Introduction
Diabetes mellitus (DM) is a widespread metabolic
disease, which is accompanied by a high incidence based on
statistics on individuals with diabetes in 200 countries and
regions between 1980 and 2014 (1). Improper treatment will cause the
body to be in a high-sugar state for a long period and can induce
various complications (2).
Intestinal disease is a common complication, manifested as
diarrhea, constipation and fecal incontinence, which seriously
affects the quality of life of patients (3,4).
Constipation is the most common symptom of diabetic bowel syndrome,
manifested as colonic dyskinesia and slow transportation, leading
to hard stools and defecation disorders (5,6).
DM can affect most parts of the gastrointestinal tract; however, to
the best of our knowledge, the specific pathogenic mechanisms are
yet not fully elucidated (7).
Previous studies have reported that intestinal dyskinesia is
closely associated with intestinal autonomic neuropathy, including
enteric nervous system (ENS) disorders (8), neuron damage and reduction
(9-11) and a loss of interstitial cells of
Cajal (12,13).
The ENS is an endogenous nervous system, including
gastrointestinal motility and sensory systems, which consists of
the submucosal and intermuscular nerve plexuses (14). The ENS is distributed in the
gastrointestinal tract in a network, and it receives and integrates
autonomic nerve signals, releases neurotransmitters, maintains
gastrointestinal movement and regulates intestinal epithelial cell
secretion (15). ENS disease can
cause colonic dysfunction and DM can cause pathological changes in
the ENS (8,16). Therefore, patients with DM often
have certain symptoms, such as constipation, diarrhea or
alternation of constipation and diarrhea (17). Enteric neural stem cells in the
ENS possess regeneration and repair functions (18,19). Except for the embryonic period,
this regenerative function of the adult intestine occurs only when
the ENS is damaged (20-22). Previous studies have revealed
that enteric neural stem cells are also present in adult intestinal
tissues (23-25), and these cells may be the source
of ENS regeneration (26).
As the most common diabetic bowel syndrome,
constipation is mainly treated with drugs; however, diet and
exercise can also exert an auxiliary treatment effect (27). Osmotic and irritant laxatives are
the two most commonly prescribed drugs (28,29). Although these can increase the
frequency of bowel movements, the treatment effect remains
unsatisfactory (30).
Furthermore, 20-40% of individuals will experience adverse side
effects, such as bloating (30).
Initially, 5-hydroxytryptamine 4 (5-HT4) receptor agonists
cisapride and tegaserod were used for the treatment of functional
bowel disease; however, these were withdrawn from the market due to
their damaging effects on cardiovascular function (31-33). 5-HT is a neurotransmitter
expressed in the central and peripheral nervous systems, and it can
regulate gastrointestinal motility and sensory functions (34). As the fourth subtype of 5-HT,
5-HT4 can regulate intestinal movement and reduce visceral
sensitivity (30). Furthermore,
5-HT4 receptors serve an important role in the growth and function
maintenance of enteric nerves (35,36).
As a selective and high-affinity 5-HT4 receptor
agonist, prucalopride can activate the 5-HT4 receptor, enhance
choline secretion, promote intestinal contraction and promote
gastrointestinal motility (37-39). Prucalopride has been used to
treat diabetic constipation and functional gastrointestinal
diseases (37,38,40,41), and it is mainly used for the
treatment of chronic constipation in women (37,38). Prucalopride was approved by the
European Medicines Agency for clinical use in 2009 and was marketed
in Germany and the UK in 2010 (42). Procabulide has few side effects
in the treatment of chronic constipation, and exerts almost no harm
to the cardiovascular system, and thus, it can also be used by
elderly individuals with cardiovascular diseases (43-46). At present, most of the research
examining this drug is focused on its clinical efficacy, and there
are few investigations on the mechanism underlying the effects of
prucalopride on improving constipation caused by DM (43-46). Understanding the mechanism via
which prucalopride improves colon changes caused by DM is of great
significance for optimizing treatment plans and identifying novel
treatment methods.
Therefore, the present study established a rat model
of DM and the rats were treated with prucalopride. The present
study aimed to investigate the alterations of enteric neural stem
cells and neurons by examining the expression levels of the markers
of neural stem cells, neurons and glial cells, as well as measuring
5-HT4 expression, in order to determine whether prucalopride, as a
5-HT4 receptor agonist, improved intestinal motility by promoting
ENS regeneration in diabetic rats.
Materials and methods
Materials
A cyanine 3 (Cy3)-conjugated goat anti-rabbit IgG
antibody (cat. no. BA1032) was obtained from Wuhan Boster
Biological Technology, Ltd. Cy3-conjugated goat anti-mouse IgG
antibody (1:200; cat. no. A10521) and FITC-conjugated goat
anti-mouse IgG antibody (1:200; cat. no. 62-6511) were purchased
from Thermo Fisher Scientific, Inc. The FITC-conjugated goat
anti-rabbit IgG antibody (1:100; cat. no. CW0114S) and
diaminobenzidine (DAB) kit (cat. no. CW0125) were purchased from
CoWin Biosciences. HRP-conjugated goat anti-rabbit IgG(H+L)
antibody (1:250; cat. no. ZB-2301) was obtained from OriGene
Technologies, Inc. Mouse anti-Ki-67 monoclonal antibody (1:250;
cat. no. bsm-33070M) and rabbit anti-SOX10 polyclonal antibody
(1:200; cat. no. bs-20563R) were purchased from BIOSS. Prucalopride
was purchased from Janssen-Cilag SpA. Rabbit anti-5-HT4 polyclonal
antibody (1:100; cat. no. DF3503) and rabbit anti-ubiquitin
thiolesterase (PGP9.5) polyclonal antibody (1:200; cat. no. AF0243)
were obtained from Affinity Biosciences. Rabbit anti-RNA-binding
protein human antigen D (HuD)+ human antigen C monoclonal antibody
(1:500; cat. no. ab184267) and rabbit anti-glial fibrillary acidic
protein (GFAP) monoclonal antibody (1:250; cat. no. ab33922) were
purchased from Abcam. Rabbit anti-Nestin polyclonal antibody
(1:200; cat. no. OM264981) was obtained from Omnimabs. A rat 5-HT
ELISA kit (cat. no. MM-0442R2) was obtained from Jiangsu Enzyme
Industry Co., Ltd. Glass beads with a 3-mm diameter were purchased
from Ziboshi Boshan Gaoqiangdu Weizhuchang.
Animals
A total of 24 male Sprague Dawley rats (6-8 weeks
old; weight, 250±20 g) were provided by Hunan SJA Laboratory Animal
Co., Ltd. All rats were housed in a room at 20-25°C with 55-70%
relative humidity and natural light. The rats had free access to
food and water. The study protocol was approved by the Ethics
Committee of The First Clinical Medical College of Nanjing Medical
University (Nanjing, China).
Establishment of the diabetic rat
model
Sprague Dawley rats were randomly divided into four
groups of 6 rats each: Control group (Control), the DM model group
(DM), the 5 µg/kg prucalopride group (DM + A) and the 10
µg/kg prucalopride group (DM + B). After fasting for 12 h,
the rats were given a single intraperitoneal injection of 1%
streptozotocin in citrate buffer (cat. no. MB1227; Dalian Meilun
Biotechnology Co., Ltd.) at a dose of 60 mg/kg. The rats in the
Control group were given an equal volume of citric acid solvent via
intraperitoneal injection. After 1 week, blood was collected
through the tail vein and the blood glucose concentration was
immediately measured using a blood glucose meter. The criterion for
successful establishment of the DM model was a blood glucose level
≥16.7 mmol/l (47). After
successful model establishment, high blood glucose levels were
maintained for 2 weeks before administration of prucalopride.
Prucalopride was dissolved in normal saline to a final
concentration of 0.5 µg/ml and then administered via gavage
daily for another 2 weeks. Finally, the rats were weighed.
Colonic transit time measurement
The colonic transit time was measured using the
glass bead discharge method (48). After the rats were anesthetized
by isoflurane inhalation at induction and maintenance doses of 5
and 2%, respectively, in an induction box, glass beads with 3-mm
diameter were placed at a depth of 2 cm from the anus, so that the
glass beads stayed in the colon. The rats implanted with the glass
beads had free access to food and water. The time required for the
rats to discharge the glass beads was recorded as the colonic
transit time.
The rats were euthanized by intraperitoneal
injection with 150 mg/kg pentobarbital sodium. The death of rats
was determined by observing whether the rats were breathing and
whether the pupils were dilated. If the breathing stopped and the
pupils were dilated, it could be judged that the rats had died. The
colons were collected and stored at −80°C for subsequent
experiments. Both frozen and paraffin-embedded samples were
prepared.
Immunohistochemical analysis
The colons were fixed in 4% paraformaldehyde for 1 h
at 4°C. Then paraffin-embedded sections of colons were prepared,
deparaffinated in xylene twice for 10 min each, hydrated in 100,
100, 95 and 80% ethanol and water for 5 min each, incubated in
citrate buffer and heated at 115°C for 2 min in a pressure cooker.
After cooling, they were washed with phosphate buffered saline,
incubated in fresh 3% hydrogen peroxide at room temperature for 10
min and rinsed. Subsequently, the sections (4-µm-thick) were
incubated in 5% BSA (cat. no. SW3015; Beijing Solarbio Science
& Technology Co., Ltd.) at 37°C for 30 min and in primary
antibody buffer at 4°C overnight. Following maintenance at room
temperature for 45 min, the sections were washed and incubated in
secondary antibody buffer at 37°C for 30 min. Sections were rinsed,
stained in DAB at room temperature for 5-10 min, incubated in
hematoxylin at room temperature for 3 min, differentiated in
hydrochloric alcohol and stained blue in ammonia water at room
temperature for 5-10 min. Subsequently, sections were washed with
water for 1 min, dehydrated, transparentized, mounted and examined
under a fluorescence microscope. Immunohistochemical images were
analyzed semi-quantitatively using Image-Pro Plus software (version
6.0; Media Cybernetics, Inc.). For each sample, three visual fields
were analyzed and a magnification of ×200 was used. The ratios of
the integrated optical density of the selected area to the color
area are presented as the semi-quantitative results.
Immunofluorescence double staining
Frozen colonic sections (-20°C; 5-µm-thick)
were washed, immersed in 0.5% Triton X-100 at room temperature for
20 min and incubated in 5% BSA at 37°C for 30 min. Sections were
then incubated in primary antibody buffer at 4°C overnight, washed
and incubated in secondary antibody buffer at 37°C for 30 min.
After washing, sections were incubated with DAPI in the dark at
room temperature for 5 min, washed, mounted and examined under a
fluorescence microscope (CKX53; Olympus Corporation).
Immunofluorescence images were analyzed semi-quantitatively using
Image-Pro Plus software (version 6.0; Media Cybernetics, Inc.). For
each sample, three visual fields were analyzed and a magnification
of ×200 was used. The ratios of the integrated optical density of
the selected area to the color area are presented as the
semi-quantitative results.
ELISA
The colons were ground and rewarmed. The levels of
5-HT were detected using a 5-HT ELISA kit according to the
manufacturer's protocol. Absorbance was measured at 450 nm.
Statistical analysis
Every experiment was repeated three times. Data are
presented as the mean ± SD. Statistical analysis was performed
using one-way ANOVA followed by Tukey post hoc test using SPSS
software (v19.0; IBM Corp.). P<0.05 was considered to indicate a
statistically significant difference.
Results
Successful establishment of the DM
model
The blood glucose levels of the rats at 1 and 3
weeks after model establishment are presented in Table I. The blood glucose levels of the
control rats were <16.7 mmol/l and the blood glucose levels of
all diabetic rats were >16.7 mmol/l, suggesting the successful
establishment of the DM model.
 | Table IBlood glucose levels of the rats at 1
and 3 weeks after model establishment. |
Table I
Blood glucose levels of the rats at 1
and 3 weeks after model establishment.
| Groups | Rat no. | Blood sugar level
at 1 week, mmol/l | Blood sugar level
at 3 weeks, mmol/la |
|---|
| Control | 1 | 7.2 | 5.4 |
| 2 | 7.9 | 6.8 |
| 3 | 5.8 | 6.0 |
| 4 | 6.0 | 7.9 |
| 5 | 6.7 | 8.2 |
| 6 | 6.4 | 5.8 |
| DM | 7 | 25.3 | 33.2 |
| 8 | 22.7 | 33.2 |
| 9 | 21.6 | 33.1 |
| 10 | 22.6 | HI |
| 11 | 23.6 | 32.3 |
| 12 | 20.8 | HI |
| DM + A | 13 | 20.0 | HI |
| 14 | 25.0 | HI |
| 15 | 21.0 | HI |
| 16 | 27.1 | HI |
| 17 | 26.0 | 27.5 |
| 18 | 25.6 | 29.4 |
| DM + B | 19 | 20.7 | HI |
| 20 | 28.7 | HI |
| 21 | 23.7 | HI |
| 22 | 29.6 | HI |
| 23 | 24.9 | HI |
| 24 | 26.9 | 31.1 |
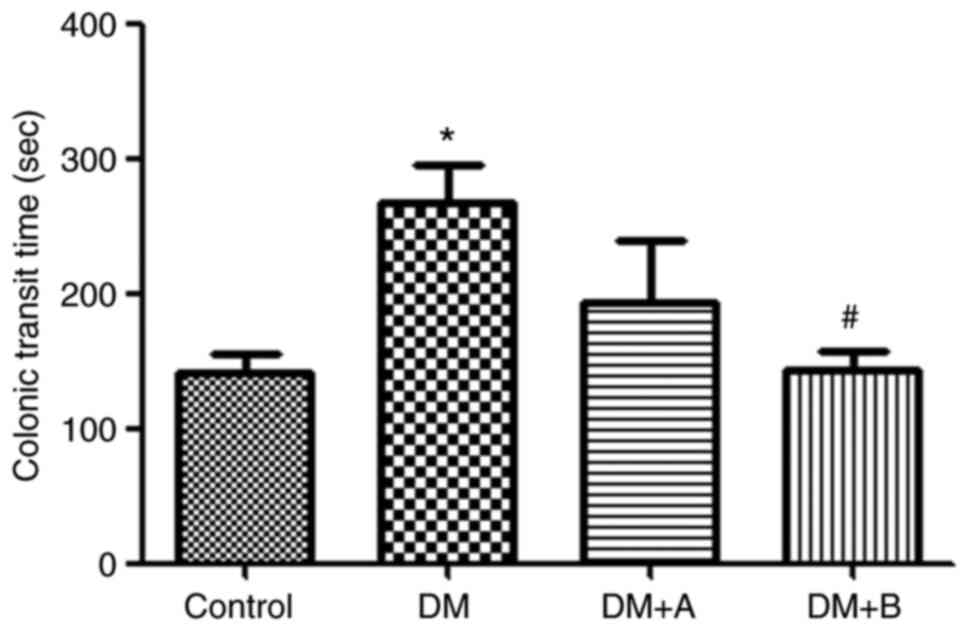
Prucalopride shortens the colonic transit
time
The colonic transit time of rats in various groups
is shown in Fig. 1. Compared
with that of rats in the Control group, the colonic transit time of
rats in the DM group was significantly prolonged (P<0.05).
Furthermore, compared with the DM group, rats treated with 5
µg/kg prucalopride (DM + A group) had a shorter colonic
transit time, although the difference was not significant. However,
after treatment with 10 µg/kg prucalopride (DM + B group),
rats had a significantly shorter colonic transit time compared with
that of rats in the DM group (P<0.05). Furthermore, the colonic
transit time of rats in the DM + B group was close to that observed
in the Control group, indicating that prucalopride treatment could
accelerate colonic movement and shorten the colonic transit
time.
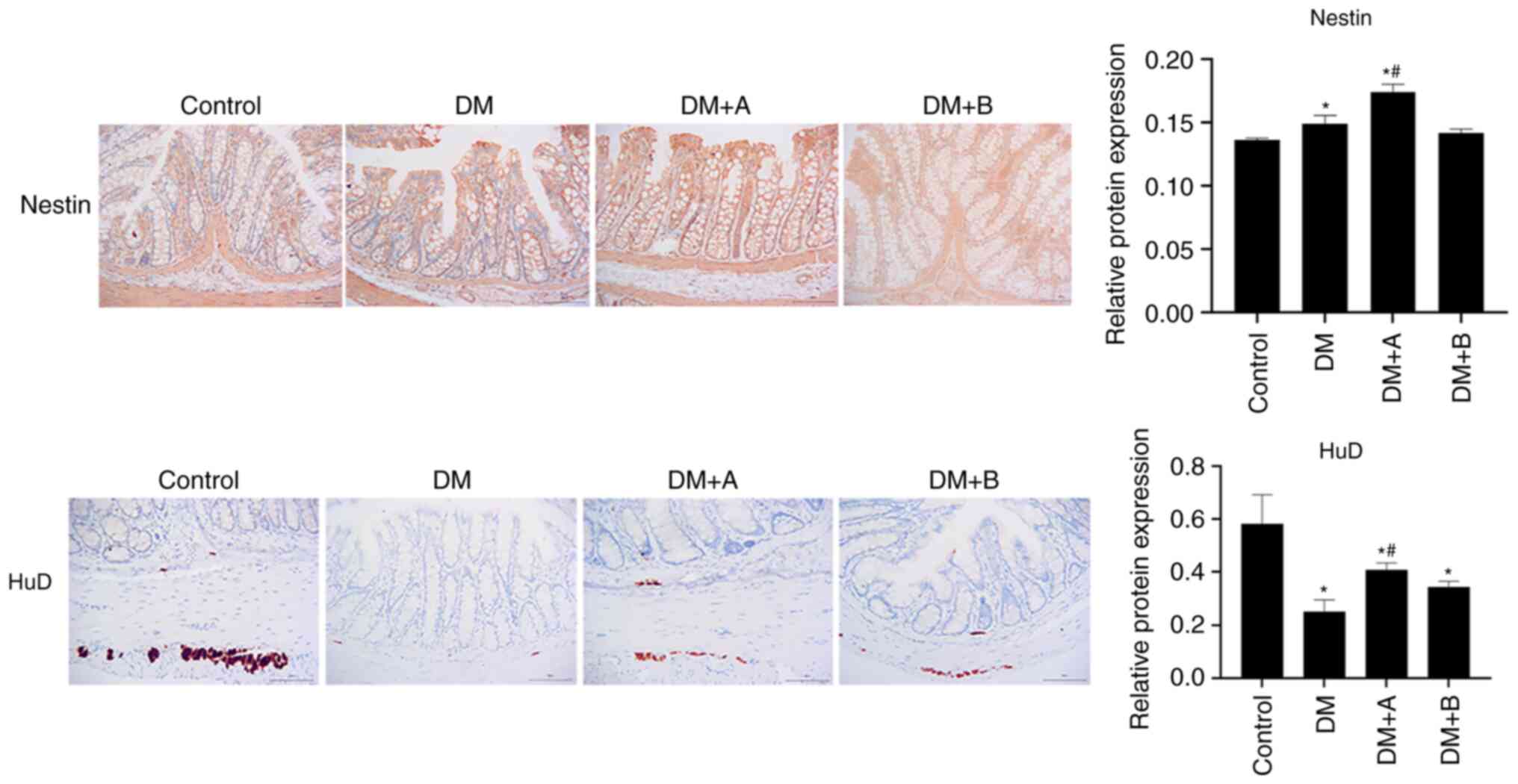
Prucalopride promotes the regeneration of
colonic neural stem cells and neurons
Nestin is a marker of enteric neural stem cells in
the ENS (24), whereas HuD is a
marker of neurons (49). The
expression levels of Nestin and HuD in various groups as determined
by immunohistochemical analysis are shown in Fig. 2. Nuclei and Nestin/HuD were
labeled as blue and brown, respectively. Nestin was mainly
expressed in the muscularis mucosae, circular muscles and columnar
epithelial cells. Compared with that in the Control group, Nestin
expression was significantly increased in the DM and DM + A groups,
and it was concentrated in columnar epithelial cells and the
mesenchyme. The expression levels of Nestin in the DM + A group
were higher than those in the DM group. There was no significant
difference in the expression levels of Nestin between the DM + B
group and the Control group. In the Control group, HuD was mainly
expressed in the junction of the longitudinal and circular muscles
of the colon, which is an area where neurons are enriched (50). Compared with the Control group,
HuD expression was decreased in the longitudinal muscle and very
little was observed in the submucosa in the DM group. In the DM + A
group, HuD appeared in the longitudinal muscle and submucosa.
Furthermore, its expression was decreased compared with that in the
Control group but was markedly higher compared with that in the DM
group. In the DM + B group, HuD expression was observed in the
longitudinal muscle and a small amount was expressed in the
submucosa. These results suggested that the ENS function of
diabetic rats was impaired and that 5 µg/kg prucalopride may
promote the regeneration of colonic neural stem cells and
neurons.
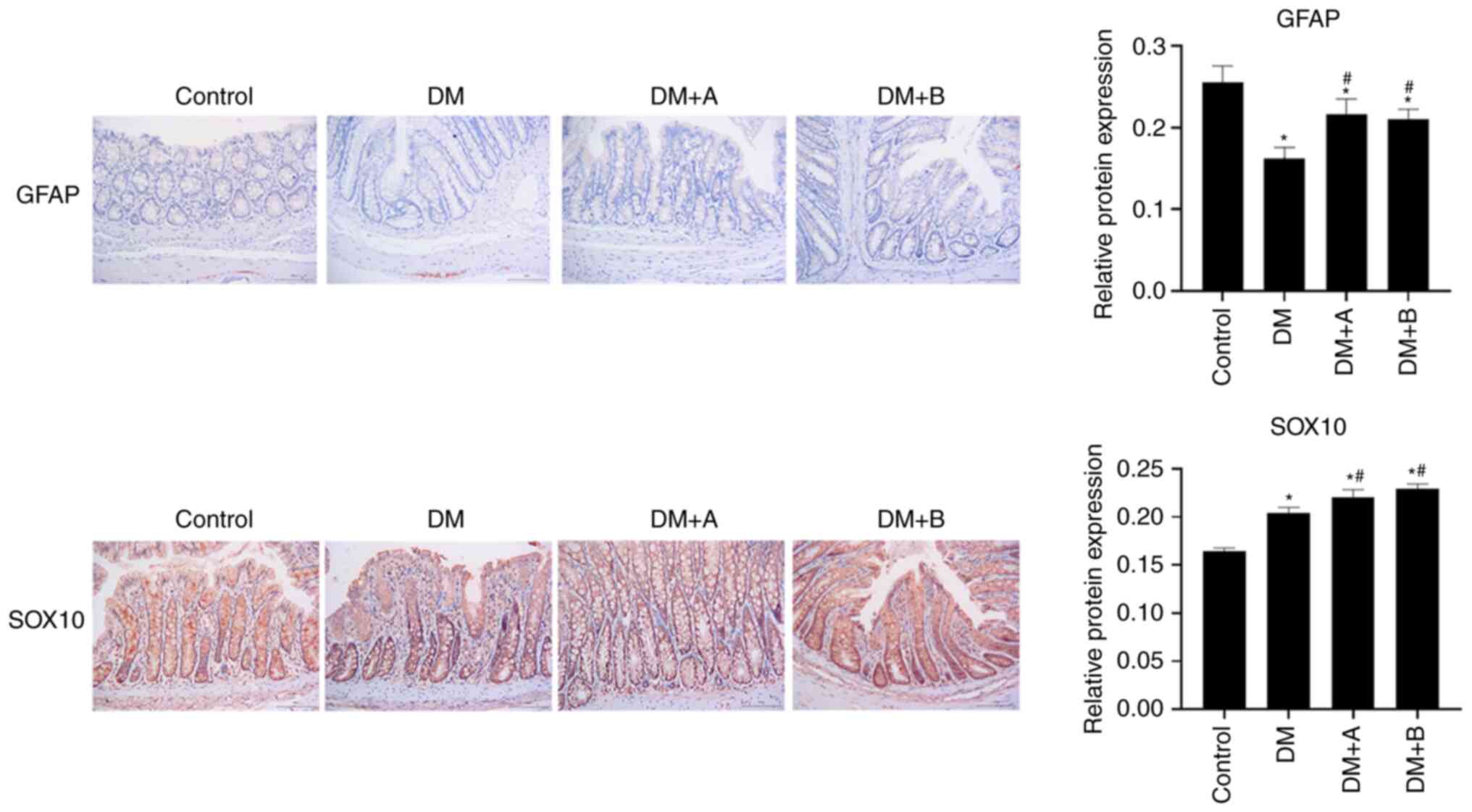
Prucalopride promotes the differentiation
of colonic neural stem cells
GFAP and SOX10 are markers of glial cells, which are
differentiated from neural stem cells (51,52). The expression levels of GFAP and
SOX10 in various groups as determined by immunohistochemical
analysis are shown in Fig. 3.
Nuclei and GFAP/SOX10 were labeled as blue and brown, respectively.
GFAP was lowly expressed in each group, and mainly found in the
longitudinal muscle and columnar epithelial cells. Compared with
the Control group, the DM, DM + A and DM + B groups exhibited
decreased expression levels of GFAP. However, GFAP expression in
the DM + A and DM + B groups was higher compared with that in the
DM group.
In the Control group, SOX10 was abundantly expressed
in the columnar epithelial cells, the nuclei of lamina propria
cells and enteraden, and it was slightly expressed in the nucleus
of the submucosa of the circular muscle. In the DM group, SOX10 was
highly expressed in the columnar epithelial cells and its
expression was significantly increased compared with that in the
Control group. In the DM + A group, SOX10 expression was
significantly higher compared with that in the DM group, and it was
mainly expressed in the columnar epithelial nuclei and enteraden.
In the DM + B group, SOX10 was expressed in small amounts in the
circular muscle; however, it was highly expressed in the columnar
epithelial cells, the nuclei of lamina propria cells and enteraden.
Furthermore, SOX10 expression in the DM + B group was higher
compared with that in the DM group. The results of SOX10 and GFAP
expression analysis indicated that prucalopride could promote the
differentiation of colonic neural stem cells, activate the
expression of glial proteins and promote the recovery of neuronal
injury to a certain extent.
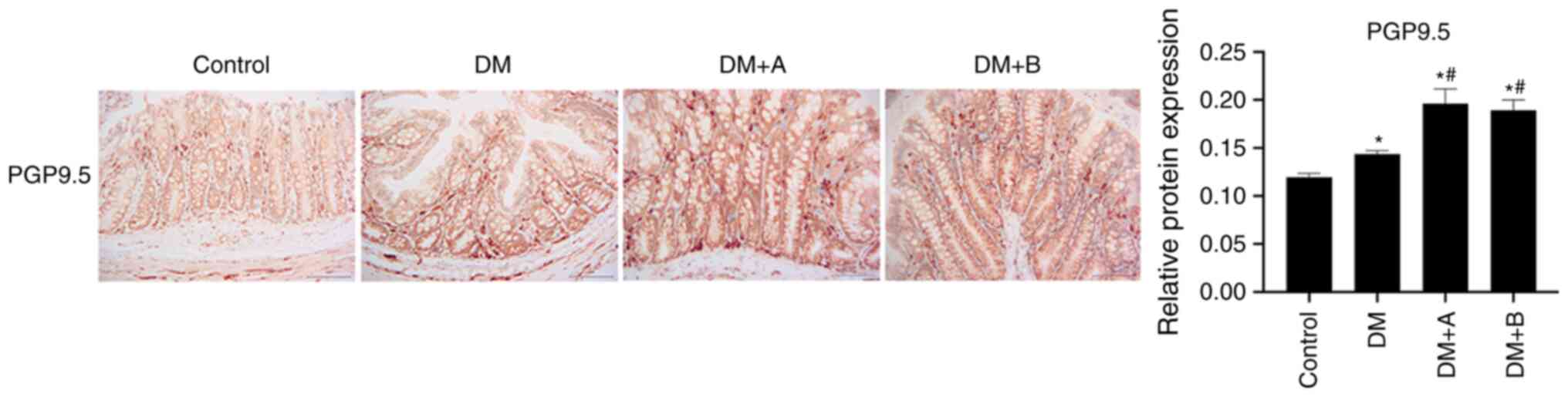
Prucalopride promotes the differentiation
of the colonic nerve node
PGP9.5 is mainly expressed in differentiated nerve
nodes, and the faster the differentiation, the higher the
expression levels of PGP9.5 (53,54). Therefore, PGP9.5 is a marker of
colonic nerve node differentiation (55). The expression levels of PGP9.5 in
various groups as determined using immunohistochemical analysis are
presented in Fig. 4. Nuclei and
PGP9.5 were labeled as blue and brown, respectively. PGP9.5 was
generally expressed in columnar epithelial cells, lamina propria,
enteraden and circular muscles, and a small amount was expressed in
the submucosa. The expression levels of PGP9.5 in the DM group were
markedly higher compared with those in the Control group. In both
the DM + A group and the DM + B group, the expression levels of
PGP9.5 were significantly increased compared with those in the
Control and DM groups. The results of PGP9.5 expression analysis
demonstrated that prucalopride was beneficial for the
differentiation of the colonic nerve node.
Prucalopride increases the secretion of
5-HT4 in the colon
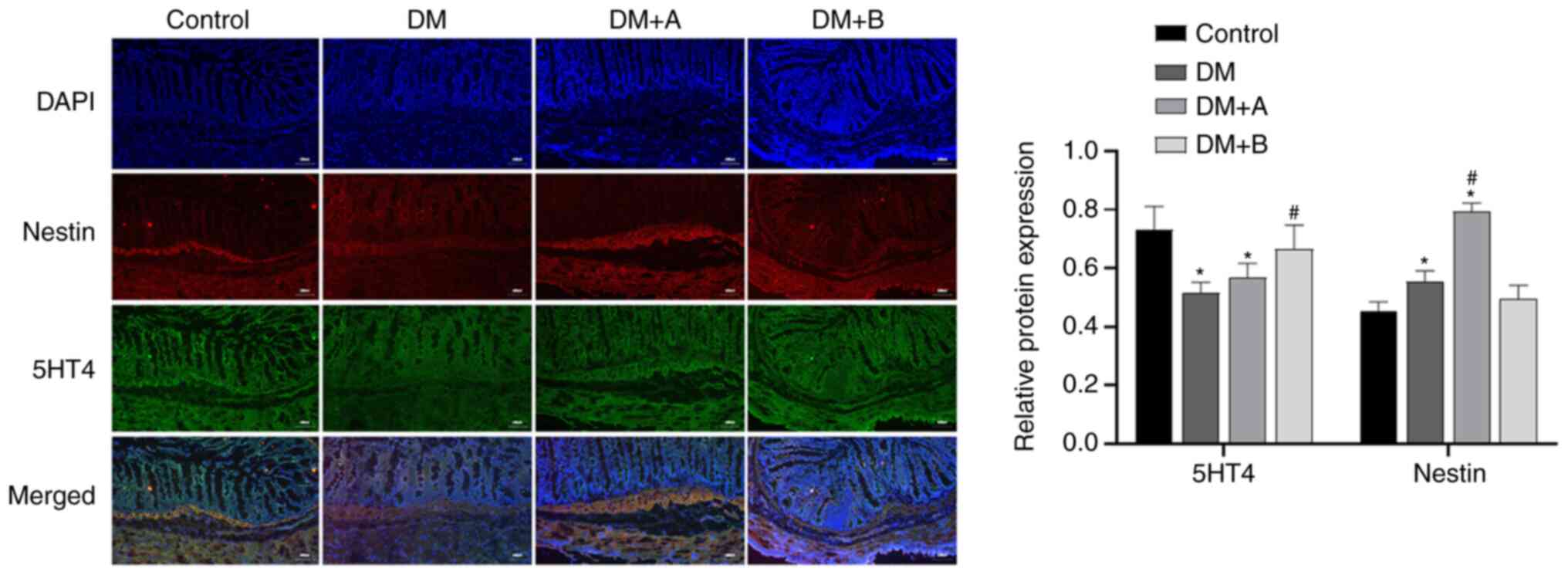
Immunofluorescence double staining images of Nestin
and 5-HT4 in the Control, DM, DM + A and DM + B groups are shown in
Fig. 5. DAPI-stained nuclei
appeared blue, while Nestin and 5-HT4 are indicated by red
fluorescence and green fluorescence, respectively. Nestin was
mainly distributed in the cytoplasm of muscularis mucosae and
circular muscles, while 5-HT4 was expressed in interstitium,
muscularis mucosae, mucosal layers and circular muscles. The main
areas in which Nestin and 5-HT4 were co-expressed included the
muscularis mucosae and circular muscles. Small amounts of these
proteins were expressed in the intercellular substance. Compared
with those in the Control group, the expression levels of 5-HT4 in
the DM and DM + A groups were significantly decreased, and the
expression levels of Nestin in the DM and DM + A groups were
significantly increased. Compared with those in the DM group, the
expression levels of Nestin in the DM + A group were significantly
increased, and the expression levels of 5-HT4 in the DM + B group
were significantly increased. Similar trends in the expression of
5-HT4 and Nestin were not observed. These results suggested that
prucalopride promoted the secretion of 5-HT4 in the colon.
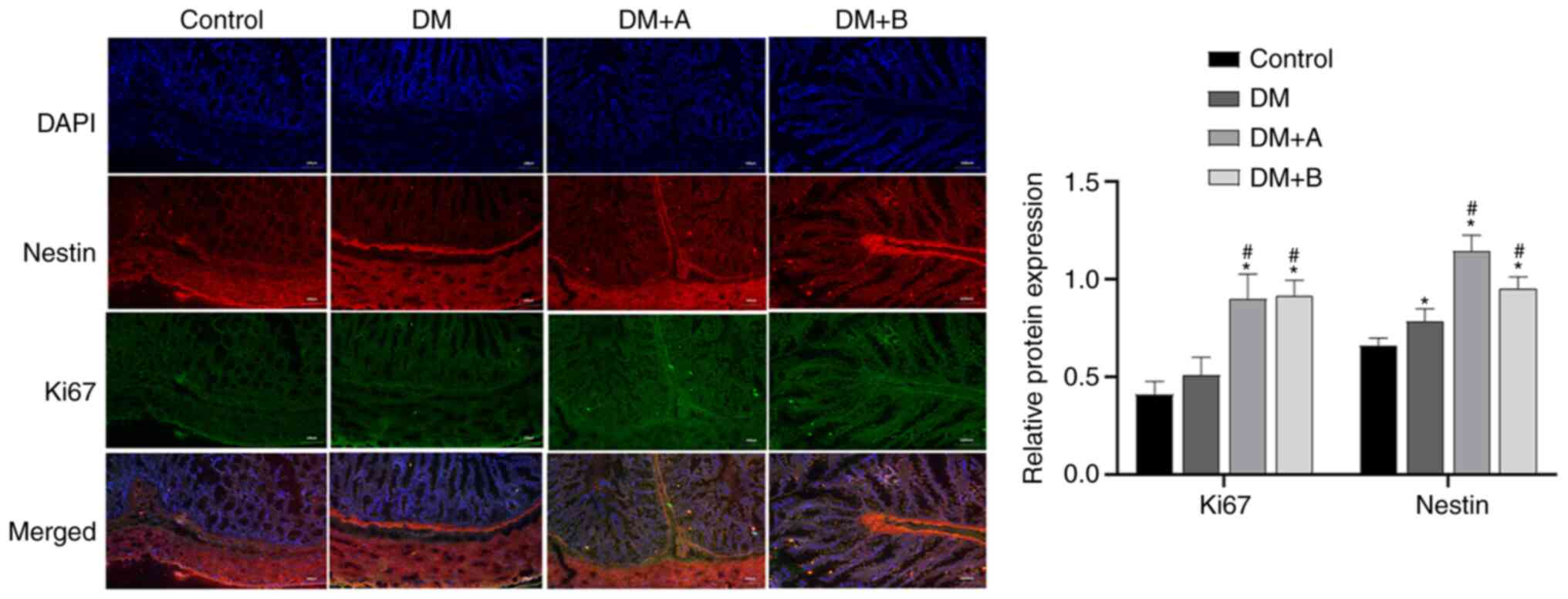
Prucalopride enhances the regeneration of
colonic neural stem cells
Ki67 is a marker of the cell proliferation rate, and
the faster the cell proliferation rate, the higher its expression
levels (56). Immunofluorescence
double staining images of Nestin and Ki67 in the Control, DM, DM +
A and DM + B groups are shown in Fig. 6. DAPI-stained nuclei appeared
blue. Nestin and Ki67 are indicated by red fluorescence and green
fluorescence, respectively. In the Control group, Nestin was mainly
distributed in the cytoplasm of muscularis mucosae and circular
muscles, and a small amount was expressed in the intercellular
substance. Ki67 was mainly expressed in the intercellular
substance, muscularis mucosae and circular muscles. The main areas
in which Nestin and Ki67 were co-expressed included the muscularis
mucosae and circular muscles. Compared with those in the Control
group, the expression levels of Nestin in the DM group were
significantly increased. The expression levels of Ki67 and Nestin
in the DM + A and DM + B groups were markedly higher compared with
those in the DM group. Furthermore, similar trends in the
expression of Ki67 and Nestin were observed. These results
indicated that prucalopride could promote the regeneration of
colonic neural stem cells.
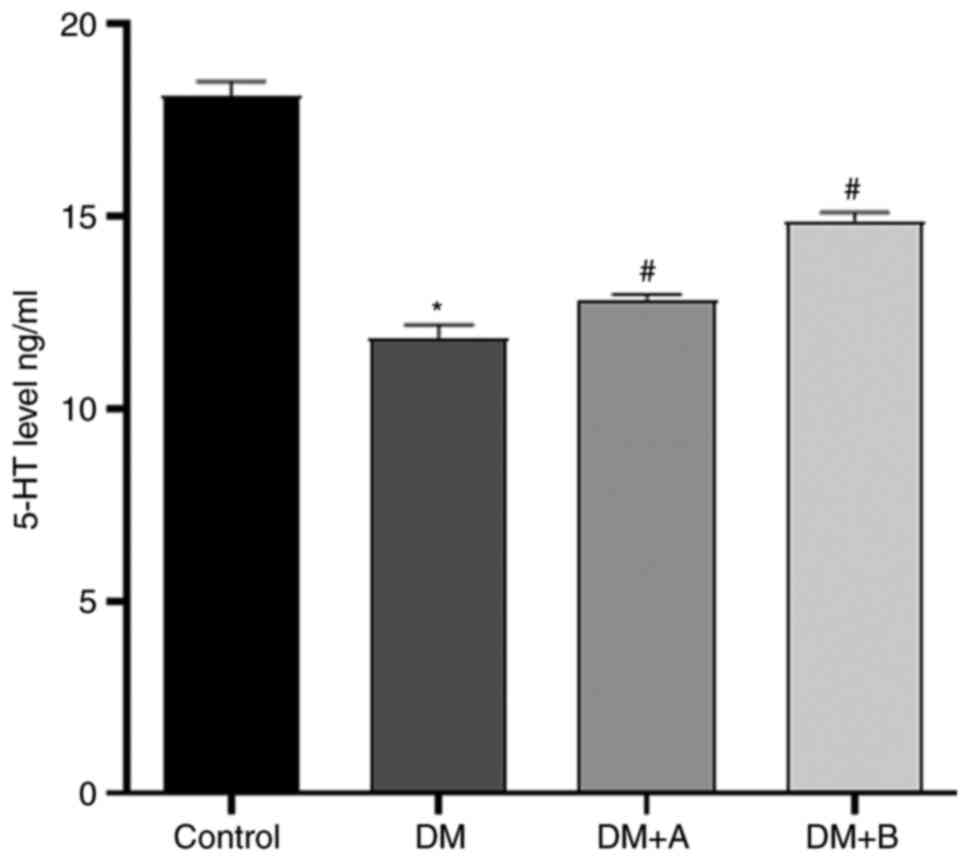
Prucalopride promotes 5-HT secretion in
the colon
The levels of 5-HT in the colon, which were measured
via ELISA, are presented in Fig.
7. Compared with those in the Control group, the levels of 5-HT
in the DM group were significantly reduced. Furthermore, the DM + A
group and the DM + B group exhibited significantly higher levels of
5-HT compared with the DM group, indicating that both 5 and 10
µg/kg prucalopride promoted 5-HT secretion in the colon.
Discussion
In animal experiments, the numbers of enteric neural
stem cells and intestinal neurons expressing Nestin are markedly
increased in normal adult mice after treatment with the 5-HT4
receptor agonists mosapride and tegaserod (39). However, the use of 5-HT4 receptor
agonists in 5-HT4 receptor-knockout mice does not increase the
number of colon neurons (39).
Furthermore, an in vitro study has demonstrated that 5-HT4
receptor agonists can promote neuron survival and reduce apoptosis
(39). Therefore, as a 5-HT4
receptor agonist, prucalopride may have an effect on enteric neural
stem cells.
Nestin is a protein specific to the central nervous
system, which is specifically expressed in undifferentiated neural
pluripotent stem cells (57,58). Neural stem cells expressing
Nestin in the ENS can differentiate into enteric neurons or glial
cells (35). When neural stem
cells differentiate into neurons and glial cells, Nestin is no
longer expressed (57).
Therefore, Nestin is considered to be a marker of enteric neural
stem cells in the ENS (24). In
the present study, the results of Nestin immunohistochemical
analysis identified that colonic stem cells of diabetic rats
possessed a regenerative function and exhibited high protein
expression levels of Nestin. Furthermore, compared with those of
the Control rats and DM rats, the expression levels of Nestin in
the DM + A group were significantly increased.
HuD can promote the mitosis of neural stem cells,
and it is an important protein involved in the differentiation and
maturation of neurons (59). HuD
is abundantly expressed in the healthy ENS and its expression is
decreased in diseased tissues with loss of intestinal function
(49). Furthermore, the loss or
reduction of HuD protein may impair embryonic development of the
ENS, which causes the absence or decrease of enteric ganglion cells
(60). The present study
demonstrated that, compared with the control group, HuD expression
was significantly decreased in the DM, DM + A and DM + B groups,
indicating that colon neurons were damaged in rats with DM.
Compared with those in the DM group, the expression levels of HuD
in the DM + A group were increased. These results suggested that
prucalopride could promote the regeneration of colonic neural stem
cells and neurons.
Neural stem cells will differentiate into neurons
and glial cells. PGP9.5 is a neuron-specific protein and it is
mainly expressed in endocrine cells of the central and peripheral
nervous systems (61). PGP9.5
regulates the cell cycle, differentiation, proliferation and
apoptosis via a non-lysosomal degradation pathway (62). Furthermore, PGP9.5 is expressed
during the differentiation of neural nodules, and it is a cancer
biomarker due to its high expression levels in various cancer
types, such as esophagus cancer and colorectal cancer (53,54). In the present study, compared
with those in the Control group, the expression levels of PGP9.5 in
the DM, DM + A and DM + B groups were significantly increased, and
its expression levels in the DM + A and DM + B groups were
significantly higher compared with those in the DM group. These
results suggested that prucalopride could promote the
differentiation of the colonic nerve node.
GFAP is expressed in astrocytes of the central
nervous system and in enteroglial cells (63). SOX10 is a transcription factor
expressed in the neural crest and peripheral nervous system, and it
is highly expressed in multiple healthy tissues, such as the
esophagus, stomach and colorectum (51,52). SOX10 serves an important role in
promoting the survival and proliferation of enteric neural crest
cells, as well as glial cell formation (62). In the present study, compared
with those in the Control group, the expression levels of GFAP in
the DM, DM + A and DM + B groups were significantly decreased, and
its expression levels in the DM + A and DM + B groups were
significantly higher compared with those in the DM group. These
results demonstrated that DM caused damage to intestinal glial
cells and decreased the numbers of glial cells, and that
prucalopride could promote the regeneration of glial cells. As a
marker of glial cells, SOX10 is abundantly expressed in healthy
tissues, but is negatively expressed in cancerous tissues (64). Compared with those in the DM
group, the expression levels of SOX10 in the DM + A and DM + B
groups were significantly increased, indicating that after colonic
glial cell injury, prucalopride could promote glial cell
regeneration.
The aforementioned results regarding the expression
levels of Nestin, HuD, PGP9.5, GFAP and SOX10 suggested that colon
cells of diabetic rats had the ability to regenerate after damage.
Furthermore, these results indicated that prucalopride may promote
the proliferation of neural stem cells and enhance the
differentiation of neural stem cells into neurons and glial cells.
Regeneration of neurons expressing PGP9.5 and HuD, and glial cells
expressing GFAP and SOX10, could repair the damaged ENS and restore
gastrointestinal motility.
Since prucalopride, as an agonist of the 5-HT4
receptor, could promote the regeneration of colonic nerve cells, it
was suggested that 5-HT4 expression may also promote the
regeneration and recovery of colonic nerve cells. In the present
study, the ELISA results indicated that the levels of 5-HT protein
were highest in the Control group, although the levels of 5-HT
protein in the DM, DM + A and DM + B groups showed an increasing
trend, indicating that prucalopride could promote the secretion of
5-HT protein in the colon. The results of immunofluorescence
double-labeling of 5-HT4 were consistent with the ELISA results,
suggesting that prucalopride may upregulate the protein expression
levels of 5-HT4. However, similar trends in the expression of 5-HT4
and Nestin were not observed.
Ki67 is a marker of cell proliferation rate, and the
faster the cell proliferation rate, the higher its expression
levels (65). The results of
immunofluorescence double-labeling of Ki67 demonstrated that the
Control group had the lowest expression levels of Ki67, and its
expression levels in the DM + A group and DM + B group were
significantly higher compared with those in the DM group.
Furthermore, similar trends in the expression of Nestin and Ki67
were observed. These findings indicated that DM caused colon damage
and initiated the regeneration of colon cells, and that
prucalopride could increase the rate of cell proliferation and
accelerate the proliferation of colon stem cells. The
aforementioned results also suggested that prucalopride could
promote the secretion of 5-HT4 in the colon, enhance 5-HT4
expression and promote the regeneration of colon stem cells.
It was subsequently examined whether prucalopride
exerted an effect on intestinal motility in diabetic rats, and
whether it promoted bowel movement. The results of colonic transit
time analysis demonstrated that the colonic transit time of
diabetic rats (DM group) was significantly longer compared with
that of the Control group. In the DM+ A group, the time for
removing the glass beads appeared to be shorter compared with that
in the DM group. Furthermore, the colonic transit time in the DM +
B group was significantly shorter compared with that in the DM
group, and it was similar to that observed in the Control group.
The treatment of the DM + B group appeared to have the best
efficacy to improve intestinal motility. Although the difference
was not significant, the treatment of DM + B group appeared to be
superior compared with that of the DM + A group in terms of
improving intestinal motility. These results suggested that
prucalopride improved intestinal motility and reduced bowel
movement time.
The results of immunohistochemical analysis
identified that the promoting effect of 5 µg/kg prucalopride
(DM + A) on colon cell regeneration was more favorable compared
with that of 10 µg/kg Prucalopride (DM + B). However, the
results of 5-HT4 expression, Ki67 expression and colonic transit
time analyses demonstrated that 10 µg/kg prucalopride had
more favorable effects on promoting 5-HT4 expression and enhancing
the intestinal motility. It was suggested that, compared with the
normal dose of prucalopride, an increase in its dose may inhibit
the rate of cell proliferation and slow cell repair. However, the
specific regulatory mechanism remains to be studied in the
future.
In conclusion, DM could cause ENS damage, which was
manifested by a decrease in neurons and glial cells, leading to
bowel movement disorders and prolonged bowel movement time. As a
5-HT4 receptor agonist, prucalopride could promote the secretion of
5-HT4 in colon cells, enhance 5-HT4 expression and improve bowel
movement function. Prucalopride could also repair colon damage by
promoting the proliferation of enteric neural stem cells, as well
as the differentiation of enteric neurons and glial cells.
Furthermore, prucalopride may improve intestinal motility by
promoting ENS regeneration in diabetic rats. These results suggest
a novel role of prucalopride in the management of diabetic
neuropathy, and this should be further validated in clinical
trials.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YW and LL designed the study, analyzed the data,
wrote the paper and confirmed the authenticity of all the raw data.
YW, XX and LL performed the experiments and collected data. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the Ethics
Committee of The First Clinical Medical College of Nanjing Medical
University (Nanjing, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare they have no competing
interests.
Acknowledgments
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant no. 81600430).
References
|
1
|
NCD Risk Factor Collaboration (NCD -RisC):
Worldwide trends in diabetes since 1980: A pooled analysis of 751
population-based studies with 4.4 million participants. Lancet.
387:1513–1530. 2016. View Article : Google Scholar
|
|
2
|
Yoo H, Choo E and Lee S: Study of
hospitalization and mortality in Korean diabetic patients using the
diabetes complications severity index. BMC Endocr Disord.
20:1222020. View Article : Google Scholar :
|
|
3
|
Horowitz M and Samsom M: Gastrointestinal
function in diabetes mellitus. Chichester: John Wiley & Sons,
Ltd; pp. 1–337. 2004
|
|
4
|
Kalra S, Zargar AH, Jain SM, Sethi B,
Chowdhury S, Singh AK, Thomas N, Unnikrishnan AG, Thakkar PB and
Malve H: Diabetes insipidus: The other diabetes. Indian J
Endocrinol Metab. 20:9–21. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Krishnan B, Babu S, Walker J, Walker AB
and Pappachan JM: Gastrointestinal complications of diabetes
mellitus. World J Diabetes. 4:51–63. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Azpiroz F and Malagelada C: Diabetic
neuropathy in the gut: Pathogenesis and diagnosis. Diabetologia.
59:404–408. 2016. View Article : Google Scholar
|
|
7
|
Ordög T, Hayashi Y and Gibbons SJ:
Cellular pathogenesis of diabetic gastroenteropathy. Minerva
Gastroenterol Dietol. 55:315–343. 2009.PubMed/NCBI
|
|
8
|
Yarandi SS and Srinivasan S: Diabetic
gastrointestinal motility disorders and the role of enteric nervous
system: Current status and future directions. Neurogastroenterol
Motil. 26:611–624. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Domènech A, Pasquinelli G, De Giorgio R,
Gori A, Bosch F, Pumarola M and Jiménez M: Morphofunctional changes
underlying intestinal dysmotility in diabetic RIP-I/hIFNβ
transgenic mice. Int J Exp Pathol. 92:400–412. 2011. View Article : Google Scholar
|
|
10
|
Du F, Wang L, Qian W and Liu S: Loss of
enteric neurons accompanied by decreased expression of GDNF and
PI3K/Akt pathway in diabetic rats. Neurogastroenterol Motil.
21:1229–e114. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Furlan MM, Molinari SL and Miranda Neto
MH: Morphoquantitative effects of acute diabetes on the myenteric
neurons of the proximal colon of adult rats. Arq Neuropsiquiatr.
60:576–581. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ordög T: Interstitial cells of Cajal in
diabetic gastroenteropathy. Neurogastroenterol Motil. 20:8–18.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin HZ, Sarosiek I, Forster J, Damjanov I,
Hou Q and McCallum RW: Association of the status of interstitial
cells of Cajal and electrogastrogram parameters, gastric emptying
and symptoms in patients with gastroparesis. Neurogastroenterol
Motility. 22:56–61. 2010.
|
|
14
|
Rao M and Gershon MD: The bowel and
beyond: The enteric nervous system in neurological disorders. Nat
Rev Gastroenterol Hepatol. 13:517–528. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lake JI and Heuckeroth RO: Enteric nervous
system development: Migration, differentiation, and disease. Am J
Physiol Gastrointest Liver Physiol. 305:G1–G24. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Farrugia G: Histologic changes in diabetic
gastroparesis. Gastroenterol Clin North Am. 44:31–38. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ihana-Sugiyama N, Nagata N, Yamamoto-Honda
R, Izawa E, Kajio H, Shimbo T, Kakei M, Uemura N, Akiyama J and
Noda M: Constipation, hard stools, fecal urgency, and incomplete
evacuation, but not diarrhea is associated with diabetes and its
related factors. World J Gastroenterol. 22:3252–3260. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Becker L, Kulkarni S, Tiwari G, Micci MA
and Pasricha PJ: Divergent fate and origin of neurosphere-like
bodies from different layers of the gut. Am J Physiol Gastrointest
Liver Physiol. 302:G958–G965. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Burns AJ and Pachnis V: Development of the
enteric nervous system: Bringing together cells, signals and genes.
Neurogastroenterol Motil. 21:100–102. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Laranjeira C, Sandgren K, Kessaris N,
Richardson W, Potocnik A, Vanden Berghe P and Pachnis V: Glial
cells in the mouse enteric nervous system can undergo neurogenesis
in response to injury. J Clin Invest. 121:3412–3424. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Joseph NM, He S, Quintana E, Kim YG, Núñez
G and Morrison SJ: Enteric glia are multipotent in culture but
primarily form glia in the adult rodent gut. J Clin Invest.
121:3398–3411. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Matsuyoshi H, Kuniyasu H, Okumura M,
Misawa H, Katsui R, Zhang GX, Obata K and Takaki M: A
5-HT(4)-receptor activation-induced neural plasticity enhances in
vivo reconstructs of enteric nerve circuit insult.
Neurogastroenterol Motil. 22:806–813.e226. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bixby S, Kruger GM, Mosher JT, Joseph NM
and Morrison SJ: Cell-intrinsic differences between stem cells from
different regions of the peripheral nervous system regulate the
generation of neural diversity. Neuron. 35:643–656. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kruger GM, Mosher JT, Bixby S, Joseph N,
Iwashita T and Morrison SJ: Neural crest stem cells persist in the
adult gut but undergo changes in self-renewal, neuronal subtype
potential, and factor responsiveness. Neuron. 35:657–669. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Heanue TA and Pachnis V: Enteric nervous
system development and Hirschsprung's disease: Advances in genetic
and stem cell studies. Nat Rev Neurosci. 8:466–479. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Burns AJ, Goldstein AM, Newgreen DF, Stamp
L, Schäfer KH, Metzger M, Hotta R, Young HM, Andrews PW, Thapar N,
et al: White paper on guidelines concerning enteric nervous system
stem cell therapy for enteric neuropathies. Dev Biol. 417:229–251.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ibrahim A, Ali RAR, Manaf MRA, Ahmad N,
Tajurruddin FW, Qin WZ, Desa SHM and Ibrahim NM: Multi-strain
probiotics (Hexbio) containing MCP BCMC strains improved
constipation and gut motility in Parkinson's disease: A randomised
controlled trial. PLoS One. 15:e02446802020. View Article : Google Scholar
|
|
28
|
Shafe AC, Lee S, Dalrymple JS and Whorwell
PJ: The LUCK study: Laxative usage in patients with GP-diagnosed
constipation in the UK, within the general population and in
pregnancy. An epidemiological study using the general practice
research database (GPRD). Therap Adv Gastroenterol. 4:343–363.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Menees SB, Guentner A, Chey SW, Saad R and
Chey WD: How Do US gastroenterologists use over-the-counter and
prescription medications in patients with gastroesophageal reflux
and chronic constipation? Am J Gastroenterol. 110:1516–1525. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gonzalez-Martinez MA, Ortiz-Olvera NX and
Mendez-Navarro J: Novel pharmacological therapies for management of
chronic constipation. J Clin Gastroenterol. 48:21–28. 2014.
View Article : Google Scholar
|
|
31
|
Hennessy S, Leonard CE, Newcomb C, Kimmel
SE and Bilker WB: Cisapride and ventricular arrhythmia. Br J Clin
Pharmacol. 66:375–385. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wysowski DK, Corken A, Gallo-Torres H,
Talarico L and Rodriguez EM: Postmarketing reports of QT
prolongation and ventricular arrhythmia in association with
cisapride and Food and Drug Administration regulatory actions. Am J
Gastroenterol. 96:1698–1703. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Thompson CA: Novartis suspends tegaserod
sales at FDA's request. Am J Health Syst Pharm. 64:10202007.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pan H and Gershon MD: Activation of
intrinsic afferent pathways in submucosal ganglia of the guinea pig
small intestine. J Neurosci. 20:3295–3309. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Belkind-Gerson J, Carreon-Rodriguez A,
Benedict LA, Steiger C, Pieretti A, Nagy N, Dietrich J and
Goldstein AM: Nestin-expressing cells in the gut give rise to
enteric neurons and glial cells. Neurogastroenterol Motil.
25:61–69.e7. 2013. View Article : Google Scholar
|
|
36
|
Liu MT, Kuan YH, Wang J, Hen R and Gershon
MD: 5-HT4 receptor-mediated neuroprotection and neurogenesis in the
enteric nervous system of adult mice. J Neurosci. 29:9683–9699.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bouras EP, Michael C, Burton DD, Thomforde
G, McKinzie S and Zinsmeister AR: Prucalopride accelerates
gastrointestinal and colonic transit in patients with constipation
without a rectal evacuation disorder. Gastroenterology.
120:354–360. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Karin S and Ken W: Chapter
35-gastrointestinal drugs. Side Eff Drugs Annu. 37:433–459. 2015.
View Article : Google Scholar
|
|
39
|
Bharucha AE, Wouters MM and Tack J:
Existing and emerging therapies for managing constipation and
diarrhea. Curr Opin Pharmacol. 37:158–166. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ueno N, Inui A and Satoh Y: The effect of
mosapride citrate on constipation in patients with diabetes.
Diabetes Res Clin Pract. 87:27–32. 2010. View Article : Google Scholar
|
|
41
|
Gershon MD and Liu MT: Serotonin and
neuroprotection in functional bowel disorders. Neurogastroenterol
Motil. 19(Suppl 2): S19–S24. 2007. View Article : Google Scholar
|
|
42
|
Bassotti G, Gambaccini D and Bellini M:
Prucalopride succinate for the treatment of constipation: An
update. Expert Rev Gastroenterol Hepatol. 10:291–300. 2016.
View Article : Google Scholar
|
|
43
|
Omer A and Quigley EMM: An update on
prucalopride in the treatment of chronic constipation. Therap Adv
Gastroenterol. 10:877–887. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Bassotti G, Usai Satta P and Bellini M:
Prucalopride for the treatment of constipation: A view from 2015
and beyond. Expert Rev Gastroenterol Hepatol. 13:257–262. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Garnock-Jones KP: Prucalopride: A review
in chronic idiopathic constipation. Drugs. 76:99–110. 2016.
View Article : Google Scholar
|
|
46
|
Daniali M, Nikfar S and Abdollahi M: An
overview of the efficacy and safety of prucalopride for the
treatment of chronic idiopathic constipation. Expert Opin
Pharmacother. 20:2073–2080. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Xie F, Lei J, Ran M, Li Y, Deng L, Feng J,
Zhong Y and Li J: Attenuation of diabetic nephropathy in diabetic
mice by fasudil through regulation of macrophage polarization. J
Diabetes Res. 2020:41269132020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
McClain J, Grubišić V, Fried D,
Gomez-Suarez RA, Leinninger GM, Sévigny J, Parpura V and Gulbransen
BD: Ca2+ responses in enteric glia are mediated by
connexin-43 hemichannels and modulate colonic transit in mice.
Gastroenterology. 146:497–507.e1. 2014. View Article : Google Scholar
|
|
49
|
Butler Tjaden NE and Trainor PA: The
developmental etiology and pathogenesis of Hirschsprung disease.
Transl Res. 162:1–15. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Nagy N and Goldstein AM: Enteric nervous
system development: A crest cell's journey from neural tube to
colon. Semin Cell Dev Biol. 66:94–106. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kiefer JC: Back to basics: Sox genes. Dev
Dyn. 236:2356–2366. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sarkar A and Hochedlinger K: The sox
family of transcription factors: Versatile regulators of stem and
progenitor cell fate. Cell Stem Cell. 12:15–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Hurst-Kennedy J, Chin LS and Li L:
Ubiquitin C-terminal hydrolase l1 in tumorigenesis. Biochem Res
Int. 2012:1237062012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Burger AM and Seth AK: The
ubiquitin-mediated protein degradation pathway in cancer:
Therapeutic implications. Eur J Cancer. 40:2217–2229. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Uesaka T, Nagashimada M and Enomoto H:
Neuronal differentiation in schwann cell lineage underlies
postnatal neurogenesis in the enteric nervous system. J Neurosci.
35:9879–9888. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Gerdes J: Ki-67 and other proliferation
markers useful for immunohistological diagnostic and prognostic
evaluations in human malignancies. Semin Cancer Biol. 1:199–206.
1990.PubMed/NCBI
|
|
57
|
Lendahl U, Zimmerman LB and Mckay RD: CNS
stem cells express a new class of intermediate filament protein.
Cell. 60:585–595. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Dahlstrand J, Lardelli M and Lendahl U:
Nestin mRNA expression correlates with the central nervous system
progenitor cell state in many, but not all, regions of developing
central nervous system. Brain Res Dev Brain Res. 84:109–129. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zheng Z, Chen B, Jin Z, Gao M, Tang C, Mao
Y, Qu Y and Liu Y: Downregulation of P2Y2 and HuD during the
development of the enteric nervous system in fetal rats with
anorectal malformations. Mol Med Rep. 20:1297–1305. 2019.PubMed/NCBI
|
|
60
|
Giorgio RD, Volta U, Stanghellini V,
Cogliandro RF, Barbara G, Corinaldesi R, Towns R, Guo C, Hong S and
Wiley JW: Neurogenic chronic intestinal pseudo-obstruction:
Antineuronal antibody-mediated activation of autophagy via Fas.
Gastroenterology. 135:601–609. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Akishima-Fukasawa Y, Ino Y, Nakanishi Y,
Miura A, Moriya Y, Kondo T, Kanai Y and Hirohashi S: Significance
of PGP9.5 expression in cancer-associated fibroblasts for prognosis
of colorectal carcinoma. Am J Clin Pathol. 134:71–79. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Kostouros A, Koliarakis I, Natsis K,
Spandidos D, Tsatsakis A and Tsiaoussis J: Large intestine
embryogenesis: Molecular pathways and related disorders (Review).
Int J Mol Med. 46:27–57. 2020.PubMed/NCBI
|
|
63
|
Gomes FC, Paulin D and Moura Neto V: Glial
fibrillary acidic protein (GFAP): Modulation by growth factors and
its implication in astrocyte differentiation. Braz J Med Biol Res.
32:619–631. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Tong X, Li L, Li X, Heng L, Zhong L, Su X,
Rong R, Hu S, Liu W, Jia B, et al: SOX10, a novel
HMG-box-containing tumor suppressor, inhibits growth and metastasis
of digestive cancers by suppressing the Wnt/β-catenin pathway.
Oncotarget. 5:10571–10583. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Miller I, Min M, Yang C, Tian C, Gookin S,
Carter D and Spencer SL: Ki67 is a graded rather than a binary
marker of proliferation versus quiescence. Cell Rep.
24:1105–1112.e5. 2018. View Article : Google Scholar : PubMed/NCBI
|