1. Introduction
Angiogenesis plays a significant role in tissue
growth, wound repair, tumor development, and metastasis. It is
controlled by growth factors, pro-angiogenic cytokines, and
neovascularization antagonists (1). Connexins (Cxs) are hexameric arrays
of tetraspan integral membrane proteins that form gap junctions
(GJs). GJs provide direct ionic and molecular communication between
neighboring cells and coordinate the exchange of chemicals and
electrical impulses between them.
Several studies have reported independent and
GJ-dependent roles of Cxs in mediating angiogenesis in various
disorders (2,3). Although there are a few reviews on
the role of Cxs in various physiological processes (4-9),
there is little discussion of how Cxs influence the angiogenic
process involved in wound repair, tumorigeneses, and cardiovascular
disorders. The purpose of the present study was to examine the
current state of knowledge regarding Cx structure, nomenclature,
function, and regulation, as well as the newly identified link
between Cxs and angiogenesis. Major Cx-mediated angio- genesis
disorders and potential therapeutic approaches were also
examined.
2. Methodology
A literature search was performed to identify
articles that discussed the role of connexins in angiogenesis. The
MEDLINE, PubMed, Scopus, and Cochrane Library databases were
searched until June 02, 2022. Individual or combined searches for
the terms 'angiogenesis', 'connexin', 'Cx', and 'gap junctions'
were performed. By scanning the references of the included studies,
additional studies were identified. Letters to the editor and
articles without abstracts were excluded.
Structure and diversity of Cxs and
GJs
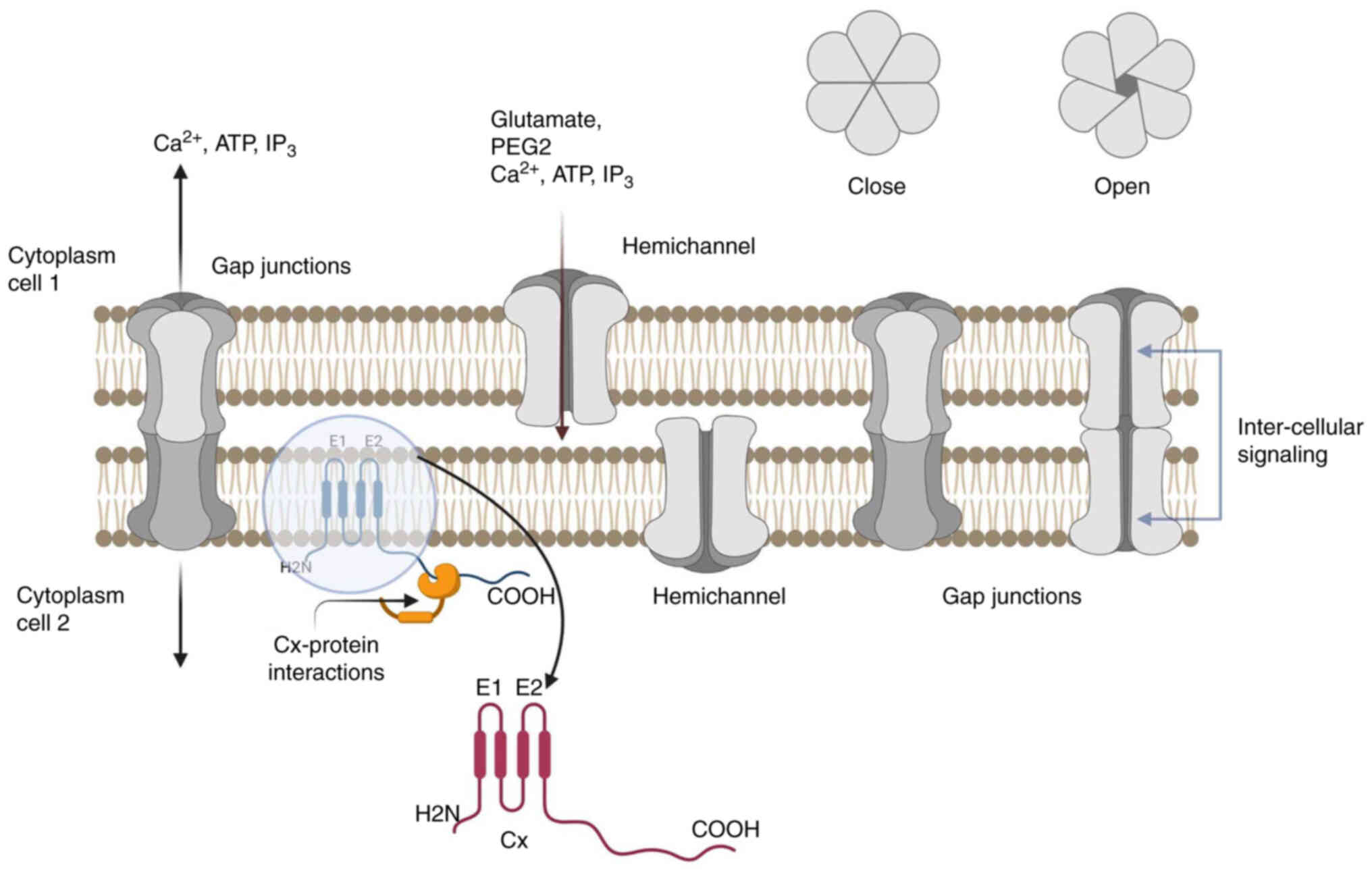
Each Cx has four hydrophobic transmembranes (M1-M4),
two extracellular areas (E1 and E2) that bind to another Cx in the
neighboring cell, and three cytoplasmic regions that correspond to
the cytoplasmic loop (CL), and the amino-terminal (NT), and
carboxy-terminal (CT) tail regions. The N-terminus,
membrane-spanning sections, and extracellular loops are consistent
throughout the structure; however, the size and structure of the CL
and the CT are not. The GJ channel is composed of two hemichannels
(or connexons), consisting of six transmembrane proteins (Cx
subunits) connected to the plasma membrane of each symmetric cell.
When two hemichannels join to produce a cell-cell conduit, one is
tilted by 30° with respect to the other. Homotypic GJs are formed
when identical Cx subunits dock, whereas heterotypic GJs are formed
when two different connexons (hemichannels) dock (10). The central cytoplasmic part and
the second extracellular domain (E2) regulate the heterotypic
adaptation of Cxs. Heterotypic channels have features that differ
from those of homotypic channels, such as unitary conductance and
gating. The permeabilities of different channels formed by
different Cxs differ, allowing secondary messengers to be
discriminated against (cyclic guanosine monophosphate,
Ca2+, or IP3) (Fig. 1).
Functional role of Cxs
Hemichannels regulate cellular responses to a wide
range of physiological, oxidative, and metabolic stressors, whereas
GJs permit intercellular trans- mission (Fig. 1). Molecules transported through
these channels are responsible for several physiological functions.
Different stimuli, including variations in voltage,
Ca2+, pH, and Cx phosphorylation, can dynamically
control gap junctional intercellular communication (GJIC) (10-15). Voltage sensitivity is critical for
controlling the intercellular connectivity of excitable cells.
Channel independence has been demonstrated in the context of
cellular proliferation, attachment, motility, apoptotic processes,
and signaling (2,12,16-19). It was also recently revealed by
the authors' research group that Cx43 levels regulate angiogenesis
in endothelial cells (ECs), irrespective of GJ function (2). Moorby and Patel conducted extensive
research on the GJ-dependent and-independent functions of Cx43 and
discovered that the carboxyl region of Cx43 mainly governs the
independent GJ function (19). It
is increasingly accepted that the effects of GJIC-independent Cxs
on carcinogenesis extend beyond proliferation and migration,
including angiogenesis and cell death (20-23).
Cxs and angiogenic processes
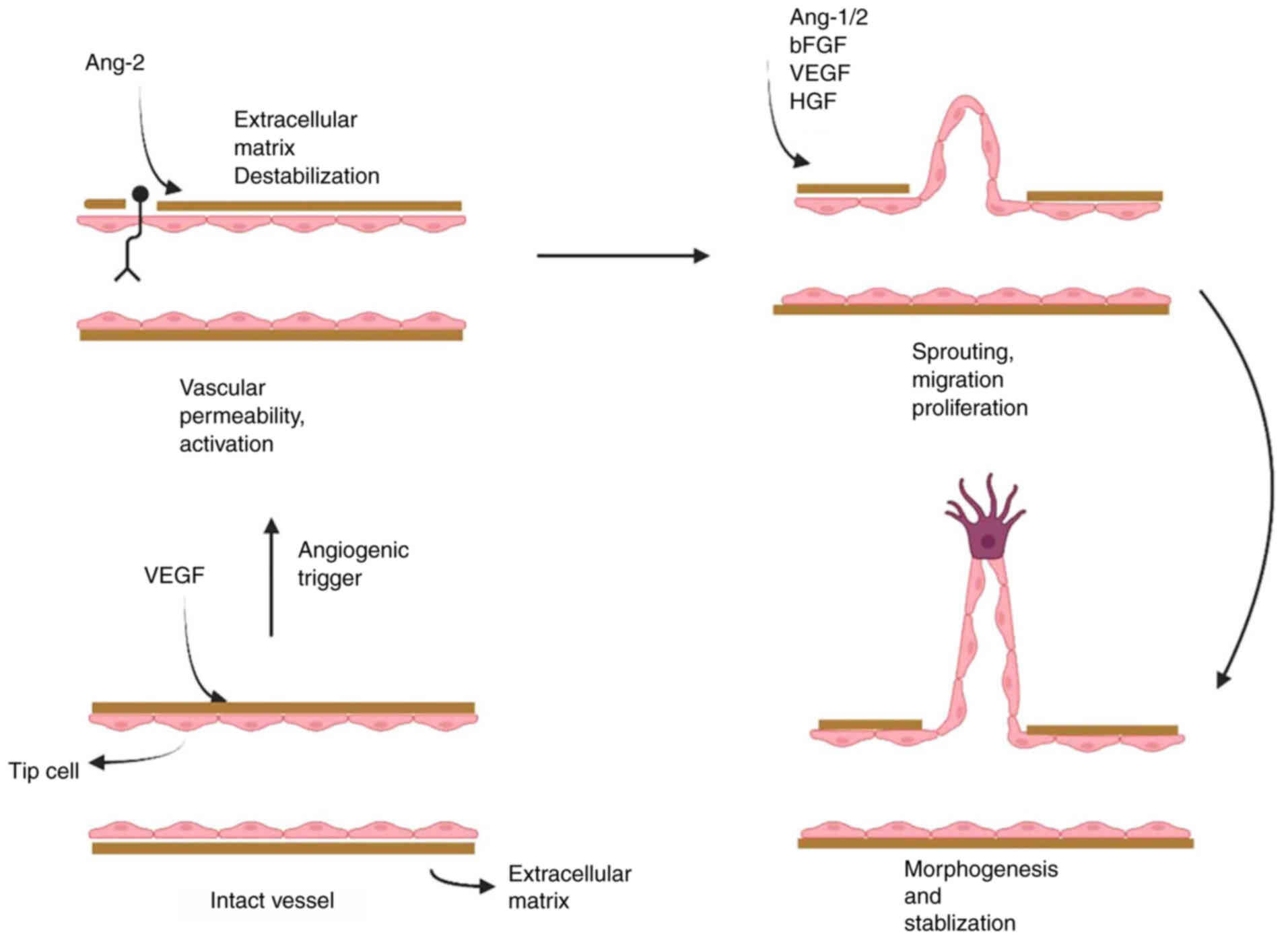
Angiogenesis plays a crucial role in tissue growth,
wound healing (WH), carcinogenesis, and metastasis (1,24).
It begins with the growth and development of preexisting vessels,
depending on a mixture of growth factors and proangiogenic
cytokines, and is regulated by various neovascularization
antagonists (Fig. 2) (25,26). Cxs have been shown to affect
angiogenic processes in various ways, including growth, transport,
and cellular stiffness (27). The
roles of Cx43, Cx37, and Cx40, which are the most prevalent Cxs
engaged in angiogenic processes, are discussed in the next section.
The expression of Cx43 expression influences the angiogenic
potential of endothelial cells independently of the GJ interaction.
Because proliferation was unchanged, it was hypothesized that the
Cx43 protein may significantly alter endothelial cell relocation,
thereby promoting angiogenesis (2).
Cx43
Decreased Cx43 expression can result in vascular
dysfunction and impaired angiogenesis (28). Cx43 is also involved in regulating
lung microvascular permeability, and its modulation is related to
endothelial monolayer permeability (29,30). Salmina et al examined
GJ-dependent neurogenesis and concluded that alterations in Cx43
expression were correlated with distinct steps in neural growth
(31). Cx43 is also upregulated
in ECs during hemodynamic stimulation-induced angiogenesis
(32). A study of the molecular
processes during human trophoblast fusion revealed that protein
kinase A-dependent phosphorylation of Cx43 enhances cell fusion
(33). Furthermore, decreased
Cx43 expression can result in improper embryo implantation and
inadequate angiogenesis (34). It
has also been found that Cx43, as a negative regulator,
participates in critical steps of WH, such as inflammation
response, remodeling of the extracellular matrix, proliferation of
epidermal/skin cells, and migration (35).
Cx37 and Cx40
Cx37 and Cx40, which are co-expressed in ECs, have
overlapping functions. Cx40 can promote EC migration, vessel
sprouting, and expansion, whereas Cx40 deficiency and inhibition
reduce angiogenesis (36).
Endothelial Cx40, according to Haefliger et al, affects the
initial phases of angiogenesis in the retina by controlling
vascularization (37). In
Cx37−/− mice, improved recovery of the hind limb was
associated with increased vasculogenesis, which resulted in greater
collateral remodeling and angiogenesis (38). Furthermore, the global deletion of
Cx37 in mice causes increased angiogenesis during tissue injury,
aiding the recovery process after ischemic injury (39). Growth inhibition mediated by Cx37
involves CT and the pore-forming domain (14). Nitric oxide affects endothelial
vasomotor activity by modulating calcium signaling (40). Cx37 and Cx40 have been shown to
uniquely control post-ischemic limb perfusion, affecting the
intensity of ischemic stress and, as a result, post-ischemic
persistence (41). Cx37
selectively affects Ang II signaling by modulating Ang II receptor
expression (42). Cx37 also
suppresses the proliferation of vascular and cancer cells.
Cx37-induced growth arrest or growth-permissive phenotypes depend
on conformational changes in Cx37 caused by phosphorylation
(43).
3. Cxs, diseases and potential
therapies
Cxs are implicated in the regulation of innate
epithelial immunity, wound repair, and inflammatory processes. The
pathophysiology of various Cx-related diseases is determined by
both the canonical and noncanonical functions of Cxs. Given the
presence of several Cxs in the endothelium, it is possible that Cxs
and immune-targeted therapies could be used synergistically. In
various pathological conditions, such as ischemia, optic nerve
damage, stroke, and spinal cord injury, communication between
junctions and hemichannels leads to secondary damage through
inflammatory processes (44).
Cx43 enhanced brain blood flow restoration in a mouse model by
regulating reparative angiogenesis during chronic cerebral
hypoperfusion (45). Due to the
variety of Cx-mediated communication and its effect on cellular
physiology and pathology, a definitive link between Cxs,
angiogenesis, and disease has not yet been identified. However, in
numerous cases, an association between aberrant Cx function,
angiogenesis, and disease has been observed. The following section
highlights the key mechanistic and therapeutic findings.
WH
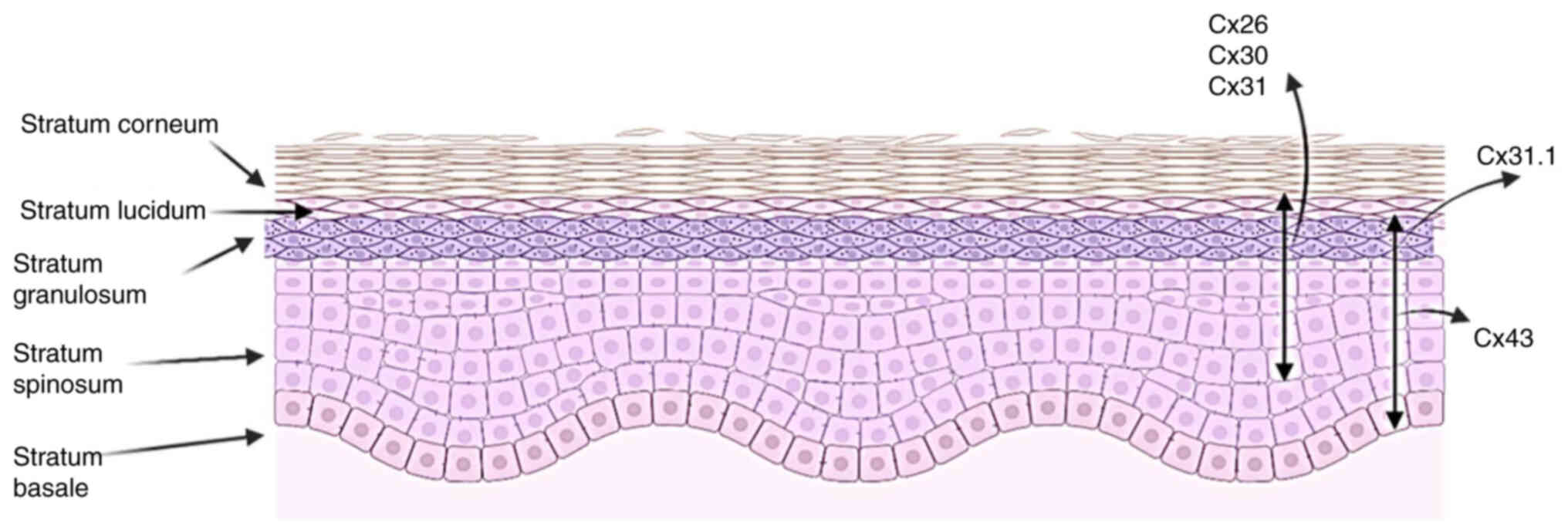
Different layers of the human epidermis express
different levels of Cxs, which are associated with a number of skin
diseases (Fig. 3). During the
early phases of WH, Cx43 has been observed to be negatively
regulated at the wound margins (46). Nitric oxide, a mediator of
vasomotion, has been reported to be a strong modulator of GJ
coupling in ECs (47). It
promotes the de novo formation of GJ by expanding the
integration of Cx40 into the plasma membrane. One of the most
evident applications that demonstrates the involvement of Cxs in
angiogenesis is the efficacy of bioactive glass (BG) in WH. In
rats, BG stimulates GJIC, which results in increased angiogenesis
and accelerates the closure of excisional wounds (48). It was recently shown that BG
affects the expression of Cx43 and ROS levels, increasing WH by
suppressing pyroptosis through the Cx43/ROS signaling pathway
(49). Cx43 remodeling is an
important event in WH that influences the cellular dynamics of
keratinocytes and fibroblasts (50). It was revealed that siRNA
knockdown of Cx43 in human microvascular endothelial cells reduced
migration in vitro, as measured by a wound assay, and
impaired aortic vessel sprouting ex vivo (16); Cx43 and the tyrosine phosphatase,
SHP-2, were also revealed to mediate endothelial cell migration,
revealing a novel interaction between Cx43 and SHP-2 that is
required for this process (16).
Mutations in Cx26, Cx30, and Cx31 are associated
with hyperproliferative skin diseases (51). Furthermore, suppression of Cx43
function affects the expression of genes associated with WH
(52). Cx mutations are
associated with epidermal dysplasia (15). Gain-of-function mutations alter
Cx-mediated calcium signaling within the epidermis; for example,
suppressing Cx43 activity in fibroblasts has been shown to increase
migration and control the expression of genes associated with WH
through the mitogen-activated protein kinase, specificity protein
1, activator protein 1, glycogen synthase kinase 3, and
transforming growth factor pathways, contributing to rapid and
scarless WH in the human gingiva (52).
Preclinical studies on peptide therapeutics, a
mimetic of Cx43 CT, have reported improvements in WH (53). Cx43 has also been reported to
counter-regulate caveolin-1 in controlling EC proliferation and
migration, and this counterregulatory effect of Cx43 could be used
in therapeutic angiogenesis (54). Morphine administration was found
to inhibit angiogenesis and delay WH by upregulating Cx43, and high
doses of morphine alter Cx43 expression by increasing fibronectin
and actin levels through the activation of transforming growth
factor signaling (55). A Cx43
mimetic peptide (TAT-Gap19) significantly upregulates matrix
metalloproteinases, tenascin-C, and vascular endothelial growth
factor (VEGF)-A (13).
Cancer
In cancer cells, intercellular communication is
aberrant, and numerous studies have suggested that dysfunctional GJ
and Cxs play a key role in this process (56). However, there appears to be a
skewed association between Cxs and cancer, with evidence suggesting
that Cxs may limit cancer cell development in certain instances
while also promoting cancer cell motility, invasion, and metastatic
dissemination in others (57,58). A key study revealed that
inhibiting Cx37 decreases tumor angiogenesis; moreover, Cx37 and
Cx40 work together to promote tumorigenesis (20). Consequently, the involvement of
Cxs and GJs in cancer is more complex than previously thought.
Breast tumor cells transplanted into heterozygous
Cx43 mice did not affect tumor growth, but greatly improved
vascularization, indicating the role of Cx43 in vessel quiescence
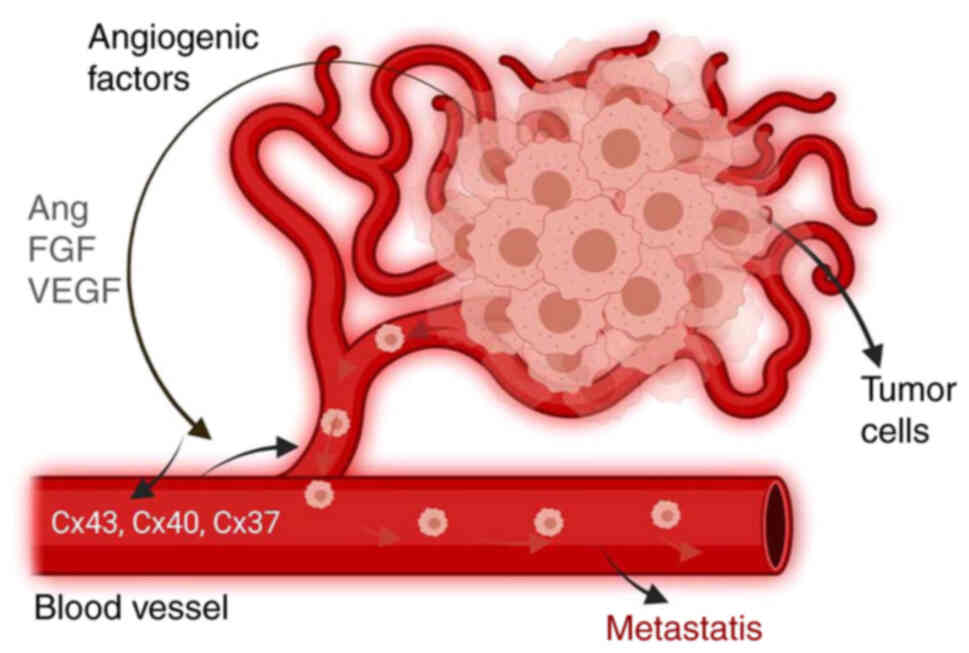
control and pathological tumor angiogenesis (22). The passage of tumor cells through
the endothelial barrier is an important step in metastasis, in
which endothelial cells adhere to the target organ by direct
cell-cell communication and paracrine activation to initiate
angiogenesis (Fig. 4). Cx46
regulates cancer stem cell and epithelial-to-mesenchymal transition
features in breast cancer cells, suggesting that it may be useful
in the development of future cancer therapeutics (59).
Intercellular communication is also required for
tumor cell trafficking across the lymphatic endothelium (60). Hemichannels have been reported to
facilitate interactions between cancer cells and blood vessels,
leading to angiogenesis. Choudhary et al revealed that
tumors downregulate Cx43 function, allowing the endothelium to
respond to angiogenic stimuli, leading to pathogenic angiogenesis
(22). The roles of Cx and Notch
endothelial signaling in coordinating the appropriate proliferation
and angiogenesis of ECs have been identified (61). It has also been shown that GJIC
inhibits tumor growth by transferring microRNAs from one EC to
surrounding tumor cells, indicating a bystander role that can be
exploited in cancer treatment (21).
Targeting Cx may be a promising therapeutic approach
for cancer (23). Exosomes
containing anti-angiogenic microRNAs released immediately through
Cx channels can prevent cancer cells from promoting angiogenesis
(21). Peptide-mediated
inhibition of Cx40 in EC is a successful anti-angiogenesis approach
that suppresses tumor angiogenesis (36). In the conditioned medium, tumor
size and vessel density in Cx43-knockdown tumor cells decreased,
indicating that Cx43 prevented tumor growth by decreasing
angiogenesis (62).
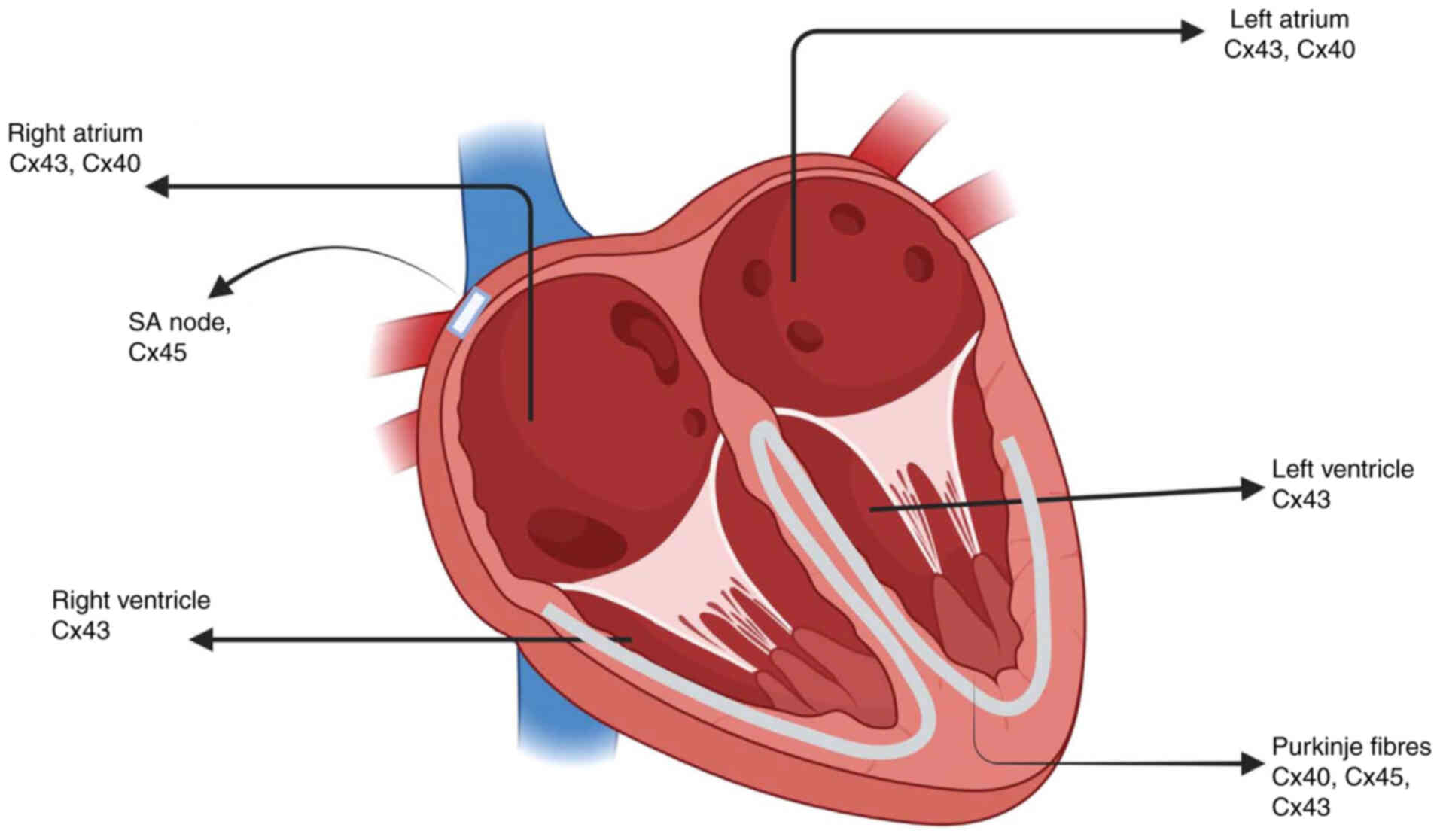
Cardiovascular disorders
Several Cxs are co-expressed in the heart; in
particular, distinct combinations of Cx40, Cx43, and Cx45 are
observed in functionally specialized cardiomyocytes (Fig. 5). GJ channels in the
cardiovascular system regulate vascular tone, which is essential
for the coordination of cell activity, by permitting the transport
of chemical messengers and energy substrates (63-65). Cxs form GJs for the transmission
of precisely choreographed current flow patterns that control the
synchronized beat of a healthy heart. Several pathophysiological
conditions, including atherosclerosis, hypertension, hypertrophy,
ischemia, and arrhythmias, have been linked to dysregulation of Cxs
in the cardiovascular system in terms of expression, function,
posttranslational modifications, and location. Ugwu et al
reported a recurring somatic Cx43-gene c.121G>T mutation as a
cause of cutaneous venous abnormalities (66). Although Cx43 levels are high in
cardiac neural crest cells, both heterozygous and homozygous
knock-in mice live long and do not exhibit symptoms of coronary
heart disease (67). Similarly,
point mutations in Cx43 were not found to cause the tetralogy of
Fallot (68).
Treatment with granulocyte colony-stimulating factor
improves arterial and capillary density and increases Cx43
expression in failing hearts (69). Through Cx43, VEGF stimulates
endothelial progenitor cells and supports vascular healing
(70). In ECs,
ischemia/reperfusion causes reactive species to disrupt Cx/pannexin
signaling mitochondrial prompt division and promote macrovesicle
release (71). Cx43 and
angiogenesis levels are higher in the exercised mouse heart,
indicating increased remodeling (72). Long-term alienation combined with
moderate environmental pressure has been associated with depressive
symptoms and aberrant expression of Cx43 and Cx45 in the left
ventricle (73). Notably,
EC-specific molecule 1 enhances the potential of induced
pluripotent stem ECs to promote angiogenesis and neovascularization
(74). Su et al determined
that preconditioning for ischemia had cardioprotective effects on
arrhythmia and myocardial recovery by upregulating
phosphatidylinositol 3-kinase-mediated Cx43 signaling (75). In a study on myometrial cell patch
transplantation to cure myocardial infarction, angiogenesis was
reported to occur in the trans- planted myometrium and Cx43
expression was observed in the transplanted patches (76). Cx43 passivation from intercellular
signaling and buildup at the mitochondrial inner membrane has been
revealed in diabetic cardiomyocytes, demonstrating that mtCx43 is
responsible for triggering aberrant contraction and disrupting
electrophysiology in cardiomyocytes (77).
Heart disease caused by myocardial tissue injury and
fibrosis is related to Cx43-based GJs. As a result, several Cx43
mimetic peptides have been proposed as potential therapeutics for
Cx43-related degenerative disorders, some even reaching human
clinical trials (78). Cx43
improves infarcted heart angiogenesis, as evidenced by higher
levels of VEGF and basic fibroblast growth factor (18). The cardioprotective properties of
expanded umbilical cord mesenchymal stem cells (MSC) were
attributed to paracrine substances that tend to enhance
angiogenesis and preserve Cx43 GJ function (75). Cx43 was found to be dispensable
for the adipogenic differentiation of early-stage MSC, although it
was protective against cell senescence (79). The survival and tube formation of
MSCs are improved by Ang II treatment and Cx43 expression (80). TEM immunogold studies on rat heart
ventricles indicated the lack of Cx26 at intercalated discs but the
presence of Cx26 at various subcellular compartments (17). It was found that after a localized
ischemic stroke, Cx43 regulated the angiogenesis of Buyang
Huanwu decoction through VEGF and Ang-1 (81). Due to the increase of tissue Cx43
and proangiogenic markers, regenerative treatment using
nanofiber-expanded hematopoietic stem cells has been reported to
have a favorable effect on rat heart function following myocardial
infarction (82).
4. Conclusions and future directions
Several studies have elucidated GJ/Cx-mediated
angiogenesis. To adequately describe the de novo blood
vessels involved in the response to tumor angiogenesis, researchers
must examine changes in the expression patterns of GJIC and Cxs in
pro-angiogenic stimuli in the neovasculature. Antiangiogenic
therapy has been shown to increase survival in human tumors;
therefore, GJ-and Cx-targeting techniques could be useful in the
development of novel medicines. Chemical blockers of Cx channels,
peptide mimics of short Cx sequences, such as Gap19/24/27/40, and
gene therapy techniques have all been shown to be extremely
effective molecular techniques for unraveling the complexity of the
function of Cxs. Future research should focus on determining the
specific molecular pathways underlying the significance of Cxs in
various diseases and designing randomized control trials for
specific therapeutic alternatives.
Availability of data and materials
Not applicable.
Authors' contributions
ZZ, WC, YL and MZ contributed to the study concept,
design, literature search and computer graphics for the figures. ZZ
wrote the manuscript. XZ revised the manuscript and was in charge
of the final approval of the manuscript prior to submission. Data
authentication is not applicable. All authors read and approved the
final manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
The present study was funded by the Natural Science Foundation
of Shenzhen University General Hospital (grant no. SUGH2019QD007 to
ZZ; grant no. SUGH2019QD014 to XZ) and the Science and Technology
Foundation of Nanshan District, Shenzhen (grant no. NS2021167 to
ZZ). The funding provided financial support for the data
collection, the analysis of the collected data and computer
graphics.
Abbreviations:
|
BG
|
bioactive glass
|
|
CL
|
cytoplasmic loop
|
|
CT
|
carboxy-terminal
|
|
ECs
|
endothelial cells
|
|
GJ
|
gap junctions
|
|
GJIC
|
Gap junctional intercellular
communication
|
|
MSC
|
mesenchymal stem cells
|
|
VEGF
|
vascular endothelial growth factor
|
|
WH
|
wound healing
|
References
|
1
|
Carmeliet P: Mechanisms of angiogenesis
and arteriogenesis. Nat Med. 6:389–395. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Koepple C, Zhou Z, Huber L, Schulte M,
Schmidt K, Gloe T, Kneser U, Schmidt VJ and de Wit C: Expression of
Connexin43 Stimulates Endothelial Angiogenesis Independently of Gap
junctional communication in vitro. Int J Mol Sci. 22:74002021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Haefliger JA, Meda P and Alonso F:
Endothelial connexins in developmental and pathological
angiogenesis. Cold Spring Harb Perspect Med. 12:a0411582022.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Qiu Y, Zheng J, Chen S and Sun Y: Connexin
mutations and hereditary diseases. Int J Mol Sci. 23:42552022.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Peracchia C and Leverone Peracchia LM:
Calmodulin-Connexin partnership in Gap junction channel
regulation-calmodulin-cork gating model. Int J Mol Sci.
22:130552021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Okamoto T, Park EJ, Kawamoto E, Usuda H,
Wada K, Taguchi A and Shimaoka M: Endothelial connexin-integrin
crosstalk in vascular inflammation. Biochim Biophys Acta Mol Basis
Dis. 1867:1661682021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Laird DW and Lampe PD: Cellular mechanisms
of connexin-based inherited diseases. Trends Cell Biol. 32:58–69.
2022. View Article : Google Scholar
|
|
8
|
King DR, Sedovy MW, Leng X, Xue J,
Lamouille S, Koval M, Isakson BE and Johnstone SR: Mechanisms of
connexin regulating peptides. Int J Mol Sci. 22:101862021.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Htet M, Nally JE, Martin PE and Dempsie Y:
New insights into pulmonary hypertension: A role for
connexin-mediated signal- ling. Int J Mol Sci. 23:3792021.
View Article : Google Scholar
|
|
10
|
Roy S, Jiang JX, Li AF and Kim D: Connexin
channel and its role in diabetic retinopathy. Prog Retin Eye Res.
61:35–59. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nielsen MS, Axelsen LN, Sorgen PL, Verma
V, Delmar M and Holstein-Rathlou NH: Gap junctions. Compr Physiol.
2:1981–2035. 2012. View Article : Google Scholar
|
|
12
|
Zhou JZ and Jiang JX: Gap junction and
hemichannel-independent actions of connexins on cell and tissue
functions-an update. FEBS Lett. 588:1186–1192. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tarzemany R, Jiang G, Jiang JX, Larjava H
and Häkkinen L: Connexin 43 hemichannels regulate the expression of
wound healing-associated genes in human gingival fibroblasts. Sci
Rep. 7:141572017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jacobsen NL, Pontifex TK, Li H, Solan JL,
Lampe PD, Sorgen PL and Burt JM: Regulation of Cx37 channel and
growth-suppressive properties by phosphorylation. J Cell Sci.
130:3308–3321. 2017.PubMed/NCBI
|
|
15
|
Cocozzelli AG and White TW: Connexin 43
mutations lead to increased hemichannel functionality in skin
disease. Int J Mol Sci. 20:61862019. View Article : Google Scholar
|
|
16
|
Mannell H, Kameritsch P, Beck H, Pfeifer
A, Pohl U and Pogoda K: Cx43 promotes endothelial cell migration
and angiogenesis via the tyrosine phosphatase SHP-2. Int J Mol Sci.
23:2942021. View Article : Google Scholar
|
|
17
|
Falleni A, Moscato S, Sabbatini ARM,
Bernardeschi M, Bianchi F, Cecchettini A and Mattii L: Subcellular
localization of connexin 26 in cardiomyocytes and in
cardiomyocyte-derived extracellular vesicles. Molecules.
26:67262021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang DG, Zhang FX, Chen ML, Zhu HJ, Yang B
and Cao KJ: Cx43 in mesenchymal stem cells promotes angiogenesis of
the infarcted heart independent of gap junctions. Mol Med Rep.
9:1095–1102. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Moorby C and Patel M: Dual functions for
connexins: Cx43 regulates growth independently of gap junction
formation. Exp Cell Res. 271:238–248. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sathiyanadan K, Alonso F, Domingos-Pereira
S, Santoro T, Hamard L, Cesson V, Meda P, Nardelli-Haefliger D and
Haefliger JA: Targeting Endothelial Connexin37 reduces angiogenesis
and decreases tumor growth. Int J Mol Sci. 23:29302022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Thuringer D, Jego G, Berthenet K, Hammann
A, Solary E and Garrido C: Gap junction-mediated transfer of
miR-145-5p from microvascular endothelial cells to colon cancer
cells inhibits angiogenesis. Oncotarget. 7:28160–28168. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Choudhary M, Naczki C, Chen W, Barlow KD,
Case LD and Metheny-Barlow LJ: Tumor-induced loss of mural Connexin
43 gap junction activity promotes endothelial proliferation. BMC
Cancer. 15:4272015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Aasen T, Leithe E, Graham SV, Kameritsch
P, Mayán MD, Mesnil M, Pogoda K and Tabernero A: Connexins in
cancer: Bridging the gap to the clinic. Oncogene. 38:4429–4451.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Distler O, Neidhart M, Gay RE and Gay S:
The molecular control of angiogenesis. Int Rev Immunol. 21:33–49.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Polverini PJ: The pathophysiology of
angiogenesis. Crit Rev Oral Biol Med. 6:230–247. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Goel S, Duda DG, Xu L, Munn LL, Boucher Y,
Fukumura D and Jain RK: Normalization of the vasculature for
treatment of cancer and other diseases. Physiol Rev. 91:1071–1121.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zefferino R, Piccoli C, Gioia SD,
Capitanio N and Conese M: Gap junction intercellular communication
in the carcinogenesis Hallmarks: Is this a phenomenon or
epiphenomenon? Cells. 8:8962019. View Article : Google Scholar :
|
|
28
|
Wang HH, Su CH, Wu YJ, Li JY, Tseng YM,
Lin YC, Hsieh CL, Tsai CH and Yeh HI: Reduction of connexin43 in
human endothelial progenitor cells impairs the angiogenic
potential. Angiogenesis. 16:553–560. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kandasamy K, Escue R, Manna J, Adebiyi A
and Parthasarathi K: Changes in endothelial connexin 43 expression
inversely correlate with microvessel permeability and VE-cadherin
expression in endotoxin-challenged lungs. Am J Physiol Lung Cell
Mol Physiol. 309:L584–L592. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
O'Donnell JJ III, Birukova AA, Beyer EC
and Birukov KG: Gap junction protein connexin43 exacerbates lung
vascular permeability. PLoS One. 9:e1009312014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Salmina AB, Morgun AV, Kuvacheva NV,
Lopatina OL, Komleva YK, Malinovskaya NA and Pozhilenkova EA:
Establishment of neurogenic microenvironment in the neurovascular
unit: The connexin 43 story. Rev Neurosci. 25:97–111. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schmidt VJ, Hilgert JG, Covi JM, Weis C,
Wietbrock JO, de Wit C, Horch RE and Kneser U: High flow conditions
increase connexin43 expression in a rat arteriovenous and
angioinductive loop model. PLoS One. 8:e787822013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gerbaud P and Pidoux G: Review: An
overview of molecular events occurring in human trophoblast fusion.
Placenta. 36(Suppl 1): S35–S42. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
He X and Chen Q: Reduced expressions of
connexin 43 and VEGF in the first-trimester tissues from women with
recurrent pregnancy loss. Reprod Biol Endocrinol. 14:462016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang XF and Cui X: Connexin 43: Key roles
in the skin. Biomed Rep. 6:605–611. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Alonso F, Domingos-Pereira S, Le Gal L,
Derré L, Meda P, Jichlinski P, Nardelli-Haefliger D and Haefliger
JA: Targeting endothelial connexin40 inhibits tumor growth by
reducing angiogenesis and improving vessel perfusion. Oncotarget.
7:14015–14028. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Haefliger JA, Allagnat F, Hamard L, Le Gal
L, Meda P, Nardelli-Haefliger D, Génot E and Alonso F: Targeting
Cx40 (Connexin40) expression or function reduces angiogenesis in
the developing mouse retina. Arterioscler Thromb Vasc Biol.
37:2136–2146. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fang JS, Angelov SN, Simon AM and Burt JM:
Cx37 deletion enhances vascular growth and facilitates ischemic
limb recovery. Am J Physiol Heart Circ Physiol. 301:H1872–H1881.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li H, Spagnol G, Pontifex TK, Burt JM and
Sorgen PL: Chemical shift assignments of the connexin37 carboxyl
terminal domain. Biomol NMR Assign. 11:137–141. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pogoda K, Füller M, Pohl U and Kameritsch
P: NO, via its target Cx37, modulates calcium signal propagation
selectively at myoendothelial gap junctions. Cell Commun Signal.
12:332014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fang JS, Angelov SN, Simon AM and Burt JM:
Cx40 is required for, and cx37 limits, postischemic hindlimb
perfusion, survival and recovery. J Vasc Res. 49:2–12. 2012.
View Article : Google Scholar
|
|
42
|
Le Gal L, Pellegrin M, Santoro T, Mazzolai
L, Kurtz A, Meda P, Wagner C and Haefliger JA: Connexin37-Dependent
mechanisms selectively contribute to modulate Angiotensin
II-Mediated Hypertension. J Am Heart Assoc. 8:e0108232019.
View Article : Google Scholar
|
|
43
|
Taylor SZ, Jacobsen NL, Pontifex TK,
Langlais P and Burt JM: Serine 319 phosphorylation is necessary and
sufficient to induce a Cx37 conformation that leads to arrested
cell cycling. J Cell Sci. 133:jcs2407212020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
O'Carroll SJ, Becker DL, Davidson JO, Gunn
AJ, Nicholson LF and Green CR: The use of connexin-based
therapeutic approaches to target inflammatory diseases. Methods Mol
Biol. 1037:519–546. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yu W, Jin H, Sun W, Nan D, Deng J, Jia J,
Yu Z and Huang Y: Connexin43 promotes angiogenesis through
activating the HIF-1α/VEGF signaling pathway under chronic cerebral
hypo- perfusion. J Cereb Blood Flow Metab. 41:2656–2675. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lorraine C, Wright CS and Martin PE:
Connexin43 plays diverse roles in co-ordinating cell migration and
wound closure events. Biochem Soc Trans. 43:482–488. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hoffmann A, Gloe T, Pohl U and Zahler S:
Nitric oxide enhances de novo formation of endothelial gap
junctions. Cardiovasc Res. 60:421–430. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Li H, He J, Yu H, Green CR and Chang J:
Bioglass promotes wound healing by affecting gap junction connexin
43 mediated endothelial cell behavior. Biomaterials. 84:64–75.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhang K, Chai B, Ji H, Chen L, Ma Y, Zhu
L, Xu J, Wu Y, Lan Y, Li H, et al: Bioglass promotes wound healing
by inhibiting endothelial cell pyroptosis through regulation of the
connexin 43/reactive oxygen species (ROS) signaling pathway. Lab
Invest. 102:90–101. 2022. View Article : Google Scholar
|
|
50
|
Faniku C, O'Shaughnessy E, Lorraine C,
Johnstone SR, Graham A, Greenhough S and Martin PEM: The connexin
mimetic peptide Gap27 and Cx43-Knockdown reveal differential roles
for Connexin43 in wound closure events in skin model systems. Int J
Mol Sci. 19:6042018. View Article : Google Scholar :
|
|
51
|
Martin PE, Easton JA, Hodgins MB and
Wright CS: Connexins: Sensors of epidermal integrity that are
therapeutic targets. FEBS Lett. 588:1304–1314. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Tarzemany R, Jiang G, Larjava H and
Häkkinen L: Expression and function of connexin 43 in human
gingival wound healing and fibroblasts. PLoS One. 10:e01155242015.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Montgomery J, Ghatnekar GS, Grek CL, Moyer
KE and Gourdie RG: Connexin 43-Based therapeutics for dermal wound
healing. Int J Mol Sci. 19:17782018. View Article : Google Scholar :
|
|
54
|
Arshad M, Conzelmann C, Riaz MA, Noll T
and Gündüz D: Inhibition of Cx43 attenuates ERK1/2 activation,
enhances the expression of Cav-1 and suppresses cell proliferation.
Int J Mol Med. 42:2811–2818. 2018.PubMed/NCBI
|
|
55
|
Wu PC, Hsu WL, Chen CL, Lam CF, Huang YB,
Huang CC, Lin MH and Lin MW: Morphine induces fibroblast activation
through Up-regulation of Connexin 43 expression: Implication of
fibrosis in wound healing. Int J Med Sci. 15:875–882. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Asencio-Barría C, Defamie N, Sáez JC,
Mesnil M and Godoy AS: Direct intercellular communications and
cancer: A snapshot of the biological roles of connexins in prostate
cancer. Cancers (Basel). 11:13702019. View Article : Google Scholar
|
|
57
|
Gleisner MA, Navarrete M, Hofmann F,
Salazar-Onfray F and Tittarelli A: Mind the Gaps in tumor immunity:
Impact of connexin-mediated intercellular connections. Front
Immunol. 8:10672017. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Graham SV, Jiang JX and Mesnil M:
Connexins and pannexins: Important players in tumorigenesis,
metastasis and potential therapeutics. Int J Mol Sci. 19:16452018.
View Article : Google Scholar :
|
|
59
|
Acuña RA, Varas-Godoy M, Herrera-Sepulveda
D and Retamal MA: Connexin46 expression enhances cancer stem cell
and Epithelial-to-Mesenchymal transition characteristics of human
breast cancer MCF-7 cells. Int J Mol Sci. 22:126042021. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Karpinich NO and Caron KM: Gap junction
coupling is required for tumor cell migration through lymphatic
endothelium. Arterioscler Thromb Vasc Biol. 35:1147–1155. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Fang JS, Coon BG, Gillis N, Chen Z, Qiu J,
Chittenden TW, Burt JM, Schwartz MA and Hirschi KK: Shear-induced
Notch-Cx37-p27 axis arrests endothelial cell cycle to enable
arterial specification. Nat Commun. 8:21492017. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Wang WK, Chen MC, Leong HF, Kuo YL, Kuo CY
and Lee CH: Connexin 43 suppresses tumor angiogenesis by
down-regulation of vascular endothelial growth factor via
hypoxic-induced factor-1α. Int J Mol Sci. 16:439–451. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Schulz R, Görge PM, Görbe A, Ferdinandy P,
Lampe PD and Leybaert L: Connexin 43 is an emerging therapeutic
target in ischemia/reperfusion injury, cardioprotection and
neuroprotection. Pharmacol Ther. 153:90–106. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Michela P, Velia V, Aldo P and Ada P: Role
of connexin 43 in cardiovascular diseases. Eur J Pharmacol.
768:71–76. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Hegner P, Lebek S, Tafelmeier M, Camboni
D, Schopka S, Schmid C, Maier LS, Arzt M and Wagner S:
Sleep-disordered breathing is independently associated with reduced
atrial connexin 43 expression. Heart Rhythm. 18:2187–2194. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Ugwu N, Atzmony L, Ellis KT, Panse G, Jain
D, Ko CJ, Nassiri N and Choate KA: Cutaneous and hepatic vascular
lesions due to a recurrent somatic GJA4 mutation reveal a pathway
for vascular malformation. HGG Adv. 2:1000282021.
|
|
67
|
Huang GY, Xie LJ, Linask KL, Zhang C, Zhao
XQ, Yang Y, Zhou GM, Wu YJ, Marquez-Rosado L, McElhinney DB, et al:
Evaluating the role of connexin43 in congenital heart disease:
Screening for mutations in patients with outflow tract anomalies
and the analysis of knock-in mouse models. J Cardiovasc Dis Res.
2:206–212. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Salameh A, Haunschild J, Bräuchle P, Peim
O, Seidel T, Reitmann M, Kostelka M, Bakhtiary F, Dhein S and
Dähnert I: On the role of the gap junction protein Cx43 (GJA1) in
human cardiac malformations with Fallot-pathology. a study on
paediatric cardiac specimen. PLoS One. 9:e953442014. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Milberg P, Klocke R, Frommeyer G, Quang
TH, Dieks K, Stypmann J, Osada N, Kuhlmann M, Fehr M, Milting H, et
al: G-CSF therapy reduces myocardial repolarization reserve in the
presence of increased arteriogenesis, angiogenesis and connexin 43
expression in an experimental model of pacing-induced heart
failure. Basic Res Cardiol. 106:995–1008. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Li L, Liu H, Xu C, Deng M, Song M, Yu X,
Xu S and Zhao X: VEGF promotes endothelial progenitor cell
differentiation and vascular repair through connexin 43. Stem Cell
Res Ther. 8:2372017. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Yu H, Kalogeris T and Korthuis RJ:
Reactive species-induced microvascular dysfunction in
ischemia/reperfusion. Free Radic Biol Med. 135:182–197. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Bellafiore M, Sivverini G, Palumbo D,
Macaluso F, Bianco A, Palma A and Farina F: Increased cx43 and
angiogenesis in exercised mouse hearts. Int J Sports Med.
28:749–755. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Grippo AJ, Moffitt JA, Henry MK, Firkins
R, Senkler J, McNeal N, Wardwell J, Scotti MA, Dotson A and Schultz
R: Altered Connexin 43 and Connexin 45 protein expression in the
heart as a function of social and environmental stress in the
prairie vole. Stress. 18:107–114. 2015. View Article : Google Scholar :
|
|
74
|
Vilà-González M, Kelaini S, Magee C,
Caines R, Campbell D, Eleftheriadou M, Cochrane A, Drehmer D,
Tsifaki M, O'Neill K, et al: Enhanced function of induced
pluripotent stem cell-derived endothelial cells through ESM1
signaling. Stem Cells. 37:226–239. 2019. View Article : Google Scholar
|
|
75
|
Su F, Zhao L, Zhang S, Wang J, Chen N,
Gong Q, Tang J, Wang H, Yao J, Wang Q, et al: Cardioprotection by
PI3K-mediated signaling is required for anti-arrhythmia and
myocardial repair in response to ischemic preconditioning in
infarcted pig hearts. Lab Invest. 95:860–871. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Taheri SA, Yeh J, Batt RE, Fang Y, Ashraf
H, Heffner R, Nemes B and Naughton J: Uterine myometrium as a cell
patch as an alternative graft for transplantation to infarcted
cardiac myocardium: A preliminary study. Int J Artif Organs.
31:62–67. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Wei X, Chang ACH, Chang H, Xu S, Xue Y,
Zhang Y, Lei M, Chang ACY and Zhang Q: Hypoglycemia-exacerbated
mitochondrial connexin 43 accumulation aggravates cardiac
dysfunction in diabetic cardiomyopathy. Front Cardiovasc Med.
9:8001852022. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Marsh SR, Williams ZJ, Pridham KJ and
Gourdie RG: Peptidic connexin43 therapeutics in cardiac reparative
medicine. J Cardiovasc Dev. 8:522021.
|
|
79
|
Shao Q, Esseltine JL, Huang T,
Novielli-Kuntz N, Ching JE, Sampson J and Laird DW: Connexin43 is
dispensable for early stage human mesenchymal stem cell adipogenic
differentiation but is protective against cell senescence.
Biomolecules. 9:4742019. View Article : Google Scholar :
|
|
80
|
Liu C, Fan Y, Zhou L, Zhu HY, Song YC, Hu
L, Wang Y and Li QP: Pretreatment of mesenchymal stem cells with
angiotensin II enhances paracrine effects, angiogenesis, gap
junction formation and therapeutic efficacy for myocardial
infarction. Int J Cardiol. 188:22–32. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Zhou Y, Zhang YX, Yang KL, Liu YL, Wu FH,
Gao YR and Liu W: Connexin 43 mediated the angiogenesis of buyang
huanwu decoction via vascular endothelial growth factor and
angiopoietin-1 after ischemic stroke. Chin J Physiol. 65:72–79.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Das H, George JC, Joseph M, Das M,
Abdulhameed N, Blitz A, Khan M, Sakthivel R, Mao HQ, Hoit BD, et
al: Stem cell therapy with overexpressed VEGF and PDGF genes
improves cardiac function in a rat infarct model. PLoS One.
4:e73252009. View Article : Google Scholar : PubMed/NCBI
|