Introduction
Distraction osteogenesis (DO) is a well-established
surgical procedure to regenerate segmental bone defects in clinical
practice (1,2), which consists of three sequential
phases: The latency phase after application of external fixation
and osteotomy, the distraction phase with the application of
regular tensile stress, and the consolidation phase for achieving
mineralized bone tissue (3).
Although a large number of studies have attempted to investigate
the underlying mechanisms of DO-induced bone regeneration from the
perspective of microRNAs (4,5),
exosomes (6,7) or various biological materials
(8), the limitations of slow
callus mineralization and lengthy treatment duration have not been
well resolved (9), and even
worse, the detailed molecular mechanism of DO in bone repair
remains poorly understood (10).
As is commonly known, bone is a highly vascularized
tissue (11) and a reduction in
vascular supply and limited nutrient availability at the injury
site causes impaired bone healing (12), which suggests that angiogenesis
is vital to the bone repair process. More importantly, growing
evidence (13,14) has demonstrated the close
spatial-temporal association and functional codependency that
exists between the processes of angiogenesis and osteogenesis
during bone repair, which is speculated to be the
angiogenic-osteogenic coupling (15). For DO, it is characterized by
robust angiogenesis in the distraction phase and subsequent
neo-osteogenesis in the later consolidation phase, which indicates
the presence of angiogenic-osteogenic coupling during the DO
process (16). Previously, a
specific vessel subtype called CD31hiEmcnhi,
with high levels of platelet and endothelial cell adhesion
molecule-1 and endomucin (Emcn), has been identified in the
skeletal system, which has been reported to actively direct bone
formation and couple angiogenesis with osteogenesis during bone
development and repair (17–19). Although
CD31hiEmcnhi vessels have been successively
found in models of osteoporosis (20), fractures (21) and bone defects (22), it still remains unclear whether
CD31hiEmcnhi vessels exist in the DO model
and whether regular tensile stress could stimulate
CD31hiEmcnhi vessel formation. Moreover,
until the present report, very few studies have been performed to
explore drugs that exert positive effects on
CD31hiEmcnhi vessel formation.
In China, it is well established that the use of
Traditional Chinese medicine, such as tonifying kidney herbs, can
exert beneficial effects on bone healing and be used as an
effective therapeutic strategy in promoting bone formation and
repair, when used in combination with other treatments (23). Total flavonoids of Rhizoma
Drynariae (TFRD), the key ingredient of the commonly known
kidney-tonifying Traditional Chinese medicine Rhizoma
Drynariae, has been demonstrated to have pharmacological
effects on the skeleton system. These actions include upregulating
the expression levels of bone morphogenetic protein 2 and
runt-related transcription factor 2 (RUNX2) (24), and promoting osteoblast
proliferation and differentiation (25). As reported previously, the active
compounds in TFRD are naringin and neoeriocitrin (26). At present, TFRD has been
developed into a Chinese patent medicine called Qianggu Capsule
(drug approval no. Z20030007, Beijing Qihuang Pharmaceutical Co.,
Ltd.). We previously reported that TFRD can accelerate bone
formation and remodeling in the distracted gap (27). Moreover, TFRD was also found to
have the potential to promote vascularization during DO (27). However, whether TFRD influences
CD31hiEmcnhi vessels and the underlying
mechanism during DO remains unknown.
Given that CD31hiEmcnhi
vessels play a critical role in coupling angiogenesis and
osteogenesis during bone development and repair, it was
hypothesized in the present study that
CD31hiEmcnhi vessels exist in the DO model
and TFRD treatment contributes to bone regeneration by stimulating
the formation of CD31hiEmcnhi vessels during
DO. The aim of the present study was to observe whether
CD31hiEmcnhi vessels exist in the DO model
and whether TFRD could promote CD31hiEmcnhi
vessel formation, and further investigate the potential mechanism
of CD31hiEmcnhi vessel-mediated bone repair
during DO.
Materials and methods
Identification for active compounds of
TFRD
The active compounds of TFRD were identified by
liquid chromatography-mass spectrometry (LC-MS) using high-
performance liquid chromatography (HPLC; TSQ Quantum™; Thermo
Fisher Scientific, Inc.) and MS (UltiMate™ 3000 RS; Thermo Fisher
Scientific, Inc.). Briefly, solution (10 µl) was subjected to HPLC
and analyzed with a UV detector (SPD-M20A; Shimadzu Corporation) at
283 nm. The mobile phase was composed of (A) methanol and (B) 0.1%
acetic acid water, with a gradient elution as follows: 0~14 min,
30-35% A; 14~22 min, 35-50% A; 22~26 min, 50-35% A; 26~35 min, and
35-35% A. The flow rate of the mobile phase was 1.0 ml/min. The
chromatographic column was a C18 (4.6×250 mm, ID, 5 µm; Merck
KGaA). The MS identification conditions were as follows: The ion
source was ESI, the atomization pressure was 35 psi, the dry gas
flow rate was 11 ml/min, the mass range of mass spectrometry was
100~1,000 amu, and the ion mode was negative. The reference
solutions were prepared directly in methanol: naringin (CAS no.
10236-47-2; batch, 23,616; Aladdin Bio-Tech Group) and
neoeriocitrin (CAS no. 13241-32-2; batch, ASB-00005190-010;
ChromaDex Standards). Working standard solutions containing the two
compounds were prepared and diluted with methanol to the
appropriate concentrations for the establishment of calibration
curves. The TFRD samples were also dissolved in methanol and
prepared as working solutions. The reference solutions and working
solutions were all prepared in dark brown calibrated flasks and
stored at 4°C. The chromatogram collection and integration of
naringin were processed by Xcalibur™ 3.0 software (Thermo Fisher
Scientific, Inc.). The peak area of naringin or neoeriocitrin was
taken as the ordinate and the concentration of naringin or
neoeriocitrin as the abscissa. The standard curve line was obtained
by linear regression analysis with Equal as the weighted
coefficient.
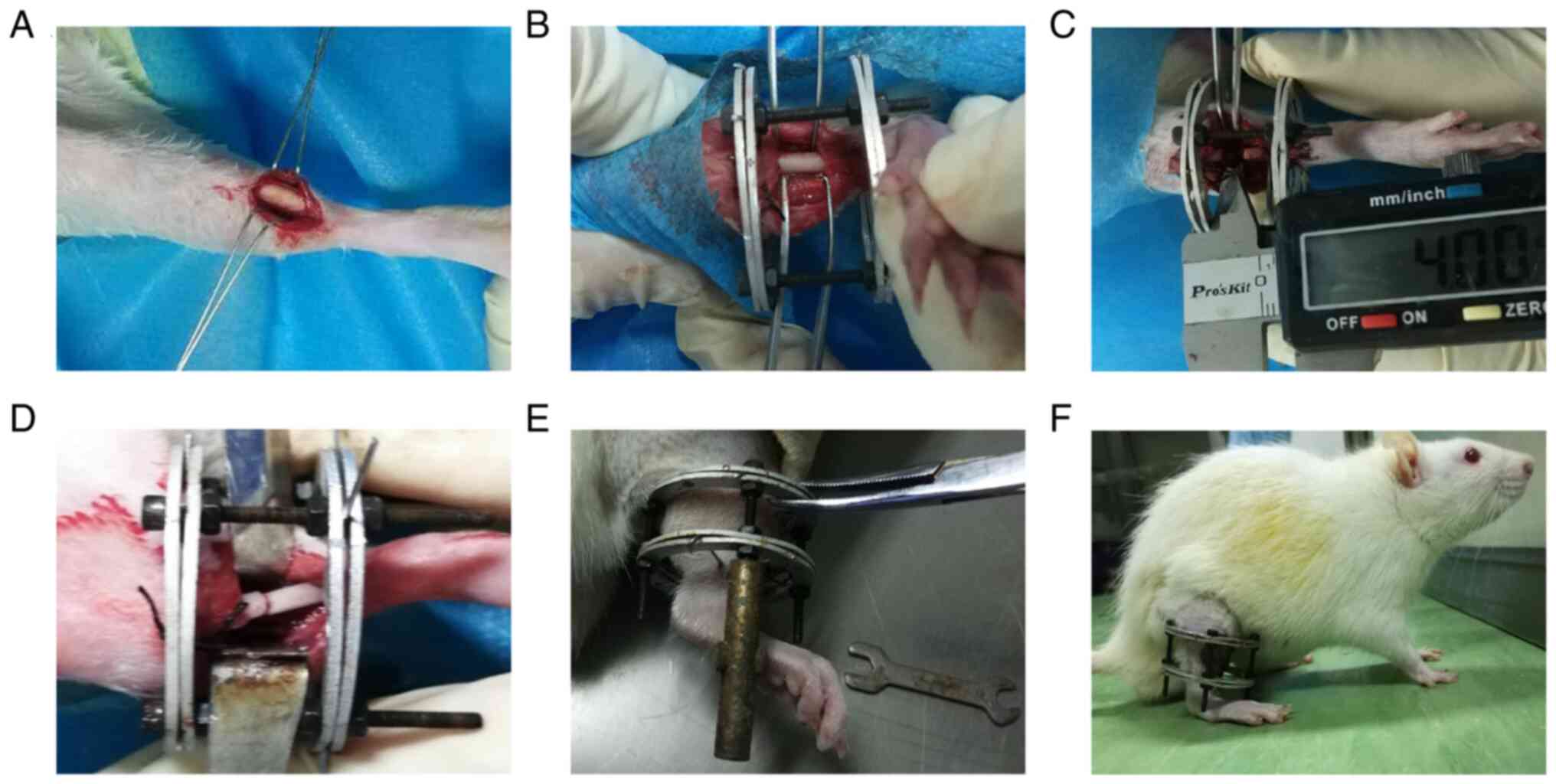
Animal surgical procedure and
experimental design
A total of 60 adult male Sprague-Dawley (SD) rats
(SIPPR-BK Experimental Animal, Ltd.) age, 20 weeks; weight, 380-420
g) were kept in specific pathogen-free housing in the laboratory
with standard conditions at 24°C in 50-70% humidity under 12/12 h
light/dark cycle. SD rats were subjected to the tibial DO model and
then randomly divided into four groups (n=15 per group): i) TFRD
group; ii) TFRD+platelet-derived growth factor
(PDGF)-BB-neutralizing antibody (Ab) group; iii) PDGF-BB-Ab group;
and iv) control group. The DO model was established as described
previously (28). In brief, as
shown in Fig. 1, after rats were
anesthetized with an intraperitoneal injection of pentobarbital (3
mg/100 g; Sigma-Aldrich; Merck KGaA), a longitudinal incision was
made in the skin distal to the right tibia crest and the bone was
exposed. Meanwhile, surgical scissors were used to cut the fibula.
Then, a custom-made circle external device was assembled and fixed
to the tibia by four 27-gauge stainless steel needles (Zhangjiagang
Baokang Medical Equipment Co., Ltd.). After stabilization,
transverse corticotomies using a Gigli saw (Zhangjiagang Baokang
Medical Equipment Co., Ltd.) were performed to create a 4-mm long
diaphyseal defect on the tibia. Thereafter, the two osteotomy
surfaces were in close contact and aligned. Finally, the wound was
irrigated with sterile saline and then closed layer by layer.
Following surgery, rats were given access to food and water ad
libitum. Amoxicillin (1.5 mg/100 g weight) and buprenorphine
(1.0 mg/kg weight) were administered intraperitoneally for 3 days
after surgery. After a 7-day latency period, the distraction
procedure was initiated at a rate of 0.1 mm/12 h until the length
of the shortened tibia induced by osteotomy was restored. Then, the
consolidation stage lasted for 4 weeks. According to a previous
study (29), rats were
administered with TFRD orally at a dose of 75 mg/kg body
weight/day, and then rats in the TFRD group were injected
intraperitoneally with an equal amount of PBS, whereas rats in the
PDGF-BB-Ab group were injected intraperitoneally with 20 g/ml
PDGF-BB-Ab (Abcam) and orally fed with an equal amount of PBS. The
TFRD+PDGF-BB-Ab group received an intraperitoneal injection of 20
g/ml PDGF-BB-Ab combined with an oral administration of TFRD at a
dose of 75 mg/kg body weight/day. The control group was subjected
to intraperitoneal and oral administration of equal amounts of PBS.
From day 1 post-surgery, TFRD was administrated once a day and
PDGF-BB-Ab was injected once a week until termination of the study.
In the present study, the rats were intraperitoneally injected with
pentobarbital (10 mg/100 g) for euthanasia. In addition, complete
cardiac and respiratory arrest, and pupil dilation were observed to
indicate that the rat had been successfully euthanized. All animal
care and experimental procedures were approved by the Institutional
Animal Ethics Committee of the First Affiliated Hospital of
Guangzhou University of Traditional Chinese Medicine (approval no.
TCMF1- 2018002; Guangzhou, China).
Micro-CT analysis
According to a previous study (30), the distracted tibia specimens
(n=3 per group) were harvested and scanned with micro-CT (SkyScan
1076; Bruker Corporation) at a resolution of 20 µm (70 kV and 130
µA radiation source with 0.5 mm aluminum filter), after
consolidation for 4 weeks. Bone tissue volume/total tissue volume
(BV/TV) inside the distracted gaps were analyzed using CTAN
software (v1.9; Bruker Corporation). The test was repeated with
three specimens.
Angiography analysis
For angiography analysis, three rats per group were
anesthetized and perfused with Microfil (MICROFIL®
MV-122; Flow Tech, Inc.) after consolidation for 4 weeks, as
described previously (7).
Briefly, the rib cage was opened after anesthetization, the
descending aorta was clamped, and the inferior vena cava was
incised. Subsequently, the vasculature was flushed with 0.9% normal
saline containing heparin sodium (100 U/ml) and 20 ml Microfil were
perfused into the left ventricle with an angiocatheter.
Subsequently, the rats were stored at 4°C overnight to ensure
polymerization of the contrast agent, after which the tibias were
dissected, fixed in 4% paraformaldehyde for 48 h at room
temperature, decalcified in 10% EDTA for 4 weeks and then imaged by
micro-CT.
Histological and immunofluorescent
analyses
At 4 weeks post-distraction, tibia specimens (n=3
per group) were taken, decalcified in 10% EDTA for 4 weeks,
dehydrated through increasing concentrations of ethanol, and then
embedded in paraffin. Thereafter, the specimens were cut into 5 µm
thick longitudinally oriented sections, and immunostaining blocking
buffer (cat. no. P0102; Beyotime Institute of Biotechnology) was
used to block sections overnight at 4°C. Some sections were
processed for hematoxylin and eosin (H&E, stained at room
temperature for 5 and 2 min, respectively), Masson's trichrome
(Weigert staining for 5 min, Masson blue for 5 min and Ponceau S
staining for 7 min at room temperature) and Safranin O staining
(Fast Green staining for 7 min and Safranin O for 20 sec at room
temperature). Other sections were used for immunofluorescent
staining. For determination of CD31hiEmcnhi
vessels, some sections were subjected to double immunofluorescence
staining for CD31 (1:50; cat. no. sc-71873; Santa Cruz
Biotechnology, Inc.) and Emcn (1:50; cat. no. sc-65495; Santa Cruz
Biotechnology, Inc.). Briefly, bone sections were stained with
individual primary antibodies overnight at 4°C, followed by
staining with secondary antibodies (1:50; cat. no. A0208, Beyotime
Institute of Biotechnology) conjugated with fluorescence at room
temperature for 1 h. Nuclei were stained with DAPI (0.5 µg/ml, 5
min at room temperature). Meanwhile, some sections were incubated
with anti-RUNX2 (1:100; cat. no. ab76956; Abcam), anti-Osterix
(OSX; 1:100; cat. no. ab22552; Abcam), anti-CD31 (1:100; cat. no.
sc-71873; Santa Cruz Biotechnology, Inc.) and anti-PDGF-BB (1;100;
cat. no. ab21234; Abcam) primary antibodies at 4°C for 24 h, and
then stained with secondary antibodies (1:50; cat. no. A0208;
Beyotime Institute of Biotechnology) conjugated with fluorescence
at room temperature for 1 h. Images were acquired with a Leica
DMI6000B fluorescence microscope (Leica Microsystems GmbH). The
number of positively stained cells or area in five random fields of
the distracted zone in three random sections from each specimen
were analyzed and all tests were repeated with three specimens.
Western blotting
Three rats in each group were randomly selected for
western blotting. In brief, after bone samples were lysed using
RIPA lysis and Extraction buffer (Nanjing KeyGen Biotech Co.,
Ltd.), a BCA protein assay kit (cat. no. ab102536; Abcam) was used
to measure the protein concentrations, according to the
manufacturer's instructions (Varioskan Flash; Thermo Fisher
Scientific, Inc.). Each protein sample (40 g) was subjected to
SDS-PAGE on a 10% gel, and then electroblotted onto a
polyvinylidene difluoride membrane (0.45 mm; EMD Millipore).
Subsequently, the membranes were blocked with 5% (w/v) non-fat dry
milk in TBS with 0.05% Tween-20 (Beyotime Institute of
Biotechnology) at room temperature for 45 min, followed by
incubation with primary anti-PDGF-BB (1:500; cat. no. ab21234;
Abcam), anti-VEGF (1:500; cat. no. sc-7269; Santa Cruz
Biotechnology, Inc.), anti-RUNX2 (1:1,000; cat. no. ab23981;
Abcam), anti-OSX (1:1,000; cat. no. ab22552; Abcam),
anti-phosphorylated (p)-AKT (1:1,000; cat. no. ab81283; Abcam),
p-PDGFR-β (1:2,000; cat. no. AF3132; Affinity Biosciences), PDGFR-β
(1:2,000; cat. no. AF6133; Affinity Biosciences), AKT (1:1,000;
cat. no. 4691; Cell Signaling Technology, Inc.), ERK (1:2,000; cat.
no. AF0155; Affinity Biosciences) and anti-p-ERK1/2 (1:1,000; cat.
no. ab214362; Abcam) antibodies at 4°C overnight. Then, the
membranes were incubated with the horseradish peroxidase-conjugated
secondary antibodies (1:5,000; cat. no. ab205718; Abcam) for 1 h at
room temperature. BeyoECL Plus reagent (cat. no. P0018S; Beyotime
Institute of Biotechnology) was used for visualization. The protein
levels were normalized against β-actin (1:1,000; cat. no. 4970;
Cell Signaling Technology, Inc.). Representative bands were
selected from the three independent experiments. Densitometric
analysis was performed using ImageJ software (v1.8.0.112; National
Institutes of Health).
Reverse transcription-quantitative
(RT-q)PCR
The newly-formed tissues inside the distracted gaps
of three rats per group were carefully and quickly collected at 4
weeks post-distraction. All procedures were performed at 4°C. The
total cellular RNA of the tissues was extracted using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.), according to the manufacturer's instructions. RNA samples
collected from the distracted tibias were examined by measuring the
ratio of the optical density at 260 and 280 nm (OD 260/280).
Samples with a ratio of 2.0 were used for reverse transcription.
cDNA was synthesized using 1 µg RNA and a RevertAid First Strand
cDNA Synthesis kit (Takara Biotechnology Co., Ltd.). RT-qPCR was
performed in triplicate using a PrimeScript™ RT reagent kit with
gDNA Eraser (cat. no. RR047A; Takara Biotechnology Co., Ltd.)
followed by a TB Green® Premix EX Taq™ II kit (cat. no.
RR820A; Takara Biotechnology Co., Ltd.). The thermocycling
conditions were as follows: Pre-denaturation at 95°C for 30 sec,
followed by 45 cycles of 95°C for 10 sec, 57°C, 58°C or 60°C for 15
sec, and 72°C for 10 sec. Cycle quantification values for the
samples were normalized to that of β-actin and the relative
expression was calculated using the 2−ΔΔCq method
(31). The primer sequences used
are listed in Table SI.
Cell culture
Endothelial precursor cells (EPCs) and bone
marrow-derived mesenchymal stem cells (BMSCs) were cultured
following previous techniques (32), with minor modifications. Briefly,
the bone marrow cavities of femurs and tibias were washed at 4°C in
0.01 M PBS. Mononuclear cells were collected from marrow suspension
and then cultured with EGM-2 MV medium (Lonza Group, Ltd.) for EPC
culture, or cultured in Mesenchymal Stem Cell Growth Medium (Lonza
Group, Ltd.) for BMSC culture. After 24 h of culture, the attached
cells were plated into a 50 ml glass flask coated with fibronectin
(Sigma-Aldrich; Merck KGaA) at a density of 1.0×106 per
liter and the medium was changed every 3 days. Cells at passage 3
were used for subsequent experiments.
Establishment of stress
conditions
As described previously (33), the stress conditions were
established using the STREX cell stretching system (ST-160; Amuza,
Inc.) to apply regular tensile stress to cells in a single,
parallel direction at an approximate ratio of 10%.
Cell migration assay
The migratory ability of EPCs was evaluated via a
Transwell migration assay (Costar; Corning, Inc.). Third passage
EPCs at a density of 1×104/well were loaded into the
upper chamber of 24-well, 8-µm pore-size Transwell plates (Corning,
Inc.) with a polycarbonate membrane that contained serum-free
endothelial growth medium(Lonza Group, Ltd.). Subsequently, medium
containing TFRD (100 µg/ml) or PDGF-BB-Ab (20 µg/ml) diluted with
low-glucose DMEM (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS was added to the lower chambers. After
co-incubation for 24 h at 37°C under 5% CO2,
non-migrated cells that remained in the upper chambers were removed
by wiping the top of the insert membranes with cotton swabs, while
the migrated cells that passed through the membrane pores were
stained with 0.5% crystal violet for 20 min at 37°C and counted
under an optical microscope (Leica Microsystems GmbH). Five random
fields of view were used to count the number of migrated cells,
this assay was performed in triplicate.
Alkaline phosphatase (ALP) staining
and activity assay
After 7 days of osteogenic induction, ALP staining
was performed to assess the osteogenic differentiation potential of
BMSCs. Briefly, BMSCs were fixed for 20 min at 37°C in 4%
paraformaldehyde, washed three times with distilled water, and
subsequently stained with the Alkaline Phosphatase Assay kit
(Beyotime Institute of Biotechnology) at 37°C for 30 min. For ALP
activity, BMSCs were lysed with lysis buffer (20 mM pH 7.5 Tris
HCl, 150 mM NaCl and 1% Triton X-100) in 96-well plates, and the
substrates and p-nitrophenol were added. ALP activity was analyzed
by determining the absorbance at 405/650 nm.
Von Kossa staining
After 21 days of osteogenic induction, calcium
mineral deposition was measured through Von Kossa staining.
Briefly, BMSCs were fixed as described above and washed for Von
Kossa staining. Following incubation in 5% silver nitrate for 10
min at 37°C, BMSCs were exposed to light for 30 min and washed in
5% sodium thiosulphate for 5 min to remove non-specific staining.
Cells were observed under an inverted microscope (Leica
Microsystems GmbH).
Matrigel tube formation assay
Matrigel Growth Factor Reduced Basement Membrane
Matrix (cat. no. 356230; Corning, Inc.) was used to assess the tube
formation of EPCs, which was thawed on ice. Then, each well was
coated with 100 µl Matrigel at 37°C for 30 min, according to the
manufacturer's instructions. EPCs (6×104 cells per well)
were seeded onto Matrigel-coated 24-well plates and cultured with
or without TFRD (100 µg/ml) at 37°C and 5% CO2 for 6 h,
some cells were pretreated with PDGF-BB-Ab (20 µg/ml) for 1 h
before TFRD treatment. After incubation for 6 h, cells were
observed with an inverted microscope (Leica Microsystems GmbH).
Total tube length in five randomly chosen fields were quantified
using ImageJ v1.8.0 software (National Institutes of Health).
Statistical analysis
All data are presented as the mean ± standard
deviation. SPSS 19.0 software (IBM Corp.) was used to analyze
experimental data. Differences among groups were assessed by
one-way analysis of variance (ANOVA), followed by a Tukey's post
hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
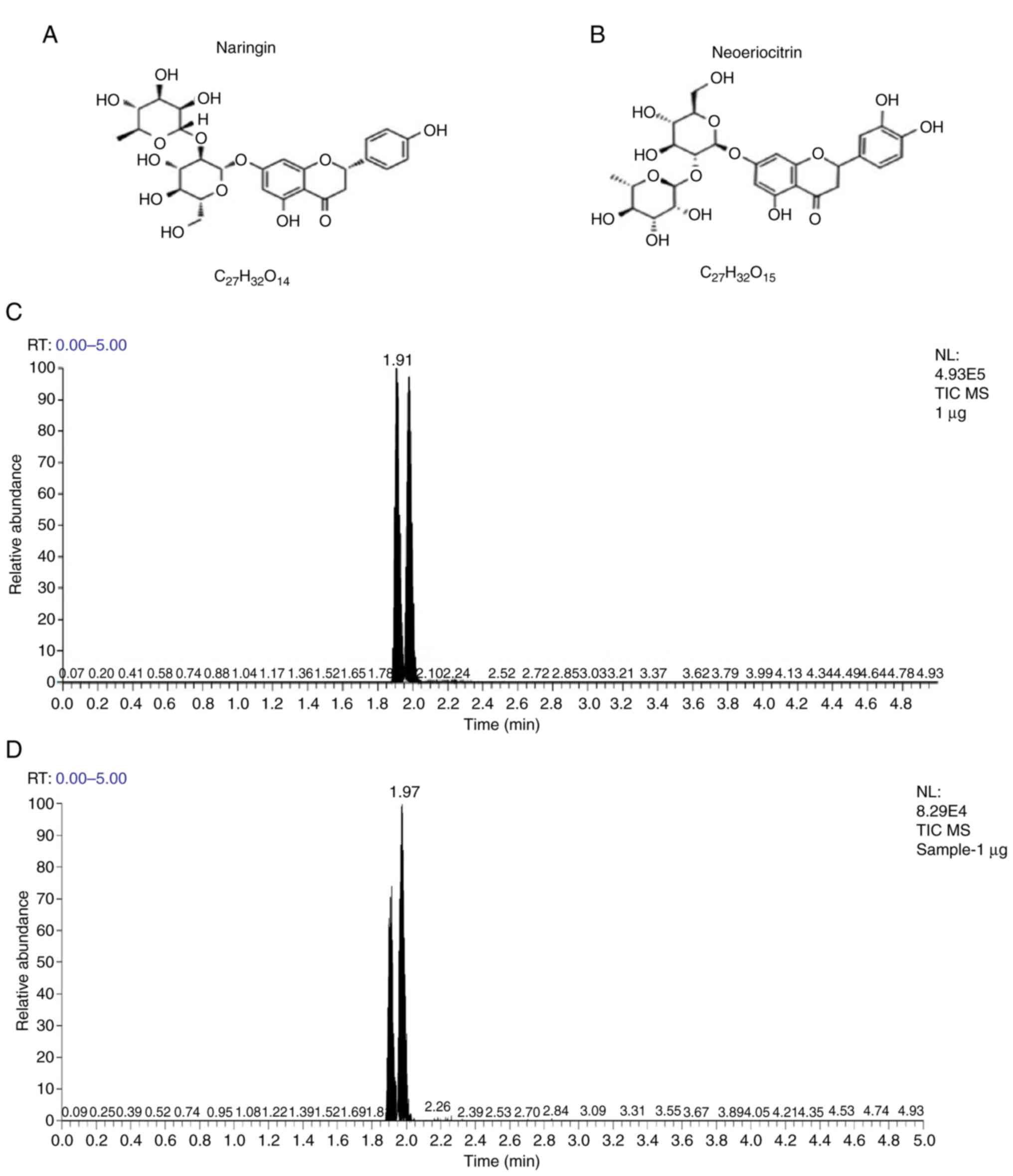
Active compounds of TFRD
The LC-MS results demonstrated that the active
ingredients of TFRD included naringin and neoeriocitrin. The
chemical structures and formulas of naringin and neoeriocitrin are
presented in Fig. 2A and B.
Based on the results in Fig. 2C and
D, the peak at the time point of ‘1.91’ and ‘1.97’ in the total
ion chromatogram of TFRD sample refers to neoeriocitrin and
naringin, respectively. As shown in the standard curve lines of
Figs. S1A-C and S2A-C, the concentrations of naringin
and neoeriocitrin in 1 µg TFRD sample were 173.082 and 131.833
ng/ml, respectively.
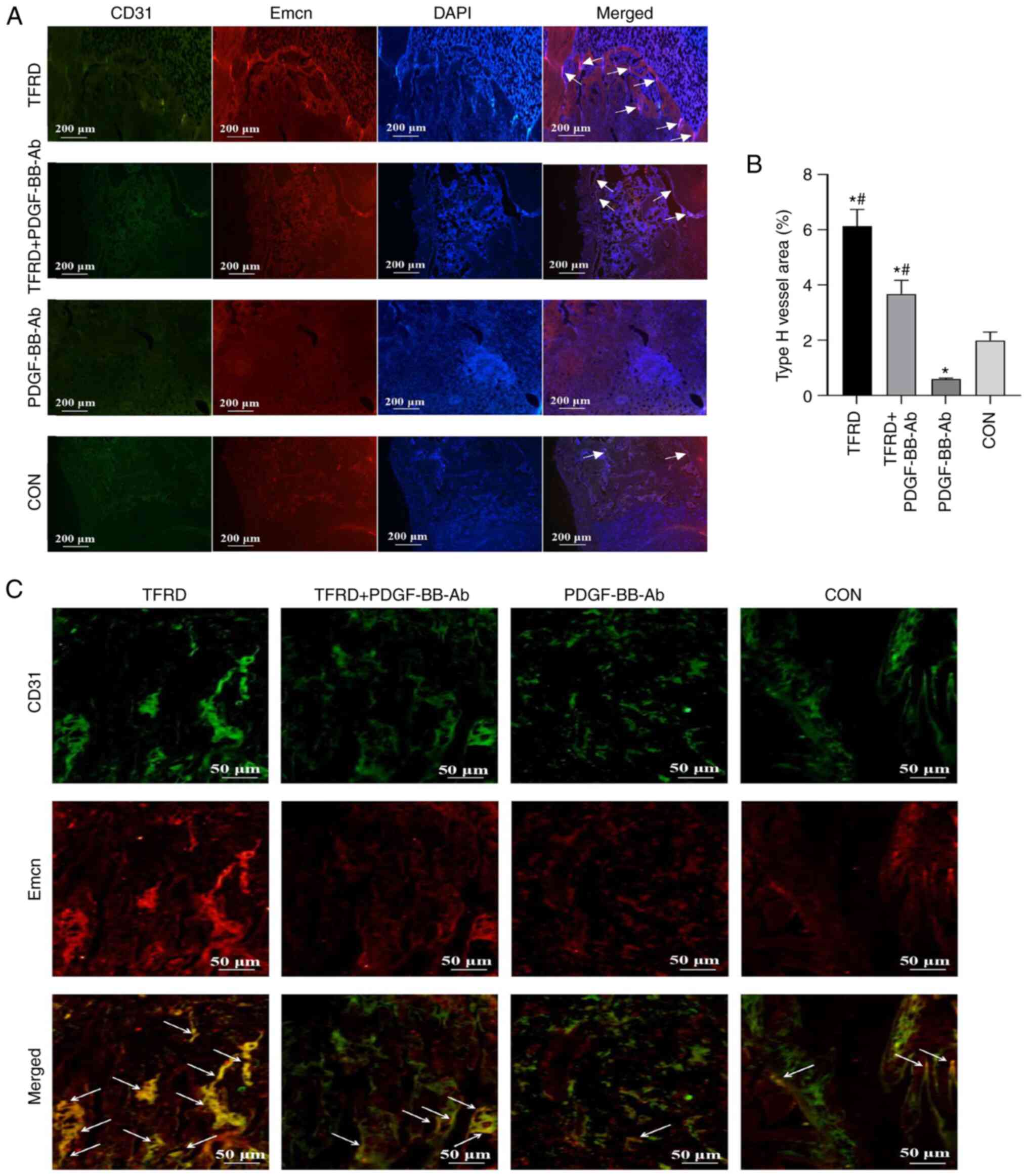
Administration of TFRD increases the
abundance of CD31hiEmcnhi vessels during DO
in rats
As shown in Fig.
3A-C and Table SII, the
CD31 and Emcn immunofluorescent staining results demonstrated the
presence of CD31hiEmcnhi vessels during the
process of DO and a higher proportion of
CD31hiEmcnhi vessels were observed in the
TFRD group compared with the control group at 4 weeks
post-distraction. Compared with the control group, a significant
decline in the abundance of CD31hiEmcnhi
vessels was observed in the PDGF-BB-Ab group. Of note, this decline
was significantly attenuated by the addition of TFRD, as
demonstrated by the significantly higher abundance of
CD31hiEmcnhi vessels observed in the
TFRD+PDGF-BB-Ab group. Thus, these results indicated that TFRD can
promote CD31hiEmcnhi vessel generation during
DO, whereas PDGF-BB-Ab suppresses the formation of
CD31hiEmcnhi vessels.
TFRD facilitates angiogenesis and
osteogenesis during DO in rats
As presented in Fig.
4A and B and Table SIII,
TFRD treatment resulted in a significant increase in angiogenesis
compared with the control and PDGF-BB-Ab groups. Similar to the
manifestation of angiogenesis, the micro-CT images indicated
increased newly-formed callus within the distracted gap in the TFRD
group than in the other three groups. For the TFRD group, complete
bony union inside the distracted gap was achieved, while partial
bony union or no obvious radiographical defect bridging occurred in
the other three groups (Fig.
4C). The BV/TV of the TFRD group was significantly higher
compared with the control and PDGF-BB-Ab groups (Fig. 4D and Table SIII). Meanwhile, histological
analyses further confirmed the aforementioned findings. The TFRD
group showed complete defect healing and had the most improved bone
connection and integration, with both newly-formed bone tissue
bridging the defects and bone marrow filling the gap, while
moderate immature new bone formation with osteoid matrix appeared
inside the distracted gaps in the TFRD+PDGF-BB-Ab group and only
chondroid matrix accompanied with fibrous connective tissues filled
the gaps in the PDGF-BB-Ab and control groups (Fig. 4E). Therefore, combined with the
results in Fig. 3, groups with
higher abundance of CD31hiEmcnhi vessels
showed increased bone and vessel formation in the distracted gaps,
whereas groups with lower abundance of
CD31hiEmcnhi vessels showed a decreased
amount of bone and vessel formation.
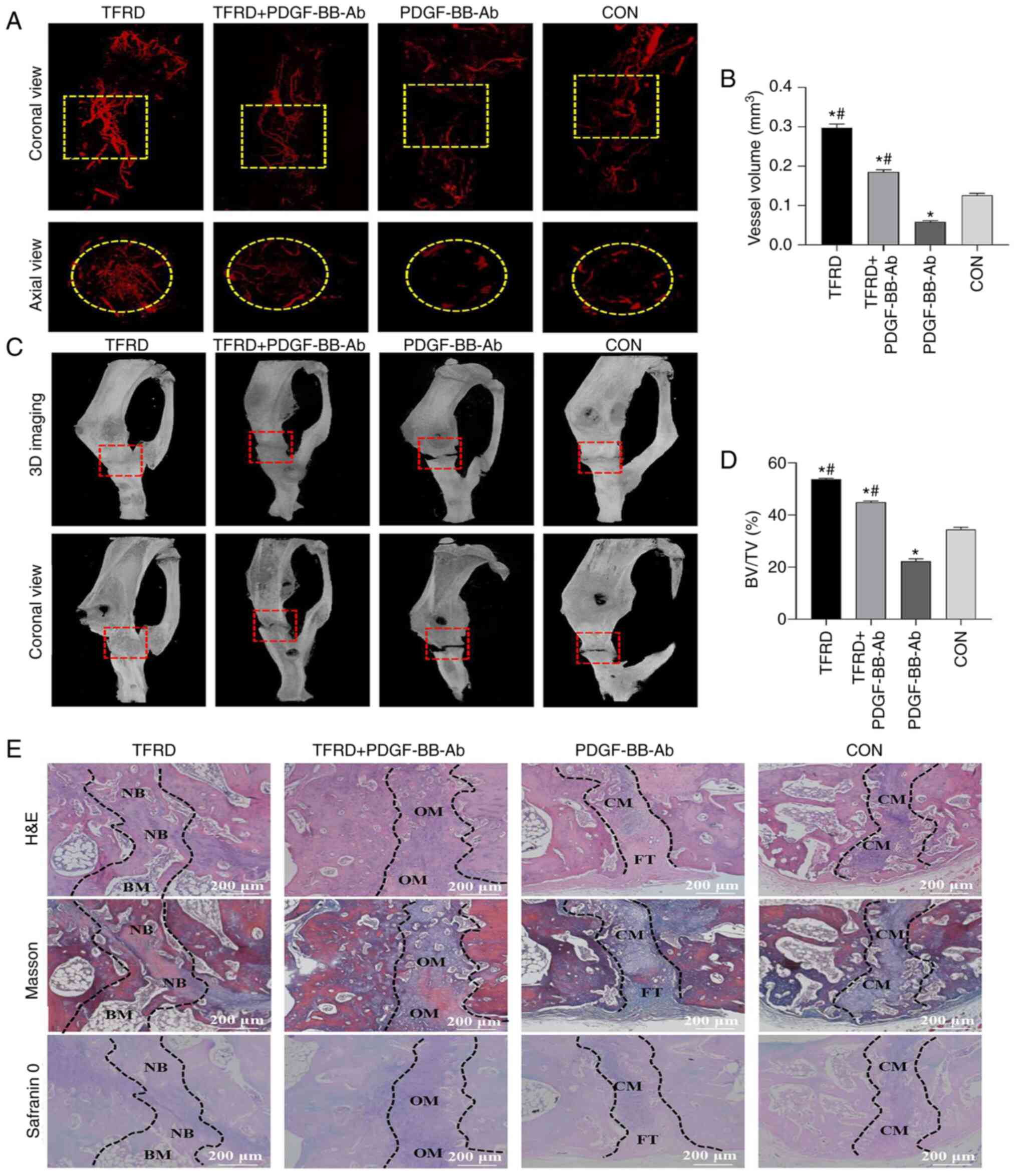 | Figure 4.TFRD facilitates angiogenesis and
osteogenesis the distraction osteogenesis process in rats. (A)
Representative angiographic images and (B) quantification of the
vessel volume inside the distracted gaps of four groups at 4 weeks
post-distraction in both coronal (top panel) and axial (lower
panel) views. Yellow dotted boxes indicate the region of interest.
(C) Representative micro-CT images and (D) quantification of bone
regeneration within the distracted gaps at 4 weeks
post-distraction. Red dotted boxes indicate the region of interest,
representing the distracted gaps. (E) Representative histological
images including H&E, Masson's trichrome and Safranin O
staining of the newly-formed tissues within the distracted gaps 4
weeks post-distraction. Scale bar, 200 µm. The regions between the
two black dotted lines represent the distracted zones. Data
represent the mean ± SD. *P<0.05 vs. control group;
#P<0.05 vs. PDGF-BB-Ab group. TFRD, total flavonoids
of Rhizoma drynariae; PDGF, platelet-derived growth factor;
BV/TV, bone tissue volume/total tissue volume; NB, newly-formed
bone; BM, bone marrow; OM, osteoid matrix; CM, chondroid matrix;
FT, fibrous tissue; H&E, hematoxylin and eosin; 3D,
three-dimensional. |
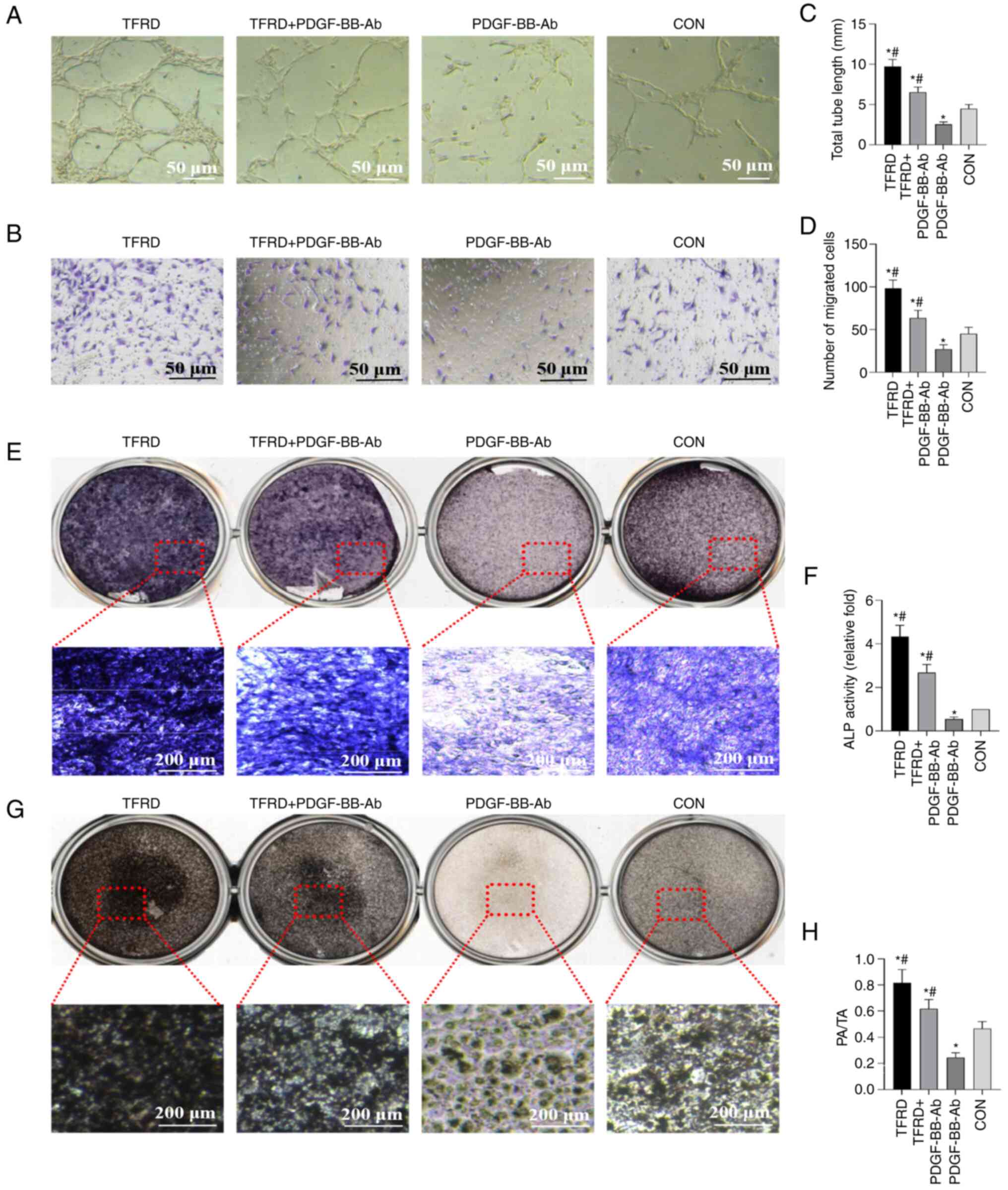
TFRD enhances the angiogenic capacity
of EPCs and the osteogenic capacity of BMSCs under stress
conditions
As revealed by the tube formation assay, the total
tube length was significantly higher following TFRD treatment,
compared with the control and PDGF-BB-Ab groups (Fig. 5A and C and Table SIV). In addition, the cell
migration assay suggested that TFRD significantly enhanced the
number of migrated cells compared with the control and PDGF-BB-Ab
groups under stress conditions (Fig.
5B and D and Table SIV).
Meanwhile, the in vitro ALP (Fig. 5E) and Von Kossa staining
(Fig. 5G) assays showed
increased staining and calcium deposits in the TFRD-treated groups
compared with the control and PDGF-BB-Ab groups under stress
conditions. Furthermore, quantitative results of ALP activity and
Von Kossa staining demonstrated that TFRD treatment significantly
increased ALP activity and calcium mineral deposition compared with
the control and PDGF-BB-Ab groups (Fig. 5F and H and Table SV). However, the aforementioned
effects were blocked by PDGF-BB-Ab. Thus, these results indicated
that TFRD significantly elevated the osteogenic capacity of BMSCs
and angiogenic capacity of EPCs under stress conditions, whereas
PDGF-BB-Ab weakened these effects.
TFRD promotes
CD31hiEmcnhi vessel formation in
angiogenic-osteogenic coupling during DO via the
PDGF-BB/platelet-derived growth factor receptor (PDGFR)-β
pathway
As shown in Fig.
3, the group treated with PDGF-BB-Ab showed the lowest
abundance of CD31hiEmcnhi vessels. More
importantly, upon downregulation of
CD31hiEmcnhi vessel expression, both
osteogenesis and angiogenesis inside the distracted gaps during DO
were suppressed, as revealed by micro-CT, angiography and
histological analyses (Fig. 4).
Furthermore, the immunofluorescent assays further verified the
radiographical and histological analyses, which demonstrated that
the expression levels of osteogenesis-related markers, including
RUNX2 and OSX, as well as the expression levels of
angiogenesis-related markers, including CD31 and PDGF-BB, were
significantly lower in the PDGF-BB-Ab group compared with the
control group, which was significantly reversed by TFRD treatment
(Fig. 6A and B and Table SVI). Additionally, the
angiogenic capacity of EPCs and osteogenic capacity of BMSCs under
stress conditions were also weakened in the PDGF-BB-Ab group
(Fig. 5). Once PDGF-BB-Ab was
administrated to rats during DO or added to the culture media under
stress conditions, the TFRD-induced positive effects on
osteogenesis and osteogenesis were also reduced. As presented in
Fig. 6C-E and Tables SVII and SVIII, the results of western blotting
and RT-qPCR analyses further verified the role of PDGF-BB in this
process, which demonstrated that TFRD significantly upregulated the
expression levels of p-PDGF-BB, VEGF, RUNX2 and OSX at the protein
and mRNA levels during DO, however, this effect was blocked by
PDGF-BB-Ab. It is of note that TFRD also contributed to the
increase of p-AKT and p-ERK1/2, which are the primary downstream
mediators of the well-known PDGF-BB pathway. Overall, the present
results demonstrated that the PDGF-BB/PDGFR-β pathway mediated the
formation of CD31hiEmcnhi vessels and TFRD
could attenuate the reduction in CD31hiEmcnhi
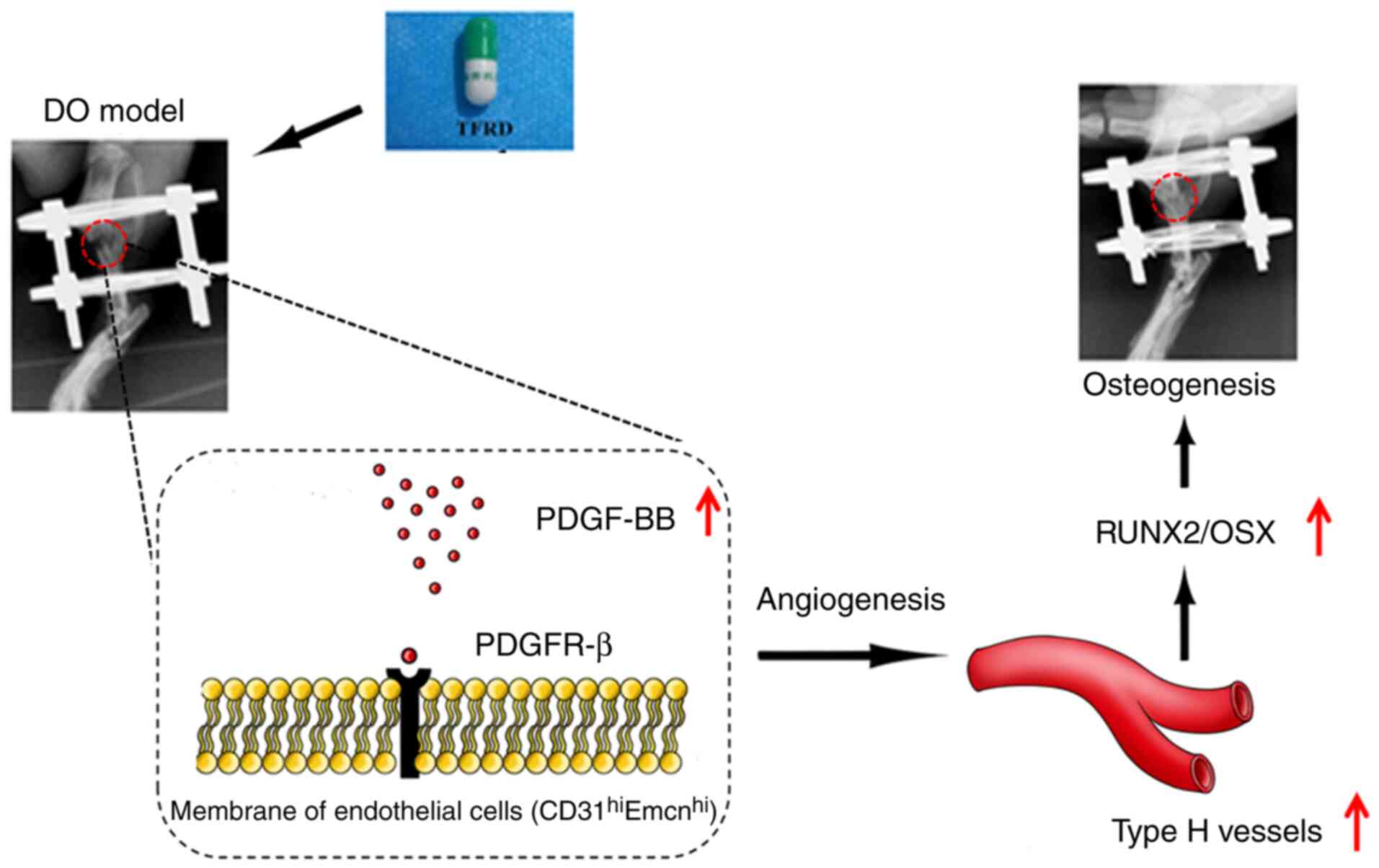
vessel generation caused by blocking PDGF-BB (Fig. 7).
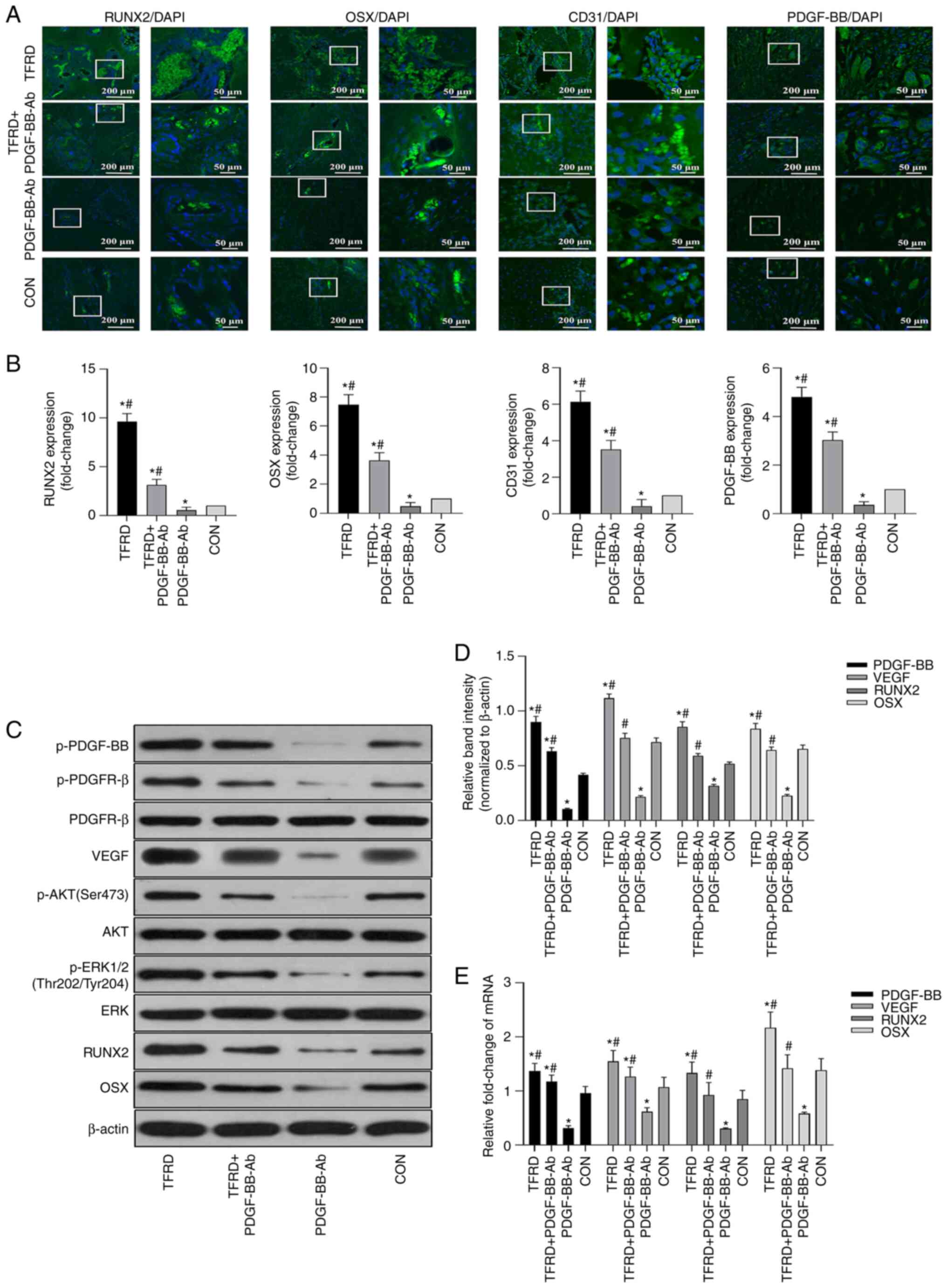 | Figure 6.TFRD promotes
CD31hiEmcnhi vessel formation in
angiogenic-osteogenic coupling during distraction osteogenesis via
the PDGF-BB/PDGFR-β pathway. (A) Representative immunofluorescence
images and (B) quantification of RUNX2, OSX, CD31 and PDGF-BB in
the distracted tibias after distraction for 4 weeks. (C)
Representative western blotting images and (D) semi-quantitative
analyses of PDGF-BB, VEGF, RUNX2, OSX as well as the
phosphorylation of AKT and ERK1/2 in the distracted tibias at 4
weeks post-distraction. (E) Quantification of mRNA expression
levels of PDGF-BB, VEGF, RUNX2 and OSX. Data represent the mean ±
SD. n=3 rats in each group from three independent experiments.
*P<0.05 vs. control group; #P<0.05 vs. PDGF-BB-Ab
group. TFRD, total flavonoids of Rhizoma drynariae; PDGF,
platelet-derived growth factor; Emcn, endomucin; PDGFR-β,
platelet-derived growth factor receptor-β; RUNX2, runt-related
transcription factor 2; OSX, Osterix; p-, phosphorylated. |
Discussion
As an endogenous bone tissue engineering technique
(34), DO has applications in
the regeneration and reconstruction of bone, however it still shows
unsatisfactory osteogenesis effects and unclear underlying
mechanisms, thus there is an urgent need to address these issues
(9). Previous studies have
primarily explored mechanical stimulation and bone-related growth
factors, thus to the best of our knowledge, the present study is
the first to investigate the regulatory functions of
CD31hiEmcnhi vessels and the role of
angiogenic-osteogenic coupling in DO.
The importance of angiogenesis in bone homeostasis
and repair has been established. Bone repair requires new blood
vessel formation and endothelial cell-derived molecular signals
(35). It is hypothesized that
the osteogenesis-promoting effect of the bone vasculature in
physiological settings is attributed to
CD31hiEmcnhi vessels (17,18) that can enhance
angiogenic-osteogenic coupling for bone repair. The abundance of
CD31hiEmcnhi vessels has been suggested as a
possible sensitive biomarker of bone mass and may represent a
potential target for promoting bone repair (36). In the present study,
CD31hiEmcnhi vessels were first identified by
co–immunofluorescence staining of CD31 and Emcn in the rat DO
model, it was also found that TFRD promoted
CD31hiEmcnhi vessel generation via the
upregulation of PDGF-BB expression, which further enhanced bone and
vessel formation during DO. As evidenced by micro-CT, angiographic
and histological arrays in the present study, the TFRD-treated
group with a high abundance of CD31hiEmcnhi
vessels were observed to have increased bone and vessel formation
in the distracted gaps, which was further confirmed by the
upregulation of RUNX2, OSX, CD31 and PDGF-BB in the
immunofluorescent arrays. Furthermore, the in vitro ALP and
Von Kossa staining assays, as well as the tube formation assay,
were performed to verify the pro-osteogenic and angiogenic effects
of TFRD on BMSCs and EPCs in the mechanical environment. It was
demonstrated that TFRD significantly elevated the osteogenic
capacity of BMSCs and the angiogenic capacity of EPCs under stress
conditions. By contrast, the PDGF-BB-Ab group, which showed a low
abundance of CD31hiEmcnhi vessels, also
demonstrated less bone and vessel formation in the distracted gaps,
and the osteogenic and angiogenic potentials of BMSCs and EPCs were
suppressed in this group,. These results indicated that PDGF-BB
mediated the formation of CD31hiEmcnhi
vessels, which was consistent with previous studies (20,37).
Of note, although several studies have reported that
PDGF-BB secreted by preosteoclasts can induce angiogenic–osteogenic
coupling by increasing the abundance of
CD31hiEmcnhi vessels in bone (20,37), the underlying mechanism of the
induction of CD31hiEmcnhi vessels in the DO
model may be different. As reported previously, EPCs, not
preosteoclasts, were found to be closely associated with the DO
process (7). Aside from
influencing the migration and osteogenic differentiation of BMSCs
(38), mechanical stimulation
could mobilize EPCs from the bone marrow into the peripheral blood
and then promote the homing of EPCs to the distracted gaps during
the distraction stage and remain in the consolidation stage
(39). EPCs that are positive
for CD31 and Emcn can differentiate into ECs and further develop
into CD31hiEmcnhi vessels, which is of great
significance to bone regeneration during DO. In addition, PDGF-BB
is proposed to induce the proliferation, migration and angiogenesis
of EPCs (40) that further
stimulate the activity of BMSCs via PDGF-BB induction (32). Moreover, it is also speculated
that PDGF-BB mobilizes cells of mesenchymal origin, stabilizes
newly formed vessels and orchestrates cellular components for
osteoblast differentiation (41). Therefore, it is hypothesized that
the underlying mechanism by which TFRD promotes the formation of
CD31hiEmcnhi vessels may be via augmenting
the activity of PDGF-BB secreted by EPCs rather than preosteoclasts
during DO.
As shown in the present study, TFRD administration
to the rats significantly increased the abundance of
CD31hiEmcnhi vessels. However, the effect was
notably blocked by PDGF-BB-Ab and blocking PDGF-BB resulted in an
obvious decrease of CD31hiEmcnhi vessels in
the DO model. Moreover, the western blotting and RT-qPCR analyses
further demonstrated that exposure of EPCs to PDGF-BB-Ab under
stress conditions downregulated the expression levels of p-PDGF-BB,
VEGF, RUNX2 and OSX. As commonly known, AKT and p-ERK1/2 are the
primary downstream mediators of the well-known PDGF-BB pathway
(42) and are involved in
angiogenesis and osteogenesis (39,43,44). The present results revealed that
the levels of activated AKT and ERK1/2 were significantly increased
after TFRD treatment, but significantly reduced by blocking
PDGF-BB, which indicated that TFRD may enhance
CD31hiEmcnhi vessel formation during DO via
the PDGF-BB-mediated signaling pathway. To the best of our
knowledge, this study is the first to demonstrate that TFRD can
enhance angiogenic-osteogenic coupling by promoting
PDGF-BB-mediated CD31hiEmcnhi vessel
generation and subsequent bone repair during DO. In addition,
although naringin and neoeriocitrin are the primary active
compounds in TFRD, the pharmacological effects of naringin and
neoeriocitrin are not completely equivalent to those of TFRD. As
reported previously, naringin has been found to exert positive
effects on proliferation and osteogenic differentiation of BMSCs
and MC3T3-E1 cells, but was found to make little difference to
proliferation and angiogenesis of EPCs (25,45,46). By contrast, our previous study
indicated that TFRD could promote callus and vessel formation
inside the bone defect gap during DO (26). More importantly, it was found in
the present study that TFRD could facilitate specific
CD31hiEmcnhi vessel formation, which is a
novel finding concerning the pharmacological effects of TFRD.
However, whether this effect is related to naringin, neoeriocitrin
or other active ingredients of TFRD remains unclear. At present,
the effect of naringin or neoeriocitrin on
CD31hiEmcnhi vessels has not yet been
reported, and little research on this topic has been performed.
Therefore, in order to improve our knowledge and investigate all
possible pharmacological effects of TFRD, it is necessary to select
naringin or neoeriocitrin as a positive control in future
studies.
In conclusion, the present study provided the first
evidence that TFRD could facilitate
CD31hiEmcnhi vessel formation and
subsequently enhance angiogenic-osteogenic coupling during DO via
the PDGF-BB/VEGF/RUNX2/OSX signaling axis. Moreover,
CD31hiEmcnhi vessels may have potential as a
novel therapeutic target for DO, and TFRD may represent a promising
drug for promoting bone regeneration in DO. Thus, the present study
offered a novel insight into the underlying mechanism of DO-induced
bone regeneration.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundations of China (grant no. 81774337), the Basic Research
Project of Science and Technology Department of Yunnan Province
(grant nos. 202101AZ070001-123, 202201AU070120), the Doctoral Fund
Project of Kunming Municipal Hospital of Traditional Chinese
Medicine, and Kunming Health Science and Technology Talent
Cultivation Project and ‘Ten Hundred Thousand’ talent project
[grant no. 2020-SW (Reserve Personnel)-52].
Availability of data and materials
All data generated or analyzed during this study
are included in this published article.
Authors' contributions
ZS, ZC and GC designed the study and prepared the
manuscript. ZS, YZ, HL, HC and MH performed the animal experiments.
YZ and ZL performed the cell experiments. ZS and ZC analyzed the
data. The research was conceived by WD and ZJ. Data acquisition was
performed by WD and YG. ZS and ZJ confirm the authenticity of all
the raw data. The custom-made circle external device was designed
by ZJ. All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
All animal care and experimental procedures were
approved by the Institutional Animal Ethics Committee of the First
Affiliated Hospital of Guangzhou University of Traditional Chinese
Medicine (approval no. TCMF1-2018002; Guangzhou, China).
Patient consent for publication
Not applicable.
Competing interests
gThe authors declare that they have no competing
interests.
References
|
1
|
Nauth A, McKee MD, Einhorn TA, Watson JT,
Li R and Schemitsch EH: Managing bone defects. J Orthop Trauma.
25:462–466. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mauffrey C, Barlow BT and Smith W:
Management of segmental bone defects. J Am Acad Orthop Surg.
23:143–153. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li W, Zhu S and Hu J: Bone regeneration is
promoted by orally administered bovine lactoferrin in a rabbit
tibial distraction osteogenesis model. Clin Orthop Relat Res.
473:2383–2393. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sun Y, Xu J, Xu L, Zhang J, Chan K, Pan X
and Li G: MiR-503 promotes bone formation in distraction
osteogenesis through suppressing smurf1 expression. Sci Rep.
7:4092017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sun YX, Zhang JF, Xu J, Xu LL, Wu TY, Wang
B, Pan XH and Li G: MiRNA-144-3p inhibits bone formation in
distraction osteogenesis through targeting Connexin 43. Oncotarget.
8:89913–89922. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jia Y, Qiu S, Xu J, Kang Q and Chai Y:
Exosomes secreted by young mesenchymal stem cells promote new bone
formation during distraction osteogenesis in older rats. Calcif
Tissue Int. 106:509–517. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jia YC, Zhu Y, Qiu S, Xu J and Chai Y:
Exosomes secreted by endothelial progenitor cells accelerate bone
regeneration during distraction osteogenesis by stimulating
angiogenesis. Stem Cell Res Ther. 10:122019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Montes-Medina L, Hernández-Fernández A,
Gutiérrez-Rivera A, Ripalda-Cemboráin P, Bitarte N, Pérez-López V,
Granero-Moltó F, Prosper F and Izeta A: Effect of bone marrow
stromal cells in combination with biomaterials in early phases of
distraction osteogenesis: An experimental study in a rabbit femur
model. Injury. 49:1979–1986. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Paley D: Problems, obstacles, and
complications of limb lengthening by the Ilizarov technique. Clin
Orthop Relat Res. 250:81–104. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dhaliwal R, Kunchur K and Farhadieh R:
Review of the cellular and biological principles of distraction
osteogenesis: An in vivo bioreactor tissue engineering model. J
Plast Reconstr Aesthet Surg. 69:e19–e26. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tomlinson RE and Silva MJ: Skeletal blood
flow in bone repair and maintenance. Bone Res. 1:311–322. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gerber HP and Ferrara N: Angiogenesis and
bone growth. Trends Cardiovasc Med. 10:223–228. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Saran U, Piperni SG and Chatterjee S: Role
of angiogenesis in bone repair. Arch Biochem Biophys. 561:109–117.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brandi ML and Collin-Osdoby P: Vascular
biology and the skeleton. J Bone Miner Res. 21:183–192. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Grosso A, Burger MG, Lunger A, Schaefer
DJ, Banfi A and Maggio ND: It takes two to tango: Coupling of
angiogenesis and osteogenesis for bone regeneration. Front Bioeng
Biotechnol. 5:682017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fang TD, Salim A, Xia W, Nacamuli RP,
Guccione S, Song HM, Carano RA, Filvaroff EH, Bednarski MD, Giaccia
AJ and Longaker MT: Angiogenesis is required for successful bone
induction during distraction osteogenesis. J Bone Miner Res.
20:1114–1124. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kusumbe AP, Ramasamy SK and Adams RH:
Coupling of angiogenesis and osteogenesis by a specific vessel
subtype in bone. Nature. 507:323–328. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ramasamy SK, Kusumbe AP, Wang L and Adams
RH: Endothelial notch activity promotes angiogenesis and
osteogenesis in bone. Nature. 507:376–380. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Peng Y, Wu S, Li Y and Crane JL: Type H
blood vessels in bone modeling and remodeling. Theranostics.
10:426–436. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang J, Yin H, Rao SS, Xie PL, Cao X, Rao
T, Liu SY, Wang ZX, Cao J, Hu Y, et al: Harmine enhances type H
vessel formation and prevents bone loss in ovariectomized mice.
Theranostics. 8:2435–2446. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu R, Yallowitz A, Qin A, Wu Z, Shin DY,
Kim JM, Debnath S, Ji G, Bostrom MP, Yang X, et al: Targeting
skeletal endothelium to ameliorate bone loss. Nat Med. 24:823–833.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang J, Gao Y, Cheng P, Li D, Jiang H, Ji
C, Zhang S, Shen C, Li J, Song Y, et al:
CD31hiEmcnhi Vessels support new trabecular
bone formation at the frontier growth area in the bone defect
repair process. Sci Rep. 7:49902017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fan YG and Zhan HS: Orthopaedics of
traditional chinese medicine. People's Medical Publishing House,
Beijing. 29–31. 2005.
|
|
24
|
Yao W, Zhang H, Jiang X, Mehmood K, Iqbal
M, Li A, Zhang J, Wang Y, Waqas M, Shen Y and Li J: Effect of total
flavonoids of on tibial dyschondroplasia by regulating BMP-2 and
Runx2 expression in chickens. Front Pharmacol. 9:12512018.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen LL, Lei LH, Ding PH, Tang Q and Wu
YM: Osteogenic effect of Drynariae rhizoma extracts and Naringin on
MC3T3-E1 cells and an induced rat alveolar bone resorption model.
Arch Oral Biol. 56:1655–1662. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Song S, Gao Z, Lei X, Niu Y, Zhang Y, Li
C, Lu Y, Wang Z and Shang P: Total flavonoids of drynariae rhizoma
prevent bone loss induced by hindlimb unloading in rats. Molecules.
22:10332017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shen Z, Jiang ZW, Li D, Zhang Y, Li ZG,
Chen HM, et al: Comparison of two types of tonifying kidney in the
mechanism of angiogenesis and osteogenesis coupling based on
distraction osteogenesis. Chin J Tradit Chin Med Pharm.
34:2150–2155. 2019.
|
|
28
|
Shen Z, Lin H, Chen G, Zhang Y, Li Z, Li
D, Xie L, Li Y, Huang F and Jiang Z: Comparison between the induced
membrane technique and distraction osteogenesis in treating
segmental bone defects: An experimental study in a rat model. PLoS
One. 14:e02268392019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Song SH, Zhai YK, Li CQ, Yu Q, Lu Y, Zhang
Y, Hua WP, Wang ZZ and Shang P: Effects of total flavonoids from
Drynariae Rhizoma prevent bone loss in vivo and in vitro. Bone Rep.
5:262–273. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yan Y, Chen H, Zhang H, Guo C, Yang K,
Chen K, Cheng R, Qian N, Sandler N, Zhang YS, et al: Vascularized
3D printed scaffolds for promoting bone regeneration. Biomaterials.
190–191. 97–110. 2019.PubMed/NCBI
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang M, Ahn W, Kim S, Hong HS, Quan C and
Son Y: Endothelial precursor cells stimulate pericyte-like coverage
of bone marrow-derived mesenchymal stem cells through
platelet-derived growth factor-BB induction, which is enhanced by
substance P. Microcirculation. 24:e123942017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Naruse K, Yamada T, Sai XR, Hamaguchi M
and Sokabe M: Pp125FAK is required for stretch dependent
morphological response of endothelial cells. Oncogene. 17:455–463.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ilizarov GA: The tension-stress effect on
the genesis and growth of tissues. Part I. The influence of
stability of fixation and soft-tissue preservation. Clin Orthop
Relat Res. 238:249–281. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Stegen S, van Gastel N and Carmeliet G:
Bringing new life to damaged bone: The importance of angiogenesis
in bone repair and regeneration. Bone. 70:19–27. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang L, Zhou F, Zhang P, Wang H, Qu Z, Jia
P, Yao Z, Shen G, Li G, Zhao G, et al: Human type H vessels are a
sensitive biomarker of bone mass. Cell Death Dis. 8:e27602017.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xie H, Cui Z, Wang L, Xia Z, Hu Y, Xian L,
Li C, Xie L, Crane J, Wan M, et al: PDGF-BB secreted by
preosteoclasts induces angiogenesis during coupling with
osteogenesis. Nat Med. 20:1270–1278. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu L, Zong C, Li B, Shen D, Tang Z, Chen
J, Zheng Q, Tong X, Gao C and Wang J: The interaction between β1
integrins and ERK1/2 in osteogenic differentiation of human
mesenchymal stem cells under fluid shear stress modelled by a
perfusion system. J Tissue Eng Regen Med. 8:85–96. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lee DY, Cho TJ, Kim JA, Lee HR, Yoo WJ,
Chung CY and Choi IH: Mobilization of endothelial progenitor cells
in fracture healing and distraction osteogenesis. Bone. 42:932–941.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang H, Yin Y, Li W, Zhao X, Yu Y, Zhu J,
Qin Z, Wang Q, Wang K, Lu W, et al: Over-expression of PDGFR-β
promotes PDGF-induced proliferation, migration, and angiogenesis of
EPCs through PI3K/Akt signaling pathway. PLoS One. 7:e305032012.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Caplan AI and Correa D: PDGF in bone
formation and regeneration: New insights into a novel mechanism
involving MSCs. J Orthop Res. 29:1795–1803. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Heldin CH, Lennartsson J and Westermark B:
Involvement of platelet-derived growth factor ligands and receptors
in tumorigenesis. J Intern Med. 283:16–44. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lee SJ, Namkoong S and Kim YM, Kim CK, Lee
H, Ha KS, Chung HT, Kwon YG and Kim YM: Fractalkine stimulates
angiogenesis by activating the Raf-1/MEK/ERK-and PI3K/Akt/
eNOS-dependent signal pathways. Am J Physiol Heart Circ Physiol.
291:H2836–H2846. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang J, Guan J, Qi X, Ding H, Yuan H, Xie
Z, Chen C, Li X, Zhang C and Huang Y: Dimethyloxaloxaloylglycine
promotes the angiogenic activity of mesenchymal stem cells derived
from iPSCs via activation of the PI3K/Akt pathway for bone
regeneration. Int J Biol Sci. 12:639–652. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kuang MJ, Zhang WH, He WW, Sun L, Ma JX,
Wang D and Ma XL: Naringin regulates bone metabolism in
glucocorticoid-induced osteonecrosis of the femoral head via the
Akt/Bad signal cascades. Chem Biol Interact. 304:97–105. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang P, Dai KR, Yan SG, Yan WQ, Zhang C,
Chen DQ, Xu B and Xu ZW: Effects of naringin on the proliferation
and osteogenic differentiation of human bone mesenchymal stem cell.
Eur J Pharmacol. 607:1–5. 2009. View Article : Google Scholar : PubMed/NCBI
|