-associated sepsis caused by Enterococcus faecalis oral
infection. Am J Pathol. 187:1998–2007. 2017. View Article : Google Scholar
|
|
54
|
Hartmann P, Seebauer CT and Schnabl B:
Alcoholic liver disease: The gut microbiome and liver cross talk.
Alcohol Clin Exp Res. 39:763–775. 2015. View Article : Google Scholar
|
|
55
|
Elamin EE, Masclee AA, Dekker J and
Jonkers DM: Ethanol metabolism and its effects on the intestinal
epithelial barrier. Nutr Rev. 71:483–499. 2013. View Article : Google Scholar
|
|
56
|
Dubinkina VB, Tyakht AV, Odintsova VY,
Yarygin KS, Kovarsky BA, Pavlenko AV, Ischenko DS, Popenko AS,
Alexeev DG, Taraskina AY, et al: Links of gut microbiota
composition with alcohol dependence syndrome and alcoholic liver
disease. Microbiome. 5:1412017. View Article : Google Scholar
|
|
57
|
Szabo G: Gut-liver axis in alcoholic liver
disease. Gastroenterology. 148:30–36. 2014. View Article : Google Scholar
|
|
58
|
Xie G, Zhong W, Zheng X, Li Q, Qiu Y, Li
H, Chen H, Zhou Z and Jia W: Chronic ethanol consumption alters
mammalian gastrointestinal content metabolites. J Proteome Res.
12:3297–3306. 2013. View Article : Google Scholar
|
|
59
|
Adachi Y, Moore LE, Bradford BU, Gao W and
Thurman RG: Antibiotics prevent liver injury in rats following
long-term exposure to ethanol. Gastroenterology. 108:218–224. 1995.
View Article : Google Scholar
|
|
60
|
Younossi ZM, Marchesini G, Pinto-Cortez H
and Petta S: Epidemiology of nonalcoholic fatty liver disease and
nonalcoholic steatohepatitis: Implications for liver
transplantation. Transplantation. 103:22–27. 2019. View Article : Google Scholar
|
|
61
|
Younossi Z, Anstee QM, Marietti M, Hardy
T, Henry L, Eslam M, George J and Bugianesi E: Global burden of
NAFLD and NASH: trends, predictions, risk factors and prevention.
Nat Rev Gastroenterol Hepatol. 15:11–20. 2018. View Article : Google Scholar
|
|
62
|
Nishikawa H and Osaki Y: Non-B, non-C
hepatocellular carcinoma (Review). Int J Oncol. 43:1333–1342. 2013.
View Article : Google Scholar
|
|
63
|
Chakraborti CK: New-found link between
microbiota and obesity. World J Gastrointest Pathophysiol.
6:110–119. 2015. View Article : Google Scholar
|
|
64
|
Leung C, Rivera L, Furness JB and Angus
PW: The role of the gut microbiota in NAFLD. Nat Rev Gastroenterol
Hepatol. 13:412–425. 2016. View Article : Google Scholar
|
|
65
|
Tomita K, Tamiya G, Ando S, Ohsumi K,
Chiyo T, Mizutani A, Kitamura N, Toda K, Kaneko T, Horie Y, et al:
Tumour necrosis factor alpha signalling through activation of
Kupffer cells plays an essential role in liver fibrosis of
non-alcoholic steatohepatitis in mice. Gut. 55:415–424. 2006.
View Article : Google Scholar
|
|
66
|
Rivera CA, Adegboyega P, van Rooijen N,
Tagalicud A, Allman M and Wallace M: Toll-like receptor-4 signaling
and Kupffer cells play pivotal roles in the pathogenesis of
non-alcoholic steatohepatitis. J Hepatol. 47:571–579. 2007.
View Article : Google Scholar
|
|
67
|
Friedman J: The long road to leptin. J
Clin Invest. 126:4727–4734. 2016. View Article : Google Scholar
|
|
68
|
Imajo K, Fujita K, Yoneda M, Nozaki Y,
Ogawa Y, Shinohara Y, Kato S, Mawatari H, Shibata W, Kitani H, et
al: Hyperresponsivity to low-dose endotoxin during progression to
nonalcoholic steatohepatitis is regulated by leptin-mediated
signaling. Cell Metab. 16:44–54. 2012. View Article : Google Scholar
|
|
69
|
Zhu L, Baker SS, Gill C, Liu W, Alkhouri
R, Baker RD and Gill SR: Characterization of gut microbiomes in
nonalcoholic steatohepatitis (NASH) patients: A connection between
endogenous alcohol and NASH. Hepatology. 57:601–609. 2013.
View Article : Google Scholar
|
|
70
|
Seki E, De Minicis S, Osterreicher CH,
Kluwe J, Osawa Y, Brenner DA and Schwabe RF: TLR4 enhances TGF-beta
signaling and hepatic fibrosis. Nat Med. 13:1324–1332. 2007.
View Article : Google Scholar
|
|
71
|
Tomita K, Teratani T, Suzuki T, Shimizu M,
Sato H, Narimatsu K, Okada Y, Kurihara C, Irie R, Yokoyama H, et
al: Free cholesterol accumulation in hepatic stellate cells:
Mechanism of liver fibrosis aggravation in nonalcoholic
steatohepatitis in mice. Hepatology. 59:154–169. 2014. View Article : Google Scholar
|
|
72
|
Gäbele E, Dostert K, Hofmann C, Wiest R,
Schölmerich J, Hellerbrand C and Obermeier F: DSS induced colitis
increases portal LPS levels and enhances hepatic inflammation and
fibrogenesis in experimental NASH. J Hepatol. 55:1391–1399. 2011.
View Article : Google Scholar
|
|
73
|
Kakiyama G, Pandak WM, Gillevet PM,
Hylemon PB, Heuman DM, Daita K, Takei H, Muto A, Nittono H, Ridlon
JM, et al: Modulation of the fecal bile acid profile by gut
microbiota in cirrhosis. J Hepatol. 58:949–955. 2013. View Article : Google Scholar
|
|
74
|
Nakanishi K, Kaji K, Kitade M, Kubo T,
Furukawa M, Saikawa S, Shimozato N, Sato S, Seki K, Kawaratani H,
et al: Exogenous administration of low-dose lipopolysaccharide
potentiates liver fibrosis in a choline-deficient
l-amino-acid-defined diet-induced murine steatohepatitis model. Int
J Mol Sci. 20:27242019. View Article : Google Scholar
|
|
75
|
Dapito DH, Mencin A, Gwak GY, Pradere JP,
Jang MK, Mederacke I, Caviglia JM, Khiabanian H, Adeyemi A,
Bataller R, et al: Promotion of hepatocellular carcinoma by the
intestinal microbiota and TLR4. Cancer Cell. 21:504–516. 2012.
View Article : Google Scholar
|
|
76
|
Riese DJ II and Cullum RL: Epiregulin:
Roles in normal physiology and cancer. Semin Cell Dev Biol.
28:49–56. 2014. View Article : Google Scholar
|
|
77
|
Qin N, Yang F, Li A, Prifti E, Chen Y,
Shao L, Guo J, Le Chatelier E, Yao J, Wu L, et al: Alterations of
the human gut microbiome in liver cirrhosis. Nature. 513:59–64.
2014. View Article : Google Scholar
|
|
78
|
U-King-Im JM, Yu E, Bartlett E, Soobrah R
and Kucharczyk W: Acute hyperammonemic encephalopathy in adults:
Imaging findings. Am J Neuroradiol. 32:413–418. 2011. View Article : Google Scholar
|
|
79
|
Bjerring PN, Eefsen M, Hansen BA and
Larsen FS: The brain in acute liver failure. A tortuous path from
hyperammonemia to cerebral edema. Metab Brain Dis. 24:5–14. 2009.
View Article : Google Scholar
|
|
80
|
Nishikawa H, Enomoto H, Ishii A, Iwata Y,
Miyamoto Y, Ishii N, Yuri Y, Hasegawa K, Nakano C, Nishimura T, et
al: Elevated serum myostatin level is associated with worse
survival in patients with liver cirrhosis. J Cachexia Sarcopenia
Muscle. 8:915–925. 2017. View Article : Google Scholar
|
|
81
|
Nishikawa H, Enomoto H, Nishiguchi S and
Iijima H: Liver cirrhosis and sarcopenia from the viewpoint of
dysbiosis. Int J Mol Sci. 21:52542020. View Article : Google Scholar
|
|
82
|
Nishikawa H, Shiraki M, Hiramatsu A,
Moriya K, Hino K and Nishiguchi S: Japan society of hepatology
guidelines for sarcopenia in liver disease (1st edition):
Recommendation from the working group for creation of sarcopenia
assessment criteria. Hepatol Res. 46:951–963. 2016. View Article : Google Scholar
|
|
83
|
Jayakumar AR, Tong XY, Curtis KM,
Ruiz-Cordero R, Abreu MT and Norenberg MD: Increased toll-like
receptor 4 in cerebral endothelial cells contributes to the
astrocyte swelling and brain edema in acute hepatic encephalopathy.
J Neurochem. 128:890–903. 2014. View Article : Google Scholar
|
|
84
|
Jayakumar AR, Rama Rao KV and Norenberg
MD: Neuroinflammation in hepatic encephalopathy: Mechanistic
aspects. J Clin Exp Hepatol. 5(Suppl 1): S21–S28. 2015. View Article : Google Scholar
|
|
85
|
Kang DJ, Betrapally NS, Ghosh SA, Sartor
RB, Hylemon PB, Gillevet PM, Sanyal AJ, Heuman DM, Carl D, Zhou H,
et al: Gut microbiota drive the development of neuroinflammatory
response in cirrhosis in mice. Hepatology. 64:1232–1248. 2016.
View Article : Google Scholar
|
|
86
|
Steib CJ, Hartmann AC, v Hesler C, Benesic
A, Hennenberg M, Bilzer M and Gerbes AL: Intraperitoneal LPS
amplifies portal hypertension in rat liver fibrosis. Lab Invest.
90:1024–1032. 2010. View Article : Google Scholar
|
|
87
|
Wiest R, Lawson M and Geuking M:
Pathological bacterial translocation in liver cirrhosis. J Hepatol.
60:197–209. 2014. View Article : Google Scholar
|
|
88
|
Labenz C, Huber Y, Kalliga E, Nagel M,
Ruckes C, Straub BK, Galle PR, Wörns MA, Anstee QM, Schuppan D and
Schattenberg JM: Predictors of advanced fibrosis in non-cirrhotic
non-alcoholic fatty liver disease in Germany. Aliment Pharmacol
Ther. 48:1109–1116. 2018. View Article : Google Scholar
|
|
89
|
Loo TM, Kamachi F, Watanabe Y, Yoshimoto
S, Kanda H, Arai Y, Nakajima-Takagi Y, Iwama A, Koga T, Sugimoto Y,
et al: Gut microbiota promotes obesity-associated liver cancer
through PGE2-mediated suppression of antitumor immunity.
Cancer Discov. 7:522–538. 2017. View Article : Google Scholar
|
|
90
|
Yoshimoto S, Loo TM, Atarashi K, Kanda H,
Sato S, Oyadomari S, Iwakura Y, Oshima K, Morita H, Hattori M, et
al: Obesity-induced gut microbial metabolite promotes liver cancer
through senescence secretome. Nature. 499:97–101. 2013. View Article : Google Scholar
|
|
91
|
He S and Sharpless NE: Senescence in
health and disease. Cell. 169:1000–1011. 2017. View Article : Google Scholar
|
|
92
|
Vernot JP: Senescence-associated
pro-inflammatory cytokines and tumor cell plasticity. Front Mol
Biosci. 7:632020. View Article : Google Scholar
|
|
93
|
Puri P, Daita K, Joyce A, Mirshahi F,
Santhekadur PK, Cazanave S, Luketic VA, Siddiqui MS, Boyett S, Min
HK, et al: The presence and severity of nonalcoholic
steatohepatitis is associated with specific changes in circulating
bile acids. Hepatology. 67:534–548. 2018. View Article : Google Scholar
|
|
94
|
Yamada S, Takashina Y, Watanabe M,
Nagamine R, Saito Y, Kamada N and Saito H: Bile acid metabolism
regulated by the gut microbiota promotes non-alcoholic
steatohepatitis-associated hepatocellular carcinoma in mice.
Oncotarget. 9:9925–9939. 2018. View Article : Google Scholar
|
|
95
|
Sajjad A, Mottershead M, Syn WK, Jones R,
Smith S and Nwokolo CU: Ciprofloxacin suppresses bacterial
overgrowth, increases fasting insulin but does not correct low
acylated ghrelin concentration in non-alcoholic steatohepatitis.
Aliment Pharmacol Ther. 22:291–299. 2005. View Article : Google Scholar
|
|
96
|
Kitagawa R, Kon K, Uchiyama A, Arai K,
Yamashina S, Kuwahara-Arai K, Kirikae T, Ueno T and Ikejima K:
Rifaximin prevents ethanol-induced liver injury in obese
KK-Ay mice through modulation of small intestinal
microbiota signature. Am J Physiol Gastrointest Liver Physiol.
317:G707–G715. 2019. View Article : Google Scholar
|
|
97
|
Kim SS, Eun JW, Cho HJ, Song DS, Kim CW,
Kim YS, Lee SW, Kim YK, Yang J, Choi J, et al: Microbiome as a
potential diagnostic and predictive biomarker in severe alcoholic
hepatitis. Aliment Pharmacol Ther. 53:540–551. 2021.
|
|
98
|
Jørgensen SF, Macpherson ME, Bjørnetrø T,
Holm K, Kummen M, Rashidi A, Michelsen AE, Lekva T, Halvorsen B,
Trøseid M, et al: Rifaximin alters gut microbiota profile, but does
not affect systemic inflammation-a randomized controlled trial in
common variable immunodeficiency. Sci Rep. 9:1672019. View Article : Google Scholar
|
|
99
|
Kaji K, Takaya H, Saikawa S, Furukawa M,
Sato S, Kawaratani H, Kitade M, Moriya K, Namisaki T, Akahane T, et
al: Rifaximin ameliorates hepatic encephalopathy and endotoxemia
without affecting the gut microbiome diversity. World J
Gastroenterol. 23:8355–8366. 2017. View Article : Google Scholar
|
|
100
|
Hijová E, Bertková I and Štofilová J:
Dietary fibre as prebiotics in nutrition. Cent Eur J Public Health.
27:251–255. 2019. View Article : Google Scholar
|
|
101
|
Ferrere G, Wrzosek L, Cailleux F, Turpin
W, Puchois V, Spatz M, Ciocan D, Rainteau D, Humbert L, Hugot C, et
al: Fecal micro-biota manipulation prevents dysbiosis and
alcohol-induced liver injury in mice. J Hepatol. 66:806–815. 2017.
View Article : Google Scholar
|
|
102
|
Kondo S, Xiao JZ, Satoh T, Odamaki T,
Takahashi S, Sugahara H, Yaeshima T, Iwatsuki K, Kamei A and Abe K:
Antiobesity effects of Bifidobacterium breve strain B-3
supplementation in a mouse model with high-fat diet-induced
obesity. Biosci Biotechnol Biochem. 74:1656–1661. 2010. View Article : Google Scholar
|
|
103
|
Minami J, Kondo S, Yanagisawa N, Odamaki
T, Xiao JZ, Abe F, Nakajima S, Hamamoto Y, Saitoh S and Shimoda T:
Oral administration of Bifidobacterium breve B-3 modifies metabolic
functions in adults with obese tendencies in a randomised
controlled trial. J Nutr Sci. 4:e172015. View Article : Google Scholar
|
|
104
|
Kondo S, Kamei A, Xiao JZ, Iwatsuki K and
Abe K: Bifidobacterium breve B-3 exerts metabolic
syndrome-suppressing effects in the liver of diet-induced obese
mice: A DNA microarray analysis. Benef Microbes. 4:247–251. 2013.
View Article : Google Scholar
|
|
105
|
Armstrong LE and Guo GL: Role of FXR in
liver inflammation during nonalcoholic steatohepatitis. Curr
Pharmacol Rep. 3:92–100. 2017. View Article : Google Scholar
|
|
106
|
Fang S, Suh JM, Reilly SM, Yu E, Osborn O,
Lackey D, Yoshihara E, Perino A, Jacinto S, Lukasheva Y, et al:
Intestinal FXR agonism promotes adipose tissue browning and reduces
obesity and insulin resistance. Nat Med. 21:159–165. 2015.
View Article : Google Scholar
|
|
107
|
Jiang C, Xie C, Lv Y, Li J, Krausz KW, Shi
J, Brocker CN, Desai D, Amin SG, Bisson WH, et al:
Intestine-selective farnesoid X receptor inhibition improves
obesity-related metabolic dysfunction. Nat Commun. 6:101662015.
View Article : Google Scholar
|
|
108
|
Kumar M, Verma V, Nagpal R, Kumar A,
Gautam SK, Behare PV, Grover CR and Aggarwal PK: Effect of
probiotic fermented milk and chlorophyllin on gene expressions and
genotoxicity during AFB1-induced hepatocellular carcinoma. Gene.
490:54–59. 2011. View Article : Google Scholar
|
|
109
|
van Nood E, Vrieze A, Nieuwdorp M, Fuentes
S, Zoetendal EG, de Vos WM, Visser CE, Kuijper EJ, Bartelsman JF,
Tijssen JG, et al: Duodenal infusion of donor feces for recurrent
Clostridium difficile. N Engl J Med. 368:407–415. 2013. View Article : Google Scholar
|
|
110
|
Cammarota G, Ianiro G and Gasbarrini A:
Fecal microbiota transplantation for the treatment of Clostridium
difficile infection: A systematic review. J Clin Gastroenterol.
48:693–702. 2014. View Article : Google Scholar
|
|
111
|
Kelly CR, Khoruts A, Staley C, Sadowsky
MJ, Abd M, Alani M, Bakow B, Curran P, McKenney J, Tisch A, et al:
Effect of fecal microbiota transplantation on recurrence in
multiply recurrent Clostridium difficile infection: A randomized
trial. Ann Intern Med. 165:609–616. 2016. View Article : Google Scholar
|
|
112
|
Liu R, Kang JD, Sartor RB, Sikaroodi M,
Fagan A, Gavis EA, Zhou H, Hylemon PB, Herzog JW, Li X, et al:
Neuroinflammation in murine cirrhosis is dependent on the gut
microbiome and is attenuated by fecal transplant. Hepatology.
71:611–626. 2020. View Article : Google Scholar
|
|
113
|
Bajaj JS, Kassam Z, Fagan A, Gavis EA, Liu
E, Cox IJ, Kheradman R, Heuman D, Wang J, Gurry T, et al: Fecal
micro-biota transplant from a rational stool donor improves hepatic
encephalopathy: A randomized clinical trial. Hepatology.
66:1727–1738. 2017. View Article : Google Scholar
|