Introduction
Atherosclerosis is a chronic inflammatory disease
characterized by proliferative lesions in the lining of the
arteries, which has a serious impact on the quality of life of
patients with the disease (1,2).
Atherosclerosis involves arterial wall thickening, sclerosis, loss
of elasticity and lumen narrowing (1,2),
and is the primary cause of the development of coronary heart
disease (CAD) (3). However, the
specific cause of atherosclerosis remains to be elucidated
(2). Extensive investigations
into the etiology of atherosclerosis have demonstrated that it is a
multi-etiological disease (4).
The collective results of previous studies demonstrated that
patients with hyperlipidemia, high blood pressure and diabetes, and
patients who smoke experience an increased risk of developing
atherosclerosis (5,6). Therapeutic methods that target
atherosclerosis can be grouped according to the severity of the
disease, including acute, general, drug-based, surgical and
Traditional Chinese medicine-based treatments (7-10). Although current strategies for
the treatment of atherosclerosis are continuously improving, the
mortality rate of atherosclerosis remains high (11). Thus, further investigations into
novel therapeutic methods for the treatment of atherosclerosis are
required.
Macrophages are common phagocytes found in the
blood, lymphatic and mammalian tissue (12), which play important roles in
normal development, homeostasis, tissue repair and in the immune
response to pathogens (13).
Previous studies have reported that macrophages use chemotaxis
induced by inflammatory factors produced during oxidized
low-density lipoprotein (ox-LDL) modification, and phagocytosis of
ox-LDLs in large quantities induces the formation of foam cells,
leading to the development of atherosclerotic lesions (14-16). The functional changes of
macrophages are closely associated with the formation and
development of atherosclerosis (14-16).
Mesenchymal stem cells (MSCs) are a type of
pluripotent stem cell with self-renewal and multidirectional
differentiation capacities (17). In addition, MSCs are safe and
effective for tissue repair and regeneration in cardiovascular
diseases, such as CAD (18).
Exosomes are small membrane vesicles (40-100 nm in diameter) that
are secreted by a variety of cells, including MSCs (19). Furthermore, exosomes are
extensively involved in intercellular communication via transport
of proteins, DNA, microRNA (miRNA/miR), long non-coding (lnc)RNA
and mRNAs (20). The results of
a previous study demonstrated that lncRNAs secreted by MSCs are
delivered into recipient cells via exosomes, participating in the
progression of CAD (21).
lncRNAs are a class of non-coding RNAs with a length
of >200 nucleotides, which are involved in various regulatory
processes, such as transcriptional silencing, transcriptional
activation, chromosome modification and intra nuclear transport
(22,23). It has previously been reported
that lncRNAs are closely associated with the occurrence,
development, prevention and treatment of CAD (24,25). In our previous study,
bioinformatics analysis revealed that LOC100129516 expression was
upregulated in peripheral blood mononuclear cells (PBMCs) from
patients with CAD, compared with PBMCs from healthy controls (in
press). In addition, LOC100129516 knockout exhibited a protective
effect on the ox-LDL-induced proliferation inhibition of human
umbilical vein endothelial cells (HUVECs).
However, the biological role of MSC-derived exosomal
LOC100129516 in atherosclerosis remains to be elucidated. In the
present study, MSC-derived exosomal-mediated delivery of small
interfering RNA (siRNA/si)-LOC100129516 increased cholesterol
excretion and suppressed intracellular lipid accumulation in THP-1
macrophage-derived foam cells and Apolipoprotein E (ApoE) knockout
(ApoE−/−) mice with atherosclerosis. These results
suggested that the exosomal-mediated delivery of si-LOC100129516
alleviated atherosclerosis development both in vitro and
in vivo. Thus, the aim of the present study was to explore
novel strategies for atherosclerosis treatment.
Materials and methods
Cell culture and construction of the foam
cell model
The human acute monocytic leukemia cell line, THP-1,
and human vascular smooth muscle cells were obtained from the
American Type Culture Collection. Human bone marrow MSCs were
purchased from Procell, Inc. The cells were cultured in RPMI-1640
medium containing 10% FBS and 2 mM glutamine (Sigma Aldrich; Merck
KGaA) in a humidified incubator supplied with 5% CO2 at
37°C. MSCs and vascular smooth muscle cells were primary cells
(commercialized), which have not been immortalized.
To construct the foam cell model, THP-1 cells or
vascular smooth muscle cells were exposed to phorbol myristate
acetate (100 nM; Sigma-Aldrich; Merck KGaA) for 24 h, and
subsequently incubated with 50 µg/ml ox-LDL for 48 h, as
described previously (26).
Reverse transcription-quantitative
(RT-q)PCR
Total RNA was extracted from cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). Subsequently, total RNA was reverse transcribed into cDNA
using an EntiLink™ 1st Strand cDNA Synthesis kit according to the
manufacturer's protocol (ELK Biotechnology, Co., Ltd.). The
temperature and duration of RT were as follows: 37°C for 60 min and
85°C for 5 min. qPCR was performed using a SYBR® Premix
Ex Taq™ II kit (ELK Biotechnology Co., Ltd.) on a 7900HT system
(Applied Biosystems; Thermo Fisher Scientific, Inc.) as follows:
60°C for 1 min, 90°C for 15 min, followed by 40 cycles of 90°C for
15 sec and 55°C for 60 sec. β-actin was used as an internal
control. The standard 2−ΔΔCq method was used for data
analysis (27). The following
primer sequences were used: β-actin forward, 5′-GTC CAC CGC AAA TGC
TTC TA-3′ and reverse, 5′-TGC TGT CAC CTT CAC CGT TC-3′; and
LOC100129516 forward, 5′-GAA AGG GGA CTC AGC CAT CAT -3′ and
reverse, 5′-TGC CAA AAA CAT TAA GTG AGG TG-3′.
Cell transfection
si-LOC100129516 and siRNA-control (ctrl) were
purchased from Guangzhou RiboBio Co., Ltd. A total of 10 nM
si-LOC100129516 or siRNA-ctrl was transfected into MSCs, THP-1
cells or vascular smooth muscle cells using
Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.) for
24 h. The sequences of siRNAs used were as follows:
si-LOC100129516, 5′-CCC AGG CTA CCA TCC CTC CAA ATA A-3′ and
siRNA-ctrl, 5′-CCC ATC GTA CCT CCC AAC CAG ATA A-3′.
Isolation and characterization of
exosomes
Supernatant from the culture of MSCs was removed and
placed into a sterile enzyme-free 15 ml centrifuge tube. The
samples were centrifuged at 2,000 × g at 4°C for 30 min to remove
cells and debris. A total of 0.5 ml Total Exosome Isolation reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) was added to the
samples. Subsequently, the exosome samples were centrifuged at
10,000 × g at 25°C for 10 min and resuspended with 100 µl 1X
PBS. The exosomes were obtained, and a BCA assay (Aspen
Biosciences, LLC) was used to detect the corresponding
concentration of the exosomes. Transmission electron microscopy
(TEM) and Nanoparticle Tracking Analysis (NTA) assays were used to
identify the exosomes, as described previously (28). Western blot analysis was used to
detect the expression levels of exosomal-specific surface
biomarkers, CD63 and tumor susceptibility gene 101 (TSG101).
Exosome labeling and uptake
THP-1 cells were treated with MSC-derived exosomes
at 37°C for 24 h. Subsequently, cells were labeled for 24 h at 4°C
with PKH26 red membrane dye (1:1,000; Biolab Co., Ltd.; cat. no.
HR9070). In addition, fluorescent phalloidin dye (Thermo Fisher
Scientific, Inc.) was used to label the cytoskeleton overnight at
4°C, and DAPI was used to stain the nuclei at room temperature for
10 min. Images were captured using a fluorescence microscope
(magnification, ×200; Olympus Corporation).
Western blot analysis
Proteins were isolated using protein lysis buffer
(Beyotime Institute of Biotechnology) and quantified using a BCA
protein assay kit. A total of 30 µg protein per lane was
loaded on a 10% SDS gel, resolved using SDS-PAGE and transferred to
a PVDF membrane. The membrane was subsequently incubated with the
following primary antibodies: Anti-CD63 (1:1,000; cat. no.
ab134045; Abcam), anti-TSG101 (1:1,000; cat. no. ab125011; Abcam),
anti-CD9 (1:1,000; cat. no. ab236630; Abcam) anti-cleaved
ccaspase-3 (1:1,000; cat. no. ab32042; Abcam), anti-peroxisome
proliferator-activated receptor γ (PPARγ; 1:1,000; cat. no.
ab178860; Abcam), anti-liver X receptor α (LXRα; 1:1,000; cat. no.
ab176323; Abcam), anti-phospholipid-transporting ATPase ABCA1
(ABCA1; 1:1,000; cat. no. ab66217; Abcam) and β-actin (1:1,000;
cat. no. ab8226; Abcam) at 4°C overnight. Following the primary
antibody incubation, membranes were incubated with HRP-conjugated
goat anti-rabbit IgG secondary antibody (1:5,000; cat. no. ab7090;
Abcam) at room temperature for 1 h. Subsequently, the protein bands
were detected using an ECL kit (Thermo Fisher Scientific,
Inc.).
ELISA
The levels of total cholesterol (TC), free
cholesterol (FC) and cholesterol ester (CE) in THP-1
macrophage-derived foam cells were measured using ELISA. The levels
of TC and low-density lipoprotein cholesterol (LDL-C) were also
measured in the plasma of mice using ELISA kits, according to the
manufacturer's protocol. The TC assay kit (cat. no. A111-1-1) and
LDL-C assay kit (cat. no. A113-1-1) were obtained from Nanjing
Jiancheng Bioengineering Institute. A Micro FC Content assay kit
(cat. no. BC1895) was purchased from Beijing Solarbio Science &
Technology Co., Ltd. The CE content was calculated using the
following equation: CE = TC content − FC content.
Observation of adipocytes
Oil red O staining was used to observe the
distribution of adipose cells in THP-1 macrophage-derived foam
cells. Oil red O was obtained from Beijing Solarbio Science &
Technology Co., Ltd. Cells were stained with Oil red O for 5 min at
room temperature. The images were observed under a fluorescence
microscope.
Flow cytometry assay
THP-1 cells were trypsinized and resuspended in
binding buffer. Then, cells were stained with 5 µl Annexin
V-FITC (BD Biosciences) and propidium iodide (PI; BD Biosciences)
in the dark at 37°C for 30 min. Flow cytometry (FACScan™; BD
Biosciences) was applied to analyze the apoptosis rate using
CellQuest™ software version 5.1 (BD Biosciences).
Nitrobenzoxadiazole-labeled cholesterol
efflux assay
THP-1 macrophage-derived foam cells were incubated
with 1 µg/ml NBD-cholesterol in RPMI-1640 medium containing
0.2% BSA for 24 h. A total of 15 µg/ml Apolipoprotein A1
(ApoA1; Sigma-Aldrich; Merck KGaA) or 50 µg/ml high-density
lipoprotein (HDL) was used to induce cholesterol efflux. A
microplate reader (Tecan Infinite M200) was used to measure
NBD-cholesterol efflux (515 nm).
In vivo model of atherosclerosis
Male ApoE−/− mice (n=24, age, 8 weeks;
weight, 20-25 g) were purchased from the Beijing Vital River
Laboratory Animal Technology Co., Ltd. Animals were housed in a
specific pathogen-free animal facility with 60% humidity at a
constant temperature of 24±2°C. To establish the in
vivoatherosclerosis model, animals were randomly divided into
four groups: i) Control group, containing ApoE−/− mice
fed on a standard 4% fat diet (cat. no. D12450B; Beijing HFK
Bioscience; ii) atherosclerosis (AS) group, containing
ApoE−/− mice fed on a high-fat diet with 60% of total
calories derived from fat (cat. no. D12492; Beijing HFK Bioscience)
and an intravenous injection of 200 µl PBS per dose; iii)
siRNA-ctrl exosome (Exo) + AS; and iv) si-LOC100129516 Exo + AS
groups, containing ApoE−/− mice fed on a high-fat diet,
with an injection of siRNA-ctrl Exo or si-LOC100129516 Exo via the
tail vein twice a week. All mice were sacrificed using
CO2 (30% volume/min) and blood samples and arteries were
collected for subsequent experiments. All animal procedures were
approved by The Committee of the First Affiliated Hospital of
Jinzhou Medical University (approval no. FAHJMU20210113). The
National Institute of Health Guide for the Care and Use of
Laboratory Animals was strictly followed (29).
Oil red O and hematoxylin and eosin
(H&E) staining of arteries
The arteries of the mice were perfused with PBS and
separated. The arteries of mice were subsequently stained with Oil
red O and H&E as described previously (30).
Statistical analysis
All results are presented as the mean ± standard
deviation. Data were analyzed using a one-way ANOVA followed by a
Tukey's post hoc test, using GraphPad Prism software (version 7.0;
GraphPad Software, Inc.). P<0.05 was considered to indicate a
statistically significant difference.
Results
LOC100129516 is upregulated in THP-1
macrophage-derived foam cells
In our previous study, results of bioinformatics
analysis demonstrated that the levels of LOC100129516 were
upregulated in PBMCs obtained from patients with CAD. In order to
identify the expression levels and role of LOC100129516 in
atherosclerosis, the levels of LOC100129516 were determined. As
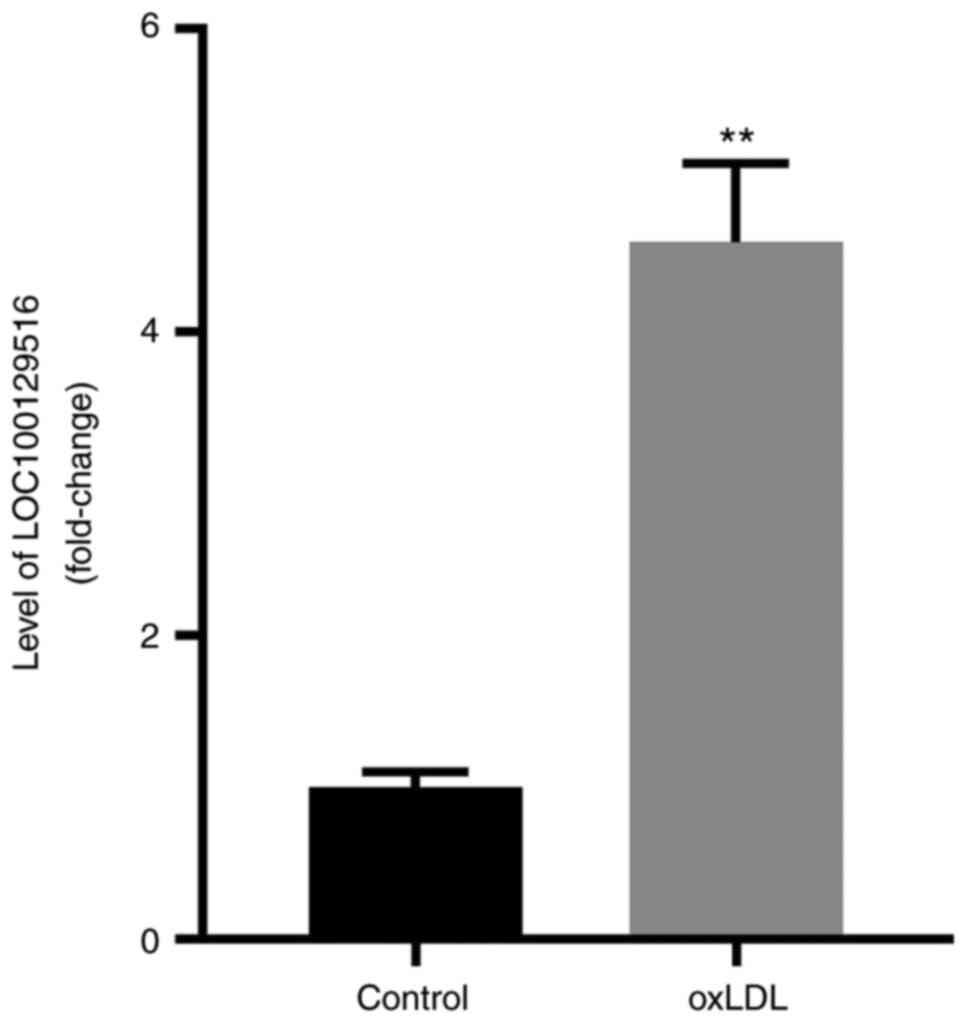
shown in Fig. 1, the expression
of LOC100129516 in THP-1 macrophage-derived foam cells was
significantly higher compared with the expression levels in THP-1
macrophages.
si-LOC100129516 is transferred from MSCs
to THP-1 macrophages via exosomes
MSC-based therapy has emerged as a cell-based
therapy for treating atherosclerosis (31). The results of a previous study
demonstrated that exosomes carrying non-coding RNAs are involved in
cell-cell communication between MSCs and macrophages (18). The present study aimed to
investigate whether si-LOC100129516 could be transferred from MSCs
to THP-1 macrophages via exosomes. As shown in Fig. 2A, transfection of si-LOC100129516
notably decreased the levels of LOC100129516 in MSCs, compared with
the siRNA-ctrl group. Furthermore, exosomes were isolated from the
culture supernatant of MSCs (MSCs-Exo), and MSCs were transfected
with siRNA-ctrl (siRNA-ctrl/MSCs-Exo) or si-LOC100129516
(si-LOC100129516/MSCs-Exo). TEM and NTA results indicated that
MSC-derived exosomes were round, phospholipid bilayer-enclosed
structures with a diameter of 40-100 nm (Fig. 2B and C). Moreover, markers of
exosomes, such as CD9, CD63 and TSG101 were detected using western
blot analysis (Fig. 2D). The
levels of LOC100129516 were significantly decreased in
si-LOC100129516/MSCs-Exo compared with that in siRNA-ctrl/MSCs-Exo
(Fig. 2E). To investigate
whether MSC-derived exosomes and MSC-derived exosomes transfected
with si-LOC100129516 were internalized by THP-1 macrophages,
exosomes were labeled with PKH26 dye and incubated with THP-1
macrophages. As demonstrated in Fig.
2F, MSC-derived exosomes were absorbed by THP-1 macrophages.
Furthermore, si-LOC100129516/MSCs-Exo significantly decreased the
levels of LOC100129516 in THP-1 macrophages (Fig. 2G). Collectively, these results
indicated that si-LOC100129516 can be transferred from MSCs to
THP-1 macrophages via exosomes.
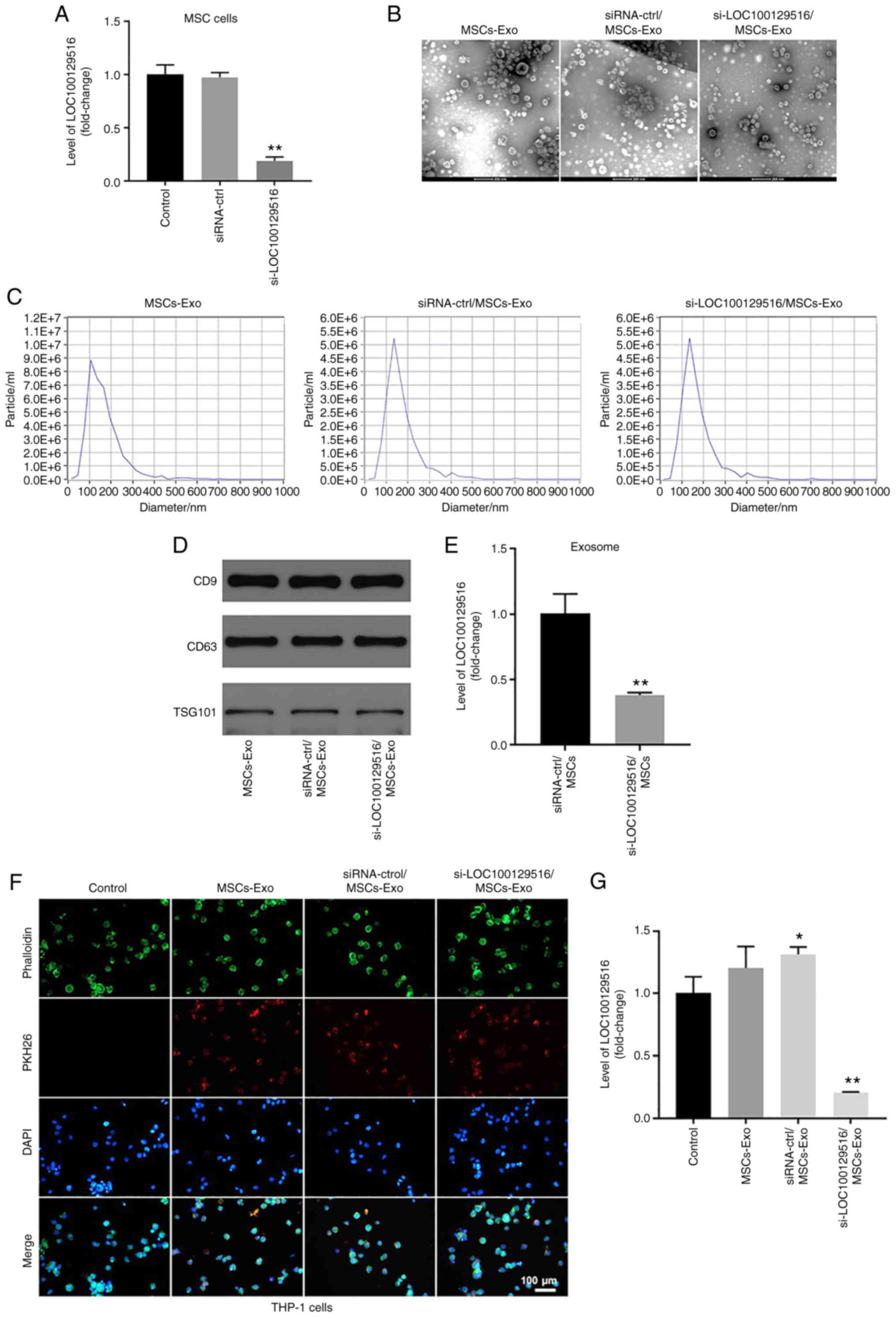 | Figure 2si-LOC100129516 can be transferred
from MSCs to THP-1 macrophages via exosomes. (A) RT-qPCR was used
to detect the levels of LOC100129516 in MSCs transfected with
si-LOC100129516 or siRNA-ctrl. (B and C) Exosomes were isolated
from MSCs that were transfected with si-LOC100129516 or siRNA-ctrl.
Transmission electron microscopy (magnification, 200×) and
Nanoparticle Tracking Analysis assays were used to identify
exosomes. (D) Western blotting was used to detect expression of
exosomal surface markers CD9, CD63 and TSG101. (E) RT-qPCR was used
to detect the level of LOC100129516 in exosomes. (F) The
MSC-derived exosomes absorbed by THP-1 macrophages were observed
under a fluorescence microscope (magnification, 200×). Red color,
exosome; green color, SHG44 cells; blue color, cell nucleus. Scale
bar, 100 µm. (G) RT-qPCR was used to detect the levels of
LOC100129516 in THP-1 macrophages after incubation with the
indicated exosomes. n=3. *P<0.05,
**P<0.01 vs. control group. siRNA, small interfering
RNA; MSC, mesenchymal stem cell; RT-qPCR, reverse
transcription-quantitative PCR; ctrl, control; TSG101, tumor
susceptibility gene 101; Exo, exosome. |
Exosomal-mediated delivery of
si-LOC100129516 inhibits apoptosis in THP-1 macrophage-derived foam
cells
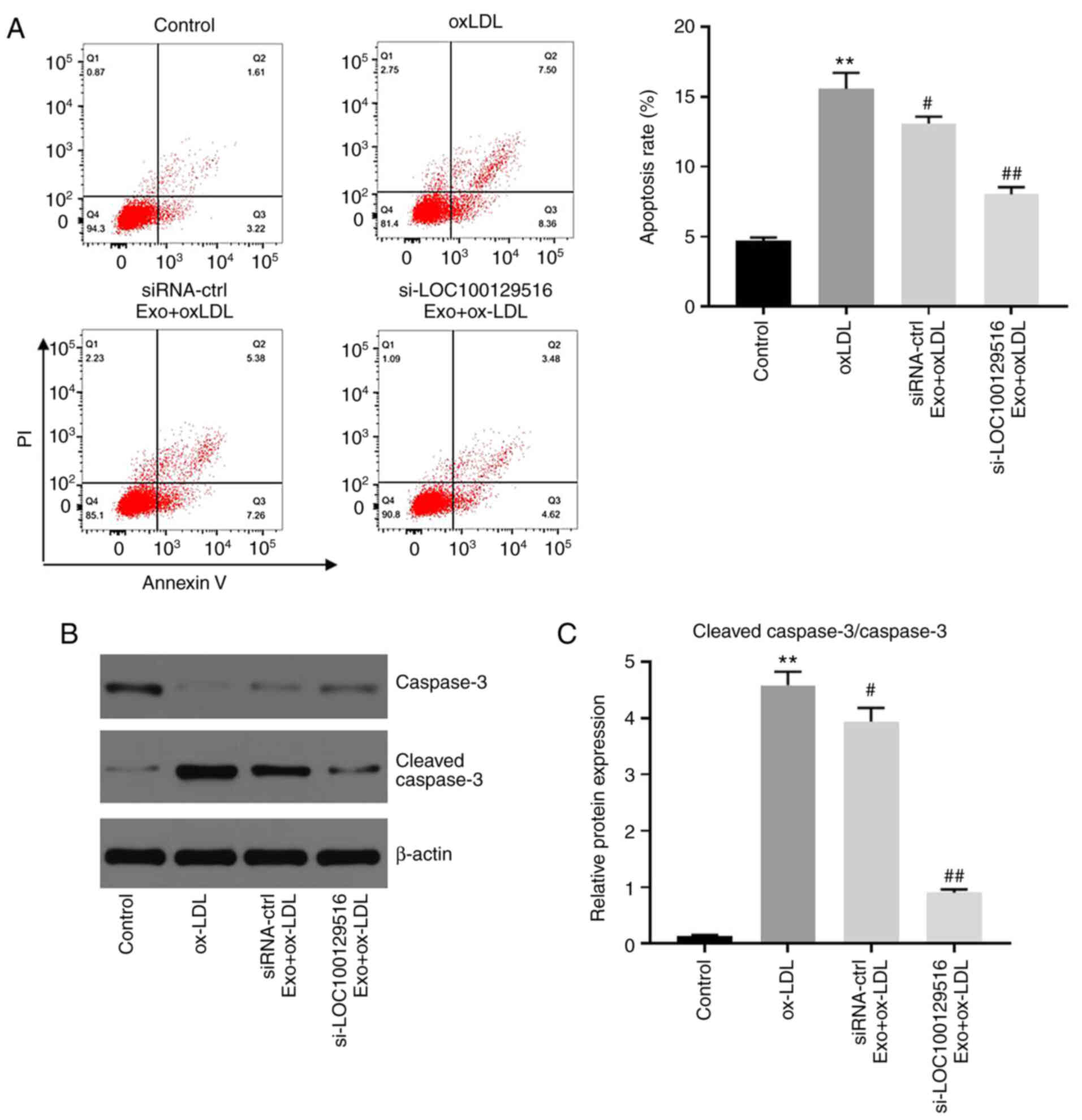
To investigate whether exosomal-mediated delivery of
si-LOC100129516 affected the apoptosis in THP-1 macrophage-derived
foam cells, flow cytometry was performed. As shown in Fig. 3A, ox-LDL significantly induced
apoptosis in THP-1 macrophages compared with the control group.
However, these changes were partially reversed by
si-LOC100129516/MSCs-Exo. In addition, si-LOC100129516/MSCs-Exo
significantly downregulated the expression of cleaved ccaspase-3 in
THP-1 macrophage-derived foam cells (Fig. 3B and C). Silencing of
LOC100129516 or si-LOC100129516/MSCs-Exo notably restored
ox-LDL-induced increase of vascular smooth muscle cell viability
(Fig. S1A), and LOC100129516
knockdown did not affect the apoptosis of THP-1 cells (Fig. S1B). These results indicated that
exosomal-mediated delivery of si-LOC100129516 inhibited the
apoptosis of THP-1 macrophage-derived foam cells.
Exosomal-mediated delivery of
si-LOC100129516 inhibits lipid accumulation in THP-1
macrophage-derived foam cells
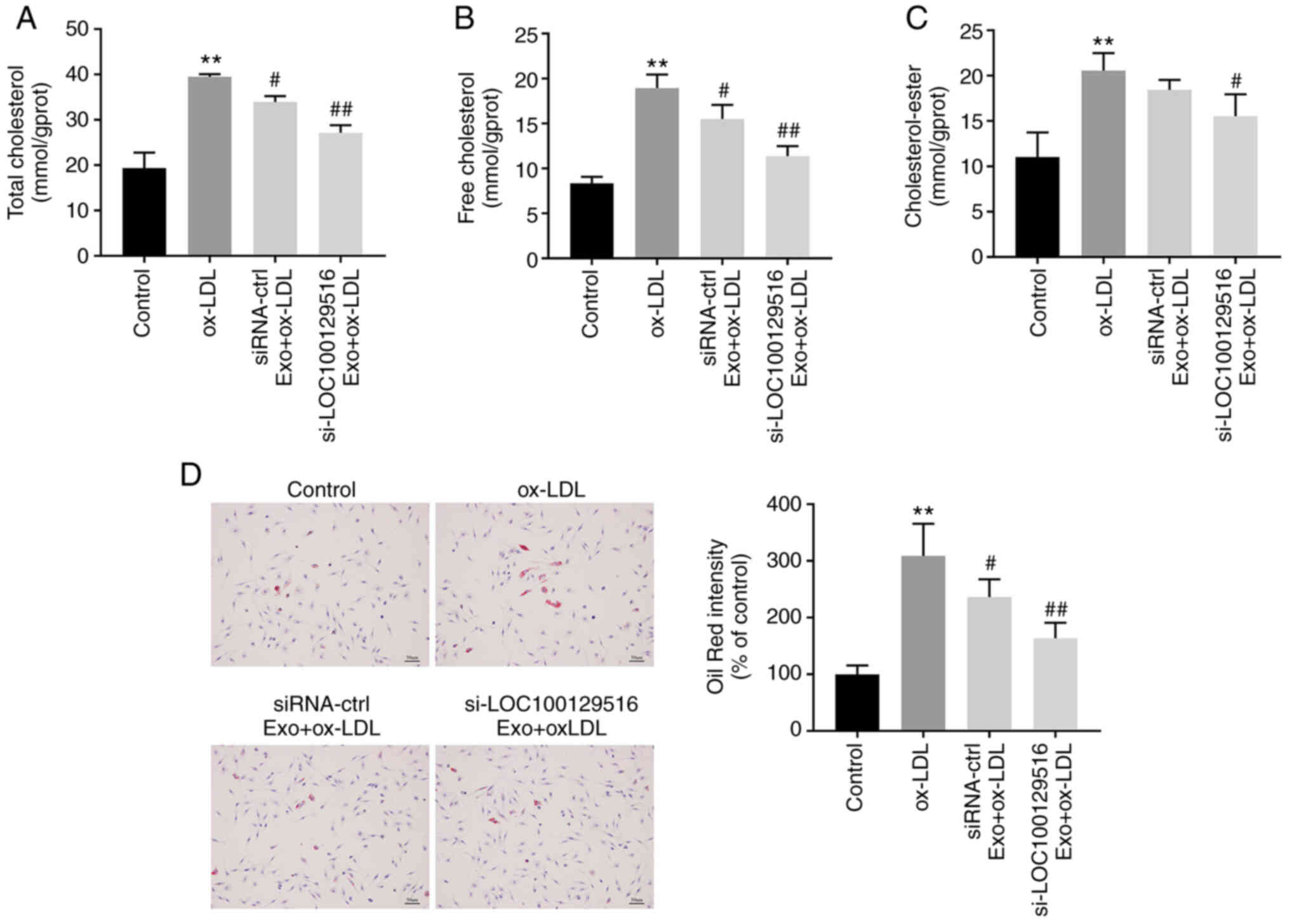
The role of exosomal-mediated delivery of
si-LOC100129516 in lipid accumulation in THP-1 macrophage-derived
foam cells was investigated. As indicated in Fig. 4A-C, ox-LDL markedly increased the
levels of TC, FC and CE in THP-1 macrophages compared with the
control group. However, these ox-LDL-induced changes were reversed
by si-LOC100129516/MSCs-Exo. In addition, the Oil-Red O staining
results indicated that ox-LDL significantly increased intracellular
lipid accumulation compared with the control group. However, this
phenomenon was also reversed in the presence of
si-LOC100129516/MSCs-Exo (Fig.
4D). Collectively, these results demonstrated that
exosomal-mediated delivery of si-LOC100129516 inhibited the lipid
accumulation in THP-1 macrophage-derived foam cells.
Exosomal-mediated delivery of
si-LOC100129516 increases cholesterol efflux in THP-1
macrophage-derived foam cells via upregulation of the
PPARγ/LXRα/ABCA1 signaling pathway
Reverse cholesterol transport (RCT) is a key process
in removing excessive cholesterol from cells (32). HDL and ApoA1 proteins serve
important roles in the RCT pathway, contributing to the efflux of
excess cellular cholesterol (33-35). Thus, the effect of
exosomal-mediated delivery of si-LOC100129516 on cholesterol efflux
was examined in the present study. As shown in Fig. 5A and B, si-LOC100129516/MSCs-Exo
markedly promoted cholesterol efflux to HDL and ApoA1. In addition,
the PPARγ/LXRα/ABCA1 signaling pathway plays an important role in
atherosclerosis progression via promotion of cholesterol efflux,
maintenance of cholesterol balance and inhibition of foam cell
formation (36,37). Thus, the protein levels of PPARγ,
LXRα and ABCA1 in THP-1 macrophage-derived foam cells were detected
using western blot analysis. As indicated in Fig. 5C-F, ox-LDL significantly
decreased the expression levels of PPARγ, LXRα and ABCA1 in THP-1
macrophages, compared with the control group. However, these
changes were partially reversed by si-LOC100129516/MSCs-Exo. Thus,
exosomal-mediated delivery of si-LOC100129516 increased cholesterol
efflux in THP-1 macrophage-derived foam cells via increasing the
activity of the PPARγ/LXRα/ABCA1 signaling pathway.
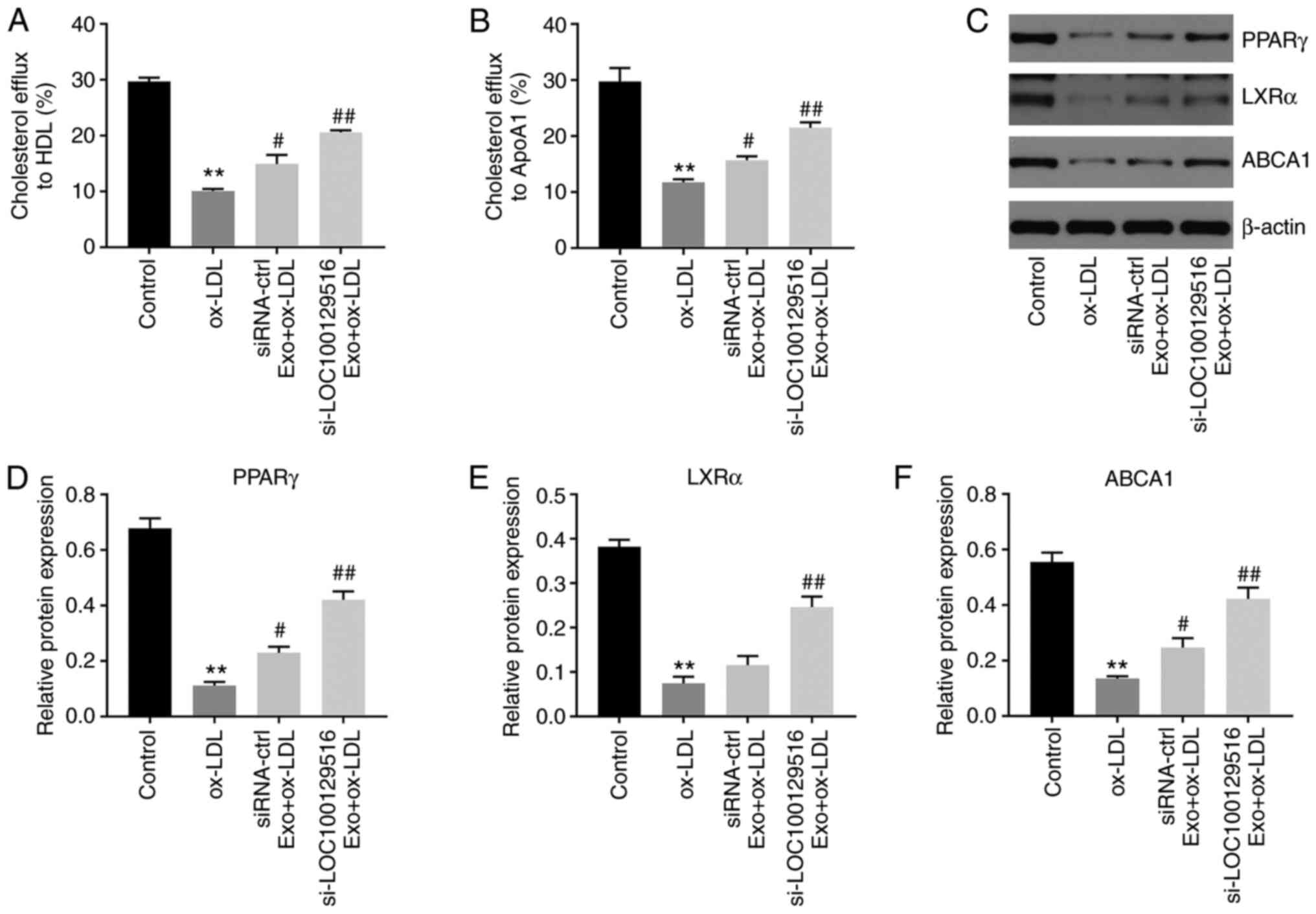 | Figure 5Exosomal si-LOC100129516 increases
cholesterol excretion in THP-1 macrophage-derived foam cells by
increasing the activity of the PPARγ/LXRα/ABCA1 signaling pathway.
MSCs were transfected with si-LOC100129516 or siRNA-ctrl. In
addition, ox-LDL-treated THP-1 macrophages were incubated with
exosomes derived from transfected MSCs. (A and B) Quantification of
cholesterol efflux to high-density lipoprotein cholesterol and
ApoA1 in cells. (C-F) Western blot assay was used to detect the
expression levels of PPARγ, LXRα and ABCA1 in cells. n=3.
**P<0.01 vs. control group; #P<0.01,
##P<0.01 vs. ox-LDL group. siRNA, small interfering
RNA; MSC, mesenchymal stem cell; ox-LDL, oxidized low density
lipoproteins; ctrl, control; PPARγ, peroxisome
proliferator-activated receptor γ; LXRα, liver X receptor α; ABCA1,
phospholipid-transporting ATPase ABCA1; APOA1, apolipoprotein A1;
Exo, exosome. |
Exosomal-mediated delivery of
si-LOC100129516 suppresses atherosclerosis progression in vivo via
upregulation of the PPARγ/LXRα/ABCA1 signaling pathway
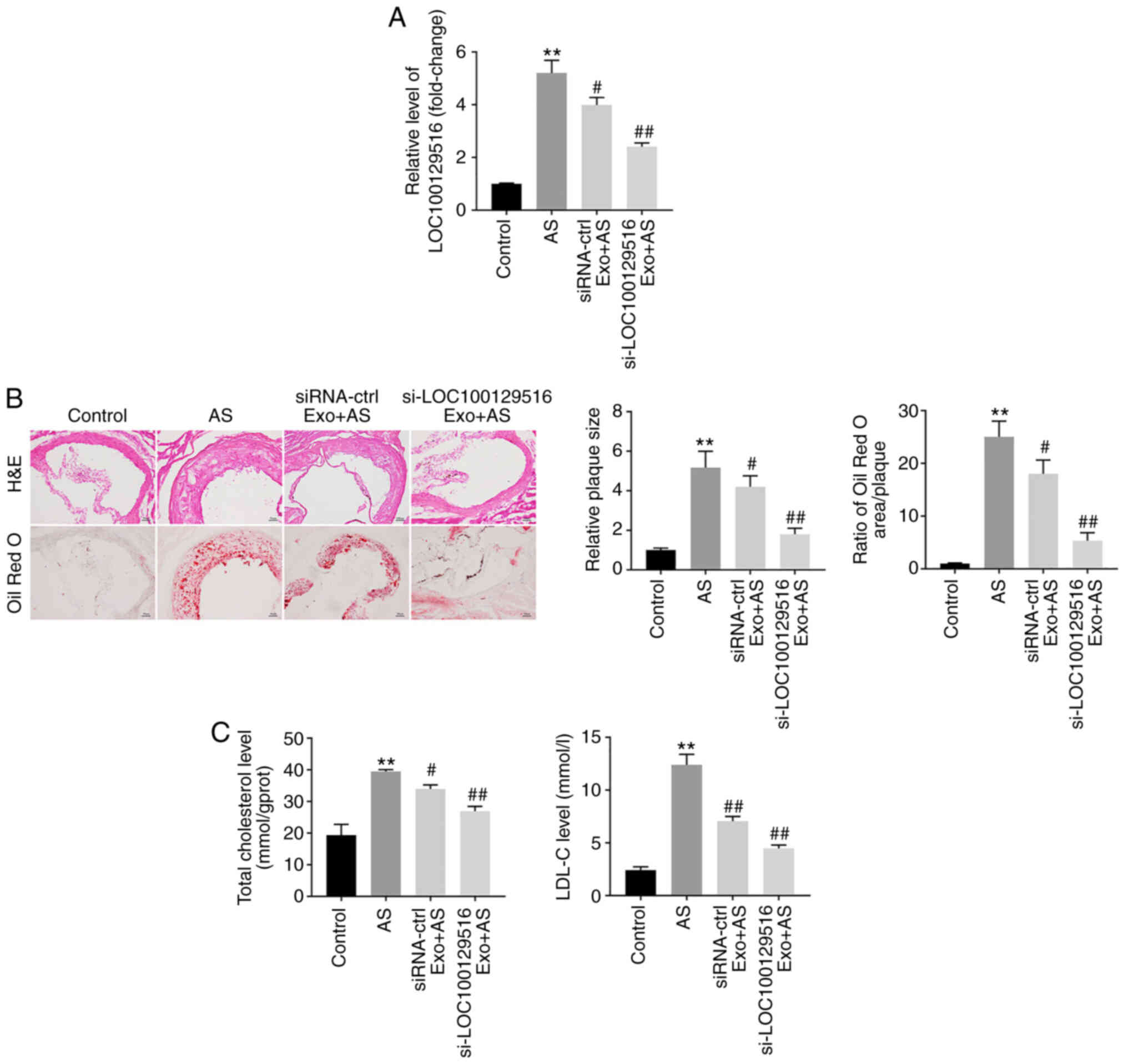
To further explore the role of exosomal-mediated
delivery of si-LOC100129516 on the progression in atherosclerosis,
a mouse model of atherosclerosis was established. As demonstrated
in Fig. 6A, the expression
levels of LOC100129516 was significantly upregulated in aortic
tissues in the atherosclerotic mouse model. However, this
phenomenon was reversed by si-LOC100129516/MSCs-Exo treatment. In
addition, mice that were fed a high-fat diet demonstrated a
distinct thickening of the blood vessel wall and notable plaque
formation in the aortic tissue. However, this effect was reversed
by si-LOC100129516/MSCs-Exo treatment (Fig. 6B). Moreover, the results of the
Oil red O staining assay revealed lipid accumulation and
atherosclerotic plaque formation in the aortic tissue of the
atherosclerotic mice compared with the control group, and this was
reversed by si-LOC100129516/MSCs-Exo treatment (Fig. 6B). si-LOC100129516/MSCs-Exo
markedly reduced the plasma levels of TC and LDL-C in
ApoE−/− mice with atherosclerosis (Fig. 6C). Furthermore,
si-LOC100129516/MSCs-Exo significantly upregulated the expression
levels of PPARγ, LXRα and ABCA1 in the aortic tissues of
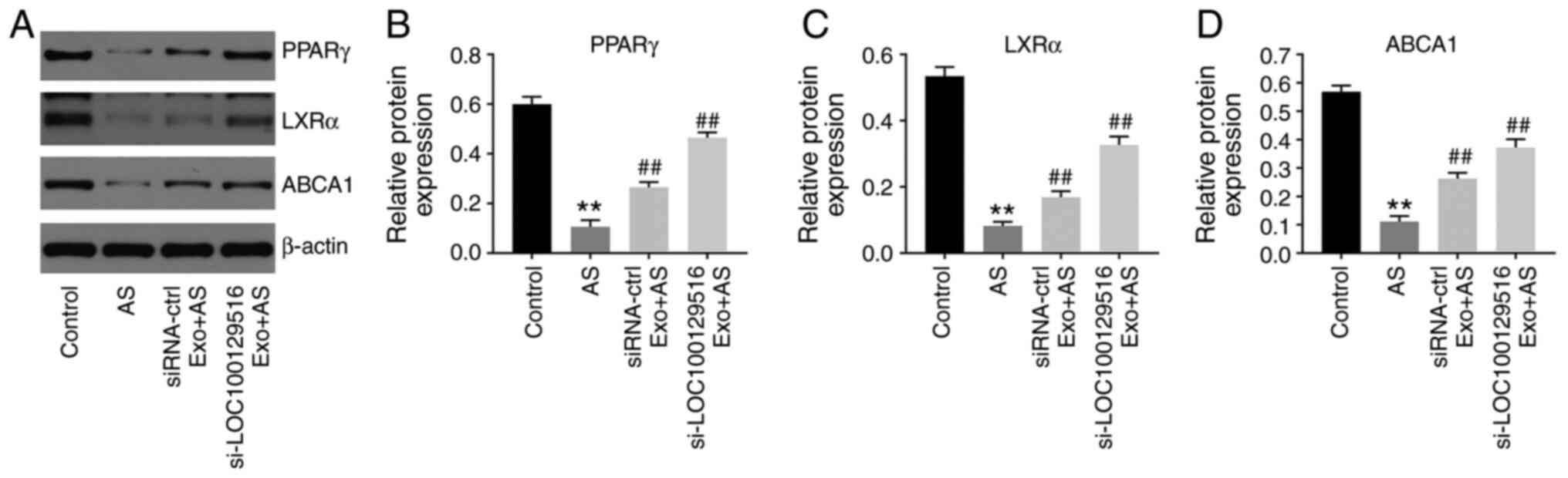
ApoE−/− mice with atherosclerosis (Fig. 7A-D). Collectively, these results
indicated that exosomal-mediated delivery of si-LOC100129516
suppressed atherosclerosis progression in vivovia
upregulation of the PPARγ/LXRα/ABCA1 signaling pathway.
Discussion
MSCs have been demonstrated to be effective for the
treatment of atherosclerosis, due to their ability to repair
tissue, in addition to their anti-inflammatory and immunological
properties (38). MSCs can be
isolated from several types of tissues, including bone marrow, the
umbilical cord, placenta, adipose tissue and human gingiva
(39,40). MSCs that are used for the
treatment of atherosclerotic plaques are primarily derived from the
bone marrow (39), but can also
be obtained the gingiva (41).
In addition, MSC-derived exosomes have been a key focus of research
for several decades (42).
Exosomes are macrovesicles 30-150 nm in size that are secreted by
MSCs via a paracrine mechanism (43). Exosomes derived from MSCs carry a
number of bioactive substances, such as proteins, miRNAs and
lncRNAs, and play an important role in the treatment of
cardiovascular diseases, such as atherosclerosis (18,43,44). A high expression level of
lncRNA-Ang362 was shown to lead to a poor prognosis in patients
with CAD (45). Moreover, Mao
et al (46) demonstrated
that MSC-derived exosomal lncRNA Krüppel-like factor 3 antisense
RNA 1 alleviated the development of myocardial infarction via the
miR-138-5p/Sirtuin-1 axis (46).
However, the functions of a number of lncRNAs in atherosclerosis
remain to be elucidated. Thus, it is necessary to identify novel
therapeutic targets for the prevention of progression of
atherosclerosis. In the present study, it was shown that exosomal
LOC100129516 derived from MSCs could suppress ox-LDL-induced HUVEC
growth inhibition. Thus, the present study was the first to explore
the function of exosomal LOC100129516 in atherosclerosis,
suggesting a novel target for the treatment of atherosclerosis.
Li et al (47) reported that the overexpression of
cyclin-dependent kinase inhibitor 2B antisense RNA 1 reduced lipid
accumulation and increased cholesterol efflux in THP-1
macrophage-derived foam cells and in atherosclerotic plaque tissues
(47). Moreover, Meng et
al (48) found that
downregulation of lncRNA growth arrest specific 5 reduced lipid
accumulation in THP-1 macrophage-derived foam cells, thus
inhibiting the progression of atherosclerosis. In the present
study, MSC-derived exosomal-mediated delivery of si-LOC100129516
significantly inhibited the apoptosis, intracellular cholesterol
accumulation and lipid accumulation of THP-1 macrophage-derived
foam cells, consistent with Meng et al (48). In the study by Meng et al
(48), GAS5 knockdown reduced
EZH2-mediated transcriptional inhibition of ABCA1 via histone
methylation, and ABCA1 served a vital role in atherosclerotic
progression (49). Consistently,
ABCA1 was found to be regulated by exosomes with downregulated
levels of LOC100129516. Therefore, the similar functions between
GAS5 and exosomes with downregulated LOC100129516 expression may
result in this similarity. Moreover, exosomal-mediated delivery of
si-LOC100129516 reduced lipid accumulation and atherosclerotic
plaque formation in ApoE−/− mice with atherosclerosis.
The results of the present study suggested that exosomal-mediated
delivery of si-LOC100129516 inhibited atherosclerosis progression
both in vitro and in vivo. Meanwhile, the present
study found that exosomal-mediated delivery of si-NC could also
reverse ox-LDL-induced HUVEC growth inhibition. This phenomenon may
result from the fact that exosomes have a targeting effect, which
can escape the phagocytosis of macrophages (50), highlighting a potential advantage
of exosomes as a mode of treatment/delivery in vivo.
PPARγ is an effective cholesterol sensor and plays a
major role in the treatment of atherosclerosis (51). In addition, PPARγ enhances
cholesterol efflux by inducing LXRα and ABCA1 transcription
(52). The results of a previous
study demonstrated that downregulation of the PPARγ/LXRα/ABCA1
signaling pathway aggravated the progression of atherosclerosis
(53). Zhao et
al(51) revealed that
miR-613 inhibited the efflux of cholesterol from THP-1
macrophage-derived foam cells by downregulating the activity of the
PPARγ/LXRα/ABCA1 signaling pathway (51). Furthermore, Wang et al
(54) demonstrated that chrysin
enhanced the efflux of cholesterol by regulating the
PPARγ/LXRα/ABCA1/ATP binding cassette subfamily G member 1
signaling pathway (54). The
results of the present study revealed that si-LOC100129516/MSCs-Exo
increased cholesterol excretion by upregulating the
PPARγ/LXRα/ABCA1 signaling pathway in THP-1 macrophage-derived foam
cells in vitro, and in ApoE−/− mice with
atherosclerosis in vivo.
The present study has some limitations as follows:
i) The advantages and disadvantages of exosomal si-LOC100129516 in
comparison to treatment with si-LOC100129516 transfection remain
unclear; and ii) more in-depth analysis of the signaling pathways
regulated by exosomal LOC100129516 in ox-LDL-treated HUVECs is
required. In conclusion, MSC-derived exosomal-mediated delivery of
si-LOC100129516 promoted cholesterol efflux and suppressed
intracellular lipid accumulation, thus alleviating the progression
of atherosclerosis by stimulating the PPARγ/LXRα/ABCA1 signaling
pathway. The results of the present study highlighted that
LOC10012951 may act as a potential target for novel therapeutic
strategies in the treatment of atherosclerosis.
Supplementary Data
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
LS made major contributions to the conception and
design of the study, as well as the drafting of the manuscript. XH
and TZ were responsible for data acquisition, data analysis, data
interpretation and manuscript revision. GT and YH made substantial
contributions to conception and design of the study, and revised
the manuscript critically for important intellectual content. All
authors have read and approved the final manuscript. YH and LS
confirmed the authenticity of all the raw data.
Ethics approval and consent to
participate
All animal procedures were approved by the Committee
of the First Affiliated Hospital of Jinzhou Medical University
(approval no. FAHJMU20210113). The National Institute of Health
Guide for the Care and Use of Laboratory Animals was strictly
followed.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Falk E: Pathogenesis of atherosclerosis. J
Am Coll Cardiol. 47:C7–C12. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tedgui A and Mallat Z: Cytokines in
atherosclerosis: Pathogenic and regulatory pathways. Physiol Rev.
86:515–581. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Weber C and Noels H: Atherosclerosis:
Current pathogenesis and therapeutic options. Nat Med.
17:1410–1422. 2011. View
Article : Google Scholar
|
|
4
|
Lechner K, von Schacky C, McKenzie AL,
Worm N, Nixdorff U, Lechner B, Kränkel N, Halle M, Krauss RM and
Scherr J: Lifestyle factors and high-risk atherosclerosis: Pathways
and mechanisms beyond traditional risk factors. Eur J Prev Cardiol.
27:394–406. 2020. View Article : Google Scholar :
|
|
5
|
Shoeibi S: Diagnostic and theranostic
microRNAs in the pathogenesis of atherosclerosis. Acta Physiol
(Oxf). 228:e133532020. View Article : Google Scholar
|
|
6
|
Kunitomo M: Oxidative stress and
atherosclerosis. Yakugaku Zasshi. 127:1997–2014. 2007.In Japanese.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li TT, Wang ZB, Li Y, Cao F, Yang BY and
Kuang HX: The mechanisms of traditional Chinese medicine underlying
the prevention and treatment of atherosclerosis. Chin J Nat Med.
17:401–412. 2019.PubMed/NCBI
|
|
8
|
Zarzycka B, Nicolaes GA and Lutgens E:
Targeting the adaptive immune system: New strategies in the
treatment of atherosclerosis. Expert Rev Clin Pharmacol. 8:297–313.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang C, Niimi M, Watanabe T, Wang Y, Liang
J and Fan J: Treatment of atherosclerosis by traditional Chinese
medicine: Questions and quandaries. Atherosclerosis. 277:136–144.
2018. View Article : Google Scholar
|
|
10
|
Vaidyanathan K and Gopalakrishnan S:
Nanomedicine in the diagnosis and treatment of atherosclerosis - a
systematic review. Cardiovasc Hematol Disord Drug Targets.
17:119–131. 2017. View Article : Google Scholar
|
|
11
|
Fasolo F, Di Gregoli K, Maegdefessel L and
Johnson JL: Non-coding RNAs in cardiovascular cell biology and
atherosclerosis. Cardiovasc Res. 115:1732–1756. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Varol C, Mildner A and Jung S:
Macrophages: Development and tissue specialization. Annu Rev
Immunol. 33:643–675. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Smigiel KS and Parks WC: Macrophages,
wound healing, and fibrosis: Recent insights. Curr Rheumatol Rep.
20:172018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kuznetsova T, Prange KHM, Glass CK and de
Winther MPJ: Transcriptional and epigenetic regulation of
macrophages in atherosclerosis. Nat Rev Cardiol. 17:216–228. 2020.
View Article : Google Scholar :
|
|
15
|
Moore KJ and Tabas I: Macrophages in the
pathogenesis of atherosclerosis. Cell. 145:341–355. 2011.
View Article : Google Scholar :
|
|
16
|
Barrett TJ: Macrophages in atherosclerosis
regression. Arterioscler Thromb Vasc Biol. 40:20–33. 2020.
View Article : Google Scholar
|
|
17
|
Uccelli A, Moretta L and Pistoia V:
Mesenchymal stem cells in health and disease. Nat Rev Immunol.
8:726–736. 2008. View
Article : Google Scholar
|
|
18
|
Li J, Xue H, Li T, Chu X, Xin D, Xiong Y,
Qiu W, Gao X, Qian M, Xu J, et al: Exosomes derived from
mesenchymal stem cells attenuate the progression of atherosclerosis
in ApoE−/− mice via miR-let7 mediated infiltration and
polarization of M2 macrophage. Biochem Biophys Res Commun.
510:565–572. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kalluri R and LeBleu VS: The biology,
function, and biomedical applications of exosomes. Science.
367:eaau69772020. View Article : Google Scholar :
|
|
20
|
Sasaki R, Kanda T, Yokosuka O, Kato N,
Matsuoka S and Moriyama M: Exosomes and hepatocellular carcinoma:
From BENCH TO BEDside. Int J Mol Sci. 20:14062019. View Article : Google Scholar
|
|
21
|
Huang P, Wang L, Li Q, Tian X, Xu J, Xu J,
Xiong Y, Chen G, Qian H, Jin C, et al: Atorvastatin enhances the
therapeutic efficacy of mesenchymal stem cells-derived exosomes in
acute myocardial infarction via up-regulating long non-coding RNA
H19. Cardiovasc Res. 116:353–367. 2020. View Article : Google Scholar
|
|
22
|
Yue Y, Li YQ, Fu S, Wu YT, Zhu L, Hua L,
Lv JY, Li YL and Yang DL: Osthole inhibits cell proliferation by
regulating the TGF-β1/Smad/p38 signaling pathways in pulmonary
arterial smooth muscle cells. Biomed Pharmacother. 121:1096402020.
View Article : Google Scholar
|
|
23
|
Charles Richard JL and Eichhorn PJA:
Platforms for investigating lncRNA functions. SLAS Technol.
23:493–506. 2018.PubMed/NCBI
|
|
24
|
Tan J, Liu S, Jiang Q, Yu T and Huang K:
lncRNA-MIAT increased in patients with coronary atherosclerotic
heart disease. Cardiol Res Pract. 2019:62801942019. View Article : Google Scholar :
|
|
25
|
Liao J, Wang J, Liu Y, Li J and Duan L:
Transcriptome sequencing of lncRNA, miRNA, mRNA and interaction
network constructing in coronary heart disease. BMC Med Genomics.
12:1242019. View Article : Google Scholar
|
|
26
|
Lin XL, Hu HJ, Liu YB, Hu XM, Fan XJ, Zou
WW, Pan YQ, Zhou WQ, Peng MW and Gu CH: Allicin induces the
upregulation of ABCA1 expression via PPARγ/LXRα signaling in THP-1
macrophage-derived foam cells. Int J Mol Med. 39:1452–1460. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
28
|
Cui Y, Fu S, Sun D, Xing J, Hou T and Wu
X: EPC-derived exosomes promote osteoclastogenesis through
lncRNA-MALAT1. J Cell Mol Med. 23:3843–3854. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
National Research Council Committee for
the Update of the Guide for the Care and Use of Laboratory Animals:
The National Academies Collection: Reports funded by National
Institutes of Health. Guide for the Care and Use of Laboratory
Animals. 8th edition. National Academies Press; Washington, DC:
2011
|
|
30
|
Shen S, Zheng X, Zhu Z, Zhao S, Zhou Q,
Song Z, Wang G and Wang Z: Silencing of GAS5 represses the
malignant progression of atherosclerosis through upregulation of
miR-135a. Biomed Pharmacother. 118:1093022019. View Article : Google Scholar
|
|
31
|
Guo Z, Zhao Z, Yang C and Song C: Transfer
of microRNA-221 from mesenchymal stem cell-derived extracellular
vesicles inhibits atherosclerotic plaque formation. Transl Res.
226:83–95. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yu XH, Zhang DW, Zheng XL and Tang CK:
Cholesterol transport system: An integrated cholesterol transport
model involved in atherosclerosis. Prog Lipid Res. 73:65–91. 2019.
View Article : Google Scholar
|
|
33
|
Ertek S: High-density lipoprotein (HDL)
dysfunction and the future of HDL. Curr Vasc Pharmacol. 16:490–498.
2018. View Article : Google Scholar
|
|
34
|
Kennedy MA, Barrera GC, Nakamura K, Baldán
A, Tarr P, Fishbein MC, Frank J, Francone OL and Edwards PA: ABCG1
has a critical role in mediating cholesterol efflux to HDL and
preventing cellular lipid accumulation. Cell Metab. 1:121–131.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Talbot CPJ, Plat J, Ritsch A and Mensink
RP: Determinants of cholesterol efflux capacity in humans. Prog
Lipid Res. 69:21–32. 2018. View Article : Google Scholar
|
|
36
|
Wang H, Yang Y, Sun X, Tian F, Guo S, Wang
W, Tian Z, Jin H, Zhang Z and Tian Y: Sonodynamic therapy-induced
foam cells apoptosis activates the phagocytic
PPARγ-LXRα-ABCA1/ABCG1 pathway and promotes cholesterol efflux in
advanced plaque. Theranostics. 8:4969–4984. 2018. View Article : Google Scholar :
|
|
37
|
Mao MJ, Hu JP, Wang C, Zhang YY and Liu P:
Effects of Chinese herbal medicine Guanxinkang on expression of
PPARγ-LXRα-ABCA1 pathway in ApoE-knockout mice with
atherosclerosis. Zhong Xi Yi Jie He Xue Bao. 10:814–820. 2012.In
Chinese. View Article : Google Scholar
|
|
38
|
Li Y, Shi G, Han Y, Shang H, Li H, Liang
W, Zhao W, Bai L and Qin C: Therapeutic potential of human
umbilical cord mesenchymal stem cells on aortic atherosclerotic
plaque in a high-fat diet rabbit model. Stem Cell Res Ther.
12:4072021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kirwin T, Gomes A, Amin R, Sufi A, Goswami
S and Wang B: Mechanisms underlying the therapeutic potential of
mesenchymal stem cells in atherosclerosis. Regen Med. 16:669–682.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hashem RM, Rashed LA, Abdelkader RM and
Hashem KS: Stem cell therapy targets the neointimal smooth muscle
cells in experimentally induced atherosclerosis: Involvement of
intracellular adhesion molecule (ICAM) and vascular cell adhesion
molecule (VCAM). Braz J Med Biol Res. 54:e108072021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang X, Huang F, Li W, Dang JL, Yuan J,
Wang J, Zeng DL, Sun CX, Liu YY, Ao Q, et al: Human gingiva-derived
mesenchymal stem cells modulate monocytes/macrophages and alleviate
atherosclerosis. Front Immunol. 9:8782018. View Article : Google Scholar
|
|
42
|
Chen S, Zhou H, Zhang B and Hu Q: Exosomal
miR-512-3p derived from mesenchymal stem cells inhibits oxidized
low-density lipoprotein-induced vascular endothelial cells
dysfunction via regulating Keap1. J Biochem Mol Toxicol. 35:1–11.
2021. View Article : Google Scholar
|
|
43
|
Yang Y, Ye Y, Su X, He J, Bai W and He X:
MSCs-derived exosomes and neuroinflammation, neurogenesis and
therapy of traumatic brain injury. Front Cell Neurosci. 11:552017.
View Article : Google Scholar :
|
|
44
|
Yu C, Tang W, Lu R, Tao Y, Ren T and Gao
Y: Human adipose-derived mesenchymal stem cells promote lymphocyte
apoptosis and alleviate atherosclerosis via miR-125b1-3p/BCL11B
signal axis. Ann Palliat Med. 10:2123–2133. 2021. View Article : Google Scholar
|
|
45
|
Wang H, Gong H, Liu Y and Feng L:
Relationship between lncRNA-Ang362 and prognosis of patients with
coronary heart disease after percutaneous coronary intervention.
Biosci Rep. 40:BSR202015242020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Mao Q, Liang XL, Zhang CL, Pang YH and Lu
YX: lncRNA KLF3-AS1 in human mesenchymal stem cell-derived exosomes
ameliorates pyroptosis of cardiomyocytes and myocardial infarction
through miR-138-5p/Sirt1 axis. Stem Cell Res Ther. 10:3932019.
View Article : Google Scholar :
|
|
47
|
Li H, Han S, Sun Q, Yao Y, Li S, Yuan C,
Zhang B, Jing B, Wu J, Song Y and Wang H: Long non-coding RNA
CDKN2B-AS1 reduces inflammatory response and promotes cholesterol
efflux in atherosclerosis by inhibiting ADAM10 expression. Aging
(Albany NY). 11:1695–1715. 2019. View Article : Google Scholar
|
|
48
|
Meng XD, Yao HH, Wang LM, Yu M, Shi S,
Yuan ZX and Liu J: Knockdown of GAS5 inhibits atherosclerosis
progression via reducing EZH2-mediated ABCA1 transcription in
ApoE−/− mice. Mol Ther Nucleic Acids. 19:84–96. 2020.
View Article : Google Scholar
|
|
49
|
Zhang ZZ, Chen JJ, Deng WY, Yu XH and Tan
WH: CTRP1 decreases ABCA1 expression and promotes lipid
accumulation through the miR-4245p/FoxO1 pathway in THP-1
macrophage-derived foam cells. Cell Biol Int. Jul 20–2021, Epub
ahead of print https://doi.org/10.1002/cbin.11666. View Article : Google Scholar
|
|
50
|
Moradi-Chaleshtori M, Shojaei S,
Mohammadi-Yeganeh S and Hashemi SM: Transfer of miRNA in
tumor-derived exosomes suppresses breast tumor cell invasion and
migration by inducing M1 polarization in macrophages. Life Sci.
282:1198002021. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhao R, Feng J and He G: miR-613 regulates
cholesterol efflux by targeting LXRα and ABCA1 in PPARγ activated
THP-1 macrophages. Biochem Biophys Res Commun. 448:329–334. 2014.
View Article : Google Scholar
|
|
52
|
Xu Y, Lai F, Xu Y, Wu Y, Liu Q, Li N, Wei
Y, Feng T, Zheng Z, Jiang W, et al: Mycophenolic acid induces
ATP-binding cassette transporter A1 (ABCA1) expression through the
PPARγ-LXRα-ABCA1 pathway. Biochem Biophys Res Commun. 414:779–782.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Gu HF, Li N, Xu ZQ, Hu L, Li H, Zhang RJ,
Chen RM, Zheng XL, Tang YL and Liao DF: Chronic unpredictable mild
stress promotes atherosclerosis via HMGB1/TLR4-mediated
downregulation of PPARγ/LXRα/ABCA1 in ApoE−/− mice.
Front Physiol. 10:1652019. View Article : Google Scholar
|
|
54
|
Wang S, Zhang X, Liu M, Luan H, Ji Y, Guo
P and Wu C: Chrysin inhibits foam cell formation through promoting
cholesterol efflux from RAW264.7 macrophages. Pharm Biol.
53:1481–1487. 2015. View Article : Google Scholar : PubMed/NCBI
|